Abstract
A number of studies have demonstrated β-amylase induction in response to abiotic stress. In the present work, a temperature response profile in 5°C increments from 45°C to 0°C showed that induction at temperature extremes was specific for two members of the gene family (BMY7 and BMY8). Both members encode proteins that possess apparent transit peptides for chloroplast stromal localization. However, induction was not observed for other key starch degrading enzymes demonstrating a rather specific response to temperature stress for BMY7 and BMY8. Time course experiments for heat shock at 40°C and cold shock at 5°C showed that β-amylase induction correlated with maltose accumulation. Maltose has the ability, as demonstrated by in vitro assays, to protect proteins, membranes, and the photosynthetic electron transport chain at physiologically relevant concentrations. Therefore, β-amylase induction and the resultant maltose accumulation may function as a compatible-solute stabilizing factor in the chloroplast stroma in response to acute temperature stress.
β-Amylase is an exoamylase that hydrolyzes α-1,4 glycosidic linkages of polyglucan chains at the nonreducing end to produce maltose (4-O-α-d-Glucopyranosyl-β-d-Glc). The reducing Glc of maltose is in the β-form, hence, the name β-amylase (Kossmann and Lloyd, 2000). The primary function of β-amylase is involvement in starch breakdown in plants (Kossmann and Lloyd, 2000). Based on in vitro studies, the physiological role of β-amylase in starch breakdown has long been controversial, primarily because it was thought to be catalytically inactive on native starch grains without ample prior digestion by other amylolytic enzymes like α-amylase (Beck and Ziegler, 1989). However, a recent study has demonstrated that β-amylase plays a major role in transitory starch breakdown (Scheidig et al., 2002). Down-regulation of a chloroplast-localized β-amylase using antisense methods resulted in a starch-excess phenotype in potato (Solanum tuberosum) leaves compared to wild-type plants (Scheidig et al., 2002). Plants deficient in chloroplastic β-amylase had reduced ability to degrade starch, especially in the dark, indicating that hydrolytic cleavage is the predominant route over that of phosphorylytic cleavage during transitory starch degradation. Additionally, the product of β-amylase is translocated to the cytosol by a recently discovered maltose translocator (Niittyla et al., 2004) to be metabolized to Glc units by cytosolic glucosyltransferases during transitory starch degradation (Lu and Sharkey, 2004; Sean et al., 2004).
β-Amylases are localized to the stroma of mesophyll cell chloroplasts (Lao et al., 1999; Scheidig et al., 2002), the vacuole (Ziegler and Beck, 1986; Datta et al., 1999), and the cytoplasm. One Arabidopsis β-amylase, designated ct-Bmy (AJ250341; BMY8), has been biochemically localized to the chloroplast stroma in import studies with isolated pea (Pisum sativum) chloroplasts and confirmed by accumulation of a β-amylase-GFP fusion protein in Arabidopsis chloroplasts (Lao et al., 1999). Database searches have revealed that Arabidopsis contains 9 genes for β-amylase (BMY1 to BMY9). Based on sequence analysis, only 3 genes BMY7, BMY8, and BMY9 appear to encode proteins with a putative transit peptide for chloroplast localization. For reasons that remain unknown, BMY1 (ram1), accounting for more than 90% of total β-amylase activity in Arabidopsis mesophyll cells, is localized to the vacuole or the secretory pathway (Monroe and Preiss, 1990).
β-Amylases exhibit a complex regulation. Expression and activity are regulated by light (Sharma and Schopfer, 1982; Harmer et al., 2000; Chandler et al., 2001; Tepperman et al., 2001), sugars (Nakamura et al., 1991; Mita et al., 1995; Maeo et al., 2001), phytohormones (Ohto et al., 1992; Wang et al., 1996), proteolytic cleavage (Hara-Nishimura et al., 1986; Sopanen and Lauriere, 1989), and abiotic stresses (Dreier et al., 1995; Nielsen et al., 1997; Datta et al., 1999; Seki et al., 2001; Sung, 2001). Regulation of β-amylase expression and activity by abiotic stress includes osmotic (Dreier et al., 1995; Datta et al., 1999), salt (Dreier et al., 1995; Datta et al., 1999), cold (Nielsen et al., 1997; Seki et al., 2001; Sung, 2001), and heat stress (Dreier et al., 1995). The regulation of β-amylase activity by osmotic stress appears to be a general response for several plant species (Dreier et al., 1995; Datta et al., 1999). Exposure of barley (Hordeum vulgare; Dreier et al., 1995), pearl millet (Pennisetum glaucum), and maize (Zea mays; Datta et al., 1999) to osmotic stress also results in the increase of β-amylase activity, which is correlated with an increase in β-amylase protein. Not surprisingly, salt stress also stimulates β-amylase protein accumulation and activity in maize and barley (Dreier et al., 1995; Datta et al., 1999).
β-Amylase expression (Seki et al., 2001; Sung, 2001) and/or activity (Nielsen et al., 1997) is induced by cold and heat stress. When Arabidopsis was cold-stressed at 4°C for 12 h, β-amylase (AJ25034; ct-Bmy or BMY8) expression increased about 14-fold (Sung, 2001), and induction was found to occur as early as 2 h of exposure to cold stress (Seki et al., 2001). Similarly, when potato tuber storage temperature was reduced from 20°C to 5°C or 3°C, β-amylase activity increased 4- to 5-fold over a 10-d period and was followed by maltose accumulation, whereas the activities of other starch degrading enzymes like α-glycosidase and endoamylase remained unchanged (Nielsen et al., 1997). Heat stress also induces β-amylase activity (Dreier et al., 1995), such that raising barley growth temperature from 25°C to 35°C results in increased β-amylase activity. However, the role of β-amylase induction during temperature shock is unknown.
A number of microarray studies with Arabidopsis have shown the chloroplast-localized β-amylases, BMY7 and BMY8, to be heat and cold shock responsive (Seki et al., 2001, 2002; Sung, 2001; Fowler and Thomashow, 2002; Kreps et al., 2002; Jung et al., 2003). Even though transcriptome profiling gives valuable information about the functionality of the genes based on expression patterns, down stream information such as changes in protein abundance or metabolite content is required to fully reveal functional roles. In this study, it is shown that β-amylase induction precedes the appearance of maltose, which has compatible solute-like protective abilities as demonstrated by in vitro assays. Therefore, stress-induced maltose accumulation may contribute to the protection of the photosynthetic electron transport chain, proteins, and membranes inside the chloroplast during acute temperature shock.
