Abstract
DNA vaccines provide an attractive technology platform against bioterrorism agents due to their safety record in humans and ease of construction, testing, and manufacture. We have designed monovalent and bivalent anthrax plasmid DNA (pDNA) vaccines encoding genetically detoxified protective antigen (PA) and lethal factor (LF) proteins and tested their immunogenicity and ability to protect rabbits from an aerosolized inhalation spore challenge. Immune responses after two or three injections of cationic lipid-formulated PA, PA plus LF, or LF pDNAs were at least equivalent to two doses of anthrax vaccine adsorbed (AVA). High titers of anti-PA, anti-LF, and neutralizing antibody to lethal toxin (Letx) were achieved in all rabbits. Eight or nine animals in each group were challenged with 100× LD50 of aerosolized anthrax spores 5 or 9 weeks after vaccination. An additional 10 animals vaccinated with PA pDNA were challenged >7 months postvaccination. All animals receiving PA or PA plus LF pDNA vaccines were protected. In addition, 5 of 9 animals receiving LF pDNA survived, and the time to death was significantly delayed in the others. Groups receiving three immunizations with PA or PA plus LF pDNA showed no increase in anti-PA, anti-LF, or Letx neutralizing antibody titers postchallenge, suggesting little or no spore germination. In contrast, titer increases were seen in AVA animals, and in surviving animals vaccinated with LF pDNA alone. Preclinical evaluation of this cationic lipid-formulated bivalent PA and LF vaccine is complete, and the vaccine has received U.S. Food and Drug Administration Investigational New Drug allowance.
The deliberate exposure of the civilian population of the United States to Bacillus anthracis spores in late 2001 revealed several gaps in the public health system's preparedness against bioterrorism. Because vaccines have proven to be one of the most successful public health measures against naturally acquired infectious diseases, it is no surprise that a top biodefense priority is to develop vaccines against weaponized microorganisms. The performance requirements of biodefense vaccines, however, are somewhat different from those for conventional vaccines. Some key features of biodefense vaccines to be developed for civilian use include (i) the rapidity by which a protective immune response can be elicited; (ii) the degree to which the vaccine, when administered postexposure, improves the clinical course of an exposed person; (iii) a very high benefit-to-risk ratio of vaccination in all segments of the population; (iv) the speed and ease of manufacture, distribution, and administration; and (v) the inherent stability of the formulated, final filled product to allow for long-term stockpiling. None of the currently licensed vaccines against select bioterrorism agents meet all of these performance requirements, including the currently licensed anthrax vaccine: “The current anthrax vaccine is difficult to standardize, is incompletely characterized, and is relatively reactogenic (probably even more so because it is administered subcutaneously), and the dose schedule is long and challenging. An anthrax vaccine free of these drawbacks is needed, and such improvements are feasible” (1).
Providing evidence of a biodefense vaccine's effectiveness by classic clinical efficacy trials is likely to be neither feasible nor ethical so these vaccines must be evaluated by the regulatory agencies in a unique way. The “Animal Rule” (www.fda.gov/cber/summaries/120600bio19KG.ppt), in which preferably two animal species are used to demonstrate the biological activity of the product, may establish effectiveness of these vaccines. For anthrax, the aerosolized spore inhalation challenge of vaccinated rabbits will likely be one of the animal models used to prove effectiveness. The studies reported here demonstrate the effectiveness of both monovalent and bivalent, cationic lipid-formulated gene-based plasmid DNA (pDNA) vaccines, using this rabbit model of anthrax.
The virulence of B. anthracis in rabbits, non-human primates, and humans is primarily the result of a multicomponent toxin secreted by the organism (2). The toxin consists of three separate gene products, designated protective antigen (PA), lethal factor (LF), and edema factor (EF), that are encoded on a 184-kb plasmid designated pXO1 (3). PA83 [735 aa, molecular weight (MW) 82,684] is a single-chain protein that binds to a mammalian ubiquitous cell surface receptor (4). Cleavage by furin (or a furin-like enzyme) results in a 63-kDa receptor-bound product (5, 6) that multimerizes and can bind both the 90-kDa LF protein and the 89-kDa EF protein, which are subsequently endocytosed as a two-component complex (7). LF (776 aa, MW 90,237) is a zinc metalloprotease that, once internalized, cleaves several isoforms of mitogen-activated protein kinase kinases (Mek1, Mek2, and MKK3) and thereby disrupts signal transduction events within the cell (8). The LF protein, which when complexed with PA is referred to as Letx, is considered responsible for the rapid lethality of anthrax spore inhalation infection (2, 9) due to extensive tissue hypoxia accompanied by pleural edema (9).
Recently, a pDNA vaccine encoding a truncated PA antigen (PA63) was shown to protect against Letx challenge in mice (10), and a plasmid encoding full-length PA protected 9 of 10 rabbits from s.c. spore challenge (11). Additionally, Price et al. (12) demonstrated that a pDNA encoding a fragment of the LF gene can contribute to, or provide protection against, Letx challenge, 5an observation subsequently extended in a small inhalation challenge rabbit study showing that a pDNA prime followed by a recombinant protein boost provided protection not seen with pDNA alone (13).
