To study the frequency with which targeted tumor sequencing results will lead to implemented change in care, this study assessed tumors from 100 patients for utility, feasibility, and limitations of genomic sequencing for genomically guided therapy or other clinical purpose in the setting of a multidisciplinary molecular tumor board. Comprehensive profiling led to implementable clinical action in 35% of tumors with genomic alterations.
Keywords: Molecular sequencing, Cancer, Tumor genomics, Molecular targeted therapy, Mutation
Abstract
Background.
The frequency with which targeted tumor sequencing results will lead to implemented change in care is unclear. Prospective assessment of the feasibility and limitations of using genomic sequencing is critically important.
Methods.
A prospective clinical study was conducted on 100 patients with diverse-histology, rare, or poor-prognosis cancers to evaluate the clinical actionability of a Clinical Laboratory Improvement Amendments (CLIA)-certified, comprehensive genomic profiling assay (FoundationOne), using formalin-fixed, paraffin-embedded tumors. The primary objectives were to assess utility, feasibility, and limitations of genomic sequencing for genomically guided therapy or other clinical purpose in the setting of a multidisciplinary molecular tumor board.
Results.
Of the tumors from the 92 patients with sufficient tissue, 88 (96%) had at least one genomic alteration (average 3.6, range 0–10). Commonly altered pathways included p53 (46%), RAS/RAF/MAPK (rat sarcoma; rapidly accelerated fibrosarcoma; mitogen-activated protein kinase) (45%), receptor tyrosine kinases/ligand (44%), PI3K/AKT/mTOR (phosphatidylinositol-4,5-bisphosphate 3-kinase; protein kinase B; mammalian target of rapamycin) (35%), transcription factors/regulators (31%), and cell cycle regulators (30%). Many low frequency but potentially actionable alterations were identified in diverse histologies. Use of comprehensive profiling led to implementable clinical action in 35% of tumors with genomic alterations, including genomically guided therapy, diagnostic modification, and trigger for germline genetic testing.
Conclusion.
Use of targeted next-generation sequencing in the setting of an institutional molecular tumor board led to implementable clinical action in more than one third of patients with rare and poor-prognosis cancers. Major barriers to implementation of genomically guided therapy were clinical status of the patient and drug access. Early and serial sequencing in the clinical course and expanded access to genomically guided early-phase clinical trials and targeted agents may increase actionability.
Implications for Practice:
Identification of key factors that facilitate use of genomic tumor testing results and implementation of genomically guided therapy may lead to enhanced benefit for patients with rare or difficult to treat cancers. Clinical use of a targeted next-generation sequencing assay in the setting of an institutional molecular tumor board led to implementable clinical action in over one third of patients with rare and poor prognosis cancers. The major barriers to implementation of genomically guided therapy were clinical status of the patient and drug access both on trial and off label. Approaches to increase actionability include early and serial sequencing in the clinical course and expanded access to genomically guided early phase clinical trials and targeted agents.
Introduction
Significant advances in sequencing technologies, “synthetic lethal” strategies to identify targets for loss of function in tumor suppressors, and pharmacologic innovations have increased both our understanding of the spectrum of alterations present in human cancers and development of therapeutics [1–5]. Although an ever-growing spectrum of genomic alterations is now therapeutically targetable, the clinical actionability of genomic testing is not known.
Widespread sequencing efforts demonstrate that most targetable genomic alterations are not malignancy specific, cannot be predicted by histology alone, are observed in “target-silent” tumors, and are useful as predictive or prognostic biomarkers [2, 6–8]. Furthermore, there is a need to identify mechanisms and alternative therapeutic strategies after development of drug resistance [9–13]. Genomic profiling may help circumvent these inherent clinical challenges by identifying drivers responsible for survival advantage and drug resistance. It also offers an attractive approach for rare tumors, given that insufficient numbers of patients are available to complete clinical trials, and establishment of guidelines for best clinical practice remains elusive.
Despite these advantages, the benefits and limitations of genomic sequencing in clinical practice requires prospective assessment. Several studies demonstrate that it is possible to obtain high-quality sequencing data from formalin-fixed, paraffin-embedded (FFPE) tissues [14, 15]. However, it remains unclear how often such genome characterization will actually lead to implemented, useful clinical interventions.
To understand the feasibility of clinical-grade genomic sequencing, we implemented a prospective, comprehensive genomic profiling protocol with an institutional, multidisciplinary molecular tumor board. Clinical action based on genomic profiling results was possible in 31% of patients in this study. Results suggest that this approach is feasible, has clinical actionability, and defines potential barriers to broader implementation.
Methods
Study Participants
One hundred patients with rare or refractory tumors, evaluated at the Rutgers Cancer Institute of New Jersey, provided informed consent for a prospective trial for point-of-care tumor genomic profiling from April 2013 to December 2013 (supplemental online Fig. 1). Relevant clinical history, pathology, and genomic profiling results were reviewed at a formal molecular tumor board (MTB). Profiling results were discussed in the context of clinical course, tumor type, mutational frequency, role in cancer biology/behavior, preplanned therapy, and considerations for genomically guided therapy. Based on consensus recommendations, those with actionable alterations were referred for clinical trials, US Food and Drug Administration (FDA)-approved therapies (on- or off-label), and genetic counseling, if appropriate, and were followed for clinical course. The MTB did not provide recommendations for nontargeted therapies, and the therapeutic approach was ultimately the treating physician’s choice. The protocol was approved by the Institutional Review Board of Rutgers Robert Wood Johnson Medical School.
Comprehensive Genomic Profiling
FFPE tissue was sent to Foundation Medicine (Cambridge, MA, https://www.foundationmedicine.com/) for next-generation sequencing using the Clinical Laboratory Improvement Amendments (CLIA)-certified FoundationOne platform [15]. Targeted sequencing of the entire coding sequence was done for 236 genes and 47 introns of 19 genes involved in fusions at a depth of ×500 to ×1,000. Alterations included point mutations, deletions, amplifications, duplications, insertions, rearrangements, and splice variants that were identified as known or likely pathogenic changes, as reported by the FoundationOne assay. Variants of unknown significance (VUS) and synonymous single-nucleotide polymorphisms (SNPs) are not included in tables depicting alterations in tumors. Calculations for “per tumor alterations” excluded VUS and SNPs.
