Abstract
MicroRNAs (miRNAs) are deregulated in cancer and have been shown to exhibit both oncogenic and tumor suppressive functions. Although the functional effects of several miRNAs have been elucidated, those of many remain to be discovered. In silico analysis identified microRNA-206 (miR-206) binding sites in the 3′-untranslated regions (3′-UTR) of both the mouse and human CCND1 gene. Cyclin D1 is a recognized oncogene involved in direct phosphorylation of the retinoblastoma (Rb) protein and promoting cell cycle transition from G1 to S. miR-206 specifically binds to the CCND1 3′-UTR and mediates reduction of both cyclin D1 protein and mRNA. Expression of miR-206 induced a G1 arrest and a decrease in cell proliferation in breast cancer cells. Ectopic expression of miRNA-resistant cyclin D1 was able to reverse the miR-206-induced decrease in cell proliferation. Therefore, we identified miR-206 as an activator of cell cycle arrest resulting in a decrease in cell proliferation that is dependent on the inhibition of cyclin D1. Interestingly, prostatic cancer (PCa) cells express low levels of miR-206 resulting in deregulated cyclin D1 expression compared with non-transformed primary prostatic epithelial cells (PrEC). Finally, we demonstrate that cyclin D1 is regulated by miR-206 in PrEC but not in PCa cells and this is due to the absence of a CCND1 3'-UTR in these cells. This suggests that miR-206-based anti-cyclin D1 targeted therapy would be beneficial in cancers where cyclin D1 is overexpressed and contains a 3′-UTR.
Introduction
MicroRNAs (miRNAs) are non-coding RNA molecules that act as negative regulators of gene expression. These small cellular RNAs bind to partially complementary sequences at the 3′-untranslated region (3′-UTR) of specific target mRNA molecules, leading to degradation or translational inhibition of target mRNAs.1 miRNAs have a fundamental role in regulating the genetic programs that dictate development, cellular differentiation and proliferation.1 In addition, multiple lines of evidence highlight miRNA deregulation in many human cancers.2, 3, 4 Despite this growing body of evidence, there is still limited information available regarding the biological targets of these miRNAs and the pathways they regulate during carcinogenesis.
MicroRNA-206 (MiR-206) was initially identified as a skeletal muscle-enriched miRNA involved in differentiation.5 However, further studies show miR-206 is expressed in other tissues. For example, miR-206 represses estrogen receptor alpha (ERα)6 and is downregulated in ERα positive breast cancer.7 These authors propose that miR-206 loss may be linked with breast cancer development. Another study indicates that miR-206 levels are low in prostate tumors compared with normal prostate.5 MiR-206 has also been shown to function as a pro-apoptotic factor in HeLa cells by targeting Notch3 signaling.8 In more recent studies, loss of miR-206 has been shown to correlate with increased metastasis and invasion in lung and laryngeal cancers.9,10 All these studies further implicate a tumor suppressor role for miR-206.
In this study, we show for the first time that miR-206 directly targets and regulates the full-length 3′-UTR of the human CCND1 gene. Indeed, this adds significantly to a recent study that demonstrates that miR-206 targets a specific fragment of the mouse Ccnd1 3′-UTR.11 Cyclin D1 is the regulatory subunit of cyclin-dependent kinase 4/6 (CDK4/6), which phosphorylates and inactivates the retinoblastoma (Rb) protein, thereby promoting progression from G1 to S phase of the cell cycle.12 The CCND1 gene is amplified and cyclin D1 protein is overexpressed in a significant proportion of human cancers including breast, prostate and colon cancer, as well as parathyroid adenoma, lymphoma and melanoma.13 Our studies reveal that miR-206 targets cyclin D1 in human breast cancer cells causing a G1 cell cycle arrest and a decrease in cell proliferation/viability. We further show that miR-206 reduces the clonogenic survival of breast cancer cells, which can be rescued by miRNA-resistant cyclin D1. Our study suggests miR-206 is a critical regulator of cell survival in breast cancer cells via cross-talk with cyclin D1. Interestingly, cyclin D1 expression is insensitive to miR-206 regulation in prostatic cancer (PCa) cells owing to the lack of a CCND1 3′-UTR. This highlights that a miR-206-based anti-cyclin D1 therapy would be specific to cancers that overexpress cyclin D1 and possess a 3′-UTR.
Results
miR-206 targets mouse and human cyclin D1
Similar to other studies we identified miR-206 as a miRNA that was significantly upregulated during C2C12 differentiation5,11 (Supplementary Figure 1). Analysis of putative targets of miR-206, using the target prediction algorithms TargetScan14 and PicTar,15 identified potential miR-206 binding sites in the CCND1 3′-UTR of mouse and human genes. To confirm that cyclin D1 is a miR-206 target, a portion of the mouse 3′-UTR of Ccnd1 containing the putative miR-206 consensus site was cloned into a luciferase reporter gene and co-transfected with either a Pre-miR miR-206 precursor molecule (miR-206; a small, chemically modified double-stranded RNA molecule designed to mimic endogenous mature miR-206), or a Pre-miR Precursor negative control (miR-ve), into 3T3-L1 cells, which do not normally express miR-206. Ectopic expression of miR-206 in 3T3-L1 cells repressed the expression of the Ccnd1 3′-UTR reporter gene construct, but not the original reporter gene construct, indicating that miR-206 post-transcriptionally regulates cyclin D1 at the 3′-UTR (Figure 1b). Furthermore, overexpression of miR-206 markedly decreased the levels of endogenous mouse cyclin D1 protein (Supplementary Figure 3A) and mRNA (Supplementary Figure 3B) in 3T3-L1 and C2C12 cells. Although these data verify previous studies that identify a miR-206 binding site at position 911–917 of the mouse Ccnd1 3′-UTR,11 they have not taken into consideration the regulation of the full-length 3′-UTR by miR-206, which would be more reflective of the endogenous Ccnd1 gene locus.
