Abstract
Aspergillus section Nidulantes includes species with striking morphological characters, such as biseriate conidiophores with brown-pigmented stipes, and if present, the production of ascomata embedded in masses of Hülle cells with often reddish brown ascospores. The majority of species in this section have a sexual state, which were named Emericella in the dual name nomenclature system. In the present study, strains belonging to subgenus Nidulantes were subjected to multilocus molecular phylogenetic analyses using internal transcribed spacer region (ITS), partial β-tubulin (BenA), calmodulin (CaM) and RNA polymerase II second largest subunit (RPB2) sequences. Nine sections are accepted in subgenus Nidulantes including the new section Cavernicolus. A polyphasic approach using morphological characters, extrolites, physiological characters and phylogeny was applied to investigate the taxonomy of section Nidulantes. Based on this approach, section Nidulantes is subdivided in seven clades and 65 species, and 10 species are described here as new. Morphological characters including colour, shape, size, and ornamentation of ascospores, shape and size of conidia and vesicles, growth temperatures are important for identifying species. Many species of section Nidulantes produce the carcinogenic mycotoxin sterigmatocystin. The most important mycotoxins in Aspergillus section Nidulantes are aflatoxins, sterigmatocystin, emestrin, fumitremorgins, asteltoxins, and paxillin while other extrolites are useful drugs or drug lead candidates such as echinocandins, mulundocandins, calbistrins, varitriols, variecolins and terrain. Aflatoxin B1 is produced by four species: A. astellatus, A. miraensis, A. olivicola, and A. venezuelensis.
Key words: Ascomycetes, Eurotiales, Multi-gene phylogeny, Sterigmatocystin
Taxonomic novelties: New section: Section Cavernicolus A.J. Chen, Frisvad & Samson
New species: Aspergillus angustatus A.J. Chen, Frisvad & Samson, A. aurantiopurpureus A.J. Chen, Frisvad & Samson, A. botswanensis A.J. Chen, Frisvad & Samson, A. dromiae A.J. Chen, Frisvad & Samson, A. israelensis A.J. Chen, Frisvad & Samson, A. latilabiatus A.J. Chen, Frisvad & Samson, A. savannensis A.J. Chen, Frisvad & Samson, A. stercorarius A.J. Chen, Frisvad & Samson, A. sulphureoviridis A.J. Chen, Frisvad & Samson, A. viridicatenatus A.J. Chen, Frisvad & Samson
Introduction
The species of Aspergillus fall into distinct clusters, which have been called “groups” by Thom and Church, 1926, Thom and Raper, 1945 and Raper & Fennell (1965). These groups do not have nomenclatural standing and therefore Gams et al. (1985) introduced formal names for these groups as subgenera and sections. Subgenus Nidulantes contained five sections, namely sections Nidulantes, Versicolores, Usti, Terrei, and Flavipedes. Several investigations were conducted for nearly 20 years to test the taxonomic hypotheses based on phenotypic analysis. Peterson (2008) and Peterson et al. (2008) assessed phylogenetic relationships across Aspergillus using four loci and they accepted sections Nidulantes, Usti, Ochraceorosei, Sparsi and three hypothetical sections Raperi, Silvati, Bispori. Varga et al., 2010a, Varga et al., 2010b introduced sections Aenei and Sparsi based on CaM, BenA and ITS sequence data, whereas Houbraken et al. (2014) accepted eight sections namely Aenei, Bispori, Cremei, Nidulantes, Ochraceorosei, Silvati, Sparsi and Usti in subgenus Nidulantes. Until now approximately 100 species have been described in this subgenus. The indoor relevant species in Aspergillus subgenus Nidulantes section Versicolores are closely related to species in section Nidulantes (Raper and Fennell, 1965, Klich, 1993, Jurjevic et al., 2012).
Aspergillus section Nidulantes accommodates Aspergillus nidulans and other species producing biseriate conidiophores with pale brown pigmented stipes, and if present, the ascomata embedded in masses of Hülle cells (Frisvad and Samson, 2004, Horie, 1978, Horie, 1979, Horie, 1980, Kong and Qi, 1986, Horie et al., 1989, Horie et al., 1990, Horie et al., 1996a, Horie et al., 1996b, Horie et al., 1998, Horie et al., 2000, Raper and Fennell, 1965, Samson and Mouchacca, 1975, Stchigel and Guarro, 1997, Thom and Raper, 1939, Zalar et al., 2008 and others). The majority of section Nidulantes species are able to produce a sexual state and those species were, in the dual name nomenclature system, assigned to the genus Emericella. Because of the adoption of the “one fungus: one name” nomenclatural system, all Emericella species have been transferred to Aspergillus (Samson et al. 2014). Most former Emericella species belong to Aspergillus subgenus Nidulantes section Nidulantes. The only exceptions are: 1) Aspergillus heterothallicus (= Emericella heterothallica), the only known heterothallic species in subgenus Nidulantes, currently classified in Aspergillus subgenus Nidulantes section Usti (Houbraken et al., 2007, Samson et al., 2011), and 2) A. bicolor (=E. bicolor), A. discophorus (=E. discophora), A. foeniculicola (=E. foeniculicola), and A. spectabilis (=E. spectabilis) classified in Aspergillus subgenus Nidulantes section Aenei (Varga et al. 2010a).
The morphology of the ascospores including colour, shape, size and ornamentation are of particular importance for species delineation and identification in Emericella (Thom and Raper, 1939, Christensen and Raper, 1978, Horie, 1980, Christensen and States, 1982, Ismail et al., 1995, Zalar et al., 2008, Matsuzawa et al., 2012, Guarro et al., 2012, Kritmitzas et al. 2013). Nowadays multiple methods are applied for species recognition and for example Frisvad & Samson (2004) applied a polyphasic analysis and described A. venezuelensis (= E. venezuelensis) based on morphological characters, extrolites and phylogenetic analyses. Using molecular phylogenetics, morphological data and growth temperatures Matsuzawa et al. (2012) discussed the species concept in Emericella and found that several species including A. nidulans (= E. nidulans), A. dentatus (= E. dentata), A. sublatus (= E. sublata), A. montenegroi (= E. montenegroi), A. nidulans var. latus (= E. nidulans var. lata), A. quadrilineatus (= E. quadrilineata), A. miyajii (= E. miyajii), A. parvathecius (= E. parvathecia) and A. acristatus (= E. acristata) were undistinguishable by phylogenetic analysis alone. Therefore, they suggested to evaluate phylogenetic, morphological and physiological characters to identify species in this genus or section.
Aspergillus section Nidulantes species are widely distributed in nature and are believed to play significant roles in decomposition processes (Raper & Fennell 1965). The most well-known species A. nidulans, with the whole genome being sequenced in 2005 (Galaghan et al. 2005), occupies a place of prominence second only to Neurospora in the field of fungal genetics, being used to study a wide range of subjects including recombination, DNA repair, mutation, cell cycle control, nucleokinesis, pathogenesis, metabolism, and experimental evolution (Pontecorvo et al., 1954, Herbert and Arst, 1976, Dean and Timberlake, 1989, Schoustra et al., 2006, Todd et al., 2007). In addition to its role as genetic model, A. nidulans has been demonstrated as causative agent of diverse infections in humans. It was identified in cases of otomycosis, mycetoma, keratitis, sinusitis and pulmonary aspergilloma and was recognised as a major cause of invasive aspergillosis (IA) in patients with chronic granulomatous disease (CGD) (Baylet et al., 1968, Doby and Kombila-Favry, 1978, Joshi et al., 1985, Segal et al., 1998, Henriet et al., 2012). Other species in section Nidulantes and Versicolores such as A. delacroxii (=A. spinulosporus), A. dentatus, A. protuberus, A. quadrilineatus, A. sublatus, A. unguis, A. sydowii, A. stellatus, A. versicolor and A. hongkongensis have also been reported in human infections (Polacheck et al., 1992, de Hoog et al., 2000, Verweij et al., 2008, Arabatzis et al., 2011, Yu et al., 2013, de Fontbrune et al., 2014, Sabino et al., 2014, Tsang et al., 2016).
Members of Aspergillus section Nidulantes produce a high number of secondary metabolites: such as aflatoxins and sterigmatocystins, echinocandins and mulundocandins, penicillins, terreins, and many others (Turner, 1971, Cole and Cox, 1981, Turner and Aldridge, 1983, Frisvad, 1985, Liu and Shen, 2011, Saito et al., 2016). In general, similar metabolites can occur in phylogenetically closely related species, for example A. variecolor (= E. variecolor), A. filifer (= E. filifera), A. stella-maris (= E. stella-maris), A. olivicola (= E. olivicola), A. venezuelensis (= E. venezuelensis) and A. astellatus (= E. astellata) all produce the octaketides shamixanthones, emericellin and arugosins, while A. pluriseminatus (= E. pluriseminata), a phylogenetically species distant from these, showed an entirely distinctive extrolite profile (Zalar et al. 2008). Anidulafungin, a semisynthetic lipopeptide antifungal drug of the echinocandin type, is derived from a fermentation product of A. spinulosporus (syn. A. nidulans var. echinulatus) (Nyfeler & Keller-Schierlein 1974), A. parvathecius, A. navahoensis, A. quadrilineatus, A. rugulosus and A. pachycristatus (= “Aspergillus nidulans var. roseus” nomen nudum) (Boeckner and Kastner, 1981, Klich et al., 2001, de la Cruz et al., 2012, Matsuzawa et al., 2012, Bills et al., 2014, Yue et al., 2015). Aflatoxin production is observed in A. astellatus (= E. astellata), A. venezuelensis (= E. venezuelensis) and A. olivicola (= E. olivicola) (Frisvad and Samson, 2004, Frisvad et al., 2004, Zalar et al., 2008). Recently, a fungal natural product aspergillomarasmine A (AMA) was identified from extracts of A. versicolor (strain WAC-138). This compound combined with a carbapenem antibiotic has therapeutic potential to address the clinical challenge of MBL (metallo-β-lactamase)-positive carbapenem-resistant Gram-negative pathogens (King et al. 2014).
In this study, we delineate the sections of Aspergillus subgenus Nidulantes using a phylogenetic analysis of a combined data set of partial ITS, β-tubulin (BenA), calmodulin (CaM) and RNA polymerase II second largest subunit (RPB2) gene sequences. Subsequently, the taxonomy of section Nidulantes was investigated using a polyphasic approach including sequence analyses, morphological and physiological characterisation, and extrolite profiles.
Material and methods
Fungal strains
Isolates used in this study were obtained from: 1) CBS, culture collection of CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; 2) IBT, culture collection of the DTU Systems Biology, Lyngby, Denmark; and 3) CGMCC, China General Microbiological Culture Collection Centre, Beijing, China). Isolates deposited in the working collection of the Applied and Industrial Mycology department (DTO) housed at CBS-KNAW were also included in this study. An overview of strains is listed in Table 1.
Table 1.
Strains used in this study.
| Species name | Section | Collection no. | Substrate and origin | GenBank accession nr. |
|||
|---|---|---|---|---|---|---|---|
| ITS | BenA | CaM | RPB2 | ||||
| Aspergillus amoenus | Nidulantes | NRRL 4838T | Berberis sp. fruit, Germany | EF652480 | JN853946 | JN854035 | JN853824 |
| A. angustatus | Nidulantes | CBS 273.65T = DTO 319-H8 | Mangifera indica root, Mali | EU448283 | AY339993 | EU443984 | KU867013 |
| A. askiburgiensis | Nidulantes | CBS 134374T = CCF 4716 = CCF 4428 = NRRL 62818 = IBT 33114 = IBT 32911 | Ex cave sediment, Czech Republic | LN873939 | LN873952 | LN873965 | LN873984 |
| A. asperescens | Nidulantes | CBS 110.51T = NRRL2252 = NRRL 4770 = ATCC 11079 = DSM 871 = IMI 046813 = QM 1946 = WB 2252 = WB 4770 = WB 5038 = IBT 19363 = DTO 021-F4 | Soil from cave, Somerset, England, UK | EF652475 | EF652299 | EF652387 | EF652211 |
| CBS 116.53 = DTO 020-G8 | Soil from cave, Wales, UK | KU866661 | KU866888 | KU866689 | KU866940 | ||
| CBS 117.53 = IBT 22590 = WB 4738 = DTO 020-G9 | Bat dung in cave, Krakow, Poland | KU866662 | KU866899 | KU866690 | KU866941 | ||
| A. astellatus | Nidulantes | CBS 261.93T = CBS 134.55 = NRRL 2396 = ATCC 16817 = IMI 61455 = IMI 61455ii = NRRL A-1634 = QM 1910 = WB 2396 = IBT 21902 = IBT 22589 = DTO 010-I7 | Leaf, South Seymour, Baltra, Ecuador | EF652446 | EF652270 | EF652358 | EF652182 |
| CBS 135.55 = NRRL 2397 = IMI 350353 = NRRL A-2295 = QM 1911 = WB 2397 = DTO 011-G9 | Dead leaf, Galapagos, Seymour Island, Ecuador | EU448273 | AY339994 | EU443975 | KU866936 | ||
| A. aurantiobrunneus | Nidulantes | CBS 465.65T = NRRL 4545 = NRRL 2775 = IMI 074897 = LCP 84.2354 = ATCC 16821 = WB 4545 = DSL 48 = IMI 139821 = IBT 22880 = DTO 047-G7 | Canvas haversack for respirator, Australia | EF652465 | EF652289 | EF652377 | EF652201 |
| A. aurantiopurpureus | Nidulantes | CBS 140608T = IBT 12601 = DTO 060-A7 | Kangaroo rat cheek pouch, Sevilette National Wildlife Refuge, New Mexico, USA | KU866588 | KU866824 | KU866711 | KU866966 |
| CBS 140609 = IBT 12604 = DTO 061-B9 | Kangaroo rat cheek pouch, Sevilette National Wildlife Refuge, New Mexico, USA | KU866593 | KU866826 | KU866716 | KU866970 | ||
| A. aureolatus | Nidulantes | CBS 190.65T = NRRL 5126 = ATCC 16810 = IMI 136527 = IMI 136527ii = WB 5126 = IBT 18471 = IBT 22670 = DTO 053-C1 | Air, Beograd, Serbia | EF652501 | EF652325 | EF652413 | EF652237 |
| CBS 138434 = DTO 131-G5 | Unknown source | KU866663 | KU866898 | KU866726 | KU866985 | ||
| A. austroafricanus | Nidulantes | NRRL 233T | Cape town, South Africa | JQ301891 | JN853963 | JN854025 | JN853814 |
| A. botswanensis | Nidulantes | CBS 314.89T = DTO 047-I4 | Forest soil, at base of Diospyros mespiliformis (ebony tree), Okavango Delta, Island Forest Area, Botswana | KU866572 | KU866812 | KU866695 | KU866949 |
| A. caespitosus | Nidulantes | CBS 103.45T= NRRL 1929 = ATCC 11256 = IMI 16034 = MUCL 13587 = NCTC 6972 = NCTC 6973 = QM 7399 = WB 1929 = IBT 10624 = DTO 053-D1 | Soil, Fayetteville, Arkansas, USA | EF652428 | EF652252 | EF652340 | EF652164 |
| CBS 654.74 = DTO 053-D3 | Desert soil, Western Desert, Egypt | KU866578 | KU866891 | KU866701 | KU866955 | ||
| IBT 4097 = DTO 325-C1 | Salt marsh soil, Kuwait | KU866669 | KU866907 | KU866789 | KU867054 | ||
| A. corrugatus | Nidulantes | CBS 191.77T = NHL 2763 = IMI 212201 = IBT 22829 = DTO 047-I9 | Soil under Saccharum officinarum, Nakorn Pathom, Thailand | KU866574 | KU866814 | KU866696 | KU866951 |
| A. creber | Nidulantes | NRRL 58592T | Indoor air sample, California, USA | JQ301889 | JN853980 | JN854043 | JN853832 |
| A. croceus | Nidulantes | CBS 134396T = CCF 4405 = NRRL 62495 = IBT 33602 | Ex cave sediment, Spain | LN873931 | LN873944 | LN873957 | LN873976 |
| A. cvjetkovicii | Nidulantes | NRRL 227T | Soil, New Jersey, USA | EF652440 | EF652264 | EF652352 | EF652176 |
| A. desertorum | Nidulantes | CBS 653.73T = IBT 21899 = NRRL 5921 = IMI 343076 = DTO 048-A1 | Grey soil, Egypt | EF652505 | EF652329 | EF652417 | EF652241 |
| CBS 654.73 = IBT 21900 = DTO 047-I1 | Grey soil, Egypt | KU866571 | KU866811 | KU866694 | KU866948 | ||
| CBS 655.73 = IBT 21901 = DTO 319-I8 | Grey soil, Egypt | KU866619 | KU866861 | KU866757 | KU867020 | ||
| A. dromiae | Nidulantes | CBS 140633T = IBT 25166 = DTO 059-H5 | Dromia erythropus (crab, Crustacea), Morro of Garapáta, Mochima Bay, Venezuela | KU866580 | KU866885 | KU866703 | KU866958 |
| CBS 140639 = IBT 20996 = DTO 325-C7 | Dromia erythropus (crab, Crustacea), Morro of Garapáta, Mochima Bay, Venezuela | KU866672 | KU866909 | KU866792 | KU867057 | ||
| A. falconensis | Nidulantes | CBS 271.91T = IFM 4997 = NHL 2999 = ATCC 76117 = IBT 14808 = DTO 048-A2 | Soil with steppe-type vegetation of Sabaneta, Falcon State, Coro City, Venezuela | KU866575 | KU866815 | KU866697 | KU866952 |
| CBS 989.72 = IBT 22830 = DTO 048-A3 | Arid soil, of recent reclamation and cultivated with corn, New Valley Region, Western Desert, Dakhla Oasis, 12 km NW of Mut, Egypt | KU866576 | KU866816 | KU866698 | KU866953 | ||
| CBS 126188 = IBT 23426 = RMF N172 = DTO 060-A1 | A1 horizon soil, Halile Rest Camp south of Dolemile Hill, Popane woodland (savanna), Namibia | KU866584 | KU866820 | KU866707 | KU866962 | ||
| IBT 25692 = DTO 061-C1 | Soil, Namibia | KU866594 | KU866827 | KU866717 | KU866971 | ||
| CBS 650.73A = DTO 319-I6 | Grey soil, Kharga Oasis, near Kharga Town, Egypt | KU866617 | KU866859 | KU866755 | KU867018 | ||
| CBS 650.73D = DTO 320-C4 | Grey soil, Kharga Oasis, near Kharga Town, Egypt | KU866631 | KU866870 | KU866769 | KU867033 | ||
| CBS 650.73B = DTO 324-D5 | Grey soil, Kharga Oasis, near Kharga Town, Egypt | KU866643 | KU866877 | KU866781 | KU867046 | ||
| CBS 650.73C = IBT 22846 = DTO 319-I7 | Grey soil, Kharga Oasis, near Kharga Town, Egypt | KU866618 | KU866860 | KU866756 | KU867019 | ||
| A. filifer | Nidulantes | CBS 113636T = IBT 23443 = DTO 011-A5 | Hypersaline water, Secovlje salterns, Adriatic coast, Slovenia | EU448277 | EF428372 | EU443973 | KU866932 |
| CBS 128791 = IFM 54282 = CBM FA-865 = DTO 098-H8 (ex-type of A. chinensis) | Kara Kuri Lake,near Mt.Kungur, Pamire plateau, Xinjiang Province, China | AB249003 | AB248345 | AB476806 | KU866982 | ||
| CBS 113637 = IBT 23438 = DTO 011-A6 | Hypersaline water, Secovlje salterns, Slovenia | EU448276 | KU866887 | EU443972 | KU866933 | ||
| CBS 114510 = IBT 28015 = DTO 011-A7 | Raisins, Catamarca Province, Tinogasta, Argentina | EU448278 | KU866896 | EU443974 | KU866934 | ||
| CBS 128790 = IFM 54232 = DTO 098-H9 | Kara Kuri Lake,near Mt.Kungur, Pamire plateau, Xinjiang Province, China | AB248963 | AB248305 | KU866724 | KU866983 | ||
| A. foveolatus | Nidulantes | CBS 279.81T = IBT 22847 = IFM 4547 = NHL 2839 = NBRC 30559 = IFO 30559 = IBT 22847 = DTO 320-D2 | Herbal drug of Tribulus terrestris, India | KX423658 | KX423622 | KX423635 | KU867034 |
| CBS 542.83 = DTO 319-I2 | Litter, Spain | KU866615 | KU866857 | KU866754 | KU867016 | ||
| A. fruticulosus | Nidulantes | CBS 486.65T = NRRL 4903 = ATCC 16823 = IMI 139279 = O-1077 = QM 8033 = WB 4903 = IBT 33973 = DTO 047-H8 | Soil, Colorado Desert, California | EF652483 | EF652307 | EF652395 | EF652219 |
| A. fructus | Nidulantes | NRRL 239T | Date fruit, California, USA | EF652449 | EF652273 | EF652361 | EF652185 |
| A. griseoaurantiacus | Nidulantes | CBS 138191T = DTO 267-D8 | House dust, Micronesia | KJ775553 | KJ775086 | KJ775357 | KU866988 |
| A. hongkongensis | Nidulantes | HKU49T = NBRC 110693 = NCPF 7870 = BCRC FU30360 | From the big toenail of a man with onychomycosis in Hong Kong, China | AB987907 | LC000552 | LC000565 | LC000578 |
| A. israelensis | Nidulantes | CBS 140627T= IBT 24293 = DTO 325-E2 | Evaporation pond, Ein Bokek, Dead Sea, Israel | KU866677 | KU866915 | KU866797 | KU867062 |
| CBS 140628 = IBT 24364 = DTO 325-E3 | Evaporation pond, Ein Bokek, Dead Sea, Israel | KU866678 | KU866916 | KU866798 | KU867063 | ||
| A. jaipurensis | Nidulantes | CBS 952.97T = IMT 378525 = FMR 6232 = IBT 23715 = DTO 320-A9 | Soil, Jaipur, Rajasthan, India | KU866623 | AY339988 | KU866761 | KU867024 |
| CBS 100253 = DTO 325-D8 = IBT 23714 | Soil, Jaipur, India | KU866675 | KU866913 | KU866795 | KU867060 | ||
| A. jensenii | Nidulantes | NRRL 58600T | Indoor air sample, Montana, USA | JQ301892 | JN854007 | JN854046 | JN853835 |
| A. latilabiatus | Nidulantes | CBS 426.93T = IBT 33959 = DTO 320-B2 | Sheep dung, Kerzaz, Algeria | KU866624 | KU866864 | KU866762 | KU867025 |
| A. latus | Nidulantes | CBS 492.65T = ATCC 16848 = IBT 22844 = IMI 074181 = NRRL 200 = QM 7425 = WB 200 = DTO 047-H2 | Unknown source | KF465768 | AB248334 | KU866693 | KU866946 |
| CBM-FA-669 (ex-type of A. montenegroi) | Soil, Brazil | – | AB248312 | AB524041 | – | ||
| CBS 140630 = IFO 30906 = IBT 19356 = IFM 4553 = DTO 338-F7 (ex-type of A. sublatus) | Geranium nepalense, Japan | KU866683 | KU866920 | KU866804 | KU867069 | ||
| CBS 236.65 = DTO 320-C1 | Fruit, South Africa | KU866628 | KU866867 | KU866766 | KU867030 | ||
| IBT 13352 = DTO 325-B9 | Cereal, Kenya | KU866668 | KU866883 | KU866788 | KU867053 | ||
| IBT 25906 = DTO 338-F9 = DTO 325-E9 | Soil under Erica sp., Zachenberg, Greenland | KU866684 | KU866921 | KU866805 | KU867070 | ||
| A. miraensis | Nidulantes | CBS 140625T = CGMCC 3.14984 = IBT 33946 = IBT 36278 = DTO 323-B2 | Roots of Polygonum macrophyllum var. stenophyllum, Nyingchi County,Tibet, China | KU866642 | KC342577 | KU866780 | KU867045 |
| A. multicolor | Nidulantes | CBS 133.54T = NRRL 4775 = ATCC 16804 = IFO 8133 = IBT 23157 = IMI 69857 = LSHBBB .356 = QM 1952 = WB 4281 = WB 4775 = DTO 053-C9 | Forest soil, Giuba River, Somalia | EF652477 | EF652301 | EF652389 | EF652213 |
| A. mulundensis | Nidulantes | CBS 140610T = DSMZ 5745 = IBT 33104 = DTO 316-C9 | Soil, Bangladesh | KU866604 | KU866833 | KU866729 | KU866989 |
| A. navahoensis | Nidulantes | CBS 351.81T = NRRL 13002 = ATCC 44663 = IMI 259971 = IMI 304939 = IBT 10950 = LCP 84.2561 = DTO 047-H7 | Soil from native sand-dune shrub, Northern Arizona, Arizona, USA | EF652424 | EF652248 | EF652336 | EF652160 |
| A. nidulans | Nidulantes | CBS 589.65T = NRRL 187 = ATCC 10074 = IHEM 3563 = IMI 126691 = IMI86806 = QM 1985 = Thom 4640.5 = WB 187 = DTO 047-H9 | Froidchapelle, Belgium | EF652427 | EF652251 | EF652339 | EF652163 |
| DTO 065-F9 | Air, pharmaceutical factory , Vienna, Austria | KU866599 | KU866831 | KU866722 | KU866977 | ||
| CBS 100522 = DTO 319-F7 | Air, university hospital, Austria | KU866605 | KU866848 | KX423636 | KU867005 | ||
| CBS 426.77 = IBT 22826 = DTO 319-H9 | Grassland soil, Saudi Arabia | KU866613 | KU866855 | KU866752 | KU867014 | ||
| CBS 100.20 = IBT 22895 = WB 4862 = IMI 091906 = LSHB Ac85 = NCPF 2182 = NCTC 3786 = WB 189 = DTO 320-B8 | Foot mycetoma, Tunisia | KU866627 | KU866866 | KU866765 | KU867029 | ||
| CBS 240.90 = DTO 320-C2 | Wound at back of head, 10 year old male, after craniotomy, Netherlands | KU866629 | KU866868 | KU866767 | KU867031 | ||
| CGMCC 3.06385 = DTO 322-H9 | Moldy bamboo, Yunnan province, China | KU866638 | KU866873 | KU866776 | KU867041 | ||
| CBS 114.63 = NRRL 4908 = ATCC 16829 = IBT 22839 = IMI 126693 = QM 8172 = WB 4908 = IBT 22839 = DTO 047-G8 (ex-type of A. dentatus) | Finger nail, Delhi, India | EF652488 | AY573552 | EF652400 | EF652224 | ||
| A. olivicola | Nidulantes | CBS 119.37T = IBT 21903 = IBT 26499 = DTO 011-A8 = DTO 002-I2 | Decaying fruit, Verona, Italy | EU448268 | AY339996 | EU443986 | KU866923 |
| CBS 597.65 = IBT 21904 = IBT 10994 = DTO 011-A9 | Fruit, Italy | EU448267 | AY339997 | EU443985 | KU866935 | ||
| CGMCC 3.00670 = DTO 322-A9 | Unknown source, Czech | KU866632 | KU866904 | KU866770 | KU867035 | ||
| A. omanensis | Nidulantes | CBM FA-700T = IFM 54275 | Forest soil, Oman | – | AB248347 | AB524047 | – |
| A. pachycristatus | Nidulantes | IFM 55265T = NBRC 104790 | Soil, Pichan, Xinjiang, China | – | AB375875 | AB524062 | – |
| IBT 10999 = DTO 060-A3 | Amaranthus flowerhead, kangaroo rat burrow, Portal Arizona, USA | KU866585 | KU866821 | KU866708 | KU866963 | ||
| IBT 23550 = NRRL 11440 = SRRC 1173 = ATCC 58397 = Lilly A42335 = DTO 060-A5 | Soil, Indiana, USA | KU866587 | KU866823 | KU866710 | KU866965 | ||
| IBT 22934 = DTO 061-C5 | Soil, La Paz, Mexico | KU866595 | KU866828 | KU866718 | KU866972 | ||
| CBS 198.88 = DTO 324-D8 | Flower head, USA | KU866644 | KU866878 | KU866782 | KU867047 | ||
| IBT 10993 = DTO 325-A6 | Amaranthus flower heads, Arizona, USA | KU866645 | KU866879 | KU866783 | KU867048 | ||
| IBT 12268 = DTO 325-B1 | Old Cotton Research Center, Phoenix, Arizona, USA | KU866666 | KU866881 | KU866786 | KU867051 | ||
| IBT 24499 = DTO 325-E4 | Saltern, Secovlje, Slovenia | KU866679 | KU866917 | KU866799 | KU867064 | ||
| IBT 28593 = DTO 325-F2 | Air in factory, Denmark | KU866680 | KU866918 | KU866800 | KU867065 | ||
| A. pluriseminatus | Nidulantes | CBS 100523T = FMR 5588 = IMI 370867 = DTO 011-H1 | Soil, Jaipur, Rajasthan, India | KU866566 | AY339989 | EU443988 | KU866937 |
| CBS 102705 = DTO 010-I8 | Soil, Jaipur, Rajasthan, India | KU866565 | KU866806 | KU866686 | KU866926 | ||
| A. protuberus | Nidulantes | CBS 602.74T = NRRL 3505 = ATCC 18990 = QM 9804 | Deteriorated rubber-coated electric cable, Yugoslavia | EF652460 | EF652284 | EF652372 | EF652196 |
| A. purpureus | Nidulantes | CBS 754.74T = NRRL 6133 = IMI 334937 = LCP 82.3323 = DTO 047-H5 | Desert soil, Egypt | EF652506 | EF652330 | EF652418 | EF652242 |
| A. puulaauensis | Nidulantes | NRRL 35641T | Dead hardwood branch, subalpine dry forest, Hawaii, USA | JQ301893 | JN853979 | JN854034 | JN853823 |
| A. qinqixianii | Nidulantes | CBS 128788T = IFM 55020 = CMB-FA-866 = DTO 098-H6 | Desert soil, Xinjiang Province, China | KU866600 | AB524360 | AB524051 | KU866980 |
| CBS 128789 = DTO 098-H7 | Desert soil, Xinjiang Province, China | KU866601 | KU866894 | KU866723 | KU866981 | ||
