Abstract
Mounting evidence highlights specific forms of psychological stress as risk factors for ill health. Particularly strong evidence indicates that childhood adversity and adulthood trauma exposure increase risk for physical and psychiatric disorders, and there is emerging evidence that inflammation may play a key role in these relationships. In a population-based sample from the Health and Retirement Study (n = 11,198, mean age 69 ± 10), we examine whether childhood adversity, adulthood trauma, and the interaction between them are associated with elevated levels of the systemic inflammatory marker high sensitivity C-reactive protein (hsCRP). All models were adjusted for age, gender, race, education, and year of data collection, as well as other possible confounds in follow-up sensitivity analyses. In our sample, 67% of individuals had experienced at least one traumatic event during adulthood, and those with childhood adversity were almost three times as likely to have experienced trauma as an adult. Childhood adversities and adulthood traumas were independently associated with elevated levels of hsCRP (β = 0.03, p = 0.01 and β = 0.05, p < 0.001, respectively). Those who had experienced both types of stress had higher levels of hsCRP than those with adulthood trauma alone, Estimate = −0.06, 95% CI [−0.003, −0.12], p = 0.04, but not compared to those with childhood adversity alone, Estimate = −0.06, 95% CI [0.03, −0.16], p = 0.19. There was no interaction between childhood and adulthood trauma exposure. To our knowledge, this is the first study to examine adulthood trauma exposure and inflammation in a large population-based sample, and the first to explore the interaction of childhood adversity and adulthood trauma with inflammation. Our study demonstrates the prevalence of trauma-related inflammation in the general population and suggests that childhood adversity and adulthood trauma are independently associated with elevated inflammation.
Keywords: childhood adversity, adulthood trauma, C-reactive protein, immune system, inflammation
1. Introduction
Exposure to traumatic psychological stress increases risk for psychiatric disorders (Brown et al., 2000; Carr et al., 2013; Turner et al., 1995) as well as physical morbidity and mortality (Glaesmer et al., 2011; Krause et al., 2004; Shonkoff et al., 2012). Particularly strong evidence links childhood stress exposure with adverse health outcomes. Adversity in childhood increases risk for adult-onset mood and anxiety disorders in the general population, and depression and post-traumatic stress disorder (PTSD) in military personnel (Calabro et al., 2003; Kessler et al., 1997). Childhood adversities also increase risk for physical illnesses including cardiovascular disease, diabetes, and metabolic disorders (Galobardes et al., 2006, Tamayo et al., 2010). Among adults, those with combat or non-combat trauma exposure had an increased rate of psychiatric disorders (Brown et al., 2000; Prigerson et al., 2002) and physical illnesses including heart failure, stroke, and autoimmune disorders (O’Donovan et al., 2015; Spitzer et al., 2009). The biological mechanisms underlying the link between trauma and poor health are not well understood, but there is evidence that inflammation may play a key role (e.g., Danese et al., 2007; O’Donovan et al. 2012).
Emerging evidence indicates that elevated inflammation is associated with psychopathology. Large bodies of research now link depressive and anxiety disorders with elevated levels of inflammation (Howren et al., 2009; O’Donovan et al., 2010; O’Donovan et al., 2013; Toker et al., 2005). PTSD has also been linked with elevated levels of inflammatory markers, including C-reactive protein (CRP) (Michopoulos et al., 2015), and higher levels of CRP predicted risk for PTSD symptoms in a prospective study of 2610 war zone–deployed Marines (Eraly et al., 2014). Evidence from animal models and from experimental human research suggests that elevated inflammation is a causal factor that promotes psychiatric symptoms rather than a mere correlate of the disorder (Dantzer et al., 2008; Eisenberger et al., 2010; Raison and Miller, 2013). Chronic low-grade inflammation is also an established risk factor for physical diseases that are associated with trauma exposure, including cardiovascular disease and autoimmune disorders (Harris et al., 1999; Libby 2002; O’Donovan et al., 2015).
Both childhood adversity and specific types of adulthood trauma exposure have been associated with elevated levels of systemic inflammation. Childhood maltreatment has been linked with elevated levels of high sensitivity C-reactive protein (hsCRP) in participants 20 years later (Danese et al., 2007). Similarly, childhood adversity, defined as having experienced one or more unusually stressful events in childhood, has been associated with elevated adulthood levels of systemic inflammation as well as shorter telomere length (Kiecolt-Glaser et al., 2011; Slopen et al., 2010; Slopen et al., 2013). Childhood adversity has also been associated with higher expression of pro-inflammatory genes in a small pilot study (n = 114) of Health and Retirement Study participants (Levine et al., 2015). Across the entire lifespan, greater cumulative exposure to different categories of trauma has also been linked with elevated hsCRP (O’Donovan et al., 2012), as have specific types of adulthood trauma such as being a prisoner of war or a victim of intimate partner violence (Dekaris et al., 1993; Woods et al., 2005). However, in contrast with the large number of studies on childhood adversity and inflammation, there are very few studies that examine the relationship between adulthood trauma exposure and inflammation.
