Abstract
Trigger factor is a molecular chaperone that is present in all species of eubacteria. It binds to the ribosomal 50S subunit near the translation exit tunnel and is thought to be the first protein to interact with nascent polypeptides emerging from the ribosome. The chaperone has a peptidyl-prolyl cis-trans isomerase (PPIase) activity that catalyzes the rate-limiting proline isomerization in the protein-folding process. We have determined the crystal structure of nearly full-length trigger factor from Vibrio cholerae by x-ray crystallography at 2.5-Å resolution. The structure is composed of two trigger-factor molecules related by a noncrystallographic two-fold symmetry axis. The monomer has an elongated shape and is folded into three domains: an N-terminal domain I that binds to the ribosome, a central domain II that contains PPIase activity, and a C-terminal domain III. The active site of the PPIase domain is occupied by a loop from domain III, suggesting that the PPIase activity of the protein could be regulated. The dimer interface is formed between domains I and III and contains residues of mixed properties. Further implications about dimerization, ribosome binding, and other functions of trigger factor are discussed.
Folding of nascent polypeptides is often assisted by molecular chaperones (1). As nascent polypeptides emerge from the ribosome exit tunnel during protein translation, they are welcomed by a committee of ribosome-associated chaperones (2). The role of these chaperones is to stabilize the unfolded conformation of nascent polypeptides before initiation of productive folding processes. In bacteria, trigger factor is thought to fulfill this function in the folding of cytosolic proteins (3–5). It interacts with both the ribosome and nascent polypeptide chains, displaying an in vitro chaperone-like activity (6, 7). Its interaction partner on the ribosome has been identified as the L23 protein that is located near the translation exit tunnel (8, 9). In addition, trigger factor can catalyze the peptidyl-prolyl cis-trans isomerization reaction, often a rate-limiting step in protein folding (10, 11). Trigger factor's function in vivo partially overlaps with that of a cytosolic chaperone DnaK (12). Deletion of the gene for trigger factor is compensated for by the presence of DnaK, and vice versa, but the deletion of both is synthetically lethal at 37°C (13–15). Recent evidence showed that bacteria lacking both chaperones can survive if grown at lower temperatures or in the presence of overexpressed GroEL, the third major chaperone system involved in bacterial protein folding (16). Genes encoding trigger factor have been identified in all bacterial genomes sequenced so far (17, 18).
Limited proteolysis of Escherichia coli trigger factor combined with circular dichroism experiments showed that trigger factor consists of three independently folded domains (19). The N domain consists of residues 1–145 (19) and is both necessary and sufficient for binding to the ribosome (20). The M domain consists of residues 148–249 (21) and bears strong sequence similarity to the human FK-506 binding protein (FKBP), a well known peptidyl-prolyl cis-trans isomerase (PPIase) (22, 23). Individually expressed M domains display PPIase activity toward short proline-containing peptides (6, 21). The remainder of the sequence makes up the C domain whose function is largely undefined (6, 13, 20). The chaperone activity of the protein has not been mapped to any particular domain but is believed to involve the N and C domains (19). Nonnative polypeptide binding to trigger factor is independent of proline residues, and PPIase activity is not required for chaperone activity (24–26). Biochemical characterization of trigger factor showed that the protein interacts with nonnative polypeptide substrates in a dynamic process, initiating rapid cycles of binding and release which are thought to prevent aggregation while allowing nascent chains freedom to engage in productive folding interactions (27).
Although the structures of the isolated N and M domains of trigger factors have been published (28, 29), the lack of a complete structure has impeded our further understanding of the function of the protein. The functional interplay between the three domains of trigger factor has remained a mystery. In particular, it is not clear how these interactions contribute to the overall function of trigger factor as a molecular chaperone. In the present study, we determined the crystal structure of nearly full-length Vibrio cholerae trigger factor at 2.5-Å resolution. Our work confirms the previous prediction that trigger factor is folded into three distinctive domains, each corresponding to different functions of the protein. The active site of the PPIase domain is occupied by a loop from the C-terminal domain. Trigger factor exists as a dimer in solution but appears to bind to the ribosome as a monomer (30). Our study shows that dimer formation involves intimate association between the ribosome-binding domain and the C-terminal domain. The closeness of the ribosome-binding loop to the dimer interface suggests that dimer formation will likely interfere with trigger factor binding to the ribosome.