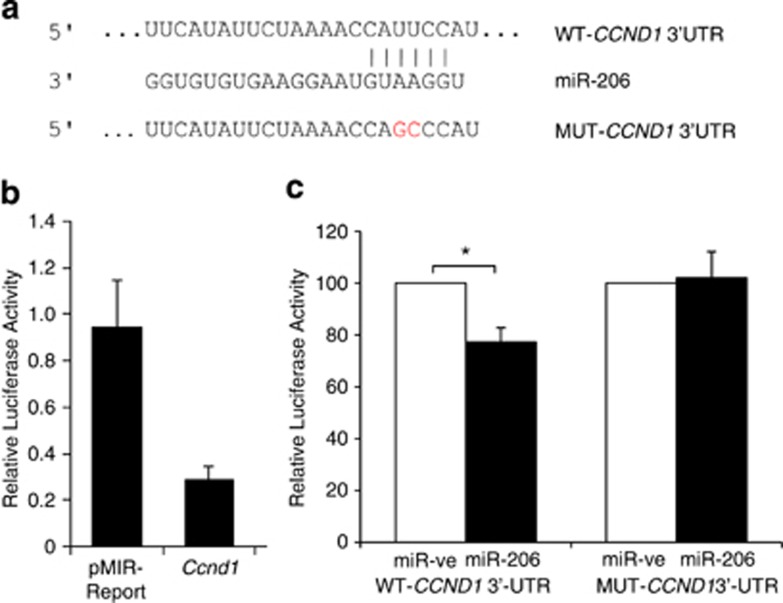
Figure 1.
miR-206 directly targets CCND1 at a specific 3′-UTR site. (a) Schematic of putative miR-206 target site within the human CCND1 3′-UTR as predicted by TargetScan and PicTar algorithms. Mutations introduced into the full-length human CCND1 3′-UTR are highlighted in red. (b) Relative luciferase activity was measured in 3T3-L1 cells co-transfected with a luciferase reporter construct containing a fragment of the mouse Ccnd1 3′-UTR encompassing the miR-206 binding site or empty pMIR-Report vector, along with miR-ve or miR-206. Data represent relative Firefly values as determined by the ratio of Firefly to Renilla luciferase activity with control miR-ve set as 1. Values are the mean±s.e.m. of three separate experiments. (c) Relative luciferase activity was measured in H1299 cells co-transfected with reporter constructs containing human full-length wild-type (WT)-CCND1 3′-UTR or mutant (MUT)-CCND1 3′-UTR along with miR-ve or miR-206. Firefly luciferase units were normalized against Renilla luciferase units to control for transfection efficiency. Percentage Firefly luciferase values were determined with control miR-ve set at 100%. Data represent the mean+s.e.m. of three independent experiments. A paired t-test was used to determine significance between miR-ve and miR-206 transfected cells (* indicates P<0.05).
Because a miR-206 target site was also observed in the human CCND1 3′-UTR at position 1000–1006 (Figure 1a),11 we used a reporter plasmid containing the full-length wild-type human CCND1 3′-UTR (WT-CCND1 3′-UTR) fused to a firefly luciferase cDNA. As human H1299 (lung carcinoma) cells had minimal endogenous miR-206 expression, this provided a good in vitro cell model system to study the interaction between exogenously expressed miR-206 and cyclin D1 (Supplementary Figure 2). These cells were transfected with miR-206 or miR-ve, and luciferase activity was measured. miR-206 overexpression significantly decreased luciferase activity driven by WT-CCND1 3′-UTR by 23%, indicating that miR-206 regulates cyclin D1 via the 3′-UTR (Figure 1c). To determine whether this effect is direct, the predicted miR-206 binding site in the 3′-UTR of WT-CCND1 was mutated by generating MUT-CCND1 3′-UTR (Figure 1a). Mutation of the miR-206 binding site renders the CCND1 3′-UTR insensitive to miR-206 repression (Figure 1c). Taken together, these data indicate that miR-206 interacts with specific elements in the 3′-UTR of human CCND1, providing an additional mechanism of cyclin D1 regulation. Overall, the above results identified mouse and human cyclin D1 as target genes of miR-206.
miR-206 regulates cyclin D1 in breast cancer cells
The CCND1 gene is amplified and cyclin D1 protein is overexpressed in a significant proportion of human cancers.13 For example, the CCND1 gene is overexpressed in 50% of human breast cancers and this is associated with breast carcinogenesis as well as the emergence of hormone therapy-resistant cancers.16 Given that miR-206 regulates cyclin D1 expression and that it has been reported that miR-206 is downregulated in breast cancer,7 we wanted to determine whether overexpression of cyclin D1 is a consequence of miR-206 loss in breast cancer cell lines. As ERα is also a direct target of miR-2066 and cyclin D1 is a downstream target of the ERα signaling pathway,17 we first wanted to rule out any indirect effect of miR-206 on cyclin D1 expression via ERα signaling. In order to ascertain if miR-206 is directly regulating the cyclin D1 3′-UTR in the absence of ERα signaling, we chose the ERα-negative breast cancer cell line MDA-MB-231. Then, to assess the contribution of ERα to this pathway, we studied the MDA-MB-231 cell line expressing the open reading frame (ORF) of ERα (MDA-MB-231-ERα).18 A decrease in cyclin D1 was observed by immunoblot analysis 48 h following miR-206 transfection (Figure 2a). This reduction is in part independent of ERα expression as cyclin D1 protein levels were repressed in MDA-MB-231 cells that do not express ERα (31±8% reduction), and also in MDA-MB-231 cells that express ERα lacking the miR-206 binding site (51±6% reduction). This 20% reduction in cyclin D1 levels mediated by miR-206 in MDA-MB-231-ERα cells may be linked to an ERα-induced increase in cyclin D1 protein expression (Figure 2a), an observation that has been previously reported.17,19,20 These data indicate that miR-206 can reduce cyclin D1 protein levels through direct targeting of cyclin D1 mRNA.
