Abstract
We have developed an in vitro model of testis development (3D-TCS) using rat testicular cells overlaid with extracellular matrix. One barrier preventing utilization of in vitro models in toxicity testing is absence of metabolic capability. Another challenge is lack of kinetic data for compounds in vitro. We characterized metabolic capabilities and investigated the kinetics of phthalate male reproductive toxicants in the 3D-TCS. Cells were treated with three phthalate diesters for 2, 8 and 24 hours. Parent compounds and metabolites were measured in cell culture media and cell lysate via mass spectrometry. Levels of monoester metabolites were used as an indication of metabolism of phthalates via lipase activity. Metabolites were detected in all treated cell media and cell lysate samples, with levels ranging from <0.5–14.7% of initial mass of parent compound. Phthalates partitioned between media and lysate in a manner consistent with each compound’s degree of lipophilicity. UDGPT activity was detected in DBP and DEP treated samples. 3D-TCS microarray data indicated gene expression for lipases and CYPP450s. Results indicate that the 3D-TCS is a metabolically active co-culture and that physiochemical properties can provide information about the kinetics of compounds in the 3D-TCS, improving our ability to interpret results from the model.
Keywords: Phthalate esters, reproductive toxicity, in vitro models, testes, metabolism, organotypic
Introduction
We have developed an in vitro model of testis development (Yu et al. 2005; Wegner et al. 2013). This three dimensional cellular co-culture system (3D-TCS) contains the rat testis cell types including: Sertoli, germ and Leydig cells grown in a three dimensional conformation facilitated by an extracellular matrix (ECM) overlay. Previous experiments have demonstrated that addition of ECM in this co-culture model results in a more physiologically stable system and that cells form a testicular-like architectural structure representative of in vivo characteristics of seminiferous tubules (Yu et al. 2005). Since the optimization of the 3D-TCS, we have demonstrated that this co-culture was able to inform specific cell signaling pathways involved in the mechanism of cadmium toxicity in the testes (Yu et al. 2008). We have also shown that this co-culture was able to discriminate between reproductively toxic and non-toxic phthalate diesters based on patterns of transcriptomic changes (Yu et al. 2009). These results highlight a promising potential for the use of the 3D-TCS in characterizing compounds for male reproductive toxicity.
One major barrier in the utilization of in vitro models in toxicity assessment is the absence of functional metabolic systems which are in place in vivo but not represented in vitro. For example, primary and cell line derived hepatocyte cultures have been shown to frequently exhibit decreased cytochrome-P450 activity compared to what is observed in vivo (Sidhu et al. 1993; Boess et al. 2003; Brown et al. 2007). This presents a problem in terms of utilizing in vitro cell models in toxicity screening of chemical compounds as many environmental toxicants require metabolic activation by enzyme systems in order to exert toxic effects. Conversely, enzyme systems may also metabolize toxicants to non-toxic metabolites. Absence of these processes in in vitro test systems makes interpretation of data obtained from these cell culture models more difficult, especially in a risk assessment context. Thus, the metabolic capability of test systems to activate or deactivate toxic compounds is crucial to interpreting in vitro data. Addressing this issue, a 2006 workshop report from the European Centre for the Validation of Alternative Methods (ECVAM) advised that basal biotransformation capabilities of in vitro test systems should be characterized before use as a tool for toxicity screening (Coecke et al. 2006). Recent reports continue to call for these studies and cite absence of in vitro metabolism in these systems in the prevention of their application, indicating that metabolic activity toward test compounds may be an important factor in interpreting toxicity data from in vitro testicular models (Hartung et al. 2011; Saldutti et al. 2013). Relevant to our in vitro testis model, there are numerous examples of biotransformation systems known to be active in the adult rat testis including cytochrome P450s, glutathione-S-transferases, aryl hydrocarbon hydroxylase, epoxide hydrolase and lipases (Dixon and Lee 1980; Holst et al. 1994). In order to appropriately interpret results of toxicity assays in the 3D-TCS, it is important to determine to what extent these and other enzyme systems are conserved in the in vitro context of this co-culture system.
Another challenge in interpretation of in vitro results is the absence of data on the kinetics of compounds in in vitro systems. In order for in vitro methodologies to be properly utilized in the risk assessment process, doses used to identify adverse effects in vitro should be relevant for in vivo exposure scenarios. Discrepancies between the nominal toxicant concentration in cell media and the concentration at the site of toxic action could arise from a number of factors, including those with quantitative impact such as binding to proteins, binding to tissue culture plastic, and evaporation of the test compound (Blaauboer 2010; Groothuis et al. 2013). Evaluations of the kinetic behavior of compounds within the 3D-TCS will be critical to understanding the effects observed in toxicity assays because these evaluations will enable us to determine dosimetry-corrected media concentrations, allowing for more accurate translation of in vitro to in vivo doses for the purposes of risk assessment.
In the current study we have used exposure to three structurally related phthalate esters to investigate metabolic capabilities and characterize the kinetics of compounds in the 3D-TCS. Some phthalate diesters are known male reproductive toxicants and exposure at low levels is widespread in many human populations (Duty et al. 2003; Bustamante-Montes et al. 2013). Effects of phthalates on male reproductive endpoints in rats have been extensively studied and include hypospadias, cryptorchidism, decreased anogenital distance, and decreased testosterone levels in the testes (Mylchreest et al. 1998; Gray et al. 2000; Clewell et al. 2010). Taken together, these male reproductive endpoints in rats have been described by the term “phthalate syndrome” (Swan 2008). Interestingly, the outcomes described by phthalate syndrome closely resemble the human Testicular Dysgenesis Syndrome (TDS) first described by Skakkebaek et al. in 2001. The origins of TDS are postulated to be due in part to early life-stage exposures to endocrine disrupting compounds such as phthalates (Skakkebaek et al. 2001). Due to rising rates of effects described by TDS in some human populations, the possibility that phthalate exposures are contributing to human male reproductive disorders remains a concern (Martino-Andrade and Chahoud 2010; Bustamante-Montes et al. 2013). In order to characterize the inherent capacity for the cells in the 3D-TCS to metabolize this important class of male reproductive toxicants, we exposed cells to two known reproductively toxic phthalates, dibutyl phthalate and diethylhexyl phthalate (DBP and DEHP, 100µM) as well as one non-reproductively toxic phthalate, diethyl phthalate (DEP, 150µM). Concentrations of parent compound and the monoester metabolite (which is thought to be the main metabolite responsible for testes toxicity in vivo) were measured in media and lysate via mass spectrometry (HPLC-ESIMS/ MS) (Clewell et al. 2010).