| A. quadrilineatus | Nidulantes | CBS 591.65T = NRRL 201 = ATCC 16816 = IMI 089351ii = IMI 89351 = IBT 22897 = LSHBA 546 = QM 7465 = Thom 4138.N8 = WB 201 = DTO 048-A9 | Soil, New Jersey | EF652433 | EF652257 | EF652345 | EF652169 |
| CBS 937.73 = IBT 23429 = DTO 020-I9 (ex-type of A. floriformis) | Desert soil, Egypt | KU866568 | KU866808 | KU866691 | KU866942 | ||
| CBS 119.55 = NRRL 2394 = NRRL A-4030 = ATCC 16839 = IBT 11111 = IMI 061453 = LCP 84.2558 = QM 1908 = WB 2394 = DTO 047-G6 (ex-type of A. nidulans var. acristatus ) | Exposed fabric, New Mexico | EF652444 | AY573549 | AB476805 | KU866945 | ||
| CBS 493.65 = NRRL 4904 = ATCC 16822 = IMI 139280 = LCP 84.2553 = QM 8034 = WB 4904 = DTO 047-H4 (Neotype of A. parvathecius) | Man skin, California | KU866570 | AB243111 | AB524048 | KU866947 | ||
| CBS 125.55 = DTO 048-A8 | Culture contaminant, Recife, Brazil | KU866577 | KU866817 | KU866699 | KU866954 | ||
| CBS 113684 = DTO 319-F9 | Nails, Uttar Pradesh | KU866607 | KU866850 | KU866746 | KU867007 | ||
| CBS 118.51 = DTO 319-G2 | Netherlands | KU866609 | KU866852 | KU866748 | KU867009 | ||
| CBS 467.88 = DTO 320-C3 | Garden soil, Spain | KU866630 | KU866869 | KU866768 | KU867032 | ||
| CGMCC 3.04661 = DTO 322-D3 | Unknown source, Japan | KU866634 | KU866871 | KU866772 | KU867037 | ||
| CGMCC 3.06393 = DTO 322-I8 | Soil, Yunnan province, China | KU866639 | KU866874 | KU866777 | KU867042 | ||
| CBS 126215 = IBT 23423 = DTO 325-D5 | Surface sandy dune soil, desert, Namibia | KU866674 | KU866912 | KU866794 | KU867059 | ||
| CBM-FA-833 (ex-type of A. miyajii) | Unknown | – | AB243110 | AB524040 | – | ||
| CBS 853.96 = IBT 28023 = DTO 320-A8 | Unknown source, Spain | KU866622 | KU866863 | KU866760 | KU867023 | ||
| A. recurvatus | Nidulantes | CBS 496.65T = NRRL 4902 = ATCC 16809 = IMI 136528 = O-566 = QM 7972 = WB 4902 = IBT 23271 = DTO 053-C8 | Dung of lizard, desert area near Blythe, California | EF652482 | EF652306 | EF652394 | EF652218 |
| CBS 126259 = RMF 7730 = DTO 195-D8 | Soil (dung, arid site, animal litter), Africa | KU866603 | KU866832 | KU866727 | KU866986 | ||
| A. rugulosus | Nidulantes | CBS 133.60T = NRRL 206 = ATCC 16820 = IMI 136775 = QM 1987 = Thom 4138.T11 = WB 206 = IBT 22820 = DTO 321-H1 | Soil, New Jersey, USA | EF652434 | EF652258 | EF652346 | EF652170 |
| CBS 200.75 = IBT 22848 = IMI 131554 = NRRL 3651 = QM 9184 = DTO 047-I8 (ex-type of A. cleistominutus ) | Soil, Kaulbhaskar, agricultural farm at Allahabad | KU866573 | KU866813 | AB476810 | KU866950 | ||
| IBT 12265 = DTO 061-D7 | Unknown source | KU866596 | KU866829 | KU866719 | KU866975 | ||
| CBS 113407 = DTO 319-F8 | Bat faecal pellet, near Gaba, Oman | KU866606 | KU866849 | KU866745 | KU867006 | ||
| CBS 117.50 = IBT 22519 = DTO 319-G1 | Manure, Thunder Bay, Ontario, Canada | KU866608 | KU866851 | KU866747 | KU867008 | ||
| CBS 130.48 = IBT 22837 = DTO 319-H5 | Unknown source | KU866611 | KU866853 | KU866750 | KU867011 | ||
| CGMCC 3.06394 = DTO 322-I9 | Corn flour, Yunnan province, China | KU866640 | KU866875 | KU866778 | KU867043 | ||
| IBT 10998 = DTO 325-A7 | Amaranthus flower heads, Denmark | KU866664 | KU866880 | KU866784 | KU867049 | ||
| IBT 13207 = DTO 325-B8 | Dipodomys ordii cheek pouch, Seviletta National Wildlife Refuge, Socorro County, New Mexico, USA | KU866667 | KU866882 | KU866787 | KU867052 | ||
| IBT 31140 = DTO 325-F3 | Saltern, Secovlje, Slovenia | KU866681 | KU866919 | KU866801 | KU867066 | ||
| A. savannensis | Nidulantes | CBS 140607T = IBT 23422 = DTO 059-H6 | A1 horizon soil, in Halili Rest Camp, south of Dolomite Hill (savanna), Namibia | KU866581 | KU866818 | KU866704 | KU866959 |
| CBS 126213 = IBT 23421 = RMF N171A = DTO 061-B8 | A1 horizon soil, Halile Rest Camp south of Dolemile Hill, Popane woodland, savanna, Namibia | KU866592 | KU866825 | KU866715 | KU866969 | ||
| A. spelunceus | Nidulantes | CBS 497.65T = NRRL 4989 = ATCC 16838 = IMI 211389 = NRRL A-3676 = QM 8898 = WB 4989 = IBT 33967 = DTO 053-C4 | Dead cane crickets, floor of Laurel Creek Cave, West Virginia | EF652490 | EF652314 | EF652226 | EF652402 |
| A. spinulosporus | Nidulantes | CBS 120.55T = NRRL 2395 = ATCC 16825 = IBT 22841 = IMI 061454 = LCP 84.2557 = QM 1909 = WB 2395 = IBT 22841 = DTO 047-G9 | Soil, Buenos Aires, Argentina | EF652445 | AY573553 | EF652357 | EF652181 |
| CBS 564.80 = IBT 22840 = IMI 250977 = TRTC 48545 = DTO 047-H1 | Culture contaminant, Canada | KU866569 | KU866809 | KU866692 | KX423662 | ||
| DTO 065-F7 | Air, pharmaceutical factory , Vienna, Austria | KU866598 | KU866830 | KU866721 | KU866976 | ||
| CGMCC 3.05277 = DTO 322-D6 | Moldy pork, Sichuan province, China | KU866635 | KU866872 | KU866773 | KU867038 | ||
| IBT 23829 = DTO 325-D9 | Indoor air, factory, Denmark | KU866676 | KU866914 | KU866796 | KU867061 | ||
| A. stella-maris | Nidulantes | CBS 113638T = IBT 23439 = DTO 011-A2 | Hypersaline water, Secovlje salterns, Slovenia | EU448269 | KU866886 | EU443978 | KU866929 |
| CBS 114378 = IBT 28013 = DTO 010-I6 | Leaf litter, National Agronomic Institute, Tunisia | EU448271 | KU866906 | EU443980 | KU866925 | ||
| CBS 113639 = IBT 23441 = DTO 011-A3 | Hypersaline water, Secovlje salterns, Slovenia | EU448270 | EF428367 | KU866687 | KU866930 | ||
| CBS 124670 = DTO 319-H1 | Finger nails, Athens, Greece | KU866610 | KU866900 | KU866749 | KU867010 | ||
| A. stellatus | Nidulantes | CBS 598.65T = NRRL 1858 = ATCC 16819 = IBT 32665 = IBT 21908 = IMI 136778 = QM 6835 = WB 1858 = IBT 32730 = DTO 327-F3 | Soil, Panama | EF652426 | EF652250 | EF652338 | EF652162 |
| CBS 668.82 = DTO 010-I5 | Seed, India | EU448281 | AY339992 | KU866685 | KU866924 | ||
| IBT 25137 = DTO 059-H2 | Mangrove tree branch with Isognomon sp., surface water, Mochima Bay, Venezuela | KU866579 | KU866889 | KU866702 | KU866957 | ||
| IBT 25113 = DTO 059-I7 | Pyura vittata (red ascidia, tunicate, urochordata), sand bottom with corals, 2-3 m deep water, 23°C, Cabruta, Mochima Bay, Venezuela | KU866583 | KU866890 | KU866706 | KU866961 | ||
| IBT 25306 = DTO 061-B5 | Mangrove tree branch with Isognomon sp., surface water, Mochima Bay, Venezuela | KU866591 | KU866895 | KU866714 | KU866968 | ||
| DTO 127-C6 | Air sample bakery, USA | KU866602 | KU866897 | KU866725 | KU866984 | ||
| CBS 136.55 = NRRL 4761 = ATCC 12069 = IMI 060316 = IMUR 256 = QM 6957 = WB 4761 = DTO 320-B6 | Laboratory contaminant, Brazil | KU866626 | AY339990 | KU866764 | KU867028 | ||
| CGMCC 3.06292 = DTO 322-F8 | Glass pane, Tonghua, Liaoning province, China | KU866636 | KU866903 | KU866774 | KU867039 | ||
| IBT 12233 = DTO 325-A9 | Cotton-field near Gila Bend, Arizona, USA | KU866665 | KU866905 | KU866785 | KU867050 | ||
| IBT 20986 = DTO 325-C6 | Gorgonie (octocoral, Coelenterata) from rocky sand bottom, Mochima Bay, Venezuela | KU866671 | KU866908 | KU866791 | KU867056 | ||
| A. stercorarius | Nidulantes | CBS 428.93T = IBT 28024 = DTO 320-B3 | Dung (Uromastix acanthinurus), Kerzaz, Sahara, Algeria | KU866625 | KU866865 | KU866763 | KU867026 |
| A. striatus | Nidulantes | CBS 592.65T = IBT 22824 = ATCC 16815 = NRRL 4699 = CBS 283.67 = IHEM 4515 = IMI 096679 = LCP 82.3319 = WB 4699 = DTO 320-D3 | Mangrove mud, Kagh Islands | EF652470 | EF652294 | EF652382 | EF652206 |
| CBS 451.75 = IBT 22822 = DTO 319-I1 | Gorakhpur, Uttar Pradesh, India | KU866614 | KU866856 | KU866753 | KU867015 | ||
| CBS 866.70 = IBT 22823 = DTO 320-A7 | Gorakhpur, Uttar Pradesh, India | KU866621 | KU866862 | KU866759 | KU867022 | ||
| A. subversicolor | Nidulantes | NRRL 58999T | Green coffee berries, India | JQ301894 | JN853970 | JN854010 | JN853799 |
| A. sulphureoviridis | Nidulantes | CBS 140626T = IBT 21868 = DTO 325-D1 | Indoor air, factory, Denmark | KU866673 | KU866911 | KU866793 | KU867058 |
| A. sydowii | Nidulantes | CBS 593.65T = NRRL 250 = IMI 211384 = NRRL 254 | Clinical Isolate, Waycross, Georgia, USA | EF652450 | EF652274 | EF652362 | EF652186 |
| A. tabacinus | Nidulantes | CBS 122718T = NRRL 4791 = IFO 4098 = QM 9766 = WB 4791 | Tobacco | EF652478 | EF652302 | EF652390 | EF652214 |
| A. tennesseensis | Nidulantes | NRRL 13150T | Toxic dairy cattle feed, Tennessee, USA | JQ301895 | JN853976 | JN854017 | JN853806 |
| A. undulatus | Nidulantes | CBS 261.88T =AS 3.4510 = IBT 28027 = DTO 011-A1 | Soil, Hubei Province, Shennongjia, China | EU448275 | EF428363 | EU443989 | KU866928 |
| CGMCC 3.00750 = DTO 322-B2 | Unknown source, Germany | KU866633 | KU866901 | KU866771 | KU867036 | ||
| CGMCC 3.06295 = DTO 322-G2 | Soil, Shennongjia, Hubei province, China | KU866637 | KU866902 | KU866775 | KU867040 | ||
| A. unguis | Nidulantes | CBS 132.55T = NRRL 2393 = ATCC 16812 = IMI 136526 = NRRL A-2391 = NRRLA-445 = QM 25B = WB 2393 = DTO 047-I5 | Shoe leather, Philadelphia, Pennsylvania | EF652443 | EF652267 | EF652355 | EF652179 |
| DTO 017-A6 | Air in factory, Vienna, Austria | KU866567 | KU866807 | KU866688 | KU866939 | ||
| CBS 131.55 = DTO 319-H6 | Unknown resource, Brazil | KU866612 | KU866854 | KU866751 | KU867012 | ||
| CBS 595.65 = NRRL 216 = ATCC 10073 = WB 216 = Thom 5706.1 = IMI 136525 = IBT 21610 = DTO 319-I5 | Man, Belgium | KU866616 | KU866858 | FN594611 | KU867017 | ||
| CBS 691.93 = DTO 320-A5 | Banana-pulp, USA | KU866620 | AB 248319 | KU866758 | KU867021 | ||
| CGMCC 3.06404 = DTO 323-A2 | Soil, Beijing, China | KU866641 | KU866876 | KU866779 | KU867044 | ||
| IBT 14723 = DTO 325-C2 | Marine derived isolate, Bahamas | KU866670 | KU866910 | KU866790 | KU867055 | ||
| A. varians | Nidulantes | CBS 505.65T = NRRL 4793 = ATCC 16836 = IFO 4114 = IMI 172297 = WB 4793 = IBT 22568 = DTO 073-B5 | Unknown source | EF652479 | EF652303 | EF652391 | EF652215 |
| IBT 12603 = DTO 063-I1 | Cork, Portugal | KU866597 | KX423620 | KU866720 | – | ||
| A. venenatus | Nidulantes | NRRL 13147T | Toxic dairy cattle feed, Tennessee, USA | JQ301896 | JN854003 | JN854014 | JN853803 |
| A. venezuelensis | Nidulantes | CBS 868.97T = IBT 20956 = DTO 011-A4 | In red mangrove, surface water, Rojo, Mochima Bay, Mochima Nat. Park, Sucre State, Venezuela | AJ874119 | AY339998 | EU443977 | KU866931 |
| A. versicolor | Nidulantes | CBS 583.65T = NRRL 238 = ATCC 9577 = IFO 33027 = IMI 229970T = JCM 10258 = QM 7478 = Thom 5519.57 = WB 238 | Unknown | EF652442 | EF652266 | EF652354 | EF652178 |
| A. violaceus | Nidulantes | CBS 138.55T = NRRL 2240 = ATCC 16813 = CECT2587 = IFO 8106 = IMI 061449ii = IMI 61449 = LCP 82.3318 = NRRL A-3156 = QM 1905 = UC4511 = WB 2240 = DTO 048-B2 | Soil, Tafo, Ghana | EF652438 | EF652262 | EF652350 | EF652174 |
| CBS 293.93 = NHL 3000 = DTO 010-I9 (ex-type of A. similis) | Soil in date palm plantation, Basrah City, Iraq | EU448279 | EF428374 | EU443987 | KU866927 | ||
| A. viridicatenatus | Nidulantes | CBS 140629T = IBT 31492 = DTO 325-F4 | Root of Gymnadenia conopsea, Denmark | KU866682 | KX423621 | KU866802 | KU867067 |
| A. aeneus | Aeni | CBS 128.54T = NRRL 4769 = ATCC 16803 = IMI 069855 = LSHBBB 355 = MUCL 13570 = QM 1945 = WB 4279 = WB 4769 | Forest soil, Modilen near Guiba River, Somalia | EF652474 | EF652298 | EF652386 | EF652210 |
| A. bicolor | Aeni | CBS 425.77T= NRRL 6364 = ATCC 36104 = IMI 216612 | Soil from Artemisia grassland, Wyoming | EF652511 | EF652335 | EF652423 | EF652247 |
| A. crustosus | Aeni | CBS 478.65T = NRRL 4988 = ATCC 16806 = IMI 135819 = NRRL A-3254 = QM 8910 = WB 4988 | Man skin scrapings, Illinois | EF652489 | EF652313 | EF652401 | EF652225 |
| A. discophorus | Aeni | CBS 469.88T = IBT 21910 = IMI 328717 = DTO 011-B1 | Soil, Spain | EU448272 | AY339999 | EU443970 | KX423661 |
| A. eburneocremeus | Aeni | CBS 130.54T= NRRL 4773 = ATCC 16802 = IMI 69856 = MUCL 13588 = QM 1949 = WB 4773 | Forest soil, Somalia | EF652476 | EF652300 | EF652388 | EF652212 |
| A. foeniculicola | Aeni | CBS 156.80T = ATCC 42155 = IMI 334933 = LCP 84.2560 = NHL 2777 | Foeniculum vulgare seed, China | EU448274 | EU443990 | EU443968 | KU867027 |
| A. heyangensis | Aeni | CBS 101751T = AS 3.4630 | Cotton seed, China | FJ491520 | FJ491521 | FJ491522 | KX423659 |
| A. karnatakaensis | Aeni | CBS 102800T = IBT 22153 | Soil under coconut palm in coffeeplantation, Karnataka | EU482441 | EU482438 | EU482431 | KU866956 |
| A. spectabilis | Aeni | CBS 429.77T = NRRL 6363 = ATCC 36105 = IMI 216611 = RMFH429 | Coal mine spoil material, Wyoming | EF652510 | EU482437 | EF652422 | EF652246 |
| A. cavernicola | Cavernicolus | CBS 117.76T = NRRL 6327 | Wall of cave, Romania | EF652508 | EF652332 | EF652420 | EF652244 |
| CBS 600.67 = ATCC 18351 = IMI 129961 = MUCL 15648 = VKM F-906 (ex-type of A. amylovorus) | Wheat starch, Ukraine | FJ531140 | FJ531161 | FJ531190 | JN121538 | ||
| A. californicus | Cavernicolus | CBS 123895T = IBT 16748 | Chamise chaparral (Adeonostoma fasciculatum) soil, San Gabriel Mountains, North of Claremont and near San Antonio Dam, California | FJ531153 | FJ531180 | FJ531128 | KU866974 |
| A. egyptiacus | Cavernicolus | CBS 656.73T = NRRL 5920 = ATCC 32114 = IMI 141415 | Sandy soil, under Olea europaea, Egypt | EF652504 | EF652328 | EF652416 | EF652240 |
| A. kassunensis | Cavernicolus | CBS 419.69T= NRRL 3752 = IMI 334938 | Soil, Syria | EF652461 | EF652285 | EF652373 | EF652197 |
| A. subsessilis | Cavernicolus | CBS 502.65T = NRRL 4905 = ATCC 16808 = IMI 135820 = QM 4905 = QM 8035 = WB 4905 | Desert soil, California, Mojave Desert, USA | EF652485 | EF652309 | EF652397 | EF652221 |
| A. bisporus | Bisporus | CBS 707.71T = NRRL 3693 = ATCC 22527 = IMI 350350 = NRRL A-17271 = QM 9700 | Soil injected into mouse, Maryland | EF661208 | EF661121 | EF661139 | EF661077 |
| A. funiculosus | Ochraceorosei | NRRL 4744T = NRRL 2550 = NRRL A-6752 | Soil, Nigeria | EF661223 | EF661112 | EF661175 | EF661078 |
| A. ochraceoroseus | Ochraceorosei | CBS 550.77T = NRRL 28622 = ATCC 38873 = SRRC1432 | Soil, Ivory Coast | EF661224 | EF661113 | EF661137 | EF661074 |
| A. rambellii | Ochraceorosei | CBS 101887T = ATCC 42001 = IBT 14580 | Soil, Ivory Coast | AJ874116 | JN217228 | KU866700 | JN121416 |
| A. silvaticus | Silvati | CBS 128.55T = ATCC 16843 = ATCC 46904 = IFO 8173 = IMI 061456 = NRRL 2398 = QM 1912 = WB 2398 | Soil, Ghana | EF652448 | EF652272 | EF652360 | EF652184 |
| A. ivoriensis | Raperi | CBS 551.77T = NRRL 22883 | Soil, Ivory Coast | EF652441 | EF652265 | EF652353 | EF652177 |
| A. raperi | Raperi | CBS 123.56T = NRRL 2641 = ATCC 16917 = IFO 6416 = IMI 70949 = NRRL 4778 = NRRL A-7462 = QM 1898 = WB 4221 = WB 4778 | Grassland soil, Zaire | EF652454 | EF652278 | EF652366 | EF652190 |
| A. amazonicus | Sparsi | CBS 124228T | Soil, Ecuador | – | FJ943939 | FJ943936 | KU866979 |
| A. anthodesmis | Sparsi | CBS 552.77T = NRRL 22884 = IMI 223070 | Soil, Ivory Coast | FJ491662 | EF661108 | FJ491648 | EF661039 |
| A. biplanus | Sparsi | CBS 468.65T = NRRL 5071 = ATCC 16858 = IMI 235602 = QM 8873 = WB 5071 | Soil, Costa Rica | EF661210 | EF661116 | EF661130 | EF661036 |
| A. conjunctus | Sparsi | CBS 476.65T = NRRL 5080 = ATCC 16796 = IMI 135421 = QM 8878 = WB 5080 | Forest soil, Costa Rica | EF661179 | EF661111 | EF661133 | EF661042 |
| A. diversus | Sparsi | CBS 480.65T = NRRL 5074 = ATCC 16849 = IMI 232882 = QM 8882 = WB 5074 | Forest soil, Costa Rica | EF661213 | EF661114 | EF661128 | EF661034 |
| A. haitiensis | Sparsi | CBS 464.91T | Soil under sage and cactus, Haiti | FJ491657 | FJ491670 | FJ491645 | KU866943 |
| A. implicatus | Sparsi | CBS 484.95T | Forest soil, Tai, Ivory Coast | FJ491656 | FJ491667 | FJ491650 | – |
| A. panamensis | Sparsi | CBS 120.45T = NRRL 1785 = ATCC 16797 = IMI 019393ii = IMI 019393iii = IMI 19393 = LSHBA .61 = NCTC 6974 = QM 6829 = QM 8897 = WB 1785 | Soil, Panama | EF661177 | EF661109 | EF661135 | EF661040 |
| A. sparsus | Sparsi | CBS 139.61T = NRRL 1933 = ATCC 16851 = IHEM 4377 = IMI 19394 = IMI 19394ii = MUCL 31314 = NCTC 6975 = QM 7470 = WB 1933 | Soil, Costa Rica | EF661181 | EF661125 | EF661173 | EF661071 |
| A. asper | Usti | CBS 140842T = NRRL 35910 = CCF 5174 | House air in Pennsylvania, USA | KT698840 | KT698838 | KT698839 | KT698842 |
| A. baeticus | Usti | NRRL 62501T = CMF ISB 2153 = CCF 4226 | Cave sediment, Spain | HE615086 | HE615092 | HE615117 | HE615124 |
| A. calidoustus | Usti | CBS 121601T | Bronchoalveolar lavage fluid, proven invasive aspergillosis; Nijmegen, The Netherlands | HE616558 | FJ624456 | HE616559 | – |
| A. carlsbadensis | Usti | CBS 123894T = IBT 14493 | Soil, New Mexico, Carlsbad Caverns National Park, Lechuquilla Cave, USA | FJ531151 | FJ531179 | FJ531126 | KU866973 |
| A. collinsii | Usti | CBS 140843T = NRRL 66196 = CCF 5175 | From an air settle plate exposed in a domestic bathroom, Fair Oaks, California | KT698845 | KT698843 | KT698844 | KT698848 |
| A. deflectus | Usti | CBS 109.55T = NRRL 2206 = ATCC 16807 = IMI 61448 = NRRL A-2700A = QM 1904 = UC4638 = WB 2206 | Soil, Brazil | EF652437 | EF652261 | EF652349 | EF652173 |
| A. elongatus | Usti | CBS 387.75T = NRRL 5176 = QM 9702 = WB 5495 | Alkaline Usar soil, India | EF652502 | EF652326 | EF652414 | EF652238 |
| A. germanicus | Usti | CBS 123887T | Indoor air, Germany | FJ531146 | FJ531172 | FJ531141 | KU866944 |
| A. granulosus | Usti | NRRL 1932T = ATCC 16837 = IMI 17278 = QM 6846 = WB 1932 | Soil, Fayetteville, Arkansas, USA | EF652430 | EF652254 | EF652342 | EF652166 |
| A. heterothallicus | Usti | CBS 488.65T = NRRL 5096 = ATCC 16847 = IMI 139277 = QM 8916 = WB 5096 | Soil, Costa Rica | EF652499 | EF652323 | EF652411 | EF652235 |
| A. insuetus | Usti | CBS 107.25T = NRRL 279 = NRRL 1726 = ATCC 1033 = IFO 4128 | Unknown | EF652457 | EF652281 | EF652369 | EF652193 |
| A. keveii | Usti | CBS 209.92T | Soil, Spain | EU076354 | EU076376 | EU076365 | KU866938 |
| A. keveioides | Usti | CBS 132737T | Soil, China | JN982704 | JN982694 | JN982684 | KX423660 |
| A. lucknowensis | Usti | CBS 449.75T = NRRL 3491 = ATCC 18607 = IMI 278379 = PIL623 = QM 9271 = WB 5377 | Alkaline Usar soil, India | EF652459 | EF652283 | EF652371 | EF652195 |
| A. minutus | Usti | NRRL 4876T | Soil, Iowa, USA | EF652393 | EF652481 | EF652305 | EF652217 |
| A. monodii | Usti | CBS 435.93T | Dung of sheep, Chad | FJ531150 | FJ531171 | FJ531142 | – |
| A. porphyreostipitatus | Usti | CBS 138203T = DTO 266-D9 | House dust, Mexico | KJ775564 | KJ775080 | KJ775338 | KU866987 |
| A. pseudodeflectus | Usti | CBS 756.74T = NRRL 6135 | Desert soil, Egypt | EF652507 | EF652331 | EF652419 | EF652243 |
| A. pseudoustus | Usti | CBS 123904T = NRRL 5856 = IBT 28161 | Stored maize, South Africa | FJ531147 | FJ531168 | FJ531129 | KU866978 |
| A. puniceus | Usti | CBS 495.65T = NRRL 5077 = ATCC 16800 = IMI 126692 = QM 9812 = WB 5077 | Soil, Costa Rica | EF652498 | EF652322 | EF652410 | EF652234 |
| A. thesauricus | Usti | NRRL 62485T = CMF ISB 2155 = 5CCF 4166 | Indoor air, Spain | HE615088 | HE615095 | HE615120 | HE615126 |
| A. turkensis | Usti | CBS 504.65T = NRRL A-3261 = NRRL 4993 = ATCC 16799 = IMI 135420 | Soil, Turkey | FJ531160 | FJ531191 | FJ531145 | EF652230 |
| A. ustus | Usti | CBS 261.67T = NRRL 275 = ATCC 1041 = ATCC 16818 = IMI 211805 = QM 7477 = WB 275 | Culture contaminant, USA | EF652455 | EF652279 | EF652367 | EF652191 |
DNA extraction, PCR amplification and sequencing
Strains were grown for 1 wk on MEA prior to DNA extraction. DNA was extracted using the UltracleanTM Microbial DNA isolation Kit (MoBio, Solana Beach, U.S.A.) and stored at −20 °C. ITS, BenA, CaM, and RPB2 were amplified and sequenced using methods and primers as previously described (Houbraken and Samson, 2011, Samson et al., 2014).
Phylogenetic analysis
The phylogenetic relationship between species was studied using a combined data set containing ITS, BenA, CaM and RPB2 sequences, individual single gene phylogenies were also generated to resolve relationships among the species. Sequence alignments were generated with MAFFT v. 7 (Katoh & Standley 2013). The most suitable substitution model was determined using FindModel (Posada & Crandall 1998). Bayesian analyses were performed with MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003). The sample frequency was set to 100 and the first 25 % of the trees removed as burn-in. Maximum likelihood analyses including 500 bootstrap replicates were run using RAxML (Gamma model of rate heterogeneity) (Stamatakis et al. 2008). Aspergillus flavipes (NRRL 302T) was used as outgroup in the Aspergillus subgenus Nidulantes phylogeny and Aspergillus ustus (CBS 261.67T) as outgroup in the section Nidulantes phylogeny. The resulting trees were visualized with FigTree v1.4.2 and annotated using Adobe Illustrator CS5. BI posterior probabilities (pp) values and bootstrap (bs) percentages of analysis are labelled at the nodes. Values less than 0.95 pp and less than 70 % bs are not shown. Branches with values more than 1 pp and 95 % bs are thickened. Newly obtained sequences were deposited in GenBank.
Morphological analysis
Macroscopic characters were studied on the agar media Czapek Yeast Autolysate agar (CYA), CYA supplemented with 5 % NaCl (CYAS), yeast extract sucrose agar (YES), creatine sucrose agar (CREA), dichloran 18 % glycerol agar (DG18), oatmeal agar (OA) and malt extract agar (MEA; Oxoid CM0059), trace elements (0.1 g ZnSO4·7H2O and 0.5 g CuSO4·5H2O in 100 ml distilled water) were added to all media to obtain stable pigment production and consistent conidial colours (Samson et al. 2010). The isolates were inoculated at three points on 90 mm plates and incubated for 7 d at 25 °C in darkness. In addition, CYA plates were incubated at 37 and 40 °C (CYA 37 °C and CYA 40 °C, respectively), while additional MEA plates were incubated at 37 °C (MEA 37 °C). After 7 d of incubation, colony diameters were recorded. Colony texture, degree of sporulation, obverse and reverse colony colours, production of soluble pigments, exudates and ascomata were determined. Acid production on CREA is indicated by a change in the pH sensitive bromocresole purple dye from purple to yellow around growing colonies. For ascomata production, OA, MEA and CYA plates were incubated at 25 °C for up to four wks.
Light microscope preparations were made from 1 wk old colonies grown on MEA, for species which do not sporulate on MEA, other media (YES, OA or DG 18) were used for preparations and were indicated in species descriptions. Ascomata, asci and ascospores were observed from OA. Lactic acid (60 %) was used as mounting fluid. Alcohol (96 %) was used to remove excess conidia and prevent air bubbles. A Zeiss Stereo Discovery V20 dissecting microscope and Zeiss AX10 Imager A2 light microscope both equipped with a Nikon DS-Ri2 camera and software NIS-Elements D v4.50 were used to capture digital images. The temperature growth profile of the strains was studied on CYA. Strains were inoculated at one point in the centre of the plates and incubated at 18, 21, 24, 27, 30, 33, 37, 40, 45 and 50 °C for 5 d in darkness. Species in the clade Versicolores were studied extensively by Jurjevic et al. (2012) and are not included here.
Cryo Scanning Electron Microscopy (cryoSEM)
Mature ascomata were harvested from 30–50 day old cultures on OA. Ascomata were crushed and ascospores were picked using a dissecting needle and carefully transferred into distilled deionized water. A drop (5 μl) of this suspension was transferred to a polycarbonate membrane (1.0 Micron, 47 mm, GE Water and Process Technologies, Trevose, PA, USA). Polycarbonate membranes were placed on filter paper circles (0.7 mm, Schleicher & Schuell) to ensure that fluid was quickly absorbed through the pores of the membranes. The quick removal of fluid resulted in an equal distribution of ascospores and also more ascospores that could be viewed from the equatorial side as compared with passive evaporation of a droplet. The polycarbonate membranes with ascospore depositions were carefully cut out with a surgical knife and transferred to an aluminium stub. After drying at room temperature for one wk, the stubs were sputter-coated with gold three times for 30 s in a JEOL JFC-1300 Auto-fine coater and then viewed using a JEOL 5600LV scanning electron microscope (JEOL, Tokyo, Japan). Electron micrographs were acquired with the F4 scan at an acceleration voltage of 10 kV.
Extrolite analysis
Representatives of 48 section Nidulantes species were analysed for extrolite production using the method originally described by Frisvad and Thrane, 1987, Frisvad and Thrane, 1993 and modified by Smedsgaard (1997), and using the UHPLC-DAD method described in Kildgaard et al. (2014) and Klitgaard et al. (2014). Strains were inoculated and incubated on CYA and YES agar for 7 d at 25 °C in darkness and subsequently three plugs were extracted as described by Smedsgaard (1997). Species in clade Versicolores (= section Versicolores) and currently described A. croceus and A. askiburgiensis (Hubka et al. 2016) are not included.
