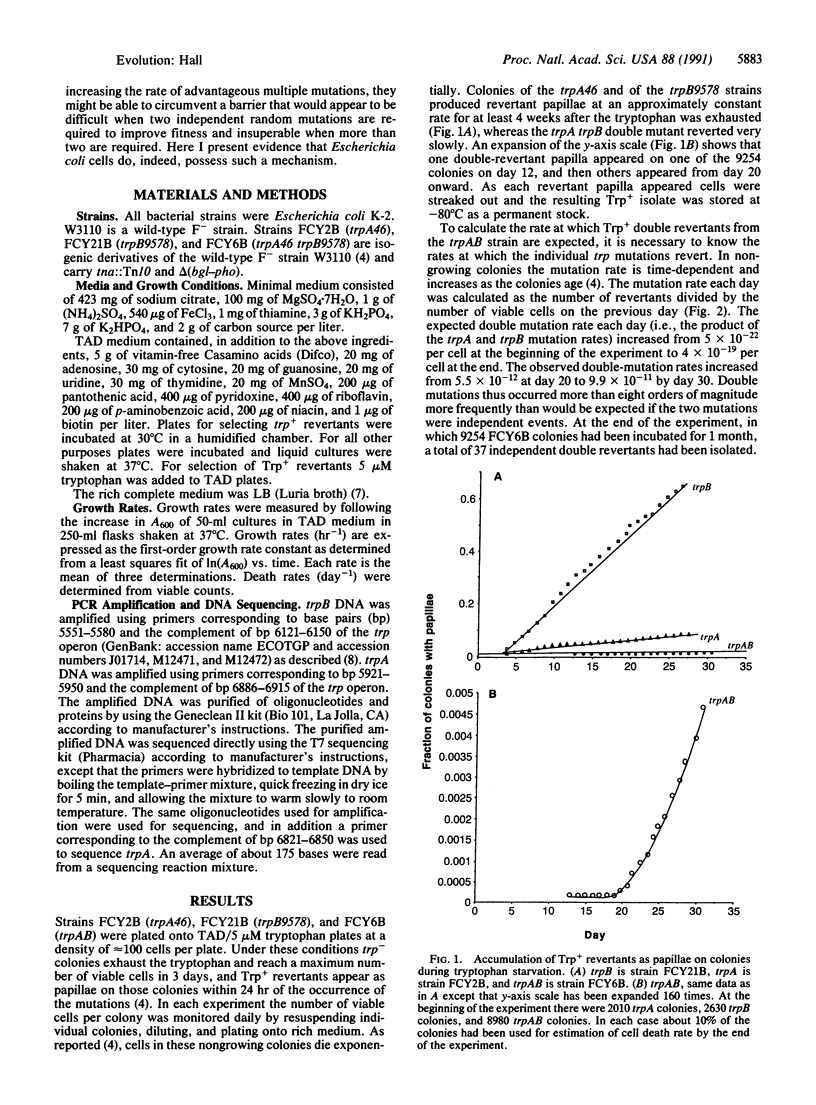
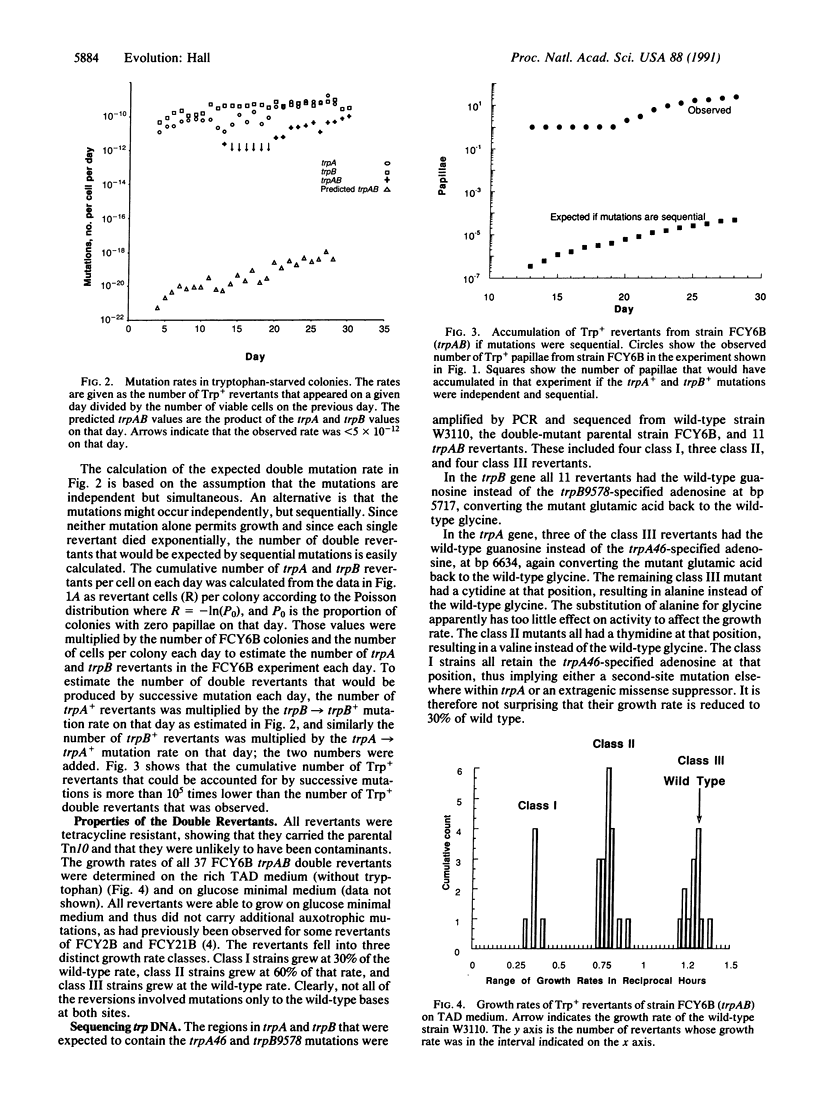
Abstract
A previous study has demonstrated that adaptive missense mutations occur in the trp operon of Escherichia coli. In this study it is shown that, under conditions of intense selection, a strain carrying missense mutations in both trpA and trpB reverts to Trp+ 10(8) times more frequently than would be expected if the two mutations were the result of independent events. Comparison of the single mutation rates with the double mutation rate and information obtained by sequencing DNA from double revertants show that neither our classical understanding of spontaneous mutation processes nor extant models for adaptive mutations can account for all of the observations. Despite a current lack of mechanistic understanding, it is clear that adaptive mutations can permit advantageous phenotypes that require multiple mutations to arise and that they appear enormously more frequently than would be expected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Davis B. D. Transcriptional bias: a non-Lamarckian mechanism for substrate-induced mutations. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5005–5009. doi: 10.1073/pnas.86.13.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics. 1988 Dec;120(4):887–897. doi: 10.1093/genetics/120.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G., Betts P. W., Wootton J. C. DNA sequence analysis of artificially evolved ebg enzyme and ebg repressor genes. Genetics. 1989 Dec;123(4):635–648. doi: 10.1093/genetics/123.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics. 1990 Sep;126(1):5–16. doi: 10.1093/genetics/126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L. L., Hall B. G. Mechanisms of activation of the cryptic cel operon of Escherichia coli K12. Genetics. 1990 Mar;124(3):473–482. doi: 10.1093/genetics/124.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W. Bacterial genetics. A unicorn in the garden. Nature. 1988 Sep 8;335(6186):112–113. doi: 10.1038/335112a0. [DOI] [PubMed] [Google Scholar]


