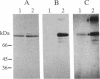
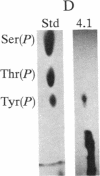
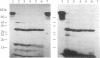
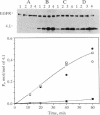
Abstract
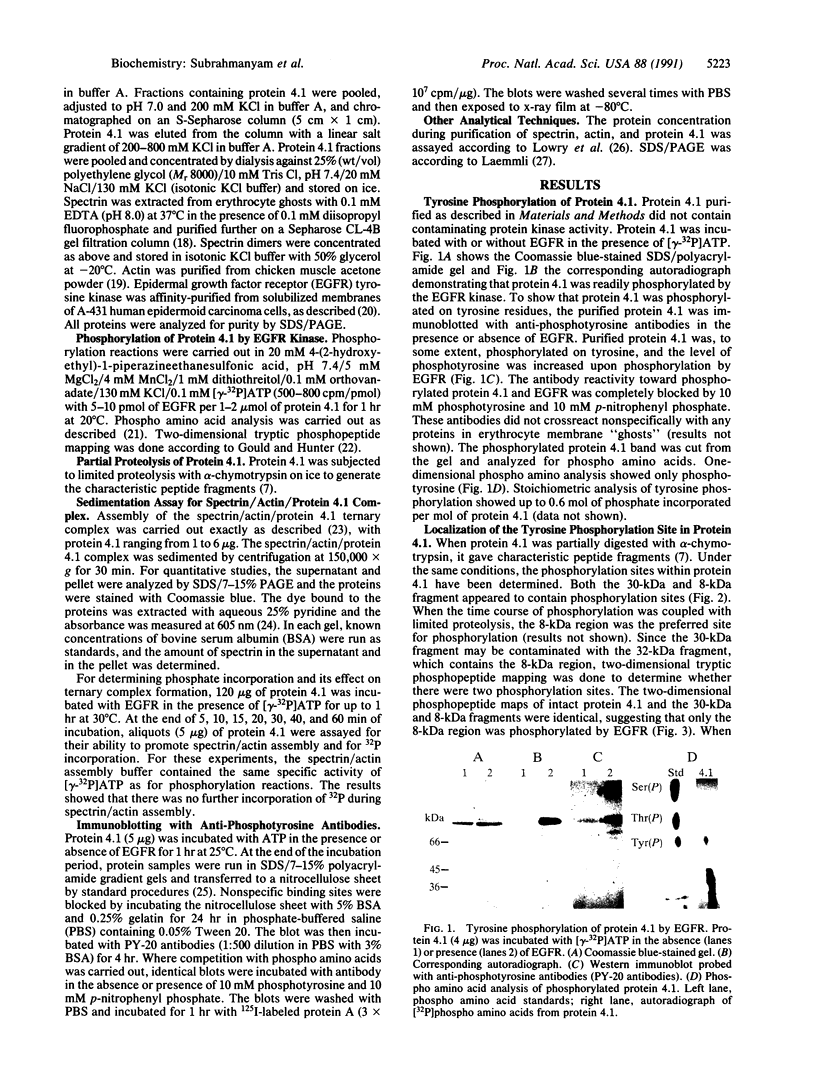
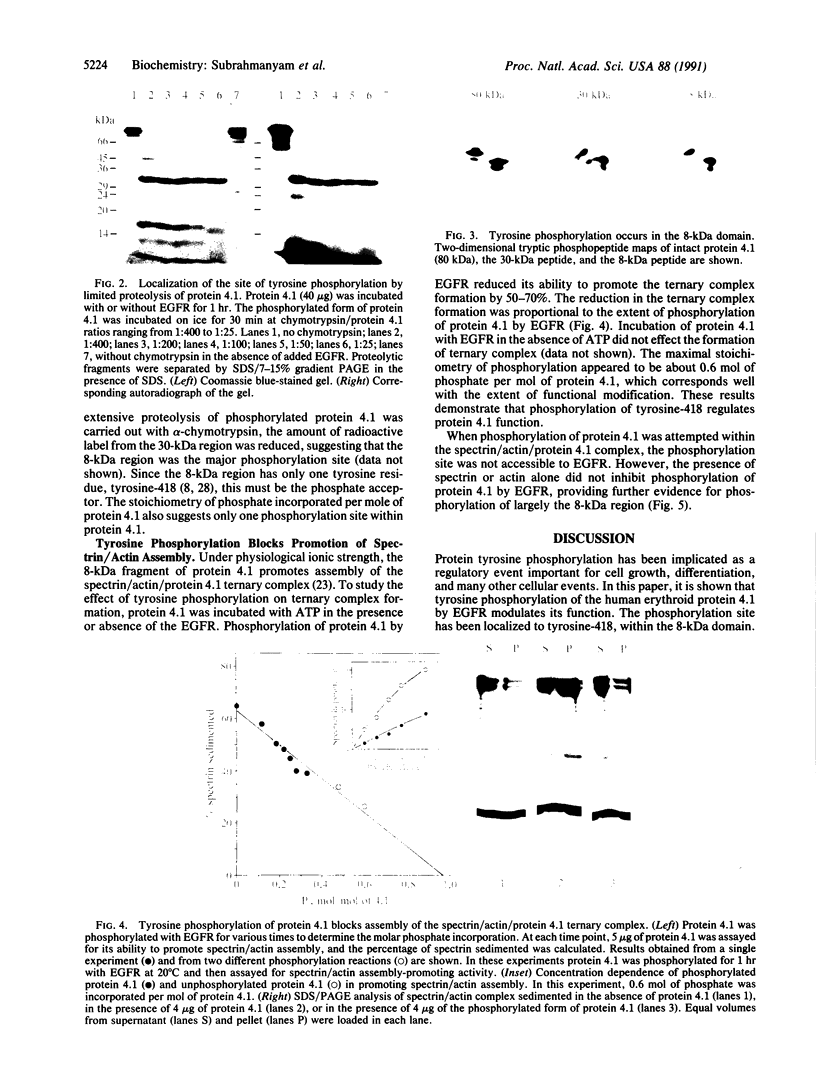
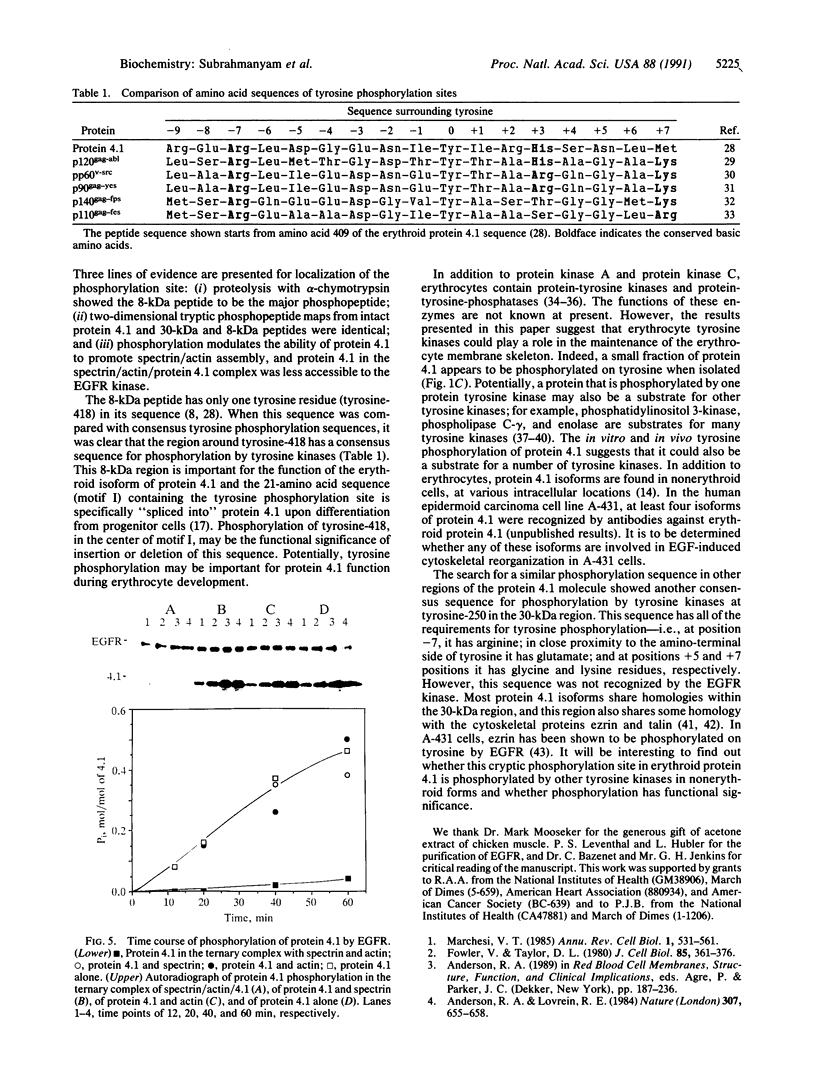
Protein 4.1 was initially characterized as a protein that regulates cytoskeletal assembly in erythrocytes. However, recent studies have shown that protein 4.1 is ubiquitous in mammalian cells. Here, we show that protein 4.1 is phosphorylated on tyrosine by the epidermal growth factor receptor (EGFR) tyrosine kinase. The phosphorylation site has been localized to the 8-kDa domain, which has one tyrosine, tyrosine-418. The 8-kDa region is required for the assembly of the spectrin/actin complex, and phosphorylation by EGFR reduced the ability of protein 4.1 to promote the assembly of the spectrin/actin/protein 4.1 ternary complex. Immunoblotting with anti-phosphotyrosine antibodies showed that purified protein 4.1 contained phosphorylated tyrosine, and this increased upon phosphorylation by EGFR. This suggests that tyrosine phosphorylation of protein 4.1 occurs in vivo and may be functionally significant. The tyrosine phosphorylation site is in the center of a sequence motif that is expressed by a differentiation-specific splicing mechanism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Correas I., Mazzucco C., Castle J. D., Marchesi V. T. Tissue-specific analogues of erythrocyte protein 4.1 retain functional domains. J Cell Biochem. 1988 Jul;37(3):269–284. doi: 10.1002/jcb.240370303. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Lovrien R. E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984 Feb 16;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Marchesi V. T. Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature. 1985 Nov 21;318(6043):295–298. doi: 10.1038/318295a0. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989 Mar;108(3):921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clari G., Moret V. Comparative characterization of membrane-associated and cytosolic Tyr-protein kinases in human erythrocytes. Eur J Biochem. 1989 Feb 15;179(3):581–588. doi: 10.1111/j.1432-1033.1989.tb14586.x. [DOI] [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Correas I., Leto T. L., Speicher D. W., Marchesi V. T. Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J Biol Chem. 1986 Mar 5;261(7):3310–3315. [PubMed] [Google Scholar]
- Correas I., Speicher D. W., Marchesi V. T. Structure of the spectrin-actin binding site of erythrocyte protein 4.1. J Biol Chem. 1986 Oct 5;261(28):13362–13366. [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Danilov Y. N., Fennell R., Ling E., Cohen C. M. Selective modulation of band 4.1 binding to erythrocyte membranes by protein kinase C. J Biol Chem. 1990 Feb 15;265(5):2556–2562. [PubMed] [Google Scholar]
- Dekowski S. A., Rybicki A., Drickamer K. A tyrosine kinase associated with the red cell membrane phosphorylates band 3. J Biol Chem. 1983 Mar 10;258(5):2750–2753. [PubMed] [Google Scholar]
- Eder P. S., Soong C. J., Tao M. Phosphorylation reduces the affinity of protein 4.1 for spectrin. Biochemistry. 1986 Apr 8;25(7):1764–1770. doi: 10.1021/bi00355a047. [DOI] [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Fowler V., Taylor D. L. Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol. 1980 May;85(2):361–376. doi: 10.1083/jcb.85.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Bretscher A., Esch F. S., Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989 Dec 20;8(13):4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Hunter T. Platelet-derived growth factor induces multisite phosphorylation of pp60c-src and increases its protein-tyrosine kinase activity. Mol Cell Biol. 1988 Aug;8(8):3345–3356. doi: 10.1128/mcb.8.8.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Laprevotte I., Galibert F., Fedele L. A., Sherr C. J. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell. 1982 Oct;30(3):775–785. doi: 10.1016/0092-8674(82)90282-3. [DOI] [PubMed] [Google Scholar]
