Abstract
The advent of click chemistry has had a profound influence on almost all branches of chemical science. This is particularly true of radiochemistry and the synthesis of agents for positron emission tomography (PET), single photon emission computed tomography (SPECT), and targeted radiotherapy. The selectivity, ease, rapidity, and modularity of click ligations make them nearly ideally suited for the construction of radiotracers, a process that often involves working with biomolecules in aqueous conditions with inexorably decaying radioisotopes. In the following pages, our goal is to provide a broad overview of the first 10 years of research at the intersection of click chemistry and radiochemistry. The discussion will focus on four areas that we believe underscore the critical advantages provided by click chemistry: (i) the use of prosthetic groups for radiolabeling reactions, (ii) the creation of coordination scaffolds for radiometals, (iii) the site-specific radiolabeling of proteins and peptides, and (iv) the development of strategies for in vivo pretargeting. Particular emphasis will be placed on the four most prevalent click reactions—the Cu-catalyzed azide-alkyne cycloaddition (CuAAC), the strainpromoted azide-alkyne cycloaddition (SPAAC), the inverse electron demand Diels-Alder reaction (IEDDA), and the Staudinger ligation—although less well-known click ligations will be discussed as well. Ultimately, it is our hope that this review will not only serve to educate readers but will also act as a springboard, inspiring synthetic chemists and radiochemists alike to harness click chemistry in even more innovative and ambitious ways as we embark upon the second decade of this fruitful collaboration.
Graphical Abstract
INTRODUCTION
A decade and a half have passed since Kolb, Finn, and Sharpless published the landmark review that introduced the concept of click chemistry.1 In the intervening years, the influence of click chemistry has grown by leaps and bounds. To wit, the number of publications with “click chemistry” in the title has grown from 6 in 2003 to 252 in 2009 to 2014 in 2015!2
In the words of the original authors, the criteria for a click chemistry ligation are as demanding as they are straightforward: 1
“The reaction must be modular, wide in scope, give very high yields, generate only inoffensive byproducts that can be removed by non-chromatographic methods, and be stereospecific (but not necessarily enantioselective). The required process characteristics include simple reaction conditions (ideally, the process should be insensitive to oxygen and water), readily availably starting materials and reagents, the use of no solvent or a solvent that is benign (such as water) or easily removed, and simple product isolation.”
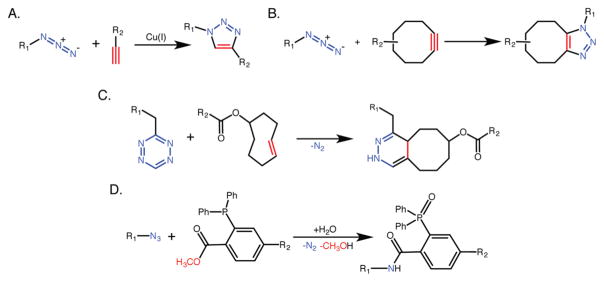
A handful of reactions that satisfy (or, at the very least, come close to satisfying) these criteria have been uncovered, including nucleophilic ring opening reactions with epoxides, aziridines, and aziridinium ions; the formation of ureas, oximes, and hydrazones via nonaldol carbonyl chemistry; and oxidative and Michael additions to carbon–carbon double bonds.3 Yet one particularly powerful reaction has emerged as the canonical click ligation and has proven remarkably useful in myriad applications: the copper-catalyzed [3 + 2] cycloaddition between an azide and a terminal alkyne (Figure 1A).4,5 More recently, Bertozzi and others have pioneered a subset of click reactions that boast an additional boundary condition: bioorthogonality.6–9 Bioorthogonal click ligations satisfy all of the requirements of standard click reactions but are also inert within biological systems. Not surprisingly, these reactions are hard to come by, yet a handful (most notably the Staudinger ligation, the strain-promoted azide–alkyne cycloaddition reaction, and the inverse electron demand Diels–Alder cycloaddition) have been developed and proven powerful in the hands of chemical biologists, biochemists, and biomedical scientists (Figure 1B–D).7,10–16
Figure 1.
Schematics of the (A) Cu-catalyzed azide–alkyne cycloaddition reaction, (B) the strain-promoted azide–alkyne cycloaddition, (C) the inverse electron demand Diels–Alder cycloaddition, and (D) the Staudinger ligation.
Click chemistry has had a paradigm-shifting influence on a wide range of chemical fields, from drug development17,18 and polymer chemistry19,20 to chemical biology21 and nanoscience.22 However, it is hard to imagine a field that has more to gain from harnessing click chemistry than radiochemistry. The principal reason for this lies in what makes radiochemistry unique: the inexorable physical decay of radioisotopes during synthesis. As a result, radiolabeling reactions—and especially radiolabeling reactions using short-lived isotopes such as 11C (t1/2 ≈ 20 min) and 68Ga (t1/2≈68 min)—must be rapid and efficient tomaximize yield as well as selective and clean to eliminate time-sapping purification steps. Furthermore, the widespread use of biomolecules as targeting vectors has also placed a premium on bioconjugation reactions that are both selective and unencumbered by water. Finally, the proliferation of an ever-growing list of prosthetic groups and radiometal chelators has made modularity a critical feature of radiosynthetic protocols as well. Remarkably, all of these traits can be found in click chemistry ligations.
In light of these benefits, it is somewhat surprising that the first publications describing radiopharmaceuticals synthesized using click chemistry came rather late: a 2006 work from Mindt et al. describing the use of click chemistry to create coordination scaffolds for 99mTc and a 2007 report from Wuest and co-workers detailing the use of the CuAAC reaction to create an 18F-labeled variant of neurotensin(8–13).23 Yet in the years since this somewhat belated start, work at the nexus of these two fields has expanded dramatically.24–27 This growth means that an exhaustive review covering every instance in which click chemistry has been applied to nuclear imaging would almost certainly be an exhausting read. Instead, in the pages that follow, it is our goal to highlight the most interesting, exciting, and useful points of intersection between click chemistry and nuclear medicine. More specifically, we will focus on the use of click chemistry for (i) radiolabeling reactions with prosthetic groups, (ii) the creation of novel chelation architectures, (iii) site-specific bioconjugation, and (iv) in vivo pretargeting. Taken together, we believe that these four areas underscore how the rapidity, efficiency, selectivity, modularity, and bioorthogonality of click chemistry have empowered radiochemists to create innovative agents for imaging and therapy. Ultimately, we sincerely hope that this review not only informs the reader about research at the intersection of chemistry and radiochemistry but also inspires new and seasoned researchers alike to apply this remarkably useful chemical technique to the development radiopharmaceuticals.
RADIOLABELING WITH PROSTHETIC GROUPS
One of the first reported, and still most extensively employed, applications of click chemistry to radiochemistry lies in the use of “clickable” prosthetic groups for radiolabeling. The everincreasing use of imaging agents based on biomolecular vectors has put a premium on radiosynthesis strategies that are both mild and selective. Put simply, peptides, proteins, and antibodies should be radiolabeled under aqueous conditions at room temperature to ensure that their structural integrity is preserved, yet critically, many radiolabeling reactions require elevated temperatures, nonaqueous solvents, or (at the very least) pH conditions outside of the physiological norm. This is especially true for 18F-radiofluorination reactions, which often require organic solvents and high temperatures.
Radiolabeled prosthetic groups provide an efficient way to circumvent these issues. Prosthetic groups are radiolabeled reactive small molecules that can be appended to biomolecules under benign conditions. Until recently, the vast majority of prosthetic groups have relied upon reactions with natural amino acids (most notably, couplings between N-hydroxysuccinimidyl (NHS) esters and lysines and Michael additions between maleimides and cysteines).28–30 Yet prosthetic groups of this ilk present a number of problems. Most concerning is the complete loss of regiochemical control during the labeling of a peptide or protein containing more than one lysine or cysteine. This, of course, can only be remedied by yield-sapping separations or the addition of time-consuming protection and deprotection steps.31 On top of this, both NHS esters and their isothiocyanate cousins are unstable under aqueous conditions, and maleimide–thiol linkages are prone to reversible substitution reactions in vivo.32
In response to these limitations, radiochemists have increasingly turned to “clickable” prosthetic groups. Not surprisingly, the canonical CuAAC ligation leads the pack. In this regard, the relative age of the reaction certainly plays a role. Yet another critical advantage of the CuAAC ligation is that its “footprint” — a 1,2,3-triazole ring — is unlikely to perturb the structure or activity of the vector: the heterocycle is both relatively small and a rigid stereoisomer of an amide linkage. At this junction, we would be remiss if we did not mention the CuAAC reaction’s lesser-known cousin: the ruthenium-catalyzed azide–alkyne cycloaddition (RuAAC).33 The RuAAC reaction produces 1,5-disubstituted 1,2,3-triazoles as opposed to the 1,4-disubstituted 1,2,3-triazoles created by the Cu-catalyzed cycloaddition. Even though it is regarded as a “click reaction”, the RuAAC ligation requires organic solvents, elevated temperatures, and inert gas atmosphere. Furthermore, the 1,5-disubstituted 1,2,3-triazoles produced by the reaction are—unlike 1,4-disubstituted 1,2,3-triazoles—metabolically active and can be degraded via enzymatic N3 oxidation to produce highly reactive and potentially toxic metabolites.34 Given both of these issues, it is not surprising that, to the best of our knowledge, the RuAAC reaction has not been applied to the synthesis of radiopharmaceuticals.
Moving back to the topic at hand, an extensive body of work has emerged on the design, synthesis, and optimization of radiolabeled CuAAC-ready building blocks. Much, although not all, of this work has focused on 18F.35–38 Indeed, a variety of radiosynthetic methods have been employed to create azide- and alkyne-bearing 18F-labeled prosthetic groups (Figure 2A).37,39,40 These tools and the CuAAC reaction have been harnessed with great success in the radiolabeling of a wide variety of vectors, including phosphonium ions,41 peptides,42–50 oligonucleotides,39,47 and proteins.27,47 This application of the CuAAC reaction is not without its flaws, however. These stem primarily from the two reagents needed to facilitate the cycloaddition: Cu(I/II) cations and a sacrificial reductant. The latter, most often ascorbic acid, can inadvertently reduce particularly fragile peptides and proteins.27 The Cu cations can be even more of a problem. Peptides and proteins (specifically serine, histidine, and arginine residues) can coordinate Cu2+ ions, resulting in structural and functional alterations to the peptide.51 For example, Pretze et al. observed the accidental formation of Cu–peptide complexes following the CuAAC-mediated ligation of an 18F-labeled, alkyne-containing prosthetic group to an azide-bearing SNEW peptide.45 The coordination of the oxidative Cu(I) species can also lead to dramatic alterations to the chelating amino acid residues, as demonstrated very recently.52 These issues are compounded even further for radiometal-containing constructs. In these cases, not only can the chelator capture the copper catalyst and prevent the reaction from happening, but residual Cu2+ ions can also outcompete the far less abundant radiometal cations for coordination by the chelator.53 On top of these coordination-related concerns, the presence of Cu+ can also increase the likelihood of undesired side reactions such as Glaser couplings or the formation of copper-acetylides.45,54,55 Some of these issues can be ameliorated through the use of Cu+-stabilizing chelators such as THPTA or N-heterocyclic carbene complexes of Cu+; however, these reagents can create their own set of complications.56–58
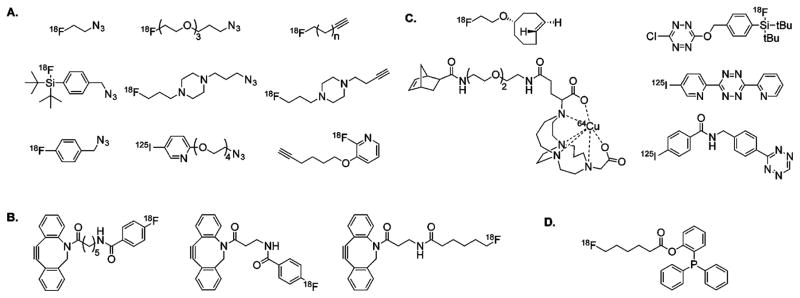
Figure 2.
An assortment of radiolabeled prosthetic groups used for the synthesis of radiopharmaceuticals via the (A) copper-catalyzed azide–alkyne cycloaddition, (B) strain-promoted azide–alkyne cycloaddition, (C) inverse electron-demand Diels–Alder cycloaddition, and (D) traceless Staudinger ligation.
In light of the limitations of the CuAAC ligation, researchers have turned to a handful of “second generation” click reactions that are both bioorthogonal and catalyst-free. The most obvious place to start is the strain-promoted azide–alkyne cycloaddition (SPAAC). The SPAAC reaction is an azide–alkyne cycloaddition in which ring strain built into a cyclic alkyne—often a dibenzocyclooctyne (DBCO)—drives the reaction and eliminates the need for a catalyst.59,60 Campbell-Verduyn et al. were among the first to use this approach for radiochemistry, creating a series of 18F-labeled bombesin derivatives via the reaction of a DBCO-modified peptide with an array of 18F-bearing, azide-containing prosthetic groups.61 Following a similar strategy, another laboratory modified a series of ανβ3-targeting RGD peptides with DBCO and radiolabeled them using an [18F]fluoro–PEG4–azide prosthetic group.50,62 In a creative twist, the authors scavenged excess unlabeled peptide using an azide-grafted resin, allowing them to achieve specific activities of up to 62.5GBq/μmol. Critically, all of the 18F-labeled peptides bore biological affinity comparable to their unlabeled cousins and were shown to be effective for the visualization ανβ3-expressing U87MG xenografts (Figure 3). Of course, radiolabeling via the SPAAC reaction goes both ways: several laboratories have created 18F-labeled cyclooctynes for the radiofluorination of azide-modified small molecules, sugars, and peptides (Figure 2B).63–65
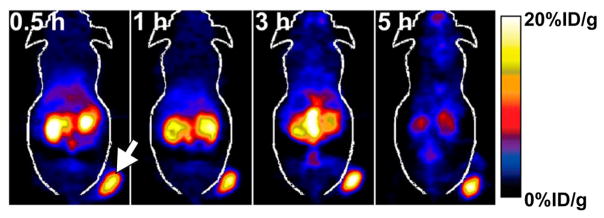
Figure 3.