Experimental Procedures
Protein Expression and Purification. V. cholerae trigger factor (VCTF) genes were amplified from genomic DNA stocks purchased from American Type Culture Collection by using the PCR method. Amplified genes were cloned into a modified pET21a vector (Novagen) such that expressed proteins contain an N-terminal histidine tag with a TEV protease cleavage site between the tag and trigger factor to facilitate protein purification. To generate high-quality diffracting crystals, a truncated form of VCTF (lacking the C-terminal 44 residues) was used in the study. E. coli BL21(DE3) cells harboring the recombinant plasmid were grown in LB medium, and protein expression was induced with 0.4 mM isopropyl β-d-thiogalactoside at 37°C for 4 h. The selenomethionine form of the protein was expressed in M9 minimal medium supplemented with 50 mg·liter–1 selenomethionine. Proteins were purified by a Co2+-Talon affinity column (Clontech), and a subsequent anion-exchange Source-Q column (Amersham Pharmacia).
Crystallization and Data Collection. Purified protein was concentrated to ≈20 mg·ml–1 in 20 mM Tris·HCl (pH 7.5) and used for sparse-matrix crystallization screening. Two conditions yielded diffraction quality crystals: 2.1 M sodium malonate at pH 7.5–9.5 and 2.7 M ammonium sulfate at pH 7.5–8.5. Protein containing selenomethionine in place of methionine was only crystallized in the sodium malonate solution. Crystals were cryoprotected for data collection under a nitrogen stream at 110 K. Crystals grown under ammonium sulfate belong to the space group I41 with unit cell constants a = b = 189.8 Å, c = 62.1 Å. Crystals grown under sodium malonate belong to the space group P21212 with unit cell constants a = 98.8 Å, b = 164.8 Å, c = 62.2 Å. In both cases, the asymmetric unit contains one VCTF dimer complex. All data were collected on DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) (5ID-D) at the Advanced Photon Source and were processed and scaled by using the program d*trek (31). Multiwavelength anomalous dispersion (MAD) data were collected from a selenomethionine derivative crystal at three different wavelengths (Table 1, which is published as supporting information on the PNAS web site).
Structure Solution and Refinement. Experimental phases were obtained by using the MAD method (32) in the space group P21212 (Table 1). Eighteen of the 22 selenium sites expected in the VCTF dimer were located. Subsequently, heavy atom parameters were refined and MAD phases were calculated with sharp (33). The initial electron density map showed the overall trace of the molecule that allowed us to define a molecular envelope and identify the twofold noncrystallographic symmetry (NCS) axis. The electron density map was then improved by density modification along with phase extension to 2.7-Å resolution. The high quality of the resulting map and the known selenium sites allowed tracing of the protein main chain and most of the side chains. All model building used the o program (34), and structure refinement procedures were carried out with the cns program (35). The initial model was refined against the MAD data set collected at the peak wavelength with strict NCS imposed between the two monomers. Once the structure was partially refined, it was used as a molecular replacement search model to obtain the structure in the I41 space group. All subsequent refinement was performed against the 2.5-Å native data set. In brief, two monomers were placed into the tetragonal cell and oriented by rigid body refinement. Initial refinement was performed with data to 3.0 Å and strict NCS. Later rounds of refinement were performed on an NCS-restrained dimer with slowly increasing high-resolution data. In the final rounds, all the data from 44.8 to 2.5 Å were used with bulk solvent and anisotropic B factor correction. The final model includes residues 1–130 and 134–378 for subunit A, residues 1–129 and 134–377 for subunit B, and a total of 109 water molecules. Residues not included in the model are those that are not visible and assumed to be disordered. The coordinates and structure factors have been deposited into the Protein Data Bank (PDB ID code 1T11).
Results and Discussion
Overall Structure. VCTF, a homologue of E. coli trigger factor with 70% sequence identity, consists of 433 amino acid residues (Fig. 1). We have crystallized a nearly full-length VCTF protein (residues 1–389), which contains the ribosome-binding domain, the PPIase domain and the majority of the functionally undefined C-terminal domain. Two different crystal forms were obtained, each belonging to a different space group (I41 and P21212). In both cases, a VCTF dimer was found in the asymmetric unit. The final refined model was at 2.5 Å with an R factor of 23.9% and an Rfree of 29.0%. Of all of the nonglycine residues, 84.8% are located in the most favored regions on the Ramachandran plot, and none are located in the disallowed regions.
Fig. 1.
