Many studies underline the clinical value of low muscle mass as a prognostic factor for adverse outcomes in cancer patients, but there is no standardized approach for measuring muscle mass. This review summarizes the methods of diagnosing low muscle mass in cancer patients, the difference between underlying syndromes (sarcopenia and cachexia), and the association with clinical outcomes.
Keywords: Low muscle mass, Cancer, Chemotherapy, Sarcopenia
Abstract
In several diseases, low muscle mass has been revealed as an unfavorable prognostic factor for outcome. Whether this holds true in patients with solid malignancies as well has increasingly been explored recently. However, this research field is severely hampered by a lack of consensus on how to determine muscle mass in cancer patients and on the definition of low muscle mass. Consequently, the prevalence of low muscle mass varies widely across several studies. Nevertheless, most studies show that, in patients with solid malignancies, low muscle mass is associated with a poor outcome. In the future, more research is needed to get better insight into the best method to determine muscle mass, the exact prognostic value of low muscle mass in diverse tumor types and stages, pathophysiology of low muscle mass in patients with cancer, and ways to intervene and improve muscle mass in patients. This review addresses the current literature on the importance of muscle mass in cancer patients and the methods of muscle measurement.
Implications for Practice:
An increasing number of studies underline the clinical value of low muscle mass as a prognostic factor for adverse outcomes in cancer patients. However, studies show large heterogeneity because of the lack of a standardized approach to measure muscle mass and the lack of reference populations. As a result, the interpretation of data and further progress are severely hampered, hindering the implementation of muscle measurement in oncological care. This review summarizes the methods of diagnosing low muscle mass in cancer patients, the difference between underlying syndromes such as sarcopenia and cachexia, and the association with clinical outcomes described so far.
Introduction
Muscle mass starts to decline around the age of 40 years, resulting in a mean loss of 8% per decade until the age of 70 [1]. Above 70 years of age, this decline accelerates to 25%–40% muscle mass loss per decade [2, 3].
Loss of muscle mass is associated with unfavorable outcomes in chronic diseases, such as liver cirrhosis [4] and cardiovascular disease [5], and is frequently present in patients with rheumatoid arthritis [6], diabetes [7], and HIV/AIDS [8]. In surgical patients, low muscle mass is associated with postoperative complications and can be used to identify the risk level of patients before surgery [9]. Recently, the role of low muscle mass has become of interest in patients with cancer. In different tumor types and treatment settings, patients with low muscle mass appear to have worse survival compared with patients without low muscle mass [10–13]. Additionally, patients with low muscle mass are more likely to experience more severe toxicities from systemic antitumor agents [14]. Many chemotherapeutic drugs are distributed to the fat-free compartment of the body [15]. Because it is associated with a decline of the fat-free compartment, low muscle mass is thought to result in relatively higher drug concentrations with all accompanying toxicities [16]. Consequently, muscle mass could be an important new prognostic factor for survival and treatment tolerability in cancer patients.
In patients with cancer, muscle loss is probably the result of both sarcopenia and processes closely linked to cachexia. Sarcopenia is a geriatric syndrome with multifactorial etiology, consisting of low muscle mass combined with low muscle strength or impaired physical performance [17]. In older adults, sarcopenia is associated with mortality [18, 19] and physical disability [20]. Cachexia is a severe wasting of both fat and muscle mass and loss of weight, mediated by systemic inflammation in the presence of a severe chronic disease [21]. Several names have been used in the literature to describe muscle status, such as sarcopenia, low muscle mass, and muscle loss. In oncological studies, the term sarcopenia is frequently used, although most studies do not report impaired muscle function and physical performance, parameters that are crucial to diagnosing sarcopenia [17].
Here, we review the current knowledge on diagnosing low muscle mass, its prevalence, and its prognostic value in cancer patients. To make the nomenclature in this review clear, we use the term low muscle mass to describe radiologically measured muscle mass (i.e., measured using radiation techniques). We use the term sarcopenia when describing the combination of radiologically measured muscle mass, impaired muscle function, and/or impaired physical performance. Because most of the literature on muscle mass and its association with outcome has been generated in studies on the elderly, special emphasis is put on the methods used in muscle measurement, which might be useful for studying the clinical relevance of low muscle mass in patients with cancer.
Sarcopenia and Aging
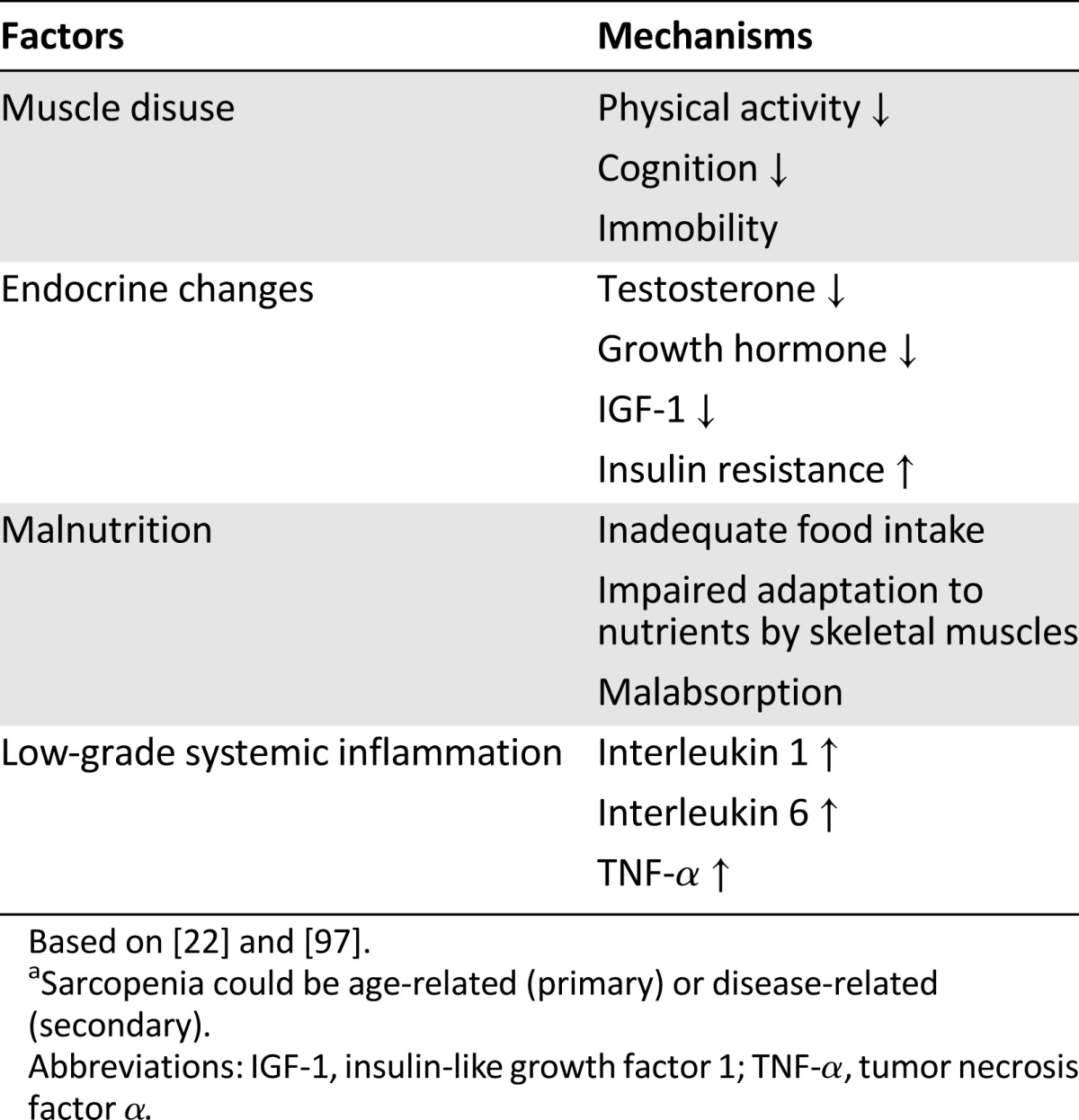
The probable mechanism of sarcopenia occurring in the elderly is an imbalance in muscle protein turnover [22], without the possibility of pointing out a single factor as the main cause (Table 1) [17]. Muscle protein synthesis decreases during aging, partly because of age-related endocrine changes, such as a reduction of sex hormones and growth factors [23]. Additionally, muscle protein breakdown increases, mainly because of age-related low-grade systemic inflammation [24], alongside other factors, such as physical inactivity and malnutrition [21]. This low-grade systemic inflammation, also called inflammaging [25], is characterized by elevated proinflammatory cytokines and is caused by age-related cell damage [25] and mitochondrial dysfunction, leading to accumulation of oxidative stress [26].
