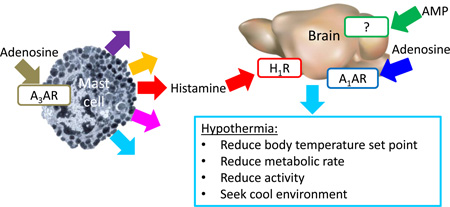
Abstract
Small mammals have the ability to enter torpor, a hypothermic, hypometabolic state, allowing impressive energy conservation. Administration of adenosine or adenosine 5'-monophosphate (AMP) can trigger a hypothermic, torpor-like state. We investigated the mechanisms for hypothermia using telemetric monitoring of body temperature in wild type and receptor knock out (Adora1−/−, Adora3−/−) mice. Confirming prior data, stimulation of the A3 adenosine receptor (AR) induced hypothermia via peripheral mast cell degranulation, histamine release, and activation of central histamine H1 receptors. In contrast, A1AR agonists and AMP both acted centrally to cause hypothermia. Commonly used, selective A1AR agonists, including N6-cyclopentyladenosine (CPA), N6-cyclohexyladenosine (CHA), and MRS5474, caused hypothermia via both A1AR and A3AR when given intraperitoneally. Intracerebroventricular dosing, low peripheral doses of Cl-ENBA [(±)-5'-chloro-5'-deoxy-N6-endo-norbornyladenosine], or using Adora3−/− mice allowed selective stimulation of A1AR. AMP-stimulated hypothermia can occur independently of A1AR, A3AR, and mast cells. A1AR and A3AR agonists and AMP cause regulated hypothermia that was characterized by a drop in total energy expenditure, physical inactivity, and preference for cooler environmental temperatures, indicating a reduced body temperature set point. Neither A1AR nor A3AR were required for fasting-induced torpor. A1AR and A3AR agonists and AMP trigger regulated hypothermia via three distinct mechanisms.
Keywords: hypothermia, adenosine, A1AR, A3AR, AMP, torpor
Graphical Abstract
1. Introduction
Mammals are endotherms, typically maintaining a warm core body temperature (Tb) of ~35 °C to 37 °C, depending on circadian fluctuations (Refinetti, 2010). Some small mammals, including mice, when exposed to an inadequate food supply in a quiet, cool environment use torpor to achieve significant energy conservation (Geiser, 2004; Melvin and Andrews, 2009). Torpor is an example of a regulated hypothermia (anapyrexia), which is characterized by a reduced Tb set point, metabolic rate, and physical activity, with Tb falling close to the environmental temperature. In regulated hypothermia multiple physiologic mechanisms are coordinated to cool the body, including vasodilation, decreased physical activity, reduced brown adipose tissue thermogenesis, and seeking a cool environment (Lute et al, 2014). Regulated hypothermia differs from hypothermia caused by cold exposure, where the body attempts, but is unable, to maintain Tb.
Hypothermia can also be caused by a wide variety of drugs and neurotransmitters (Clark and Lipton, 1985), although in most cases it is not documented if there is a reduction in Tb set point. Clinically, hypothermia is routinely employed to minimize tissue damage after hypoxic or ischemic injury (Arrich et al, 2012; Azzopardi et al, 2014). The hypothermia is typically induced with surface cooling. However, pharmacological reduction of the Tb might minimize undesired compensatory mechanisms (e.g., sympathetic activation, shivering). Development of a drug regimen that produces controlled, regulated hypothermia and avoids activating counter-regulatory responses would likely be of clinical utility (Drew et al, 2015; Tupone et al, 2014).
The hypothermic effect of adenosine was first reported in 1931 (Bennet and Drury, 1931). Once multiple adenosine receptor (AR) subtypes were identified, the hypothermia was attributed to action at A1AR, likely within the brain (Anderson et al, 1994). Peripheral dosing of N6-cyclohexyladenosine (CHA; see Fig. S1 for structures) elicited hypothermia, which was lost in A1AR knock out (Adora1−/−) mice (Johansson et al, 2001). CHA also caused hypothermia when infused directly into the nucleus of the solitary tract in rats (Tupone et al, 2013). However, some data suggested a contribution from non-A1AR mechanisms, as only partial attenuation of hypothermia caused by N6-R-phenylisopropyladenosine (R-PIA) was seen in Adora1−/− mice (Yang et al, 2007).
More recently, it has become clear that adenosine agonists also induce hypothermia in mice via A3AR. A critical observation was that hypothermia caused by R-PIA was attenuated in A3AR knock out (Adora3−/−) mice (Yang et al, 2010). In rodents, A3AR is expressed on immune cells (Borea et al, 2015) and A3AR agonists trigger mast cell degranulation (Auchampach et al, 1997; Fozard et al, 1996; Salvatore et al, 2000). We have recently shown that A3AR agonists activate peripheral mast cells releasing histamine, which then acts on central histamine H1 receptors to lower the Tb set point (Carlin et al, 2016).
Adenosine 5′-monophosphate (AMP) is a proposed natural regulator of torpor and injection of a large dose of AMP (500–3500 mg/kg, i.p.) causes hypothermia (Zhang et al, 2006). Given the dose size, one might consider if some of the effects of AMP are due to its conversion to free adenosine (Rittiner et al, 2012; Swoap et al, 2007). The observation that AMP’s hypothermic effects remain intact in mice lacking any one of the four AR subtypes, indicates that AMP is not acting non-redundantly via a single AR (Daniels et al, 2010). However, AMP-induced hypothermia was reported to be blocked by infusion of an A1AR antagonist in the pre-optic area (Muzzi et al, 2013). The mechanism by which AMP causes hypothermia is currently unclear.
While studying hypothermia caused by A3AR agonists, we observed that some nucleoside derivatives commonly used as A1AR agonists also had activity at the A3AR. This prompted the current re-examination and comparison of A1AR agonists, A3AR agonists, and AMP. Our data suggest that each of these can trigger hypothermia in mice via different mechanisms.
2. Materials and Methods
2.1 Mice
Male C57BL/6J and KitW-sh/W-sh mice (Stock #012861) (Grimbaldeston et al, 2005; Nigrovic et al, 2008) were obtained from the Jackson Laboratory. Adora1−/− mice on a C57BL/6J background were provided by Dr. Jurgen Schnermann (Sun et al, 2001) and genotyped by PCR (Adora1 reverse common primer 5'-ACATGGGGGTTGAACAGAGA, Adora1 forward primer 5'-AGCTGGCTACCGCTACACAT, and Neo forward primer 5'-TCTGGATTCATCGACTGTGG), producing 302 bp wild type and ~900 bp null allele products. Adora3−/− mice on a C57BL/6 background made by Merck (Salvatore et al, 2000) were provided by Dr. Stephen Tilley and genotyped by PCR (Adora3 reverse common primer 5'-ACTGGCCCATACACAACCTG, Adora3 forward primer 5'-AGACAATGAAATAGACGGTGGTG, and Neo forward primer 5'-ATGGAAGGATTGGAGCTACG), producing 208 bp wild type and ~400 bp null allele products. Mice were singly housed at ~22 °C with a 12:12-h light-dark cycle. Chow (NIH-07, Envigo Inc, Madison, WI) and water were available ad libitum. Mice were studied ≥ 7 days after any operation or prior treatment. Reuse of mice tends to reduce physical activity levels, presumably due to acclimatization. No specific effort was made to acclimatize mice to handling in individual experiments. Studies were approved by the Animal Care and Use Committee of National Institute of Diabetes and Digestive and Kidney Diseases.
2.2 Drugs
The following compounds (vehicle) were purchased from Sigma (St. Louis, MO) or Tocris (Minneapolis, MN): Cl-ENBA, (±)-5'-chloro-5'-deoxy-N6-endo-norbornyladenosine (Franchetti et al, 2009; Trivedi et al, 1989) (10% DMSO in saline); CHA, N6-cyclohexyladenosine (dissolved in DMSO, then diluted to 10% DMSO with saline); CPA, N6-cyclopentyladenosine (saline); pyrilamine (saline); AMP, adenosine 5'-monophosphate (saline); CCPA, 2-chloro-N6-cyclopentyladenosine. MRS5474, (1R,2R,3S,5S)-4-(2-chloro-6-((dicyclopropylmethyl)amino)-9H-purin-9-yl)bicyclo[3.1.0]hexane-2,3-diol, (dissolved in DMSO, then diluted with 9 volumes 30% PEG400) was synthesized as described (Tosh et al, 2012b).
2.3 Adenosine Receptor Binding Affinities
Binding affinity for mouse A1AR, A2AAR, and A3ARs was measured as described (Kreckler et al, 2006) using membranes from human embryonic kidney (HEK)-293 cells stably expressing individual recombinant mouse adenosine receptors and using the agonists [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-methyluronamide ([125I]AB-MECA; A1AR and A3AR) and [3H]CGS21680 (A2AAR) as radioligands. Nonspecific binding was defined using 100 µM adenosine-5'-N-ethylcarboxamide (NECA). Ki values were obtained using the Cheng-Prusoff equation from IC50 values calculated by non-linear regression analysis of specific binding data using GraphPad Prism software (San Diego, CA).
2.4 Central infusions
Mice were anesthetized with ketamine/xylazine (80/10 mg/kg, i.p.). Sterile guide cannulas (5.25 mm, 26 gauge, Plastics One, Roanoke, VA) were unilaterally implanted into the lateral ventricle (coordinates relative to bregma: −0.34 mm anterior, 1.0 mm lateral, and +1.7 mm ventral) and fixed with dental cement (Parkell, Edgewood, NY). Compounds in 5 µl were infused (0.5 µl/min) through a 33 gauge cannula protruding 0.5 mm past the tip of the guide cannula using PE-50 tubing fitted to a 5 µl syringe (Hamilton, Reno, NV). Cannula positions were verified by postmortem histological analysis.
