Abstract
The opaque2 (o2) mutation increases the Lys content of maize (Zea mays) endosperm by reducing the synthesis of zein storage proteins and increasing the accumulation of other types of cellular proteins. Elongation factor 1A (eEF1A) is one of these proteins, and its concentration is highly correlated with the amount of other Lys-containing proteins in the endosperm. We investigated the basis for this relationship by comparing patterns of protein accumulation and gene expression between a high (Oh51Ao2) and a low (Oh545o2) eEF1A inbred, as well as between high and low eEF1A recombinant inbred lines obtained from their cross. The content of α-zein and several cytoskeletal proteins was measured in high and low eEF1A inbred lines, and the levels of these proteins were found to correlate with that of eEF1A. To extend this analysis, we used an endosperm expressed sequence tag microarray to examine steady-state levels of RNA transcripts in developing endosperm of these genotypes. We identified about 120 genes coordinately regulated in association with eEF1A content. These genes encode proteins involved in several biological structures and processes, including the actin cytoskeleton, the endoplasmic reticulum, and the protein synthesis apparatus. Thus, higher levels of eEF1A in o2 mutants may be related to a more extensive cytoskeletal network surrounding the rough endoplasmic reticulum and increased synthesis of cytoskeleton-associated proteins, all of which contribute significantly to the Lys content of the endosperm.
The discovery that the opaque2 (o2) mutation almost doubles the Lys content of maize (Zea mays) endosperm (Mertz et al., 1964) led to extensive efforts to develop genotypes with better nutritional quality for human and animal diets. Unfortunately, the inferior agronomic traits associated with this mutation, including reduced yield and protein content, soft kernels, and pathogen susceptibility, made it difficult to develop o2 in breeding programs (Glover and Mertz, 1987). To overcome these problems, maize breeders at International Center for Development of Maize and Wheat, Mexico (Villegas et al., 1992) and the University of Natal, South Africa (Gevers and Lake, 1992) developed modified o2 mutants called Quality Protein Maize (QPM). These genotypes combine the improved nutritional quality trait of o2 with those responsible for vitreous endosperm and normal yield. However, additional improvement of protein quality is required for Quality Protein Maize to meet the minimal Lys content (5 mg/100 mg of protein) recommended for human nutrition (Young et al., 1998).
Several studies have documented a broad range of variability in Lys content among normal, o2, and modified o2 maize genotypes (Moro et al., 1996; Zarkadas et al., 2000), showing that the effect of the mutation is highly dependent on the genetic background. These studies suggested the Lys content of the kernel can be improved by appropriate selection. However, identification of more nutritional maize genotypes has been limited by knowledge of the genetic and biochemical basis for Lys accumulation in the endosperm. O2 encodes a basic domain/Leu zipper transcription factor that regulates the expression of a number of genes, including the highly abundant α-zeins (Kodrzycki et al., 1989; Schmidt et al., 1990). Defects in O2 lead to a significant reduction in α-zein synthesis and a pleiotropic increase in the synthesis of several Lys-rich, nonzein proteins (Damerval and Devienne, 1993; Habben et al., 1993). Both of these effects contribute to the increased Lys content of the endosperm (Moro et al., 1996), but the molecular mechanisms responsible for the larger amount of Lys-rich proteins are unknown.
Habben et al. (1993) investigated the nature of the Lys-containing proteins that are increased in o2 and found that elongation factor 1A (eEF1A), a protein synthesis factor, is highly increased compared to wild type. Analysis of a large number of normal and o2 inbreds with extensive variability in Lys and eEF1A content showed this protein is highly correlated (r = 0.9) with the protein-bound Lys of the endosperm (Habben et al., 1995; Moro et al., 1996). However, the biological basis of this relationship is unclear. eEF1A contains 11% Lys, but this protein only accounts for 2.3% of the Lys in W64Ao2 endosperm (Sun et al., 1997). This suggests a stoichiometric relationship exists between eEF1A and the other major proteins contributing to the Lys content of the grain.
One possible explanation for the relationship between eEF1A and other Lys-rich proteins in maize endosperm is that eEF1A is a component of the cytoskeleton (Durso and Cyr, 1994) that can bind both actin filaments (Yang et al., 1990) and microtubules (Kuriyama et al., 1990; Ohta et al., 1990). Biochemical characterization of eEF1A from maize endosperm revealed this protein binds F-actin (Sun et al., 1997; Lopez-Valenzuela et al., 2003) and immunolocalization showed eEF1A is associated with an F-actin network that surrounds the rough endoplasmic reticulum (RER) at sites where protein bodies are forming (Clore et al., 1996). Wang et al. (2001) found a quantitative trait locus linked with eEF1A content that mapped with a cluster of 22-kD α-zein genes. Based on this association, they proposed a positive correlation between eEF1A content and the surface area of protein bodies, hence the network of cytoskeletal proteins surrounding the RER in the endosperm. Thus, the level of eEF1A may reflect the amount of proteins that are components of the cytoskeleton in maize endosperm.
This study was initiated to identify proteins that vary in abundance in parallel with eEF1A. The inbred lines Oh51Ao2 (high eEF1A) and Oh545o2 (low eEF1A), as well as high and low eEF1A recombinant inbred lines (RILs) derived from their cross, were used for ELISA measurement of endosperm proteins and for mRNA transcript profiling with cDNA microarrays. The results of this study revealed a number of genes that are coordinately regulated with eEF1A content, several of which encode proteins associated with the actin cytoskeleton and the endoplasmic reticulum (ER), as well as components of the protein synthesis machinery.
RESULTS
Development of Recombinant Inbred Lines with High and Low eEF1A Content
To monitor patterns of gene expression and protein synthesis in high and low eEF1A genotypes, we developed a set of 75 RILs by single seed descent from a cross between Oh51Ao2, a high eEF1A inbred, and Oh545o2, a low eEF1A inbred. After six generations of self-pollination, each of the inbred lines appeared to be homogeneous based on phenotypic uniformity in the field. The level of eEF1A in these lines at the F6 generation was measured by ELISA and found to exist in a 2-fold concentration range, similar to that observed in the F3 (Wang et al., 2001). The variation in eEF1A content of these genetically fixed and related lines allowed us to study gene expression patterns and their relationship to endosperm Lys content in o2 mutants.
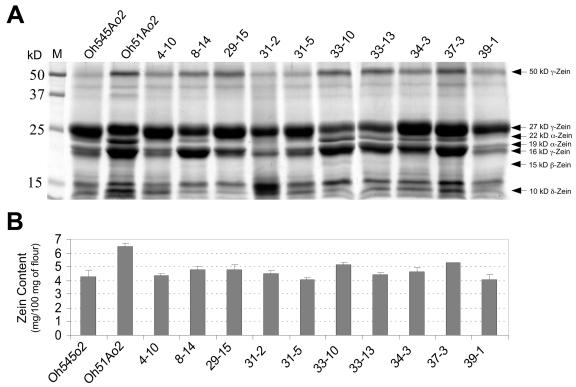
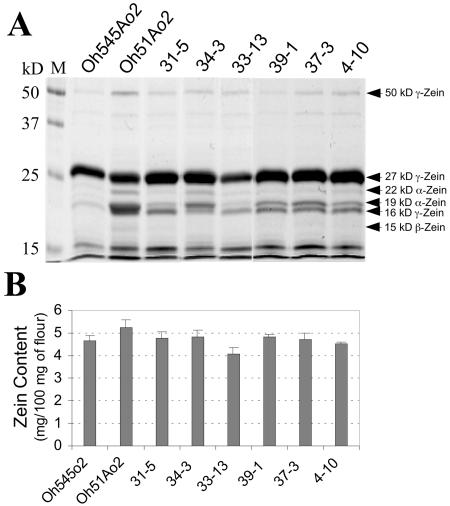
The parental inbreds, Oh545o2 and Oh51Ao2, and a subset of 10 RILs representing the range of eEF1A variation were first compared for total zein and nonzein protein content in mature endosperm. Protein extracts from equal amounts of endosperm flour of each genotype were partitioned into zein and nonzein fractions (Wallace et al., 1990). Figure 1A illustrates a Coomassie Blue-stained gel showing the nature and relative abundance of various zein proteins in the parental inbreds and the RILs. The accumulation of subfamilies of zein proteins is quite different between Oh545o2 and Oh51Ao2; the amount of 19- and 22-kD α-zeins is much lower in Oh545o2 than Oh51Ao2 (Fig. 1A), and this appears to account for most of the 50% decrease in total zein content in this inbred compared to Oh51Ao2 (Fig. 1B). The amount of 50-kD γ-zein is less in Oh545o2 than Oh51Ao2, while the amount of 27-kD γ-zein appears to be slightly greater in this inbred compared to Oh51Ao2. The relative abundance of 16-kD γ-zein could not be estimated, because this protein migrated too close to the 19-kD α-zeins in the gel. The abundance of 15-kD β-zein was too low to be compared among these inbreds. The 10-kD δ-zein appears to be less abundant in Oh545o2 than Oh51Ao2.
Figure 1.
SDS-PAGE and quantification of zeins in mature endosperm of Oh545o2, Oh51Ao2, and 10 RILs with high and low eEF1A contents. A, Total zein extracts were prepared from 750 μg of endosperm flour of the indicated genotypes, separated by 12.5% (w/v) SDS-PAGE, and stained with Coomassie Brilliant Blue R-250. Prestained molecular mass standards are shown on the left. B, Total zein content was estimated using BCA reagent and BSA as standard. The values are the average of two independent extractions in which three measurements were taken.