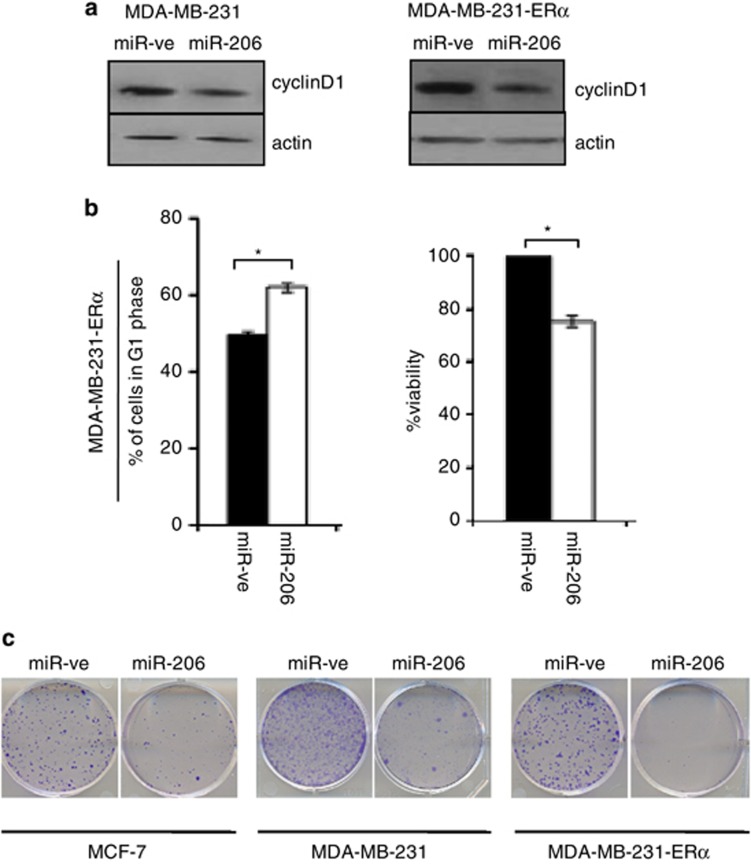
Figure 2.
miR-206 inhibits cyclin D1 expression in breast cancer cells. MDA-MB-231, MDA-MB-231-ERα or MCF-7 cells were reverse transfected with miR-ve or miR-206. (a) Forty-eight hours later, protein extracts were prepared and western blot analysis was performed to determine cyclin D1 protein levels. Beta-actin served as a loading control. Immunoblots are representative of three independent experiments. (b) Seventy-two hours later, cells were fixed, stained with propidium iodide and cell cycle distribution was assessed by flow cytometry. Graphical representation of percentage of cells in G1 phase following transfection with miR-ve (black bar) or mir-206 (white bar). Data represent the mean±s.e.m. of three independent experiments, and a paired t-test was used to determine the significance between miR-ve and miR-206 expressing cells (left panel). Cell viability was determined by MTT assay. Values represent the mean±s.e.m. of three independent experiments and a paired t-test was performed between miR-ve and miR-206 transfected cells (right panel). (c) Forty-eight hours later cells were trypsinized, counted and 500 cells were plated in a 10-cm dish and analyzed for clonogenic survival.
Cyclin D1 along with its binding partners CDK4/6 regulates G1 to S phase transition during the cell cycle.12 Knockdown of cyclin D1 by RNA interference induces a G1 arrest in breast cancer cells.21 Hence, we set out to determine whether the miR-206-induced decrease in cyclin D1 protein expression caused a G1 arrest. Propidium iodide staining and analysis by flow cytometry demonstrated that overexpression of miR-206 in MDA-MB-231-ERα cells caused a significant increase in the percentage of cells in G1 phase of the cell cycle (~12%) (Figure 2b). This also occurred in C2C12 myoblasts, whereby an increase of 26±2% in the number of cells in G1 and a corresponding decrease of 26±6% in the number of cells in S phase was observed in miR-206 expressing cells (Supplementary Figure 4A). Apoptosis induction caused by cyclin D1 knockdown and by overexpression of miR-206 has been previously reported.8,22 We did not observe an increase in apoptosis upon miR-206 overexpression in our studies as determined by assessing the sub G1 population (data not shown).
Inhibition of cyclin D1 by siRNA in breast cancer cells also causes a decrease in cell proliferation.21,23 We used an MTT assay to determine the effect of cyclin D1 knockdown by miR-206 on cell proliferation/viability. MiR-ve or miR-206 was overexpressed in MDA-MB-231-ERα cells and 72 h later cell viability was determined by MTT assay. MiR-206 caused a significant decrease in cell viability/proliferation of MDA-MB-231-ERα cells (>20%) (Figure 2b). In addition, ectopic expression of miR-206 in C2C12 myoblasts and 3T3-L1 caused a 42% and 39% decrease in cell number, respectively, compared with miR-ve expressing cells (Supplementary Figure 4B). Taken together, these data suggest miR-206 is a critical regulator of survival in these cellular systems.
Cell cycle arrest and/or a decrease in cell proliferation/viability can reduce the ability of cells to form colonies. Therefore, we compared the clonogenic survival of miR-206 expressing breast cancer cells with miR-ve expressing cells. As mentioned above, Cyclin D1 is an ERα-regulated gene6 and ERα is itself a mir-206 target,17 therefore to study the ERα-independent effect of miR-206 on cyclin D1 regulation, we carried out these experiments in MDA-MB-231 and MDA-MB-231-ERα cells. MiR-206 causes a decrease in the clonogenic survival of MCF-7, MDA-MB-231 and MDA-MB-231-ERα cells (Figure 2c). Hence, we demonstrate that this miR-206-induced phenotype is comparatively independent of ERα signaling as reduced colony formation is observed in the ERα-negative cell line, as well as cells that express miRNA-resistant ERα.