Childhood adversity appears to increase vulnerability to the effects of later stressful events. For example, stress sensitization models hypothesize that childhood adversity may sensitize individuals to psychiatric psychopathology by lowering their tolerance to later stressors (Hammen et al., 2000). One longitudinal study of young women showed that those with a history of childhood adversities were more likely to become depressed following less total stress than women without a similar history, after controlling for previous depression and current chronic stressful conditions (Hammen et al., 2000). Relatedly, adults with a history of childhood adversity or trauma are more likely to develop PTSD following subsequent exposure to adulthood trauma (Breslau et al., 1999; Brewin et al., 2000; Pratchett et al., 2011). The stress sensitivity associated with childhood trauma may be particularly potent in those with genetic vulnerability; both the 5-HTTLPR genotype and FKBP5 polymorphisms have been shown to interact with childhood adversity and adulthood trauma to predict adult PTSD (Binder et al., 2008; Xie et al., 2009). While the mechanisms of stress sensitization are unclear, alterations of hypothalamic-pituitary-adrenal (HPA) axis functioning may play a role (Gunnar 1998, Heim et al., 2001; Miller et al., 2011). For instance, early life stress has been shown to induce sensitization of corticotropin-releasing factor (Heim et al., 2001). This sensitization is associated with pro-inflammatory tendencies that may be exacerbated by behavioral proclivities such as hypervigilance, poor social relationships, and adverse health behaviors, which are themselves associated with childhood adversity (Miller et al., 2011). To our knowledge, no population-based studies have examined whether people who have experienced childhood adversity are more susceptible to inflammation associated with adulthood trauma exposure.
In the present study, we examine whether exposure to childhood adversity, adulthood trauma, and their interaction are associated with elevated inflammation in a large population-based sample of Americans over the age of 50. Advantages of a population-based study were that they allowed for estimation of the distribution and prevalence of childhood adversity and adulthood trauma, minimization of confounders to exposures and outcomes, and maximization of external validity and generalizability. We analyzed both the presence and the number of childhood adversities and adulthood traumas, because the number of adverse experiences can increase risk for inflammation and for the development of physical and mental disorders (Coker et al., 2005; McLaughlin et al., 2010; O’Donovan et al., 2012). We also considered the contribution of health behaviors, as both childhood trauma and specific types of adulthood trauma have been associated with smoking, alcohol use, obesity, and physical and mental health conditions (Dube et al., 2003; Kaysen et al., 2007; Kessler et al., 1997; Pizarro et al., 2006; Vieweg et al., 2007; Williamson et al., 2002). These health behaviors have in turn been linked to elevated inflammation (O’Connor and Irwin, 2010). Inflammation was measured using hsCRP, an acute-phase reactant and nonspecific marker of inflammation that is secreted by the liver and adipose tissues (Libby 2002). Current evidence supports the use of hs-CRP as the analyte of choice for systemic inflammation, after considering the various analytes’ stabilities; the analytes’ assay precision, accuracy, and availability; and the availability of standards for proper assay calibration (Pearson et al., 2003). Given prior research linking childhood adversity and adulthood trauma to elevated systemic inflammation, we predicted that exposure to childhood adversity and adulthood trauma would independently be associated with higher levels of hsCRP. As previous studies suggest that childhood adversity may increase vulnerability to adulthood stressors through HPA axis sensitization and pro-inflammatory activity, we aimed to assess the relative contribution of childhood adversity and adulthood trauma to inflammation by assigning study members to 1 of 4 groups as follows: no history of childhood adversity or adulthood trauma; history of childhood adversity but no adulthood trauma; no history of childhood adversity but history of adulthood trauma; history of both childhood adversity and adulthood trauma. We hypothesized that those with a history of both childhood adversity and adulthood trauma would have elevated levels of inflammation compared to those with a history of childhood adversity or adulthood trauma alone. Those with neither a history childhood adversity nor adulthood trauma would have the lowest levels of inflammation. We further predicted that there would be a significant interaction between the two types of stress.
2. Methods
2.1 Participants
Participants were drawn from the Health and Retirement Study (HRS), a longitudinal study of a population-based sample of more than 20,000 Americans over the age of 50. The target population for the original HRS cohort includes all adults in the contiguous United States born during the years 1931–1941 who reside in households, with a 2:1 oversample of African-American and Hispanic populations. The original sample has been refreshed with new birth cohorts over the years. Starting in 2006, the study implemented a psychosocial questionnaire that included assessments of childhood adversity and adulthood trauma (Clarke et al., 2008). In the same year, the study also implemented biomarker assessments (Crimmins et al., 2013), which were completed by half of the participants in 2006, and half of the participants in 2008. For our analyses, we used the combined data from 2006 and 2008. We selected participants from the original sample who had 1) completed the measures for either childhood adversity or adulthood trauma, and 2) had valid hsCRP values in either 2006 or 2008. A total of 11,198 participants met these criteria.
2.2 Measures
2.2.1 Childhood Adversity
Childhood adversity was evaluated at baseline using all items from the measure developed by Krause, Shaw, and Cairney that were assessed in both 2006 and 2008 (Krause et al., 2004). Respondents were presented with three adverse events and asked whether they experienced each one before the age of 18. These included: having to do a year of school over again, having parents who drank or used drugs so often that it caused problems in the family, and being physically abused by a parent. An unweighted sum of the three events was used to gauge the level of childhood adversity.
2.2.2 Adulthood Trauma
Adulthood trauma was assessed at baseline using all available items from the measure developed by Krause, Shaw, and Cairney (Krause et al., 2004). Respondents were presented with seven potentially traumatic events and asked whether they experienced each one at any point in their lifetime after the age of 18. These included: having had a child who died; having been in a major fire, flood, earthquake or natural disaster; having fired a weapon in combat or been fired upon in combat; ever having a spouse, partner, or child addicted to drugs or alcohol; having been a victim of a serious physical attack or assault; ever having a life-threatening illness or accident; and ever having a spouse or child experience a life-threatening illness or accident. An unweighted sum of the seven traumas was used to gauge the level of adulthood trauma.
