Abstract
Cancer research depends on the use of human cell lines for both the in vitro (culture) and in vivo (xenograft) analysis of tumor progression and treatment. However, the extent to which cultured preparations of human cancer lines display similar properties in vivo, where important host factors may influence tumor biology, remains unclear. Here, we address this question by conducting a functional proteomic analysis of the human breast cancer line MDA-MB-231 grown in culture and as orthotopic xenograft tumors in the mammary fad pad of immunodeficient mice. Using a suite of activity-based chemical probes, we identified carcinoma (human) enzyme activities that were expressed selectively in culture or in xenograft tumors. Likewise, distinct groups of stromal (mouse) enzyme activities were found that either infiltrated or were excluded from xenograft tumors, indicating a contribution by specific host components to breast cancer development. MDA-MB-231 cells isolated from tumors exhibited profound differences in their enzyme activity profiles compared with the parent cell line, including the dramatic posttranscriptional up-regulation of the serine proteases urokinase plasminogen activator and tissue plasminogen activator and down-regulation of the glycolytic enzyme phosphofructokinase. These altered enzyme activity profiles correlated with significantly greater tumor growth rates and metastases for xenograft-derived MDA-MB-231 cells upon reintroduction into mice. Collectively, these data indicate that the in vivo environment of the mouse mammary fat pad cultivates the growth of human breast cancer cells with elevated tumorigenic properties and highlight the value of activity-based protein profiling for identifying proteomic signatures that depict such changes in cancer cell biology.
Tumorigenesis involves an intricate crosstalk between cancer cells and the nontumor (stromal) environment (1–3). Host or stromal factors can be both supportive and responsive to tumors and have been shown to play key roles in fostering the development of cancer in several experimental models. For example, both carcinoma-associated fibroblasts (4) and fibroblasts subjected to irradiation (5) have been found to promote transformation of nontumorigenic epithelial cells. Likewise, the deletion of specific host proteases (6) or protease inhibitors (7) impedes tumor progression in vivo. For breast cancer, in particular, the orthotopic host environment appears to play a uniquely supportive role, as the mouse mammary gland and surrounding mesenchymal tissue, the mammary fat pad (mfp), allow human breast cancer cells to form tumors more readily than nonorthotopic sites [e.g., the subcutis (8)]. Although an intimate relationship clearly exists between the growing tumor and surrounding stromal environment, the molecular changes induced in cancer cells by host factors remain largely unknown.
Determining the impact of stromal influence on the molecular and cellular properties of tumor cells is especially relevant for the study of human cancer cell lines, which are commonly examined both in culture and in mouse xenograft models to compare, for example, their proliferation/growth rates (9) and pharmacological responses (10, 11). Several cancer lines display context-dependent differences in behavior, exhibiting aggressive/invasive properties in culture, but failing to form tumors in mice (9), which suggests an as of yet ill-defined dependence on the host environment for growth in vivo. Further confounding the relationship between the in vitro properties of human cancer lines and their tumorigenicity in animal models is the fact that many of these lines are heterogeneous (12), which may result in different subpopulations of cells dominating growth in distinct environments. In vivo selection strategies have been used to identify variants of human cancer lines that show altered gene expression profiles that correlate with enhanced metastatic properties in mice (12, 13). In these studies, however, the molecular characteristics of primary tumors were not examined, leaving several important questions unanswered. For example, do specific components of the surrounding stroma infiltrate the primary tumor, and if so, at what stage during tumor development are such host–cancer interactions observed? Similarly, when during the course of tumor growth do changes in the molecular profiles of cancer cells occur and are these alterations maintained after removal from the host environment? Finally, do the biological properties of cancer cells explanted from primary tumors differ from those of the original parental line?
Here, we address these questions by conducting a comparative functional proteomic analysis of the human breast cancer line MDA-MB-231 grown in culture or during and after tumor formation in the mfp of immunodeficient mice. By using a chemical proteomic approach known as activity-based protein profiling (ABPP) (14–16), in which a suite of active site-directed probes are used to measure changes in the activity of many enzymes in parallel, we show the following: (i) MDA-MB-231 xenograft tumors possess a discrete set of stromal-derived enzyme activities distinguishable from carcinoma enzymes by mass spectrometry (MS) based on their respective species of origin (mouse or human); (ii) MDA-MB-231 cells, after passage in vivo, exhibit dramatic posttranscriptional changes in the activity of specific enzymes, including proteases and glycolytic enzymes; and (iii) these alterations in the enzyme activity profiles of mfp-cultivated MDA-MB-231 cells correlate with enhanced tumor growth rates and metastasis upon reintroduction of these cells in vivo. These findings highlight the profound influence of host environment on the molecular and cellular properties of tumor cells and intimate that the behavior of human cancer cell lines grown in vivo may vary considerably from their characteristics in culture.
