Abstract
Rho-associated kinase 2 (ROCK2) determines the balance between human T helper 17 (TH17) cells and regulatory T (Treg) cells. We investigated its role in the generation of T follicular helper (TFH) cellswhich provide help necessary to generate antibody-producing B cells in normal and autoimmune conditionsWe found that targeting ROCK2 in normal human T cells or peripheral blood mononuclear cells (PBMCs) from patients with active systemic lupus erythematosus (SLE) decreased the number and function of Tfh cells induced by activation ex vivo. Moreover, inhibition of ROCK2 activity concomitantly decreased the abundance of the transcriptional regulator Bcl6 and increased that of Blimp1 by xxx concurrent regulation of the binding of signal transducer and activator of transcription 3 (STAT3) and STAT5 to the promoters of the genes Bcl6 and PRDM1, respectively. In the MRL/lpr murine model of SLE, oral administration of the selective ROCK2 inhibitor KD025 resulted in a two-fold reduction in the numbers of Tfh cells and antibody-producing plasma cells in the spleen, as well as a decrease in the size of splenic germinal centers, sites of interaction between TFH cells and B cells. KD025-treated mice showed a substantial improvement in both histological and clinical scores compared to those of untreated mice, and had reduced amounts of Bcl6 and phosphorylated STAT3, as well as increased STAT5 phosphorylation. Together, these data suggest that ROCK2 signaling plays a critical role in controlling the development of Tfh cells induced by autoimmune conditions through reciprocal regulation of STAT3 and STAT5 activation.
Introduction
T follicular helper (TFH) cells are an essential component of protective humoral immune responses that support B cell activation, and differentiation in germinal centers within secondary lymphoid organs (1–3). However, aberrant Tfh cell activity can also lead to autoimmune disorders, such as systemic lupus erythematosus (SLE), which is characterized by an increased number of self-reactive mature B cells and high amounts of auto-antibodies (antibodies against self-antigens) (4–6). Tfh cells express the B cell zone–homing chemokine receptor CXC-chemokine receptor 5 (CXCR5), high amounts of Programmed cell Death protein 1 (PD1) and Inducible T-cell COStimulator (ICOS), but have decreased amounts of the T cell zone–homing receptor CC-chemokine receptor 7 (CCR7) (7–9). Both the differentiation and function of TFH cells depend on activating signals from pro-inflammatory cytokines, such as interleukin-6 (IL-6) and IL-21 and co-stimulatory molecules, including ICOS and CD40 ligand (CD40L), (10–12), and require the activation of transcription factors, including signal transducer and activator of transcription 3 (STAT3) and B cell lymphoma 6 (Bcl6) (13–15). The generation of Tfh cells is inhibited by IL-2–stimulated STAT5 signaling, which induces expression of PRDM1 [the gene encoding B lymphocyte–induced maturation protein 1 (Blimp1)] to suppress Bcl6 function (16, 17). Although it appears that Tfh cells are oppositely regulated by activation of the STAT3-Bcl6 and STAT5-Blimp1 pathways (18), the upstream signaling events involved in controlling the precise balance between these transcriptional programs in health and disease states remain enigmatic.
The Rho kinase family members, consisting of Rho-associated kinase 1 (ROCK1) and ROCK2, play a central role in the control of intracellular signaling cascades involved in the regulation of cytoskeletal reorganization and the acquisition of the appropriate effector phenotype in T cells (19, 20). Specifically, ROCK2 is critical in induction of IL-21 and IL-17 secretion by T cells and the development of autoimmunity in both mice and humans (21–23). Additionally, ROCK activity in T cells is increased in patients with SLE compared to that in healthy controls (24). Here, we found that ROCK2 played an instrumental and previously unidentified role in the increased number and function of Tfh cells induced by autoimmune conditions. We demonstrated this role in vitro in experiments with T cells from healthy individuals; in vivo with the MRL/lpr mouse model of SLE; and in peripheral blood mononuclear cells (PBMCs) purified from active SLE patients and stimulated ex vivo. Furthermore, the ROCK2-mediated generation of Tfh cells consistently involves a competitive antagonism of STAT3 and STAT5 activation across the aforementioned experimental systems, and represents a previously uncharacterized paradigm of therapy for Tfh-driven autoimmune disorders.