Table 1.
Etiological factors of sarcopeniaa
Middle-aged men (40–50 years old) have more muscle mass than women of the same age [1]. However, because of faster deterioration of muscle mass and muscle strength in men compared with women, at older age, men experience more absolute muscle loss and relatively greater losses of both muscle mass and muscle strength than women [2, 3]. A specific subgroup is patients suffering from sarcopenic obesity. Sarcopenic obesity is not just the combination of obesity and low muscle mass, but the result of unfavorable metabolic changes leading to both obesity and low muscle mass [27]. These patients may form a particularly worse prognostic group for adverse outcomes.
Diagnosing Sarcopenia in Geriatric Patients
Nowadays, deterioration of muscle mass alone is considered insufficient to establish the diagnosis of sarcopenia. A prospective cohort study in 2,292 community-dwelling elderly subjects showed that muscle strength had a higher association with mortality than muscle mass [28], whereas there was no linear correlation between muscle loss and reduced muscle strength [17]. Longitudinal studies reported dissociations in time between loss of muscle mass and loss of muscle strength [29], with muscle strength deteriorating more rapidly than muscle mass [20].
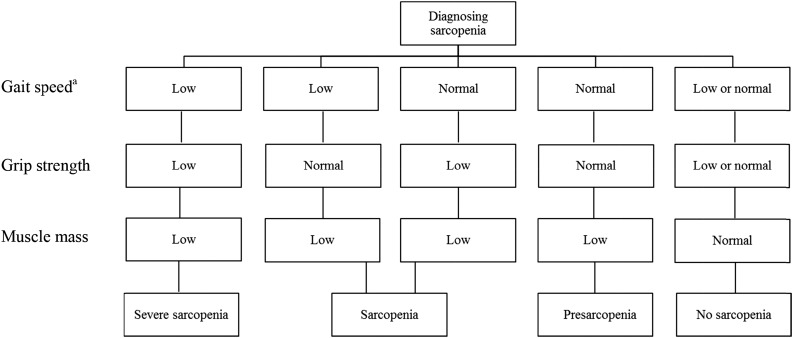
Therefore, it is recommended by the European Working Group on Sarcopenia in Older People (EWGSOP) to include muscle strength and physical performance in addition to muscle mass to diagnose sarcopenia in the elderly (Fig. 1) [17, 20, 21, 30]. A crucial shortcoming of this recommendation, however, is that no advice has been given on how to measure muscle mass and strength or which cutoff values should be used to define sarcopenia. Consequently, consensus about a definition of sarcopenia has not yet been reached, and various methods and definitions are used [17, 31]. Studies comparing the various definitions diagnosing sarcopenia, which includes muscle mass, muscle strength, and physical performance, showed a large variation of 0%–20% in the prevalence of sarcopenia in different populations [32–34].
Figure 1.
Diagnostic algorithm for sarcopenia according to the European Working Group on Sarcopenia in Older People [17]. aLow gait speed, ≤0.8 m/second; normal gait speed, >0.8 m/second.
Cachexia
In contrast to sarcopenia, cachexia is not caused by aging itself, but is a result of metabolic changes due to disease [35]. Cachexia is a combination of weight loss, muscle and adipose tissue loss, anorexia [35], hyperglycemia, hyperlipidemia, and anemia [36] (the two most widely used definitions of cachexia are listed in Table 2). Factors contributing to these metabolic changes and muscle protein degradation are proinflammatory cytokines, whereas in cancer patients, tumor metabolism contributes as well [36].
Table 2.
Definitions of cachexia

Both sarcopenia and cachexia feature the combined loss of muscle mass and muscle function; thus, distinguishing between these two syndromes in one patient can be difficult or even impossible [21, 37]. However, certain clinical features are more pathognomonic for cachexia, such as weight loss in a short time frame in combination with the failure of nutrition support [36, 38] and abnormal biochemistry. In general, muscle mass measurement alone, or even in combination with muscle function, is not sufficient to differentiate sarcopenia and cachexia; knowledge regarding metabolic state is essential [37].
Determination of Muscle Mass in Noncancer Patients
Muscle mass is part of the fat-free mass (FFM) of the human body. Total body mass consists of several compartments: fat mass (FM), water, protein, and bone, with the latter three forming the fat-free compartment [39]. Body composition analyses focus on measuring these compartments individually rather than simply measuring total body weight. Distinguishing between the measurement of FM and FFM can be important, because alterations in both compartments do not occur synchronically [40]. Furthermore, body mass index [41] and body surface area [42] cannot be relied on to detect total lean body mass or muscle mass, which indicates the need to assess these conditions separately.
