Abstract
A vaccine that would engage the mucosal immune system against a broad range of HIV-1 subtypes and prevent epithelial transmission is highly desirable. Here we report fusing the mucosal targeting B subunit of cholera toxin to the conserved galactosylceramide-binding domain (including the ELDKWA-neutralizing epitope) of the HIV-1 gp41 envelope protein, which mediates the transcytosis of HIV-1 across the mucosal epithelia. Chimeric protein expressed in bacteria or plants assembled into oligomers that were capable of binding galactosyl-ceramide and GM1 gangliosides. Mucosal (intranasal) administration in mice of the purified chimeric protein followed by an i.p. boost resulted in transcytosis-neutralizing serum IgG and mucosal IgA responses and induced immunological memory. Plant production of mucosally targeted immunogens could be particularly useful for immunization programs in developing countries, where desirable product traits include low cost of manufacture, heat stability, and needle-free delivery.
Despite the success of extensive AIDS prevention programs and powerful antiretroviral drug therapies (1), it is generally agreed that these efforts will have to be combined with effective microbicides and vaccines, but the design and testing of such vaccines have proven to be complex (2, 3). An HIV-1 prophylactic vaccine of the future may ultimately be a multicomponent subunit vaccine aimed at blocking multiple steps of viral infection. Because HIV-1 transmission occurs most commonly through exposure of mucosal surfaces to HIV-1-infected secretions (4), a component of such a vaccine should engage the mucosal immune system to interfere with early steps of mucosal viral transmission and potential receptors. It is still unclear how HIV-1 particles or cell-associated virus penetrates the mucosa and initiate infection, but several non-mutually exclusive mechanisms for HIV-1 transmission across genital epithelia have been proposed (5, 6). In simple epithelia (e.g., intestinal and endometrial columnar epithelia), transmission depends on specific interactions between the viral envelope proteins gp120 and gp41 and the cerebroside galactosyl-ceramide (GalCer; refs. 6 and 7), an important component of endocytotic “raft” membrane microdomains. Rather than fusion and infection, these interactions lead to transcytosis of the virus across the epithelial barrier and its trapping by submucosal dendritic cells, which disseminate it to their target CD4+ cells (6, 8). Recently, the GalCer-binding domain of gp41 was mapped to a highly conserved, partially α-helical region of gp41 (“P1” peptide, residues 649–684, Fig. 1), extending between the gp120 cap and the transmembrane domain (9–11). This domain spans conserved epitopes including the ELDKWA (“Katinger”) sequence, the target of the broadly neutralizing human monoclonal IgG, 2F5 (Fig. 1; refs. 12–14). In addition to direct targeting of HIV-1 by neutralizing Abs (15), HIV-1 transcytosis can be blocked by disruption of rafts and by Abs against the ELDKWA epitope (7, 8, 10, 11).
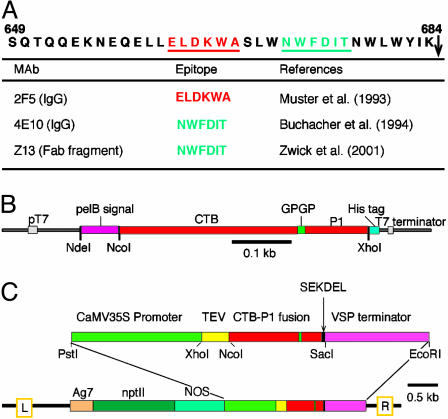
Fig. 1.
The P1 peptide and CTB-P1 expression vectors. (A) Sequence of the P1 peptide (HIV-1 isolate 593 clone 1 from Haiti, U08444). An arrow indicates the beginning of the gp41 transmembrane domain. HIV-1 cross-clade neutralizing epitopes (underlined) and their corresponding Abs are listed. (B) The relevant region of the CTB-P1 bacterial expression vector, pTM101. (C) pTM095, the CTB-P1 plant expression vector. Ca35S, cauliflower mosaic virus 35S promoter; TEV, the translation enhancer region of tobacco etch virus; VSP, the 3′-UTR of the soybean vegetative storage protein; npt II, the kanamycin resistance selectable marker; NOS, the nopline synthase promoter; Ag7, the nopline synthase polyadenylation signal.
Four lines of evidence suggest that Abs contribute to resistance to mucosal transmission of HIV-1. First, immunization of macaques with inactivated simian immunodeficiency virus targeted to mucosal or lymph node sites resulted in protection against both systemic and mucosal simian immunodeficiency virus challenge, and protection was directly correlated to the number of submucosal IgA-secreting cells (16). Second, passive immunization with 2F5 Abs protected macaques from a mucosal challenge (17, 18). Similarly, vaginal application of the gp120-directed b12 Ab was highly effective in protecting monkeys from a vaginal challenge (19). Third, anti-HIV-1 secretory IgA, isolated from mucosal secretions of infected individuals, can block transcytosis of HIV-1 and infection of CD4+ cells ex vivo (20, 21) and the neutralization potential of these Abs was greater than the paired IgGs (21). Fourth, transcytosis and infection neutralizing secretory IgAs have been detected in highly exposed persistently seronegative individuals. Significantly, these secretory IgAs are mostly directed against gp41 and the ELDKWA epitope (22–26). These mucosal Ab responses (together with cellular responses) are strongly suggestive of an efficient “natural” vaccination in these individuals.