Results
Specific inhibition of ROCK2 reduces the percentage of human Tfh cells through opposing regulation of STAT3 and STAT5 transcriptional activity
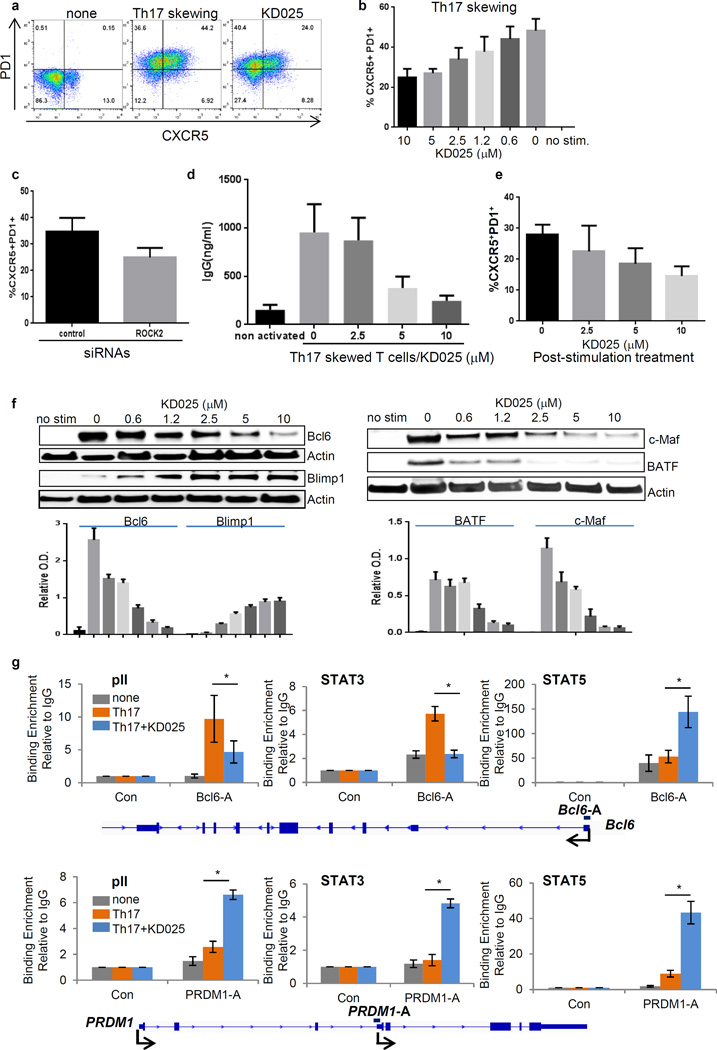
CXCR5+ Tfh cells represent a heterogeneous subset of CD4+ T cells that are localized in germinal centers in secondary lymphoid organs and are also found circulating in the peripheral blood (1, 18). Studies demonstrated that functional human Tfh cells can be generated in vitro by stimulation of the T cell receptor (TCR) of naïve CD4+ T cells in the presence of proi-nflammatory cytokines, such as transforming growth factor–β (TGF-β), interleukin-1β (IL-1β), and IL-6 (25), which are essential for the generation (skewing) of human Th17 cells from naïve CD4+ T cells in vitro (26). Indeed, these in vitro–generated Tfh cells share similarities with Th17 cells, such as their high abundances of RAR-related orphan receptor gamma t (RORγt)), IL-17, and IL-21 (25). Specific inhibition of ROCK2 decreases the amounts of both IL-17 and IL-21 produced by human peripheral CD4+ T cells that are activated under Th17 cell–skewing conditions, such as stimulation with anti-CD3 and anti-CD28 antibodies in the presence of both IL-1β and TGF-β (22). Here, we report that this activation protocol gave rise to a subset of cells that were characterized by high amounts of Tfh cell–associated markers, including CXCR5, PD1, ICOS, and CD40L, as well as low amounts of CCR7 compared to those of nonactivated cells (fig. S1A). After 2 days of culture, approximately 70% of the IL-21–producing cells were within the CXCR5+PD1+ population (fig. S1B). Moreover, neutralization of IL-21 signaling during the stimulation process led to a twofold reduction in the percentage of CXCR5+PD1+ cells within the population (fig. S1C). In addition, the CXCR5+PD1+ICOS+CD40L+CCR7− subset of cells promoted autologous B cells in cocultures to secrete antibodies in the presence of the antigen staphylococcal enterotoxin B (SEB), confirming the Tfh cell–like phenotype of the cells activated under Th17 cell–skewing conditions in vitro (fig. S1D).
We next found that the selective ROCK2 inhibitor KD025 dose-dependently decreased the percentage of human CXCR5+PD1+ICOS+CD40L+ cells (of total CD4+ T cells) generated by Th17 cell–skewing conditions (Fig. 1, A and B; fig. S2A). KD025 is an ATP-competitive inhibitor and is 100-fold more selective for ROCK2 than for ROCK1 (22, 27). To further confirm the role of ROCK2 in the KD025-depedendent effects on CXCR5+PD1+ cells, we used ROCK2-specific small interfering RNA (siRNA), which reduced the amount of ROCK2 protein in human CD4+ T cells by 70% (fig. S2B) and decreased the percentage of CXCR5+PD1+ T cells (Fig. 1C). This reduction in the relative size of the CXCR5+PD1+ subset correlated with a dose-dependent decrease in the number of IL-21–producing cells (fig. S2C) and limited the ability of these cells to induce autologous B cells to secrete antibody in vitro (Fig. 1D). Furthermore, KD025 decreased the percentage of CXCR5+PD1+ T cells even in the presence of IL-21 (fig. S3A), during longer periods (5 days) of Th17 cell–skewing conditions (fig. S3B), or when it was added to cells after they had been stimulated (Fig. 1E).
Fig. 1. ROCK2 is required for the induction of human Tfh cells through its reciprocal regulation of STAT3 and STAT5 transcriptional activity.
(A to H) Human peripheral blood CD4+ T cells were treated with the selective ROCK2 inhibitor KD025 (A, B, and D to H) or were transfected with control or ROCK2-specific siRNAs (C) and then were left unstimulated or were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies in combination with IL-1β and TGF-β (for Th17-type skewing) for 48 hours. (E) Some cells were stimulated under Th17-type skewing conditions for 5 days, treated with or without KD025, and then re-stimulated for 48 hours. (A to C and E) The percentages of CXCR5+PD1+ cells were determined by flow cytometric analysis, whereas cell extracts were analyzed by Western blotting with antibodies against the indicated proteins (F). (D) Treated T cells were co-cultured with autologous B cells for 7 days in the presence of SEB (0.05 ng/ml), and IgG secretion was determined by ELISA. (G and H) ChIP assays were performed with normal rabbit IgG, anti-RNA pII, anti-STAT3, and anti-STAT5 antibodies. Data are means ± SEM of six (B, G, H), five (C, E), or three (D) experiments. Western blots in (F) are from a single experiment and are representative of three independent experiments, whereas data in bar graphs are means ± SEM of three independent experiments. Statistical analysis was performed with the Wilcoxon test. The Bonferroni method was used for controlling for multiplicity (B). *P < 0.05.
