Abstract
Metallothioneins are small, ubiquitous Cys-rich proteins known to be involved in reactive oxygen species (ROS) scavenging and metal homeostasis. We found that the expression of a metallothionein gene (OsMT2b) was synergically down-regulated by OsRac1 and rice (Oryza sativa) blast-derived elicitors. Transgenic plants overexpressing OsMT2b showed increased susceptibility to bacterial blight and blast fungus. OsMT2b-overexpressing cells showed reduced elicitor-induced hydrogen peroxide production. In contrast, homozygous OsMT2b::Tos17-inserted mutant and OsMT2b-RNAi-silenced transgenic cells showed significantly higher elicitor-induced hydrogen peroxide production than the wild-type cells. In vitro assay showed that recombinant OsMT2b protein possessed superoxide- and hydroxyl radical-scavenging activities. Taken together, these results showed that OsMT2b is an ROS scavenger and its expression is down-regulated by OsRac1, thus potentiating ROS, which function as signals in resistance response. The results suggest that OsRac1 plays a dual role as an inducer of ROS production and a suppressor of ROS scavenging.
Plants are constantly exposed to the onslaught of potential pathogens. To ward off pathogens plants have developed a battery of defense strategies. The primary defense of plants is preformed physical barriers, which include thick cuticle and cell wall, antimicrobial proteins, and metabolites (Hammond-Kosack and Jones, 2000). However, when these defenses fail, inducible defenses are invoked. These defense responses are triggered by the specific recognition of the pathogen by plant disease resistance genes. This recognition leads to a hypersensitive response, which is often associated with rapid ion fluxes, protein phosphorylation, reactive oxygen species (ROS) accumulation, and induction of defense-related gene expression (Hammond-Kosack and Jones, 1996; Heath, 2000).
ROS accumulation during the early stages of plant defense signaling is known as oxidative burst (Doke, 1983). Although ROS accumulation alone is insufficient to trigger disease resistance (Heath, 2000; Huckelhoven and Kogel, 2003), it plays a central role in plant defense against pathogens (Lamb and Dixon, 1997). ROS may directly repel invading pathogens or serve as signaling molecules that activate defense response (Hammond-Kosack and Jones, 1996). In contrast, ROS resulting from biotic and abiotic stresses can cause severe cellular damage and are thus tightly regulated and detoxified by complex enzymatic and nonenzymatic mechanisms (Mittler, 2002). Plants need to distinguish between ROS produced under environmental stresses and pathogen-induced signals to invoke suitable responses. The regulatory mechanisms underlying such a distinction are totally unknown.
Small GTPases are important molecular switches or molecular timers that regulate signal transduction in eukaryotes (Yang, 2002). Depending on incoming signals, they undergo conformational change, cycling between the GTP-bound active form and the GDP-bound inactive form. For Ras-like GTPases, which include Rac, the exchange of GDP for GTP occurs spontaneously at low rates, but is catalyzed in vivo by guanine exchange factors. On the other hand, GTP is hydrolyzed to GDP by the intrinsic GTPase activity of small GTPases, which is increased by GTPase-activating proteins (Vetter and Wittinghofer, 2001). In plants, the Rac GTPases, which are also known as Rops, are thought to be involved in ROS production through the activation of NADPH oxidase (Yang, 2002). Previous studies showed that Racs/Rops play key roles in developmental processes (Yang, 2002), tolerance to hypoxia (Baxter-Burrell et al., 2002), abscisic acid signaling (Lemichez et al., 1999; Zheng et al., 2002), auxin signaling (Tao et al., 2002), and defense signaling (Park et al., 2000; Schiene et al., 2000; Ono et al., 2001; Agrawal et al., 2003). Some of these functions of Rac GTPases are mediated by ROS (Park et al., 2000; Ono et al., 2001; Baxter-Burrell et al., 2002).
Previously, we have shown that the small GTPase OsRac1 plays a key role in ROS production and cell death during plant defense signaling (Kawasaki et al., 1999; Ono et al., 2001). Rac-mediated defense signaling in other plants has also been reported (Park et al., 2000; Schiene et al., 2000). In this study, we characterized an ROS scavenger, the metallothionein OsMT2b. Metallothioneins are low-Mr (6–7 kD), Cys-rich, metal-binding proteins. Metallothionein proteins and genes have been identified in diverse organisms including plants, animals, and fungi, and even in the prokaryote Synechococcus (Cobbett and Goldsbrough, 2002). Metallothioneins are known to be involved in ROS scavenging and metal homeostasis (Thornalley and Vasak, 1985; Hussain et al., 1996; Cobbett and Goldsbrough, 2002). Although metallothioneins in animals have been the subject of intensive studies due to their roles in animal physiology and toxicology (Cai et al., 1999), the function of metallothioneins in plants has remained unknown. Here, we show that OsMT2b is an ROS scavenger, confirming that plant metallothioneins possess functions similar to their animal counterparts. We also found that the expression of OsMT2b was down-regulated by OsRac1 and that perturbation of this down-regulation by overexpression of OsMT2b in transgenic plants resulted in increased susceptibility to pathogens. Furthermore, our results suggest that OsRac1 is a key redox regulator that plays a dual role as an inducer of ROS production and a suppressor of ROS scavenging.
RESULTS
Fluorescent Differential Display Analyses and RNase Protection Assays of Differentially Expressed Transcripts
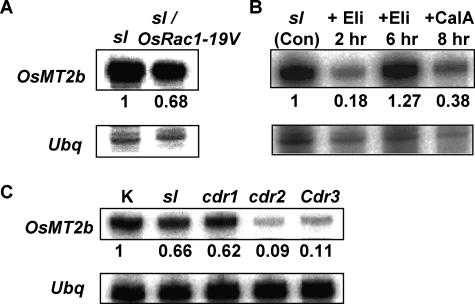
Previously, we found that overexpression of the constitutively active Pro35S:OsRac1-19V in rice (Oryza sativa) induced cell death and potentiated calyculin A- and elicitor-induced ROS production and disease resistance (Kawasaki et al., 1999; Ono et al., 2001; Suharsono et al., 2002). To study the signal transduction downstream of the small GTPase OsRac1, we compared the expression profile of mRNA transcripts in the Sekiguchi lesion (sl) mimic mutant (Kiyosawa, 1970) and in an sl mutant overexpressing the constitutively active Pro35S:OsRac1-19V, using fluorescent differential display (FDD) analysis. The sl mutation is recessive, and the lesions are induced by many stimuli, including pathogen infection (Kiyosawa, 1970). The sl mutant is known to show enhanced hydrogen peroxide production and resistance to blast infection in a light-dependent manner (Arase et al., 2000), and these phenotypes are further enhanced by the overexpression of Pro35S:OsRac1-19V (Kawasaki et al., 1999). In this study, we found that the expression of a previously uncharacterized metallothionein gene was down-regulated in Pro35S:OsRac1-19V-overexpressing sl cells (Fig. 1A). We designated the metallothionein gene as OsMT2b, based on the nomenclature proposed by Cobbett and Goldsbrough (2002; Fig. 2A).