Results
Phylogeny
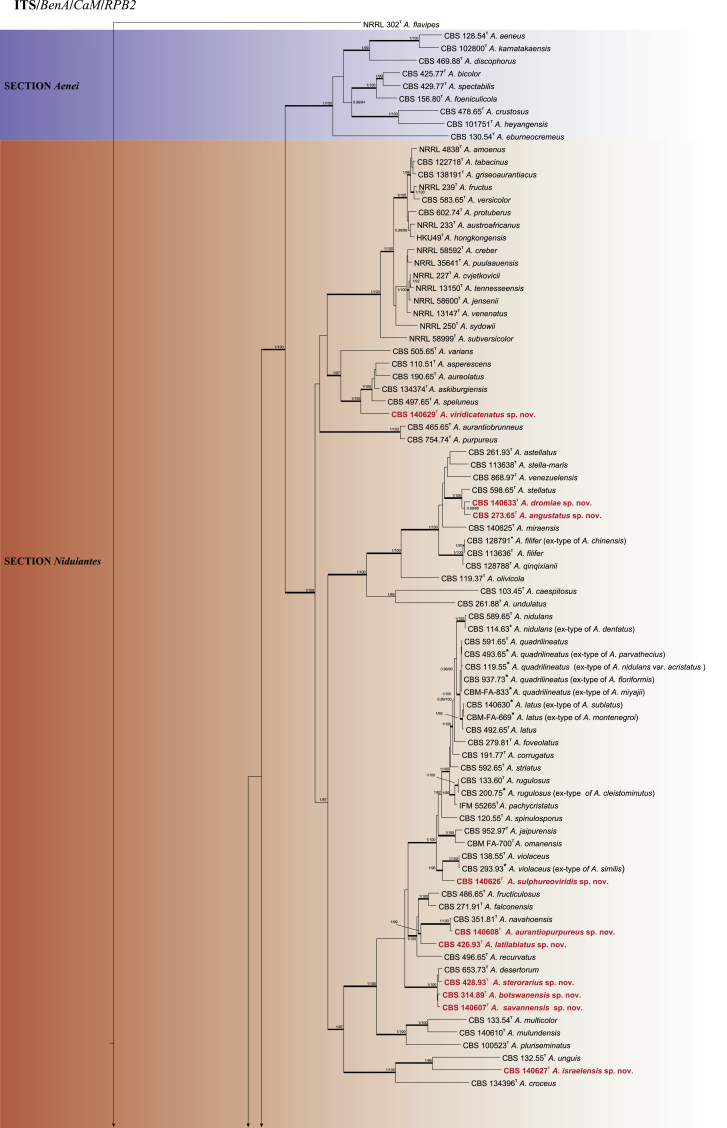
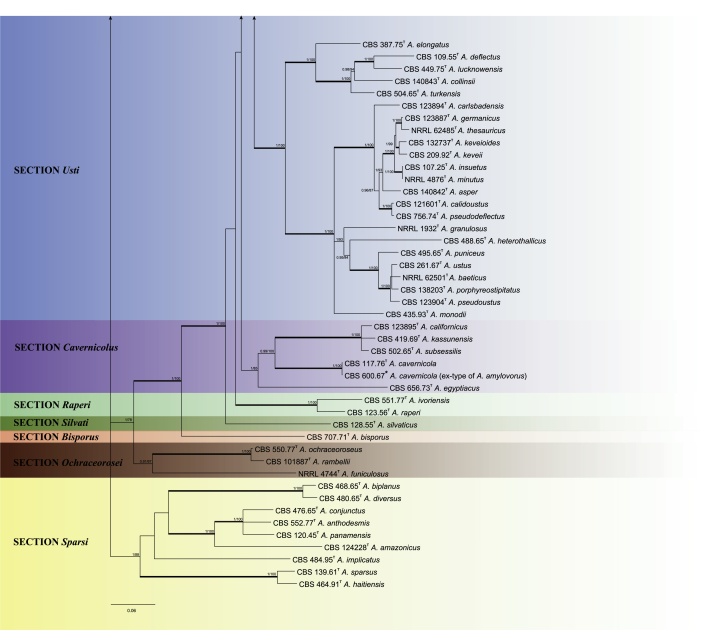
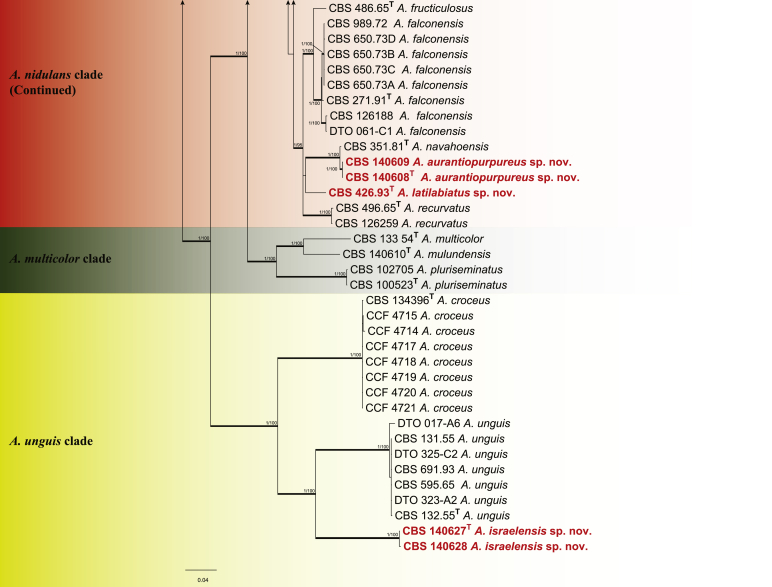
The phylogenetic relationships among Aspergillus subgenus Nidulantes species were studied using concatenated sequence data of four loci: ITS, BenA, CaM and RPB2. In total, 130 ex-type strains were included in the analysis and the total length of the aligned data set was 2483 characters, containing 498, 527, 537 and 921 bp for ITS, BenA, CaM and RPB2 respectively. For Bayesian analyses, GTR+G model was used for ITS, BenA, CaM and RPB2. Fig. 1 shows the results of the analysis and reveals the presence of nine lineages in subgenus Nidulantes. These lineages are treated here as sections, namely Aenei, Nidulantes, Usti, Raperi, Silvati, Bispori, Ochraceorosei, Sparsi and the newly introduced section Cavernicolus. The members of sections Nidulantes and Versicolores form a well-supported group (1 pp, 100 % ML), which is in agreement with previous studies (Peterson, 2008, Peterson et al., 2008). On the basis of the phylogenetic analysis we follow Hubka et al. (2016) and include Versicolores within section Nidulantes. Based on our results, 65 species are well resolved in section Nidulantes. Section Cavernicolus (1 pp, 85 % ML) contains five species previously assigned to section Usti, namely A. californicus, A. cavernicola, A. egyptiacus, A. kassunensis and A. subsessilis. Most of species in this section produce short conidiophores, except A. californicus, which produces long, light brown conidiophores, resembling typical section Usti species (Samson et al. 2011). Aspergillus funiculosus included in section Sparsi by Peterson (2008), clusters with A. ochraceorosues and A. rambellii with poor bootstrap and Bayesian statistics.
Fig. 1.
Phylogenetic tree of subgenus Nidulantes inferred from concatenated 4 loci: ITS, BenA, CaM and RPB2. Branches with values more than 1 pp and 95% bs are thickened. The phylogram is rooted with Aspergillus flavipes (NRRL 302T).
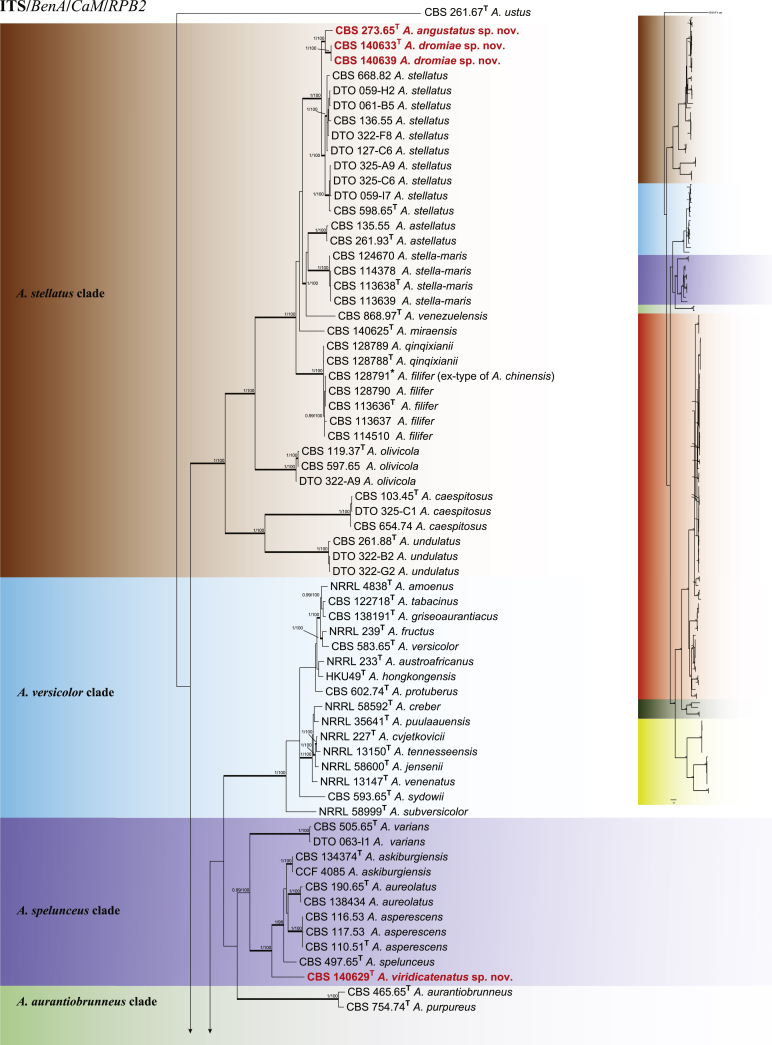
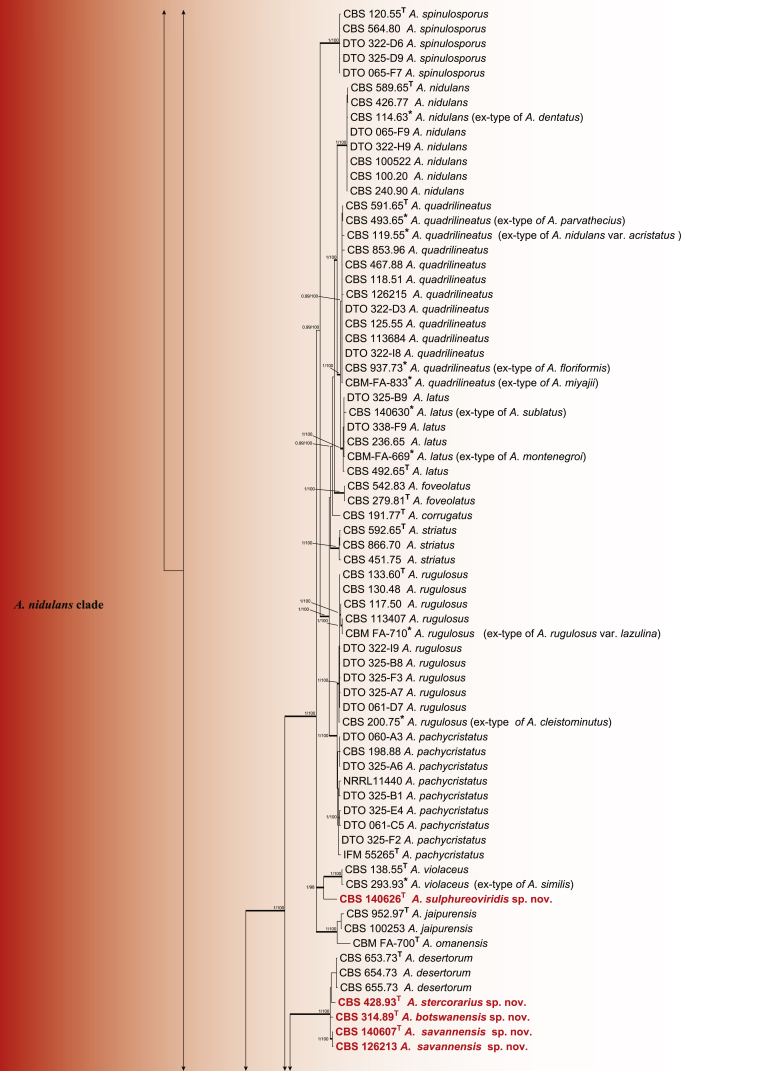
To define relationships within section Nidulantes, an aligned concatenated data set with a total length of 2,400 characters (ITS 533; BenA 472; CaM 505; RPB2 890 bp) was analysed. For Bayesian analysis, GTR+G was used for BenA, CaM and ITS and K2P+G for RPB2. Members of section Nidulantes are resolved into seven well supported clades (Fig. 2). The A. nidulans clade contains 23 species including the type species of section Nidulantes-A. nidulans. Aspergillus dentatus is phylogenetically identical with A. nidulans and therefore considered a synonym. Similarly, four species (A. parvathecius, A. nidulans var. acristatus, A. floriformis and A. miyajii) are synonymised with A. quadrilineatus. Aspergillus sublatus and A. montenegroi are synonymised with A. latus; A. rugulosus var. lazulina and A. cleistominutus are synonymised with A. rugulosus; A. similis is synonymised with A. violaceus. The relation between the clades A. aurantiobrunneus, A. spelunceus and A. versicolor are uncertain, A. aurantiobrunneus clade clusters outside clades A. spelunceus and A. versicolor in the subgenus phylogeny (Fig. 1), while it clusters with A. spelunceus clade in the section phylogeny (Fig. 2), both of the phylograms do not have bootstrap and Bayesian statistics. The A. stellatus clade contains species with either stellate or appendaged ascospores. Aspergillus chinensis is considered a synonym of A. filifer based on phylogenetic and morphological characters as suggested by Matsuzawa et al. (2012) and Hubka et al. (2016).
