Abstract
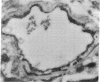
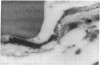
It is generally assumed that there is symmetric distribution of the glucose transporter on the lumenal and ablumenal membranes of the brain capillary endothelial cell that makes up the blood-brain barrier (BBB) in vivo. However, the presence of brain endothelial tight junctions allows for asymmetric distribution of BBB plasma membrane proteins. Glucose transporter isoform 1 (GLUT-1), the principal glucose transporter at the BBB, was assessed in rat brain in the present studies using immunogold electron microscopy. The distribution of the immunoreactive GLUT-1 protein on the endothelial lumenal membrane, the ablumenal membrane, and the cytoplasmic compartment was 12%, 48%, and 40%, respectively, and no significant immunolabeling of the neuropil was measurable. These studies suggest (i) that GLUT-1 is asymmetrically distributed on the BBB plasma membrane with an approximately 4-fold greater abundance on the ablumenal membrane as compared to the lumenal membrane; (ii) that approximately 40% of the endothelial glucose transporter protein is contained within the cytoplasmic space, which provides a mechanism for rapid up-regulation of the transporter by altered distribution of transporter between cytoplasmic and plasma membrane compartments; and (iii) that no significant labeling of neuropil is found with antisera directed against the GLUT-1 protein. These studies also suggest mechanisms of regulation of glucose transport from blood to brain that involve differential distribution of the BBB glucose transporter in subcellular compartments of brain capillary endothelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L., Lundahl P. C-terminal-specific monoclonal antibodies against the human red cell glucose transporter. Epitope localization with synthetic peptides. J Biol Chem. 1988 Aug 15;263(23):11414–11420. [PubMed] [Google Scholar]
- Bagley P. R., Tucker S. P., Nolan C., Lindsay J. G., Davies A., Baldwin S. A., Cremer J. E., Cunningham V. J. Anatomical mapping of glucose transporter protein and pyruvate dehydrogenase in rat brain: an immunogold study. Brain Res. 1989 Oct 16;499(2):214–224. doi: 10.1016/0006-8993(89)90769-5. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Goldstein G. W. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science. 1978 Oct 13;202(4364):225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Gibbs E. M., Lienhard G. E., Slot J. W., Geuze H. J. Insulin-induced translocation of glucose transporters from post-Golgi compartments to the plasma membrane of 3T3-L1 adipocytes. J Cell Biol. 1988 Jan;106(1):69–76. doi: 10.1083/jcb.106.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado R. J., Pardridge W. M. The brain-type glucose transporter mRNA is specifically expressed at the blood-brain barrier. Biochem Biophys Res Commun. 1990 Jan 15;166(1):174–179. doi: 10.1016/0006-291x(90)91927-k. [DOI] [PubMed] [Google Scholar]
- Brightman M. W. Morphology of blood-brain interfaces. Exp Eye Res. 1977;25 (Suppl):1–25. doi: 10.1016/s0014-4835(77)80008-0. [DOI] [PubMed] [Google Scholar]
- Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965 Nov;181(1):103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham V. J., Hargreaves R. J., Pelling D., Moorhouse S. R. Regional blood-brain glucose transfer in the rat: a novel double-membrane kinetic analysis. J Cereb Blood Flow Metab. 1986 Jun;6(3):305–314. doi: 10.1038/jcbfm.1986.53. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart D. Z., LeVasseur R. J., Broderius M. A., Drewes L. R. Glucose transporter localization in brain using light and electron immunocytochemistry. J Neurosci Res. 1989 Apr;22(4):464–472. doi: 10.1002/jnr.490220413. [DOI] [PubMed] [Google Scholar]
