This study examined the prevalence of and factors contributing to cancer-related fatigue (CRF) among cancer patients in China. The results suggest that effective management of sleep disturbance and physical exercise (the two changeable contributing factors of CRF) may reduce CRF and thus could be used as references for CRF management.
Keywords: Cancer-related fatigue, Prevalence, Associated factors
Abstract
Background.
Cancer-related fatigue (CRF) is a subjective and distressing symptom, and its associated factors in developing countries remain ambiguous. The goal of this study was to determine the prevalence of and factors associated with CRF among cancer patients in China.
Methods.
This study was designed as a cross-sectional study to determine the prevalence of and factors associated with CRF among cancer patients in eastern China, regardless of their diagnoses. Data were collected by using a questionnaire survey (including demographic information and brief fatigue inventory) after informed written consent was obtained. A chi-square test was used to analyze the correlations between single categorical factors and CRF, and multiple logistic regression analysis was used to evaluate the associations of potential risk factors with the presence of CRF.
Results.
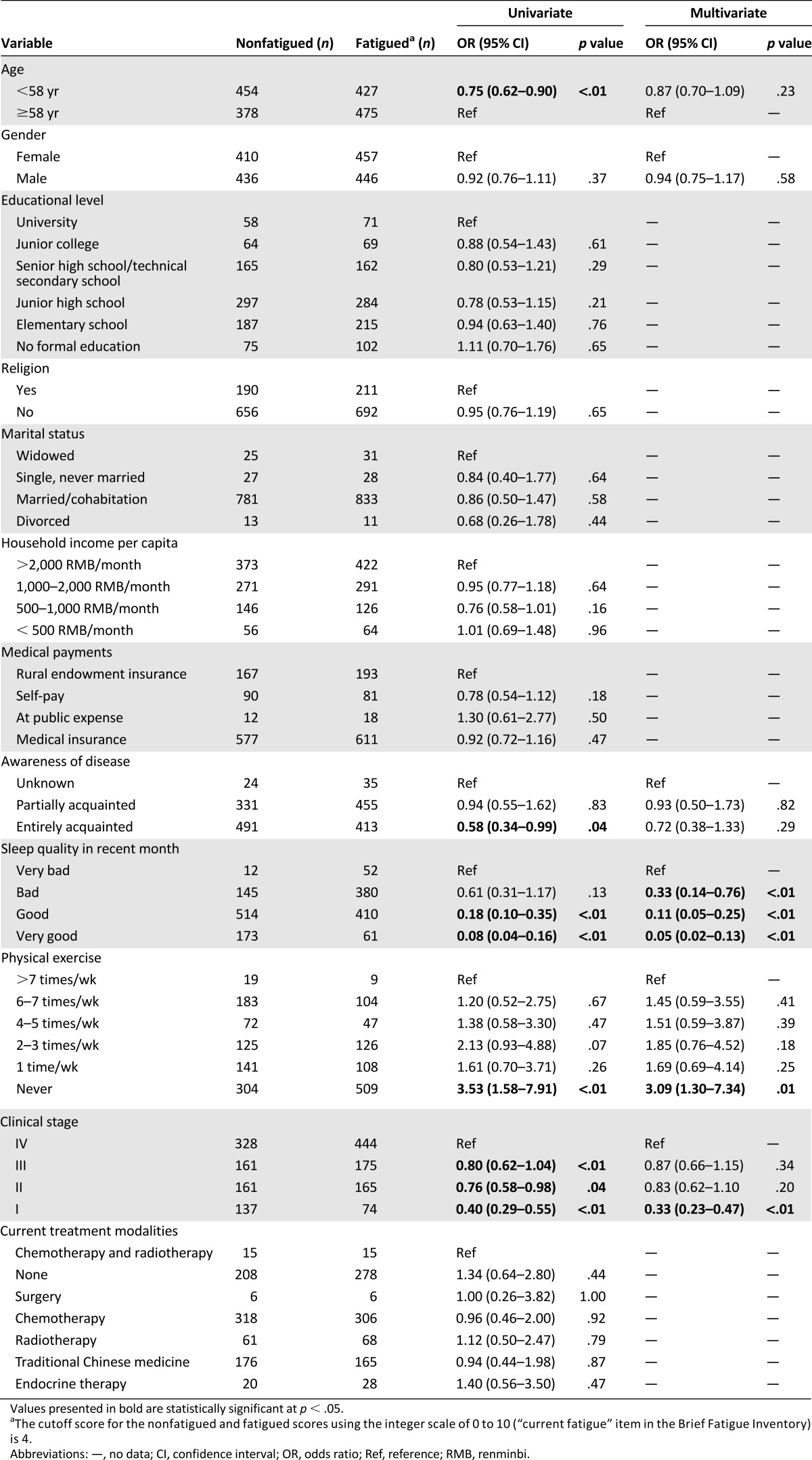
Out of a total population of 1,938 cancer patients, 1,749 had completed the study questionnaire; 52.07% (n = 904) reported clinically significant fatigue (score ≥4 on Brief Fatigue Inventory). Four hundred twenty-seven (48.47%) patients younger than age 58 years (the median age) and 475 (55.69%) patients age 58 years or older reported clinically significant fatigue. In multivariate analysis, higher sleep quality (p < .01) was negatively associated with CRF, whereas never engaging in physical exercise (p < .01) and higher clinical stage of cancer (p < .01) were positively associated factors that could increase the odds of CRF.
