Abstract
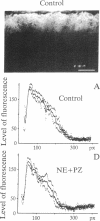
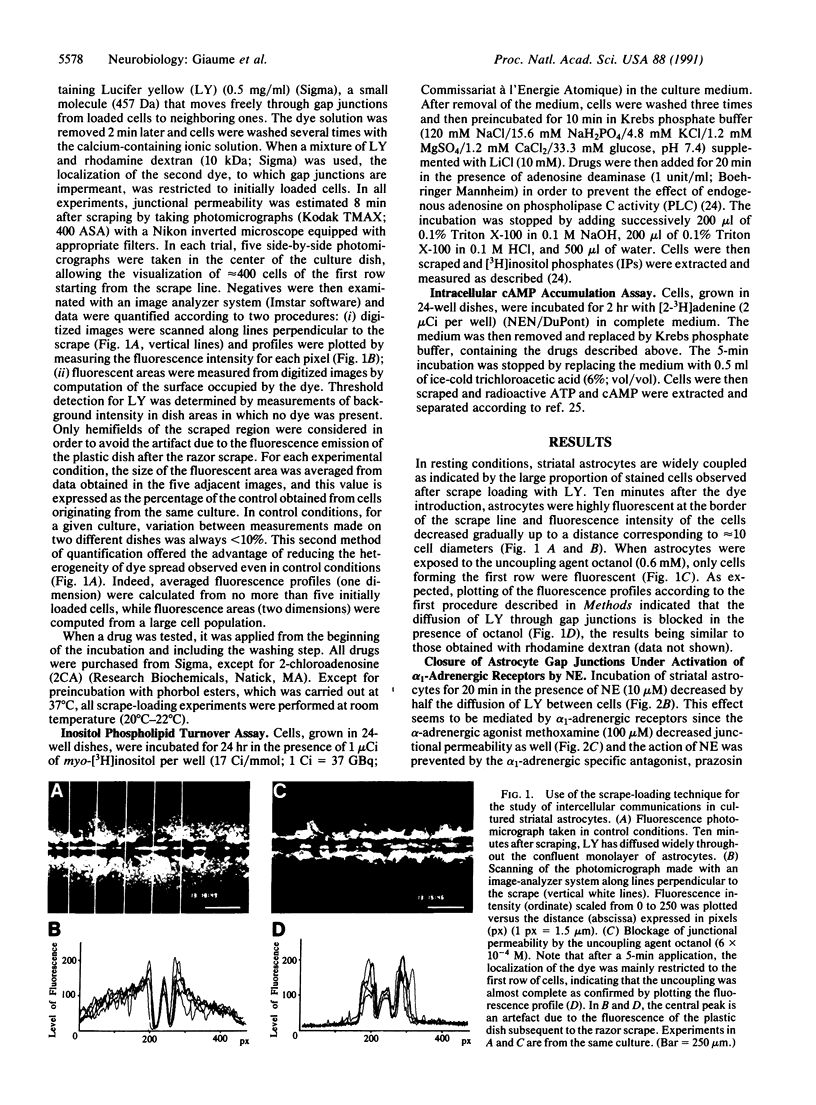
The permeability of gap junctions in cultured striatal astrocytes was investigated by the scrape-loading/dyetransfer technique. Prolonged application of norepinephrine (NE) (10 microM) reduced by half the extent of dye (Lucifer yellow) spread. This effect was linked to the activation of alpha 1-adrenergic receptors since it was mimicked by methoxamine and antagonized by prazosin. The adenosine agonist 2-chloroadenosine (10 microM), which potentiates the NE-evoked activation of phospholipase C (PLC) in striatal astrocytes, also potentiated the NE-evoked closure of gap junctions, the effect being as important as that observed with the uncoupling agent octanol. Measurements of inositol phospholipid turnover performed in identical experimental conditions revealed a close relationship between the extent of PLC activation and the magnitude of the uncoupling process. The effect of NE was mimicked by both phorbol ester and arachidonic acid, suggesting that biochemical events linked to PLC stimulation such as protein kinase C activation and/or eicosanoid production are likely involved in the NE-induced uncoupling. In addition, in the presence of a cAMP phosphodiesterase inhibitor, the stimulation of beta-adrenergic receptors by isoproterenol (10 microM) led to a large increase in cAMP accumulation correlated with an extension of dye diffusion. This observation suggests that junctional permeability could also be controlled by a cAMP-dependent mechanism. Altogether these results indicate that intercellular communication between cultured astrocytes can be regulated by different second messenger pathways as a result of the action of neurotransmitters on their receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Enkvist M. O., Holopainen I. Activators of protein kinase C and phenylephrine depolarize the astrocyte membrane by reducing the K+ permeability. Neurosci Lett. 1988 Oct 17;92(3):265–269. doi: 10.1016/0304-3940(88)90600-3. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Benardo L. S., Prince D. A. Carbon dioxide sensitivity of dye coupling among glia and neurons of the neocortex. J Neurosci. 1984 May;4(5):1324–1330. doi: 10.1523/JNEUROSCI.04-05-01324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- DeGeorge J. J., Morell P., McCarthy K. D., Lapetina E. G. Adrenergic and cholinergic stimulation of arachidonate and phosphatidate metabolism in cultured astroglial cells. Neurochem Res. 1986 Jul;11(7):1061–1071. doi: 10.1007/BF00965594. [DOI] [PubMed] [Google Scholar]
- Dermietzel R., Traub O., Hwang T. K., Beyer E., Bennett M. V., Spray D. C., Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek F. E., Gribkoff V. K., Olson J. E., Hertzberg E. L. Reduction of dye coupling in glial cultures by microinjection of antibodies against the liver gap junction polypeptide. Brain Res. 1988 Jan 26;439(1-2):275–280. doi: 10.1016/0006-8993(88)91484-9. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Takeda K., Brehm P. Activation of a calcium-dependent photoprotein by chemical signalling through gap junctions. Nature. 1987 Jan 1;325(6099):60–62. doi: 10.1038/325060a0. [DOI] [PubMed] [Google Scholar]
- Fischer G., Kettenmann H. Cultured astrocytes form a syncytium after maturation. Exp Cell Res. 1985 Aug;159(2):273–279. doi: 10.1016/s0014-4827(85)80001-x. [DOI] [PubMed] [Google Scholar]
- Giaume C., Randriamampita C., Trautmann A. Arachidonic acid closes gap junction channels in rat lacrimal glands. Pflugers Arch. 1989 Jan;413(3):273–279. doi: 10.1007/BF00583541. [DOI] [PubMed] [Google Scholar]
