Abstract
The common developmental origin of endothelial and hematopoietic cells is manifested by coexpression of several cell surface receptors. Adult murine bone marrow (BM) long-term repopulating hematopoietic stem cells (LT-HSCs), endowed with the highest repopulation and self-renewal potential, express endothelial protein C receptor (EPCR), which is used as a marker to isolate them. EPCR/PAR1 signaling in endothelial cells has anticoagulant and anti-inflammatory roles, while thrombin/PAR1 signaling induces coagulation and inflammation. Recent studies define two new PAR1-mediated signaling cascades that regulate EPCR+ LT-HSC BM retention and egress. EPCR/PAR1 signaling facilitates LT-HSC BM repopulation, retention, survival, and chemotherapy resistance by restricting nitric oxide (NO) production, maintaining NOlow LT-HSC BM retention with increased VLA4 expression, affinity, and adhesion. Conversely, acute stress and clinical mobilization upregulate thrombin generation and activate different PAR1 signaling which overcomes BM EPCR+ LT-HSC retention, inducing their recruitment to the bloodstream. Thrombin/PAR1 signaling induces NO generation, TACE-mediated EPCR shedding, and upregulation of CXCR4 and PAR1, leading to CXCL12-mediated stem and progenitor cell mobilization. This review discusses new roles for factors traditionally viewed as coagulation related, which independently act in the BM to regulate PAR1 signaling in bone- and blood-forming progenitor cells, navigating their fate by controlling NO production.
Keywords: hematopoietic stem cells, HSC mobilization, bone marrow retention, aPC/EPCR/PAR1 signaling, thrombomodulin, CXCL12/CXCR4, nitric oxide
Introduction
Long-term repopulating hematopoietic stem cells (LT-HSCs) maintain continued blood cell production and host immunity throughout life and respond to demands due to inflammation, injury and blood loss. The bone marrow (BM) is the main site of adult hematopoiesis and the majority of LT-HSCs remain confined to the BM microenvironment in a quiescent non-motile mode via adhesive interactions.1–3 The hallmark of LT-HSCs is their durable BM repopulation potential in functional transplantation assays, which requires high self-renewal ability, homing, and developmental potential. Previous studies revealed that a very small number of hematopoietic stem and progenitor cells (HSPCs) circulate in the peripheral blood as part of homeostasis and can reconstitute hematopoiesis in lethally irradiated transplanted recipients.4 The physiologic process of HSPC egress from the BM to the circulation is accelerated by mobilizing agents, which mimic inflammatory and alarm situations, as HSPCs are mostly harvested from the peripheral blood rather than the BM for clinical stem cell transplantation.5
Emerging evidence shows that hemostatic factors, traditionally viewed as coagulation and inflammation related, also independently control hematopoietic stem cells in the murine BM.6–8 The hemostatic system was originally discovered as a response to acute and transient tissue injury, balancing between procoagulant and anticoagulant forces to prevent blood loss and to control intravascular thrombosis.9 The procoagulant cascades include platelet and fibrin clot formation while the anticoagulant activities include inhibitors of coagulation and the fibrinolytic system.
Thrombin is among the most potent procoagulant factors acting through proteolytic cleavage of cell surface protease-activated receptor-1 (PAR1), and its activity induces proinflammatory and proapoptotic responses. PAR1, which belongs to a family of G protein–coupled cell surface receptors, is expressed by many cell types, including BM endothelial and stromal cells,10 leukocytes,11 and blood-12 and bone-forming progenitors.13 Conversely, anticoagulant and anti-inflammatory responses are induced by binding of protease-activated protein C (aPC) to the endothelial protein C receptor (EPCR), enabling endothelial cell PAR1 signaling that is anti-inflammatory and cytoprotective, due to cleavage of PAR1 at a site different from thrombin.14–16
EPCR was originally identified and cloned as an endothelial-specific receptor together with its major ligand aPC.17 EPCR plays a critical role in supporting aPC-mediated anticoagulant and cytoprotective signaling. Binding of aPC to EPCR facilitates PAR1 cleavage and signaling, initiating anti-inflammatory and antiapoptotic responses and protection of endothelial barrier integrity.18 Recently, it was shown that EPCR is also expressed by some BM HSC, suggesting additional new roles for this receptor. It has been demonstrated that a minority of HSCs in the murine fetal liver and adult BM, endowed with the highest BM long-term repopulation potential, also express EPCR, which was used as a marker to isolate them.19–21 EPCR+ LT-HSCs functionally exhibit durable self-renewal potential at the single transplanted stem cell level.22
This review will outline the complex and multiplayer regulation of hematopoietic stem cell maintenance in the BM microenvironment. We will discuss the bifunctional roles of PAR1 signaling in the context of hematopoietic stem cell function (i.e., BM repopulation and retention versus recruitment to the blood circulation and development). We will highlight the importance of dynamic PAR1 signaling, both for hematopoietic and for bone-forming stem cell migration and development as well as for protection from myelotoxic insult via control of nitric oxide (NO) generation. Cooperation between osteoclast/osteoblast-mediated bone turnover and aPC/EPCR/PAR1 versus thrombin/PAR1 signaling in regulating balanced stem cell function under both normal and acute stress conditions will be discussed.
LT-HSCs and preservation of endothelial elements
Before circulation is established during embryonic development, primitive blood-forming stem cells reside within the yolk sac and the aorta–gonad–mesonephros (AGM) region. Once the vascular system develops, the lack of sufficient stromal support facilitates initial hematopoietic seeding of the fetal liver, where HSCs undergo extensive proliferation and differentiation to all fetal blood cell lineages.23–25 In the final stages of embryonic development, HSCs migrate from the fetal liver via the blood circulation, across the physical blood–BM endothelial cell barrier, and home to their stromal niches, in a CXCR4/CXCL12-dependent manner.26 Close physical association between the developing primary blood cell and endothelial lineages in the yolk sac initially suggested the “hemangioblast” concept, in which the hematopoietic cells and endothelial cells share a common precursor during development.27,28 Indeed, hematopoietic and endothelial markers have extensive overlapping protein expression pattern in the AGM, including VE-cadherin,28 Runx1,29 eNOS,30 CD31,29 CD34, and CD45.31 New cell-tracing technique challenged the ancestor hemangioblast concept and suggested earlier segregation of hematopoietic stem cells from specific blood vessel endothelial cells.32 While the hierarchy of transcription factors that guide hematopoietic development is incompletely defined, lineage tracing may provide new avenues for studying HSC developmental hierarchy and offer new perspectives into the origins of HSCs.
Recent reports reveal that the close association between the hematopoietic and vascular systems also continues into postnatal life. These studies have shown that endothelial cells, including those isolated from adult BM, are capable of supporting long-term hematopoiesis in vitro.33 Importantly, in vivo studies have demonstrated that BM endothelial cells are essential for hematopoietic recovery from lethal total-body irradiation and for transplanted stem cell self-renewal and BM repopulation.34,35 Recent advances in imaging technologies have greatly advanced our understanding of the association between vasculature organization and HSC localization in the murine BM. The marrow microenvironment is highly vascularized, containing large blood vessels and sinusoids. Interestingly, some adult BM LT-HSCs were located in perivascular niches, adjacent to endothelial cells, in postneonatal life.36,37. Nonetheless, these niches are not fully characterized and could also depend on critical contributions from nonvascular cells, such as αSMA+ macrophages,38 stromal precursors,39 and CXCL12-expressing CAR cells.40,41
While the ultimate consequence of the endothelial-to-hematopoietic transition during ontogeny is downregulation of the endothelial program in blood-forming stem cells and their progeny,42 BM-retained adult LT-HSCs also preserve and express some endothelial markers. Vascular cell adhesion molecule 1 (VCAM1) and endothelial cell–selective adhesion molecule-1 (ESAM1) are related adhesion molecules first described and identified on endothelial cells but are also upregulated in LT-HSCs, both at the transcript and protein levels.43 VCAM1 interactions with the integrin α4β1 (also termed VLA4) mediate cell–cell interactions in multiple cell types, and both VCAM1 and integrin α4β1 inhibition have been implicated in LT-HSC mobilization44 and their activity is essential for their homing to the BM.45,46
Single-cell analysis showed that a minority of phenotypically defined BM LT-HSCs also express von Willebrand factor (vWF), previously thought to be exclusively expressed by megakaryocytes, platelets, and the endothelium.47 vWF+ HSCs identify a primitive BM HSC population capable of stable long-term myeloid- and megakaryocyte-biased reconstitution supporting platelet production.47 vWF is central for platelet aggregation, hemostasis, and thrombus formation. Recently, it became evident that vWF plays multiple roles in vascular biology, controlling smooth muscle cell proliferation, vascular inflammation, and angiogenesis.48 While the ultimate role of vWF in LT-HSCs has yet to be determined, it is conceivable that vWF might be secreted by HSCs themselves to contribute to their regulation by ITGA2B-dependent adhesion49 in a self-primed specific niche. Providing unique adhesion ligands might also pave the way for LT-HSC expansion and skewing towards injury-responsive differentiation with megakaryocyte- and platelet-biased progenitor expansion.