A diverse pattern of zein accumulation was observed in the RILs developed from the Oh51Ao2 by Oh545o2 cross (Fig. 1A), but some of these lines closely resembled the parental inbreds. For example, the zein profiles of RILs 4-10, 31-5, and 39-1 are very similar to that of Oh545o2 (Fig. 1A), and the amount of total zein in these lines is also similar to Oh545o2 (Fig. 1B). On the other hand, the zein profiles of RILs 34-3 and 37-3 are qualitatively similar to that of Oh51Ao2 (Fig. 1A), but the amount of total zein in these lines is 20% to 25% less than in Oh51Ao2 (Fig. 1B). This is mainly because of a reduction in α-zeins, although there appeared to be a slight increase in the synthesis of the 27-kD γ-zein (Fig. 1A).
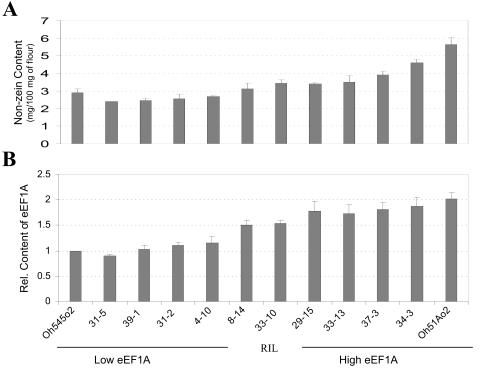
We measured the nonzein protein content in endosperms of these inbreds using the Bradford assay, and the level of eEF1A by ELISA (Fig. 2). There is about 2-fold more nonzein protein in Oh51Ao2 (5.7 mg/100 mg of flour) than Oh545o2 (2.8 mg/100 mg of flour; Fig. 2A). The nonzein content of the RILs ranged from about 2.5 mg/100 mg of flour in 31-5 to about 4.5 mg/100 mg of flour in 34-3 (Fig. 2A). The pattern of eEF1A accumulation in the parental inbreds and the RILs (Fig. 2B) was similar to that observed for nonzein content (Fig. 2A). The difference in eEF1A content between Oh545o2 and Oh51Ao2 is about 2-fold, and the range of eEF1A contents in the RILs was also about 2-fold. As with nonzein content, the lines with the lowest and highest eEF1A content were RILs 31-5 and 34-3, respectively (Fig. 2B). It is worth noting that these two lines also have zein profiles similar to those of the corresponding low and high eEF1A parents (Fig. 1A).
Figure 2.
Nonzein quantification and ELISA measurement of eEF1A content in mature endosperm of Oh51Ao2, Oh545o2, and 10 RILs with high and low eEF1A content (see Fig. 1). Protein extracts were prepared from endosperm flour as described in “Materials and Methods.” A, Nonzein content was estimated using Bradford reagent and BSA as standard. The order of samples is the same as that identified in B. B, ELISA measurement of eEF1A was conducted as described in “Materials and Methods,” and the results were normalized to the values of the Oh545o2 inbred. In both A and B, the results are the average of two independent extractions in which three measurements were taken.
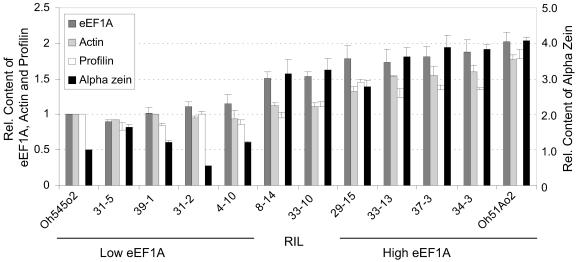
To test the hypothesis that proteins associated with the cytoskeleton were present in higher concentrations in genotypes with increased eEF1A content (Wang et al., 2001), the levels of eEF1A, actin, profilin, and α-zein were measured in mature endosperms of Oh545o2, Oh51Ao2, and 10 of their RILs by ELISA (Fig. 3). This analysis showed that the relative contents of actin and profilin closely paralleled those of eEF1A. Actin and profilin proteins were about 1.8-fold higher in Oh51Ao2 than Oh545o2, and the differences in their protein levels in RIL 34-3 compared to RIL 31-5 were about 1.9- and 1.7-fold greater, respectively. The relative amounts of eEF1A shown in Figure 3 correspond to those in Figure 2, but they are included here for reference. The levels of α-tubulin could not be determined in mature endosperms, perhaps because of protein instability. The relative content of α-zein was determined using antibodies that do not distinguish between the 19- and 22-kD subclasses. There was about four times more α-zein in Oh51Ao2 than Oh545o2 and about two times more α-zein in RIL 34-3 than RIL 31-5 (Fig. 3). Except for RIL 31-2, which had only about 50% of the α-zein content of Oh545o2, the levels of α-zein proteins correlated with those of eEF1A. In contrast, the levels of 27-kD γ-zein did not correlate with the levels of eEF1A (data not shown).
Figure 3.
ELISA measurement of eEF1A, actin, profilin, and α-zein content in mature endosperm of Oh51Ao2, Oh545o2, and 10 RILs with high and low eEF1A content. Protein extracts were prepared from endosperm flour. ELISA measurements of the indicated proteins were conducted as described in “Materials and Methods,” and the results were normalized to the values of the Oh545o2 inbred. The results are the average of two independent experiments in which three measurements were taken.
Microarray Analysis
To investigate the pattern of gene expression more broadly in high and low eEF1A genotypes, selected inbreds were analyzed using an endosperm cDNA microarray from the Maize Gene Discovery Project (Fernandes et al., 2002). Poly(A)+ RNA was extracted from 18 d-after-pollination (DAP) endosperms of the high eEF1A inbred lines, Oh51Ao2 and RIL 34-3, and the low eEF1A inbreds, Oh5454o2 and RIL 31-5. This stage of development (18-DAP) was chosen because most endosperm cells are differentiated and have high levels of gene expression, as reflected by large amounts of starch and storage protein synthesis (Demason, 1997). For each genotype, two RNA samples were prepared from two different ears to account for biological variability. Following poly(A)+ isolation, cDNA was synthesized and labeled with Cy3- and Cy5-dUTP. For comparison of each high and low eEF1A inbred, we used two RNA samples with reciprocal labeling, for a total of four slides that resulted in eight replicates because the array was duplicated on each slide. DNA microarray hybridization data were normalized as described in “Materials and Methods” and the fluorescence intensity ratio of the two probes was determined. The expression ratio from the eight replicates was used for statistical analysis. All the replicate slides were analyzed in parallel, and only the probes with an adjusted probability value q < 0.05 were considered significant.
We initially identified 540 probes that showed statistically significant differences in gene expression levels for both the parental inbreds and the RILs (see supplemental data, available at www.plantphysiol.org), of which about 343 corresponded to zein sequences, mainly 19-kD α-zeins. The 605 microarray is not normalized, and consequently these probes are highly redundant. A significant amount of redundancy was also found for a number of other genes. This array contains about 2,800 tentative unique genes among 5,534 expressed sequence tags (ESTs; Fernandes et al., 2002). However, the probe redundancy allowed us to accurately document some measurements, because in many cases different ESTs of the same gene showed similar changes in expression levels. In some cases, multiple ESTs corresponding to the same gene showed slightly different expression ratios, which may have resulted from differences in hybridization efficiency due to variable target/probe length and overall base-pair composition (Xu et al., 2001). After correction for redundancy, about 120 genes encoding proteins of known function were found to be differentially expressed, the majority of which were up-regulated in high eEF1A genotypes.
Table I shows a summary of the genes that changed expression in parallel with eEF1A content. The functional classification of these genes was based on their identification by BLAST, which corresponded to the most similar sequences in GenBank. Proteins encoded by these genes include several structural and metabolic proteins, such as enzymes involved in amino acid, carbohydrate, and cell wall metabolism, as well as storage proteins, proteins involved in stress responses, and components of the actin cytoskeleton, signal transduction pathways and the transcription/translation machinery.
Table I.