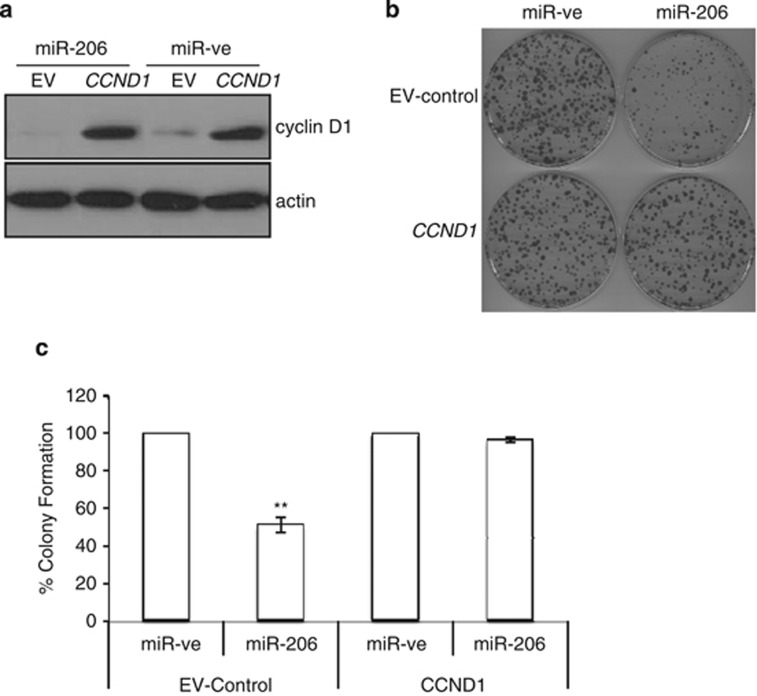
To definitively establish that regulation of cyclin D1 contributes to the miR-206-induced decrease in clonogenic survival, MDA-MB-231 cells were transiently transfected with a vector containing the human CCND1 ORF (and thus lacking the full-length 3′-UTR and inherent miR-206 regulatory elements), or empty vector control (EV-control). Cells were then transfected with miR-ve or miR-206 and the ability of cells to form colonies was determined. As shown in Figure 3a, overexpression of miR-206 caused a decrease in cyclin D1 protein levels in EV-control transfected cells and a 50% decrease in the ability of MDA-MB-231 cells to form colonies. However, miR-206 did not reduce the expression of exogenous cyclin D1 that lacks a 3′-UTR, and miR-206 regulatory elements. Importantly, the miR-206-induced decrease in clonogenic survival of MDA-MB-231 cells was reversed by co-expression of the CCND1 ORF, which is insensitive to miR-206 regulation (Figure 3b and c). Taken together, these data indicate that cyclin D1 is a critical parameter regulated by miR-206 essential for breast cancer cell survival.
Figure 3.
miRNA-resistant cyclin D1 reverses the miR-206-induced decrease in cell survival. MDA-MB-236 cells were co-transfected with a CCND1 expression vector or empty vector control and miR-ve or miR-206. Twenty-four hours later, cells were (a) harvested for western blot analysis. (b) Analyzed for clonogenic survival. n=3 **P=0.019. (c) Graphical representation of three independent experiments. Values are mean±s.e.m. Percentage cell survival was determined with control miR-ve set at 100%. A paired t-test was used to determine the significance between miR-ve and miR-206 expressing cells, **P=0.006.
Cyclin D1 in PCa is insensitive to miR-206 control
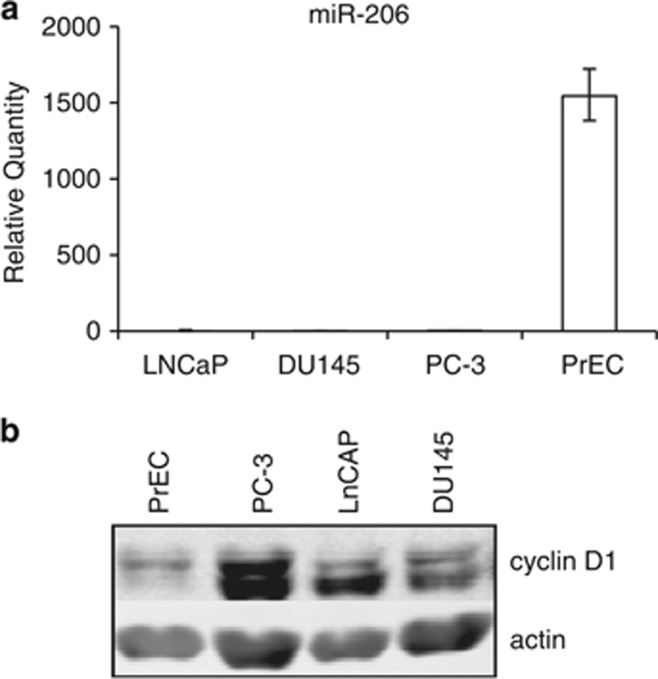
A link between cyclin D1 overexpression and CCND1 amplification has also been reported in prostate cancer (PCa).24,25 Hence, we aimed to establish if similar to breast cancer, cyclin D1 overexpression in prostate cancer is a result of miR-206 loss. In the first instance we compared the expression of miR-206 between non-transformed human primary prostatic epithelial cells (PrECs) and various PCa cell lines. MiR-206 levels were significantly higher in PrECs compared with the PCa cells, DU145, PC-3 and LNCaP cells (Figure 4a). This is consistent with a tumor suppressor role for miR-206, whereby levels are high in non-transformed cells and expression is low in cancer cells.5,9,10 We aimed to establish if miR-206 inversely correlated with cyclin D1 levels in these prostatic cell lines. Figure 4 indicates that miR-206 levels inversely correlate with cyclin D1 protein levels (Figure 4b). These data suggest that reduced miR-206 expression and a corresponding deregulation of cyclin D1 expression may be one of the mechanisms that contributes to prostatic tumourigenesis.