2.5 Body Temperature and Activity Telemetry and Indirect Calorimetry
Tb and activity were measured continuously by telemetry (Starr Life Sciences, Oakmont, PA) using ER4000 energizer/ receivers, G2 E-mitters implanted intraperitoneally, and VitalView software with data collected each minute. Invalid E-mitter data points (defined as a change of ≥ 1 °C in one minute) were replaced by interpolation using flanking data. Any Tb ≤ 24 °C were scored as 24 °C. Unless noted otherwise, the average Tb response was calculated using the first 60 min after agonist dosing. Hypothermia duration is the duration of the interval between dosing and 300 min during which core temperature is <35 °C. Activity is the sum of counts from 10–60 min after A1AR agonist administration. Inhibitors were dosed 15 min before agonists. Indirect calorimetry was performed as described (Carlin et al, 2016). Experiments were performed at ~22 °C. Occasionally a mouse did not become hypothermic with a treatment that generally caused hypothermia. In these cases, the same mouse was retested ≥ 7 days later and routinely became hypothermic and the second data set was used.
2.6 Temperature Gradient
Environmental temperature preference was measured using an in-house apparatus by continuous video monitoring of the position of the mouse in a 45 cm thermal gradient, nominally 15 °C to 35 °C (Carlin et al, 2016). Data are reported as mean ± SEM. Significance (two-tailed p < 0.05) was determined by t test or ANOVA followed by post hoc Holm-Sidak multiple comparison tests.
3. Results
3.1 CHA induces hypothermia via both A1AR and A3AR
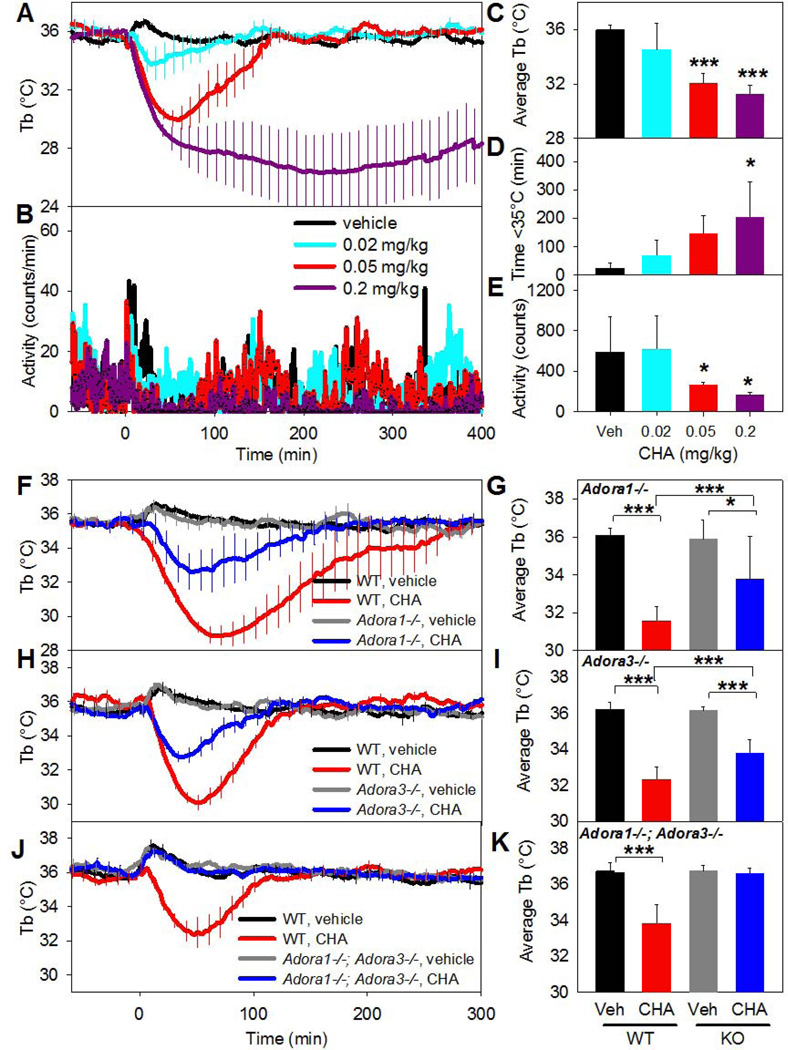
We investigated the ability of systemically-dosed adenosine agonists to cause hypothermia. CHA is ~290-fold selective for binding to mouse A1AR over A3AR (Table 1). With vehicle treatment, dosing-associated handling caused an increase in Tb and activity that lasted about an hour. Treatment of C57BL/6J mice with CHA (0.02–0.2 mg/kg, i.p.) caused a drop in Tb, accompanied by reduced physical activity (Fig. 1A–E). The Tb and physical activity decrease and the increase in duration of hypothermia were all dose-dependent.
Table 1.
Ligand binding affinity at adenosine receptors.
Ki, nM (or % inhibition at 10 µM)
| A1AR | Mouse A2AAR |
A3AR | A1AR | Human A2AAR |
A3AR | |
|---|---|---|---|---|---|---|
| Agonists | ||||||
| IB-MECAa,b | 5.9 | ~1000 | 0.087 | 51 | 2900 | 1.8 |
| Cl-IB-MECAa,b | 35 | ~10,000 | 0.18 | 220 | 5400 | 1.4 |
| MRS5474c,d | 3.20±0.05 | 34±9% | 1056±251 | 50 | 3950 | 470 |
| MRS5698e,e | 14% | 27% | 3.1 | 6% | 41% | 3.5 |
| MRS5841f,f | 15% | 1% | 11 | 16% | 7% | 1.9 |
| MRS5980g,g | 38% | 7% | 36 | 6% | 24% | 0.70 |
| CCPAh,i | 0.27 | 988 | 16 | 0.83 | 2270 | 43 |
| CPAc,i | 0.22±0.01 | 808±89 | 534±14 | 2.3 | 794 | 43 |
| CHAc,j | 2.15±0.37 | 1695±60 | 611±12 | 2.4 | 1390 | 73 |
| SPAc,c | 1.08±0.04 | 33±2% | 3111±183 | 7.92±1.55 | 3778±890 | 246±21 |
| Cl-ENBAc,k | 0.20±0.01 | 3985±358 | 2414±325 | 0.51 | 1340 | 1290 |
| CGS21680c,i | 193±38 | 10±2 | 48±11 | 289 | 27 | 67 |
| Antagonists | ||||||
| MRS1523n,l | 5,330 | 0% | 702 | >10,000 | 3660 | 19 |
| MRS1191c,l | 0% | 0% | 32±3% | >10,000 | >10,000 | 31 |
| SCH442416c,m | 765±36 | 1.27±0.04 | 6±2% | 35% | 4.1 | 67% |
| DPCPXn,i | 1.5 | 598 | 0% | 3.9 | 129 | 3960 |
Bold indicates a Ki ≥100-fold lower than the other two receptors of the same species. Superscripts indicate the references for literature data (mouse, human). SEMs are included for experimental results.
current results
Fig. 1. Systemic CHA causes hypothermia and decreased physical activity through both A1AR and A3AR.
(A, B) Tb and physical activity response to the indicated CHA doses injected i.p. into C57BL/6J mice. (C–E) Effect of CHA on average Tb (0–60 min), duration of hypothermia, and physical activity (10–60 min). Data are mean ± SEM, n=5/group; every tenth SEM is shown in A and SEMs were omitted in B for visual clarity. (F,G) Tb response to CHA (0.05 mg/kg, i.p.) or vehicle in C57BL/6J (WT) and Adora1−/− (KO) mice. (H,I) Tb response to CHA (0.05 mg/kg, i.p.) or vehicle in C57BL/6J (WT) and Adora3−/− (KO) mice. (J,K) Tb response to CHA (0.05 mg/kg, i.p.) or vehicle in C57BL/6J (WT) and Adora1−/−;Adora3−/− (DKO) mice. In F–K, data are mean ± SEM, n=5–10/group in a crossover design; every tenth SEM is shown in F, H, and J; * p<0.05, *** p<0.001.
The in vivo specificity of CHA was examined using Adora1−/− and Adora3−/− mice. Hypothermia elicited by CHA (0.05 mg/kg, i.p.) was attenuated in both Adora1−/− and Adora3−/− mice, but was completely lost in Adora1−/−;Adora3−/− double knock out mice (Fig. 1F–K). These data demonstrate that the hypothermic effect of this low dose of CHA is contributed by agonism at both A1AR and A3AR.
3.2 MRS5474 induces hypothermia via A3AR
Truncated nucleoside MRS5474, which contains a bicyclic substitute for ribose, is a moderately selective, full A1AR agonist that is well tolerated in vivo (Tosh et al, 2012b) and is ~280-fold selective for binding to mouse A1AR over A3AR (Table 1). It caused dose-dependent hypothermia and hypoactivity (Fig. S2A,B and data not shown). Treatment of Adora1−/− and Adora3−/− mice with MRS5474 (3 mg/kg, i.p.) demonstrated that the hypothermia was via A3AR, without a clear contribution from A1AR (Fig. S2C–F). Since centrally-active dopamine D2-like receptor agonists cause hypothermia (Nunes et al, 1991), we investigated the effect of raclopride, a D2-like receptor antagonist. Hypothermia induced by MRS5474 (3 mg/kg, i.p.) was not inhibited by pretreatment with raclopride (1 mg/kg, i.p.; data not shown).
3.3 CPA induces hypothermia via A3AR more than A1AR
CPA is another widely used adenosine agonist with better selectivity (~2400-fold) for binding to mouse A1AR over A3AR (Table 1). CPA caused a dose-dependent (0.03–3 mg/kg, i.p.) hypothermia and reduction in physical activity in C57BL/6J mice (Fig. S3A,B and data not shown). However, hypothermia induced by CPA (0.3 mg/kg, i.p.) was not significantly attenuated in Adora1−/− mice and was attenuated but still present in Adora3−/− mice (Fig. S3C–F). These data suggest that the hypothermic effects of this dose of CPA are via agonism at A3AR, likely with a lesser contribution from A1AR.