Materials and Methods
Preparation of three dimensional co-culture model for testis (3D-TCS)
Protocol for the preparation of 3D-TCS has been explained in detail elsewhere (Yu et al. 2005; Wegner et al. 2013). Briefly, testes were dissected from 5-day-old male pups obtained from mated Sprague–Dawley rats (Charles River Laboratories, Wilmington, USA). Sequential enzymatic digestions were used to create a single cell suspension containing primarily Sertoli, germ and Leydig cells. Cells were suspended in a solution containing serum-free Eagle’s Minimal Essential Medium (Invitrogen, Carlsbad, CA) containing 0.1nM nonessential amino acids, 1mM sodium pyruvate, 3mM sodium lactate, 1% ITS culture supplement (BD Biosciences, Bedford, MA). Cells were then plated at a density of 1.6×106 cells/35mm plate followed by the addition of extracellular matrix medium (Matrigel™). Phthalate solutions with a final concentration of 100µM (DBP, DEHP) or 150µM (DEP) diluted in dimethyl sulfoxide (DMSO) were then added directly to the culture medium 48 h after initial plating of cells. Final concentrations of DMSO were <0.2%. Doses were selected based on those that caused minimal cytotoxic impact observed in previous experiments. DBP (Sigma #D2270, 99% purity), DEHP (Sigma #4-8557, 99% purity), DEP (Sigma #524972, 99.5% purity) and DMSO (Sigma #D1435) were obtained from Sigma-Aldrich (MO, USA). Phthalates are ubiquitous and have been shown to have the potential to contaminate plastic lab supplies (Reid 2007). In addition, non-enzymatic breakdown to monoesters can occur for phthalates in vitro and in the environment (Daniel and Bratt 1974; Xu et al. 2008; Lertsirisopon et al. 2009). In order to address this issue, dishes with only Matrigel™ and phthalates added (no cells) were plated in parallel in order to detect any background levels of phthalate metabolites in absence of cells (“Matrigel™ controls”).
Sample collection and processing
Dishes containing cells (plus Matrigel™) or Matrigel™ controls were exposed to phthalate for 2, 8 and 24 hours (n=3). At the end of each exposure period, media (~2mL) was collected from each plate, placed on dry ice and stored at −80°C. Plates were rinsed 3X with 1mL phosphate buffered saline followed by addition of 100µL of cell lysis buffer. Plates were then scraped with a cell scraper and cell lysates (lysis buffer plus scraped material) were collected. Cell lysates were immediately placed on dry ice and then stored at −80°C until further analysis. All samples were thawed, sonicated (Heat Systems Ultrasonics™, Farmingdale, New York) for 15 seconds total then centrifuged for 5 minutes at 16,000×G to remove cellular debris. Supernatants were then placed back at −80°C before analysis via mass spectrometry. Total protein levels for cell lysate samples were determined using commercially available colorimetric assay (BioRad Laboratories, #500-0006).
Detection of parent compounds and monoester metabolites in 3D-TCS samples
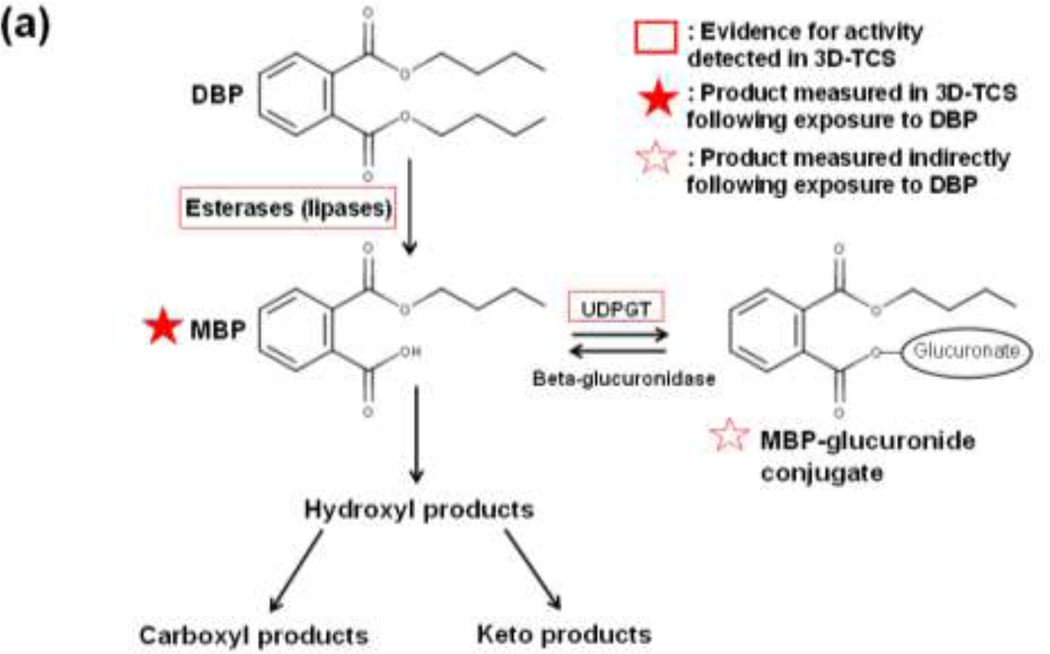
The metabolic pathways for the three phthalates used in this study are shown in Figure 1 (a–c) (Albro 1986; Taylor 2001; Hauser and Calafat 2005; Frederiksen et al. 2007). Phthalate diester parent compounds are converted to monoester metabolites in the phase I reaction followed by glucuronidation in phase II. Parent compounds and monoester metabolites were measured directly while the glucuronidated monoesters were measured indirectly by calculating differences in monoester levels pre-and post-glucuronidase deconjugation. The methods used to detect parent compounds and monoester metabolites were adapted from Centers for Disease Control method for detecting phthalates in urine (Calafat 2010). This method uses high performance liquid chromatography-electrosprayionization-tandem mass spectrometry (HPLC-ESI-MS/MS) in order to quantitatively detect phthalate parent compounds and monoester metabolites. Analysis was performed with an Agilent 6410 LC-MS-MS System. The column used was a Synergi Polar-RP 80A (Phenomenex™). Phthalate standards were obtained from AccuStandard, Inc. (New Haven, CT). Total and unconjugated levels of monoester metabolites were measured after incubation of samples with or without glucuronidase for 90 minutes at 37°C. The difference in metabolite levels measured with and without glucurionidase (after subtracting background levels detected in matrigel controls) was used to generate an estimate of the total amount of glucuronidated metabolite in each sample.
Fig. 1. Pathways of phthalate metabolism. Metabolism pathways are shown for the three phthalate diesters used in the current study: (a) dibutyl phthalate (DBP). (b) di(ethylhexyl) phthalate (DEHP) and (c) diethyl phthalate (DEP).
Phthalate parent compounds are initially converted to monoester metabolites by a class of enzymes known as lipases. This phase I reaction is followed by either phase II glucuronidation or the formation of alternative metabolic products.