Given these considerations, we developed and tested a series of anthrax pDNA vaccine constructs. Three PA and LF pDNAs formulated with cationic lipid warranted further investigation in a lethal spore challenge study in rabbits. This work demonstrated that formulated pDNA vaccines induce complete, long-term protection, and that an LF pDNA administered alone provides partial protection in a model in which death results a few days after inhalation exposure (14). In contrast to the anthrax vaccine adsorbed (AVA) and LF pDNA alone groups, the formulated PA or bivalent PA plus LF pDNA vaccines generated a protective response at peak titer that seemed to block germination of the spores or sufficient bacterial replication to cause a postchallenge increase in anti-PA-, anti-LF-, or Letx-neutralizing titers.
Materials and Methods
Plasmids. PA83Δfurin. The nucleotide sequence encoding B. anthracis PA83 protein (GenBank accession no. AF306782, nt 49–2343) was codon-optimized with the backtranslation tool at www.syntheticgenes.com by using the Homo sapiens codon frequency table at www.kazusa.or.jp/codon. The PA construct (GenBank accession no. AY428556) was chemically synthesized (Retrogen, San Diego) to include an amino terminal human tissue plasminogen activator (hTPA) leader peptide (replacing the Bacillus leader peptide) fused to a PA83 sequence (amino acids 30–764) with the furin cleavage site deleted (SRKKRS, amino acids 192–197) (6, 15). This construct, designated PA83Δ furin, was cloned into the mammalian expression vector VR1012 for these studies (16).
LF[I] and LF[I–III]. The LF coding sequences used in this study were derived from the B. anthracis LF93 protein sequence (GenBank accession no. M30210, nt 784–3114), codon-optimized, and chemically synthesized as above to include the hTPA leader peptide. The LF domain I–III (17) was PCR amplified from this clone by using a forward (5′-GAGCTTGATATCGCCACCATGGATGC-3′) and reverse (5′-GAACCTGGATCCCTACACCACCTTGGCGTCGATG-3′) primer pair to amplify the 1,740-bp fragment (GenBank accession no. AY428557) encoding the hTPA leader peptide fused to LF amino acids 34–583. The LF domain [I] was also derived from the LF93 plasmid by PCR amplification using forward (5′-GAGCTTGATATCGCCACCATGGATGC-3′) and reverse (5′-CCATACGGATCCTCACTGGTCTTTCAGTTCCTCCA-3′) primer pairs to amplify an 876-bp fragment (GenBank accession no. AY428558) encoding an hTPA leader peptide fused to LF amino acids 34–295. Both LF genes were cloned into the VR1012 vector.
Plasmid DNA Preparation. Plasmid DNA was prepared from overnight cultures of transformed XL-2 Blue bacteria (Stratagene) in Terrific Broth (Invitrogen) plus 50 μg/ml kanamycin sulfate (Sigma) and processed by using Endo-free Giga kits (Qiagen, Valencia, CA).
Vaxfectin and DMRIE/DOPE Formulations. One milliliter of sterile water for irrigation (SWFI) was added to a vial containing a dried film of 3.75 μmol each of a 1:1 mixture of cationic lipid and colipid and vortex mixed for 5 min. Vaxfectin consists of GAP-DMORIE [(±)-N-(3-aminopropyl)-N,N-dimethyl-2,3-bis(cis-9-tetradecenyloxy)-1-propanaminium bromide)] plus DPyPE (1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine). DMRIE/DOPE consists of DMRIE [(±)-N-(2-hydroxyethyl)-N,N-dimethyl-2,3-bis(tetradecyloxy)-1-propanaminium bromide] plus DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine). The liposome suspension was diluted to 1.5 mM with SWFI and added to an equal volume of pDNA (2 mg/ml in 2× PBS) and vortex mixed briefly. The final molar ratio of all formulations was 4:1, DNA/cationic lipid.
Rabbit Intramuscular Vaccination. Two- to five-kilogram female New Zealand White rabbits were injected bilaterally in the quadriceps muscle with 1 ml (1 mg/ml) of pDNA formulated with Vaxfectin or DMRIE/DOPE (0.5 ml per leg). Rabbits vaccinated with PA, LF, or vector received 1 mg of that pDNA whereas rabbits coinjected with PA plus LF pDNAs received a mixture of 0.5 mg of each plasmid. Groups of rabbits receiving three doses were injected on days 0, 28, and 56; rabbits receiving only two doses were injected on study days 0 and 28. Rabbits immunized with AVA (a gift from BioPort Corporation, Lansing, MI, to D.G.) were injected unilaterally with 50 μl of AVA diluted to 0.5 ml in PBS on days 28 and 56. Prebleeds and biweekly postvaccination bleeds were taken for all groups for analysis of serum antibodies.
Anti-PA and Anti-LF Binding Antibody Titers. Anti-PA and anti-LF IgG antibody titers were determined by end-point dilution ELISA (18). Briefly, 96-well plates were coated with 1 μg/ml PA or LF protein (List Biological Laboratories, Campbell, CA). After blocking overnight, serum samples were serially diluted and incubated for 16 h at 4°C. Bound antibodies were detected with alkaline phosphatase-conjugated anti-rabbit IgG (1:4,000, H+L, Jackson ImmunoResearch) before adding the PNPP substrate (Sigma) and measuring absorbance at 450 nm (A450). The average of duplicate wells was used to determine the end-point titer, defined as the reciprocal of the highest dilution giving a reading ≥3 times the control wells without serum.