Genomic Profile Clustering
For unsupervised clustering, each of the 92 samples had at least one detected genomic alteration representing only 118 of the possible 236 genes evaluated. A bidirectional, agglomerative hierarchical clustering with Jaccard’s distance measure was performed. Choice of distance measure was motivated by the sparseness of the data but also allowed analysis to ignore a type and a number of genomic alterations within a gene or a pathway for a given sample. Data were treated as binary (0 = no alteration, 1 = alteration). The clustering and visualization were done with a modified version of “heatmap.2” from the R package “gplots” (R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/foundation/) [16]; the corresponding dissimilarity structure was produced by “dist” with binary distance measure.
Results
Genomic Spectrum Reflects Both Intertumoral and Tumor Type–Specific Commonalities
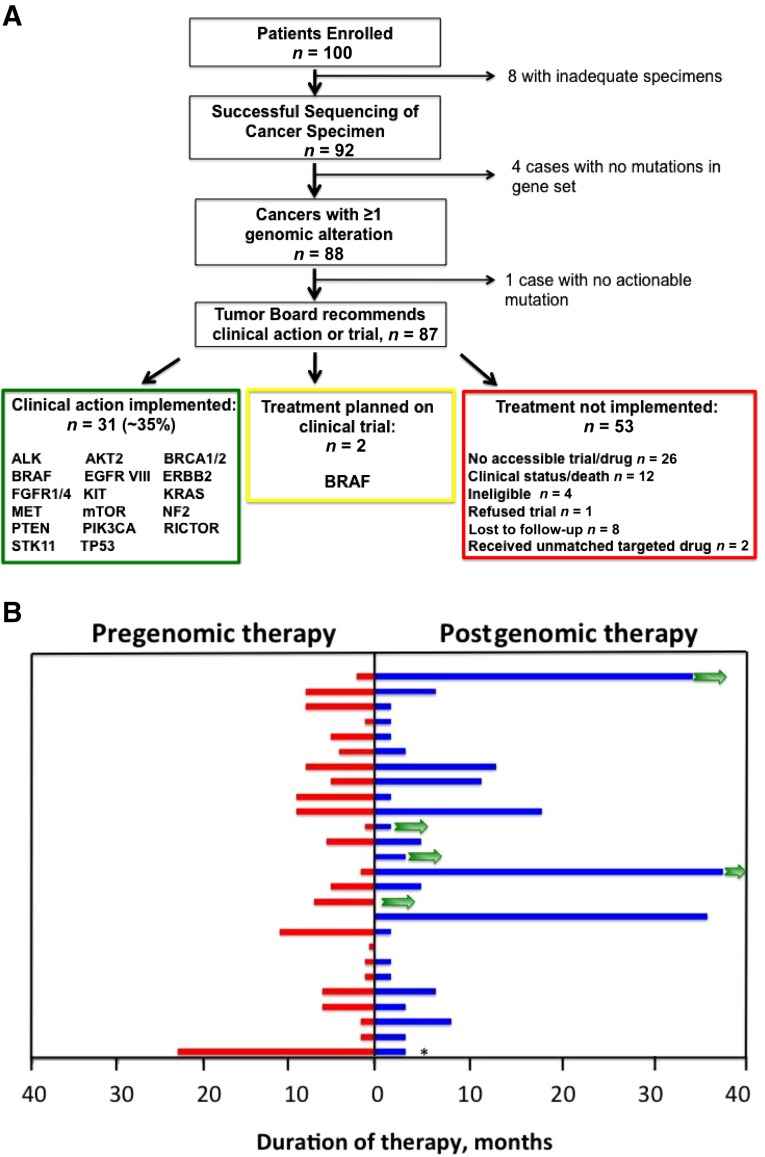
Four pediatric and 96 adult patients with rare histology or refractory cancers underwent tumor genomic profiling for a varied spectrum of epithelial and mesenchymal origin tumors (supplemental online Figs. 1, 2; supplemental online Table 1). At enrollment, 91% of patients were stage IV and had received a mean of 2.5 prior therapies. Sixty-five percent of patients were alive with disease at a year or more after testing, with a mean overall survival of 129 days after enrollment. Of the 100 patients enrolled, eight did not have genomic profiling because of inadequate tissue amount, poor tissue/DNA quality, or the patient came off study. Ninety-two patients had successful genomic analysis, with 88 (96%) having at least one genomic alteration; the average number of alterations per tumor was 3.6 (range 0–10) (Fig. 1A), with urothelial and endometrial cancers displaying the highest mutational burden (supplemental online Fig. 2).
Figure 1.
Tumor genomic profiling consort diagram and patient outcomes. (A): Comprehensive genomic profiling of tumors from 100 patients with rare or refractory cancers. Time from genomic testing to generation of formal recommendations averaged 3–4 weeks. Clinical action included enrollment in a therapeutic clinical trial, FDA-approved therapy, off-label use of approved therapy, discontinuation of ineffective targeted therapy, germline mutation testing, and diagnostic reclassification. Genes with alterations for which clinical action was implemented are listed in the respective categories. Two patients are being screened for participation in clinical trial with targeted therapy. Two patients with action implemented had targeted therapy prescribed but were then lost to follow-up. (B): Duration of therapy in individual patients before genomic profiling and with targeted therapy (not significant). Comparisons are for nontargeted therapy before genomic profiling (red) and duration of targeted therapy after genomic profiling (blue). Green arrows indicate patients with ongoing therapy. Last patient was on combined therapy with targeted agent, which was discontinued (∗) at 5 months for side effects. Patient has ongoing response with monotherapy.
Abbreviation: FDA, U.S. Food and Drug Administration.