Statistics for phthalate parent compound and metabolite analysis
For cell media and cell lysate samples, pairwise t-tests were used to identify significant differences in both total and glucuronidated monoester metabolite levels (in ng/mL) from corresponding Matrigel™ controls at each time point (p<0.05). In order to identify significant differences in partitioning of phthalate parent compounds between cell media and cell lysate fractions, t-tests were used to detect significant differences in the levels of phthalates observed in media and lysate at 2, 8 and 24 hours (p<0.05).
Transcriptomic analysis of metabolic gene expression (lipases and CYP450s)
In order to further characterize the metabolic capabilities of the 3D-TCS, datasets consisting of microarray gene expression from the 3D-TCS and in vivo fetal rat testes were utilized. In vitro data were obtained from a study in which 3D-TCS cells were exposed to 100µM of seven phthalates (benzyl butyl phthalate, dibutyl phthalate, diethyhexyl phthalate, dipentyl phthalate, diethyl phthalate, dimethyl phthalate or dioctyl terephthalate) or vehicle control (DMSO) (Yu et al. 2009). In vivo microarray data were obtained from testes of fetal rats for which dams were exposed to the same seven phthalates or control (corn oil via oral gavage) from gestational days 12–19. Detailed methods for each of these studies are described elsewhere (Yu et al. 2009) (Liu et al. 2005). Arrays used for in vitro data were Affymetrix Rat 230 2.0 and in vivo data were 230 (A and B chips), which contain the same probes. All gene expression intensities were normalized to log2 (fold-change/mean of controls) using the corresponding control for each dataset.
In order to investigate gene expression for key metabolic genes present in the 3DTCS, the microarray data were queried for gene expression signals for lipases and CYP450s. We identified expression signals for 16 lipase genes and 10 CYP450s with relevance to male reproductive development and/or steroid and lipid metabolism which were changed in the 3D-TCS by at least one phthalate exposure (F-test, FDR<0.1). Gene expression data for the corresponding lipases and CYPs were identified in the in vivo fetal rat testes data for comparison. We then used the Gene Ontology (GO) Consortium database to identify GO terms (biological processes, cellular components and molecular functions) related to testes or male reproductive processes In order to characterize relevant functions of these lipases and CYP450s in the 3D-TCS model.
Results
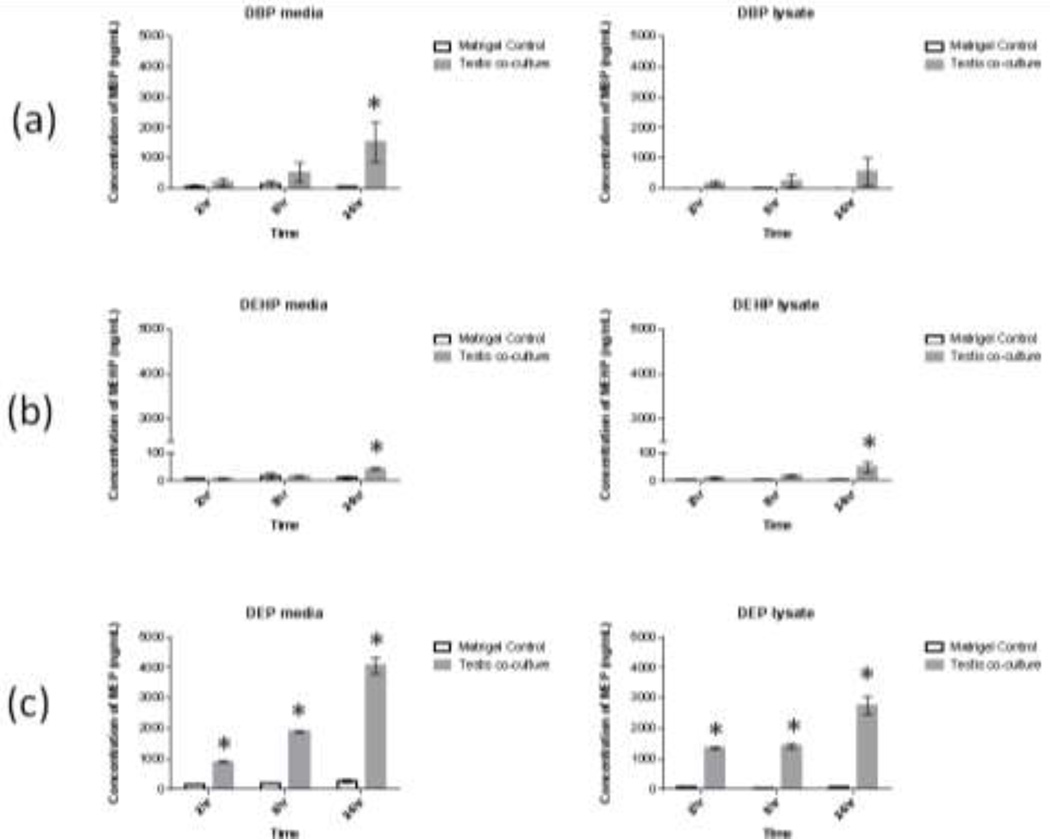
Levels of total monoester metabolite (free plus glucuronidated) in cell media and cell lysate for all samples and timepoints are shown in Fig 2. (a–c). The levels of monoester metabolites detected after 24 hours varied by phthalate, with average levels ranging from 44 (MEHP) to 4,065 (MEP) ng/mL in cell media and 49 (MEHP) to 2,760ng/mL (MEP) in cell lysate. On a mass balance basis, total metabolite levels accounted for 0.5% (DEHP) to 14.7% (DEP) of the original mass of the parent compound after 24 hours (see Suppl. Fig. 1 for mass balance calculations).
Fig. 2. Detected levels of phthalate metabolites in testis co-culture after exposure to parent compounds.
Testis co-cultures (and matrigel-only controls) were exposed to 100µM concentrations of either (a) dibutyl phthalate (DBP) or (b) bis(2-ethylhexyl) phthalate (DHEP) or (c) 150µM diethyl phthalate (DEP). After 2, 8 or 24 hours cell media, cell lysate and corresponding matrigel-only control samples were collected and analyzed for levels of monoester metabolites. Bars show means +/− s.e. (n=3 biological replicates for phthalate exposures and 2 replicate matrigel preparations). *indicates significant difference from corresponding matrigel control (p<0.05, t-test).
Metabolism analysis
Detected levels of monoester metabolites were used as an indication of phase I metabolism of phthalate diesters to monoesters by non-specific lipases. Levels of monoester metabolite detected in the 3D-TCS were compared with metabolic activity of normal in vivo rat and human testes tissues. Measured rates of lipase activity for these tissues obtained from the literature were used for this comparison (Terner et al. 1975; Murase et al. 1982; Choi et al. 2012). The reported rates of lipase activity from in vivo testes were compared with the rates of monoester metabolite formation over time in our culture in vitro. As shown in Table 1, the rates of lipase activity estimated based on formation of monoester metabolites in our culture were within the ranges of activity levels reported in the literature for DBP and DEP, while the rate of DEHP was below this range (~17% of the reported rate of DEHP metabolism in human testes tissue).