Gene array studies have revealed that the anticoagulant and anti-inflammatory EPCR is highly expressed predominantly in purified LT-HSCs obtained from murine fetal liver and adult BM but not in common lymphoid or myeloid progenitor cells.50,51. Furthermore, isolation of primitive fetal liver and adult BM LT-HSCs on the basis of surface EPCR expression followed by transplantation assays revealed that EPCR+ LT-HSCs have the highest hematopoietic reconstitution activity.19–21 Single-cell transplantations of EPCR+Sca-1high/CD150+CD48− (SLAM) cells isolated from adult murine BM defined a highly purified population of LT-HSCs exhibiting durable self-renewal potential.22 Interestingly, while EPCR expression is a clear endothelial characteristic,52,53 it has also been identified as a stem cell marker in other tissues,12 including mammary stem cells,54 and its function is crucial for regulating integrin α4β1 in breast cancer stem cells and for tumor progression.55 Of note, atypical EPCR expression by BM stem and progenitor cells was observed in the S129 (129S1/SvlmJ) mouse strain (preliminary results, data not shown), indicating that different mouse strains might have different EPCR expression and function.
Although the association of the zymogen protein C (PC) with EPCR greatly enhances activation to the serine protease–activated protein C (aPC) with crucial intravascular anticoagulant functions, ligation of EPCR by aPC is key to altering signaling pathways resulting in stabilization and protection of the endothelial blood barrier.56 The zymogen PC is synthesized predominantly in the liver and is proteolytically activated by the thrombin–thrombomodulin (TM) complex.57 Preservation of EPCR expression among different stem cell lineages suggests that EPCR may provide stem cell signals that maintain the undifferentiated stemness phenotype. Current evidence indicates that the aPC/EPCR pathway plays an important role in both fetal stem cell survival21 and adult BM LT-HSC retention and protection from myelotoxicity,6 ensuring preservation of the BM stem cell pool throughout adult life.
BM niches are major sites for stem cell maintenance
LT-HSCs reside in specialized niches in the BM, where stromal and hematopoietic cells provide support for adhesion of the stem cell pool, maintaining their undifferentiated primitive state.1,38,40 The BM microenvironment is considered to be a negative regulator of stem cell migration,58 proliferation, and differentiation, as part of the mechanisms to preserve the dormant BM HSC pool while maintaining their developmental potential.59 Signals produced as a consequence of the dynamic osteoclast/osteoblast bone turnover by BM-derived cells, such as osteoblasts,60 mesenchymal progenitor cells, endothelial cells, and myeloid cells, coordinately regenerate the BM, making it the preferred site for adult LT-HSCs.1,61–63 Despite technology breakthroughs in high-resolution in vivo imaging techniques, the exact location of BM LT-HSCs and the dynamic nature, cellular composition, and structure of their niches remain controversial topics.1
Another fundamental open question that remains is whether different niches exist for quiescent and for cycling LT-HSCs in the BM. Current understanding suggests that both endosteal (provided by bone-lining cells) and perivascular niches coexist in the BM, comprising stromal and hematopoietic cells that provide membrane-bound and secreted factors to promote LT-HSC retention, maintenance, and development.1 Considering the complexity of the BM architecture, it might be possible that these two proposed distinct niches might be in fact closely associated and cooperate to provide mutual signals to promote BM LT-HSC protection and regulation. Adding layers of complexity, accumulating evidence implies that several types of endothelial cells and blood vessels make up the stem cell vascular niche.2 While the vascular sinusoidal niche has been implicated in HSC maintenance and regeneration,36,64 recent studies suggest that the HSC vascular niche may not be limited to sinusoids, since phenotypically defined LT-HSCs are also found adjacent to non-sinusoidal vascular structures, including arteries and arterioles adjacent or in close proximity to bone.56 Whether or not considerable overlap exists between HSC vascular microenvironments, an interesting question is whether all sinusoids or arterioles can functionally participate in promoting LT-HSC maintenance and protection in the BM, or if only specialized domains within these vascular structures can do so.
Recent observations suggest that fetal and adult BM EPCR+ LT-HSCs are located in close proximity to BM endothelial subpopulations that highly express thrombomodulin (TM).6,7,21 Generated thrombin typically forms a tight complex with TM that converts PC to its activated form, aPC.57 Importantly, TM+ endothelial cells in the adult murine BM also stained for aPC and EPCR+ LT-HSC were found to be located adjacent to aPC-enriched endothelial areas6 (Fig. 1). Interestingly, similarly to endothelial cells,14,15 binding of the anticoagulant protease aPC to EPCR, which is functional on the LT-HSC surface, induced PAR1 signaling,6 most likely by cleaving a site different from the canonical thrombin cleavage site, as has been demonstrated for endothelial cells. Signals coming from aPC through the EPCR/PAR1 axis promote LT-HSC retention in the BM, thereby providing protection from DNA-damaging agents and preservation of the HSC pool size in the murine adult BM.6 It appears that within the BM endothelial microenvironment, only a specialized type of endothelial cell, defined as TM6,7 and aPC-expressing6 cells, can efficiently and specifically maintain EPCR+ LT-HSCs in the BM. The interplay of TM and aPC in specialized BM microenvironments was found to be physiologically relevant to allow accelerated stem cell recovery from radiation-induced hematopoietic suppression and death.7 While the relevant targets of aPC allowing radioprotective activity remain unknown,7 EPCR signaling was found to be essential for stem cell protection from chemotherapy-induced hematology failure and death.6
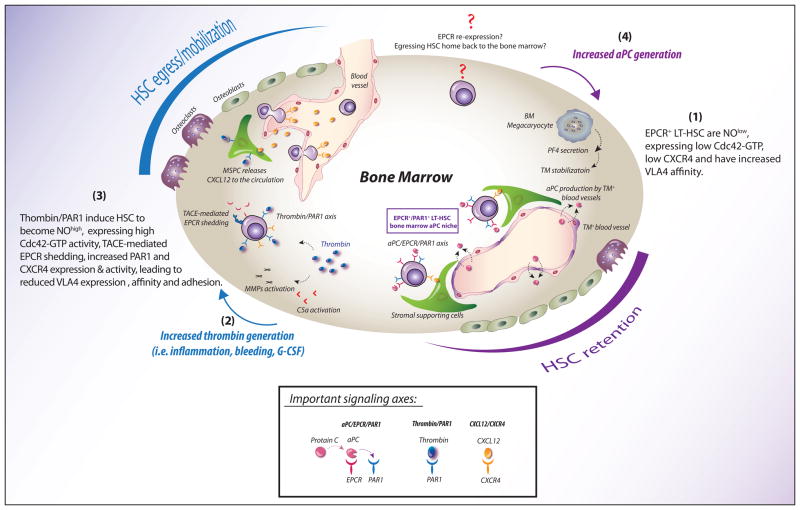
Figure 1.
PAR1 signaling balances EPCR+ long-term repopulating stem cell BM retention and trafficking. (1) TM+/aPC+ endothelial cells form an anticoagulant niche for EPCR+ LT-HSCs in the BM. Binding of the anticoagulant protease aPC to EPCR on the LT-HSC surface induces PAR1 signaling, leading to the restriction of nitric oxide (NO) production and thereby initiating Cdc42–GDP polarity, downregulation of its GTP activity, and VLA4-dependent adhesion. The TM/aPC pathway may be linked to BM megakaryocytes, which secrete PF4, stabilizing the thrombin–TM complex, thereby promoting aPC generation and induction of EPCR+ LT-HSC retention. (2) Physiologic stress, inflammation, injury, and cytokines transiently increase thrombin generation, which in turn activates extrinsic metalloproteases, such as C5a and MMPs, promoting HSC detachment from the BM. (3) The thrombin–PAR1 axis initiates NO generation, causing LT-HSCs to become NOhigh, leading to enhanced Cdc42–GTP activity and reduced VLA4 affinity and promoting TACE-mediated EPCR shedding. In parallel, thrombin also induces PAR1-dependent CXCL12 secretion from BM stromal cells into the circulation, followed by PAR1 and CXCR4 upregulation on the surface of BM HSPCs, leading to increased CXCL12-induced chemoattraction of responsive HSPCs to the blood. (4) It remains to be determined which signals initiate aPC generation in the BM and how EPCR− stem cells home back to the BM. How do circulating and mobilized HSCs, which lack surface EPCR expression (but may express intracellular EPCR), re-express EPCR upon their BM homing, lodgment, and repopulation?