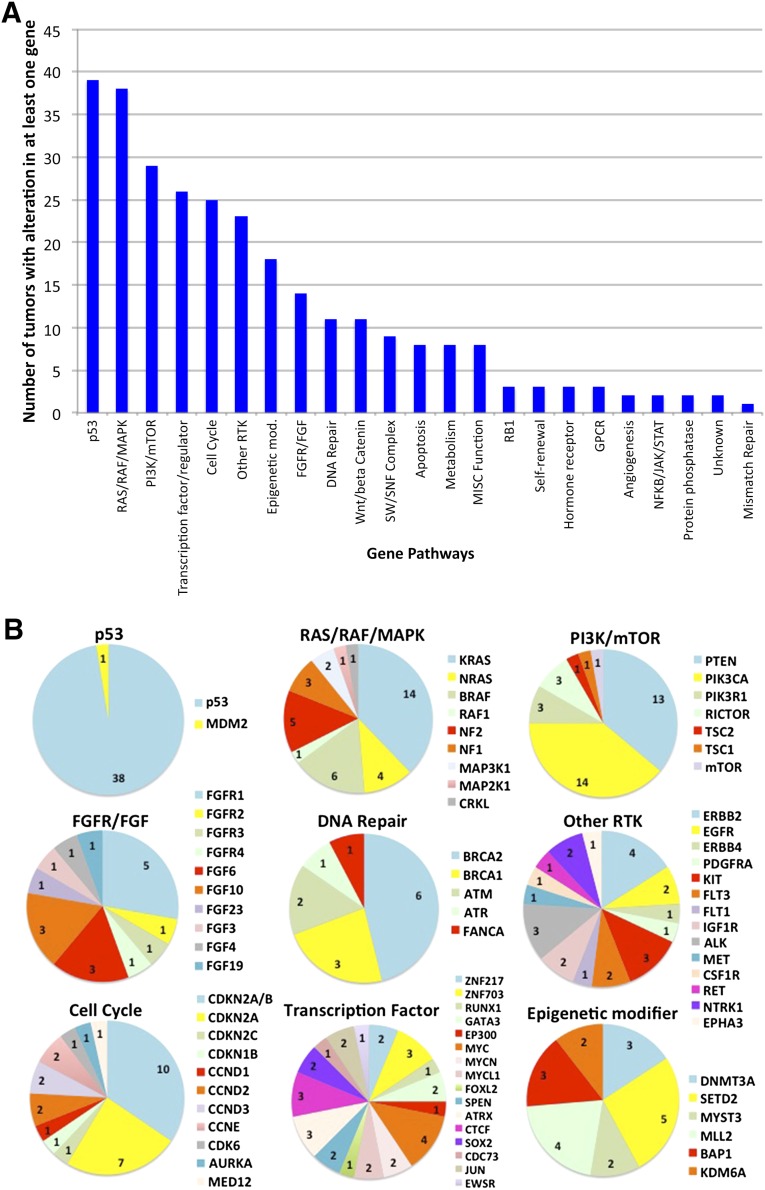
The most commonly altered genes included TP53 (41%), CDKN2A/B (cyclin-dependent kinase inhibitor 2A/B) (22%), KRAS (16%), PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit a) (15%), PTEN (phosphatase and tensin homolog) (14%), and BRAF (7%). There was also a large set of low-frequency but actionable changes in tyrosine kinase genes (ALK [anaplastic lymphoma kinase], KIT [stem cell growth factor receptor], ERBB2 [erb-B2 receptor tyrosine kinase 2], FGFR4 [fibroblast growth factor receptor 4], KDR [vascular endothelial growth factor receptor 2], MET [hepatocyte growth factor receptor], NTRK1 [neurotrophic tyrosine kinase receptor 1], PDGFR [platelet derived growth factor receptor]) and tumor suppressor genes (BRCA1, BRCA2 [breast cancer type 1, breast cancer type 2]). The panel of gene alterations was sorted into functional pathways (supplemental online Tables 2, 3), including the most common: p53 (46%), RAS/RAF/MAPK (45%), PI3K/AKT/mTOR (35%), transcription factors (31%), cell cycle (30%), and receptor tyrosine kinases (RTKs) (27%; excluded fibroblast growth factor [FGF] pathway alterations) (Fig. 2A). Functional pathways, such as p53, RAS/RAF/MAPK, PI3K/AKT/mTOR, cell cycle, FGFR/FGF, Wnt (wingless-related integration site)/β-catenin, and DNA repair have profiles in which one or more altered genes are enriched (Fig. 2B). In contrast, transcription factors and other RTKs have a wider spectrum of affected genes but may reflect tissue specificity based on tumor type. Furthermore, some tumor subtypes correlated with a higher probability of harboring specific alterations (e.g., BRAF-altered papillary thyroid cancers).
Figure 2.
Mutational landscape identified in 92 different tumors based on functional pathways. (A): Types of alterations and number of alterations were reflective of tumor subtypes profiled. (B): For 9 of the 10 most frequent pathways, the frequency of genes with alterations within those pathways occurring is depicted. Wnt/β-catenin is not shown but had only two genes represented: APC (adenomatous polyposis coli) (n = 8) and MSH6 (MutS Homolog 6) (n = 1). Numbers represent the frequency of alterations affecting that gene.
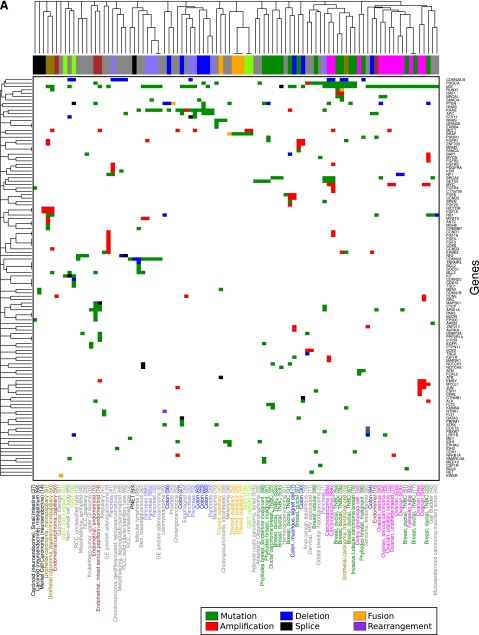
As tumor subtype may reflect mutational landscape and this study profiled diverse tumor types, unsupervised clustering of tumors with at least one genomic alteration was performed based on gene (Fig. 3A) and pathway (Fig. 3B) alterations. Notably, some tumor subtypes clustered more closely together than tumors of differing subtypes. Serial specimens clustered more closely to prior specimens than to similar tumors from other patients, suggesting preservation of specific patterns of mutations. Triple-negative breast cancers clustered closely with ovarian cancers (primarily high-grade serous), confirming prior data that these tumors may have similar underlying biology. Pathway clustering demonstrated that, despite diversity in specific mutations, certain pathways are commonly altered in diverse tumors (e.g., MAPK, PI3K), and these paired pathway anomalies are relevant for future design of multidrug approaches.