Coronal PET images of a NOD/SCID mouse bearing a GLP-1R-positive insulinoma xenograft (white arrow) collected 0.5, 1, 3, and 5 h after the injection of an 18F-labeled Exendin-4 radiotracer synthesized using a “clickable” prosthetic group. Adapted and reprinted with permission from Wu et al., copyright 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
The SPAAC reaction has also been used for radioiodinations and radiometalations. Choi et al., for example, used a DBCO-bearing cRGD peptide and a prosthetic group composed of a PEG4–azide moiety grafted to an 125I-labeled pyridine to create an 125I-labeled cRGD.66 Evans et al. labeled an azide-modified DOTA with 68Ga for the radiometalation of several DBCO-modified peptides.53 Likewise, the Anderson group has conjugated DIBO-bearing copper chelators to an azide-modified cetuximab antibody and an azide-bearing somatostatin analogue.67,68
Despite its utility, the SPAAC ligation has one critical limitation: its dibenzocyclooctatriazole “footprint”. The work of Hausner and co-workers provides a particularly useful cautionary example.69 Here, the authors radiolabeled an azide-modified A20FMDV2-peptide using an 18F-labeled variant of DBCO. While in vitro experiments confirmed that the 18F-labeled peptide retained its affinity and specificity for ανβ6-expressing cells, in vivo imaging suggested that the bulky and hydrophobic benzocyclooctatriazole footprint introduced by the SPAAC ligation led to dramatic changes in the pharmacokinetics of the tracer and significantly impaired its uptake in ανβ6-expressing xenografts.
The inverse electron demand Diels–Alder (IEDDA) cycloaddition between tetrazine (Tz) and a dienophile, most commonly trans-cyclooctene (TCO) but also norbornene (NB), has also provided fertile ground for the development of prosthetic groups. Like the SPAAC ligation, the IEDDA reaction is bioorthogonal and proceeds without a catalyst. The principal advantage of the IEDDA ligation is its extraordinary speed (vide infra), which makes it particularly well suited for applications with short-lived radioisotopes. In 2010, the laboratories of Fox and Conti reported the first 18F-labeled TCO (Figure 2C).70 This prosthetic group was used for the rapid (t < 5 min) radiolabeling of a range of tetrazine-bearing peptides, including RGD and the GLP agonist Exendin.71–73 The 18F-labeled Exendin proved particularly promising, enabling the PET imaging of GLP-1R-positive insulinoma xenografts in mice. The same 18F–TCO was also used to great effect by Weissleder and co-workers for labeling a Tz-bearing analog of the PARP1 inhibitor AZD2281. In this work, however, the authors added a creative wrinkle: removing unlabeled AZD2281–Tz using a TCO-coated magnetic resin.74,75 Finally, a number of 18F-labeled tetrazines have also been synthesized, but the in vivo use of radiopharmaceuticals created using these moieties has thus far remained somewhat sparing.76,77
The utility of the IEDDA reaction extends beyond radiofluorination. 53 To wit, a handful of radioiodinated tetrazine constructs have been successfully developed (Figure 2C). Albu et al., for example, synthesized an 125I-labeled tetrazine and conjugated this building block to a TCO-modified anti-VEGFR2 antibody.78 Interestingly, in vivo studies using this tracer revealed an additional benefit of this approach: the 125I-labeled antibody proved to be more than 10-fold more stable to deiodination over 48 h compared to traditionally radioiodinated analogs. More recently, Choi et al. used a similar strategy for the radiolabeleling of both a cRGD peptide and human serum albumin (HSA).79 The 125I-labeled HSA displayed impressive in vivo behavior, with a deiodination rate reduced by 50-fold compared to constructs created via traditional radioiodination. In 2011, Zeglis et al. employed the IEDDA reaction to create a modular strategy for the bioconjugation of a trastuzumab–TCO immunoconjugate with Tz–desferrioxamine (for 89Zr4+) and Tz–DOTA (for 64Cu2+).80 More recently, Kumar and co-workers harnessed the IEDDA reaction to circumvent the incompatibility of antibodies with the high temperatures required to radiolabel the CB-TE2A-1C chelator with 64Cu.81 To this end, the authors modified the chelator with a norbornene moiety and grafted tetrazine onto an anti-PSMA antibody (YPSMA). After radiolabeling of the chelator-NB building block with 64Cu at 85 °C, the 64Cu–CBTE2A1C-NB synthon was attached to YPSMA–Tz under mild conditions, and the 64Cu-labeled radioimmunoconjugate was successfully deployed for the PET imaging of PSMA-expressing tumors in a murine model of prostate cancer.
Although the rapidity of the IEDDA reaction provides a marked improvement over the sluggish SPAAC ligation, it fails to solve one of the latter’s major issues: a bulky, hydrophobic footprint. As we have discussed, the SPAAC reaction leaves a benzocyclooctatriazole moiety in its wake. The IEDDA ligation creates an equally large footprint: a bicyclic [6.4.0] ring system. Both structures have the potential to interfere with the biological activity and pharmacokinetics of vectors, particularly small molecules and short peptides. The traceless version of the Staudinger ligation offers an exciting alternative (Figure 4A). This ligation relies on an initial reaction between a phosphine-based moiety and an azide followed by a rearrangement that produces a simple amide linkage. Along these lines, the radiolabeling of peptides with 18F has been achieved via the reaction between (diphenylphosphanyl)methanethiol thioester-bearing peptides and an 18F-labeled azide as well as that between a radiolabeled 2-(diphenylphosphanyl)phenol ester with an azide-bearing peptide (Figure 2D).82–84 Unfortunately, however, the traceless Staudinger ligation requires high temperatures (90–130 °C) to achieve speeds that are compatible with short-lived isotopes. This undoubtedly limits its utility with fragile small molecules, peptides, and proteins; however, we are optimistic about the potential applications of this elegant transformation with longer-lived isotopes.
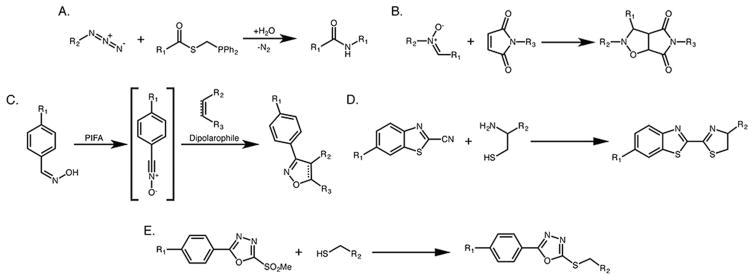
Figure 4.
Schematics of an assortment of click chemistry ligations (beyond those depicted in Figure 1) used for prosthetic group radiolabelings: (A) traceless Staudinger ligation, (B) nitrone–alkene cycloaddition, (C) nitrile–oxide cycloaddition, (D) 1,2-aminothiol–cyanobenzothiazole condensation, and (E) phenyloxadiazole methylsulfone–thiol conjugation.
Finally, a handful of other, less-well-known click ligations have made sparing yet interesting appearances in the literature of prosthetic groups. In 2012, Zlatopolskiy et al. reported the formation of a reactive nitrone from 18F-fluorobenzaldehyde and phenylhydroxylamine.85 The authors showed that this 18F-labeled nitrone could undergo a [3 + 2] cycloaddition with a maleimide, resulting in quantitative conversion in less than 15 min at 80 °C (Figure 4B). It must be said, however, these reaction conditions leave much to be desired when it comes to labeling biomolecules. Later the same year, the same group probed the potential of cycloaddition reactions between nitriloxides and dipolarophiles (Figure 4C).86 An 18F-labeled nitriloxide was synthesized from 18F-p-fluorobenzaldehyde and reacted with a series of dipolarophiles, producing quantitative conversions in <10 min at 40 °C. However, these reactions were performed in alcohol, and no data was presented regarding the feasibility of this transformation under aqueous conditions. Recently, other groups have harnessed the reactivity of 2-cyanobenzothiazoles toward 1,2-aminothiols to radiolabel peptides and proteins containing N-terminal cysteines (Figure 4D).87,88 To this end, 18F-labeled 2-cyanobenzothiazoles were synthesized and appended to RGD and diRGD peptides bearing N-terminal cysteines as well as a genetically engineered variant of luciferase with a cysteine at the N-terminus. Lastly, just this year, Chiotellis et al. have explored phenyloxadiazole methylsulfones (PODS) as more stable alternatives to maleimides for conjugations with thiols (Figure 4E).89 In this work, an 18F-labeled PODS was used to radiolabel both a cysteine-bearing peptide and a cysteine-modified affibody, and the resulting constructs were used to HER2-positive tumors in a mouse model of breast cancer.
CREATING COORDINATION SCAFFOLDS
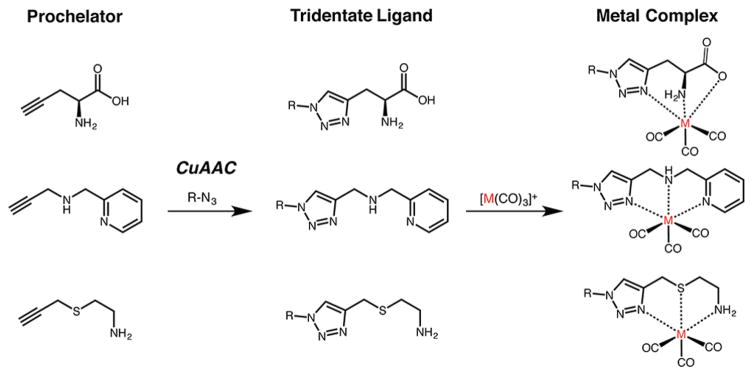
The use of click chemistry to create radiometal chelation architectures provides one of the best examples of the unique modularity conferred by this synthetic approach.90,91 Easily the best known of these methods, dubbed “click-to-chelate” by its inventors, was introduced in 2006 by Mindt et al. (Figure 5).92–94 This strategy employs the CuI-catalyzed azide–alkyne cycloaddition (CuAAC) reaction to attach small molecule “pro-chelators” to peptides and small molecules. However, the 1,2,3-triazole produced by the click ligation becomes far more than just a simple link between the subunits of the construct. Indeed, the heterocycle forms an integral part of a tripodal coordination scaffold capable of the rapid chelation of [M(CO)3]+ synthons, in which M can be the γ-emitting radiometal 99mTc (t1/2 = 6.01 h) or the β-emitting radiometal 188Re (t1/2 = 16.98 h). In this way, “click-to-chelate” facilitates the creation of a chelator and its subsequent radiometalation in a rapid, robust, and reproducible one-pot reaction. This is particularly important given the mercurial coordination chemistry of 99mTc.
Figure 5.
“Click-to-chelate” approach: a variety of prochelators exhibiting electron-donating groups undergo the CuI-catalyzed azide–alkyne cycloaddition with an azide to from a tridentate ligand that can coordinate an organometallic [M(CO)3]+ synthon.
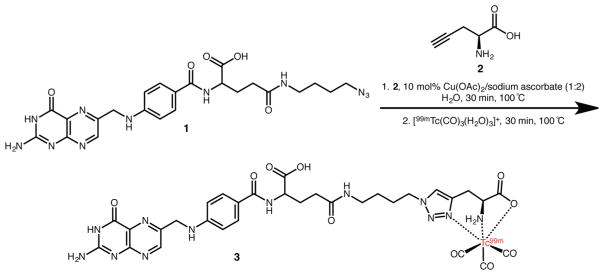
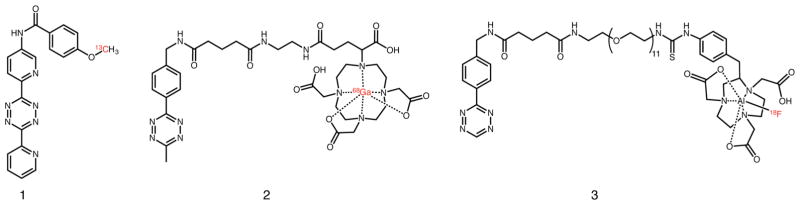
In their initial proof-of-concept report, the authors created seven different tripodal scaffolds—including N3, N2S, and N2O ligand architectures—using a series of azide-modified small molecules. Subsequent labeling with M(CO3) [M = Re, 99mTc] synthons resulted in a series of highly stable, low-spin d6-complexes despite differences in the size, molecular charge, and hydrophilicity of the prochelator.92–95 The creation of a 99mTc-labeled variant of folate using “click-to-chelate” provides an excellent example of the approach (Figure 6). The 1,2,3-triazole ring formed in the first phase of the reaction between the azide-bearing folate construct (1) and the alkyne-modified amino acid (2) not only connects the pro-chelator to the folate vector but also serves as an essential part of the N2O coordination scaffold for the [99mTc(CO)3]+ moiety. The incubation of the chelator-bearing construct with [99mTc(CO)3(H2O)3]+ reproducibly yields 99mTc-labeled folate (3) in high yield and specific activity.92
Figure 6.
Advantages of the one-pot “click-to-chelate” approach are particularly apparent in the context of synthetically challenging probes such as this 99mTc-labeled folate radiopharmaceutical (3).
