Excessive daytime sleepiness is common,1 yet opportunities to learn about sleep medicine in medical school are rare; a survey in 1998 indicated that undergraduate courses devoted a median of five minutes to sleep and its disorders.2 In this review we provide an update on the biology, diagnosis, and management of narcolepsy—an important, yet often misdiagnosed, cause of sleepiness that has seen exciting recent advances. We also briefly outline the other principal causes of daytime sleepiness and aim to equip the general reader with a practical approach to the assessment of patients who complain of excessive daytime sleepiness.
Sources and selection criteria
This paper is based on a literature search conducted to produce evidence based guidelines on the diagnosis and management of narcolepsy in adults and children.3 We searched Medline, Embase, the Cochrane Collaboration, and two specialist sleep literature resources for abstracts with the key word “narcolepsy.” We read the full text of relevant papers and hand searched these for other relevant material. A multidisciplinary working party prepared the guidelines, and a group of 10 independent experts later reviewed them. These guidelines can be downloaded from the news section of www.sleeping.org.uk (accessed July 2004).
Clinical features of narcolepsy
Narcolepsy is a chronic neurological disorder affecting sleep regulation and causing excessive sleepiness and, in most cases, cataplexy (brief attacks of weakness on emotional arousal).3,4 The excessive sleepiness of narcolepsy comprises both a background feeling of sleepiness present much of the time and a strong, sometime irresistible, urge to sleep recurring at intervals through the day. This desire is heightened by conducive, monotonous circumstances, but naps at inappropriate times—such as during meals—are characteristic. The naps of narcolepsy usually last from minutes to an hour and occur a few times each day. Cataplexy refers to partial or generalised, almost invariably bilateral, loss of skeletal muscle tone and power in response to emotion, especially amusement, anger, and elation. Generalised attacks can lead to collapse. Awareness is usually preserved throughout the attacks, which usually last for less than a minute and can occur up to several times a day. Irregular twitching of the limbs or face during attacks of cataplexy can easily be mistaken for epilepsy.
Summary points
A structured sleep history is the key to the assessment of daytime sleepiness
The Epworth sleepiness scale provides a useful measure of daytime sleepiness
Assessment in a sleep laboratory is often helpful in finding the cause of excessive daytime sleepiness
Hypocretin-1 and hypocretin-2 are recently described neurotransmitters that help to regulate the sleep-wake cycle; their levels in the brain are reduced in narcolepsy with cataplexy
Moderately effective treatments are available for daytime sleepiness and cataplexy
Approaches to the diagnosis and treatment of narcolepsy are likely to change rapidly over the next few years
Several other symptoms are common: sleep paralysis (inability to move for a minute or two at the beginning or end of sleep), hypnagogic hallucinations (vivid, dream-like experiences at the start of sleep), disturbed nocturnal sleep (associated with an increased rate of behavioural abnormalities in sleep, such as sleep walking), and automatic behaviour (the continued error prone performance of a task at a time of mounting sleepiness). Secondary symptoms related to sleepiness include visual blurring, diplopia, and difficulties with memory and concentration. In combination, the symptoms of narcolepsy often have a major impact on relationships, education, employment, driving, mood, and quality of life.5
Sleepiness is the most common presenting complaint of patients with narcolepsy. The onset of the associated symptoms may be delayed, sometimes by several years, but occasionally precedes the appearance of excessive daytime sleepiness (see “A patient's perspective”). The combination of excessive daytime sleepiness and unambiguous cataplexy strongly suggests the diagnosis of narcolepsy. The other symptoms mentioned are less specific for the diagnosis. Once established, the symptoms of narcolepsy generally persist for life.