2.2.3 Covariates
Self-report questionnaires were administered to all participants to gather information on demographics and health behaviors. Race was categorized as Caucasian, African-American, or other. As in previous HRS studies on childhood adversity and trauma (Levine et al., 2015), education was assessed by asking participants whether they had completed at least nine years of formal education. Smoking was assessed by asking participants whether or not they currently smoked cigarettes. Body mass index (BMI) was assessed by dividing participants’ self-reported weight (kg) by the square of their self-reported height (m2). Alcohol use was measured by asking participants the number of days per week they drank alcohol, averaged over the past three months. Physical activity was evaluated by asking participants whether or not they participated in vigorous activity more than once a week. Overall health was calculated as the unweighted sum of the number of current or prior diagnoses of all chronic health conditions documented in the HRS study (Fisher et al., 2005): hypertension, diabetes mellitus, cancer (excluding minor skin cancer), chronic lung disease (excluding asthma), heart conditions (heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems), stroke, arthritis, and psychiatric problems. All covariates were assessed in 2006.
2.2.4 High sensitivity C-reactive protein (hsCRP)
Blood samples were obtained from participants by cleaning a finger with an alcohol prep pad and pricking the finger with a lancet. Samples were collected on a blood spot card, air-dried for 10 to 15 minutes, and placed in foil pouches. The samples were then mailed to the University of Vermont and assayed for hsCRP using a standard enzyme-linked immunosorbent assay (ELISA). The hsCRP assay had a lower limit of detection of 0.04 mg/L, with an within-assay coefficient of variability (CV) of 8% and an inter-assay CV of 11%.
In our study, original blood spot hsCRP values were used for all primary analyses. In sensitivity analyses to exclude acute inflammation, calculated whole-blood equivalent hsCRP values were used to exclude participants with hsCRP values greater than 10 mg/L (see Section 2.3). To calculate whole-blood equivalent hsCRP values, the blood spot hsCRP value from HRS was regressed onto the whole-blood equivalent hsCRP value from The National Health and Nutrition Examination Survey (NHANES) according to HRS study protocol (Crimmins et al., 2013). As both HRS and NHANES are population-based studies that are intended to represent the non-institutional U.S. population, their distributions of hsCRP were assumed to be similar. The log-transformed value of both assays were determined at 100 weighted percentiles, and the HRS blood-spot values were then transformed onto the whole-blood NHANES values after adjustment for between-lab differences. The blood spot hsCRP values and whole-blood hsCRP values were strongly correlated (Pearson R = 0.99) and linearly related (HRS hsCRP value = 0.370 + NHANES hsCRP value x 1.077). Further details of this calculation are documented elsewhere (Crimmins et al., 2013).
2.3 Analytic strategy
Adversity was examined as both continuous (total number) and dichotomous (exposed vs. not exposed) measures. A series of hierarchical linear regression models were used to separately assess the association between level of childhood adversity and hsCRP, and level of adulthood trauma and hsCRP. Due to the few number of individuals with three childhood adverse events (n = 79), we regrouped the number of childhood adverse events as 0, 1, and 2+. Similarly, due to the few number of individuals with 6 or 7 adulthood trauma events (n = 25 and n = 2 respectively), we regrouped the number of adulthood trauma events as 0, 1, 2, 3, 4, and 5+. Adversity scores were skewed towards 0. Raw hsCRP values were not normally distributed and we therefore completed a log-transformation of hsCRP to approximate a more normal distribution.
To assess the interaction of childhood adversity and adulthood trauma on hsCRP, we used analysis of covariance (ANCOVA) models. Childhood adversity was characterized as absent (0 childhood adverse events, 7761 participants) or present (1 or more childhood adverse events, 3261 participants); similarly, adulthood trauma was characterized as absent (0 adulthood trauma events, 3489 participants) or present (1 or more adulthood trauma events, 7248 participants). Finally, participants were categorized into four groups: 1) no childhood adversity or adulthood trauma; 2) childhood adversity and no adulthood trauma; 3) adulthood trauma and no childhood adversity; and 4) childhood adversity and adulthood trauma, and we examined whether hsCRP levels differed among the four groups. In follow-up planned comparisons, we examine whether individuals who experienced both childhood adversity and adulthood trauma had higher levels of hsCRP than the other three groups.
All models, unless otherwise specified, were adjusted for age, gender, race, education, and year of data collection (2006 or 2008), which all had a 98% response rate or higher. In follow-up analyses, models were further adjusted for health behaviors that were significantly associated with either childhood adversity or adulthood trauma at a significance level of p < 0.05. Recent evidence indicates that hsCRP levels increase with age (Wener et al., 2000) and that levels above 10 mg/L can be observed even in cases of chronic inflammation, particularly among those who are female, obese, have low income, and use sex hormones (Ishii et al., 2012). Nonetheless, we ran sensitivity analyses excluding all participants with NHANES whole-blood equivalent hsCRP values greater than 10 mg/L in order to exclude those with possible acute inflammation due to infection or injury. The threshold for statistical significance was set at p < .05. All statistical analyses were performed with SPSS 22.0.
3. Results
3.1 Participant Characteristics
Sample characteristics for participants with complete data for childhood adversity or adulthood trauma in addition to valid hsCRP values are described in Table 1. The average participant was 69 years old, with a standard deviation of 10 years. 39% of participants were male and 84% were white. 67% had experienced at least one adulthood trauma. Participants had an average childhood adversity score of 0.37 ± 0.62 and an average adulthood trauma score of 1.25 ± 1.20. This difference in means partly reflects the difference in number of items in the two scales.
Table 1.