Figure 4.
Relationship between miR-206 and cyclin D1 expression in normal and cancer prostate cells. RNA was prepared from LNCaP, DU145, PC-3 and PrEC cells. (a) Levels of miR-206 were determined by real-time quantitative PCR (RT-qPCR). RNU24 levels were used as a loading control. Values represent mean±s.e.m. (b) Protein extracts were prepared from LNCaP, PC-3, DU145 and PrEC cells and analyzed by western blot for cyclin D1 expression. Beta-actin served as a loading control.
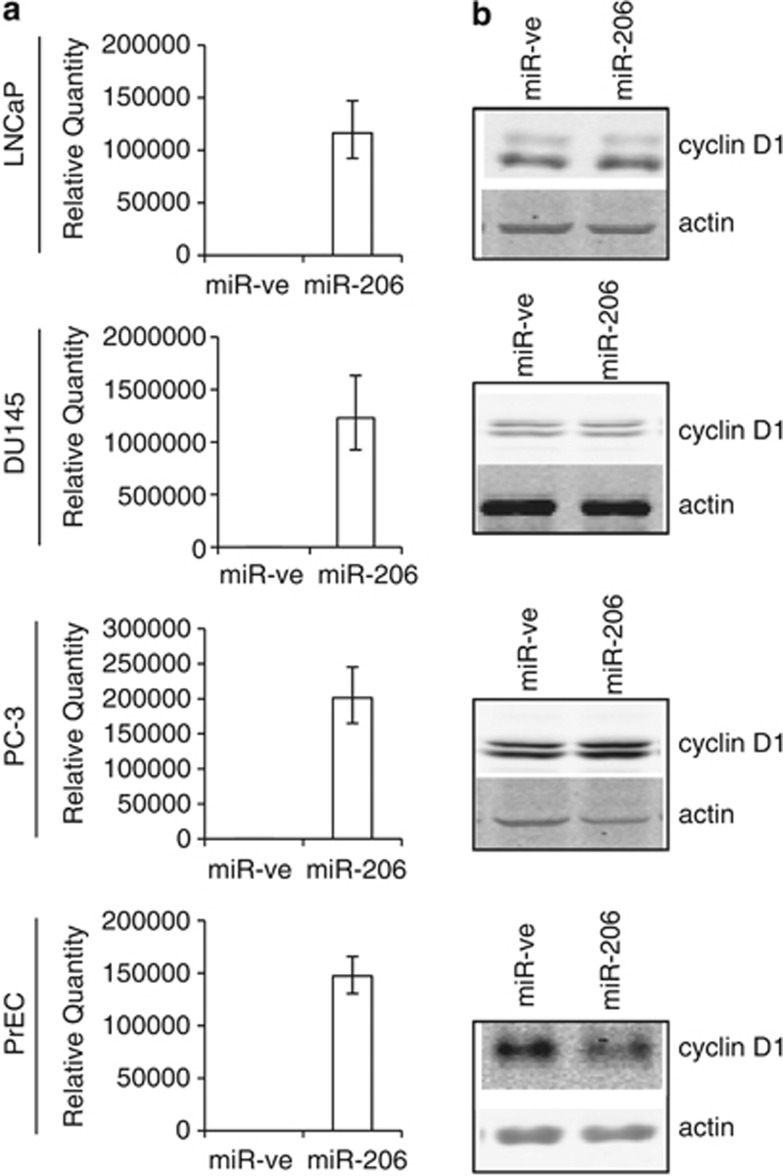
To investigate if miR-206 can modulate endogenous cyclin D1 expression in prostatic cells, we examined the effects of miR-206 overexpression on cyclin D1 protein levels in PrECs and PCa cell lines. Upon transfection, miR-206 was overexpressed efficiently in all cell lines as determined by RT-qPCR (Figure 5a). Notably, miR-206 overexpression did not reduce cyclin D1 protein levels in any of the PCa cell lines, but caused a reduction in cyclin D1 protein expression in primary PrECs (Figure 5b). Taken together, these data indicate that overexpression of miR-206 in primary mouse and human cells (for example, C2C12, 3T3-L1, and PrECs) as well as human breast cancer cells (for example, MDA-MB-231) causes a decrease in cyclin D1 protein levels, but the expression of cyclin D1 in PCa cells is insensitive to miR-206 regulation.
Figure 5.
Differential effects of miR-206 in primary and prostate cancer cell lines. LNCaP, DU145, PC-3 and PrEC cells were reverse transfected with miR-ve or miR-206 for 48 h. (a) RNA extracts were prepared and miR-206 levels were determined by qRT-PCR. RNU24 levels were used as a loading control. (b) CSK extracts were prepared and cyclin D1 protein levels were determined by western blot analysis. Actin served as a loading control.