Materials and Methods
Human Breast Cancer Xenografts. The parental MDA-MB-231 human breast cancer cell line (American Type Culture Collection) was cultured in Leibovitz L-15 medium supplemented with 10% FCS (complete medium). Cells were injected orthotopically into the mfp of 6-week-old female C.B-17 SCID mice (Taconic Farms) as described (ref. 17; see Supporting Text, which is published as supporting information on the PNAS web site, for more details).
Proteome Labeling and Quantification of Enzyme Activities. For proteomic analysis mice were killed at different time points, and xenograft tumors and control mfp samples (harvested from the contralateral side) were excised. Tumor and mfp tissue were Dounce-homogenized in 50 mM Tris·HCl (pH 8.0), followed by centrifugation at 100,000 × g to provide a soluble proteome fraction (supernatant) and a particulate fraction (pellet). Proteomic fractions were analyzed with ABPP probes as described (refs. 18–20; see Supporting Text for more details).
Isolation and Identification of Probe-Labeled Enzyme Activities. Isolation of enzyme activities was achieved by using trifunctional probes (21) containing both a rhodamine and biotin moiety and avidin-based affinity purification procedures (see Supporting Text for more details).
Preparation of Xenograft Tumor-Derived MDA-MB-231 Cells. A well established (10 weeks postinjection) MDA-MB-231 xenograft tumor growing in the mouse mfp was removed aseptically and minced with a razor blade; tumor pieces were transferred into tissue culture flasks with complete medium. After 1 week of culture, when abundant adherent tumor cells were visible, floating tumor debris was removed. Attached cells were then expanded to constitute a cell population referred to here as 231mfp cells. Northern blotting and Western blotting were conducted according to standard laboratory procedures. Anti-tissue plasminogen activator and anti-urokinase plasminogen activator (anti-uPA) antibodies were purchased from American Diagnostica (Greenwich, CT) and diluted to 1 μg/ml for Western blotting.
Results and Discussion
ABPP of Human Breast Cancer Line Xenografts. In previous studies (20), we used ABPP to profile enzyme activities in cultured preparations of human cancer cell lines, which constitute a homogeneous and renewable source of cancer cells. These experiments identified proteomic signatures containing both known (e.g., uPA) and novel (e.g., KIAA1363) enzyme activities that classified cancer lines into subtypes based on key biological properties, such as tissue of origin and/or state of invasiveness. Nonetheless, how these enzyme activity profiles relate to the functional state of cancer cells in vivo, where a variety of host (stromal) factors may affect tumor development, remains unknown. To address this important issue, we established orthotopic xenograft tumors of the human breast cancer line MDA-MB-231 in the mfp of immunodeficient SCID mice and compared their enzyme activity profiles with those of cultured preparations of these cells. We hypothesized that the mixed-species nature of this xenograft model might permit the simultaneous discovery of carcinoma (human)- and stromal (mouse)-derived enzyme activities associated with breast tumor growth in vivo. Indeed, on average, mouse and human orthologues share ≈80% sequence identity (22), which should, in general, enable their discrimination by MS analysis.
MDA-MB-231 xenograft tumors and control mfp tissue were collected for ABPP analysis over an 11-week time course (two to four mice per week). ABPP was carried out with rhodamine-coupled fluorophosphonate (FP) and sulfonate ester (SE) probes, which target serine hydrolases (SHs) (14, 20, 23) and several groups of metabolic enzymes [e.g., GSTs, sugar kinases, and aldehyde dehydrogenases (ALDHs)] (19, 21, 24, 25), respectively. Tumor and control mfp samples were separated into soluble and membrane fractions before treatment with ABPP probes. Fluorescently labeled proteins were then separated by 1D SDS/PAGE and visualized/quantified by direct in-gel fluorescence scanning as described (20). In parallel experiments, trifunctional (rhodamine- and biotin-coupled) ABPP probes (21) were used to affinity-isolate enzyme activities showing expression patterns of interest, which permitted their molecular identification by liquid chromatography-tandem MS (MS/MS) methods.