Fig. 2.
Phylogenetic tree of section Nidulantes inferred from concatenated 4 loci: ITS, BenA, CaM and RPB2. Branches with values more than 1 pp and 95% bs are thickened. The phylogram is rooted with Aspergillus ustus (CBS 261.67T).
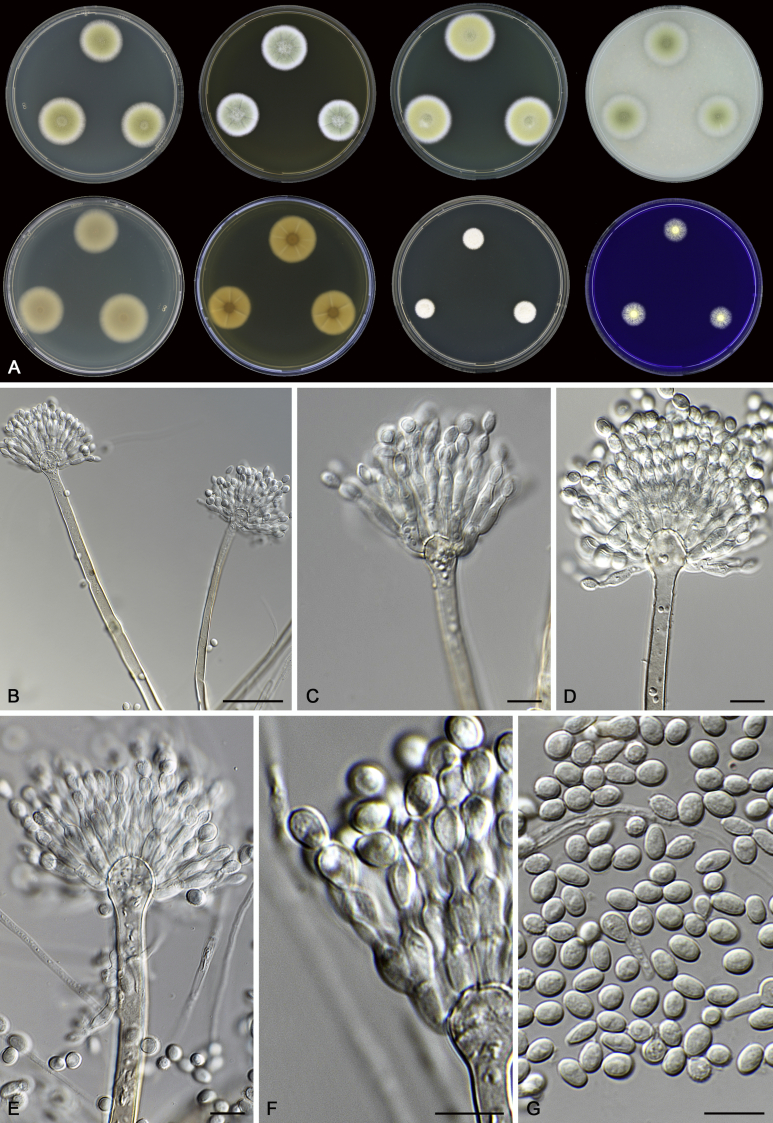
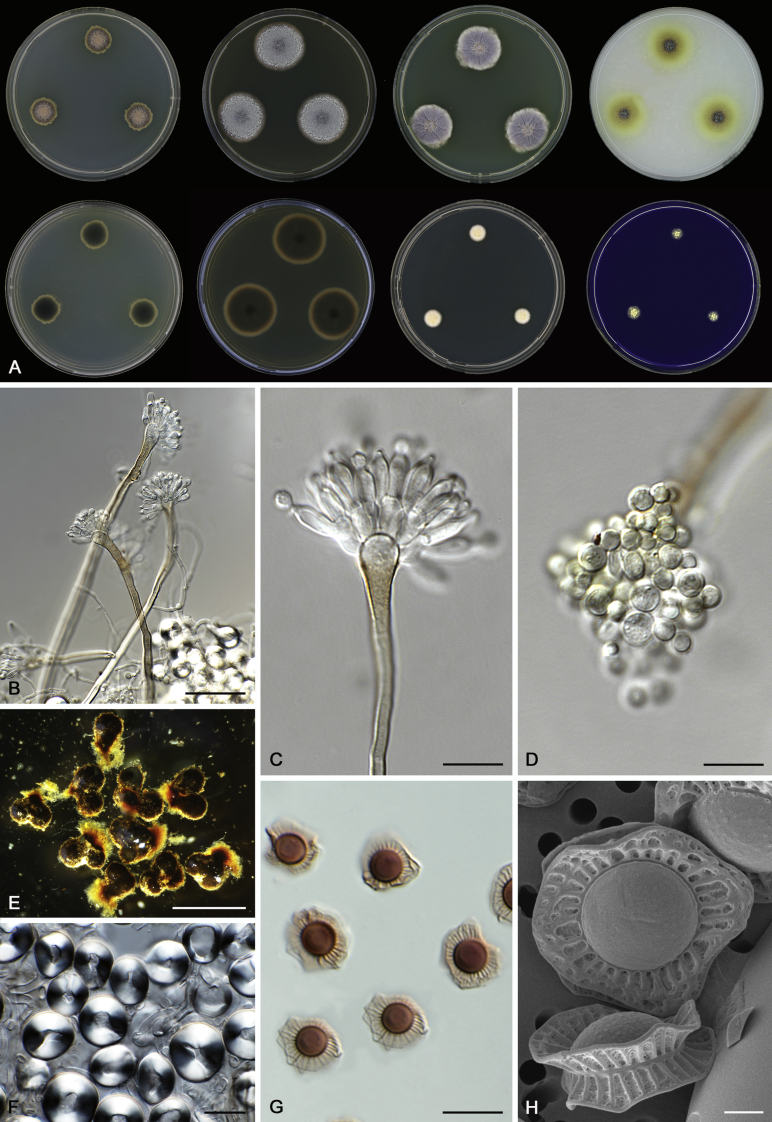
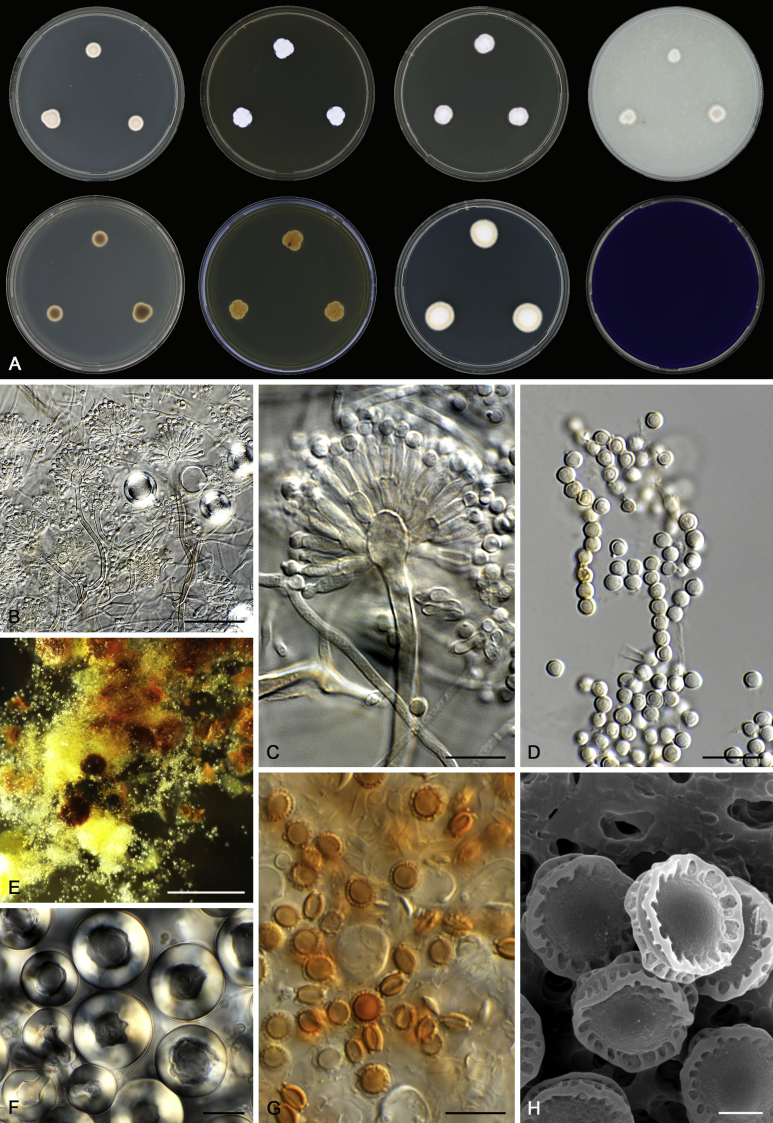
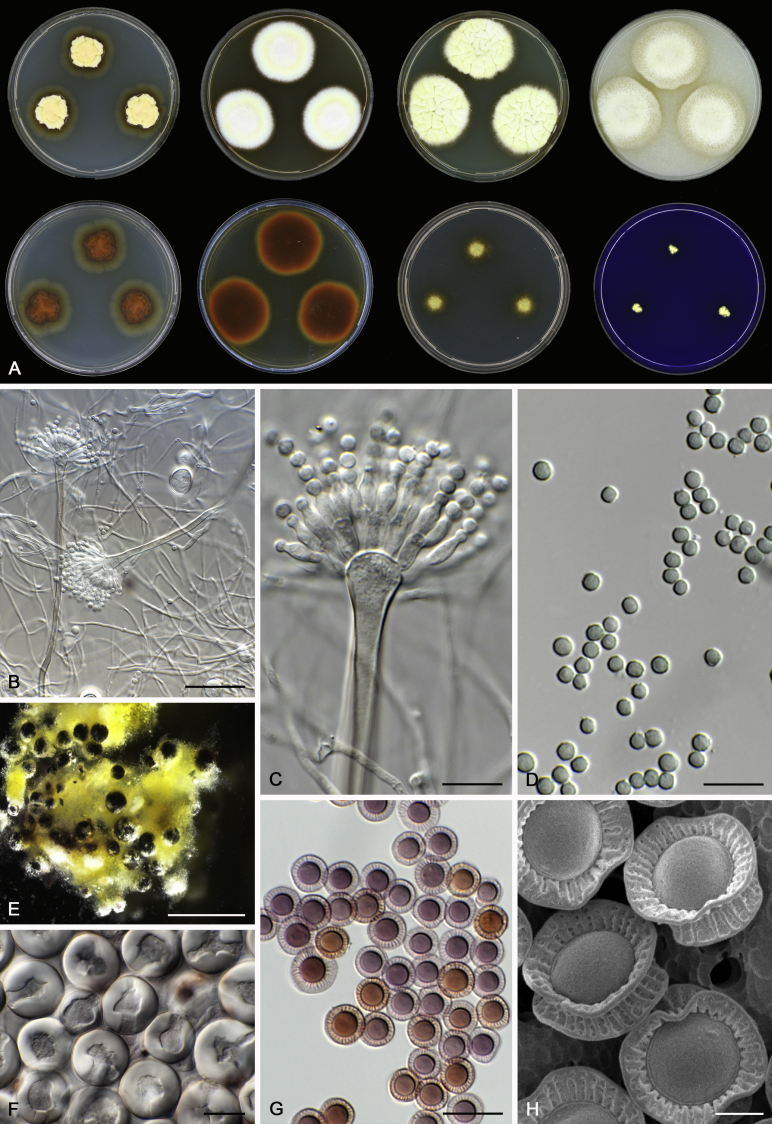
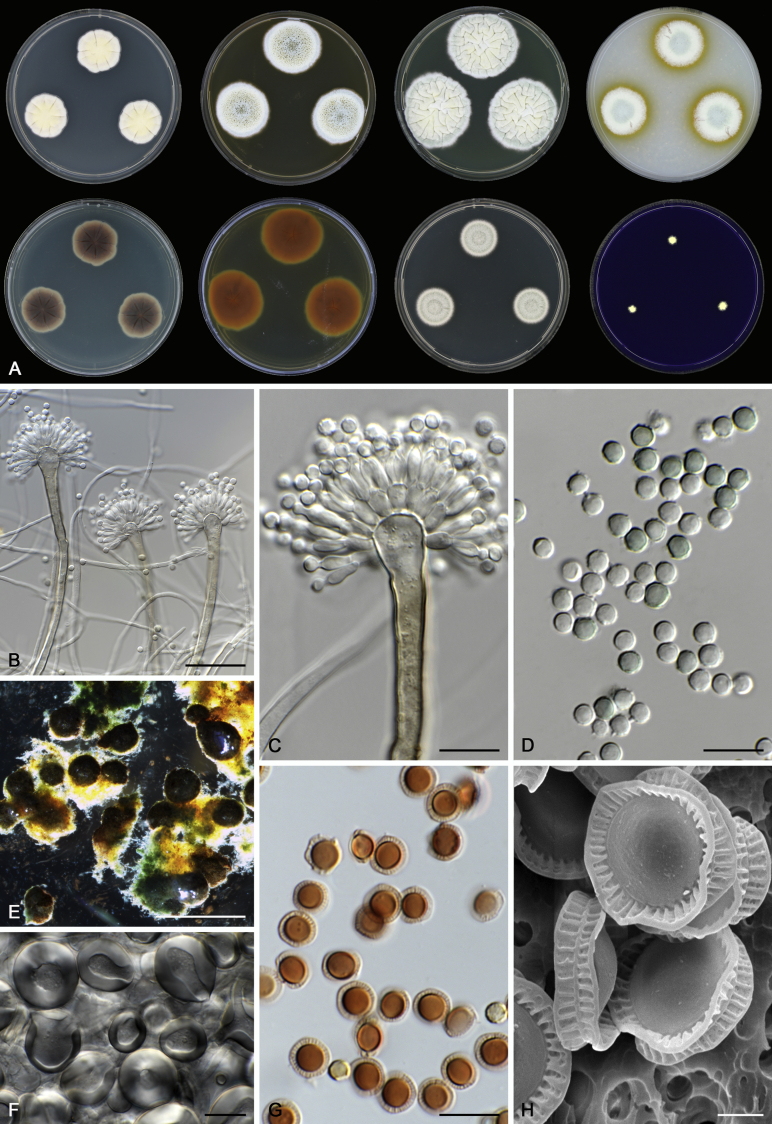
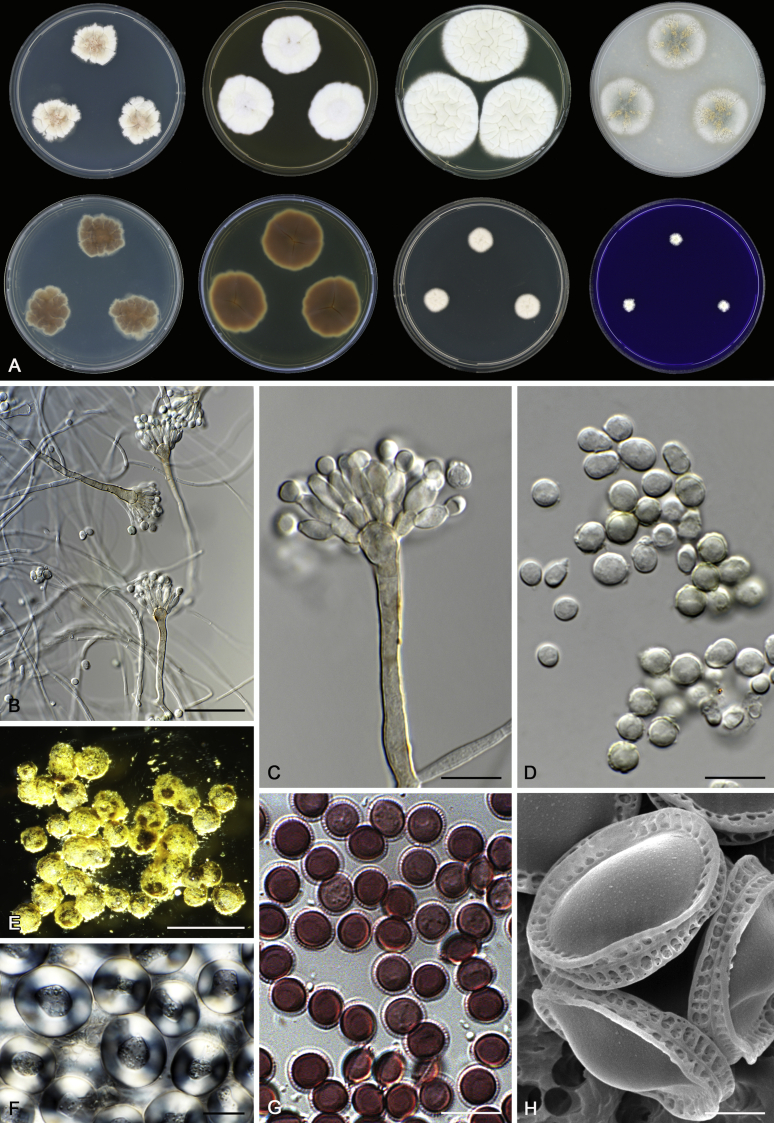
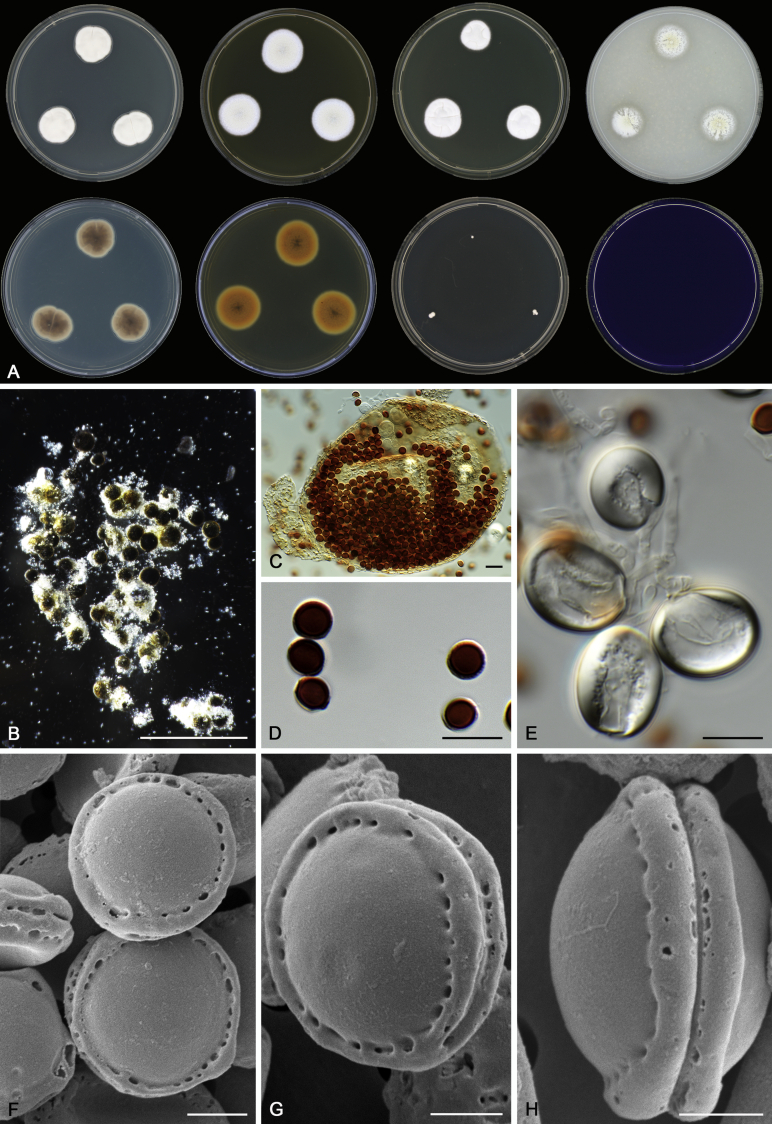
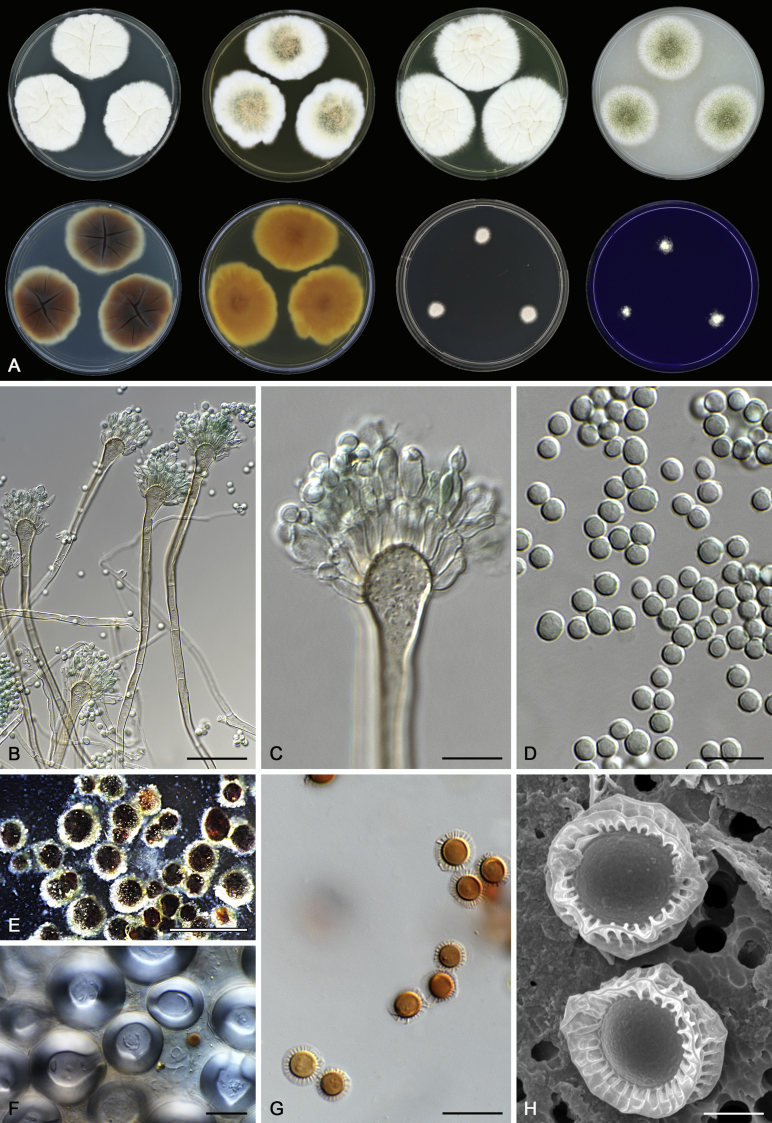
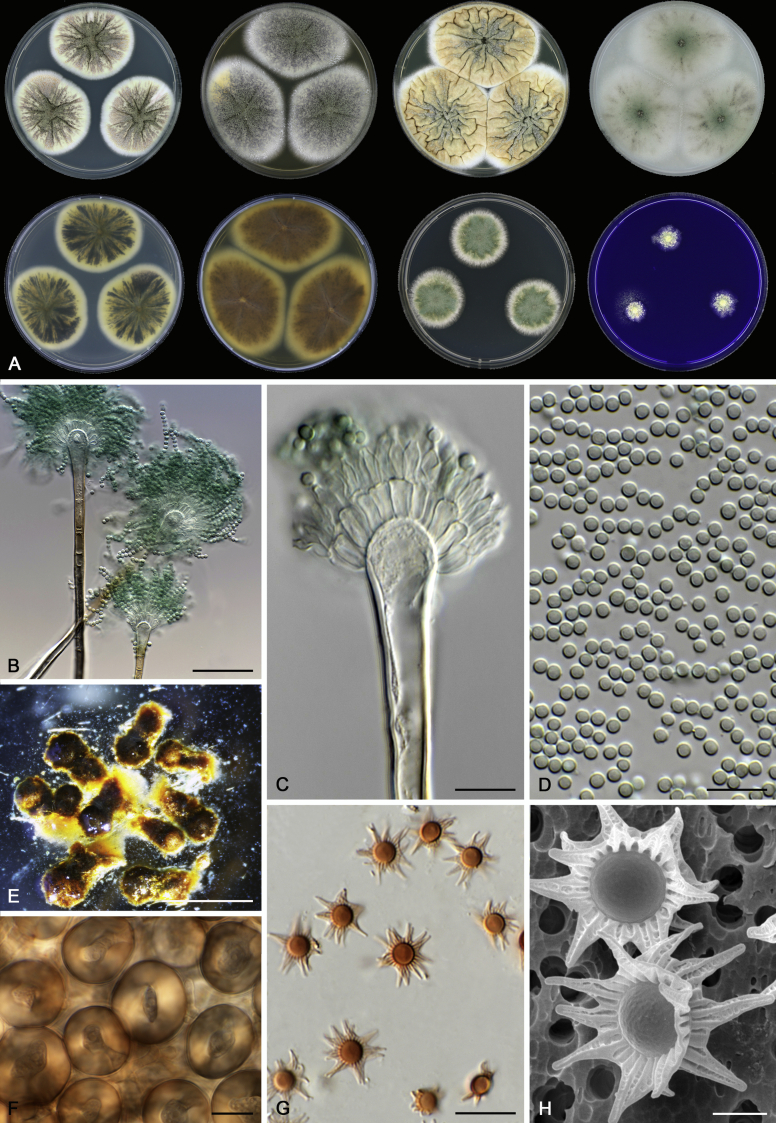
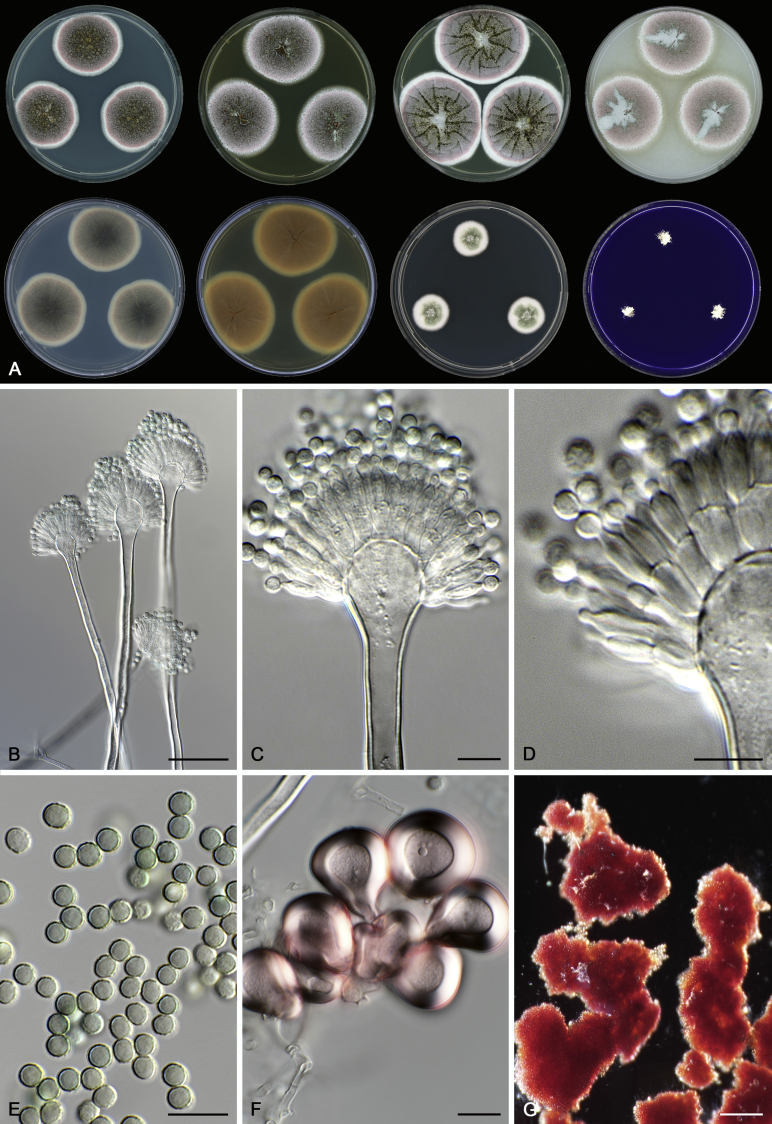
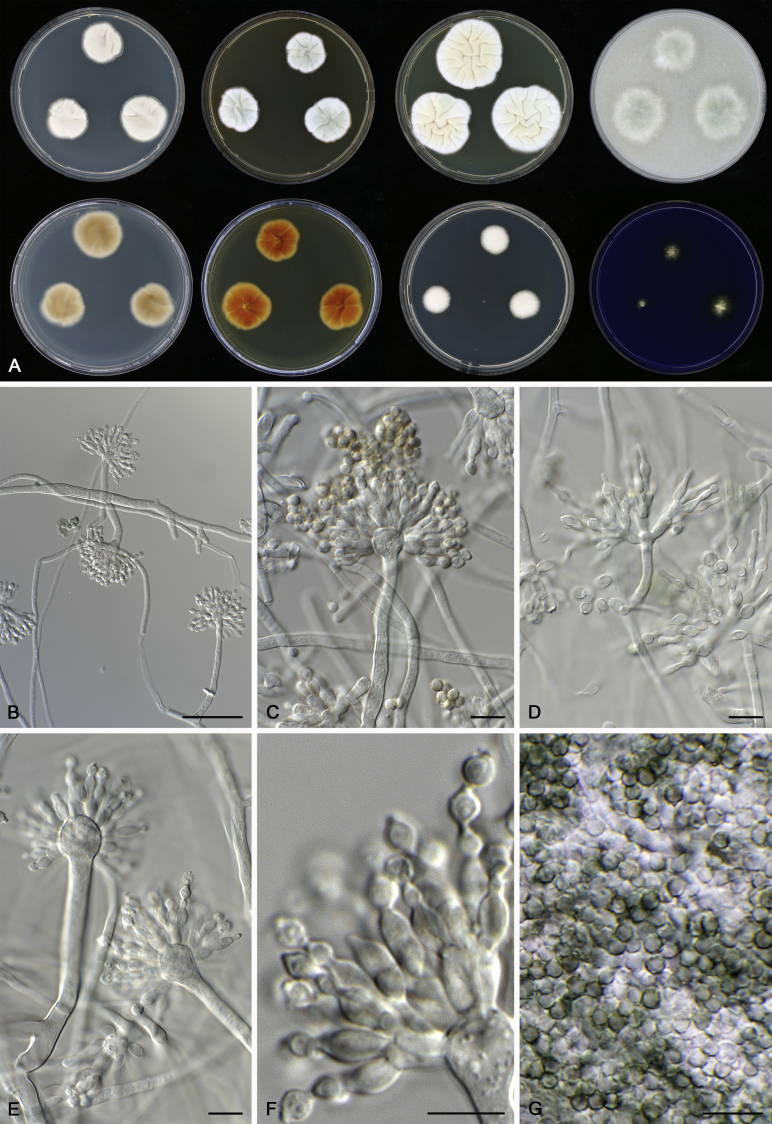
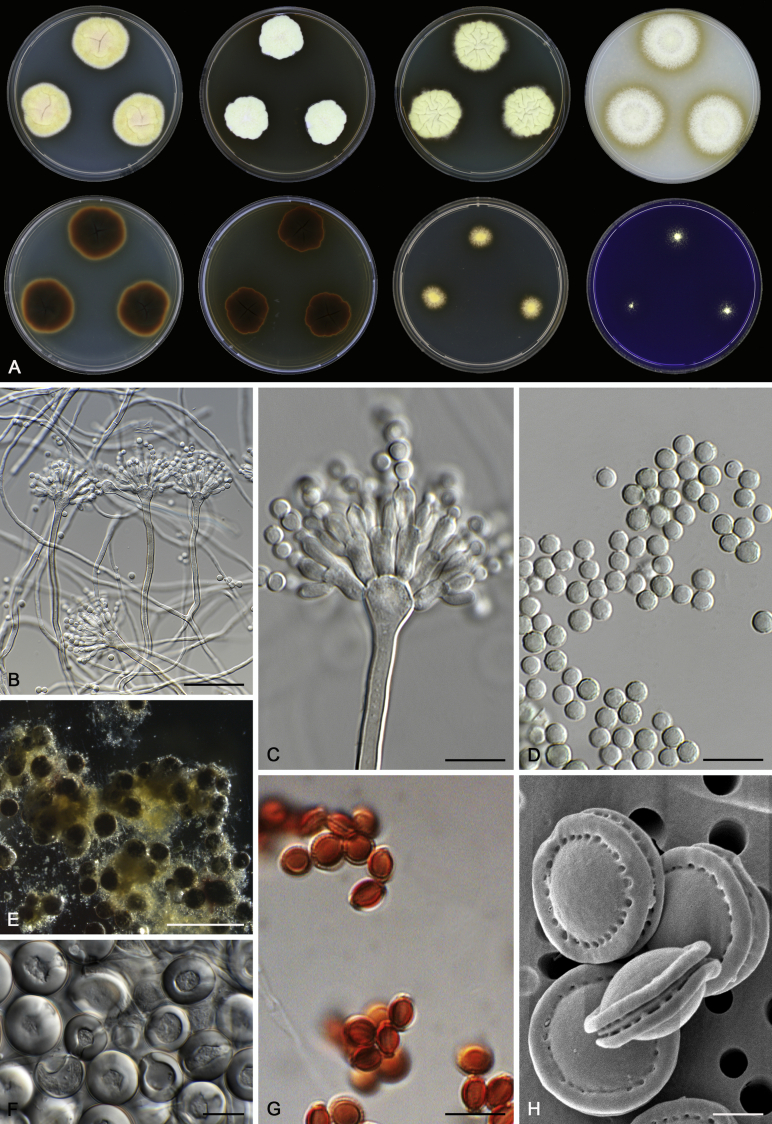
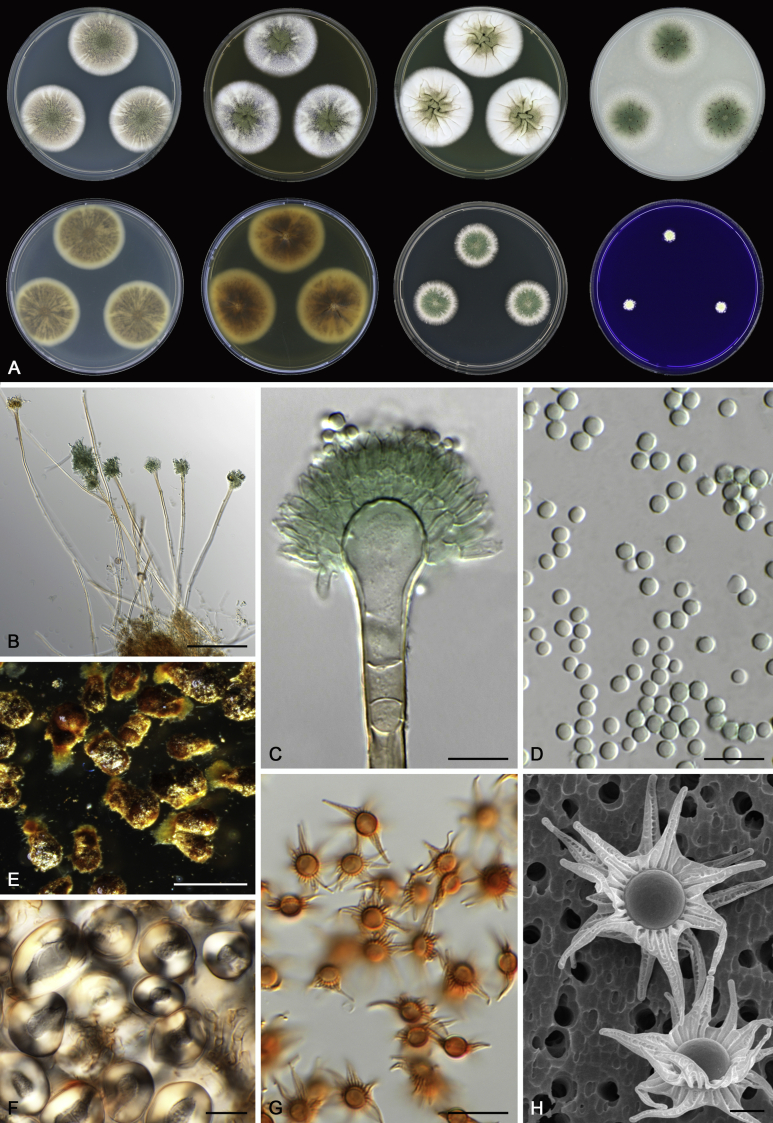
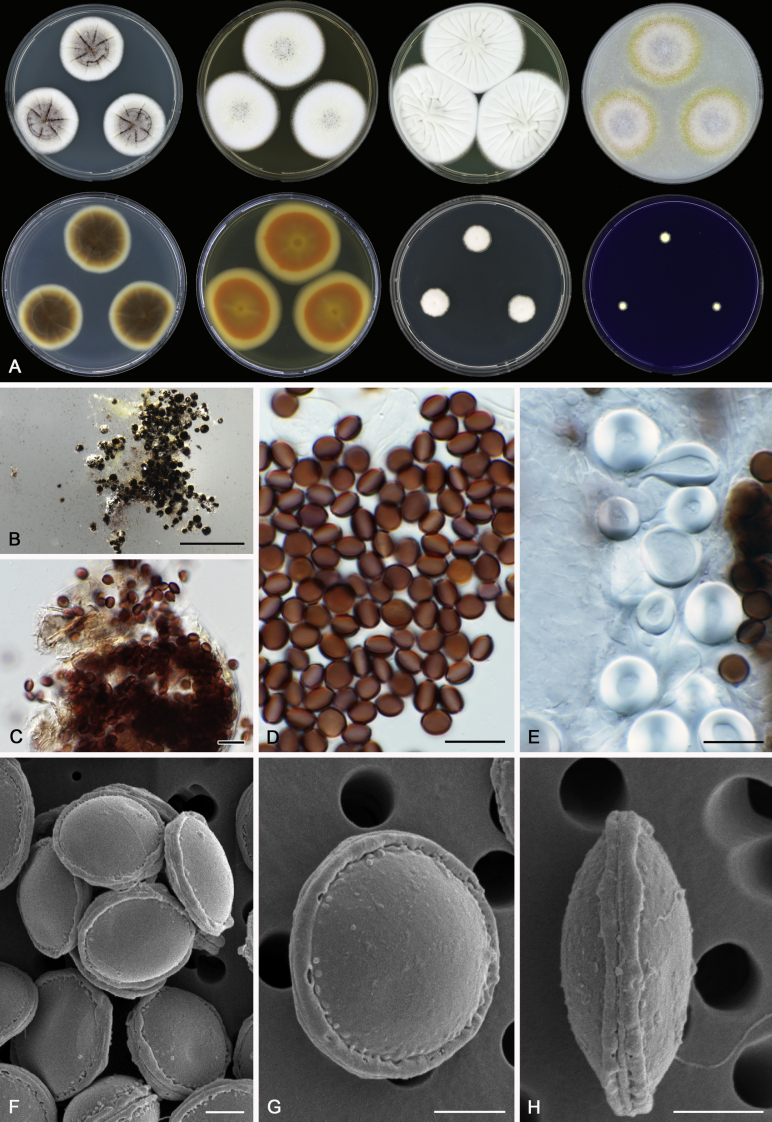
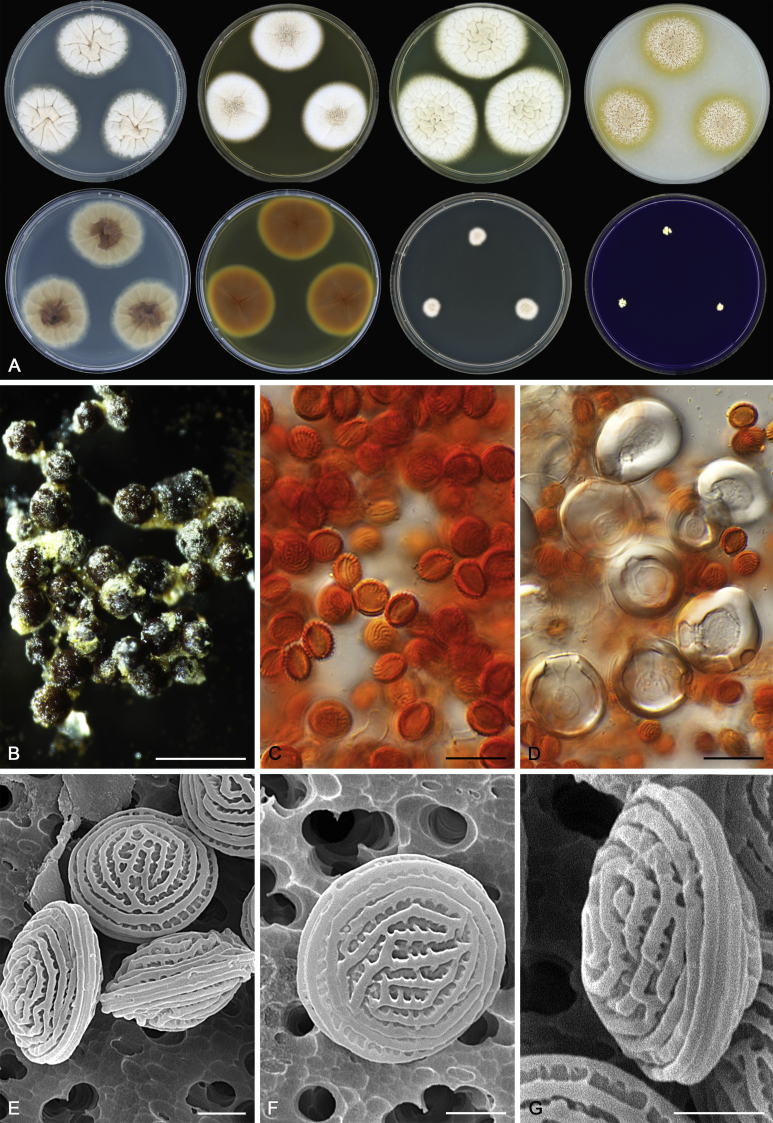
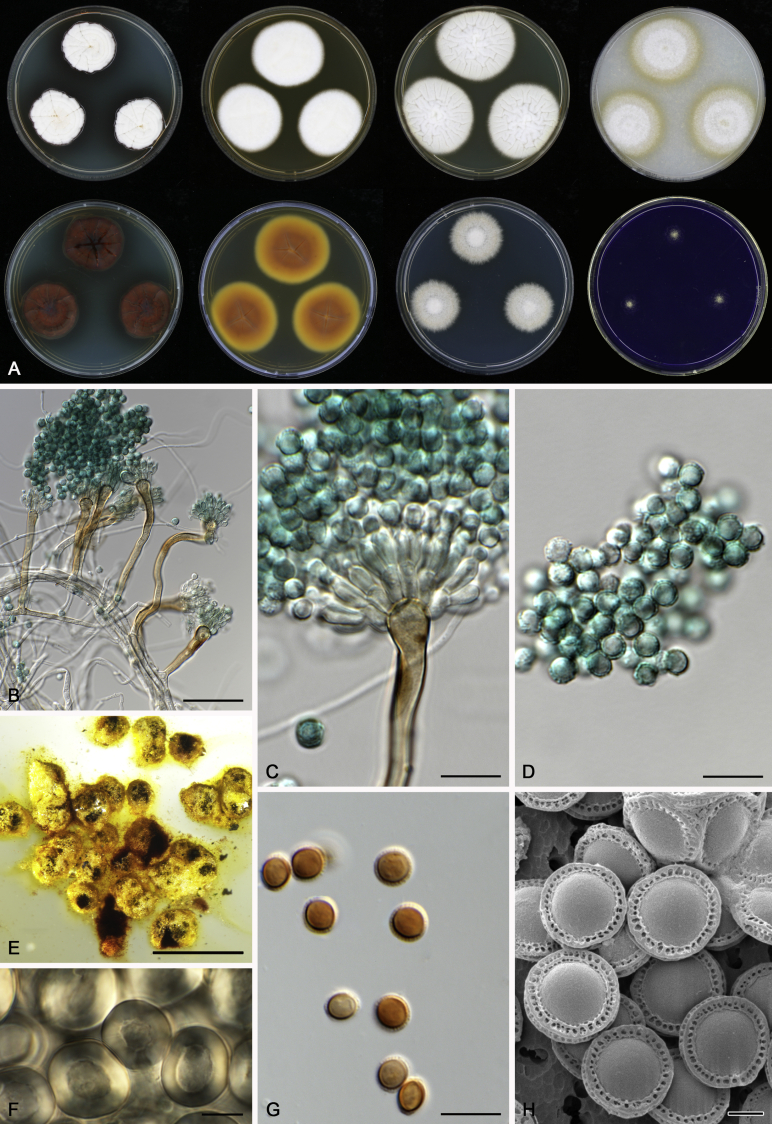
Morphology
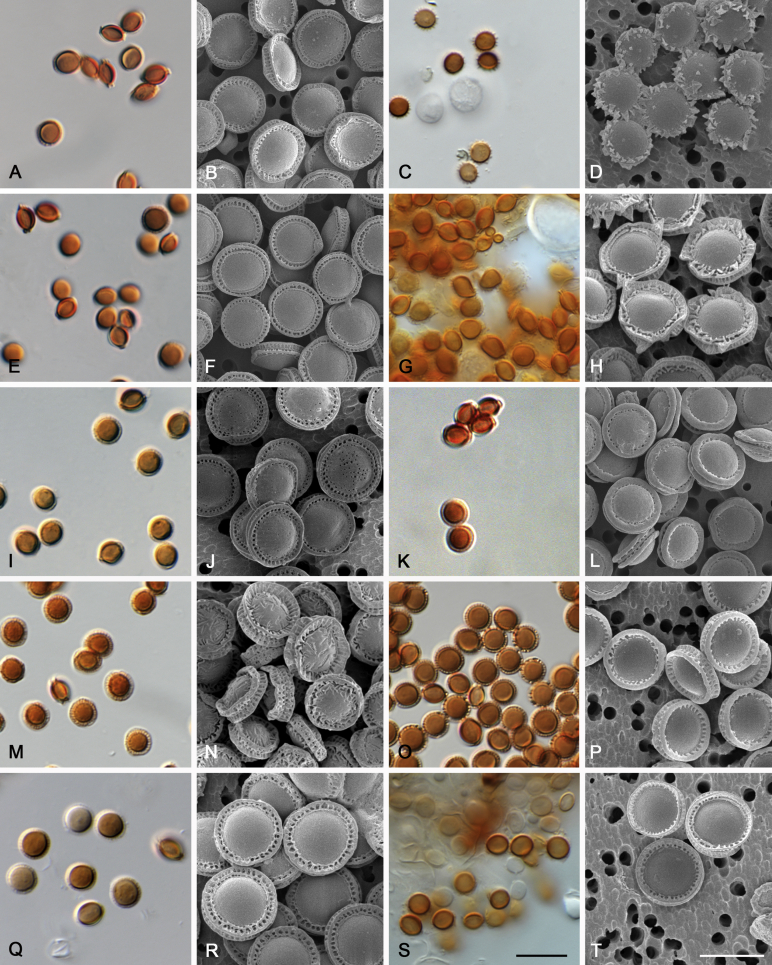
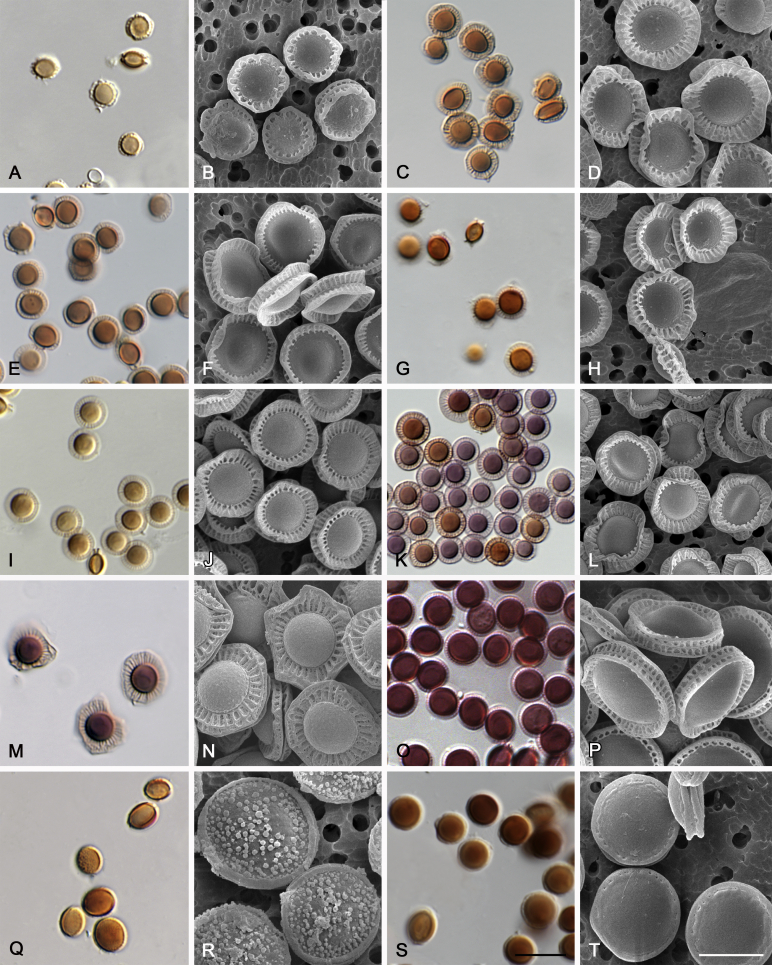
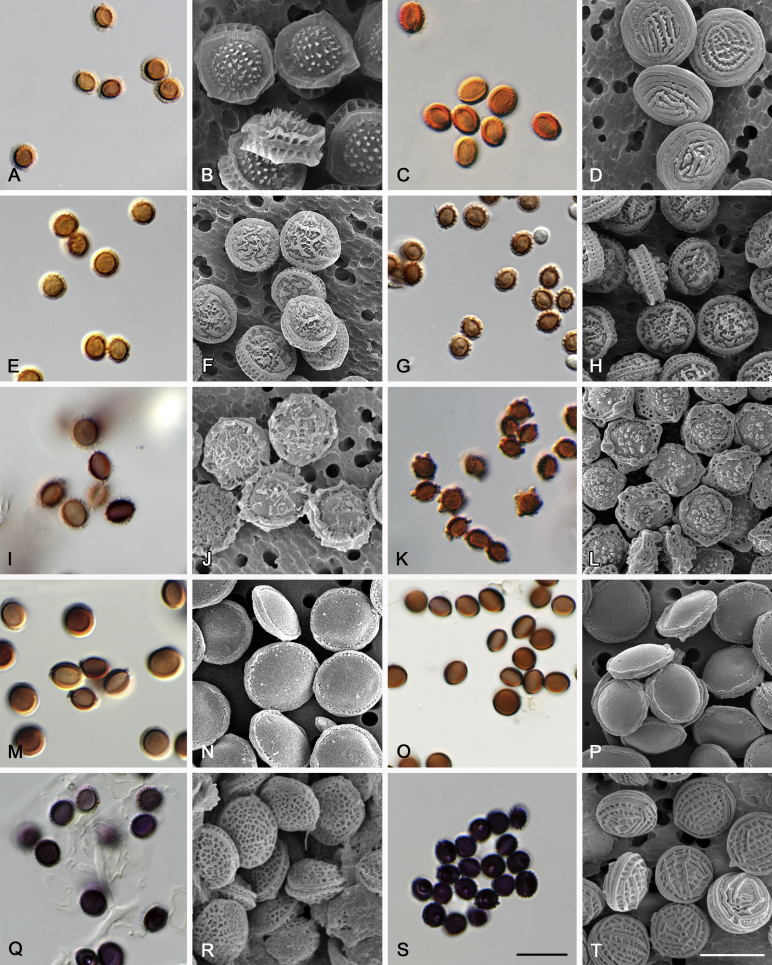
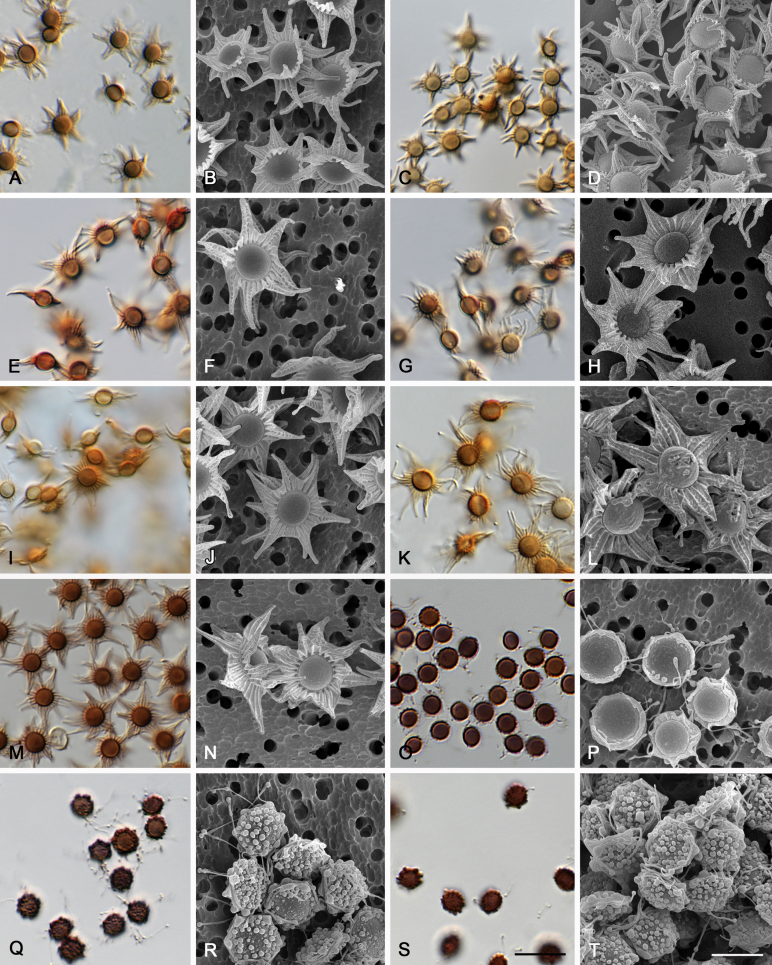
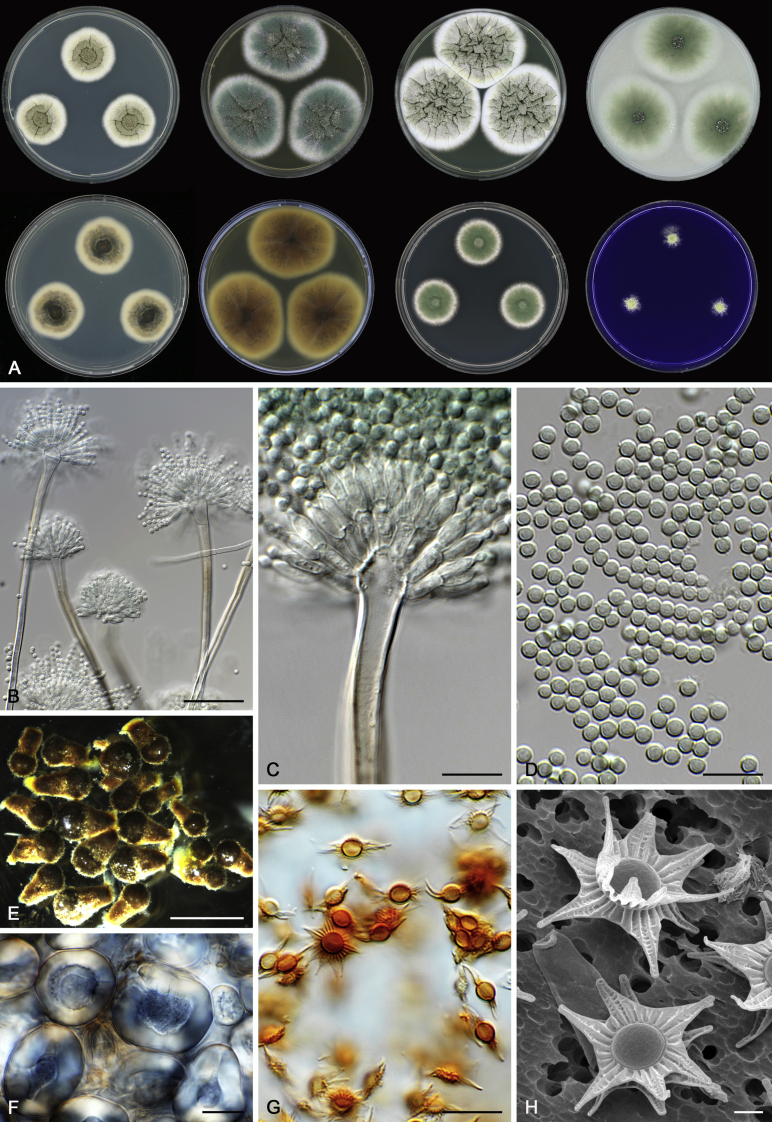
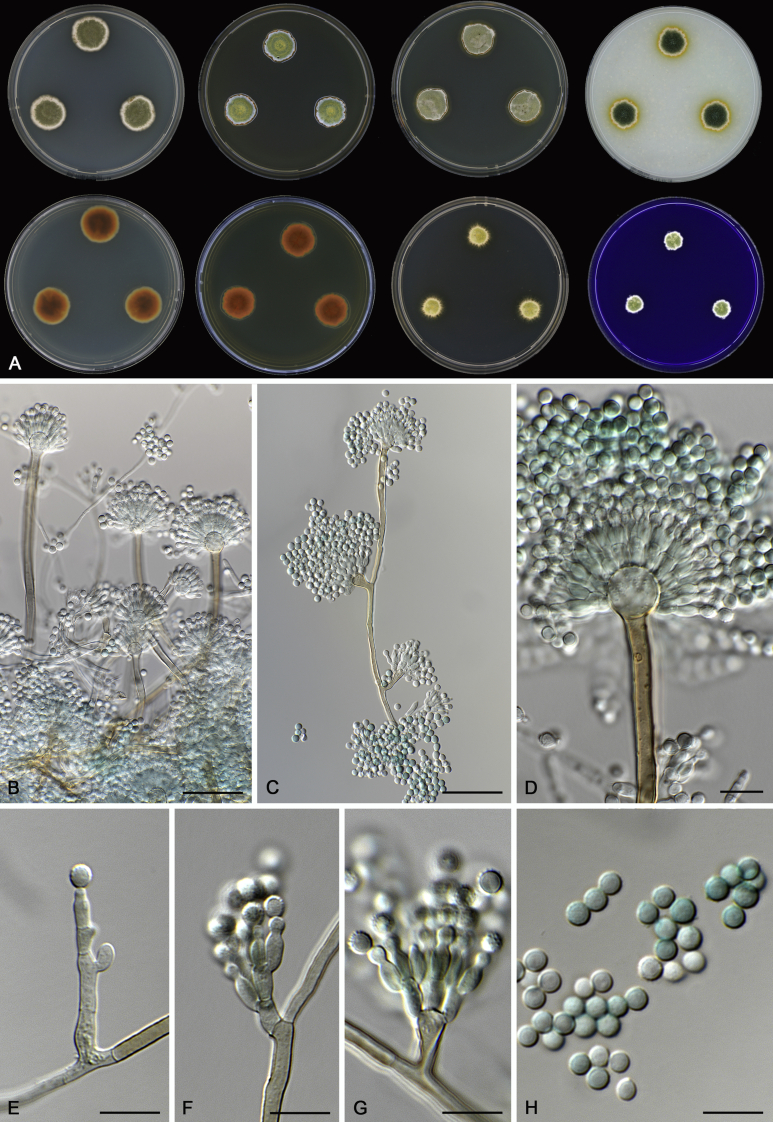
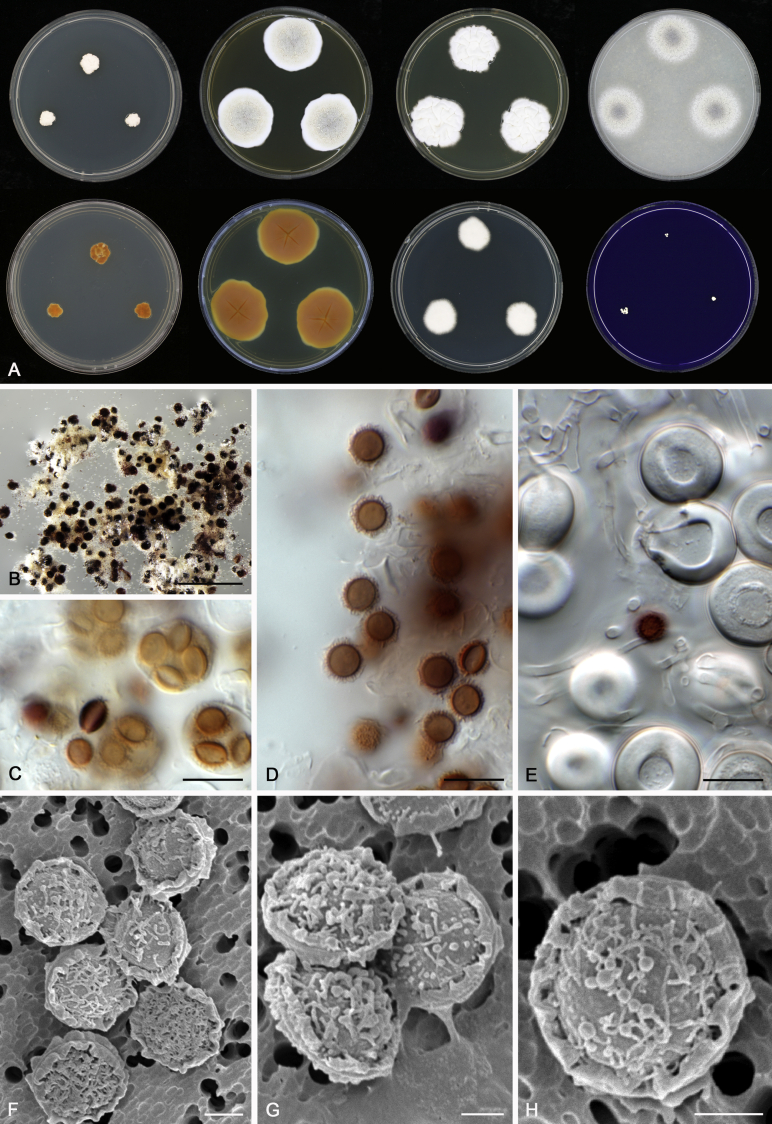
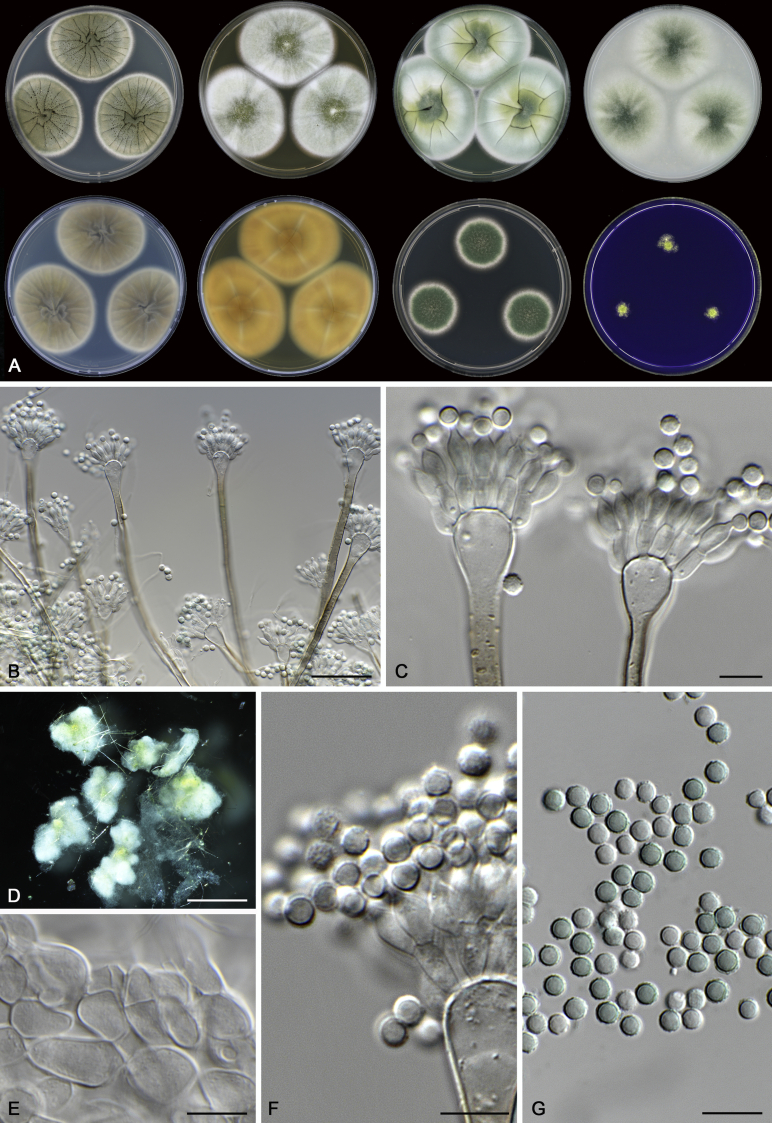
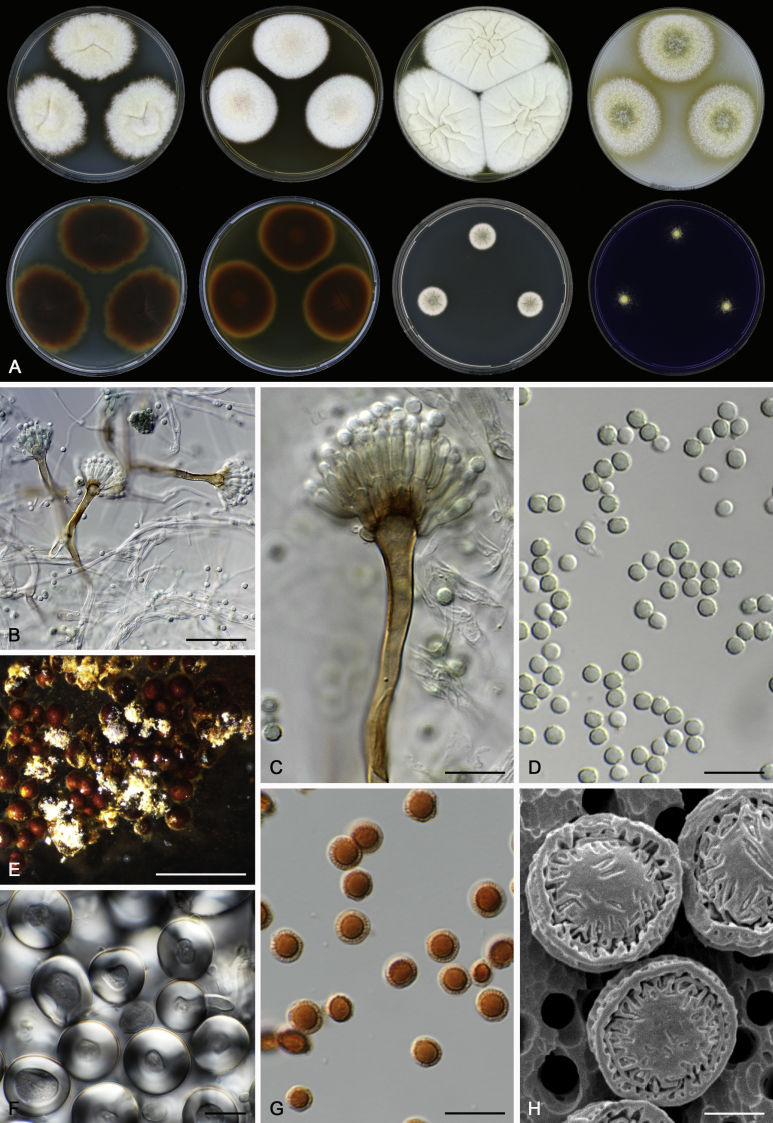
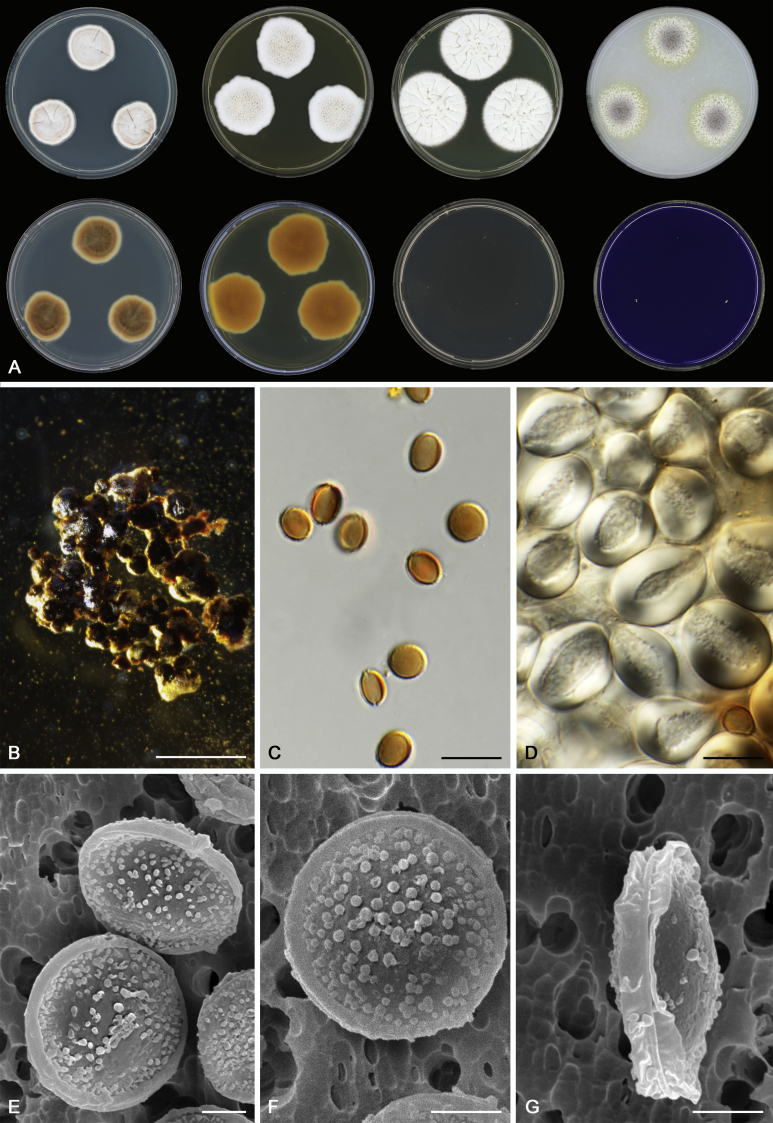
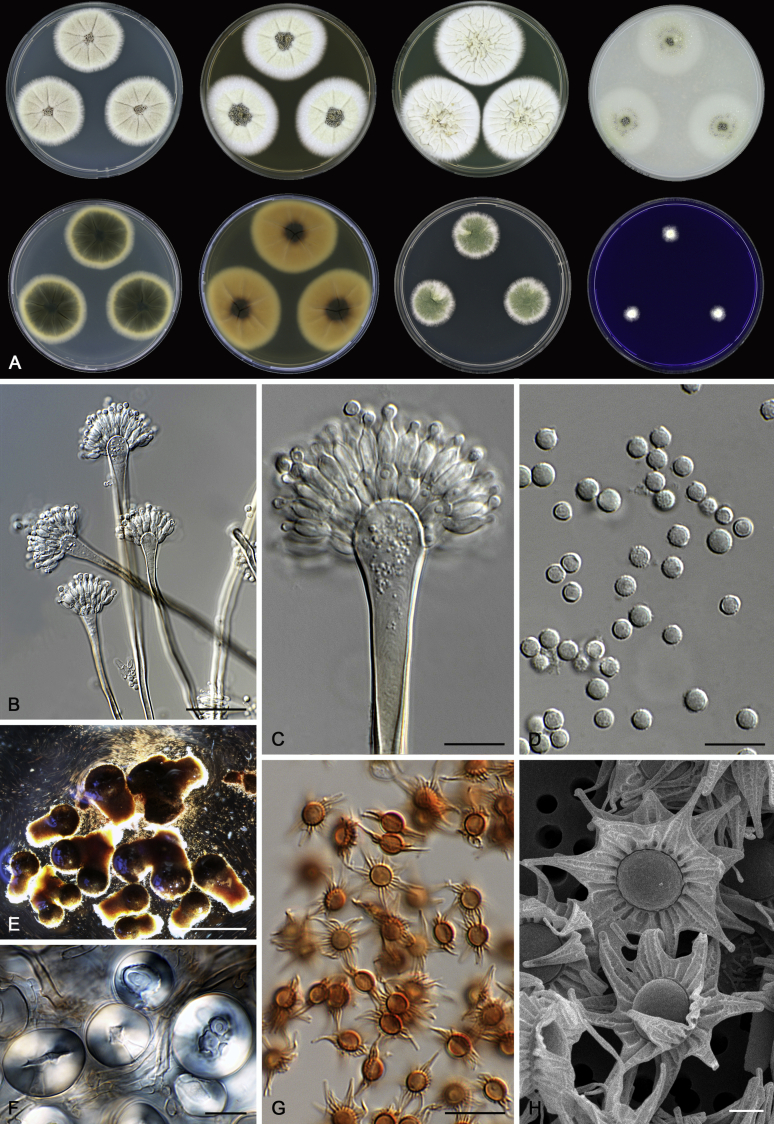
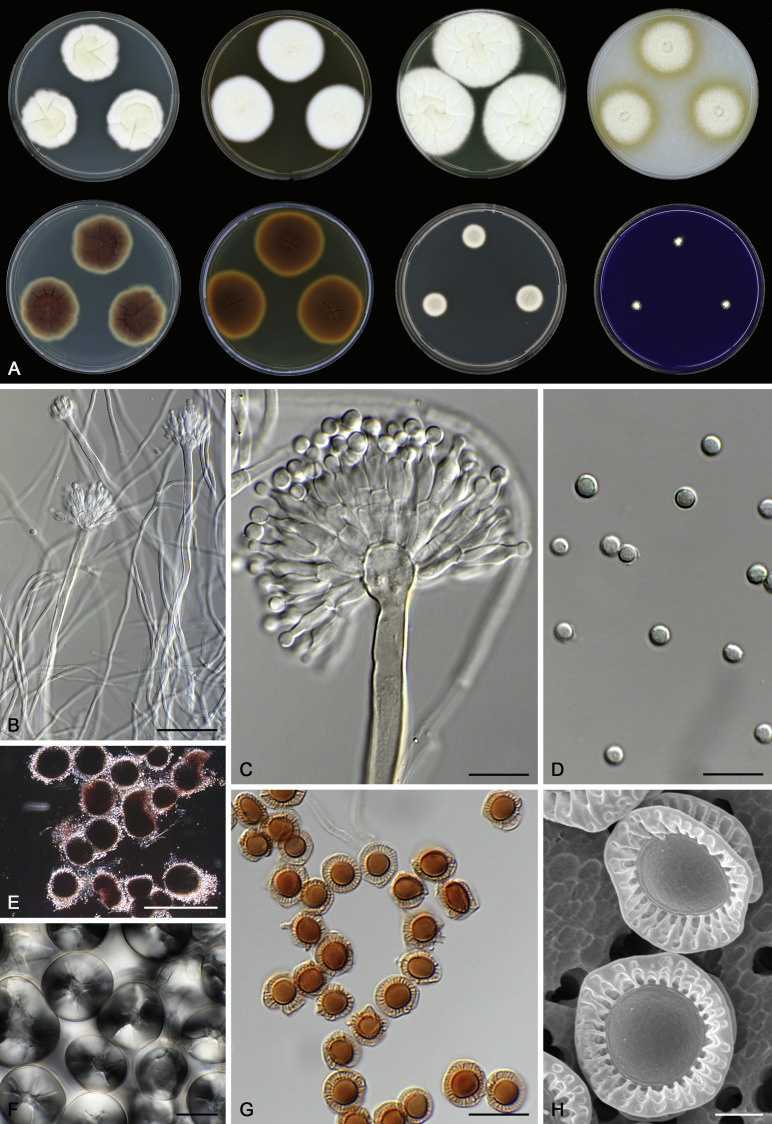
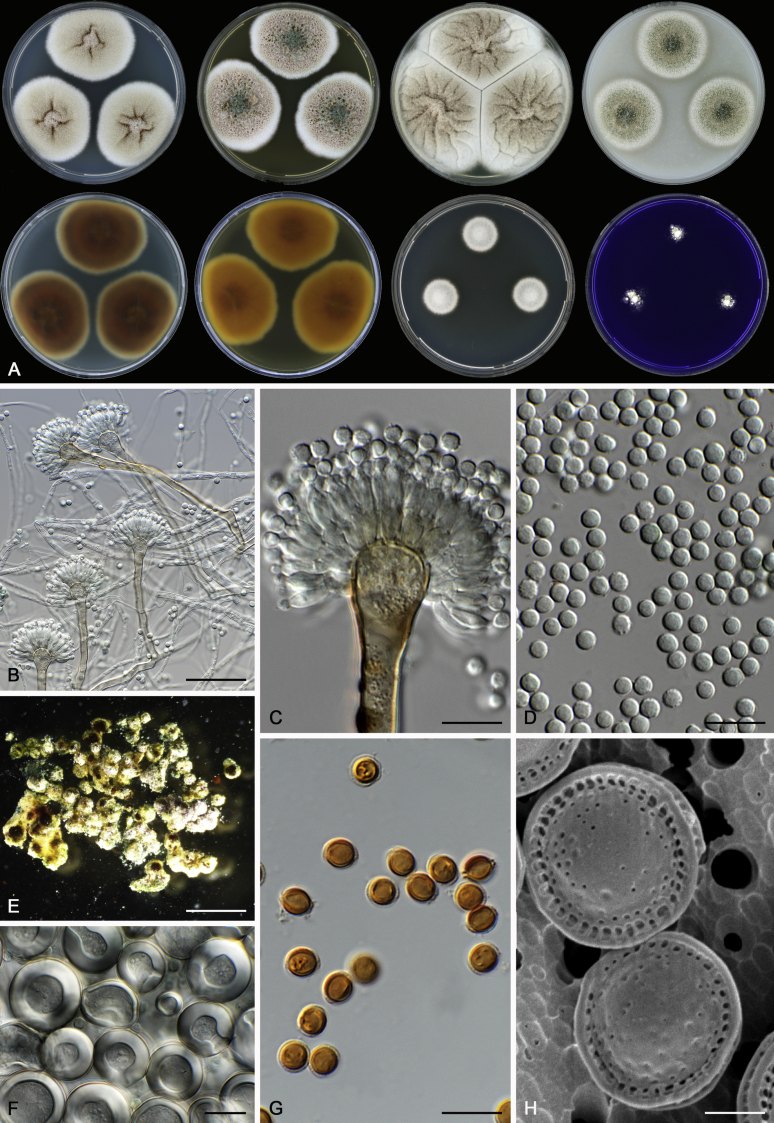
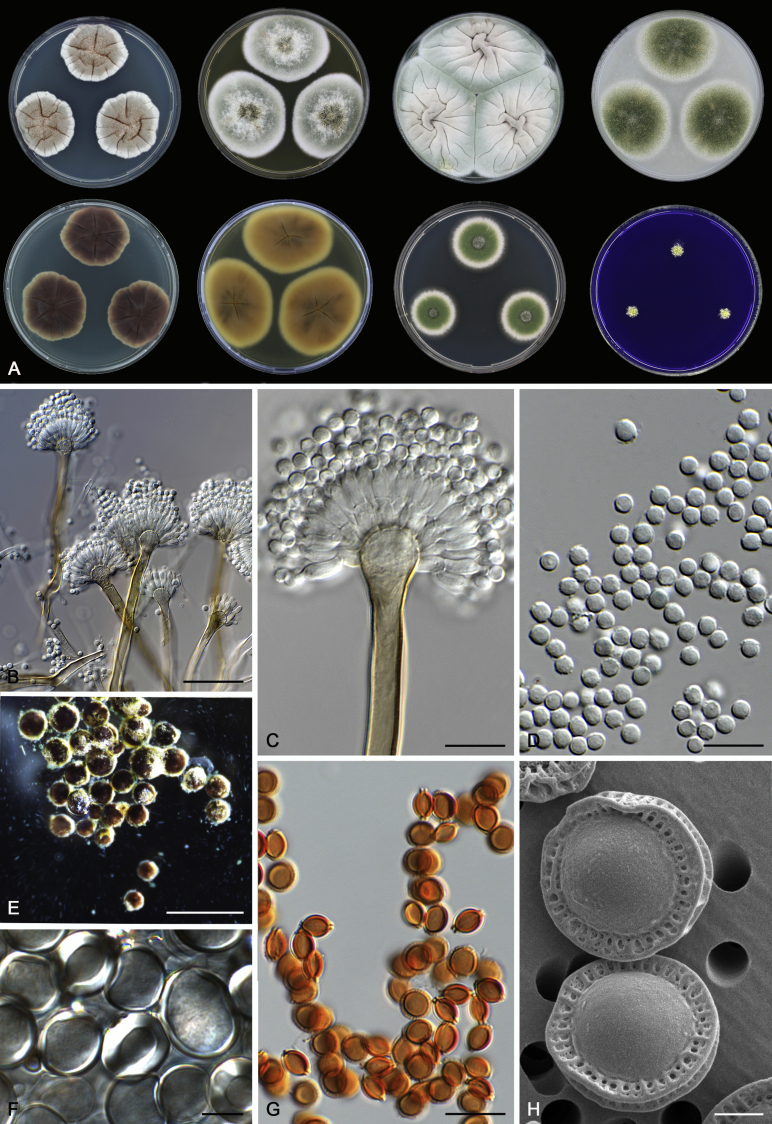
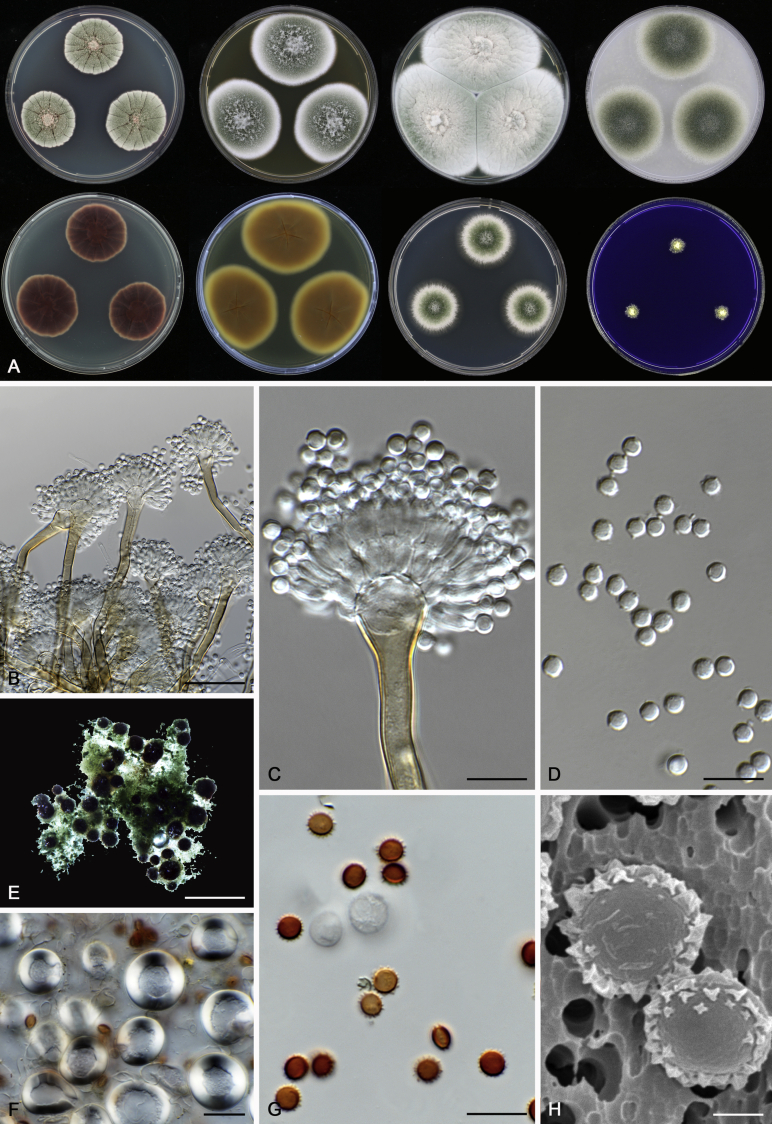
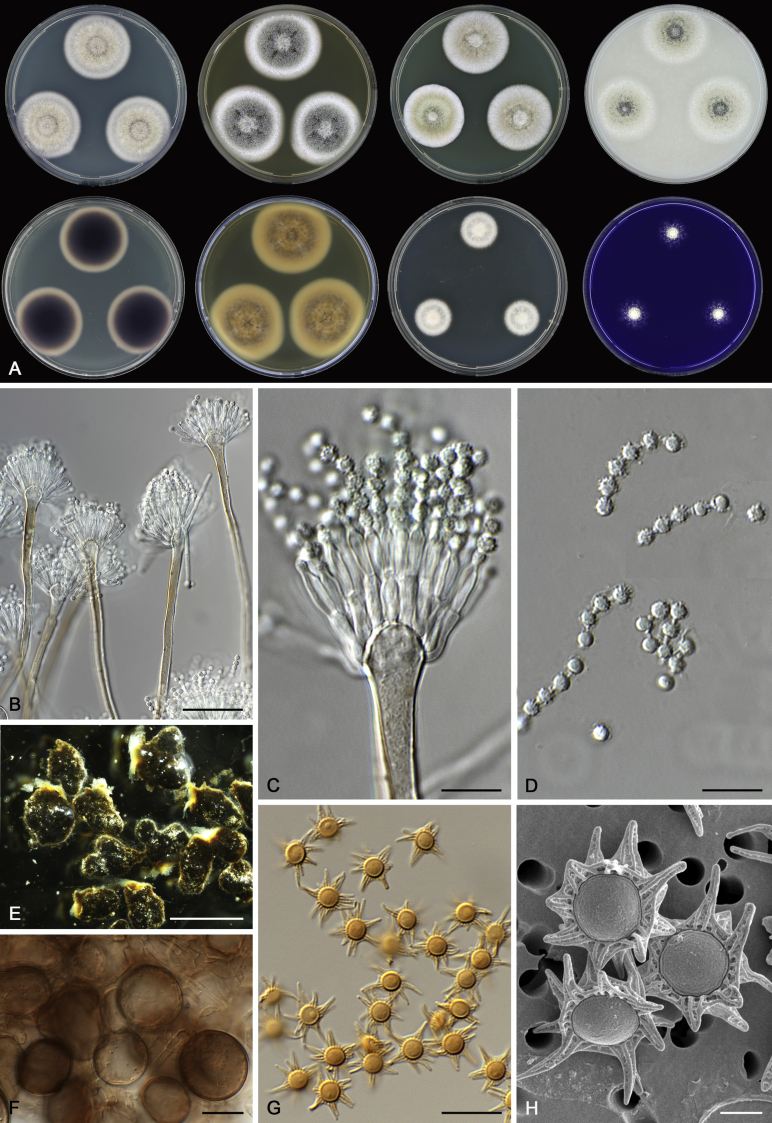
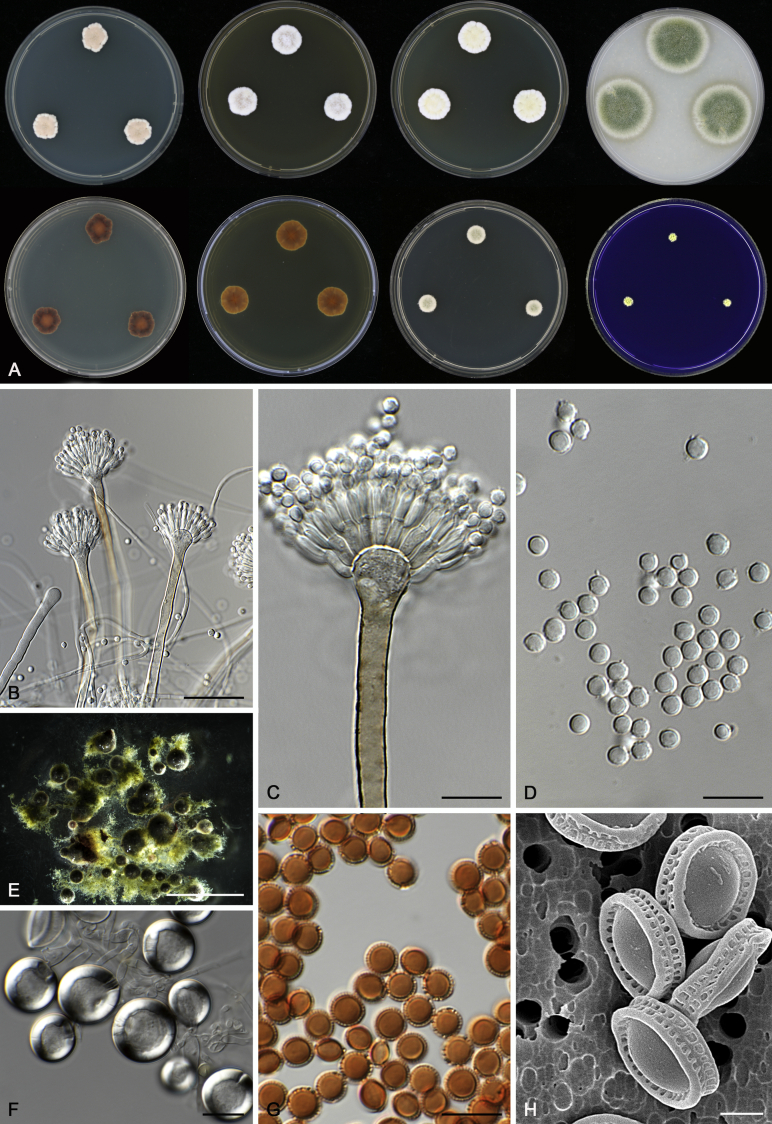
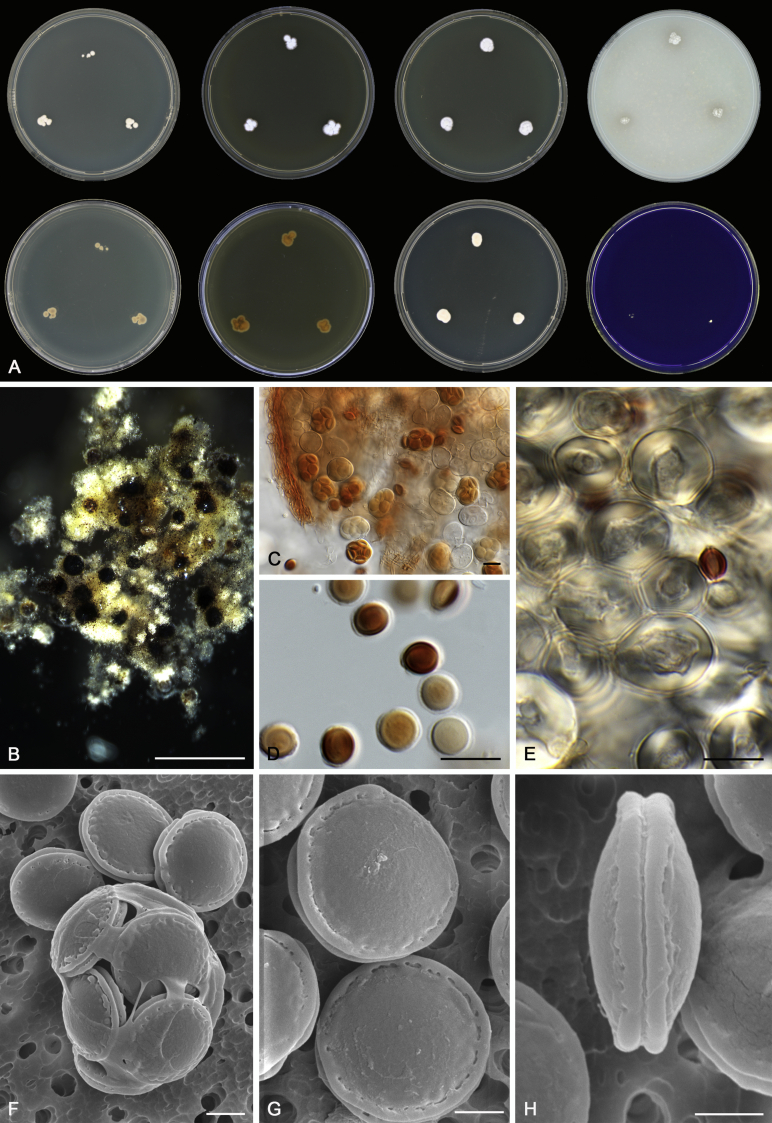
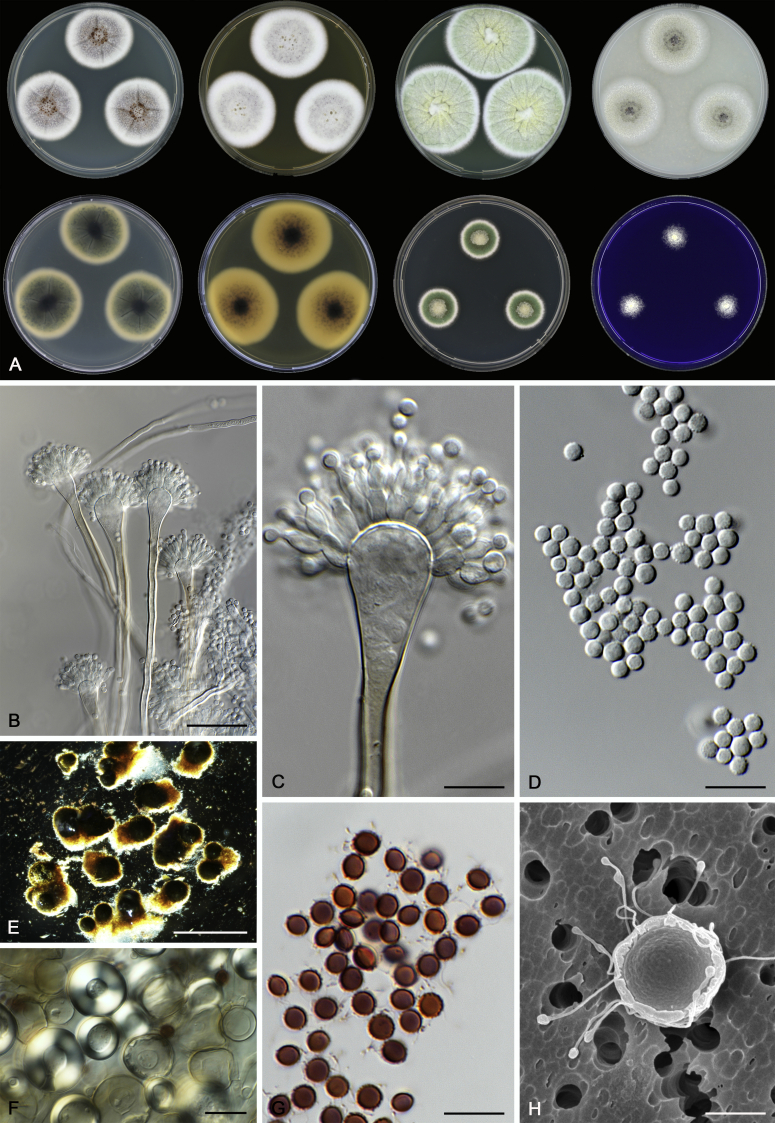
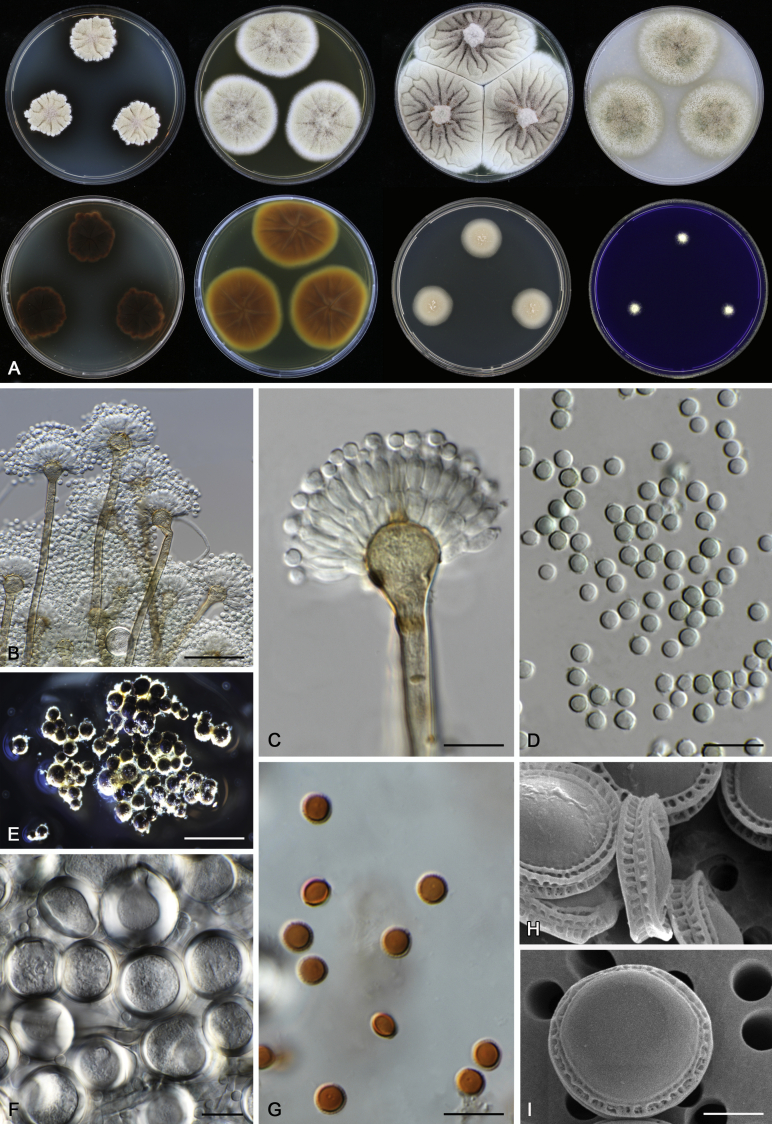
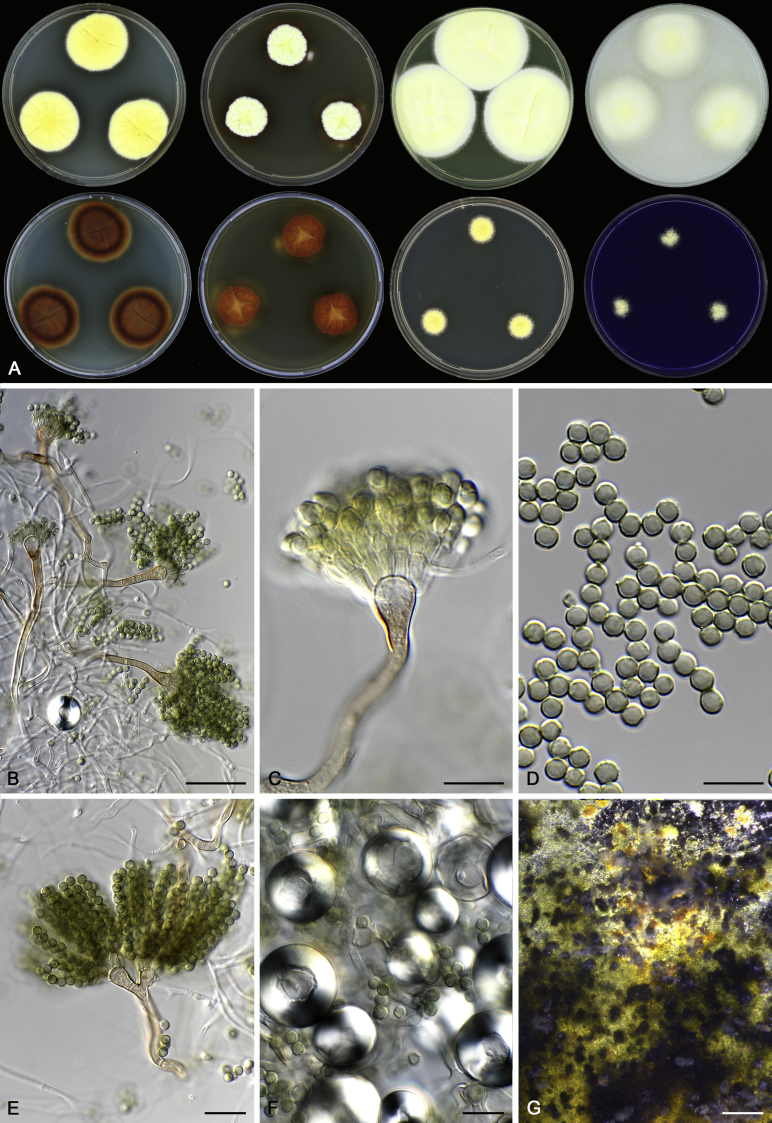
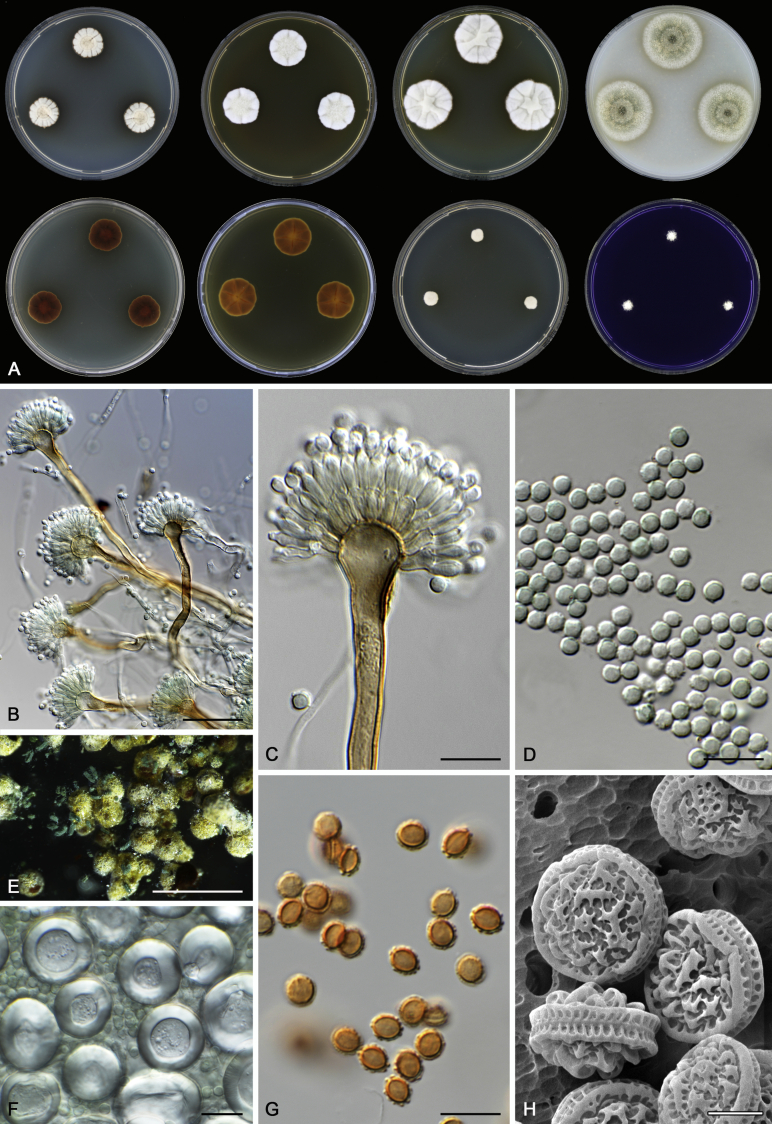
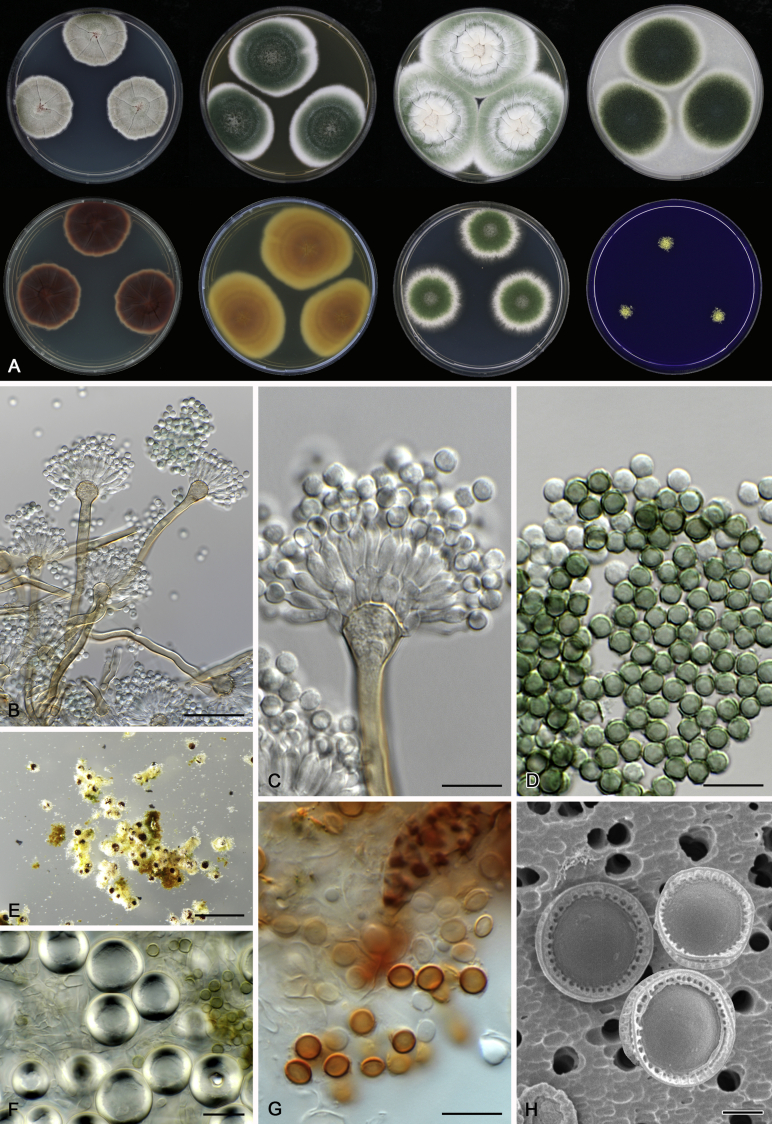
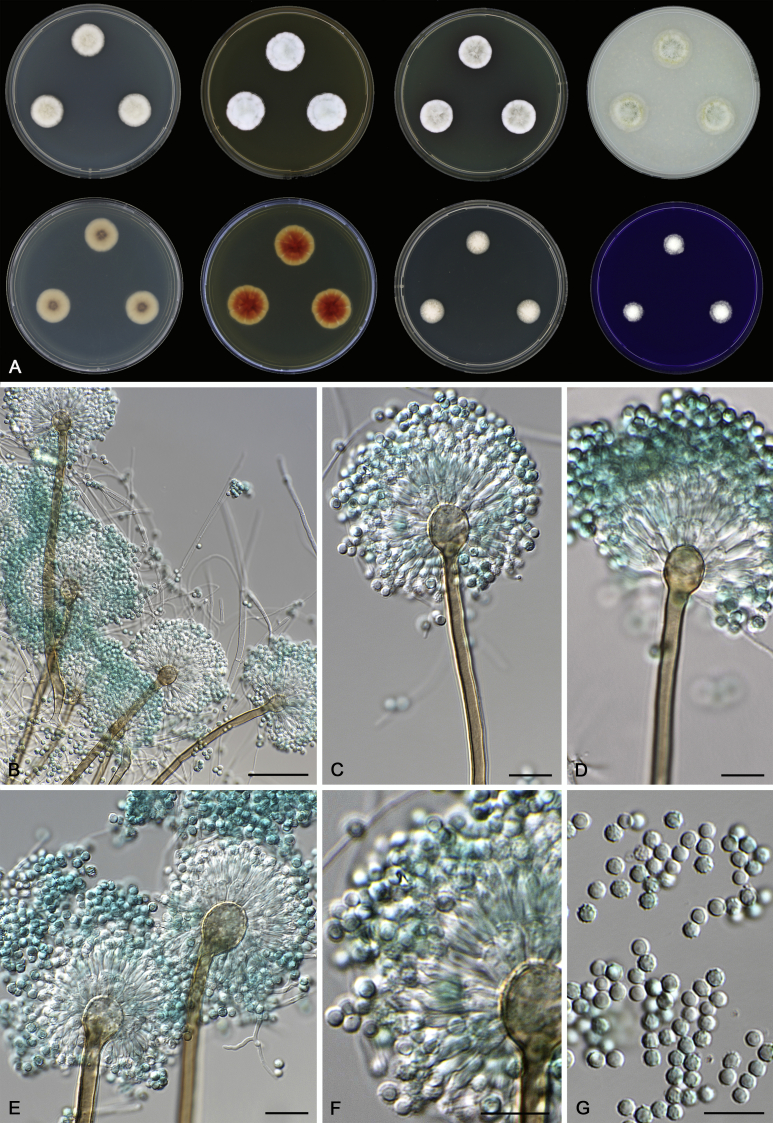
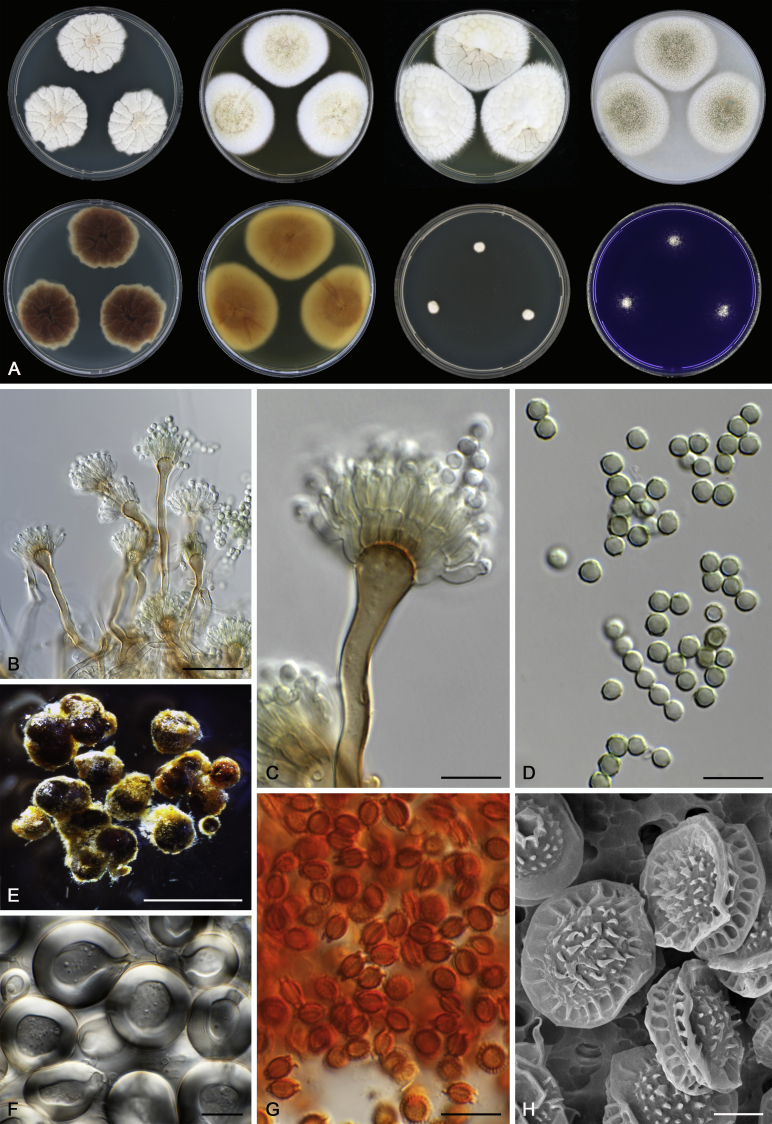
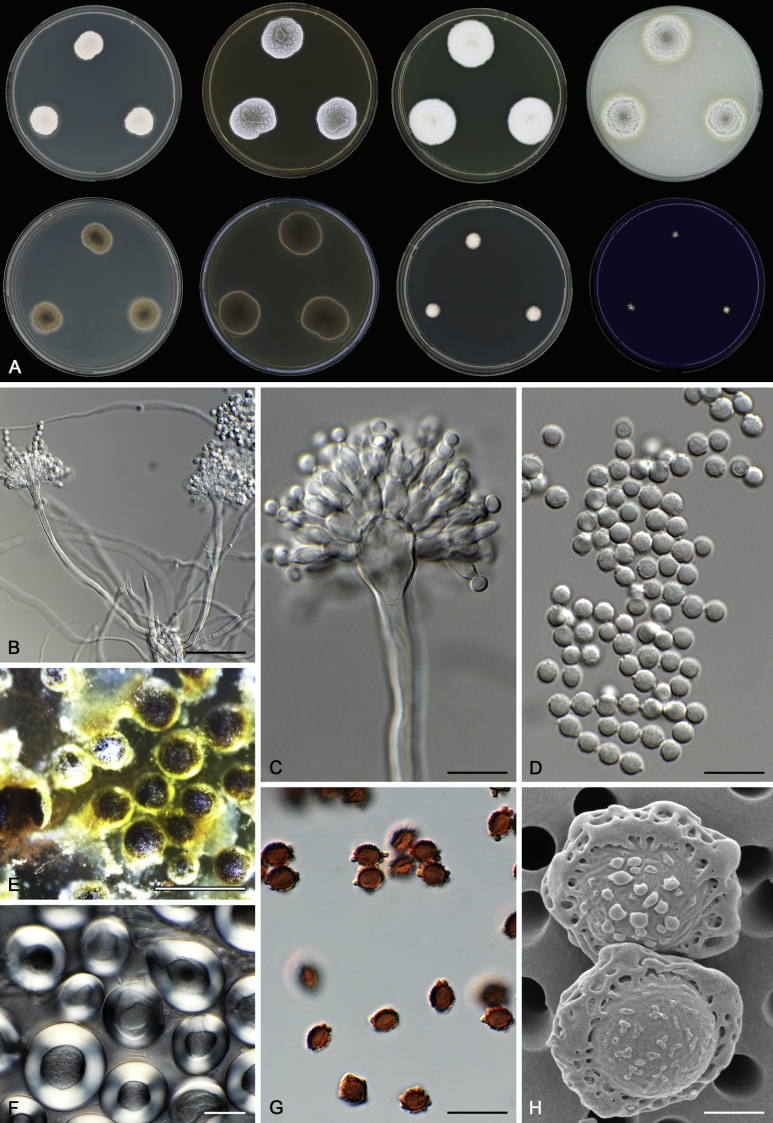
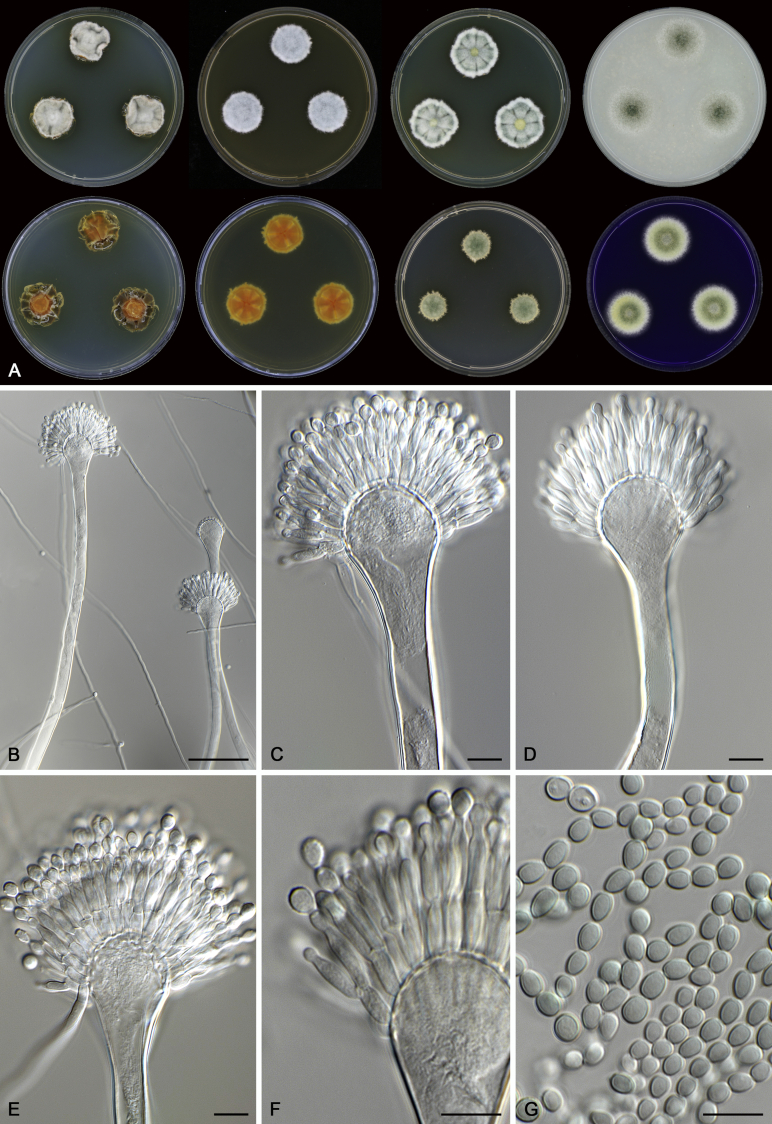
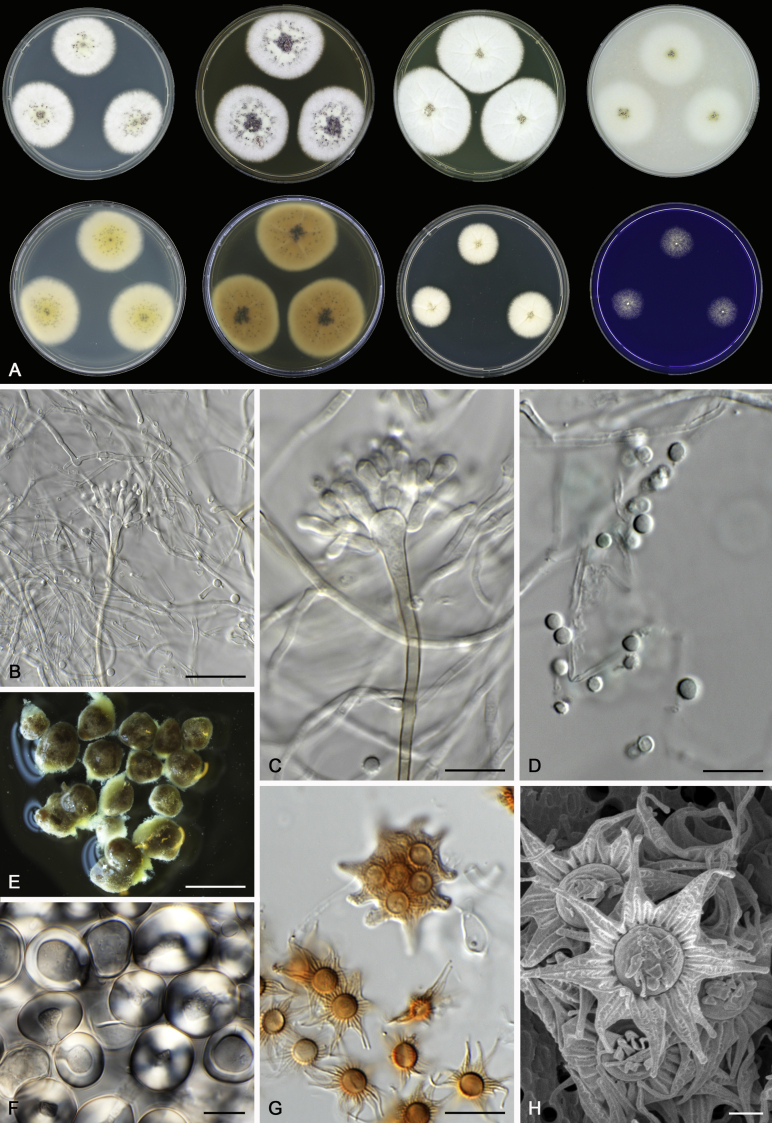
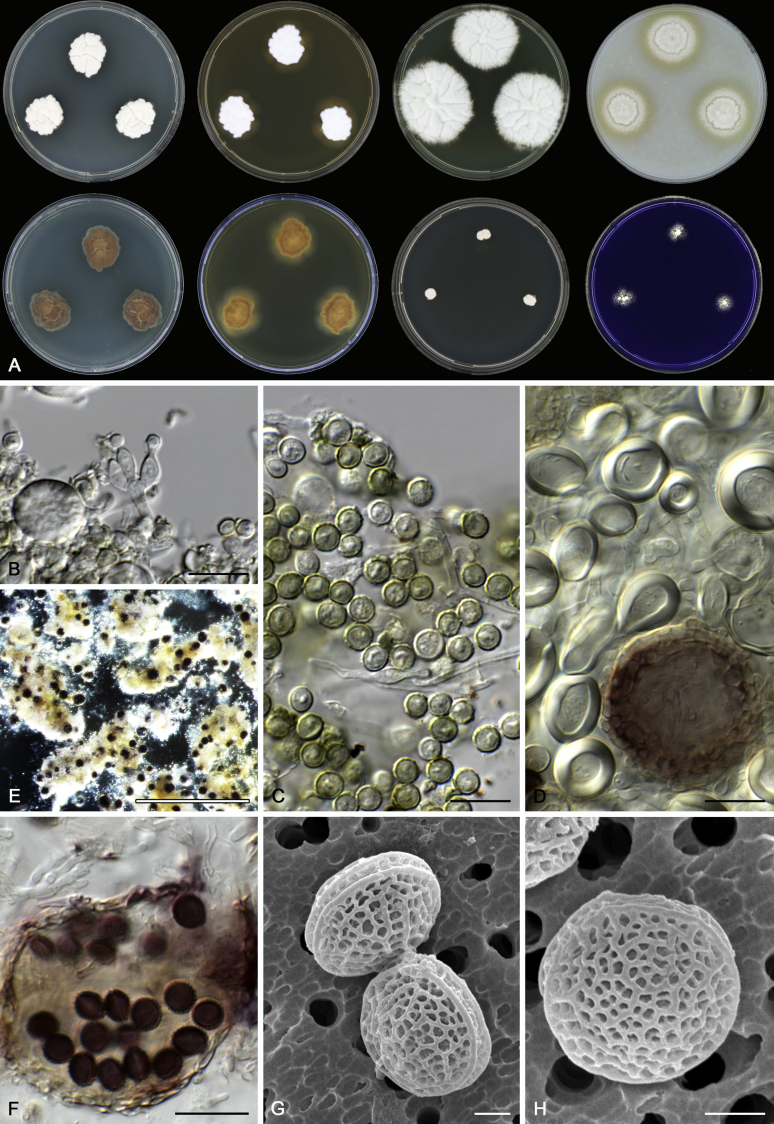
Morphological characters of Aspergillus section Nidulantes are summarised in Table 2, Table 3, Table 4. Ascospores can be globose, subglobose, stellate or appendaged (Fig. 3, Fig. 4, Fig. 5, Fig. 6). The ornamentation on the ascospore convex is informative for species identification. For example in the A. nidulans clade, the ascospore ornamentation can be irregularly wrinkled (A. corrugatus), finely pitted (A. foveolatus), rugulose (A. rugulosus) or echinulate (A. spinulosporus). Ascospore crests are two in number in most species, four crests are only observed in A. quadrilineatus. Ascospore colour is also taxonomically informative, for example the violet ascospores can easily differentiate A. violaceus from other section Nidulantes species (Fig. 5). In A. aurantiopurpureus, orange ascospores can turn to violet in older cultures (Fig. 4), which is firstly observed in section Nidulantes. However, ascospore colour can be variable in some species. Peintner & Rainer (1999) reported an isolate of A. nidulans (CBS 100522) with blue ascospores; another example is A. miraensis, which was originally described with violet ascospores (Zhang et al. 2013), but shows orange to reddish brown ascospores in our study. Ascomata, when present, are mostly 200–600 μm, but it may be highly variable depending on the media, and in some species like A. quadrilineatus and A. violaceus, variable size of ascomata were observed in different strains even under same cultivation condition. Thus the ascoma size is not recommended as a distinguishing feature.