- Kartner N., Ling V. Multidrug resistance in cancer. Sci Am. 1989 Mar;260(3):44–51. doi: 10.1038/scientificamerican0389-44. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Orkand R. K., Schachner M. Coupling among identified cells in mammalian nervous system cultures. J Neurosci. 1983 Mar;3(3):506–516. doi: 10.1523/JNEUROSCI.03-03-00506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Ransom B. R. Electrical coupling between astrocytes and between oligodendrocytes studied in mammalian cell cultures. Glia. 1988;1(1):64–73. doi: 10.1002/glia.440010108. [DOI] [PubMed] [Google Scholar]
- Lawrence T. S., Beers W. H., Gilula N. B. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978 Apr 6;272(5653):501–506. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- Lerea L. S., McCarthy K. D. Astroglial cells in vitro are heterogeneous with respect to expression of the alpha 1-adrenergic receptor. Glia. 1989;2(3):135–147. doi: 10.1002/glia.440020302. [DOI] [PubMed] [Google Scholar]
- Lowenstein W. R. Regulation of cell-to-cell communication by phosphorylation. Biochem Soc Symp. 1985;50:43–58. [PubMed] [Google Scholar]
- Massa P. T., Mugnaini E. Cell-cell junctional interactions and characteristic plasma membrane features of cultured rat glial cells. Neuroscience. 1985 Feb;14(2):695–709. doi: 10.1016/0306-4522(85)90320-3. [DOI] [PubMed] [Google Scholar]
- Mobley P., Greengard P. Evidence for widespread effects of noradrenaline on axon terminals in the rat frontal cortex. Proc Natl Acad Sci U S A. 1985 Feb;82(3):945–947. doi: 10.1073/pnas.82.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S., Pearce B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience. 1987 Aug;22(2):381–394. doi: 10.1016/0306-4522(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Physiological modulation of gap junction permeability. J Exp Biol. 1986 Sep;124:993–114. [PubMed] [Google Scholar]
- Pearce B., Cambray-Deakin M., Morrow C., Grimble J., Murphy S. Activation of muscarinic and of alpha 1-adrenergic receptors on astrocytes results in the accumulation of inositol phosphates. J Neurochem. 1985 Nov;45(5):1534–1540. doi: 10.1111/j.1471-4159.1985.tb07224.x. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Shiosaka S., Yamamoto T., Hertzberg E. L., Nagy J. I. Gap junction protein in rat hippocampus: correlative light and electron microscope immunohistochemical localization. J Comp Neurol. 1989 Mar 8;281(2):282–297. doi: 10.1002/cne.902810210. [DOI] [PubMed] [Google Scholar]
- Stone E. A., Ariano M. A. Are glial cells targets of the central noradrenergic system? A review of the evidence. Brain Res Brain Res Rev. 1989 Oct-Dec;14(4):297–309. doi: 10.1016/0165-0173(89)90015-5. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin G. P., Marriott D. R., Cholewinski A. J. Astrocyte heterogeneity. Trends Neurosci. 1990 Feb;13(2):43–46. doi: 10.1016/0166-2236(90)90065-i. [DOI] [PubMed] [Google Scholar]
- Yong V. W., Kim S. U., Kim M. W., Shin D. H. Growth factors for human glial cells in culture. Glia. 1988;1(2):113–123. doi: 10.1002/glia.440010203. [DOI] [PubMed] [Google Scholar]
- el Aoumari A., Fromaget C., Dupont E., Reggio H., Durbec P., Briand J. P., Böller K., Kreitman B., Gros D. Conservation of a cytoplasmic carboxy-terminal domain of connexin 43, a gap junctional protein, in mammal heart and brain. J Membr Biol. 1990 May;115(3):229–240. doi: 10.1007/BF01868638. [DOI] [PubMed] [Google Scholar]
- el-Etr M., Cordier J., Glowinski J., Premont J. A neuroglial cooperativity is required for the potentiation by 2-chloroadenosine of the muscarinic-sensitive phospholipase C in the striatum. J Neurosci. 1989 May;9(5):1473–1480. doi: 10.1523/JNEUROSCI.09-05-01473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Fouly M. H., Trosko J. E., Chang C. C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987 Feb;168(2):422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]