In geriatric studies, measurement of muscle mass is mostly performed by dual energy x-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA) [30]. In oncological studies, computed tomography (CT) imaging is most often used. Other options are magnetic resonance imaging (MRI) [31] and ultrasound [43]. CT imaging and MRI are regarded as gold standards for muscle mass measurement [30, 44] after validation in cadavers (r = .99) [45]. Although DEXA has shown high correlation with MRI images (r = .94) [46], to the best of our knowledge, DEXA has never been validated in cadaver studies, and its accuracy decreases in obese patients [47]. Furthermore, a study in advanced cancer patients showed that appendicular skeletal muscle mass obtained from DEXA and muscle cross-sectional area at the lumbar vertebra 3 (L3) level measured by CT showed a moderate correlation (r = .70), but with a large difference in agreement after Bland-Altman analysis [48]. BIA is less accurate when measuring tissues with heterogeneous composition [30, 49, 50], often leading to overestimation of measured muscle mass [51]. Considering this and the high availability of CT images in cancer patients, we recommend CT imaging as the “gold standard” method to assess skeletal muscle in cancer patients.
Muscle mass measurement using DEXA in noncancer patients has been described in two ways, with different cutoff points to define low muscle mass. In the first method, appendicular skeletal muscle mass (ASM), which is the sum of the muscle mass of all limbs [52], is corrected for height. In one study, low muscle mass is defined as ASM two standard deviations below ASM in young adults, aged 30 years (mean), resulting in cutoffs of 7.26 kg/m2 for men and 5.45 kg/m2 for women [53]; these cutoff points are frequently used in studies. In other cutoff points commonly used, low muscle mass is defined as the 20th percentile of ASM in community-dwelling elderly subjects, resulting in cutoffs of 7.23 kg/m2 for men and 5.67 kg/m2 for women [54], which shows high similarity. In the second method, ASM is corrected for both height and fat mass by using linear regression. The residuals of the regression were used to identify the difference between expected muscle mass and true muscle mass. Low muscle mass was defined as the 20th percentile of the distribution of the residuals [54]. However, despite using 20th percentiles in both methods, almost 50% of a population of community-dwelling elderly subjects were identified as having low muscle mass by one method, but not by the other [54]. This discrepancy clearly stresses the high need for a standardized approach to measure muscle mass.
Determination of Low Muscle Mass in Cancer Patients
Little is known about the pathophysiology of low muscle mass in cancer patients [55]. Etiological factors seen during aging, such as physical inactivity and increased levels of proinflammatory cytokines, also contribute to cancer-related muscle wasting; however, the main cause is probably increased activity of the ubiquitin-proteasome system (UPS), resulting in increased muscle protein degradation. This can be present without the other determinants of cachexia, such as weight loss, metabolic changes, and loss of muscle and adipose tissue. Furthermore, cancer treatment frequently leads to vomiting, inappropriate food intake, and lack of physical activity, which can also result in the loss of both fat and muscle tissue [56]. In addition, corticosteroids, which are frequently used in cancer patients, stimulate the UPS and cause insulin resistance, both leading to muscle proteolysis [57].
To measure muscle mass in cancer patients, CT imaging instead of DEXA is mostly used because of its high availability, given its frequent use to evaluate tumor growth. However, DEXA could be a valuable alternative to measure muscle mass in cancer patients. Unfortunately, as holds true for muscle measurement in the elderly, consensus for determining muscle mass by CT scanning is lacking.
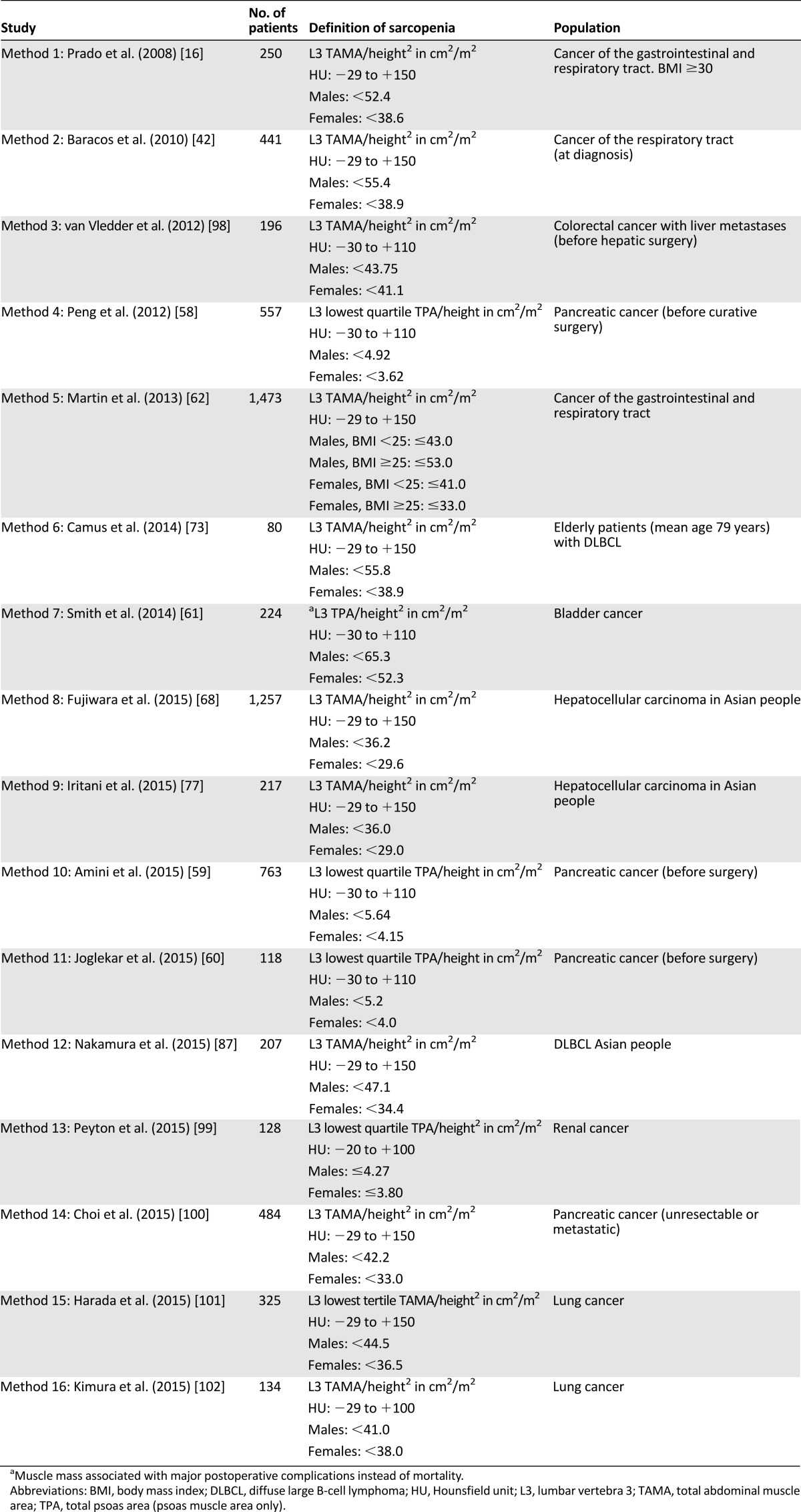
Muscle mass has been determined by measuring either the total psoas cross-sectional area (TPA) at the L3 level [58–61] or the total abdominal muscle area (TAMA) at the L3 level [16, 42, 62, 63]. The TAMA at L3 is highly correlated with the total body muscle mass (r = .924) [64]. The TAMA is corrected for height, resulting in a skeletal muscle index (cm2/m2). Therefore, muscle mass quantification can be performed easily by using only one slice, avoiding analyses of multiple images and larger surfaces being exposed to radiation.