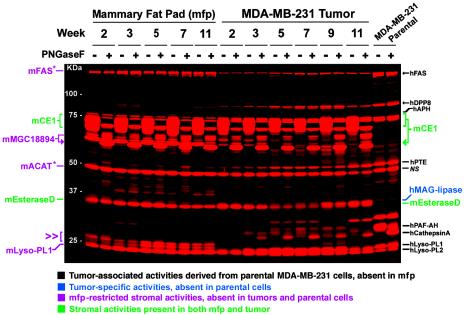
SH Activity Profiles of Human Breast Cancer Xenograft Proteomes. Fig. 1 shows a representative in-gel fluorescence analysis of the SH activity profiles of MDA-MB-231 xenograft tumor and contralateral mfp-soluble proteomes (see Fig. 7, which is published as supporting information on the PNAS web site, for profiles of membrane proteomes). To account for the diffuse labeling of several glycosylated enzyme activities, a portion of each FP-labeled proteome was deglycosylated by treatment with peptide: N-glycosidase F (PNGaseF) before separation by SDS/PAGE, increasing the resolution of these proteins [for example, see mouse carboxylesterase-1 (mCE-1); Fig. 2A]. Although many of the soluble and membrane SH activities expressed in cultured MDA-MB-231 cells were maintained in xenograft tumors (Fig. 1, enzymes labeled in black, and Fig. 7), several additional activities were also present in these tumors. For example, two soluble enzyme activities present in the mfp were also found in tumor tissue throughout the 11-week time course (Fig. 1, enzymes labeled in green). These ≈34- and ≈72-kDa SH activities were identified by MS analysis as the mouse orthologues of CE-1 and esterase D, respectively. In contrast, the activities of several other SHs displayed an mfp-restricted distribution, including the mouse enzymes MGC18894 (an uncharacterized CE) and lysophospholipase A1 (Fig. 1, labeled in magenta). These studies also detected a ≈35-kDa soluble SH activity that showed selective expression in xenograft tumors but not in mfp or cultured preparations of MDA-MB-231 cells. This enzyme activity was identified as human monoacylglycerol lipase (Fig. 1, labeled in blue), indicating that the expression of certain carcinoma enzyme activities depends on the in vivo tumor microenvironment.
Fig. 1.
SH activity profiles of MDA-MB-231 xenograft tumors and mfp. Representative in-gel fluorescence analysis of SH activity profiles obtained from reactions of soluble proteomes from MDA-MB-231 xenograft tumors and contralateral mfp samples with a rhodamine-tagged FP probe. The identities of enzymes are listed on either side of the gel and are color-coded based on their respective expression patterns (see Table 1 for full names of enzymes). Each proteome sample was analyzed both before and after deglycosylation by treatment with peptide: N-glycosidase F (PNGaseF) to enhance resolution. The double arrowhead highlights mfp-restricted stromal activities that were not identified. Asterisks indicate enzyme activities that were only observed by MS analysis in the mfp, but their absence in xenograft tumors could not be unambiguously determined because of comigration with carcinoma-derived enzyme activities. NS, nonspecific target of FP probes. For a Coomassie blue-stained gel image of these proteomes, see Fig. 11, which is published as supporting information on the PNAS web site. mACAT, mouse acyl-CoA thioesterase; mFAS, mouse fatty acid synthase; mLyso-PL1, mouse lysophospholipase A1; hMAG-lipase, human monoacylglycerol lipase.
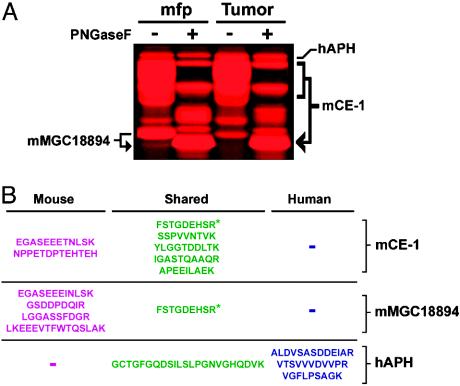
Fig. 2.
General MS-based strategy to distinguish stromal (mouse) and carcinoma (human) enzyme activities in xenograft tumors. (A) Expanded view of representative FP-labeled tumor and mfp SH activities showcasing the increased resolution that is achieved for glycosylated enzyme activities (e.g., mCE-1) after treatment with peptide: N-glycosidase F (PNGaseF). Identities of enzyme activities are shown on either side of the gel: hAPH, human acyl-peptide hydrolase; and mMGC18894, uncharacterized mCE. (B) Tryptic peptide maps for representative SH activities shown in A. Blue, peptides unique to human orthologue; magenta, peptides unique to mouse orthologue; green, peptide shared between mouse and human orthologues. The asterisk highlights a peptide that is shared between CE-1 and MGC18894 enzymes.