In subsequent work, this technique was applied to peptides as well as an array of other biologically active small molecules such as sugars, nucleosides, and steroids.96–100 Fernandez et al., for example, developed a 99mTc-labeled glucose derivative as an imaging probe for glucose metabolism.97 Similarly, Struthers et al. developed an elegant one-pot “click-to-chelate” synthesis of a 99mTc-labeled thymidine analogue as a SPECT surrogate for the clinically successful proliferation marker 18F–FLT.98 Taken together, this work clearly demonstrates that 99mTc-labeled tracers created using the “click-to-chelate” methodology demonstrate in vivo behavior that is comparable, and in some cases superior, to the current “gold standard” chelators for [99mTc-(CO)3]+: Nτ-derivatized histidine and Nα-acetylated histidine. Indeed, studies using 99mTc-labeled folate revealed that the click-to-chelate approach furnished compounds in purities and radiochemical yields equal to those achieved using traditional radiolabeling techniques. Furthermore, in this work, the click-to-chelate approach did not alter biodistribution patterns or pharmacodynamic parameters such as receptor affinities and selectivities. Finally, the superiority of the click-to-chelate methodology becomes most obvious in the context of synthetically challenging molecules. In the case of the azide-modified folate construct, for example, the differences in synthetic effort and yield are striking: “click-to-chelate” furnished an 99mTc-labeled tracer in 80% overall yield in 8 steps, whereas 10 steps were required to muster approximately 1% yield with a histidine-based chelator.92
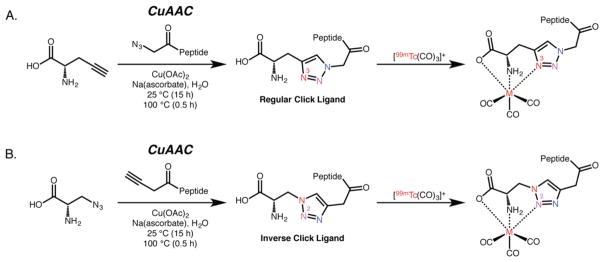
From a chemical standpoint, it is important to note that the inherent asymmetry of the CuAAC reaction means that two different 1,2,3-triazoles can be formed when linking the vector and the chelator (Figure 7).93,95 In the first, the “regular click ligand”, the pro-chelator bears the alkyne moiety while the vector contains the azide group, and the N3 atom of the triazole participates in the coordination of 99mTc. In the second, the “inverse click ligand”, the pro-chelator boasts the azide moiety while the vector wields the alkyne group, and the N2 atom of the triazole participates in the coordination of 99mTc. Somewhat surprisingly, the two different chelation environments display quite different behavior when radiolabeled with [99mTc(CO)3]+ and [188Re(CO)3]+, with the “inverse click ligand” offering significantly lower labeling efficiency and decreased in vivo stability.93 Although a concrete explanation for this phenomenon remains elusive, the most likely hypothesis points to the decreased electron density in the N2 position compared to the N3 site.
Figure 7.
The asymmetry of the CuAAC reaction creates two different coordination scaffolds depending on whether the prochelator contains the alkyne or azide functionality. The “regular click ligand” (A) is a more effective chelator for [99mTc(CO)3]+ and [188Re(CO)3]+ than the “inverse click ligand” (B).
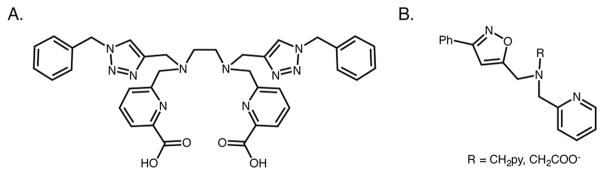
Before moving on, it is worth noting that a handful of other groups have also used click chemistry in the synthesis of radiometal chelators. Bailey et al., for example, used the CuAAC reaction in the synthesis H4azapa: a carboxypyridine-based chelator for 111In3+ and 177Lu3+ (Figure 8A).91 In addition, Bottorff et al. have developed a synthetic strategy to generate isoxazole ligands via click chemistry (Figure 8B).101 Yet in the end, it is undeniable that the “click-to-chelate” methodology represents the gold standard in this area. Indeed, this approach not only provides a cardinal example of the modularity and flexibility provided by click chemistry but also stands as one of the most useful and innovative developments in 99mTc chemistry of the past decade.2–4
Figure 8.
Structures of the acyclic H2azapa (A) and isoxazole (B) chelators for diagnostic and therapeutic radiometals such as 67Ga, 64Cu, 111In (A), and 99mTc (B), respectively. The isoxazole ligand (B) was synthesized via click chemistry using the Cu-free 1,3-dipolar cycloaddition between an alkyne and an oxime.
SITE-SPECIFIC BIOCONJUGATION
The selectivity and bioorthogonality of click chemistry have also been leveraged for the site-specific modification of proteins and antibodies. This process has become ubiquitous in the synthesis of biomolecular therapeutics such as antibody-drug conjugates, and it is increasingly important in the creation of radiolabeled probes as well. Until recently, the overwhelming majority of bioconjugation methods were predicated on ligations between reactive bifunctional probes—e.g., N-hydroxysuccinimide-bearing chelators or maleimide-modified toxins—and amino acids within the biomolecule, most often lysines and cysteines. While these methods are undeniably simple, they are far from precise. Control over the location and frequency of these ligations is impossible because proteins have multiple copies of these amino acids distributed throughout their structures. As a result, these bioconjugation strategies produce constructs that are both heterogeneous and poorly defined. Furthermore, random conjugation strategies can decrease the reactivity of constructs if the cargo is inadvertently appended to the target-binding domains of the biomolecule.
In response to these issues, significant effort has been dedicated to the creation of strategies for the site-specific bioconjugation of proteins and antibodies. A wide variety of methods have been developed, including variants predicated on the selective reduction of disulfide bridges and the oxidative manipulation of the heavy chain glycans. Yet regardless of the exact strategy, a wealth of preclinical data makes the bottom line clear: site-specifically labeled proteins and antibodies are more homogeneous, better defined, and exhibit superior in vivo behavior compared to constructs synthesized using traditional, random bioconjugation techniques.102–105 A handful of the most promising site-specific bioconjugation strategies combine the selectivity of enzymatic reactions with the modularity of chemical ligations. Generally speaking, these chemoenzymatic strategies have two steps. In the first, an enzyme is used to site-specifically incorporate a substrate bearing a reactive handle into the biomolecule. Then, in the second, a cargo bearing a complementary reactive handle is appended to its partner in the biomolecule. In this context, the selectivity and bioorthogonality of click chemistry are particularly valuable, as the two handles must only react with each other and not the enzyme or biomolecule.
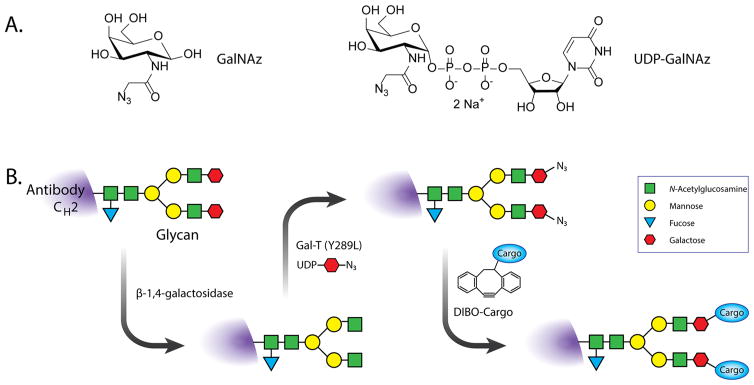
A recently developed strategy for the site-specific modification of the heavy chain glycans (the biantennary sugar chains attached to the CH2 domains of the FC region of antibodies) provides an excellent example of a chemoenzymatic approach that employs the SPAAC ligation. Inspired by the work of Hsieh-Wilson and Qasba, this methodology employs two enzymes and has three steps: (i) the removal of the terminal sugars of the heavy chain glycans using β-(1,4)-galactosidase; (ii) the incorporation of azide-modified galactose residues (GalNAz) into the sugar chains with a promiscuous galactosyltransferase [GalT-(Y289L)]; and (iii) the attachment of dibenzocyclooctyne (DIBO)-bearing cargoes to the azide-presenting sugars (Figure 9).106–108 Ultimately, this approach has the potential to yield highly homogeneous and well-defined immunoconjugates carrying up to four cargo molecules per antibody. In their initial report, Zeglis et al. used a DIBO-modified desferrioxamine (DFO) to create a site-specifically modified, 89Zr-labeled radioimmunoconjugate based on the PSMA-targeting antibody J591. In subsequent work, the authors demonstrated the modularity and flexibility of this approach through the development of a series of immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of colorectal and pancreatic cancer. Importantly, in all three cases, the in vivo performance of the site-specifically modified imaging agents was equivalent, and in some respects superior, to that of analogous constructs synthesized using traditional techniques.109,110 More recently, Geel et al. reported an interesting variation on this theme.111 In this work, EndoS, an enzyme that trims each glycans chain down to its innermost residues, is used instead of β-(1,4)-galactosidase. This change ultimately produces an immunoconjugate with two azides per antibody after treatment with GalT(Y289L) and GalNAz. To date, this strategy has only been used in conjunction with DIBOmodified chemotherapeutics, but the modularity of the SPAAC ligation could easily facilitate the adaptation of this approach to the synthesis of radiopharmaceuticals.
Figure 9.
(A) The structures of N-azidoacetylgalactosamine (GalNAz; left) and the enzyme substrate UDP–GalNAz (right); (B) schematic of the methodology for the chemoenzymatic incorporation of azide moieties in the heavy-chain glycans as well as the subsequent SPAAC-mediated grafting of cargo molecules.
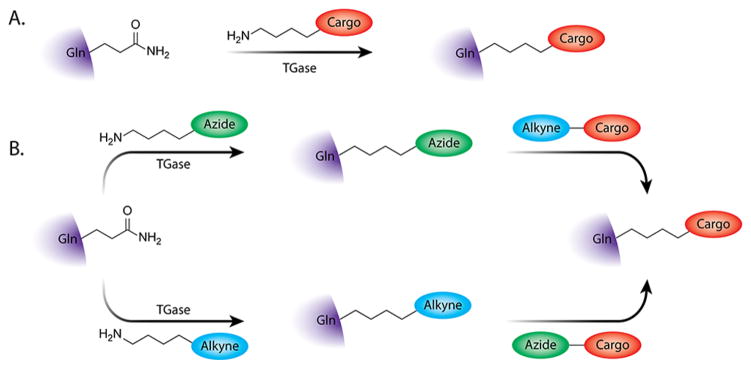
Shifting gears to another family of enzymes, transglutaminases catalyze the formation of isopeptide bonds between the acyl functionality of glutamine residues and primary amines. While antibodies certainly contain multiple glutamine residues, transglutaminases have been found to react exclusively with the Q295 glutamines within the CH2 domain of deglycosylated or aglycosylated IgGs. This unique reactivity has led a number of laboratories (most notably, that of Roger Schibli at ETH Zurich) to harness these enzymes for the site-specific modification of antibodies. One-step and two-step approaches have been developed (Figure 10). In the former, transglutaminase is used to directly append cadaverine-modified cargoes to the antibody. This method was used by Jeger et al. to site-specifically append two DFO chelators to a deglycosylated variant of the L1CAM-targeting antibody chCE7 for radiometalation with 64Cu, 67Ga and 89Zr.112 In an interesting twist, this group used transglutaminase to modify amutant version of the chCE7 antibody that contained two additional glutamines in place of the N297 residues, thereby creating an immunoconjugate with four DFO/mAb. More germane to the topic at hand, transglutaminase has also been used to modify proteins with azide- and cyclooctyne-modified cadeverines that can then be reacted via the SPAAC ligation with DIBO- or azide-bearing cargoes, respectively.113,114 In a very recent proof-of-concept study, Puthenveetil et al. have used this strategy to label a model antibody with both Cy5.5 and BODIPY fluorophores. 115 While this approach has not yet been applied to radioimmunoconjugates, it could easily be adapted to create a modular route for the conjugation of radiometal chelators.
Figure 10.
Schematic of the one-step (A) and SPAAC-mediated two-step (B) approaches to the bioconjugation of antibodies using transglutaminase (TGase).
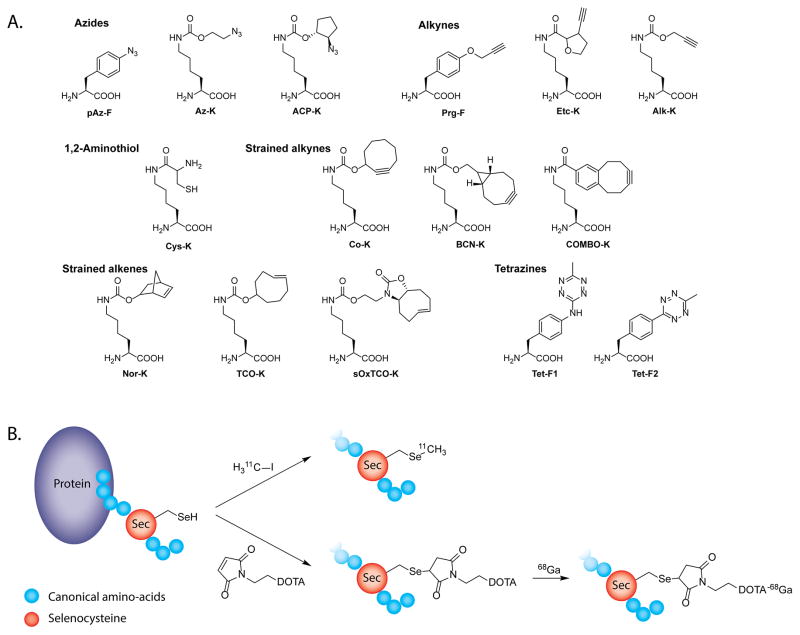
The last bioconjugation technique that we will discuss relies not on post-translational modifications but, rather, on harnessing the cell’s translational machinery itself. The expansion of the genetic code to enable the incorporation of unnatural and noncanonical amino acids (uAAs and ncAAs, respectively) into proteins has quickly become a vital component of the molecular biologist’s toolkit. The union of this technology and click chemistry has proven particularly powerful. p-Azido-L-phenylalanine (pAzF) is one of the most commonly used uAAs, and residues bearing trans-cyclooctene, tetrazine, cyclooctyne, and norbornene groups have been incorporated into proteins as well (Figure 11A).116–119 pAzF and the SPAAC ligation have been used to create both antibody-drug conjugates and immunoglobulins modified with fluorophores. Yet until very recently, only one instance of the use of this technology to create a radiolabeled compound had been reported. In this work, Wållberg et al. developed affibodies containing selenocysteine, a natural ncAA, and exploited the unique reactivity of this residue with maleimides and iodomethane to site-specifically radiolabel the vectors with both 68Ga and 11C (Figure 11B).120 Finally, just prior to the submission of this review, Wu et al. reported the first example of a radioimmunoconjugate created using an uAA. In this case, the authors incorporated an azide-bearing lysine residue (Az–K) into the heavy chain of the anti-CD20 antibody Rituximab and subsequently used the SPAAC ligation to attach a DIBO-bearing DOTA to the azide-containing immunoconjugate. 121 The site-specifically modified antibody showed in vitro and in vivo behavior comparable with an analogous construct synthesized using traditional methods. Ultimately, we are confident that more laboratories will use genetic engineering and click chemistry for the synthesis of radiopharmaceuticals as the technology underlying the former becomes more widely accessible and less technically demanding.