Characteristics of participants by presence of childhood adversity and adulthood trauma
| Characteristic | Childhood Adversity | p value | Adulthood Trauma | p value | ||
|---|---|---|---|---|---|---|
| Absent n = 7761 |
Present n = 3261 |
Absent n = 3489 |
Present n = 7248 |
|||
| Age | 69.36 (10.35) | 66.87 (9.95) | <0.001** | 67.78 (10.48) | 68.84 (10.16) | <0.001** |
|
| ||||||
| Male sex, n (%) | 2877 (37.6) | 1455 (45.2) | <0.001** | 1311 (38.0) | 2935 (41.0) | 0.003** |
|
| ||||||
| White race, n (%) | 6445 (84.2) | 2732 (84.8) | 0.113 | 2905 (84.3) | 6086 (85.1) | 0.302 |
|
| ||||||
| Data collection 2006, n (%) | 4045 (52.1) | 1532 (47.0) | <0.001** | 1770 (50.7) | 3661 (50.5) | 0.831 |
|
| ||||||
| < 9 years of education, n (%) | 644 (8.3) | 289 (9.0) | 0.312 | 319 (9.3) | 577 (8.0) | 0.038* |
|
| ||||||
| Health related behaviors | ||||||
|
| ||||||
| Current smoker, n (%) | 841 (10.8) | 490 (15.0) | 0.001** | 392 (11.2) | 914 (12.6) | 0.975 |
|
| ||||||
| BMI | 29.16 (14.15) | 30.24 (16.03) | <0.001** | 29.24 (14.58) | 29.58 (14.62) | 0.259 |
|
| ||||||
| Alcohol use | 2.03 (2.43) | 2.01 (2.45) | 0.694 | 2.05 (2.38) | 2.02 (2.46) | 0.550 |
|
| ||||||
| Physical inactivity, n (%) | 5921 (76.3) | 2524 (77.4) | 0.214 | 2632 (75.4) | 5572 (76.9) | 0.097 |
|
| ||||||
| Physical and mental health conditions | 2.23 (1.44) | 2.44 (1.51) | <0.001** | 1.93 (1.35) | 2.37 (1.49) | <0.001** |
Notes. Values reported as Mean (Standard Deviation) unless otherwise indicated. BMI = body mass index. P values are based on Pearson chi squared for ratios and t-tests for continuous variables.
p < .05,
p < .01.
Presence of childhood adversity was associated with older age, male sex, and data collection in 2008, but was not associated with race, education, alcohol use, or physical inactivity. Presence of adulthood trauma was associated with younger age, male sex, and having at least nine years of education, but was not associated with race, data collection year, smoking status, BMI, alcohol use, or physical inactivity. While both childhood adversity and adulthood trauma were associated with a higher number of physical and mental health conditions as predicted, only childhood adversity was associated with adverse health behaviors, specifically current smoking and higher BMI.
3.2 Association between Childhood Adversity and Adulthood Trauma
Notably, presence of childhood adversity was associated with presence of adulthood trauma (β = 0.10, p < 0.001). In fact, those exposed to childhood adversity had a 2.86 fold increased prevalence of adulthood trauma compared to those without a similar history (n = 2322 vs. n = 811). Moreover, participants who reported a higher number of childhood adversities (i.e., a higher dose of childhood adversity) also reported a higher number of adulthood trauma (β = 0.16, p < 0.001).
3.3 Childhood Adversity and Inflammation
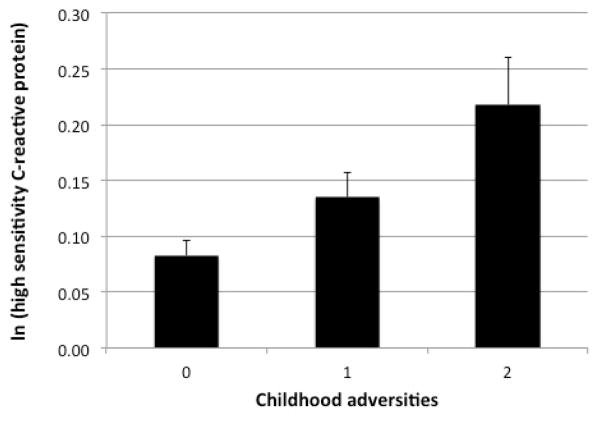
In unadjusted models, both the presence of one or more childhood adversities and the total number of adverse childhood events were significantly associated with elevated levels of hsCRP (β = 0.03, p = 0.006 and β = 0.03, p = 0.001 respectively). Similarly, in adjusted models, both the presence of one or more childhood adversities and the total number of adverse childhood events were significantly associated with elevated levels of hsCRP (β = 0.03, p = 0.002 and β = 0.03, p = 0.001 respectively). Both associations remained significant after further adjustment for adulthood trauma (β = 0.02, p = 0.02 and β = 0.03, p = 0.01 respectively) (Figure 1).
Figure 1.
Relationship between childhood adversities and ln(high sensitivity C-reactive protein)
Notes. Error Bars: +/− 1 SE
3.4 Adulthood Trauma and Inflammation
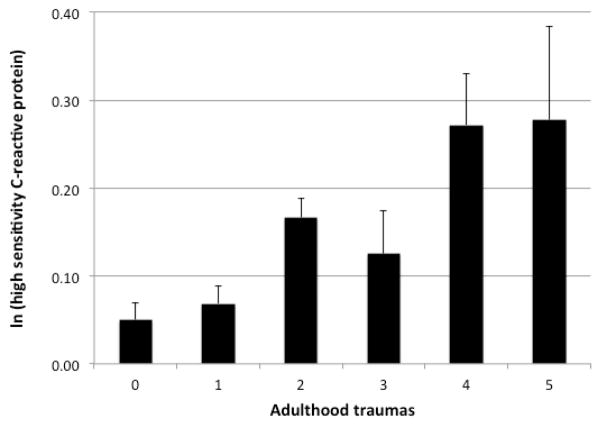
In unadjusted models, both the presence of one or more adulthood traumas and the total number of adulthood traumas were significantly associated with elevated levels of hsCRP (β = 0.03, p = 0.002 and β = 0.05 p < 0.001 respectively). Similarly, in adjusted models, both the presence of one or more adulthood traumas and the total number of adulthood traumas were significantly associated with elevated levels of hsCRP (β = 0.03, p = 0.001 and β = 0.06, p < 0.001 respectively). Associations remained significant after further adjusting for childhood adversity (β = 0.03, p = 0.002 and β = 0.05, p < 0.001 respectively) (Figure 2).