Figure 1.
Expression of OsMT2b in transgenic OsRac1-19V and lesion mimic mutant cells. OsMT2b expression levels were analyzed by RNase protection assays using total RNA isolated from (A) NT lesion mimic mutant sl and sl cells overexpressing Pro35S:OsRac1-19V (sl/OsRac1-19V), (B) sl cells treated with N-acetylchitooligosaccharide elicitor (3 μg/mL; Eli) and calyculin A (10 μm; CalA), and (C) wild-type cv Kinmaze (K) and lesion mimic mutants sl, cdr1, cdr2, and Cdr3 cells. The numbers below the upper sections (OsMT2b) indicate the relative band intensity after normalization with those of the ubiquitin (Ubq; lower sections).
Figure 2.
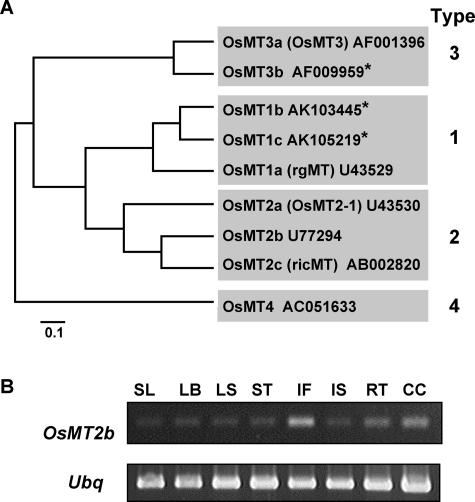
OsMT2b, a novel rice metallothionein. A, A phylogenetic tree of rice metallothioneins with a proposed new nomenclature based on Cobbett and Goldsbrough (2002). Brackets indicate previously designated names. The numbers following rice metallothionein genes indicate the GenBank accession numbers of the corresponding genes. Asterisk indicates new metallothionein found from database search. B, Differential expression of OsMT2b in seedlings (SL), leaf blades (LB), leaf sheaths (LS), stems (ST), immature inflorescences (IF), immature seeds (IS), roots (RT), and cultured cells (CC) of rice, analyzed by reverse transcription-PCR.
Metallothioneins are known to function as ROS scavengers (Thornalley and Vasak, 1985; Hussain et al., 1996), confer protection against DNA damage (Chubatsu and Meneghini, 1993), and function as negative regulators of apoptosis (Shimoda et al., 2003) in animals, but are completely uncharacterized in plants. Therefore, we further studied the function of OsMT2b and the possible role of OsRac1 in the regulation of OsMT2b expression during defense signaling.
To examine if the down-regulation of OsMT2b expression is involved in defense signaling, we treated sl cells with an N-acetylchitooligosaccharide elicitor and an inhibitor of protein phosphatase I, calyculin A. In rice cells, N-acetylchitooligosaccharide elicitor and calyculin A are known to elicit genetic and physiological responses related to defense, such as the induction of defense-related gene expression and rapid ROS production (Kuchitsu et al., 1995; Nishizawa et al., 1999; Takahashi et al., 1999). We found that OsMTb expression was down-regulated in calyculin A-treated sl cells and was transiently down-regulated in N-acetylchitooligosaccharide elicitor-treated sl cells. (Fig. 1B).
Previously, Takahashi et al. (1999) showed that, at the lesion-positive stage, the nonallelic cdr1, cdr2, and Cdr3 lesion mimic mutants showed significant resistance to virulent rice blast (race 007) and that cdr1 and cdr2 cells are hyper-responsive to calyculin A-induced ROS production. The calyculin A-induced ROS can be inhibited by diphenylene iodonium, an inhibitor of neutrophil NADPH oxidase. Therefore, to test if the enhanced ROS production and resistance to pathogen in these lesion mimic mutants are related to reduced levels of OsMT2b expression, we compared the basal level of OsMT2b expression in these mutants with that in the wild-type cultivar (cv) Kinmaze. We found that OsMT2b expression was reduced in all lesion-mimic mutants studied (sl, cdr1, cdr2, and Cdr3) compared to that of the wild type (Fig. 1C). Taken together, the results suggest that the down-regulation of OsMT2b expression may be involved in defense signaling, and that the use of the wild-type cv Kinmaze (which has a higher basal level of OsMT2b expression), instead of the sl mutant, would be better for studying the down-regulation of OsMT2b expression.
Phylogeny of the Rice Metallothionein Family and Differential Expression of OsMT2b in Rice Tissue
According to the classification proposed by Cobbett and Goldsbrough (2002), plant metallothioneins are divided into four types. Type 1 and 2 metallothioneins contain two Cys-rich domains which are separated by a spacer region. The Cys-rich domain in Type 1 metallothioneins contains six Cys-Xaa-Cys motifs (where Xaa is an amino acid other than Cys) that are distributed equally among the two domains. In contrast, in Type 2 metallothioneins, the first pair of Cys is a Cys-Cys motif, and a highly conserved N-terminal sequence, MSCCGGNCGCS, is present. Type 3 and 4 metallothioneins contain 3 and 4 Cys-rich domains, respectively (Cobbett and Goldsbrough, 2002). Through database searches, we found 9 rice metallothionein genes, including three that have not been reported (Fig. 2A). Due to the discovery of these new metallothionein genes, we propose a new nomenclature for rice metallothioneins, as shown in Figure 2A, following the classification of Robinson et al. (1993) and Cobbett and Goldsbrough (2002). According to this classification, OsMT2b is a Type 2 metallothionein. The predicted amino acid sequences of rice metallothionein genes aligned and analyzed using Clustal analysis of the GeneWorks software (version 2.5.1, Intelligenetics, Mountain View, CA), and the phylogeny is shown as a rooted UPGMA tree in Figure 2A.