Figure 11.
(A) Examples of unnatural amino acids bearing bioorthogonally reactive functional groups; (B) schematic of the site-specific incorporation of the noncanonical amino acid selenocysteine into proteins and its subsequent radiolabeling with 11C and 68Ga.
IN VIVO PRETARGETING
The next area of discussion, in vivo pretargeting, places the bioorthogonality and speed of click chemistry on center stage. To provide some background, in vivo pretargeting strategies have been developed in direct response to a core limitation of radiolabeled antibodies. Immunoglobulins are extraordinarily promising vectors for nuclear medicine due to their exquisite affinity and selectivity for their molecular targets. However, because antibodies have multiday biological half-lives, they must necessarily be labeled with isotopes with multiday physical half-lives such as 124I (t1/2 ≈ 4.2 days) or 89Zr (t1/2 ≈ 3.2 days) to create effective radioimmunoconjugates.122 Unfortunately, however, this combination of lengthy circulation times and slow radioactive decay can create prohibitively high radiation dose rates to healthy organs.
Pretargeting methodologies seek to circumvent this problem by decoupling the antibody from the radioisotope and injecting the two components separately, in essence synthesizing the radioimmunoconjugate at the target tissue itself. A pair of components form the core of any pretargeting strategy: a small molecule radiolabeled hapten and an antibody capable of binding both an antigen and said hapten. The antibody is injected first and is given a number of days to accumulate at the target site and clear from the blood. After this interval, the radiolabeled hapten is administered. Because it is a small molecule, the hapten travels through the bloodstream quickly, either combining with its immunoconjugate partner or clearing from the body. This approach offers two distinct advantages over traditional immunoconjugates.123,124 First, the rapid clearance of any unreacted radioligand limits the activity concentrations in and radiation dose to healthy organs. Second, and more importantly, this strategy facilitates the use of short-lived radioisotopes—e.g., 64Cu (t1/2 = 12.7 h), 18F (t1/2 = 109 min), and 68Ga (t1/2 = 68 min)—that would normally be incompatible with antibody-based vectors. The latter trait not only produces a dosimetric benefit but also has the potential to accelerate imaging workflows. A variety of approaches to in vivo pretargeting have been attempted, including the use of streptavidin-modified antibodies and biotin-based radioligands,125–128 genetically engineered bispecific antibodies capable of binding radiometal chelate complexes,124,129–131 and antibodies and radioligands conjugated to complementary oligonucleotide strands.132–134 While all of these strategies have proven promising in the preclinical arena, their ultimate clinical implementation has been derailed somewhat by intrinsic issues such as the immunogenicity of streptavidin-modified bioconjugates.
In the end, it is not surprising that bioorthogonal click chemistry has attracted attention as a tool for in vivo pretargeting. Indeed, the selectivity, speed, and, above all, bioorthogonality of these reactions make them seem almost perfectly suited to the task. Attempts at in vivo pretargeting have been made using a variety of bioorthogonal click ligations. In 2011, for instance, Vugts et al. described the development of a pretargeting approach based on the Staudinger ligation between an azide-bearing antibody and phosphine-containing small molecule probes labeled with 68Ga, 89Zr, 177Lu, and 123I.135 In this work, the authors reported that the Staudinger ligation product could not be observed in vivo, leading to the conclusion that in vivo pretargeting with the Staudinger ligation is not possible due to the reaction’s sluggish kinetics, the inherent instability of the phosphine radioligands, or a combination thereof. The SPAAC reaction also has a history of in vivo use dating back to the groundbreaking work in zebrafish performed by Carolyn Bertozzi’s laboratory.136,137 Van den Bosch et al. investigated the feasibility of the SPAAC reaction for in vivo pretargeting using an 125I- and azide-bearing Rituximab immunoconjugate (125I–Rtx–N3) and 177Lu-labeled cyclooctyne radioligands.138 Unfortunately, however, dual-isotope biodistribution experiments revealed disappointing activity concentrations of 177Lu in the target tissue, suggesting that the somewhat slow reaction kinetics of the SPAAC ligation limit its application in vivo. Intriguingly, however, this position has been countered by recent work on the use of nanoparticles for SPAAC-mediated in vivo pretargeting (vide infra).139
Over the past 5 years, one of the newest additions of the click chemistry toolbox, the inverse electron demand Diels–Alder (IEDDA) reaction, has proven particularly well suited for pretargeting. 10,24,140,141 Like the Staudinger and SPAAC ligations, the IEDDA reaction is catalyst-free, clean, selective, and bioorthogonal. From a pretargeting perspective, the true advantage of the IEDDA cycloaddition is speed. The first-order rate constants for reactions between 1,2,4,5-tetrazines (Tz) and trans-cyclooctenes (TCO) hover in the range of 104–105M−1 s−1. In contrast, the rate constants for the Staudinger and SPAAC ligations are orders of magnitude slower: approximately 0.002 and 0.07M−1 s−1, respectively.142,143 Surely, this added speed could play a pivotal roll in the feasibility of click chemistry in the in vivo environment.
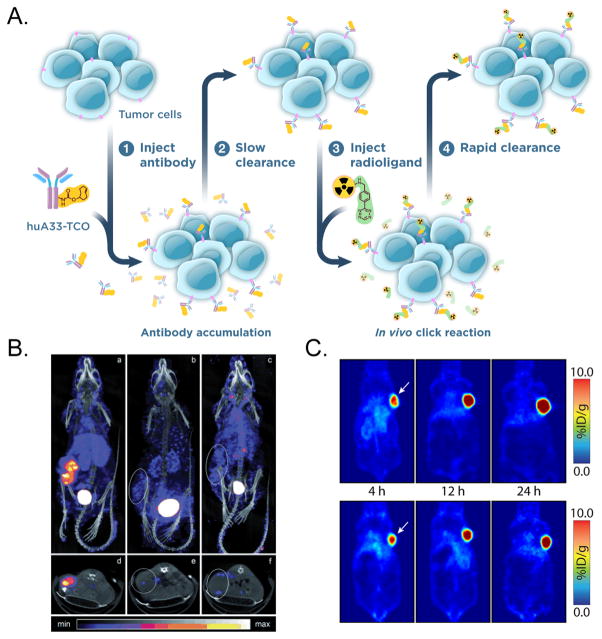
The vast majority of IEDDA-based pretargeting approaches employ a TCO-labeled antibody and a tetrazine-based radioligand (Figure 12A). Rossin et al. were the first to report a pretargeting strategy based on the ligation. The authors successfully employed a TCO-labeled immunoconjugate (CC49-TCO) and an 111In-labeled dipyridyltetrazine radioligand to facilitate the pretargeted SPECT imaging of TAG72-expressing colorectal cancer xenografts (Figure 12B).144 Since this initial report, this group has continued to be a pioneer in the field, producing investigations on alternative trans-cyclooctene moieties,145 tetrazine-bearing clearing agents,146 and pretargeting with antibody fragments and affibodies.147,148 In parallel to the Dutch work, Zeglis et al. have developed and optimized a 64Cu-based pretargeting approach for the PET imaging of colorectal carcinoma that produces images with excellent quality and contrast at only a fraction of the radiation dose to healthy tissue created by traditional radioimmunoconjugates (Figure 12C).108,149,150 Even more recently, Houghton,151 Meyer,152 and colleagues used 5B1-TCO, a CA19.9-targeting immunoconjugate, as well as 64Cu- and 18F-labeled tetrazines to demonstrate the feasibility of the pretargeted imaging of an antigen that is both shed and internalized. Other laboratories have contributed to the advent of IEDDA-based pretargeting as well, developing 11C-, 68Ga-, and 99mTc-labeled tetrazine radioligands153–156 as well as 18F- and tetrazine-labeled nanoparticles (Figure 13).157 Finally, Rossin et al. very recently expanded the scope of this methodology even further by harnessing the newly developed IEDDA pyridazine elimination reaction to trigger the selective in vivo cleavage of tumor-bound antibody-drug conjugates (ADCs).158 This innovative “click-to-release” approach has the potential to add a powerful new tool to the arsenal of ADC therapies.
Figure 12.
(A) Schematic of the in vivo pretargeting approach using the IEDDA reaction. (B) Rossin et al. developed the first pretargeting approach using the in vivo IEDDA reaction to visualize TAG72-expressing LS174T xenografts (left) via SPECT imaging. To this end, a Tz-modified 111In-DOTA radioligand was administered 24 h after the injection of a TCO-bearing immunoconjugate of the CC49 antibody. The use of either unmodified CC49 antibody (middle) or rituximab (no affinity for TAG72, right) instead of CC49-TCO confirmed that the accumulation of 111In in the tumor was a product of in vivo click chemistry. (C) In a study conducted by Zeglis et al., pretargeted PET imaging was performed in mice bearing A33 antigen expressing SW1222 human colorectal carcinoma xenografts. To this end, the mice were first administered a TCO-bearing immunoconjugate of the A33 antigen-targeting antibody huA33 (huA33-TCO) followed, after a 24 h interval, by a 64Cu- and Tz-modified radioligand (64Cu–Tz–SarAr). Panels A and C were reprinted with permission from Zeglis et al., copyright 2015 American Chemical Society. Panel B was reprinted with permission from Rossin et al., copyright 2010 John Wiley and Sons.
Figure 13.
Structures of selected tetrazines radiolabeled with the short-lives radionuclides 11C (t1/2 ≈ 20 min; 1), 68Ga (t1/2 ≈ 68 min; 2), and 18F (t1/2 ≈ 110 min; 3).
Of course, the extremely promising preclinical results for IEDDA-based pretargeting beg the question: will it work in humans? Not surprisingly, some legitimate concerns have been leveled on this front (most notably, the dramatic increase in blood volume upon moving from mice to humans). Although no trials have yet been reported, a number of laboratories are currently working toward bringing these exciting technologies to the clinic, and the field is collectively hopeful that the speed, selectivity, and bioorthogonality of the IEDDA reaction will be up to the task.
EMERGING APPLICATIONS
Of course, the four areas we have discussed so far are not the only points of intersection between click chemistry and radiopharmaceutical science. Indeed, the past few years have played witness to the increasingly innovative use of click chemistry in the synthesis of radiopharmaceuticals. For example, click chemistry has played an important role in the advent of nanoparticles as vectors for molecular imaging.159 In this regard, the modularity, selectivity, and chemically mild nature of click chemistry have proven especially useful. Along these lines, Zeng et al. used the strain-promoted alkyne–azide cycloaddition (SPAAC) to modify the surface of azide-bearing shell-cross-linked nanoparticles with ~500 64Cu–DOTA moieties per particle, ultimately achieving specific activities of up to 975 Ci/μmol.160 Similarly, Lee et al. reported the synthesis and in vivo evaluation of 64Cu-labeled chitosan nanoparticles constructed via the SPAAC reaction between 64Cu–DOTA–DBCO prosthetic groups and azide-modified chitosan NPs (Figure 14).161 Click chemistry has also been used to enable pretargeted imaging using nanoparticulate vectors. For instance, Lee et al. have developed an SPAAC-based pretargeting strategy based on mesoporous silica nanoparticles (MSN).139 In this work, DBCO-modified mesoporous silica nanoparticles were injected into mice bearing U87MG tumors. A total of 24 h later, the same mice were injected with an 18F-labeled, azide-functionalized radioligand. This strategy successfully enabled the noninvasive visualization of tumor tissue (up to 1.4% ID/g at 2 h post-injection) with promising tumor-to-background contrast, a truly remarkable result given the somewhat sluggish kinetics of the SPAAC ligation.
Figure 14.
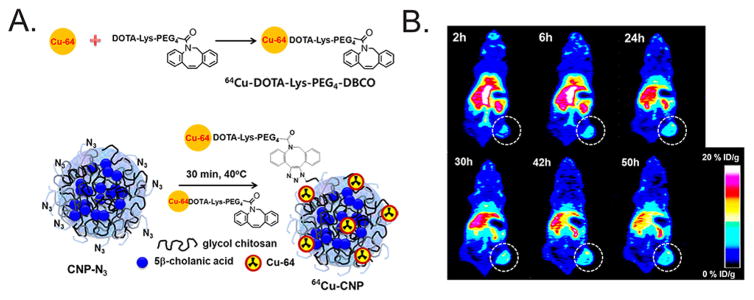
(A) The SPAAC ligation was employed to combine 64Cu–DOTA–Lys–PEG4–DBCO prosthetic groups and azide-modified chitosan nanoparticles (CNP) to create 64Cu-labeled CNPs. (B) MicroPET images of mice bearing SCC-7 xenografts acquired 2–50 h after the injection of the 64Cu–CNPs (250 μCi, 200 μg per mouse) reveal tumoral activity concentrations of up to 11.3 ± 1.3% ID/g at 50 h post-injection. Reprinted with permission from Lee et al., copyright 2013 American Chemical Society.
