Abstract
Background/Aims
Recently, loss-of-function mutations in the MKRN3 gene have been implicated in the etiology of familial central precocious puberty (CPP) in both sexes. We aimed to analyze the frequency of MKRN3 mutations in boys with CPP and to compare the clinical and hormonal features of boys with and without MKRN3 mutations.
Methods
This was a retrospective review of clinical, hormonal and genetic features of 20 male patients with idiopathic CPP evaluated at an academic medical center. The entire coding regions of MKRN3, KISS1 and KISS1R genes were sequenced.
Results
We studied 20 boys from 17 families with CPP. All of them had normal brain magnetic resonance imaging. Eight boys from 5 families harbored four distinct heterozygous MKRN3 mutations predicted to be deleterious for protein function, p.Ala162Glyfs * 14, p.Arg213Glyfs * 73, p.Arg- 328Cys and p.Arg365Ser. One boy carried a previously described KISS1-activating mutation (p.Pro74Ser). The frequency of MKRN3 mutations among these boys with idiopathic CPP was significantly higher than previously reported female data (40 vs. 6.4%, respectively, p < 0.001). Boys with MKRN3 mutations had typical clinical and hormonal features of CPP. Notably, they had later pubertal onset than boys without MKRN3 abnormalities (median age 8.2 vs. 7.0 years, respectively, p = 0.033).
Conclusion
We demonstrated a high frequency of MKRN3 mutations in boys with CPP, previously classified as idiopathic, suggesting the importance of genetic analysis in this group. The boys with CPP due to MKRN3 mutations had classical features of CPP, but with puberty initiation at a borderline age.
Keywords: MKRN3 gene, Male precocious puberty, Gonadotropin-releasing hormone, Genetics
Introduction
Normal human puberty is triggered by the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus to stimulate the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary, which leads to the consequent activation of gonadal function [1]. During the childhood years, neurons secreting GnRH are subjected to persistent transsynaptic inhibition by mechanisms that are not completely understood [2]. The initiation of puberty requires loss of inhibitory inputs and gain in excitatory inputs to the complex GnRH neuronal network [2, 3]. The beginning of puberty in boys is marked by testicular enlargement, assessed as testicular volume >4 ml or testicular length >2.5 cm (genital Tanner stage 2) [1]. A mean age at male pubertal onset of 11.6 years has been described in past decades, but more recent studies have suggested that boys are entering puberty earlier [4–6].
Sexual precocity in boys is defined by the development of secondary sexual characteristics before the age of 9 years [7]. The most common mechanism of progressive precocious puberty is early activation of the hypothalamic-pituitary-gonadal axis (central precocious puberty, CPP) [1]. Diagnostic confirmation of CPP relies on the demonstration of pubertal basal and/or GnRH-stimulated LH levels [1, 8]. CPP may result from a central nervous system (CNS) lesion, such as hypothalamic hamartoma, astrocytoma, arachnoid cyst or hydrocephalus [1, 9]. When no cause is identified, it is called idiopathic CPP [1]. Interestingly, idiopathic CPP is almost 10 times less frequent in boys than in girls [10, 11]. Among boys with CPP, a higher prevalence of CNS lesions has been demonstrated (40–90%), indicating the need for brain magnetic resonance imaging (MRI) in all boys with CPP [1, 9, 11, 12]. However, a recent study suggested that the number of cases of male idiopathic CPP is increasing over time [13].
The factors that regulate the onset of puberty remain a mystery. Certainly, genetic, nutritional, environmental, socioeconomic and epigenetic factors are important regulators of the initiation of puberty [2, 14–16]. The role of genetic factors in pubertal timing is illustrated by the occurrence of familial CPP, which is defined by the presence of more than one affected member in a family [16]. Currently, loss-of-function mutations in the makorin ring finger protein 3 (MKRN3) gene are the most frequent known genetic cause of familial CPP, affecting both sexes [17–19]. Other than mutations in MKRN3, only one other genetic defect in association with male idiopathic CPP has been reported, in a boy with a gain-of-function mutation in KISS1 [20].
Limited research has been conducted in boys with CPP in the absence of CNS abnormalities. To date, little clinical and hormonal data from males with CPP or early puberty caused by MKRN3 mutations have been reported [17, 21–23]. The aims of the present study were to describe the clinical, hormonal and genetic features of boys with CPP due to MKRN3 mutations, to compare their phenotypes with that of boys with idiopathic CPP, and to assess the frequency of MKRN3 mutations in boys with idiopathic CPP.
Patients and Methods
A total of 254 consecutive patients (234 girls and 20 boys) with CPP were referred for clinical and/or genetic evaluation to the Endocrinology Unit at São Paulo University from 1998 to 2015. In all of them, CNS abnormalities were excluded by MRI. Boys with idiopathic CPP had different origins (8 Brazilians, 3 Greeks, 3 Turkish, 3 Argentineans, 2 Americans and 1 Belgian). Nine of them (9/20, 45%) had first-degree relatives with a history of premature sexual development. Patients 10 and 11 were brothers from a Brazilian family, and patients 15, 16 and 17 were also brothers from an Argentinean family ( table 1 ). Idiopathic CPP in boys was diagnosed based on the presence of genital Tanner stage 2 (testicular volume >4 ml or length >2.5 cm) before the age of 9 years, pubertal basal and/or GnRH-stimulated LH levels, and normal brain MRI [8, 24–26]. Six male cases (patients 9 to 14, table 1 ) have been previously reported [17, 20]. This study was approved by the local ethics committee, and written informed consent was obtained from the parents of the children.