We propose that mucosal Abs blocking the transcytosis across the epithelial barrier (and subsequent CD4+ cell infection) could afford additional protection from HIV-1 and that a mucosally targeted P1 peptide (gp41 residues 649–684), spanning not only the ELDKWA epitope but the entire GalCer-binding domain, could elicit such immune responses. The ELDKWA epitope seems to be a potential target for neutralization by Abs (27), but, elicitation of Abs against it, especially mucosal Abs with transcytosis and infection neutralizing activities, has proved difficult and reported successes are scant (27, 28). Here, we explore the potential of a chimeric protein comprised of the P1 peptide fused to the GM1-ganglioside-binding B subunit of the cholera toxin (CTB) to fulfill the requirements of contextual integrity of this gp41 domain, mucosal targeting, and immunogenicity. Our approach combines a mucosally targeted immunogen, CTB-P1, with an unique production system, namely transgenic plants (29–32), that may enable the creation of safe, effective, needle-free, and lower cost recombinant HIV-1 vaccines.
Materials and Methods
Vector Construction. A plant-expression-optimized synthetic CTB gene (GenBank accession no. AY475128) was a kind gift of Hugh Mason. Overlapping oligonucleotides corresponding to the 3′-sequence region of the CTB gene, a GPGP linker, the P1 peptide of HIV-1 MN isolate (GenBank accession no. AF075722), and an endoplasmic reticulum retention signal (SEKDEL) were PCR assembled (33). The resulting PCR product was cloned (TOPO-TA, Invitrogen), its sequence confirmed, and then was used to replace a HindIII–SacI fragment of the CTB gene to create pTM071. For Escherichia coli expression, the CTB-P1 fusion gene (GenBank accession no. AY727035) was cloned into pET-22b(+) (Novagen) to form pTM101 (Fig. 1B). For plant expression, the CTB-P1 and CTB genes were cloned into plant expression vectors described before (34) to yield, respectively, pTM095 and pTM096 (Fig. 1C).
Expression of CTB-P1 in Bacteria. Fusion protein was expressed in E. coli BL21 (DE3) (Novagen). At 2 h after induction, the cells were collected and resuspended in PBS, homogenized (French press), and centrifuged. The pellet was resuspended in 4 M Urea, 20 mM Tris (pH 7.9), 5 mM imidazole, and 0.5 M NaCl, and CTB-P1 was purified (His·Bind resin, Novagen), extensively dialyzed against water, and stored at –80°C until use.
Transient Expression of CTB-P1 in Nicotiana benthamiana. Mid-logarithmic cultures of Agrobacterium tumefaciens LBA4404 harboring pTM095 or pTM096 were harvested, and bacteria were resuspended to OD600 = 0.2 in 10 mM Mes (pH 5.5) and 200 μM acetosyringone. The suspension was injected into fully expanded N. benthamiana leaves by using a needle-less syringe. After a 2-day cultivation, the marked infiltrated areas of leaves were excised and homogenized in extraction buffer (25 mM Na-phosphate, pH 7.8/100 mM NaCl/1 mM EDTA/1% Triton X-100/3 μg/ml leupeptin/50 mM Na-ascorbate) in a FastPrep machine (Bio 101).
Biochemical Analyses. Preparations of CTB-P1, CTB (Biomol, Plymouth Meeting, PA), and P1 peptide (synthesized on a PerSeptive Biosystems 9050 synthesizer) were analyzed by SDS/PAGE either under denaturing or nondenaturing conditions (no DTT in sample buffer and no boiling). After electrophoresis, proteins were transferred to a poly(vinylidene difluoride) membrane, immunodecorated, and detected by chemiluminescence (ECL+, Amersham Pharmacia). CTB was detected by goat anti-CTB antiserum (List Biological Laboratories, Campbell, CA) and rabbit anti-goat IgG-peroxidase (Sigma). P1 was detected with 2F5 Abs, goat anti-human IgG biotin (Rockland, Gilbertsville, PA), and streptavidin-peroxidase (Calbiochem). GalCer blot analysis was carried out as described (11). Sandwich or GM1 ELISA was done essentially as described (34) by using anti-CT Ab (Sigma) or GM1 (Sigma-Aldrich), respectively, for capture. ELISA was developed with Sigma FAST OPD (o-phenylenediamine dihydrochloride) substrate (Sigma), and A490 was read (SpectraMax 340PC, Molecular Devices).
Immunization of Mice with CTB-P1. Female BALB/c mice (7-wk-old, n = 6) were immunized intranasally (i.n.) with CTB (10 μg, List Biological Laboratories), purified recombinant CTB-P1 produced in E. coli (14 μg), or CTB-P1 (14 μg) plus CT (1 μg, List Biological Laboratories) on weeks 0, 1, 2, 3, 4, and 7. Dose was administered in 10 μl into each nostril. Mice were i.p. injected with subimmunogenic dose of immunogens on week 9 (P1, 1 μg) and week 10 (CTB-P1, 3.5 μg). CTB-P1 was quantified by ELISA by using P1 as a standard, taking into account the Mr of the chimera. Serum, fecal pellets, and vaginal secretions were collected on the indicated days. Fecal extracts were prepared by soaking seven pellets (≈100 mg) in 1 ml of PBS containing 0.02% Na-azide for 30 min at 4°C with occasional vortex and cleared by centrifugation (15,000 × g). Vaginal secretions were collected by adsorbing to Ultracell surgical sponges (Prescott's) after a saline lavage (50 μl). The sponges were then soaked in 450 μl of PBSTM (1% dry milk, 0.05% Tween-20 in PBS). The experimental protocol involving mice was approved by the Arizona State University Animal Care and Use Committee.