Sequence alignment of bacterial trigger factors. The sequences of trigger factors from Bacillus subtilis, Haemophilus influenzae, Mycoplasma genitalium, E. coli, and V. cholerae were aligned by using clustalw (43). Invariant residues are indicated with red letters. Secondary structures are shown below the sequence: α-helices are drawn as cylinders, β-strands are drawn as arrows, other elements appear as solid lines, and structurally unobserved residues in the midsequence and at the C terminus are shown as dashed lines. Elements in domain I are blue, elements in domain II are green, and elements in domain III are red.
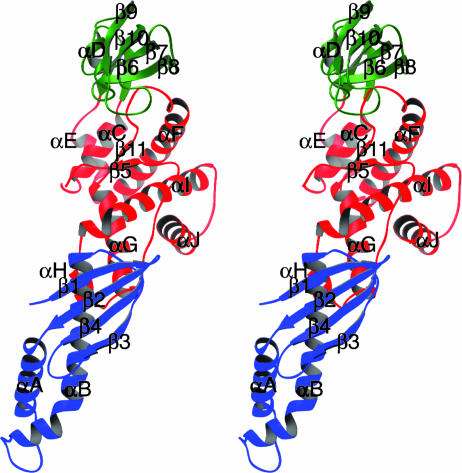
The structure shows trigger factor has an overall elongated shape and is folded into three distinctive structural domains (Fig. 2). Domain I (residues 1–110; Fig. 2, blue) is of mixed α/β-fold, whereas domain II (residues 150–246; Fig. 2, green) is almost entirely β-sheet and domain III (residues 111–149 and 247–378; Fig. 2, red) is predominantly α-helical. Domain I roughly corresponds to the N domain, which has been shown to be responsible for ribosome binding. Domain II is nearly identical with the M domain whose structure adopts an FKBP-like fold (36). Domain III corresponds to the C domain but also contains elements from the N domain. Domain III occupies the center of the molecule and makes contact with the other two domains. Domains I and II are spatially distant and do not interact with one another.
Fig. 2.
A stereo ribbon diagram of a trigger-factor monomer. Secondary structure elements in three domains of trigger factor are colored and labeled as in Fig. 1 (domain I, blue; domain II, green; and domain III, red). α-helices are drawn as coils, β-strands are drawn as arrows, and other elements are drawn as tubes. This figure was prepared with the program ribbons (44).
Ribosome Binding. Domain I is composed primarily of a four-stranded antiparallel β-sheet containing strands β1, β2, β4, and β3, in that order, and two α-helices (αA and αB) inserted between strands β2 and β3 (Fig. 2). The ribosome-binding motif is located on a flexible loop that connects the two helices. The domain appears to be independently folded, held together by a hydrophobic core with the long helix αB forming the central scaffold. The lower half of helix αB packs against the shorter helix αA and residues from an extended turn between strands β3 and β4 of the β-sheet. The upper half of helix αB packs against the four-stranded β-sheet. It also forms one of the few interdomain contacts in the VCTF structure with helix αH from domain III.
A functionally critical part of domain I is the ribosome-binding loop, which contains the conserved GFRxGxxP ribosome-binding motif, or “TF signature” (8). It is positioned between helices αA and αB at the spatial end of the molecule. In our current structure, this region has relatively poor electron density. Several side chains could not be built into the model with confidence and have been excluded from the final model. The poor electron density combined with relatively high temperature factors indicates a high degree of structural flexibility in this region. This flexibility would allow the ribosome-binding motif to adopt an efficient binding conformation in the presence of L23, the ribosomal binding partner for trigger factor.
Peptidyl-prolyl cis-trans Isomerase. Domain II of VCTF, defined here as residues 150–247, is nearly identical with the original definition of the M domain (19, 21), which is necessary and sufficient for the PPIase activity of trigger factor. The structure of domain II adopts a classic FKBP-like fold (37) consisting of a core four-stranded β-sheet (strands β7–β10) and a short helix αD inserted between strands β8 and β9 (Fig. 2). It is nearly superimposable to the NMR structure of the M. genitalium trigger-factor M domain (29), with an rms deviation of 1.80 Å for 85 main-chain Cα atoms. Domain II is connected to domain I by a long L-shaped loop followed by helix αC. Both structure elements are an integral part of domain III.
