Abstract
Objective
To determine the association between sleep quality and lower urinary tract symptom (LUTS) severity in men working non-standard shifts, a population at risk for poor sleep quality.
Materials and Methods
Men who presented to a single andrology clinic between July and October 2014 and worked non-standard shifts completed the International Prostate Symptom Score (IPSS) and responded to questions regarding their work habits, sleep-quality, and physical/cognitive function. We assessed the relationship between age, sleep-quality, physical/cognitive function and severity of LUTS.
Results
228 men with a mean±SD age of 41.8±5.7 (range 21–76) years reported working non-standard shifts with the majority working these for more than 1 year (81%). Men with difficulties falling asleep reported more severe LUTS than men who did not have difficulty falling asleep (IPSS score 9 vs. 6, p<0.001). Men who reported difficulty staying asleep or falling back asleep after awakening also reported more severe LUTS (IPSS scores 6 vs. 13, p=0.004; 5 vs. 13, p<0.001, respectively). Men with a decreased sense of wellbeing or decreased physical/cognitive function also reported more severe LUTS (IPSS score 6 vs. 9, p<0.0010; 6 vs. 10, p=0.016, respectively). All findings were independent of subject age.
Conclusion
Men working non-standard shifts who have difficulty falling asleep, staying asleep, and falling back asleep report more severe LUTS than men without similar sleep difficulties. Men with a decreased sense of wellbeing or decreased physical/cognitive function also report worse LUTS. These findings implicate sleep quality as a possible risk factor for LUTS symptom severity.
MeSH Key Words: lower urinary tract symptoms, LUTS, sleep, shift work disorder, prostate
INTRODUCTION
Lower urinary tract symptoms (LUTS) are one of the most common and problematic medical conditions in older men. LUTS may be present in up to 97% of men ≥ 65 years old and are associated with worse health quality.1 Sequelae of LUTS include nocturia and consequent sleep disturbances, and more severe LUTS increases the risk for impaired sleep quality.2 Conversely, improvement in LUTS results in improved sleep.3 Impaired sleep quality may contribute to numerous other conditions including hypertension, diabetes mellitus, arteriosclerosis,4 and cardiovascular events.5 However, the relationship between sleep quality and LUTS remains unclear.
Sleep quality can be affected by abnormal sleep schedules, and shift workers often cannot maintain a normal sleep pattern as a result of their work schedule. Shift workers comprise 15–25% of the workforce and often develop impaired sleep quality, with 10–32% developing shift work disorder (SWD).6 SWD is a circadian rhythm disorder characterized by insomnia and sleepiness in people whose working hours overlap those of a normal sleep period, typically at night. Patients with SWD are more likely to have decreased daytime alertness and impaired cognitive function,7 higher body mass indices (BMIs), cholesterol levels, triglycerides, and rates of hypertension than non-shift workers.7, 8 The prevalence of depression is also greater in night shift workers when compared with daytime workers. The mechanisms underlying development of SWD sequelae are unclear, although obesity,9 inflammation,10 autonomic dysregulation,11 and metabolic dysregulation12 can all result from sleep impairment; dysregulated neurotransmission may also result from SWD.13, 14 Since LUTS can arise from these same factors,12, 15 impaired sleep quality may not only be a consequence of, but also an exacerbating factor for LUTS.
Whether impaired sleep quality predisposes to LUTS or vice versa (bidirectional causality) has not been extensively studied. However, evidence suggests that poor sleep quality increases the risk of developing LUTS within 5 years of diagnosis.15 Here, we identify a relationship between the level of sleep quality and severity of LUTS in male non-standard shift workers.
METHODS
All men presenting to a single academic men’s health clinic between July and October 2014 were asked to complete an electronic survey about their work schedules, specifically regarding whether or not they worked non-standard shifts. Survey respondents were not selected for presenting symptoms, age, or race. As defined in the literature, men who work non-standard shifts begin work before 7 a.m. or after 2 p.m., regularly rotate between standard and non-standard formats, or regularly work hours outside of a standard 7 a.m. to 6 p.m. workday.16 Men who work non-standard shifts were asked how long they have been working these shifts, and were asked to complete the validated International Prostate Symptom Score (IPSS) questionnaire,17 as well as non-validated questionnaires to inform quality of life and cognitive function (Supplementary Data Figure 1).