Importantly, the first cutoff points for TAMA measurement using the single slice technique were computed in an obese population [16], but the prevalence of obesity in later studies using these cutoff points varied from 14% [65] to 57% [66]. Several studies established their own cutoff points for both TAMA and TPA, resulting in a large variation of diagnosing low muscle mass (Table 3). Furthermore, cutoff points for low muscle mass are mostly established by optimum stratification to detect the association with mortality, but the sensitivity to detect survival differences is higher in obese patients [62]. However, few studies report the distribution of muscle loss according to body mass index (BMI) groups in cancer patients.
Table 3.
Cutoff points for low muscle mass associated with mortality
Importantly, the first cutoff points for TAMA measurement using the single slice technique were computed in an obese population, but the prevalence of obesity in later studies using these cutoff points varied from 14% to 57%.
Prevalence of Low Muscle Mass in Cancer Patients
Several large studies have reported on the prevalence of low muscle mass in cancer patients [42, 62, 67, 68]. Most studies used TAMA and TPA to describe the prevalence of low muscle mass. The prevalence of low muscle mass using TPA seems to be somewhat lower compared with the measurement of TAMA (Table 4). However, because there is no standard method for the quantification of muscle mass by CT imaging, reported prevalence of low muscle mass is difficult to compare and are highly dependent on the used definition for muscle measurement. Furthermore, the level of correlation between TPA and TAMA is unknown, which makes it hard to compare these results with other studies. Table 4 describes the reported prevalence of low muscle mass and the used definition per cancer type. The prevalence of low muscle mass was highly variable across cancer types, ranging from 5% to 89%.
Table 4.
Prevalence of low muscle mass in patients with cancer using CT imaging
In cancer patients, low muscle mass more often occurs in patients older than 65 years, although is not restricted to the elderly [16]. In patients with tumors of the respiratory and gastrointestinal tract, 68% of the patients with low muscle mass were older than 65 years. Among the group of patients without low muscle mass, 45% were above 65 years old [16]. Knowledge is lacking about the prognostic value of low muscle mass in different age groups in the presence of malignancy. According to gender, no difference in the prevalence of low muscle mass is objectivated [68–71]. Compared with women, however, susceptibility for muscle loss and adverse outcomes related to low muscle mass in males has been described on multiple occasions in patients with cancer [69, 72–74] and without cancer [19, 75].
In addition to taking into account gender and BMI, stratification of cutoff points for low muscle mass by ethnicity should be considered. It has been reported that the muscle mass of young healthy Chinese men was 17% lower than in Caucasian men [76]. In studies investigating Asian populations, lower cutoff points for low muscle mass are applied [68, 77] (Table 3).
Adding Functional Assessments to Muscle Mass Measurements in Cancer Patients
Importantly—like what has been done in studies in the elderly and according to the previously mentioned EWGSOP guidelines— the prognostic value of determining muscle mass can potentially be increased by adding functional tests to muscle mass measurement in cancer patients. Physical performance is mostly described by using the Eastern Cooperative Oncology Group (ECOG) performance score in cancer patients. Although a high ECOG performance score correlated well with impaired physical function according to geriatric assessment, 38% of the patients with low ECOG performance scores were limited in instrumental activities of daily living, which possibly would require additional parameters to assess functional status [78].
However, so far, only two studies among cancer patients combined muscle mass determination and functional assessments (gait speed and handgrip strength) according to the EWGSOP guidelines, and they reported that the combination of radiological muscle mass and functional assessments had more predictive power for postoperative complications than radiological muscle mass alone in patients with colorectal cancer [12] and gastric cancer [79]. Although the EWGSOP guidelines do not recommend devices to determine handgrip strength, the Jamar hand dynamometer (Lafayette Instrument Co., Lafayette, IN, http://www.lafayetteinstrument.com) is the most widely used and is considered the gold standard to measure handgrip strength [80].
Functional tests actually reflect muscle quality, and several mechanisms of muscle quality are reported in the literature, such as decrease in muscle fiber size and number (resulting in reduced gait speed), reduction of muscle fiber contractility (resulting in reduced strength), mitochondrial dysfunction, and microfatty or macrofatty infiltration of muscle. Further research is warranted to determine whether adding functional tests improves the clinical value of muscle mass determination in cancer patients and, if so, to establish the most appropriate way to determine muscle quality.
Association of Low Muscle Mass With Chemotherapeutic Toxicity and Survival
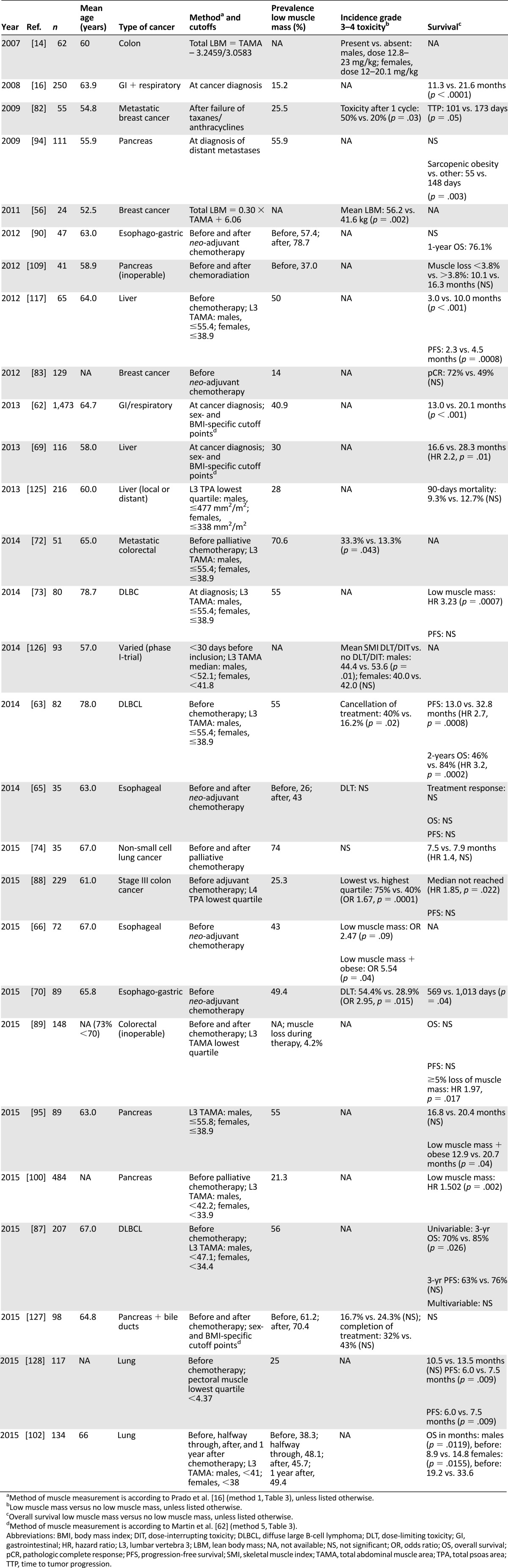
Low muscle mass might be of emerging clinical significance in the oncology field because of its association with clinical endpoints such as toxicity and cancer-related mortality [81]. A summary of studies reporting on the prognostic value of low muscle mass for survival and treatment toxicity in cancer patients undergoing chemotherapeutic treatment is provided in Table 5. The studies are characterized by a variation of muscle-measurement methods, and the knowledge regarding prognosis is described per cancer type.