To study the expression pattern of OsMT2b in different rice tissues, we examined the expression of OsMT2b in tissues from rice seedlings and mature plants by reverse transcription-PCR (Fig. 2B). The expression of OsMT2b was detected in most tissues studied (i.e. immature seeds, leaves of seedling, leaf sheaths, and leaf blades), and higher expression level was detected in immature inflorescences, roots, and cultured cells (Fig. 2B). The tissue expression pattern of OsMT2b was different from that of other Type 2 metallothioneins, namely OsMT2c, which is highly expressed in stem, leaf blades, and leaf sheaths (Yu et al., 1998), and OsMT2a, which is highly expressed in the mature and senescent leaves (Hsieh et al., 1996). The expression of Type 2 metallothioneins appears to be differentially regulated and possibly complement one another.
Synergistic Down-Regulation of OsMT2b Expression by OsRac1 and Sphingolipid Elicitor
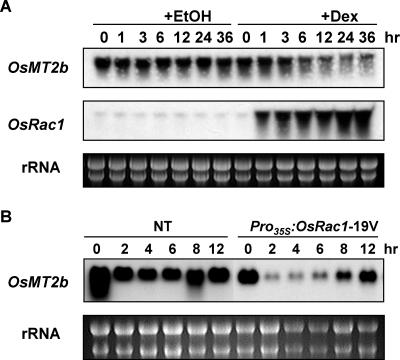
To confirm that OsRac1 is involved in the down-regulation of OsMT2b expression, we cloned the constitutively active OsRac1-19V in a dexamethasone (Dex)-inducible pTA7002 binary vector (Aoyama and Chua, 1997) to give pDEX-OsRac1, and generated a Dex-inducible transgenic cell culture. In these cells, when OsRac1-19V expression was induced by Dex treatment, OsMT2b mRNA was clearly reduced 12 h after Dex treatment, but unaffected in the negative control ethanol-treated cells (Fig. 3A). The results confirmed the role of OsRac1 in the down-regulation of OsMT2b expression, and showed that the Dex-inducible system worked well in rice cell culture.
Figure 3.
Synergistic down-regulation of OsMT2b mRNA by OsRac1 and sphingolipid elicitor. A, Time course RNA gel-blot analyses of OsMT2b expression in Dex-inducible OsRac1-19V cells. The expression of OsRac1-19V gene was induced by Dex (30 μm), and ethanol (ethanol) was used as a negative control. B, Time course RNA gel-blot analyses of OsMT2b expression in NT and Pro35S:OsRac1-19V-overexpressing cells treated with sphingolipid elicitor (10 μg/mL).
To further study if the down-regulation of OsMT2b expression is related to defense signaling, nontransformant cultured cells (NT) and Pro35S:OsRac1-19V-overexpressing cells (Ono et al., 2001; Suharsono et al., 2002) were treated with a sphingolipid elicitor which was purified from the cell wall of rice blast (Koga et al., 1998; Umemura et al., 2002). We found that the basal level of OsMT2b expression was lower in the Pro35S:OsRac1-19V-overexpressing cells than in NT cells. We also found that the expression of OsMT2b was transiently down-regulated between 2 to 6 h after elicitor treatment in both the NT and Pro35S:OsRac1-19V-overexpressing cells, similar to the treatment with the N-acetylchitooligosaccharide elicitor (Fig. 1B). It should be noted that the reported timing of the oxidative burst induced by the sphingolipid and N-acetylchitooligosaccharide elicitors (Ono et al., 2001; Suharsono et al., 2002; Tsukada et al., 2002) coincided with the timing of OsMT2b down-regulation (Figs. 1B and 3B). Furthermore, in the Pro35S:OsRac1-19V-overexpressing cells, OsMT2b mRNA was more drastically reduced, compared to that in the NT cells, indicating that OsRac1 and the sphingolipid elicitor synergistically down-regulated OsMT2b expression (Fig. 3B). Taken together, these results suggest that the down-regulation of OsMT2b expression is involved in defense signaling.
Increased Susceptibility of OsMT2b-Overexpressing Plants to Bacterial Blight and Blast Infection
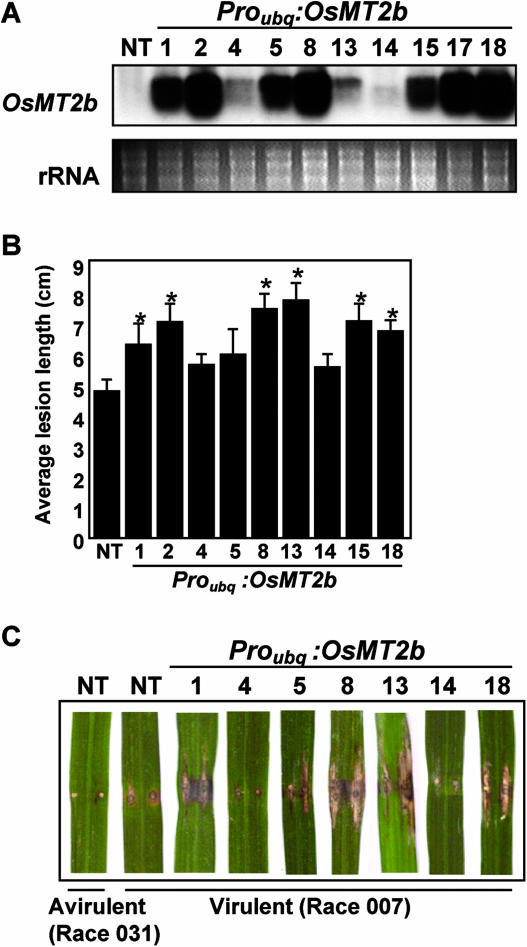
To examine if the down-regulation of OsMT2b expression is involved in defense signaling, we overexpressed OsMT2b in rice plants (Fig. 4A) and tested their response to virulent bacterial blight and blast infections. We found that OsMT2b-overexpressing plants showed increased susceptibility to both bacterial blight and blast infection, as indicated by the increased spreading of disease lesions (Fig. 4, B and C). Generally, this increased susceptibility also correlated with high OsMT2b mRNA levels (r2 = 0.655, P < 0.05, data not shown) in the susceptible transgenic lines (Fig. 4A), excluding transgenic line 13. The lack of concordance in line 13 could be due to that it was anomalous due to unknown reasons or that manipulation of OsMT2b expression level alone significantly modulates susceptibility to the pathogen but is insufficient to determine the level of susceptibility. However, in general, the results indicate that failure of the plants to down-regulate OsMT2b expression resulted in increased susceptibility to pathogens.