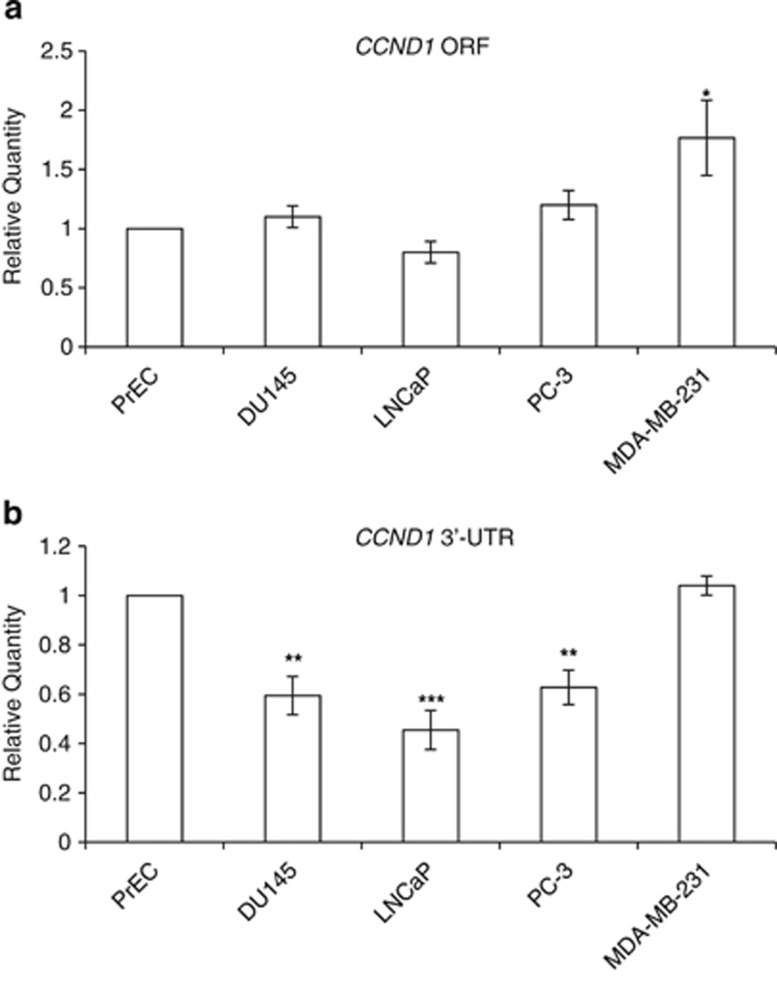
This opens the question, ‘Why can miR-206 regulate cyclin D1 in non-transformed prostatic cells but not in PCa cells?' It has been established that cancer cell lines express mRNA isoforms with shorter 3′-UTRs, leading to a subsequent loss of miRNA-mediated repression.26 Indeed, these authors detected higher amounts of shorter cyclin D1 mRNA in lung, breast and colon cancer cells compared with non-transformed cell lines. To investigate whether cyclin D1 in PCa cells has a shorter 3′-UTR that does not contain the miR-206 binding site, we prepared RNA from PrECs, DU145, LNCaP and PC-3 cells and performed RT-qPCR using primers that amplified the CCND1 ORF or the CCND1 3′-UTR (see Supplementary Figure 5). Cyclin D1 in MDA-MB-231 cells is sensitive to miR-206 regulation, therefore we also prepared RNA from these cells to represent a positive control. Comparable levels of cyclin D1 mRNA containing the ORF was detected in all prostatic cell lines indicating that higher levels of cyclin D1 expression is not the reason why miR-206 in ineffective in PCa cell lines (Figure 6a). However, PCa cell lines do express significantly lower levels of cyclin D1 mRNA containing a 3′-UTR when compared with primary non-transformed PrECs or MDA-MB-231 cells (Figure 6b). This selective expression of cyclin D1 mRNA lacking the 3′-UTR explains why cyclin D1 protein expression is insensitive to miR-206 regulation in PCa cells, and suggests an additional mechanism to promote tumourigenicity by blocking the tumor suppressor function of miR-206.
Figure 6.
Prostatic cancer cells lack a CCND1 3′-UTR. RNA from PrEC, DU145, LNCaP, PC-3 and MDA-MB-231 cells was reversed transcribed into cDNA. qPCR was used to amplify (a) the CCND1 ORF and (b) the CCND1 3′-UTR from these various cell lines. Shown is the average relative quantity (±s.e.m.) of four experiments normalized to TATA-binding protein (TBP) and to the amount of RNA expressed in PrEC.
Discussion
In this study, we have demonstrated that the tumor suppressor miR-206 regulates cyclin D1 and that some cancers have established mechanisms to evade the miR-206 pathway. Cyclin D1 is a well-established oncogene in many different cancers.13 We show for the first time that miR-206 directly targets full-length human CCND1 3′-UTR. In addition, we show overexpression of miR-206 in breast cancer and non-cancer cells universally downregulate cyclin D1 protein expression, resulting in an arrest in G1 phase of the cell cycle and a decrease in cell proliferation/viability. Finally, we show that several PCa cell lines have either lost miR-206 expression and/or selectively express cyclin D1 mRNA lacking the miRNA 3'UTR regulatory elements, resulting in deregulated cyclin D1 expression that can contribute to their tumourigenic state.
MiR-206 was initially described as a muscle-specific miRNA, and we have corroborated previous studies that show miR-206 is upregulated during differentiation of C2C12 cells and targets mouse cyclin D1.5,11 However it is now accepted that miR-206 is present and functions in other tissues. For example, miR-206 has been shown to target notch3 and inhibit apoptosis in a cervical cancer cell line.8 Additionally, miR-206 levels are high in ERα-negative cells compared with ERα-positive human breast cancer tissues, suggesting that miR-206 is a key regulator of ERα in breast carcinogenesis.7 Indeed, miR-206 downregulates ERα mRNA and protein levels in ERα-positive breast cancer cells resulting in inhibition of cell growth.6 In an effort to study the role of miR-206 directly on cyclin D1 regulation, we conducted our experiments in ERα-negative breast cancer cells (MDA-MB-231) as well as ERα-negative cells reconstituted with miRNA-resistant ERα. These measures allowed us to conclude that the miR-206-induced G1 cell cycle arrest as well as the decease in cell proliferation/viability is independent of ERα signaling. However, miR-206 has multiple cellular targets, thus the miR-206-induced decrease in clonogenic survival in breast cancer cells may be dependent or independent on cyclin D1 function. Another study shows that miR-206 targets cyclin D1 in lung cancer; however, they do not definitely show that the miR-206-induced decrease in cell proliferation that they observed was a direct consequence of cyclin D1 repression.11 Here we demonstrate that we can inhibit this miR-206-induced phenotype with exogenous cyclin D1 that is resistant to miR-206 regulation.
