Abstract
Introduction
For decades, progestins have been included in hormone therapies (HT) prescribed to women to offset the risk of unopposed estrogen-induced endometrial hyperplasia. However, the potential effects on cognition of subcategories of clinically used progestins have been largely unexplored.
Methods
In two studies, the present investigation evaluated the cognitive effects of norethindrone acetate (NETA), levonorgestrel (LEVO), and medroxyprogesterone acetate (MPA) on the water radial-arm maze (WRAM) and Morris water maze (MM) in middle-aged ovariectomized rats.
Results
In Study 1, six-weeks of a high-dose NETA treatment impaired learning and delayed retention on the WRAM, and impaired reference memory on the MM. Low-dose NETA treatment impaired delayed retention on the WRAM. In Study 2, high-dose NETA treatment was reduced to four-weeks and compared to MPA and LEVO. As previously shown, MPA impaired working memory performance during the lattermost portion of testing, at the highest working memory load, impaired delayed retention on the WRAM, and impaired reference memory on the MM. NETA also impaired performance on these WRAM and MM measures. Interestingly, LEVO did not impair performance, but instead enhanced learning on the WRAM.
Conclusions
The current study corroborates previous evidence that the most commonly prescribed FDA-approved progestin for HT, MPA, impairs learning and memory in the ovariectomized middle-aged rat. When progestins from two different subcategories were investigated, NETA impaired learning and memory similarly to MPA, but LEVO enhanced learning. Future research is warranted to determine LEVO’s potential as an ideal progestin for optimal health in women, including for cognition.
Keywords: Hormone Therapy, Progestin, Aging, Menopause, Memory, Cognition
1. Introduction
A progestin, a synthetic form of progesterone, is included in hormone therapy (HT) prescribed to women with a uterus, as well as in hormonal contraceptive formulations. The need to develop this class of steroids was due to the poor bioavailability and short biological half-life of the natural hormone, although micronized progesterone somewhat overcomes these caveats [1]. The necessity to include a progestin in hormone therapies and contraceptives is well documented, as it offsets the increased risk of endometrial hyperplasia related to unopposed estrogen treatment [2, 3]. However, the potential risks to cognition and other crucial biological processes that progestins pose have been largely unexplored. There is indirect evidence via the large, placebo-controlled, randomized WHIMS that the most commonly prescribed FDA-approved progestin for HT, medroxyprogesterone acetate (MPA; [4]), may adversely affect cognition. Indeed, a series of studies found that the combination HT Prempro (conjugated equine estrogens; CEE, plus MPA) was associated with a significant increased risk of dementia [5], while CEE-only HT produced a nonsignificant increase in risk of dementia (p = 0.18), compared to those taking placebo [6]. Further, MPA is the only active ingredient in the contraceptive Depo Provera, and a documented case study revealed amnestic effects associated with its use [7]. In animal models, MPA has been associated with memory impairments across the adult lifespan [8–11] as well as adverse cellular effects in vitro [12–14]. Given the evidence that use of MPA in HT and contraceptives has potentially deleterious effects, it is imperative to determine an alternative progestin that is safer for women’s health, not only to prevent endometrial hyperplasia, but also to protect the brain and its function.
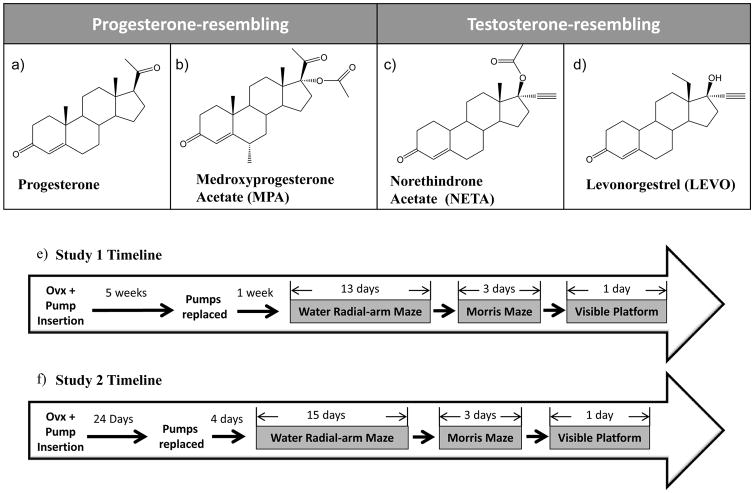
Based on chemical structure, synthetic progestins either resemble progesterone or testosterone [15, 16]. MPA is an example of a progesterone-resembling progestin (Figure 1b). Testosterone-resembling progestins can be further subcategorized based on chemical structure, and each subcategory contains a progestin that is FDA-approved for HT (norethindrone acetate, or NETA in Figure 1c, and levonorgestrel, or LEVO in Figure 1d) [16, 17]. These differences in chemical structure have functional consequences on relative binding affinities for progesterone and androgen receptors. LEVO and NETA have higher affinity for the androgen receptor than MPA, with LEVO being the highest (LEVO>NETA> MPA; [18]). LEVO also has the highest affinity for the progesterone receptor, followed by MPA and then NETA (LEVO>MPA>NETA); none of the progestins bind to the estrogen receptor [18]. However, little is known about whether these differences in chemical structure and relative binding affinities for steroid receptors affect biological processes such as cognition.
Figure 1.
Chemical structures of progesterone-resembling progestogens: (a) progesterone (b) medroxyprogesterone acetate (MPA), and testosterone-resembling progestins: (c) norethindrone acetate (NETA) (d) levonorgesterel (LEVO). Timeline for (e) Study 1 and (f) Study 2 summarizing duration of treatment and behavior testing.
Having already established a progesterone-resembling progestin (MPA) as being cognitively impairing, as well as detrimental to several other aspects of health, it is reasonable to explore the safety of other frequently used progestins in comparison to MPA. Use of NETA as the progestin component of HT is on the rise, especially as many women choose custom compounds and off-label hormonal contraceptives for HT [19–22]. Despite its widespread use, there have been limited scientific investigations on the effects of NETA-containing HT (paired with ethinyl- or 17β-estradiol) on cognition and brain function in healthy menopausal women. In these studies NETA-containing HT, was associated with greater brain activity during a memory test [23, 24], protection from memory decline after one and two years of treatment [25], or no impact on self-report of memory and concentration difficulties [26]. While these investigations are encouraging in that they indicate either null or potentially beneficial cognitive effects of combination therapies including NETA, they did not determine whether the progestin component specifically had effects on the brain or memory. Indeed, it still remains to be methodologically evaluated whether or how NETA alone impacts the brain and its function, and whether there are other progestins that more positively affect brain health and cognitive function.