Figure 2.
Relationship between adulthood traumas and ln(high sensitivity C-reactive protein)
Notes. Error Bars: +/− 1 SE
3.5 Interaction of Childhood Adversity and Adulthood Trauma
Although childhood adversity and adulthood trauma were both significantly associated with hsCRP (see 3.2 and 3.3), we found no significant interaction between childhood adversity and adulthood trauma in either unadjusted or adjusted models, F(1, 10564) = 0.02, p = 0.89 and F(1, 10052) = 0.17, p = 0.69, respectively. Using MODPROBE (Hayes and Matthes, 2009), we also examined whether there was an interaction between continuous childhood adversity and adulthood trauma scores on hsCRP. Again, we found no evidence for an interaction in either unadjusted or adjusted models (β = −0.005, SE = 0.02, p = 0.75 and β = −0.01, SE = 0.02, p = 0.47, respectively).
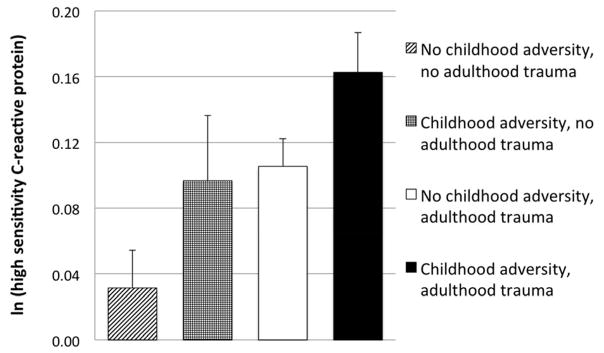
We were a priori interested in differences in inflammation among the four groups of participants with or without childhood adversity and adulthood trauma. The groups included participants with 1) no childhood adversity or adulthood trauma (n = 2636, 25%); 2) childhood adversity and no adulthood trauma (n = 811, 8%); 3) adulthood trauma and no childhood adversity (n = 4799, 45%); and 4) childhood adversity and adulthood trauma (n = 2322, 22%). Our analyses revealed significant differences in levels of hsCRP among these groups, F(3, 10384) = 6.565, p < 0.001. Planned comparisons revealed that participants exposed to both childhood adversity and adulthood trauma had significantly higher levels of hsCRP (ln(M) = 0.16, SD = 1.15) than those with neither childhood adversity nor adulthood trauma (ln(M) = 0.03, SD = 1.18), Estimate = −0.15, 95% CI [−0.08, −0.21], p < 0.001, and compared to those with adulthood trauma alone (ln(M) = 0.11, SD = 1.16), Estimate = −0.06, 95% CI [−0.003, −0.12], p = 0.04. However, although participants with childhood adversity and adulthood trauma had higher levels of hsCRP than those with childhood adversity alone, there were no significant differences in hsCRP between these two groups (ln(M) = 0.10, SD = 1.13), Estimate = −0.06, 95% CI [0.03, −0.16], p = 0.19, (Figure 3). Thus, these data suggest that compared to those without a similar history, individuals with childhood adversity are more likely to have higher levels of inflammation when further exposed to adulthood trauma.
Figure 3.
Relationship between presence or absence of childhood adversity and adulthood trauma and ln(high sensitivity C-reactive protein)
Notes. Error Bars: +/− 1 SE
3.6 Sensitivity Analyses
Because hsCRP levels above 10 mg/L may be an indicator of acute inflammation rather than chronic low-grade systemic inflammation (Pearson et al., 2003), we excluded these cases from follow-up analyses. After excluding individuals with hsCRP levels > 10 mg/L (n = 1099), the presence and number of childhood adverse events remained significantly associated with hsCRP (β = 0.03, p = 0.003 and β = 0.03, p = 0.002, respectively), as did presence and number of adulthood traumas (β = 0.02, p = 0.02 and β = 0.04, p < 0.001, respectively). The interaction was still not statistically significant, F(1, 10052) = 0.03; p = 0.86. The difference in levels of hsCRP among the four groups of participants with or without childhood adversity and adulthood trauma remained significant, F(3, 10052) = 4.14, p = 0.006, and the pattern of results in our planned comparisons did not change.
Because childhood adversity was associated with smoking and higher BMI in this sample (Table 1), we reran the analyses controlling for these factors. The presence and number of childhood adversities remained associated with hsCRP (β = 0.04, p = 0.005 and β = 0.04, p = 0.003 respectively) and the interaction was still not statistically significant, F(1, 5778) = 0.163; p = 0.69). The difference in levels of hsCRP among the four groups of participants with or without childhood adversity and adulthood trauma remained significant, F(3, 5778) = 5.93, p < 0.001, and the pattern of results in our planned comparisons did not change.
4. Discussion
4.1 Summary and Significance
Our analysis of data from 11,198 participants in the population-based Health and Retirement Study indicates that exposures to childhood adversity and adulthood trauma are independently associated with elevated inflammation as indexed by hsCRP. To our knowledge, this is the first study to examine the relationship between adulthood trauma exposure and inflammation in a large population-based sample of participants in old age, the first to determine if childhood adversity and adulthood trauma are independently associated with elevated inflammation, and the first to explore the interaction between childhood adversity and adulthood trauma. Although the interaction between childhood adversity and adulthood trauma was not significant, we did find that individuals with both childhood adversity and adulthood trauma had higher levels of inflammation than those with adulthood trauma alone, but not compared to those with childhood adversity alone. These data suggest that early life experiences may influence susceptibility to trauma-associated inflammation in adulthood.