Table 1.
Clinical, hormonal and genetic features of 20 boys from 17 families with idiopathic CPP
| Family No. |
Patient No. |
Origin | Initial clinical manifestation (age, years) |
Time of diagnosis
|
BA years |
LH, IU/l
|
Basal FSH, IU/l |
T, ng/dl |
Hormone assay |
Familial case |
Genetic analysis |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| age years |
genital Tanner stage |
BMI Z-score |
basal | after GnRH |
||||||||||
| I | 1 | Turkey | Penis enlargement and pubarche (2.4) | 3.4 | 2 | 4.0 | 5 | 6.7 | n.a. | 5.9 | 42 | ICMA | No | – |
|
| ||||||||||||||
| II | 2 | Turkey | Penis enlargement (0.9) | 1.4 | 2 | n.a. | 3 | 1.2 | 23.7 | 5.1 | 297 | ICMA | No | – |
|
| ||||||||||||||
| III | 3 | Turkey | Penis enlargement and pubarche (4.0) | 4.2 | 2 | 2.0 | 5 | 6.0 | n.a. | 3.8 | 160 | ECLIA | No | – |
|
| ||||||||||||||
| IV | 4 | Brazil | Testicular enlargement and pubarche (8.0) | 10.1 | 3 | 0.2 | 13 | 0.9 | 8.4 | 2.5 | 68 | IFMA | No | – |
|
| ||||||||||||||
| V | 5 | Brazil | Pubarche (7.0) and testicular enlargement (9.0) | 9.8 | 2 | n.a. | n.a. | 0.7 | 18.0 | 1.0 | 75.7 | IFMA | No | – |
|
| ||||||||||||||
| VI | 6 | Brazil | Testicular enlargement and pubarche (8.0) | 10.4 | 3 | 0.9 | 13 | 2.5 | n.a. | 1.3 | 163 | IFMA | No | – |
|
| ||||||||||||||
| VII | 7 | Brazil | Testicular enlargement (7.0) and pubarche (9.0) | 8.7 | 2 | 0.3 | 8 | 1.2 | 33.4 | 2.5 | 19 | IFMA | No | – |
|
| ||||||||||||||
| VIII | 8 | Brazil | Pubarche (2.0), testicular and penis enlargement (3.0) | 5.1 | 4 | 1.0 | 13 | 1.1 | n.a. | 1.1 | 92 | IFMA | No | – |
|
| ||||||||||||||
| IX | 9 | Brazil | Pubarche and penis enlargement (1.0) | 1.4 | 2 | n.a. | 3 | 11.5 | 47.2 | 8.3 | 600 | RIA | No | KISS1(p.Pro74Ser) |
|
| ||||||||||||||
| X | 10 | Brazil | Testicular enlargement and pubarche (5.9) | 8.1 | 3 | 0.9 | 10 | 1.2 | 6.7 | 1.5 | 116 | ICMA | Yes | MKRN3 (p.Ala162Glyfs*14) |
| 11 | Testicular enlargement and pubarche (unknown) | 9.7 | 3 | 1.4 | 9.7 | 1.6 | 10.9 | 0.8 | 548 | ICMA | ||||
|
| ||||||||||||||
| XI | 12 | Belgium | Testicular enlargement and pubarche (unknown) | 9.7 | 3 | 1.2 | 12 | 2.0 | 19.5 | 4.4 | 67 | ICMA | Yes | MKRN3 (p.Arg365Ser) |
|
| ||||||||||||||
| XII | 13 | USA | Testicular enlargement and pubarche (8.5) | 8.8 | 3 | n.a. | 11.5 | 4.1 | n.a. | 3.1 | 216 | ICMA | Yes | MKRN3 (p.Ala162Glyfs*14) |
|
| ||||||||||||||
| XIII | 14 | USA | Testicular enlargement (8.0) | 8.7 | 3 | 2.2 | 11 | 2.9 | 20.0 | 2.5 | 78 | ICMA | Yes | MKRN3 (p.Arg213Glyfs*73) |
|
| ||||||||||||||
| XIV | 15 | Argentina | Testicular enlargement (7.5) | 8.4 | 3 | 1.18 | 10 | 1.1 | 10.2 | 1.5 | 466 | ICMA | Yes | MKRN3 (p.Arg328Cys) |
| 16 | Testicular enlargement (8.5) | 9.8 | 3 | 0.19 | 11 | 1.4 | n.a. | 2.4 | 200 | ICMA | ||||
| 17 | Testicular enlargement (8.6) | 8.6 | 2 | 0.14 | 9 | 0.7 | n.a. | 1.2 | <10 | ICMA | ||||
|
| ||||||||||||||
| XV | 18 | Greece | Pubarche and testicular enlargement (unknown) | 9.5 | 3 | 2.4 | 12 | 4.7 | 21.5 | 5.5 | 40.9 | ECLIA | No | – |
|
| ||||||||||||||
| XVI | 19 | Greece | Pubarche and testicular enlargement (7.8) | 8.8 | 2 | 1.7 | 10.5 | 1.2 | 14.2 | 1.9 | 27.7 | ECLIA | Yes | – |
|
| ||||||||||||||
| XVII | 20 | Greece | Pubarche and testicular enlargement (8.0) | 10 | 4 | 1.7 | 13 | 1.5 | 17.5 | 0.7 | 243 | ECLIA | No | – |
BA = Bone age; T = testosterone; n.a. = not available.