Conclusion.
The results of this study suggest that effective management of the two changeable contributing factors of CRF may reduce CRF and thus could be used as references for CRF management.
Implications for Practice:
The two modifiable factors of cancer-related fatigue (CRF)—sleep disturbance and physical exercise—should be specifically assessed and managed to mitigate CRF.
Abstract
摘要
背景. 癌症相关疲乏(CRF)是一种令人痛苦的主观症状, 其在发展中国家的相关因素仍不明确。本研究旨在确定CRF在中国癌症患者中的流行情况及相关因素。
方法. 本研究为横断面研究, 旨在确定CRF在中国华东地区癌症患者(无论其诊断)中的流行情况和相关因素。在患者签署知情同意书后, 使用问卷调查(包括人口统计学信息和简明疲乏量表)收集数据。使用卡方检验分析单个分类因素与CRF之间的相关性, 使用多元logistic回归分析评价潜在危险因素与发生CRF的相关性。
结果. 共纳入1 938例癌症患者, 其中1 749例完成了研究问卷调查, 52.07%(n=904)的患者报告了具有临床意义的疲乏(简明疲乏量表评分≥4)。427例(48.47%)<58岁(中位年龄)的患者和475例(55.69%)≥58岁的患者报告了有临床意义的疲乏。多变量分析显示睡眠质量较好与CRF呈负相关(P<0.01), 而从未参与体育锻炼(P<0.01)、癌症临床分期较高(P<0.01)是可以增加CRF发生风险的正相关因素。
结论. 本研究结果提示对2个可改变的CRF促进因素进行有效管理可能减少CRF, 因此可作为CRF管理的指标。The Oncologist 2016;21:1349–1354
对临床实践的提示: 应对癌症相关疲乏(CRF)的两个可改善因素(睡眠障碍及体育锻炼)进行专门评估和管理以缓解CRF。
Introduction
The occurrence of cancer has increased recently, and developing countries account for approximately 57% of cancer cases worldwide [1]. With the continued development of cancer diagnostic and therapeutic technologies, patient survival duration has been significantly extended. However, improvements in the quality of life of cancer patients have fallen short of expectations because of cancer-related fatigue (CRF), one of the most common cancer-related symptoms [2]. CRF can also be a barrier to cancer survivors’ return to work, thus imposing an enormous burden on society [3]. Therefore, it is necessary to identify and minimize the factors contributing to CRF.