Letx Neutralization Titers. Letx neutralizing antibody titers were measured by the J774A.1 mouse macrophage cytotoxicity assay (12). Two-fold dilutions of serum in DMEM (DMEM/10% FBS/1% sodium pyruvate) were incubated with an equal volume of Letx (500 ng/ml PA plus 400 ng/ml LF in DMEM) for 1 h at room temperature. The Letx plus serum mixture was added to wells containing 3 × 104 J774A.1 cells (plated 1 day before) for 4 h at 37°C, then replaced with 0.1 ml DMEM and 0.05 ml XTT reagent (Cell Proliferation Kit II, Roche Molecular Biochemicals). Cell viability was determined by measuring the A450 after 16 h. The A450 of 100% viable cells was calculated from the average of four wells receiving no Letx. The average of duplicate samples was used to calculate titers, defined as the reciprocal of the highest dilution of serum that gives an A450 ≥ 90% of the value of wells receiving no Letx.
Anthrax Aerosolized Spore Challenge. In a blinded study at Battelle Memorial Institute (West Jefferson, OH) under BSL-3 conditions, selected rabbits (see Table 2 legend) were randomly divided into four groups and challenged with a target dose of 100× the LD50 of aerosolized B. anthracis spores, Ames strain, on successive days. Each group contained pDNA-vaccinated, AVA-vaccinated, vector controls, and naive rabbits. Samples of the spore-containing aerosol were collected to calculate the dose of exposure (1 Ames LD50 equals 105,000 colony-forming units) (13). After challenge, rabbits were observed for 21 days for signs of B. anthracis infection, and serum from surviving animals was collected 7 and 21 days postchallenge.
Table 2. B. anthracis inhalation spore challenge results.
| Group | Vaccination | Survival | Prechallenge Letx neut titer, median ± SD | Inhaled spore dose, median LD50* ± SD (LD50 Range) | Days to death, median ± SD |
|---|---|---|---|---|---|
| 1 | PA83Δfurin | 8/8 | 5120 ± 6110 | 98 ± 28 (56-129) | >21 |
| 2 | PA83Δfurin/DM-DP | 8/8 | 2560 ± 3055 | 116 ± 69 (56-238) | >21 |
| 3 | PA83Δfurin, 2 inj. | 8/8 | 1076 ± 634 | 94 ± 74 (52-252) | >21 |
| 4† | PA83Δfurin | 10/10 | 844 ± 843 | 93 ± 40 (27-155) | >21 |
| 5 | PA83Δfurin plus LF[I], 2inj. | 8/8 | 415 ± 199 | 95 ± 43 (72-205) | >21 |
| 6 | PA83Δfurin plus LF[I-III] | 8/8 | 3948 ± 1325 | 113 ± 47 (65-192) | >21 |
| 7 | LF[I-III] | 5/9 | 453 ± 339 | 112 ± 67 (46-241) | 5.3 ± 1.5 |
| 8 | Vector | 0/5 | <20 | 90 ± 35 (58-144) | 2.6 ± 0.5 |
| 9 | AVA, 2 inj. | 4/4 | 1280 ± 805 | 135 ± 34 (107-176) | >21 |
| Naive rabbits | 0/12 | ND | 110 ± 49 (57-208) | 2.6 ± 0.7 | |
| † | Naive rabbits | 0/5 | ND | 112 ± 72 (34-198) | 2.8 ± 1.4 |
Rabbits were selected for challenge on week 13 by calculating the mean Letx neutralization (neut) titer for each group and excluding one rabbit with a higher and lower titer than the mean but always including the highest and lowest titer animals. DM-DP, DMRIE-DOPE; inj, injections.
1 LD50 = 105,000 Ames spores.
All 10 group 4 rabbits and five naive animals were challenged on week 40.
Statistical Analysis. Statistical analysis was by paired Student's t test, one-way ANOVA with pairwise comparisons, and two-way ANOVA with pairwise comparisons. P < 0.05 was considered significant. Analyses were performed with statistical analysis system 8.02 (SAS Institute, Cary, NC).
Results
Expression Cassette Optimization. To optimize the pDNA expression cassette for PA and LF, five parameters were evaluated: (i) full-length vs. subdomains; (ii) wild-type nucleotide sequence vs. human codon-optimized sequence; (iii) wild-type-deduced amino acid sequence vs. sequence deleted of predicted N-linked glycosylation sites; (iv) deduced amino acid sequence with vs. without predicted enzymatic processing or active sites; and (v) deduced amino acid sequence with vs. without predicted multimerization or conformational-dependent domains. In all, six PA pDNAs and seven LF pDNAs were constructed (Fig. 4, which is published as supporting information on the PNAS web site). Comparison of pDNAs by in vitro expression assays of transfected mammalian cells and by immunogenicity assays (ELISA and Letx neutralization) of serum from mice injected i.m. with formulated pDNA (Table 3 and Fig. 5, which are published as supporting information on the PNAS web site) indicated that a codon-optimized, noncleavable, full-length PA (PA83Δfurin) and either of two truncated LF (LF[I] or LF [I–III]) expression plasmids were worthy of further evaluation in rabbits, a well accepted model to test immunogenicity and protection induced by candidate anthrax vaccines (14). A 150-rabbit study was conducted to test the immunogenicity of PA and LF pDNAs, combinations of PA and LF pDNAs, and delivery using several formulations. The portion of the study that was advanced to aerosolized inhalation spore challenge is presented here.