Table 1.
Estimated rate of total lipase activity in testis co-culture: Comparison with rates reported in testis tissue in the literature
| Reaction | Mean (SD) reaction rate (picomoles/mg protein/minute) |
| DEHP -> MEHP | 0.5 (0.3) |
| DBP -> MBP | 57 (20) |
| DEP -> MEP | 242 (113) |
| Range of calculated metabolic rate for phthalates in the testis co-culture: 0.5–242 picomoles/mg protein/min. | |
| Reaction | Reported reaction rate (picomoles/mg protein/minute) |
| Conversion DEHP->MEHP (Human testes, subcellular fractions)a |
~3 |
| Lipoprotein lipase activity (Rat testes, whole tissue)b | 18 |
| Triglyceride lipase activity (rat germ cells)c | 283 |
| Reported rates for lipases and phthalate metabolism in rat and human testes in literature: ~3–283 picomoles/mg protein/min. | |
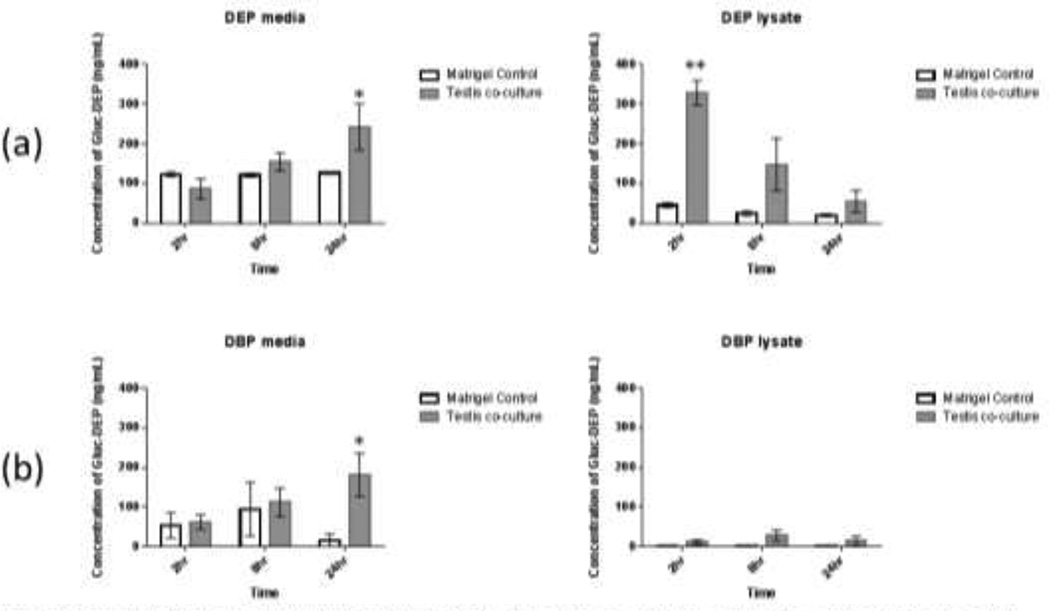
Levels of glucuronidation were estimated by measuring the levels of monoester metabolites pre- and post-incubation with glucuronidase, with the estimated levels of glucuronidated metabolites equal to the difference between the two values. Fig. 3 (a+b) shows the estimated levels of glucuronidation for DBP and DEP treated cells. Levels of glucuronidated metabolite significantly increased in DBP and DEP cell media over 24 hours, with average concentrations reaching 243 and 181 ng/mL, respectively. Significant levels of glucuronidated metabolite were also detected in DEP lysate after 2 hours (329 ng/mL) followed by decreased levels after 8 and 24 hours. No detectable levels of glucuronidation were observed for DEHP treatments.
Fig. 3. Concentration of glucuronidated metabolite in testis co-culture cell media and cell lysate after treatment with phthalate esters for 24 hours.
Tests co-cultures were treated with (a) diethyl phthalate (DEP, 150µM) or (b) dibutyl phthalate (DBP, 100µM). Levels of monoester metabolites were measured before and after glucuronidase treatment in order to determine levels of glucuronidated metabolites in each sample. Bars show mean +/− s.e. (n=3) *: p<0.05, **: p<0.001 (t-test vs. corresponding matrigel control)
In order to compare to levels of conjugated metabolites detected in co-culture samples with rates observed for in vivo testes, the rates of UGT activity calculated for 3DTCS samples (normalized by protein levels and time) were compared with rates of UGT activity reported for rat testes in the literature. We specifically examined the rates of glucuronidation of bisphenol A (BPA) and 1-napthol (reported in nanomoles glucuronidated/mg protein/hour) measured by Yokota, et al. (2002) in rat testes microsomes. As summarized in Table 2, the estimated rates in the 3D-TCS for DBP and DEP exposures were two orders of magnitude lower than the rates reported by (Yokota et al. 1999).
Table 2.