Responses were collected electronically, and only responses with complete questionnaire data were included for analysis. We associated sleep quality (falling asleep, staying asleep, and getting back to sleep), quality of life, and cognitive/physical function with IPSS data. Because age is an important risk factor for LUTS,18 we compared subject ages across all response groups to determine whether or not the relationship between sleep, quality of life and LUTS were independent of age. We compared means using Student’s t-test, MANOVA, and linear regression where appropriate. SPSS version 22 (IBM Corporation, Somers, NY) was used for all statistical analyses, with p<0.05 considered statistically significant.
RESULTS
We hypothesized that sleep quality would impact LUTS severity, as it has been shown to exacerbate numerous other conditions. We asked men who work non-standard shifts to report their quality of sleep and LUTS severity using the IPSS. A total of 228 men reported working non-standard shifts and were included in the study with a mean±SD age of 41.8±5.7 (range 21–76) years (Table 1). Men reported working an average of 3.8±1.9 non-standard shifts per week, and 81% of men (n=186) reported working non-standard shifts for greater than 1 year. The average IPSS score for non-standard shift workers was 6.0±5.6 with an average quality of life score of 1.4±1.4.
Table 1.
Cohort Demographics
| Variable | Value |
|---|---|
| Age (mean ± SD) (years) | 41.8 ± 5.7 |