Summary of microarray results
| EST ID | Function | Oh51Ao2/Oh545o2
|
34-3/31-5
|
Top BLAST Hit
|
|
|---|---|---|---|---|---|
| Fold Change | Fold Change | BLASTN | BLASTX | ||
| Amino Acid Metabolism | |||||
| AI673901 | Acetolactate synthase (EC 4.1.3.18) precursor | 6.444 | 2.203 | gi|21213093 | gi|22139 |
| AI664892 | Putative ketol-acid reductoisomerase | 5.618 | 1.831 | gi|21206785 | gi|34911874 |
| AI676992 | Putative lipoamide dehydrogenase | 3.935 | 2.071 | gi|21207606 | gi|34894958 |
| AI665281 | Putative sarcosine oxidase | 2.361 | 1.736 | gi|21209418 | gi|34894598 |
| AI664857 | Homo-Cys S-methyltransferase-1e | 2.169 | 2.098 | gi|10732784 | gi|10732785 |
| AI668313 | Putative acetolactate synthase | 2.131 | 1.718 | gi|21208121 | gi|30685071 |
| Carbohydrate Metabolism | |||||
| AI833375 | Putative Suc cleavage protein | 4.856 | 1.792 | gi|21216346 | gi|7488353 |
| AI668434 | Pyruvate dehydrogenase E1 α-subunit | 3.227 | 1.846 | gi|21209231 | gi|3851005 |
| AI677414 | Putative phosphofructokinase α-subunit | 3.210 | 1.776 | gi|21209419 | gi|29647436 |
| AI746218 | Pyruvate, orthophosphate dikinase | 2.959 | 1.698 | gi|21206738 | gi|168586 |
| AI677069 | Suc synthase (Sus1) | 1.751 | 1.630 | gi|514945 | gi|514946 |
| Cell Wall Synthesis/Turnover | |||||
| AI833405 | Putative Hyp-rich glycoprotein | 6.237 | 2.068 | gi|21207317 | gi|34394453 |
| AI677486 | Putative 2-dehydro-3-deoxyphosphooctonate aldolase | 3.640 | 1.867 | gi|21217159 | gi|29367487 |
| AI712112 | Putative α-l-arabinofuranosidase/β-d-xylosidase | 3.062 | 1.803 | gi|21216968 | gi|38344900 |
| Cytoskeleton | |||||
| AI740034 | Putative actin | 5.248 | 2.134 | gi|21206665 | gi|24496452 |
| AI740030 | Putative actin | 4.535 | 1.787 | gi|21210184 | gi|28301928 |
| AI677353 | Actin-1 (MAc1) | 0.307 | 0.243 | gi|21207800 | gi|113220 |
| Molecular Chaperonin | |||||
| AI668124 | Putative 82-kD heat shock protein | 5.310 | 1.714 | gi|21206616 | gi|7546186 |
| AI833418 | Putative heat shock hsp20 protein | 4.404 | 1.601 | AZM3_4963a | gi|15221027 |
| AI833616 | Protein disulfide isomerase | 3.996 | 1.826 | gi|21211194 | gi|625148 |
| AI745903 | Putative cyclophilin ROC7 | 3.568 | 1.657 | gi|21206974 | gi|168461 |
| AI665058 | Putative calmodulin-binding heat shock protein | 2.894 | 1.592 | gi|21211155 | gi|7489106 |
| AI820388 | DnaJ-related protein ZMDJ1 | 2.187 | 1.621 | NHb | gi|2984709 |
| Membrane Transport/Function | |||||
| AI665354 | Voltage-dependent anion channel protein 1a | 5.115 | 2.257 | gi|21207948 | gi|5929928 |
| AI670271 | Putative translocase inner membrane-like protein | 4.681 | 2.138 | gi|21211219 | gi|34394571 |
| AI665496 | Adenine nucleotide translocator | 3.874 | 1.876 | gi|22161 | gi|22162 |
| AI665590 | Putative ethylene-responsive small GTP-binding protein | 3.467 | 1.888 | gi|21213302 | gi|33146687 |
| AI668193 | H+-pyrophosphatase | 3.294 | 1.760 | gi|1049254 | gi|1049255 |
| AI668232 | Putative nodulin N21 family protein | 2.929 | 1.766 | AZM3_44524 | gi|15238103 |
| AI714547 | Putative YGL010w-like protein | 2.906 | 1.822 | gi|21211893 | gi|2982301 |
| AI820289 | Putative acyl carrier protein | 2.884 | 1.718 | gi|21211812 | gi|453189 |
| AI668423 | Putative lipid transfer protein | 2.876 | 1.623 | AZM3_33030 | gi|21553541 |
| AI820380 | Toc34-2 | 2.346 | 1.797 | gi|7259223 | gi|7259224 |
| AI712220 | Putative phosphate/phosphoenolpyruvate translocator | 2.233 | 1.694 | gi|21213398 | gi|27476065 |
| AI668512 | Small basic membrane integral protein ZmSIP2-1 | 2.114 | 1.684 | gi|13447816 | NH |
| AI676864 | Putative amino acid transport protein | 1.638 | 1.923 | gi|21213436 | gi|38345408 |
| Metabolism/Oxidoreductase | |||||
| AI665560 | Putative cytochrome P450 | 6.384 | 1.772 | gi|27464999 | gi|17980361 |
| AI712147 | Met adenosyltransferase 1-like | 4.426 | 2.381 | gi|19172416 | NH |
| AI668366 | Putative succinyl-CoA ligase α-subunit | 3.995 | 1.713 | gi|21207397 | gi|34393512 |
| AI670285 | DWARF3 | 2.866 | 1.840 | gi|21209514 | gi|987267 |
| AI668359 | Putative aluminum induced protein wali7 | 2.657 | 1.650 | gi|21209487 | gi|7489671 |
| AI759056 | Putative nicotianamine aminotransferase A | 2.370 | 1.967 | gi|21207437 | gi|6498122 |
| AI746000 | Putative oxidase | 2.321 | 1.701 | TC198800c | gi|21105122 |
| AI739897 | Putative 5′-phosphoribosyl-5-aminoimidazole synthetase | 2.221 | 1.774 | gi|21214521 | gi|34897250 |
| AI670559 | Ferredoxin III (Fd) isoprotein | 0.407 | 2.058 | gi|168472 | gi|168473 |
| AI677085 | Ferredoxin III, chloroplast precursor | 0.405 | 1.731 | gi|21207226 | gi|168473 |
| Lipid Metabolism | |||||
| AI795367 | Putative acyl-CoA synthetase | 3.978 | 2.198 | AZM3_44857 | gi|27754483 |
| AI665554 | Putative GDSL family lipase | 3.244 | 1.810 | AZM3_16045 | gi|7486138 |
| Protein Turnover | |||||
| AI711859 | Putative 26S proteasome triple-A ATPase subunit4 | 5.336 | 1.950 | gi|21213166 | gi|11094192 |
| AI746055 | Putative 26S proteasome non-ATPase chain S5a | 3.875 | 1.945 | gi|21206642 | gi|11281550 |
| AI714537 | Putative Clp protease ATP-binding subunit | 3.764 | 1.649 | gi|21212366 | gi|18423503 |
| AI665136 | Ubiquitin conjugating enzyme | 2.789 | 1.603 | gi|21215571 | gi|2668744 |
| AI739846 | Putative Clp protease | 2.699 | 1.822 | gi|21211614 | gi|1168978 |
| AI657385 | Putative proteasome subunit α-type 1 | 2.349 | 1.661 | gi|21208015 | gi|1709758 |
| AI668422 | Putative ubiquitin-specific protease | 1.907 | 1.670 | gi|21207637 | gi|11993471 |
| AI714629 | Putative SGT1 | 1.648 | 1.748 | gi|21207770 | gi|2984709 |
| Seed Protein | |||||
| AI657407 | 19-kD α zeine | 10.663 | 1.830 | gi|16305120 | gi|16305121 |
| AI664915 | 15-kD β zeine | 6.062 | 0.421 | gi|16305114 | gi|16305115 |
| AI668413 | 22-kD α zeine | 5.255 | 2.651 | gi|22215 | gi|22216 |
| AI711821 | α Globulin | 2.189 | 1.628 | gi|16305141 | gi|16305142 |
| AI673885 | 18-kD δ zein | 1.995 | 0.282 | gi|16305116 | gi|16305117 |
| AI746160 | 16-kD γ zein | 1.909 | 1.817 | gi|16305110 | gi|168666 |
| AI657420 | 10-kD δ zeine | 0.213 | 0.179 | gi|340933 | gi|511870 |
| Signal Transduction | |||||
| AI737044 | Putative polo-like kinase | 5.574 | 1.748 | gi|21214227 | gi|11340595 |
| AI674011 | Putative phosphoprotein phosphatase (EC 3.1.3.16) | 5.517 | 1.850 | gi|14333242 | gi|11358619 |
| AI740053 | Putative GAST1-like protein | 4.876 | 2.250 | gi|21439749 | gi|21439750 |
| AI664994 | Putative Ca2+-binding EF-hand protein | 4.570 | 1.736 | gi|21214443 | gi|18409649 |
| AI665571 | Putative phytosulfokine peptide precursor (PSK1) | 3.887 | 2.169 | gi|23466412 | gi|23466413 |
| AI670625 | Putative phytosulfokine peptide precursor | 3.531 | 1.784 | gi|21211096 | gi|23466413 |
| AI711744 | Protein phosphatase 2C | 2.870 | 1.815 | gi|21206843 | gi|20146110 |
| AI711829 | Protein phosphatase type-2C | 2.740 | 1.892 | NH | gi|12003990 |
| AI673979 | Putative cell elongation protein DIMINUTO | 2.387 | 2.045 | gi|21207143 | gi|37534052 |
| AI746070 | Putative protein phosphatase | 2.152 | 1.635 | gi|21210550 | gi|26006493 |
| AI746164 | Putative transducin/WD-40 repeat protein | 1.980 | 1.856 | gi|21213317 | gi|18416416 |
| AI711873 | VPS29-like phosphoesterase-related protein | 0.243 | 0.188 | gi|21207398 | gi|7487365 |
| Stress/Defense Response | |||||
| AI668414 | Putative proteinase inhibitor | 5.514 | 2.227 | gi|21211819 | gi|7451428 |
| AI665244 | Cytosolic ascorbate peroxidase | 4.341 | 2.037 | gi|600115 | gi|600116 |
| AI657458 | β-glucosidase aggregating factor | 1.882 | 2.029 | gi|21209445 | gi|9313027 |
| Transcription/RNA Processing | |||||
| AI820389 | Putative nucleolar autoantigen | 7.101 | 1.983 | gi|21215410 | gi|15232342 |
| AI664855 | Putative RNA binding protein Rp120 | 6.557 | 1.803 | gi|21217062 | gi|32492578 |
| AI677310 | Putative heterogeneous nuclear ribonucleoprotein A1 | 5.713 | 2.112 | gi|21211013 | gi|38175737 |
| AI746091 | Translation initiation factor 5A | 4.882 | 1.934 | gi|2668737 | gi|2668738 |
| AI795508 | Putative ribonucleoprotein F | 4.455 | 2.010 | gi|21211198 | gi|10177116 |
| AI833840 | ABA-inducible gene for Gly-rich protein | 3.941 | 1.640 | gi|22312 | NH |
