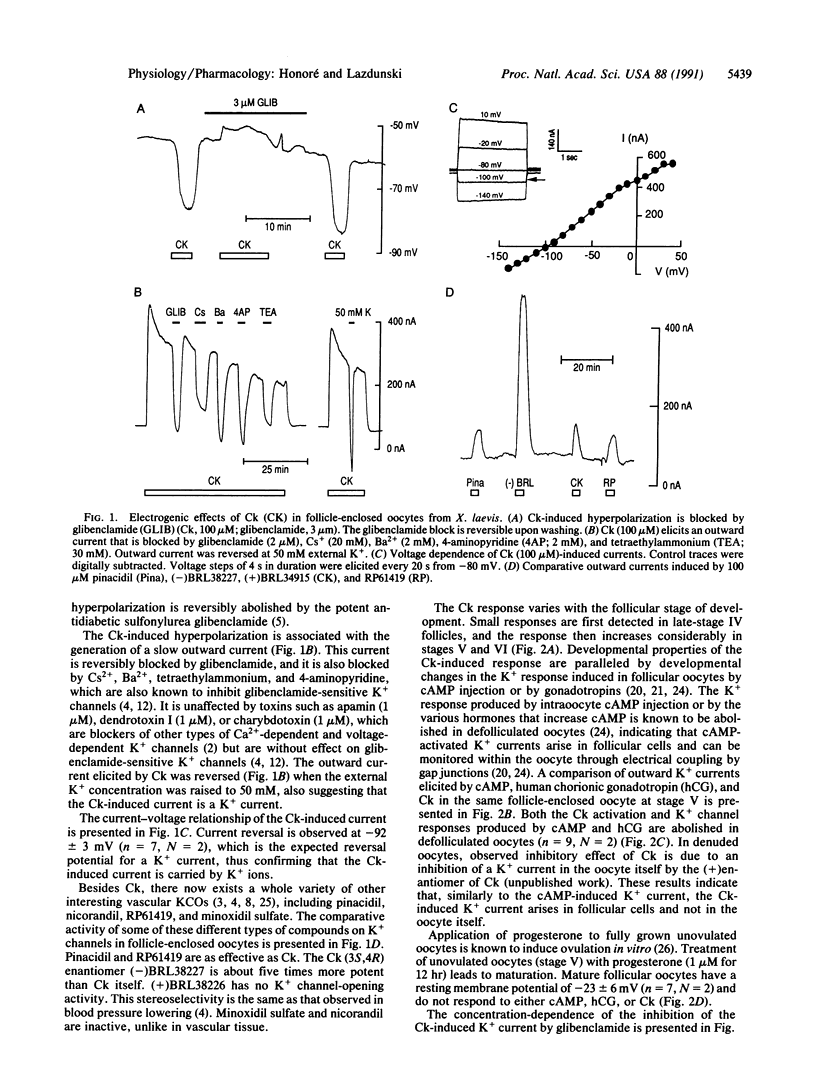
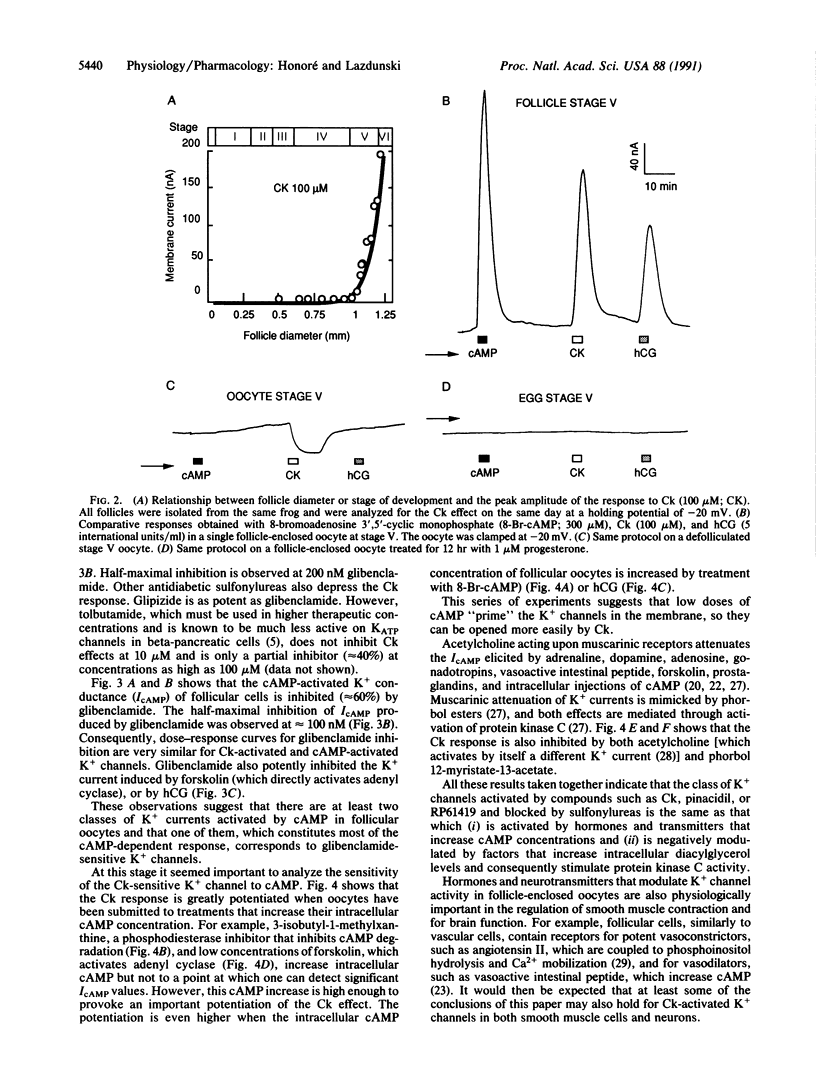
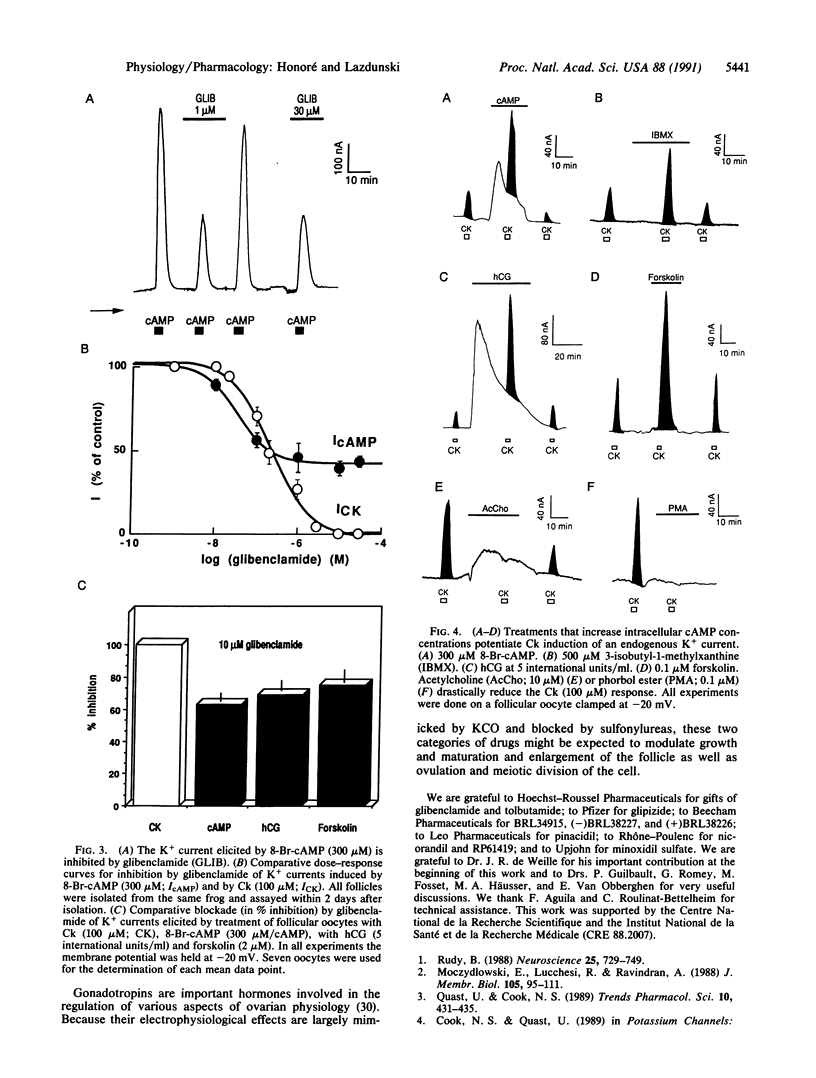
Abstract
Follicular oocytes from Xenopus laevis contain K+ channels activated by members of the recently recognized class of vasorelaxants that include cromakalim and pinacidil and blocked by antidiabetic sulfonylureas, such as glibenclamide. These channels are situated on the adherent follicular cells and are not present in denuded oocytes. Cromakalim-activated K+ channels are also activated by increases in intracellular cAMP, and cAMP-activated K+ channels are blocked by glibenclamide. Although cromakalim and cAMP effects are synergistic, cromakalim activation of K+ channels is drastically reduced or abolished by treatments that stimulate protein kinase C (e.g., muscarinic effectors, phorbol esters). Gonadotropins, known to play an essential role in ovarian physiology, also activate cromakalim and sulfonylurea-sensitive K+ channels. Follicular oocytes constitute an excellent system for studying regulation of cromakalim-sensitive K+ channels that are important in relation to a variety of disease processes, such as cardiovascular dysfunction and asthma, as well as brain function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amoroso S., Schmid-Antomarchi H., Fosset M., Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990 Feb 16;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M., Lass Y. Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3',5'-cyclic monophosphate. J Physiol. 