Table 2.
Most important micromorphological characters for non-ascosporic Aspergillussection Nidulantes species (μm).
| Species name | Conidiophores | Vesicles | Metulae | Phialides | Conidia |
|---|---|---|---|---|---|
| Aspergillus askiburgiensis1 | 40–180 × 3-8.5 | 5.5–18.5 | 4–6 | 5–8 | 2.5–4 (–4.5) |
| A. asperescens | 200–400 × 6–8 | 8–15 | 6–9 × 3–4 | 7.5–9 × 3–4 | 4–7 × 3–5 |
| A. aureolatus | 80–200 × 4–5.5 | 9–12 | 5–8.5 × 2–4 | 5–7 × 2.5–3 | 3.5–5 |
| A. caespitosus | 200–300 × 3–6 | 10–15 | 5–8 × 3–3.5 | 6.5–8 × 3–4.5 | 3–4 |
| A. croceus1 | 90–200 × 3.5–5 | 7–15 | 7.5–10.5 | (6.5–) 7–9 (–9.5) | 2–3 (–3.5) |
| A. israelensis | 90–160 × 3.5–4.5 | 7–10 | 5–8 × 2.5–3.5 | 6–8 × 2–2.5 | 2.5–3.5 |
| A. multicolor | 300–350 × 5–7 | 16–20 | 6–10 × 3–4 | 8–9 × 2.5–3 | 3.5–5.5 |
| A. mulundensis | 33–70 × 2.5–4.5 | 5.5–15 | 5–7 × 2.5–4.5 | 6–7.5 × 2.5–4 | 2.5–3.5 |
| A. recurvatus | 40–150 × 3.5–4.5 | 8–10 | 5–6 × 2.5–3.5 | 4.5–5.5 × 2–3 | 3–4.5 |
| A. spelunceus | 130–300 × 4–6 | 7–11 | 4–6.5 × 2.5–3.5 | 5.5–7.5 × 2–2.5 | 2.5–3.5 |
| A. unguis | 50–100 × 3–5 | 8–10 | 5–7 × 2.5–3.5 | 5–9 × 2–2.5 | 2.5–4 |
| A. varians | 600–1200 × 7–12 | 20–30 | 7–10 × 3.5–4.5 | 8–12 × 3–4 | 4–6 × 3.5–4 |
| A. viridicatenatus | 120–270 × 5–6 | 10–15 | 6–9 × 2.5–3.5 | 6–9.5 × 2.5–3.5 | 3–5 × 2.5–4 |
| A. amoenus1 (A. versicolor clade) | (35–) 100–600 (–1100) × (2.5–) 4–7 (–8) | (4–) 7–17 (–21) | 3–6 (–8) × 2.5–4 (–5.5) | (5–) 6–8 (–11) × 2–3 | 2.5–3.5 (–5) |
| A. austroafricanus1 (A. versicolor clade) | (40–) 100–350 (–500) × 3–5 (–6) | (4–) 6–12 (–15) | 3–7 (–9) × 2.5–4.5 | (4–) 5–7 (–9) × (2–) 2.5–3 (–4) | 2.5–3.5 (–4.5) |
| A. creber1 (A. versicolor clade) | (10–) 70–450 (–650) × (3–) 4–7 (–8) | (4–) 7–17 (–25) | (3–) 4–6 (–8) × 2.5–4.5 | (4–) 5–8 (–10) × 2–3 (–4) | (2.5–) 3–4 (–9) |
| A. cvjetkovicii1 (A. versicolor clade) | (40–) 200–700 (–850) × (3–) 4–7 (–8) | (5–) 9–18 (–23) | 3–6 (–8) × 2.5–4.5 | 5–8 (–10) × 2–3 (–4) | (2–) 2.5–3.5 (–5) |
| A. fructus1 (A. versicolor clade) | (50–) 150–400 (–500) × 4–7 | (6–) 9–17 (–21) | (2–) 3–7 (–9) × 2.5–4.5 (–7) | (5–) 6–8 (–11) × 2–3 (–4) | (2–) 2.5–3.5 (–4.5) |
| A. griseoaurantiacus1 (A. versicolor clade) | 100–500 × 3.5–8 | (3.5–) 9–18 (–26.5) | 4–10 × 3–5.5 | 5.5–7 × 2.5–3.5 | 2.5–4 × 2–3 |
| A. hongkongensis1 (A. versicolor clade) | >100 | 15 | – | – | 2 |
| A. jensenii1 (A. versicolor clade) | (45–) 200–700 (–1000) × (3–) 4–7 (–8) | (5–) 7–16 (–22) | 3–8 × 2.5–4 (–5) | (4–) 5–8 (–11) × 2–3 | (2.5–) 3–4.5 (–7) |
| A. protuberus1 (A. versicolor clade) | (120–) 300–800 (–1250) × 4–10 | (6–) 10–24 (–27) | (3–) 4–7 (–8) × 2.5–4.5 (–5.5) | (4–) 5–8 (–11) × 2–3 (–3.5) | (2–) 2.5–3.5 (–5) |
| A. puulaauensis1 (A. versicolor clade) | (35–) 100–500 (–700) × (3–) 4–7 | (5–) 8–18 (–21) | (3–) 4–7 (–9) × 2.5–4 | 5–7 (–10) × 2–3 | (2.5–) 3–4 (–5.5) |
| A. subversicolor1 (A. versicolor clade) | (60–) 250–450 (–550) × 4–7 (–10) | (6–) 10–17 (–22) | (3–) 4–7 (–9) × (2–) 2.5–4 | 5–8 (–10) × 2–3 | (2.5–) 3–4 (–7) |
| A. sydowii1 (A. versicolor clade) | 100–500 × 4–7 | 5–10 (–15) | 6–7 × 2–3 | 7–10 × 2–2.5 | 2.5–3 (–5) |
| A. tabacinus1 (A. versicolor clade) | (70–) 300–700 (–900) × 4–8 (–9) | (5–) 8–15 (–22) | 3–8 (–9) × 2.5–4.5 (–5.5) | 5–8 (–11) × 2–3 (–3.5) | (2.5–) 3–4 (–7) |
| A. tennesseensis1 (A. versicolor clade) | (35–) 100–300 (–400) × 4–7 | (7–) 10–16 (–18) | 4–6 (–8) × 2.5–4 | 5–8 (–11) × 2–3 | (2.5–) 3–4 (–8) |
| A. venenatus1 (A. versicolor clade) | (20–) 100–400 (–500) × 4–7 | (6–) 9–17 (–21) | (3–) 4–7 (–9) × 2.5–4 (–5) | (5–) 6–8 (–11) × 2–3 (–3.5) | 3–4 (–6) |