Another type of frequently used testosterone-resembling progestin that is of a different chemical structure class than NETA is LEVO. LEVO is a part of only one FDA-approved HT regimen [19, 27] but is also rising in popularity as many women choose off-label hormonal contraceptives for peri- and post- menopausal HT [20, 22]. There has also been limited scientific investigation of the effects of LEVO-containing HT on cognition in healthy menopausal women, with one study (paired with estradiol valerate) showing benefits to attention, processing speed, and memory [28]. While this encouraging result suggests a beneficial cognitive effect of combination therapies including LEVO, there are no methodological investigations of how LEVO alone impacts cognition in either menopausal women or rodent models of menopause or aging. In young, reproductively intact female rats LEVO treatment enhanced spatial memory [29], indicating promise for a potentially positive profile for brain function with menopause and aging. Further, differences in chemical structure from the cognitively-impairing progestin MPA, and their global use, make LEVO and NETA worthy candidates for more scientific studies evaluating cognitive effects. In two separate studies, the current investigation aimed to evaluate the effects of NETA, LEVO, and MPA on cognition in the middle-aged ovariectomized (Ovx) rat as compared to vehicle. Additionally, in light of the recent recommendation by the Food and Drug Administration (FDA) to use HT for the shortest time possible to alleviate menopausal symptoms [30], we explored a between study comparison of treatment duration with a reduction in duration in Study 2.
Study 1
This study was designed to investigate whether the progestin NETA, given tonically, alters cognitive performance in the middle-aged Ovx rat.
2. Material and Methods
2.1 Subjects
Subjects were 33 twelve month old, Fisher-344 female rats born and raised at the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN). Animals were acclimated for several weeks and pair housed with an identical treatment assigned cage-mate in the Arizona State University (ASU) animal facility. All animals had exposure to food and water ad-lib, and were maintained on a 12-h light/dark cycle at 23°C. Procedures were approved by the ASU IACUC committee and adhered to NIH standards.
2.2 Ovariectomy and Hormone Treatment
Rats underwent Ovx at 12 months of age and divided into three treatment groups. Each rat was randomly assigned to either vehicle (propylene glycol, Sigma-Aldrich, St. Louise, MO) or one of two doses of NETA (Steraloids, Inc., Newport, RI), administered via an Alzet osmotic pump (2006; Durect Co., Cupertine, CA). Treatment groups included: Ovx+Vehicle (n=8), Ovx+NETA-Low (4μg/day, n=9), and Ovx+NETA-High (20μg/day, n=5). The low and high doses of NETA were based on doses given to women taking NETA in HT; 1mg in FemHRT and 5mg in Aygestin [19], respectively, adapted for body weight of the rat1. Ovx was performed on all rats while under isoflurane inhalation. Bilateral dorsolateral incisions were made in the skin and peritoneum, followed by ligature and removal of the ovaries and tips of the uterine horns. Muscle and skin were then sutured. At the time of surgery, Alzet osmotic pumps containing propylene glycol alone for vehicle animals, or propylene glycol plus the appropriate dose of NETA for drug-assigned animals, were inserted under the skin at the scruff of the neck. This pump model released hormone for a 6 week duration. After surgery, rats received Rimadyl (5 mg/mL/kg) for pain and saline (2 mL) to prevent dehydration. Animals underwent pump reinsertion surgery 35 days after Ovx. Behavioral testing began 42 days after the first pump insertion (7 days after the second pump re-insertion; Figure 1e). Thus, hormone administration continued throughout behavior testing and sacrifice.
2.3 Vaginal Smears and Uterine Weights
Vaginal smears were performed twice between 35–40 days after Ovx. Each smear was identified as proestrus, estrous, metestrus or diestrus, per prior protocols [31, 32]. At sacrifice, uteri from all subjects were removed, trimmed of fat and weighed immediately (wet weight) in order to examine drug effects.
2.4 General Considerations for Statistics on Behavioral Data
For behavior assessments, data were analyzed separately for each maze. Specific days, trials, and blocking details are given below for each maze. First, in order to determine learning on each maze, an omnibus ANOVA including all groups was ran to investigate a significant main effect of all Days and/or Trials. For Treatment effects, since our interest was to determine whether each dose enhanced or impaired performance relative to the Ovx-Vehicle group, two-tailed, two-group planned comparisons were conducted as detailed below with each maze. For measures with significant group differences between a NETA treatment group and the Vehicle group, further comparisons were made between NETA dose groups to further interpret dose effects. Alpha was set at p ≤ 0.05, and p ≤ 0.10 was interpreted as a marginal effect.
2.5 Water Radial-Arm Maze
Subjects were tested on the eight-arm, win-shift, water radial-arm maze (WRAM) to evaluate spatial working and reference memory. The WRAM also allowed us to evaluate performance as working memory load increased (e.g., [33–36]). Four of the eight arms contained escape platforms that were hidden under the water surface. The platform locations remained fixed throughout the experiment but were counterbalanced between subjects. Subjects were introduced to the maze in the start arm and had three minutes (m) to locate a platform. After escaping onto a platform, the rat remained there for 15 seconds (s), and was then returned to its heated cage until its next trial. The inter-trial interval (ITI) was 30s. During the ITI, the just-found platform was removed from the maze. The subject was placed again into the start arm and allowed to locate another platform until all four platforms were found. Thus, there were four trials in each daily session, with the number of platformed arms reduced by one on each consecutive trial. The working memory system was increasingly taxed as trials progressed, allowing us to evaluate working memory load. Subjects reached asymptotic performance after receiving one session per day for 12 consecutive days. On day 13 a six hour delay was instilled between trials 2 and 3 to evaluate delayed memory retention.
Error quantification and blocking of days/trials were based on prior studies (e.g., [34–38]). A mark on the outside of the arm (11 cm into the arm) delineated the point of arm entry, as measured by the crossing of the tip of a rat’s snout. Working and reference memory errors were quantified using the orthogonal measures as defined by Jarrard, et al. [39], as done previously in WRAM studies [35, 36]. Working memory correct (WMC) errors were the number of first and repeat entries into any arm where the rat had already found the platform that day. WMC errors cannot be committed on Trial 1 because no platform has yet been found. Reference memory (RM) errors were counted the first time a rat entered an arm that never contained a platform. Working memory incorrect (WMI) errors were the number of repeat entries into an arm that never contained a platform (RM arm).
Data were blocked into days 2–6, which is considered the acquisition phase, and days 7–12, which is considered the asymptotic phase. Blocking data into these two phases is the traditional protocol for analyzing WRAM data [33, 34, 36–38]. For WMC, WMI, and RM errors, the Ovx-Vehicle group was compared to each Ovx-progestin group using planned comparison two-group repeated measures ANOVAs. To analyze effects of the 6 hour delay, Day 12 Trial 3 was considered the baseline day/trial (the last day of regular testing) and this was compared to the post delay trial given on Day 13, Trial 3. This was done to examine memory retention after a six hour delay. Each group was analyzed separately using a 2-within (baseline trial and post-delay trial) repeated measures ANOVA as done previously [40].