1984 Jul;352:551–574. doi: 10.1113/jphysiol.1984.sp015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Lotan I., Gillo B., Lester H. A., Lass Y. Acetylcholine and phorbol esters inhibit potassium currents evoked by adenosine and cAMP in Xenopus oocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6001–6005. doi: 10.1073/pnas.82.17.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulus-secretion coupling. Biochim Biophys Acta. 1991 Mar 7;1071(1):67–82. doi: 10.1016/0304-4157(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Structure-activity relationships of K+ channel openers. Trends Pharmacol Sci. 1990 Oct;11(10):417–422. doi: 10.1016/0165-6147(90)90149-3. [DOI] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Leguern S., Cavero I. The potassium channel opener cromakalim (BRL 34915) activates ATP-dependent K+ channels in isolated cardiac myocytes. Biochem Biophys Res Commun. 1988 Jul 29;154(2):620–625. doi: 10.1016/0006-291x(88)90184-2. [DOI] [PubMed] [Google Scholar]
- Gandolfo G., Gottesmann C., Bidard J. N., Lazdunski M. Subtypes of K+ channels differentiated by the effect of K+ channel openers upon K+ channel blocker-induced seizures. Brain Res. 1989 Aug 21;495(1):189–192. doi: 10.1016/0006-8993(89)91236-5. [DOI] [PubMed] [Google Scholar]
- Gandolfo G., Romettino S., Gottesmann C., van Luijtelaar G., Coenen A., Bidard J. N., Lazdunski M. K+ channel openers decrease seizures in genetically epileptic rats. Eur J Pharmacol. 1989 Aug 11;167(1):181–183. doi: 10.1016/0014-2999(89)90762-0. [DOI] [PubMed] [Google Scholar]