- Horne W. C., Leto T. L., Marchesi V. T. Differential phosphorylation of multiple sites in protein 4.1 and protein 4.9 by phorbol ester-activated and cyclic AMP-dependent protein kinases. J Biol Chem. 1985 Aug 5;260(16):9073–9076. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Ling E., Danilov Y. N., Cohen C. M. Modulation of red cell band 4.1 function by cAMP-dependent kinase and protein kinase C phosphorylation. J Biol Chem. 1988 Feb 15;263(5):2209–2216. [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mohamed A. H., Steck T. L. Band 3 tyrosine kinase. Association with the human erythrocyte membrane. J Biol Chem. 1986 Feb 25;261(6):2804–2809. [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Pasternack G. R., Racusen R. H. Erythrocyte protein 4.1 binds and regulates myosin. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9712–9716. doi: 10.1073/pnas.86.24.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. J., Ades S. E., Singer S. J., Hynes R. O. Sequence and domain structure of talin. Nature. 1990 Oct 18;347(6294):685–689. doi: 10.1038/347685a0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tang T. K., Leto T. L., Correas I., Alonso M. A., Marchesi V. T., Benz E. J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Qin Z., Leto T., Marchesi V. T., Benz E. J., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990 Mar;110(3):617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W., Bertics P. J., Gill G. N. Immunoaffinity purification of the epidermal growth factor receptor. Stoichiometry of binding and kinetics of self-phosphorylation. J Biol Chem. 1984 Dec 10;259(23):14631–14636. [PubMed] [Google Scholar]