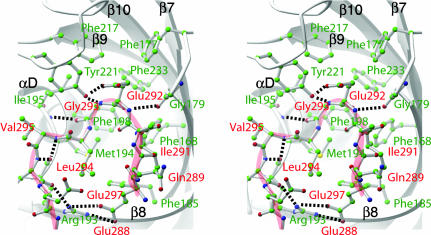
As in FKBP and the M. genitalium M domain, the β-sheet is highly curved. The PPIase active site is located on the concave side of the sheet, enclosed by the loops between strands β7 and β8, between strands β9 and β10, and between strand β8 and helix αD (Fig. 3). The sequences of all three structural elements are highly conserved within the trigger factor family. The overall character of the active site is aromatic, including conserved residues Phe-168, Phe-177, Met-194, Ile-195, Phe-198, Val-215, Phe-217, Pro-218, Tyr-221, and Phe-233. Three of them, Ile-195, Tyr-221, and Phe-233, are strictly conserved in the FKBP family as well. Surprisingly, the active site is occupied by an insertion loop originating from domain III (Fig. 3). This insertion loop consists of Gln-291 through Glu-301 and connects helices αF and αG. Most of the interactions between the loop and PPIase active site involve main-chain hydrogen bond interactions. For example, the carbonyl oxygen of residue Arg-193 forms a pair of hydrogen bonds with the amide nitrogen of Asn-298 and the carbonyl oxygen of Lys-296. More striking is the interaction between the absolutely conserved Tyr-221, whose tyrosyl hydroxyl group forms a pair of hydrogen bonds with the main chain amide nitrogen of Gly-293 and side chain of Glu-292. Mutation of Tyr-221 into a phenylalanine abolishes 85% of the PPIase activity of trigger factor, suggesting its important role in catalysis (25). The corresponding tyrosine in the active site of FKBP binds the FK506 substrate by a similar hydrogen bond and appears to lower the energy barrier for peptide bond flipping (36).
Fig. 3.
Interaction between the PPIase active site of domain II and the insertion loop from domain III. A close-up stereo view of the PPIase active site is shown. Domain II (PPIase) is shown as gray ribbon drawings. Side chains of residues that form the substrate-binding pocket are shown as ball-and-stick models (labeled in green). Note the overall hydrophobic nature of the pocket. The entire insertion loop (Glu-288 to Asn-298) is also shown in ball-and-stick rendition (labeled in red) superimposed with a light-red coil to facilitate chain tracing. Salt bridges and hydrogen bonds are depicted as dashed lines. Note that the catalytically important Tyr-221 makes a main chain contact with the amide group of Gly-293. This figure was prepared with the program ribbons (44).
Because binding of the insertion loop at the PPIase active site blocks substrate access, domain II needs to dissociate from the insertion loop to exhibit high levels of PPIase activity observed in vitro (6). This high level of activity is likely to be achieved by an en bloc movement of domain II. Domain II is linked to the rest of the molecule by a pair of antiparallel β-strands (β6 and the C-terminal part of β10). Limited proteolysis experiments showed that E. coli trigger factor was cut at Arg-145 and Glu-251, both of which are located at the linker (19). This finding suggests that the linker region is exposed and potentially flexible. Therefore, it is likely that the linker serves as a hinge to allow the domain II movement needed for catalyzing the PPIase reaction. Our structural analysis also suggests a high degree of motion for domain II. In fact, only one of the two domains of VCTF dimer in the asymmetric unit of the P21212 space group can be traced unambiguously.
All trigger factors known to date contain an FKBP-like domain and exhibit in vitro PPIase activity. Although the PPIase activity of trigger factor is important for supporting the growth of bacteria at low temperature (38), and recent evidence also shows that it is essential for the secretion and maturation of a cysteine protease in Streptococcus pyogenes (39), it is not clear to what extent this enzyme activity is required for trigger factor's role as a ribosome-associated chaperone. Several lines of evidence suggest that the PPIase domain is not directly involved in recognizing and binding nascent polypeptide chains. First, unfolded proteins that competitively inhibited trigger factor's chaperone activity did not affect its PPIase activity (11). Second, PPIase active site mutants of trigger factor retained their binding affinity for nonnative polypeptides and their chaperone activity (25). Third, the isolated PPIase domain is ineffective in catalyzing the refolding of a proline-containing polypeptide and unfolded proteins do not inhibit the reaction (11). Our structure adds additional support to the hypothesis. The hydrophobic substrate-binding site of the PPIase domain is plugged by the insertion loop from domain III, making it physically inaccessible to nascent polypeptides. Once a polypeptide is bound to trigger factor, the insertion loop may dissociate from the PPIase domain and expose the active site. A number of interactions could cause such a conformational change in trigger factor, including interaction with substrate polypeptide or ribosome binding. In the absence of such a signal, the insertion loop would remain bound to prevent erroneous substrate binding.