| AI833456 | Putative ETTIN-like protein 2 | 3.921 | 2.092 | gi|21209173 | gi|19352035 |
| AI673982 | Putative DNA-binding protein | 3.488 | 2.126 | gi|21207037 | gi|7447216 |
| AI711809 | CRP1 | 3.479 | 1.685 | NH | gi|3289002 |
| AI746090 | Putative CAF protein | 3.295 | 1.652 | gi|21216651 | gi|34902240 |
| AI833470 | Putative RNA polymerase II subunit Rpb10 | 3.190 | 1.909 | gi|21207629 | gi|30682258 |
| AI665397 | Gly-rich proteine | 3.170 | 1.802 | gi|22292 | NH |
| AI746156 | Histone H3 | 3.143 | 2.135 | AZM3_80817 | gi|122087 |
| AI759090 | Putative OsNAC6-like protein | 3.110 | 1.734 | gi|21213270 | gi|25458553 |
| AI795298 | Putative cohesin-like protein | 2.949 | 1.856 | gi|21213668 | gi|32309542 |
| AI833442 | Putative zinc finger transcription factor | 2.828 | 1.708 | gi|21211573 | gi|34906436 |
| AI712222 | Putative snRNP associated protein | 2.826 | 1.815 | gi|21209956 | gi|33146808 |
| AI667754 | Histone H3 gene (H3C2) | 2.730 | 1.792 | gi|168494 | gi|168493 |
| AI833929 | Putative nucleolar protein | 2.710 | 2.054 | gi|21209364 | gi|34899158 |
| AI665498 | DNA binding protein S1FA | 2.704 | 1.844 | gi|21208641 | gi|1173349 |
| AI746026 | Fertilization-independent endosperm protein 1 | 2.648 | 1.949 | gi|13444304 | gi|28192549 |
| AI670631 | Putative RNA polymerase II fifth largest subunit | 2.631 | 1.737 | AZM3_23172 | gi|15233533 |
| AI833836 | Putative nuclear RNA binding protein A | 2.430 | 1.768 | gi|21206684 | gi|34910712 |
| Translation | |||||
| AI668514 | Putative eukaryotic initiation factor 3B | 11.434 | 2.024 | gi|21209497 | gi|37537002 |
| AI820279 | Putative 60S ribosomal protein L37a | 6.959 | 2.034 | gi|14332189 | gi|34911166 |
| AI676990 | Putative 60S ribosomal protein L13a | 6.482 | 2.217 | gi|21213066 | gi|21326116 |
| AI711802 | Putative ribosomal protein S29 | 4.636 | 2.135 | gi|21213327 | gi|34915198 |
| AI665388 | Putative translational elongation factor Tu | 4.229 | 1.819 | gi|21208587 | gi|11181616 |
| AI711673 | Putative ribosomal protein L27 | 3.433 | 1.694 | gi|21207613 | gi|37536910 |
| AI665325 | Putative 40S ribosomal protein S2 | 3.332 | 1.729 | gi|21207125 | gi|30103021 |
| AI759043 | Mitochondrial 26S rRNA | 3.264 | 1.601 | gi|342661 | NAd |
| AI833872 | Putative ribosomal protein S15 | 2.912 | 1.720 | gi|21212912 | gi|34897190 |
| AI820275 | Putative ribosomal protein L18a | 2.899 | 1.832 | gi|21206699 | gi|34909590 |
| AI676941 | Acidic ribosomal protein P0 | 2.837 | 1.928 | gi|1550813 | gi|1550814 |
| AI668357 | Putative elongation factor EF-2 | 2.788 | 2.053 | gi|21216529 | gi|38344860 |
| AI746166 | 25S rRNA | 2.472 | 1.785 | gi|13397940 | NA |
| AI745842 | Putative fibrillarin 2 | 2.256 | 1.943 | gi|21206882 | gi|18416588 |
| AI668498 | Ribosomal protein L39 | 1.985 | 1.878 | gi|1177368 | gi|1177369 |
| AI746161 | Putative translation elongation factor eEF-1 βe | 1.799 | 1.714 | gi|14332585 | gi|38175744 |
| AI677351 | 40S ribosomal protein S6 | 0.309 | 0.248 | gi|21207495 | gi|1172818 |
| AI711882 | Putative ribosomal protein L18a | 0.244 | 0.174 | gi|21206699 | gi|34909590 |
Accession numbers beginning with AZM3 were obtained from the maize genomics consortium genome survey build 3.0.
NH, No significant hits obtained.
Accession numbers beginning with TC are obtained from The Institute for Genomic Research maize gene index version 10.0.
NA, Not applicable.
Expression ratios represent an average value for more than one spot.
Among genes encoding seed storage proteins, 19- and 22-kD α-zeins showed the largest difference in expression between high and low eEF1A genotypes. The transcript levels of these genes were about 10- and 5-fold higher, respectively, in Oh51Ao2 than Oh545o2 (Table I). The 19- and 22-kD α-zeins are encoded by multigene families, and our results most likely are representative of the gene subfamilies, due to cross-hybridization among the members (Marks et al., 1985). The transcript level of the 10-kD δ-zein correlated inversely with that of eEF1A; the amount of 10-kD δ-zein mRNA was about five times lower in high versus low eEF1A genotypes.
The mRNA levels of genes encoding enzymes involved in the synthesis of branched amino acids, such as acetolactate synthase and ketol-acid reductoisomerase, were severalfold greater in high compared to low eEF1A genotypes. Similarly, several genes involved in carbohydrate metabolism were up-regulated in the high eEF1A genotypes. Genes encoding some of the proteins in these two groups were reported to be direct or indirect targets of O2 regulation (Damerval and Le Guilloux, 1998).
Two genes encoding actin were significantly up-regulated in high eEF1A genotypes (Table I). Conversely, one actin probe (MAc1) was down-regulated in high eEF1A genotypes. The nucleotide sequence identity between MAc1 and the other actin sequences is about 60%, so it is unlikely their transcripts cross-hybridized. Other actin genes represented in the array that were not altered in expression included Maz83 (GenBank accession AI677580), Maz87 (AI665295), Maz89 (AI745940), and Maz95 (AI677101). The transcript for Maz87 was significantly increased in the high eEF1A parental inbred but not in the RILs. There were no significant differences in the expression levels of Maz83 in high and low eEF1A genotypes, while the hybridization signals of Maz89 and Maz95 were similar to background.
One group of genes shown in Table I with altered expression associated with eEF1A has been implicated in stress responses and includes ascorbate peroxidase, a proteinase inhibitor, a Gly-rich (cell wall) protein and molecular chaperones, such as cyclophilin, heat shock proteins and protein disulfide isomerase. Other genes that were up-regulated in high eEF1A genotypes included several sequences encoding phosphatases, kinases, ribosomal proteins, histones, and protein synthesis factors.
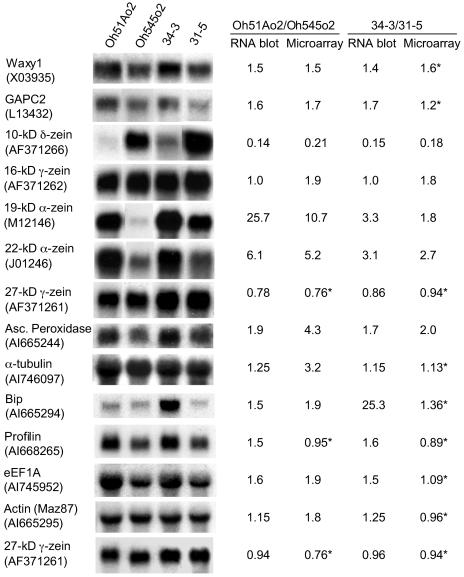
RNA-Blot Analysis
To validate and extend the results of the microarray hybridization, the transcript levels of 13 genes were investigated by RNA gel-blot analysis using total RNA prepared from the same samples used for the microarray experiments (Fig. 4). The intensities of the radioactive signals were normalized to the 27-kD γ-zein RNA, because its level was found to be similar among these genotypes at 18-DAP. For most of these genes, the expression level determined by RNA gel-blot analysis correlated with that detected with microarrays. According to microarray data, the expression ratios of eEF1A, granule-bound starch synthase (Waxy1), and glyceraldehyde-3-phosphate dehydrogenase (GAPC2) were between 1.5- and 1.9-fold greater in the high versus the low eEF1A parental genotypes, while these genes were not found to be significantly changed in the RIL genotypes. The reason that the expression differences in the RIL genotypes were not statistically significant was related to the fact that the data obtained from one slide (no. 76) had markedly higher variance than the others, which reduced the ability to detect significant changes between the RILs. The expression ratios between 1.4- and 1.7-fold greater, measured by RNA gel-blot analyses of these genes, were consistent with the values from the microarray analysis of the parental genotypes. The expression levels determined by microarray hybridization for actin (Maz87) and α-tubulin were 1.8- and 3.2-fold greater, respectively, in high compared to low eEF1A parental inbreds; however, the differences in gene expression detected by gel-blot analysis were approximately 1.2-fold larger. In the case of ascorbate peroxidase, the expression ratio obtained by microarray hybridization with Oh51Ao2 versus Oh545o2 was 4.3, while the expression ratio obtained by gel-blot analysis was 1.9. In spite of the discrepancy in the RNA expression ratios for ascorbate peroxidase, the pattern of expression in high and low eEF1A genotypes was consistent. Conversely, according to microarray data, the expression ratio of Bip in RIL 34-3 versus 31-5 was not statistically significant, while by gel-blot analysis the ratio was 25.3. It is difficult to explain the unexpectedly high level of Bip mRNA in RIL 34-3, but this result was confirmed in multiple RNA blots.