| Number of Days Per Week Working Non-standard Shifts (mean ± SD) |
3.8 ± 1.9 |
| Duration of Non-Standard Shift Work (N(%)) | |
| < 1 month | 16 (7%) |
| 1–6 months | 17 (7%) |
| 7–12 months | 9 (4%) |
| 1–5 years | 60 (26%) |
| > 5 years | 126 (55%) |
| IPSS Score (mean ± SD) | 6.0 ± 5.6 |
| IPSS Quality of Life Score (mean ± SD) | 1.4 ± 1.4 |
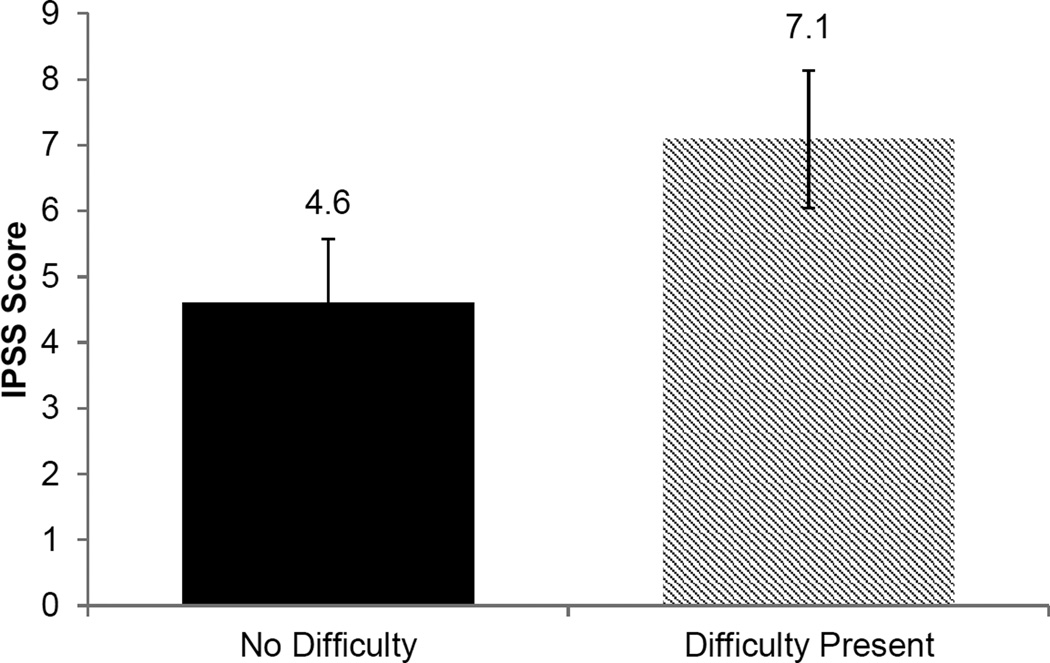
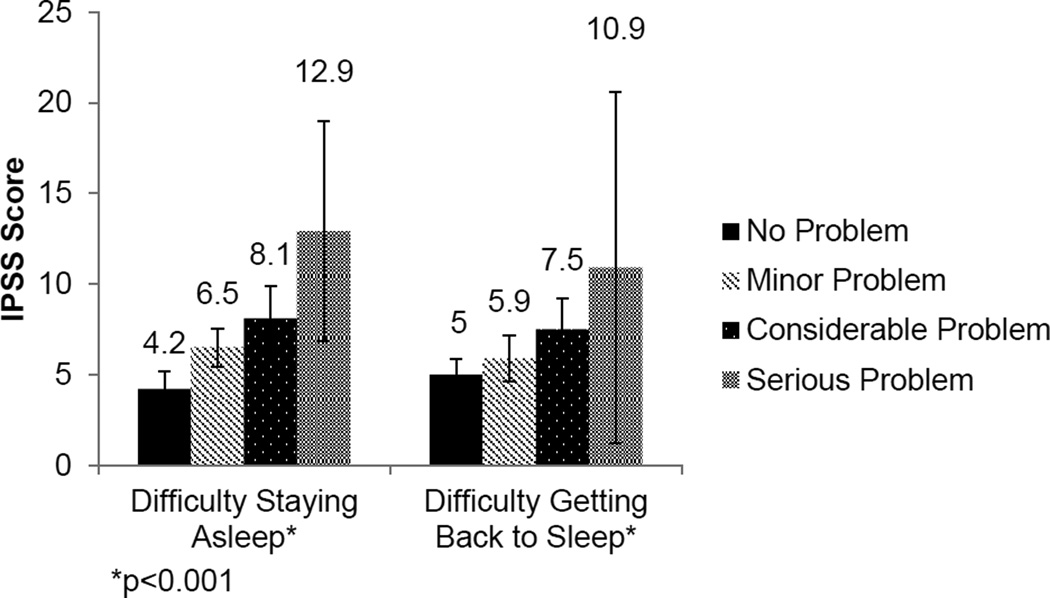
To identify poor sleep as a potential risk factor for LUTS severity, we used sleep quality as the independent variable. Men who reported difficulty falling asleep had more severe LUTS than men without difficulty falling asleep (IPSS score 7.1 vs. 4.6, p<0.001) (Figure 1). Because of the independent associations between age, sleep quality, and LUTS, we confirmed that the relationship between impaired sleep quality and LUTS severity was independent of subject age. Importantly, there was no difference in mean age between men who did not report difficulty falling asleep or men with such difficulties (43.1 vs. 41.7 years old, p=0.08). A direct relationship was observed between LUTS severity and difficulty staying asleep or falling back asleep (p<0.001) (Figure 2). Similarly, these findings were independent of subject age across all levels of sleep difficulties (p>0.05).
Figure 1.
Relationship between difficulty falling asleep and LUTS severity (p<0.001).
Figure 2.
Relationship between difficulty staying asleep or falling back asleep and LUTS severity (p<0.001).