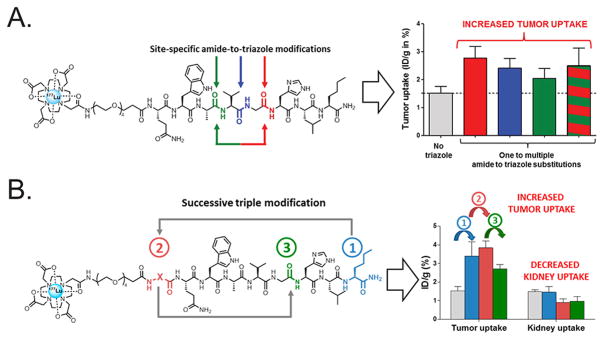
Shifting gears somewhat, a number of recent reports have emerged in which click chemistry (and the triazole-forming reactions, in particular) has been harnessed to enhance the in vivo stability of peptide-based imaging agents.162,163 1,2,3-Triazoles possess two critical physicochemical similarities to the amide bonds that normally link amino acids: planarity and the ability to act as hydrogen bond acceptors.163,164 Yet unlike amide bonds, triazoles are resistant to protease or peptidase metabolism in vivo. As a result, radiolabeled peptides in which triazole linkages replace some of the traditional amide bonds offer enticing prospects for nuclear imaging. Just last year, Valverde et al.165 demonstrated the potential of this approach by synthesizing a series of triazole-containing, 177Lu-labeled peptidomimetic radiotracers that target the gastrin-releasing peptide receptor (GRPr) (Figure 15A). In vivo studies revealed a significant increase in the in vivo stability of the triazole-containing compounds, a result that is likely responsible for the observation that the amide-to-triazole substituted derivatives exhibited an approximately 2-fold increase in tumor uptake. Very recently, the same group applied this methodology to bombesin-derived peptides, aiming to create tracers with improved tumor-to-kidney activity concentration ratios.166 This work demonstrated that the triazole-containing constructs boast improved (~5-fold) serum stability without sacrificing binding affinity. In vivo biodistribution experiments in mice bearing antigen-expressing PC3 and AR42J xenografts further revealed that the backbone-modified constructs possessed superior in vivo properties (Figure 15B).166
Figure 15.
(A) A series of 177Lu-labeled peptidomimetics containing 1,4-disubstituted 1,2,3-triazole moieties were shown to exhibit significantly increased proteolytic stability while retaining nanomolar affinity for GRPr. These alterations are likely responsible for ~2-fold increases in the uptake of the tracers in target-expressing PC3 xenografts compared to their triazole-lacking cousins. (B) The same methodology was successfully applied to synthesize a series of GRPr-targeting bombesin derivatives that boast high tumor-to-kidney activity concentration ratios, a critical feature in the design of therapeutic radiopharmaceuticals. Panel A was reprinted with permission from Valverde et al., copyright 2015 American Chemical Society. Panel B was reprinted with permission from reference Valverde et al., copyright 2016 American Chemical Society.
Finally, a very recent paper from Thurber and co-workers (although admittedly one that focuses on fluorescence rather than nuclear imaging agents) describes a complementary way to use click chemistry to enhance the in vivo stability of peptides.167,168 In this work, the authors use an innovative “double-click” approach that simultaneously enables the conjugation of a fluorophore to the peptide and creates an internal cross-link that stabilizes the α-helical structure of the peptide.168 The authors were able to demonstrate that the “double-clicked” peptides exhibited improved metabolic stability compared to analogous constructs. Furthermore, in vivo studies in C57BL/6 mice revealed that the click-stabilized peptides possessed increased protease resistance and significantly enhanced bioavailability.
CONCLUSIONS AND FUTURE DIRECTIONS
In the preceding pages, we have highlighted what we believe to be the most important and innovative advances from the first 10 years of research at the intersection of click chemistry and radiopharmaceutical chemistry. The bulk of this work has been concentrated in four areas: (i) the radiolabeling of molecules using prosthetic groups, (ii) the assembly of radiometal coordination architectures, (iii) the site-specific modification of immunoglobulins, and (iv) the creation of in vivo pretargeting strategies. Of course, in each, the details differ. Far more important, though, is that in every case, the refrain remains the same: the intrinsic selectivity and modularity of click chemistry can—and very often do—dramatically improve the construction of radiopharmaceuticals.
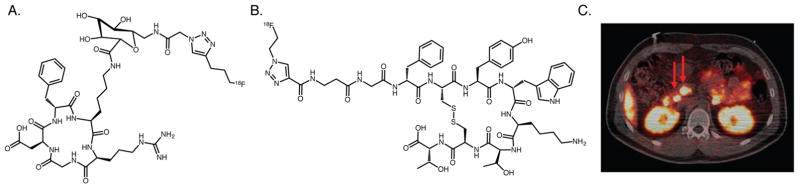
However, despite a wealth of preclinical data, click-based radiopharmaceuticals seem to have stalled just short of the clinic. This is somewhat surprising given the manifold advantages click chemistry offers for the construction of radiolabeled agents. For example, click chemistry could dramatically streamline the logistics of clinical probe production, as a single “clickable” prosthetic group could be used in the production of multiple radiotracers. Yet still, only a small handful of click-based radiopharmaceuticals have been the subject of clinical trials, most notably the αvβ3-targeting peptide 18F–RGD–K5 and the somatostatin receptor targeting peptide 18F–fluoroethyltriazole–Tyr3–octreotate (Figure 16).169–171 These two agents certainly represent a great start, but they must be considered just that: a start. We believe that as we move into the field’s second decade, clinical translation must be the top priority. To be sure, pushing back the frontiers of basic preclinical radiochemistry will remain vital. Yet ultimately, only the clinical translation of a variety of click-based probes will demonstrate once and for all the utility of this chemical technology in nuclear medicine.
Figure 16.
(A) Structure of 18F–RGD–K5; (B) structure of 18F–fluoroethyltriazole–Tyr3–octreotate; (C) 18F–fluoroethyltriazole–Tyr3–octreotate PET–CT image of a patient with multiple endocrine neoplasia type 1 syndrome and multiple pancreatic neuroendocrine tumors (red arrows). Adapted and reprinted with permission from Dubash et al., copyright 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Acknowledgments
The authors are grateful for the generous financial support of the National Institutes of Health (4R00CA178205-02, B.M.Z.; R01CA204167, B.M.Z. and J.S.L.; P30CA08748, MSKCC), the TeamConnor Childhood Cancer Foundation (B.M.Z.), the National Institute on Minority Health and Health Disparities (G12MD007599; B.M.Z.), the Tow Foundation Fellowship Program in Molecular Imaging and Nanotechnology (J.S.L.), and Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Center for Experimental Therapeutics at Memorial Sloan Kettering Cancer Center (J.S.L.).
Footnotes
The authors declare no competing financial interest.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem, Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed June 28, 2016];Web of Science. http://apps.webofknowledge.com/
- 3.Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 4.Bock VD, Hiemstra H, van Maarseveen JH. Cu-I-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur J Org Chem. 2005;2006:51–68. [Google Scholar]
- 5.Hein JE, Fokin VV. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem Soc Rev. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew Chem, Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sletten EM, Bertozzi CR. From mechanism to mouse: A tale of two bioorthogonal reactions. Acc Chem Res. 2011;44:666–676. doi: 10.1021/ar200148z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best MD. Click chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 9.McKay CS, Finn MG. Click chemistry in complex mixtures: Bioorthogonal bioconjugation. Chem Biol. 2014;21:1075–1101. doi: 10.1016/j.chembiol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse electron demand Diels-Alder reactivity. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon CG, Mackey JL, Jewett JC, Sletten EM, Houk KN, Bertozzi CR. Reactivity of biarylazacyclooctynones in copper-free click chemistry. J Am Chem Soc. 2012;134:9199–9208. doi: 10.1021/ja3000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schilling CI, Jung N, Biskup M, Schepers U, Braese S. Bioconjugation via azide-Staudinger ligation: an overview. Chem Soc Rev. 2011;40:4840–4871. doi: 10.1039/c0cs00123f. [DOI] [PubMed] [Google Scholar]
- 13.Kohn M, Breinbauer R. The Staudinger ligation - A gift to chemical biology’. Angew Chem, Int Ed. 2004;43:3106–3116. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]
- 14.van Berkel SS, van Eldijk MB, van Hest JCM. Staudinger ligation as a method for bioconjugation. Angew Chem, Int Ed. 2011;50:8806–8827. doi: 10.1002/anie.201008102. [DOI] [PubMed] [Google Scholar]
- 15.Devaraj NK, Weissleder R. Biomedical applications of tetrazine cycloadditions. Acc Chem Res. 2011;44:816–827. doi: 10.1021/ar200037t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seckute J, Devaraj NK. Expanding room for tetrazine ligations in the in vivo chemistry toolbox. Curr Opin Chem Biol. 2013;17:761–767. doi: 10.1016/j.cbpa.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discovery Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 18.Thirumurugan P, Matosiuk D, Jozwiak K. Click chemistry for drug development and diverse chemical-biology applications. Chem Rev. 2013;113:4905–4979. doi: 10.1021/cr200409f. [DOI] [PubMed] [Google Scholar]
- 19.Hoyle CE, Lowe AB, Bowman CN. Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev. 2010;39:1355–1387. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 20.Golas PL, Matyjaszewski K. Marrying click chemistry with polymerization: expanding the scope of polymeric materials. Chem Soc Rev. 2010;39:1338–1354. doi: 10.1039/b901978m. [DOI] [PubMed] [Google Scholar]
- 21.Mamidyala SK, Finn MG. In situ click chemistry: probing the binding landscapes of biological molecules. Chem Soc Rev. 2010;39:1252–1261. doi: 10.1039/b901969n. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Sanchez A, Perez-Baena I, Pomposo JA. Advances in click chemistry for single-chain nanoparticle construction. Molecules. 2013;18:3339–3355. doi: 10.3390/molecules18033339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramenda T, Bergmann R, Wuest F. Synthesis of F-18-labeled neurotensin(8–13) via copper-mediated 1,3-dipolar 3 + 2 cycloaddition reaction. Letters in Drug Design & Discovery. 2007;4:279–285. [Google Scholar]
- 24.Zeng D, Zeglis BM, Lewis JS, Anderson CJ. The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J Nucl Med. 2013;54:829–832. doi: 10.2967/jnumed.112.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waengler C, Schirrmacher R, Bartenstein P, Waengler B. Click-chemistry reactions in radiopharmaceutical chemistry: fast & easy introduction of radiolabels into biomolecules for in vivo imaging. Curr Med Chem. 2010;17:1092–1116. doi: 10.2174/092986710790820615. [DOI] [PubMed] [Google Scholar]
- 26.Mamat C, Ramenda T, Wuest FR. Recent applications of click chemistry for the synthesis of radiotracers for molecular imaging. Mini-Rev Org Chem. 2009;6:21–34. [Google Scholar]
- 27.Ramenda T, Kniess T, Bergmann R, Steinbach J, Wuest F. Radiolabelling of proteins with fluorine-18 via click chemistry. Chem Commun. 2009:7521–7523. doi: 10.1039/b916075b. [DOI] [PubMed] [Google Scholar]
- 28.Wuest F. Aspects of positron emission tomography radiochemistry as relevant for food chemistry. Amino Acids. 2005;29:323–339. doi: 10.1007/s00726-005-0201-1. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Conti PS. Radiopharmaceutical chemistry for positron emission tomography. Adv Drug Delivery Rev. 2010;62:1031–1051. doi: 10.1016/j.addr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Shen B, Chin FT, Cheng Z. Recent progress in radiofluorination of peptides for PET molecular imaging. Curr Org Synth. 2011;8:584–592. [Google Scholar]
- 31.Kuchar M, Pretze M, Kniess T, Steinbach J, Pietzsch J, Löser R. Site-selective radiolabeling of peptides by 18F-fluorobenzoylation with [18F]SFB in solution and on solid phase: a comparative study. Amino Acids. 2012;43:1431–1443. doi: 10.1007/s00726-012-1216-z. [DOI] [PubMed] [Google Scholar]
- 32.Shi B, Greaney MF. Reversible Michael addition of thiols as a new tool for dynamic combinatorial chemistry. Chem Commun. 2005:886–888. doi: 10.1039/b414300k. [DOI] [PubMed] [Google Scholar]