Domain III. Domain III is composed of residues 111–149 and 251–378. It is roughly equivalent to the previously defined C domain, but contains the L-shaped loop and the following helix αC that connects domains I and II (Fig. 2). It is situated at the center of the molecule with domains I and II on different sides. Helices αC, αE, αF, αG, and αH appear to be primarily structural in nature, forming a tightly packed hydrophobic core. Helix αC also serves to connect domain I to domain II. The end of helix αG along with helix αH and the intervening small loop interact with the β-sheet and helix αB of domain I. Residues immediately following helix αF form the insertion loop (see above), which is followed by a long stretch of amino acids lacking regular secondary structure that run parallel to helix αF down to the beginning of helix αG.
The C-terminal 55 amino acids are not present in our structure, of which 44 were genetically truncated from the C terminus to produce high-quality diffracting crystals. Although it is difficult to estimate the impact these 44 residues have on the rest of the trigger factor structure, it is unlikely that the absence of the C-terminal segment will change the overall fold of the protein. Based on the location of our current C terminus, the missing segment will likely project away from the main body of the structure and form an autonomous subdomain. Clearly, a full-length structure of trigger factor is needed to properly address the structure and function of the extreme C terminus of the protein.
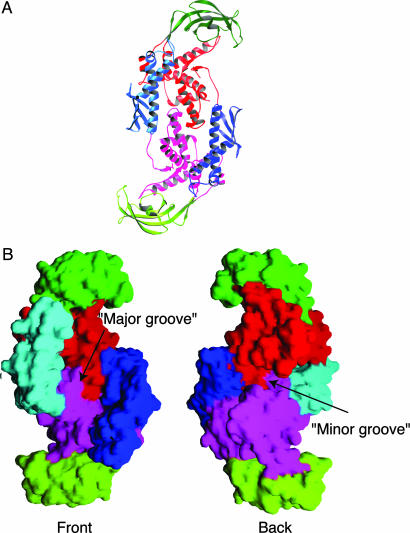
Structure of the Dimer and Dimer Interface. Trigger factor undergoes a monomer-dimer equilibrium in solution with an approximate Kd of 18 μM (30). The asymmetric unit of the crystal contains two monomers related by a noncrystallographic twofold symmetry axis. Inspection of crystal packing suggests that one pair of monomers represents the physiological dimer in solution (Fig. 4A). Each monomer in this pair buries 8.6% of its total solvent-accessible surface (1,810 Å2 as calculated by the program cns) in intermolecular contacts, an area that is in the range expected for intersubunit contacts in oligomeric proteins (as little as 5% for larger proteins and up to 20% for smaller ones) (40). All other molecular pairs involved in crystalline contacts bury <4.9% of solvent-accessible surface. The same dimer interface is observed in both crystal forms.
Fig. 4.
The structure of the trigger-factor dimer. (A) A ribbon drawing of the trigger-factor dimer looking down the twofold axis of symmetry. Secondary structure elements in one of the trigger-factor subunits are colored as in Fig. 2. Rotating this subunit ≈90° around a vertical axis (bring the left half into the page and the right half out of the page) produces the molecule in Fig. 2. Corresponding elements in the other subunit are colored similarly but in a slightly different shade. This figure was prepared with the program ribbons (44). (B) Molecular surface representation of the trigger factor dimer. (Left) Front view of the molecule (same orientation as in A). (Right) Back view of the molecule. Two grooves on the surface of the molecule are indicated. This figure was prepared with the program grasp (45).
Domains I and III mediate the dimer formation of trigger factor by wrapping around each other (Fig. 4A). The overall shape of the dimer resembles that of a right-handed double-stranded DNA molecule with a notable “major groove” in the front and a less prominent “minor groove” at the back (Fig. 4B). The “major groove” is lined by domains I and II and floored by domain III from both subunits. The width of the groove is on average ≈40 Å. The formation of the dimer places domain I close to domain II of the symmetry-related subunit, but no direct interaction occurs between the two. Therefore, even in the context of the dimer, domain II can still assume the aforementioned en bloc movement.