Epidemiology
The prevalence of narcolepsy with cataplexy in European populations has been estimated at 3-5 per 10 0006-8; this is approximately a quarter of the prevalence of multiple sclerosis in the United Kingdom. A recent study suggests that inclusion of patients without cataplexy would increase this estimate by around a third.8 The disorder most commonly starts in the teens or 20s, but it can present as early as 2 years of age or in middle age. Men and women are roughly equally affected. Family members are at an increased risk of the disorder, which occurs in 1-2% of first degree relatives, but a clear cut family history is unusual.9
Neurobiology of sleep and narcolepsy
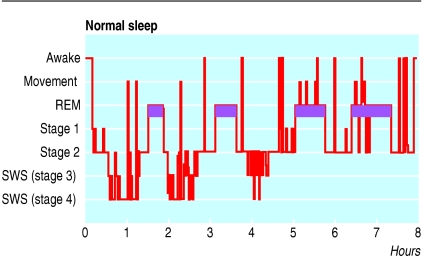
Normal sleep is a structured process.10,11 In the first hour of sleep in adults, brain activity descends through a series of stereotyped stages into “slow wave sleep,” dominated by slow electrical activity in the electroencephalogram. After a period of slow wave sleep, the brain's electrical activity re-ascends through these stages towards a state with electrical appearances similar to those of wakefulness, accompanied by rapid eye movements and profound relaxation of limb muscles; a sleeper woken in this stage of sleep is likely to report a dream. This cycle repeats itself four or five times in the course of the night, with decreasing amounts of slow wave sleep and increasing amounts of rapid eye movement sleep in successive cycles (fig 1).
Fig 1.
Sleep stage recording from a normal polysomnogram. This record shows the normal cyclical alternation of sleep stages over the course of the night. Purple bars indicate rapid eye movement (REM) sleep. Note the decreasing amounts of slow wave sleep (SWS) and increasing amounts of REM sleep over the course of the night
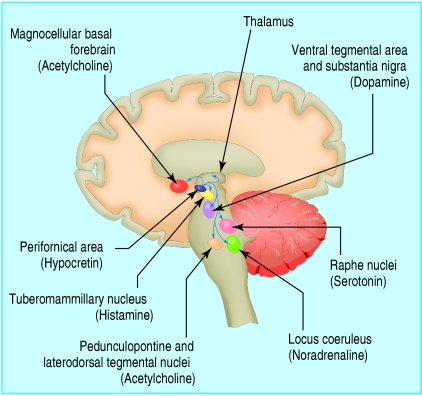
These processes are regulated from the brain stem, thalamus, and basal forebrain by an interacting set of neuronal systems whose neurotransmitters include noradrenaline, serotonin, acetylcholine, histamine, and the recently discovered hypocretins. Figure 2 emphasises the role of the recently discovered hypocretin system.
Fig 2.
Hypocretin system. The sleep-wake cycle is governed by a complex, multilevel neuronal system in the brain stem, thalamus, hypothalamus, and basal forebrain. Neurones in the hypothalamus producing hypocretin stabilise the activity of other key neuronal groups involved in the control of sleep and waking: these nuclei and their principal neurotransmitters are shown here in highly schematic fashion
After the description of the syndrome at the close of the 19th century,12 the scientific understanding of narcolepsy developed in three phases. Firstly, in the 1960s, many of the features of narcolepsy were shown to reflect a dysregulation of rapid eye movement sleep: people with narcolepsy enter rapid eye movement sleep more quickly than usual when they fall asleep (sometimes immediately13); and cataplexy, sleep paralysis, and hypnagogic hallucinations all represent intrusions of phenomena of rapid eye movement sleep (muscular atonia and dream imagery) into wakefulness. Secondly, in the 1980s, workers in Japan discovered that many people with narcolepsy have the tissue type HLA DR2.14 The closest association is with HLA DQB1*0602, present in 95% of narcoleptic patients with cataplexy; it is found in around 40% of patients with narcolepsy without cataplexy and in 18-35% of the general population.15 The importance of the HLA association is uncertain, but it suggests that autoimmunity may have a role in the disorder.