- 33.Boren BC, Narayan S, Rasmussen LK, Zhang L, Zhao HT, Lin ZY, Jia GC, Fokin VV. Ruthenium-catalyzed azide-alkyne cycloaddition: Scope and mechanism. J Am Chem Soc. 2008;130:8923–8930. doi: 10.1021/ja0749993. [DOI] [PubMed] [Google Scholar]
- 34.Massarotti A, Aprile S, Mercalli V, Del Grosso E, Grosa G, Sorba G, Tron GC. Are 1,4-and 1,5-disubstituted 1,2,3-triazoles good pharmacophoric groups? Chem Med Chem. 2014;9:2497–2508. doi: 10.1002/cmdc.201402233. [DOI] [PubMed] [Google Scholar]
- 35.Glaser M, Robins EG. ‘Click labelling’ in PET radiochemistry. J Labelled Compd Radiopharm. 2009;52:407–414. [Google Scholar]
- 36.Pretze M, Pietzsch D, Mamat C. Recent trends in bioorthogonal click-radiolabeling reactions using fluorine-18. Molecules. 2013;18:8618. doi: 10.3390/molecules18078618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kettenbach K, Schieferstein H, Ross TL. 18FLabeling using click cycloadditions. Bio Med Res Int. 2014;2014:16. doi: 10.1155/2014/361329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazari PP, Schulz J, Vimont D, Chadha N, Allard M, Szlosek-Pinaud M, Fouquet E, Mishra AK. A new SiF-dipropargyl glycerol scaffold as a versatile prosthetic group to design dimeric radioligands: Synthesis of the F-18 BMPPSiF tracer to image serotonin receptors. ChemMedChem. 2014;9:337–349. doi: 10.1002/cmdc.201300458. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Jacobson O, Avdic D, Rotstein BH, Weiss ID, Collier L, Chen X, Vasdev N, Liang SH. Orthostabilized 18F-azido click agents and their application in PET imaging with single-stranded DNA aptamers. Angew Chem, Int Ed. 2015;54:12777–12781. doi: 10.1002/anie.201505927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernard-Gauthier V, Wängler C, Schirrmacher E, Kostikov A, Jurkschat K, Wängler B, Schirrmacher R. 18F-Labeled silicon-based fluoride acceptors: Potential opportunities for novel positron emitting radiopharmaceuticals. BioMed Res Int. 2014;2014:20. doi: 10.1155/2014/454503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haslop A, Wells L, Gee A, Plisson C, Long N. One-pot multi-tracer synthesis of novel 18F-labeled PET imaging agents. Mol Pharmaceutics. 2014;11:3818–3822. doi: 10.1021/mp500324n. [DOI] [PubMed] [Google Scholar]
- 42.Glaser M, Årstad E. Click Labeling” with 2-[18F]fluoroethylazide for positron emission tomography. Bioconjugate Chem. 2007;18:989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 43.Iddon L, Leyton J, Indrevoll B, Glaser M, Robins EG, George AJT, Cuthbertson A, Luthra SK, Aboagye EO. Synthesis and in vitro evaluation of [18F]fluoroethyl triazole labelled [Tyr3]octreotate analogues using click chemistry. Bioorg Med Chem Lett. 2011;21:3122–3127. doi: 10.1016/j.bmcl.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Pretze M, Mamat C. Automated preparation of [18F]AFP and [18F]BFP: Two novel bifunctional 18F-labeling building blocks for Huisgen-click. J Fluorine Chem. 2013;150:25–35. [Google Scholar]
- 45.Pretze M, Kuchar M, Bergmann R, Steinbach J, Pietzsch J, Mamat C. An efficient bioorthogonal strategy using CuAAC click chemistry for radiofluorinations of SNEW peptides and the role of copper depletion. ChemMedChem. 2013;8:935–945. doi: 10.1002/cmdc.201300053. [DOI] [PubMed] [Google Scholar]
- 46.Mirfeizi L, Walsh J, Kolb H, Campbell-Verduyn L, Dierckx RA, Feringa BL, Elsinga PH, de Groot T, Sannen I, Bormans G, et al. Synthesis of [18F]RGD-K5 by catalyzed [3 + 2] cycloaddition for imaging integrin αvβ3 expression in vivo. Nucl Med Biol. 2013;40:710–716. doi: 10.1016/j.nucmedbio.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Ramenda T, Steinbach J, Wuest F. 4-[18F]Fluoro-N-methyl-N-(propyl-2-yn-1-yl)benzenesulfonamide ([18F]F-SA): a versatile building block for labeling of peptides, proteins and oligonucleotides with fluorine-18 via Cu(I)-mediated click chemistry. Amino Acids. 2013;44:1167–1180. doi: 10.1007/s00726-012-1450-4. [DOI] [PubMed] [Google Scholar]
- 48.Roberts MP, Pham TQ, Doan J, Jiang CD, Hambley TW, Greguric I, Fraser BH. Radiosynthesis and ‘click’ conjugation of ethynyl-4-[18F]-fluorobenzene — an improved [18F]-synthon for indirect radiolabeling. J Labelled Compd Radiopharm. 2015;58:473–478. doi: 10.1002/jlcr.3354. [DOI] [PubMed] [Google Scholar]
- 49.Hofmann S, Maschauer S, Kuwert T, Beck-Sickinger AG, Prante O. Synthesis and in vitro and in vivo evaluation of an 18F-labeled neuropeptide Y analogue for imaging of breast cancer by PET. Mol Pharmaceutics. 2015;12:1121–1130. doi: 10.1021/mp500601z. [DOI] [PubMed] [Google Scholar]
- 50.Sachin K, Jadhav VH, Kim EM, Kim HL, Lee SB, Jeong HJ, Lim ST, Sohn MH, Kim DW. F-18 Labeling protocol of peptides based on chemically orthogonal strain-promoted cycloaddition under physiologically friendly reaction conditions. Bioconjugate Chem. 2012;23:1680–1686. doi: 10.1021/bc3002425. [DOI] [PubMed] [Google Scholar]
- 51.Chikira M. DNA-fiber EPR spectroscopy as a tool to study DNA–metal complex interactions: DNA binding of hydrated Cu(II) ions and Cu(II) complexes of amino acids and peptides. J Inorg Biochem. 2008;102:1016–1024. doi: 10.1016/j.jinorgbio.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Cai H, He J, Chen H, Lam S, Cai T, Zhu Z, Bark SJ, Cai C. Extent of the oxidative side reactions to peptides and proteins during the CuAAC reaction. Bioconjugate Chem. 2016;27:2315–2322. doi: 10.1021/acs.bioconjchem.6b00267. [DOI] [PubMed] [Google Scholar]
- 53.Evans HL, Carroll L, Aboagye EO, Spivey AC. Bioorthogonal chemistry for 68Ga radiolabelling of DOTA-containing compounds. J Labelled Compd Radiopharm. 2014;57:291–297. doi: 10.1002/jlcr.3153. [DOI] [PubMed] [Google Scholar]
- 54.Glaser C. Beiträge zur kenntniss des acetenylbenzols. Ber Dtsch Chem Ges. 1869;2:422–424. [Google Scholar]
- 55.Kobus D, Giesen Y, Ullrich R, Backes H, Neumaier B. A fully automated two-step synthesis of an 18F-labelled tyrosine kinase inhibitor for EGFR kinase activity imaging in tumors. Appl Radiat Isot. 2009;67:1977–1984. doi: 10.1016/j.apradiso.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 56.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 57.Wang W, Wu J, Xia C, Li F. Reusable ammonium salt-tagged NHC-Cu(i) complexes: preparation and catalytic application in the three component click reaction. Green Chem. 2011;13:3440–3445. [Google Scholar]
- 58.Gaulier C, Hospital A, Legeret B, Delmas AF, Aucagne V, Cisnetti F, Gautier A. A water soluble CuI-NHC for CuAAC ligation of unprotected peptides under open air conditions. Chem Commun. 2012;48:4005–4007. doi: 10.1039/c2cc30515a. [DOI] [PubMed] [Google Scholar]
- 59.van Hest JCM, van Delft FL. Protein modification by strain-promoted alkyne–azide cycloaddition. Chem-BioChem. 2011;12:1309–1312. doi: 10.1002/cbic.201100206. [DOI] [PubMed] [Google Scholar]
- 60.Debets MF, van Berkel SS, Dommerholt J, Dirks AJ, Rutjes FPJT, van Delft FL. Bioconjugation with strained alkenes and alkynes. Acc Chem Res. 2011;44:805–815. doi: 10.1021/ar200059z. [DOI] [PubMed] [Google Scholar]
- 61.Campbell-Verduyn LS, Mirfeizi L, Schoonen AK, Dierckx RA, Elsinga PH, Feringa BL. Strain-Promoted Copper-Free “Click” Chemistry for 18F Radiolabeling of Bombesin. Angew Chem, Int Ed. 2011;50:11117–11120. doi: 10.1002/anie.201105547. [DOI] [PubMed] [Google Scholar]
- 62.Kim HL, Sachin K, Jeong HJ, Choi W, Lee HS, Kim DW. F-18 labeled RGD probse based on bioorthogonal strain-promoted click reaction for PET imaging. ACS Med Chem Lett. 2015;6:402–407. doi: 10.1021/ml500464f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carpenter RD, Hausner SH, Sutcliffe JL. Copper-free click for PET: Rapid 1,3-dipolar cycloadditions with a fluorine-18 cyclooctyne. ACS Med Chem Lett. 2011;2:885–889. doi: 10.1021/ml200187j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouvet V, Wuest M, Wuest F. Copper-free click chemistry with the short-lived positron emitter fluorine-18. Org Biomol Chem. 2011;9:7393–7399. doi: 10.1039/c1ob06034a. [DOI] [PubMed] [Google Scholar]
- 65.Arumugam S, Chin J, Schirrmacher R, Popik VV, Kostikov AP. [18F]Azadibenzocyclooctyne ([18F]ADIBO): A biocompatible radioactive labeling synthon for peptides using catalyst free [3 + 2] cycloaddition. Bioorg Med Chem Lett. 2011;21:6987–6991. doi: 10.1016/j.bmcl.2011.09.126. [DOI] [PubMed] [Google Scholar]
- 66.Choi MH, Shim HE, Nam YR, Kim HR, Kang JA, Lee DE, Park SH, Choi DS, Jang BS, Jeon J. Synthesis and evaluation of an 125I-labeled azide prosthetic group for efficient and bioorthogonal radiolabeling of cyclooctyne-group containing molecules using copper-free click reaction. Bioorg Med Chem Lett. 2016;26:875–878. doi: 10.1016/j.bmcl.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 67.Cai Z, Ouyang Q, Zeng D, Nguyen KN, Modi J, Wang L, White AG, Rogers BE, Xie XQ, Anderson CJ. 64Cu-labeled somatostatin analogues conjugated with cross-bridged phosphonate-based chelators via strain-promoted click chemistry for PET imaging: In silico through in vivo studies. J Med Chem. 2014;57:6019–6029. doi: 10.1021/jm500416f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng D, Guo Y, White AG, Cai Z, Modi J, Ferdani R, Anderson CJ. Comparison of conjugation strategies of cross-bridged macrocyclic chelators with cetuximab for copper-64 radiolabeling and PET imaging of EGFR in colorectal tumor-bearing mice. Mol Pharmaceutics. 2014;11:3980–3987. doi: 10.1021/mp500004m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hausner SH, Carpenter RD, Bauer N, Sutcliffe JL. Evaluation of an integrin αvβ6-specific peptide labeled with [18F]fluorine by copper-free, strain-promoted click chemistry. Nucl Med Biol. 2013;40:233–239. doi: 10.1016/j.nucmedbio.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Li Z, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, Fox JM. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem Commun. 2010;46:8043–8045. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S, Hassink M, Selvaraj R, Yap L-P, Park R, Wang H, Chen X, Fox JM, Li Z, Conti PS. Efficient 18F labeling of cysteine-containing peptides and proteins using tetrazine-transcyclooctene ligation. Mol Imaging. 2013;12:2121128. [PMC free article] [PubMed] [Google Scholar]
- 72.Selvaraj R, Liu S, Hassink M, Huang C-w, Yap L-p, Park R, Fox JM, Li Z, Conti PS. Tetrazine-trans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Bioorg Med Chem Lett. 2011;21:5011–5014. doi: 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, Todorov I, Fox JM, Li Z, Shively JE, et al. Development and evaluation of 18F-TTCO-Cys40-exendin-4: A PET probe for imaging transplanted islets. J Nucl Med. 2013;54:244–251. doi: 10.2967/jnumed.112.109694. [DOI] [PubMed] [Google Scholar]
- 74.Keliher EJ, Reiner T, Turetsky A, Hilderbrand SA, Weissleder R. High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors. ChemMedChem. 2011;6:424–427. doi: 10.1002/cmdc.201000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Synthese und In-vivo-Bildgebung eines 18F-markierten PARP1-Inhibitors mithilfe eines chemisch orthogonalen, Abfangreagens-gestützten Hochdurchsatzverfahrens. Angew Chem. 2011;123:1963–1966. [Google Scholar]
- 76.Zhu J, Li S, Wangler C, Wangler B, Bruce Lennox R, Schirrmacher R. Synthesis of 3-chloro-6-((4-(di-tert-butyl-[18F]fluorosilyl)-benzyl)oxy)-1,2,4,5-tetrazine ([18F]SiFA-OTz) for rapid tetrazine-based 18F-radiolabeling. Chem Commun. 2015;51:12415–12418. doi: 10.1039/c5cc03623b. [DOI] [PubMed] [Google Scholar]
- 77.Rashidian M, Keliher EJ, Dougan M, Juras PK, Cavallari M, Wojtkiewicz GR, Jacobsen JT, Edens JG, Tas JMJ, Victora G, et al. Use of F-18–2-fluorodeoxyglucose to label antibody fragments for immuno-positron emission tomography of pancreatic cancer. ACS Cent Sci. 2015;1:142–147. doi: 10.1021/acscentsci.5b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albu SA, Al-Karmi SA, Vito A, Dzandzi JPK, Zlitni A, Beckford-Vera D, Blacker M, Janzen N, Patel RM, Capretta A, et al. 125I-Tetrazines and inverse electron demand Diels-Alder chemistry: A convenient radioiodination strategy for biomolecule labeling, screening, and biodistribution studies. Bioconjugate Chem. 2016;27:207–216. doi: 10.1021/acs.bioconjchem.5b00609. [DOI] [PubMed] [Google Scholar]