Determination of IgG and IgA Titers in Serum, Fecal, and Vaginal Samples. ELISA plates were coated with 0.5 μg/ml P1. Threefold serially diluted samples starting from 1:100 for serum, 1:2 for fecal extract, and 1:1 for vaginal lavage in PBSTM were applied onto the plates and incubated for 1 h at 37°C. Serum IgGs and mucosal IgAs were detected by peroxidase conjugate of anti-mouse IgG (Calbiochem) and anti-mouse IgA (Sigma), respectively. Endpoint titers were determined as the reciprocal of the dilution factor of sample giving background levels of OD450. Statistical analysis of data was done by the nonparametric Kruskal–Wallis test followed by multiple comparisons (SNK analog test).
Characterization of Ig-Neutralizing Activity of HIV-1 Transcytosis. Transcytosis assays were described (11, 20). In brief, epithelial cells (intestinal carcinoma HT29 clone 19 or endometrial cell line HEC-1) were grown as a confluent monolayer on permeable support. Samples of Ig were partially purified by ammonium sulfate (0–50% saturation fraction was collected for serum Igs and 50–80% fraction for mucosal Igs) and then dialyzed against RPMI medium 1640, 10% FCS. Cells (106/40 μl) infected with HIV-1NDK were incubated with Ig-containing samples for 60 min at 4°C and then inoculated at the apical pole of epithelial cells (final volume was 150 μl, and final dilution of the Ig-containing samples was 1:25). Transcytosis was allowed to proceed for 110 min at 37°C. Viral load in the basolateral chamber was estimated from the p24 content (p24 kit, Coulter). Transcytosis was expressed as percentage of control, i.e., transcytosis observed in absence of Ig fraction. For analysis of the P1 reversion of the neutralizing activity, samples were incubated overnight at 4°C with 125 μM P1 [critical concentration for dimerization (10)], extensively dialyzed to remove unbound P1 and assayed as described above. IgA and IgG were depleted from fecal samples by using biotinylated-goat anti-mouse IgA and IgG, respectively, followed by binding to 25 μl of streptavidin-agarose beads (Neutravidin, Pierce). Beads were washed with 100 mM glycine (pH 4.0) at 4°C and centrifuged, and the supernatant fluid containing the released IgA and IgG was immediately neutralized to pH 7.4 with 1 M Tris (pH 8). Experiments were repeated at least twice for all conditions with each of the two cell types and results shown reflect the mean of at least four repeats.
Results
CTB-P1 Assembles into Functional Oligomers. To facilitate the testing of our approach, we created vectors for the expression of CTB-P1 fusion protein in bacteria and in plants (Fig. 1 B and C). Samples of affinity-purified fusion protein produced in E. coli were resolved by nondenaturing SDS/PAGE and revealed two major bands corresponding, respectively, to monomers and oligomers of CTB-P1, with oligomers comprising 30–50% of the total chimeric protein (Fig. 2A). The oligomeric CTB-P1 band is positioned at ≈100 kDa when resolved on a 16% gel under nondenaturing conditions. When resolved on a 4–20% gradient gel, the apparent molecular mass is closer to that predicted by the sequence (80 kDa, data not shown). The CTB-P1 monomers and oligomers tended to precipitate readily out of solution. Because this was typical of the chimera but not of CTB (not shown), we assume that the hydrophobic P1 lends this property to the chimera and enables it to form large aggregates in detergent-free neutral pH aqueous solutions.
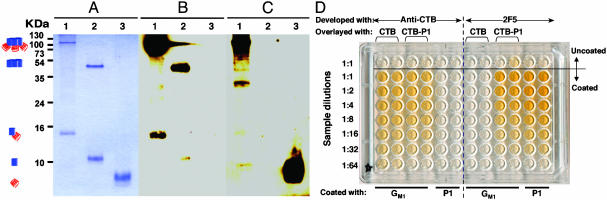
Fig. 2.
E. coli-produced CTB-P1 assembles into oligomers, retains its relevant antigenic determinants and sphingolipid-binding capacity. (A–C) Western blot analysis. Lane 1, affinity purified CTB-P1; lane 2, CTB, and lane 3, P1 peptide. Proteins were resolved by nondenaturing SDS/PAGE, visualized by Coomassie staining (A) or transferred to a poly(vinylidene difluoride) membrane and immunodecorated with specific anti-CTB (B) or anti-P1 (C) Abs. Symbols on the left mark the position of oligomeric and monomeric CTB-P1 and CTB, and free P1. (D) GM1 ganglioside ELISA. The indicated wells were coated with gangliosides, P1, or left uncoated. Serially diluted samples of CTB or CTB-P1 were applied to the indicated wells and then were reacted with either anti-CTB or anti-P1 Abs, as indicated.
Specific Abs against CTB reacted with oligomeric and monomeric CTB-P1 as well as with CTB (Fig. 2B). Similarly the anti-P1 2F5 mAbs reacted with the synthetic P1 peptide and with the CTB-P1 fusion (Fig. 2C). Interestingly, the 2F5 Ab reacted more strongly with oligomeric CTB-P1 as compared to the monomer, suggesting that the neutralizing epitope is available for interactions within the chimeric CTB-P1 oligomer context.