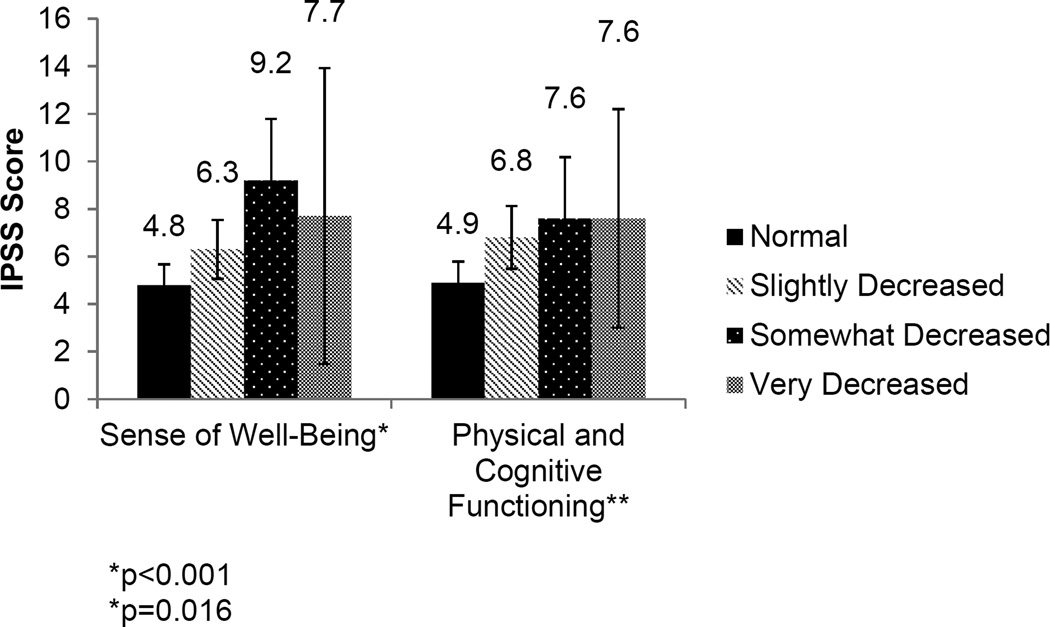
Because physical and psychological wellbeing increases the risk for other conditions and because LUTS have been associated with decreased emotional and physical well-being, we examined the relationship between self-reported sense of wellbeing or physical/cognitive function and LUTS severity. Men who reported a decreased sense of wellbeing or decreased physical and cognitive function had more severe LUTS than men who did not report such changes (p<0.001 and p=0.016, respectively) (Figure 3). Importantly, neither sense of wellbeing nor physical or cognitive function were associated with age in the cohort. These findings suggest a potential role for sleep quality and physical/mental wellbeing on the severity of LUTS.
Figure 3.
Relationship between sense of wellbeing or physical/cognitive function and LUTS severity (p<0.001, p=0.016).
DISCUSSION
While the prevalence of LUTS varies based on sampling methods and diagnostic criteria, these symptoms are common and can negatively impact quality of life.19 We identified an inverse relationship between sleep quality and LUTS severity in men working non-standard shifts. Importantly, the majority of men in our cohort reported working non-standard shifts for more than a year, increasing the likelihood that the impact of suboptimal sleep had already manifested. LUTS can reduce sleep quality, resulting in repeated awakening at night to urinate. Poor sleep, in turn, has numerous negative effects on quality of life and can increase the risk of depression, dysthymia, cardiovascular pathology, musculoskeletal pain,20, 21 and can have negative impacts on mood, subjective well-being, and overall functioning.20 LUTS can result from numerous conditions, including prostatic hyperplasia as well as systemic conditions such as cardiac failure, diabetes mellitus, sleep apnea, spinal cord injury, Parkinson’s disease, and medications, emotional and psychological factors, and patient lifestyle.22 Because of the relationship between poor sleep and the development or exacerbation of many common diseases, poor sleep may also exacerbate LUTS. Identifying sleep quality as an exacerbating factor for, rather than merely a result of, LUTS can provide an opportunity for intervention in affected men.
Our data suggest a bidirectional association between sleep quality and LUTS; we observed that men working non-standard shifts who reported difficulty falling asleep had more severe LUTS than men who were able to fall sleep without difficulty. This finding suggests that nighttime awakening resulting from nocturia is not the sole factor leading to impaired sleep quality in these men. Other data support a bidirectional relationship between LUTS severity and sleep quality, as patients with poor sleep quality have an increased incidence of nocturia, and significant nocturia and urinary frequency are risk factors for an undiagnosed sleep disorder.15, 23 Sleep is important in maintaining a healthy autonomic nervous system and metabolism, and poor sleep can lead to impaired neural control and metabolic abnormalities, both of which are important in regulating the smooth muscle tone in the urinary tract.12–14, 24 It is tempting to speculate that several homeostatic mechanisms including the neural and metabolic pathways that are important for normal urinary function, and are impaired by poor sleep, may be a potential mechanism for how sleep quality may impact LUTS severity. Improvement in sleep habits and other lifestyle factors, including increased exercise, can reduce the incidence of nocturia and result in improved sleep quality and may reduce LUTS severity.25
The psychological impact on disease may often be overlooked as a cause of symptom development or exacerbation. Men in our cohort who reported a worse sense of wellbeing or impaired physical/cognitive function also reported more severe LUTS. The impact of shift work on physical and cognitive function has been described, with night shift workers having more severe fatigue than daytime workers, leading to reduced alertness and work performance as well as temporary declines in cognitive function.26 The psychological impact of impaired sleep resulting from shift work is not limited to fatigue-related causes. Nurses who work night shifts report more psychological issues including anxiety, obsessive-compulsive traits, somatization, interpersonal sensitivity, and paranoid symptoms, than nurses working during the day.27 The mechanism by which impaired sleep leads to increased psychological stress that exacerbates urinary symptoms is complex and incompletely defined. However, psychological stress activates the hypothalamic-pituitary-adrenal axis and may disrupt brain-bladder signaling at the level of the urothelium, potentially predisposing to LUTS.28–30 Thus, LUTS, poor sleep quality, and impaired physical and cognitive function are likely intertwined, and amelioration of symptoms in one category may result in improvement in the others.