Figure 3.
Hierarchical clustering of tumors with at least one genomic alteration by gene (A) and functional pathway (B). Tumor subtypes are represented in colored text below heat map and with corresponding boxes above heat map. In (A), box color (rows) within heat map depicts alteration in gene by type of genomic alteration; in (B), it depicts alteration in genes in functional pathway by type of genomic alteration. Alteration key: red, amplification; green, mutation; blue, deletion; purple, rearrangement; black, splice; orange, fusion; white, no alteration. The remaining colors are multiple alteration subtypes. Numbers in parentheses represent specimen number, and a, b, and c represent serial specimens.
Genomic Profiling Alters Clinical Management
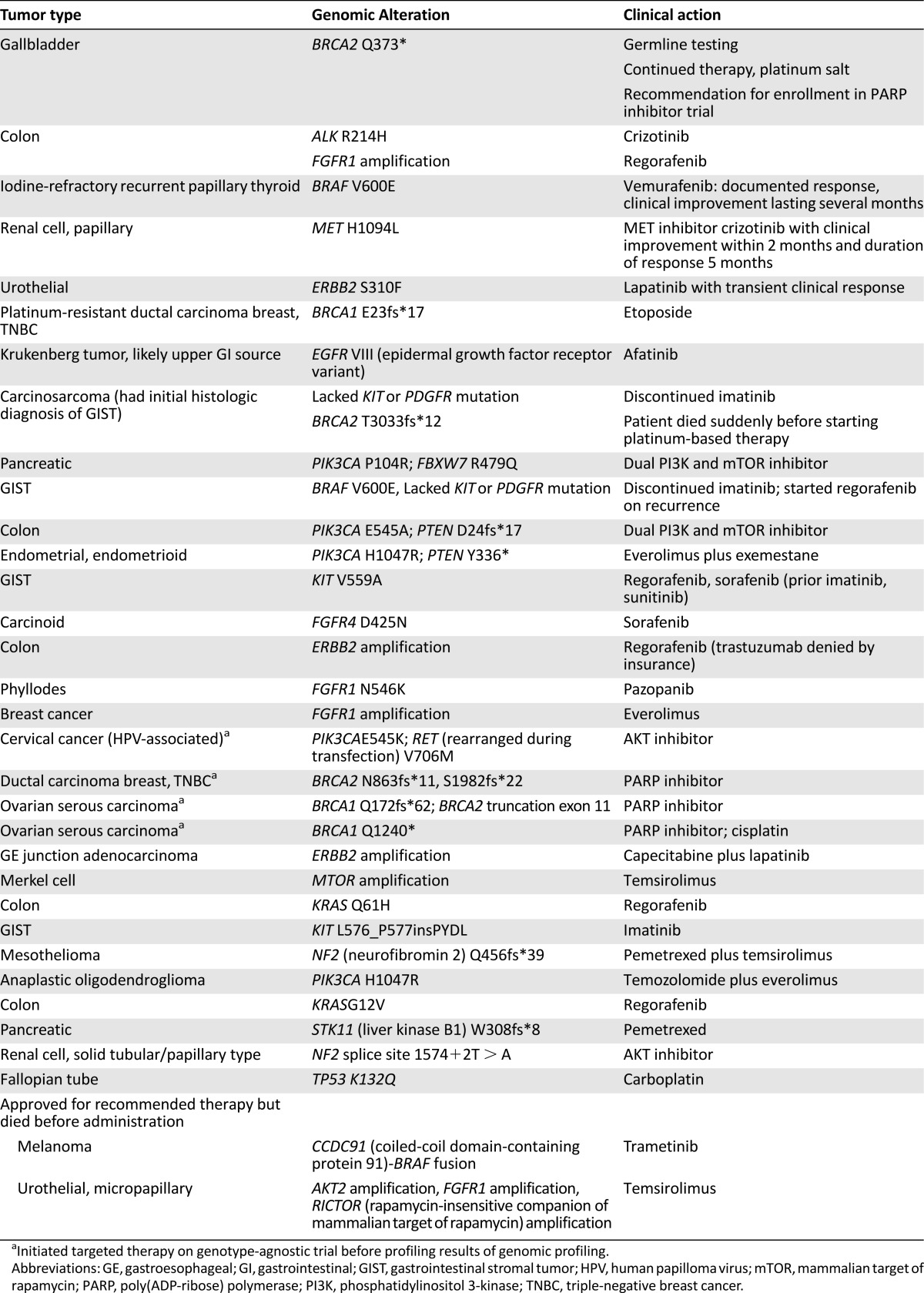
The majority of tumors had alterations for which the MTB had recommended action (Fig. 1A). Of the 88 cases with identified alterations, 87 had clinically relevant alterations. The critical question was, how many of these patients with potentially actionable genomic alterations identified actually had a change in clinical management based on sequencing results? Thirty-one patients had implementation of genomically guided therapy. Another two patients are under screening for genomically guided clinical trials. Thus, 35% of patients with actionable alterations, and ∼31% of study patients overall, received genomically guided therapy.
Management decisions based on genomic results (Table 1) included referral to clinical trials, use of FDA-approved drugs, and off-label use of targeted therapy when clinical trials were unavailable (e.g., vemurafenib for BRAF V600E-mutated papillary thyroid cancer, lapatinib for ERBB2-mutated urothelial cancer, platinum for BRCA1/2 mutations). Examples of clinical responses included an intestinal carcinoid with FGFR4 mutation with resolution of bulky mediastinal lymphadenopathy and stability of retroperitoneal lymph nodes with sorafenib for 23 months; and an ongoing, 21-month stable response to everolimus in a heavily pretreated male breast cancer with 8p11 amplification. A patient with metastatic papillary renal cell carcinoma with MET H1094L had objective clinical improvement within 2 months of initiating crizotinib and is described elsewhere [17].