We compared CTB-P1 to CTB in their potential to bind GM1 gangliosides by ELISA by using ganglioside-coated plates (Fig. 2D). Both proteins could bind GM1 gangliosides and were recognized by anti-CTB Abs, but only the chimera and the free P1 peptide (directly bound to the plate) were detected by the 2F5 Abs. Thus the CTB-P1 retains not only its immunological signature but also its ability to bind to the cellular receptor, which will facilitate its mucosal targeting. The P1 peptide was shown to bind in vitro and in cell cultures to its cellular receptor, the cerebroside GalCer. Importantly, the P1 peptide presented as a fusion protein on CTB was able to bind GalCer (data not shown). The preservation of this important functionality of P1 lends further support to the structural integrity of the important epitopes it contains.
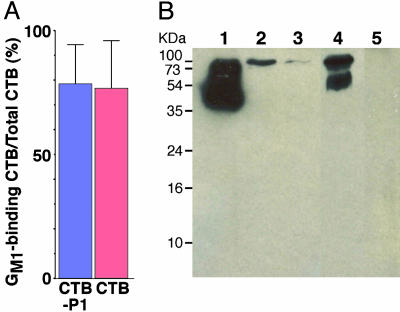
Plant Production of CTB-P1. To demonstrate the ability of plants to produce and assemble CTB-P1, we constructed a plant expression vector (Fig. 1C). The coding region included the native CTB signal peptide, which was shown before to enable export of proteins to the endoplasmic reticulum (35), the plant-expression optimized CTB-P1 fusion gene, and an endoplasmic reticulum retention signal (SEKDEL, refs. 35 and 36). As a control, a similar vector directing the expression of CTB was also studied. We transiently expressed these construct by A. tumefaciens infiltration of N. benthamiana leaves. Proteins were extracted and were assayed by GM1 ELISA and CTB sandwich ELISA (Fig. 3A) and by Western blotting (Fig. 3B). These assays demonstrated that >80% of the CTB and CTB-P1 produced by the system were assembled into functional oligomers, as compared to only ≈30% in the bacterial production system. Importantly the electrophoretic mobility of the bacterial- and plant-derived chimeric proteins was the same (Fig. 3B and data not shown). Interestingly, both commercial (bacterially produced) and plant-derived CTB is resolved into two bands, an ≈55 kDa and an ≈100 kDa, probably reflecting the previously reported potential of this protein to form decameric structures (37).
Fig. 3.
Plant-produced CTB-P1 retains its relevant antigenic determinants and sphingolipid-binding capacity. CTB and CTB-P1 were transiently expressed in N. benthamiana leaves. (A) Percentage of GM1 binding (assayed by ganglioside ELISA) per total CTB (determined by anti-CTB sandwich ELISA). CTB-P1, n = 5; CTB, n = 3. (B) Western blot analysis of recombinant CTB and CTB-P1. Total soluble leaf proteins were resolved by nondenaturing SDS/PAGE, transferred to a poly(vinylidene difluoride) membrane, and reacted with anti-CTB Abs. Lane 1, commercial CTB; lanes 2 and 3, CTB-P1 leaves; lane 4, CTB leaf; and lane 5, control leaf.
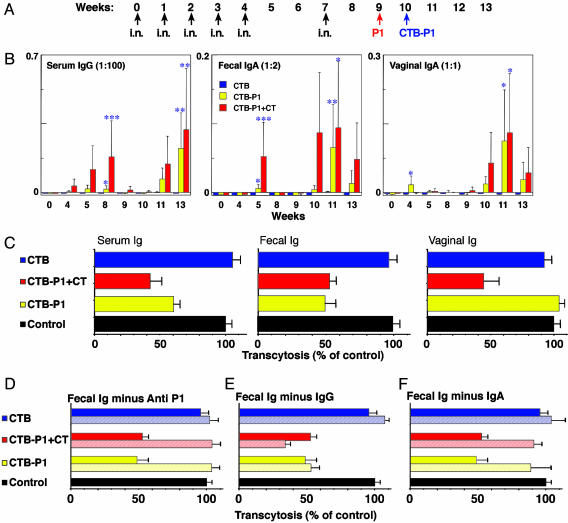
Mucosal and Systemic Immunogenicity of CTB-P1. To test the mucosal immunogenicity of the E. coli produced CTB-P1, three groups of mice were immunized i.n. with CTB, CTB-P1, or CTB-P1 plus CT (groups 1–3, respectively). Mice were immunized (i.n.) once a week for 5 consecutive wk (Fig. 4A). Mice were boosted (week 7, same doses) by the nasal route and then by subimmunogenic doses of the antigen by i.p. injection on week 9 (P1 peptide) and week 10 (CTB-P1). Whereas all of the mice developed a strong humoral response against CTB (data not shown), only mice that received the chimeric protein or the chimeric protein plus CT responded by producing serum and mucosal P1-specific Abs (Fig. 4B). Mucosal IgA Abs were detected in fecal pellets and vaginal secretions only after 4–5wk of immunization, although their level was lower than the respective serum IgG samples. The IgA response persisted for a shorter time as compared to the serum IgG response (Fig. 4B and data not shown).
Fig. 4.