The designation of enzyme activities as human or mouse was accomplished by liquid chromatography-electrospray tandem MS (MS/MS) analysis of their corresponding tryptic peptide maps. Fig. 2B shows the labeling profile and tryptic peptides for three representative SH activities identified in xenograft tumor and mfp tissue. In each case, at least three unique peptides were identified that allowed for unambiguous assignment of the enzymes as stromal/mouse (MGC18894 and CE-1) or tumor/human (APH) in origin. For a more complete list of the tryptic peptides corresponding to each enzyme identified in this study, see Table 1, which is published as supporting information on the PNAS web site.
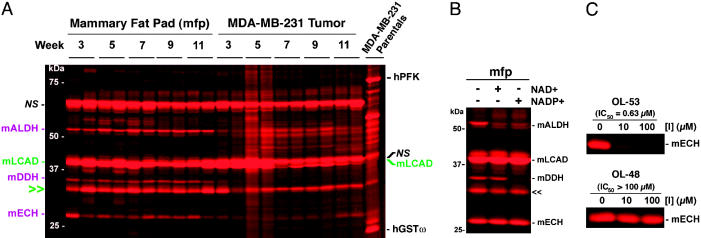
Metabolic Enzyme Activity Profiles of Human Breast Cancer Xenograft Proteomes. We next profiled MDA-MB-231 xenograft tumor and mfp control tissues with a rhodamine-tagged phenyl SE probe (phenyl SE) that targets several different metabolic enzymes in an active site-directed manner (25). Many of the SE-reactive enzyme activities observed in cultured MDA-MB-231 cells, including platelet-type phosphofructokinase (PFK) (21) and GSTω (19), were not detected in MDA-MB-231 xenograft tumors, even at the earliest time points examined (Fig. 3A), suggesting that they were down-regulated in vivo.
Fig. 3.
Metabolic enzyme activities labeled by a phenyl SE probe in xenograft tumor and mfp tissue. (A) Representative in-gel fluorescence analysis of enzyme activity profiles obtained from reactions of soluble proteomes from MDA-MB-231 xenograft tumors and contralateral mfp samples with a rhodamine-tagged phenyl SE probe (two independent tumors shown per week). Identities of enzymes are listed on either side of the gel and are color-coded based on their respective expression patterns [black, parental MDA-MB-231 enzyme activities; magenta, mfp-restricted enzyme activities; green, enzyme activities expressed in both mfp and tumor (double arrowhead denotes unidentified SE-reactive protein)]. mLCAD, mouse long-chain acyl-CoA dehydrogenase-1. NS refers to nonspecific SE reactivities as determined by lack of heat-sensitive labeling (data not shown). (B) Indirect identification of SE-labeled enzymes by competitive profiling with cofactors. The inhibition of labeling of 55- and 36-kDa enzymes by NAD+/NADP+ and NADP+, respectively, indicates that these proteins correspond to a mALDH and mDDH, respectively. (C) Indirect identification of an SE-labeled enzyme by competitive profiling with inhibitors. The inhibition of labeling of a 30-kDa enzyme by the trifluoromethyl ketone agent OL-53, but not the related α-keto heterocycle agent OL-48, provides evidence that this protein is mECH, which has been shown to be inhibited selectively by the former agent with an IC50 value of 0.63 μM (27). See ref. 27 for the structures of OL-48 and OL-53.
Other SE-labeled enzyme activities were detected in mfp tissue, the majority of which were not observed in xenograft tumors (Fig. 3A). The identification of these presumably stromal enzyme activities by standard avidin enrichment-MS analysis proved challenging because of the low quantities of mouse mfp proteome. As an alternative strategy to ascertain the identity of enzyme activities in the mfp, we tested the ability of various cofactors/inhibitors to compete the SE labeling of specific proteins in this tissue. The SE reactivity of a 55-kDa enzyme activity was blocked by NAD+ or NADP+ (Fig. 3B), indicating that it is likely a member of the ALDH family, which is labeled by SE probes (19, 24). Likewise, the SE labeling of a 36-kDa enzyme activity was specifically inhibited by NADP+ (Fig. 3B), indicating that this protein is the SE target dihydrodiol dehydogenase (DDH), which selectively utilizes this cofactor for catalysis (26). Finally, a 30-kDa SE target was identified as enoyl-CoA hydratase-1 (ECH-1) by blocking its labeling with an ECH-1 selective inhibitor (27) (Fig. 3C). Together, these findings highlight the value of competitive ABPP methods, which enabled the molecular classification of three SE targets by using <100 μgof total protein from mouse mfp. In contrast to these enzymes, which were restricted in expression to the mfp, an additional 37-kDa SE target was observed in both mfp and tumor samples and was identified as long-chain acyl-CoA dehydrogenase-1.