RESULTS
Heat and Cold Shock Elicit Specific β-Amylase Gene Induction
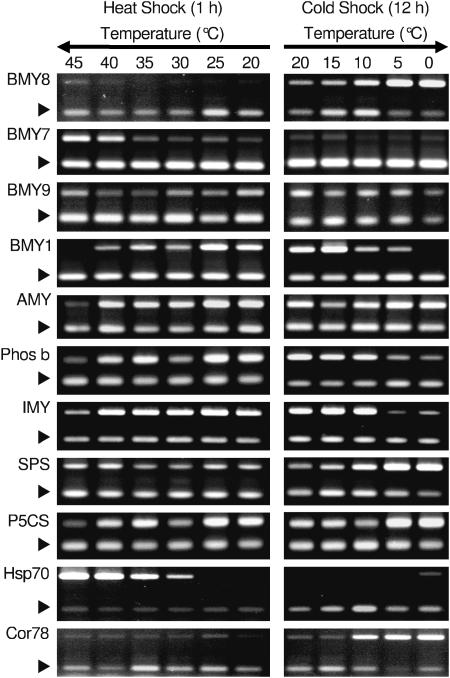
A step-up and step-down experiment was performed to determine how temperature influences β-amylase expression. Plants were exposed to temperatures in 5°C increments from 20°C to 45°C for 1 h in the step-up experiment (Fig. 1). For the step-down experiment, plants were exposed to temperatures in 5°C decrements from 20°C to 0°C for 12 h (Fig. 1). The different exposure times for the step-up and step-down treatments were approximated to equalize the influence of the temperature differential on kinetics based on the Arrhenius equation relationship for respiratory processes (Yelenosky and Guy, 1977) for the temperature extremes of 40° and 5°C. Gene-specific transcript levels were evaluated by reverse transcription (RT)-PCR. Hsp70 (At3g12580) was used as a control for heat treatment, and its expression gradually increased as temperature increased (Fig. 1). Likewise, Cor78/rd29A (At5g52310) was used as a control for cold shock treatment, and its expression showed slight induction at 10°C and strong induction at 5°C and 0°C (Fig. 1). The chloroplast-targeted β-amylase (ct-bmy, BMY8; Fig. 1) showed the greatest expression at 5°C and 0°C increasing by 15- and 13-fold, respectively. Expression was more modestly induced at 10°C, approximately 7-fold. During heat shock, expression was unchanged. Conversely, the expression of another putative chloroplast-targeted β-amylase (BMY7) was increased at 40°C and 45°C, 11- and 8-fold, respectively. BMY7 expression did not change in response to cold shock temperatures down to 0°C. A third predicted chloroplast-localized β-amylase (BMY9) did not exhibit temperature-regulated modulation of expression, but instead was expressed at all temperatures from 45°C to 0°C.
Figure 1.
RT-PCR analysis of selected genes following heat and cold shock. Sixteen-day-old Arabidopsis plants were grown at 20°C and exposed to heat shock at 25°C, 30°C, 35°C, 40°C, and 45°C for 1 h and cold shock at 15°C, 10°C, 5°C, and 0°C for 12 h. Control plants were kept at 20°C. The arrowheads indicate the 18S rRNA internal control. Representative images are shown from one of the three experiments. BMY1 β-amylase 1 (At4g15210); BMY7, β-amylase 7 (At3g23920); BMY8, β-amylase 8 (At4g17090); BMY9, β-amylase 9 (At4g00490); AMY, α-amylase (At1g69830); IMY, isoamylase (At2g39930); Phos b, phosphorylase b (At3g29320); P5CS, δ-1-pyrroline 5-carboxylase synthetase (At2g39795); SPS, sucrose-phosphate synthase (AL391222); Hsp70, heat shock protein 70 (At3g12580); Cor78, low-temperature-induced protein 78 (At5g52310); 18S rRNA (At3g41768).
The vacuolar form accounts for the major β-amylase activity in Arabidopsis tissues. Expression of the vacuolar form (BMY1) was largely unchanged during heat stress, except at 45°C where its mRNA became undetectable. Expression of BMY1 during cold shock decreased 5-fold at 10°C and 5°C and became undetectable at 0°C.
To determine whether modulation of BMY7 and BMY8 expressions is a general stress response of genes for starch degrading enzymes, expression profiles for key enzymes in starch degradation pathways were examined in the step-up and step-down RT-PCR experiments (Fig. 1). α-Amylase (AMY1) was repressed 3-fold at 40°C and 5-fold at 45°C; however, its expression was only induced 2-fold at 10°C and 5°C. Phosphorylase b (Phos b) expression was unchanged under heat shock and repressed 5-fold at 5°C and 0°C. Debranching enzyme, or isoamylase (IMY), expression was repressed 4-fold at 45°C, but unchanged at all other heat shock temperatures. During cold shock, IMY expression was unchanged except at 0°C where it was repressed 3-fold. Therefore, the expression patterns of BMY7 and BMY8 under a variety of heat (from 20°C to 45°C) and cold (from 20°C to 0°C) shock temperatures appear to involve specific responses relative to other genes of starch degradation pathways.
Sucrose phosphate synthase (SPS) and δ-1-pyroline 5-carboxylase synthetase (P5CS) expression profiles were also compared with β-amylase expression patterns because their transcripts were known to accumulate during exposure to cold shock temperatures. When the temperature was lowered, SPS expression levels increased (Fig. 1) 3-, 7-, and 10-fold at 10°C, 5°C, and 0°C, respectively. During heat shock, SPS expression was slightly increased. P5CS expression was induced 2.5-fold during cold shock, but its expression was unchanged during heat shock, except for a slight decrease at 40°C and 45°C (Fig. 1).
β-Amylase Expression Is Correlated with Maltose Accumulation
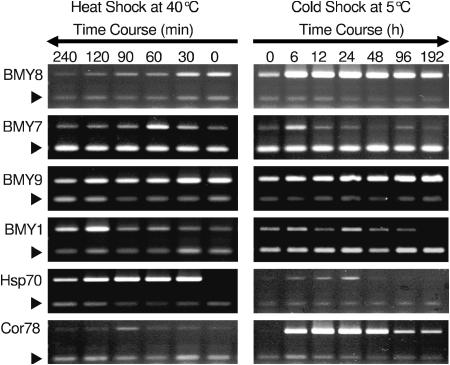
Based on step-up and step-down experiments, expression of β-amylase induction was found to be greatest at 40°C and 5°C. A time course RT-PCR (Fig. 2) study was done to determine the induction kinetics for the β-amylases upon exposure to heat and cold stress. Confirming the previous RT-PCR analysis, under cold shock at 5°C, BMY8 expression was induced dramatically as early as 6 h and peaked at 24 h then gradually decreased but remained higher than control levels at 192 h. BMY8 expression during heat shock at 40°C was repressed after 30 min. In contrast, BMY7 expression peaked at 60 min of exposure to 40°C and then decreased. It did not show any significant change in expression in response to cold shock. At 5°C, BMY7 expression seemed to be induced at 6 h; however, this response was seen only in one replication out of the three (Fig. 2). In agreement with the step-up and step-down experiment, BMY9 failed to show significant changes in expression during heat shock, but BMY1 expression was induced 7-fold at 2 and 4 h of heat shock. Expression of BMY9 and BMY1 was unaffected by cold shock (Fig. 2). Hsp70 was used as a control for the heat treatment and its expression peaked at 30 to 60 min upon exposure to 40°C. Under cold shock conditions, Hsp70 also exhibited a slight induction until 24 h, when its expression became undetectable. Cor 78/rd29A was used as a cold shock treatment control. It showed a very similar expression profile to BMY8 during cold shock but no significant changes in response to heat shock conditions.
Figure 2.
A time course study of RT-PCR analysis of selected β-amylase genes under heat and cold shock. Eighteen-day-old Arabidopsis plants grown at 20°C were exposed to 40°C for 0, 30, 60, 120, and 240 min for heat shock and to 5°C for 0, 6, 24, 48, 96, and 192 h. Controls were kept at 20°C. The arrowheads indicate the 18S rRNA internal control. Representative images are shown from one of the three experiments.