A related agonist, CCPA, is often used as a more selective A1AR agonist. However, we found that its binding affinity at the A3AR would make it even less desirable than CPA for use in mouse (Table 1). In general, there is a need to reexamine the affinities of widely used AR ligand probes across species (Alnouri et al, 2015). Therefore, we compare the affinities of various agonists and antagonists at human and mouse receptors. Some previous studies of adenosine and hypothermia used DPCPX as a selective antagonist of the A1AR. However, the selectivity ratios of DPCPX vary significantly between species (Table 1 and (Alnouri et al, 2015)).
3.4 In vivo Cl-ENBA is a more selective A1AR agonist
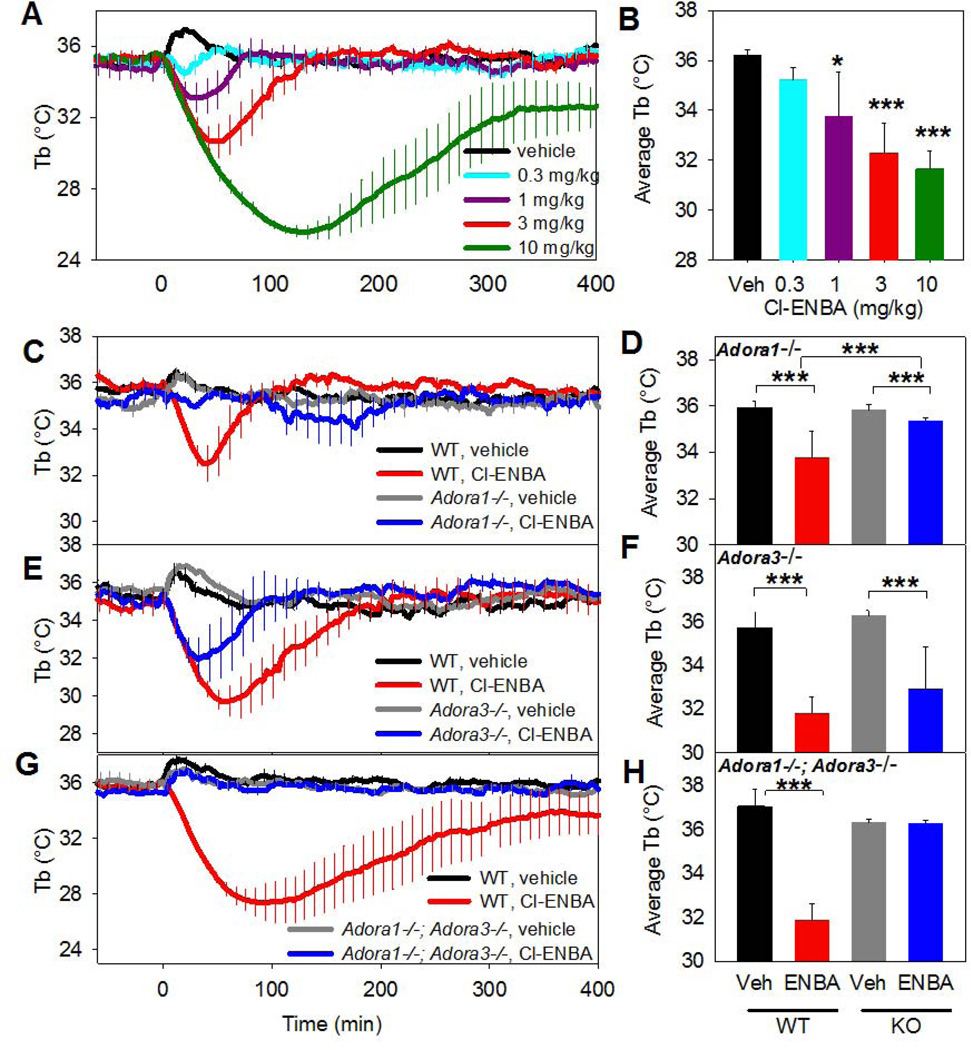
Cl-ENBA is the most selective A1AR agonist described, with ~2600-fold selectivity for binding to human A1AR vs. A3AR (Franchetti et al, 2009; Trivedi et al, 1989) and ~10,000-fold selectivity for mouse A1AR over A3AR (Table 1). Cl-ENBA (0.3–10 mg/kg, i.p.) caused dose-dependent hypothermia and hypoactivity (Fig. 2A,B and data not shown). Cl-ENBA (3 mg/kg, i.p.) hypothermia was not affected by pretreatment with raclopride (1 mg/kg, i.p.; data not shown). It was largely abolished in Adora1−/− mice (Fig. 2C,D), remained present in Adora3−/− mice (Fig. 2E,F), and was completely lost in Adora1−/−;Adora3−/− mice (Fig. 2G,H). These data (and see 3.5) demonstrate that the hypothermic effects of Cl-ENBA are mainly mediated via A1AR, but at higher doses there is probably also a contribution from A3AR.
Fig. 2. Systemic Cl-ENBA acts largely via A1AR to induce hypothermia.
(A,B) Tb response to the indicated Cl-ENBA dose injected i.p. into C57BL/6J mice. (C,D) Tb response to Cl-ENBA (3 mg/kg, i.p.) or vehicle in C57BL/6J (WT) and Adora1−/− (KO) mice. (E,F) Tb response to Cl-ENBA (3 mg/kg, i.p.) or vehicle in C57BL/6J (WT) and Adora3−/− (KO) mice. (G,H) Tb response to Cl-ENBA (3 mg/kg, i.p.) or vehicle in C57BL/6J (WT) and Adora1−/−;Adora3−/− (KO) mice. Data are mean ± SEM, n=3–6/group in a crossover design; every tenth SEM is shown in C, E, and G; * p<0.05*** p<0.001.
3.5 A1AR agonist-induced hypothermia occurs via central sites, while A3AR agonist-mediated hypothermia requires mast cells
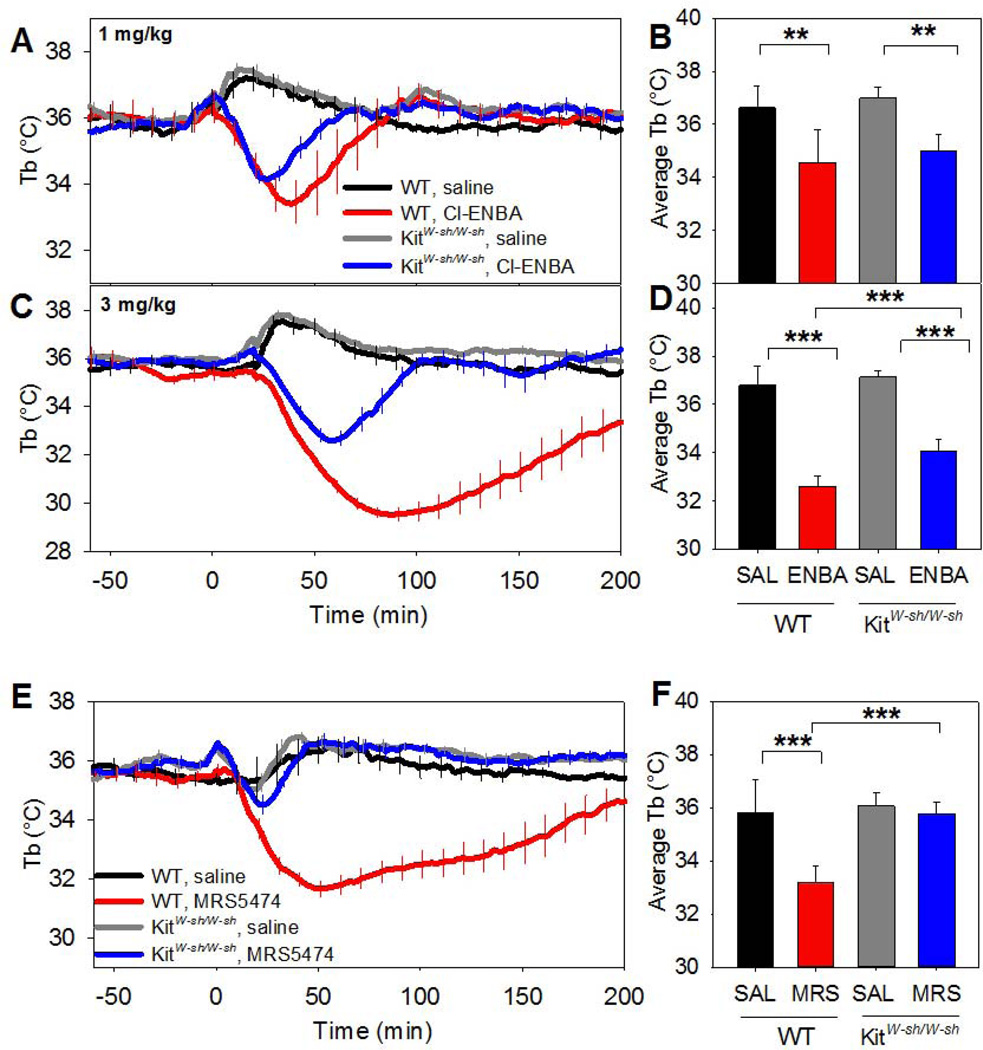
The poor in vivo A1AR selectivity of systemic CHA and CPA prompted us to re-examine the role of mast cells and histamine in A1AR and A3AR agonist-induced hypothermia. Hypothermia from A3AR agonists is caused by histamine release from activated mast cells (Carlin et al, 2016), while a role for mast cells in A1AR agonist-induced hypothermia has not been reported. Hypothermia from systemic Cl-ENBA at a dose of 1 mg/kg was largely intact in mast cell-deficient KitW-sh/W-sh mice (Fig. 3A,B), while the hypothermia with 3 mg/kg was significantly attenuated (Fig. 3C,D). In contrast, the hypothermic effect of MRS5474, at a dose that is selective for A3AR, was abolished in KitW-sh/W-sh mice (Fig. 3E,F). Pyrilamine, an histamine H1R antagonist, inhibited hypothermia from this dose, but did not block A1AR agonist-induced hypothermia (Fig. S4A–D). These results show that, in contrast to A3AR-mediated hypothermia, A1AR hypothermia does not require mast cells or H1R, and suggest that Cl-ENBA at 1 mg/kg i.p., but not 3 mg/kg, is relatively selective for A1AR.