Hormone Assays
Serum LH, FSH and testosterone concentrations were measured by ultrasensitive assays (immunofluorometric assay – IFMA, immunochemiluminometric assay – ICMA or electrochemiluminometric assay – ECLIA) with good correlation among them. The interassay and intraassay coefficients of variation were 5% or less for all assays. For IFMA, serum LH and FSH concentrations were determined by commercial, solid phase, two-site fluoroimmunometric assays (FIA; AutoDELFIA hLH Spec and AutoDELFIA hFSH; Wallac Oy, Turku, Finland), and serum testosterone concentrations were measured by commercial solid-phase FIAs (AutoDELFIA Testosterone; Wallac Oy). Functional sensitivity was set at 0.6 IU/l for LH, 1.0 IU/l for FSH and 14 ng/dl for testosterone. For ICMA, LH and FSH were measured using an Immulite 1000 automated system and commercial kits (Diagnostic Products Corp., Medlab, Los Angeles, Calif., USA). Sensitivity was set at 0.1 IU/l for both LH and FSH and 19 ng/dl for testosterone [26]. Finally, for ECLIA, commercial kits for LH (Ref. 11732234) and FSH (Ref. 11775863) (Roche Diagnostics GmbH, Mannheim, Germany) were used and assessed in the modular Cobas e601 analyzer. The functional sensitivities of both LH and FSH assays were 0.1 U/l, according to the second WHO IS 80/552 for LH and the second IRP 78/549 for FSH. For total testosterone, functional sensitivity was set at 12 ng/dl. For the acute GnRH stimulation test, serum LH was measured at –15, 0, 15, 30, 45 and 60 min after i.v. administration of 100 μg of GnRH. Basal LH levels >0.6 U/l (IFMA) or 0.2 U/l (ICMA and ECLIA) were considered as pubertal levels, and a GnRH-stimulated LH peak >9.6 U/l (IFMA) or 5.0 U/l (ICMA and ECLIA) was considered as a pubertal response [8, 24–26]. In only 1 case (patient 9, table 1 ), was the hormonal profile measured by radioimmunoassay (RIA), and a GnRH-stimulated LH peak >25 U/l was considered as a pubertal response [20]. Basal testosterone levels higher than 19 ng/dl (IFMA), 14 ng/dl (ICMA and ECLIA) or 30 ng/dl (RIA) were considered as pubertal values.
Genetic Analyses
Genomic DNA was extracted from peripheral blood leukocytes from all patients using standard procedures. The single exon of MKRN3 (GenBank accession No. NC_000015.9) was amplified by PCR followed by automated sequencing of the products in all 20 boys [27]. The boys without mutations in the coding sequence of MKRN3 were screened for abnormalities in the promoter region of MKRN3. A 1,000-bp region (−750 to +350 in relation to the transcriptional start site) of the MKRN3 gene, including putative transcription factor motifs (PEA3, SRE, SRF, C/EBP, AP2, testis-R), was amplified by PCR followed by automated sequencing.
Additionally, all patients were screened for mutations in KISS1 and KISS1R genes [20, 28]. The promoter region and the three exons of the KISS1 gene (GenBank accession No. NC_000001.10) were amplified by PCR followed by automated sequencing of the products [20]. The entire coding region and the exon-intron boundaries of KISS1R (GenBank accession number NC_000019.9) were also amplified by PCR and sequenced on an automated sequencer [28].
Two databases (1000 Genomes and NHLBI EVS) were used to exclude common variants (minor allele frequency >1%) [29, 30]. Computational algorithms (PolyPhen-2, SIFT, Panther and MutationTaster) were used to predict the pathogenicity of the variants [31, 32].
Statistical Analyses
Data are presented as median and range. The nonparametric Mann-Whitney U test was used to compare clinical and hormonal data between patients with and without MKRN3 mutations.
A previously reported female cohort assembled by São Paulo University composed of 234 girls from 221 families with idiopathic CPP was used for statistical comparison between boys and girls [17, 27]. MKRN3 mutations were identified in 15 girls (11 of them were unrelated) in this large cohort. The χ2 test was applied to analyze the association between categorical variables, considering both the entire group and only index cases. Statistical analyses were performed using the software Sigmastat for Windows 3.5, and statistical significance was set at p < 0.05.
Results
The clinical and hormonal features of the 20 boys with idiopathic CPP are described in table 1. The median age at pubertal onset in these boys was 7.5 years (ranging from 0.9 to 8.6 years). Testicular enlargement and pubarche were reported as the first signs of puberty in 45% of the boys. At the time of first evaluation (median age 8.7 years), genital Tanner stage 3 was observed in 50% of the boys. The median Δbone age – chronological age was 1.7 years (ranging from 0 to 7.9 years). The median body mass index (BMI) Z-score was 1.2 (ranging from 0.1 to 4.0). All boys had pubertal basal LH levels (median 1.4 IU/l, ranging from 0.7 to 6.7 IU/l). The median GnRH-stimulated LH peak was 17.7 IU/l (ranging from 6.7 to 33.4 IU/l). The median testosterone level was 104 ng/dl (ranging from 19 to 548 ng/dl). One patient (patient 17, table 1 ) had prepubertal testosterone level despite pubertal basal LH, and he was excluded from the testosterone level analysis.