Table 5.
Prognostic impact of low muscle mass in patients undergoing chemotherapeutic treatment
Breast Cancer
After the introduction of muscle mass measurement using CT imaging, the first study that reported on the association of low muscle mass and oncological outcome was conducted among 55 younger patients with metastatic breast cancer and a mean age of 55 years [82]. All patients received a fixed dose of capecitabine, and toxicity was determined after one cycle to avoid the influence of treatment adjustments. Patients with low muscle mass had a calculated higher capecitabine dose per kilogram of lean body mass and had a three times greater risk of chemotherapeutic toxicity, such as diarrhea and stomatitis. Moreover, low muscle mass was the only independent predictor of toxicity in a model with age, body surface area, and ECOG performance score. In the same study, low muscle mass was associated with a shorter time to tumor progression (62 vs. 105 days). The authors mentioned that chemotherapeutic dose interruption or reduction for toxicity, which was more prevalent in patients with low muscle mass, could be responsible for a shorter time to tumor progression. Alternatively, the low muscle mass before starting treatment itself could be a sign of aggressive or advanced underlying disease [82].
In contrast, patients with localized breast cancer and low muscle mass achieved higher rates of pathological complete response after neo-adjuvant chemotherapeutic treatment compared with those without low muscle mass [83]. It is possible that patients with low muscle mass received a relatively higher dose of chemotherapeutic agents per kilogram of lean body mass (LBM), resulting in better chemotherapeutic efficacy on tumor eradication [83]. The systemic clearance and systemic drug levels of hydrophilic chemotherapeutic agents correlate well with the LBM [15, 84], and in patients with low LBM in relation to their length and weight, a lower volume of distribution of chemotherapeutic drugs can be seen, resulting in higher systemic drug levels and consequently more chemotherapeutic toxicity [14, 85, 86]. Likewise, it can be hypothesized that patients with low muscle mass will also have higher systemic drug levels, which might lead not only to more toxicity, but to better antitumor effects as well, in this case translated into a higher pathological response rate in patients with low muscle mass.
Non-Hodgkin Lymphoma
Three studies described the prognosis of patients with non-Hodgkin lymphoma undergoing chemotherapeutic treatment [63, 73, 87]. Only one study investigated the association between low muscle mass and treatment tolerability. Low muscle mass was a predictive factor of cancellation of chemotherapy compared with normal muscle mass, although the reasons for treatment interruption were not mentioned (40% vs. 16%, p = .02) [63]. In all studies, muscle mass was measured before the start of chemotherapy, and low muscle mass was associated with a worse overall survival compared with patients with normal muscle masses. In one study, the unfavorable survival effect was only detected in males [87].
Gastrointestinal Tumors
In colorectal cancer, low muscle mass was associated with a higher incidence of grade 3–4 chemotherapeutic toxicity during both adjuvant [88] and palliative treatment [72, 89]. Furthermore, most studies report impaired overall survival in patients with low muscle mass. Two studies showed an association between low muscle mass and mortality due to disease progression in patients with stage III colon cancer receiving adjuvant chemotherapy (hazard ratio [HR] 1.85, p = .022) [88] and in a large cohort of 1,473 patients (HR 1.34, p < .001) [62]. A third study showed that muscle loss greater than 5% during chemotherapy resulted in a two times higher mortality rate [89]. Remarkably, no association with recurrence-free survival was reported [88, 89].
In colorectal cancer, low muscle mass was associated with a higher incidence of grade 3–4 chemotherapeutic toxicity during both adjuvant and palliative treatment. Furthermore, most studies report impaired overall survival in patients with low muscle mass.
In patients with esophageal cancer, the association of low muscle mass with a higher incidence of chemotherapeutic toxicity was further confirmed [66, 70]. Among obese patients, those with low muscle mass had a five times higher risk of treatment toxicity compared with obese patients without low muscle mass before neo-adjuvant chemotherapy. Risk of toxicity did not reach significance in patients with low muscle mass and normal weight, also indicating that especially obese patients with low muscle mass are the worst prognostic group [66]. Another study containing 47 patients with esophago-gastric cancer reported that 57% were diagnosed with low muscle mass before the start of neo-adjuvant chemotherapy, and these patients suffered further reduction of muscle mass during chemotherapy. There was no association with reduced completion of chemotherapy or mortality, although this should be interpreted with caution, because the study was not powered to detect differences in clinical outcome [90].
Only one study determined the association between muscle mass and survival in patients with gastric cancer [91]. In this study, involving 152 patients before surgery, low muscle mass was not associated with mortality during hospital admission and 6-month mortality, although other studies have reported a higher incidence of postoperative complications in patients with low muscle mass after gastrectomy [92, 93].
Pancreatic and Hepatocellular Cancer
Associations of low muscle mass with treatment toxicity in patients with pancreatic and hepatocellular cancer have not been described yet. In patients with pancreatic cancer in a palliative setting, impaired overall survival has been reported in obese patients with low muscle mass. Of note, these results mostly could not be extended to patients with low muscle mass and normal weight [94, 95] (Table 5). In patients with hepatocellular carcinoma, low muscle mass was associated with both overall and progression-free survival [69, 96].