Bcl6 is a key transcription factor that mediates the differentiation of naïve CD4+ T cells into Tfh cells, and it is activated through STAT3 signaling and inhibited through STAT5 signaling (28–30). We found that the treatment of T cells cultured under TH17 cell–skewing conditions with KD025 reduced the abundance of Bcl6, as well as those of Basic Leucine Zipper ATF-like Transcription Factor (BATF) and Musculoaponeurotic Fibrosarcoma oncogene homolog (c-MAF), and increased Blimp1 abundance in a dose-dependent manner (Fig. 1F). To test whether the ROCK2-mediated contrasting regulation of Bcl6 and Blimp1 abundances involved the regulation of STAT3 and STAT5 binding to the promoters of both Bcl6 and PRDM1 (which encodes Blimp1), we performed chromatin immunoprecipitation (ChIP) assays. In CD4+ T cells activated for 2 days in Th17 cell–skewing conditions, both STAT3 and RNA polymerase II (pII) were enriched at the Bcl6 promoter compared to the control site (a region downstream to uncoupled protein 1 (UCP1) gene) in human T cells (Fig. 1G). Moreover, KD025 substantially decreased the extent of binding of STAT3 and pII to the same site, while increasing the extent of STAT5 binding threefold (Fig. 1G). In addition, knocking down STAT3 with a specific siRNA in CD4+ T cells resulted in an ~80% reduction in the amount of Bcl6 and decreased the percentage of CXCR5+PD1+ T cells in the culture (fig. S4A). These data suggest that in human T cells under Th17 cell–skewing conditions, both STAT3 (positively) and STAT5 (negatively) contribute to the ROCK2-mediated regulation of Bcl6 abundance.
Treatment of Th17 cell–skewed cells with KD025 resulted in three- and five-fold increases, respectively, in the amounts of pII and STAT5 that bound to the PRDM1 promoter compared to the amounts of these transcription factors that bound in untreated cells (Fig. 1H). In addition, we detected the binding of STAT3 to the promoter of PRDM1 in human CD4+ T cells (Fig. 1H). Consistent with a positive role for STAT3 in the regulation of PRDM1 expression in mouse B and T cells (31), the extent of STAT3 binding to the PRDM1 promoter was further increased in human CD4+ T cells treated with KD025 compared to that in cells cultured under Th17-skewing conditions (Fig. 1H), despite the overall decrease in the extent of phosphorylation of STAT3 (fig. S4B). In addition, STAT3-specific siRNA reduced the abundance of Blimp1 in transfected human T cells (fig. S4C). Thus, under Th17 cell–skewing conditions, the targeting of ROCK2 oppositely regulated the transcriptional activities of STAT3 and STAT5 to reduce Bcl6 abundance and increase Blimp1 abundance in human T cells.
Human Tfh cells generated under TH17 cell–skewing, but not TH1 cell–skewing, conditions are dependent on ROCK2 signaling
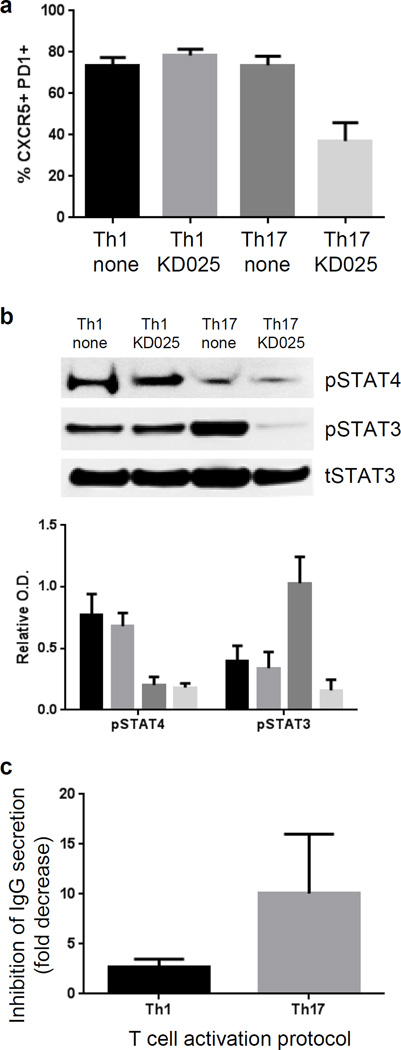
It has been suggested that TGF-β plays opposing roles in the regulation of Tfh cells in mice and humans. In mice, TGF-β decreases the amounts of proteins that are required for Tfh cell generation and function including IL-21, ICOS, and Bcl6 (15, 32). In contrast, TGF-β in combination with IL-23 or IL-12 promotes the differentiation of naïve human CD4+ T cells into Tfh subsets that are dependent on STAT3 or STAT4 signaling in Th17 cell– and Th1 cell–skewing conditions, respectively (25). Furthermore, accumulating evidence also indicates that the percentage of the Th17 cell–type, but not the Th1 cell–type, of Tfh cells is increased in the circulation of patients with autoimmune disorders, including SLE and Sjogren’s syndrome (2). Because ROCK proteins are implicated in TGF-β-induced signaling (33), and because inhibition of ROCK2 activity specifically reduces the phosphorylation of STAT3, but not STAT4 (22), it is therefore possible that ROCK2 contributes differently to the Th17-type cytokine– and Th1-type cytokine–dependent generation of Tfh cells. Through the activation protocol described by Schmitt et al. (25), we found that KD025 led to a twofold reduction in the percentage of CXCR5+PD1+ cells induced by activation under TH17 cell–skewing conditions (that is, in the presence of IL-1β, TGF-β, IL-6, and IL-23), but had no effect in a culture that favors the generation of Th1 cell–type Tfh cells (that is, in the presence of IL-1β, TGF-β, IL-6, and IL-12; Fig. 2A). Moreover, KD025 decreased the extent of phosphorylation of STAT3 in cells activated under TH17 cell-skewing conditions, as well as the ability of the cells to induce immunoglobulin G (IgG) secretion from B cells, but KD025 had no effect on STAT4 phosphorylation induced by activation under Th1 cell–skewing conditions (Fig. 2, B and C). Together, these data suggest that ROCK2 signaling specifically contributes to the generation of a Th17-type cytokine–stimulated subset of Tfh cells through the activation of STAT3, but had a limited effect on theSTAT4-dependent subset of Tfh cells.