2.6 Morris Maze
The MM allowed us to evaluate spatial reference memory [41, 42]. Rats were tested for six trials/day for three days. A tub (188 cm diameter) was filled with black water made opaque with non-toxic paint and a platform (10 cm wide) was hidden in a fixed location. The rat was introduced to the maze from the North, South, East, or West location, denoted by a mark on the outside of the maze. The rat had 60s to locate the hidden platform, which remained in a fixed location (Northeast [NE] quadrant) throughout testing. Once the rat found the platform, they remained there for 15s. The rat was then placed into its heated cage until its next trial with an approximate ITI of 10m. After all test trials on day 3, a 60s probe trial with no platform was given to evaluate whether rats localized the platform to the spatial location. A camera suspended above the maze tracked each rat’s path for each trial, and Ethovision 6 (Noldus Instruments) was used to analyze each rat’s path.
MM analyses evaluated distance (cm) and latency (s) to the platform by comparing Ovx-Vehicle to each progestin group using planned comparison two-group repeated measures ANOVAs. Probe trial data were analyzed as percent distance in the NE quadrant, which previously contained the platform, compared to the diagonally opposite quadrant (SW) using ANOVA [43]. If a rat successfully localized the platform to the spatial location they were expected to spend the greatest percent distance in the NE vs. SW quadrant [44]. To evaluate potential Treatment effects, or Treatment x Quadrant interactions, an omnibus ANOVA with Treatment as the between variable, and Quadrant as repeated-measures, was used.
2.7 Visible Platform Maze
The Visible Platform Maze was used to confirm that all subjects had intact vision and could perform the procedural components of a swim task without difficulty for interpretability of WRAM and MM group differences [8, 9, 40]. A rectangular tub (39 × 23 in) filled with clear water contained a black platform (10 cm wide) that was elevated above the water surface, and extramaze cues within the room were covered with opaque curtains. The platform location for each trial varied spatially semi-randomly but the drop off location remained the same across trials. Rats had to escape on the platform protruding from the water, and were given 8 trials. The dependent variable was latency (s) to the platform.
3. Results
3.1 Vaginal Smears and Uterine Weights
All animals, regardless of treatment group, exhibited diestrous vaginal smears with primarily leukocytic cells. Uterine weights were analyzed using ANOVA with Treatment as the between variable. Mean ± SE uterine weights were: Ovx+Vehicle (0.163 ± 0.038), Ovx+NETA-Low (0.114 ± 0.006), and Ovx+NETA-High (0.152 ±.038). Uterine weights did not differ between treatment groups [F(2, 19) = 0.869; p = 0.44]. These results are in agreement with the known non-estrogenic activity of NETA and confirm lack of estrogenic stimulation from the ovaries in the Ovx+Vehicle group (e.g., [1]).
3.2 Water Radial-arm Maze
3.2.1 Acquisition and Asymptotic Testing Phases
Across Days 2–12 (combined acquisition and asymptotic testing phases) there was a Day main effect for the omnibus ANOVA for each type of error [WMC: F (10, 190) = 3.564; p < 0.001; WMI: F (10, 190) = 2.913; p < 0.01; RM: F (10, 190) = 3.661; p < 0.001], with errors decreasing across days, demonstrating learning of the task.
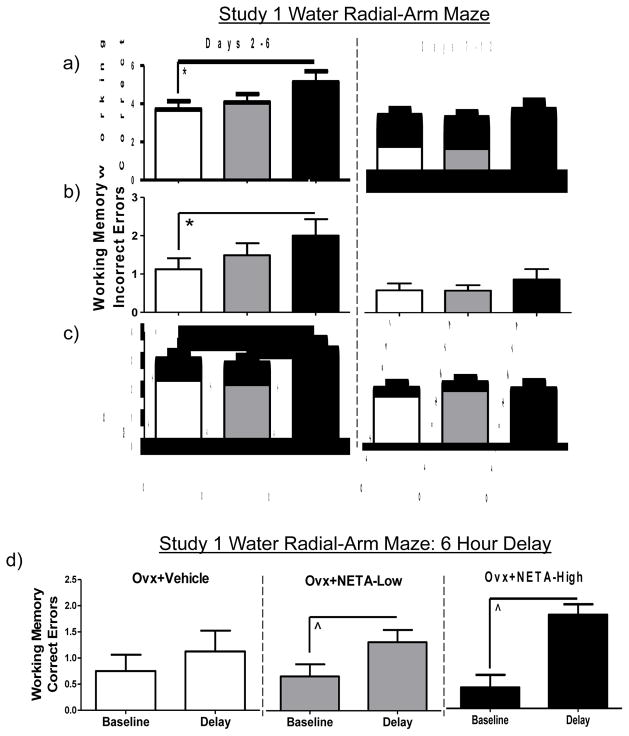
For the acquisition phase, there was a main effect of Treatment for the Ovx+Vehicle vs. Ovx+NETA-High comparison for WMC [F (1, 11) = 7.18; p < 0.05 (Figure 2a)], WMI [F (1, 11) = 8.76; p < 0.05 (Figure 2b)], and RM [F (1, 11) = 4.84; p < 0.05 (Figure 2c)], with the high NETA dosed group committing more of all three error types and therefore, showing impaired performance. RM was the only measure where the Ovx+NETA-High group also committed more errors than the Ovx+NETA-Low group [F (1, 12) = 5.93; p < 0.05 (Figure 2c)]. There were no main effects of Treatment for Ovx+Vehicle vs. Ovx+NETA-Low on the acquisition phase or either NETA dose for the asymptotic phase.
Figure 2.
Study 1 Mean error scores (+SE) collapsed across all trials on the water radial-arm maze during the learning (Days 2–6) and asymptotic (Days 7–12) testing phases: (a) Working Memory Correct errors, (b) Working Memory Incorrect errors, and (c) Reference Memory errors. (d) Mean Working Memory Correct error scores (+SE) for baseline (last day of regular testing trial 3) vs. delay (trial 3 immediately following a 6 hour delay). *p < 0.05; ^p < 0.05 Baseline vs. Delay
3.2.2 Delay Testing
To evaluate which groups were impaired by the 6 hour delay, we compared performance on Day 12 Trial 3 (baseline), to Day 13 Trial 3 (post-delay). For WMC errors, on Trial 3, the trial immediately following the delay, the Ovx+NETA-Low and the Ovx+NETA-High groups each made more errors as compared to baseline [Ovx+NETA-Low: t (8) = 4.00; p < 0.05; Ovx+NETA-High: t (4) = 3.5; p < 0.05 (Figure 2d)], indicating that the delay impaired performance in both NETA groups. This delay-induced decrement was not observed in Vehicle treated rats (p = 0.57). There were no delay-induced effects for WMI or RM errors.