E. coli produced CTB-P1 elicits transcytosis-neutralizing Abs in mice. (A) Immunization regimen. Mice were immunized i.n. (black arrows) with CTB (group 1), CTB-P1 (group 2), or CTB-P1 plus CT (group 3) and then by an i.p. injection of P1 (red arrow) and CTB-P1 (blue arrow). (B) Serum and mucosal anti-P1 Abs detected in mice of groups 2 and 3, but not in group 1. Shown are average net values (mean ± SEM). Symbols indicate statistical significance estimated by Kruskal-Wallis and SNK analog tests: *, P < 0.010; **, P < 0.025; and ***, P < 0.05. (C) Abs raised against CTB-P1 inhibit HIV-1 transcytosis. Transcytosis neutralizing activities of enriched Ig fractions from serum, feces and unfractionated vaginal secretions of the two best responding mice from groups 2 and 3 were compared to fractions obtained from a group 1 mouse (expressed as percentage of control). (D–F) Neutralization is due to P1-specific IgA. Fecal Ig fractions were preincubated with P1 to block P1-specific Ab activity, resulting in abrogation of neutralizing activity (D, hatched bars). In separate experiments, fecal IgG-depleted fractions, containing mostly IgA, retained their neutralizing activity (E, hatched bars), whereas IgA depleted fractions, containing mostly IgG, lost their neutralizing potential (F, hatched bars).
The serum anti-P1 IgG response peaked on week 8 and declined thereafter (Fig. 4B). Parenteral administration (i.p.) of synthetic P1 peptide followed by an i.p. injection of CTB-P1, boosted the response, and 2 wk after that administration, the Ab titers of the two best responders exceeded the titer at the peak of the priming response and continued to rise. Importantly, some of the non- or low-responding mice exhibited significant responses after the boost and the total number of responders increased from 1 to 4 in group 2 and from 1 to 3 in group 3 (primed with CTB-P1 and CTB-P1 plus CT, respectively). By week 13, mean anti-P1 Ab responses of groups 2 and 3 reached statistical significance when compared to the CTB control (P < 0.025). At the peak of the response, endpoint titers for serum anti-P1 IgG for all of the mice in the CTB-P1 group were 244–17,516 (geometric mean titer = 940). For the three responding mice in the CTB-P1 plus CT group, the endpoint titers were 634–83,641 (geometric mean titer = 4,372). In contrast, mice of group 1 that were primed with CTB did not respond to the i.p. booster shot, demonstrating that the amount of antigen delivered was subimmunogenic for priming but was sufficient to recall the immune memory of the mucosally primed mice. Similarly, the mucosal IgA responses in both vaginal and gastrointestinal secretions, declined after i.n. administration but were boosted by the i.p. injection. In fact, the post-boosting responses exceeded the responses achieved by the priming treatment, which proves the establishment of local and systemic immune memory. Of interest is the fact a single i.p. injection of P1 peptide was able to induce modest mucosal anti-P1 IgA responses in mice previously primed with i.n. administration of CTB-P1, but had no effect on serum IgG levels in these mice.
CTB-P1 Elicits Transcytosis-Blocking Abs. To test the functionality of the Abs raised by CTB-P1, we assayed the capacity of the various Ig-containing samples (serum, vaginal secretion, and feces) to neutralize the transcytosis of HIV-1NDK induced by virus-infected CD4+ T cells, in an ex vivo model of tight epithelium (8). To this end, serum, vaginal secretion, and fecal extract samples were collected at week 13 of the immunization from the best responding mice of the CTB-P1 and CTB-P1 plus CT and a representative control CTB-immunized mouse. Abs present in these samples were partially purified, incubated with the HIV-1-infected cells, and inoculated on the luminal surface of the epithelium to initiate HIV-1 transcytosis. Fecal and serum Ig from either the CTB-P1- or CTB-P1 plus CT-immunized mice were shown to significantly block HIV transcytosis (Fig. 4C). In contrast vaginal Ig blocked transcytosis only when induced by the CTB-P1 plus CT immunization protocol, suggesting that the Ab response is compartmentalized (38). Interestingly, Ig in fecal and vaginal secretion exhibited a neutralizing activity with a transcytosis ID50 as low as 6.5 and 0.135 μg/ml, respectively.
To show the P1 specificity of this neutralizing activity, fecal Ig fractions were incubated with saturating levels of P1 peptide to block P1-specific Ab. As a result, the transcytosis-neutralizing activity of the fecal Ig was entirely reversed (Fig. 4D, hatched bars). Finally, IgG and IgA were, respectively, depleted from the fecal Ig samples and resulting fraction assayed functionally against HIV-1 transcytosis. Although IgG-depleted fractions retained their neutralizing activity (Fig. 4E, hatched lines), the IgA depleted sample completely lost it (Fig. 4F, hatched bars). This result indicates that the neutralizing Abs present in feces are P1-specific IgAs.
Discussion
The ELDKWA epitope of the envelope protein gp41 attracted much attention since its initial characterization by Katinger and coworkers (39). It is well conserved among the different HIV-1 clades, and ELDKWA-specific Abs can neutralize infection of CD4+ cells (39). More recently, it was shown that the region surrounding this epitope (corresponding to the P1 peptide, Fig. 1) mediates viral interaction with the epithelial cell receptor of HIV-1, GalCer (7, 10, 11). This interaction may be important for the nonfusion processes that enable HIV-1 to cross simple epithelia and can be blocked by the 2F5 IgG, ELDKWA-specific IgA from infected or highly exposed persistently seronegative individuals (20, 21). The ability of anti-ELDKWA Abs to block viral transmission in vivo by passive immunization in the macaque model (17, 18) further illuminates the importance of inducing Abs against this region as part of a prophylactic HIV-1 vaccine. However, elicitation of an Ab response against the linear ELDKWA epitope has proved difficult (28, 40).