The TM/aPC pathway may also be linked to BM megakaryocytes that have been shown to negatively regulate BM HSC proliferation via the secretion of platelet factor 4 (PF4).65,66 PF4 (also termed CXCL4) stabilizes TM and promotes aPC generation,67 suggesting that megakaryocytes may participate in the establishment of the stem cell niches for BM EPCR+ cells, potentially enhancing aPC generation and thereby ensuring EPCR+ LT-HSC retention and protection.6 While the role of aPC in this process remains to be formally demonstrated, supportive evidence shows that phenotypically defined Lin− SLAM cells are frequently located adjacent to marrow megakaryocytes,66 and, under chemotherapeutic stress conditions, PF4 secretion by BM megakaryocytes increased HSC levels65 (Fig. 1). The coexpression of TM on BM EPCR+ LT-HSCs, but not by stem cells in the bloodstream, suggests that aPC generation might not only take place in the surrounding BM endothelial microenvironment, but also by the EPCR+ LT-HSCs themselves.6,7
aPC/EPCR/PAR1 signaling regulates LT-HSC retention in the BM
The adult bone cavity is organized in a highly complex architecture of cellular components, including mature and immature leukocytes, surrounded by mesenchymal cell lineages. The current paradigm suggests that only a single stem cell occupies a niche in the BM microenvironment rather than stem cells being clustered together. This clonal paradigm suggests that a single stem cell exploits the marrow microenvironmental resources, therefore stem cell expansion is functionally and mechanically limited, maintaining their slow cycling status. Such restricted cycling in turn provides protection from myelotoxicity and exhaustion due to extensive inflammatory conditions and during age-dependent functional decline.
A key additional concept is that mutual interactions between HSCs and their stromal supporting cells exert inhibitory feedback on their proliferation and differentiation, keeping HSCs dormant in a nonmotile mode.59,68 The chemokine receptor CXCR4 is essential for quiescence of LT-HSCs in the murine BM, suggesting that membrane-bound CXCL12 expressed by BM stromal cells induces adhesion and retention of CXCR4+ LT-HSCs.40. Conditional deletion of CXCL1269 or CXCR440,70 leads to increased cycling and exhaustion of the stem cell pool, as well as loss of BM retention and protection from DNA damaging agents. While CXCR4 is essential for LT-HSC quiescence40, BM EPCR+ LT-HSCs express only low levels of surface CXCR4,6 suggesting that low CXCR4 receptor signaling requires additional synergistic signaling cascades to maintain EPCR+ LT-HSC BM retention. EPCR-expressing LT-HSCs exhibit increased in vitro adhesion to fibronectin (FN)-bound CXCL12 and reduced migration ability to soluble CXCL12, in correlation with their BM retention. Short in vitro aPC prestimulation further increased their CXCL12-induced adhesion, demonstrating cooperation between EPCR and CXCR4 in LT-HSC adhesion and suggesting a synergistic role for these receptors in LT-HSC BM retention.6 Interestingly, inhibitory effects of aPC/EPCR on leukocyte migration have also been documented in human activated neutrophils expressing EPCR in neutrophil chemotaxis triggered by interleukin-8.71
In BM LT-HSCs, the aPC/EPCR pathway induces a PAR1 signaling cascade leading to restriction of nitric oxide (NO) production, thereby initiating Cdc42 downregulation and increased VLA4-dependent adhesion. HSCs expressing low levels of EPCR or HSCs pretreated with EPCR-neutralizing antibody fail to compete with normal untreated EPCR+ LT-HSCs in competitive BM transplantation into irradiated hosts.6 In support of the crucial role of ligand binding to EPCR in maintaining functional LT-HSCs in the BM, mice harboring a disabling single mutation in the aPC-binding domain of EPCR develop splenomegaly as a result of hematological BM failure.72
The cell adhesion receptor integrin VLA4 (α4β1) binds fibronectin and VCAM-1 and is crucial for CXCL12/CXCR4-mediated BM HSC retention.44,73 VLA4 is expressed by most leukocytes, as well as some non-hematopoietic cells,74 while its expression is higher on EPCR+ LT-HSCs as compared to EPCR− progenitor cells. VLA4 affinity was enhanced as a consequence of aPC/EPCR signaling in LT-HSCs, providing a clear mechanism for their preferential location within the adherent BM environment6 (Fig. 1). It has long been proposed that VLA4 expression by circulating and BM-retained LT-HSCs might be important for binding and detachment of stem cells within the human BM microenvironment. Strong expression of VLA4 was mainly found on human BM HSPCs, while circulating cells following G-CSF–induced clinical stem cell mobilization express low levels of VLA4. Surprisingly, EPCR expression during steady state was restricted to the murine BM and was also absent from circulating HSPCs. Moreover, genetically modified mice strains with only low levels of EPCR (EPCRlow)6 or deficient in VLA4 have higher numbers of circulating HSPCs.6,75,76.
While it has yet to be determined whether EPCR signaling also regulates VLA4 transcription and surface expression, these observations indicate that, apart from regulating the VLA4 affinity state, EPCR and VLA4 might have a tightly regulated cross talk to enable LT-HSC retention in the BM. Since adhesive interactions are crucial for retention of HSPC in their BM niches, targeting these molecules is expected to induce mobilization. Indeed, administration of neutralizing antibodies to the integrin VLA4 induced LT-repopulating HSC mobilization.77 Similarly, blocking EPCR signaling by neutralizing EPCR antibody treatment induced CXCR4 upregulation and reduced VLA4 affinity, inducing mobilization of HSCs that expressed high levels of EPCR in the circulation.6 The surprisingly overlapping roles of EPCR and VLA4 in enhancing LT-HSC adhesion and BM retention is further supported by the preliminary observation demonstrating that EPCR supports LT-HSCs homing specifically to the BM.6 Upon injection, EPCR-expressing LT-HSCs rapidly appear in the BM in an EPCR-dependent manner, while EPCR blockage had no effect on HSC homing to other hematopoietic organs, such as the spleen. This unique homing pattern of LT-HSCs to the BM was previously reported in studies demonstrating that cells lacking the integrin α4 exhibited impaired homing to the BM, but not to the spleen.75 In addition to the homing defect, an impaired expansion and BM repopulation ability was even more pronounced in transplanted α4 integrin–null cells, which was restricted to the BM compartment and not to the spleen.75 The idea that the BM and the spleen might provide different signals for HSC maintenance and development requires additional mechanistic investigation. However, preliminary results reveal that the murine spleen lacks TM+ endothelial cells, which are needed for aPC generation.78 This difference may explain the unique BM periarterial microenvironments that are needed to attract and to retain EPCR+ LT-HSCs with high VLA4 affinity and adhesions.6
Adhesion and retention of LT-HSCs not only requires the activity of adhesion molecules, but also requires significant changes in regulators of the actin cytoskeleton. The small GTPases Rac1, Rac2, Cdc42, and RhoA are involved in adhesion signaling of hematopoietic cells.79,80 Cdc42 is a member of the Rho GTPase family that cycles between the GTP-bound active and the GDP-bound inactive states and thus acts as a binary molecular switch to activate responses for a variety of extracellular stimuli, which can be diffusible factors, signals on neighboring cells, and/or signals from the extracellular matrix.81 Cdc42 activation levels influence cell adhesion, migration, division. and polarity establishment.82
Regulation of Cdc42 activity and its polar distribution in HSPCs is crucial for stem cell adhesion and BM repopulation potential.83,84 Deficiency in Cdc42 results in increased HSC cycling, impaired homing, and retention, leading to massive stem cell mobilization.85 Conversely, mice expressing elevated levels of active Cdc42–GTP also exhibit reduced adhesion, impaired directional migration, and defective short-term and long-term engraftment.83 Taking these results together, it appears that either very high or very low levels of Cdc42 correlate with impaired HSPC retention in the BM and that balanced Cdc42 activity ultimately determines HSPC retention. Low Cdc42 activity was found to be essential to allow EPCR+ LT-HSC retention in the BM, and aPC/EPCR signaling reduced Cdc42-GTP levels (Fig. 1).6
Moreover, as Cdc42 activity was found to be correlated with its cellular distribution,86 EPCR-expressing LT-HSCs demonstrate polar Cdc42 distribution while EPCR− progenitor cells exhibit a random distribution of Cdc42 within the cell.6 Cellular polarity has been tightly linked to proper cell function in general and particularly to stem cell function.87 A study reported asymmetric segregation of Numb, which inhibits Notch signaling in murine HSCs, suggesting that Numb polarity is important for HSC self-renewal.88 Moreover, the vast majority of immature human CD34+ cells acquire a polarized cell shape when cultured in the presence of stem cell–supporting cytokines, such as stem cell factor and thrombopoietin.89 Furthermore, this polarization pattern is accompanied by a redistribution and polarization of several lipid rafts and adhesion molecules, such as CD133 and ICAM1.90. Polarizable myosin IIB contributes to human CD34+ stem cell asymmetric division, thereby facilitating stem cell self-renewal without expansion. As a consequence, inhibiting myosin II expands LT-HSCs.91 It is therefore conceivable that EPCR through its ability to establish Cdc42 polarity and restrict Cdc42 activity regulates not only LT-HSC retention, but also HSC self-renewal potential.