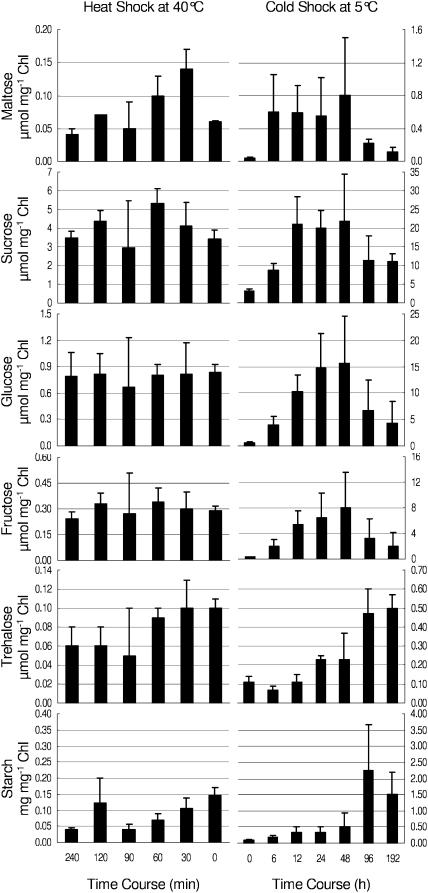
Leaf tissue maltose content (Fig. 3) was measured to determine whether maltose accumulation paralleled changes in β-amylase expression. Soluble sugar analysis revealed that maltose content doubled from 0.06 to 0.14 μmol mg−1 Chl within 30 min exposure to 40°C, remained constant until 60 min, and then decreased back to control levels at 4 h. During cold shock, the maltose content showed a dramatic increase from 0.04 (control) to 0.60 μmol mg−1 Chl in the first 6 h then continued to accumulate to 0.80 μmol mg−1 Chl by 48 h. Afterward, maltose decreased to 0.11 μmol mg−1 Chl by 192 h. The maltose accumulation profile (Fig. 3) was similar to that of Suc, Glc, and Fru. Given that the concentrations of a number of soluble sugars were altered in response to temperature shock, the proportion of maltose at 30 min of heat shock and at 6 h of cold shock was increased relative to the total soluble sugar content. Interestingly, when total soluble sugar content began decreasing, trehalose content began increasing about 96 h after cold shock. In contrast, trehalose content slightly decreased after 90 min of exposure to heat shock.
Figure 3.
Carbohydrate profiles for time course experiments for heat at 40°C and cold at 5°C. Eighteen-day-old Arabidopsis plants were grown at 20°C then exposed to 40°C for 0, 30, 60, 120, and 240 min for heat shock and to 5°C for 0, 6, 24, 48, 96, and 192 h. Control plants were kept at 20°C. Error bars indicate sd of the mean for three experiments.
To determine whether maltose accumulates within chloroplasts during temperature stress, intact chloroplasts needed to be isolated. Most species, including Arabidopsis, are not well suited for isolation of intact chloroplasts. Currently, pea and spinach are widely used for intact chloroplast isolation. Therefore, as a proxy for Arabidopsis, we chose to study isolated pea chloroplast. Intact chloroplasts were isolated from 10-d-old pea leaves exposed to 40°C for 30 min or to 5°C for 24 h, and Glc, trehalose, Fru, and maltose contents were determined (Table I). After 24 h at 5°C, maltose content in pea chloroplasts was about 2.5-fold greater than chloroplasts from 20°C grown seedlings, but under heat shock, a change in maltose content was not detected.
Table I.
Soluble sugar content of the isolated pea chloroplast fraction from control and temperature-shocked plants
| Treatment | Maltose | Glucose | Fructose | Trehalose |
|---|---|---|---|---|
| μmol mg−1 Chl | μmol mg−1 Chl | μmol mg−1 Chl | μmol mg−1 Chl | |
| Control 18°C | 0.002 ± 0.001 | 0.017 ± 0.005 | 0.005 ± 0.003 | 0.001 ± 0.001 |
| Cold Shock 5°C 24 h | 0.005 ± 0.001 | 0.046 ± 0.011 | 0.015 ± 0.002 | 0.002 ± 0.001 |
| Heat Shock 40°C 30 min | 0.001 ± 0.000 | 0.028 ± 0.004 | 0.011 ± 0.005 | 0.002 ± 0.001 |
Starch content (Fig. 3) was determined to better understand its relationship with maltose accumulation. Starch content increased with time and was correlated with accumulation of maltose at about 48 h of cold shock. Conversely, during heat shock, starch content decreased over time from 0.15 to 0.04 mg mg−1 Chl. The decrease in starch content could be related to accelerated metabolism due to elevated temperature.
β-Amylase protein levels were analyzed to determine whether a correlation between protein abundance and gene expression profiles existed. Monoclonal antibodies specific for unique β-amylase C-terminal sequences of each chloroplast-localized β-amylase were prepared using synthetic peptides. Selected monoclonal antibodies were tested for their specificity against purified recombinant GST β-amylase proteins. Monoclonal antibodies specific for BMY7 and BMY9 and a polyclonal mouse serum that recognized all three BMY7, BMY8, and BMY9 proteins were used for western analysis. Protein levels were found to be very low for BMY7, BMY8, and BMY9. BMY7 protein levels gradually decreased in response to heat shock and remained constant under cold shock paralleling its expression profile (data not shown). BMY9 protein levels remained unchanged in response to heat and cold shock also mirroring expression patterns (data not shown). Unfortunately, informative western blots were not obtained for BMY8 proteins levels using the polyclonal mouse serum.
Total leaf β-amylase enzyme activity was measured to distinguish whether activity is correlated with changes in β-amylase gene expression. Total leaf β-amylase enzyme activity remained constant during heat and cold shock conditions (data not shown). To rule out the possibility that changes in chloroplastic β-amylase activity, which is about 10% of the total, may have been obscured in total β-amylase activity measurements, pea chloroplasts were isolated to determine the influence of temperature on chloroplastic β-amylase activity. Pea plants were heat shocked at 40°C for 30 min and cold shocked at 5°C for 24 h and chloroplasts isolated. Total pea chloroplastic β-amylase activity did not show any striking change in response to heat or cold shock (two replications done for each treatment; data not shown).
Maltose Has Compatible Solute Properties
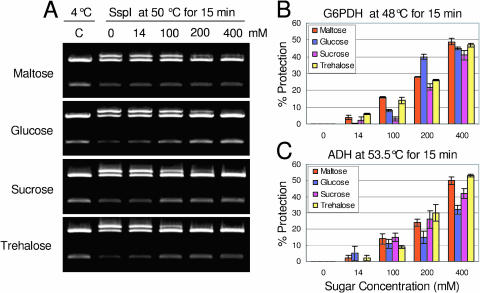
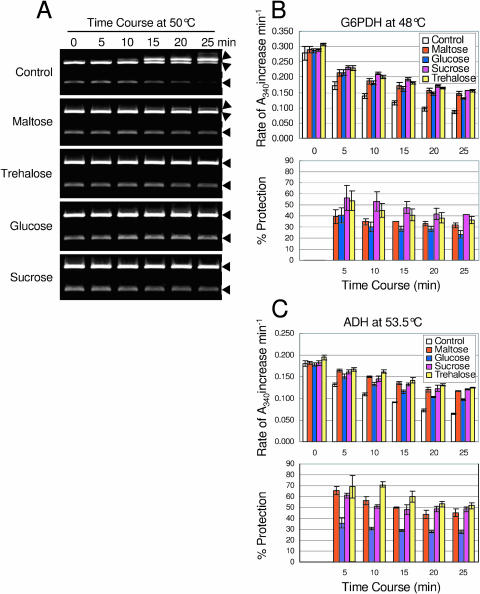
Compatible solutes stabilize proteins and membranes and contribute to cell osmotic potential during stress. The compatible solute properties of maltose were tested for three different enzymes: SspI, Glc 6 phosphate dehydrogenase (G6PDH), and alcohol dehydrogenase (ADH). The restriction enzyme, SspI (Fig. 4A, lane C), cleaves the substrate plasmid (pGEX-4T-2; 4.97 kb, Pharmacia Biotech, Piscataway, NJ) twice, leading to 1- and 4-kb plasmid fragments when the enzyme is fully functional. If the enzyme is compromised by high temperature, it does not completely digest the plasmid but produces a singly cleaved plasmid fragment of 5 kb (top band) and double cut fragments of 1 and 4 kb (Fig. 4A, lane 0, mm sugars). As a result, three bands are produced instead of two. We considered that when the intensity of the 5-kb band (singly cleaved) and the 4-kb band was equal, the enzyme had lost 50% activity. Ssp1 was exposed to 50°C for 15 min (Fig. 4A) in the absence of maltose (0 mm as a control) and in the presence of (14, 100, 200, and 400 mm) maltose. The 5-kb band, which represented the loss of activity, was quantified to determine relative protection. When the enzyme was fully active, the 5-kb band was not visible (Fig. 4A, lane C), but its intensity increased with loss of enzyme activity (lane 2; 0 mm sugars). At an estimated physiological concentration (14 mm), maltose was able to partially protect SspI function. Not surprisingly, this protection increased with increasing maltose concentration (Fig. 4A). At 200 and 400 mm, the intensity of the top band was much reduced compared to 0 mm maltose.