CRF is a subjective and distressing symptom experienced by nearly all cancer patients [4, 5]; it does not result from activity or exertion and is not relieved by sleep or rest [6]. Spichiger et al. [7] examined 103 hospitalized patients with advanced cancer and found that younger patients and patients with lower functional status, depression, or anemia experienced greater fatigue. In addition, CRF is also correlated with a high body mass index [8, 9] and white blood cell count [8], increased limb volume [8], low levels of physical activity [8–11], higher tumor grade [9, 12–15], and sleep disturbances [12, 14]. Because significant discrepancies exist among these studies (e.g., some studies show associations between sociodemographic data and fatigue [7, 12], whereas others indicate the opposite [9, 13]) and because most of this research (except for two studies) has been conducted in developed countries, the factors associated with CRF in developing countries remain ambiguous. Therefore, additional relevant research is warranted.
The purpose of this study was to examine the prevalence of and factors contributing to CRF among cancer patients in China and to provide a basis for future CRF management.
Materials and Methods
Ethical Considerations
Ethical clearance was obtained from the Research Ethics Committee of the School of Nursing, Fudan University, Shanghai, China. Participants were explicitly informed that the data produced in the survey would be confidential, would not affect their treatment, and would be used only for academic research purposes. Written informed consent was obtained from all participants, who were informed that participation was voluntary and that they could withdraw at any time during the survey.
Study Design and Sampling Frame
This study used a cross-sectional design. Convenience sampling of cancer patients from general hospitals in Shanghai, Suzhou, Wuxi, and Nantong was conducted simultaneously from October 2013 to June 2014. The inclusion criteria included the following: (a) a pathology- or cytology-based diagnosis, regardless of cancer type and treatment; (b) age ≥18 years; (c) no cognitive impairment; and (d) a willingness to participate, as specified by the written consent form. The sample size was calculated on the basis of results from a pilot investigation by using estimation methods for a cross-sectional survey (i.e., n = tɑ2 [P(1 − P)/δ2], where n is sample size, tɑ is the confidence level of the study findings, P is the estimating prevalence, and δ is the maximal likely error that can be tolerated. The occurrence rate of clinically significant fatigue was 24% [16], and the two-tailed α level was set at 0.05; the admissible error δ was set at 0.1π; therefore, n = 1.962(0.24[1 − 0.24])/(0.1 × 0.24)2 = 1,266. Considering a 20% nonresponse rate, a minimum of 1,583 patients were required for the study.
Data Collection
Data were collected by using a questionnaire survey after informed written consent was obtained. The researchers instructed patients on how to fill out the questionnaire, and the patients then did so accordingly.
The questionnaire consisted of the following two parts.
Demographic information
Personal characteristics (including age, gender, ethnicity, educational level, religion, marital status, household income per capita, medical insurance, sleep quality, awareness of disease, and physical activity) and disease-related information (including disease diagnosis, diagnosis time, clinical stage, and current treatment) were collected in this part. The personal characteristics were supplied by the patients themselves, and the disease information was obtained from medical records. Sleep quality in the past month and awareness of disease were self-reported (very bad/bad/good/very good and unknown/partially acquainted/entirely acquainted, respectively). Aerobic exercise, resistance exercise/muscle strengthening, and stretching exercise were considered as the forms of physical activity, and the intensity, duration, and frequency of the exercise were assessed by the patients’ self-report.
Brief Fatigue Inventory
The Brief Fatigue Inventory (BFI) was initially developed by Mendoza et al. [17], and the instrument used in this study was the Chinese version [18]. It includes nine items, with the first three assessing the “now,” “usual,” and “worst” levels of fatigue during the past 24 hours. Fatigue severity was evaluated by using an integer scale of 0 (no fatigue) to 10 (fatigue as bad as you can imagine), which was recommended by National Comprehensive Cancer Network (NCCN) guidelines for screening and re-evaluation of cancer related fatigue, with the cutoff score for clinically significant fatigue being 4 [2]. The internal consistency reliabilities (Cronbach α) of the first three items in the Chinese version is 0.92 [18]. The remaining six items assess the extent to which fatigue has interfered with daily life during the past 24 hours in terms of general activity, mood, walking ability, normal work, relationships with other people, and enjoyment of life. The internal consistency reliabilities (Cronbach α) of the remaining six items in the Chinese version is 0.90 [18]. This study primarily explored fatigue severity using the first three items of the BFI.