Table 1.
Genomic alterations for which clinical action was implemented.
For patients receiving genomically guided therapy, the mean number of treatments implemented postsequencing was 1.5, whereas patients who did not receive targeted therapy despite target identification had a mean of only 0.3 treatments (p = .0001). Of patients who received targeted therapy, duration on targeted therapy was 7.6 months (Fig. 1B) compared with 4.2 months for patients not receiving any targeted therapy (unpublished data, p = .07). However, this includes patients who did not receive any therapy postsequencing. For those patients who were able to receive any nontargeted therapy, duration on nontargeted therapy was 6.1 months. This was not statistically different from the duration for those who received targeted therapy. One patient was on combined therapy including a targeted agent, but the latter had to be discontinued at 5 months because of significant side effects (Fig. 1B). The patient has ongoing response with nontargeted monotherapy. Within the group receiving postgenomic, nontargeted therapy, two patients had a 24-month duration of this therapy, both for the treatment of breast cancer. Of these, one had a triple-negative breast cancer with a BRCA2 variant, for which she received a platinum-based regimen on clinical trial. The second patient had a hormonally responsive breast cancer and was receiving a combination of anastrozole plus fulvestrant as second-line therapy in the metastatic setting. Inclusion of these patients substantially increased the duration on nontargeted therapy, from 4.8 months to 6.1 months. Therefore, the ability to document increased duration of postgenomic therapy compared with pregenomic therapy is highly dependent on tumor type as well as availability of effective standard and targeted therapies for specific tumor types. As there are currently five patients with ongoing treatment with targeted therapy, the mean duration on genomically guided therapy would be expected to increase with continued follow-up.
Fifty-three patients with potentially actionable genomic changes did not receive genomically guided therapy primarily because of lack of availability of a clinical trial or access to an FDA-approved drug (n = 26). An additional 12 patients did not receive treatment because of deteriorating clinical status. The remaining patients were ineligible for clinical trials because of comorbid conditions (n = 4), refused trial participation (n = 1), received nonmatched targeted drugs (n = 2), or were lost to follow-up (n = 8).
Tumor Genomic Profiling Augments the Cancer Diagnostic Armamentarium
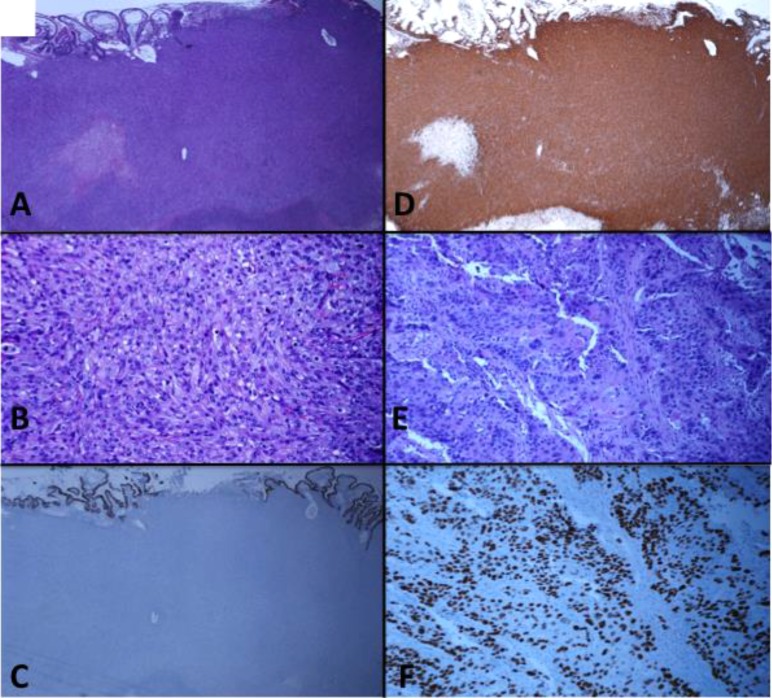
Several patients with synchronous, histology-discordant tumors at different sites had genomic analysis of all sites. A diagnostically challenging referral of a synchronous, small bowel tumor and pelvic mass with unique histologic appearance initially led to diagnosis of atypical gastrointestinal stromal tumor and ovarian squamous cell carcinoma, respectively, despite noticeably absent CD117 expression (Fig. 4). The former represented a high-grade sarcoma (Figs. 4A–4D), whereas the ovarian mass showed squamous cell carcinoma (Figs. 4E, 4F). Genomic profiling at both sites revealed identical alterations in six genes (supplemental online Table 2). Of six alterations private to the pelvic mass, four occurred within an amplicon containing the putative driver gene, CCND1 (cyclin D1). The spectrum of alterations was highly suggestive of both arising from the same Müllerian precursor, displaying both sarcomatous and carcinomatous components indicative of carcinosarcoma.
Figure 4.
Genomic profiling aids in the diagnostic analysis of two anatomically distinct tumors from a single patient and ultimately suggests that tumors arose from the same precursor. Abdominal mass (A–D) shows a high-grade, vimentin-positive sarcoma with predominant epithelioid appearance and focal necrosis. (A, B): hematoxylin and eosin, low and high power, respectively; (C, D): low power, pancytokeratin and vimentin, respectively. The ovarian mass (E, F) shows a poorly differentiated, p40-positive squamous cell carcinoma. (E): Hematoxylin and eosin, high power. (F): p40, low power. CD117 expression was absent (data not shown).
An additional patient initially presented with a pancreatic adenocarcinoma and a synchronous mucinous lung cancer that was believed to be a second unrelated primary. Sequencing of both tumor specimens showed identical mutations in KRAS and TP53, again suggesting that these arose from pancreatic adenocarcinoma as a common precursor. Had this information been known at diagnosis, the patient may have been spared surgical resection.