Genetic Findings
Four MKRN3 mutations were detected in 8 boys (patients 10 to 17, table 1 ) from 5 families with CPP. Three mutations have been reported previously, including 2 frameshift (p.Ala162Glyfs * 14 and p.Arg213Glyfs * 73) and one missense (p.Arg365Ser) mutations [17]. Automated sequencing of MKRN3 revealed one novel missense variant (p.Arg328Cys) in the Argentinean family, comprised of 3 brothers with CPP. All four MKRN3 variants were not identified in population database (1000 Genomes and NHLBI EVS) and were predicted to be ‘damaging’ or ‘disease causing’ by four different in silico programs, suggesting deleterious effect. A KISS1-activating mutation (p.Pro74Ser) was previously identified in one boy with sporadic CPP (patient 9, table 1 ) [20]. The other 11 boys did not have any detectable rare coding variants (minor allele frequency <1%) in MKRN3, KISS1 or KISS1R. The study of the promoter regions of MKRN3 and KISS1 revealed no rare variants in this group of boys.
Clinical Features of CPP Boys with MKRN3 Defects
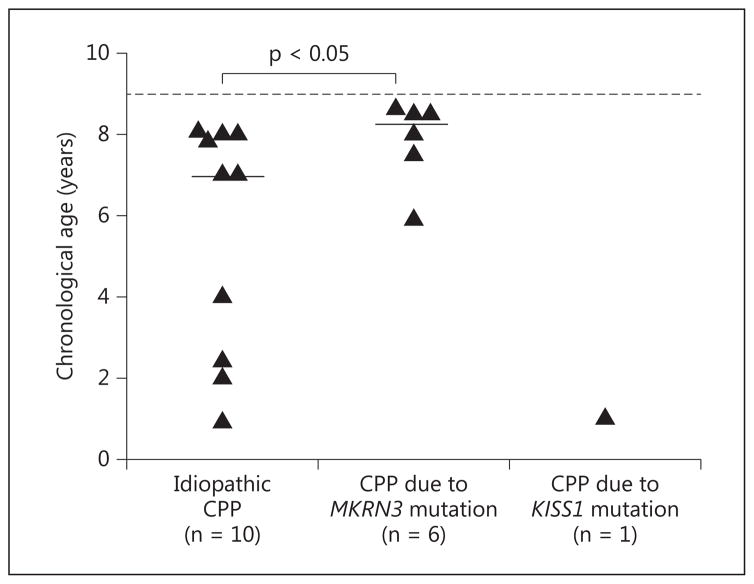
The age at puberty initiation in the 8 boys with MKRN3 mutations ranged from 5.9 to 8.6 years (median 8.2 years). Four of them exhibited testicular enlargement and pubarche as the first signs of puberty by medical records, whereas the remaining 4 patients initially presented with only testicular enlargement. At the time of first evaluation, their ages ranged from 8.1 to 9.8 years (median 8.7 years). The Δbone age – chronological age ranged from 0 to 2.7 years (median 1.7 years). The median BMI Z-score was 1.2 in this group, ranging from 0.1 to 2.2. Median basal and GnRH-stimulated LH levels were 1.5 IU/l (ranging from 0.7 to 4.1 IU/l) and 10.9 IU/l (ranging from 6.7 to 20 IU/l), respectively. The median testosterone level was 200 ng/dl (ranging from 67 to 548 ng/dl). Except for the significantly later pubertal onset in the boys with MKRN3 mutations (median 8.2 years, ranging from 5.9 to 8.6 years) compared to those without MKRN3 mutations (median 7.0 years, ranging from 0.9 to 8.0 years), there were no other differences between their clinical and hormonal features ( table 2 ; fig. 1 ).
Table 2.
Comparison between clinical and hormonal features of boys with and without MKRN3 mutations
| MKRN3 mutations (n = 8) | Idiopathic CPP (n = 11) | p value | |
|---|---|---|---|
| Age at pubertal onset, years | 8.2 (5.9 – 8.6) | 7.0 (0.9 – 8.0) | 0.033 |
| Age at first evaluation, years | 8.7 (8.1 – 9.8) | 8.7 (1.4 – 10.4) | 0.817 |
| BMI, Z-score | 1.2 (0.14 – 2.2) | 1.7 (0.2 – 4.0) | 0.314 |
| Bone age advancement, years | 1.7 (0 – 2.7) | 1.7 (0 – 7.9) | 0.383 |
| Basal LH, IU/l | 1.5 (0.7 – 4.1) | 1.2 (0.7 – 6.7) | 1.000 |
| LH after GnRH, IU/l | 10.9 (6.7 – 20) | 18 (8.4 – 33.4) | 0.268 |
| Basal FSH, IU/l | 1.9 (0.8 – 4.4) | 2.5 (0.7 – 5.9) | 0.649 |
| Testosterone, ng/dl | 200 (67 – 548) | 75.7 (19 – 297) | 0.103 |
Data are presented as median (range). The boy with a MKRN3 mutation and testosterone level <10 ng/dl (patient 17, table 1) was excluded from the testosterone level analysis.
Fig. 1.
Age at puberty onset in boys with idiopathic and genetic CPP. This information was not clear in 3 of 20 cases, which were excluded from the figure. The dashed line indicates the lower age limit for normal male puberty onset (9.0 years). The one boy with a KISS1 mutation started puberty at 1.0 year of age. The short horizontal lines indicate the median age at puberty onset (7.0 years in the boys with idiopathic CPP and 8.2 years in those with MKRN3 mutations). The boys with MKRN3 mutations had a significantly later pubertal onset compared to those with idiopathic CPP. p value as assessed with Mann-Whitney U test.
Frequency of MKRN3 Mutations in Boys with Idiopathic CPP
Considering the entire group, the frequency of MKRN3 mutations among the 20 boys with idiopathic CPP was significantly higher than the female data [8 of 20 boys (40%) vs. 15 of 234 girls (6.4%), p < 0.001, by χ2 test]. When only index cases were considered in this analysis, 3 boys of 17 families (17.6%) versus 11 girls of 221 families (5%) carried MKRN3 mutations (p = 0.05). Of note, 2 boys (patients 12 and 14, table 1 ) with MKRN3 mutations belonged to two families whose index cases were females, and they were not included in the latter analysis.