The third phase of discovery began with the discovery in the late 1990s, by two independent groups of researchers, of a novel hypothalamic peptide neurotransmitter, named “orexin” by one group (as it seemed to stimulate appetite in rats) and “hypocretin” by the other group (as it was found in the hypothalamus and resembled secretin).16,17 In 1999 an inherited abnormality in the membrane receptor for one of the two forms of the hypocretin molecule, hypocretin-2, was shown to cause genetically determined canine narcolepsy.18 In 2000 Nishino et al reported that concentrations of hypocretin-1 were markedly reduced in the cerebrospinal fluid of people with narcolepsy associated with cataplexy.19 Subsequent reports have confirmed this finding and shown depletion of hypocretin-1 and hypocretin-2 in brains from patients with narcolepsy examined after death.20,21 In broad terms, among other roles in homeostasis, the hypocretins seem to “stabilise” conscious states and prevent inappropriate switches; their depletion helps to explain both the rapid transitions between wakefulness and rapid eye movement sleep and the tendency for these states to fragment in narcolepsy.22 The relation between narcolepsy with cataplexy and narcolepsy without cataplexy is not yet clear; reduced levels of cerebrospinal fluid hypocretin are found consistently only in the first.23
Box 1: Other major causes of excessive daytime sleepiness
Insufficient sleep at night—diagnostic clues include improvement with extended sleep (at weekends, on holidays)
Obstructive sleep apnoea—clues include snoring, witnessed apnoea
Circadian rhythm disorders—for example, shift work, jet lag, advanced and delayed sleep phase syndrome
Idiopathic hypersomnolence—clues include prolonged unrefreshing nocturnal sleep, long daytime naps
Periodic limb movement disorder—clues include restless legs in the evening, repetitive limb movements in sleep
Depression—clues include low mood, lack of pleasure
Head injury—history should provide clues
Other medical disorders—for example, chronic pain disturbing sleep
Drugs—such as hypnotic drugs, anticonvulsants
Differential diagnosis of excessive daytime sleepiness
The differential diagnosis of excessive daytime sleepiness occurring in isolation is wide.1,3 Narcolepsy is an important cause, although by no means the most common. Box 1 lists the main possibilities and diagnostic pointers. Excessive daytime sleepiness occurring in conjunction with unambiguous cataplexy is almost always due to narcolepsy. Very rarely, narcolepsy can be symptomatic of another underlying disorder of the central nervous system, usually a structural lesion involving the region of the hypothalamus. Additional symptoms and signs of endocrine or neurological disorder are likely to be present. Occasionally, epilepsy can be mistaken for narcolepsy, and factitious simulation of narcolepsy has been described.3 However, failure to recognise narcolepsy, with delayed diagnosis or misdiagnosis, is the more common error. Narcolepsy has been mistaken for epilepsy, chronic fatigue syndrome, and schizophrenia.3
Clinical assessment of excessive daytime sleepiness
History
History taking remains the key to the diagnosis of excessive daytime sleepiness. A brief, structured history should address the questions listed in box 2. The Epworth sleepiness scale (box 3) may be helpful.
Examination
A physical examination is seldom informative. However, obesity and causes of upper airway obstruction may be clues to a diagnosis of obstructive sleep apnoea. Endocrine or neurological signs may point to an underlying neurological cause of sleepiness (other than idiopathic narcolepsy).
Box 2: Taking a sleep history
Is the patient genuinely sleepy or just tired? The Epworth sleepiness scale (box 3) is an extremely helpful instrument for quantifying daytime sleepiness.24 Scores > 11 suggest abnormal sleepiness
Is the patient getting regular and sufficient sleep? Check on bed time, wake time, sleep quality and pattern (for example, shift work)
Does the patient snore heavily or stop breathing during sleep? If so, consider obstructive sleep apnoea
Is there a history of short lived weakness on emotional arousal? If so, consider narcolepsy
Is there a history of uncomfortable, fidgety legs in the evenings? If so, consider restless legs syndrome
Is there evidence of depression (low mood or lack of pleasure in life)? If so, excessive daytime sleepiness might be symptomatic of a mood disorder
Might prescribed or recreational drugs be contributing to sleepiness?
Are there other potentially relevant medical conditions disturbing sleep, such as chronic pain?
Where possible, a history should be taken from the bed partner
Investigations
Investigations need specialist expertise and equipment and should be tailored to the suspected cause of excessive daytime sleepiness. Whether every patient with suspected narcolepsy needs formal evaluation in a sleep laboratory is a matter for debate.3,25 Although no single definitive laboratory test for narcolepsy is available, we believe that gathering objective data at the outset by using sleep tests is wise. These tests should include an assessment of overnight sleep quality, typically with polysomnography, and an evaluation of daytime sleepiness on the following day, using the multiple sleep latency test. This test offers the patient four
Box 3: Epworth sleepiness scale
Patients are asked to rate their level of sleepiness in normal daytime situations. They are asked to grade from 0 to 3 their likelihood of dozing or falling asleep, in contrast to just feeling tired or five opportunities to sleep in conducive circumstances at two hourly intervals through the day, allowing measurement of the latency of sleep and the onset of rapid eye movement sleep. Around 70% of people with narcolepsy have a mean sleep latency of less than eight minutes and two or more sleeps with a rapid eye movement sleep latency of less than 15 minutes. The presence of excessive daytime sleepiness, cataplexy, and a positive multiple sleep latency test allows a definite diagnosis of narcolepsy. The presence of two of these features makes the diagnosis probable.