Statistical Analysis
The data were analyzed by using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com). The prevalence of CRF was estimated for the overall population and for each age and sex group. Multiple logistic regression models were developed with CRF as the dichotomous dependent variable and relevant predictors as covariates. If the p value was <.10 in univariate models, these possible predictors were included in multiple logistic regression models. All probabilities quoted were two-sided and were considered statistically significant at p < .05.
Results
Patient Characteristics
In this study, 1,938 patients participated in the survey, 1,749 effective questionnaires were collected, and 189 questionnaires were not included because the key clinical data (such as pathological or cytological data for diagnosis) were missing in the medical records of these patients. Of all participants, 99.54% were of the Han nationality. The distributions of sociodemographic and disease characteristics of the participants are shown in Table 1.
Table 1.
Sociodemographic characteristics of the participants
Prevalence of Cancer-Related Fatigue
Of 1,749 patients, 904 (52.07%) experienced clinically significant fatigue (≥4). Four hundred fifty-seven female patients (52.71%), 446 male patients (50.57%), 427 patients younger than age 58 years (the median age) (48.47%), and 475 patients aged ≥58 years (55.69%) reported clinically significant fatigue (Table 2).
Table 2.
Univariate analyses of risk factors of cancer-related fatigue
Factors Associated With CRF Prevalence
Table 2 shows the univariate and multivariate associations of cancer-related fatigue in the study population. In univariate analysis, increasing age, lower awareness of disease, poor sleep quality, the absence of physical exercise, and higher clinical stage of cancer were significantly associated with a higher prevalence of cancer-related fatigue (p < .05). Other factors, such as gender, education level, religion, marital status, household income per capita, medical payments, and current treatment modalities, were not significantly associated with the prevalence of cancer-related fatigue. In multivariate analysis, low sleep quality (p < .01), never performing physical exercise (p < .01), and higher clinical stage (p < .01) were significantly associated with an increasing prevalence of cancer-related fatigue.
Discussion
In this survey, 52.07% of 1,749 cancer patients experienced clinically significant fatigue. Disease- or treatment-specific factors and sociodemographic factors were included in the unconditional logistic regression analysis, yet only sleep quality, physical exercise, and clinical stage of cancer were correlated with CRF. Meanwhile, other factors, such as awareness of one’s disease, household income per capita, medical payments, current treatment modalities, and marital status, showed no correlation with CRF.
Among all cancer patients, 30%–75% experience sleep/wake disturbances [19], which can clearly aggravate CRF [20]. In this survey, sleep quality showed a significant association with clinically significant fatigue, in accord with previous research [12, 14]. In addition, good or very good sleep quality can alleviate CRF to some extent, whereas there was no significant difference between bad and very bad sleep quality. Therefore, sufficient attention should be paid to the management of sleep/wake disturbances.
Haas [21] reported that physical activity levels were associated with CRF, and lower levels of physical activity lead to CRF [8–11]. In this study, no particular exercise frequency was found to exacerbate CRF when compared with other frequencies of physical exercise. However, there was no statistically significant difference among the five exercise frequencies, except between two to three times per week and six to seven times per week (odds ratio, 1.77; 95% confidence interval, 1.26–2.50). In this survey, the most common mode of physical exercise reported by the participants was home-based walking at a tolerated speed without any exercise prescription, and the exercise duration, intensity, and frequency were all reported by the patients themselves with no professional supervision, which may partially explain this result. The optimal frequency and intensity of exercise should be discussed with medical professionals [22].
The preceding two factors were modifiable factors associated with CRF; a less controllable factor, clinical stage, also significantly influenced CRF. In addition, a higher clinical stage correlated with a greater level of CRF.