| A. versicolor1 (A. versicolor clade) | (45–) 200–750 (–1050) × (4–) 5–8 (–12) | (6–) 9–17 (–20) | 3–6 (–9) × 2.5–4.5 | (4–) 5–7 (–11) × 2–3 | (2–) 2.5–3.5 (–6.5) |
1Data derived from Jurjevic et al., 2012, Visagie et al., 2014, Hubka et al., 2016, Tsang et al., 2016.
Table 3.
Most important micromorphological characters for Aspergillus section Nidulantes species with stellate ascospores (μm).
| Species name | Teleomorphic characters |
Anamorphic characters |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascomata | Hülle cells | Ascospore colour | Ascospore size | Spore bodies | Ornamentation of convex surfaces | Undissected part of crests | Extentions | Conidiophores | Vesicles | Metulae | Phialides | Conidia | |
| A. angustatus | 430–780 | 17–35 | Orange to reddish brown | 9–12 | 3–4 × 3–3.5 | Smooth | 0.5–1 | 1.5–3 | 200–400 × 4.5–6 | 8–12 | 6–8 × 3–4.5 | 7–8.5 × 2.5–3.5 | 3–4.5 |
| A. dromiae | 450–800 | 16–31 | Orange to reddish brown | 11–15 | 3–4.5 × 3.5–4.5 | Smooth | 1–1.5 | 2–3 | 300–410 × 4.5–6.5 | 12–17 | 6–8 × 3–4.5 | 6.5–10 × 3.5–4.5 | 3.5–4.5 |
| A. miraensis | 320–600 | 14–22 | Orange to reddish brown or violet | 8–10 | 2–4 × 2–3 | Smooth or verrucose | 0.7–1 | 1.5–2.5 | 300–500 × 5–6 | 12–15 | 5–8 × 3–4 | 6–8 × 2–3.5 | 2–3.5 |
| A. olivicola | 400–770 | 15–28 | Orange to reddish brown | 7.5–11 | 3–4.5 × 3–4 | Smooth | 0.4–0.7 | 1–3 | 150–340 × 4–5.5 | 8–15 | 7.5–10.5 × 2–3.5 | 7.5–12.5 × 1.5–3 | 2–3.5 |
| A. pluriseminatus1 | 80–250 | 10–22 | Violet brown | – | 7–9 × 6–7 | Tuberculate | – | – | – | – | – | – | – |
| A. stella-maris | 370–770 | 16–22 | Orange to reddish brown | 13–16 | 3–4.5 × 2.5–4.5 | Smooth | 1–1.5 | 3–4.5 | 300–800 × 3.5–7 | 9–20 | 5–9 × 3–4 | 6–9 × 2–3.5 | 3–4 |
| A. stellatus | 300–600 | 11.5–25.5 | Orange to reddish brown | 10–14 | 3.5–4×3–4 | Smooth | 0.5–1 | 2.5–4 | 320–610 × 4.5–6.5 | 13.5–18.5 | 4–7.5 × 3.5–4 | 6–8.5 × 2.5–3.5 | 2.5–3 |
| A. venezuelensis | 400–1000 | 12–21.5 | Orange to reddish brown | 12.5–19.5 | 4–5×3.5–4.5 | Covered with triangular flap | 1–1.2 | 2.5–4 | 65–130 × 2–3 | 5.5–7 | 4–5 × 2.5–3.5 | 6–7 × 2.5–3.5 | 2.5–4 |
1Data derived from Stchigel & Guarro 1997.
Table 4.
Most important micromorphological characters for Aspergillus section Nidulantes species with globose or appendiged ascospores (μm).
| Species name | Teleomorphic characters |
Anamorphic characters |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascomata | Hülle cells | Ascospore color | Spore bodies | Ornamentation of convex surfaces | Crests | Conidiophores | Vesicles | Metulae | Phialides | Conidia | |
| Aspergillus astellatus | 330–500 | 15–27 | Reddish brown | 5.5–6 × 3.5–5 | Smooth | 2–3.5 | 80–200 × 3–4 | 5–7 | 4.5–5.5 × 2–3 | 4.5–5 × 2–4 | 2.5–6 |
| A. aurantiobrunneus | 60–300 | 14–25 | Light orange | 4–5 × 3.5–4.5 | Smooth | 0.8–1 | 50–200 × 3.5–4.5 | 7–12 | 4–6 × 2.5–3.5 | 6.5–7.5 × 2.5–3 | 2.5–3.5 |
| A. aurantiopurpureus | 200–320 | 11.5–20.5 | Orange or reddish brown, later turns to violet | 3.5–4.5 × 3–3.5 | Smooth | 0.8–1.2 | 130–260 × 3.5–5 | 8–11.5 | 5–6 × 2.5–4 | 5–6.5 × 3–3.5 | 3–3.5 |
| A. botswanensis | 90–180 | 12–16.5 | Brown | 5–6 × 3.5–5 | Tuberculate to reticulate | 0.4–0.8 | – | – | – | – | – |
| A. corrugatus | 200–360 | 16–23 | Orange to reddish brown | 3.5–4.5 × 3.5–4 | Irregularly wrinkled | 0.5–1 | 40–120 × 3.5–5.5 | 8–10 | 5–8 × 2.5–4 | 6–8 × 2.5–3.5 | 2.5–3.5 |
| A. desertorum | 100–300 | 10–25 | Reddish brown | 6.5–7.5 × 6–7.5 | Tuberculate | 0.5 | – | – | – | – | – |