3.3 Morris Maze
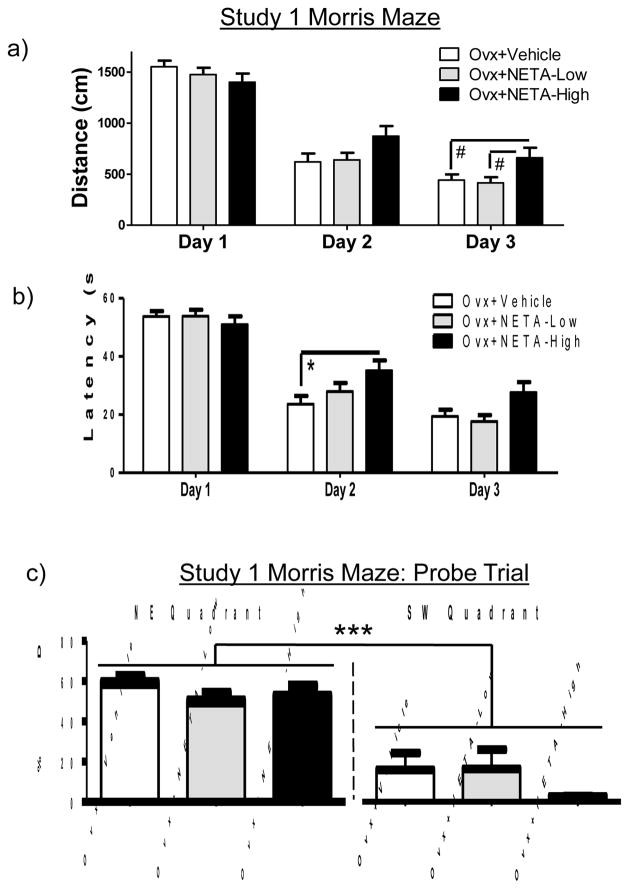
There was a Day main effect for the omnibus ANOVAs for distance [F (2, 38) = 155.797; p < 0.0001 (Figure 3a)] and latency [F (2, 38) = 109.17; p < 0.0001 (Figure 3b)], with scores decreasing across days, demonstrating learning of the task. For the Ovx+Vehicle vs. Ovx+NETA-High comparison, there was a Day x Treatment interaction that was significant for distance [F (2, 22) = 3.537; p < 0.05] and marginal for latency [F (2, 22) = 3.123; p < 0.10]. Interrogating the days individually revealed significantly longer latency to the platform for Ovx+NETA-High animals as compared to Ovx+Vehicle animals on Day 2 [F (1, 11) = 5.091; p < 0.05 (Figure 3b)] and marginally longer distance on Day 3 [F (1, 11) = 3.25; p < 0.10 (Figure 3a)]. The Ovx+NETA-High animals also swam a marginally longer distance than the Ovx+NETA-Low animals on Day 3 [F (1, 12) = 3.28; p < 0.10 (Figure 3a)]. The Day x Treatment interactions were not significant for the Ovx+Vehicle vs. Ovx+NETA-Low comparison, and there were no Treatment main effects for either dose.
Figure 3.
Study 1 Morris maze mean (+SE) (a) Distance scores in centimeters and (b) Latency scores in seconds across all days of testing (Days 1–3). (c) Mean percent Distance in the target NE quadrant as compared to the opposite SW quadrant during the probe trial. *p <.05; #p <.10; ***p <.0001 NE quadrant vs. SW quadrant
For the probe trial, a higher percent distance was spent in the previously platformed (NE) versus the opposite (SW) quadrant [Quadrant main effect: F (1, 19) = 77.77; p < 0.0001 (Figure 3c)]. This pattern did not differ by Treatment [null Quadrant x Treatment interaction], suggesting all groups localized the platform location by the end of testing.
3.4 Visible Platform
There was a main effect of Trial [F (5, 95) = 7.985; p < 0.0001 (Supplemental Figure 1)], with latency decreasing across trials. There were no Treatment effects when collapsing across all trials or for individual trials for the Ovx+Vehicle vs. Ovx+NETA-Low comparison, or for the Ovx+Vehicle vs. Ovx+NETA-High comparison. There were also no Treatment x Trial interactions for either NETA group compared to Vehicle. The average time to find the platform was 12s by the last trial of testing, confirming visual and motor competence for solving a water-escape swim task.
Study 2
In Study 1, the progestin NETA impaired cognition in the middle-age Ovx rat. Study 2 was designed to compare NETA to MPA, which we have previously shown to impair cognition in middle-aged and aged Ovx rats [9]. Study 2 also examined the cognitive effects of LEVO, a progestin novel to cognitive testing in a menopausal rat model and of a different chemical structure category than both NETA and MPA. Additionally, in light of the recent FDA recommendation to use HT for the shortest time possible to alleviate menopausal symptoms [30], consideration was taken to model the approximate treatment duration likely to occur under this new guideline. The most common menopausal symptoms that lead to physician consultations are vasomotor [45]. Through meta-analysis of duration of menopausal vasomotor symptoms, Politi and colleagues [45] determined that bothersome symptoms sharply declined three years after the final menstrual period. Approximate calculations attempting to translate three years in a women’s life into an appropriate treatment duration in our rat’s life resulted in four weeks [46, 47]. Thus, four weeks was chosen as the treatment duration for Study 2. This change in treatment duration allowed for between study comparisons of our previous 6–8 week treatment durations for NETA (Study 1) and MPA [8, 9].
4. Material and Methods
4.1 Subjects and Ovariectomy
Subjects were 34 twelve month old, Fisher-344 female rats born and raised at the National Institute on Aging colony at Harlan Laboratories (Indianapolis, IN). Acclimation, housing, food and water, light/dark, Ovx, and pump insertion procedures were identical to Study 1.
4.2 Hormone Treatment
Rats were randomly assigned to one of four treatment groups, with vehicle (propylene glycol, n=8), MPA (0.7mg/day, n=8; Sigma-Aldrich), NETA-High (20μg/day, n=9), and LEVO (0.06 mg/day, n=9; Sigma-Aldrich). The dose of MPA was based on our previous study [8]. The LEVO dose was based on a dose given to women taking LEVO clinically in Climara Pro [27], adapted for body weight of the rat2. Animals underwent pump re-insertion surgery 24 days after Ovx. Behavioral testing began 28 days after the first pump insertion (4 days after the second pump re-insertion; Figure 1f). Thus, hormone administration continued throughout behavior testing and sacrifice.
4.3 General Considerations for Statistics on Behavioral Data
Statistical analyses were identical to Study 1 except where specified for specific mazes below. For Treatment effects, since our interest was to determine whether each progestin enhanced or impaired performance relative to the Ovx-Vehicle group, two-tailed, two-group planned comparisons were conducted, as detailed below with each maze. For measures with significant group differences between a progestin treatment group and the Vehicle group, further comparisons were made between progestin groups to further interpret group differences. Alpha was set at p ≤ 0.05, and p ≤ 0.10 was interpreted as a marginal effect.
4.4 Water Radial-Arm Maze
WRAM procedure was identical to Study 1, except that subjects required 14 days of regular testing to reach asymptotic performance, which we have noted before with progestogen treatment in our lab [40]. Given the slower learning curve, a less demanding delay of four hours was chosen on day 15, also as previously done with progestogen treatment [8]. Quantification and blocking of errors was identical to Study 1 but with a longer asymptotic phase (days 7–14). Statistical analyses were identical to Study 1. The replication analysis of MPA-induced impairments on the final day of regular testing, day 14, at the highest working memory load (Trial 4), was one-tailed for the Ovx+Vehicle vs. Ovx+MPA comparison [8] and two-tailed for the Ovx+Vehicle vs. Ovx+NETA-High and Ovx+Vehicle vs. Ovx+LEVO comparisons.