Thrombin/PAR1 signaling regulates HSC mobilization
As part of the host defense, the BM reservoir of immature and maturing leukocytes effectively adjusts to address alarm situations induced by injury, bleeding, inflammation, and DNA damage. These processes are tightly regulated to avoid overproduction of leukocytes and exhaustion of the BM stem cell pool, yet to allow rapid and efficient generation of blood and immune cells on demand. Bidirectional trafficking between the BM and the periphery, referred to as homing, steady-state egress, stress-induced recruitment, and clinical mobilization, is a hallmark of HSPC physiology. Although the vast majority of HSPCs reside in the BM, a small amount of HSPCs continuously egress from the BM to the peripheral blood and are dramatically increased during stress conditions.92 Such systemic and stress-induced changes include the detachment of primitive stem cells and immature progenitors from their anchored BM niches, followed by increased motility and massive recruitment of HSPCs and maturing leukocytes to the circulation.93 Several clinical protocols mimicking physiological stress conditions were developed to enable the use of mobilized HSPCs in order to reconstitute hematopoiesis in ablated patients. Clinical mobilizing agents that induce BM HSPC migration include chemotherapy drugs such as cyclophosphamide or repeated injections of the myeloid cytokine G-CSF, mimicking stress-induced HSPC recruitment through proinflammatory signals.94
An expanding line of evidence suggests that PAR1 signaling critically regulates BM HSPC trafficking. Physiologic stress, inflammation, injury, and cytokines transiently increase thrombin generation as part of the inflammatory process.95,96 Early observations indicated that the powerful mobilizing cytokine G-CSF might in fact induce a hypercoagulant state in healthy donors, as evidenced by increased thrombin generation, and consequently carry a risk of thrombosis.97,98. The expression of the major thrombin receptor PAR1 assessed by microarrays was found to be 3.3-fold higher in G-CSF–mobilized human CD34+ stem and progenitor cells compared with steady-state BM CD34+ HSPCs.99 These observations paved the way for the hypothesis that stress-induced, accelerated thrombin generation may promote PAR1+ HSPC exit from the BM, and that the higher PAR1 surface expression by the mobilized human CD34+ stem and progenitor cells has motility-supporting functions. In the murine hematopoietic system, mimicking acute stress by injecting high levels of thrombin into mice shows that active thrombin enters the BM very rapidly, activating PAR1 signal transduction pathways in BM HSPCs and stromal cells in parallel, inducing PAR1-dependent HSPC mobilization and recruitment of LT-HSCs into the bloodstream.6
The chemokine CXCL12 is a powerful chemotactic factor for HSPCs, which express the major CXCL12 receptor CXCR4.100 CXCL12/CXCR4 interaction is an essential signal pathway in the tightly regulated process of LT-HSC trafficking and allows quiescent BM retention and protection from chemotherapy-induced cell death.40 Modulating the balanced CXCL12/CXCR4 interaction by the thrombin/PAR1 pathway in vivo was found to provide the essential signal to enhance HSPC recruitment from the BM. CXCL12 is produced by many stromal BM resident cell types,39,40,100–102 including stem cell niche–supporting mesenchymal stem cells (MSC) and endothelial cells.103 Basal cell surface expression of CXCL12 by BM stromal cells is required for HSPC homeostatic adhesion and maintenance, acting via CXCR4 to ensure LT-HSC quiescence and BM retention in a nonmotile mode.40
Unlike membrane-bound stromal and endothelial CXCL12, which induces LT-HSC adhesion, retention, and quiescence, secreted soluble CXCL12 is a potent chemotactic factor leading to HSPCs egress and mobilization to the blood circulation.100,104 Perturbed CXCL12/CXCR4 signaling in the BM leads to mobilization, which is associated with CXCL12 secretion by marrow stromal cells and its release into the circulation. In parallel, HSPCs in the BM gain increased surface CXCR4 expression and motility, followed by their enhanced migration to the periphery.100,104,105 Rapid HSPC mobilization is a fast process that does not involve stem cell expansion and proliferation. One agent to rapidly mobilize HSC is AMD3100, which was characterized as a CXCR4 antagonist according to its ability to inhibit migration of enriched BM mononuclear cells towards a gradient of CXCL12 in vitro.106,107 Consistent with the notion that stem cells detachment from their nursing microenvironment and recruitment to the circulation is an active process, AMD3100 also has a dominant agonistic effect by mediating rapid CXCL12 secretion from murine BM CXCR4+ stromal and endothelial cells, followed by rapid release of CXCL12 into the circulation and induction of CXCR4-dependent HSPC mobilization.100
Thrombin proteolytic activity in vivo induces rapid PAR1-dependent CXCL12 secretion from BM stromal cells into the circulation, followed by PAR1 and CXCR4 upregulation on the surface of BM HSPCs, leading to increased CXCL12-induced chemoattraction of responsive HSPCs to the blood6 (Fig. 1). Evidence for thrombin in the blood plasma during G-CSF and AMD3100 mobilization further demonstrates the complexity of HSPC motility regulatory mechanisms.108 It is therefore conceivable that increased thrombin levels are a common mechanism to switch the BM EPCR- and CXCR4-mediated retention to rapid and prolonged PAR1-mediated CXCL12 secretion and CXCR4-induced HSPC mobilization. Clinically, the timing of stem cell harvest with respect to thrombin peak levels in the plasma may yield higher levels of mobilized HSPCs, leading to better therapeutic outcomes.
Current data strongly implicate metalloproteinases and other proteolytic enzymes as part of the stem cell migration and mobilization machinery by inactivating BM-derived growth factors, chemokines, and extracellular matrix responsible for hematopoietic stem cell adhesion and retention. Proteolytic degradation in turn facilitates enhanced movement across the physical barrier of the BM extracellular matrix.109–111 It has been suggested that high levels of thrombin can prime the migration of HSPCs towards a gradient of CXCL12, enabled by upregulation of proteolytic enzymes.112 Thrombin induced MMP-9 secretion and upregulation of MT1-MMP expression in human cord blood CD34+ cells, leading to enhanced chemoinvasion of HSPCs towards a low CXCL12 gradient.112 G-CSF–mobilized human CD34+ HSPCs also have increased surface MT1-MMP expression and function and reduced levels of its inhibitor RECK, possibly induced by thrombin.113 Thrombin was also found to be a potent inducer of MMP-9 secretion from human monocytes,114 facilitating recruitment of leukocytes to inflammatory sites,115 further demonstrating the potential role of thrombin/PAR1 signaling in mediating enhanced HSPC motility by regulating proteolytic enzyme activation.
Increased thrombin levels in the blood plasma can also facilitate the activation of the complement cascade, leading to enhanced stem cell recruitment from the BM.116 Interestingly, direct inhibition of thrombin with the drug refludan significantly attenuated HSPC mobilization after G-CSF and AMD3100 administration. Reduced AMD3100-induced HSPC mobilization following thrombin inhibition may imply that thrombin/PAR1 signaling contributes to mobilization through CXCR4.108 Notably, in another study, G-CSF–induced progenitor cell mobilization in PAR1-deficient F2r−/− mice was found to be higher than in wild-type mice, which was attributed to defective bone structure and stromal signaling.48 One should consider that the altered BM microenvironment may induce alternative mobilization pathways that can substitute for the loss of PAR1 signaling. However, since these mice have higher basal levels of circulating HSPCs,6 the overall G-CSF–induced mobilization index is reduced. The altered HSPC BM retention is most probably due to the defective BM microenvironment,8 since PAR1 signaling is also required for EPCR+ LT-HSC BM retention as well as for BM stromal cells.