Confirmation in Rabbits of in Vivo Biological Activity of the Expression Cassette. Total anti-PA and anti-LF antibody titers and Letx neutralizing antibody titers were used to confirm the immunogenicity of the PA and LF expression cassettes, respectively. Groups of rabbits (n = 10) received two or three monthly injections of cationic lipid-formulated PA, either alone (Table 1, groups 1–4) or in combination with one of two LF pDNAs (groups 5 and 6). All six groups achieved end-point antibody titers to PA in the range of 105 to 106. Rabbits injected with a Vaxfectin-formulated LF pDNA, either alone (group 7), or in combination with PA pDNA (groups 5 and 6), generated similarly high anti-LF antibody titers (105 to 106) (Fig. 1B). Formulated vector pDNA-injected animals (group 8) had no detectable antibody titers to either PA or LF (data not shown). Together, these data indicate that both the PA and the LF pDNAs generate anti-PA and anti-LF antibody responses, respectively, when injected alone (1 mg) or coinjected (0.5 mg per plasmid). Furthermore, coinjection of PA and LF pDNAs does not cause detectable interference in the immunogenicity of either of the pDNAs (e.g., for PA, compare Fig. 1 A, groups 1 and 6; for LF, compare Fig. 1B, groups 6 and 7).
Table 1. Rabbit vaccination experimental groups.
| Group | pDNA injected | n | Vaccination, weeks |
|---|---|---|---|
| 1 | PA83Δfurin | 10 | 0, 4, 8 |
| 2 | PA83Δfurin DMRIE/DOPE | 10 | 0, 4, 8 |
| 3 | PA83Δfurin | 10 | 0, 4 |
| 4 | PA83Δfurin | 10 | 0, 4, 8 |
| 5 | PA83Δfurin plus LF[I] | 10 | 0, 4 |
| 6 | PA83Δfurin plus LF[I-III] | 10 | 0, 4, 8 |
| 7 | LF[I-III] | 10 | 0, 4, 8 |
| 8 | Vector | 5 | 0, 4, 8 |
| 9 | AVA | 4 | 4, 8 |
Rabbits were injected with pDNA (0.5 mg/0.5 ml per leg) formulated with Vaxfectin (groups 1 and 3-8), or DMRIE/DOPE (group 2). Group 9 animals were vaccinated with 50 liters of AVA diluted to 0.5 ml in PBS. Group 4 rabbits were monitored monthly to assess the duration of the immune response and long-term protection.
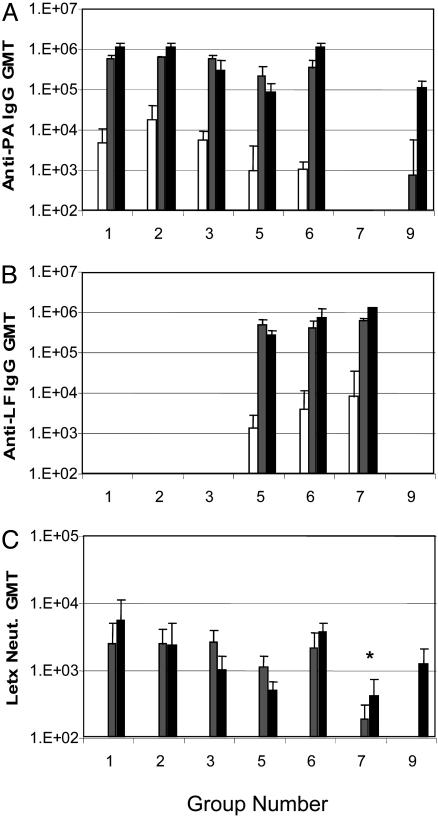
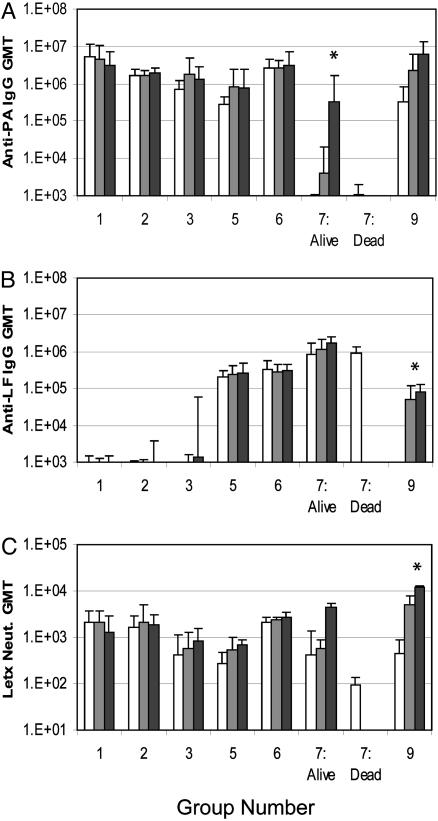
Fig. 1.
Immune responses in vaccinated rabbits. Shown are group geometric mean titers (GMT) at 2 weeks (□), 6 weeks (▒), and 10 weeks (▪). Shown are anti-PA IgG titer (A), anti-LF IgG titer (B), and Letx neutralization titer (C). Group 9 (AVA) was vaccinated on weeks 4 and 8; therefore, the 10-week value is 2 weeks after the second vaccination, an equivalent time point to the 6-week values for groups 1–8. No titer was detected in vector-immunized rabbits (group 8). *, Group 7 (LF[I-III]) was statistically lower than all other groups given three injections (groups 1, 2, and 6).