Rate of UGT activity in vitro (testis co-culture) and in vivo (rat testicular microsomes)
| Substrate | Rate of glucuronidation (nanomoles/mg protein/hour) |
|---|---|
| Testes co-culture (current study) |
|
| MBP | 0.16 |
| MEP | 0.18 |
| Testes tissue (values reported in literature)1 |
|
| BPA | 60 |
| 1-napthol | 48 |
Data obtained from: Yokota, et al. (2002), J. Biochem. 132, 265–270
Gene expression analysis for metabolic genes
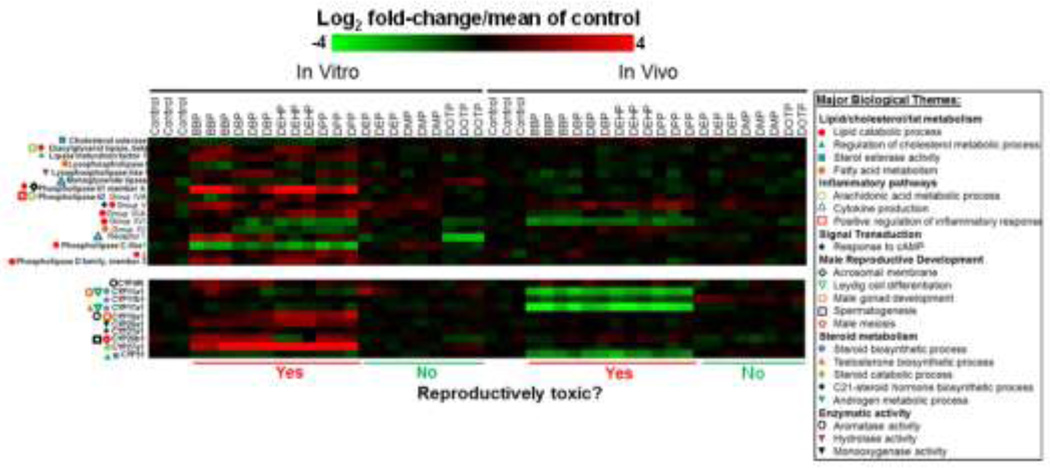
Fig. 4 shows changes in gene expression for 16 lipase and 10 CYP450 genes in the 3D-TCS after a 24 hour exposure to seven different phthalates. We observed a distinct profile of lipase gene changes (both up and downregulation) for reproductively toxic phthalate exposures which was quite distinct from non-reproductively toxic phthalate exposures. Gene Ontology defined categories were used to identify relevant functional processes associated with each gene. The 16 lipases are associated with a number of major biological themes including lipid metabolism (GO:16402 lipid catabolic process, GO:8203 cholesterol metabolic process, GO: 4771 sterol esterase activity), inflammatory processes (GO:19369 arachidonic acid metabolism, GO:1816 cytokine production, GO:6954 regulation of inflammatory response), intracellular signaling (GO:51591 response to cAMP) and male reproductive development (GO:2080 acrosomal membrane). Similarly, we observed phthalate induced gene expression patterns for 10 CYP450 enzymes in our cell culture model. These 10 CYP450s are associated with a number of major biological themes related to steroid metabolism (GO:6694 steroid biosynthetic process), lipid/cholesterol metabolism (GO:90181 regulation of cholesterol metabolism) and male reproductive development (GO: 33327 Leydig cell differentiation, GO:7140 male meiosis). Similar to the results reported for the lipase genes, reproductively toxic phthalate exposures impacted gene expression for this class enzymes differently than non-reproductively toxic phthalates.
Fig. 4. Heatmaps showing changes in gene expression for 16 lipases (top) and 10 cytochrome P450’s (bottom) after phthalate exposure, both in the testis co-culture (in vitro) and in rat fetal testes (in vivo) after exposure to reproductively toxic and non-toxic phthalate esters.
These genes belong to multiple Gene Ontology categories covering biological themes relevant to male reproductive processes (e.g. lipid/steroid metabolism. male reproductive development and aromatase activity). In the key to the right major biological themes are listed in bold with specific Gene Ontology terms listed below next to symbols.
Phthalate kinetics in the 3D-TCS
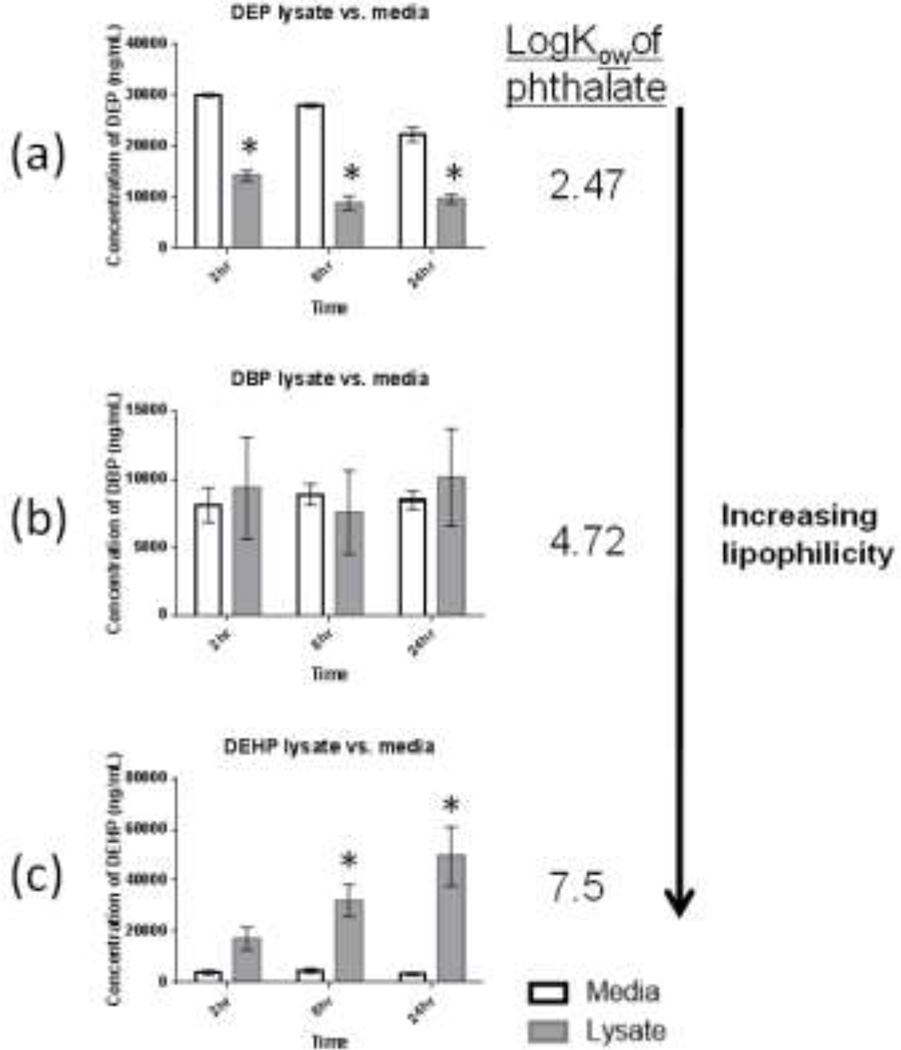
In order to characterize the kinetics of phthalates in our co-culture model, we determined the extent that physiochemical properties such as logKow could be used to predict the kinetics of phthalates in the 3D-TCS. The three phthalates used in this study had a range of logKow values: 2.47 (DEP), 4.72 (DBP) and 7.5 (DEHP). We predicted that the compound with the highest logKow (i.e. the most lipophilic compound) would partition more to the cell lysate fraction compared to the cell media. As shown in Fig. 5, the three phthalate diesters differentially partitioned between cell media and cell lysate, following our hypothesis that differences in lipophilicity would lead to different kinetics of compounds in the co-culture system. The concentration of DEHP was significantly higher in the cell lysate versus the cell media fraction at 8 and 24 hour timepoints, with average DEHP concentrations of 124 and 8µg/mL in cell lysate and cell media, respectively, after 24 hours. In contrast, DEP maintained a significantly higher concentration in the cell media compared to cell lysate. There appeared to be no discernible tendency for DBP to concentrate in either fraction, as the concentrations in cell media and lysate were not significantly different at any timepoint.
Fig. 5. Concentrations of 3 phthaiate diesters detected in testis co-culture cell media or cell lysate after exposure to either DBP, DEHP (100µM) or DEP (150µM).