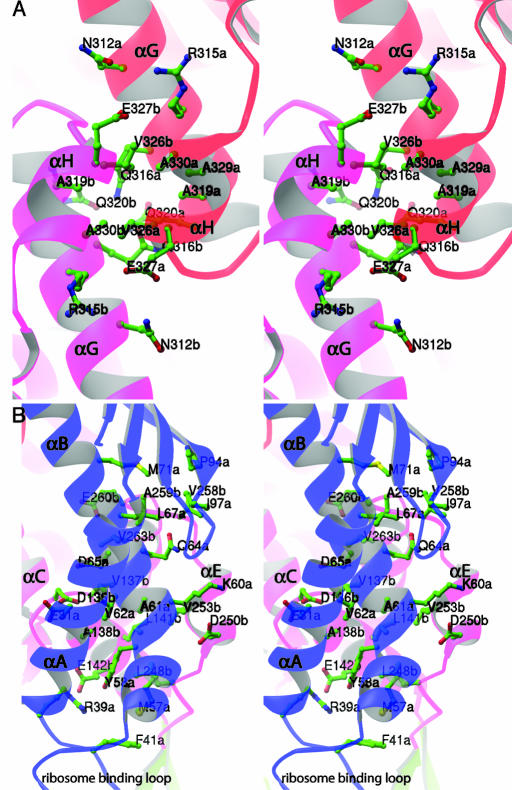
The interactions across the dimer interface are of mixed nature, including both polar and nonpolar interactions. Five helices are particularly involved in the dimerization: helix αA, helix αB, helix αC, helix αE, and helix–turn–helix αG-αH. In the center of the structure, two symmetry-related helix–turn–helix αG-αH segments from domain III interact with each other (Fig. 5A). On either side, the interface is formed between helix αA, helix αB (domain I) of one subunit and helix αC, helix αE (domain III) of the second symmetrical subunit (Fig. 5B). The three interfaces are not continuous leaving two apparent holes in the middle of the structure.
Fig. 5.
The dimer interface of trigger factor. (A) An enlarged stereo view of the residues involved in interactions between αG-αH helix–turn–helix motifs of two symmetry-related domains III. (B) An enlarged stereo view of residues involved in the dimer interface between helices αA and αB of domain I of one subunit, and helices αC and αE of domain III of the other subunit. Only residues that are involved in salt bridges, hydrogen bonds, or van der Waals interactions are shown. Note the position of the ribosome-binding loop relative to the dimer interface. Both figures were prepared with the program ribbons (44).
Because the ribosome-binding loop is located between helix αA and helix αB, the interaction between domain I and domain III across the dimer interface places the loop very close to the opposite domain III, suggesting that a dimer structure of trigger factor is incompatible with its binding to the ribosome. This is consistent with previous observations that trigger factor binds to the ribosome as a monomer in the absence of translating polypeptide (30, 41). Although the precise role of the trigger factor dimer is still unclear, recent data suggested that trigger factor is released from the ribosome together with nascent polypeptide chain in the bacterial cytosol during its reaction cycle (42). Although trigger factor has a strong tendency to dimerize when not bound to the ribosome, it remains to be seen whether the dimer structure constitutes the functional state of trigger factor.
In summary, we have shown that trigger factor has an overall protein fold that is organized into three functionally unique domains: domain I (ribosome-binding and dimerization), domain II (PPIase), and domain III (dimerization). Although structures of isolated domains have been previously studied, the complete structure of trigger factor provides a first look at how these three domains interplay with each other. We have shown that the PPIase domain has an active site arrangement similar to that of the FKBP family. However, the active site here is blocked by an insertion loop from domain III. Trigger factor dimerizes through the interaction between domains I and III. In particular, dimerization appears to be incompatible with ribosome binding, supporting that the trigger factor binds to the ribosome as a monomer. Together with other recent discoveries in the field, we are beginning to advance our understanding of the molecular mechanism of trigger factor on a structural level.
Supplementary Material
Acknowledgments
We thank J. Stuckey for maintaining the x-ray facility at the University of Michigan Medical School and Life Sciences Institute; K. Yoshino for assistance at the early stage of the project; D. Peisach for help in data collection and crystallography; and Z. Wawrzak for access and help at Advanced Photon Source DuPont–Northwestern–Dow Collaborative Access Team (APS DND-CAT) 5ID-D beamline. DND-CAT is supported by the E.I. DuPont de Nemours & Co., the Dow Chemical Company, the U.S. National Science Foundation through Grant DMR-9304725, and the State of Illinois through the Department of Commerce and Board of Higher Education Grant IBHE HECA NWU 96. This work was supported by National Institutes of Health Grant R01-GM60997 (to Z.X.) and the University of Michigan Biological Scholar Program. Z.X. is a Pew Scholar in Biomedical Sciences.