- 79.Choi MH, Shim HE, Yun SJ, Kim HR, Mushtaq S, Lee CH, Park SH, Choi DS, Lee DE, Byun EB, et al. Highly efficient method for 125I-radiolabeling of biomolecules using inverse-electron-demand Diels–Alder reaction. Bioorg Med Chem. 2016;24:2589–2594. doi: 10.1016/j.bmc.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 80.Zeglis BM, Mohindra P, Weissmann GI, Divilov V, Hilderbrand SA, Weissleder R, Lewis JS. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjugate Chem. 2011;22:2048–2059. doi: 10.1021/bc200288d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar A, Hao G, Liu L, Ramezani S, Hsieh JT, Öz OK, Sun X. Click-chemistry strategy for labeling antibodies with copper-64 via a cross-bridged tetraazamacrocyclic chelator scaffold. Bioconjugate Chem. 2015;26:782–789. doi: 10.1021/acs.bioconjchem.5b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaeta A, Woodcraft J, Plant S, Goggi J, Jones P, Battle M, Trigg W, Luthra SK, Glaser M. Use of 2-[18F]fluoroethylazide for the Staudinger ligation – Preparation and characterisation of GABAA receptor binding 4-quinolones. Bioorg Med Chem Lett. 2010;20:4649–4652. doi: 10.1016/j.bmcl.2010.05.106. [DOI] [PubMed] [Google Scholar]
- 83.Carroll L, Boldon S, Bejot R, Moore JE, Declerck J, Gouverneur V. The traceless Staudinger ligation for indirect 18F-radiolabelling. Org Biomol Chem. 2011;9:136–140. doi: 10.1039/c0ob00564a. [DOI] [PubMed] [Google Scholar]
- 84.Pretze M, Wuest F, Peppel T, Köckerling M, Mamat C. The traceless Staudinger ligation with fluorine-18: a novel and versatile labeling technique for the synthesis of PET-radiotracers. Tetrahedron Lett. 2010;51:6410–6414. [Google Scholar]
- 85.Zlatopolskiy BD, Kandler R, Mottaghy FM, Neumaier B. C-(4-[18F]fluorophenyl)-N-phenyl nitrone: A novel 18F-labeled building block for metal free [3 + 2]cycloaddition. Appl Radiat Isot. 2012;70:184–192. doi: 10.1016/j.apradiso.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Zlatopolskiy BD, Kandler R, Kobus D, Mottaghy FM, Neumaier B. Beyond azide-alkyne click reaction: easy access to 18F-labelled compounds via nitrile oxide cycloadditions. Chem Commun. 2012;48:7134–7136. doi: 10.1039/c2cc31335a. [DOI] [PubMed] [Google Scholar]
- 87.Jeon J, Shen B, Xiong L, Miao Z, Lee KH, Rao J, Chin FT. Efficient method for site-specific 18F-labeling of biomolecules using the rapid condensation reaction between 2-cyanobenzothiazole and cysteine. Bioconjugate Chem. 2012;23:1902–1908. doi: 10.1021/bc300273m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inkster JAH, Colin DJ, Seimbille Y. A novel 2-cyanobenzothiazole-based 18F prosthetic group for conjugation to 1,2-aminothiol-bearing targeting vectors. Org Biomol Chem. 2015;13:3667–3676. doi: 10.1039/c4ob02637c. [DOI] [PubMed] [Google Scholar]
- 89.Chiotellis A, Sladojevich F, Mu L, Muller Herde A, Valverde IE, Tolmachev V, Schibli R, Ametamey SM, Mindt TL. Novel chemoselective 18F-radiolabeling of thiol-containing biomolecules under mild aqueous conditions. Chem Commun. 2016;52:6083–6086. doi: 10.1039/c6cc01982j. [DOI] [PubMed] [Google Scholar]
- 90.Lebedev AY, Holland JP, Lewis JS. Clickable bifunctional radiometal chelates for peptide labeling. Chem Commun. 2010;46:1706–1708. doi: 10.1039/b924784j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey GA, Price EW, Zeglis BM, Ferreira CL, Boros E, Lacasse MJ, Patrick BO, Lewis JS, Adam MJ, Orvig C. H(2)azapa: a versatile acyclic multifunctional chelator for Ga-67, Cu-64, In-111, and Lu-177. Inorg Chem. 2012;51:12575–12589. doi: 10.1021/ic302225z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mindt TL, Muller C, Melis M, de Jong M, Schibli R. “Click-to-Chelate”: In vitro and in vivo comparison of a Tc-99m(CO)(3)-labeled N(tau)-histidine folate derivative with its isostructural, clicked 1,2,3-triazole analogue. Bioconjugate Chem. 2008;19:1689–1695. doi: 10.1021/bc800183r. [DOI] [PubMed] [Google Scholar]
- 93.Mindt TL, Struthers H, Brans L, Anguelov T, Schweinsberg C, Maes V, Tourwe D, Schibli R. “Click to chelate”: Synthesis and installation of metal chelates into biomolecules in a single step. J Am Chem Soc. 2006;128:15096–15097. doi: 10.1021/ja066779f. [DOI] [PubMed] [Google Scholar]
- 94.Struthers H, Spingler B, Mindt TL, Schibli R. “Click-to-Chelate”: Design and incorporation of triazole-containing metal-chelating systems into biomolecules of diagnostic and therapeutic interest. Chem - Eur J. 2008;14:6173–6183. doi: 10.1002/chem.200702024. [DOI] [PubMed] [Google Scholar]
- 95.Kluba CA, Mindt TL. Click-to-chelate: Development of technetium and rhenium-tricarbonyl labeled radiopharmaceuticals. Molecules. 2013;18:3206–3226. doi: 10.3390/molecules18033206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kluba CA, Bauman A, Valverde IE, Vomstein S, Mindt TL. Dual-targeting conjugates designed to improve the efficacy of radiolabeled peptides. Org Biomol Chem. 2012;10:7594–7602. doi: 10.1039/c2ob26127h. [DOI] [PubMed] [Google Scholar]
- 97.Fernandez S, Crocamo N, Incerti M, Giglio J, Scarone L, Rey A. Preparation and preliminary bioevaluation of a 99mTc(CO)3-glucose derivative prepared by a click chemistry route. J Labelled Compd Radiopharm. 2012;55:274–280. [Google Scholar]
- 98.Struthers H, Viertl D, Kosinski M, Spingler B, Buchegger F, Schibli R. Charge dependent substrate activity of C3′ and N3 functionalized, organometallic technetium and rhenium-labeled thymidine derivatives toward human thymidine kinase 1. Bioconjugate Chem. 2010;21:622–634. doi: 10.1021/bc900380n. [DOI] [PubMed] [Google Scholar]
- 99.Burai R, Ramesh C, Nayak TK, Dennis MK, Bryant BK, Prossnitz ER, Arterburn JB. Synthesis and characterization of tricarbonyl-Re/Tc(I) chelate probes targeting the G protein-coupled estrogen receptor GPER/GPR30. PLoS One. 2012;7:e46861. doi: 10.1371/journal.pone.0046861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasten BB, Ma XW, Liu HG, Hayes TR, Barnes CL, Qi SB, Cheng K, Bottorff SC, Slocumb WS, Wang J, et al. Clickable, hydrophilic ligand for fac-M-I(CO)(3) (+) (M = Re/Tc-99m) applied in an S-functionalized alpha-MSH peptide. Bioconjugate Chem. 2014;25:579–592. doi: 10.1021/bc5000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bottorff SC, Kasten BB, Stojakovic J, Moore AL, MacGillivray LR, Benny PD. Cu-free 1,3-dipolar cycloaddition click reactions to form isoxazole linkers in chelating ligands for fac-M(CO)(3) (+) centers (M = Re, Tc-99m) Inorg Chem. 2014;53:1943–1945. doi: 10.1021/ic402825t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. mAbs. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Vught R, Pieters RJ, Breukink E. Site-specific functionalization of proteins and their applications to therapeutic antibodies. Comput Struct Biotechnol J. 2014;9:e201402001. doi: 10.5936/csbj.201402001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Behrens CR, Liu B. Methods for site-specific drug conjugation to antibodies. mAbs. 2014;6:46–53. doi: 10.4161/mabs.26632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agarwal P, Bertozzi CR. Site-specific antibody–drug conjugates: The nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjugate Chem. 2015;26:176–192. doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramakrishnan B, Qasba PK. Structure-based design of β1,4-galactosyltransferase I (β4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity: Point mutation broadens β4Gal-T1 donor specificity. J Biol Chem. 2002;277:20833–20839. doi: 10.1074/jbc.M111183200. [DOI] [PubMed] [Google Scholar]
- 107.Boeggeman E, Ramakrishnan B, Pasek M, Manzoni M, Puri A, Loomis KH, Waybright TJ, Qasba PK. Site specific conjugation of fluoroprobes to the remodeled Fc N-glycans of monoclonal antibodies using mutant glycosyltransferases: Application for cell surface antigen detection. Bioconjugate Chem. 2009;20:1228–1236. doi: 10.1021/bc900103p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cook BE, Adumeau P, Membreno R, Carnazza KE, Brand C, Reiner T, Agnew BJ, Lewis JS, Zeglis BM. Pretargeted PET imaging using a site-specifically labeled immunoconjugate. Bioconjugate Chem. 2016;27:1789–1795. doi: 10.1021/acs.bioconjchem.6b00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeglis BM, Davis CB, Abdel-Atti D, Carlin SD, Chen A, Aggeler R, Agnew BJ, Lewis JS. Chemoenzymatic strategy for the synthesis of site-specifically labeled immunoconjugates for multimodal PET and optical imaging. Bioconjugate Chem. 2014;25:2123–2128. doi: 10.1021/bc500499h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Houghton JL, Zeglis BM, Abdel-Atti D, Aggeler R, Sawada R, Agnew BJ, Scholz WW, Lewis JS. Site-specifically labeled CA19.9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proc Natl Acad Sci U S A. 2015;112:15850–15855. doi: 10.1073/pnas.1506542112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Geel R, Wijdeven MA, Heesbeen R, Verkade JMM, Wasiel AA, van Berkel SS, van Delft FL. Chemoenzymatic conjugation of toxic payloads to the globally conserved N-glycan of native mAbs provides homogeneous and highly efficacious antibody–drug conjugates. Bioconjugate Chem. 2015;26:2233–2242. doi: 10.1021/acs.bioconjchem.5b00224. [DOI] [PubMed] [Google Scholar]
- 112.Jeger S, Zimmermann K, Blanc A, Grünberg J, Honer M, Hunziker P, Struthers H, Schibli R. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew Chem, Int Ed. 2010;49:9995–9997. doi: 10.1002/anie.201004243. [DOI] [PubMed] [Google Scholar]
- 113.van Geel R, Debets MF, Löwik DWPM, Pruijn GJM, Boelens WC. Detection of transglutaminase activity using click chemistry. Amino Acids. 2012;43:1251–1263. doi: 10.1007/s00726-011-1198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gundersen MT, Keillor JW, Pelletier JN. Microbial transglutaminase displays broad acyl-acceptor substrate specificity. Appl Microbiol Biotechnol. 2014;98:219–230. doi: 10.1007/s00253-013-4886-x. [DOI] [PubMed] [Google Scholar]
- 115.Puthenveetil S, Musto S, Loganzo F, Tumey LN, O’Donnell CJ, Graziani E. Development of solid-phase site-specific conjugation and its application toward generation of dual labeled antibody and Fab drug conjugates. Bioconjugate Chem. 2016;27:1030–1039. doi: 10.1021/acs.bioconjchem.6b00054. [DOI] [PubMed] [Google Scholar]
- 116.Lang K, Chin JW. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem Rev. 2014;114:4764–4806. doi: 10.1021/cr400355w. [DOI] [PubMed] [Google Scholar]
- 117.Blizzard RJ, Backus DR, Brown W, Bazewicz CG, Li Y, Mehl RA. Ideal bioorthogonal reactions using a site-specifically encoded tetrazine amino acid. J Am Chem Soc. 2015;137:10044–10047. doi: 10.1021/jacs.5b03275. [DOI] [PubMed] [Google Scholar]
- 118.Machida T, Lang K, Xue L, Chin JW, Winssinger N. Site-specific glycoconjugation of protein via bioorthogonal tetrazine cycloaddition with a genetically encoded trans-cyclooctene or bicyclononyne. Bioconjugate Chem. 2015;26:802–806. doi: 10.1021/acs.bioconjchem.5b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kozma E, Nikić I, Varga BR, Aramburu IV, Kang JH, Fackler OT, Lemke EA, Kele P. Hydrophilic transcyclooctenylated noncanonical amino acids for fast intracellular protein labeling. ChemBioChem. 2016;17:1518–1524. doi: 10.1002/cbic.201600284. [DOI] [PubMed] [Google Scholar]
- 120.Wållberg H, Grafström J, Cheng Q, Lu L, Martinsson Ahlzén HS, Samén E, Thorell JO, Johansson K, Dunås F, Olofsson MH, et al. HER2-Positive tumors imaged within 1 h using a site-specifically 11C-labeled Sel-tagged affibody molecule. J Nucl Med. 2012;53:1446–1453. doi: 10.2967/jnumed.111.102194. [DOI] [PubMed] [Google Scholar]
- 121.Wu Y, Zhu H, Zhang B, Liu F, Chen J, Wang Y, Wang Y, Zhang Z, Wu L, Si L, et al. Synthesis of site-specific radiolabeled antibodies for radioimmunotherapy via genetic code expansion. Bioconjugate Chem. 2016;27:2460–2468. doi: 10.1021/acs.bioconjchem.6b00412. [DOI] [PubMed] [Google Scholar]
- 122.Wu AM. Antibodies and antimatter: the resurgence of immuno-PET. J Nucl Med. 2009;50:2–5. doi: 10.2967/jnumed.108.056887. [DOI] [PubMed] [Google Scholar]