- Gelband C. H., Lodge N. J., Van Breemen C. A Ca2+-activated K+ channel from rabbit aorta: modulation by cromakalim. Eur J Pharmacol. 1989 Aug 22;167(2):201–210. doi: 10.1016/0014-2999(89)90580-3. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weston A. H. Cromakalim, nicorandil and pinacidil: novel drugs which open potassium channels in smooth muscle. Gen Pharmacol. 1989;20(1):1–9. doi: 10.1016/0306-3623(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Membrane currents elicited by prostaglandins, atrial natriuretic factor and oxytocin in follicle-enclosed Xenopus oocytes. J Physiol. 1989 Sep;416:623–643. doi: 10.1113/jphysiol.1989.sp017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. Glucose-regulated potassium channels are sweet news for neurobiologists. Trends Neurosci. 1990 Jun;13(6):197–199. doi: 10.1016/0166-2236(90)90158-7. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sandberg K., Bor M., Ji H., Markwick A., Millan M. A., Catt K. J. Angiotensin II-induced calcium mobilization in oocytes by signal transfer through gap junctions. Science. 1990 Jul 20;249(4966):298–301. doi: 10.1126/science.2374929. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., Amoroso S., Fosset M., Lazdunski M. K+ channel openers activate brain sulfonylurea-sensitive K+ channels and block neurosecretion. Proc Natl Acad Sci U S A. 1990 May;87(9):3489–3492. doi: 10.1073/pnas.87.9.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Van Renterghem C. Cyclic adenosine monophosphate, calcium, acetylcholine and the current induced by adenosine in the Xenopus oocyte. J Physiol. 1986 May;374:551–569. doi: 10.1113/jphysiol.1986.sp016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Hormonal activation of ionic currents in follicle-enclosed Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4135–4139. doi: 10.1073/pnas.84.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]