Fig. 2. STAT3-dependent, but not STAT4-dependent, human Tfh cells are induced by ROCK2 signaling.
(A to C) Naïve CD4+ T cells were stimulated for 4 days with anti-CD3 and anti-CD28 monoclonal antibodies in the presence of IL-1β, TGF-β, and IL-6 in combination with IL-12 (for Th1-type skewing conditions) or IL-23 (for Th17-type skewing conditions) in the presence or absence of 10 µM KD025. (A) The percentages of CXCR5+PD1+ cells were determined by flow cytometry. (B) Top: Cell extracts were analyzed by Western blotting with antibodies against the indicated proteins. Bottom: Densitometric analysis of the indicated band intensities was performed. (C) The amount of secreted IgG was determined by ELISA after culturing the indicated T cells with autologous B cells for 7 days in the presence of SEB (0.05 ng/ml). Data in (A), (C), and the bar chart in (B) are means ± SEM of three independent experiments. Western blots in (B) are from a single experiment and are representative of three independent experiments. Statistical analysis was performed with the Wilcoxon test.
Oral administration of KD025 decreases the number of Tfh cells and blocks disease progression in the Mrl/lpr mouse model of lupus
To assess the potential of ROCK2 inhibition to decrease Tfh cell numbers in vivo, we used the MRL/lpr mouse, which spontaneously develops a severe systemic autoimmune disease similar to that of SLE patients (34) and exhibits an increase in the number of Tfh cells during disease progression (35). Additionally, increased ROCK2 activation is observed in stimulated CD4+ T cells from MRL/lpr mice (21). We found that oral administration of KD025 beginning at 11 weeks of age substantially ameliorated disease progression as demonstrated by reductions in proteinuria, kidney histology scores, and concentrations of urea nitrogen and anti-dsDNA antibodies in the blood (Fig. 3, A to C and fig. S5A) compared to those of mice treated with vehicle control. The therapeutic effect of KD025 was comparable to the effect of the general immunosuppressive agent cyclophosphamide (Fig. 3A); however, KD025 did not decrease the total number of lymphocytes in the spleen at the end of the treatment on week 17 to the same extent as did cyclophosphamide (Fig. 3D). Furthermore, KD025 markedly reduced the percentage of IL-21+ cells (fig. S5B) as well as of both Tfh cells (CD4+CXCR5+PD1+) and mature plasma cells (B220lowCD138hi), which was accompanied by a reduction in the size of germinal centers in the spleen compared with those of vehicle-treated mice (Fig. 3, E to G). This finding was consistent with a decrease in the percentage of CD4+CXCR5+PD1+ mouse T cells treated with KD025 in vitro (fig. S5C). The aforementioned cellular changes in vivo were complemented by a decrease in the amounts of pSTAT3 and BCL6 in total spleenocytes in addition to an increase in the amount of pSTAT5 (Fig. 3H). This latter change in pSTAT5 abundance was not detected in splenocytes from cyclophosphamide-treated animals (Fig. 3H). Thus, in contrast to causing general immune suppression in vivo, targeted ROCK2 inhibition decreased the percentage of Tfh cells through differential regulation of STAT3 and STAT5 phosphorylation in the MRL/lpr mouse model of lupus.
Fig. 3. Treatment of MRL/lpr mice with KD025 decreases the percentage of CXCR5+PD1+ Tfh cells through a STAT3- and STAT5-dependent mechanism.
(A to H) MRL/lpr mice (15 per group) were orally treated with vehicle or KD025 (100 mg/kg) once a day or were intraperitoneally treated once a week with cyclophosphamide (50 mg/kg) beginning on week 11 and continuing until week 17. (A) The mean disease score ± SEM was defined by the extent of proteinuria. The concentrations of blood urea nitrogen (B) and anti-dsDNA antibodies (C) in serum were measured at the end of the study. (D to F) Splenocytes were analyzed by flow cytometry to determine the total numbers of cells (D), the percentage of CXCR5+PD1+ cells (E), and the percentage of plasma cells (F). (G) Representative sections of the spleens of the indicated mice at the end of the study were subjected to H&E staining. Arrows indicate GCs. (H) Total spleenocytes were analyzed by Western blotting with antibodies against pSTAT3 (n = 5), Bcl6 (n = 5), and pSTAT5 (n = 4). P values were calculated with the Mann-Whitney test.
Targeted ROCK2 inhibition decreases pSTAT3 and Bcl6 abundances while increasing the amounts of pSTAT5 and Blimp1 in PBMCs purified from patients with active SLE
The percentage of blood cells that are Tfh cells is increased in patients with active SLE compared to that in normal healthy donors (24, 36, 37). Tfh cells are also found in T cell and B cell aggregates and in ectopic germinal centers in the kidneys of patients with lupus nephritis, which is suggestive of their pathogenic role in the most prevalent severe manifestation of human SLE (38). Furthermore, PBMCs from active SLE patients have increased ROCK activity, consistent with a previously reported role for ROCK2 signaling in the induction of IL-17 and IL-21 secretion (21, 22, 24). We therefore obtained PBMCs from six active SLE patients who exhibited from mild to severe disease severity (fig. S6A), and we stimulated the cells ex vivo under Th17 cell–skewing conditions in the presence or absence of KD025. Similar to its effects on cells derived from healthy individuals, KD025 decreased the percentages of IL-17– and IL-21–secreting cells from SLE patients by 60 and 70%, respectively, at the highest concentration of inhibitor used, and by 30 and 40%, respectively, at the lowest concentration (fig. S6B). Moreover, under the same conditions, KD025 markedly reduced the percentage of CXCR5+PD1+ T cells in culture in a dose-dependent manner (Fig. 4A). In addition, the treatment of SLE patient–derived PBMCs with KD025 substantially decreased the phosphorylation of STAT3 and markedly reduced the amount of Bcl6 (Fig. 4, B and C). Consistent with the findings observed in cells from healthy individuals, KD025 led to three-fold increases in pSTAT5 and Blimp1 abundances in SLE patient–derived cells cultured under Th17 cell–skewing conditions (Fig. 4, D and E). These data suggest that ROCK2 contributes to the regulation of CXCR5+PD1+ Tfh cells derived from normal and SLE patient populations through a common molecular mechanism that involves concurrent regulation of the phosphorylation of STAT3 and STAT5 and the abundance of Bcl6 and Blimp1.