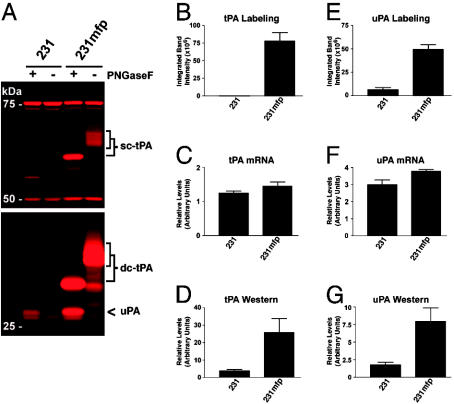
ABPP of mfp-Cultivated MDA-MB-231 Cells. MDA-MB-231 cells grown in vivo in the mouse mammary fad pad were isolated from xenograft tumors and recultured to provide a propagatable subpopulation of cells referred to as 231mfp cells. Comparative ABPP of 231mfp and parental MDA-MB-231 cells revealed dramatic differences in the levels of discrete enzyme activities. For example, several FP-labeled enzyme activities were highly up-regulated in the conditioned media of 231mfp cells (Fig. 4A). These enzymes were identified as uPA and the single- and double-chain forms of tissue plasminogen activator (tPA). Additionally, multiple cytosolic SE-labeled enzyme activities were down-regulated in 231mfp cells, including PFK (Fig. 8, which is published as supporting information on the PNAS web site), indicating that the reduced expression of these enzyme activities in xenograft tumors (Fig. 3A) was maintained in culture. Notably, these differentially expressed enzyme activities stood out against an otherwise similar background of enzyme activity profiles for the 231mfp and parental MDA-MB-231 cells, as exemplified by their nearly identical soluble and membrane FP-reactivity profiles (Fig. 9, which is published as supporting information on the PNAS web site). Also consistent with the general similarity of these two MDA-MB-231 cell variants, they did not differ in the expression of the uPA receptor or other cell surface molecules implicated in tumor stroma interactions (2), including the EGF receptor and integrin-β1, as determined by flow cytometry (data not shown). Finally, it is noteworthy that the 231mfp and parental MDA-MB-231 cells also displayed equivalent levels of GSTω (Fig. 8), an SE target that was not observed in xenograft tumors, indicating that the expression of this enzyme activity was reconstituted upon culturing 231mfp cells.
Fig. 4.
Posttranscriptional up-regulation of secreted serine protease activities in mfp-cultivated MDA-MB-231 cells (231mfp cells). (A) Representative in-gel fluorescence analysis of SH activity profiles of the secreted (conditioned media) proteomes of parental MDA-MB-231 and 231mfp cells showing the dramatic elevation in the activity of the serine proteases uPA and tPA [both single chain (sc) and double chain (dc) forms] in 231mfp cells. (B and E) Quantification of levels of active tPA and uPA as measured by in-gel fluorescence scanning. Data reported as averages ± standard errors (SE); n = 3 per group. (C and F) Relative mRNA levels for tPA and uPA as measured by Northern blotting (n = 3 per group; values expressed in arbitrary units normalized to an internal 7S RNA control). (D and G) Relative protein levels for tPA and uPA as measured by Western blotting (n = 3 per group).
Northern blotting revealed that the parental MDA-MB-231 and 231mfp lines expressed similar levels of tPA and uPA transcripts (Fig. 4 C and F), whereas Western blotting showed that the expression of both proteases (Fig. 4 D and G), like their activity (Fig. 4 B and E), was highly elevated in 231mfp cells. These findings indicate that tPA and uPA expression/activity are regulated by a posttranscriptional or posttranslational mechanism in MDA-MB-231 cells. In this regard, mRNA levels for the endogenous tPA/uPA inhibitor, plasminogen activator inhibitor-1 were ≈4-fold decreased in 231mfp cells (Fig. 10, which is published as supporting information on the PNAS web site), suggesting that the elevated tPA/uPA expression and activity in these cells may reflect, at least in part, the down-regulation of this protease inhibitor. Indeed, both tPA and uPA are rapidly cleared by cells after complexation with plasminogen activator inhibitor-1 (28, 29). Discordance was also observed between the quantity of activity and transcript for the SE target PFK, for which equivalent mRNA levels were detected in parental MDA-MB-231 and 231mfp cells, but activity was observed only in the parental cells (Fig. 9). Together these findings demonstrate that posttranscriptional and/or posttranslational events play a major role in determining the functional state of enzymes in cancer cell proteomes.