- 123.Honarvar H, Westerlund K, Altai M, Sandstrom M, Orlova A, Tolmachev V, Karlstrom AE. Feasibility of affibody molecule-based PNA-mediated radionuclide pretargeting of malignant tumors. Theranostics. 2016;6:93–103. doi: 10.7150/thno.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharkey RM, Karacay H, Cardillo TM, Chang CH, McBride WJ, Rossi EA, Horak ID, Goldenberg DM. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin Cancer Res. 2005;11:7109S–7121S. doi: 10.1158/1078-0432.CCR-1004-0009. [DOI] [PubMed] [Google Scholar]
- 125.Hnatowich DJ, Virzi F, Rusckowski M. Investigation of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294–1302. [PubMed] [Google Scholar]
- 126.Casalini P, Luison E, Menard S, Colnaghi MI, Paganelli G, Canevari S. Tumor pretargeting: Role of avidin/streptavidin on monoclonal antibody internalization. J Nucl Med. 1997;38:1378–1381. [PubMed] [Google Scholar]
- 127.Wilbur DS, Pathare PM, Hamlin DK, Stayton PS, To R, Klumb LA, Buhler KR, Vessella RL. Development of new biotin/streptavidin reagents for pretargeting. Biomol Eng. 1999;16:113–118. doi: 10.1016/s1050-3862(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 128.Hamblett KJ, Press OW, Meyer DL, Hamlin DK, Axworthy D, Wilbur DS, Stayton PS. Role of biotin-binding affinity in streptavidin-based pretargeted radioimmunotherapy of lymphoma. Bioconjugate Chem. 2005;16:131–138. doi: 10.1021/bc034049g. [DOI] [PubMed] [Google Scholar]
- 129.McBride WJ, Zanzonico P, Sharkey RM, Noren C, Karacay H, Rossi EA, Losman MJ, Brard PY, Chang CH, Larson SM, et al. Bispecific antibody pretargeting PET (ImmunoPET) with an I-124-labeled hapten-peptide. J Nucl Med. 2006;47:1678–1688. [PubMed] [Google Scholar]
- 130.Sharkey RM, Cardillo TM, Rossi EA, Chang CH, Karacay H, McBride WJ, Hansen HJ, Horak ID, Goldenberg DM. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11:1250–1255. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 131.Reilly RM. Radioimmunotherapy of solid tumors: The promise of pretargeting strategies using bispecific antibodies and radiolabeled haptens. J Nucl Med. 2006;47:196–199. [PubMed] [Google Scholar]
- 132.Kuijpers WHA, Bos ES, Kaspersen FM, Veeneman GH, Vanboeckel CAA. Specific recognition of antibody oligonucleotide conjugates by radiolabeled antisense nucleotides - a novel approach for 2-step radioimmunotherapy of cancer. Bioconjugate Chem. 1993;4:94–102. doi: 10.1021/bc00019a013. [DOI] [PubMed] [Google Scholar]
- 133.Mardirossian G, Rusckowski M, Chang F, Qu T, Lei K, Egholm M, Hnatowich DJ. In vivo hybridization of technetium-99m-labeled peptide nucleic acid (PNA) J Nucl Med. 1996;37:924–924. [PubMed] [Google Scholar]
- 134.Liu GZ, Mang’era K, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using Tc-99m-labeled morpholino, a DNA analog. J Nucl Med. 2002;43:384–391. [PubMed] [Google Scholar]
- 135.Vugts DJ, Vervoort A, Stigter-van Walsum M, Visser GWM, Robillard MS, Versteegen RM, Vulders RCM, Herscheid JDM, van Dongen GAMS. Synthesis of phosphine and antibody-azide probes for in vivo Staudinger ligation in a pretargeted imaging and therapy approach. Bioconjugate Chem. 2011;22:2072–2081. doi: 10.1021/bc200298v. [DOI] [PubMed] [Google Scholar]
- 136.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Laughlin ST, Bertozzi CR. Imaging the glycome. Proc Natl Acad Sci U S A. 2009;106:12–17. doi: 10.1073/pnas.0811481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van den Bosch SM, Rossin R, Verkerk PR, ten Hoeve W, Janssen HM, Lub J, Robillard MS. Evaluation of strained alkynes for Cu-free click reaction in live mice. Nucl Med Biol. 2013;40:415–423. doi: 10.1016/j.nucmedbio.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 139.Lee SB, Kim HL, Jeong HJ, Lim ST, Sohn MH, Kim DW. Mesoporous silica nanoparticle pretargeting for PET imaging based on a rapid bioorthogonal reaction in a living body. Angew Chem, Int Ed. 2013;52:10549–10552. doi: 10.1002/anie.201304026. [DOI] [PubMed] [Google Scholar]
- 140.Knight JC, Cornelissen B. Bioorthogonal chemistry: implications for pretargeted nuclear (PET/SPECT) imaging and therapy. Am J Nucl Med Mol Imaging. 2014;4:96–113. [PMC free article] [PubMed] [Google Scholar]
- 141.Reiner T, Zeglis BM. The inverse electron demand Diels-Alder click reaction in radiochemistry. J Labelled Compd Radiopharm. 2014;57:285–290. doi: 10.1002/jlcr.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. A comparative study of bioorthogonal reactions with azides. ACS Chem Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 143.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rossin R, Verkerk PR, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS. In vivo chemistry for pretargeted tumor imaging in live mice. Angew Chem, Int Ed. 2010;49:3375–3378. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 145.Rossin R, van Duijnhoven SMJ, Lappchen T, van den Bosch SM, Robillard MS. Trans-cyclooctene tag with improved properties for tumor pretargeting with the Diels-Alder reaction. Mol Pharmaceutics. 2014;11:3090–3096. doi: 10.1021/mp500275a. [DOI] [PubMed] [Google Scholar]
- 146.Rossin R, Lappchen T, van den Bosch SM, Laforest R, Robillard MS. Diels-alder reaction for tumor pretargeting: In vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J Nucl Med. 2013;54:1989–1995. doi: 10.2967/jnumed.113.123745. [DOI] [PubMed] [Google Scholar]
- 147.van Duijnhoven SMJ, Rossin R, van den Bosch SM, Wheatcroft MP, Hudson PJ, Robillard MS. Diabody pretargeting with click chemistry in vivo. J Nucl Med. 2015;56:1422–1428. doi: 10.2967/jnumed.115.159145. [DOI] [PubMed] [Google Scholar]
- 148.Altai M, Perols A, Tsourma M, Mitran B, Honarvar H, Robillard M, Rossin R, ten Hoeve W, Lubberink M, Orlova A, et al. Feasibility of affibody-based bioorthogonal chemistry mediated radionuclide pretargeting. J Nucl Med. 2016;57:431–436. doi: 10.2967/jnumed.115.162248. [DOI] [PubMed] [Google Scholar]
- 149.Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, Weissleder R, Lewis JS. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J Nucl Med. 2013;54:1389–1396. doi: 10.2967/jnumed.112.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zeglis BM, Brand C, Abdel-Atti D, Carnazza KE, Cook BE, Carlin S, Reiner T, Lewis JS. Optimization of a pretargeted strategy for the PET imaging of colorectal carcinoma via the modulation of radioligand phramacokinetics. Mol Pharmaceutics. 2015;12:3575–3587. doi: 10.1021/acs.molpharmaceut.5b00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Houghton JL, Zeglis BM, Abdel-Atti D, Sawada R, Scholz WW, Lewis JS. Pretargeted immuno-PET of pancreatic cancer: Overcoming circulating antigen and internalized antibody to reduce radiation doses. J Nucl Med. 2016;57:453–459. doi: 10.2967/jnumed.115.163824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meyer JP, Houghton JL, Kozlowski P, Abdel-Atti D, Reiner T, Pillarsetty NVK, Scholz WW, Zeglis BM, Lewis JS. F-18-Based pretargeted PET imaging based on bioorthogonal Diels-Alder click chemistry. Bioconjugate Chem. 2016;27:298–301. doi: 10.1021/acs.bioconjchem.5b00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Herth MM, Andersen VL, Lehel S, Madsen J, Knudsen GM, Kristensen JL. Development of a C-11-labeled tetrazine for rapid tetrazine-trans-cyclooctene ligation. Chem Commun. 2013;49:3805–3807. doi: 10.1039/c3cc41027g. [DOI] [PubMed] [Google Scholar]
- 154.Denk C, Svatunek D, Mairinger S, Stanek J, Filip T, Matscheko D, Kuntner C, Wanek T, Mikula H. Design, synthesis, and evaluation of a low-molecular-weight C-11-labeled tetrazine for pretargeted PET imaging applying bioorthogonal in vivo click chemistry. Bioconjugate Chem. 2016;27:1707–1712. doi: 10.1021/acs.bioconjchem.6b00234. [DOI] [PubMed] [Google Scholar]
- 155.Evans HL, Nguyen QD, Carroll LS, Kaliszczak M, Twyman FJ, Spivey AC, Aboagye EO. A bioorthogonal Ga-68-labelling strategy for rapid in vivo imaging. Chem Commun. 2014;50:9557–9560. doi: 10.1039/c4cc03903c. [DOI] [PubMed] [Google Scholar]
- 156.Garcia MF, Zhang X, Shah M, Newton-Northup J, Cabral P, Cerecetto H, Quinn T. Tc-99m-bioorthogonal click chemistry reagent for in vivo pretargeted imaging. Bioorg Med Chem. 2016;24:1209–1215. doi: 10.1016/j.bmc.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Devaraj NK, Thurber GM, Keliher EJ, Marinelli B, Weissleder R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc Natl Acad Sci U S A. 2012;109:4762–4767. doi: 10.1073/pnas.1113466109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rossin R, van Duijnhoven SMJ, ten Hoeve W, Janssen HM, Kleijn LHJ, Hoeben FJM, Versteegen RM, Robillard MS. Triggered drug release from an antibody-drug conjugate using fast “click-to-release” chemistry in mice. Bioconjugate Chem. 2016;27:1697–1706. doi: 10.1021/acs.bioconjchem.6b00231. [DOI] [PubMed] [Google Scholar]
- 159.Avti PK, Maysinger D, Kakkar A. Alkyne-azide “click” chemistry in designing nanocarriers for applications in biology. Molecules. 2013;18:9531–9549. doi: 10.3390/molecules18089531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeng D, Lee NS, Liu Y, Zhou D, Dence CS, Wooley KL, Katzenellenbogen JA, Welch MJ. 64)Cu core-labeled nanoparticles with high specific activity via metal-free click chemistry. ACS Nano. 2012;6:5209–19. doi: 10.1021/nn300974s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee DE, Na JH, Lee S, Kang CM, Kim HN, Han SJ, Kim H, Choe YS, Jung KH, Lee KC, et al. Facile method to radiolabel glycol chitosan nanoparticles with Cu-64 via copper-free click chemistry for microPET imaging. Mol Pharmaceutics. 2013;10:2190–2198. doi: 10.1021/mp300601r. [DOI] [PubMed] [Google Scholar]
- 162.Fuaad AA, Azmi F, Skwarczynski M, Toth I. Peptide conjugation via CuAAC ‘click’ chemistry. Molecules. 2013;18:13148–13174. doi: 10.3390/molecules181113148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li HY, Aneja R, Chaiken I. Click chemistry in peptide-based drug design. Molecules. 2013;18:9797–9817. doi: 10.3390/molecules18089797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mascarin A, Valverde IE, Vomstein S, Mindt TL. 1,2,3-Triazole stabilized neurotensin-based radiopeptidomimetics for improved tumor targeting. Bioconjugate Chem. 2015;26:2143–2152. doi: 10.1021/acs.bioconjchem.5b00444. [DOI] [PubMed] [Google Scholar]
- 165.Valverde TE, Vomstein S, Fischer CA, Mascarin A, Mindt TL. Probing the backbone function of tumor targeting peptides by an amide-to-triazole substitution strategy. J Med Chem. 2015;58:7475–7484. doi: 10.1021/acs.jmedchem.5b00994. [DOI] [PubMed] [Google Scholar]
- 166.Valverde IE, Vomstein S, Mindt TL. Toward the optimization of bombesin-based radiotracers for tumor targeting. J Med Chem. 2016;59:3867–3877. doi: 10.1021/acs.jmedchem.6b00025. [DOI] [PubMed] [Google Scholar]
- 167.Zhang L, Navaratna T, Liao J, Thurber GM. Dual-purpose linker for alpha helix stabilization and imaging agent conjugation to glucagon-like peptide-1 receptor ligands. Bioconjugate Chem. 2015;26:329–337. doi: 10.1021/bc500584t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang L, Navaratna T, Thurber GM. A helix-stabilizing linker improves subcutaneous bioavailability of a helical peptide independent of linker lipophilicity. Bioconjugate Chem. 2016;27:1663–1672. doi: 10.1021/acs.bioconjchem.6b00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Doss M, Kolb HC, Zhang JJ, Belanger MJ, Stubbs JB, Stabin MG, Hostetler ED, Alpaugh RK, von Mehren M, Walsh JC, et al. Biodistribution and radiation dosimetry of the integrin marker F-18-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J Nucl Med. 2012;53:787–795. doi: 10.2967/jnumed.111.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leyton J, Iddon L, Perumal M, Indrevoll B, Glaser M, Robins E, George AJT, Cuthbertson A, Luthra SK, Aboagye EO. Targeting somatostatin receptors: Preclinical evaluation of novel F-18-fluoroethyltriazole-Tyr(3)-octreotate analogs for PET. J Nucl Med. 2011;52:1441–1448. doi: 10.2967/jnumed.111.088906. [DOI] [PubMed] [Google Scholar]
- 171.Dubash SR, Keat N, Mapelli P, Twyman F, Carroll L, Kozlowski K, Al-Nahhas A, Saleem A, Huiban M, Janisch R, et al. Clinical translation of a click-labeled 18F-octreotate radioligand for imaging neuroendocrine tumors. J Nucl Med. 2016;57:1207–13. doi: 10.2967/jnumed.115.169532. [DOI] [PubMed] [Google Scholar]