Figure 4.
In vitro compatible solute assay for three enzymes. SspI, G6PDH, and ADH were exposed to heat for 15 min in the absence and presence of a variety of concentrations of maltose, Glc, Suc, and trehalose. A, SspI cleaves double stranded DNA. In this assay, functional SspI cuts the pGEX-4T-2 plasmid twice to produce 2 bands of 3.77 kb and 1.19 kb. Heat damaged SspI cuts the plasmid only once to yield a 5-kb open circle (top band). Protection of the enzyme by soluble sugars is indicated by a reduction in the intensity of the 5-kb band. B, G6PDH. C, ADH.
Besides maltose, identical concentrations of Suc, trehalose, and Glc were also tested to compare the performance of maltose as a compatible solute (Fig. 4A). Maltose performed as well as trehalose at physiological concentration. At 200 mm, trehalose showed 100% protection, while maltose showed 80% to 90% protection. At 400 mm, Glc, trehalose, and Suc showed 100% protection, while maltose showed 85% to 90% protection. In addition, protection of SspI activity (Fig. 5A) in the presence of soluble sugars maltose, trehalose, Glc, and Suc (400 mm) can be maintained for at least 25 min at 50°C, while in the absence of sugars, the enzyme lost almost 90% of its activity over this time. Again trehalose, Glc, and Suc show 100% protection, while maltose shows approximately 90% protection.
Figure 5.
In vitro time course compatible solute assay for three enzymes. SspI, G6PDH, and ADH were exposed to heat for 25 min in the absence and presence of 400 mm maltose, Glc, Suc, and trehalose. A, SspI, arrowheads show the 4- and 5-kb bands. B, G6PDH. C, ADH.
To test whether protection was general, compatible solute assays were done using G6PDH, which uses d-Glc-6-P and NAD(P)+ as substrates to produce d-glucono-δ-lactone-P and NAD(P)H. Maltose performed as well as the other compatible solute sugars even at physiological concentration (Fig. 4B). The same performance was seen (Fig. 5B) when the time was extended to 25 min in the presence of 400 mm of the above sugars.
ADH utilizes RCH2OH and NAD+ as substrates to produce RCHO, NADH, and H+ in a reversible reaction. ADH was heat stressed (Fig. 4C) in the absence and presence of 14, 100, 200, and 400 mm maltose, Glc, trehalose, and Suc. Similar to that of the SspI and G6PDH compatible solute assays, maltose showed an equal level of protection for ADH. The same was true when the time was extended to 25 min at 53.5°C (Fig. 5C).
Maltose Can Function as a Chloroplast Stromal Compatible Solute in Vitro
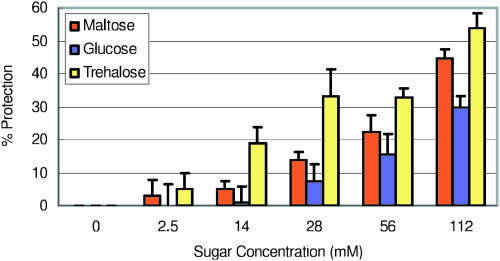
Maltose was tested in vitro for compatible solute properties using thylakoid membranes for the functionality of the electron transport chain against heat denaturation (Fig. 6) and freezing stress (Fig. 7). Maltose was compared against trehalose and Glc. Pea thylakoids were exposed to 40°C for 4 min (Fig. 6), where 30% of electron transport chain activity remained in the absence of compatible solute sugars. Electron transport chain activity was followed by reduction of a redox dye 2,6-dichlorophenolindophenol (DCPIP), which accepts electrons from QB of PSII and FeSA of PSI (Häder and Tevini, 1987). Photosynthetic electron transport chain activity was protected 3% and 5% at physiological concentrations of 2.5 and 14 mm maltose, respectively, during heat shock. When the concentration of maltose increased further, electron transport activity was preserved up to 45% at 112 mm. This level of protection was very comparable to trehalose at the same concentration. Glc showed 30% protection at 112 mm.
Figure 6.
Electron transport chain activity of isolated thylakoids in the absence and presence of soluble sugars during heat shock at 40°C for 4 min. Electron transport was evaluated by DCPIP reduction.
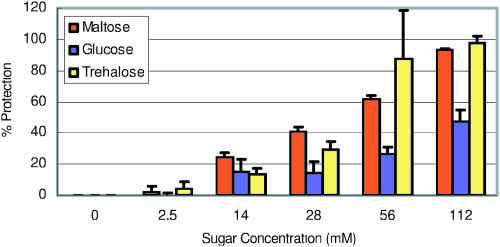
Figure 7.
Electron transport chain activity of isolated thylakoids in the absence and presence of soluble sugars following freezing stress at −15°C for 20 h. Electron transport was evaluated by DCPIP reduction.
Pea thylakoids were frozen (Fig. 7) at −15°C for 20 h, which caused a 60% reduction of electron transport chain activity. Similar to heat shock, protection by maltose was seen at physiologically relevant maltose concentrations 2.5 and 14 mm preserving 2 and 24% activity, respectively. This protection increased gradually to 93% with the increasing concentration of maltose and was very similar to that of trehalose. Glc concentration at 112 mm gave 47% protection, which was much less than that of maltose or trehalose.
DISCUSSION
The RT-PCR analyses presented here are in good agreement with several published microarray studies (Seki et al., 2001; Sung, 2001; Fowler and Thomashow, 2002; Kreps et al., 2002; Seki et al., 2002) where a chloroplastic β-amylase (ct-bmy, BMY8 At4g17090) was shown to be induced during cold shock. In response to heat shock, BMY7 (At3g23920) encoding a protein with a putative chloroplast transit peptide is induced, and this is consistent with a previous microarray study from our laboratory (Sung, 2001). Temperature stress induction apparently is specific to these two β-amylases, but not for all other members of the β-amylase gene family. The expression of other genes of the starch degradation pathway were either unchanged or repressed during heat and cold shock, except for the cold shock induction of α-amylase. These data suggest that β-amylase (BMY7 and BMY8) stress induction was unique and not a common response of starch degrading enzymes to temperature shock.
β-Amylase activity is known to increase in response to heat stress (Dreier et al., 1995) and cold stress (Nielsen et al., 1997). In contrast, Arabidopsis total β-amylase activity of leaf tissue was constant during heat shock at 40°C for a period of 4 h, and during cold shock at 5°C for a period of 8 d. Yet, total leaf β-amylase activity may not represent actual changes in chloroplastic β-amylase activity because 90% of the total β-amylase activity is extra-chloroplastic in Arabidopsis (Monroe and Preiss, 1990). With isolated chloroplasts from heat shocked or cold shocked pea leaves, β-amylase activity slightly increased in response to heat shock but remained unchanged during cold shock (data not shown). It is conceivable that the increase in β-amylase enzyme activity in pea chloroplast could be species specific under stress conditions. Nevertheless, in Arabidopsis and pea, temperature stress resulted in an increase in maltose content similar to that observed for cold stressed potato tubers (Nielsen et al., 1997).