Grades
0=would never doze
1=slight chance of dozing
2=moderate chance of dozing
3=high chance of dozing
Situations
Sitting and reading
Watching television
Sitting inactive in a public place, such as a theatre or meeting
As a passenger in a car for an hour without a break
Lying down to rest in the afternoon
Sitting and talking to someone
Sitting quietly after lunch (without alcohol)
In a car, while stopped in traffic
A total score > 11 suggests a high probability of a sleep problem, and referral should be considered, after asking relevant questions to exclude other obvious causes such as insufficient sleep or depression
HLA typing and toxicology for amphetamines (in rare cases in which patients are suspected of simulating narcolepsy to obtain a supply) are occasionally helpful. Measurement of hypocretin concentrations in cerebrospinal fluid may become a standard diagnostic tool in narcolepsy, but at present it remains a research technique.23
Management of narcolepsy
General management
Narcolepsy is a lifelong condition with wide ranging implications. Relevant and accurate information should be made available to the patient, relatives, schools, employers, and medical professionals. Narcolepsy is compatible with success both at school and in the work-place, but schools should be encouraged to arrange appropriate schedules for children with narcolepsy, and career choices should take account of the possible hazards caused by excessive daytime sleepiness and cataplexy. The consensus is that regular nocturnal sleep habits and attention to sleep hygiene help to minimise excessive daytime sleepiness, and some evidence shows that planned naps can be used to optimise daytime performance.26 In the United Kingdom, people with narcolepsy are required by law to let the Driver and Vehicle Licensing Authority know about the diagnosis and are generally well advised to refrain from driving until the DVLA has reached a decision on their case. Holders of ordinary group 1 licences will be permitted to drive once “satisfactory control of symptoms” is achieved; people with narcolepsy are generally considered unfit to hold group 2 (heavy goods vehicle and bus) licences, although exceptions can be made.
Drug management
Sleepiness and cataplexy are the symptoms of narcolepsy that most often merit treatment with drugs. The decision to treat should always be guided by a discussion of the anticipated benefits and side effects with the patient.
Sleepiness—Excessive daytime sleepiness is reduced by amphetamine-like stimulants (usually dexamfetamine, which is licensed for this use, or methylphenidate) and the relatively recent “wake promoter” modafinil (also licensed for this use) (table). Little doubt exists about the efficacy of the amphetamine-like drugs, although the formal evidence base for their use is slim, reflecting the early date of their introduction.3 The use of modafinil is supported by up to date randomised controlled trials.3,27 Amphetamine-like drugs and modafinil have not been compared head to head in a randomised controlled trial, nor has combined use of the two classes of drug been formally studied. Advantages of amphetamine-like drugs include long experience, low cost, and possibly an action against cataplexy and higher efficacy; modafinil has the advantage that tolerance does not develop (which can occur with amphetamines) and possibly has a lower rate of side effects. Common side effects of amphetamine-like drugs include irritability and insomnia; modafinil may cause headache, nausea, and rhinitis and may interact with the oral contraceptive pill, necessitating a dose of at least 50 μg oestrogen.
Table 1.
Treatment of daytime sleepiness in narcolepsy
| Drug | Usual total daily dose (mg) | Usual frequency (times/day)* |
|---|---|---|
| Dexamfetamine | 10-60 | 2-4 |
| Methylphenidate | 20-60 | 2 |
| Modafinil | 200-400 | 1-2 |
Routine use of drugs late in the day is inadvisable as it may disturb nocturnal sleep. Adjustment of doses and timings to fit in with individual needs is often helpful.