Discussion
Muscle mass loss occurs during aging, and in cancer patients it is possibly due to cachexia-associated processes. Accordingly, in cancer patients, low muscle mass is prevalent across all ages, but particularly in the elderly. The number of studies in cancer patients investigating the relationship between low muscle mass and clinical outcome is rapidly increasing, and promising results on the use of muscle mass measurement as a prognostic factor have been reported.
However, there are a few limitations. There is no consensus on a standard approach to measure muscle mass, and different cutoff points and devices are used. Furthermore, the current terminology of muscle mass in the literature is confusing. Radiological low muscle mass is part of the sarcopenia syndrome and is often called sarcopenia. However, sarcopenia is more than low muscle mass alone and consists of a triad of radiological low muscle mass, low muscle strength, and impaired physical performance [17]. Unfortunately, there is also no consensus on a definition of sarcopenia. Although low muscle mass seems like a good prognostic marker, it is possible that the prognostic value can be improved further by measuring muscle function and physical performance. This needs further investigation. Functional measures such as gait speed and handgrip strength are easy to perform, but are not yet widely available in oncological care. Nevertheless, many studies report a prognostic significance on measuring muscle mass, and establishing low muscle mass as a prognostic factor could be a valuable addition in estimating treatment risks and survival effects.
In cancer patients, CT imaging is mostly used to measure muscle mass, but a reference population for this evaluation has never been described. Consequently, the prevalence of low muscle mass and/or sarcopenia and their association with clinical outcomes is highly variable across the different studies and difficult to put into perspective. Studies are needed to construct reference populations for muscle mass measurement by CT imaging, adjusted for age, gender, race, and body mass index. Furthermore, the usage of devices to measure muscle mass, such as DEXA, which, like CT imaging, are widely available, could be explored in cancer patients. DEXA is highly available across cancer patients, in particular in postmenopausal, hormone receptor-positive breast cancer patients treated with endocrine therapy, and reference populations for muscle measurement using this device are well described.
To further study the prognostic value of low muscle mass in cancer patients, investigating the pathophysiology of muscle loss and the accompanying functional impairments in cancer patients is crucial. Studies investigating the impact of other well-known etiological factors of low muscle mass—such as low androgen levels, physical inactivity, and impaired nutritional intake—or on the effects of antitumor agents or comedication frequently used in cancer patients—such as corticosteroids—on muscle mass in cancer patients are lacking. Better insight into the mechanisms underlying low muscle mass in cancer patients is crucial because this might provide strategies to improve muscle mass and function and thereby potentially improve outcomes. Current treatment strategies to increase muscle mass in the elderly mainly involve resistance training and stimulation of nutritional intake [97]. Another possibility might be the investigation of low androgen levels in cancer patients with low muscle mass and whether this can be used as an intervention strategy. However, whether such strategies actually work in cancer patients—and, if so, what the optimal timing of such strategies should be—remains to be established.
Conclusion
Low muscle mass among cancer patients seems to be an important prognostic factor for outcomes in terms of treatment-induced toxicity and survival, but consensus about a definition of impaired muscle mass and a standardized approach to measure this are urgently warranted. Functional tests need to be measured to use the term sarcopenia, but the added value of these tests in cancer patients are yet to be established. Until a consensus on these items has been reached, reported prevalence of low muscle mass in populations and between cancer sites remains difficult to put into perspective. Consensus about a definition of low muscle mass and knowledge about its prognostic value and the underlying mechanisms are likely to contribute to strategies to develop a more personalized treatment approach and to novel interventions improving outcome.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This work was funded by the ORAS Foundation (Oncological Research Albert Schweitzer Hospital) and the Leerhuis of the Albert Schweitzer Hospital, Dordrecht, The Netherlands.
Author Contributions
Conception/Design: Hánah N. Rier, Agnes Jager, Stefan Sleijfer, Andrea B. Maier, Mark-David Levin
Provision of study material or patients: Hánah N. Rier, Agnes Jager, Stefan Sleijfer, Mark-David Levin
Collection and/or assembly of data: Hánah N. Rier
Data analysis and interpretation: Hánah N. Rier, Agnes Jager, Stefan Sleijfer, Mark-David Levin
Manuscript writing: Hánah N. Rier, Agnes Jager, Stefan Sleijfer, Andrea B. Maier, Mark-David Levin
Final approval of manuscript: Hánah N. Rier, Agnes Jager, Stefan Sleijfer, Andrea B. Maier, Mark-David Levin
Disclosures
Mark-David Levin: Abbvie, Roche (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Kitamura I, Koda M, Otsuka R, et al. Six-year longitudinal changes in body composition of middle-aged and elderly Japanese: Age and sex differences in appendicular skeletal muscle mass. Geriatr Gerontol Int. 2014;14:354–361. doi: 10.1111/ggi.12109. [DOI] [PubMed] [Google Scholar]
- 2.Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Kalafateli M, Konstantakis C, Thomopoulos K, et al. Impact of muscle wasting on survival in patients with liver cirrhosis. World J Gastroenterol. 2015;21:7357–7361. doi: 10.3748/wjg.v21.i24.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins JL, Whincup PH, Morris RW, et al. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Oliveira Nunes Teixeira V, Filippin LI, Viacava PR, et al. Muscle wasting in collagen-induced arthritis and disuse atrophy. Exp Biol Med (Maywood) 2013;238:1421–1430. doi: 10.1177/1535370213505961. [DOI] [PubMed] [Google Scholar]
- 7.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto Neto LF, Sales MC, Scaramussa ES, et al. Human immunodeficiency virus infection and its association with sarcopenia. Braz J Infect Dis. 2016;20:99–102. doi: 10.1016/j.bjid.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J Surg Oncol. 2015;112:503–509. doi: 10.1002/jso.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yip C, Dinkel C, Mahajan A, et al. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging. 2015;6:489–497. doi: 10.1007/s13244-015-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol. 2016;54:2–10. doi: 10.1016/j.semcdb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Huang DD, Wang SL, Zhuang CL, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17:O256–O264. doi: 10.1111/codi.13067. [DOI] [PubMed] [Google Scholar]
- 13.Malietzis G, Johns N, Al-Hassi HO, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263:320–325. doi: 10.1097/SLA.0000000000001113. [DOI] [PubMed] [Google Scholar]
- 14.Prado CM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–3268. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- 15.Gusella M, Toso S, Ferrazzi E, et al. Relationships between body composition parameters and fluorouracil pharmacokinetics. Br J Clin Pharmacol. 2002;54:131–139. doi: 10.1046/j.1365-2125.2002.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM, et al. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging. 2013;17:259–262. doi: 10.1007/s12603-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilSIRENTE study. Age Ageing. 2013;42:203–209. doi: 10.1093/ageing/afs194. [DOI] [PubMed] [Google Scholar]