| A. falconensis | 300–700 | 14–25 | Orange to reddish brown | 4–6 × 3–3.5 | Smooth | 1–2 | 75–240 × 4–6.5 | 8–10 | 6–10 × 2–3.5 | 6–9 × 2–4 | 2.5–4 |
| A. foveolatus | 100–280 | 7–21.5 | Orange to reddish brown | 4–5 × 3.5–4.5 | Finely pitted | 0.5–1 | 40–200 × 4.5–6.5 | 12–15 | 5–7 × 2–4 | 6–8 × 2–3 | 3–4.5 |
| A. fruticulosus | 230–500 | 10–20 | Orange to reddish brown | 4.5–5.5 × 3–5 | Smooth | 0.8–1 | 40–200 × 4–6 | 8–12 | 5–6 × 3–4.5 | 6–9 × 2–3.5 | 3.5–4 |
| A. jaipurensis | 150–500 | 14–25 | Purplish red | 6–7.5 × 5.5–6 | Smooth | 0.8–1 | 30–100 × 4–6 | 7–9 | 5–6.5 × 2.5–3.5 | 5–7 × 2–3.5 | 4–6.5 × 3–4.5 |
| A. latilabiatus | 100–160 | 13–24 | Brown | 5.5–7 × 4.5–6 | Smooth | 0.5–1 | – | – | – | – | – |
| A. latus | 150–400 | 14–30 | Light orange, orange or reddish brown | 3.5–5 × 3–5 | Smooth, incompletely reticulate or ribbed | 1–1.5 | 150–300 × 4.5–5.6 | 10–12.5 | 3.5–8 × 3–4 | 6.5–11 × 2–3.5 | 2.5–5 |
| A. navahoensis | 140–400 | 13–23 | Orange to reddish brown | 3.5–4.5 × 3–3.5 | Smooth | 0.7–1 | 35–150 × 2.5–3 | 6–8 | 6–9.5 × 3–4.5 | 6–8 × 2.5–3 | 3.5–4.5 |
| A. nidulans | 150–420 | 12–20 | Orange to reddish brown | 3.5–5 × 3–4.5 | Smooth | 0.5–1 (entire or dentate) | 70–220 × 5–8 | 8–14.5 | 5–8 × 2.5–4.5 | 6–8 × 2.5–3.5 | 3–4 |
| A. omanensis1 | 180–370 | 10–35 | Brownish red | 4.5–5.5 × 4–4.5 including crests | Tuberculate or verruculose | 1 | 50–120 × 4–7 | 10–14 | 4–7 × 2–3 | 5–8 × 2–4 | 4–5.5 |
| A. pachycristatus | 200–500 | 11–21 | Orange to reddish brown | 4–5 × 3.5–4 | Smooth | 0.7–1 | 150–260 × 5–6 | 8–12 | 5.5–7.5 × 2.5–4 | 6–9 × 2.5–3.5 | 3–4 |
| A. purpureus | 90–200 | 8–20 | Brown | 6–7 × 4.5–5 | Smooth | 0.3–0.6 | 40–50 × 2.5–5* | 6–8* | 3.5–6 × 2.5–3.5* | 6–8 × 2.5–3* | 3.5–5.5 × 1.5–2* |
| A. quadrilineatus | 100–700 | 10–24 | Orange to reddish brown | 4–4.5 × 3–4.5 | Smooth | 0.5–1 (entire, defective or with irregular protuberance) | 50–150 × 4–5.5 | 10–13 | 5–7 × 2–4.5 | 5–7 × 2–4 | 3–4 |
| A. rugulosus | 220–350 | 14–24 | Orange, greyish violet, reddish purple or brownish red | 4–4.5 × 3.5–4 | Rugulose | 0.5–0.6 | 50–200 × 5–6 | 8–12 | 7–8 × 3–3.5 | 6–7 × 2.5–3 | 3–4 |
| A. savannensis | 65–120 | 11–16.5 | Orange to reddish brown | 4–5 × 3.5–4 | Smooth | 0.5–1 | 85–190 × 5–7 | 8–15.5 | 4.5–8 × 3.5–4.5 | 7.5–9 × 3–4 | 3.5–5 |
| A. spinulosporus | 200–550 | 15–30 | Orange to reddish brown | 3.5–4.5 × 3–4.5 | Echinulate | 0.8–1 | 70–120 × 5–6 | 9–11 | 6–8 × 3–4 | 6–8.5 × 2–3 | 3–4 |
| A. stercorarius | 70–150 | 8–14.5 | Brown | 4.5–6 × 3.5–4.5 | Smooth | 0.3–0.4 | – | – | – | – | – |
| A. striatus | 180–500 | 14–23 | Orange | 6–7 × 5–5.5 | With concentric thickenings | – | – | – | – | – | – |
| A. sulphureoviridis | 350–600 | 10–22.5 | Orange to reddish brown | 4.5–5.5 × 3.5–4.5 | Smooth | 0.8–1.2 | 30–80 × 3–5 | 7–10 | 6.5–8.5 × 2.5–3.5 | 6.5–7.5 × 3–4 | 3.5–5 |
| A. undulatus | 300–500 | 14–26 | Brown | 4–4.5 × 3.5–4 | Tuberculate | Low part: 0.3–0.7; high part: 0.8–1.3 | 80–150 × 3–4.5 | 7–11 | 5.5–7.5 × 2.5–3 | 6.5–7.5 × 2–2.5 | 3–4 |
| A. violaceus | 25–190 | 6–26 | Violet | 4–6.5 × 3–5 | Reticulate intertwined | <0.3 | 30–50 × 3–41 | 5–61 | 6–7.5 × 3–3.51 | 5–6 × 2–2.51 | 2.8–3.31 |
| A. filifer | 220–660 | 13–24 | Brown | 3.5–4.5 × 3–4 | Tuberculate | 0.5–1.2 (with filiform appendages | 120–250 × 3–5 | 7–13 | 7–10 × 3–5 | 7–11 × 2–4 | 3–4 |
| A. qinqixianii | 200–510 | 16–24 | Brown | 3.5–4.5 × 3–4 | Smooth | 0.5 (with filiform appendages) | 120–280 × 3–5 | 7–12 | 4–8 × 3–5 | 7–8 × 2–4 | 3–4 |
1Data derived from Fennell and Raper, 1955, Samson and Mouchacca, 1975, Horie and Udagawa, 1995.
Fig. 3.