The geometric mean anti-PA titers of 105 in rabbits after two injections with formulated PA pDNA were comparable to anti-PA titers in the four animals (group 9) given two 50-μl injections of AVA previously shown to protect rabbits from aerosolized spore challenge (19). Indeed, the anti-PA and anti-LF titers and the Letx neutralization titers were sufficiently high after two injections to justify forgoing the third injection in two groups of animals (group 3, PA pDNA alone; and group 5, PA pDNA plus LF[I] pDNA) to compare the protective immune response generated after 2 injections of formulated pDNAs with two injections of AVA.
The anti-PA titers in groups 3 and 5 and the anti-LF titer in group 5 declined slightly, but not significantly, 6 weeks after the second injection (Fig. 1 A and B). In agreement with a previous cationic lipid-formulated pDNA study (18), the third DNA dose did not increase the anti-PA or -LF titers significantly, suggesting that 0.5 mg of PA or LF pDNA maximizes the response in these rabbits after two injections.
Letx neutralizing antibody titers were measured in serum from all animals after the second and third (where relevant) injections by using the J774A.1 cytotoxicity assay (12). Similar to total binding antibody titers, all rabbits injected with PA pDNA alone or in combination with LF pDNA achieved significant neutralizing antibody titers after two injections (Fig. 1C) that were similar to the titers achieved after two injections of AVA. These neutralizing titers were minimally boosted with a third injection of PA pDNA and/or LF pDNA. Only a moderate decline in Letx neutralizing antibody titers was observed at the later time points in groups 3 and 5 given only two injections of pDNA. Animals administered LF pDNA alone achieved a group geometric mean Letx neutralization titer significantly below all of the other pDNA groups given three injections, suggesting that the high titer of anti-LF binding antibody in these animals was not as effective as anti-PA antibodies in neutralizing Letx (Fig. 1).
Demonstration of Protective Immunity by Aerosolized Inhalation Challenge. Eight rabbits selected from groups 1–3, 5, and 6, nine rabbits from group 7, five blank vector-injected rabbits (group 8), all four AVA-vaccinated rabbits (group 9), and 12 naive animals were subjected to an inhalation challenge targeted at 100× the rabbit LD50 aerosolized spores ≈5 weeks after the third injection (study day 90+). All 40 rabbits vaccinated with PA pDNA, either alone (groups 1, 2 and 3) or in combination with LF pDNA (groups 5 and 6) survived challenge (Table 2), including all 16 animals that received only two injections of pDNA. The four rabbits vaccinated twice with AVA also survived. None of the 12 naive rabbits and none of the five rabbits injected with formulated vector pDNA (group 8) survived challenge. Protection was also achieved in five of the nine animals injected three times with LF pDNA (group 7), demonstrating that an immune response to LF does confer protection, but not as complete as the protection elicited by PA immunization. The average time to death was 5.3 days for the four nonsurviving rabbits in group 7, which, when compared with the 2.6-day average survival of control animals (P = 0.035), further suggests that LF provided measurable, although incomplete, protective immunity.
Immune Responses After Exposure to Pathogen. To determine the effect of spore challenge, serum antibodies were assayed in all surviving rabbits 7 and 21 days postchallenge (Fig. 2). Spore challenge boosted the mean anti-PA antibody titer >10-fold in AVA-vaccinated rabbits (group 9) and 2–3 orders of magnitude (P = 0.035) in surviving rabbits vaccinated with LF pDNA alone (Fig. 2 A, group 7). This result is consistent with the germination of spores in these rabbits resulting in sufficient Letx production to boost their anti-PA titer. In contrast, rabbits vaccinated with two or three injections of PA pDNA alone (groups 1, 2, and 3) or in combination with LF pDNA (groups 5 and 6) showed no significant increase in anti-PA antibody titers postchallenge (Fig. 2 A).
Fig. 2.
Immune responses in rabbits post-anthrax spore challenge. GMTs for pDNA and AVA vaccinated rabbits pre- (□), 1-week post- (▒), and 3-weeks post- (▪) B. anthracis inhalation spore challenge. Shown are anti-PA IgG titers (A), anti-LF IgG titers (B), and Letx neutralization titers (C). The GMT of the five rabbits in the LF[I-III] group that survived challenge are graphed separately from the four nonsurvivors. *, Statistically significant increase in 3-week postchallenge titers.
With respect to anti-LF antibody responses, AVA-vaccinated animals (group 9) developed a significant anti-LF antibody titer postchallenge (P = 0.038), again consistent with the germination of spores in these rabbits (Fig. 2B). Importantly, no measurable anti-LF antibody titer was induced postchallenge in animals vaccinated three times with PA pDNA alone (Fig. 2B, groups 1 and 2). Even in rabbits immunized only twice with PA pDNA (group 3), the minimal anti-LF titer seen on day 21 postchallenge was due to a response in just two of the eight rabbits. No significant increases in postchallenge anti-LF titers were observed in rabbits vaccinated with LF pDNA (Fig. 2B, groups 5 and 6, and survivors in group 7).