- 20.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Walrand S, Guillet C, Salles J, et al. Physiopathological mechanism of sarcopenia. Clin Geriatr Med. 2011;27:365–385. doi: 10.1016/j.cger.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 24.Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 26.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohara K. Sarcopenic obesity in aging population: Current status and future directions for research. Endocrine. 2014;45:15–25. doi: 10.1007/s12020-013-9992-0. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 29.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 30.Cesari M, Vellas B. Sarcopenia: A novel clinical condition or still a matter for research? J Am Med Dir Assoc. 2012;13:766–767. doi: 10.1016/j.jamda.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Cooper C, Fielding R, Visser M, et al. Tools in the assessment of sarcopenia. Calcif Tissue Int. 2013;93:201–210. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijlsma AY, Meskers CG, Ling CH, et al. Defining sarcopenia: The impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–881. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaudart C, Reginster JY, Slomian J, et al. Prevalence of sarcopenia: The impact of different diagnostic cut-off limits. J Musculoskelet Neuronal Interact. 2014;14:425–431. [PubMed] [Google Scholar]
- 34.Reijnierse EM, Trappenburg MC, Leter MJ, et al. The impact of different diagnostic criteria on the prevalence of sarcopenia in healthy elderly participants and geriatric outpatients. Gerontology. 2015;61:491–496. doi: 10.1159/000377699. [DOI] [PubMed] [Google Scholar]
- 35.Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- 36.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 37.Muscaritoli M, Lucia S, Molfino A, et al. Muscle atrophy in aging and chronic diseases: Is it sarcopenia or cachexia? Intern Emerg Med. 2013;8:553–560. doi: 10.1007/s11739-012-0807-8. [DOI] [PubMed] [Google Scholar]
- 38.Evans WJ, Morley JE, Argilés J, et al. Cachexia: A new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro SM, Kehayias JJ. Sarcopenia and the analysis of body composition. Adv Nutr. 2014;5:260–267. doi: 10.3945/an.113.005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St-Onge MP, Gallagher D. Body composition changes with aging: The cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–155. doi: 10.1016/j.nut.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenny AM, Dawson L, Kleppinger A, et al. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J Gerontol A Biol Sci Med Sci. 2003;58:M436–M440. doi: 10.1093/gerona/58.5.m436. [DOI] [PubMed] [Google Scholar]
- 42.Baracos VE, Reiman T, Mourtzakis M, et al. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 43.Mourtzakis M, Wischmeyer P. Bedside ultrasound measurement of skeletal muscle. Curr Opin Clin Nutr Metab Care. 2014;17:389–395. doi: 10.1097/MCO.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 44.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 45.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Wang Z, Lohman T, et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr. 2007;137:2775–2780. doi: 10.1093/jn/137.12.2775. [DOI] [PubMed] [Google Scholar]
- 47.Bredella MA, Ghomi RH, Thomas BJ, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 2010;18:2227–2233. doi: 10.1038/oby.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kilgour RD, Cardiff K, Rosenthall L, et al. Use of prediction equations to determine the accuracy of whole-body fat and fat-free mass and appendicular skeletal muscle mass measurements from a single abdominal image using computed tomography in advanced cancer patients. Appl Physiol Nutr Metab. 2016;41:70–75. doi: 10.1139/apnm-2015-0068. [DOI] [PubMed] [Google Scholar]
- 49.Mijnarends DM, Meijers JM, Halfens RJ, et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: A systematic review. J Am Med Dir Assoc. 2013;14:170–178. doi: 10.1016/j.jamda.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beaudart C, Reginster JY, Slomian J, et al. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol. 2015;61:31–37. doi: 10.1016/j.exger.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Heymsfield SB, Lichtman S, Baumgartner RN, et al. Body composition of humans: comparison of two improved four-compartment models that differ in expense, technical complexity, and radiation exposure. Am J Clin Nutr. 1990;52:52–58. doi: 10.1093/ajcn/52.1.52. [DOI] [PubMed] [Google Scholar]
- 53.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 54.Newman AB, Kupelian V, Visser M, et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 55.Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol. 2013;45:2215–2229. doi: 10.1016/j.biocel.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 56.Prado CM, Lima IS, Baracos VE, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67:93–101. doi: 10.1007/s00280-010-1288-y. [DOI] [PubMed] [Google Scholar]
- 57.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 58.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amini N, Spolverato G, Gupta R, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: A new tool to assess sarcopenia [published correction appears in J Gastrointest Surg 2016 20:1082] J Gastrointest Surg. 2015;19:1593–1602. doi: 10.1007/s11605-015-2835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joglekar S, Asghar A, Mott SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith AB, Deal AM, Yu H, et al. Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol. 2014;191:1714–1720. doi: 10.1016/j.juro.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 62.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 63.Lanic H, Kraut-Tauzia J, Modzelewski R, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. 2014;55:817–823. doi: 10.3109/10428194.2013.816421. [DOI] [PubMed] [Google Scholar]
- 64.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 65.Yip C, Goh V, Davies A, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 66.Anandavadivelan P, Brismar TB, Nilsson M, et al. Sarcopenic obesity: A probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr. 2016;35:724–730. doi: 10.1016/j.clnu.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 67.Veasey Rodrigues H, Baracos VE, Wheler JJ, et al. Body composition and survival in the early clinical trials setting. Eur J Cancer. 2013;49:3068–3075. doi: 10.1016/j.ejca.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 68.Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 69.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–870. doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 70.Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41:333–338. doi: 10.1016/j.ejso.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 71.van Vugt JL, Braam HJ, van Oudheusden TR, et al. Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer [published correction appears in Ann Surg Oncol 2015 22:S1610] Ann Surg Oncol. 2015;22:3625–3631. doi: 10.1245/s10434-015-4429-z. [DOI] [PubMed] [Google Scholar]
- 72.Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 73.Camus V, Lanic H, Kraut J, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol. 2014;93:9–18. doi: 10.1111/ejh.12285. [DOI] [PubMed] [Google Scholar]
- 74.Stene GB, Helbostad JL, Amundsen T, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015;54:340–348. doi: 10.3109/0284186X.2014.953259. [DOI] [PubMed] [Google Scholar]
- 75.Batsis JA, Mackenzie TA, Barre LK, et al. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 76.Lau EM, Lynn HS, Woo JW, et al. Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005;60:213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 77.Iritani S, Imai K, Takai K, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. 2015;50:323–332. doi: 10.1007/s00535-014-0964-9. [DOI] [PubMed] [Google Scholar]