Kim et al. [23] found that employment and low income were also associated with fatigue; however, in this study, this association was not statistically significant. Furthermore, there was no association between educational level or marital status and CRF.
Our findings were based on a relatively large study sample, and many possible influencing factors were included in the statistical analysis, such as sociodemographic factors and disease- and treatment-specific factors. However, in the chemotherapy regimen analysis, the result was not very clear because of the small sample size for each treatment; the result should be confirmed by increasing the sample size in future studies. The inclusion criteria might cause selection bias because only patients who were willing to participate were included. Thus, the results of this study may not universally apply to all cancer patients during this period. In this study, we used BFI as a screening tool, an instrument that may not be as accurate as criteria based on the International Classification of Diseases, 10th revision. We assessed sleep quality, exercise, and awareness mainly on the basis of the patients’ self-report. In addition to these factors included in our study, other cancer-related symptoms (e.g., pain [24], depression and anxiety [25], and nausea [10]) and objective markers (e.g., increased total white cell count [26], lower sodium [27], lower hemoglobin [<10 g/dL] [27], and higher levels of C-reactive protein [28]) were significantly correlated with CRF. However, the current study did not include these factors, which can be further explored in future studies.
Conclusion
Two negatively associated factors (sleep quality and physical exercise) and one positively associated factor (higher clinical stage of cancer) were determined to be factors influencing CRF prevalence. The results of this study suggest that effective management of the two modifiable contributing factors of CRF (sleep disturbance and physical exercise) may be conducive to CRF management.
Acknowledgments
We express our heartfelt gratitude to Nantong Tumor Hospital and to the data collectors and study participants. This study would not have been possible without their contributions. This study was supported by Health-X Seed Funding (China Medical Board Grant 13-131) from the Global Health Institute, Fudan University.
Footnotes
For Further Reading: Elisabeth C.W. Neefjes, Maurice J.D.L. van der Vorst et al. Aiming for a Better Understanding and Management of Cancer-Related Fatigue. The Oncologist 2013;18:1135–1143.
Implications for Practice: Cancer-related fatigue (CRF) is a common problem in patients with cancer and has a major impact on quality of life. The causes of CRF are multifactorial and not fully understood. To get a better insight into the underlying mechanisms and the potential treatment possibilities of CRF, we provide an overview of currently available literature on this subject. Because current treatment options other than antitumor therapy for some of the patients are scarce and only directed at symptoms, further investigation of CRF is warranted to develop rational treatment options.
Author Contributions
Conception/Design: Li Tian, Yan Hu
Provision of study material or patients: Li Tian, Hui L. Li, Xiao J. Zhang, Shu J. Qian
Collection and/or assembly of data: Li Tian, Lu Lin, Hui L. Li, Ke J. Chen
Data analysis and interpretation: Li Tian, Lu Lin, Ke J. Chen, Yan Hu
Manuscript writing: Li Tian, Lu Lin, Yan Hu
Final approval of manuscript: Yan Hu
Disclosures
The authors indicated no financial relationships.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Berger AM, Abernethy AP, Atkinson A, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 3.Islam T, Dahlui M, Majid HA, et al. Factors associated with return to work of breast cancer survivors: A systematic review. BMC Public Health. 2014;14(suppl 3):S8. doi: 10.1186/1471-2458-14-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horneber M, Fischer I, Dimeo F, et al. Cancer-related fatigue: Epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109:161–171; quiz 172. doi: 10.3238/arztebl.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell SA, Beck SL, Hood LE, et al. Putting evidence into practice: Evidence-based interventions for fatigue during and following cancer and its treatment. Clin J Oncol Nurs. 2007;11:99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- 6.Narayanan V, Koshy C. Fatigue in cancer: A review of literature. Indian J Palliat Care. 2009;15:19–25. doi: 10.4103/0973-1075.53507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spichiger E, Müller-Fröhlich C, Denhaerynck K, et al. Prevalence and contributors to fatigue in individuals hospitalized with advanced cancer: A prospective, observational study. Int J Nurs Stud. 2012;49:1146–1154. doi: 10.1016/j.ijnurstu.