In line with prior work, we found that both the presence and cumulative exposure to childhood adversity or adulthood trauma were associated with higher levels of hsCRP. Our results extend findings linking adverse childhood events with elevated levels of inflammation (Danese et al., 2007; Kiecolt-Glaser et al., 2011) to a large population-based sample of 11,198 individuals over the age of 50. As chronic, low-grade inflammation is associated with increased morbidity and mortality, inflammation may be a key mechanism by which childhood adversity confers increased risk for developing psychiatric and physical illnesses (Kessler et al., 1997; Tamayo et al., 2010). Our results support studies that show a dose-dependent effect of number of childhood adversities on inflammation (Danese et al., 2007, Kessler et al., 1997; Taylor et al., 2006). However, other studies suggest that childhood adversity may be correlated with poor mental health due to the severity rather than number of adversities experienced (Schilling et al., 2008), and we did not have information on the severity of adversities in our study. As with childhood adversity, our study also supports and extends research linking specific adulthood trauma exposures with elevated inflammation (Dekaris et al., 1993; Woods et al., 2005). We found a dose-dependent association between adulthood trauma and inflammation. As a dose-dependent effect of specific adulthood traumas on physical and mental health has been shown in the literature (Coker et al., 2005; Mollica et al., 1998), our findings suggest that inflammation may play a role in poor health outcomes associated with cumulative adulthood trauma exposures.
The interaction between childhood adversity and adulthood trauma was not significant, indicating that the relationship between presence of childhood adversity and inflammation did not depend on the presence of adulthood trauma. Independent associations between childhood adversity and adulthood trauma with inflammation emphasize that stress may be associated with elevated inflammation throughout a person’s lifetime. However, in our sample, those who had experienced childhood adversity had a 2.86 fold increased prevalence of adulthood trauma compared to those without a similar history, and the number of childhood adversities was associated with increased number of adulthood traumas. Our findings suggest that there is an important interplay between the two types of stress, and support prior work indicating that childhood adversity is a significant risk factor for later assault (Gladstone et al., 2004; Widom et al., 2008). For instance, one study found that children who had been victims of maltreatment or crime had a 2.2–6.9 fold increased risk of any type of subsequent victimization one year later (Finkelhor et al., 2007), and that those who had experienced four or more types of victimization (“poly-victims”) had a particularly high risk of persisting poly-victimization (Finkelhor et al., 2007).
Despite the non-significant interaction between childhood adversity and adulthood trauma, participants who experienced both types of stress had higher levels of inflammation than those with adulthood trauma without childhood adversity, as well as those with neither childhood adversity nor adulthood adversity. This finding is in line with previous work indicating that cumulative trauma exposure across the lifespan is associated with elevated levels of inflammation (O’Donovan et al., 2012). Our results also complement studies showing that those with childhood adversity and adulthood depression and anxiety have HPA axis hyperreactivity and elevated levels of inflammation when compared to those with adulthood psychiatric symptoms alone (Danese et al., 2008; Heim et al., 2000). However, our findings also revealed that individuals with childhood adversity alone did not significantly differ in levels of hsCRP compared to those with the combination of childhood adversity and adulthood trauma. This result supports prior work highlighting childhood as a sensitive period of development during which adverse events have particularly potent effects on inflammation (Miller et al., 2011).
There are a number of potential factors that may contribute to the potent effects of childhood adversity. First, childhood adversity is associated with sensitization of the HPA axis to mild stresses in adulthood (Gunnar 1998; Heim et al., 2000; Heim et al., 2001). For instance, women with a history of childhood abuse have increased pituitary-adrenal and autonomic responses to stress when compared to controls (Heim et al., 2000). Furthermore, one study showed that a history of childhood abuse was associated with cortisol reactivity to acute stress, and that the cortisol reactivity was further enhanced when additional trauma was experienced in adulthood (Heim et al., 2002). Early life stressors in the form of low socioeconomic status have also been associated with resistance to glucocorticoid signaling in adulthood (Miller et al., 2009). As cortisol is an anti-inflammatory hormone, glucocorticoid resistance associated with childhood adversity may contribute to elevated inflammation with stress in adulthood. Second, epigenetic regulation in early life by childhood adversity may affect neurodevelopment through modulation of the leukocyte glucocorticoid receptor and alteration of HPA function (McGowan 2013; Tyrka et al, 2012; Xie et al, 2010). Third, women with childhood abuse-related PTSD show greater expression of target genes for the NF-κB/Rel family of transcription factors, which increase secretions of pro-inflammatory cytokines that in turn promote the production of CRP (Calabro et al., 2003; Ganter et al., 1989; Pace et al., 2012).
In our study, only childhood adversity was associated with adverse health behaviors, specifically smoking and BMI. Although specific types of adulthood trauma such as combat-related PTSD and domestic violence have been linked with greater risk for adverse health behaviors (Beckman et al., 1995; Kaysen et al., 2007; Pizarro et al., 2006; Vieweg et al., 2007), adulthood trauma exposure was not associated with health behaviors in our sample. This suggests that compared to adulthood trauma, childhood adversity may be a stronger risk factor for adverse health behaviors throughout the lifespan. Although the association between childhood adversity and inflammation was independent of health behaviors in our study, the increased rates of smoking and higher BMI associated with childhood adversity are well known predictors of ill health (Guh et al., 2009; Office of the Surgeon General 2004).
