Abstract
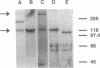
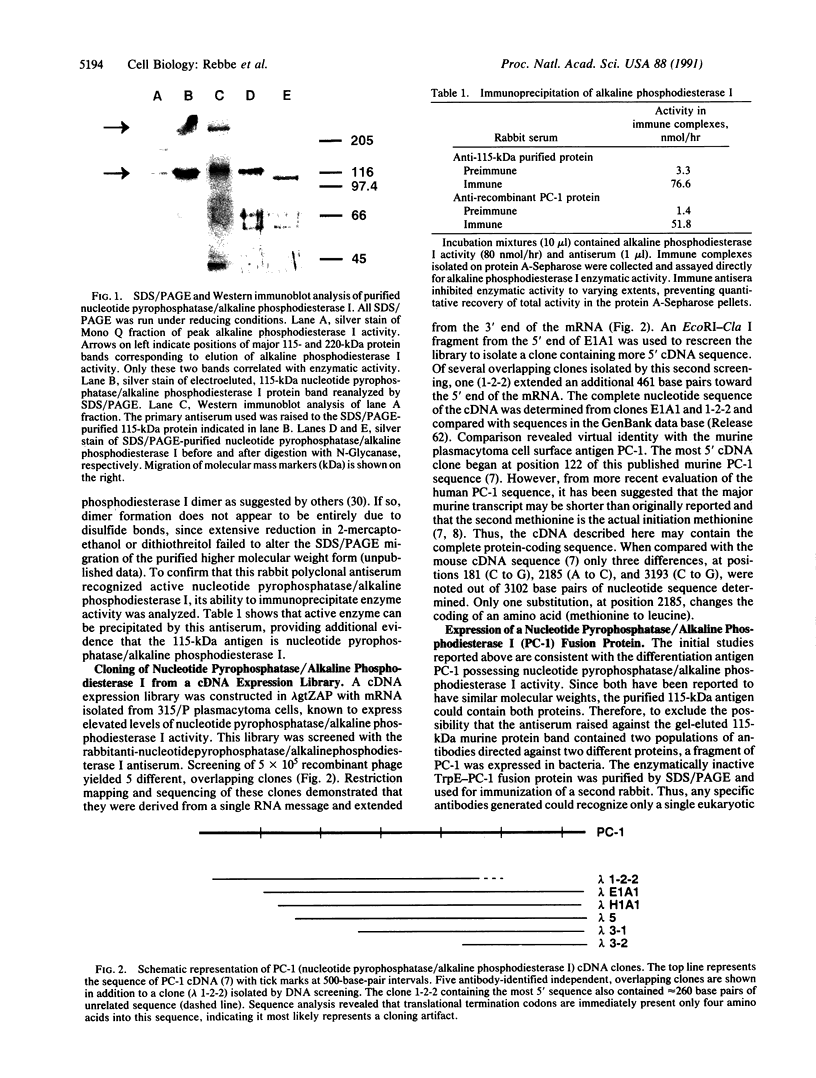
The protein responsible for both nucleotide pyrophosphatase (EC 3.6.1.9) and alkaline phosphodiesterase I (EC 3.1.4.1) activities was purified from MOPC 315 plasmacytoma cells. A single SDS/PAGE-purified 115-kDa protein band was used to produce a rabbit polyclonal antiserum. This antibody preparation precipitated alkaline phosphodiesterase I activity, indicating that the SDS/PAGE-purified protein was nucleotide pyrophosphatase/alkaline phosphodiesterase I. When used for Western blot analysis, the antiserum detected a 115-kDa protein as well as a 220-kDa protein band. Multiple overlapping cDNA clones were isolated from a cDNA expression library screened with this anti-nucleotide pyrophosphatase/alkaline phosphodiesterase I antiserum. Sequence analysis indicated that the isolated cDNA clones encoded PC-1, a murine plasma cell differentiation antigen. To confirm the suspected enzymatic identity of PC-1, a recombinant PC-1 fusion protein was expressed in bacteria, purified, and used to produce another rabbit polyclonal antiserum. This antiserum likewise immunoprecipitated alkaline phosphodiesterase I activity and recognized the 115-kDa and 220-kDa proteins in Western blot analyses of cell extracts. Furthermore, expression of nucleotide pyrophosphatase/alkaline phosphodiesterase I corresponded directly with mRNA and protein levels of PC-1 in cells known to express different levels of nucleotide pyrophosphatase/alkaline phosphodiesterase I activity. Finally, steroid induction of enzymatic activity was mirrored by levels of PC-1 mRNA and protein expression. Together, these data indicate that the plasma cell differentiation antigen PC-1 is a membrane-bound enzyme, nucleotide pyrophosphatase/alkaline phosphodiesterase I.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffet G., Rissel M., Guillouzo A. Modulation of alkaline phosphodiesterase I in cultured rat hepatocytes. Biochim Biophys Acta. 1987 Apr 22;928(2):144–151. doi: 10.1016/0167-4889(87)90115-7. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Rearick J. I., Paulson J. C., Prieels J. P., Sadler J. E., Hill R. L. Biosynthesis of mammalian glycoproteins. Glycosylation pathways in the synthesis of the nonreducing terminal sequences. J Biol Chem. 1979 Dec 25;254(24):12531–12534. [PubMed] [Google Scholar]
- Bischoff E., Tran-Thi T. A., Decker K. F. Nucleotide pyrophosphatase of rat liver. A comparative study on the enzymes solubilized and purified from plasma membrane and endoplasmic reticulum. Eur J Biochem. 1975 Feb 21;51(2):353–361. doi: 10.1111/j.1432-1033.1975.tb03935.x. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Buckley M. F., Loveland K. A., McKinstry W. J., Garson O. M., Goding J. W. Plasma cell membrane glycoprotein PC-1. cDNA cloning of the human molecule, amino acid sequence, and chromosomal location. J Biol Chem. 1990 Oct 15;265(29):17506–17511. [PubMed] [Google Scholar]
- Burg D. L., Feldbush T. L. Late events in B cell activation. Expression of membrane alkaline phosphatase activity. J Immunol. 1989 Jan 15;142(2):381–387. [PubMed] [Google Scholar]
- Byrd J. C., Fearney F. J., Kim Y. S. Rat intestinal nucleotide-sugar pyrophosphatase. Localization, partial purification, and substrate specificity. J Biol Chem. 1985 Jun 25;260(12):7474–7480. [PubMed] [Google Scholar]
- Evans W. H., Hood D. O., Gurd J. W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem J. 1973 Dec;135(4):819–826. doi: 10.1042/bj1350819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Harahap A. R., Goding J. W. Distribution of the murine plasma cell antigen PC-1 in non-lymphoid tissues. J Immunol. 1988 Oct 1;141(7):2317–2320. [PubMed] [Google Scholar]