Figure 4.
Increased susceptibility of OsMT2b-overexpressing plants to bacterial blight and rice blast infections. A, RNA gel-blot analysis of OsMT2b expression in mature leaf blade of transgenic plants that overexpress OsMT2b. B, Disease lesion length on leaf blades 14 d after bacterial blight Xanthomonas oryzae (race 1) inoculation. C, Disease lesion on leaf blades 21 d after rice blast inoculation. Numbers 1, 2, 4, 5, 8, 13, 14, 15, 17, and 18 represent transgenic lines. Bars indicate the mean obtained from 7 to 9 infected leaves. Error bars indicate SEM, and asterisks indicate statistically significant differences from the NT as analyzed by the Student's t test (P < 0.05).
OsMT2b::Tos17 plants with homozygous and heterozygous Tos17 insertions and OsMT2b-RNAi transgenic plants that showed a significant reduction in OsMT2b expression suffered moderate to developmental defects and, thus, could not be tested for their response to pathogen infection. The homozygous OsMT2b::Tos17 plants withered at the juvenile stage, while the heterozygotes showed premature yellowing (data not shown).
Function of OsMT2b as an ROS Scavenger
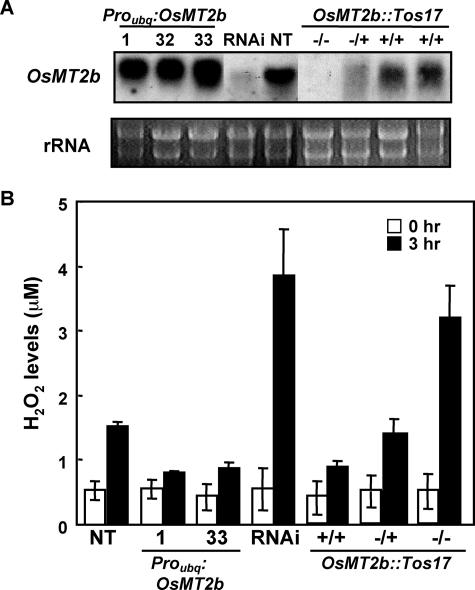
To study the function of OsMT2b, we first examined the response to the sphingolipid elicitor of transgenic cell cultures with high and low OsMT2b expressions. OsMT2b-overxpressing cells showed significantly lower elicitor-induced hydrogen peroxide production than the cv Kinmaze NT cells (Student's t test, P < 0.05; Fig. 5, A and B). In contrast, the OsMT2b-RNAi-silenced transgenic cells showed significantly higher elicitor-induced hydrogen peroxide production than the NT cells. Similarly, the homozygous OsMT2b::Tos17-inserted cells also showed significantly higher elicitor-induced hydrogen peroxide production compared to the wild-type (+/+) cv Nipponbare sibling (Student's t test, P < 0.01; Fig. 5, A and B). These results suggest that OsMT2b may function as an ROS scavenger.
Figure 5.
Effects of OsMT2b expression levels on elicitor-induced hydrogen peroxide production. A, RNA gel-blot analysis of total RNA isolated from OsMT2b- and OsMT2b-RNAi-overexpressing cells and OsMT2b::Tos17 cells. B, Hydrogen peroxide production in sphingolipid elicitor-treated cells is inversely correlated with the OsMT2b expression level. Numbers 1, 32, and 33 represent OsMT2b-overexpressing transgenic cell lines. Bars indicate the mean obtained from 7 to 9 experiments, and error bars indicate SEM.
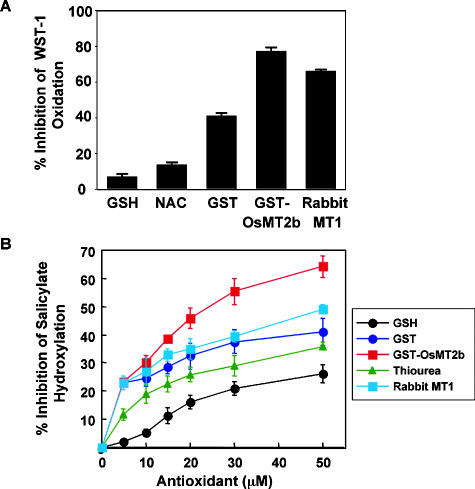
To confirm this, we produced a recombinant glutathione S-transferase (GST)-OsMT2b fusion and measured its ability to inhibit superoxide- and hydroxyl radicals-mediated oxidation in vitro. GST-OsMT2b showed higher antioxidant activity against superoxide and hydroxyl radicals than known antioxidants such as reduced glutathione (GSH), N-acetylcysteine, and thiourea at the same concentrations (Thornalley and Vasak, 1985; Kelner et al., 1990; Hussain et al., 1996; Jones et al., 2002; Fig. 6). The antioxidant activity of GST-OsMT2b was comparable to that of the rabbit metallothionein MT1, which is a known ROS scavenger (Thornalley and Vasak, 1985).
Figure 6.
Function of OsMT2b as an ROS scavenger. Comparison of ROS scavenging activity between GST-OsMT2b and other known antioxidants (A and B). A, Inhibition of superoxide-mediated WST-1 reduction by antioxidants (100 μm). Bars indicate the mean obtained from 7 to 9 samples and error bars indicate SEM. B, Inhibition of hydroxyl radical-mediated salicylate hydroxylation by antioxidants. Values are the mean obtained from 2 to 4 experiments, and error bars indicate SEM.
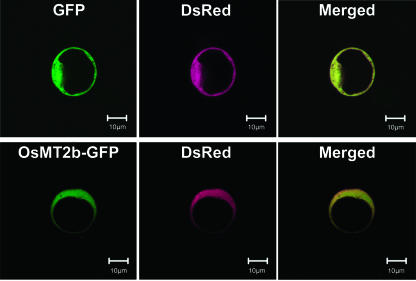
To identify the cellular compartment in which OsMT2b functions, we translationally fused OsMT2b to a green fluorescent protein (GFP) gene (Chiu et al., 1996) and used it for transient rice protoplast transformation. As shown in Figure 7, OsMT2b-GFP was localized to the cytosol of transformed protoplasts, indicating that OsMT2b mainly functions as an ROS scavenger in the cytosol.
Figure 7.