Abbreviations: MAD, multiwavelength anomalous dispersion; VCTF, Vibrio cholerae trigger factor; FKBP, FK-506 binding protein; PPIase, peptidyl-prolyl cis-trans isomerase.
Data deposition: The coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 1T11).
References
- 1.Hartl, F. U. & Hayer-Hartl, M. (2002) Science 295, 1852–1858. [DOI] [PubMed] [Google Scholar]
- 2.Bukau, B., Deuerling, E., Pfund, C. & Craig, E. A. (2000) Cell 101, 119–122. [DOI] [PubMed] [Google Scholar]
- 3.Lecker, S., Lill, R., Ziegelhoffer, T., Georgopoulos, C., Bassford, P. J., Jr., Kumamoto, C. A. & Wickner, W. (1989) EMBO J. 8, 2703–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hesterkamp, T., Hauser, S., Lutcke, H. & Bukau, B. (1996) Proc. Natl. Acad. Sci. USA 93, 4437–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang, G. C., Li, Z. Y., Zhou, J. M. & Fischer, G. (2000) Protein Sci. 9, 1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoller, G., Rucknagel, K. P., Nierhaus, K. H., Schmid, F. X., Fischer, G. & Rahfeld, J. U. (1995) EMBO J. 14, 4939–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maier, R., Eckert, B., Scholz, C., Lilie, H. & Schmid, F. X. (2003) J. Mol. Biol. 326, 585–592. [DOI] [PubMed] [Google Scholar]
- 8.Kramer, G., Rauch, T., Rist, W., Vorderwulbecke, S., Patzelt, H., Schulze-Specking, A., Ban, N., Deuerling, E. & Bukau, B. (2002) Nature 419, 171–174. [DOI] [PubMed] [Google Scholar]
- 9.Ban, N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. (2000) Science 289, 905–920. [DOI] [PubMed] [Google Scholar]
- 10.Hesterkamp, T. & Bukau, B. (1996) FEBS Lett. 385, 67–71. [DOI] [PubMed] [Google Scholar]
- 11.Scholz, C., Stoller, G., Zarnt, T., Fischer, G. & Schmid, F. X. (1997) EMBO J. 16, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deuerling, E., Patzelt, H., Vorderwulbecke, S., Rauch, T., Kramer, G., Schaffitzel, E., Mogk, A., Schulze-Specking, A., Langen, H. & Bukau, B. (2003) Mol. Microbiol. 47, 1317–1328. [DOI] [PubMed] [Google Scholar]
- 13.Deuerling, E., Schulze-Specking, A., Tomoyasu, T., Mogk, A. & Bukau, B. (1999) Nature 400, 693–696. [DOI] [PubMed] [Google Scholar]
- 14.Teter, S. A., Houry, W. A., Ang, D., Tradler, T., Rockabrand, D., Fischer, G., Blum, P., Georgopoulos, C. & Hartl, F. U. (1999) Cell 97, 755–765. [DOI] [PubMed] [Google Scholar]
- 15.Schaffitzel, E., Rudiger, S., Bukau, B. & Deuerling, E. (2001) Biol. Chem. 382, 1235–1243. [DOI] [PubMed] [Google Scholar]
- 16.Vorderwulbecke, S., Kramer, G., Merz, F., Kurz, T. A., Rauch, T., Zachmann-Brand, B., Bukau, B. & Deuerling, E. (2004) FEBS Lett. 559, 181–187. [DOI] [PubMed] [Google Scholar]
- 17.Gothel, S. F., Scholz, C., Schmid, F. X. & Marahiel, M. A. (1998) Biochemistry 37, 13392–13399. [DOI] [PubMed] [Google Scholar]
- 18.Lyon, W. R., Gibson, C. M. & Caparon, M. G. (1998) EMBO J. 17, 6263–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarnt, T., Tradler, T., Stoller, G., Scholz, C., Schmid, F. X. & Fischer, G. (1997) J. Mol. Biol. 271, 827–837. [DOI] [PubMed] [Google Scholar]
- 20.Hesterkamp, T., Deuerling, E. & Bukau, B. (1997) J. Biol. Chem. 272, 21865–21871. [DOI] [PubMed] [Google Scholar]
- 21.Stoller, G., Tradler, T., Rucknagel, K. P., Rahfeld, J. U. & Fischer, G. (1996) FEBS Lett. 384, 117–122. [DOI] [PubMed] [Google Scholar]
- 22.Callebaut, I. & Mornon, J. P. (1995) FEBS Lett. 374, 211–215. [DOI] [PubMed] [Google Scholar]