- Harshman S., Stoller D., Harshman D. L. Increase in plasma membrane phosphodiesterase activity in contact-inhibited 3T3 cells and in phenotypically reverted SV-3T3 cells. J Membr Biol. 1979 Oct 15;50(2):165–175. doi: 10.1007/BF01868946. [DOI] [PubMed] [Google Scholar]
- Hickman S., Wong-Yip Y. P., Rebbe N. F., Greco J. M. Formation of lipid-linked oligosaccharides by MOPC 315 plasmacytoma cells. Decreased synthesis by a nonsecretory variant. J Biol Chem. 1985 May 25;260(10):6098–6106. [PubMed] [Google Scholar]
- Johnson R. A., Welden J. Characteristics of the enzymatic hydrolysis of 5'-adenylylimidodiphosphate: implications for the study of adenylate cyclase. Arch Biochem Biophys. 1977 Sep;183(1):216–227. doi: 10.1016/0003-9861(77)90435-0. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Quackenbush E. J., Ronco P., Verroust P., Carrel S., Letarte M. Common acute lymphoblastic leukemia antigen expressed on leukemia and melanoma cell lines has neutral endopeptidase activity. J Clin Invest. 1989 Feb;83(2):713–717. doi: 10.1172/JCI113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massaia M., Perrin L., Bianchi A., Ruedi J., Attisano C., Altieri D., Rijkers G. T., Thompson L. F. Human T cell activation. Synergy between CD73 (ecto-5'-nucleotidase) and signals delivered through CD3 and CD2 molecules. J Immunol. 1990 Sep 15;145(6):1664–1674. [PubMed] [Google Scholar]
- Márquez C., De la Hera A., Leonardo E., Pezzi L., Strasser A., Martínez-A C. Identity of PB76 differentiation antigen and lymphocyte alkaline phosphatase. Eur J Immunol. 1990 Apr;20(4):947–950. doi: 10.1002/eji.1830200436. [DOI] [PubMed] [Google Scholar]
- Nakabayashi T., Ikezawa H. Alkaline phosphodiesterase I release from eucaryotic plasma membranes by phosphatidylinositol-specific phospholipase C. I. The release from rat organs. J Biochem. 1986 Mar;99(3):703–712. doi: 10.1093/oxfordjournals.jbchem.a135529. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Rebbe N. F., Hickman S. Modulation of nucleotide pyrophosphatase in plasmacytoma cells. Biochem Biophys Res Commun. 1991 Mar 15;175(2):637–644. doi: 10.1016/0006-291x(91)91613-h. [DOI] [PubMed] [Google Scholar]
- Rebbe N. F., Hickman W. S., Ley T. J., Stafford D. W., Hickman S. Nucleotide sequence and regulation of a human 90-kDa heat shock protein gene. J Biol Chem. 1989 Sep 5;264(25):15006–15011. [PubMed] [Google Scholar]
- Rousseau G. G., Amar-Costesec A., Verhaegen M., Granner D. K. Glucocorticoid hormones increase the activity of plasma membrane alkaline phosphodiesterase I in rat hepatoma cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1005–1009. doi: 10.1073/pnas.77.2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H., Diltz C. D., Fischer E. H., Walsh K. A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry. 1988 Nov 29;27(24):8695–8701. doi: 10.1021/bi00424a001. [DOI] [PubMed] [Google Scholar]
- Uckun F. M. Regulation of human B-cell ontogeny. Blood. 1990 Nov 15;76(10):1908–1923. [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Ye R. D., Wun T. C., Sadler J. E. cDNA cloning and expression in Escherichia coli of a plasminogen activator inhibitor from human placenta. J Biol Chem. 1987 Mar 15;262(8):3718–3725. [PubMed] [Google Scholar]
- van Driel I. R., Goding J. W. Plasma cell membrane glycoprotein PC-1. Primary structure deduced from cDNA clones. J Biol Chem. 1987 Apr 5;262(10):4882–4887. [PubMed] [Google Scholar]