4.2 Limitations
Our results should be interpreted in the light of some important study limitations. First, the cross-sectional study design limits our ability to make causal inferences about potential mechanisms of elevated inflammation. Second, elevated levels of hsCRP, while predictive of disease risk, are non-specific (Watson et al., 2012). While hs-CRP has been reported as the analyte of choice for systemic inflammation, it is only one of many markers of inflammation (Pearson et al., 2003). Third, no information on the severity or duration of stressful events was available in this study; these facets of stress exposure may play an important role in the relationship between trauma and inflammation. We were also unable to assess the role of different types of stressors or the specific features of those events. For instance, intentional traumas that involving the deliberate infliction of harm have been shown to confer worse health outcomes than non-intentional traumas (Santiago et al., 2013). Fourth, both childhood adversity and adulthood trauma were retrospective and self-reported in this cohort. Current states may bias such reports and bias our models (Maughan and Rutter, 1997). Finally, the measures for childhood adversity and adulthood trauma available in the study were not standardized measures, contained a limited number of items, and to our knowledge, have not been compared to validated questionnaires or interviews.
4.3 Conclusions
Our study indicates that both childhood adversity and adulthood trauma are independently associated with elevated inflammation, which in turn could be a mechanism for increased morbidity and mortality in stress-exposed individuals. Moreover, we found that individuals who experienced both childhood adversity and adulthood trauma had higher levels of inflammation than those exposed to adulthood trauma who reported no childhood adversity. Strikingly, we were able to observe an association between distal stressful events and hsCRP in a population of adults over the age of 50. This observation is in line with prior research showing that associations between childhood adversity and psychopathology in adolescence, early adulthood, and mid-life generally did not attenuate with age (Clark et al., 2010), and in line with previous research linking lifetime trauma exposure with elevated inflammation (O’Donovan et al., 2012). Overall, these data highlight the potential for long-term gain from interventions that 1) prevent trauma exposure, especially during childhood, and 2) for those already exposed, identify and reduce the lingering deleterious effects of trauma exposure on inflammation using behavioral or pharmacological interventions as warranted. Given the high prevalence of trauma exposure in our population-based sample, particularly during adulthood, the ill effects of trauma-related inflammation are of large public health significance.
Highlights.
High prevalence of trauma exposure in population-based sample
Independent association of childhood adversity and elevated inflammation in old age
Independent association of adulthood trauma and elevated inflammation in old age
No interaction of childhood adversity and adulthood trauma with inflammation
Inflammation may mediate relationship between trauma exposure and ill health
Acknowledgments
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and was conducted by the University of Michigan. AOD was supported by a National Center for Advancing Translational Sciences Career Development Award at the University of California, San Francisco (KL2 TR000143) and a Society in Science—Branco Weiss Fellowship. JEL was supported by the UCSF Dean’s Office Medical Student Research Program. The authors would like to acknowledge Tom Metzler for statistical support in preparation of this manuscript.
Footnotes
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- Beckham JC, Roodman AA, Shipley RH, Hertzberg MA, Cunha GH, Kudler HS, Levin ED, Rose JE, Fairbank JA. Smoking in Vietnam combat veterans with post-traumatic stress disorder. J Trauma Stress. 1995;8:461–472. doi: 10.1007/BF02102970. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit Area Survey of Trauma. Am J Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Brown ES, Fulton MK, Wilkeson A, Petty F. The psychiatric sequelae of civilian trauma. Compr Psychiatry. 2000;41:19–23. doi: 10.1016/s0010-440x(00)90126-3. [DOI] [PubMed] [Google Scholar]
- Calabró P, Willerson JT, Yeh ETH. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- Carr CP, Martins CMS, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Clark C, Caldwell T, Power C, Stansfeld SA. Does the Influence of Childhood Adversity on Psychopathology Persist Across the Lifecourse? A 45-Year Prospective Epidemiologic Study. Annals of Epidemiology. 2010;20:385–394. doi: 10.1016/j.annepidem.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Clarke P, Fisher G, House J, Smith J, Weir D. Guide to Content of the HRS Psychosocial Leave-Behind Participant Lifestyle Questionnaires: 2004 & 2006. Ann Arbor, MI: University of Michigan; 2008. [Google Scholar]
- Crimmins E, Faul J, Kim JK, et al. Documentation of Biomarkers in the 2006 and 2008 Health and Retirement Study. Ann Arbor, MI: University of Michigan; 2013. [Google Scholar]
- Coker AL, Smith PH, Fadden MK. Intimate partner violence and disabilities among women attending family practice clinics. J Womens Health (Larchmt) 2005;14:829–838. doi: 10.1089/jwh.2005.14.829. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaris D, Sabioncello A, Mazuran R, Rabatić S, Svoboda-Beusan I, Racunica NL, Tomasić J. Multiple changes of immunologic parameters in prisoners of war. Assessments after release from a camp in Manjaca, Bosnia. JAMA. 1993;270:595–599. [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Preventive Medicine. 2003;37:268–277. doi: 10.1016/S0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG Marine Resiliency Study Team. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D, Ormrod RK, Turner HA. Re-victimization patterns in a national longitudinal sample of children and youth. Child Abuse & Neglect. 2007;31:479–502. doi: 10.1016/j.chiabu.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Fisher G, Faul J, Weir D, Wallace B. Documentation of Chronic Disease Measures in the Heath and Retirement Study (HRS/AHEAD) Ann Arbor, MI: University of Michigan; 2005. [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Ganter U, Arcone R, Toniatti C, Morrone G, Ciliberto G. Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. EMBO J. 1989;8:3773–3779. doi: 10.1002/j.1460-2075.1989.tb08554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Mitchell PB, Malhi GS, Wilhelm K, Austin MP. Implications of childhood trauma for depressed women: an analysis of pathways from childhood sexual abuse to deliberate self-harm and revictimization. Am J Psychiatry. 2004;161:1417–1425. doi: 10.1176/appi.ajp.161.8.1417. [DOI] [PubMed] [Google Scholar]