Fig. 3. A1AR-mediated hypothermia is partially intact and A3AR-mediated hypothermia is abolished in KitW-sh/W-sh mice.
(A,B) Tb response to Cl-ENBA (1 mg/kg, i.p.) in KitW-sh/W-sh mice. (C,D) Tb response to Cl-ENBA (3 mg/kg, i.p.) in KitW-sh/W-sh mice. (E,F) Tb response to MRS5474 (3 mg/kg, i.p.) in KitW-sh/W-sh mice. Data are mean ± SEM, n=4–6/group in a crossover design; every tenth SEM is shown in A, C, and E; ** p<0.01.
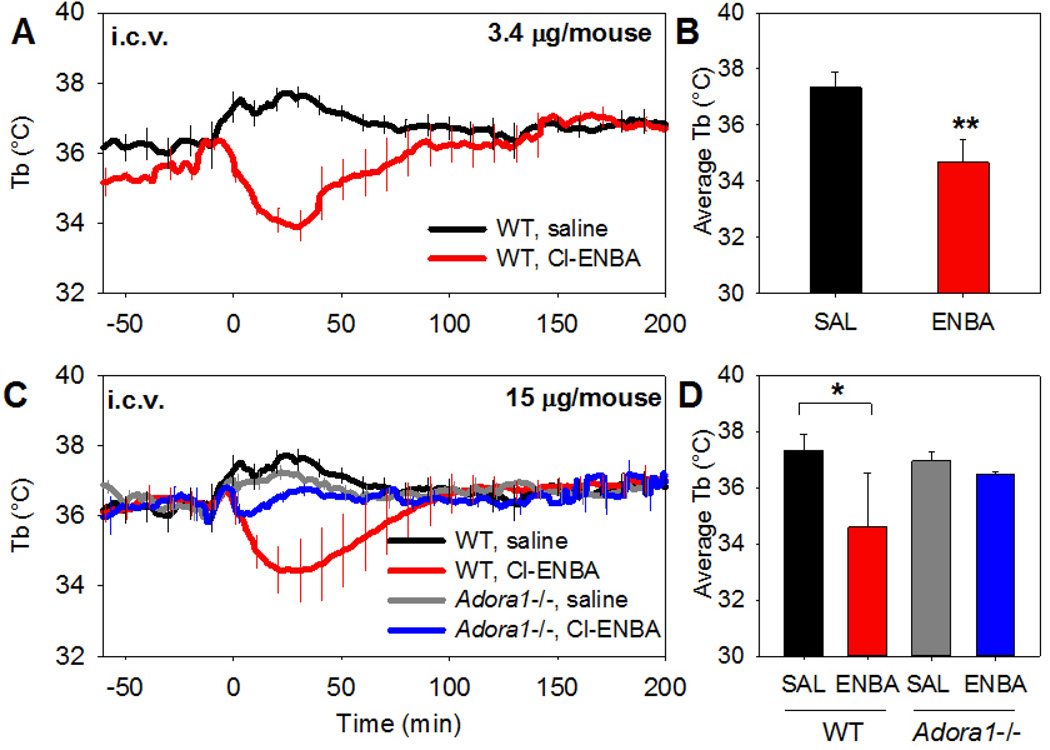
We next examined the site of A1AR agonist action to induce hypothermia. Cl-ENBA infused intracerebroventricularly (i.c.v.) at 3.4 µg/mouse (~0.12 mg/kg) caused a drop in Tb comparable to 1 mg/kg given systemically (Fig. 4A,B). The effect of i.c.v. Cl-ENBA was greatly diminished in Adora1−/− mice (Fig. 4C,D). These results indicate that Cl-ENBA is acting via central A1AR.
Fig. 4. Cl-ENBA causes hypothermia via central A1AR.
(A,B) Tb response to Cl-ENBA (3.4 µg/mouse) or vehicle injected i.c.v. into C57BL/6J. (C,D) Tb response to Cl-ENBA (15 µg/mouse) or vehicle injected i.c.v. into C57BL/6J or Adora1−/− mice. Data are mean ± SEM, n=3–6/group; every tenth SEM is shown in A, C; * p<0.05.
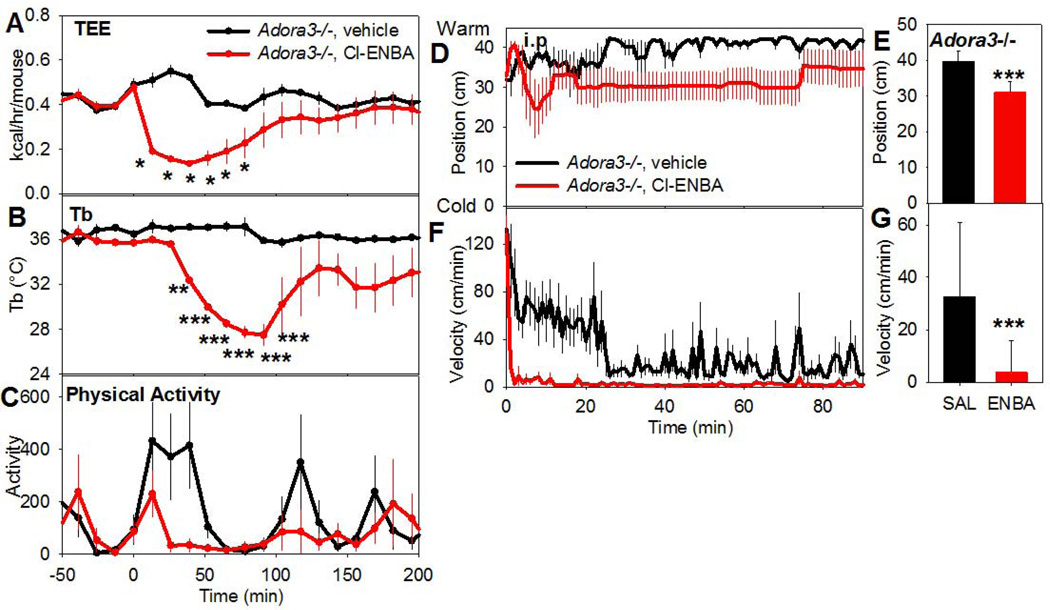
To avoid confounding due to the modest in vivo receptor specificity of the A1AR agonists, we used Adora3−/− mice to investigate mechanistic aspects of A1AR-driven hypothermia. Cl-ENBA administration reduced metabolic rate and physical activity before the Tb nadir (Fig. 5A–C). Additionally, Adora3−/− mice treated with Cl-ENBA had a modest preference for a cooler place in a thermal gradient (Fig. 5D–G). These results suggest that there is a reduced Tb set point and coordinated physiologic response to an A1AR agonist.
Fig. 5. Systemic Cl-ENBA-induced hypothermia is accompanied by a reduced metabolic rate and preference for a cooler environment.
The effect of Cl-ENBA (3 mg/kg, i.p.) or vehicle in Adora3−/− mice on (A) total energy expenditure (TEE), (B) Tb, and (C) physical activity. The TEE falls before Tb (nadir ~50 min vs ~100 min for Tb). Data are mean ± SEM, n=5/group. Position (D,E) and activity (F,G) of Adora3−/− mice treated with Cl-ENBA (3 mg/kg, i.p.) or vehicle and placed in a thermal gradient. Mean ± SEM, n=6/group, *p<0.05, ***p<0.001.
3.6 Neither A1AR nor A3AR is necessary for fasting-induced torpor
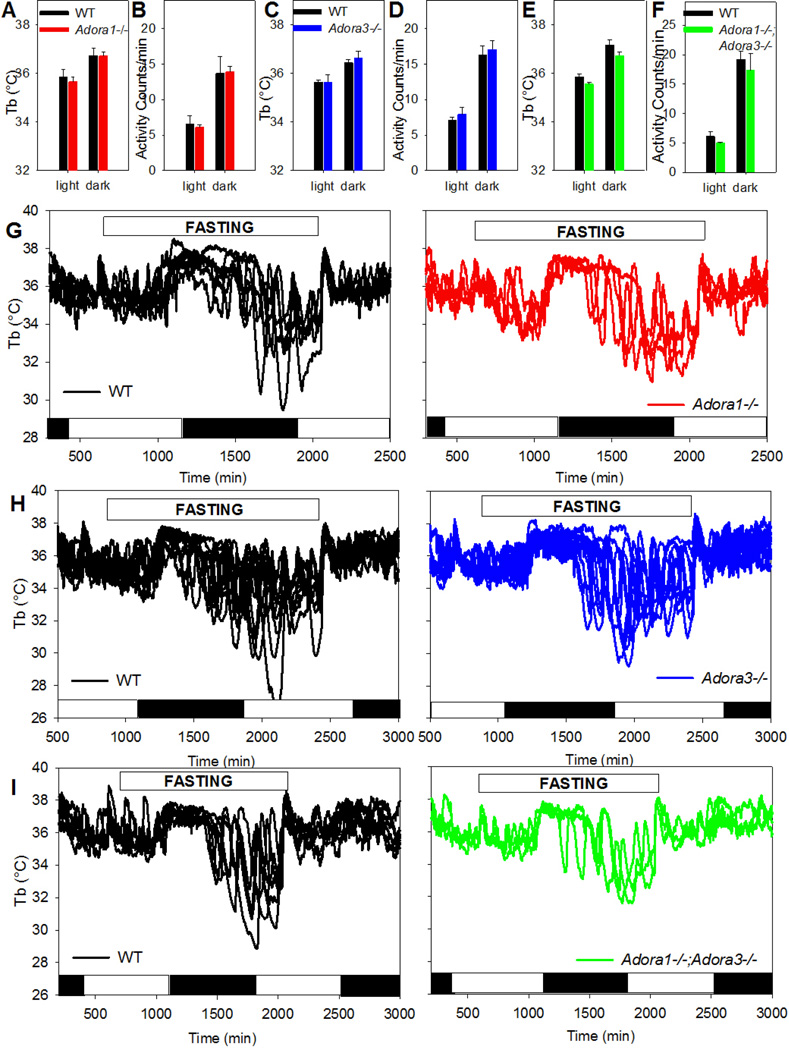
To better understand the role of adenosine in thermal physiology, baseline Tb and physical activity in light and dark phase were measured in Adora1−/−, Adora3−/−, and Adora1−/−;Adora3−/− mice. There were no clear differences between littermate control and Adora1−/− or Adora3−/− mice or Adora1−/−;Adora3−/− and age-matched C57BL/6J mice (Fig. 6A–F). A 24-hour fast elicited torpor in both Adora1−/− and Adora3−/− mice (Fig. 6G–H). In a test for receptor redundancy between A1AR and A3AR, Adora1−/−;Adora3−/− double knockout mice were found to enter fasting-induced hypothermia (Fig. 6I). These data demonstrate that neither A1AR nor A3AR, individually or in combination, is required for fasting-induced torpor.