- Gjedde A., Christensen O. Estimates of Michaelis-Menten constants for the two membranes of the brain endothelium. J Cereb Blood Flow Metab. 1984 Jun;4(2):241–249. doi: 10.1038/jcbfm.1984.33. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Mans A. M., Davis D. W., Hibbard L. S., Lu D. M. Glucose availability to individual cerebral structures is correlated to glucose metabolism. J Neurochem. 1983 Apr;40(4):1013–1018. doi: 10.1111/j.1471-4159.1983.tb08086.x. [DOI] [PubMed] [Google Scholar]
- Jacquez J. A. Red blood cell as glucose carrier: significance for placental and cerebral glucose transfer. Am J Physiol. 1984 Mar;246(3 Pt 2):R289–R298. doi: 10.1152/ajpregu.1984.246.3.R289. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D., Wu J. S., Belt J. A., Paterson A. R. Photoaffinity labeling of the human erythrocyte glucose transporter with 8-azidoadenosine. J Biol Chem. 1986 Aug 25;261(24):11077–11085. [PubMed] [Google Scholar]
- Kalaria R. N., Gravina S. A., Schmidley J. W., Perry G., Harik S. I. The glucose transporter of the human brain and blood-brain barrier. Ann Neurol. 1988 Dec;24(6):757–764. doi: 10.1002/ana.410240610. [DOI] [PubMed] [Google Scholar]
- Knudsen G. M., Pettigrew K. D., Paulson O. B., Hertz M. M., Patlak C. S. Kinetic analysis of blood-brain barrier transport of D-glucose in man: quantitative evaluation in the presence of tracer backflux and capillary heterogeneity. Microvasc Res. 1990 Jan;39(1):28–49. doi: 10.1016/0026-2862(90)90057-x. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971 Dec;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Pardridge W. M., Braun L. D., Crane P. D. Measurement of cerebral glucose utilization using washout after carotid injection in the rat. J Neurochem. 1982 May;38(5):1413–1418. doi: 10.1111/j.1471-4159.1982.tb07920.x. [DOI] [PubMed] [Google Scholar]
- Orci L., Unger R. H., Ravazzola M., Ogawa A., Komiya I., Baetens D., Lodish H. F., Thorens B. Reduced beta-cell glucose transporter in new onset diabetic BB rats. J Clin Invest. 1990 Nov;86(5):1615–1622. doi: 10.1172/JCI114883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer J. R., Setchell B. P. Cerebral glucose transport and oxygen consumption in sheep and rabbits. J Physiol. 1973 Sep;233(3):529–551. doi: 10.1113/jphysiol.1973.sp010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Boado R. J., Farrell C. R. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990 Oct 15;265(29):18035–18040. [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Kinetics of blood-brain transport of hexoses. Biochim Biophys Acta. 1975 Mar 25;382(3):377–392. doi: 10.1016/0005-2736(75)90279-5. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Triguero D., Farrell C. R. Downregulation of blood-brain barrier glucose transporter in experimental diabetes. Diabetes. 1990 Sep;39(9):1040–1044. doi: 10.2337/diab.39.9.1040. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Triguero D., Yang J., Cancilla P. A. Comparison of in vitro and in vivo models of drug transcytosis through the blood-brain barrier. J Pharmacol Exp Ther. 1990 May;253(2):884–891. [PubMed] [Google Scholar]
- Ramlal T., Sarabia V., Bilan P. J., Klip A. Insulin-mediated translocation of glucose transporters from intracellular membranes to plasma membranes: sole mechanism of stimulation of glucose transport in L6 muscle cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1329–1335. doi: 10.1016/s0006-291x(88)81020-9. [DOI] [PubMed] [Google Scholar]
- Vogt B., Mushack J., Seffer E., Häring H. U. The phorbol ester TPA induces a translocation of the insulin sensitive glucose carrier (GLUT4) in fat cells. Biochem Biophys Res Commun. 1990 May 16;168(3):1089–1094. doi: 10.1016/0006-291x(90)91141-e. [DOI] [PubMed] [Google Scholar]
- Vorbrodt A. W. Demonstration of anionic sites on the luminal and abluminal fronts of endothelial cells with poly-L-lysine-gold complex. J Histochem Cytochem. 1987 Nov;35(11):1261–1266. doi: 10.1177/35.11.3655325. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Baldwin S. A., Davies A., Martin S., Pasternak C. A. Cellular stress induces a redistribution of the glucose transporter. FASEB J. 1990 Apr 1;4(6):1634–1637. doi: 10.1096/fasebj.4.6.2156742. [DOI] [PubMed] [Google Scholar]