Box 4: Ongoing research
GHB UK study (further information from Adrian.Williams@gstt.sthames.nhs.uk)
Oxford study of psychosocial aspects of narcolepsy in childhood (data to be presented at ESRS meeting, Prague 2004)
Use of hypocretin in diagnosis
Development of hypocretin related drugs for use in treatment
Cataplexy—Cataplexy is reduced by antidepressant drugs, which suppress rapid eye movement sleep.3 Clomipramine at doses of 30-150 mg/day has been used most widely and is licensed for this indication (up to 75 mg/day), but evidence also exists that fluoxetine, other selective serotonin reuptake inhibitors, and the combined noradrenaline and serotonin reuptake inhibitor venlafaxine are effective.
Better treatments are needed for both excessive daytime sleepiness and cataplexy. The possible use of selegiline merits further study. A trial of gammahydroxybutyrate, which is now licensed in the United States for cataplexy, is under way in the United Kingdom. Hypocretin replacement may prove feasible in the future.
Additional educational resources
Websites
British Sleep Society (www.sleeping.org.uk)—the website of the main British organisation promoting clinical sleep research
Primary Care Sleep Group (www.primarycaresleep.com)—a website for primary care health professionals, providing information about sleep disorders
European Sleep Research Society (www.esrs.org)—the website of a major international sleep research organisation
Stanford Centre for Narcolepsy (www.med.stanford.edu/school/Psychiatry/narcolepsy)—a website providing information about narcolepsy and current research
Patient information resources
Narcolepsy Association UK (www.narcolepsy.org.uk)—website of the main British narcolepsy patient organisation
Narcolepsy Action for Positive Practical Solutions (www.napps.cwc.net)—website of an alternative patient support group
A patient's perspective
I have suffered from narcolepsy for 35 years. Onset was gradual, with attacks of cataplexy at the end of the week, perhaps when I relaxed. As these became more frequent, I began to experience frightening episodes of sleep paralysis on waking, terrifying hypnagogic hallucinations, and daytime sleepiness. Once the diagnosis was made, treatment proved highly effective, but when my medication became temporarily unavailable I was suspended from work as a surgeon for six months. I had to modify my lifestyle considerably after the diagnosis. My sleepiness worsens as the day progresses. Visits to the theatre, cinema, or even parents' evenings are impossible. Concentration on academic activities and boring meetings provokes sleep. Falling off one's chair is guaranteed to halt proceedings. Fortunately, I gained my academic qualifications before the onset of the condition.
Surprisingly, physical and practical work poses no problem: I have never had an attack when driving or operating, but 10 minutes after stopping such activity is a fraught period. My major problem now is cataplexy associated with emotion, usually laughter.
Close friends and relatives recognise prodromal signs of altered speech and convulsive jaw movements and support me if there is nothing convenient for me to lean on. Sleep paralysis and hallucinations have stopped with age.
Referral
Patients with suspected narcolepsy should be referred to a sleep disorders service, or its closest local equivalent, for diagnosis. Once the diagnosis is made, patients should have at least an annual review by a clinician who is knowledgeable about the disorder and its evolving treatment. The fascinating area of sleep disorders medicine needs considerable development in the United Kingdom. Box 4 shows areas of ongoing research.
We thank John Towers for his help, our colleagues for their comments, and Taylor Patten Communications for editorial support.
Contributors: All authors contributed to the literature review and the writing of the guidelines on which this clinical review is based. AZ drafted this article, which was reviewed and edited by the guidelines working party. AZ is the guarantor.
Funding: The creation of the guidelines was funded by an unrestricted educational grant to Taylor Patten Communications by Cephalon UK, the manufacturers of modafinil.
Competing interests: Taylor Patten Communications paid travel expenses and honoraria to the working party members. ND received partial support for attendance at an international meeting on sleep apnoea from Cephalon and has had research on modafinil in sleep apnoea funded by Cephalon. JH has received travel grants from Cephalon. Cephalon has sponsored AM to attend conferences; he was runner-up in the Hospital Doctor sleep unit of the year award sponsored by Cephalon. IS has received travel grants and speakers' honoraria from Cephalon. SW received an honorarium from Cephalon for speaking to their sales force. Cephalon has supported ZZ to attend a sleep disorder conference and part funded a technician in her department to attend a conference. AZ is supported by the Health Foundation.