- Glaesmer H, Brähler E, Gündel H, Riedel-Heller SG. The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. Psychosom Med. 2011;73:401–406. doi: 10.1097/PSY.0b013e31821b47e8. [DOI] [PubMed] [Google Scholar]
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Prev Med. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. J Consult Clin Psychol. 2000;68:782–787. [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Health and Retirement Study. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number U01AG009740) Ann Arbor, MI: [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Ishii S, Karlamangla AS, Bote M, Irwin MR, Jacobs DR, Cho HJ, Seeman TE. Gender, obesity and repeated elevation of C-reactive protein: data from the CARDIA cohort. PLoS ONE. 2012;7:e36062. doi: 10.1371/journal.pone.0036062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen D, Dillworth TM, Simpson T, Waldrop A, Larimer ME, Resick PA. Domestic Violence and Alcohol Use: Trauma-related Symptoms and Motives for Drinking. Addict Behav. 2007;32:1272–1283. doi: 10.1016/j.addbeh.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause N, Shaw BA, Cairney J. A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychol Aging. 2004;19:637–648. doi: 10.1037/0882-7974.19.4.637. [DOI] [PubMed] [Google Scholar]
- Levine ME, Cole SW, Weir DR, Crimmins EM. Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med. 2015;130:16–22. doi: 10.1016/j.socscimed.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Maughan B, Rutter M. Retrospective Reporting of Childhood Adversity: Issues in Assessing Long-Term Recall. Journal of Personality Disorders. 1997;11:19–33. doi: 10.1521/pedi.1997.11.1.19. [DOI] [PubMed] [Google Scholar]
- McGowan PO. Epigenomic Mechanisms of Early Adversity and HPA Dysfunction: Considerations for PTSD Research. Front Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry. 2015;172:353–362. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. PNAS. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica RF, McInnes K, Poole C, Tor S. Dose-effect relationships of trauma to symptoms of depression and post-traumatic stress disorder among Cambodian survivors of mass violence. Br J Psychiatry. 1998;173:482–488. doi: 10.1192/bjp.173.6.482. [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Irwin MR. Links between behavioral factors and inflammation. Clin Pharmacol Ther. 2010;87:479–482. doi: 10.1038/clpt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, Neylan TC. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin M-T, O’Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behav Immun. 2012;26:642–649. doi: 10.1016/j.bbi.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, O’Farrelly C, Malone KM. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013;30:307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- Office of the Surgeon General (US), Office on Smoking and Health (US) The Health Consequences of Smoking: A Report of the Surgeon General, Reports of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta (GA): 2004. [Google Scholar]
- Pace TWW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-κB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of Inflammation and Cardiovascular Disease Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pizarro J, Silver RC, Prause J. Physical and Mental Health Costs of Traumatic War Experiences Among Civil War Veterans. Arch Gen Psychiatry. 2006;63:193–200. doi: 10.1001/archpsyc.63.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchett LC, Yehuda R. Foundations of posttraumatic stress disorder: does early life trauma lead to adult posttraumatic stress disorder? Dev Psychopathol. 2011;23:477–491. doi: 10.1017/S0954579411000186. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Rosenheck RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposure among US men. Am J Public Health. 2002;92:59–63. doi: 10.2105/ajph.92.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Role of inflammation in depression: implications for phenomenology, pathophysiology and treatment. Mod Trends Pharmacopsychiatri. 2013;28:33–48. doi: 10.1159/000343966. [DOI] [PubMed] [Google Scholar]
- Santiago PN, Ursano RJ, Gray CL, Pynoos RS, Spiegel D, Lewis-Fernandez R, Friedman MJ, Fullerton CS. A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: intentional and non-intentional traumatic events. PLoS ONE. 2013;8:e59236. doi: 10.1371/journal.pone.0059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Gore S. The impact of cumulative childhood adversity on young adult mental health: Measures, models, and interpretations. Social Science & Medicine. 2008;66:1140–1151. doi: 10.1016/j.socscimed.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR. Early life Adversity and Inflammation in African Americans and Whites in the Midlife in the United States Survey. Psychosom Med. 2010;72:694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Völzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med. 2009;71:1012–1017. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- Tamayo T, Christian H, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health. 2010;10:525. doi: 10.1186/1471-2458-10-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, Melamed S. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J Occup Health Psychol. 2005;10:344–362. doi: 10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Lifetime traumas and mental health: the significance of cumulative adversity. J Health Soc Behav. 1995;36:360–376. [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood Adversity and Epigenetic Modulation of the Leukocyte Glucocorticoid Receptor: Preliminary Findings in Healthy Adults. PLoS ONE. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg WVR, Julius DA, Bates J, Quinn JF, Fernandez A, Hasnain M, Pandurangi AK. Posttraumatic stress disorder as a risk factor for obesity among male military veterans. Acta Psychiatr Scand. 2007;116:483–487. doi: 10.1111/j.1600-0447.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- Watson J, Round A, Hamilton W. Raised inflammatory markers. BMJ. 2012;344:e454. doi: 10.1136/bmj.e454. [DOI] [PubMed] [Google Scholar]
- Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2000;27:2351–2359. [PubMed] [Google Scholar]
- Widom CS, Czaja SJ, Dutton MA. Childhood victimization and lifetime revictimization. Child Abuse & Neglect. 2008;32:785–796. doi: 10.1016/j.chiabu.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]
- Woods AB, Page GG, O’Campo P, Pugh LC, Ford D, Campbell JC. The Mediation Effect of Posttraumatic Stress Disorder Symptoms on the Relationship of Intimate Partner Violence and IFN-γ Levels. Am J Community Psychol. 2005;36:159–175. doi: 10.1007/s10464-005-6240-7. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive Effect of Stressful Life Events and the Serotonin Transporter 5-HTTLPR Genotype on Posttraumatic Stress Disorder Diagnosis in 2 Independent Populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with Childhood Adversity on Risk for Post-Traumatic Stress Disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]