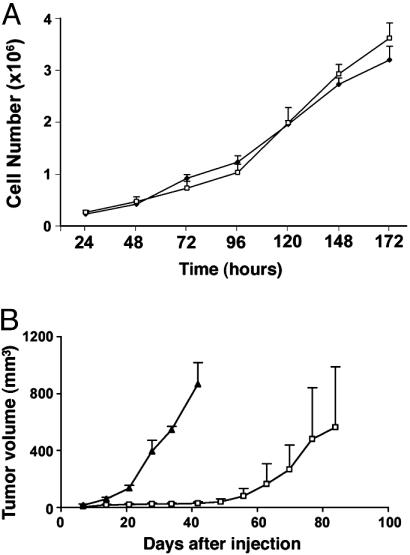
231mfp Cells Exhibit Enhanced Tumor Growth Rates and Metastasis In Vivo. We next compared the biological properties of parental MDA-MB-231 and 231mfp cells in culture and in vivo. Under standard cell culture conditions, these MDA-MB-231 variants exhibited similar growth rates, with doubling times of 36.3 ± 3.7 h and 35.0 ± 2.9 h, respectively (Fig. 5A), and equivalent invasiveness as determined by Matrigel assays (data not shown). In contrast to these in vitro results, 231mfp cells were found to show a dramatic increase in tumor growth rates in vivo. Whereas parental MDA-MB-231 tumors underwent a lag time of 5–7 weeks after injection before growing progressively, 231mfp cells produced fast-growing tumors without any apparent lag time (Fig. 5B). Furthermore, tumors from 231mfp xenografts were larger, weighing 1,245 ± 292 mg at 6 weeks after injection compared with a weight of 882 ± 681 mg at 12 weeks after injection for parental MDA-MB-231 tumors. Finally, 231mfp tumors also produced significantly more lung metastases than parental MDA-MB-231 tumors of equivalent weight (231mfp = 352 + 116 metastatic foci per tumor; MDA-MB-231 = 108 + 75 metastatic foci per tumor; P = 0.01, planned comparison; n = 4 tumors per group). Together, these data indicate that the environment of the mouse mfp selected for, or supported the growth of, a subpopulation of MDA-MB-231 cells that display enhanced tumorigenic properties in vivo.
Fig. 5.
Comparison of in vitro and in vivo growth rates of parental MDA-MB-231 and 231mfp cells. (A) In vitro growth rates of cultured parental MDA-MB-231 (□) and 231mfp (♦) cells, with doubling times averaged from three independent experiments. (B) In vivo growth rates of parental MDA-MB-231 (□) and 231mfp (▴) xenograft tumors grown in the mfp of immunodeficient mice (n = 4 per group).
Conclusions
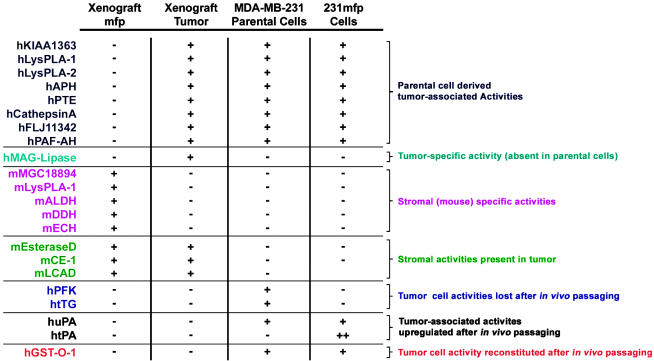
Using a suite of chemical probes for ABPP, we have comparatively profiled human breast cancer cells grown in culture and during and after in vivo passage as orthotopic xenograft tumors in the mfp of immunodeficient mice. These functional proteomic studies identified at least seven types of enzyme activities with distinct expression patterns (Fig. 6): (i) tumor-associated activities derived from parental MDA-MB-231 cells (e.g., hCathepsin A); (ii) tumor-specific activities (absent in both parental cells and mfp; e.g., human monoacylglycerol lipase); (iii) stromal activities excluded from tumors (e.g., mMGC18894); (iv) stromal activities present in tumors (e.g., mCE-1); (v) carcinoma activities expressed selectively in the parental line (e.g., hPFK); (vi) carcinoma activities expressed selectively after in vivo passaging (e.g., huPA and htPA); and (vii) carcinoma activities reconstituted after in vivo passaging (e.g., hGSTω). Together, the myriad changes observed in the MDA-MB-231 proteome after cultivation in the mouse mfp point to the existence of a subpopulation of breast cancer cells (231mfp cells) that exhibits increased tumor growth rates and metastasis in vivo. Although it remains to be determined whether the differentially expressed enzyme activities identified by ABPP contribute to the enhanced tumorigenic properties of 231mfp cells, it is nonetheless significant that many of the most dramatic alterations in enzyme activities occurred as a result of posttranscriptional events (e.g., the up-regulation of tPA and uPA activity in 231mfp cells). These findings indicate that large-magnitude changes in cancer cell proteomes may occur without corresponding alterations in transcript expression and thus underscore the value of ABPP for measuring the functional outcome of posttranscriptional and posttranslational events that ultimately govern enzyme activity in vivo.