Apart from initiating microenvironmental changes, such as CXCL12 secretion and complement activation to enhance HSPC mobilization, thrombin through the PAR1 axis has a major role in directing the exit of HSCs with the highest long-term repopulation potential. Thrombin–PAR1 signaling rapidly induces shedding of EPCR from the surface of LT-HSCs, dependent on the activation of the metallopeptidase TACE/ADAM176 (Figs. 1 and 2). Interestingly, BM EPCR+ LT-HSCs highly express TACE/ADAM17 and in particular its nonactivated precursor containing the inhibitory prodomain motif. Thrombin activation of PAR1 induces rapid activation of TACE/ADAM17, as demonstrated by reduced expression of the inhibitory prodomain, leading to EPCR shedding from the surface of BM LT-HSCs. This in turn inactivates the EPCR-mediated retention of LT-HSCs, directing their migration to the blood circulation.6 Of interest, mice lacking TACE/ADAM17 exhibited hypercellularity in the BM and extramedullary hematopoiesis in the spleen and liver.117 This BM-failure phenotype may suggest that EPCR shedding and recycling on the surface of LT-HSCs in an essential process to allow normal hematopoiesis.117 Additional work will be required to determine whether TACE/ADAM17 activation is also required to allow clinical G-CSF– and AMD3100-induced HSC mobilization and whether direct activation of TACE might be an efficient strategy to induce specific mobilization of EPCR-expressing LT-HSCs.
Figure 2.
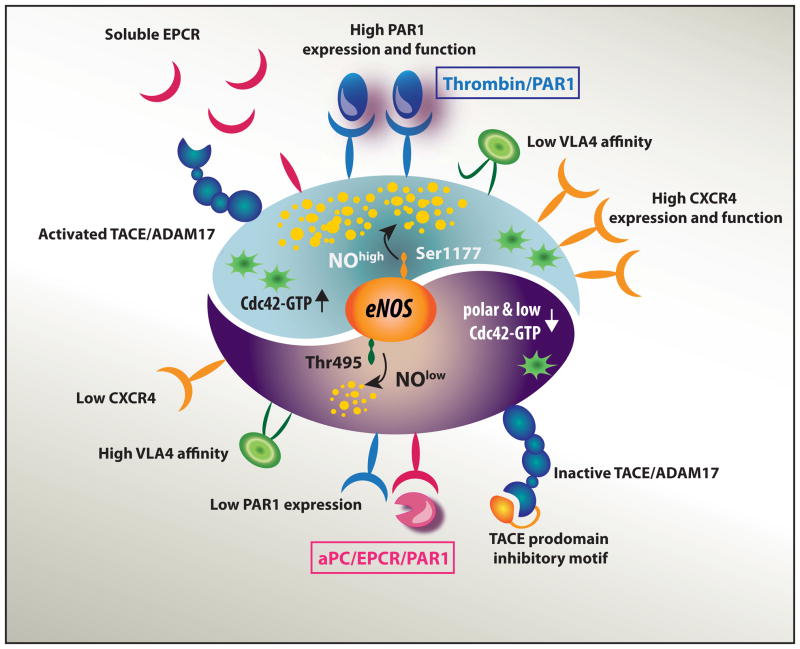
The two faces of PAR1 signaling pathways: the nitric oxide switch in BM EPCR+ long-term repopulating stem cells. PAR1 acts as rheostat that, when activated by thrombin or aPC, induces two different signaling cascades, which balance LT-HSC BM retention versus trafficking to the blood circulation by switching the intracellular nitric oxide (NO) generation machinery. The aPC–EPCR–PAR1 axis (bottom) restricts NO generation, preserving the stem cells in a NOlow state that leads to reduced surface CXCR4 expression, polar Cdc42 with reduced GTP activity, and increased VLA4-mediated adhesion, thus promoting LT-HSC retention in the BM. The thrombin–PAR1 axis (top) initiates NO generation, leading to increased PAR1 and CXCR4 expression and function, enhanced Cdc42-GTP activity loading, and TACE/ADAM17 activation and EPCR shedding, thus promoting HSC trafficking.
Nitric oxide levels balance LT-HSC retention and trafficking
PAR1 acts as rheostat that, when activated by thrombin or aPC, induces distinct cascades of events, essentially balancing HSC BM localization versus trafficking to the blood circulation. By switching the intracellular nitric oxide (NO) generation machinery to either “on” or “off,” PAR1 signaling distinctly regulates LT-HSC migratory fate6 (Fig. 2). NO is a small diffusible metabolite that serves as a cellular messenger in numerous biological systems. NO can either act within the cell in which it is produced or, by short-range release, affect adjacent cells. Evidently, the aPC–EPCR–PAR1 axis restricts NO generation, preserving the stem cells in a NOlow state that leads to reduced surface CXCR4 expression, reduced Cdc42 activity, and increased VLA4-mediated adhesion. To the contrary, the thrombin–PAR1 axis initiates NO generation, leading to increased CXCR4 expression, enhanced Cdc42–GTP loading, and TACE/ADAM17 activation. These events cumulate in EPCR shedding and reduced BM adhesion and retention (Fig. 2). Balancing the NO levels in stem cells through differential PAR1 signaling is orchestrated by endothelial NO synthase (eNOS) activity.6 NO signaling is essential for the initiation of hematopoiesis during development.30,118 eNOS (Nos3) is expressed by HSCs in the murine AGM30 and in adult BM,6 further linking the hematopoietic and endothelial developmental programs.
Direct treatment with NO donors, such as SNAP, can bypass thrombin/PAR1 signaling, leading to CXCL12 secretion, PAR1 and CXCR4 upregulation, and rapid HSPC recruitment to the bloodstream.6 In support of these results in the mouse, human cord blood CD34+ progenitor cells pretreated with NO donors also exhibited increased surface CXCR4 expression and function.119 Many effects of NO are mediated through its canonical receptor, the soluble guanylyl cyclase generating cGMP. Classically, elevated NO content activates the cGMP signaling pathway, rapidly downmodulating the affinity state of VLA4 and integrin-dependent cell adhesion.120 NO plays an expanding functional role in protein S-nitrosylation, a reversible posttranslational modification of protein cysteines. Reduced VLA4 affinity may involve S-nitrosylation of cytoskeletal proteins and integrins, leading to integrin inactivation and cell detachment.120 Similarly, NO has been implicated in the activation of TACE/ADAM17 by inducing S-nitrosylation of the inhibitory motif of the TACE prodomain.121 Thus, NO production induced by the thrombin–PAR1 axis might activate both canonical cGMP signaling and noncanonical protein modification, allowing TACE-mediated EPCR shedding and reduced VLA4 affinity, thereby facilitating HSC recruitment. The stromal contribution of NO generation is further demonstrated in the context of endothelial progenitor cell mobilization, which is dependent on BM stromal eNOS activity involving MMP-9 proteolytic activity.122
NO production by the adult bone stroma is thought to have a positive and supportive effect on hematopoiesis;123 however, a collective set of evidence indicates that NO production is detrimental to hematopoietic recovery and survival after radiation124 and chemotherapy,6 causing death. By switching the eNOS phosphorylation status, aPC–EPCR–PAR1 signaling induces eNOS phosphorylation at its negative regulatory site, which restricts NO generation, and promotes eNOS dephosphorylation in its positive regulatory site, thereby preserving the EPCR-expressing LT-HSCs in a low-NO state. Low-NO conditions are essential to maintain the stem cells retained in the marrow cavity and to protect them from chemotherapy-induced death. Pharmacological inhibition of NO generation by the eNOS inhibitor L-NAME expands the LT-HSC pool in the BM.6 Furthermore, exposure of mice to NOS inhibitors, either directly or after irradiation and BM transplantation, increases the number of stem cells in the BM.124 Adding another layer of complexity, restriction of NO generation was reported to block differentiation of human CD34+ HSCs while maintaining their proliferation potential.125 Future studies will be required to determine the series of events leading to LT-HSC expansion following inhibition of NO generation and whether the increased stem cell pool size is a cell-autonomous effect or supported by the surrounding increase in available stromal niche cells. These observations may imply that NO can be a possible target for improved stem cell expansion and BM transplantation protocols.