Fig. 4. KD025 decreases the abundances of pSTAT3 and Bcl6 while increasing the abundances of pSTAT5 and Blimp1 in PBMCs from patients with active SLE.
(A to C) PBMCs from six active SLE patients were treated with the indicated concentrations of KD025 for 1 hour and then were stimulated under Th17-type skewing conditions. (A) The percentage of CXCR5+PD1+ cells was determined by flow cytometric analysis. (B to E) The cells were analyzed by Western blotting with antibodies specific for (B) pSTAT3 (n = 5), (C) Bcl6 (n = 4), (D) pSTAT5 (n = 3), and (E) Blimp1 (n = 3). Statistical analysis was performed with the Wilcoxon test.
Discussion
Tfh cells provide the initiating events for the antigen-driven selection of B cells during the generation of antibody responses and appear to determine germinal center selection (39). Whereas an increase in Tfh cell number during immune responses against foreign antigen might be expected, these increases may affect a change in the nature of antibody being produced by expending B-cell clones which recognize more than the dominant foreign antigens and therefore can facilitate the production of autoantibodies and development of autoimmunity (2, 6, 18). Therefore, Tfh cell function must be precisely controlled during the host immune response.
ROCK2 signaling promotes the secretion of pro-inflammatory cytokines and shifting the balance between Th17 cell and Treg cell subsets (21, 23), but the role of ROCK2 in controlling Tfh cells is unclear. We report here that the Th17 cell–skewing of human T cells in vitro generated a subset of functional Tfh cells that were characterized by high amounts of CXCR5, PD1, ICOS, and CD40L, and a low abundance of the receptor CCR7 (fig. S1A). Through either pharmacological or siRNA-mediated inhibition of ROCK2, we found that this subset of human Tfh cells was dependent on ROCK2 signaling as well as on intact IL-21 and STAT3 signaling (Fig. 1A to E; fig. S1C; fig. S2B; fig. S4A). STAT4 signaling, together with that of STAT3, is required for generation and function of Tfh cells in humans (25). We found that treatment naïve CD4+ T cells with the selective and orally available ROCK2 inhibitor KD025 reduced the percentage and function of Tfh cells differentiated in vitro by IL-23 via STAT3-dependent mechanism, but not IL-12-induced Tfh cells which are dependent on STAT4 signaling (Fig. 2). This finding correlated with the lack of effect of KD025 on STAT4 phosphorylation in human T cells (22). The percentage of Th17 cell–type Tfh cells is also markedly increased in the peripheral blood of patients with various autoimmune diseases, including SLE and Sjogren’s syndrome (40, 41), whereas the Th1 cell-type of Tfh cells is underrepresented in such diseases. These observations suggest that Th17-type Tfh cells specifically contribute to the pathogenesis of certain autoimmune diseases, whereas Th1-type Tfh cells might have a role in the regulation of protective humoral responses under normal conditions (2). Thus, it is possible that targeting of Th17-type cytokine–dependent Tfh cells by selective ROCK2 inhibition could effectively attenuate autoimmune responses and affect the subdominant B cell autoantibody repertoire while preserving the ability of the immune system to provide a protective humoral response against foreign pathogens. These findings suggest a previously uncharacterized paradigm of therapeutic approach for Tfh cell–dependent diseases.
Proinflammatory cytokines, such as IL-1, IL-6 and IL-21, stimulate Tfh cell function and development through STAT3 and Bcl6 (8, 10, 13–15, 25, 28). Conversely, IL-2 signaling has an inhibitory effect on Tfh cell function through the activation of STAT5 and Blimp1, which both antagonize Bcl6 activity (3, 15–17). We found that targeted inhibition of ROCK2 decreased the abundance of Tfh cell–associated transcription factors, such as Bcl6, BATF, and c-Maf, while increasing the amount of Blimp1 (Fig. 1F). We also showed that, together with a reduction in Bcl6 abundance, targeted ROCK2 inhibition also decreased STAT3 and increased STAT5 binding to the Bcl6 and IL-21 promoters (Fig. 1G and fig. S7). Thus, the ROCK2-mediated opposing modulation of STAT3 and STAT5 phosphorylation led to differential regulation of the activity of these transcription factors in human T cells. This correlates with the previously reported differential effects of STAT3 and STAT5 on Bcl6 expression and Bcl6 protein abundance in mouse lymphocytes and cancer cell lines (30, 42). Note that although STAT3 phosphorylation is inhibited in KD025-treated cells (22), we found that the binding of both STAT3 and STAT5 to the PRDM1 promoter was markedly increased by KD025 in human Th17-skewed T cells (Fig. 1H). In mice, PRDM1 expression is induced by the IL-21–STAT3 and IL-2–STAT5 signaling pathways (15–17, 31) and is suppressed by Bcl6 (43), which leads to changes in Blimp1 abundance. Because KD025 decreases both IL-21 production and STAT3 phosphorylation in human T cells (22), targeted ROCK2 inhibition increases Blimp1 protein abundance mainly through STAT5 activation and a simultaneous decrease in Bcl6 abundance during Th17 cell–skewing in vitro (30).