Fig. 6.
Enzymes identified by ABPP and their distribution among MDA-MB-231 xenograft and cell culture samples. See Table 1 for a list of the tryptic peptides that were used to assign the identity of these enzymes.
Some of the potential shortcomings of current methods for ABPP were also revealed during the course of these studies. For example, ABPP failed to detect any secreted enzyme activities in xenograft tumors, even though several of these proteins (e.g., tPA and uPA) were identified at high levels in the conditioned media of cultured breast cancer cells. Although it is possible that these secreted serine protease activities were inactivated in vivo, thereby precluding detection by ABPP, these enzymes may have also been diluted into the extracellular milieu of the in vivo tumor environment, resulting in levels effectively below the sensitivity limit of 1D gel-based ABPP detection methods. The limited resolution of 1D gel analysis also prevented, in certain regions of the gel, the unambiguous determination of the potential presence of stromal enzyme activities in xenograft tumors due to comigration with carcinoma-derived enzyme activities (e.g., mouse fatty acid synthase and mouse acyl-CoA thioesterase in Fig. 1). Although future efforts to implement gel-free strategies for ABPP (25) should assist in both the detection and resolution of low-abundance enzyme activities in complex biological samples, such as xenograft tumors, it should still be noted that 1D gel analysis possesses several advantages for ABPP, most notably of which is exceptional throughput, permitting the rapid comparative analysis of large panels of proteomes.
In summary, we have shown, by using functional proteomic techniques, that the in vivo environment of the mouse mfp cultivates the growth of human breast cancer cells with distinct molecular and cellular properties. How might such changes arise? One possibility is that host (stromal) factors induce alterations in the proteomic expression pattern of MDA-MB-231 cells. Alternatively, considering that many cancer cell lines are heterogeneous (12, 13), the in vivo environment may support the expansion of a rare subpopulation of MDA-MB-231 cells that comes to dominate the xenograft tumor. Irrespective of the underlying mechanism(s), the distinct enzyme activity profiles of MDA-MB-231 cells grown in vitro (culture) and in vivo (xenograft tumors) suggest that these cells represent two fundamentally different populations (designated here as parental and 231mfp, respectively). Considering further that the 231mfp cells exhibit superior tumorigenic properties in mice, we propose that these cells may represent a preferred model for future investigations of breast cancer growth in vivo. More generally, these findings suggest that studies carried out on human cancer cell lines in culture, such as measurements of proliferation rates, invasiveness, and drug sensitivity, may not be predictive of the behavior of these lines in vivo. A potentially more effective strategy may be to passage cancer lines through mice before analysis in culture, thus enabling the selection of subpopulations of cancer cells more capable of forming tumors in vivo.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants CA087660 (to B.F.C.), CA085405 (to B.M.M.), and RR11823 (to J.R.Y.), the California Breast Cancer Research Foundation (N.J. and B.F.C.), and The Skaggs Institute for Chemical Biology.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ABPP, activity-based protein profiling; ALDH, aldehyde dehydrogenase; CE, carboxylesterase; DDH, dihydrodiol dehydrogenase; ECH, enoyl-CoA hydratase; FP, fluorophosphonate; mfp, mammary fat pad; h, human; m, mouse; PFK, phosphofructokinase; SH, serine hydrolase, SE, sulfonate ester; tPA, tissue plasminogen activator; uPA, urokinase plasminogen activator.