Discussion
This study reveals the importance of genetic analysis in boys with idiopathic CPP, especially in those with a family history of premature sexual development. Since the discovery that MKRN3 deficiency causes familial CPP, 5 other boys with CPP or early puberty due to mutations in this gene have been described [21–23, 33, 34]. To date, there are 10 unrelated families that include boys presenting with CPP or early puberty caused by MKRN3 defects ( table 3 ). MKRN3, an imprinted gene located on the long arm of chromosome 15 (Prader-Willi critical region), encodes makorin ring finger protein 3, which is the first factor with an inhibitory effect on GnRH secretion. The MKRN3 protein is derived only from RNA transcribed from the paternally inherited copy of the gene due to maternal imprinting. Segregation analysis of the families with CPP due to MKRN3 defects clearly demonstrated an autosomal dominant inheritance with complete penetrance [18].
Table 3.
MKRN3 mutations identified in boys with CPP
| Family No. | Origin | MKRN3 mutation | Location of MKRN3 mutation in the protein | Affected boys, n | Age at pubertal onset, years | Reference | |
|---|---|---|---|---|---|---|---|
| DNA | protein | ||||||
| 1 | Brazil | 475_476insC | Ala162Glyfs*14 | N-terminal | 2 | 5.9 Unknown | 17 |
| 2 | Belgium | 1095G>T | Arg365Ser | C-terminal (C3HC4 RING motif) | 1 | Unknown | 17 |
| 3 | USA | 475_476insC | Ala162Glyfs*14 | N-terminal | 1 | 8.5 | 17 |
| 4 | USA | 637delC | Arg213Glyfs*73 | N-terminal | 1 | 8.0 | 17 |
| 5 | Greece | 1018T>G | Cys340Gly | C-terminal (C3HC4 RING motif) | 1 | Unknown | 23 |
| 6 | Germany | 331G>T | Glu111* | N-terminal (C3H motif) | 1 | ~9.0 | 21 |
| 7 | Israel | 1260T>G | His420Gln | C-terminal (C3H motif) | 1 | Unknown | 22 |
| 8 | Denmark | 1034G>A | Arg345His | C-terminal (C3HC4 RING motif) | 1 | Unknown | 34 |
| 9 | Korea | 841C>T | Gln281* | MKRN-specific Cys-His domain |
1 | Unknown | 33 |
| 10 | Argentina | 982C>T | Arg328Cys | C-terminal (C3HC4 RING motif) | 3 | 7.5 8.5 8.6 |
Current study |
Boys from families 1, 2, 3, 4 and 10 were included in the current study. In family 10, father’s DNA was not available for segregation analysis. He reported early puberty. In the other 9 families, the affected boys inherited the mutations from their fathers, as expected for the pattern of inheritance.
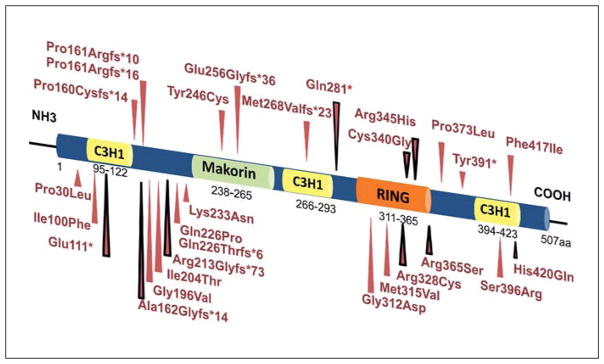
Herein, MKRN3 mutations were detected in 5 of 17 families (29.4%) with male idiopathic CPP cases. Four different heterozygous loss-of-function MKRN3 mutations were identified in 8 boys from 5 families. Two of these mutations were frameshift mutations (p.Ala162-Glyfs * 14 and p.Arg213Glyfs * 73), resulting in premature stop codons. The other two were missense variants (p. Arg328Cys and p.Arg365Ser), located in the C3HC4 RING motif of MKRN3, a putative ubiquitin ligase protein domain. Both missense mutations were predicted to be deleterious to protein function. Interestingly, Settas et al. [23] reported that two MKRN3 missense mutations (p.Cys340Gly and p.Arg365Ser), both residing in the C3HC4 RING motif, disrupt the three-dimensional structure of the protein, emphasizing the functional effects of these defects. The p.Arg328Cys mutation is also located in the same MKRN3 domain, suggesting a similar consequence to protein function. All current MKRN3 loss-of-function mutations identified in CPP patients are illustrated in figure 2.
Fig. 2.
Schematic protein structure of MKRN3 and locations of loss-of-function mutations identified in patients with CPP. The three C3H zinc finger motifs are shown in yellow, the C3HC4 RING finger motif in orange, and the MKRN-specific Cys-His domain in green. The numbers correspond to the amino acid positions in the protein. Red arrows indicate the location of all described MKRN3 mutations in patients with CPP; black-red arrows indicate the mutations identified in boys with CPP.