The molecular mechanism of targeted ROCK2 inhibition in Tfh cell–dependent autoimmunity was further confirmed in vivo by demonstrating that oral administration of KD025 substantially decreased STAT3 phosphorylation and Bcl6 abundance, while increasing STAT5 phosphorylation, in the spleens of MRL/lpr mice. These changes correlated with a marked decrease in the numbers of Tfh cells and mature B cells, with the sequelae of reduced germinal center size (Fig. 3, D to G) followed by prevention of kidney damage and disease progression (Fig. 3, A to C). The therapeutic effect of targeted ROCK2 inhibition in vivo was comparable to the effect of the general immunosuppressive agent cyclophosphamide (Fig. 3, A and B; fig. S5A). However, in contrast to the five-fold reduction in the total number of lymphocytes in the spleens of cyclophosphamide-treated mice, KD025 reduced cell numbers comparably to those in naïve mice (Fig. 3D). The concentration of KD025 in the plasma of treated MRL/lpr mice (fig. S8) was comparable to the concentrations used for ex vivo cultures of PBMCs from patients with active SLE (Fig. 4). In addition, these concentrations were reached in healthy individuals during oral administration of the drug in a Phase 1 clinical trial in which a marked decrease in the secretion of IL-21 and IL-17 was observed (22). These data suggest that patients with SLE or other Tfh cell–dependent autoimmune disorders could potentially benefit from a simultaneous shift in the Th17 cell–Treg cell balance as well as from the decrease in the size of the Th17 cell–dependent Tfh cell population by KD025.
In conclusion, our data suggest that ROCK2 plays an essential role in controlling a subset of Tfh cells through a mechanism that involves the reciprocal regulation of the activation of STAT3 and STAT5 and the abundances of Bcl6 and Blimp1 in T cells from healthy individuals and PBMCs derived from patients with active SLE, as well as in the MRL/lpr mouse model of SLE. These data provide insights into the molecular pathways involved in the regulation of Tfh cells and underscore the therapeutic potential of targeted ROCK2 inhibition in Tfh-cell driven autoimmune disorders.
Materials and Methods
Cell purification and activation
Total or naïve CD4+ T cells were purified from the peripheral blood of healthy human donors between the ages of 16 and 75 years (New York Blood Center, NY, NY) as previously described (22). Briefly, whole blood was incubated with RosetteSep™ human CD4+ T cell enrichment cocktail (StemCell Technologies) for 20 min at 22°C. The remaining un-sedimented cells were loaded onto Ficoll-Paque Plus (Amersham Bioscience), isolated by density centrifugation, and washed with phosphate-buffered saline (PBS). Untouched naïve human CD4+ T cells were purified from human blood with the EasySep Human Naïve CD4+ T Cell Enrichment Kit (Stem Cell #19155), after purification of PBMC by ficoll, to >98% purity according to the manufacturer’s instructions. B cells were purified with the Rosette Sep B cell Enrichment kit (Stem Cell #15064) to >95% purity and were frozen at −80°C in 90% FBS, 10% DMSO. PBMCs from six patients with active SLE (fig. S6A) were purchased from Sanguine Biosciences. Total CD4+ T cells were activated with immobilized anti-CD3 and anti-CD28 monoclonal antibodies (eBioscience; both at 5 µg/ml) in the presence of IL-1β (50 ng/ml) and either TGF-β (5 ng/ml) or IL-21 (5 or 25 ng/ml) (R&D Systems Inc.). Naïve CD4+ T cells were cultured with IL-1β (10 ng/ml), TGF-β (5 ng/m), and IL-6 (25 ng/ml) in combination with IL-12 (1 ng/ml) or IL-23 (25 ng/ml) (eBioscience) for four days to induce the generation of Th1-type and Th17-type Tfh cells, respectively, as previously described (25). All media used for cell culture contained RPMI supplemented with 10% FBS, L-glutamine, penicillin, streptomycin and sodium pyruvate. For experiments in which IL-21 was blocked during cell skewing, recombinant human IL-21R Fc chimera protein (R&D Systems, 991-R2-100) was used. After activation, the cells were analyzed by flow cytometry to determine the surface abundances of CXCR5, PD1, ICOS, CD40L, and CCR7, as well as the intracellular amounts of IL-17 and IL-21. Cells extracts were analyzed by Western blotting according to standard protocols.
Differentiation of mouse Tfh cells in vitro
The protocol used was the same as that described previously (44). Briefly, naïve mouse CD4+ T cells were purified from C57BL/6 mouse spleens with the EasySep Mouse Naïve CD4+ T Cell Isolation Kit (#19765; StemCell Technologies). Cells were then incubated for three days in wells coated with anti-mouse CD3 (#16-0031-85, eBioscience, San Diego, CA) and anti-mouse CD28 (#16-0281-85, eBioscience) antibodies (both at 8 µg/ml) in the presence of anti-mouse IFN-γ (#MAB485-100, R&D Systems Inc.) and anti-mouse IL-4 (#MAB404-100, R&D Systems Inc.) antibodies together with either mouse IL-21 (10 ng/ml; MN #594-ML-010, R&D Systems Inc.) or mouse IL-6 (10 ng/ml; #406-ML-005, R&D Systems Inc.) and with DMSO (as a vehicle control) or with 10 µM KD025.
T and B cell coculture assays
T cells were activated and treated with cytokines as described earlier. After two (for total CD4+ T cells) or four (for naïve CD4+ T cells) days of activation, cells were either sorted on the basis of forward scatter (FSC) and side scatter (SSC) on a FACS ARIA II cell sorter at the NYU School of Medicine Flow Cytometry Core Facility based on large size or, alternatively, anti-CD3/28 magnetic Dynabeads were removed from cell culture by two rounds of magnetic separation. The cells were washed, counted, and then combined with thawed B cells from the same donor in a 96-well U-bottom plate. Each well contained 2.5 × 103 T cells and 4 × 104 B cells. Low Endotoxin Staphylococcal Enterotoxin B (SEB)(Toxin Technologies) was used final concentration: 0.05 ng/ml in a total of 200 µl/well. After 7 or 14 days, the contents of the wells were centrifuged and the supernatants were analyzed for total IgG concentrations with a Human IgG ELISA Kit (#E88-104, Bethyl Laboratories).