Figure 4.
RNA gel-blot analysis for comparison with microarray hybridization data. Lanes contained 10 μg of total RNA extracted from 18-DAP endosperms of Oh51Ao2, Oh545o2, and their RILs: 34-3 (high eEF1A) and 31-5 (low eEF1A). Blots were hybridized with 32P-labeled probes corresponding to the genes indicated. GenBank accession numbers of EST-specific probes are indicated in parentheses. The values given are the ratio of signals between Oh51Ao2 and Oh545o2, as well as RILs 34-3 and 31-5, normalized relative to the 27-kD γ-zein RNA signal. For each gene, the expression ratio of high versus low eEF1A genotypes is compared to the ratio obtained by microarray hybridization for that accession (* expression ratio not significantly different according to Statistical Analysis for Microarray program).
The expression of profilin was also determined by RNA gel-blot analysis, and it was found to be about 1.5-fold higher in Oh51Ao2 and RIL 34-3 compared to Oh545o2 and RIL 31-5 (Fig. 4). Profilin transcript levels were not found to be significantly different between high and low eEF1A genotypes in the microarray experiments, because the signal was too close to background.
Patterns of Protein Synthesis in Developing Endosperm
To further assess the gene expression results obtained by RNA hybridization, we measured protein accumulation by ELISA in 18-DAP endosperms of the genotypes used for transcript profiling. In addition, we analyzed four RILs that differ in eEF1A content. Protein extracts from equal amounts of lyophilized endosperm of each genotype were partitioned into zein and nonzein fractions (Wallace et al., 1990) and analyzed using SDS-PAGE. Figure 5A illustrates a Coomassie Blue-stained gel showing the relative abundance of zein proteins in 18-DAP endosperm of the parental inbreds and six RILs. The level of 27-kD γ-zein appeared to be similar between Oh545o2 and Oh51Ao2 and between RILs 31-5 and 34-3, although the amount of this protein was greater in the RILs. These observations were in agreement with the RNA levels obtained by microarray hybridization and RNA-blot analysis. The accumulation of 19- and 22-kD α-zein proteins was greater in Oh51Ao2 than Oh545o2 (Fig. 5A) and correlated with the RNA hybridization results (Fig. 4). Based on Coomassie Blue staining, the differences in α-zein content in RILs 31-5 and 34-3 were not as obvious as for the parents, consistent with the smaller differences in RNA transcript levels measured for these lines compared to Oh545o2 and Oh51Ao2 (Fig. 4). Except for RIL 33-13, the accumulation of zein proteins in RILs 39-1, 37-3, and 4-10 at 18-DAP appeared to be very similar. Figure 5B shows there were only small differences in total zein content at this stage of development for the six genotypes. The amount of total zein ranged from about 4.1 mg/100 mg of flour in RIL 33-13 to about 5.2 mg/100 mg of flour in Oh51Ao2.
Figure 5.
SDS-PAGE and quantification of total zein in developing endosperm of Oh545o2, Oh51Ao2, and six RILs with high and low eEF1A content. A, For each lane, total zein extracts were prepared from 750 μg of lyophilized 18-DAP endosperms of the indicated genotypes, separated by 12.5% (w/v) SDS-PAGE, and stained with Coomassie Blue R. Prestained molecular mass standards are shown on the left. B, Total zein content was estimated using BCA reagent with BSA as standard. The results are the average of two independent extractions in which three measurements were taken.
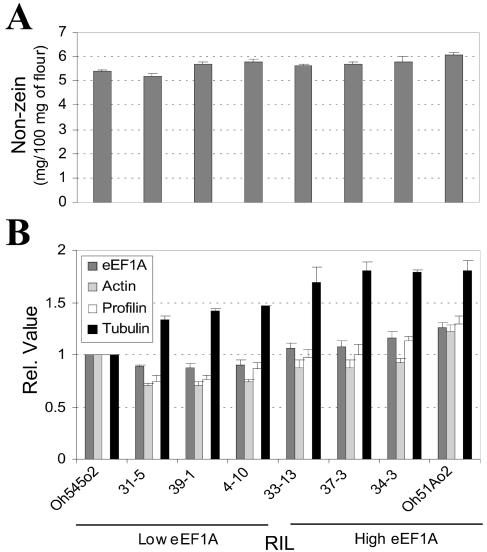
The nonzein content in 18-DAP endosperms of Oh545o2, Oh51Ao2, and the six RILs was measured by Bradford assay and the relative levels of eEF1A, actin, profilin, and α-tubulin by ELISA. Figure 6A shows the nonzein content at this stage of development was similar in all genotypes and ranged from about 5 mg/100 mg of flour in RIL 31-5 to about 6 mg/100 mg of flour in Oh51Ao2. Figure 6B shows the ELISA quantification of eEF1A, actin, profilin, and α-tubulin in these genotypes. The parental inbreds and the RILs were organized according to their relative eEF1A content. In general, the relative level of eEF1A, actin, and profilin in the RILs was lower than in the high and low eEF1A parents. There was about 1.3-fold more eEF1A in Oh51Ao2 than in Oh545o2. The difference in eEF1A content between the highest and lowest RILs, 34-3 and 31-5, was also about 1.3-fold. The levels of actin, profilin, and α-tubulin protein paralleled those of eEF1A (Fig. 6B). The amount of these proteins in Oh51Ao2 compared to Oh545o2 was about 1.2-, 1.3-, and 1.8-fold greater, respectively, and these ratios were significantly different (Student's t test, P < 0.01). Among the RILs, the greatest difference in the amount of these proteins was in RIL 34-3 (high eEF1A) and RIL 31-5 (low eEF1A), where the levels of actin, profilin, and α-tubulin were about 1.3-, 1.5-, and 1.4-fold higher in RIL 34-3 than in RIL 31-5, respectively, and were significantly different (Student's t test, P < 0.01).
Figure 6.
Nonzein quantification and ELISA measurement of eEF1A, actin, profilin, and α-tubulin content in developing endosperm of Oh51Ao2, Oh545o2, and six RILs with high and low eEF1A content. Protein extracts were prepared from lyophilized 18-DAP endosperms. A, Nonzein content was estimated using the Bradford reagent with BSA as standard. B, ELISA measurements of the indicated proteins were conducted as described in “Materials and Methods,” and the results were normalized to the values of the Oh545o2 inbred. In both A and B, the results are the average of two independent experiments in which three measurements were taken.
DISCUSSION
Prior studies of the relationship between eEF1A and Lys content indicated that there might be an increase in cytoskeleton and cytoskeleton-associated proteins in o2 mutants. For this study, we selected the maize inbreds Oh51Ao2 and Oh545o2, because they have among the highest degree of variability reported for eEF1A content (Moro et al., 1996). We also analyzed RILs created from a cross between these inbreds, as they showed a range of variation for eEF1A content between the parental genotypes (approximately 2-fold). These differences allowed us to compare multiple, genetically fixed, and related inbred lines for patterns of protein accumulation and gene expression relative to eEF1A protein content. We measured the accumulation of selected proteins by ELISA in multiple high and low eEF1A RILs at 18-DAP and maturity, which confirmed that cytoskeletal proteins were increased in high eEF1A genotypes. Microarray analysis of these genotypes also showed changes in several cytoskeleton and cytoskeleton-associated proteins. Unfortunately, the cost of the microarray experiments prevented us from analyzing more genotypes than the parents and one high and one low eEF1A RIL.
Using an endosperm EST microarray, we were able to conduct a broad survey of genes that change in expression parallel with eEF1A in high and low eEF1A genotypes. Our goal was to identify genes with an expression level that was higher in Oh51Ao2 compared to Oh545o2, and higher in RIL 34-3 compared to RIL 31-5. We tested the microarray results for several genes by RNA gel-blot analysis and generally found a good correspondence between the expression levels estimated by the two techniques. In a few cases, the differences in RNA levels detected by gel-blot analysis and microarray hybridization did not correspond. This may have been a consequence of inherent differences in the stringency of the two hybridization procedures or the specific alleles present, or absent, on the microarray. However, the general consistency between the results obtained by the RNA gel-blot analysis and microarray hybridization largely confirmed the utility of using the EST microarray to identify genes differentially expressed in high and low eEF1A genotypes.
The microarray hybridization identified about 120 genes coordinately increased with eEF1A (Table I). These genes encode proteins involved in a variety of cellular processes, and several of them have been previously reported to be differentially expressed at the transcript and protein level in wild type and o2 mutant inbreds, although not necessarily in parallel with eEF1A. For example, the levels of α-zeins, pyruvate orthophosphate dikinase, and acetolactate synthase were decreased in o2 mutants compared to wild type (Damerval and Le Guilloux, 1998; Hunter et al., 2002), while that of eEF1A was increased (Habben et al., 1993; Sun et al., 1997). For some enzymes involved in carbohydrate metabolism, such as glyceraldehyde-3-phosphate dehydrogenase and sorbitol dehydrogenase, earlier studies showed these proteins are increased in o2 mutants (Damerval and Le Guilloux, 1998).