We demonstrated that boys with CPP due to MKRN3 mutations started pubertal development at a borderline early age (median 8.2 years) when considering the lower limit age of 9 years for normal male puberty onset. A similar chronological age at puberty onset in association with MKRN3 mutations was reported in other recent studies [21, 23, 34]. In contrast, a median age at puberty onset of 6.0 years has been described in girls with CPP caused by MKRN3 mutations [18, 35]. Therefore, MKRN3 deficiency has a smaller impact on puberty onset in boys, with affected boys manifesting CPP at an older age than girls. Nevertheless, it must be noted that the determination of age at pubertal onset in boys is a challenge since testicular enlargement is not as obvious as thelarche and menarche in girls [10]. The borderline early age at pubertal onset in these boys with MKRN3 mutations can compromise the precise identification of puberty by parents and general pediatrics, and therefore leading to an underestimated incidence of CPP in this group. Commonly, male patients remember only late events of puberty, such as the age at initiation of full facial shaving and the age at voice change [16, 34, 36, 37].
The median age at puberty onset in CPP boys with MKRN3 mutations also differs strikingly from that described in boys with other causes of premature pubertal development. Boys with CPP due to hypothalamic hamartoma classically manifested physical signs of puberty before 2 years of age [38]. Moreover, the one boy with a gain-of-function mutation in the KISS1 gene had very early pubertal onset (1.0 year) [20]. In addition, testotoxicosis or familial male-limited precocious puberty, a gonadotropin-independent cause of precocious puberty that exclusively affects boys, usually occurs before 4 years of age [39, 40].
The basal and GnRH-stimulated LH levels were similar in boys with and without MKRN3 mutations. The serum testosterone levels in boys with MKRN3 abnormalities ranged from 67 to 548 ng/dl. Notably, 1 boy who carried the p.Arg328Cys mutation (patient 17, table 1 ) had a prepubertal testosterone level at the first medical evaluation. This was likely because he was at a very early stage of pubertal development at the time of assessment. Treatment was initiated at genital Tanner stage 2, with testes of 4–5 ml, basal LH 0.7 IU/l and basal FSH 1.2 IU/l. At this stage, testicular enlargement is mainly due to Sertoli cell proliferation, and there is very little Leydig cell activity (FSH predominates over LH), explaining the low testosterone level in this case.
The observation that MKRN3 mutations represented a frequent cause of male CPP led us to hypothesize that boys were more likely than girls to carry MKRN3 mutations. Indeed, the prevalence of MKRN3 mutations in boys with unknown cause of CPP (8 of 20 boys, 40%) was significantly higher compared to a large cohort of CPP girls (15 of 234 girls, 6.4%) (p < 0.001). However, when only index cases were considered, a higher prevalence of MKRN3 mutations in male patients (17.6%) compared to female patients (5.0%) was found with a borderline difference (p = 0.05). These findings might indicate that male CPP without CNS abnormalities has a greater probability of a genetic etiology.
We do not yet understand why boys with idiopathic CPP may have a higher prevalence of MKRN3 mutations than girls. Although the mechanism by which the loss of MKRN3 results in early puberty initiation is still not fully understood, the expression pattern of Mkrn3 in the hypothalamus of mice and the ubiquitin ligase protein structure suggest that MKRN3 is inhibiting GnRH secretion during childhood [17, 19]. The similar expression pattern of Mkrn3 in the arcuate nucleus of male and female mice suggests that Mkrn3 inhibitory tonus is present in both sexes but does not entirely rule out a sex-specific action of MKRN3. It is well known that girls undergo puberty initiation at an earlier age than boys and have a higher incidence of CPP [4, 11, 41]. Conversely, boys have a higher incidence of delayed puberty [41]. Based on these observations, we can speculate that the inhibition of GnRH during childhood is weaker in girls than in boys, and that as a result girls are more prone to the consequences of disruption of the ‘brake’ restraining puberty initiation.
MKRN3 loss-of-function mutations have been associated with normal CNS MRI in both sexes [17, 27, 33, 34]. In affected boys, the later puberty onset also makes organic, structural causes less likely. These recent observations could modify the clinical decision-making for performing a CNS MRI in this group. In particular, in boys with familial CPP, the genetic study of the MKRN3 gene could precede the brain MRI. In these cases, MRI should be postponed (nonmutated cases) or completely avoided (mutated cases). Furthermore, this genetic study is less costly than a brain MRI, an imaging exam that frequently requires anesthesia in children [42].
Clearly, recent human studies support that MKRN3 is a strong component of puberty regulation in both sexes [17–19, 43]. Currently, MKRN3 mutations represent a prevalent cause of familial CPP. In conclusion, this study suggested a higher prevalence of MKRN3 mutations in boys than in girls with idiopathic CPP. Remarkably, the affected boys had classical features of CPP with pubertal onset at a borderline age.
Acknowledgments
We thank the following pediatric endocrinologists for sending CPP cases for genetic analyses at São Paulo University: Carlos Alberto Longui and Cristiane Kochi (Departamento de Pediatria, Faculdade de Ciências Médicas da Santa Casa de São Paulo, Brazil); Priscila C. Gagliardi (Division of Endocrinology, Diabetes and Metabolism, Nemours Children’s Clinic, Jacksonville, USA); Francis de Zegher (Department of Pediatrics, University Hospital Gasthuisberg, University of Leuven, Belgium); Sonir R. Antonini and Ana Claudia S. Reis (Departamento de Puericultura e Pediatria, Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo, Brazil); George Chrousos, Evangelia Charmandari and Christie Kourkouti (Division of Endocrinology, Metabolism and Diabetes, First Department of Pediatrics, University of Athens Medical School, Greece); Korcan Demir (Division of Pediatric Endocrinology, Children’s Hospital, Gaziantep, Turkey).