Two possibilities could explain maltose accumulation without an increase in β-amylase activity. Under nonstressed conditions, substrate starch may be a limiting factor in maltose accumulation. The Michaelis constant (Km) of plant β-amylases for soluble starch for the nonreducing end ranges from 1.67 to 6.8 mg mL−1 (Doehlert et al., 1982; Lizotte et al., 1990). β-Amylase is also competitively inhibited by its product maltose, and the Ki for maltose is estimated at 11.5 mm (Lizotte et al., 1990). If Arabidopsis chloroplast-localized β-amylases were constrained by a high Km, increasing starch content under cold stress (Fig. 3) could increase maltose content without any increase in overall β-amylase activity until catalysis approached substrate saturation or was inhibited by its end product maltose. In Arabidopsis, the estimated starch level approximates the Km (1.5–2.3 mg mL−1). The starch levels increase gradually under cold shock to 7.9 mg mL−1 by 48 h, 34 mg mL−1 at 96 h and then decrease to 22.9 mg mL−1 at 192 h. It would be plausible that the increase in starch content at 5°C would result in the increased maltose content until 48 h. Then, competitive inhibition by maltose could limit further maltose accumulation after 48 h even though starch content continues to increase. Another possibility for maltose accumulation may be to repress maltase expression leading to decreased maltase protein and activity in the chloroplast. One study reports that chloroplast-localized maltase activity (Sun et al., 1995) exists in pea plants; nevertheless, no sequence information is available, and current database searches have not yet revealed a candidate α-glucosidase or maltase with a predicted chloroplast target signal from Arabidopsis. In contrast, a mutation in a single-copy maltose translocator gene (Niittyla et al., 2004) leads to maltose accumulation in the chloroplast. It appears that maltose is broken down to its Glc units by cytosolic glucosyltransferases (Niittyla et al., 2004), not by a chloroplastic maltase. Unpublished microarray and metabolic profiling data indicate that the expression of the maltose translocator gene does not significantly change when maltose accumulation occurs under heat and cold shock. Therefore, during temperature shock, maltose accumulation is probably due to the action of β-amylase.
The likely place for maltose to accumulate during acute stress is the chloroplast stroma. There it could act as a compatible solute to protect stromal proteins and the functionality of the thylakoid membrane for the photosynthetic electron transport chain. Compatible solutes (osmoprotectants, osmolytes) are low Mr organic molecules that at high concentrations are not toxic to cells (Yancey et al., 1982; Sakamoto et al., 1998). Estimates of maltose concentrations from this work, if localized exclusively within the stroma, could reach as high as 15 mm, a concentration that would be sufficient to provide significant compatible solute benefit. Therefore, maltose as well as the other sugars that accumulate would have positive effects on the functionality of proteins, membranes, and membrane-associated processes, such as the photosynthetic electron transport chain, against compromise by heat (Fig. 6) and freezing stress (Fig. 7).
The role of β-amylase in stress tolerance can be tested in vivo using BMY7 and BMY8 knockout mutants and examining their tolerance to temperature stress. Maltose content of knockouts can be measured to determine whether a correlation exists between decreased maltose content and decreased chloroplast stress tolerance. The findings presented here indicate that maltose accumulation may be an important factor to help plants cope with acute temperature stress and perhaps during long-term temperature stress as well. Because maltose production is a single step reaction, plants with adequate starch levels could produce significant quantities in the stroma in a very short time to contribute to the protection of chloroplast membranes and proteins. Such a short-term protective benefit would give plants additional time to produce their complement of long-term stress related proteins and metabolites, which requires significant modification and retailoring of metabolism and physiology.
MATERIAL AND METHODS
Plant Growth, and Heat and Cold Shock Treatment
Arabidopsis (variety Columbia) seeds were stratified at 4°C for 3 d and grown in Fafard 2 mix soil (Canadian sphagnum peat (55%), perlite, and vermiculite) with a 15/9 h light/dark cycle at 20°C ± 2°C for 16 to 18 d. The irradiance was 25 to 40 μmol m−2 s−1 photosynthetically active radiation (PAR). Six days before the experiment, irradiance was increased to 90 to 140 μmol m−2 s−1 PAR. Plants were exposed to the same irradiance 90 to 140 μmol m−2 s−1 PAR during heat and cold shock.
In the step-up and step-down experiments, 16-d-old Arabidopsis plants were exposed to 25°C, 30°C, 35°C, 40°C, and 45°C for 1 h heat shock and 15°C, 10°C, 5°C, and 0°C for 12 h cold shock, and in both experiments, 20°C grown plants were used as a control. The temperature treatment was begun and the initial leaf samples (0 time control) were taken at 8 am, 2 h after the lights were on at 6 am. Heat shock leaf samples were taken at 9 am and cold shock leaf samples were taken at 10 pm, 1 h before the end of the photoperiod. Three independent experiments were conducted. Leaf samples were collected, flash frozen in liquid nitrogen, and stored at −80°C for subsequent RNA extraction and RT-PCR analyses.
In the time course experiment, 18-d-old Arabidopsis plants were exposed to 40°C for 0, 30, 60, 120, and 240 min for heat shock and to 5°C for 0, 6, 24, 48, 96, and 192 h for cold shock. Three independent experiments were conducted. Leaf samples were taken 2 h after the lights were on for RNA extraction for RT-PCR analysis, carbohydrate analysis, western blot, enzyme assay, and chlorophyll analysis.
Pea seeds variety Progress number 9 (J. W. Jung Seed, Randolph, WI) were soaked in flowing water for about 8 h before planting. The next day, imbibed seeds were planted on vermiculite and placed in a controlled environment at 18°C ± 2°C 150 μmol m−2 s−1 PAR with a 12/12 light/dark cycle for 9 to 10 d. After 10 d, pea plants were subjected to cold shock 5°C for 24 h and 40°C for 30 min. The aerial portions of the plants was collected from two independent experiments, and chloroplasts were immediately isolated.
RNA Extraction
Heat- and cold-shocked Arabidopsis leaves were flash frozen in liquid nitrogen and stored at −80°C. The leaf tissues were ground to a fine powder in liquid nitrogen using a mortar and pestle, then extracted using Qiagen RNeasy plant mini kits (Qiagen, Valencia, CA) according to the manufacturer's protocol. RNA was quantified using a UV spectrophotometer and stored at −80°C.
Reverse Transcription-PCR
“Ready to Go” RT-PCR Beads (Amersham Pharmacia Biotech, Uppsala) were used for RT-PCR. Each 25 μL reverse transcription (RT)-PCR included 1 unit Taq DNA polymerase, 10 mm pH 9 Tris-HCl, 60 mm KCl, 1.5 mm MgCl2, 200 μm each dNTP, Moloney Murine Leukemia virus reverse transcriptase, RNAguard RNase inhibitor (porcine), stabilizers, DNase and RNase-free BSA, 1 μg primer deoxy nucleotide (pd(N)6) for the first strand synthesis, 0.4 μm each gene specific forward and reverse primers (Table II), 0.1 μm each 18 S rRNA forward and reverse primer (internal loading control), 0.3 μm each 3′ terminal dideoxy 18S rRNA forward and reverse primers, and 16 ng total RNA. A variety of PCR cycles 25, 30, 35, and 40 PCR cycle were tested and adjusted to each gene to make sure DNA amplification was still in a linear phase at the termination of the reaction. Additionally, the total RNA for RT-PCR came from plants that were treated on three different occasions. After addition of total RNA and pd(N)6, the reaction was incubated at 42°C for 30 min for the first strand cDNA synthesis. Gene specific and 18S rRNA primers (as a loading control) were added, and PCR amplification was carried out with a Stratagene Robocycler (Stratagene, La Jolla, CA). The first PCR cycle was 95°C for 5 min, 95°C for 30 s, 52°C for 1 min, 72°C for 1 min and the second cycle was 95°C for 30 s, 52°C for 1 min, 72°C for 1 min. The second cycle was repeated 25 times for BMY8, Cor78, Hsp70; 30 times for BMY1, BMY9, AMY1, P5CS (8 ng total RNA), Phos b; and 35 times for BMY7, IMY, SPS. The final cycle was 72°C for 7 min. Afterward, PCR products were kept at 5°C. PCR products were fractionated in a 1% agarose gel. Gels were stained with ethidium bromide and digitally photographed with an IS-1000 Digital Imaging System (Alpha Innotech, San Leandro, CA).