A final indication of the magnitude and quality of the immune response generated with pDNA is that no significant increases in postchallenge Letx neutralizing antibody titers were observed in any pDNA-vaccinated rabbits. In contrast, a significant increase (P = 0.043) in neutralizing antibody titer was seen for the four AVA-vaccinated rabbits (group 9) 21 days postchallenge.
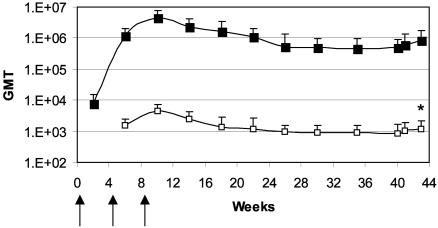
Duration of Protective Immunity. To determine the kinetics and durability of the immune response, group 4 rabbits injected three times with Vaxfectin-formulated PA pDNA were not challenged at week 12, but rather were monitored for an additional 7 months before spore challenge. Anti-PA and Letx neutralization titers were measured monthly and showed similar profiles (Fig. 3): titers peaked at week 10 (2 weeks after the third injection) and then declined somewhat until week 26. From week 26 to the time of challenge, the titers were essentially unchanged. At week 40, all 10 rabbits were challenged as previously described and survived, whereas all five naive animals died (Table 2). In contrast to the eight group 1 rabbits that were vaccinated three times with Vaxfectin-formulated PA pDNA and challenged at week 12, spore challenge induced a significant increase in the Letx neutralization titer (Fig. 3, P = 0.024) in the group 4 rabbits, suggesting that there was limited spore germination after challenge in these animals. This postchallenge increase in Letx neutralization titer, however, was smaller than the increase seen in AVA- (group 9) or LF pDNA-vaccinated (group 7) rabbits challenged at week 12 (Fig. 2C).
Fig. 3.
Long-term persistence of immune response and postchallenge response. Group 4 rabbits were vaccinated with PA pDNA (designated by arrows) and monitored for anti-PA (▪) and Letx neutralization (□) GMT. All rabbits were challenged on week 40 (32 weeks after the last immunization) and survived.
Discussion
This study demonstrates that i.m. injection of cationic lipid-formulated pDNA vaccines directed against B. anthracis Letx protect rabbits from a 100× LD50 aerosolized anthrax spore challenge. Aerosolized spore challenge is the gold standard for anthrax vaccine efficacy because it exposes the animal to the agent and expected mode of delivery anticipated in the event of a bioterrorist attack. Although there may be advantages for mucosal vaccines to be delivered by other routes (20), our data clearly show that i.m. delivered pDNA vaccines can protect against pathogenic spore exposure across a mucosal surface. Protective immunity was achieved with either two or three injections of formulated pDNA encoding detoxified PA or PA plus LF. This study also demonstrates in a relevant animal model that vaccination with LF pDNA alone can generate protective immunity. In addition, rabbits vaccinated with formulated PA pDNA achieved a durable immune response that protected them from lethal spore challenge 7 months postvaccination.
Formulated pDNA compared favorably with a 50-μl dose of AVA vaccine in this rabbit study. This dose of AVA is 1/10 the human dose, which we chose based on previous work demonstrating complete protection of rabbits from spore challenge (19). Analysis of pre- vs. postchallenge total binding and neutralizing antibody titers in animals receiving three doses of PA pDNA or PA plus LF pDNA suggests that pDNA, but not two 50-μl doses of AVA, provided sterile immunity. The sterile immunity seen in vaccinated animals at peak titer was not seen in animals challenged 7.5 months postvaccination, indicating that sterile immunity is a time-limited immunological state. Support for the mechanism by which sterile immunity can be achieved with anti-toxin antibodies comes from studies of opsonization of anthrax spores: anti-PA antibodies have been shown to opsonize anthrax spores, leading to their uptake and destruction in macrophages (21, 22). Rabbits having sufficient anti-PA antibodies to limit spore germination and bacterial growth would be predicted to have anti-PA, anti-LF, or Letx neutralization titers that do not change postchallenge.
Immune responses in rabbits injected twice with a 50-μl dose of AVA (group 9), PA pDNA (group 3), or PA plus LF pDNAs (group 5) were all of a similar magnitude before challenge. However, after challenge, the AVA animals demonstrated the largest increase in anti-PA, anti-LF, and Letx neutralizing titers, suggesting that the AVA group allowed more spore germination and concomitant Letx production. This result is dramatically shown in the AVA group by the increase in mean anti-LF titers from background levels before challenge to 81,000 postchallenge. The only postchallenge increase of similar magnitude was the anti-PA response in rabbits receiving LF pDNA alone (group 7). Animals receiving three 1-mg doses of PA pDNA alone (groups 1 and 2) showed no increase in total PA binding and neutralizing antibody titers at 7 and 21 days postchallenge. In addition, these animals generated no challenge-induced antibody to LF. Similarly, in animals receiving three injections of PA plus LF pDNAs (group 6), postchallenge PA- or LF-binding antibody titers and neutralizing antibody titers did not increase. Together, these data suggest that rabbits vaccinated three times with PA pDNA-containing vaccines develop a robust protective immune response that prevented sufficient spore germination to boost preexisting antibody responses.