4.5 Morris Water Maze
MM procedure was identical to Study 1. Additional group differences were investigated for distance and latency within each day separately to evaluate potential replications from Study 1. These analyses were one-tailed for the Ovx+Vehicle vs. Ovx+NETA-High and Ovx+Vehicle vs. Ovx+MPA comparisons, as NETA-High previously impaired MM performance in Study 1 and MPA impaired MM performance in prior publications from our lab [8, 9]. The analyses were two-tailed for the Ovx+Vehicle vs. Ovx+LEVO comparisons.
4.6 Vaginal Smears and Uterine Weights
Vaginal smears were performed identical to Study 1, except that they were done 22 and 23 days after Ovx. All other procedures were identical to Study 1.
5. Results
5.1 Vaginal Smears and Uterine Weights
All animals, regardless of treatment group, exhibited diestrous vaginal smears with primarily leukocytic cells. For uterine weights there was a Treatment main effect [F (3, 30) = 4.698; p < 0.01]. The Ovx+MPA animals had heavier uteri than Ovx+Vehicle animals [t (14) = 5.43; p < 0.05; Table 1], replicating previous findings [8, 9]; however, no visual evidence of hyperplasia/ballooning was noted. The Ovx+MPA animal’s uteri were also significantly heavier than Ovx+LEVO animals [t (15) = 6.224; p < 0.05; Table 1] and marginally heavier than Ovx+NETA-High animals [t (15) = 3.583; p < 0.10; Table 1]. Neither the Ovx+NETA-High nor the Ovx+LEVO group differed from Ovx+Vehicle (Table 1).
Table 1.
Study 2 Uterine Weights
| Treatment | Weight (g) |
|---|---|
| Ovx+Vehicle | 0.160 ± 0.01 |
| Ovx+MPA | 0.251 ± 0.04*# |
| Ovx+LEVO | 0.157 ± 0.01 |
| Ovx+NETA-High | 0.180 ± 0.01 |
p < 0.05 vs. Ovx+Vehicle and Ovx.+LEVO;
p < 0.10 vs. Ovx+NETA-High
5.2 Water Radial-Arm Maze
5.2.1 Acquisition and Asymptotic Testing Phases
Across Days 2–14 (combined acquisition and asymptotic testing phases) there was a Day main effect for the omnibus ANOVA for each type of error [WMC: F (12, 360) = 18.93; p < 0.0001; WMI: F (12, 360) = 22.711; p < 0.0001; RM: F (23, 360) = 12.956; p < 0.0001], with errors decreasing across days, demonstrating learning of the task.
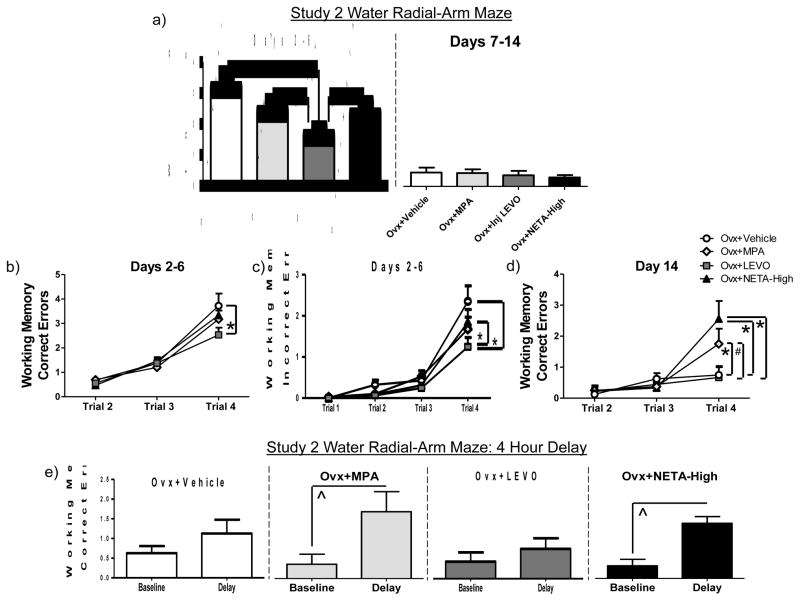
For the acquisition phase, there was a main effect of Treatment for the Ovx+Vehicle vs. Ovx+LEVO comparison for WMI [F (1, 15) = 10.50; p < 0.01 (Figure 4a)], with the LEVO group committing fewer errors and therefore, showing enhanced performance. The Ovx+LEVO group committed fewer WMI errors than the Ovx+MPA group [F (1, 15) = 5.183; p < 0.05 (Figure 4a)] and the Ovx+NETA-High group [F (1, 16) = 5.437; p < 0.05 (Figure 4a)]. The difference between Ovx+Vehicle and Ovx+LEVO animals was also evident at Trial 4, the highest working memory load, for WMC [F (1, 15) = 4.49; p = 0.05 (Figure 4b)] and WMI errors [F (1, 15) = 8.07; p < 0.01 (Figure 4c)]. The difference between Ovx+LEVO and Ovx+NETA animals also reached significance for Trial 4 WMI errors [F (1, 16) = 4.405; p = 0.05 (Figure 4c)]. There were no effects of LEVO on RM errors for the acquisition phase or any of the three error types for the asymptotic phase. For each of three error types, neither the high NETA dose nor MPA differed from Vehicle on the acquisition or asymptotic phases yielding no main effect or interactions.
Figure 4.
Study 2 (a) Mean error scores (+SE) collapsed across all trials on the water radial-arm maze during the learning (Days 2–6) and asymptotic (Days 7–14) testing phases for Working Memory Incorrect errors. Working memory load effect during the learning testing phase (Days 2–6) for (b) Working Memory Correct errors and (c) Working Memory Incorrect errors, and (d) during the last day of regular testing (Day 14) for Working Memory Correct errors. (e) Mean Working Memory Correct error scores (+SE) for baseline (last day of regular testing trial 3) vs. delay (trial 3 immediately following a 4 hour delay). *p ≤ 0.05; #p <.10; ^p < 0.05 Baseline vs. Delay
5.2.2 Last Day of Regular Testing
In order to examine a potential replication of our previously published MPA-induced impairment [8], we investigated group differences on the last day of regular testing, at the highest working memory load. Replicating prior findings, for Trial 4 on Day 14, there was a main effect of Treatment for the Ovx+Vehicle vs. Ovx+MPA comparison for WMC errors [t (14) = 1.81; p < 0.05 (Figure 4d)], with the MPA group committing more errors and therefore, showing impaired performance. The Ovx+MPA group made marginally more errors than the Ovx+LEVO group [t (15) = 1.78; p < 0.10 (Figure 4d)]. There was also a main effect of Treatment for the Ovx+Vehicle vs. Ovx+NETA-High comparison for WMC errors on Trial 4, Day 14 [t (15) = 2.73; p < 0.05 (Figure 4d)], with the NETA-High group making more errors. The NETA-High group also made more errors than the LEVO group on this measure [t (16) = 2.74; p < 0.05 (Figure 4d)]. There were no main effects of treatment between Ovx+Vehicle and Ovx+MPA or Ovx+Vehicle and Ovx+NETA-High for WMI or RM Errors. For each of the three error types, LEVO had no effect on the last day of regular testing, at the highest working memory load as compared to Vehicle.