Table II.
List of primers used in RT-PCR reactions
| Target Gene | MIPS | Primer No. | Primers 5′ to 3′ | |
|---|---|---|---|---|
| BMY1 | At4g15210 | CG363 | ACGCCGGAGAATACAATG | F |
| CG364 | CAACGGCACAATCTCATG | R | ||
| BMY7 | At3g23920 | CG305 | GACACCCAGTTCAAAA | F |
| CG306 | CTCAACTTCTTCCCGACA | R | ||
| BMY8 | At4g17090 | CG307 | GGAACAAGCGGACCTCAT | F |
| CG308 | TCTCAGCGATCTTGCCTT | R | ||
| BMY9 | At4g00490 | CG382 | GCTGGCAGGCGTAACACT | F |
| CG383 | CGGTTTGAGGAGTTGTAGAAG | R | ||
| IMY | At2g39930 | CG351 | CGTCTTGAACCACACAGC | F |
| CG352 | GCAAAGTCTCCCTCCTCT | R | ||
| AMY | At1g69830 | CG345 | CCAGGGTAGAGGAAACAA | F |
| CG346 | TCGAAGAAGACCGCTGGT | R | ||
| PHOS b | At3g29320 | CG315 | AAGATGAAGGAAATGAGTG | F |
| CG316 | CATCTTTTCTGGTCTCGG | R | ||
| P5CS | At2g39795 | CG353 | GGACCAAGGGCAAGTAAG | F |
| CG354 | AGCCCATCCTCCTCTGTG | R | ||
| SPS | At5g11110 | CG321 | AATGACAATATCTGAGACTC | F |
| CG322 | ACCACATTCTTTAGCCTC | R | ||
| RD29A or Cor78 | At5g52310 | CG309 | CTTTGACTCTGTTCTCGGT | F |
| CG310 | GTTGTCAGTTTCTCCGCC | R | ||
| Hsp70 | At3g12580 | CG258 | TCAAGCGGATAAGAGTCACT | F |
| CG259 | CTCGTCCGGGTTAATGCT | R | ||
| 18S rRNA | At3g41768 | CG359 | GGAGCGATTTGTCTGGTT | F |
| CG360 | TGATGACTCGCGCTTACT | R | ||
| 18S rRNA 3′ dideoxy | At3g41768 | CG361 | GGAGCGATTTGTCTGGTT-3′ | F |
| CG362 | TGATGACTCGCGCTTACT-3′ | R |
β-Amylase Enzyme Assay
Heat and cold shocked Arabidopsis leaves from three independent experiments were flash frozen in liquid nitrogen and ground to a fine powder using mortar and pestle. Crude extracts were prepared in an extraction buffer (50 mm Tris and 1 mm Na2EDTA pH 6.2), thoroughly mixed, and centrifuged for 10 min at 10,000g in a JA-18.1 rotor using a Beckman J2-21 centrifuge at 4°C. The supernatant was used for an enzyme activity assay using a β-amylase assay kit (Megazyme, Bray, Ireland) according to the manufacturer's protocol. The β-amylase substrate solution (100 μL) was preincubated at 40°C for approximately 5 min, then 100 μL crude enzyme extract was added to the substrate solution, mixed, and incubated at 40°C for exactly 10 min. The control included a substrate solution and extraction buffer instead of plant extract. After 10 min, 1500 μL of stop buffer (1% [w/v] Trisma base) was added to stop the reaction. Production of p-nitrophenyl was measured at A410 spectrophotometrically. In this assay, the specific artificial substrate p-nitrophenyl maltopentaoside (PNPG5) was used, which is resistant to cleavage by α-amylases for p-nitrophenyl production. Total protein content was quantified using a Bradford assay at A595.
Western-Blot Analysis
Monoclonal antibodies were prepared using synthetic peptides that were unique (C-terminal) for each of the three β-amylases, namely BMY7, BMY8, and BMY9. Peptide sequences were as follows: BMY7 (At3g23920) EGRDSHCREEVEREAEHFVHC, BMY8 (At4g17090) KNMKEGGHGRRLSKEDTTGSDLC, and BMY9 (At4g00490) ESQNFKEFERFLKRMGEAVC. The peptides were cross-linked individually to the carrier protein, keyhole limpet hemocyanin, through the C-terminal Cys sulfhydryl. DNA sequences for cDNAs of all three β-amylases were cloned into pGEX-4T-2 or pGEX-2T vectors (Pharmacia Biotec). The expressed recombinant proteins were used for screening hybridoma cell lines and cross-reactivity tests. The monoclonal antibody for BMY7 did not cross-react with the other recombinant β-amylases BMY8 and BMY9, and similarly the monoclonal antibody for BMY9 did not cross-react with the other recombinant β-amylases BMY7 and BMY8.
Monoclonal antibodies were obtained for BMY9 and BMY7, 3H10-3D6 and 4F5-5G10, respectively. A monoclonal antibody for BMY8 was not obtained. However, a mouse serum that recognizes all three β-amylases was obtained.
Heat and cold shocked Arabidospis leaves were flash frozen in liquid nitrogen and stored at −80°C. Leaves from three independent experiments were ground to a fine powder in liquid nitrogen using mortar and pestle. Total protein from leaf tissue was prepared in an extraction buffer containing 50 mm Tris-HCl pH 7.0, 0.1 mm Na2EDTA, 5 mm dithiotheitol, 1 mm PMSF, 1 μg/mL leupeptin, and 1 μg/mL pepstatin A. Protein content was quantified by Bradford assay at A595. Fifteen μg total protein was loaded on to 10% polyacrylamide gel and run at 100 V for 15 min and then 200 V for 45 min. Proteins were transferred to polyvinylidene difluoride membrane by semidry-blotter (Bio-Rad, Hercules, CA), according to the manufacturer's protocol. Because the abundance of β-amylase protein was too low for the conventional western-blot staining detection system, we used the more sensitive ABC staining kit from Pierce (Rockford, IL). ABC western staining was done according to the manufacturer's protocol. Control gels for equal loading were stained with Coomassie Blue.
Chlorophyll Extraction
Chlorophyll content was determined by the method of Bruinsma (1963). Chlorophyll was extracted from 10 mg freeze-dried leaves prepared from three independent experiments using 1 mL of 80% acetone at 4°C overnight in a 2-mL screw cap micro tube in the dark. Chlorophyll content was quantified spectrophotometrically at A645 and A663.
Carbohydrate Analysis
Eighteen-day-old cold and heat shocked Arabidopsis plants from three independent experiments were harvested, flash frozen in liquid nitrogen, and freeze-dried. Lactose at 200 μm was added to samples as an internal standard at the beginning of extraction to normalize the data due to losses during the extraction procedure and due to changes in the HPLC detection system. Soluble sugars were extracted five times using hot 80% aqueous ethanol from 30 mg (dry-weight) of aerial tissue, including leaves and stems. Ethanol insoluble materials were saved for starch analysis. Ethanol was evaporated at 80°C, and the remaining aqueous solution was lyophilized and resuspended in distilled water. To collect soluble neutral sugars, extracts were passed though an Amberlite ion-exchange column with 1 meq/exchange capacity at room temperature. The flow-through was freeze-dried. Monosaccharides and disaccharides were separated using an HPLC with Dionex CarboPac PA10 column. Samples injected on a PA 10 column were separated at a flow rate of 1.0 mL/min with a step gradient of NaOH from 10 to 200 mm in 50 min. The step gradient was in the following order: 10 mm NaOH for 15 min, 80 mm NaOH for 5 min, 140 mm NaOH for 10 min, 200 mm NaOH for 10 min for column regeneration, and 10 mm NaOH for 10 min for column reequilibration. Monosaccharides (Fru and Glc), disaccharides (Suc, trehalose, maltose), and the sugar alcohol form of maltose (maltitol) were quantified.