Fig. 6. A1AR or A3AR is not required for fasting-induced hypothermia.
Baseline Tb (A) and activity (B) in WT (n=6) and Adora1−/− (n=4) littermates during the light or dark phase. Baseline Tb (C) and activity (D) in WT (n=5) and Adora3−/− (n=4) littermates during the light or dark phase. Baseline Tb (E) and activity (F) in C57BL/6J (n=6) and Adora1−/−;Adora3−/− (n=3) mice. during the light or dark phase. Individual traces of Tb in (G) WT and Adora1−/− littermates, (H) WT and Adora3−/− littermates, and (I) C57BL/6J and Adora1−/−;Adora3−/− mice during a 24 hour fast. White and black bars indicate light and dark phases, respectively. Data are mean ± SEM.
3.7 AMP does not require A1AR or A3AR to induce hypothermia and can act centrally
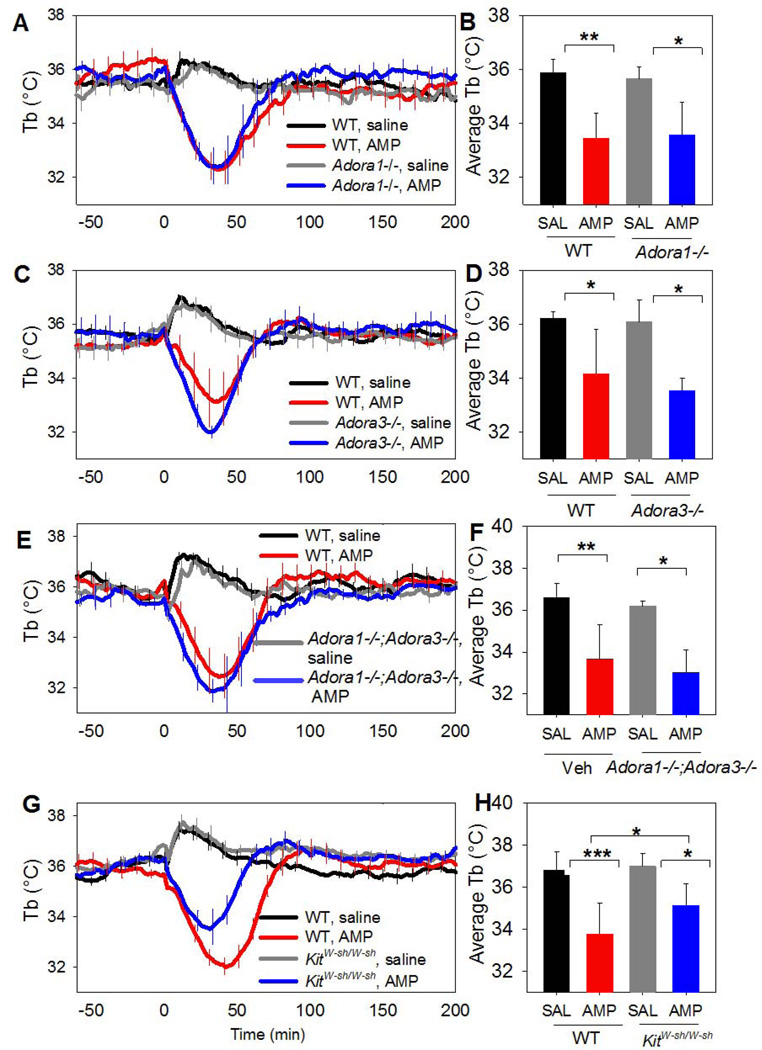
Large AMP doses (500–3500 mg/kg, i.p.) induce torpor (Zhang et al, 2006). We studied a modest dose (100 mg/kg, i.p.) to minimize possible effects of impurities or breakdown products. The AMP-induced hypothermia was intact in Adora1−/− and Adora3−/− mice (Fig. 7A–D). It was also intact in Adora1−/−;Adora3−/− double knockout and partially intact in KitW-sh/W-sh mice (Fig. 7E–H). Pretreatment of Adora1−/− mice with the brain-penetrant H1R antagonist pyrilamine (10 mg/kg, i.p.) did not ablate the hypothermia (data not shown). Similarly, pretreatment with raclopride (2 mg/kg, i.p.) did not block the hypothermia (data not shown). These data demonstrate that the hypothermic effects of AMP do not require mast cells, A1AR, A3AR, H1R, D2R, or the combination of A1AR/A3AR.
Fig. 7. Systemic AMP causes hypothermia independent of A1AR, A3AR, and mast cells.
(A,B) Tb response to AMP (100 mg/kg, i.p.) in C57BL/6J (WT) and Adora1−/− (KO) mice (n=6/group). (C,D) Tb response to AMP (100 mg/kg, i.p.) in C57BL/6J (WT) or Adora3−/− mice (n=4/group). (E,F) Tb response to AMP (100 mg/kg, i.p.) in C57BL/6J and Adora1−/−;Adora3−/− mice (n=5–6/group). (G,H) Tb response to AMP (100 mg/kg, i.p.) in C57BL/6J (WT) or KitW-sh/Wsh mice (n=7–8/group). Data are mean ± SEM; every tenth SEM is shown in A, C, E, and G; * p<0.05, ** p<0.01, *** p<0.001.
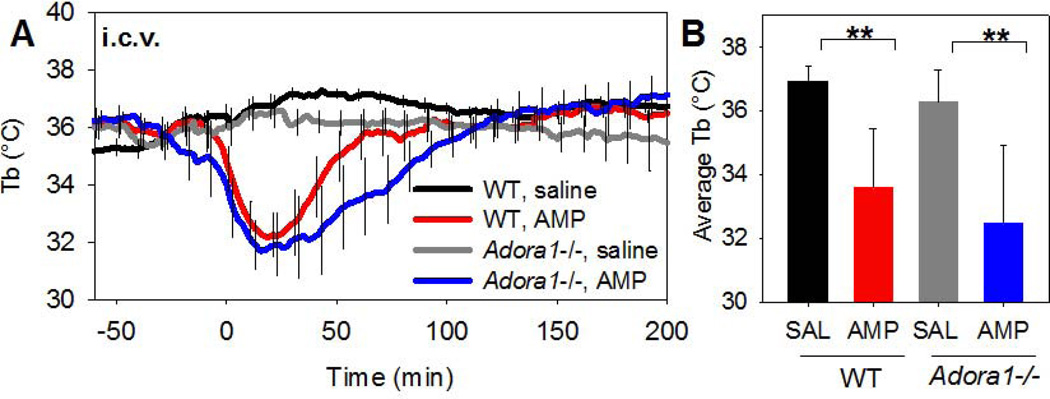
To investigate the site of action where AMP acts to cause hypothermia, AMP was infused i.c.v. into C57BL/6J mice. A low i.c.v. dose (100 µg/mouse, ~3.3 mg/kg) induced a drop in Tb that was comparable to a 30-fold larger (100 mg/kg) systemic dose and the hypothermia was not attenuated in Adora1−/− mice (Fig. 8A,B).
Fig. 8. Centrally-administered AMP induces hypothermia.
(A,B) Tb response to 100 µg (~3.3 mg/kg) AMP injected i.c.v. into C57BL/6J mice or Adora1−/− (n=4/group). Data are mean ± SEM; every tenth SEM is shown; * p<0.05, ** p<0.01.
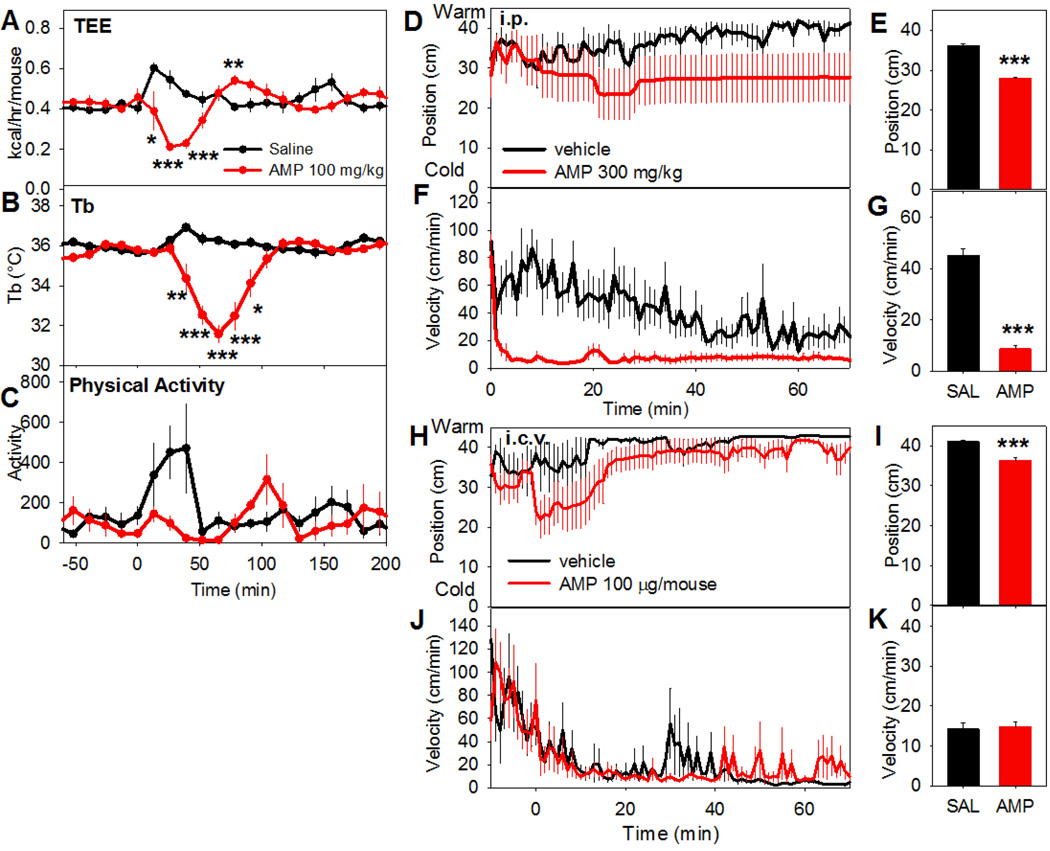
Mice treated with AMP (100 mg/kg, i.p.) demonstrated a drop in metabolic rate that preceded the hypothermia (Fig. 9A-C). We did not observe a clear change in ambient temperature preference at this dose (not shown). With a higher dose (300 mg/kg, i.p.), the mice generally moved to a cooler region of a thermal gradient and became immobile (Fig. 9D–G). When AMP was administered centrally (100 µg/mouse, ~3.3 mg/kg, i.c.v.), there was a similar movement toward cooler environmental temperature but without a significant drop in activity (Fig. 9H–K). These findings are consistent with AMP acting in the brain to reduce the Tb set point.