Testis cells were exposed to DBP, DEHP or DEP for 2, 8 or 24 hours. Values are mean and standard errors for 3 replicate treatments. Bars show mean +/− s.e. (n=3) * indicates average concentration in cell lysate samples were significantly different then concentration in cell media at corresponding time point (t-test p<0.05).
Discussion
Uncertainty about the metabolic capabilities in cell culture models is one of the challenges in the utilization of in vitro models in toxicity screening. We have demonstrated that metabolism of phthalate diesters is occurring in our model of male testis development (3D-TCS), as evidenced by detection of monoester metabolites in cell lysate and cell media. Phthalates were metabolized at different rates over time and levels of metabolites ranged from <0.1% −13% of initial concentration of phthalate parent compound. The phase I reaction of phthalate metabolism (see Figures 1. a–c) produces the monoester metabolites and is catalyzed by a class of enzymes called lipases. These enzymes also play important roles in lipid metabolism and in male reproductive development (Holst et al. 1994; Chung et al. 2001). The rate of metabolism observed (in units of picomoles/mg of protein/minute) for DBP and DEP was within the range of reported rates for lipase activity in rat testes and for human testes subcellular fractions, while the rate of metabolism of DEHP was about ~17% of the lowest of the reported rates (see Table 1). Interestingly, the rates of monoester metabolite formation across the three phthalates appeared to follow a trend similar to that reported by Lake et al., who observed in rat and human intestinal preparations, that phthalates with shorter alkyl side chain length were metabolized at a faster rate compared to those with longer side chains. For example, DBP was metabolized at a slower rate than DEP (Lake et al. 1977). Further experiments will be needed to confirm this observation.
We also measured levels of glucuronidated monoester metabolites indirectly by calculating the difference in metabolite levels observed before and after incubation with glucuronidase. Glucuronidation is a phase II reaction catalyzed by a group of enzymes known as UDP-glucuronosyltransferases (UGTs). This group of enzymes plays a role in the metabolism of both endogenous and xenobiotic compounds by converting lipophilic substrates into water-soluble compounds, facilitating excretion. UGTs are expressed in a number of organs in the rat, including the Sertoli cells in the testes and in testicular Leydig cells of humans (Shah and McLachlan 1976; Brands et al. 2000; Uhlen et al. 2010). In our cultures, the average estimated rates for UGT activity toward MBP and MEP (metabolites of DBP and DEP) after 24 hours were 0.16 and 0.18 nanomoles/mg protein/hour, respectively. By comparison, these rates are lower than rates of glucuronidation reported for various other compounds in the in vivo rat testicular homogenates. For example, Yokota et al. (2002) found a rate of 60 and 48 nanomoles/mg protein/min. in rat testes microsomes when bisphenol A and 1-napthol were used as a substrates, respectively (Yokota et al. 2002). There are several potential explanations for these results including differences in activity toward substrates (e.g. activity toward BPA may be higher than toward MBP for certain UGT’s) as well as kinetic factors (MBP and MEP may not have sufficient uptake into cells to induce similar levels of UGT activity). In contrast to MBP and MEP, no detectable levels of MEHP conjugation were observed in our in vitro model. These results are consistent with observations in rat DEHP exposures in vivo, in which MEHP has mostly been observed in its free form and is not thought to be conjugated in any significant amount (Keys et al. 2000; Kurata et al. 2012).
In addition to the indication of lipase activity suggested by the formation of phthalate metabolites in the 3D-TCS, we demonstrated that gene expression signals were present for these enzymes and we compared these data with fetal rat testes in vivo. Both in vitro and in vivo data showed that gene expression for lipase genes were significantly impacted by exposure to reproductively toxic phthalates, consistent with what has been shown in previous studies. For example, DEHP has been shown to inhibit the activity of lysosomal acid lipase in rat liver fractions and increase lipoprotein lipase activity in rat epididymal tissue. MEHP, the toxic metabolite of DEHP, has been shown to increase expression of hormone sensitive lipase transcript in mouse Leydig tumor cells (Teruyoshi 1986; Gunnarsson et al. 2008; Martinelli et al. 2010). In our 3D-TCS model, we observed a trend of significant downregulation for lysosomal acid lipase after exposure to developmentally toxic phthalates and a significant increase in lipoprotein lipase expression. Phthalate impacts on lipases have a number of implications for elucidating phthalate effects on testes function in vivo. Lipases regulate lipid metabolism and one of the modes of action for phthalates in the disruption of lipid profiles in the testes, which in turn can lead to altered steroid levels. We queried the Gene Ontology database to identify additional biological processes and molecular functions associated with these genes, which revealed more functions relevant to male reproductive development. For example, in addition to their well-known roles in lipid metabolism, several of the affected lipases were shown to be involved inflammatory processes such as cytokine production (GO:1816) and arachidonic acid metabolism (GO:19369). These processes have important implications for male reproductive toxicity pathways due to the regulatory roles that some cytokines and arachidonic acid play in the regulation of steroidogenesis (Romanelli et al. 1995; Hedger and Meinhardt 2003; Iovannisci et al. 2007). When comparing changes occurring in these lipases in vivo and in vitro, we observed that relatively fewer changes were present in the in vivo testes. There are a number of reasons for this, including the differences in times of exposure in vivo and in vitro as well as kinetic and metabolic differences between the two model systems. The gene expression changes observed in the in vitro testes model after 24 hours may represent an early response to phthalate exposure that is not maintained after the repeating dosing performed for the in vivo data. For example, similar to what we observed in our model, Lahousse, et al. (2006), observed increased expression of Pla1a (phospholipase A1, member A) in in vivo rat testes 12 hours after exposure to the MEHP, the active metabolite of DEHP (Lahousse et al. 2006).
In addition to lipases CYP450s represent another important class of enzymes with numerous functions including metabolism of exogenous compounds and endogenous metabolism of steroids, cholesterol or lipids (Nebert and Russell 2002). Gene expression signals for 10 CYP450s involved in endogenous metabolic processes related to male reproductive development, such as steroid hormone metabolism (Cyp11a1, Cyp17a1 and Cyp19a1) and development of male gametes (Cyp26b1) were detected in the 3D-TCS. Exposure to reproductively toxic phthalates differentially affected the expression of these CYPs compared to exposure to non-reproductively toxic phthalates. We saw a similar pattern of differential expression for the reproductively toxic phthalate exposures in the in vivo testes, although the specific CYPs affected and pattern of expression changes were divergent. These results indicate that the 3D-TCS is a metabolically active culture that is able to metabolize exogenous compounds (phthalates), and expresses transcriptomic signals for important endogenous metabolic functions. These classes of enzymes which have important roles in the proper development of male reproductive organs. Characterizing responses mediated by these enzymes will aid in the elucidation of toxicity pathways, facilitating linkages to in vivo pathways using the 3D-TCS.