Serial Genomic Sequencing Demonstrates Molecular Evolution
Nine patients had analysis of serial samples at different time points, with a mean concordance rate of 59% (range, 0%–100%) (supplemental online Table 2). For example, a patient with metastatic, triple-negative breast cancer had analysis of three specimens: primary site and brain metastasis, both of which responded to platinum-based chemotherapy, and recurrent, platinum-resistant cancer that progressed locally (supplemental online Fig. 3). All specimens from this patient had a core set of mutations (i.e., BRCA1, known germline), TP53, PIK3CA, and RUNX1-ATLN (runt-related transcription factor 1; atlastin GTPase 1) fusion. Both the brain metastasis and recurrent tumor had additional private mutations, suggesting evolution from a common precursor. Concordance was higher between the two breast sites (80%) than with the brain specimen (50%). Notably, the brain metastasis carried an ERBB2 amplification not observed in either breast specimen. No reversion mutation/internal deletion was noted in BRCA1, despite this known mechanism of resistance for poly(ADP-ribose) polymerase and platinum agents [18–20]. A complex genomic event cannot be ruled out. Thus, serial analysis of specimens lends insight into tumor evolution and heterogeneity. This finding also underscores the importance of analyzing recurrent metastatic disease that progressed after initial response to targeted agents, as potentially actionable targets may be gained or lost as a result of tumor drift or molecular evolution, particularly under selection pressure.
Tumor Sequencing Can Lead to Germline Testing
Genomic sequencing may raise the possibility of germline mutation testing and is disclosed to participants during consent. At the MTB, allele frequency and tumor purity are routinely analyzed when the possibility of germline variants is raised. In this cohort, previously unknown alterations in BRCA1/2 were suggested by tumor sequencing in four patients. In two of these patients with BRCA2 mutations, further testing showed no evidence of a germline BRCA2 mutation. Two other patients were confirmed to have germline BRCA1/2 mutations and received genetic counseling.
Known alterations in BRCA1 or BRCA2 were confirmed in four additional cases without evidence of reversion mutations. One patient with an activating MET mutation in a papillary renal cell cancer was also informed of the possibility of germline mutation. Overall, 10% of tumors from this cohort exhibited alterations in known cancer susceptibility genes. Germline testing was recommended based on tumor genomic profiling and resulted in identifying three individuals with no prior knowledge of their carrier status.
Tumor genomic profiling without matched normal tissue has the potential to mistakenly identify germline variants as somatic tumor mutations. Recent reports have suggested that paired normal/tumor tissue analysis is necessary to eliminate false-positive calls of germline variants [21, 22]. In this study, bioinformatics analysis using allele frequency, estimated tumor purity, and ploidy was used to flag potential germline variants [23]. Of 317 variants identified as pathogenic or likely pathogenic, 29 were flagged as potentially germline (supplemental online Table 4). Of these, nine variants (e.g., in BRCA1/2) were clearly identified as likely germline at the MTB discussion and referred to genetic counseling. Several variants (in RB and TP53) were eliminated as germline variants on further manual analysis. The remaining potential germline variants result in a potential false-positive rate of 5.7% (18 of 317). Analysis of measured allele frequency, tumor purity, and read depth data by our MTB allows identification of many of these potential germline variants and is now routinely done. As such, our MTB recommends genetic testing for identified potential germline variants when placed in the context of tumor type, personal history, and family history.
Discussion
Advances in sequencing technologies have led to the ability to perform clinical-grade, high-depth sequencing of cancer gene panels to identify clinically relevant tumor genomic alterations. To determine its clinical actionability, we implemented a comprehensive genomic profiling protocol with an integrated institutional, multidisciplinary MTB. We successfully performed genomic profiling in 92% of rare or poor-prognosis cancers from patients who had exhausted standard therapies, and this testing led to actual change in clinical management in 31% of patients. Importantly, our study also identified recurrent and unsuspected targetable genomic alterations, consistent with findings by other groups [24–33]. Some differences between studies may reflect the spectrum of frequency of tumor types as well as methodology and gene coverage in sequencing panels.
The main limitation for genomic profiling not leading to clinical action was lack of drug access/availability of clinical trials, similar to a recent report [31]. In some cases, clinical trials were available but included only a specific tumor subtype, suggesting a need to significantly increase access through eligibility. However, mutational profiles should be placed in context of the biology of cell/tissue origin, as exemplified by differences in response to vemurafenib for BRAF V600E mutant melanoma versus BRAF V600E mutant colorectal cancer. BRAF V600E remains a genomic target and driver in colon cancer; however, to effectively target it in colon cancer, feedback pathways mediating activation of EGFR and rapid resistance have to be addressed [26, 34–36]. Multigene predictors of response for targeted therapy may need to be considered [37].
Clinical deterioration in 12% of patients precluded enrollment on clinical trials, which strongly supports tumor sequencing earlier in the disease course to maximize the window of benefit from genomically guided therapy. Our data show that repeat biopsies upon tumor progression, especially after therapies that may induce significant selection pressure, are critical to identify tumor evolution and mechanisms of resistance [38–44]. Thus, there is a need to do genomic analysis “early and often” in the course of treatment.
Although many validated drivers commonly found in diverse tumor types do not have targeted therapy available even in early-phase trials, much ongoing drug development may identify approaches for these alterations that could reach the clinic in the future and increase eligibility for genomically guided therapy. During the course of this study, clinical trials of IDH inhibitors became available, rendering IDH1/2 (isocitrate dehydrogenase 1/2) alterations actionable [45]. Moreover, characterization of novel gene alterations found in individual tumors is vital, given that specific alterations may confer differential protein functionality or drug sensitivity, similar to our novel BRAF fusion identified in a melanoma that may be resistant to currently available BRAF inhibitors [46].