This work was supported by: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (to D.S.B. and M.C.S.); Fundação de Amparo à Pesquisa do Estado de São Paulo Grant 2013/06391-1 (to D.B.M.); Fundação de Amparo à Pesquisa do Estado de São Paulo Grant 13/03236-5 (to A.C.L.), and National Institutes of Health R01 HD082314 (to U.B.K.) and F05 HD072773 (to A.P.A.).
Footnotes
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Carel JC, Leger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358:2366–2377. doi: 10.1056/NEJMcp0800459. [DOI] [PubMed] [Google Scholar]
- 2.Ojeda SR, Lomniczi A. Puberty in 2013: unravelling the mystery of puberty. Nat Rev Endocrinol. 2014;10:67–69. doi: 10.1038/nrendo.2013.233. [DOI] [PubMed] [Google Scholar]
- 3.Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 4.Susman EJ, Houts RM, Steinberg L, Belsky J, Cauffman E, Dehart G, Friedman SL, Roisman GI, Halpern-Felsher BL. Longitudinal development of secondary sexual characteristics in girls and boys between ages 91/2 and 151/2 years. Arch Pediatr Adolesc Med. 2010;164:166–173. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, Wasserman R, Serwint JR, Smitherman L, Reiter EO. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130:e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- 6.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner HG, Otten BJ. Precocious puberty in boys. N Engl J Med. 1999;341:1763–1765. doi: 10.1056/NEJM199912023412311. [DOI] [PubMed] [Google Scholar]
- 8.Macedo DB, Cukier P, Mendonca BB, Latronico AC, Brito VN. Advances in the etiology, diagnosis and treatment of central precocious puberty (in Portuguese) Arq Bras Endocrinol Metabol. 2014;58:108–117. doi: 10.1590/0004-2730000002931. [DOI] [PubMed] [Google Scholar]
- 9.Choi KH, Chung SJ, Kang MJ, Yoon JY, Lee JE, Lee YA, Shin CH, Yang SW. Boys with precocious or early puberty: incidence of pathological brain magnetic resonance imaging findings and factors related to newly developed brain lesions. Ann Pediatr Endocrinol Metab. 2013;18:183–190. doi: 10.6065/apem.2013.18.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pigneur B, Trivin C, Brauner R. Idiopathic central precocious puberty in 28 boys. Med Sci Monit. 2008;14:CR10–CR14. [PubMed] [Google Scholar]
- 11.Bridges NA, Christopher JA, Hindmarsh PC, Brook CG. Sexual precocity: sex incidence and aetiology. Arch Dis Child. 1994;70:116–118. doi: 10.1136/adc.70.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Sanctis V, Corrias A, Rizzo V, Bertelloni S, Urso L, Galluzzi F, Pasquino AM, Pozzan G, Guarneri MP, Cisternino M, De Luca F, Gargantini L, Pilotta A, Sposito M, Tonini G. Etiology of central precocious puberty in males: the results of the Italian Study Group for Physiopathology of Puberty. J Pediatr Endocrinol Metab. 2000;13(suppl 1):687–693. doi: 10.1515/jpem.2000.13.s1.687. [DOI] [PubMed] [Google Scholar]
- 13.Alikasifoglu A, Vuralli D, Gonc EN, Ozon A, Kandemir N. Changing etiological trends in male precocious puberty: evaluation of 100 cases with central precocious puberty over the last decade. Horm Res Paediatr. 2015;83:340–344. doi: 10.1159/000377678. [DOI] [PubMed] [Google Scholar]
- 14.Palmert MR, Boepple PA. Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab. 2001;86:2364–2368. doi: 10.1210/jcem.86.6.7603. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Park HK, Ko JH, Kim YJ, Hwang JS. Impact of body mass index on luteinizing hormone secretion in gonadotropin-releasing hormone stimulation tests of boys experiencing precocious puberty. Neuroendocrinology. 2013;97:225–231. doi: 10.1159/000342342. [DOI] [PubMed] [Google Scholar]
- 16.de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89:1794–1800. doi: 10.1210/jc.2003-030361. [DOI] [PubMed] [Google Scholar]
- 17.Abreu AP, Dauber A, Macedo DB, Noel SD, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368:2467–2475. doi: 10.1056/NEJMoa1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulcao Macedo D, Nahime Brito V, Latronico AC. New causes of central precocious puberty: the role of genetic factors. Neuroendocrinology. 2014;100:1–8. doi: 10.1159/000366282. [DOI] [PubMed] [Google Scholar]
- 19.Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. A new pathway in the control of the initiation of puberty: the MKRN3 gene. J Mol Endocrinol. 2015;54:R131–R139. doi: 10.1530/JME-14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, Bianco SD, Kuohung W, Xu S, Gryngarten M, Escobar ME, Arnhold IJ, Mendonca BB, Kaiser UB, Latronico AC. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95:2276–2280. doi: 10.1210/jc.2009-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiner F, Gohlke B, Hamm M, Korsch E, Woelfle J. MKRN3 mutations in familial central precocious puberty. Horm Res Paediatr. 2014;82:122–126. doi: 10.1159/000362815. [DOI] [PubMed] [Google Scholar]
- 22.de Vries L, Gat-Yablonski G, Dror N, Singer A, Phillip M. A novel MKRN3 missense mutation causing familial precocious puberty. Hum Reprod. 2014;29:2838–2843. doi: 10.1093/humrep/deu256. [DOI] [PubMed] [Google Scholar]
- 23.Settas N, Dacou-Voutetakis C, Karantza M, Kanaka-Gantenbein C, Chrousos GP, Voutetakis A. Central precocious puberty in a girl and early puberty in her brother caused by a novel mutation in the MKRN3 gene. J Clin Endocrinol Metab. 2014;99:E647–E651. doi: 10.1210/jc.2013-4084. [DOI] [PubMed] [Google Scholar]
- 24.Brito VN, Batista MC, Borges MF, Latronico AC, Kohek MB, Thirone AC, Jorge BH, Arnhold IJ, Mendonca BB. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab. 1999;84:3539–3544. doi: 10.1210/jcem.84.10.6024. [DOI] [PubMed] [Google Scholar]
- 25.Neely EK, Hintz RL, Wilson DM, Lee PA, Gautier T, Argente J, Stene M. Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr. 1995;127:40–46. doi: 10.1016/s0022-3476(95)70254-7. [DOI] [PubMed] [Google Scholar]
- 26.Resende EA, Lara BH, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab. 2007;92:1424–1429. doi: 10.1210/jc.2006-1569. [DOI] [PubMed] [Google Scholar]
- 27.Macedo DB, Abreu AP, Reis AC, Montenegro LR, et al. Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene makorin ring finger 3. J Clin Endocrinol Metab. 2014;99:E1097–E1103. doi: 10.1210/jc.2013-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358:709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.San Lucas FA, Wang G, Scheet P, Peng B. Integrated annotation and analysis of genetic variants from next-generation sequencing studies with variant tools. Bioinformatics. 2012;28:421–422. doi: 10.1093/bioinformatics/btr667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Heart, Lung, and Blood Institute. Exome variant server, NHLBI GO Exome Sequencing Project (ESP) http://evsgswashingtonedu/EVS/2013.