- 78.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 79.Wang H, Li TL, Hsia S, et al. Skeletal muscle atrophy is attenuated in tumor-bearing mice under chemotherapy by treatment with fish oil and selenium. Oncotarget. 2015;6:7758–7773. doi: 10.18632/oncotarget.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 81.Prado CM, Maia YL, Ormsbee M, et al. Assessment of nutritional status in cancer--the relationship between body composition and pharmacokinetics. Anticancer Agents Med Chem. 2013;13:1197–1203. doi: 10.2174/18715206113139990322. [DOI] [PubMed] [Google Scholar]
- 82.Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 83.Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. The Oncologist. 2012;17:1240–1245. doi: 10.1634/theoncologist.2012-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26:292–307. doi: 10.2165/00003088-199426040-00005. [DOI] [PubMed] [Google Scholar]
- 85.Ali R, Baracos VE, Sawyer MB, et al. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016;5:607–616. doi: 10.1002/cam4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sjøblom B, Grønberg BH, Benth JS, et al. Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer. 2015;90:85–91. doi: 10.1016/j.lungcan.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura N, Hara T, Shibata Y, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol. 2015;94:2043–2053. doi: 10.1007/s00277-015-2499-4. [DOI] [PubMed] [Google Scholar]
- 88.Jung HW, Kim JW, Kim JY, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015;23:687–694. doi: 10.1007/s00520-014-2418-6. [DOI] [PubMed] [Google Scholar]
- 89.Miyamoto Y, Baba Y, Sakamoto Y, et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One. 2015;10:e0129742. doi: 10.1371/journal.pone.0129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31:74–77. doi: 10.1016/j.clnu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112:403–407. doi: 10.1002/jso.24015. [DOI] [PubMed] [Google Scholar]
- 92.Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: A prospective study. Ann Surg Oncol. 2016;23:556–564. doi: 10.1245/s10434-015-4887-3. [DOI] [PubMed] [Google Scholar]
- 93.Fukuda Y, Yamamoto K, Hirao M, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2016;19:986–993. doi: 10.1007/s10120-015-0546-4. [DOI] [PubMed] [Google Scholar]
- 94.Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 95.Cooper AB, Slack R, Fogelman D, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:2416–2423. doi: 10.1245/s10434-014-4285-2. [DOI] [PubMed] [Google Scholar]
- 96.Mir O, Coriat R, Boudou-Rouquette P, et al. Gemcitabine and oxaliplatin as second-line treatment in patients with hepatocellular carcinoma pre-treated with sorafenib. Med Oncol. 2012;29:2793–2799. doi: 10.1007/s12032-012-0208-x. [DOI] [PubMed] [Google Scholar]
- 97.Ali S, Garcia JM. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—a mini-review. Gerontology. 2014;60:294–305. doi: 10.1159/000356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 99.Peyton CC, Heavner MG, Rague JT, et al. Does sarcopenia impact complications and overall survival in patients undergoing radical nephrectomy for stage III and IV kidney cancer? J Endourol. 2016;30:229–236. doi: 10.1089/end.2015.0492. [DOI] [PubMed] [Google Scholar]
- 100.Choi Y, Oh DY, Kim TY, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One. 2015;10:e0139749. doi: 10.1371/journal.pone.0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harada K, Ida S, Baba Y, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 2015 doi: 10.1111/dote.12381. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 102.Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015;23:1699–1708. doi: 10.1007/s00520-014-2534-3. [DOI] [PubMed] [Google Scholar]
- 103.Kim EY, Kim YS, Park I, et al. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol. 2015;10:1795–1799. doi: 10.1097/JTO.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 104.Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 106.Broughman JR, Williams GR, Deal AM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. J Geriatr Oncol. 2015;6:442–445. doi: 10.1016/j.jgo.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thoresen L, Frykholm G, Lydersen S, et al. The association of nutritional assessment criteria with health-related quality of life in patients with advanced colorectal carcinoma. Eur J Cancer Care (Engl) 2012;21:505–516. doi: 10.1111/j.1365-2354.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 108.Thoresen L, Frykholm G, Lydersen S, et al. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin Nutr. 2013;32:65–72. doi: 10.1016/j.clnu.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Dalal S, Hui D, Bidaut L, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: A pilot study. J Pain Symptom Manage. 2012;44:181–191. doi: 10.1016/j.jpainsymman.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Di Sebastiano KM, Yang L, Zbuk K, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br J Nutr. 2013;109:302–312. doi: 10.1017/S0007114512001067. [DOI] [PubMed] [Google Scholar]
- 111.Wesseltoft-Rao N, Hjermstad MJ, Ikdahl T, et al. Comparing two classifications of cancer cachexia and their association with survival in patients with unresected pancreatic cancer. Nutr Cancer. 2015;67:472–480. doi: 10.1080/01635581.2015.1004728. [DOI] [PubMed] [Google Scholar]
- 112.Antoun S, Birdsell L, Sawyer MB, et al. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: Results from a placebo-controlled study. J Clin Oncol. 2010;28:1054–1060. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 113.Huillard O, Mir O, Peyromaure M, et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer. 2013;108:1034–1041. doi: 10.1038/bjc.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cushen SJ, Power DG, Teo MY, et al. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000061. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 115.Sharma P, Zargar-Shoshtari K, Caracciolo JT, et al. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol Oncol. 2015;33:339.e17–339.e23. doi: 10.1016/j.urolonc.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 116.Tamandl D, Paireder M, Asari R, et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 2016;26:1359–1367. doi: 10.1007/s00330-015-3963-1. [DOI] [PubMed] [Google Scholar]
- 117.Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. 2012;7:e37563. doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 119.Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015;261:1173–1183. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 120.Imai K, Takai K, Hanai T, et al. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int J Mol Sci. 2015;16:9612–9624. doi: 10.3390/ijms16059612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nault JC, Pigneur F, Nelson AC, et al. Visceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitors. Dig Liver Dis. 2015;47:869–876. doi: 10.1016/j.dld.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 122.Kamachi S, Mizuta T, Otsuka T, et al. Sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment. Hepatol Res. 2016;46:201–208. doi: 10.1111/hepr.12562. [DOI] [PubMed] [Google Scholar]
- 123.Psutka SP, Boorjian SA, Moynagh MR, et al. Mortality after radical cystectomy: impact of obesity versus adiposity after adjusting for skeletal muscle wasting. J Urol. 2015;193:1507–1513. doi: 10.1016/j.juro.2014.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fukushima H, Yokoyama M, Nakanishi Y, et al. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One. 2015;10:e0115895. doi: 10.1371/journal.pone.0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dodson RM, Firoozmand A, Hyder O, et al. Impact of sarcopenia on outcomes following intra-arterial therapy of hepatic malignancies. J Gastrointest Surg. 2013;17:2123–2132. doi: 10.1007/s11605-013-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cousin S, Hollebecque A, Koscielny S, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs. 2014;32:382–387. doi: 10.1007/s10637-013-0053-6. [DOI] [PubMed] [Google Scholar]
- 127.Rollins KE, Tewari N, Ackner A, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2015 doi: 10.1016/j.clnu.2015.08.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 128.Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer. 2016;24:2075–2084. doi: 10.1007/s00520-015-2997-x. [DOI] [PubMed] [Google Scholar]