5.2.3 Delay Testing
To evaluate which groups were impaired by the 4 hour delay, we compared performance on Day 14 Trial 3 (baseline), to Day 15 Trial 3 (post-delay). For WMC errors, on Trial 3, the trial immediately following the delay, the Ovx+MPA [t (7) = 2.43; p < 0.05] and Ovx+NETA-High [t (8) = 4.26; p < 0.005] groups made significantly more errors as compared to baseline (Figure 4e), indicating a delay-induced impairment in performance with MPA and high dose NETA treatment. Ovx+Vehicle and Ovx+ LEVO animals were not impaired by the delay. There were no delay-induced effects for WMI or RM errors.
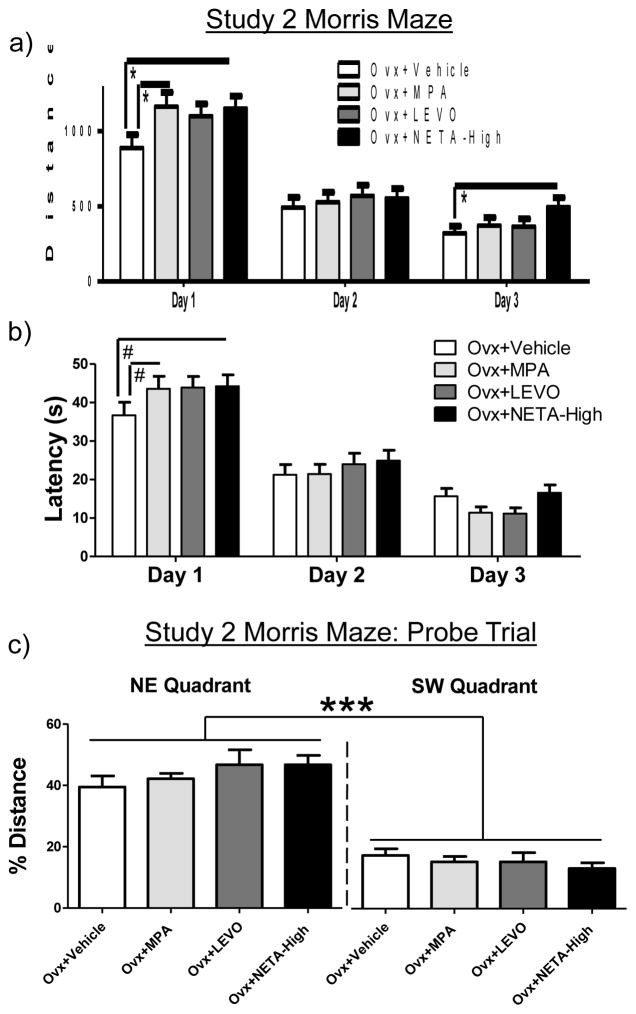
5.3 Morris Water Maze
There were Day main effects for distance [F (2, 60) = 105.499; p < 0.0001 (Figure 5a)] and latency [F (2, 60) = 123.906; p < 0.0001 (Figure 5b)] for the omnibus ANOVA, with scores decreasing across days, demonstrating learning of the task. For Day 1, there was a main effect of Treatment for distance for Ovx+Vehicle vs. Ovx+NETA-High [F (1, 15) = 3.76; p < 0.05 (Figure 5a)] and Ovx+Vehicle vs. Ovx+MPA [F (1, 14) = 3.76; p < 0.05 (Figure 5a)], with the NETA-High and MPA groups swimming longer distances to the platform, showing impaired performance. The latency effects were marginal for each comparison [Ovx+Vehicle vs. Ovx+NETA-High: F (1, 15) = 2.328; p < 0.10 (Figure 5b); Ovx+Vehicle vs. Ovx+MPA: F (1, 14) = 2.57; p < 0.10 (Figure 5b)]. For NETA-treated animals this impairment, with respect to Ovx+Vehicle animals, persisted and was present on Day 3 for distance [F (1, 15) = 3.72; p < 0.05 (Figure 5a)], but not for latency. There were no differences between Ovx+Vehicle and Ovx+MPA for Day 3. There was no effect of LEVO treatment on any day of MM testing.
Figure 5.
Study 2 Morris maze mean (+SE) (a) Distance scores in centimeters and (b) Latency scores in seconds across all days of testing (Days 1–3). (c) Mean percent Distance in the target NE quadrant as compared to the opposite SW quadrant during the probe trial. *p < 0.05; #p <.10; ***p < 0.001 NE quadrant vs. SW quadrant
For the probe trial, a higher percent distance was spent in the previously platformed (NE) versus the opposite (SW) quadrant [Quadrant main effect: F (1, 40) = 106.1; p < 0.0001 (Figure 5c)]. This pattern did not differ by Treatment [null Quadrant x Treatment interaction], suggesting that all groups localized the platform location by the end of testing.
5.4 Visible Platform
There was a main effect of Trial [F (5, 150) = 9.521; p < 0.0001 (Supplemental Figure 2)], with latency decreasing across trials. There were no Treatment effects when collapsing across all trials or for individual trials for MPA, NETA, or LEVO with respect to Vehicle for the visible platform task. There were also no Treatment x Trial interactions for any progestin group compared to Vehicle. The average time to find the platform was 10s by the last trial of testing, confirming visual and motor competence for solving a water-escape swim task.
6. Discussion
The effectiveness of clinically used progestins in offsetting the increased risk of endometrial hyperplasia associated with unopposed estrogen treatment is well documented [2, 3]; indeed, progestins are an important component of exogenous hormone regimens for both contraceptives and HT. However, little is known about the potential risks to cognition. The current study corroborates previous evidence [9] that the most commonly used FDA-approved progestin for HT, MPA, impairs learning and memory in the Ovx middle-aged rat. The herein study also presents for the first time conditions under which two other types of frequently used progestins for HT and contraception, NETA and LEVO, improve and/or impair cognition in the Ovx middle-age rat.