Bone remodeling and hemostasis cross talk modulates HSC maintenance
Residing in bone trabecular regions with high remodeling rates, HSCs are durably exposed to signals of bone formation and resorption. During fetal development, HSC lodgment to the BM is synchronized with BM vascularization; however in Osx−/− mice lacking osteolineage (bone) cells, HSCs fail to durably repopulate the marrow of ablated recipients, demonstrating the need for osteolineage cells in HSC function.126 Consistent with this concept, ectopic generation of HSC niches was successful only in the presence of endochondral ossification, a process leading to bone formation.127 Many factors expressed by mesenchymal stem cells and their maturing osteolineage cell progeny are endowed with HSC-preservation potential.128 Thus, bone formation appears to be associated with HSC preservation and niche assembly. Osteoblast and osteoclast activities are coupled to maintain net bone remodeling throughout life. Bone formation is defective in newborn oc/oc mice due to the lack of functional bone-resorbing osteoclasts.60 These mice have reduced BM HSC pools, impaired osteoblast differentiation, and defective BM HSC niches. Consequently, homing of normal HSCs to the BM of oc/oc mice is reduced. Restoring BM niches and HSC function by rescue of osteoclast activity demonstrates that osteoclasts are required for normal formation of HSC niches in newborn mice.60,129
Bone resorption is accompanied by extensive activity of osteoclast-secreted degrading enzymes, among which cathepsin K is essential. We have previously shown that cathepsin K cleaves endosteal membrane–bound stem cell factor, CXCL12, and osteopontin, all essential components of BM HSC niches with major stem cell–regulation activities.101 Destruction of these retention-mediating molecules deactivates the adhesion machinery and releases HSCs from the BM. Hence, HSC mobilization (e.g., by G-CSF, bleeding, bacterial LPS) is associated with increased osteoclast maturation and activity in wild-type mice, whereas reduced mobilization capacity was obtained in mice exhibiting defective osteoclast adhesion and activity.101 Interestingly, young op/op mice have normal HSCs but lack the cytokine M-CSF, osteoclast activity, and bone cavities.130 These mice had enhanced G-CSF–induced HSPC mobilization and a lack of stem cell protection and death induced by 5-fluorouracil chemotherapy, most probably due to lack of BM stem cell niches.130
Are hemostatic factors, which are active in bone-remodeling cells, involved in regulation of HSCs in their niches? As discussed earlier in this review, aPC/EPCR signaling in HSCs facilitates their retention in their established BM niches. Interestingly, EPCR expression in the BM is not limited to LT-HSCs, as preosteoblasts and osteoblasts also functionally express EPCR.131,132 EPCR expression by osteoblasts might also participate in bone turnover, as aPC stimulates osteoblast proliferation via EPCR132 and administration of aPC together with bone morphogenetic protein 2 in mice induced ectopic bone formation with higher rates of osteoclast differentiation and vascularization, all implicated in bone-remodeling processes.131 Further evidence linking hemostatic regulation with bone physiology demonstrated that long-term oral anticoagulant therapy in children is associated with osteopenia and a risk of future development of osteoporosis,133 possibly indicating the need for balanced production of anticoagulant factors and normal bone turnover.
As for procoagulant factors, as far back in 1916, the BM was shown to be a source of the thrombin precursor, prothrombin (PT);134 however, the producing cell types were not well characterized. Recent data show that PT is mainly present in the newly formed bone matrix of the metaphyseal trabecular bone (a region rich in HSCs), in close association to osteoclasts. MMP-9+ osteoclasts contain PT, and PT expression is increased during osteoclast differentiation.135 Thus, PT is available in the plasma, in osteoclasts, and is also incorporated into the newly formed bone, which makes it available as a source for thrombin generation during bone remodeling. Of interest, BM stromal progenitor cells, as well as bone-lining cells, also express PT (unpublished data), demonstrating an additional source for thrombin availability in the BM. The effects of prothrombin conversion to thrombin in the marrow cavity on LT-HSC physiology remain to be determined.
Tissue factor (TF) is a major initiator of thrombin generation in vivo. TF is expressed in various myeloid cells, monocytes, and macrophages. Under homeostatic conditions, TF is expressed at low levels in monocytes. In the context of innate immune responses, TF is upregulated by inflammatory mediators, such as LPS.136,137 In vitro, RANKL-differentiated osteoclasts also express TF and coagulation factor Xa, which convert PT to thrombin.138 TF plays a non-hemostatic role as well and regulates innate immune function through PAR2 signaling, also implicated in inflammation.139. Enhanced HSC mobilization and osteoclast activation during inflammation presumably involves TF activity in osteoclasts to locally produce thrombin, which releases HSCs from their niches. On the other hand, thrombin inhibits osteoclast differentiation through a non-proteolytic mechanism,140 which may be a regulatory mechanism to shut down the inflammatory response.
PAR1, the major thrombin receptor, also plays a role in bone repair. Reduced new bone formation and increased osteoclasts invading the damaged bone were observed in PAR1-deficent mice. Thrombin treatment dose-dependently induced proliferation of BM stromal cells from wild-type mice, but not from PAR1-deficent mice, demonstrating the requirement for PAR1 for bone healing.141 Since PAR1 is activated by plasmin and the fibrinolytic system is necessary for bone repair,56 PAR1 signaling involving proteases other than thrombin may contribute to homeostatic maintenance of the HSC during bone remodeling. Indeed, Par1 knockout mice suffer from an abnormal bone phenotype.8,142. Taken together, factors of the coagulation system cooperate within the bones in non-hemostatic pathways to orchestrate bone-remodeling processes and stem cell niche regulation during steady-state and inflammatory conditions, along with pro- and anticoagulant signaling. These mediators act together to harness HSCs to the BM or navigate them out from this organ as part of host immunity and hematopoiesis.
Concluding remarks and perspectives
“Well, you can go on looking forward… There may be many unexpected feasts ahead of you.”
J.R.R. Tolkien.
Since the first cloning and identification of EPCR as the physiological receptor for the powerful anticoagulant aPC143 and the discovery of EPCR/aPC/PAR1 anticoagulant signaling,14 additional evidence emerged for a close interaction between the coagulation systems and inflammation, cellular metabolism, angiogenesis, and innate immunity.144–146 Blood coagulation and primary hemostasis evolved as important defense mechanisms to prevent bleeding,147 and a primary function of the vascular TM/aPC/EPCR pathway is to counterbalance intravascular thrombosis and maintain endothelial quiescence. With new functions emerging in our understanding of EPCR in BM LT-HSCs and the challenge of deciphering the mechanisms of the tight regulation by distinct PAR1 signaling,6 new questions arise on the relevance of the hemostatic system for human stem cell physiology, and future studies will enhance our understanding of the role of PAR1 and NO signaling in clinical G-CSF– and AMD3100-induced HSPC mobilization. As was previously suggested, PAR1 expression might be a good predictor of the efficacy of stem cell mobilization,99 and further strategies to manipulate PAR1 signaling and its downstream effectors might be beneficial in individuals who do not respond efficiently to currently mobilization protocols and are therefore termed poor mobilizers. Another fundamental question is whether imbalanced coagulant activity, often observed in elderly patients, might be a relevant target to improve HSPC mobilization and BM transplantation.
Stem cells in the BM were characterized as NOlow (Ref. 6) and reactive oxygen species (ROS)low-expressing cells.148 While it has yet to be determined whether there is an overlap between the two cell populations, it was previously suggested that high levels of ROS are reversible, and reduction of ROS levels in the stem cell would induce reduced cycling and restore long-term repopulation potential.148 Since circulating cells express higher levels of NO,6 one can speculate that restoration of low NO levels might be beneficial to improve stem cell retention potential upon BM transplantation.
The leukemic stem cell paradigm refers to the ability of a subpopulation of cancer cells to initiate tumorigenesis by undergoing self-renewal and differentiation. It becomes clear that the high frequency of relapse after conventional cytotoxic chemotherapies predicts that leukemic stem cells are resistant to standard therapy, suggesting that targeting the stem cell population will likely lead to improved patient outcomes. It is also becoming evident that the BM microenvironment is linked with primary leukemic stem cell resistance to therapy. The potential for unraveling how EPCR signaling and the anticoagulant microenvironment participates in malignant hematological disorders might provide new approaches for improved cancer therapy.