Starch content was determined by the method of Li et al. (1965). After soluble sugar extraction, the ethanol insoluble residue was vacuum-dried. Starch was solubilized in boiling water for 15 min. The supernatant was reacted with iodine-potassium iodide (0.1%), and color density was measured at A620 using a spectrophotometer. Potato starch was used as the standard to estimate starch quantity.
Compatible Solute Assay for Maltose
Compatible solute assays for SspI, G6PDH, and ADH were repeated three times with the exception of the G6PDH time course experiment that was repeated twice. SspI (New England Biolabs, Beverly, MA) is a bacterial restriction enzyme that cleaves double stranded DNA. In this assay, it cuts the pGEX-4T-2 circular plasmid twice and generates 3.77-kb and 1.19-kb bands in the reaction buffer (50 mm NaCl, 100 mm Tris-HCL, 10 mm MgCl2, 0.025% Triton X-100 pH 7.5 at 25°C). Five units of SspI in the reaction buffer in the absence of maltose were incubated at 50°C for 15 min where 50% of the enzyme activity was lost. Aliquots were incubated at 50°C in the presence of 14, 100, 200, and 400 mm maltose. A substrate, 0.5 μg of pGEX-4T-2 circular plasmid, was added to the reaction mixture and incubated at 37°C for 1 h. The control reaction did not include maltose and was not exposed to heat treatment to show the full enzyme activity. Twenty-two μL of a 50 μL reaction was loaded on a 1% agarose gel. The gel was run at 100 V for 30 min and stained with ethidium bromide to visualize the product. A 5-kb band representing a single double stranded cut of plasmid was quantified to assess the degree of protection by maltose. Scion Image for Windows (Scion, Frederick, MD, http://www.scioncorp.com) was used to quantify the intensity of the ethidium bromide stained DNA bands from the inverse images of the gels (Reidler, 2000).
The same assay was performed using Suc, trehalose, and Glc to compare the ability of maltose as a compatible solute. A time-course experiment was conducted where SspI was treated in the absence and in the presence of 400 mm maltose, Glc, Suc, and trehalose at 50°C for 0, 5, 10, 15, 20, and 25 min.
Lyophilized G6PDH from Leuconostoc mesenteroides was purchased from Worthington Biochemical Company (Lakewood, NJ). G6PDH in 50 mm Tris-HCl pH 7.8 was incubated at 48°C for 15 min, which reduced activity by 50%. G6PDH in 50 mm Tris-HCl pH 7.8 was heated in the absence and presence of 14, 100, 200, and 400 mm maltose, trehalose, Glc, and Suc. The control did not include any sugar and was not given a heat treatment. The reaction (1.5 mL) included 2.97 mm MgCl2, 50 mm Tris-HCl pH 7.8, 0.6 mm β-NADP+ (freshly prepared), 10 mm Glc-6-P, and 595 ng heat shocked G6PDH. Production of NADPH was followed for 3 min at A340 using a Lambda 3A UV/VIS spectrophotometer (Perkin-Elmer, Foster City, CA). A time-course experiment was conducted where G6PDH was treated in the absence and in the presence of 400 mm maltose, Glc, Suc, and trehalose at 48°C for 0, 5, 10, 15, 20, and 25 min.
Lyophilized ADH from yeast 300 U/mg was purchased from Calbiochem (San Diego). ADH in 50 mm potassium phosphate buffer pH 7.6 was exposed to 53.5°C for 15 min with a loss of 50% activity. ADH in 50 mm potassium phosphate buffer pH 7.6 was heated in the absence and presence of 14, 100, 200, and 400 mm maltose, trehalose, Glc, and Suc. The control did not include any sugar and was not given a heat treatment. Reaction volume 1.5 mL included 333 mm ethanol, 50 mm potassium phosphate pH 7.6, 4.15 mm β-NAD+ (freshly prepared), and 500 ng heated ADH. Production of NADH was followed for 20 s at A340 using a Lambda 3A UV/VIS spectrophotometer (Perkin-Elmer). A time course experiment was conducted where ADH was treated in the absence and in the presence of 400 mm maltose, Glc, Suc, and trehalose at 53.5°C for 0, 5, 10, 15, 20, and 25 min.
Pea Chloroplast Isolation
Pea chloroplasts were isolated according to Cline et al. (1993). Fifty g of aerial portions of 10-d-old pea seedlings were chopped into small pieces and placed into 200 mL of ice cold GR-buffer (50 mm HEPES adjusted to pH 7.5 with KOH, 0.33 m sorbitol, 1 mm MgCl2, 1 mm MnCl2, 2 mm EDTA, 5 mm Na-ascorbate, 1% BSA) and homogenized using a polytron. The homogenate was filtered though 1 layer of miracloth and centrifuged at 2,000g for 3 min in a swing-out rotor. The plastid containing pellet was resuspended in GR buffer, overlayed on a Percoll gradient, and centrifuged at 2,000g for 15 min in a swing-out rotor. Intact plastids were collected, diluted three times with buffer (50 mm HEPES/KOH pH 8, 0.33 m sorbitol), and pelleted 1,500g for 5 min. The pellet was resuspended in 25 mL of buffer (50 mm HEPES/KOH pH 8, 0.33 m sorbitol), and chlorophyll content was quantified.
Thylakoid Isolation
Thylakoids were isolated according to Santarius (1996). The isolated chloroplasts were ruptured by resuspending them at 1 mg/mL in 10 mm HEPES/KOH, pH 8, 5 mm MgCl2, and incubating for 2 min on ice. Wash buffer 1:1 (70 mm KCl, 30 mm NaNO3, 20 mm K2SO4, 5 mm MgCl2, and 5 mm HEPES/KOH pH 7.5) was added, and the mixture was centrifuged 3,300g for 8 min in a swing-out rotor to collect thylakoid membranes. The pellet was washed at 1 mg/mL in wash buffer and centrifuged at 3,300g for 8 min. Thylakoids were resuspended in wash buffer corresponding to 1 mg/mL chlorophyll.
In Vitro Freezing Stress and Heat Shock of Electron Transport Chain
Aliquots of 0.2 mL thylakoid in wash buffer (70 mm KCl, 30 mm NaNO3, 20 mm K2SO4, 5 mm MgCl2, and 5 mm HEPES/KOH pH 7.5) corresponding to 0.5 mg/mL chlorophyll were exposed to 40°C for 4 min in the absence and presence of 2.5, 14, 28, 56, and 112 mm maltose, Glc, and trehalose. Full activity of the whole electron transport chain was measured after isolation of thylakoid membranes. Five independent experiments with three replications each were conducted.
Aliquots of 0.2 mL thylakoid in wash buffer (70 mm KCl, 30 mm NaNO3, 20 mm K2SO4, 5 mm MgCl2, and 5 mm HEPES/KOH pH 7.5) corresponding to 0.5 mg/mL chlorophyll were exposed to freezing stress at −15°C for 20 h in absence and presence of 2.5, 14, 28, 56, and 112 mm maltose, Glc, and trehalose. Full activity of the whole electron transport chain was measured after isolation of thylakoid membranes. Four independent experiments with three replications each were done.
The whole electron transport chain assay with DCPIP was done according to Allen and Holmes (1986). The assay buffer included 0.1 m sorbitol, 50 mm HEPES-KOH pH 7.6, 5 mm NaCl, 5 mm MgCl2, 0.1 mm DCPIP. The reaction was mixed after addition of thylakoid membranes corresponding to 25 μg mL−1 chlorophyll and illuminated for 10 s at 200 μmol photons m−2 s−1 light intensity. Activity of the electron transport chain was determined by assaying the reduction of the redox dye DCPIP at A595.