In Study 1, there was a dose effect of NETA for cognitive impairment. Both the low and high doses of NETA impaired memory retention with a six hour delay on the WRAM, a detrimental effect not seen in vehicle-treated rats. However, the high dose NETA treatment impaired working and reference memory performance during learning on the WRAM and reference memory performance on the MM, as compared to vehicle treatment, and in some cases, as compared to low dose NETA treatment as well. In Study 2 this same high dose of NETA was investigated under a shorter duration of treatment, modeling the approximate HT treatment duration likely to occur in women under the new FDA recommendation to use HT for the shortest time possible to alleviate menopausal symptoms [30]. In Study 2 the high dose of NETA was also compared to a previously established cognitive impairing dose of MPA [8] and another testosterone-resembling progestin of a different chemical structure class than NETA, LEVO. We replicated our previous finding that MPA impaired working memory performance on the last day of regular testing, at the highest working memory load, and after a delay on the WRAM [8]. The high dose of NETA also impaired performance on these WRAM measures. Interestingly, LEVO did not impair performance at any time during testing, but instead enhanced learning on the WRAM, as compared to all other treatment groups. Both MPA and NETA showed a reference memory impairment on the MM. The magnitude of cognitive impairment seen with the high dose of NETA in Study 1 was not observed in Study 2, when a shorter duration treatment was implemented. The cognitive impairments associated with the high dose of NETA in Study 2 were more subtle than in Study 1. Lastly, our past [8, 9] and present results with MPA treatment clearly show the detrimental effect of this progesterone-resembling progestin on cognition at variable treatment durations. Table 2 summarizes the behavior effects from each study.
Table 2.
Conclusions Summary
| Study 1 – 6 Week Treatment | |
|---|---|
| Treatment | Outcome |
| NETA-Low | Impaired delayed retention |
| NETA-High | Impaired learning, delayed retention, and reference memory |
| Study 2 – 4 Week Treatment | |
|---|---|
| Treatment | Outcome |
| MPA | Impaired at highest working memory load, delayed retention, and reference memory |
| NETA-High | Impaired at highest working memory load, delayed retention, and reference memory |
| LEVO | Enhanced learning |
The present investigation of unopposed NETA effects on cognition in a menopausal rodent model sheds light on previous findings with menopausal women. NETA in combination with ethinyl- or 17β-estradiol has been shown to enhance brain activation during a memory test [23, 24] and protect from memory decline [48]. Our findings in combination with these clinical studies in women suggest estrogens in combination HT may outweigh negative effects of NETA. This hypothesis warrants future investigations evaluating NETA and estrogens alone versus in combination. Alternatively, NETA may interact with the endogenous hormone milieu produced by menopausal ovaries in women in a way that alters the cognitive effect of the drug from those seen here with Ovx rats. We have previously shown that surgical vs. transitional modes of menopause in rats result in disparate cognitive effects of HT (CEE), possibly due to interaction with hormones released from the residual transitional menopausal ovary [49]. Delineating how NETA treatment interacts with exogenous estrogens and endogenous post-menopausal hormones is essential to optimizing its use for menopausal women.
The current study is the first to investigate the cognitive effects of LEVO in a menopausal rodent model. Here we found that LEVO enhanced spatial learning on the WRAM, supporting our hypothesis that progestins with a different chemical structure (Figure 1d) than the cognitively impairing MPA (an acetylated pregnane derivative, Figure 1b) can enhance cognition in the middle-age Ovx rat. Previous research in young, reproductively intact female rats, has also shown LEVO-induced spatial memory enhancements, but non-spatial memory impairments [29]. While more research is needed to better understand how LEVO impacts different cognitive domains, these results are encouraging for the potential of LEVO to enhance brain function in post-menopausal women. Pharmacologically, LEVO has higher relative binding affinities for both the progesterone and androgen receptors than NETA and MPA [18]. Activation of both receptors initiates the neuroprotective MAPK/ERK signaling pathway [16, 50], and Liu and colleagues [51] demonstrated LEVO to be the only of these three progestins to induce this neuroprotective pathway. More research is warranted to determine if this is the mechanism of the cognitive enhancing effects of LEVO. Nevertheless, this is an exciting finding for women who have chosen the one LEVO-containing HT or LEVO-containing hormonal contraceptives for off-label use [19, 22]
Finally, this is now our third report showing that MPA when given alone impairs cognition in the rodent model. Limiting the duration of treatment did not reduce the magnitude of the cognitive effect, as findings from Braden et al. [8] were replicated. Other rodent studies have shown that long-term treatment of 17β-estradiol plus MPA led to impaired spatial memory performance, as compared to 17β-estradiol, in Ovx rats [10, 11]. Additionally, in young Ovx mice, MPA treatment reversed the cognitive benefits seen with progesterone alone [52]. In other basic science work, MPA has attenuated or blocked the beneficial effects of 17β-estradiol on neuronal health in hippocampal cell cultures [12, 53], and exacerbated glutamate-induced toxicity when given alone [13]. Further, our findings are corroborated clinically by a case study of amnesia corresponding with the use of MPA in the contraceptive depo provera [7] and indirect evidence, via the combined estrogen alone and estrogen+progestin trials, that the addition of MPA to HT increases risk of dementia [5, 6]. Although still the most common FDA-approved progestin for HT [4], MPA use sharply declined after the detrimental findings form the WHI [54], and has not regained popularity in the face of custom compound and off label HT use [21].
7. Conclusions
It is critical to find a progestin option for women taking HT or hormonal contraceptives that will protect against estrogen induced endometrial hyperplasia but will not have adverse effects on cognition, such as those seen with MPA. Here, we present preclinical evidence that LEVO may be a safer alternative for cognitive health than both MPA and NETA. At this time, LEVO is included in only one FDA-approved HT preparation [27]; our work suggests that further evaluation of LEVO is warranted for cognitive and brain effects in animal models and in women, and that these evaluations could bring desperately needed novel avenues of treatment for HT. Future research must be done to better understand how this progestin interacts with estrogens for cognition both in the clinic and in animal models, as well as the mechanisms behind divergent effects on cognition of different chemical classes of progestins.
Supplementary Material
Highlights.
Clinically-used progestins in hormone therapy differ by chemical structure class.
Two types of progestins impair learning and memory in the menopausal rat model.
One progestin enhances learning in the menopausal rat model.
The progestin that enhances learning may be a better choice for menopausal women.
Acknowledgments
This research was funded by grants awarded to HAB-N from the National Institute on Aging (AG028084), state of Arizona, ADHS, and the Arizona Alzheimer’s Disease Core Center.
Footnotes
mg/kg calculations were based on a 70kg woman.