Rabbits that received two injections of Vaxfectin-formulated pDNA were protected from challenge. However, serum binding and neutralizing antibody titers decreased during the 8- to 9-week period after their second injection. Therefore, we measured the duration of the protective immune response generated in rabbits after vaccination with pDNA. In the 10 animals followed for >7 months after their third vaccination, binding and neutralizing antibody titers plateaued ≈3 months after the third injection and remained stable for the duration of this 7.5-month postvaccination period. At this plateau level, the immune response still protected all 10 rabbits from a 100× LD50 spore challenge. However, in contrast to rabbits challenged at peak titer, a 1.7-fold significant increase in mean Letx neutralizing titer postchallenge was seen, indicating sufficient spore generation to boost the preexisting immune response in these rabbits.
Although anti-LF antibodies were less protective than anti-PA antibodies, there are several reasons to advance a bivalent PA plus LF anthrax vaccine. A vaccine able to neutralize both components of Letx may protect against genetically engineered PA- or LF-containing toxins. Additionally, a recent study (23) demonstrating that LF (without PA) is able to permeate mammalian cells is a clear indication that the unchecked release of LF into serum cannot be considered harmless. Finally, the possible added benefit provided by coinjecting the LF pDNA did not compromise the immune response generated by the PA pDNA.
The prevention of anthrax disease is likely to be augmented by both the innate and cellular arms of the immune system, in addition to a protective humoral response. DNA vaccines have been shown to induce both CD4+ and CD8+ T cells (24). Such responses are likely to be induced by our DNA approach and may contribute to the control of disease, especially in the host attempt to clear infected cells and macrophages. Additionally, the combination of cationic lipids and DNA has been shown to activate an innate immune response that has antitumor activity (25), and such a response in the setting of a spore challenge may be critical in tipping the balance in favor of the host during the hours and days after exposure.
To date, the antibody responses in humans vaccinated with naked pDNA-based products have not been sufficient to warrant further product development (26). The Vaxfectin and DMRIE/DOPE cationic lipid formulations used in this study have not yet been tested in humans but have been shown to significantly enhance antigen-specific antibody responses to pDNA vaccines in animals (18, 27–29), possibly by the induction of cytokines like IFN-γ, IL-12, and IL-6. The two cationic lipid formulations performed equally well in this study and offer an attractive option to exploit the utility of pDNA technology. The advantages of a pDNA vaccine approach are that (i) target immunogens can be genetically, and thus specifically, stably and uniformly detoxified; (ii) a large number of different constructs and combinations of immunogens (i.e., multivalent vaccines) can be rapidly developed and tested; (iii) a range of formulations suitable for use in humans can be tested that can provide the optimal balance of humoral and cellular immune responses without immunopathology; (iv) pDNA does not require the handling of the pathogen or any mammalian cell substrates (with the associated risk of adventitious agents) at any point in the manufacturing process; and (v) stable and scalable pDNA vaccines can be rapidly transitioned to the clinic, requiring only DNA sequence information and minor manufacturing process development (24).
The rabbit studies described herein are part of an anthrax vaccine development project that has progressed from plasmid design and construction through in vivo immunogenicity and inhalation spore challenge, resulting in product selection, preclinical safety studies, and U.S. FDA Investigational New Drug allowance. This progress has all been accomplished within a 28-month span. The uniformity of DNA vaccine technology suggests that this product development timeframe can be repeated with other biodefense vaccine targets. Therefore, DNA vaccines provide a valuable technology for the rapid development of safe and efficacious vaccines needed for biodefense and emerging infectious diseases.
Supplementary Material
Acknowledgments
We thank Alain Rolland, Larry Smith, and Alan Engbring for review of this manuscript and Margaret Hogan for help with the figures. This study was partially funded by National Institutes of Health Small Business Technology Transfer Grant 1R41AI053060-01.
Abbreviations: pDNA, plasmid DNA; LF, lethal factor; PA, protective antigen; AVA, anthrax vaccine adsorbed; Letx, anthrax lethal toxin; DMRIE, (±)-N-(2-hydroxyethyl)-N,N-dimethyl-2,3-bis(tetradecyloxy)-1-propanaminium bromide; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; GMT, geometric mean titer; hTPA, human tissue plasminogen activator.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY428556–AY428558).
