Abstract
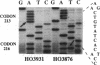
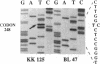
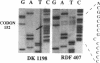
We have investigated the frequency of p53 mutations in B- and T-cell human lymphoid malignancies, including acute lymphoblastic leukemia, the major subtypes of non-Hodgkin lymphoma, and chronic lymphocytic leukemia. p53 exons 5-9 were studied by using genomic DNA from 197 primary tumors and 27 cell lines by single-strand conformation polymorphism analysis and by direct sequencing of PCR-amplified fragments. Mutations were found associated with (i) Burkitt lymphoma (9/27 biopsies; 17/27 cell lines) and its leukemic counterpart L3-type B-cell acute lymphoblastic leukemia (5/9), both of which also carry activated c-myc oncogenes, and (ii) B-cell chronic lymphocytic leukemia (6/40) and, in particular, its stage of progression known as Richter's transformation (3/7). Mutations were not found at any significant frequency in other types of non-Hodgkin lymphoma or acute lymphoblastic leukemia. In many cases, only the mutated allele was detectable, implying loss of the normal allele. These results suggest that (i) significant differences in the frequency of p53 mutations are present among subtypes of neoplasms derived from the same tissue; (ii) p53 may play a role in tumor progression in B-cell chronic lymphocytic leukemia; (iii) the presence of both p53 loss/inactivation and c-myc oncogene activation may be important in the pathogenesis of Burkitt lymphoma and its leukemic form L3-type B-cell acute lymphoblastic leukemia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Benjamin D., Magrath I. T., Maguire R., Janus C., Todd H. D., Parsons R. G. Immunoglobulin secretion by cell lines derived from African and American undifferentiated lymphomas of Burkitt's and non-Burkitt's type. J Immunol. 1982 Sep;129(3):1336–1342. [PubMed] [Google Scholar]
- Buchman V. L., Chumakov P. M., Ninkina N. N., Samarina O. P., Georgiev G. P. A variation in the structure of the protein-coding region of the human p53 gene. Gene. 1988 Oct 30;70(2):245–252. doi: 10.1016/0378-1119(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Cabanillas F., Pathak S., Trujillo J., Manning J., Katz R., McLaughlin P., Velasquez W. S., Hagemeister F. B., Goodacre A., Cork A. Frequent nonrandom chromosome abnormalities in 27 patients with untreated large cell lymphoma and immunoblastic lymphoma. Cancer Res. 1988 Oct 1;48(19):5557–5564. [PubMed] [Google Scholar]
- Cheng J., Haas M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol Cell Biol. 1990 Oct;10(10):5502–5509. doi: 10.1128/mcb.10.10.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass E. C., Magrath I. T., Lee E. C., Whang-Peng J. Cytogenetic studies in non-African Burkitt lymphoma. Blood. 1980 Jan;55(1):148–155. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Halevy O., Michalovitz D., Oren M. Different tumor-derived p53 mutants exhibit distinct biological activities. Science. 1990 Oct 5;250(4977):113–116. doi: 10.1126/science.2218501. [DOI] [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iman D. S., Harris C. C. Oncogenes and tumor suppressor genes in human lung carcinogenesis. Crit Rev Oncog. 1991;2(2):161–171. [PubMed] [Google Scholar]
- Jonveaux P., Berger R. Chromosomal deletions in non-Hodgkin's malignant lymphomas. Cancer Genet Cytogenet. 1990 Oct 15;49(2):265–269. doi: 10.1016/0165-4608(90)90151-y. [DOI] [PubMed] [Google Scholar]
- Juliusson G., Oscier D. G., Fitchett M., Ross F. M., Stockdill G., Mackie M. J., Parker A. C., Castoldi G. L., Guneo A., Knuutila S. Prognostic subgroups in B-cell chronic lymphocytic leukemia defined by specific chromosomal abnormalities. N Engl J Med. 1990 Sep 13;323(11):720–724. doi: 10.1056/NEJM199009133231105. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Pelicci P. G., Dalla-Favera R. T-cell receptor beta chain gene rearrangements: genetic markers of T-cell lineage and clonality. Hum Pathol. 1986 Jun;17(6):546–551. doi: 10.1016/s0046-8177(86)80125-3. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Krolewski J. J., Dalla-Favera R. Molecular genetic approaches in the diagnosis and classification of lymphoid malignancies. Hematol Pathol. 1989;3(2):45–61. [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigueur A., Maltby V., Mock D., Rossant J., Pawson T., Bernstein A. High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol Cell Biol. 1989 Sep;9(9):3982–3991. doi: 10.1128/mcb.9.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir G. M., Preud'homme J. L., Bernheim A., Berger R. Correlation between immunoglobulin light chain expression and variant translocation in Burkitt's lymphoma. Nature. 1982 Jul 29;298(5873):474–476. doi: 10.1038/298474a0. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Merry D., Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci U S A. 1986 Jan;83(1):130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A., Knowles D. M., Greco A., McCormick F., Dalla-Favera R. Analysis of RAS oncogene mutations in human lymphoid malignancies. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9268–9272. doi: 10.1073/pnas.85.23.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Prokocimer M., Shaklai M., Bassat H. B., Wolf D., Goldfinger N., Rotter V. Expression of p53 in human leukemia and lymphoma. Blood. 1986 Jul;68(1):113–118. [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [PubMed] [Google Scholar]
- Takahashi T., D'Amico D., Chiba I., Buchhagen D. L., Minna J. D. Identification of intronic point mutations as an alternative mechanism for p53 inactivation in lung cancer. J Clin Invest. 1990 Jul;86(1):363–369. doi: 10.1172/JCI114710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]