mg/kg calculations were based on a 70kg woman.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sitruk-Ware R. Hormonal replacement therapy. Rev Endocr Metab Disord. 2002;3(3):243–56. doi: 10.1023/a:1020028510797. [DOI] [PubMed] [Google Scholar]
- 2.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293(23):1164–7. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- 3.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293(23):1167–70. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]
- 4.Sood R, Shuster L, Smith R, Vincent A, Jatoi A. Counseling postmenopausal women about bioidentical hormones: ten discussion points for practicing physicians. J Am Board Fam Med. 2011;24(2):202–10. doi: 10.3122/jabfm.2011.02.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 6.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel A, Fahim G. Do Depot Medroxyprogesterone Acetate Contraceptive Injections Cause Mood Changes and Memory Impairment? Primary Psychiatry. 2005;12(4):59–60. [Google Scholar]
- 8.Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93(3):444–53. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braden BB, Garcia AN, Mennenga SE, Prokai L, Villa SR, Acosta JI, Lefort N, Simard AR, Bimonte-Nelson HA. Cognitive-impairing effects of medroxyprogesterone acetate in the rat: independent and interactive effects across time. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowry NC, Pardon LP, Yates MA, Juraska JM. Effects of long-term treatment with 17 beta-estradiol and medroxyprogesterone acetate on water maze performance in middle aged female rats. Horm Behav. 2010;58(2):200–7. doi: 10.1016/j.yhbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okojie AK, Oyekunle OA. Depo-provera effects on Wistar rat performance in the Y-maze. Metabolic brain disease. 2014;29(2):529–31. doi: 10.1007/s11011-013-9460-9. [DOI] [PubMed] [Google Scholar]
- 12.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143(1):205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 13.Nilsen J, Morales A, Brinton RD. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol. 2006;22(7):355–61. doi: 10.1080/09513590600863337. [DOI] [PubMed] [Google Scholar]
- 14.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68(10–13):879–90. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313–39. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss GP IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) The nomenclature of steroids. Recommendations 1989. Eur J Biochem. 1989;186(3):429–58. [PubMed] [Google Scholar]
- 18.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Curtis MG, Overholt S, Hopkins MP. Glass’ Office Gynecology. 6. Lippincott, Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 20.Murphy PA, Brixner D. Hormonal contraceptive discontinuation patterns according to formulation: investigation of associations in an administrative claims database. Contraception. 2008;77(4):257–63. doi: 10.1016/j.contraception.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.T.N.A.M. Society. [accessed April 13, 2016];More Women Now Using Compounded Hormones without Understanding the Risks. 2015 < http://www.menopause.org/docs/default-source/2015/cbht-use-trends-gaps.pdf>.
- 22.Ikhena DE, Johnson JV. What are the options for providing contraception to perimenopausal women? Menopausal Medicine. 2012;20(3):S1–6. [Google Scholar]
- 23.Smith, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab. 2006;91(11):4476–81. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persad CC, Zubieta JK, Love T, Wang H, Tkaczyk A, Smith YR. Enhanced neuroactivation during verbal memory processing in postmenopausal women receiving short-term hormone therapy. Fertil Steril. 2009;92(1):197–204. doi: 10.1016/j.fertnstert.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierney MC, Oh P, Moineddin R, Greenblatt EM, Snow WG, Fisher RH, Iazzetta J, Hyslop PS, MacLusky NJ. A randomized double-blind trial of the effects of hormone therapy on delayed verbal recall in older women. Psychoneuroendocrinology. 2009;34(7):1065–74. doi: 10.1016/j.psyneuen.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Gambacciani M, Ciaponi M, Cappagli B, Monteleone P, Benussi C, Bevilacqua G, Genazzani AR. Effects of low-dose, continuous combined estradiol and noretisterone acetate on menopausal quality of life in early postmenopausal women. Maturitas. 2003;44(2):157–63. doi: 10.1016/s0378-5122(02)00327-4. [DOI] [PubMed] [Google Scholar]
- 27.T.N.A.M. Society. [accessed April 11, 2016];Approved Prescription Products for Menopausal Symptoms in the United States and Canada. 2016 < http://www.menopause.org/docs/default-source/2014/nams-ht-tables.pdf>.
- 28.Rudolph I, Zimmermann T, Kaminski K, Jandova K, Borovsky B, Ahrendt HJ, Golbs S. Changes in psychic and somatic well-being and cognitive capabilities of peri- and postmenopausal women after the use of a hormone replacement drug containing estradiol valerate and levonorgestrel. Methods and findings in experimental and clinical pharmacology. 2000;22(1):51–6. doi: 10.1358/mf.2000.22.1.795841. [DOI] [PubMed] [Google Scholar]
- 29.Simone J, Bogue EA, Bhatti DL, Day LE, Farr NA, Grossman AM, Holmes PV. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology. 2015;62:265–78. doi: 10.1016/j.psyneuen.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 30.U.S.F.a.D. Administration. [accessed April 11, 2016];Menopause and Hormones: Common Questions. 2015 < http://www.fda.gov/ForConsumers/ByAudience/ForWomen/ucm118624.htm>.
- 31.Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009 doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 33.Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161–73. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 34.Bimonte HA, Denenberg VH. Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav. 2000;68(4):495–9. doi: 10.1016/s0031-9384(99)00201-2. [DOI] [PubMed] [Google Scholar]
- 35.Bimonte HA, Granholm AC, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neurosci Lett. 2002;328(1):50–4. doi: 10.1016/s0304-3940(02)00442-1. [DOI] [PubMed] [Google Scholar]
- 36.Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70(3–4):311–7. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- 37.Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785(2):236–44. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- 38.Hyde LA, Sherman GF, Hoplight BJ, Denenberg VH. Working memory deficits in BXSB mice with neocortical ectopias. Physiol Behav. 2000;70(1–2):1–5. doi: 10.1016/s0031-9384(00)00239-0. [DOI] [PubMed] [Google Scholar]
- 39.Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98(6):946–54. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- 40.Braden BB, Kingston ML, Koenig EN, Lavery CN, Tsang CW, Bimonte-Nelson HA. The GABAA antagonist bicuculline attenuates progesterone-induced memory impairments in middle-aged ovariectomized rats. Front Aging Neurosci. 2015;7:149. doi: 10.3389/fnagi.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24(1):229–42. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- 42.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 43.Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008 doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stavnezer AJ, Hyde LA, Bimonte HA, Armstrong CM, Denenberg VH. Differential learning strategies in spatial and nonspatial versions of the Morris water maze in the C57BL/6J inbred mouse strain. Behav Brain Res. 2002;133(2):261–70. doi: 10.1016/s0166-4328(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 45.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med. 2008;23(9):1507–13. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sengupta P. The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med. 2013;4(6):624–30. [PMC free article] [PubMed] [Google Scholar]
- 47.Sass B, Rabstein LS, Madison R, Nims RM, Peters RL, Kelloff GJ. Incidence of spontaneous neoplasms in F344 rats throughout the natural life-span. Journal of the National Cancer Institute. 1975;54(6):1449–56. doi: 10.1093/jnci/54.6.1449. [DOI] [PubMed] [Google Scholar]
- 48.Tierny J, Gyulassy A, Simon E, Pascucci V. Loop surgery for volumetric meshes: Reeb graphs reduced to contour trees. IEEE Trans Vis Comput Graph. 2009;15(6):1177–84. doi: 10.1109/TVCG.2009.163. [DOI] [PubMed] [Google Scholar]
- 49.Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151(8):3795–804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. Journal of neurochemistry. 2005;94(6):1639–51. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Zhao L, She H, Chen S, Wang JM, Wong C, McClure K, Sitruk-Ware R, Brinton RD. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology. 2010;151(12):5782–94. doi: 10.1210/en.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frye CA, Koonce CJ, Walf AA. Mnemonic effects of progesterone to mice require formation of 3alpha,5alpha-THP. Neuroreport. 2010;21(8):590–5. doi: 10.1097/WNR.0b013e32833a7e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002;13(6):825–30. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- 54.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.