Target actionability reflects drug availability in current clinical trials [31, 32]. A recent report demonstrated that use of an 11- to 50-gene hotspot mutation panel led to an 11% rate of enrollment onto genotype-guided trials, the majority of which were driven by alterations in PIK3CA/AKT1/PTEN/BRAF [31]. Our study used a panel targeting a larger range of genomic events and was integrated with an MTB. This may have led to the higher actionability rate, although similar targets were identified. Our study confirms that clinical-grade genomic analysis is feasible and can guide clinical decision-making in a subset of patients. Similar obstacles to implementation of genomically guided therapy were identified, including lack of trials, poor clinical status, and lack of drug access. There is a tradeoff between breadth and depth of sequencing by current assays [47], and it is not yet clear what the optimal approach and platform is for tumor genomic analysis. Expectations are that with further advances, it will be possible to perform more comprehensive sequencing in greater depth. Improvements in target-drug matching and efficacy of newer drugs may lead to superior outcomes on targeted therapy.
A critical component of this protocol was implementation of an integrated, institutional MTB. The MTB brings together a multidisciplinary group of professionals to discuss optimal, genomically guided clinical options for each patient, a vital point as the landscape of specific alterations as predictive markers continues to evolve. For example, PIK3CA and PTEN alterations were initially thought to be potential markers for response to mTOR inhibitors, but were recently shown not to be the case in some settings [48]. MTB affords clinicians an avenue for better understanding of assay strengths/limitations, result interpretation/applicability, and clinical implementation, which may then translate into greater uptake of genomic testing and improved physician-patient communication [49, 50]. The systematic multispecialty overview at the MTB also enhances successful drug acquisition for off-label use of the rapidly evolving spectrum of targeted agents, especially important for rare tumors lacking established standards of care.
Although this study highlights the potential of point-of-care tumor sequencing, the ultimate question is whether it will lead to improved survival and/or quality of life for cancer patients. This approach can only lead to improved patient outcome if partnered with development of novel targeted agents, expanded trial portfolios, and access to clinical trials. Further advances in directed use of single or combinatorial targeted therapy will be needed to translate promise of tumor genomic characterization into improved patient outcomes.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank the patients; Natasha Nicholson and Frances Di Clemente for administrative support for the Molecular Tumor Board; and services provided by the Biospecimen Repository Service and the Office for Human Research Service. This work was supported by Hugs for Brady, The Val Skinner Foundation, National Institutes of Health Grant P30CA072720, and a generous gift to the Genetics Diagnostics to Cancer Treatment Program of the Rutgers Cancer Institute of New Jersey and Rutgers University Cell and DNA Repository Infinite Biologics.
Author Contributions
Conception/Design: Kim M. Hirshfield, Robert S. DiPaola, Lorna Rodriguez-Rodriguez, Shridar Ganesan
Provision of study material or patients: Kim M. Hirshfield, Mark N. Stein, Susan Murphy, Hetal Vig, John Glod, Rebecca A. Moss, Lauri Goodell, Howard Kaufman, Elizabeth Poplin, Janice Mehnert, Antoinette R. Tan, Joseph R. Bertino, Joseph Aisner, Robert S. DiPaola, Lorna Rodriguez-Rodriguez, Shridar Ganesan
Collection and/or assembly of data: Kim M. Hirshfield, Denis Tolkunov, Hua Zhong, Siraj M. Ali, Mark N. Stein, Susan Murphy, Hetal Vig, David Foran, Roman Yelensky, Norma A. Palma, James X. Sun, Vincent A. Miller, Philip J. Stephens, Jeffrey S. Ross, Lorna Rodriguez-Rodriguez, Shridar Ganesan
Data analysis and interpretation: Kim M. Hirshfield, Denis Tolkunov, Hua Zhong, Siraj M. Ali, Mark N. Stein, Susan Murphy, Hetal Vig, Alexei Vazquez, John Glod, Rebecca A. Moss, Vladimir Belyi, Chang S. Chan, Suzie Chen, Lauri Goodell, David Foran, Roman Yelensky, Norma A. Palma, James X. Sun, Vincent A. Miller, Philip J. Stephens, Jeffrey S. Ross, Howard Kaufman, Elizabeth Poplin, Janice Mehnert, Antoinette R. Tan, Joseph R. Bertino, Joseph Aisner, Robert S. DiPaola, Lorna Rodriguez-Rodriguez, Shridar Ganesan
Manuscript writing: Kim M. Hirshfield, Denis Tolkunov, Hua Zhong, Siraj M. Ali, Mark N. Stein, Susan Murphy, Hetal Vig, Alexei Vazquez, John Glod, Rebecca A. Moss, Vladimir Belyi, Chang S. Chan, Suzie Chen, Lauri Goodell, David Foran, Roman Yelensky, Norma A. Palma, James X. Sun, Vincent A. Miller, Philip J. Stephens, Jeffrey S. Ross, Howard Kaufman, Elizabeth Poplin, Janice Mehnert, Antoinette R. Tan, Joseph R. Bertino, Joseph Aisner, Robert S. DiPaola, Lorna Rodriguez-Rodriguez, Shridar Ganesan
Final approval of manuscript: Kim M. Hirshfield, Denis Tolkunov, Hua Zhong, Siraj M. Ali, Mark N. Stein, Susan Murphy, Hetal Vig, Alexei Vazquez, John Glod, Rebecca A. Moss, Vladimir Belyi, Chang S. Chan, Suzie Chen, Lauri Goodell, David Foran, Roman Yelensky, Norma A. Palma, James X. Sun, Vincent A. Miller, Philip J. Stephens, Jeffrey S. Ross, Howard Kaufman, Elizabeth Poplin, Janice Mehnert, Antoinette R. Tan, Joseph R. Bertino, Joseph Aisner, Robert S. DiPaola, Lorna Rodriguez-Rodriguez, Shridar Ganesan
Disclosures
Siraj M. Ali: Foundation Medicine (E, OI, IP); Mark N. Stein: Jansen, Astellas, Medivation, Pfizer, Merck, Oncoceutics (RF); Rebecca A. Moss: Bristol-Myers Squibb (E, OI); Lauri Goodell: Johnson & Johnson (OI); Roman Yelensky: Foundation Medicine (E, OI); Norma A. Palma: Foundation Medicine (E); James X. Sun: Foundation Medicine (E, OI); Vincent A. Miller: Foundation Medicine (E, OI); Philip J. Stephens: Foundation Medicine (E, OI); Jeffrey S. Ross: Foundation Medicine (RF, E, OI); Howard Kaufman: Amgen, EMD Serono, Merck, Prometheus, Sanofi (C/A), Merck (H); Joseph Aisner: Bristol-Myers Squibb, Merck-Serona (C/A); Shridar Ganesan: Novartis, Inspirata Inc. (C/A), Inspirata Inc. (OI, IP). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 5.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Morrison C, Wang L, et al. Identification of somatic mutations in non-small cell lung carcinomas using whole-exome sequencing. Carcinogenesis. 2012;33:1270–1276. doi: 10.1093/carcin/bgs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett MT, Lenkiewicz E, Evers L, et al. Clonal evolution and therapeutic resistance in solid tumors. Front Pharmacol. 2013;4:2. doi: 10.3389/fphar.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H, Moriceau G, Kong X, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cepero V, Sierra JR, Corso S, et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 2010;70:7580–7590. doi: 10.1158/0008-5472.CAN-10-0436. [DOI] [PubMed] [Google Scholar]
- 13.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warnes GR. gplots: Various R Programming Tools for Plotting Data. Available at https://cran.r-project.org/web/packages/gplots/index.html. Accessed July 25, 2016.