- 31.Frousios K, Iliopoulos CS, Schlitt T, Simpson MA. Predicting the functional consequences of non-synonymous DNA sequence variants – evaluation of bioinformatics tools and development of a consensus strategy. Genomics. 2013;102:223–228. doi: 10.1016/j.ygeno.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Min L, Nie M, Zhang A, Wen J, Noel SD, Lee V, Carroll RS, Kaiser UB. Computational analysis of missense variants of G protein-coupled receptors involved in the neuroendocrine regulation of reproduction. Neuroendocrinology. 2016;103:230–239. doi: 10.1159/000435884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HS, Jin HS, Shim YS, Jeong HR, Kwon E, Choi V, Kim MC, Chung IS, Jeong SY, Hwang JS. Low frequency of MKRN3 mutations in central precocious puberty among Korean girls. Horm Metab Res. 2015;48:118–122. doi: 10.1055/s-0035-1548938. [DOI] [PubMed] [Google Scholar]
- 34.Kansakoski J, Raivio T, Juul A, Tommiska J. A missense mutation in MKRN3 in a Danish girl with central precocious puberty and her brother with early puberty. Pediatr Res. 2015;78:709–711. doi: 10.1038/pr.2015.159. [DOI] [PubMed] [Google Scholar]
- 35.Simon D, Ba I, Mekhail N, Ecosse E, Paulsen A, Zenaty D, Houang M, Jesuran Perelroizen M, de Filippo GP, Salerno M, Simonin G, Reynaud R, Carel JC, Leger J, de Roux N. Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol. 2015;174:1–8. doi: 10.1530/EJE-15-0488. [DOI] [PubMed] [Google Scholar]
- 36.Juul A, Magnusdottir S, Scheike T, Prytz S, Skakkebaek NE. Age at voice break in Danish boys: effects of pre-pubertal body mass index and secular trend. Int J Androl. 2007;30:537–542. doi: 10.1111/j.1365-2605.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 37.Harries ML, Walker JM, Williams DM, Hawkins S, Hughes IA. Changes in the male voice at puberty. Arch Dis Child. 1997;77:445–447. doi: 10.1136/adc.77.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Brito VN, Latronico AC, Arnhold IJ, Lo LS, Domenice S, Albano MC, Fragoso MC, Mendonca BB. Treatment of gonadotropin dependent precocious puberty due to hypothalamic hamartoma with gonadotropin releasing hormone agonist depot. Arch Dis Child. 1999;80:231–234. doi: 10.1136/adc.80.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schedewie HK, Reiter EO, Beitins IZ, Seyed S, Wooten VD, Jimenez JF, Aiman EJ, DeVane GW, Redman JF, Elders MJ. Testicular Leydig cell hyperplasia as a cause of familial sexual precocity. J Clin Endocrinol Metab. 1981;52:271–278. doi: 10.1210/jcem-52-2-271. [DOI] [PubMed] [Google Scholar]
- 40.Reiter EO, Brown RS, Longcope C, Beitins IZ. Male-limited familial precocious puberty in three generations. Apparent Leydig-cell autonomy and elevated glycoprotein hormone alpha subunit. N Engl J Med. 1984;311:515–519. doi: 10.1056/NEJM198408233110807. [DOI] [PubMed] [Google Scholar]
- 41.Bianco SD. A potential mechanism for the sexual dimorphism in the onset of puberty and incidence of idiopathic central precocious puberty in children: sex-specific kiss-peptin as an integrator of puberty signals. Front Endocrinol (Lausanne) 2012;3:149. doi: 10.3389/fendo.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4:265–274. [Google Scholar]
- 43.Hagen CP, Sorensen K, Mieritz MG, Johannsen TH, Almstrup K, Juul A. Circulating MKRN3 levels decline prior to pubertal onset and through puberty: a longitudinal study of healthy girls. J Clin Endocrinol Metab. 2015;100:1920–1926. doi: 10.1210/jc.2014-4462. [DOI] [PubMed] [Google Scholar]