The association between age and LUTS severity is well known, with a large epidemiological study of 4,737 Australian men providing an effective group to which other populations of similarly aged men can be compared. IPSS scores increased from 2.7 in healthy men 40–49 years old with no reproductive health issues to 6.2 in men ≥70 years old.18 Despite the relatively young mean age of men in our cohort (42 years), the average IPSS score (6.0) was closer to that of men ≥70 years old. Men with the most severe issues with sleep, quality of life, and cognitive function had more significant LUTS, which were independent of age. This contrast between IPSS scores and symptoms in night shift workers, when compared with the above healthy age-matched male cohort supports a direct relationship between sleep quality and LUTS. Nevertheless, direct comparisons between our cohort and other patient populations are limited by variations between these groups.
Our study has several key strengths and limitations that merit discussion. The large sample size of non-standard shift workers who maintained this schedule for a long duration (81% >1 year) increases the likelihood that reported symptoms are related to work schedules. In our study, the majority of IPSS symptoms were significantly worse in men with sleep dysfunction. However, we were unable to identify a particular relationship between a specific symptom or cluster of symptoms that further associated with poor sleep. The lack of association between age and sleep quality supports the conclusion that impaired sleep and worsening LUTS are not solely a function of age. This study was limited by its retrospective design and the inherent limitations associated with this approach, as well as a lack of data regarding many of the comorbidities and lifestyle factors that may impact LUTS, limiting our ability to comment on the impact of these variables on our findings. The use of non-standardized questionnaires regarding sleep symptoms, quality of life, and cognitive function limits the findings of this study. This study is also limited by the lack of a comparison group of standard or daytime shift workers, and men with diagnosed SWD and those without. Presence of a control group would have improved the strength of findings and will be an important component in future studies on this subject. It is also important to discuss that the difference between a clinical and statistical difference in IPSS scores is important in this context. The correlation and trend associating worse sleep quality with worse IPSS score suggests that in some patients, sleep dysfunction may be related to their urinary symptoms. Future work will incorporate these comparisons and further our understanding of how impaired sleep may exacerbate LUTS.
CONCLUSION
Men working non-standard shifts who have difficulty falling asleep, staying asleep, and falling back asleep report more severe LUTS than men who do not have similar sleep difficulties. Men who report a decreased sense of wellbeing and decreased physical/cognitive function also have more severe LUTS. These findings implicate sleep quality as a potential risk factor for LUTS severity, suggesting that poor sleep may be a causal factor and not just a consequence of LUTS.
Supplementary Material
Supplement 1 – Shift work questionnaire.