Acknowledgments
The studies discussed in this review were partially supported by the Israel Science Foundation (851/13), the Ernest and Bonnie Beutler Research Program of Excellence in Genomic Medicine and FP7-HEALTH-2010 (CELL-PID 261387), the Weizmann - University of Michigan - Technion Collaboration Program, NIH Grant HL-60742, and the Humboldt Foundation.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125:2621–2629. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–714. [PubMed] [Google Scholar]
- 5.Bonig H, Papayannopoulou T. Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol. 2012;904:1–14. doi: 10.1007/978-1-61779-943-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gur-Cohen S, et al. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat Med. 2015;21:1307–1317. doi: 10.1038/nm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiger H, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–1129. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronovich A, et al. A novel role for factor VIII and thrombin/PAR1 in regulating hematopoiesis and its interplay with the bone structure. Blood. 2013;122:2562–2571. doi: 10.1182/blood-2012-08-447458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman M. Remodeling the blood coagulation cascade. Journal of thrombosis and thrombolysis. 2003;16:17–20. doi: 10.1023/B:THRO.0000014588.95061.28. [DOI] [PubMed] [Google Scholar]
- 10.Karp JM, et al. Thrombin mediated migration of osteogenic cells. Bone. 2005;37:337–348. doi: 10.1016/j.bone.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Colognato R, et al. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–2652. doi: 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- 12.Ramalho-Santos M, et al. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 13.Ho IA, et al. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells. 2009;27:1366–1375. doi: 10.1002/stem.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riewald M, et al. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 15.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 16.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125:2898–2907. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 18.Gleeson EM, O’Donnell JSR, Preston J. The endothelial cell protein C receptor: cell surface conductor of cytoprotective coagulation factor signaling. Cell Mol Life Sci. 69:717–726. doi: 10.1007/s00018-011-0825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balazs AB, et al. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107:2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kent DG, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113:6342–6350. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki H, et al. Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood. 2010;116:544–553. doi: 10.1182/blood-2009-08-240903. [DOI] [PubMed] [Google Scholar]
- 22.Wilson NK, et al. Combined Single-Cell Functional and Gene Expression Analysis Resolves Heterogeneity within Stem Cell Populations. Cell Stem Cell. 2015;20:00162–00169. doi: 10.1016/j.stem.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 24.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 25.Ghiaur G, et al. Rac1 is essential for intraembryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells. Blood. 2008;111:3313–3321. doi: 10.1182/blood-2007-08-110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ara T, et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 27.Huber TL, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 28.Zovein AC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North TE, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 30.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong JW. Molecular and developmental biology of the hemangioblast. Dev Dyn. 2008;237:1218–1231. doi: 10.1002/dvdy.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padron-Barthe L, et al. Clonal analysis identifies hemogenic endothelium as the source of the blood-endothelial common lineage in the mouse embryo. Blood. 2014;124:2523–2532. doi: 10.1182/blood-2013-12-545939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi H, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature cell biology. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludin A, et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13:1072–1082. doi: 10.1038/ni.2408. [DOI] [PubMed] [Google Scholar]
- 39.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugiyama T, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Taoudi S, et al. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132:4179–4191. doi: 10.1242/dev.01974. [DOI] [PubMed] [Google Scholar]
- 43.Forsberg EC, et al. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood. 1998;91:2231–2239. [PubMed] [Google Scholar]
- 45.Papayannopoulou T. Mechanisms of stem-/progenitor-cell mobilization: the anti-VLA-4 paradigm. Semin Hematol. 2000;37:11–18. doi: 10.1016/s0037-1963(00)90084-2. [DOI] [PubMed] [Google Scholar]
- 46.Papayannopoulou T, et al. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanjuan-Pla A, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- 48.Lenting PJ, et al. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428–2437. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
- 49.Lombardo VT, et al. Independent modulation of von Willebrand factor and fibrinogen binding to the platelet membrane glycoprotein IIb/IIIa complex as demonstrated by monoclonal antibody. J Clin Invest. 1985;76:1950–1958. doi: 10.1172/JCI112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanova NB, et al. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 51.Akashi K, et al. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood. 2003;101:383–389. doi: 10.1182/blood-2002-06-1780. [DOI] [PubMed] [Google Scholar]
- 52.Regan LM, et al. The endothelial cell protein C receptor. Inhibition of activated protein C anticoagulant function without modulation of reaction with proteinase inhibitors. J Biol Chem. 1996;271:17499–17503. doi: 10.1074/jbc.271.29.17499. [DOI] [PubMed] [Google Scholar]
- 53.Laszik Z, et al. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation. 1997;96:3633–3640. doi: 10.1161/01.cir.96.10.3633. [DOI] [PubMed] [Google Scholar]
- 54.Wang D, et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature. 2015;517:81–84. doi: 10.1038/nature13851. [DOI] [PubMed] [Google Scholar]
- 55.Schaffner F, et al. Endothelial protein C receptor function in murine and human breast cancer development. PLoS One. 2013;8:e61071. doi: 10.1371/journal.pone.0061071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bae JS, et al. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stearns-Kurosawa DJ, et al. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster K, et al. Different Motile Behaviors of Human Hematopoietic Stem versus Progenitor Cells at the Osteoblastic Niche. Stem cell reports. 2015;5:690–701. doi: 10.1016/j.stemcr.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zipori D, Sasson T. Adherent cells from mouse bone marrow inhibit the formation of colony stimulating factor (CSF) induced myeloid colonies. Exp Hematol. 1980;8:816–817. [PubMed] [Google Scholar]
- 60.Mansour A, et al. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao M, Li L. Regulation of hematopoietic stem cells in the niche. Science China Life sciences. 2015;58:1209–1215. doi: 10.1007/s11427-015-4960-y. [DOI] [PubMed] [Google Scholar]
- 62.Park D, Sykes DB, Scadden DT. The hematopoietic stem cell niche. Frontiers in bioscience. 2012;17:30–39. doi: 10.2741/3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acar M, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao M, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20:1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 66.Bruns I, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slungaard A, et al. Platelet factor 4 enhances generation of activated protein C in vitro and in vivo. Blood. 2003;102:146–151. doi: 10.1182/blood-2002-11-3529. [DOI] [PubMed] [Google Scholar]
- 68.Wilson A, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tzeng YS, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 70.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sturn DH, et al. Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 2003;102:1499–1505. doi: 10.1182/blood-2002-12-3880. [DOI] [PubMed] [Google Scholar]
- 72.Pepler L, et al. Characterization of mice harboring a variant of EPCR with impaired ability to bind protein C: Novel role of EPCR in hematopoiesis. Blood. 2015;126:673–682. doi: 10.1182/blood-2014-02-558940. [DOI] [PubMed] [Google Scholar]
- 73.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemler ME, et al. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 75.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ulyanova T, et al. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106:86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci U S A. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gur Cohen S, et al. EPCR/PAR1 signaling navigates long-term repopulating hematopoietic stem cell bone marrow homing to thrombomodulin-enriched blood vessels. ASH Annual Meeting. 2015 Vol. Abstract. [Google Scholar]
- 79.Cancelas JA, Williams DA. Rho GTPases in hematopoietic stem cell functions. Current opinion in hematology. 2009;16:249–254. doi: 10.1097/MOH.0b013e32832c4b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, Zheng Y. Cdc42: a signal coordinator in hematopoietic stem cell maintenance. Cell Cycle. 2007;6:1445–1450. [PubMed] [Google Scholar]
- 81.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 82.Etienne-Manneville S. Cdc42--the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 83.Wang L, et al. Genetic deletion of Cdc42GAP reveals a role of Cdc42 in erythropoiesis and hematopoietic stem/progenitor cell survival, adhesion, and engraftment. Blood. 2006;107:98–105. doi: 10.1182/blood-2005-05-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geiger H, Koehler A, Gunzer M. Stem cells, aging, niche, adhesion and Cdc42: a model for changes in cell-cell interactions and hematopoietic stem cell aging. Cell Cycle. 2007;6:884–887. doi: 10.4161/cc.6.8.4131. [DOI] [PubMed] [Google Scholar]