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Gerber LH, Stout N, McGarvey C, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer. 2011;19:1581–1591. doi: 10.1007/s00520-010-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz SL, Dalton SO, Christensen J, et al. Factors correlated with fatigue in breast cancer survivors undergoing a rehabilitation course, Denmark, 2002-2005. Psychooncology. 2011;20:352–360. doi: 10.1002/pon.1739. [DOI] [PubMed] [Google Scholar]
- 10.Purcell A, Fleming J, Bennett S, et al. A multidimensional examination of correlates of fatigue during radiotherapy. Cancer. 2010;116:529–537. doi: 10.1002/cncr.24731. [DOI] [PubMed] [Google Scholar]
- 11.Seo Y, Oh H, Seo W. Causal relationships among factors associated with cancer-related fatigue. Eur J Oncol Nurs. 2010;14:380–386. doi: 10.1016/j.ejon.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Banthia R, Malcarne VL, Ko CM, et al. Fatigued breast cancer survivors: The role of sleep quality, depressed mood, stage and age. Psychol Health. 2009;24:965–980. doi: 10.1080/08870440802110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein D, Bennett BK, Webber K, et al. Cancer-related fatigue in women with breast cancer: Outcomes of a 5-year prospective cohort study. J Clin Oncol. 2012;30:1805–1812. doi: 10.1200/JCO.2011.34.6148. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Zhang Q, Kang X, et al. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: A cross-sectional study. BMC Cancer. 2010;10:453. doi: 10.1186/1471-2407-10-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundh Hagelin C, Wengström Y, Fürst CJ. Patterns of fatigue related to advanced disease and radiotherapy in patients with cancer-a comparative cross-sectional study of fatigue intensity and characteristics. Support Care Cancer. 2009;17:519–526. doi: 10.1007/s00520-008-0502-5. [DOI] [PubMed] [Google Scholar]
- 16.Temel JS, Pirl WF, Recklitis CJ, et al. Feasibility and validity of a one-item fatigue screen in a thoracic oncology clinic. J Thorac Oncol. 2006;1:454–459. [PubMed] [Google Scholar]
- 17.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Wang XS, Hao XS, Wang Y, et al. Validation study of the Chinese version of the Brief Fatigue Inventory (BFI-C) J Pain Symptom Manage. 2004;27:322–332. doi: 10.1016/j.jpainsymman.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Berger AM, Parker KP, Young-McCaughan S, et al. Sleep wake disturbances in people with cancer and their caregivers: State of the science. Oncol Nurs Forum. 2005;32:E98–E126. doi: 10.1188/05.ONF.E98-E126. [DOI] [PubMed] [Google Scholar]
- 20.Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. The Oncologist. 2007;12(suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 21.Haas BK. Fatigue, self-efficacy, physical activity, and quality of life in women with breast cancer. Cancer Nurs. 2011;34:322–334. doi: 10.1097/NCC.0b013e3181f9a300. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson D, Diorio C, Beyene J, et al. Effect of exercise on cancer-related fatigue: A meta-analysis. Am J Phys Med Rehabil. 2014;93:675–686. doi: 10.1097/PHM.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Son BH, Hwang SY, et al. Fatigue and depression in disease-free breast cancer survivors: Prevalence, correlates, and association with quality of life. J Pain Symptom Manage. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: Covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29:333–337. doi: 10.1037/a0018836. [J] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50:440–447. doi: 10.1176/appi.psy.50.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexander S, Minton O, Andrews P, et al. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romito F, Montanaro R, Corvasce C, et al. Is cancer-related fatigue more strongly correlated to haematological or to psychological factors in cancer patients? Support Care Cancer. 2008;16:943–946. doi: 10.1007/s00520-007-0357-1. [DOI] [PubMed] [Google Scholar]
- 28.Pertl MM, Hevey D, Boyle NT, et al. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–119. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]