Abbreviations
- LUTS
Lower Urinary Tract Symptoms
- SWD
Shift Work Disorder
- BMI
Body Mass Index
- IPSS
International Prostate Symptom Score
REFERENCES
- 1.Taylor BC, Wilt TJ, Fink HA, et al. Prevalence, severity, and health correlates of lower urinary tract symptoms among older men: the MrOS study. Urology. 2006;68:804–809. doi: 10.1016/j.urology.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Helfand BT, McVary KT, Meleth S, et al. The relationship between lower urinary tract symptom severity and sleep disturbance in the CAMUS trial. The Journal of urology. 2011;185:2223–2228. doi: 10.1016/j.juro.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Helfand BT, Lee JY, Sharp V, et al. Associations between improvements in lower urinary tract symptoms and sleep disturbance over time in the CAMUS trial. The Journal of urology. 2012;188:2288–2293. doi: 10.1016/j.juro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 4.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Archives of internal medicine. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33:1037–1042. doi: 10.1093/sleep/33.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep medicine reviews. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Vogel M, Braungardt T, Meyer W, Schneider W. The effects of shift work on physical and mental health. Journal of neural transmission. 2012;119:1121–1132. doi: 10.1007/s00702-012-0800-4. [DOI] [PubMed] [Google Scholar]
- 8.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocrine reviews. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 9.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nature and science of sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best practice & research. Clinical endocrinology & metabolism. 2010;24:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gacci M, Vignozzi L, Sebastianelli A, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate cancer and prostatic diseases. 2013;16:101–106. doi: 10.1038/pcan.2012.44. [DOI] [PubMed] [Google Scholar]
- 13.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JH, Ginovart N, Boovariwala A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Archives of general psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 15.Araujo AB, Yaggi HK, Yang M, McVary KT, Fang SC, Bliwise DL. Sleep related problems and urological symptoms: testing the hypothesis of bidirectionality in a longitudinal, population based study. The Journal of urology. 2014;191:100–106. doi: 10.1016/j.juro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Presser HB. Job, family, and gender: determinants of nonstandard work schedules among employed Americans in 1991. Demography. 1995;32:577–598. [PubMed] [Google Scholar]
- 17.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of urology. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 18.Holden CA, McLachlan RI, Pitts M, et al. Men in Australia Telephone Survey (MATeS): a national survey of the reproductive health and concerns of middle-aged and older Australian men. Lancet. 2005;366:218–224. doi: 10.1016/S0140-6736(05)66911-5. [DOI] [PubMed] [Google Scholar]
- 19.Milsom ICR. Incontinence. 5th. Paris: International Consultation on Urological Diseases and European Association of Urology; 2013. [Google Scholar]
- 20.Bliwise DL, Rosen RC, Baum N. Impact of nocturia on sleep and quality of life: a brief, selected review for the International Consultation on Incontinence Research Society (ICI-RS) nocturia think tank. Neurourology and urodynamics. 2014;33(Suppl 1):S15–S18. doi: 10.1002/nau.22585. [DOI] [PubMed] [Google Scholar]
- 21.Parthasarathy S, Fitzgerald M, Goodwin JL, Unruh M, Guerra S, Quan SF. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PloS one. 2012;7:e30969. doi: 10.1371/journal.pone.0030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gratzke C, Bachmann A, Descazeaud A, et al. EAU Guidelines on the Assessment of Non-neurogenic Male Lower Urinary Tract Symptoms including Benign Prostatic Obstruction. European urology. 2015;67:1099–1109. doi: 10.1016/j.eururo.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 23.Rai A, Nimeh T, Sood A, et al. Could nocturia be an indicator of an undiagnosed sleep disorder in male veterans? Urology. 2015;85:641–647. doi: 10.1016/j.urology.2014.10.055. [DOI] [PubMed] [Google Scholar]
- 24.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 25.Soda T, Masui K, Okuno H, Terai A, Ogawa O, Yoshimura K. Efficacy of nondrug lifestyle measures for the treatment of nocturia. The Journal of urology. 2010;184:1000–1004. doi: 10.1016/j.juro.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Takeyama H, Matsumoto S, Murata K, et al. Effects of the length and timing of nighttime naps on task performance and physiological function. Revista de saude publica. 2004;38(Suppl):32–37. doi: 10.1590/s0034-89102004000700006. [DOI] [PubMed] [Google Scholar]
- 27.Selvi Y, Aydin A, Boysan M, Atli A, Agargun MY, Besiroglu L. Associations between chronotype, sleep quality, suicidality, and depressive symptoms in patients with major depression and healthy controls. Chronobiology international. 2010;27:1813–1828. doi: 10.3109/07420528.2010.516380. [DOI] [PubMed] [Google Scholar]
- 28.Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology. 2011;94:12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- 29.Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Experimental neurology. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aberg KM, Radek KA, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. The Journal of clinical investigation. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1 – Shift work questionnaire.