References
- 1.Joellenbeck, L., Zwanziger, L. L., Durch, J. S. & Strom, B. L., eds. (2002) The Anthrax Vaccine: Is It Safe? Does It Work? (Natl. Acad. Press, Washington, DC). [PubMed]
- 2.Dixon, T. C., Meselson, M., Guillemin, J. & Hanna, P. C. (1999) N. Engl. J. Med. 341, 815–826. [DOI] [PubMed] [Google Scholar]
- 3.Mock, M. & Mignot, T. (2003) Cell Microbiol. 5, 15–23. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, K. A., Mogridge, J., Mourez, M., Collier, R. J. & Young, J. A. (2001) Nature 414, 225–229. [DOI] [PubMed] [Google Scholar]
- 5.Klimpel, K. R., Molloy, S. S., Thomas, G. & Leppla, S. H. (1992) Proc. Natl. Acad. Sci. USA 89, 10277–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh, Y., Chaudhary, V. K. & Leppla, S. H. (1989) J. Biol. Chem. 264, 19103–19107. [PubMed] [Google Scholar]
- 7.Mogridge, J., Cunningham, K., Lacy, D. B., Mourez, M. & Collier, R. J. (2002) Proc. Natl. Acad. Sci. USA 99, 7045–7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duesbery, N. S., Webb, C. P., Leppla, S. H., Gordon, V. M., Klimpel, K. R., Copeland, T. D., Ahn, N. G., Oskarsson, M. K., Fukasawa, K., Paull, K. D. & Vande Woude, G. F. (1998) Science 280, 734–737. [DOI] [PubMed] [Google Scholar]
- 9.Moayeri, M., Haines, D., Young, H. A. & Leppla, S. H. (2003) J. Clin. Invest. 112, 670–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu, M. L., Leppla, S. H. & Klinman, D. M. (1999) Vaccine 17, 340–344. [DOI] [PubMed] [Google Scholar]
- 11.Riemenschneider, J., Garrison, A., Geisbert, J., Jahrling, P., Hevey, M., Negley, D., Schmaljohn, A., Lee, J., Hart, M. K., Vanderzanden, L., et al. (2003) Vaccine 21, 4071–4080. [DOI] [PubMed] [Google Scholar]
- 12.Price, B. M., Liner, A. L., Park, S., Leppla, S. H., Mateczun, A. & Galloway, D. R. (2001) Infect. Immun. 69, 4509–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galloway, D., Liner, A., Legutki, J., Mateczun, A., Barnewall, R. & Estep, J. (2004) Vaccine 22, 1604–1608. [DOI] [PubMed] [Google Scholar]
- 14.Zaucha, G. M., Pitt, L. M., Estep, J., Ivins, B. E. & Friedlander, A. M. (1998) Arch. Pathol. Lab. Med. 122, 982–992. [PubMed] [Google Scholar]
- 15.Brossier, F., Weber-Levy, M., Mock, M. & Sirard, J. C. (2000) Infect. Immun. 68, 1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartikka, J., Sawdey, M., Cornefert-Jensen, F., Margalith, M., Barnhart, K., Nolasco, M., Vahlsing, H. L., Meek, J., Marquet, M., Hobart, P., et al. (1996) Hum. Gene Ther. 7, 1205–1217. [DOI] [PubMed] [Google Scholar]
- 17.Pannifer, A. D., Wong, T. Y., Schwarzenbacher, R., Renatus, M., Petosa, C., Bienkowska, J., Lacy, D. B., Collier, R. J., Park, S., Leppla, S. H., et al. (2001) Nature 414, 229–233. [DOI] [PubMed] [Google Scholar]
- 18.Hartikka, J., Bozoukova, V., Ferrari, M., Sukhu, L., Enas, J., Sawdey, M., Wloch, M. K., Tonsky, K., Norman, J., Manthorpe, M. & Wheeler, C. J. (2001) Vaccine 19, 1911–1923. [DOI] [PubMed] [Google Scholar]
- 19.Pitt, M. L., Little, S. F., Ivins, B. E., Fellows, P., Barth, J., Hewetson, J., Gibbs, P., Dertzbaugh, M. & Friedlander, A. M. (2001) Vaccine 19, 4768–4773. [DOI] [PubMed] [Google Scholar]
- 20.Boyaka, P. N., Tafaro, A., Fischer, R., Leppla, S. H., Fujihashi, K. & McGhee, J. R. (2003) J. Immunol. 170, 5636–5643. [DOI] [PubMed] [Google Scholar]
- 21.Welkos, S., Little, S., Friedlander, A., Fritz, D. & Fellows, P. (2001) Microbiology 147, 1677–1685. [DOI] [PubMed] [Google Scholar]
- 22.Welkos, S., Friedlander, A., Weeks, S., Little, S. & Mendelson, I. (2002) J. Med. Microbiol. 51, 821–831. [DOI] [PubMed] [Google Scholar]
- 23.Kushner, N., Zhang, D., Touzjian, N., Essex, M., Lieberman, J. & Lu, Y. (2003) Proc. Natl. Acad. Sci. USA 100, 6652–6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly, J. J., Ulmer, J. B. & Liu, M. A. (1998) Dev. Biol. Stand. 95, 43–53. [PubMed] [Google Scholar]
- 25.Horton, H. M., Dorigo, O., Hernandez, P., Anderson, D., Berek, J. S. & Parker, S. E. (1999) J. Immunol. 163, 6378–6385. [PubMed] [Google Scholar]
- 26.Srivastava, I. K. & Liu, M. A. (2003) Ann. Intern. Med. 138, 550–559. [DOI] [PubMed] [Google Scholar]
- 27.Reyes, L., Hartikka, J., Bozoukova, V., Sukhu, L., Nishioka, W., Singh, G., Ferrari, M., Enas, J., Wheeler, C. J., Manthorpe, M. & Wloch, M. K. (2001) Vaccine 19, 3778–3786. [DOI] [PubMed] [Google Scholar]
- 28.Fischer, L., Tronel, J. P., Minke, J., Barzu, S., Baudu, P. & Audonnet, J. C. (2003) Vaccine 21, 1099–1102. [DOI] [PubMed] [Google Scholar]
- 29.D'Souza, S., Rosseels, V., Denis, O., Tanghe, A., De Smet, N., Jurion, F., Palfliet, K., Castiglioni, N., Vanonckelen, A., Wheeler, C. & Huygen, K. (2002) Infect. Immun. 70, 3681–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.