Unlike previous immunization studies, our approach was to construct a mucosal-targeted immunogen, which would comprise several of the neutralizing epitopes present on this exposed region of gp41, including the ELDKWA sequence. Because, the context in which this linear epitope is presented affects its processing by antigen-presenting cells (2), we focused on the minimal region of gp41 that allows GalCer binding (i.e., P1) and is assumed to preserve important features of the native structure (10). Preventing this specific and crucial lectin-like interaction is necessary, and seemingly sufficient, to block HIV-1 transcytosis across the epithelial barrier and infection of CD4+ cells. However, the P1 peptide is poorly immunogenic by itself, i.p. (Fig. 4B), i.n. or orally (N.M. and T.S.M., unpublished data). To allow the efficient presentation of the P1 peptide to the mucosa-associated lymphoid tissue, while maintaining the structural integrity of the P1 peptide, we created a translational fusion between the C terminus of CTB and the peptide. The epithelial-binding subunits of bacterial enterotoxins such as CTB (41, 42) and the homologous B subunit of the heat labile toxin of E. coli (31, 34, 43) are known to be potent mucosal immunogens, which can efficiently present fused antigenic determinants to the immune system. Based on the ability of CTB-P1, but not CTB, to bind GalCer in vitro (data not shown), and the aggregation of CTB-P1 oligomers into larger oligomeric structures (Figs. 2 and 3), we assume that self-association of the P1 domain and consequent reconstitution of the lectin-binding site are also possible in the context of the fusion protein, similar to the case of the free peptide (and presumably the corresponding region of gp41). Thus, the CTB-P1 immunogen presented here contains a modality, which simple ELDKWA epitope fusions lack (40).
In contrast to the free P1 peptide, CTB-P1 induced mucosal and systemic immune responses directed against the P1 peptide, indicating that the CTB-P1 fusion strategy for mucosal targeting was successful. However, we cannot rule out the possibility that contaminating E. coli lipopolysaccharides, potentially present in the CTB-P1 preparation, could contribute to its stronger immunogenicity. The fusion protein elicited strong priming responses in only some of the treated mice, and the presence of the strong mucosal adjuvant CT did not enhance its effect. This may be due to the marginal dose chosen for the experiments presented here. In fact, intranasal administration of a three time higher dose of CTB-P1 can induce stronger mucosal and serum Ab responses (N.M. and T.S.M., unpublished data). The actual dose could even be lower considering that only one-third of the protein is properly assembled. However, even at the lower dose used here, most mice were sensitized as is evident by the ability of subimmunogenic boosting dose of the antigen delivered parenterally to generate systemic and local Ab responses that were higher than the priming events. In particular, individual mice for which appreciable immune responses were not observed after the series of intranasal immunizations, did respond to the i.p. booster shot. Such responses were not obtained in control mice that were not primed by the CTB-P1 immunogen. That a significant boosting effect was not demonstrated by the mucosal route can be due to the particulars of the immunization regimen chosen here, but it cannot be ruled out that the synergism of the mixed-route sensitization has an important, if obscure, biological basis.
Significantly, i.n. immunization resulted in local responses in two distinct mucosal effector sites: the urogenital and the gastrointestinal tracts, the primary portals of HIV-1 transmission, responses which were, however, transient. Relatively fast decline of vaginal and fecal IgA levels as compared to serum IgG in response to nonreplicating mucosal vaccine is not uncommon. However, it is important that mice were able to mount a fast mucosal response after re-exposure to the antigen, indicating that immune memory was established not only for the serum, but also for mucosal secretions. These results suggest that frequent boosting of the response may be required to maintain high level of Abs to protect against exposure through the mucosal route. Interestingly, results obtained with highly exposed persistently seronegative individuals, demonstrated that both secretory IgA levels and cellular immune responses waned down and HIV-1 infection rate went up upon a change in lifestyle that was accompanied by a reduction in exposure to the virus (44, 45). Thus, maintaining adequate mucosal Ab concentrations for protection from viral transmission through vaccination may require frequent mucosal boosting, necessitating abundant supplies of recombinant antigen at a low cost.
A significant feature of the Abs elicited by CTB-P1 was their ability to inhibit the transcytotic process of the virus, one of the potential pathways epithelial transmission of HIV-1. Both immunization protocols (CTB-P1 ± CT) also were able to induce serum Ig with HIV-1-neutralizing activities against transcytosis. However, at the mucosal level, the presence of the mucosal adjuvant CT has a profound impact on the induction of neutralizing IgA in vaginal secretions, because transcytosis-neutralizing Abs were induced only in its presence, confirming the compartmentalization of the mucosal Ab response.
In recent years, the idea of subunit vaccine production in plants has gained momentum with the successful outcome of several phase I/II clinical trials (reviewed in refs. 29, 30, and 32). The attractive feature of transgenic plants is that they combine a cost-effective production system with a safe and efficacious delivery system that targets the gut-associated lymphoid tissue. The use of minimally processed, room temperature resistant, and mucosally delivered immunogens is especially valuable when elicitation of mucosal Abs is the object and when frequent boosting is anticipated, as is the case presented here. Although several attempts in expression of HIV-1 antigens in plants have been reported (28, 46–48), none focused on the lectin domain of gp41 in its entirety. Our results demonstrate the ability of plants to produce and correctly assemble CTB-P1 oligomers. Stable transgenic plant lines are in development and will enable evaluation of the safety and efficacy of CTB-P1 in preclinical models, an important step toward a needle-free vaccine against HIV-1.