Cyclin D1 is also overexpressed in prostate cancer.24 We found that miR-206 levels inversely correlate with cyclin D1 protein levels in prostate cells, such that miR-206 levels are high and cyclin D1 levels are low in non-transformed prostatic cells, whereas miR-206 levels are low and cyclin D1 levels are high in PCa cells. Similar to other cellular systems overexpression of miR-206 in non-transformed PrECs causes a reduction in cyclin D1 protein levels. Intriguingly, cyclin D1 is insensitive to miR-206 regulation in PCa cells. Our data indicate this differential effect of miR-206 on cyclin D1 is due to the absence of a 3′-UTR in PCa cells rendering cyclin D1 insensitive to miR-206 regulation.
As cyclin D1 is activated in many cancers, this has prompted much focus on the development of anti-cyclin D1 therapy.13 As well as the classical cell cycle regulator role of cyclin D1, it is becoming increasingly clear that cyclin D1 conveys CDK-independent functions. Recent findings indicate that cyclin D1 regulates transcription factors, histone acetylation, cellular metabolism and cell migration.13,16 It is suspected that these additional novel functions of cyclin D1 contribute to tumourigenesis. This would suggest direct targeting of the cyclin D1 gene, RNA, or protein may be a more successful approach in treating cyclin D1-dependent cancers rather than the conventional strategy of finding small molecule CDK inhibitors. Our results suggest that miR-206-based anti-cyclin D1-targeted therapy would be beneficial in cancers where cyclin D1 is overexpressed and contains a 3′-UTR.
Materials and methods
Cell culture
MCF-7, MDA-MB-231, PC-3, DU145 and LNCaP cells were obtained from ATCC (Rockville, MD, USA). MDA-MB-231 cells overexpressing ERα (MDA-MB-231-ERα) were kindly provided by Dr George Reid (EMBL-Heidelberg). MCF-7, MDA-MB-231, MDA-MB-231 ERα, PC-3 and DU145 were maintained in DMEM medium (Sigma Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (Life Technologies/Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma Aldrich). LNCaP cells were maintained in RPMI medium (Sigma Aldrich) supplemented with 10% fetal bovine serum (FBS) (Life Technologies/Invitrogen), 100 U/ml penicillin and 100 mg/ml streptomycin (Sigma Aldrich). Clonetics Primary Prostate Epithelial Cells (PrEC) were obtained from Lonza (Walkersville, MD, USA) and maintained in Prostate Epithelial Cell Medium BulletKit, Clonetics, PrEGM, BulletKit (CC-3166) containing the following growth supplements: bovine pituitary extract, Hydrocortisone, human epidermal growth factor, Epinephrine, Transferrin, Insulin, Retinoic Acid, Triiodothyronine, and GA-1000. All cells were cultured at 37 °C and 5% CO2.
miRNA overexpression
miRNA-206 was overexpressed by reverse transfection of 100nM Pre-miR miR-206 precursor molecule (miR-206; Life Technologies/Ambion, Carlsbad, CA, USA) using siPORT NeoFX transfection reagent (Life Technologies/Ambion) as per manufacturer's instructions and compared with Pre-miR negative control (miR-ve). This is a random sequence Pre-miR molecule that has been extensively validated in cell lines and tissues to have no effect on known miRNA function (Life Technologies/Ambion-AM17110).
Luciferase reporter assay
Luciferase reporter plasmid containing the full-length human cyclin D1 3′-UTR was obtained from Switchgear Genomics (Carlsbad, CA, USA). The putative miR-206 target site in the cyclin D1 3′-UTR was mutated using the QuikChange XL Site-Directed Mutatgenesis Kit (Stratagene, La Jolla, CA, USA). H1299 cells were co-transfected with 100 ng wild-type or mutant cyclin D1 3′-UTR luciferase reporter and 5 ng Renilla luciferase reporter using FuGENE HD (Roche Applied Science, Indianapolis, IN, USA). Twenty-four hours, later cells were reverse transfected with 5 nM miR-ve or miR-206 using siPORT NeoFX transfection agent (Life Technologies/Ambion) as per manufacturer's instructions. Cells were incubated for a further 24 h and luciferase activity was assessed using the Dual-Luciferase Assay System (Promega, Fitchburg, WI, USA) as per manufacturer's instructions. Firefly luciferase units were normalized against Renilla luciferase units to control for transfection efficiency.
RNA and protein analyses
Total RNA was prepared by the TRI Reagent extraction method (Life Technologies/Ambion) according to manufacturer's instructions. miRNA and mRNA analyses were performed by real-time quantitative PCR (RT-qPCR) using TaqMan MicroRNA Assays and TaqMan Gene Expression Assays respectively (Life Technologies/Applied Biosystems, Carlsbad, CA, USA). Relative quantification was performed using RNU24 as endogenous control for miRNAs and TATA-box binding protein for mRNAs. Western blot on protein extracts were performed as described in Barkley et al.27 In brief, samples were normalized for protein content, then separated by SDS-PAGE, transferred to nitrocellulose and analyzed by immune-blotting with the following antibodies: rabbit anti-cyclin D1 (Cell Signaling Technology, Danvers, MA, USA), mouse monoclonal anti-beta-actin (Sigma Aldrich) and anti-α-tubulin (Santa Cruz Biotechnology, Dallas, TX, USA).