One explanation for the relationship between eEF1A and endosperm Lys content is that eEF1A is part of an elaborate cytoskeletal network that surrounds the RER, particularly at sites where protein bodies are forming (Clore et al., 1996; Reuzeau et al., 1997). Other Lys-rich proteins that exist in association with this cytoskeletal network may be increased as the consequence of a greater protein body surface area (Sun et al., 1997). Oh51Ao2 has more α-zein and also appears to have a larger number of small protein bodies (larger RER surface area) than Oh545o2 (Wang et al., 2001), which could result in a more extensive ER-associated cytoskeletal network. Consistent with this hypothesis, our microarray results indicated the expression of α-zeins and a number of genes encoding actin-associated and ER proteins are coordinately increased in high eEF1A genotypes (Table I). In addition, the levels of α-zeins and some cytoskeletal proteins corresponded well with those of eEF1A in the parental inbreds and some high and low eEF1A RILs (Figs. 3 and 6). This could explain a significant portion of the increased eEF1A content in Oh51Ao2 and its related high eEF1A RILs. Microscopy and biochemical studies will be required to examine the postulated ultrastructural differences in protein body size and number and ER development in RILs with differing levels of eEF1A.
The RNA expression data also showed that several genes encoding carbohydrate-metabolizing enzymes were up-regulated in high eEF1A genotypes. Interestingly, some of these enzymes, such as Suc synthase (Carneiro, 1998; Winter et al., 1998), glyceraldehyde-3-phosphate dehydrogenase, and Fru-1,6-bisphosphate aldolase were recently reported to be associated with the cytoskeleton (Azama et al., 2003). The Lys content of these proteins varies from about 5% to 8.5%, and their masses, together with those of structural cytoskeletal proteins (actin, tubulin, and eEF1A), were reported to contribute significantly (approximately 67%) to the total endosperm Lys content of W64Ao2 (Azama et al., 2003). Several endosperm proteins found to associate with actin in yeast two-hybrid interactions (Carneiro, 1998) included a Ser-Thr protein kinase, 60S acidic ribosomal protein, E2 ubiquitin-conjugating enzyme, and genes encoding these proteins were also up-regulated in high eEF1A genotypes (Table I).
In a previous study, we characterized isoforms of eEF1A from developing maize endosperm and found that they differ in their F-actin binding activities (Lopez-Valenzuela et al., 2003). The isoform that binds actin most efficiently was the predominant form accumulated in endosperm of the high eEF1A inbreds, Oh51o2, and RIL 34-3, which may reflect enhanced cytoskeleton formation and therefore increased synthesis of cytoskeleton-associated proteins in these genotypes. Consistent with this hypothesis, our results show the mRNA levels of several genes encoding ribosomal proteins and protein synthesis factors are significantly higher in Oh51Ao2 and RIL 34-3, compared to Oh545o2 and RIL 31-5 (Table I). It is known that a significant proportion of polysomes in maize endosperm exist in a cytoskeleton-bound state and several plant translation factors have been shown to interact directly with cytoskeletal polymers (for review, see Browning, 1996; Hesketh and Pryme, 1991). Altogether, approximately 25% of the significantly changed genes shown in Table I have implied or proven associations with the cytoskeleton.
Proteins that directly regulate the dynamics of the actin (e.g. ARP2/3 complex, fimbrin, capping protein, or profilin) and microtubule cytoskeletal networks (e.g. katanin, p65, Mor1, or Tangled protein) are conspicuously absent from the microarray analysis. There are several factors that contributed to this. First, because the array was printed from randomly chosen cDNA clones from an endosperm library, many of these regulatory proteins were not represented. This is due to the great abundance of storage protein sequences, but it could also reflect that many of these proteins are not highly expressed in maize endosperm. Second, regulatory proteins that are present on the microarray, such as profilin, actin depolymerizing factor, katanin, and a putative centromeric microtubule binding protein, had signal intensities that were close to background levels; consequently, this made it difficult to discriminate significant differences in gene expression. Finally, some proteins, α- and β-tubulin for example, were significantly changed only in the parental inbred lines; it is unclear whether such proteins can be considered to contribute to the increased Lys content of high eEF1A lines, since they were not significantly different in the RILs.
Differences in transcript levels between genotypes are often consistent with variation in protein levels, although this need not be the case. Furthermore, differences in RNA levels in developing maize seeds need not necessarily be related to protein content in the mature seed. However, it is the latter that ultimately determines the Lys content of the grain. The microarray analysis was predicated on the hypothesis that by measuring differences in transcript levels at mid-development (18-DAP), we could identify proteins linked with eEF1A concentration in mature maize endosperm. To a large extent, this proved to be correct. At 18-DAP, the differences in eEF1A protein between Oh51Ao2 and Oh545o2 and between RIL 34-3 and RIL 31-5 (approximately 1.3-fold; Fig. 6B) were similar to those measured at the RNA transcript level by microarrays and RNA gel-blot analysis (1.6–1.9-fold; Table I; Fig. 4). According to microarray hybridization (Table I), two actin RNA transcripts were more than 2-fold increased in Oh51Ao2 and RIL 34-3 (high eEF1A) compared to Oh545o2 and RIL 31-5 (low eEF1A), while only about 1.2-fold differences in actin transcript levels were measured by RNA-blot analysis for a third actin gene (Fig. 4). The protein measurements indicated there was about 20% to 30% more actin in Oh51Ao2 and RIL 34-3 (high eEF1A) compared to Oh545o2 and RIL 31-5 (low eEF1A) at 18-DAP (Fig. 5B). The values obtained for α-tubulin protein content (1.8-fold greater in Oh51Ao2 than Oh545o2 and 1.4-fold greater in RIL 34-3 than RIL 31-5) fell between the microarray results (approximately 3-fold; Table I) and the 1.2-fold difference obtained by RNA-blot analysis (Fig. 4). Differences in profilin RNA levels were not apparent by microarray hybridization, but differences in the amount of this protein in Oh51Ao2 versus Oh545o2 and RIL 34-3 versus RIL 31-5, 1.3- and 1.5-fold, respectively, were in good agreement with the transcript levels determined by gel-blot analysis (approximately 1.5-fold; Fig. 3). In mature endosperm, the differences in eEF1A and actin protein content between Oh51Ao2 and Oh545o2 and between RIL 34-3 and RIL 31-5 (approximately 2-fold; Fig. 6) were similar to those measured at the RNA transcript level by microarrays (Table I), while the differences in α-zein protein content (2- to 4-fold) were smaller than those measured at the transcript level in 18-DAP endosperm (Table I; Fig. 3). Thus, in general there was a good relationship between RNA transcript levels at 18-DAP and protein levels in developing and mature endosperm.
In conclusion, the results of this study support the hypothesis that eEF1A concentration in maize endosperm is related to the concentration of cytoskeleton-associated protein components, and this may contribute to the high correlation between eEF1A and the concentration of protein-bound Lys in endosperm (Habben et al., 1995). Cytoskeleton-associated proteins are not as abundant as zeins, but combined, their masses appear to make a substantial contribution to the Lys content of the grain (Azama et al., 2003). By selecting for maize genotypes, particularly Quality Protein Maize lines (Moro et al., 1996; Zarkadas et al., 2000) with high levels of eEF1A, it should be possible to create maize kernels that meet the Lys requirement for humans and other monogastric animals.
MATERIALS AND METHODS
Plant Materials
The maize (Zea mays) inbred lines Oh51Ao2 (high eEF1A content) and Oh545o2 (low eEF1A content) were obtained from Crow's Hybrid Corn, Milford, IL. F1 and F2 progeny were created from a cross between these two inbreds (Wang et al., 2001), and the RILs were created by single seed descent from the F2 progeny. Parental inbreds and RILs were grown at the University of Arizona West Agricultural Center in Tucson, AZ, and at the University of Florida, Gainesville, FL. Developing kernels were harvested at 18-DAP, frozen in liquid nitrogen, and stored at −80°C until use. Prior to RNA extraction, the embryo and pericarp were removed by hand dissection and the endosperm placed in liquid nitrogen.
RNA Purification and Labeling
Total RNA was extracted from 18-DAP developing endosperms. Frozen endosperms were ground with a mortar and pestle, and the powder was homogenized in one volume of NTES buffer (20 mm Tris, pH 8.0, 100 mm NaCl, 10 mm EDTA, 1% [w/v] SDS) and one volume of phenol/chloroform saturated with Tris pH 8.0. This extraction buffer allowed the precipitation and removal of starch, which is highly abundant in 18-DAP endosperm. Following centrifugation, nucleic acids were precipitated from the aqueous phase with three volumes of ethanol and resuspended in diethyl pyrocarbonate (DEPC) water. RNA was precipitated with three volumes of 4 m LiCl at −20ºC for 4 h, resuspended in DEPC water, and purified using TRIzol (Invitrogen, Carlsbad, CA). Poly(A)+ RNA was purified from total RNA using a Qiagen oligotex mRNA kit (Qiagen, Valencia, CA). RNA quantity was estimated by UV absorption at 260 nm, and its quality was assessed by visual inspection following agarose gel electrophoresis. For synthesis of fluorescently labeled cDNA targets, 2 μg of poly(A)+ RNA was first hybridized to 1 μg oligo(dT) primer at 65°C for 10 min. The reaction was completed by addition of 1× reverse transcription buffer (50 mm Tris-HCl, pH 8.3, 75 mm KCl, 3 mm MgCl2), 500 μm dNTPs (dATP, dCTP, dGTP), 100 μm dTTP, 50 μm Cy3- or Cy5-dUTP, 10 mm dithiothreitol (DTT), and 400 units Superscript II reverse transcriptase (Invitrogen), followed by incubation at 42°C for 90 min.