Acknowledgments
We thank Dr. Ken Cline for making his lab available to isolate chloroplasts, Dr. Carole Dabney-Smith for providing isolated pea chloroplast for the subsequent thylakoid isolation, Dr. Denise Tieman, Scott McMillen, and Dr. Bradley Hayes for soluble sugar analysis of pea chloroplasts and Arabidopsis, Jeff Rollins for access to the ELISA plate reader, and Dale Haskell and Cameron Schiller for critical review of this manuscript.
This work was supported by the USDA NRI (grant nos. 2000–35100–9532 and 2002–35100–12110 to C.L.G.) and by the Institute of Food and Agricultural Sciences at the University of Florida.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040808.
References
- Allen JF, Holmes NG (1986) Electron transport and redox titration. In MF Hipkins, NR Baker, eds, Photosynthesis Energy Transduction, Practical Approach Series. IRL Press, Oxford, pp 103–141
- Beck E, Ziegler P (1989) Biosynthesis and degradation of starch in higher plants. Annu Rev Plant Physiol Plant Mol Biol 40: 95–117 [Google Scholar]
- Bruinsma J (1963) The quantitative analysis of chlorophylls a and b in plant extracts. Photochem Photobiol 2: 241–249 [Google Scholar]
- Chandler JW, Apel K, Melzer S (2001) A novel putative beta-amylase gene and Atb-Amy from Arabidopsis thaliana are circadian regulated. Plant Sci 161: 1019–1024 [Google Scholar]
- Cline K, Henry R, Li CJ, Yuan J (1993) Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J 12: 4105–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Selvi MT, Seetharama N, Sharma R (1999) Stress-mediated enhancement of beta-amylase activity in pearl millet and maize leaves is dependent on light. J Plant Physiol 154: 657–664 [Google Scholar]
- Doehlert DC, Duke SH, Anderson L (1982) Beta-amylases from alfalfa (Medicago sativa L.) roots. Plant Physiol 69: 1096–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier W, Schnarrenberger C, Börner T (1995) Light- and stress-dependent enhancement of amylolytic activities in white and green barley leaves: beta-amylases are stress-induced proteins. J Plant Physiol 145: 342–348 [Google Scholar]
- Fowler S, Thomashow MF (2002) Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14: 1675–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häder D-P, Tevini M (1987) General Photobiology. Pergamon Press, New York
- Hara-Nishimura I, Nishimura M, Daussant J (1986) Conversion of free beta-amylase to bound beta-amylase on starch granules in the barley endosperm during desiccation phase of seed development. Protoplasma 134: 149–153 [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290: 2110–2113 [DOI] [PubMed] [Google Scholar]
- Jung S-H, Lee J-Y, Lee D-H (2003) Use of SAGE technology to reveal changes in gene expression in Arabidopsis leaves undergoing cold stress. Plant Mol Biol 52: 553–567 [DOI] [PubMed] [Google Scholar]
- Kossmann J, Lloyd J (2000) Understanding and influencing starch biochemistry. Crit Rev Biochem Mol Biol 35: 141–196 [PubMed] [Google Scholar]
- Kreps JA, Wu Y, Chang SH, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt osmotic and cold stress. Plant Physiol 130: 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao NT, Schoneveld O, Mould RM, Hibberd JM, Gray JC, Kavanagh TA (1999) An Arabidopsis gene encoding a chloroplast-targeted beta-amylase. Plant J 20: 519–527 [DOI] [PubMed] [Google Scholar]
- Li PH, Weiser CJ, van Huystee R (1965) Changes in metabolites of red-osier dogwood during cold acclimation. J Am Soc Hortic Sci 86: 723–730 [Google Scholar]
- Lizotte PA, Henson CA, Duke SH (1990) Purification and characterization of pea epicotyl beta-amylase. Plant Physiol 92: 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sharkey TD (2004) The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 218: 466–473 [DOI] [PubMed] [Google Scholar]
- Maeo K, Tomiya T, Hayashi K, Akaike M, Morikami A, Ishiguro S, Nakamura K (2001) Sugar-responsible elements in the promoter of a gene for beta-amylase of sweet potato. Plant Mol Biol 46: 627–637 [DOI] [PubMed] [Google Scholar]
- Mita S, Suzuki-Fujii K, Nakamura K (1995) Sugar-inducible expression of a gene for beta-amylase in Arabidopsis thaliana. Plant Physiol 107: 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe JD, Preiss J (1990) Purification of a β-amylase that accumulates in Arabidopsis thaliana mutants defective in starch metabolism. Plant Physiol 94: 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Ohto M, Yoshida N, Nakamura K (1991) Sucrose-induced accumulation of beta-amylase occurs concomitant with the accumulation of starch and sporamin in leaf-petiole cuttings of sweet potato. Plant Physiol 96: 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TM, Deiting U, Stitt M (1997) A beta-amylase in potato tubers is induced by storage at low temperature. Plant Physiol 113: 503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittyla T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- Ohto M-A, Nakamura-Kito K, Nakamura K (1992) Induction of expression of genes coding for sporamin and beta-amylase by polygalacturonic acid in leaf-petiole cuttings of sweet potato. Plant Physiol 99: 422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidler JA (2000) Low cost gel analysis. Methods Mol Biol 132: 277–288 [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Alia, Murata N, Murata A (1998) Metabolic engineering of rice leading to biosynthesis of glycine betaine to salt and cold. Plant Mol Biol 38: 1011–1019 [DOI] [PubMed] [Google Scholar]
- Santarius KA (1996) Freezing of isolated thylakoid membranes in complex media. X. Interactions among various low molecular weight cryoprotectants. Cryobiology 33: 118–126 [Google Scholar]
- Scheidig A, Fröhlich A, Schulze S, Lloyd JR, Kossmann J (2002) Downregulation of a chloroplast-targeted beta-amylase leads to starch-excess phenotype in leaves. Plant J 30: 581–591 [DOI] [PubMed] [Google Scholar]
- Sean EW, Weber APM, Sharkey TD (2004) Maltose is the major form of carbon exported from the chloroplast at night. Planta 218: 474–482 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, et al (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31: 279–292 [DOI] [PubMed] [Google Scholar]
- Sharma R, Schopfer P (1982) Sequential control of phytochome-mediated synthesis de novo of beta-amylase in cotyledons of mustard (Sinapis alba L.) seedlings. Planta 155: 183–189 [DOI] [PubMed] [Google Scholar]
- Sopanen T, Lauriere C (1989) Release and activity of bound beta-amylase in germinating barley grain. Plant Physiol 89: 244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Duke SH, Henson CA (1995) The role of pea chloroplast (alpha) glucosidase in transitory starch degradation. Plant Physiol 108: 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DY (2001) Characterization of Arabidopsis heat shock protein 70 (Hsp70) gene family and microarray analysis of gene expression in response to temperature extremes. PhD thesis. University of Florida, Gainesville, FL
- Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH (2001) Multiple transcription-factor genes are early targets of phytochome A signaling. Proc Natl Acad Sci USA 98: 9437–9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-M, Lue W-L, Eimert K, Chen J (1996) Phytohormone-regulated beta-amylase gene expression in rice. Plant Mol Biol 31: 975–982 [DOI] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217: 1214–1222 [DOI] [PubMed] [Google Scholar]
- Yelenosky G, Guy CL (1977) Carbohydrate accumulation in leaves and stems of “Valencia” orange at progressively colder temperatures. Bot Gaz 138: 13–17 [Google Scholar]
- Ziegler P, Beck E (1986) Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplast. Plant Physiol 82: 1119–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]