- 85.Yang L, et al. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc Natl Acad Sci U S A. 2007;104:5091–5096. doi: 10.1073/pnas.0610819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Florian MC, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Florian MC, Geiger H. Concise review: polarity in stem cells, disease, and aging. Stem Cells. 2010;28:1623–1629. doi: 10.1002/stem.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu M, et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell. 2007;1:541–554. doi: 10.1016/j.stem.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giebel B, et al. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104:2332–2338. doi: 10.1182/blood-2004-02-0511. [DOI] [PubMed] [Google Scholar]
- 90.Giebel B, et al. Primitive human hematopoietic cells give rise to differentially specified daughter cells upon their initial cell division. Blood. 2006;107:2146–2152. doi: 10.1182/blood-2005-08-3139. [DOI] [PubMed] [Google Scholar]
- 91.Shin JW, et al. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014;14:81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 93.Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- 94.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 95.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 96.Riewald M, Ruf W. Science review: role of coagulation protease cascades in sepsis. Critical care. 2003;7:123–129. doi: 10.1186/cc1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naina HV, et al. Low risk of symptomatic venous thromboembolic events during growth factor administration for PBSC mobilization. Bone Marrow Transpl. 2011;46:291–293. doi: 10.1038/bmt.2010.106. [DOI] [PubMed] [Google Scholar]
- 98.LeBlanc R, et al. A prospective study of G-CSF effects on hemostasis in allogeneic blood stem cell donors. Bone Marrow Transplant. 1999;23:991–996. doi: 10.1038/sj.bmt.1701756. [DOI] [PubMed] [Google Scholar]
- 99.Steidl U, et al. Gene expression profiling identifies significant differences between the molecular phenotypes of bone marrow-derived and circulating human CD34+ hematopoietic stem cells. Blood. 2002;99:2037–2044. doi: 10.1182/blood.v99.6.2037. [DOI] [PubMed] [Google Scholar]
- 100.Dar A, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286–1296. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 102.Dar A, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- 103.Imai K, et al. Selective secretion of chemoattractants for haemopoietic progenitor cells by bone marrow endothelial cells: a possible role in homing of haemopoietic progenitor cells to bone marrow. Br J Haematol. 1999;106:905–911. doi: 10.1046/j.1365-2141.1999.01644.x. [DOI] [PubMed] [Google Scholar]
- 104.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 105.Christopher MJ, et al. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hatse S, et al. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 107.Devine SM, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 108.Borkowska S, et al. Novel evidence that crosstalk between the complement, coagulation and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs) Leukemia. 2014;28:2148–2154. doi: 10.1038/leu.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golan K, et al. MT1-MMP and RECK: opposite and essential roles in hematopoietic stem and progenitor cell retention and migration. Journal of molecular medicine. 2011;89:1167–1174. doi: 10.1007/s00109-011-0792-9. [DOI] [PubMed] [Google Scholar]
- 110.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 111.Klein G, Schmal O, Aicher WK. Matrix metalloproteinases in stem cell mobilization. Matrix biology : journal of the International Society for Matrix Biology. 2015;44–46:175–183. doi: 10.1016/j.matbio.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 112.Shirvaikar N, et al. Hyaluronic Acid and Thrombin Upregulate MT1-MMP through PI3K and Rac-1 Signaling and Prime the Homing-Related Responses of Cord Blood Hematopoietic Stem/Progenitor Cells. Stem Cells Dev. 2010;20:19–30. doi: 10.1089/scd.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Vagima Y, et al. MT1-MMP and RECK are involved in human CD34+ progenitor cell retention, egress, and mobilization. J Clin Invest. 2009;119:492–503. doi: 10.1172/JCI36541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang CJ, et al. Thrombin regulates matrix metalloproteinase-9 expression in human monocytes. Biochem Biophys Res Commun. 2009;385:241–246. doi: 10.1016/j.bbrc.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 115.Suk K, Cha S. Thrombin-induced interleukin-8 production and its regulation by interferon-gamma and prostaglandin E2 in human monocytic U937 cells. Immunol Lett. 1999;67:223–227. doi: 10.1016/s0165-2478(99)00015-2. [DOI] [PubMed] [Google Scholar]
- 116.Ratajczak MZ, et al. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 117.Horiuchi K, et al. Conditional inactivation of TACE by a Sox9 promoter leads to osteoporosis and increased granulopoiesis via dysregulation of IL-17 and G-CSF. J Immunol. 2009;182:2093–2101. doi: 10.4049/jimmunol.0802491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adamo L, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y, et al. Identification of CXCR4 as a new nitric oxide-regulated gene in human CD34+ cells. Stem Cells. 2007;25:211–219. doi: 10.1634/stemcells.2006-0468. [DOI] [PubMed] [Google Scholar]
- 120.Chigaev A, Smagley Y, Sklar LA. Nitric oxide/cGMP pathway signaling actively down-regulates alpha4beta1-integrin affinity: an unexpected mechanism for inducing cell de-adhesion. BMC Immunol. 2011;12:1471–2172. doi: 10.1186/1471-2172-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Z, et al. Activation of tumor necrosis factor-alpha-converting enzyme-mediated ectodomain shedding by nitric oxide. J Biol Chem. 2000;275:15839–15844. doi: 10.1074/jbc.M000604200. [DOI] [PubMed] [Google Scholar]
- 122.Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 123.Krasnov P, et al. Neuronal nitric oxide synthase contributes to the regulation of hematopoiesis. Mol Med. 2008;14:141–149. doi: 10.2119/2007-00011.Krasnov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Michurina T, et al. Nitric oxide is a regulator of hematopoietic stem cell activity. Mol Ther. 2004;10:241–248. doi: 10.1016/j.ymthe.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 125.Tiribuzi R, et al. Nitric oxide depletion alters hematopoietic stem cell commitment toward immunogenic dendritic cells. Biochim Biophys Acta. 2013;1830:2830–2838. doi: 10.1016/j.bbagen.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 126.Coskun S, et al. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 2014;9:581–590. doi: 10.1016/j.celrep.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 129.Blin-Wakkach C, Rouleau M, Wakkach A. Roles of osteoclasts in the control of medullary hematopoietic niches. Archives of biochemistry and biophysics. 2014;561:29–37. doi: 10.1016/j.abb.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 130.Miyamoto K, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen K, et al. Activated protein C (APC) can increase bone anabolism via a protease-activated receptor (PAR)1/2 dependent mechanism. J Orthop Res. 2014;32:1549–1556. doi: 10.1002/jor.22726. [DOI] [PubMed] [Google Scholar]
- 132.Kurata T, et al. Activated protein C stimulates osteoblast proliferation via endothelial protein C receptor. Thromb Res. 2010;125:184–191. doi: 10.1016/j.thromres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 133.Avgeri M, et al. Assessment of bone mineral density and markers of bone turnover in children under long-term oral anticoagulant therapy. J Pediatr Hematol Oncol. 2008;30:592–597. doi: 10.1097/MPH.0b013e31817541a8. [DOI] [PubMed] [Google Scholar]
- 134.Drinker CK, Drinker KR, Kreutzmann HA. The Factors Concerned in the Appearance of Nucleated Red Blood Corpuscles in the Peripheral Blood : Ii. Influence of Procedures Designed to Increase the Rate of Blood Flow through the Blood-Forming Organs-Hemorrhage and Infusion. J Exp Med. 1918;27:383–397. doi: 10.1084/jem.27.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karlstrom E, et al. Localization and expression of prothrombin in rodent osteoclasts and long bones. Calcif Tissue Int. 2011;88:179–188. doi: 10.1007/s00223-010-9443-3. [DOI] [PubMed] [Google Scholar]
- 136.Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;367284:20. doi: 10.4061/2011/367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bode M, Mackman N. Regulation of tissue factor gene expression in monocytes and endothelial cells: Thromboxane A2 as a new player. Vascul Pharmacol. 2014;62:57–62. doi: 10.1016/j.vph.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karlstrom E, et al. RANKL induces components of the extrinsic coagulation pathway in osteoclasts. Biochem Biophys Res Commun. 2010;394:593–599. doi: 10.1016/j.bbrc.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 139.Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012;34:133–149. doi: 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- 140.Sivagurunathan S, et al. Thrombin inhibits osteoclast differentiation through a non-proteolytic mechanism. J Mol Endocrinol. 2013;50:347–359. doi: 10.1530/JME-12-0177. [DOI] [PubMed] [Google Scholar]
- 141.Song SJ, et al. The role of protease-activated receptor-1 in bone healing. Am J Pathol. 2005;166:857–868. doi: 10.1016/S0002-9440(10)62306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tudpor K, et al. Thrombin receptor deficiency leads to a high bone mass phenotype by decreasing the RANKL/OPG ratio. Bone. 2015;72:14–22. doi: 10.1016/j.bone.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 143.Fukudome K, Esmon CT. Molecular cloning and expression of murine and bovine endothelial cell protein C/activated protein C receptor (EPCR). The structural and functional conservation in human, bovine, and murine EPCR. J Biol Chem. 1995;270:5571–5577. doi: 10.1074/jbc.270.10.5571. [DOI] [PubMed] [Google Scholar]
- 144.Liang HP, et al. EPCR-dependent PAR2 activation by the blood coagulation initiation complex regulates LPS-triggered interferon responses in mice. Blood. 2015;125:2845–2854. doi: 10.1182/blood-2014-11-610717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Disse J, et al. The endothelial protein C receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors. J Biol Chem. 2011;286:5756–5767. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liang HP, et al. Coagulation factor V mediates inhibition of tissue factor signaling by activated protein C in mice. Blood. 2015;126:2415–2423. doi: 10.1182/blood-2015-05-644401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Doolittle RF. Step-by-step evolution of vertebrate blood coagulation. Cold Spring Harb Symp Quant Biol. 2009;74:35–40. doi: 10.1101/sqb.2009.74.001. [DOI] [PubMed] [Google Scholar]
- 148.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]