Acknowledgments
We thank Jacquelyn Kilbourne for her expert technical support. We thank Hugh Mason and Larry Zeitlin for critical reading of the manuscript. This work was supported in part by a seed grant from the Biodesign Institute at Arizona State University (to T.S.M.), by National Institutes of Health Cooperative Agreement U19AI62150 (to C.J.A. and T.S.M.), and by Agence Nationale de Recherches sur le SIDA and SIDACTION-Ensemble contre le SIDA (to M.B.). N.M was supported in part by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad (awarded in 2004).
Abbreviations: CT, cholera toxin; CTB, B subunit of CT; GalCer, galactosyl-ceramide; i.n., intranasally.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY727035).
References
- 1.Valdiserri, R. O., Ogden, L. L. & McCray, E. (2003) Nat. Med. 9, 881–886. [DOI] [PubMed] [Google Scholar]
- 2.Letvin, N. L., Barouch, D. H. & Montefiori, D. C. (2002) Annu. Rev. Immunol. 20, 73–99. [DOI] [PubMed] [Google Scholar]
- 3.McMichael, A. J. & Hanke, T. (2003) Nat. Med. 9, 874–880. [DOI] [PubMed] [Google Scholar]
- 4.Pope, M. & Haase, A. T. (2003) Nat. Med. 9, 847–852. [DOI] [PubMed] [Google Scholar]
- 5.Miller, C. J. & Shattock, R. J. (2003) Microbes Infect. 5, 59–67. [DOI] [PubMed] [Google Scholar]
- 6.Bomsel, M. & David, V. (2002) Nat. Med. 8, 114–116. [DOI] [PubMed] [Google Scholar]
- 7.Meng, G., Wei, X., Wu, X., Sellers, M. T., Decker, J. M., Moldoveanu, Z., Orenstein, J. M., Graham, M. F., Kappes, J. C., Mestecky, J., et al. (2002) Nat. Med. 8, 150–156. [DOI] [PubMed] [Google Scholar]
- 8.Bomsel, M. (1997) Nat. Med. 3, 42–47. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt, R. & Sodroski, J. (1998) Science 280, 1884–1888. [DOI] [PubMed] [Google Scholar]
- 10.Alfsen, A. & Bomsel, M. (2002) J. Biol. Chem. 8, 25649–25659. [DOI] [PubMed] [Google Scholar]
- 11.Alfsen, A., Iniguez, P., Bouguyon, E. & Bomsel, M. (2001) J. Immunol. 166, 6257–6265. [DOI] [PubMed] [Google Scholar]
- 12.Purtscher, M., Trkola, A., Grassauer, A., Schulz, P. M., Klima, A., Dopper, S., Gruber, G., Buchacher, A., Muster, T. & Katinger, H. (1996) AIDS 10, 587–593. [DOI] [PubMed] [Google Scholar]
- 13.Buchacher, A., Predl, R., Strutzenberger, K., Steinfellner, W., Trkola, A., Purtscher, M., Gruber, G., Tauer, C., Steindl, F., Jungbauer, A., et al. (1994) AIDS Res. Hum. Retroviruses 10, 359–369. [DOI] [PubMed] [Google Scholar]
- 14.Zwick, M. B., Labrijn, A. F., Wang, M., Spenlehauer, C., Saphire, E. O., Binley, J. M., Moore, J. P., Stiegler, G., Katinger, H., Burton, D. R., et al. (2001) J. Virol. 75, 10892–10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, Q., Frank, I., Williams, V., Santos, J. J., Watts, P., Griffin, G. E., Moore, J. P., Pope, M. & Shattock, R. J. (2004) J. Exp. Med. 199, 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehner, T., Bergmeier, L. A., Panagiotidi, C., Tao, L., Brookes, R., Klavinskis, L. S., Walker, P., Walker, J., Ward, R. G., Hussain, L. & et al. (1992) Science 258, 1365–1369. [DOI] [PubMed] [Google Scholar]
- 17.Mascola, J. R., Stiegler, G., VanCott, T. C., Katinger, H., Carpenter, C. B., Hanson, C. E., Beary, H., Hayes, D., Frankel, S. S., Birx, D. L. & Lewis, M. G. (2000) Nat. Med. 6, 207–210. [DOI] [PubMed] [Google Scholar]
- 18.Baba, T. W., Liska, V., Hofmann-Lehmann, R., Vlasak, J., Xu, W., Ayehunie, S., Cavacini, L. A., Posner, M. R., Katinger, H., Stiegler, G., et al. (2000) Nat. Med. 6, 200–206. [DOI] [PubMed] [Google Scholar]
- 19.Veazey, R. S., Shattock, R. J., Pope, M., Kirijan, J. C., Jones, J., Hu, Q., Ketas, T., Marx, P. A., Klasse, P. J., Burton, D. R. & Moore, J. P. (2003) Nat. Med. 9, 343–346. [DOI] [PubMed] [Google Scholar]
- 20.Bomsel, M., Heyman, M., Hocini, H., Lagaye, S., Belec, L., Dupont, C. & Desgranges, C. (1998) Immunity 9, 277–287. [DOI] [PubMed] [Google Scholar]
- 21.Hocini, H., Bélec, L., Iscaki, S., Garin, B., Pillot, J., Becquart, P. & Bomsel, M. (1997) AIDS Res. Hum. Retroviruses 13, 1179–1185. [DOI] [PubMed] [Google Scholar]
- 22.Mazzoli, S., Trabattoni, D., Lo Caputo, S., Piconi, S., Ble, C., Meacci, F., Ruzzante, S., Salvi, A., Semplici, F., Longhi, R., et al. (1997) Nat. Med. 3, 1250–1257. [DOI] [PubMed] [Google Scholar]