Another challenge in using in vitro toxicity models is uncertainty regarding the kinetics of compounds. Evaporation, binding to tissue culture plastics and binding to serum protein have all been identified as factors that could lead to differences between nominal concentrations of test compound in cell media and concentrations observed at cellular targets. Prediction of kinetic behaviors of test compounds in vitro will allow for better interpretation of data generated in these model systems (Groothuis et al. 2013). In the present study, we showed that logKow (a measure of lipophilicity) was able to predict phthalate diester partitioning between media and cell lysate fractions in the 3D-TCS. This suggests that physiochemical properties are at least partially driving phthalate kinetics in this model.
In summary, phthalate monoester metabolites were detected in cell media and cell lysate samples in the 3D-TCS. These results, along with observed transcriptomic responses, provide evidence for the presence of functional lipases and CYP450s (enzymes involved in important metabolic processes in the testes) in our testis co-culture (Lobo et al. 2009). In addition we have obtained important information about the kinetic behavior of phthalates in the 3D-TCS. Our results will greatly contribute to the optimization of accurate dosimetry in order to determine the in vitro concentrations which correlate with concentrations in testis tissue in vivo under relevant exposure scenarios and more accurately interpret toxicity data obtained from our model.
Supplementary Material
Highlights.
Metabolism and kinetics of three phthalate esters were investigated in an in vitro model of male testes development
Metabolism of phthalates is occurring in the testes model, evidenced by detection of metabolites in cells and cell media
LogKow was able to predict phthalate diester partitioning between media and cells
Gene expression signals for key metabolic enzymes (CYPP450s and lipases) were reflective of in vivo conditions and responses to phthalates
Results indicate that this testes model is metabolically active and that physiochemical properties can predict phthalate kinetics in the model
Acknowledgments
This work was supported by in part by the UW NIEHS Center for Ecogenetics and Environmental Health (5 P30 ES007033), UW EPA Center for Predictive Toxicology (RD-83573801), US-FDA (FDA: 1U01FD004242), NIEHS training grant (T32ES007032) and the NIH Center on Human Development and Disability (1 U54 HD083091-01). We gratefully acknowledge Kevin Gaido for generously providing the in vivo transcriptomic data. We would additionally like to thank Kirk Van Ness (Institute for Risk Analysis and Risk Communication, University of Washington) for his helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albro PW. Absorption, metabolism, and excretion of di(2-ethylhexyl) phthalate by rats and mice. Environ Health Perspect. 1986;65:293–298. doi: 10.1289/ehp.8665293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauboer BJ. Biokinetic modeling and in vitro-in vivo extrapolations. J Toxicol Environ Health B Crit Rev. 2010;13(2–4):242–252. doi: 10.1080/10937404.2010.483940. [DOI] [PubMed] [Google Scholar]
- Boess F, Kamber M, et al. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci. 2003;73(2):386–402. doi: 10.1093/toxsci/kfg064. [DOI] [PubMed] [Google Scholar]
- Brands A, Munzel PA, et al. In situ hybridization studies of UDP-glucuronosyltransferase UGT1A6 expression in rat testis and brain. Biochem Pharmacol. 2000;59(11):1441–1444. doi: 10.1016/s0006-2952(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 5.Brown HS, Griffin M, et al. Evaluation of cryopreserved human hepatocytes as an alternative in vitro system to microsomes for the prediction of metabolic clearance. Drug Metab Dispos. 2007;35(2):293–301. doi: 10.1124/dmd.106.011569. [DOI] [PubMed] [Google Scholar]
- Bustamante-Montes LP, Hernandez-Valero MA, et al. Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. J Dev Orig Health Dis. 2013;4(4) doi: 10.1017/S2040174413000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. Phthalate Metabolites in Urine, Method No: 6306.03. C. f. D. C. a. Prevention. 2010 [Google Scholar]
- Choi K, Joo H, et al. In vitro metabolism of di(2-ethylhexyl) phthalate (DEHP) by various tissues and cytochrome P450s of human and rat. Toxicol In Vitro. 2012;26(2):315–322. doi: 10.1016/j.tiv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Chung S, Wang SP, et al. Infertility and testicular defects in hormone-sensitive lipase-deficient mice. Endocrinology. 2001;142(10):4272–4281. doi: 10.1210/endo.142.10.8424. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Campbell JL, et al. Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol In Vitro. 2010;24(1):327–334. doi: 10.1016/j.tiv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Coecke S, Ahr H, et al. Metabolism: a bottleneck in in vitro toxicological test development. The report and recommendations of ECVAM workshop 54. Altern Lab Anim. 2006;34(1):49–84. doi: 10.1177/026119290603400113. [DOI] [PubMed] [Google Scholar]
- Daniel JW, Bratt H. The absorption, metabolism and tissue distribution of di(2-ethylhexyl)phthalate in rats. Toxicology. 1974;2(1):51–65. doi: 10.1016/0300-483x(74)90042-0. [DOI] [PubMed] [Google Scholar]
- Dixon RL, Lee IP. Pharmacokinetic and adaptation factors involved in testicular toxicity. Fed Proc. 1980;39(1):66–72. [PubMed] [Google Scholar]
- Duty SM, Silva MJ, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003;14(3):269–277. [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, et al. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51(7):899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Groothuis FA, Heringa MB, et al. Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology. 2013 doi: 10.1016/j.tox.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Gunnarsson D, Leffler P, et al. Mono-(2-ethylhexyl) phthalate stimulates basal steroidogenesis by a cAMP-independent mechanism in mouse gonadal cells of both sexes. Reproduction. 2008;135(5):693–703. doi: 10.1530/REP-07-0460. [DOI] [PubMed] [Google Scholar]
- Hartung T, Blaauboer BJ, et al. An expert consortium review of the EC-commissioned report "alternative (Non-Animal) methods for cosmetics testing: current status and future prospects - 2010". ALTEX. 2011;28(3):183–209. doi: 10.14573/altex.2011.3.183. [DOI] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62(11):806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58(1):1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- Holst LS, Hoffmann AM, et al. Localization of hormone-sensitive lipase to rat Sertoli cells and its expression in developing and degenerating testes. FEBS Lett. 1994;355(2):125–130. doi: 10.1016/0014-5793(94)01185-0. [DOI] [PubMed] [Google Scholar]
