Abstract
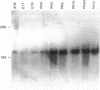
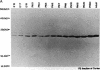
The developmental expression and subcellular distribution of the neuron-specific 25-kDa synaptosomal protein (SNAP-25) were investigated by using Northern (RNA) blots, immunoblots, and immunocytochemistry. Both SNAP-25 protein and mRNA were present at low levels in embryonic day 15 rat brain, and levels of both increased during early postnatal maturation. Developmental immunoblots with antipeptide antisera demonstrated that a 25-kDa peptide was the major isoform in brain, and this form increased steadily from embryonic day 15 through adulthood. A second 27-kDa immunoreactive isoform was present in brain only during early development. Immunoblots of two-dimensional SDS/polyacrylamide gels revealed the presence of a predominant 25-kDa isoform of SNAP-25 in adult brain. Immunocytochemical studies indicated that as immunoreactivity for SNAP-25 increased during development, the cellular localization of SNAP-25 immunoreactivity concomitantly shifted from axons and cell bodies to presynaptic terminals. These data suggest that the SNAP-25 protein shifts in subcellular localization during development and may play a role in the establishment and stabilization of specific presynaptic terminals in brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billingsley M. L., Polli J. W., Balaban C. D., Kincaid R. L. Developmental expression of calmodulin-dependent cyclic nucleotide phosphodiesterase in rat brain. Brain Res Dev Brain Res. 1990 May 1;53(2):253–263. doi: 10.1016/0165-3806(90)90015-q. [DOI] [PubMed] [Google Scholar]
- Bixby J. L., Reichardt L. F. The expression and localization of synaptic vesicle antigens at neuromuscular junctions in vitro. J Neurosci. 1985 Nov;5(11):3070–3080. doi: 10.1523/JNEUROSCI.05-11-03070.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Branks P. L., Wilson M. C. Patterns of gene expression in the murine brain revealed by in situ hybridization of brain-specific mRNAs. Brain Res. 1986 Jul;387(1):1–16. doi: 10.1016/0169-328x(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Catsicas S., Larhammar D., Blomqvist A., Sanna P. P., Milner R. J., Wilson M. C. Expression of a conserved cell-type-specific protein in nerve terminals coincides with synaptogenesis. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):785–789. doi: 10.1073/pnas.88.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Geddes J. W., Hess E. J., Hart R. A., Kesslak J. P., Cotman C. W., Wilson M. C. Lesions of hippocampal circuitry define synaptosomal-associated protein-25 (SNAP-25) as a novel presynaptic marker. Neuroscience. 1990;38(2):515–525. doi: 10.1016/0306-4522(90)90047-8. [DOI] [PubMed] [Google Scholar]
- Haas C. A., DeGennaro L. J. Multiple synapsin I messenger RNAs are differentially regulated during neuronal development. J Cell Biol. 1988 Jan;106(1):195–203. doi: 10.1083/jcb.106.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Developmental changes in morphology and molecular composition of isolated synaptic junctional structures. Brain Res. 1981 Feb 16;206(2):251–257. doi: 10.1016/0006-8993(81)90531-x. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. The cell biology of the nerve terminal. Neuron. 1988 Aug;1(6):431–438. doi: 10.1016/0896-6273(88)90174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus P., Betz H., Rehm H. Expression of synaptophysin during postnatal development of the mouse brain. J Neurochem. 1986 Oct;47(4):1302–1304. doi: 10.1111/j.1471-4159.1986.tb00754.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli J. W., Patanow C. M., Billingsley M. L. Developmental expression of neuronal calmodulin-binding proteins in rat brain. Brain Res Dev Brain Res. 1990 Apr 1;53(1):62–70. doi: 10.1016/0165-3806(90)90124-h. [DOI] [PubMed] [Google Scholar]
- Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase. Adv Second Messenger Phosphoprotein Res. 1988;22:39–112. [PubMed] [Google Scholar]
- Südhof T. C., Baumert M., Perin M. S., Jahn R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron. 1989 May;2(5):1475–1481. doi: 10.1016/0896-6273(89)90193-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]