- 17.Stein MN, Hirshfield KM, Zhong H, et al. Response to crizotinib in a patient with MET-mutant papillary renal cell cancer after progression on tivantinib. Eur Urol. 2015;67:353–354. doi: 10.1016/j.eururo.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai W, Swisher EM, Jacquemont C, et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69:6381–6386. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7:1353–1359. doi: 10.4161/cc.7.10.5930. [DOI] [PubMed] [Google Scholar]
- 21.Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med. 2015;7:283ra53. doi: 10.1126/scitranslmed.aaa7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Frampton G, Wang K, et al. A computational method for somatic vs. germ-line variant status determination from targeted next-generation sequencing of clinical cancer specimens without a matched normal control. Cancer Res. 2014;74(suppl 19):1893a. [Google Scholar]
- 24.André F, Bachelot T, Commo F, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: A multicentre, prospective trial (SAFIR01/UNICANCER) Lancet Oncol. 2014;15:267–274. doi: 10.1016/S1470-2045(13)70611-9. [DOI] [PubMed] [Google Scholar]
- 25.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herter-Sprie GS, Greulich H, Wong KK. Activating mutations in ERBB2 and their impact on diagnostics and treatment. Front Oncol. 2013;3:86. doi: 10.3389/fonc.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balschun K, Haag J, Wenke AK, et al. KRAS, NRAS, PIK3CA exon 20, and BRAF genotypes in synchronous and metachronous primary colorectal cancers: Diagnostic and therapeutic implications. J Mol Diagn. 2011;13:436–445. doi: 10.1016/j.jmoldx.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lechner M, Frampton GM, Fenton T, et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors. Genome Med. 2013;5:49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirshfield KM, Aisner J, Ali SM, et al. Targeted treatment of RAI-resistant metastatic thyroid cancer postcomprehensive genomic profiling. J Clin Oncol. 2014;32(suppl):e22070a. [Google Scholar]
- 31.Meric-Bernstam F, Brusco L, Shaw K, et al. Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J Clin Oncol. 2015;33:2753–2762. doi: 10.1200/JCO.2014.60.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boland GM, Piha-Paul SA, Subbiah V, et al. Clinical next generation sequencing to identify actionable aberrations in a phase I program. Oncotarget. 2015;6:20099–20110. doi: 10.18632/oncotarget.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beltran H, Eng K, Mosquera JM, et al. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 2015;1:466–474. doi: 10.1001/jamaoncol.2015.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 35.Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21:1313–1320. doi: 10.1158/1078-0432.CCR-14-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabchy A, Eltonsy N, Housman DE, et al. Systematic identification of combinatorial drivers and targets in cancer cell lines. PLoS One. 2013;8:e60339. doi: 10.1371/journal.pone.0060339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balko JM, Giltnane JM, Wang K, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meric-Bernstam F, Frampton GM, Ferrer-Lozano J, et al. Concordance of genomic alterations between primary and recurrent breast cancer. Mol Cancer Ther. 2014;13:1382–1389. doi: 10.1158/1535-7163.MCT-13-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Axelrod M, Gordon VL, Conaway M, et al. Combinatorial drug screening identifies compensatory pathway interactions and adaptive resistance mechanisms. Oncotarget. 2013;4:622–635. doi: 10.18632/oncotarget.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abel EV, Basile KJ, Kugel CH, 3rd, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest. 2013;123:2155–2168. doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vignot S, Frampton GM, Soria JC, et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol. 2013;31:2167–2172. doi: 10.1200/JCO.2012.47.7737. [DOI] [PubMed] [Google Scholar]
- 44.Peeters M, Oliner KS, Parker A, et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res. 2013;19:1902–1912. doi: 10.1158/1078-0432.CCR-12-1913. [DOI] [PubMed] [Google Scholar]
- 45.Stein E, Tallman M, Pollyea DA, et al. Clinical safety and activity in a phase I trial of AG-221, a first in class, potent inhibitor of the IDH2-mutant protein, in patients with IDH2 mutant positive advanced hematologic malignancies. Cancer Res. 2014;74(suppl 19):CT103a. [Google Scholar]
- 46.Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci USA. 2013;110:5957–5962. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sims D, Sudbery I, Ilott NE, et al. Sequencing depth and coverage: Key considerations in genomic analyses. Nat Rev Genet. 2014;15:121–132. doi: 10.1038/nrg3642. [DOI] [PubMed] [Google Scholar]
- 48.Janku F, Hong DS, Fu S, et al. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Reports. 2014;6:377–387. doi: 10.1016/j.celrep.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller FA, Hayeems RZ, Bytautas JP, et al. Testing personalized medicine: Patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. Eur J Hum Genet. 2014;22:391–395. doi: 10.1038/ejhg.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray SW, Hicks-Courant K, Cronin A, et al. Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol. 2014;32:1317–1323. doi: 10.1200/JCO.2013.52.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.