Fig. 9. AMP-induced hypothermia is accompanied by a reduced metabolic rate and preference for a cooler environment.
The effect of AMP (100 mg/kg, i.p.) or vehicle in C57BL/6J mice on (A) total energy expenditure (TEE), (B) Tb, and (C) physical activity in a calorimetry chamber. The TEE falls before Tb (nadir ~40 min vs ~60 min). Data are mean ± SEM, n=5/group. Position (D,E) and activity (F,G) of C57BL/6J mice treated with AMP (300 mg/kg, i.p.) or vehicle and placed in a thermal gradient. Position (H,I) and activity (J,K) of C57BL/6J mice treated with AMP (100 µg, i.c.v.) or vehicle and placed in a thermal gradient. Mean ± SEM, n=5–6/group, *p<0.05, ***p<0.001
4. Discussion
We demonstrate at least three distinct adenosine-related routes to trigger hypothermia in mice (Fig. S5): 1) activation of central A1AR, 2) peripheral mast cell activation by A3AR agonists, releasing histamine, which stimulates histamine H1 receptors, and 3) a central AMP-mediated mechanism. Additionally, we report that some agonists commonly used as A1AR probes also activate A3AR in vivo.
4.1 Suboptimal in vivo A1AR agonist selectivity
Much understanding of adenosine physiology has been acquired with selective ligands (Fredholm, 2014) (Table 1). CHA and CPA were developed to be selective for A1AR vs “A2AR” (Moos et al, 1985). The subsequent identification of four ARs and the generation of receptor-ablated animals now permit improved dissection of the specific receptors mediating adenosine actions. To our initial surprise, CHA, MRS5474, and CPA all produced hypothermia via A3AR, despite their much higher affinity for A1AR over A3AR. This is presumably due to higher drug exposure at peripheral mast cell A3AR, with poor availability at brain A1AR. This is striking, with a 2000-fold advantage in binding affinity being nullified by pharmacokinetic considerations. We did not measure peripheral A1AR effects of these agonists (eg., on heart rate, blood pressure, or kidney function (Sun et al, 2001; Vallon et al, 2006)) for comparison with the peripheral mast cell A3AR effect.
This observation suggests a reexamination of prior in vivo studies using CHA and CPA as A1AR probes. In mice, CHA is typically used at i.p. doses of 0.2 to 1 mg/kg, with ED50s in locomotor assays of 0.3 to 1.7 mg/kg (Akula and Kulkarni, 2014; Heffner et al, 1989; Zarrindast et al, 1993). Doses of 0.1 to 1 mg/kg are reported in other species (Jinka et al, 2010; Jinka et al, 2015; Olson et al, 2013). We demonstrate that both A1AR and A3AR contribute to hypothermia at a CHA dose of 0.05 mg/kg. Similarly, CPA is typically used in mice at doses of 0.2 to 1 mg/kg i.p., with ED50s in locomotor assays of 1.0 to 1.7 mg/kg (El Yacoubi et al, 2000; Heffner et al, 1989; Listos et al, 2011; Von Lubitz et al, 1993). Comparable doses (0.1 to 1 mg/kg) are used in rats (Heurteaux et al, 1995; O'Neill et al, 2014; Ramos-Zepeda and Herrero, 2013). Our results with 0.3 mg/kg show that both A1AR and A3AR contribute to CHA-induced hypothermia. Thus, commonly used i.p. doses of both CPA and CHA stimulate both A1AR and A3AR and some effects attributed to A1AR may actually be due completely, or in part, to A3AR. This is of particular concern when the pharmacodynamic effect requires brain-penetration for an A1AR ligand but only peripheral exposure for A3AR. A non-brain penetrant agonist, SPA, was found to be highly selective for the mouse A1AR (~3000-fold vs A3AR) and could be useful in future in vivo studies.
There have been clinical trials of A1AR agonists for conditions such as diabetic foot pain, diabetes, glaucoma, and cardiac arrhythmias, many of which have failed (Elzein and Zablocki, 2008). Other envisioned therapeutic applications of A1AR agonists are based on reported neuroprotective, antiseizure, anti-nociceptive, sleep promoting, and antidepressant effects (Tosh et al, 2012b). The fact that some in vivo actions of agonists, that appear selective in in vitro receptor assays, also might include A3AR effects, should be considered in future development of such agonists. A3AR agonists display some similar effects, such as neuroprotection and antinociception (Janes et al, 2016), which might contribute to efficacy of nominal A1AR agonists and would be difficult to separate mechanistically in clinical trials. The A3AR is expressed at relatively low levels in the mouse hippocampus, cerebellum and medulla (Janes et al, 2016; Yaar et al, 2005). Thus, although we found no effects of centrally administered A3AR agonists on Tb, there may be effects of this receptor on CNS function.
Cl-ENBA is a more selective A1AR agonist than CPA, CHA, and MRS5474 in the mouse for binding (Franchetti et al, 2009; Trivedi et al, 1989) (Table 1) and in vivo hypothermia. At 1 mg/kg i.p., Cl-ENBA was largely selective for A1AR, although at 3 mg/kg some of the effect was contributed by A3AR. Cl-ENBA is anti-nociceptive in the mouse at 0.5 mg/kg (Luongo et al, 2012), suggesting that this effect is via A1AR. Cl-ENBA’s greater selectivity makes it a preferred agonist for investigating in vivo A1AR physiology, at least in mice.
The Adora1−/− and Adora3−/− mice were essential for assessing in vivo ligand selectivity. In the absence of sufficiently selective A1AR agonists, using Adora3−/− mice eliminates confounding A3AR effects. Adenosine agonist affinities and selectivities can vary profoundly among species (Table 1) (Alnouri et al, 2015). Adenosine physiology can also vary by species--for example, A3AR is not present in some human mast cells (Rudich et al, 2012). In summary, the results particularly suggest caution when studying in vivo effects of A1AR when using i.p. dosing.
4.2 A3AR Hypothermia
A3AR agonists activate peripheral mast cells, releasing histamine, which then acts on central histamine H1 receptors to lower the Tb set point (Carlin et al, 2016). Here we examined the hypothermic effects of MRS5474 (Tosh et al, 2012b) and found that at 3 mg/kg i.p., MRS5474 is acting via A3AR. The hypothermia is lost in KitW-sh/W-sh mice, confirming that A3AR agonism is acting via mast cells. Since histamine is reported to both increase and decrease Tb (Tabarean, 2016), further study is required.
The kinetics of hypothermia induction via A3AR and A1AR are similar, despite the peripheral vs central sites of agonist action. The likely explanation is that heat loss (and thus hypothermia) occurs slowly compared to the rapid increase in circulating histamine and quick neural transmission.
4.3 A1AR and regulated hypothermia
The poor in vivo A1AR selectivity of peripherally-dosed agonists raises concern that some physiology attributed to A1AR might actually be due to A3AR. For hypothermia, the lack of a Tb effect by brain A3AR agonist indicates that studies using central agonist dosing are probably studying A1AR effects. Thus, the hypothermia elicited by local CHA delivery to the anterior hypothalamus of Syrian hamsters (Shintani et al, 2005) or to the nucleus of the solitary tract of rats (Tupone et al, 2013) remain plausibly attributed to A1AR. A recent rat study used brain-penetrant CHA to produce A1AR hypothermia combined with a non-brain-penetrant antagonist (8-(p-sulfophenyl)theophylline) to block peripheral A1AR-mediated bradycardia (Jinka et al, 2015). We interpret this experiment as additionally having hypothermia from peripheral CHA activation of rat mast cell A3AR (Fozard et al, 1996), which may not be blocked by 8-(p-sulfophenyl) theophylline (Gao et al, 2001).
4.4 AMP Hypothermia
Circadian fluctuation of plasma AMP concentration is a proposed endogenous regulator of torpor in rodents (Zhang et al, 2006). Large exogenous doses of AMP (500 to 3500 mg/kg) cause torpor and have protective effects in various injury models (Miao et al, 2015; Miao et al, 2012; Tao et al, 2011; Wang et al, 2014). There is no clearly identified receptor for AMP (although AMP has been proposed to be an A1AR ligand (Rittiner et al, 2012)). In our studies, AMP-induced hypothermia did not require mast cells, A1AR, A3AR, H1R, D2R-like, or a combination of A1AR/A3AR. Other nucleotides, such as ATP, ADP and cyclic-AMP, have hypothermic effects when injected into the hypothalamic region in cats (Dascombe and Milton, 1975), although the mechanisms have not been defined.