3-(4,5–dimethylthiazol–2–yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cells were reverse transfected with 100 nM miR-ve or miR-206 using siPORT NeoFX transfection agent (Life Technologies/Ambion) as per manufacturer's instructions. MTT assay was performed 72 h post transfection. Briefly, 0.1 volume of MTT at a concentration of 5 mg/ml was added to cells. Cells were incubated at 37 oC for 2 h, 1 volume stop mix (40% dimethyl formamide, 20% SDS) was then added to stop the reduction and solubilize formazan. Cells were incubated for a further 1 h with mixing. Formazan was dissolved by pipetting and absorbance was measured at 550 nm using the Wallac plate reader (Perkin-Elmer, Waltham, MA, USA).
Cell Cycle analysis by flow cytometry
Cell cycle distribution was assessed by analysis of propidium iodide stained cells. Briefly, miR-ve and miR-206 expressing cells were harvested by trypsinization followed by fixation in 35% ethanol and stored at 4 °C overnight. Cells were washed in PBS, pelleted and resuspended in 200 μl of 50 μg/ml propidium iodide/8 μg/ml RNAse A solution for 30 min at room temperature in the dark. Following this, results were acquired by flow cytometry (FACScanto, BD Bioscience, San Jose, CA, USA) and analyzed by FACSdiva software (BD Bioscience).
Colony formation assay
Cells were reverse transfected with miR-ve or miR-206 (as described above). Twenty-four hours later, miR-ve and miR-206 expressing cells were trypsinized, counted and replated at a density of 500 cells/10-cm dish. Ten days later, colonies resulting from the surviving cells were fixed, stained with crystal violet and counted. MDA-MB-236 cells were transfected with a Cyclin D1 expression vector or empty vector control using FuGENE HD. The full-length Cyclin D1 expression vector was obtained from Dr Cyrus Vaziri (University of North Carolina). Twenty-four hours later, transfected cells were reverse transfected with miR-ve or miR-206 and colony formation performed as described above.
Statistical analysis
All data shown are representative of experiments that were repeated at least three times with similar results on each separate occasion. Data are shown as mean±s.e.m. Data were compared by the paired Student's t-test. A P-value of <0.05 was considered statistically significant.
Acknowledgments
This work was directly supported by a Medical Research Charities Group/Health Research Board/Irish Cancer Society Joint funding Prostate Cancer grant [PCI11BAR to LRB] and by a Flight Attendant Medical Research Institute (FAMRI) grant [072101 to LRB].
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis)
Supplementary Material
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857–866. [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007; 449: 682–688. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008; 451147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 2006; 174: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol 2007; 21: 1132–1147. [DOI] [PubMed] [Google Scholar]
- Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. miR-206 expression is down-regulated in estrogen receptor alpha-positive human breast cancer. Cancer Res 2008; 68: 5004–5008. [DOI] [PubMed] [Google Scholar]
- Song G, Zhang Y, Wang L. MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem 2009; 284: 31921–31927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ling C, Bai Y, Zhao J. MicroRNA-206 is associated with invasion and metastasis of lung cancer. Anat Rec (Hoboken) 2011; 294: 88–92. [DOI] [PubMed] [Google Scholar]
- Zhang T, Liu M, Wang C, Lin C, Sun Y, Jin D. Down-regulation of MiR-206 promotes proliferation and invasion of laryngeal cancer by regulating VEGF expression. Anticancer Res 2011; 31: 3859–3863. [PubMed] [Google Scholar]
- Alteri A, De Vito F, Messina G, Pompili M, Calconi A, Visca P et al. Cyclin D1 is a major target of miR-206 in cell differentiation and transformation. Cell Cycle 2013; 12: 3781–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev 1993; 7: 812–821. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Lamb J. The activities of cyclin D1 that drive tumorigenesis. Trends Mol Med 2004; 10: 158–162. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120: 15–20. [DOI] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ et al. Combinatorial microRNA target predictions. Nat Genet 2005; 37: 495–500. [DOI] [PubMed] [Google Scholar]
- Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol 2005; 23: 4215–4224. [DOI] [PubMed] [Google Scholar]
- Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev 2006; 20: 2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D et al. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell 2003; 11: 695–707. [DOI] [PubMed] [Google Scholar]
- Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker MG, Truss M et al. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene 1996; 12: 2315–2324. [PubMed] [Google Scholar]
- Foster JS, Wimalasena J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol Endocrinol 1996; 10: 488–498. [DOI] [PubMed] [Google Scholar]
- Grillo M, Bott MJ, Khandke N, McGinnis JP, Miranda M, Meyyappan M et al. Validation of cyclin D1/CDK4 as an anticancer drug target in MCF-7 breast cancer cells: effect of regulated overexpression of cyclin D1 and siRNA-mediated inhibition of endogenous cyclin D1 and CDK4 expression. Breast Cancer Res Treat 2006; 95: 185–194. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Q, Cui Y, Liu ZY, Zhao W, Wang CL et al. Knockdown of cyclin D1 inhibits proliferation, induces apoptosis, and attenuates the invasive capacity of human glioblastoma cells. J Neurooncol 2012; 106: 473–484. [DOI] [PubMed] [Google Scholar]
- Wei M, Zhu L, Li Y, Chen W, Han B, Wang Z et al. Knocking down cyclin D1b inhibits breast cancer cell growth and suppresses tumor development in a breast cancer model. Cancer Sci 2011; 102: 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res 1999; 59: 803–806. [PubMed] [Google Scholar]
- Han EK, Lim JT, Arber N, Rubin MA, Xing WQ, Weinstein IB. Cyclin D1 expression in human prostate carcinoma cell lines and primary tumors. Prostate 1998; 35: 95–101. [DOI] [PubMed] [Google Scholar]
- Mayr C, Bartel DP. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 2009; 138: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley LR, Palle K, Durando M, Day TA, Gurkar A, Kakusho N et al. c-Jun N-terminal kinase-mediated Rad18 phosphorylation facilitates Poleta recruitment to stalled replication forks. Mol Biol Cell 2012; 23: 1943–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.