References
- 1.Guilleminault C, Brooks SN. Excessive daytime sleepiness. Brain 2001;124: 1482-91. [DOI] [PubMed] [Google Scholar]
- 2.Stores G, Crawford C. Medical student education in sleep and its disorders. J R Coll Physicians Lond 1998;32: 149-53. [PMC free article] [PubMed] [Google Scholar]
- 3.Britton T, Douglas N, Hansen A, Hicks J, Howard R, Meredith A, et al. Guidelines on the diagnosis and management of narcolepsy in adults and children. Ashtead: Taylor Patten Communications, 2002 (available at www.sleeping.org.uk or www.primarycaresleep.com).
- 4.Overeem S, Mignot E, van Dijk JG, Lammers GJ. Narcolepsy: clinical features, new pathophysiologic insights and future perspectives. J Clin Neurophysiol 2001;18: 78-105. [DOI] [PubMed] [Google Scholar]
- 5.Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res 2001;10: 75-81. [DOI] [PubMed] [Google Scholar]
- 6.Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikila K, Koskimies K, et al. The prevalence of narcolepsy: an epidemiological study of the Finnish twin cohort. Ann Neurol 1994;35: 709-16. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM, Priest RG, Caulet M, Guilleminault C. Hypnagogic and hypnopompic hallucinations: pathological phenomena? Br J Psychiatry 1996;169: 459-67. [DOI] [PubMed] [Google Scholar]
- 8.Silber MH, Krahn LE, Pankratz VS. The epidemiology of narcolepsy in Olmstead County, Minnesota: a population-based study. Sleep 2002;25: 197-202. [DOI] [PubMed] [Google Scholar]
- 9.Mignot E. Genetic and familial aspects of narcolepsy. Neurology 1998;50(suppl 1): 516-22. [DOI] [PubMed] [Google Scholar]
- 10.Pace-Schott EF, Hobson JA. The neurobiology of sleep. Nat Rev Neurosci 2002;3: 591-605. [DOI] [PubMed] [Google Scholar]
- 11.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep. Nat Rev Neurosci 2002;3: 670-93. [DOI] [PubMed] [Google Scholar]
- 12.Gelineau JBE. De la narcolepsie. Gazette des hopitaux de Paris 1880: 626-8, 635-7.
- 13.Rechtschaffen A, Wolpert E, Dement WC, Mitchell SA, Fisher C. Nocturnal sleep of narcoleptics. Electroencephalogr Clin Neurophysiol 1963;15: 599-609. [DOI] [PubMed] [Google Scholar]
- 14.Juji T, Satake M, Honda Y, Doi Y. HLA antigens in Japanese patients with narcolepsy: all the patients were DR2 positive. Tissue Antigens 1984;24: 316-9. [DOI] [PubMed] [Google Scholar]
- 15.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep 1997;20: 1012-20. [PubMed] [Google Scholar]
- 16.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behaviour. Cell 1998;92: 573-85. [DOI] [PubMed] [Google Scholar]
- 17.De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 1998;95: 322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999;98: 365-76. [DOI] [PubMed] [Google Scholar]
- 19.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy [letter]. Lancet 2000;355: 39-40. [DOI] [PubMed] [Google Scholar]
- 20.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalised absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000;6: 991-7. [DOI] [PubMed] [Google Scholar]
- 21.Thannickal TC, Moore RY, Nienhuls R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced numbers of hypocretin neurons in human narcolepsy. Neuron 2000;27: 469-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scammell T. The neurobiology, diagnosis and treatment of narcolepsy. Ann Neurol 2003;53: 154-66. [DOI] [PubMed] [Google Scholar]
- 23.Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 2002;59: 1553-62. [DOI] [PubMed] [Google Scholar]
- 24.Johns MJ. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;6: 540-5. [DOI] [PubMed] [Google Scholar]
- 25.Standard of Practice Committee, American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy. Sleep 2001;24: 451-66. [PubMed] [Google Scholar]
- 26.Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001;24: 385-91. [DOI] [PubMed] [Google Scholar]
- 27.US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology 2000;54: 1166-75. [DOI] [PubMed] [Google Scholar]