Proposed mechanisms for AMP-induced hypometabolism are that AMP uptake into erythrocytes causes increased 2,3-bisphosphoglycerate levels and reduced oxygen transport (Daniels et al, 2010) and/or that the AMP activates an intracellular energy depletion sensor, AMP-kinase (Melvin et al, 2009). The observation that metabolic rate reduction precedes the drop in Tb (Daniels et al, 2010) and our results that relatively small amounts of i.c.v. AMP elicit hypothermia and AMP-treated mice chose a cool environment, all indicate that AMP acts centrally to reduce the Tb set point, suggesting direct or indirect action at the hypothalamus. Muzzi et al. have shown that AMP injected into the mouse preoptic area induces hypothermia and that AMP reduces firing activity of preoptic area neurons in a DPCPX-sensitive manner (Muzzi et al, 2013). These observations and the Adora1−/− data can be reconciled by hypothesizing that AMP causes hypothermia via both A1AR-dependent and A1AR-independent mechanisms.
4.5 Fasting-induced hypothermia does not require A1AR or A3AR
Mice have a large surface area/volume ratio and therefore a disproportionally high energetic cost of staying warm, so they enter torpor to conserve energy (Abreu-Vieira et al, 2015; Hudson and Scott, 1979; Swoap, 2008). It is likely that the control of torpor is complex, integrating information on nutritional status, energy needs, and environmental situation. For example, leptin deficiency sensitizes to induction of torpor, but replacement of leptin in adipose-deficient mice does not prevent fasting-induced torpor (Gavrilova et al, 1999; Himms-Hagen, 1985).
There is much support for A1AR having a central role in regulating torpor, including daily torpor in mice (Iliff and Swoap, 2012), seasonal torpor in arctic ground squirrel (Jinka et al, 2011), and hibernation in Syrian hamsters (Tamura et al, 2005). These conclusions are based on pharmacologic manipulation, showing torpor induction with agonist and exit with antagonist treatment. However, the observation that mice lacking A1AR (or A3AR, or both) enter torpor upon fasting demonstrates that A1AR is not required. However, due to the intrinsic variability of fasting-induced hypothermia and the size of our dataset, we cannot rule out quantitative or probabilistic effects of A1AR and/or A3AR on this process. This illustrates the utility of the knock out mice, highlights the limitations of purely pharmacologic approaches, and suggests searching for the other regulators of torpor-related states. Presumably, like leptin, A1AR signaling is one of multiple inputs that are integrated to cause entry into (or exit from) torpor.
4.6 Limitations
Hypothermia can vary with inter-experiment differences, particularly in duration (with larger standard errors at longer times after dosing). Possible contributing factors include mouse age, weight, and adiposity, acclimation to handling, season, environmental temperature, and the noise/activity level in the animal facility. To assure that this variability does not compromise the conclusions, we focus on the all-or-none effects and have not attempted to quantitate the fraction of a mixed effect that is due to A1AR versus A3AR.
While knockout mice are excellent probes of in vivo ligand specificity, compensation for receptor loss may occur. No compensatory changes in Adora1−/− or Adora3−/− mice have been described (Johansson et al, 2001; Salvatore et al, 2000), but it is possible that undetected compensatory changes do occur. Importantly, there may also be redundant sites of action, for example AMP may act at both A1AR and other site(s).
4.7 Common themes in A1AR, A3AR, and AMP Hypothermia
A1AR and A3AR agonists and AMP all trigger a regulated hypothermia that is not inhibited by D2R antagonist. Yet the three act in different places in the body, with different ligands and ligand affinities. Extracellular adenosine (and AMP) can be thought of as indicators of physiologically unfavorable situations or ‘extreme physiology’ (Fredholm, 2014). It would be advantageous for the body to be able to broadly sense many forms of pathology and funnel the signals to common response mechanisms such as hypothermia. The ability to go into torpor is a widely conserved behavior that reduces injury, conserves energy, and increases survival. Understanding the neural regulation of hypothermia will identify signaling pathways and may lead to improved pharmacologic approaches to hypothermia induction and maintenance in clinical practice.
Supplementary Material
Highlights.
Some commonly used A1AR agonists are not selective; they act at A3AR
A1AR, A3AR, and AMP are three distinct adenosine-related inducers of hypothermia
Neither A1AR nor A3AR is required for fasting-induced torpor
AMP can cause hypothermia via the brain, independent of A1AR and A3AR
Acknowledgments
We thank Jurgen Schnermann for discussions. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases [ZIA DK075063; ZIA DK031117] and by the National Heart, Lung and Blood Institute [R01Hl077707].
Abbreviations
- i.p.
intraperitoneal
- i.c.v.
intracerebroventricular
- AxAR
adenosine x receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds
AMP, adenosine 5'-monophosphate
CCPA, 2-chloro-N6-cyclopentyladenosine
CHA, N6-cyclohexyladenosine
Cl-ENBA, (±)-5'-chloro-5'-deoxy-N6-endo-norbornyladenosine
CPA, N6-cyclopentyladenosine
MRS5474, (1R,2R,3S,5S)-4-(2-chloro-6-((dicyclopropylmethyl)amino)-9H-purin-9-yl)bicyclo[3.1.0]hexane-2,3-diol
R-PIA, N6-R-phenylisopropyladenosine
SPA, N6-(p-sulfo-phenyl)adenosine
References
- Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab. 2015;4(6):461–470. doi: 10.1016/j.molmet.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula KK, Kulkarni SK. Effect of curcumin against pentylenetetrazol-induced seizure threshold in mice: possible involvement of adenosine A1 receptors. Phytother Res. 2014;28(5):714–721. doi: 10.1002/ptr.5048. [DOI] [PubMed] [Google Scholar]
- Alnouri MW, Jepards S, Casari A, Schiedel AC, Hinz S, Muller CE. Selectivity is species-dependent: Characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015;11(3):389–407. doi: 10.1007/s11302-015-9460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Sheehan MJ, Strong P. Characterization of the adenosine receptors mediating hypothermia in the conscious mouse. Br J Pharmacol. 1994;113(4):1386–1390. doi: 10.1111/j.1476-5381.1994.tb17151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database of Systematic Reviews. 2012;9 doi: 10.1002/14651858.CD004128.pub3. Art. No.: CD004128. [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, et al. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5'-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res. 1997;80(6):800–809. doi: 10.1161/01.res.80.6.800. [DOI] [PubMed] [Google Scholar]
- Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. The New England journal of medicine. 2014;371(2):140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- Bennet DW, Drury AN. Further observations relating to the physiological activity of adenine compounds. J Physiol. 1931;72(3):288–320. doi: 10.1113/jphysiol.1931.sp002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, et al. The A3 adenosine receptor: history and perspectives. Pharmacol Rev. 2015;67(1):74–102. doi: 10.1124/pr.113.008540. [DOI] [PubMed] [Google Scholar]
- Carlin JL, Tosh DK, Xiao C, Pinol RA, Chen Z, Salvemini D, et al. Peripheral Adenosine A3 Receptor Activation Causes Regulated Hypothermia in Mice That Is Dependent on Central Histamine H1 Receptors. J Pharmacol Exp Ther. 2016;356(2):475–483. doi: 10.1124/jpet.115.229872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WG, Lipton JM. Changes in body temperature after administration of acetylcholine, histamine, morphine, prostaglandins and related agents: II. Neurosci Biobehav Rev. 1985;9(3):479–552. doi: 10.1016/0149-7634(85)90023-5. [DOI] [PubMed] [Google Scholar]
- Daniels IS, Zhang J, O'Brien WG, 3rd, Tao Z, Miki T, Zhao Z, et al. A role of erythrocytes in adenosine monophosphate initiation of hypometabolism in mammals. The Journal of biological chemistry. 2010;285(27):20716–20723. doi: 10.1074/jbc.M109.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascombe MJ, Milton AS. The effects of cyclic adenosine 3',5'-monophosphate and other adenine nucleotides on body temperature. J Physiol. 1975;250(1):143–160. doi: 10.1113/jphysiol.1975.sp011046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew KL, Romanovsky AA, Stephen TK, Tupone D, Williams RH. Future approaches to therapeutic hypothermia: a symposium report. Temperature (Austin) 2015;2(2):168–171. doi: 10.4161/23328940.2014.976512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Menard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. British journal of pharmacology. 2000;129(7):1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert opinion on investigational drugs. 2008;17(12):1901–1910. doi: 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Valentine HL, Stone GA, Williams M. Adenosine A1 Receptors in Mammalian Brain: Species Differences in Their Intreration with Agonists and Antagonists. Drug Dev Res. 1986;9:85–93. [Google Scholar]
- Fozard JR, Pfannkuche HJ, Schuurman HJ. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur J Pharmacol. 1996;298(3):293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- Franchetti P, Cappellacci L, Vita P, Petrelli R, Lavecchia A, Kachler S, et al. N6-Cycloalkyl- and N6-bicycloalkyl-C5'(C2')-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J Med Chem. 2009;52(8):2393–2406. doi: 10.1021/jm801456g. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine--a physiological or pathophysiological agent? J Mol Med (Berl) 2014;92(3):201–206. doi: 10.1007/s00109-013-1101-6. [DOI] [PubMed] [Google Scholar]
- Gao Z, Li BS, Day YJ, Linden J. A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol Pharmacol. 2001;59(1):76–82. doi: 10.1124/mol.59.1.76. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Blaustein JB, Gross AS, Melman N, Jacobson KA. N6-Substituted adenosine derivatives: selectivity, efficacy, and species differences at A3 adenosine receptors. Biochem Pharmacol. 2003;65(10):1675–1684. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, et al. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 1999;96(25):14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, et al. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5'-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319(3):1200–1210. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167(3):835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner TG, Wiley JN, Williams AE, Bruns RF, Coughenour LL, Downs DA. Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology. 1989;98(1):31–37. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci U S A. 1995;92(10):4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J. Food restriction increases torpor and improves brown adipose tissue thermogenesis in ob/ob mice. Am J Physiol. 1985;248(5 Pt 1):E531–E539. doi: 10.1152/ajpendo.1985.248.5.E531. [DOI] [PubMed] [Google Scholar]