Cytosolic localization of GFP-OsMT2b in rice protoplasts. Rice protoplasts were cotransfected with plasmids pUbq:DsRed and p35S:OsMT2b-GFP (lower section) or p35S:GFP (upper section) by electroporation. OsMT2b-GFP and DsRed fluorescences colocalized in the cytoplasm of rice protoplasts.
DISCUSSION
Animal metallothioneins have been the subject of intensive research, especially in relation to their role of metallothionein in metal detoxification, homeostasis, and protection against oxidative damage (for reviews, see Sato and Bremner, 1993; Cai et al., 1999; Coyle et al., 2002). However, reports related to plant metallothioneins are few, by comparison. Recently, increasing numbers of reports indicate that plant metallothioneins may play important functions, as they do in animals (Robinson et al., 1993; Cobbett and Goldsbrough, 2002). Following the recent discoveries of numerous novel plant metallothioneins, a unified nomenclature becomes essential. Here, we have built on the classification system established by Robinson et al. (1993) and Cobbett and Goldsbrough (2002), and proposed a nomenclature for rice metallothioneins.
To date, studies of plant metallothioneins are mainly limited to analyses of gene expression in different tissues and of responses to various metal treatments (Cobbett and Goldsbrough, 2002; Coyle et al., 2002); functional studies are scarce. In this study, we found that the rice metallothionein OsMT2b protein may function as an ROS scavenger, supporting the notion, at the biochemical level, that plant metallothioneins play roles as important as those of their animal counterparts.
In plant defense signaling, rapid ROS accumulation following host-pathogen recognition is known to be a key factor in disease resistance (Hammond-Kosack and Jones, 1996). However, under oxidative stress, plants typically respond by activating a complex system of enzymatic and nonenzymatic ROS scavengers for maintaining redox homeostasis and protection against oxidative damage (Mittler, 2002). Therefore, activation of ROS scavengers during defense signaling would diminish ROS accumulation during the oxidative burst phase, and would thus be detrimental to disease resistance.
Numerous reports have shown that plants with reduced levels of ROS scavengers exihibit increased disease resistance. In tobacco (Nicotiana tabacum), expression of cytosolic ascorbate peroxidase is suppressed at the posttranscriptional level during pathogen-induced programmed cell death (Mittler et al., 1998). Transgenic tobacco plants with reduced expression of cytosolic ascorbate peroxidase or catalase are more tolerant or hyper-responsive to pathogen infection (Takahashi et al., 1997; Chamnongpol et al., 1998; Mittler et al., 1999). Furthermore, the mRNA levels of catalases are also reduced in the elicitor-infiltrated zone of tobacco leaf (Dorey et al., 1998). In barley (Hordeum vulgare) resistant to powdery mildew, the change in total GSH levels and the GSH/oxidized glutathione ratio indicate that a concerted regulation of the redox state of the pathogen-infected cell may be a key factor in disease resistance (Vanacker et al., 2000). Furthermore, during systemic acquired resistance, reduction of specific Cys residues in key regulators, such as NPR1 and the basic domain/Leu zipper TGA transcription regulators, TGA1, TGA2, and TGA3, is required for salicylic acid-dependent systemic acquired resistance (Despres et al., 2003; Johnson et al., 2003; Mou et al., 2003). Mou et al. (2003) also found a biphasic change in redox state in plants treated with 2,6-dichloroisonicotinic acid (salicylic acid analog) and infected with avirulent pathogen; this is similar to the findings of Vanacker et al. (2000).
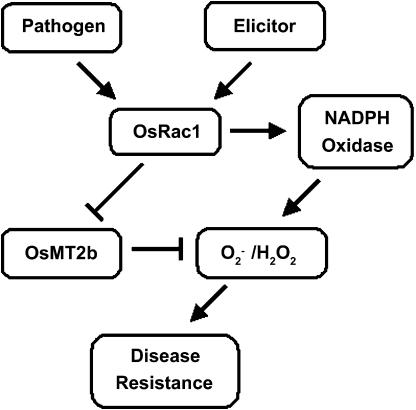
In this study, down-regulation of OsMT2b expression was observed during the oxidative burst phase in elictor-treated cells. Interestingly, overexpression of OsMT2b resulted in increased susceptibility of the transgenic plants to bacterial blight and blast fungus. Taken together, the results suggest that metallothionein may participate in redox regulation, and its levels may be transiently suppressed, thereby augmenting ROS accumulation and defense signaling (Fig. 8). Furthermore, our results agree with those results of a recent microarray analysis by Akimoto-Tomiyama et al. (2003), which showed that the expression of OsMT2b (BLAST hit, P93733) and OsMT3b (BLAST hit, AF147786) were down-regulated in N-acetylchitooligosaccharide elicitor-treated rice cells. The expression of other redox-related genes, including a monodehydroascorbate reductase (BLAST hit, AB026731) and a thioredoxin (BLAST hit, AB053294), is reduced in rice cells 2 h after the N-acetylchitooligosaccharide elicitor treatment (Akimoto-Tomiyama et al., 2003), supporting the notion that down-regulation of ROS scavengers during oxidative burst is important for defense signaling.
Figure 8.
A model depicting the dual role of OsRac1 in inducing ROS production and down-regulating OsMT2b expression during defense signaling in rice. Recognition of signals from pathogens and elicitors activates OsRac1, which in turn induces NADPH oxidase-mediated ROS production. In parallel to ROS production, the expression of the ROS scavenger OsMT2b gene is down-regulated, thereby potentiating ROS accumulation and defense signaling.
On the other hand, hydrogen peroxide may synergize with nitric oxide (NO) to promote cell death (Delledonne et al., 2001). Metallothioneins are also known to scavenge NO (Schwarz et al., 1995), and thus the down-regulation of OsMT2b expression may potentiate the effect of NO. Alternatively, the function of metallothionein could be linked to its well-known metal chelation ability. A decrease in metallothionein levels could result in increased free transition metal ions, thus allowing hydroxyl radicals to be formed more readily through the Fenton or Haber-Weiss reactions (Briat, 2002). Therefore, down-regulation of OsMT2b expression becomes favorable for ROS accumulation. In addition to metallothionein, other ROS scavengers are also suppressed during defense signaling. A redox regulatory mechanism that coordinately suppresses ROS scavengers during oxidative burst may exist in plants.