Microarray Hybridization and Analysis
Glass slides of the maize endosperm 605.03 microarray were obtained from the Maize Gene Discovery Project (Fernandes et al., 2002). A detailed description of the array design can be found at http://zmdb.iastate.edu/zmdb/microarray. Hybridization was conducted following the protocols described by Fernandes et al. (2002) and the details available at http://zmdb.iastate.edu/zmdb/microarray/protocols.html. The slides were analyzed with a ScanArray 3000 scanner (GSI Lumonics, Watertown, MA) for both channel 1 (Cy3) and 2 (Cy5) at 10-μm resolution. Photomultiplier settings were adjusted manually to reduce the percentage of spots on the array with saturated signal values and to obtain a Cy3:Cy5 signal ratio close to one for the control genes. For each high versus low eEF1A genotype comparison, we used two slides, one with direct and one with reverse labeling. Thus, for each probe a total of two RNA samples, two slides per sample and two arrays per slide, resulted in eight intensity values for analysis. The image files obtained were analyzed using Imagene software (Biodiscovery, Marina del Rey, CA). Quality control measurements produced by the Imagene software were used to ensure that only spots of good quality were used in the analysis. Further analysis and normalization of the data were performed using the BioConductor suite of microarray analysis programs (Dudoit et al., 2003). Net signal intensity was computed from the median signal minus the local background. The intensity data were normalized using the variance stabilized normalization function in BioConductor (Huber et al., 2002). The normalized data were exported to Microsoft Excel for statistical analysis of differential expression using the Statistical Analysis for Microarray Excel plug-in (Tusher et al., 2001) to minimize the false discovery rate due to multiple hypothesis testing. Prior to analysis of the data, the intensities for each subarray were placed in separate columns, resulting in a total of eight intensity values for each cDNA in the array. The cutoff value of the corrected probability (q) for significant differences was q < 0.05, which resulted in a median number of false positives less than 0.1% for each dataset.
The identity and function of each EST that was significantly changed was confirmed manually by BLAST analysis. BLASTN searches were performed against the GenBank plant nonredundant database release 138 with an expectation cutoff of 1 × 10−10. BLASTX searches were performed against the nonredundant protein database with an expectation cutoff of 1 × 10−4. In cases where the most significant nucleotide hit was to an uncharacterized maize sequence, unannotated mRNA sequences contributed by the maize mapping project for example, and the identity was greater than 95%, the complete sequence of the longer sequence in the database was used for a BLASTX search. Finally, any sequences that did not produce any significant hits were searched against the maize genomics consortium release 3.0 database of contigs from methyl-filtered and high-Cot sequences, as well as the Institute for Genomic Research maize gene index release 14. If the sequence identity of the hits from these databases were greater than 95%, the contig sequence was used to search the nonredundant protein database as described above.
RNA-Blot Hybridization
RNA (10 μg of total RNA per sample) was separated by electrophoresis in 1.2% agarose gels containing 2.2 m formaldehyde (Lehrach et al., 1977) and blotted onto nylon membranes (MSI, Westboro, MA). DNA probes were obtained by labeling with α-32P-dCTP using a random prime method, according to the manufacturer (Invitrogen). EST-specific cDNAs were obtained from the corresponding 605 EST clones by PCR amplification as described by Fernandes et al. (2002) and their identity confirmed by partial sequencing. Primer sequences used for amplification of the 605 EST clones were as follows: forward 5′-CTGCAGTAATACGACTCACTATAG-3′ and reverse 5′-CTATTCGATGATGAAGATACC-3′. The cDNAs corresponding to zeins, glyceraldehyde 3-phosphate dehydrogenase (GAPC2), and granule-bound starch synthase (Waxy1) were obtained by PCR using gene-specific primers. Amplified DNA products were analyzed by agarose gel electrophoresis and purified using the GeneClean III kit from Amersham (Piscataway, NJ). The RNA gel blots were hybridized using the indicated 32P-labeled probes. As a loading control, the blots were hybridized with a 32P-labeled cDNA corresponding to the 27-kD γ-zein. Hybridizations were performed at 65ºC in a solution containing 1.5× sodium chloride/sodium phosphate/EDTA, pH 7.7, 6% PEG 8000, 1% SDS, 1% nonfat dried milk, 0.1% (v/v) DEPC, and 30 μg mL−1 freshly denatured salmon sperm DNA (Sabelli and Shewry, 1993). After hybridization, the blots were washed once with 1× SSC and 0.1% SDS at room temperature, then twice with 1× SSC and 0.1% SDS for 15 min at 65ºC, and then a final wash with 0.1× SSC and 0.1% SDS for 15 min at 65ºC. Quantification of hybridization signals was obtained using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Blots were stripped between hybridizations with boiled 0.5% SDS, followed by a 20-min incubation at 65°C.
Zein and Nonzein Protein Extraction, Quantification, and SDS-PAGE Analysis
Protein fractions were obtained from mature endosperms or lyophilized, developing endosperms as described by Wallace et al. (1990). Fifty milligrams of flour was extracted overnight with 1 mL of borate buffer (12.5 mm Na2B4O7, 1% [w/v] SDS, 2% 2-mercaptoethanol, pH 10) at 37°C, and the soluble proteins were partitioned into zeins (supernatant) and nonzeins (pellet) by the addition of absolute ethanol to a final concentration of 70% (v/v). Zein proteins (equivalent to an extract from 15 mg of flour) were lyophilized and resuspended in 100 μL of deionized water. Nonzein proteins obtained from the same amount of flour were resuspended in 100 μL of 8 m urea.
The concentration of zein proteins was determined by the bicinchoninic acid (BCA) method (Brown et al., 1989), using the BCA protein assay reagents from Pierce (Rockford, IL). The concentration of nonzein proteins was determined according to Bradford (1976), using the Bradford protein assay reagent from Bio-Rad Laboratories (Hercules, CA). Bovine serum albumin (BSA) was used as a standard for both zein and nonzein quantification. The results reported (mg of protein/100 mg of flour) correspond to the average of two independent experiments in which three measurements were taken. Five microliters of the original zein extract were used for analysis with 12.5% (w/v) SDS-PAGE, and the gel was stained with Coomassie Blue R.
ELISA Measurements
Endosperm protein extraction and zein/nonzein fractionation were performed as described by Wallace et al. (1990). ELISA of eEF1A was similar to that described by Habben et al. (1995), Moro et al. (1996), and Wang et al. (2001). Total endosperm protein extract was diluted 1,000-fold in carbonate coating buffer (CCB; 0.1 m Na2CO3/NaHCO3, pH 9.6), and 150 μL of the sample was loaded in the well of an ELISA plate (Immulon2, Dynatech Laboratories, Chantilly, VA). A multichannel pipette was used to mix 50 μL of the sample with 100 μL of CCB to make four 3-fold dilutions into adjacent wells containing CCB. The protein was allowed to bind to the plate overnight at 4°C. Subsequently, the wells were washed twice using PBS plus Tween 20 (PBST, 0.05 m phosphate, 145 mm NaCl, 0.05% [v/v] Tween 20, pH 7.4) and 100 μL of rabbit eEF1A antiserum (Habben et al., 1995) diluted 1:1,000 in PBST was added and incubated for 3 h. After incubation, the primary antibody was removed, the wells were washed twice with PBST, and the secondary antibody, goat anti-rabbit IgG alkaline conjugate (Sigma Chemical, St. Louis) diluted 1:1000 in PBST, was added and allowed to bind for 2 h. After removal of the secondary antibody, the wells were washed twice with PBST and 200 μL of the alkaline phosphatase substrate (Sigma) diluted in diethanolamine buffer (50 mm diethanolamine, pH 9.8) was added. The color reaction was allowed to develop for 30 to 45 min, and the absorbance was read at 420 nm with a micro plate reader (MRX, Dynatech).
An ELISA to measure actin, profilin, and α-tubulin was performed as for eEF1A, but with some modifications. Nonzein pellets from 15 mg of flour were resuspended in 100 μL of 8 m urea. The nonzein protein solution was diluted 2,500-, 4,000-, and 10-fold in CCB for actin, profilin, and α-tubulin, respectively. Two hundred microliters of the samples were loaded in the well of the ELISA plate, and 100 μL were mixed with 100 μL of CCB to make four 2-fold dilutions into adjacent wells. The rest of the procedure was similar to that described for eEF1A. The primary antibodies were from rabbit antiserum produced against actin and profilin from maize pollen (Gibbon et al., 1998; Gibbon et al., 1999) and recombinant α-tubulin of human origin (Santa Cruz Biotechnology, Santa Cruz, CA); they were diluted 1:500, 1:500, and 1:50, respectively, in PBST.
The ELISA for α-zein was similar to the procedure described by Moro et al. (1996), with some modifications. Following the initial extraction of endosperm flour, the protein extract was diluted 20,000-fold and used to make four 2-fold dilutions into adjacent wells of the ELISA plate. The dilution and antigen binding steps were done using a 40% (v/v) ethanol, 10% (v/v) acetic acid solution (Wallace et al., 1990), and the primary antibody (rabbit anti-α-zein) dilution was 1:2,000 in PBST. The range of protein concentration for all the ELISA assays was such that the relationship between absorbance and relative antigen concentration was linear, and a regression analysis was performed. The slope of the regression is proportional to the antigen concentration, and it was used to measure the relative protein content. The results were normalized to the values of the Oh545o2 inbred and represent the average of two independent experiments in which three measurements were taken.