- 23.Tradler, T., Stoller, G., Rucknagel, K. P., Schierhorn, A., Rahfeld, J. U. & Fischer, G. (1997) FEBS Lett. 407, 184–190. [DOI] [PubMed] [Google Scholar]
- 24.Scholz, C., Mucke, M., Rape, M., Pecht, A., Pahl, A., Bang, H. & Schmid, F. X. (1998) J. Mol. Biol. 277, 723–732. [DOI] [PubMed] [Google Scholar]
- 25.Kramer, G., Patzelt, H., Rauch, T., Kurz, T. A., Vorderwulbecke, S., Bukau, B. & Deuerling, E. (2004) J. Biol. Chem. 279, 14165–14170. [DOI] [PubMed] [Google Scholar]
- 26.Li, Z. Y., Liu, C. P., Zhu, L. Q., Jing, G. Z. & Zhou, J. M. (2001) FEBS Lett. 506, 108–112. [DOI] [PubMed] [Google Scholar]
- 27.Maier, R., Scholz, C. & Schmid, F. X. (2001) J. Mol. Biol. 314, 1181–1190. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen, O. & Gajhede, M. (2003) Structure (London) 11, 1547–1556. [DOI] [PubMed] [Google Scholar]
- 29.Vogtherr, M., Jacobs, D. M., Parac, T. N., Maurer, M., Pahl, A., Saxena, K., Ruterjans, H., Griesinger, C. & Fiebig, K. M. (2002) J. Mol. Biol. 318, 1097–1115. [DOI] [PubMed] [Google Scholar]
- 30.Patzelt, H., Kramer, G., Rauch, T., Schonfeld, H. J., Bukau, B. & Deuerling, E. (2002) Biol. Chem. 383, 1611–1619. [DOI] [PubMed] [Google Scholar]
- 31.Pflugrath, J. W. (1999) Acta Crystallogr. D Biol. Crystallogr 55, Part 10, 1718–1725. [DOI] [PubMed] [Google Scholar]
- 32.Hendrickson, W. A. & Ogata, C. M. (1997) Methods Enzymol. 276, 494–523. [DOI] [PubMed] [Google Scholar]
- 33.Fortelle, E. d. L. & Bricogne, G. (1998) Methods Enzymol. 276, 472–494. [DOI] [PubMed] [Google Scholar]
- 34.Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard. (1991) Acta Crystallogr. A 47, Part 2, 110–119. [DOI] [PubMed] [Google Scholar]
- 35.Brunger, A. T., Adams, P. D., Clore, G. M., Delano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, N., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905–921. [DOI] [PubMed] [Google Scholar]
- 36.Van Duyne, G. D., Standaert, R. F., Karplus, P. A., Schreiber, S. L. & Clardy, J. (1993) J. Mol. Biol. 229, 105–124. [DOI] [PubMed] [Google Scholar]
- 37.Van Duyne, G. D., Standaert, R. F., Karplus, P. A., Schreiber, S. L. & Clardy, J. (1991) Science 252, 839–842. [DOI] [PubMed] [Google Scholar]
- 38.Schiene-Fischer, C., Habazettl, J., Tradler, T. & Fischer, G. (2002) Biol. Chem. 383, 1865–1873. [DOI] [PubMed] [Google Scholar]
- 39.Lyon, W. R. & Caparon, M. G. (2003) J. Bacteriol. 185, 3661–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janin, J. & Chothia, C. (1990) J. Biol. Chem. 265, 16027–16030. [PubMed] [Google Scholar]
- 41.Patzelt, H., Rudiger, S., Brehmer, D., Kramer, G., Vorderwulbecke, S., Schaffitzel, E., Waitz, A., Hesterkamp, T., Dong, L., Schneider-Mergener, J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 14244–142449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agashe, V. R., Guha, S., Chang, H. C., Genevaux, P., Hayer-Hartl, M., Stemp, M., Georgopoulos, C., Hartl, F. U. & Barral, J. M. (2004) Cell 117, 199–209. [DOI] [PubMed] [Google Scholar]
- 43.Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994) Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carson, M. (1997) Methods Enzymol. 277, 493–505. [PubMed] [Google Scholar]
- 45.Nicholls, A., Sharp, K. A. & Honig, B. (1991) Proteins 11, 281–296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.