- 23.Kaul, R., Trabattoni, D., Bwayo, J. J., Arienti, D., Zagliani, A., Mwangi, F. M., Kariuki, C., Ngugi, E. N., MacDonald, K. S., Ball, T. B., et al. (1999) AIDS 13, 23–29. [DOI] [PubMed] [Google Scholar]
- 24.Devito, C., Broliden, K., Kaul, R., Svensson, L., Johansen, K., Kiama, P., Kimani, J., Lopalco, L., Piconi, S., Bwayo, J. J., et al. (2000) J. Immunol. 165, 5170–5176. [DOI] [PubMed] [Google Scholar]
- 25.Devito, C., Hinkula, J., Kaul, R., Lopalco, L., Bwayo, J. J., Plummer, F., Clerici, M. & Broliden, K. (2000) AIDS 14, 1917–1920. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni, P. S., Butera, S. T. & Duerr, A. C. (2003) AIDS Rev. 5, 87–103. [PubMed] [Google Scholar]
- 27.Zolla-Pazner, S. (2004) Nat. Rev. Immunol. 4, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marusic, C., Rizza, P., Lattanzi, L., Mancini, C., Spada, M., Belardelli, F., Benvenuto, E. & Capone, I. (2001) J. Virol. 75, 8434–8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason, H. S., Warzecha, H., Mor, T. & Arntzen, C. J. (2002) Trends Mol. Med. 8, 324–329. [DOI] [PubMed] [Google Scholar]
- 30.Mor, T. S. & Mason, H. S. (2004) in Handbook of Plant Biotechnology, eds. Christou, P. & Klee, H. (Wiley, New York).
- 31.Tacket, C. O., Mason, H. S., Losonsky, G., Clements, J. D., Wasserman, S. S., Levine, M. M. & Arntzen, C. J. (1998) Nat. Med. 4, 607–609. [DOI] [PubMed] [Google Scholar]
- 32.Koprowski, H. & Yusibov, V. (2001) Vaccine 19, 2735–2741. [DOI] [PubMed] [Google Scholar]
- 33.Stemmer, W. P. C., Crameri, A., Ha, K. D., Brennan, T. M. & Heyneker, H. L. (1995) Gene 164, 49–53. [DOI] [PubMed] [Google Scholar]
- 34.Haq, T. A., Mason, H. S., Clements, J. D. & Arntzen, C. J. (1995) Science 268, 714–716. [DOI] [PubMed] [Google Scholar]
- 35.Arakawa, T., Chong, D. K., Merritt, J. L. & Langridge, W. H. (1997) Transgenic Res. 6, 403–413. [DOI] [PubMed] [Google Scholar]
- 36.Wandelt, C., Khan, M., Craig, S., Schroeder, H., Spencer, D. & Higgins, T. (1992) Plant J. 2, 181–192. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda, Y., Matano, K., Asai, T. & Tochikubo, K. (1998) FEMS Immunol. Med. Microbiol. 20, 311–318. [DOI] [PubMed] [Google Scholar]
- 38.Brandtzaeg, P., Farstad, I. N. & Haraldsen, G. (1999) Immunol. Today 20, 267–277. [DOI] [PubMed] [Google Scholar]
- 39.Muster, T., Steindl, F., Purtscher, M., Trkola, A., Klima, A., Himmler, G., Ruker, F. & Katinger, H. (1993) J. Virol. 67, 6642–6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coëffier, E., Clément, J. M., Cussac, V., Khodaei Boorane, N., Jehanno, M., Rojas, M., Dridi, A., Latour, M., El Habib, R., Barré Sinoussi, F., et al. (2000) Vaccine 19, 684–693. [DOI] [PubMed] [Google Scholar]
- 41.Millar, D. G., Hirst, T. R. & Snider, D. P. (2001) Infect. Immun. 69, 3476–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu, J. & Langridge, W. H. (2001) Nat. Biotechnol. 19, 548–552. [DOI] [PubMed] [Google Scholar]
- 43.Lipscombe, M., Charles, I. G., Roberts, M., Dougan, G., Tite, J. & Fairweather, N. F. (1991) Mol. Microbiol. 5, 1385–1392. [DOI] [PubMed] [Google Scholar]
- 44.Mazzoli, S., Lopalco, L., Salvi, A., Trabattoni, D., Lo Caputo, S., Semplici, F., Biasin, M., Bl, C., Cosma, A., Pastori, C., et al. (1999) J. Infect. Dis. 180, 871–875. [DOI] [PubMed] [Google Scholar]
- 45.Kaul, R., Rowland-Jones, S. L., Kimani, J., Dong, T., Yang, H. B., Kiama, P., Rostron, T., Njagi, E., Bwayo, J. J., MacDonald, K. S., et al. (2001) J. Clin. Invest. 107, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusibov, V., Modelska, A., Steplewski, K., Agadjanyan, M., Weiner, D., Hooper, D. C. & Koprowski, H. (1997) Proc. Natl. Acad. Sci. USA 94, 5784–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, G. G., Rodrigues, L., Rovinski, B. & White, K. A. (2002) Mol. Biotechnol. 20, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogers, W. M., Bergmeier, L. A., Ma, J., Oostermeijer, H., Wang, Y., Kelly, C. G., Ten Haaft, P., Singh, M., Heeney, J. L. & Lehner, T. (2004) AIDS 18, 25–36. [DOI] [PubMed] [Google Scholar]