The mechanism by which OsRac1 down-regulates OsMT2b remains to be studied. The synergistic effect of sphingolipid elicitor in the down-regulation of OsMT2b in Pro35S:OsRac1-19V transgenic cells suggests that some unknown effectors, which are activated by the elicitor, are needed for strong down-regulation of OsMT2b. The mechanism of the down-regulation is probably independent of ROS because in the wild-type cells, elicitor-induced down-regulation of OsMT2b was unaffected by diphenylene iodonium (data not shown). Furthermore, the down-regulation was not observed in hydrogen peroxide-treated cells (data not shown).
This study also supports the idea that OsRac1 is a key redox regulator in defense signaling (Kawasaki et al., 1999; Ono et al., 2001) and provides an insight into how transient ROS accumulation and down-regulation of ROS scavengers can be coordinated. Although Rac GTPases are known to alter redox levels through activation of NADPH oxidase-mediated ROS production (Bokoch and Diebold, 2002), the dual function of a Rac GTPase as, additionally, a negative regulator of ROS scavenger, shown in this study, is novel. Down-regulation of several ROS scavengers in response to elicitors and pathogens have been reported, but the associated regulatory mechanisms are completely unknown. The novel dual function of OsRac1 should not come as a complete surprise, however, because this dual activity would greatly enhance the function of Rac as a redox regulator. Furthermore, a Rac/Rop can aptly fit the role of a redox regulator when transient ROS accumulation is required, because after the oxidative burst, Rac/Rop can be rapidly inactivated by a GTPase-activating protein, which is activated by ROS (Baxter-Burrell et al., 2002).
MATERIALS AND METHODS
Plant Materials and Pathogen Inoculations
A japonica cv Kinmaze of rice, carrying the blast disease resistance gene Pi-a and lesion mimic mutants sl, cdr1, cdr2, and Cdr3 of Kinmaze background (Kiyosawa, 1970; Marchetti et al., 1983; Takahashi et al., 1999), were used in this study. Rice suspension cultures expressing the constitutively active OsRac1 have been described previously (Kawasaki et al., 1999; Ono et al., 2001). Rice seeds in the cv Nipponbare background carrying the retrotransposon Tos17 insertion in the OsMT2b gene (NE7013) were kindly provided by Drs. Hirohiko Hirochika and Akio Miyao (Institute of the Society for Techno-Innovation of Agriculture, Forestry and Fisheries, Tsukuba, Japan). In plant line NE7013, the Tos17 is inserted in the second exon of OsMT2b (Tos17 Mutant Panel, http://pc7080.abr.affrc.go.jp/~miyao/pub/tos17/). The OsMT2b::Tos17 insertion was confirmed by PCR using genomic DNA and combinations of Tos17-specific (T17-1F: 5′-CTATGTGCCCTCCGAGCTACAAG-3′; T17-1R: 5′- GAGGTTGCTTAGCAGTGAAACGC-3′) and OsMT2b-specific (MT2b-1F: 5′-CAATTCTTGAGCTCAATCAGC-3′; MT2b-1R: 5′-ACACACGCACACACTGACAAC-3′) primers.
Magnaporthe grisea 2403-1 (race 007) is virulent and TH67-22 (race 031) is avirulent on cv Kinmaze. The growth conditions of blast fungus and the method for infection of leaf blades have been described previously (Takahashi et al., 1999; Ono et al., 2001). For bacterial blight Xanthomonas oryzae inoculations, leaves were inoculated with the Japanese Xoo race 1, which is virulent on cv Kinmaze, by the clipping method (Kauffman et al., 1973).
RNA Analyses
Total RNA was isolated and FDD was performed as described previously (Kuno et al., 2000), with modifications as described by Hayama et al. (2002). The PCR products were electrophoresed on a 4% polyacrylamide gel and detected with FMBIO II Multi-View (Takara, Kyoto). Candidate bands from FDD were excised from the gel and reamplified using the same PCR conditions, and cloned into the pGEM-T vector (Promega, Madison, WI). The cloned inserts were used as probes for Southern-blot analysis of the original PCR products from FDD, to remove false positives. The expression of the transcripts was analyzed by RNase protection assay, and gel images were analyzed and quantitated with an image analyzer BAS 2000 (Fuji Photo Film, Tokyo), according to Hayama et al. (2002).
RNA gel-blot analyses were performed according to Sambrook et al. (1989). RNA-blot analyses were performed using the Gene Images AlkPhos Direct labeling system and the CDP-Star chemiluminescent detection reagent (Amersham Pharmacia Biotech, Uppsala). Hybridization was performed at 65°C and stringent washes at 70°C, based on conditions recommended by the manufacturer. For making DNA probes, primers specific for OsMT2b (MT2b-1F and MT2b-1R; see above) and OsRac1 (Rac1F: 5′-AGATAGGGCCTATCTTGCTGATCATC-3′; Rac1R: 5′-CTAGAGTTTCCTCCTAGCTGCAAGC-3′) were used for PCR amplification.
DNA Constructs and Rice Transformation
Agrobacterium-mediated transformation of rice calli was performed as described previously (Hiei et al., 1994). Plants were regenerated from transformed calli by selecting for hygromycin resistance. A full-length cDNA clone of OsMT2b isolated from a rice vegetative meristem cDNA library was kindly provided by Dr. Junko Kyozuka (University of Tokyo) and subcloned into the p2K1+ vector under the control of the maize Ubq1 promoter. The OsMT2b-RNAi construct was made by cloning a DNA fragment containing two full-length OsMT2b cDNAs, in inverse orientation and separated by a GFP sequence linker, into the p2K1+ vector under the control of the maize Ubq1 promoter.
Quantitation of ROS
Quantitation of sphingolipid elicitor-induced hydrogen peroxide production was performed according to He et al. (2000), with modifications described by Suharsono et al. (2002).
For superoxide scavenging assays, we used the WST SOD Assay Kit (Dojindo, Kumamoto, Japan). Reactions in 200 mL were performed in microplates according to the manufacturer's instruction. The in vitro superoxide assay system is based on the reduction of a water-soluble sulfonated tetrazolium salt, WST-1, by superoxide generated from a hypoxanthine/xanthine oxidase system. Inhibition of WST-1 reduction by an antioxidant is represented by the percentage decrease in A450 compared to an uninhibited (control) assay (Peskin and Winterbourn, 2000).
For hydroxyl radical scavenging assays, antioxidant-mediated competitive inhibition of the salicylate hydroxylation by hydroxyl radicals was performed as described previously (Smirnoff and Cumbes, 1989).