Inhibitors and RNA interference
The selective ROCK2 inhibitor KD025 [formerly known as Slx-2119 (27)] was dissolved in DMSO. KD025 was found previously to have no substantial activity against 300 intracellular kinases (including ROCK1) and surface receptors (22). Mixtures of four ON-TARGETplus SMARTpool siRNAs specific to ROCK2 (L-004610-00) or STAT3 (L-003544-00) and control siRNA (D-001810-10-20) were purchased from Dharmacon (Thermo Fisher Scientific Inc.). Transfection of freshly purified T cells with these siRNAs was performed with the human T cell Nucleofector kit (Amaxa Biosystems, Lonza). Transfection efficiency was determined by evaluating the amounts of ROCK2 and STAT3 proteins by Western blotting analysis 48 hours after transfection.
Western blotting analysis
Total cell lysates, or nuclear and cytoplasmic extracts were prepared and analyzed for protein content. Sample buffer was then added, and, after boiling, samples, containing equal amounts of proteins, were resolved by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked and incubated with the appropriate antibodies overnight. Anti-ROCK2 antibody (HPA007459) was purchased from Sigma-Aldrich and anti β-actin antibody (4970S) was purchased from Cell Signaling. Anti-pSTAT3 (#4113), anti-STAT3 (#4904), anti-pSTAT5 (#4322S), anti-Bcl6 (#14895), anti-Blimp1 (#9115) and anti-BATF (#8638) antibodies were purchased from Cell Signaling Technologies. Anti c-Maf antibody was obtained from Santa Cruz Biotechnology Inc. Immunoreactive protein bands were visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies and an enhanced ECL system. Quantification of band intensities was performed with NIH ImageJ software.
ChIP assays
Peripheral blood CD4+ T cells were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies, IL-1β (50 ng/ml), and TGF-β (5 ng/ml) for 48 hours in the absence or presence of 10 µM KD025. Cells were then harvested and treated with 1% formaldehyde for 10 min, quenched with 0.125 M glycine, washed with cold PBS and lysed in buffer A [50 mM hepes (pH 7.5), 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% Sodium-deoxycholate, 0.1% SDS and 1× protease inhibitor]. Lysates were sonicated and subjected to centrifugation, and supernatants were incubated overnight at 4°C with protein G-Dynabeads that were pre-conjugated with the appropriate antibodies. The immunoprecipitated Dynabeads were washed with buffer B [20 mM Tris-HCl (pH 8.1), 500 mM NaCl, 2 mM EDTA, 1% Triton X-100 and 0.1% SDS] and then with TE buffer before being eluted with buffer C (1% SDS in TE) at 65°C for 1 hour. The eluates were then incubated at 65°C for 6 hours or overnight to reverse cross-linking before being purified with ChIP DNA clean and concentrator (Zymo Research). Binding of factors were determined by real-time PCR with designed primer sets.
Mouse model of lupus
MRL/lpr mice were obtained from Jackson Laboratories (Stock No: 000485), and all in vivo studies were performed by Hooke Labs. All of the mice used in the experiments were kept under specific pathogen–free conditions. KD025 (100 mg/kg) was administered to mice orally once a day for 6 weeks beginning from 11 weeks of age. Mice (15 per group) were monitored weekly for proteinuria with Chemstrip 2GP (Roche # 200743). Scoring for proteinuria was performed as follows: 0, no protein; 1, protein of > 30 mg/dl; 2, protein of 30 to 100 mg/dl; 3, protein of 100 to 500 mg/dl; 4, protein > 500 mg/dl. The plasma concentrations of anti-dsDNA antibodies were measured with an anti-mouse dsDNA antibody ELISA kit (Biovendor #RSHAKRDD061R). Blood urea nitrogen was tested with a urease/Glutamate Dehydrogenase (GLDH) assay kit (Olympus Reagents) and was performed by IDEXX Laboratories. Histological and Flow analyses of kidneys and spleens were performed at the end of the treatment (week 17).
Histological analysis of kidneys and spleens
At the end of the study, one kidney from each mouse was collected and analyzed histologically. Two PAS-stained sections were analyzed from each mouse. Histological analysis was performed by a pathologist blinded to the experimental groups and all readouts. Histological findings were graded on a 0–4 scale as follows: 0-no lesions; 1-minimal thickening of mesangium; 2- increased mesangium thickness and glomerular cellularity; 3-increased mesangium thickness and glomerular cellularity with inflammatory exudates and capsular adhesions; 4-glomerular architecture obliterated in more than 70% of glomeruli. Half of the spleen from each mouse was fixed in formalin and analyzed histologically. One H&E-stained section was analyzed from each mouse. The diameter of 10 randomly selected spleen follicles was measured for each mouse and the average calculated. The number of spleen follicles per viewing field was counted in three viewing fields and the average calculated for each mouse.