- Hudson JW, Scott IM. Daily torpor in the laboratory mouse, mus musculus var. albino. Physiol Zool. 1979;52(2):205–218. [Google Scholar]
- Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol. 2012;303(5):R477–R484. doi: 10.1152/ajpregu.00081.2012. [DOI] [PubMed] [Google Scholar]
- Janes K, Symons-Liguori AM, Jacobson KA, Salvemini D. Identification of A3 adenosine receptor agonists as novel non-narcotic analgesics. Br J Pharmacol. 2016;173(8):1253–1267. doi: 10.1111/bph.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Carlson ZA, Moore JT, Drew KL. Altered thermoregulation via sensitization of A1 adenosine receptors in dietary-restricted rats. Psychopharmacology. 2010;209(3):217–224. doi: 10.1007/s00213-010-1778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Combs VM, Drew KL. Translating Drug-Induced Hibernation to Therapeutic Hypothermia. ACS Chem Neurosci. 2015;6(6):899–904. doi: 10.1021/acschemneuro.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Toien O, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J Neurosci. 2011;31(30):10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98(16):9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, et al. Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 1998;357(1):1–9. doi: 10.1007/pl00005131. [DOI] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317(1):172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- Kumar TS, Mishra S, Deflorian F, Yoo LS, Phan K, Kecskes M, et al. Molecular probes for the A2A adenosine receptor based on a pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine scaffold. Bioorg Med Chem Lett. 2011;21(9):2740–2745. doi: 10.1016/j.bmcl.2010.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang BT, Urso M, Zambraski E, Jacobson KA. Adenosine A3 receptors in muscle protection. In: Borea PA, editor. A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics. Springer; 2010. pp. 257–280. [Google Scholar]
- Listos J, Talarek S, Poleszak E, Wrobel A, Fidecka S. Attenuating effect of adenosine receptor agonists on the development of behavioral sensitization induced by sporadic treatment with morphine. Pharmacology, biochemistry, and behavior. 2011;98(3):356–361. doi: 10.1016/j.pbb.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Luongo L, Petrelli R, Gatta L, Giordano C, Guida F, Vita P, et al. 5'-Chloro-5'-deoxy-(+/−)-ENBA, a potent and selective adenosine A(1) receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules. 2012;17(12):13712–13726. doi: 10.3390/molecules171213712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lute B, Jou W, Lateef DM, Goldgof M, Xiao C, Pinol RA, et al. Biphasic effect of melanocortin agonists on metabolic rate and body temperature. Cell Metab. 2014;20(2):333–345. doi: 10.1016/j.cmet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin RG, Andrews MT. Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol Metab. 2009;20(10):490–498. doi: 10.1016/j.tem.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YF, Wu H, Yang SF, Dai J, Qiu YM, Tao ZY, et al. 5'-adenosine monophosphate-induced hypothermia attenuates brain ischemia/reperfusion injury in a rat model by inhibiting the inflammatory response. Mediators Inflamm. 2015;2015:520745. doi: 10.1155/2015/520745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Lu S, Du N, Guo W, Zhang J, Song Y, et al. Hypothermia induced by adenosine 5'-monophosphate attenuates acute lung injury induced by LPS in rats. Mediators Inflamm. 2012;2012:459617. doi: 10.1155/2012/459617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos WH, Szotek DS, Bruns RF. N6-cycloalkyladenosines. Potent, A1-selective adenosine agonists. J Med Chem. 1985;28(10):1383–1384. doi: 10.1021/jm00148a001. [DOI] [PubMed] [Google Scholar]
- Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, et al. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab. 2013;33(2):183–190. doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173(6):1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes JL, Sharif NA, Michel AD, Whiting RL. Dopamine D2-receptors mediate hypothermia in mice: ICV and IP effects of agonists and antagonists. Neurochem Res. 1991;16(10):1167–1174. doi: 10.1007/BF00966597. [DOI] [PubMed] [Google Scholar]
- O'Neill CE, Hobson BD, Levis SC, Bachtell RK. Persistent reduction of cocaine seeking by pharmacological manipulation of adenosine A1 and A 2A receptors during extinction training in rats. Psychopharmacology. 2014;231(16):3179–3188. doi: 10.1007/s00213-014-3489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JM, Jinka TR, Larson LK, Danielson JJ, Moore JT, Carpluck J, et al. Circannual rhythm in body temperature, torpor, and sensitivity to A(1) adenosine receptor agonist in arctic ground squirrels. J Biol Rhythms. 2013;28(3):201–207. doi: 10.1177/0748730413490667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletta S, Tosh DK, Finley A, Gizewski ET, Moss SM, Gao ZG, et al. Rational design of sulfonated A3 adenosine receptor-selective nucleosides as pharmacological tools to study chronic neuropathic pain. J Med Chem. 2013;56(14):5949–5963. doi: 10.1021/jm4007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Zepeda G, Herrero JF. Interaction of the adenosine A1 receptor agonist N6-cyclopentyladenosine (CPA) and opioid receptors in spinal cord nociceptive reflexes. Life sciences. 2013;93(5–6):233–239. doi: 10.1016/j.lfs.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Refinetti R. The circadian rhythm of body temperature. Front Biosci. 2010;15:564–594. doi: 10.2741/3634. [DOI] [PubMed] [Google Scholar]
- Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, et al. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287(8):5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudich N, Ravid K, Sagi-Eisenberg R. Mast cell adenosine receptors function: a focus on the a3 adenosine receptor and inflammation. Front Immunol. 2012;3:134. doi: 10.3389/fimmu.2012.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275(6):4429–4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- Shintani M, Tamura Y, Monden M, Shiomi H. Characterization of N(6)-cyclohexyladenosine-induced hypothermia in Syrian hamsters. J Pharmacol Sci. 2005;97(3):451–454. doi: 10.1254/jphs.sc0040178. [DOI] [PubMed] [Google Scholar]
- Stone GA, Jarvis MF, Sills MA, Weeks B, Snowhill EW, Williams M. Species differences in high-affinity adenosine A2 binding sites in striatal membranes from mammalian brain. Drug Dev Res. 1988;15(1):31–46. [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98(17):9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ. The pharmacology and molecular mechanisms underlying temperature regulation and torpor. Biochem Pharmacol. 2008;76(7):817–824. doi: 10.1016/j.bcp.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R468–R473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
- Tabarean IV. Histamine receptor signaling in energy homeostasis. Neuropharmacology. 2016;106:13–19. doi: 10.1016/j.neuropharm.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H. Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain research. 2005;1045(1–2):88–96. doi: 10.1016/j.brainres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Tao Z, Zhao Z, Lee CC. 5'- Adenosine monophosphate induced hypothermia reduces early stage myocardial ischemia/reperfusion injury in a mouse model. Am J Transl Res. 2011;3(4):351–361. [PMC free article] [PubMed] [Google Scholar]
- Tchilibon S, Joshi BV, Kim SK, Duong HT, Gao ZG, Jacobson KA. (N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists. J Med Chem. 2005;48(6):1745–1758. doi: 10.1021/jm049580r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh DK, Deflorian F, Phan K, Gao ZG, Wan TC, Gizewski E, et al. Structure-guided design of A(3) adenosine receptor-selective nucleosides: combination of 2-arylethynyl and bicyclo[3.1.0]hexane substitutions. J Med Chem. 2012a;55(10):4847–4860. doi: 10.1021/jm300396n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh DK, Finley A, Paoletta S, Moss SM, Gao ZG, Gizewski ET, et al. In vivo phenotypic screening for treating chronic neuropathic pain: modification of C2-arylethynyl group of conformationally constrained A3 adenosine receptor agonists. J Med Chem. 2014;57(23):9901–9914. doi: 10.1021/jm501021n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh DK, Paoletta S, Deflorian F, Phan K, Moss SM, Gao ZG, et al. Structural sweet spot for A1 adenosine receptor activation by truncated (N)-methanocarba nucleosides: receptor docking and potent anticonvulsant activity. J Med Chem. 2012b;55(18):8075–8090. doi: 10.1021/jm300965a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi BK, Bridges AJ, Patt WC, Priebe SR, Bruns RF. N6-bicycloalkyladenosines with unusually high potency and selectivity for the adenosine A1 receptor. Journal of medicinal chemistry. 1989;32(1):8–11. doi: 10.1021/jm00121a002. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci. 2013;33(36):14512–14525. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: potential clinical applications for altering BAT thermogenesis. Front Neurosci. 2014;8:14. doi: 10.3389/fnins.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86(3):901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DK, Paul IA, Carter M, Jacobson KA. Effects of N6-cyclopentyl adenosine and 8-cyclopentyl-1,3-dipropylxanthine on N-methyl-D-aspartate induced seizures in mice. European journal of pharmacology. 1993;249(3):265–270. doi: 10.1016/0014-2999(93)90521-i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo W, Li Y, Pan X, Lv W, Cui L, et al. Hypothermia induced by adenosine 5'-monophosphate attenuates injury in an L-arginine-induced acute pancreatitis rat model. J Gastroenterol Hepatol. 2014;29(4):742–748. doi: 10.1111/jgh.12448. [DOI] [PubMed] [Google Scholar]
- Yaar R, Jones MR, Chen JF, Ravid K. Animal models for the study of adenosine receptor function. J Cell Physiol. 2005;202(1):9–20. doi: 10.1002/jcp.20138. [DOI] [PubMed] [Google Scholar]
- Yang JN, Tiselius C, Dare E, Johansson B, Valen G, Fredholm BB. Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors. Acta Physiol (Oxf) 2007;190(1):63–75. doi: 10.1111/j.1365-201X.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- Yang JN, Wang Y, Garcia-Roves PM, Bjornholm M, Fredholm BB. Adenosine A(3) receptors regulate heart rate, motor activity and body temperature. Acta Physiol (Oxf) 2010;199(2):221–230. doi: 10.1111/j.1748-1716.2010.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Modabber M, Sabetkasai M. Influences of different adenosine receptor subtypes on catalepsy in mice. Psychopharmacology. 1993;113(2):257–261. doi: 10.1007/BF02245707. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439(7074):340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.