Production of Recombinant OsMT2b
A PCR fragment containing the entire open reading frame of OsMT2b was cloned into the BamHI site of the Escherichia coli expression vector pGEX4T-1 (Amersham Pharmacia Biotech) to give GST-OsMT2b. To overexpress GST-OsMT2b and the control GST proteins, the pGEX plasmids were used to transform BL21(DE3) E. coli cells. Overnight cultures of the transformant E. coli cells were diluted 1:50 in fresh Luria-Bertani medium supplemented with 100 μg/mL carbenicillin. Transformant E. coli cells were grown to A600 0.8 at 37°C before expression of the recombinant proteins was induced by addition of 1 mm isopropyl β-d-thiogalactoside, followed by growth at 25°C for 4 h. For expression of GST-OsMT2b, the culture was also supplemented 100 μg/mL of ZnCl2. The cells were harvested by centrifugation and lysed by sonication, as described previously (Valls et al., 2001). GST and GST-OsMT2b proteins in the recovered supernatant were purifed by batch affinity chromatography with glutathione-Sepharose 4B (Amersham Pharmacia Biotech) according to the manufacturer's instruction. The purified proteins were dialyzed with 3 changes against 500 volumes phosphate-buffered saline overnight at 4°C and concentrated by Centriprep Concentrators (Amicon, Beverly, MA) with a cut-off of 10 kD. To prevent protein oxidation, the buffer solutions were bubbled with pure nitrogen gas in all the purification steps.
GFP Fusion and Transient Expression in Rice Protoplast
The GFP sequence derived from sGFP-S65T (Chiu et al., 1996) was fused to the C terminus of OsMT2b. Rice Oc protoplasts were prepared as described previously (Kyozuka and Shimamoto, 1991). Protoplasts (5 × 106/mL) were mixed with 10 μg of test plasmid and electroporated using a Gene Pulser (Bio-Rad Laboratories, Hercules, CA). After a 16-hr incubation at 30°C, the protoplasts were examined under a confocal laser scanning microscope (LSM510; Zeiss, Jena, Germany) using a 63× oil immersion lens with dual-line switching excitation (488 nm for GFP, 543 nm for DsRed) and emission (BP 505–530 nm for GFP, LP 560 nm for DsRed) filter sets. The DsRed gene was subcloned from plasmid pDsRed1-1 (a gift from BD Biosciences CLONTECH, Tokyo).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Dr. Philip Mullineaux for suggestions, Drs. Takashi Aoyama and Nam-Hai Chua for the pTA7002 vector, and Dr. Ian Smith for reading the manuscript. We thank the members of the Laboratory of Plant Molecular Genetics at Nara Institute of Science and Technology for comments and participation in discussions.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.036384.
References
- Agrawal GK, Iwahashi H, Rakwal R (2003) Small GTPase ‘Rop’: molecular switch for plant defense responses. FEBS Lett 546: 173–180 [DOI] [PubMed] [Google Scholar]
- Akimoto-Tomiyama C, Sakata K, Yazaki J, Nakamura K, Fujii F, Shimbo K, Yamamoto K, Sasaki T, Kishimoto N, Kikuchi S, et al (2003) Rice gene expression in response to N-acetylchitooligosaccharide elicitor: comprehensive analysis by DNA microarray with randomly selected ESTs. Plant Mol Biol 52: 537–551 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Chua N-H (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11: 605–612 [DOI] [PubMed] [Google Scholar]
- Arase S, Fujita K, Uehara T, Honda Y, Isota J (2000) Light-enhanced resistance to Magnaporthe grisea infection in the rice Sekiguchi lesion mutant. J Phytopathol 148: 197–203 [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028 [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Diebold BA (2002) Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100: 2692–2696 [DOI] [PubMed] [Google Scholar]
- Briat J-F (2002) Metal ion-activated oxidative stress and its control. In D Inze, M Van Montagu, eds, Oxidative Stress in Plants. Taylor and Francis, London, pp 171–189
- Cai L, Satoh M, Tohyama C, Cherian MG (1999) Metallothionein in radiation exposure: its induction and protective role. Toxicology 132: 85–98 [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H Jr, Van Montagu M, Inze D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA 95: 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6: 325–330 [DOI] [PubMed] [Google Scholar]
- Chubatsu LS, Meneghini R (1993) Metallothionein protects DNA from oxidative damage. Biochem J 291: 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59: 627–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98: 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15: 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to hyphal wall components. Physiol Plant Pathol 23: 345–357 [Google Scholar]
- Dorey S, Baillieul F, Saindrenan P, Fritig B,j Kauffmann S (1998) Tobacco class I and II catalases are differentially expressed during elicitor-induced hypersensitive cell death and localized acquired resistance. Mol Plant Microbe Interact 11: 1102–1109 [Google Scholar]
- Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8: 1773–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG (2000) Response to plant pathogens. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. ASPP Press, Rockville, MD, pp 1102–1156
- Hayama R, Izawa T, Shimamoto K (2002) Isolation of rice genes possibly involved in the photoperiodic control of flowering by a fluorescent differential display method. Plant Cell Physiol 43: 494–504 [DOI] [PubMed] [Google Scholar]
- He Z, Wang Z-Y, Li J, Zhu Q, Lamb C, Ronald P, Chory J (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288: 2360–2363 [DOI] [PubMed] [Google Scholar]
- Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44: 321–334 [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282 [DOI] [PubMed] [Google Scholar]
- Hsieh HM, Liu WK, Chang A, Huang PC (1996) RNA expression patterns of a type 2 metallothionein-like gene from rice. Plant Mol Biol 32: 525–529 [DOI] [PubMed] [Google Scholar]
- Huckelhoven R, Kogel KH (2003) Reactive oxygen intermediates in plant-microbe interactions: who is who in powdery mildew resistance? Planta 216: 891–902 [DOI] [PubMed] [Google Scholar]
- Hussain S, Slikker W Jr, Ali SF (1996) Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem Int 29: 145–152 [DOI] [PubMed] [Google Scholar]
- Johnson C, Boden E, Arias J (2003) Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 15: 1846–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Lawrence A, Wardman P, Burkitt MJ (2002) Electron paramagnetic resonance spin trapping investigation into the kinetics of glutathione oxidation by the superoxide radical: re-evaluation of the rate constant. Free Radic Biol Med 32: 982–990 [DOI] [PubMed] [Google Scholar]
- Kauffman HE, Reddy APK, Hsieh SPH, Merca SD (1973) Improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis Rep 57: 537–541 [Google Scholar]
- Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimamoto K (1999) The small GTP-binding protein Rac is a regulator of cell death in plants. Proc Natl Acad Sci USA 96: 10922–10926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner MJ, Bagnell R, Welch KJ (1990) Thioureas react with superoxide radicals to yield a sulfhydryl compound. Explanation for protective effect against paraquat. J Biol Chem 265: 1306–1311 [PubMed] [Google Scholar]
- Kiyosawa S (1970) Inheritance of a particular sensitivity of the rice variety, Sekiguchi Asahi, to pathogens and chemicals, and linkage relationship with blast resistance genes. Bull Natl Inst Agric Sci Ser D (Physiol Genet) 21: 61–71 [Google Scholar]
- Koga J, Yamauchi T, Shimura M, Ogawa N, Oshima K, Umemura K, Kikuchi M, Ogasawara N (1998) Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. J Biol Chem 273: 31985–31991 [DOI] [PubMed] [Google Scholar]
- Kuchitsu K, Kosaka H, Shiga T, Shibuya N (1995) EPR evidence for generation of hydroxyl radical triggered by N-acetylchitooligosaccharide elicitor and a protein phosphatase inhibitor in suspension-cultured rice cells. Protoplasma 188: 138–142 [Google Scholar]
- Kuno N, Muramatsu T, Hamazato F, Furuya M (2000) Identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis using a fluorescent differential display technique. Plant Physiol 122: 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyozuka J, Shimamoto K (1991) Transformation and regeneration of rice protoplasts. In K Lindsey, ed, Plant Tissue Culture Manual B1. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–17
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua N-H (1999) Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev 15: 1808–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MA, Bollich CN, Uecker FA (1983) Spontaneous occurrence of the Sekiguchi lesion in two American rice lines: its induction, inheritance, and utilization. Phytopathology 73: 603–606 [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–441 [DOI] [PubMed] [Google Scholar]
- Mittler R, Feng X, Cohen M (1998) Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen-induced programmed cell death in tobacco. Plant Cell 10: 461–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Herr EH, Orvar BL, van Camp W, Willekens H, Inze D, Ellis BE (1999) Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc Natl Acad Sci USA 96: 14165–14170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Nishizawa Y, Kawakami A, Hibi T, He DY, Shibuya N, Minami E (1999) Regulation of chitinase gene expression in suspension-cultured rice cells by N-acetylchitooligosaccharides: differences in the signal transduction pathways leading to the activation of elicitor-responsive genes. Plant Mol Biol 39: 907–914 [DOI] [PubMed] [Google Scholar]
- Ono E, Wong H-L, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K (2001) Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 98: 759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Choi HJ, Lee S, Lee T, Yang Z, Lee Y (2000) Rac-related GTP-binding protein in elicitor-induced reactive oxygen generation by suspension-cultured soybean cells. Plant Physiol 124: 725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin AV, Winterbourn CC (2000) A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin Chim Acta 293: 157–166 [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Tommey AM, Kuske C, Jackson PJ (1993) Plant metallothioneins. Biochem J 295: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radic Biol Med 14: 325–337 [DOI] [PubMed] [Google Scholar]
- Schiene K, Puhler A, Niehaus K (2000) Transgenic tobacco plants that express an antisense construct derived from a Medicago sativa cDNA encoding a Rac-related small GTP-binding protein fail to develop necrotic lesions upon elicitor infiltration. Mol Gen Genet 263: 761–770 [DOI] [PubMed] [Google Scholar]
- Schwarz MA, Lazo JS, Yalowich JC, Allen WP, Whitmore M, Bergonia HA, Tzeng E, Billiar TR, Robbins PD, Lancaster JR Jr, et al (1995) Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proc Natl Acad Sci USA 92: 4452–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda R, Aachanzar WE, Qu W, Nagamine T, Takagi H, Mori M, Waalkes MP (2003) Metallothionein is a potential negative regulator of apoptosis. Toxicol Sci 73: 294–300 [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28: 1057–1060 [Google Scholar]
- Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2002) The heterotrimeric Gα protein subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 99: 13307–13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kawasaki T, Henmi K, Shii K, Kodama O, Satoh H, Shimamoto K (1999) Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant J 17: 535–545 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Chen Z, Du H, Liu Y, Klessig DF (1997) Development of necrosis and activation of disease resistance in transgenic tobacco plants with severely reduced catalase levels. Plant J 11: 993–1005 [DOI] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu H-M (2002) Plant Rac-like GTPases are activated by auxin and mediate auxin responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, Vasak M (1985) Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta 827: 36–44 [DOI] [PubMed] [Google Scholar]
- Tsukada K, Ishizaka M, Fujisawa Y, Iwasaki Y, Yamaguchi T, Minami E, Shibuya N (2002) Rice receptor for chitin oligosaccharide elicitor does not couple to heterotrimeric G-protein: elicitor responses of suspension cultured rice cells from Daikoku dwarf (d1) mutants lacking a functional G-protein α-subunit. Physiol Plant 116: 373–382 [Google Scholar]
- Umemura K, Ogawa N, Koga J, Iwata M, Usami H (2002) Elicitor activity of cerebroside, a sphingolipid elicitor, in cell suspension cultures of rice. Plant Cell Physiol 43: 778–784 [DOI] [PubMed] [Google Scholar]
- Valls M, Bofill R, Gonzalez-Duarte R, Gonzalez-Duarte P, Capdevila M, Atrian S (2001) A new insight into metallothionein (MT) classification and evolution: the in vivo and in vitro metal binding features of Homarus americanus recombinant MT. J Biol Chem 276: 32835–32843 [DOI] [PubMed] [Google Scholar]
- Vanacker H, Carver TL, Foyer CH (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol 123: 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294: 1299–1304 [DOI] [PubMed] [Google Scholar]
- Yang Z (2002) Small GTPases: versatile signaling switches in plants. Plant Cell 14: S375–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LH, Umeda M, Liu JY, Zhao NM, Uchimiya H (1998) A novel MT gene of rice plants is strongly expressed in the node portion of the stem. Gene 206: 29–35 [DOI] [PubMed] [Google Scholar]
- Zheng ZL, Nafisi M, Tam A, Li H, Crowell DN, Chary SN, Schroeder JI, Shen J, Yang Z (2002) The plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 14: 2787–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]