Flow cytometry
All flow cytometric analysis was performed with a Millipore Guava EasyCyte 8HT flow cytometer. For intracellular staining of cytokines, cells were stimulated for 4 hours with Cell Stimulation Cocktail (00-4975-03; eBioscience), which contains phorbol 12-myristate 13-acetate (PMA), ionomycin, and protein transport inhibitor, and then were fixed and permeabilized. Viability was assessed with a viability dye that stained dead cells (eBioscience 65-0865-18). Staining of human cells was performed with the following fluorescently conjugated antibodies: CD4 PerCP eFluor 710 (Clone SK3), PD-1 FITC (Clone MH4), CXCR5 PE (Clone MU5UBEE), ICOS PerCP eFluor 710 (Clone ISA-3), CCR7 PE Cy7 (Clone3D12), IL-21 eFluor 660 (Clone eBio3A3-N2), and IL-17 FITC (Clone eBio64DEC17). Staining of mouse cells was performed with the following antibodies and reagents: CD4 PerCP eFluor 710 (Clone RM4-5), PD-1 FITC (Clone J43), CXCR5 Biotin (Clone SPRCL5), streptavidin PE, CD19 (Clone eBio1D3) PE Cy5.5, B220 FITC (Clone RA3-6B2), and CD138 Biotin (Clone 281-2).
Statistics
Data were analyzed by Mann-Whitney-Wilcoxon test with GraphPad Prism Software. To control for multiple comparisons, each P value was adjusted with a Bonferroni correction relative to the number of comparisons in the corresponding figure.
Supplementary Material
Acknowledgments
We thank S. Martomo for providing assistance with the designing of the T and B cell coculture assay, J. MacDougall for assistance with statistical analysis, and L. Witte and J. Ryan for discussions and critical reading of the manuscript.
Funding: B.B. is supported by P01 AI 056299, P01 CA142106, and the Leukemia Society Translational Research grant 6462-15.
Footnotes
Supplementary Materials
Fig. S1. The TH17 cell–like skewing of human CD4+ T cells induces a TFH cell–like phenotype.
Fig. S2. ROCK2 is required for generation and function of human TFH cells.
Fig. S3. KD025 decreases the percentage of CXCR5+PD1+ T cells in the presence of IL-21 in vitro.
Fig. S4. STAT3 is required for Bcl6 and Blimp1 in human TFH cells.
Fig. S5. KD025 stops the progression of kidney damage in MRL/lpr mice.
Fig. S6. KD025 inhibits IL-17 and IL-21 secretion by stimulated PBMCs from active SLE patients.
Fig. S7. KD025 oppositely regulates STAT3 and STAT5 binding to the IL-21 promoter
Fig. S8. Pharmacokinetic analysis of MRL/lpr mice treated with KD025
Author contributions: J.M.W., W.C., R.F., J.R.T., S.M., and A.Z.-Z. designed the research; J.M.W., W.C., M.S.N., A.T., S.M., and A.Z.-Z. performed the research; J.M.W., W.C., R.F., S.M., B.R.B., and A.Z.-Z. analyzed data; and S.D.W. and A.Z.-Z. wrote the paper.
Competing interests: J.M.W., W.C., M.S.N., A.T., J.R.T., and A.Z.-Z. are employees of the Kadmon Research Institute.
References and Notes
- 1.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 2.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 4.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 5.Wu HY, Center EM, Tsokos GC, Weiner HL. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25−LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus. 2009;18:586–596. doi: 10.1177/0961203308100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 11.Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 13.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, Arkwright PD, Kreins AY, Averbuch D, Engelhard D, Magdorf K, Kilic SS, Minegishi Y, Nonoyama S, French MA, Choo S, Smart JM, Peake J, Wong M, Gray P, Cook MC, Fulcher DA, Casanova JL, Deenick EK, Tangye SG. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 17.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 20.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, Bhagat G, Pernis AB. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120:3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Mo R, Depoil D, Rao N, Liu B, Wei J, Lucas S, Koslow M, Roche M, Schueller O, Weiss S, Poyurovsky MV, Tonra J, Hippen KL, Dustin ML, Blazar BR, Liu CJ, Waksal SD. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A. 2014;111:16814–16819. doi: 10.1073/pnas.1414189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanin-Zhorov A, Waksal SD. ROCKing cytokine secretion balance in human T cells. Cytokine. 2015;72:224–225. doi: 10.1016/j.cyto.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Isgro J, Gupta S, Jacek E, Pavri T, Duculan R, Kim M, Kirou KA, Salmon JE, Pernis AB. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65:1592–1602. doi: 10.1002/art.37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, McGonigle S, Paradise E, Sweetnam P, Fink LM, Vozenin-Brotons MC, Hauer-Jensen M. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinolysis. 2008;19:709–718. doi: 10.1097/MBC.0b013e32830b2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi YS, Yang JA, Crotty S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Curr Opin Immunol. 2013;25:366–372. doi: 10.1016/j.coi.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao W, Spolski R, Li P, Du N, West EE, Ren M, Mitra S, Leonard WJ. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc Natl Acad Sci U S A. 2014;111:3508–3513. doi: 10.1073/pnas.1301138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, Ozato K, Levy DE, Nutt SL, Calame K, Leonard WJ. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, Nakajima H. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Yang J, Chu Y, Wang J, Guan M, Zhu X, Xue Y, Zou H. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in Lupus-prone MRL/lpr mice. PLoS One. 2013;8:e62855. doi: 10.1371/journal.pone.0062855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest. 2015;125:2220–2227. doi: 10.1172/JCI78087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, Maurouard T, Dougall D, Davizon ES, Dumortier H, Douchet I, Raffray L, Richez C, Lazaro E, Duffau P, Truchetet ME, Khoryati L, Mercie P, Couzi L, Merville P, Schaeverbeke T, Viallard JF, Pellegrin JL, Moreau JF, Muller S, Zurawski S, Coffman RL, Pascual V, Ueno H, Blanco P. OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response. Immunity. 2015;42:1159–1170. doi: 10.1016/j.immuni.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186:1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 40.Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One. 2013;8:e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XY, Wu ZB, Ding J, Zheng ZH, Li XY, Chen LN, Zhu P. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjogren's syndrome. Biochem Biophys Res Commun. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 42.Walker SR, Nelson EA, Yeh JE, Pinello L, Yuan GC, Frank DA. STAT5 outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Mol Cell Biol. 2013;33:2879–2890. doi: 10.1128/MCB.01620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.