Supplementary Material
Acknowledgments
We thank Dr. Monika Dalal for technical advice in the early stages of this work and Dr. Rangasamy Elumalai for providing EST clones through the Maize Gene Discovery Project. We thank Dr. David Galbraith for access to his microarray facility in the Department of Plant Sciences at the University of Arizona. We give special thanks to Dr. Larkin Curtis Hannah for growing some of these materials at the University of Florida and providing us with developing kernels. J.A.L.V. was the recipient of a graduate fellowship from Consejo Nacional de Ciencia y Tecnologia, Mexico.
This work was supported by the U.S. Department of Agriculture National Research Initiative (grant no. NRI–981427 to B.A.L.), by the Department of Energy (grant no. DE–96ER20242 to B.A.L.), and by the National Science Foundation (grant no. DBI–9872657 to B.A.L.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.042259.
References
- Azama K, Abe S, Sugimoto H, Davies E (2003) Lysine-containing proteins in maize endosperm: a major contribution from cytoskeleton-associated carbohydrate-metabolizing enzymes. Planta 217: 628–638 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brown RE, Jarvis KL, Hyland KJ (1989) Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem 180: 136–139 [DOI] [PubMed] [Google Scholar]
- Browning KS (1996) The plant translational apparatus. Plant Mol Biol 32: 107–144 [DOI] [PubMed] [Google Scholar]
- Carneiro N (1998) Patterns of gene expression in maize endosperm: characterization of the eEF1A gene family. PhD thesis. University of Arizona, Tucson
- Clore AM, Dannenhoffer JM, Larkins BA (1996) EF-1 alpha is associated with a cytoskeletal network surrounding protein bodies in maize endosperm cells. Plant Cell 8: 2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerval C, Devienne D (1993) Quantification of dominance for proteins pleiotropically affected by opaque-2 in maize. Heredity 70: 38–51 [Google Scholar]
- Damerval C, Le Guilloux M (1998) Characterization of novel proteins affected by the o2 mutation and expressed during maize endosperm development. Mol Gen Genet 257: 354–361 [DOI] [PubMed] [Google Scholar]
- Demason DA (1997) Endosperm structure and development. In BA Larkins, IK Vasil, eds, Cellular and Molecular Biology of Plant Seed Development. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 73–115
- Dudoit S, Gentleman RC, Quackenbush J (2003) Open source software for the analysis of microarray data. Biotechniques 34: S45–S51 [PubMed] [Google Scholar]
- Durso NA, Cyr RJ (1994) Beyond translation—elongation factor-1 alpha and the cytoskeleton. Protoplasma 180: 99–105 [Google Scholar]
- Fernandes J, Brendel V, Gai X, Lal S, Chandler VL, Elumalai RP, Galbraith DW, Pierson EA, Walbot V (2002) Comparison of RNA expression profiles based on maize expressed sequence tag frequency analysis and micro-array hybridization. Plant Physiol 128: 896–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers HO, Lake JK (1992) Development of modified opaque-2 maize in South Africa. In ET Mertz, ed, Quality Protein Maize. The American Society of Cereal Chemists, St. Paul, pp 49–78
- Gibbon BC, Kovar DR, Staiger CJ (1999) Latrunculin B has different effects on pollen germination and tube growth. Plant Cell 11: 2349–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon BC, Zonia LE, Kovar DR, Hussey PJ, Staiger CJ (1998) Pollen profilin function depends on interaction with proline-rich motifs. Plant Cell 10: 981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DV, Mertz ET (1987) Corn. In RA Olson, KT Frey, eds, Nutritional Quality of Cereal Grains: Genetic and Agronomic Improvement. ASA-CSSA-SSSA, Madison, WI, pp 183–336
- Habben JE, Kirleis AW, Larkins BA (1993) The origin of lysine-containing proteins in opaque-2 maize endosperm. Plant Mol Biol 23: 825–838 [DOI] [PubMed] [Google Scholar]
- Habben JE, Moro GL, Hunter BG, Hamaker BR, Larkins BA (1995) Elongation factor 1-alpha concentration is highly correlated with the lysine content of maize endosperm. Proc Natl Acad Sci USA 92: 8640–8644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh JE, Pryme IF (1991) Interaction between messenger-RNA, ribosomes and the cytoskeleton. Biochem J 277: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W, von Heydebreck A, Sueltmann H, Poustka A, Vingron M (2002) Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18: S96–S104 [DOI] [PubMed] [Google Scholar]
- Hunter BG, Beatty MK, Singletary GW, Hamaker BR, Dilkes BP, Larkins BA, Jung R (2002) Maize opaque endosperm mutations create extensive changes in patterns of gene expression. Plant Cell 14: 2591–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodrzycki R, Boston RS, Larkins BA (1989) The opaque-2 mutation of maize differentially reduces zein gene transcription. Plant Cell 1: 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Savereide P, Lefebvre P, Dasgupta S (1990) The predicted amino-acid-sequence of a centrosphere protein in dividing sea-urchin eggs is similar to elongation-factor (Ef-1α). J Cell Sci 95: 231–236 [DOI] [PubMed] [Google Scholar]
- Lehrach H, Diamond D, Wozney JM, Boedtker H (1977) RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 16: 4743–4751 [DOI] [PubMed] [Google Scholar]
- Lopez-Valenzuela JA, Gibbon BC, Hughes PA, Dreher TW, Larkins BA (2003) eEF1A isoforms change in abundance and actin-binding activity during maize endosperm development. Plant Physiol 133: 1285–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MD, Lindell JS, Larkins BA (1985) Nucleotide sequence analysis of zein mRNAs from maize endosperm. J Biol Chem 260: 16451–16459 [PubMed] [Google Scholar]
- Mertz ET, Nelson OE, Bates LS (1964) Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145: 279–280 [DOI] [PubMed] [Google Scholar]
- Moro GL, Habben JE, Hamaker BR, Larkins BA (1996) Characterization of the variability in lysine content for normal and opaque2 maize endosperm. Crop Sci 36: 1651–1659 [Google Scholar]
- Ohta K, Toriyama M, Miyazaki M, Murofushi H, Hosoda S, Endo S, Sakai H (1990) The mitotic apparatus-associated 51-Kda protein from sea-urchin eggs is a GTP-binding protein and is immunologically related to yeast polypeptide elongation factor-1α. J Biol Chem 265: 3240–3247 [PubMed] [Google Scholar]
- Reuzeau C, Doolittle KW, McNally JG, Pickard BG (1997) Covisualization in living onion cells of putative integrin, putative spectrin, actin, putative intermediate filaments, and other proteins at the cell membrane and in an endomembrane sheath. Protoplasma 199: 173–197 [DOI] [PubMed] [Google Scholar]
- Sabelli PA, Shewry PR (1993) Nucleic acid blotting and hybridisation. Methods Plant Biochem 40: 79–100 [Google Scholar]
- Schmidt RJ, Burr FA, Aukerman MJ, Burr B (1990) Maize regulatory gene Opaque-2 encodes a protein with a “leucine-zipper” motif that binds to zein DNA. Proc Natl Acad Sci USA 87: 46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Carneiro N, Clore AM, Moro GL, Habben JE, Larkins BA (1997) Characterization of maize elongation factor 1A and its relationship to protein quality in the endosperm. Plant Physiol 115: 1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher V, Tibshirani R, Chu C (2001) Significance analysis of microarrays applied to ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas E, Vasal SK, Bjarnarson M (1992) Quality protein maize: what is it and how was it developed. In ET Mertz, ed, Quality Protein Maize. The American Society of Cereal Chemists, St. Paul, pp 27–48
- Wallace JC, Lopes MA, Paiva E, Larkins BA (1990) New methods for extraction and quantitation of zeins reveal a high content of gamma-zein in modified opaque-2 maize. Plant Physiol 92: 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Woo YM, Kim CS, Larkins BA (2001) Quantitative trait locus mapping of loci influencing elongation factor 1alpha content in maize endosperm. Plant Physiol 125: 1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1998) Identification of sucrose synthase as an actin-binding protein. FEBS Lett 430: 205–208 [DOI] [PubMed] [Google Scholar]
- Xu W, Bak S, Decker A, Paquette SM, Feyereisen R, Galbraith DW (2001) Microarray-based analysis of gene expression in very large gene families: the cytochrome P450 gene superfamily of Arabidopsis thaliana. Gene 272: 61–74 [DOI] [PubMed] [Google Scholar]
- Yang F, Demma M, Warren V, Dharmawardhane S, Condeelis J (1990) Identification of an actin-binding protein from Dictyostelium as elongation factor 1α. Nature 347: 494–496 [DOI] [PubMed] [Google Scholar]
- Young VR, Scrimshaw NS, Pellet PL (1998) Significance of dietary protein source in human nutrition: animal and/or plant proteins? In JC Waterlow, DG Armstrong, L Fowden, R Riley, eds, Feeding a World Population of More than Eight Billion People. Oxford University Press in association with Rank Prize Funds, New York, pp 205–221
- Zarkadas CG, Hamilton RI, Yu ZR, Choi VK, Khanizadeh S, Rose NG, Pattison PL (2000) Assessment of the protein quality of 15 new northern adapted cultivars of quality protein maize using amino acid analysis. J Agric Food Chem 48: 5351–5361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.