References
- 1.Wiseman, B. S. & Werb, Z. (2002) Science 296, 1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell, M. J., Radisky, D. C., Rizki, A., Weaver, V. M. & Peterson, O. W. (2002) Differentiation (Berlin) 70, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tlsty, T. D. & Hein, P. W. (2001) Curr. Opin. Gen. Dev. 11, 54–59. [DOI] [PubMed] [Google Scholar]
- 4.Olumi, A., Grossfeld, G., Hayward, S., Carroll, P., Tlsty, T. D. & Cunha, G. (1999) Cancer Res. 59, 5002–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barcellos-Hoff, M. & Ravani, S. (2000) Cancer Res. 60, 1254–1260. [PubMed] [Google Scholar]
- 6.Shapiro, R. L., Duquete, J. G., Roses, D. F., Numes, I., Harris, M. N., Kamino, H., Wilson E. L. & Rifkin, D. B. (1996) Cancer Res. 56, 3597–3604. [PubMed] [Google Scholar]
- 7.Bajou, K., Noel, A., Gerald, R. D., Masson, V., Brunner, N., Holst-Hansen, C., Skobe, M., Fusenig, N. E., Carmeliet, P., Collen, D. & Foidart, J. M. (1998) Nat. Med. 4, 923–928. [DOI] [PubMed] [Google Scholar]
- 8.Felding-Habermann, B., O'Toole, T. E., Smith, J. W., Fransvea, E., Ruggeri, Z. M., Ginsberg, M. H., Hughes, P. E., Pampori, N., Shattil, S. J., Saven, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson, E. W., Paik, S., Brunner, N., Sommers, C. L., Zugmaier, G., Clarke, R., Shima, T. B., Torri, J., Donahue, S., Lippman, M. E., et al. (1992) J. Cell Phys. 150, 534–544. [DOI] [PubMed] [Google Scholar]
- 10.Bissery, M. C., Nohynek, G., Sanderink, G. J. & Lavelle, F. (1995) Anti-Cancer Drugs 6, 339–355. [DOI] [PubMed] [Google Scholar]
- 11.Sliwkowski, M. X., Lofgren, J. A., Lewis, G. D., Hotaling, T. E., Fendly, B. M. & Fox, J. A. (1999) Semin. Oncol. 26, 60–70. [PubMed] [Google Scholar]
- 12.Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo, C., Guise, T. A. & Massague, J. (2003) Cancer Cell 3, 537–549. [DOI] [PubMed] [Google Scholar]
- 13.Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. (2000) Nature 406, 532–535. [DOI] [PubMed] [Google Scholar]
- 14.Liu, Y., Patricelli, M. P. & Cravatt, B. F. (1999) Proc. Natl. Acad. Sci. USA 96, 14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessani, N. & Cravatt, B. F. (2004) Curr. Opin. Chem. Biol. 8, 54–59. [DOI] [PubMed] [Google Scholar]
- 16.Speers, A. E. & Cravatt, B. F. (2004) Chembiochem 5, 41–47. [DOI] [PubMed] [Google Scholar]
- 17.Price, J. E., Polyzos, A., Zhang, R. D. & Daniels, L. M. (1990) Cancer Res. 50, 717–721. [PubMed] [Google Scholar]
- 18.Patricelli, M. P., Giang, D. K., Stamp, L. M. & Burbaum, J. J. (2001) Proteomics 1, 1067–1071. [DOI] [PubMed] [Google Scholar]
- 19.Adam, G. C., Sorensen, E. J. & Cravatt, B. F. (2002) Nat. Biotechnol. 20, 805–809. [DOI] [PubMed] [Google Scholar]
- 20.Jessani, N., Liu, Y., Humphrey, M. & Cravatt, B. F. (2002) Proc. Natl. Acad. Sci. USA 99, 10335–10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam, G. C., Sorensen, E. J. & Cravatt, B. F. (2002) Mol. Cell. Proteomics 1, 828–835. [DOI] [PubMed] [Google Scholar]
- 22.Mouse Genome Sequencing Consortium (2002) Nature 420, 520–562. [DOI] [PubMed] [Google Scholar]
- 23.Kidd, D., Liu, Y. & Cravatt, B. F. (2001) Biochemistry 40, 4005–4015. [DOI] [PubMed] [Google Scholar]
- 24.Adam, G. C., Cravatt, B. F. & Sorensen, E. J. (2001) Chem. Biol. 8, 81–95. [DOI] [PubMed] [Google Scholar]
- 25.Adam, G. C., Burbaum, J. J., Kozarich, J. W., Patricelli, M. P. & Cravatt, B. F. (2004) J. Am. Chem. Soc. 126, 1363–1368. [DOI] [PubMed] [Google Scholar]
- 26.Asada, Y., Aoki, S., Ishikura, S., Usami, N. & Hara, A. (2000) Biochem. Biophys. Res. Commun. 278, 333–337. [DOI] [PubMed] [Google Scholar]
- 27.Leung, D., Hardouin, C., Boger, D. L. & Cravatt, B. F. (2003) Nat. Biotechnol. 21, 687–691. [DOI] [PubMed] [Google Scholar]
- 28.Orth, K., Madison, E. L., Gething, M. J., Sambrook, J. F. & Herz, J. (1992) Proc. Natl. Acad. Sci. USA 89, 7422–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nykjaer, A., Petersen, C. M., Moller, B., Jensen, P. H., Moestrup, S. K., Holtet, T. L., Etzerodt, M., Thogersen, H. C., Munch, M., Andreasen, P. A. & Gliemann, J. (1992) J. Biol. Chem. 267, 14543–14546. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.