- Iovannisci DM, Lammer EJ, et al. Association between a leukotriene C4 synthase gene promoter polymorphism and coronary artery calcium in young women: the Muscatine Study. Arterioscler Thromb Vasc Biol. 2007;27(2):394–399. doi: 10.1161/01.ATV.0000252680.72734.10. [DOI] [PubMed] [Google Scholar]
- Keys DA, Wallace DG, et al. Quantitative evaluation of alternative mechanisms of blood disposition of di(n-butyl) phthalate and mono(n-butyl) phthalate in rats. Toxicol Sci. 2000;53(2):173–184. doi: 10.1093/toxsci/53.2.173. [DOI] [PubMed] [Google Scholar]
- Kurata Y, Makinodan F, et al. Metabolism of di (2-ethylhexyl) phthalate (DEHP): comparative study in juvenile and fetal marmosets and rats. J Toxicol Sci. 2012;37(1):33–49. doi: 10.2131/jts.37.33. [DOI] [PubMed] [Google Scholar]
- Lahousse SA, Wallace DG, et al. Testicular gene expression profiling following prepubertal rat mono-(2-ethylhexyl) phthalate exposure suggests a common initial genetic response at fetal and prepubertal ages. Toxicol Sci. 2006;93(2):369–381. doi: 10.1093/toxsci/kfl049. [DOI] [PubMed] [Google Scholar]
- Lake BG, Phillips JC, et al. The in vitro hydrolysis of some phthalate diesters by hepatic and intestinal preparations from various species. Toxicol Appl Pharmacol. 1977;39(2):239–248. doi: 10.1016/0041-008x(77)90157-0. [DOI] [PubMed] [Google Scholar]
- Lertsirisopon R, Soda S, et al. Abiotic degradation of four phthalic acid esters in aqueous phase under natural sunlight irradiation. J Environ Sci (China) 2009;21(3):285–290. doi: 10.1016/s1001-0742(08)62265-2. [DOI] [PubMed] [Google Scholar]
- Liu K, Lehmann KP, et al. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod. 2005;73(1):180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- Lobo MV, Huerta L, et al. Hormone-sensitive lipase expression and IHC localization in the rat ovary, oviduct, and uterus. J Histochem Cytochem. 2009;57(1):51–60. doi: 10.1369/jhc.2008.951996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli MI, Mocchiutti NO, et al. Effect of di(2-ethylhexyl) phthalate (DEHP) on lipolysis and lipoprotein lipase activities in adipose tissue of rats. Hum Exp Toxicol. 2010;29(9):739–745. doi: 10.1177/0960327110361750. [DOI] [PubMed] [Google Scholar]
- Martino-Andrade AJ, Chahoud I. Reproductive toxicity of phthalate esters. Mol Nutr Food Res. 2010;54(1):148–157. doi: 10.1002/mnfr.200800312. [DOI] [PubMed] [Google Scholar]
- Murase T, Yamada N, et al. Triacylglycerol lipases in adrenals and testes of rats. J Biochem. 1982;92(3):817–821. doi: 10.1093/oxfordjournals.jbchem.a133994. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, et al. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43(1):47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360(9340):1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- Reid AM, Brougham Concepta A, Fogarty Andrew M, Roche James J. An investigation into possible sources of phthalate contamination in the environmental analytical laboratory. International Journal of Environmental Analytical Chemistry. 2007;87(2):125–133. [Google Scholar]
- Romanelli F, Valenca M, et al. Arachidonic acid and its metabolites effects on testosterone production by rat Leydig cells. J Endocrinol Invest. 1995;18(3):186–193. doi: 10.1007/BF03347801. [DOI] [PubMed] [Google Scholar]
- Saldutti LP, Beyer 2 Bruce K, Breslin 3 William, Brown 4 Terry R, Chapin 5 Robert E, Campion 5 BE Sarah, Faustman 7 Elaine, Foster 8 Paul MD, Hartung 9 Thomas, et al. In Vitro Testicular Toxicity Models: Opportunities for Advancement via Biomedical Engineering Techniques. Altern Lab Anim. 2013;30(3):353–377. doi: 10.14573/altex.2013.3.353. [DOI] [PubMed] [Google Scholar]
- Shah HC, McLachlan JA. The fate of diethylstilbestrol in the pregnant mouse. J Pharmacol Exp Ther. 1976;197(3):687–696. [PubMed] [Google Scholar]
- Sidhu JS, Farin FM, et al. Influence of extracellular matrix overlay on phenobarbital-mediated induction of CYP2B1, 2B2, and 3A1 genes in primary adult rat hepatocyte culture. Arch Biochem Biophys. 1993;301(1):103–113. doi: 10.1006/abbi.1993.1121. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, et al. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16(5):972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108(2):177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Toxicological Profile for Di-n-butyl Phthalate. A. f. T. S. a. D. R. U.S. Department Of Health And Human Services; 2001. [Google Scholar]
- Terner C, MacLaughlin J, et al. Changes in lipase and phosphatase activities of rat spermatozoa in transit from the caput to the cauda epididymidis. J Reprod Fertil. 1975;45(1):1–8. doi: 10.1530/jrf.0.0450001. [DOI] [PubMed] [Google Scholar]
- Teruyoshi Y, Enomoto Noriyuki, Kuzuhara Shoji. Effects of Phthalate Esters on Liver Lysosomal Acid Lipase and Acid Esterase In Vitro. Agric. Biol. Chem. 1986;6(50):1653–1654. [Google Scholar]
- Uhlen M. The Human Protein Atlas. Retrieved 2/23/15, 2015, from http://www.proteinatlas.org/ENSG00000167165-UGT1A6/tissue. [Google Scholar]
- Uhlen M, Oksvold P, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28(12):1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Wegner S, Hong S, et al. Preparation of rodent testis co-cultures. Curr Protoc Toxicol. 2013;Chapter 16(Unit 16):10. doi: 10.1002/0471140856.tx1610s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Li F, et al. Occurrence and degradation characteristics of dibutyl phthalate (DBP) and di-(2-ethylhexyl) phthalate (DEHP) in typical agricultural soils of China. Sci Total Environ. 393(2–3):333–340. doi: 10.1016/j.scitotenv.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Yokota H, Iwano H, et al. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J. 1999;340(Pt 2):405–409. [PMC free article] [PubMed] [Google Scholar]
- Yokota H, Kunimasa Y, et al. Effects on extrahepatic UDP-glucuronosyltransferases in hypophysectomized rat. J Biochem. 2002;132(2):265–270. doi: 10.1093/oxfordjournals.jbchem.a003220. [DOI] [PubMed] [Google Scholar]
- Yu X, Hong S, et al. Cadmium-induced activation of stress signaling pathways, disruption of ubiquitin-dependent protein degradation and apoptosis in primary rat Sertoli cell-gonocyte cocultures. Toxicol Sci. 2008;104(2):385–396. doi: 10.1093/toxsci/kfn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Hong S, et al. Improving in vitro Sertoli cell/gonocyte co-culture model for assessing male reproductive toxicity: Lessons learned from comparisons of cytotoxicity versus genomic responses to phthalates. Toxicol Appl Pharmacol. 2009;239(3):325–336. doi: 10.1016/j.taap.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Sidhu JS, et al. Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat Sertoli cell/gonocyte co-cultures: an improved in vitro model for assessment of male reproductive toxicity. Toxicol Sci. 2005;84(2):378–393. doi: 10.1093/toxsci/kfi085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.