Range of ascospore phenotypes. A,B. Aspergillus nidulans CBS 589.65T. C,D. A. nidulans CBS 114.63 (ex-type of A. dentatus). E,F. A. quadrilineatus CBS 591.65T. G,H. A. quadrilineatus CBS 853.96. I,J. A. foveolatus CBS 279.81T. K,L. A. navahoensis CBS 351.81T. M,N. A. corrugatus CBS 191.77T. O,P. A. pachycristatus NRRL 11440. Q,R. A. sulphureoviridis CBS 140626T. S,T. A. savannensis CBS 140607T. Scale bars: S = 10 μm, applies to A,C,E,G,I,K,M,O,Q; T = 5 μm, applies to B,D,F,H,J,L,N,P,R. Pictures were arranged according to ascospore colour, shape and ornamentation.
Fig. 4.
Range of ascospore phenotypes. A,B. Aspergillus aurantiobrunneus CBS 465.65T. C,D. A. falconensis CBS 271.91T. E,F. A. fruticulosus CBS 486.65T. G,H . A. latus CBS 492.65T. I,J. A. latus CBS 140630 (ex-type of A. sublatus). K,L. A. aurantiopurpureus CBS 140608T. M,N. A. astellatus CBS 261.93T. O,P. A. jaipurensis CBS 952.97T. Q,R. A. desertorum CBS 653.73T. S,T. A. purpureus CBS 754.74T. Scale bars: S = 10 μm, applies to A,C,E,G,I,K,M,O,Q; T = 5 μm, applies to B,D,F,H,J,L,N,P,R. Pictures were arranged according to ascospore colour, shape and ornamentation.
Fig. 5.
Range of ascospore phenotypes. A,B. Aspergillus spinulosporus CBS 120.55T. C,D. A. striatus CBS 592.65T. E,F. A. rugulosus CBS 133.60T. G,H . A. rugulosus CBS 200.75 (ex-type of A. cleistominutus). I,J. A. botswanensis CBS 314.89T. K,L. A. undulatus CBS 261.88T. M,N. A. latilabiatus CBS 426.93T. O,P. A. stercorarius CBS 428.93T. Q,R. A. violaceus CBS 138.55T. S,T. A. violaceus CBS 293.93 (ex-type of A. similis). Scale bars: S = 10 μm, applies to A,C,E,G,I,K,M,O,Q; T = 5 μm, applies to B,D,F,H,J,L,N,P,R. Pictures were arranged according to ascospore colour, shape and ornamentation.
Fig. 6.
Range of ascospore phenotypes. A,B. Aspergillus miraensis CBS 140625T. C,D. A. olivicola CBS 119.37T. E,F. A. stella–maris CBS 113638T. G,H. A. dromiae CBS 140633T. I,J. A. angustatus CBS 273.65T. K,L. A. venezuelensis CBS 868.97T. M,N. A. stellatus CBS 598.65T. O,P. A. qinqixianii CBS 128788T. Q,R. A. filifer CBS 113636T. S,T. A. filifer CBS 128791 (ex-type of A. chinensis). Scale bars: S = 10 μm, applies to A,C,E,G,I,K,M,O,Q; T = 5 μm, applies to B,D,F,H,J,L,N,P,R. Pictures were arranged according to ascospore colour, shape and ornamentation.
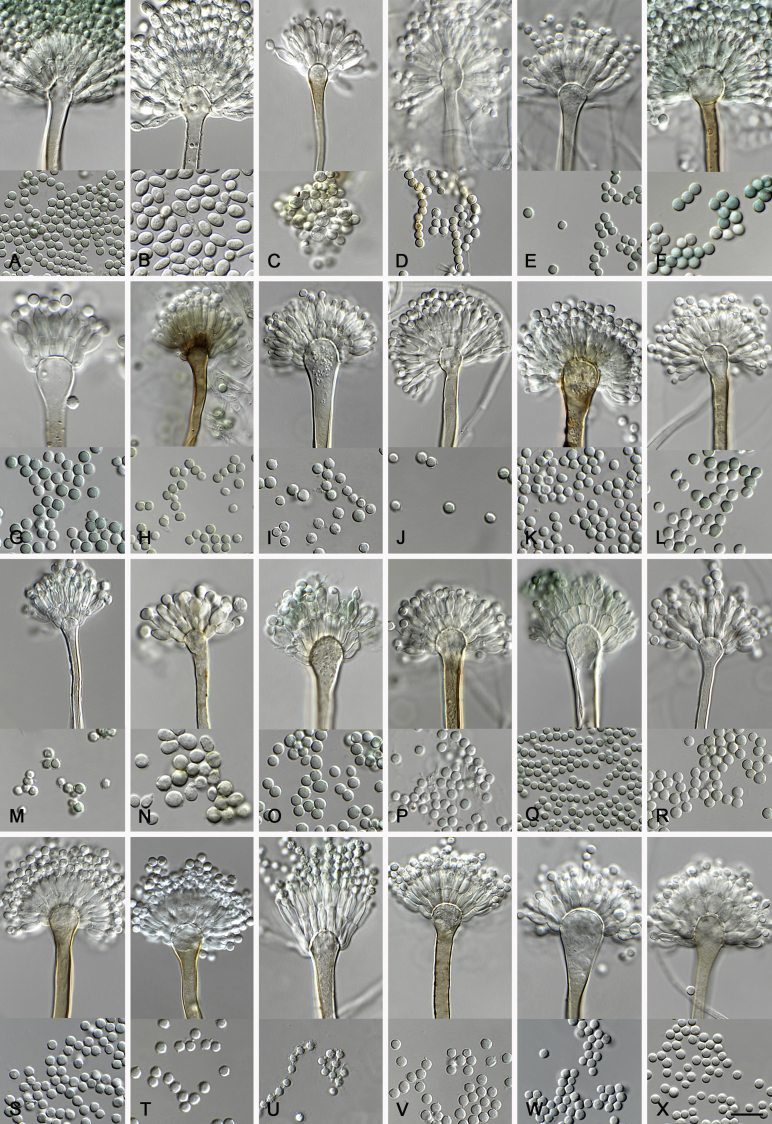
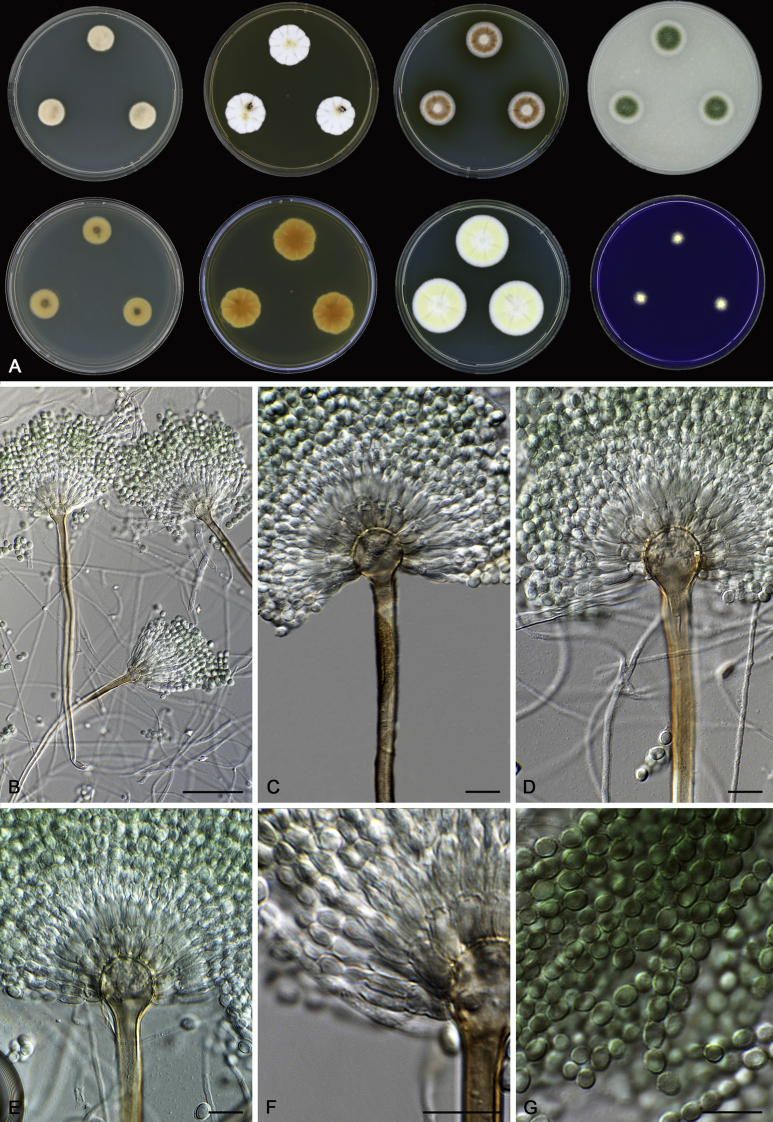
In general, species in Aspergillus section Nidulantes produce more or less brown-pigmented conidiophores, typically smooth but occasionally showing surface protuberances. Vesicles are usually globose, subglobose or subclavate, narrower than 30 μm. Conidia are typically globose and echinulate, green in mass, in some cases (A. asperescens and A. varians) conidia are ellipsoidal (Fig. 7, Fig. 8). For non-ascosporic species, size and shape of conidiophores and conidia are taxonomically informative (Table 2).
Fig. 7.
Range of conidiophore and conidia phenotypes. A. A. angustatus CBS 273.65T. B. A. asperescens CBS 110.51T. C. A. astellatus CBS 261.93T. D. A. aurantiobrunneus CBS 465.65T. E. A. aurantiopurpureus CBS 140608T. F. A. aureolatus CBS 190.65T. G. A. caespitosus CBS 103.45T. H. A. corrugatus CBS 191.77T. I. A. dromiae CBS 140633T. J. A. falconensis CBS 271.91T. K. A. foveolatus CBS 279.81T. L. A. fruticulosus CBS 486.65T. M. A. israelensis CBS 140627T. N. A. jaipurensis CBS 952.97T. O. A. latus CBS 492.65T. P. A. latus CBS 140630 (extype of A. sublatus). Q. A. miraensis CBS 140625T. R. A. navahoensis CBS 351.81T. S. A. nidulans CBS 589.65T T. A. nidulans CBS 114.63 (ex-type of A. dentatus). U. A. olivicola CBS 119.37T. V. A. pachycristatus NRRL 11440. W. A. qinqixianii CBS 128788T. X. A. filifer CBS 113636T. Scale bar: X = 10 μm, applies to A–W.
Fig. 8.
Range of conidiophore and conidia phenotypes. A. A. quadrilineatus CBS 591.65T. B. A. quadrilineatus CBS 853.96. C. A. recurvatus CBS 496.65T. D. A. rugulosus CBS 133.60T. E. A. savannensis CBS 140607T. F. A. spelunceus CBS 497.65T. G. A. spinulosporus CBS 120.55T. H. A. stellatus CBS 598.65T. I. A. sulphureoviridis CBS 140626T. J. A. stella–maris CBS 113638T. K. A. undulatus CBS 261.88T. L. A. unguis CBS 132.55T. M. A. venezuelensis CBS 868.97T. N. A. viridicatenatus CBS 140629T. O. A. mulundensis CBS 140610T. P. A. multicolor CBS 133.54T. Q. A. varians CBS 505.65T. Scale bar: Q = 10 μm, applies to A–P.
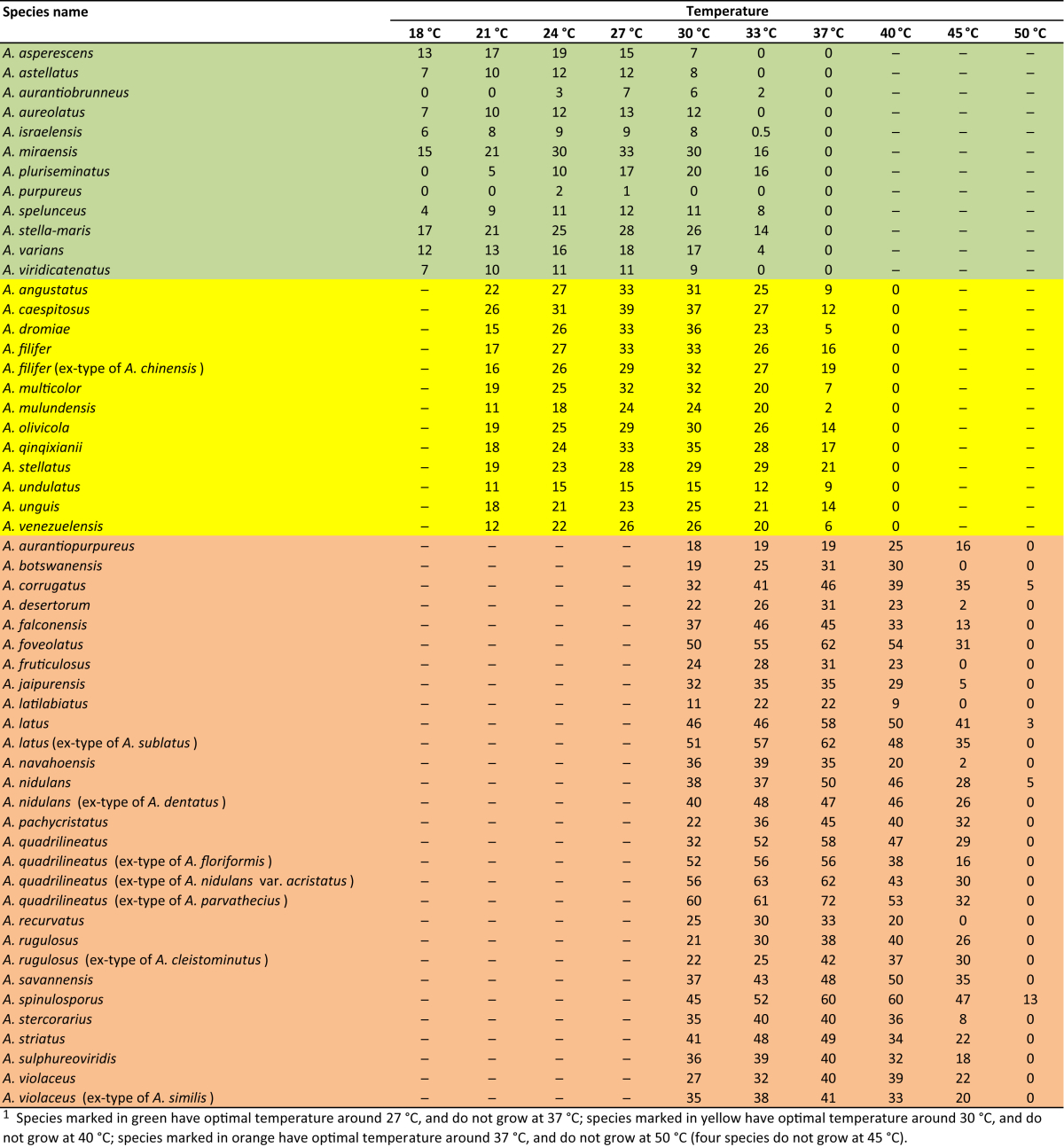
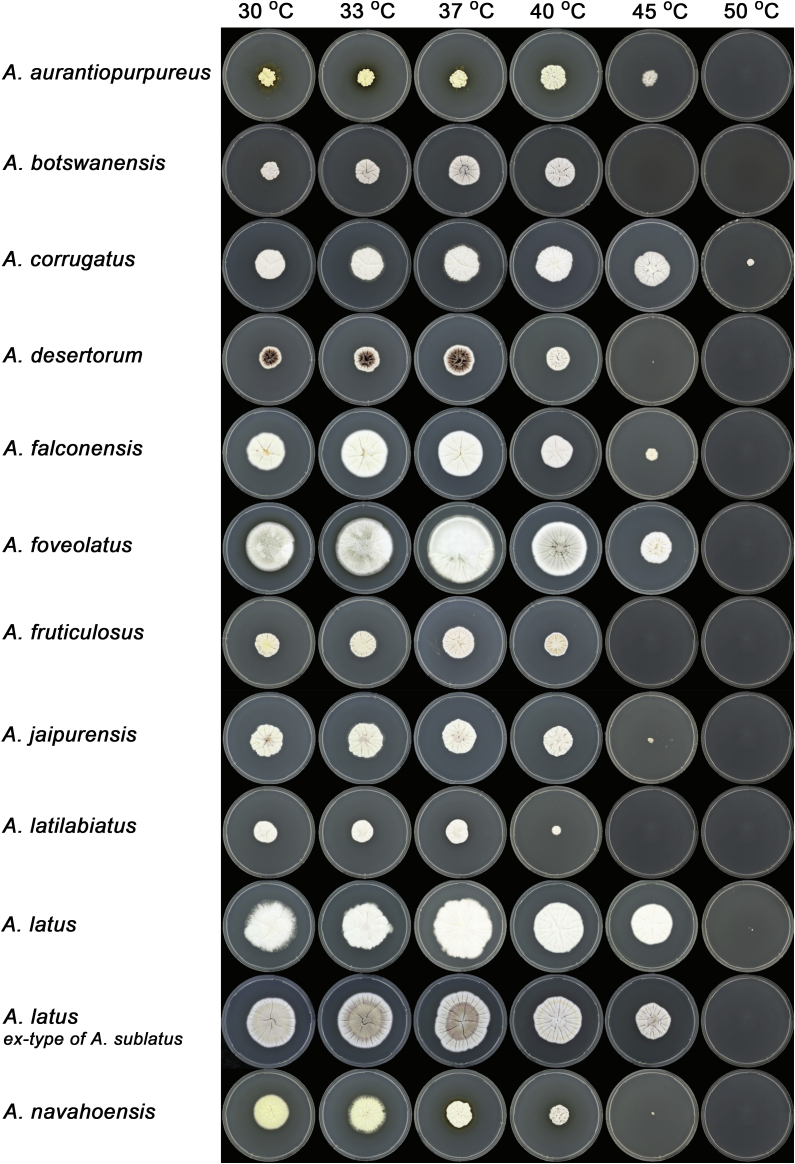
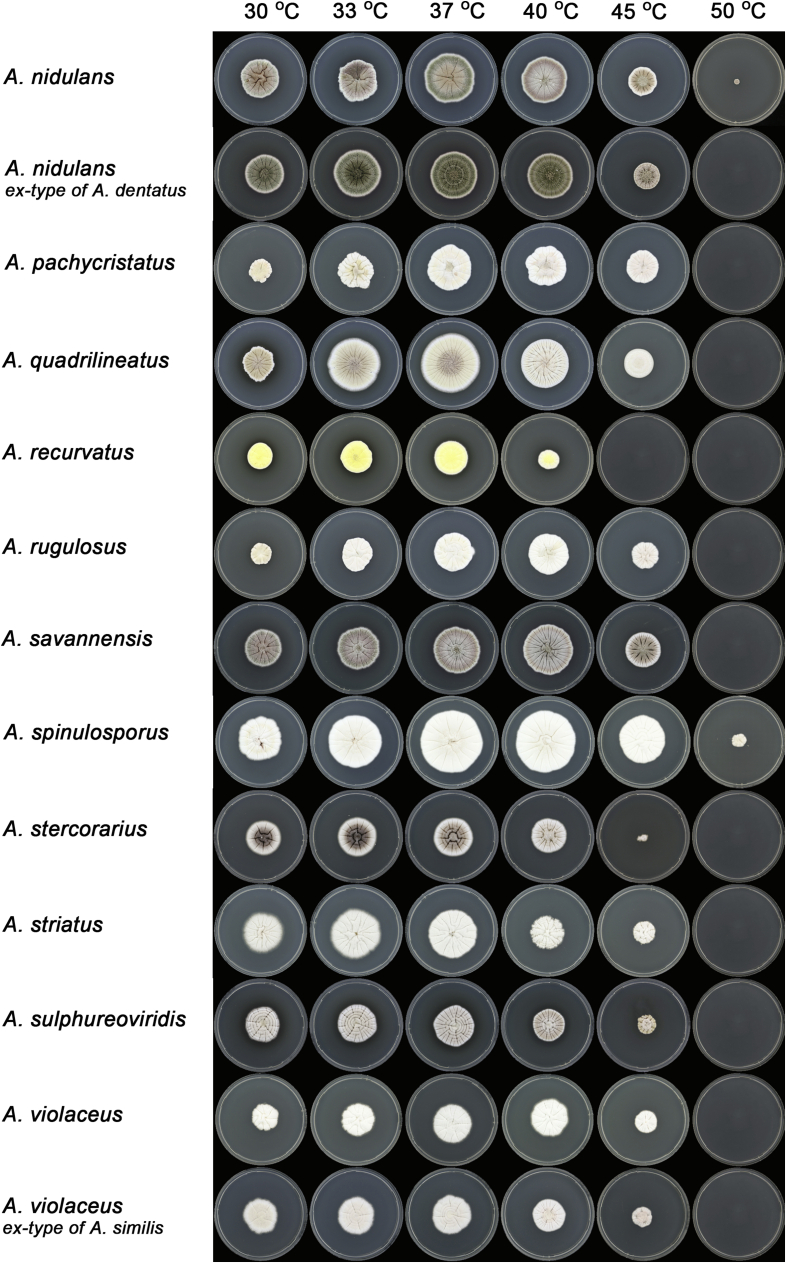
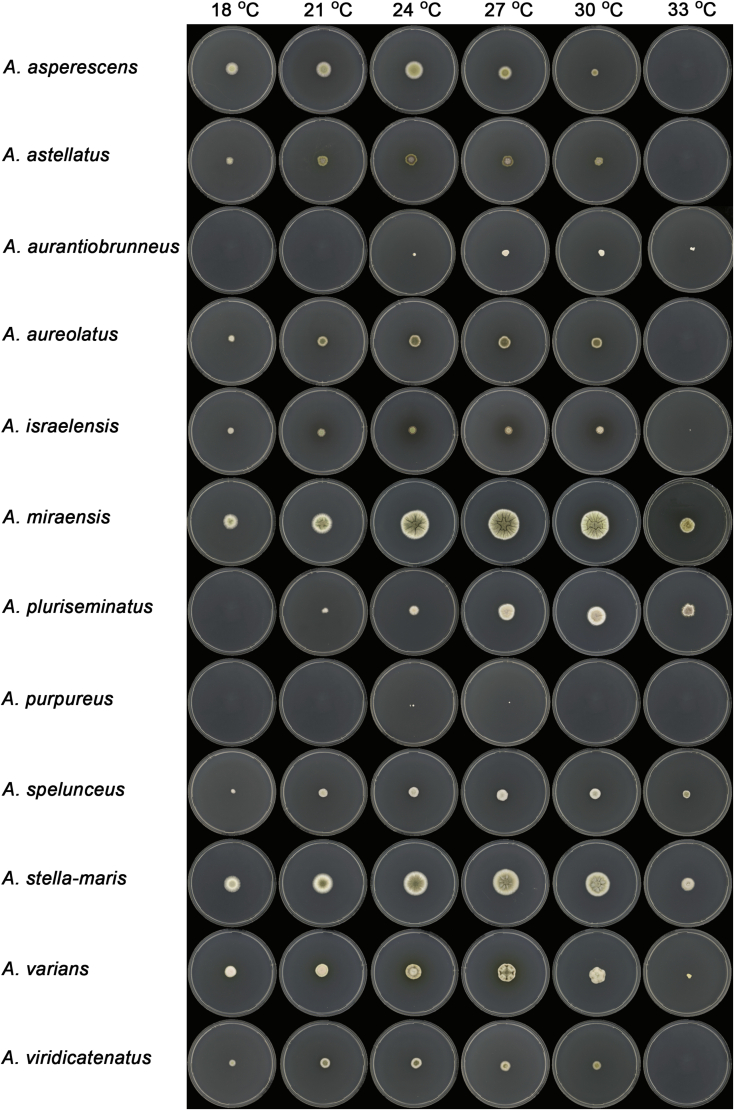
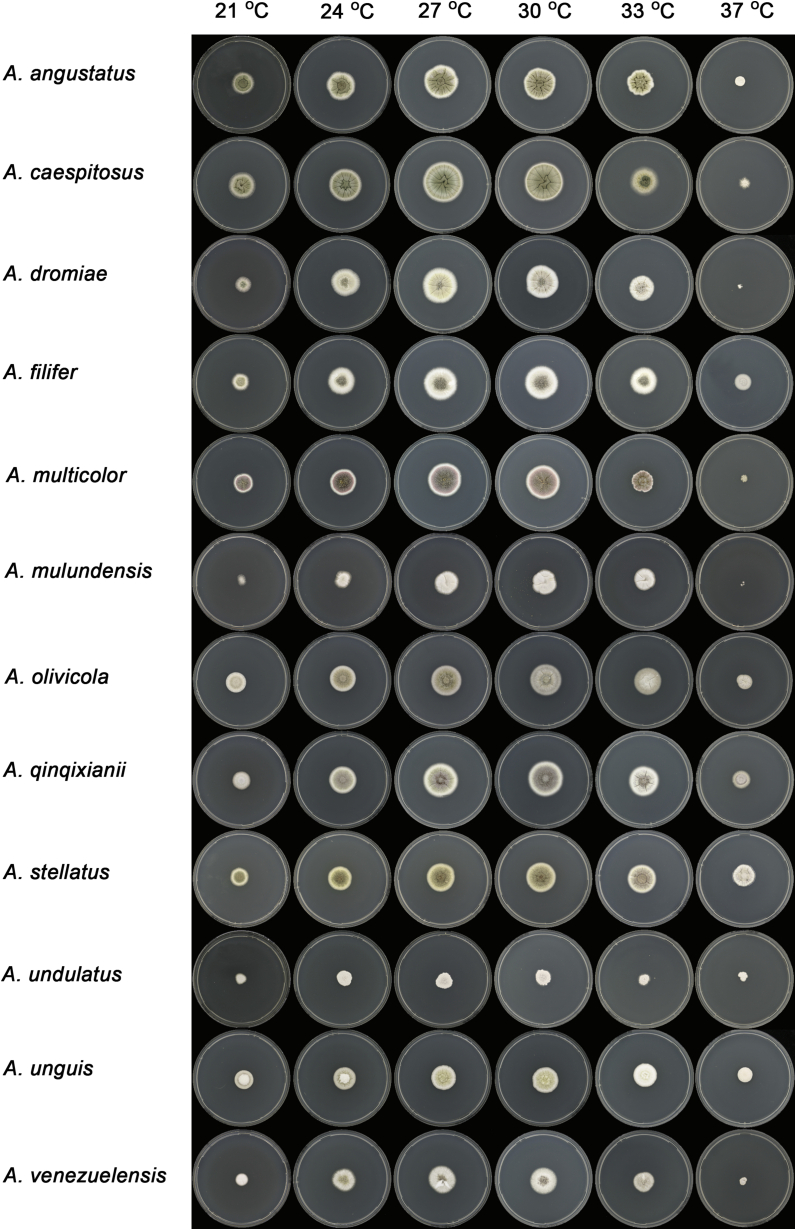
Macromorphology including temperature growth profile, production of cleistothecia, mycelium colour, sporulation, soluble pigments, and exudate is also important distinguishing character. Species within the A. nidulans clade grow optimally at 37°C but do not grow at 50 °C (Table 5, Fig. 9, Fig. 10), while species in the other six clades cannot grow at 40 °C, some species such as A. asperescens, A. aureolatus, A. pluriseminatus, A. spelunceus and A. varians cannot grow at 36 °C (Table 5, Fig. 11, Fig. 12).
Table 5.
Temperature profiles (5 days, in mm) on CYA for Aspergillus section Nidulantes species1.
Fig. 9.
Temperature growth profile of Aspergillus sect. Nidulantes species on CYA, from left to right 30, 33, 37, 40, 45, 50 °C.
Fig. 10.
Temperature growth profile of Aspergillus sect. Nidulantes species on CYA, from left to right 30, 33, 37, 40, 45, 50 °C.
Fig. 11.
Temperature growth profile of Aspergillus sect. Nidulantes species on CYA, from left to right 18, 21, 24, 27, 30, 33 °C.
Fig. 12.
Temperature growth profile of Aspergillus sect. Nidulantes species on CYA, from left to right 21, 24, 27, 30, 33, 37 °C.
Extrolites
Fourty eight species were analysed for extrolites and produced several shared or unique small molecule extrolites and often had species specific profiles (mentioned after each species description). An overview of reported extrolites from section Nidulantes species is shown in Table 6. Sterigmatocystins, shamixanthones, and violaceols are common to many species and are also found in some species from sections Usti and Aenei (Houbraken et al., 2007, Varga et al., 2010a, Samson et al., 2011). The shamixanthones are produced by 19 species in section Nidulantes. The ascospore / Hülle cell-associate metabolite asperthecin is produced by 20 species in the section. The desertorin polyketides are produced by 13 species, while violaceol polyketides are found in 19 species. The falconensins and falconensons are produced by the closely related species A. aurantiopurpureus, A. falconensis, A. fruticulosus, A. navahoensis and A. revurvatus. Emericellin is found in 18 species, asperugin polyketides is produced by 14 species and the shikimic derived emerins are formed by two species. The dithiodiketopiparazine mycotoxin emestrin is produced by six closely related species: A. foveolatus, A. jaipurensis, A. quadrilineatus, A. rugulosus, A. striatus and A. violaceus. The important antibiotic echinocandin and mulundocandin producers include A. mulundensis, A. navahoensis, A. pachycristatus, A. quadrilineatus, and A. rugulosus (Bills et al., 2016, de la Cruz et al., 2012). One of the originally reported producers was first identified as A. spinulosporus (as Emericella echinulata), but was later re-identified as A. rugulosus (Emericella rugulosa) (Dreyfuss 1986).
Table 6.
Extrolites reported from species of Aspergillus section Nidulantes.
| Species | Extrolites reported | Reference |
|---|---|---|
| Aspergillus askiburgiensis | Sterigmatocystin, versicolorins, cf. monascorubramin | Hubka et al. 2016 |
| A. astellatus | Aflatoxin B1 | Frisvad et al. 2004 |
| Asperthecin | Frisvad 1985 | |
| Austin, dehydroaustin | Simpson et al. 1982 (as “variant” of “Aspergillus variecolor”) | |
| 2-(3,4-dihydroxyhepta-1,5-dienyl)-6-methoxybenzyl alcohol & terrein | Dunn & Johnstone 1979 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Sterigmatocystin | Frisvad et al. 2004 | |
| Tajixanthone, shamixanthone | Ahmed et al. 1992 | |
| A. aurantiobrunneus | Emeremophiline | Fujimoto et al. 2000 |
| Emericolin A-D, variecolin, variecolol | Yoganathan et al. 2004 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Sterigmatocystin | Rabie et al. 1977 | |
| Variecoacetal A & B | Yoganathan et al. 2004 | |
| Variecolactone, variecolin, variecolol | Fujimoto et al., 2000, Yoganathan et al., 2004 | |
| A. caespitosus | Asperline, (5S,6S)-5,6-dihydro-5-acetoxy-6-(1,2-trans-propenyl)-2H-pyran-2-one, (5S,6S)5,6-dihydro-5-acetoxy-6-(1,2-trans-epoxy-propyl)-2H-pyran-2-one | Mizuba et al. 1975 |
| Fumitremorgin B, C, verruculogen | Schroeder et al., 1975, Steyn et al., 1981 | |
| Penicillin G | Dulaney, 1947b, Gill-Carey, 1949 | |
| Cyclopiamine B | Steyn et al. 1981 | |
| 6-methoxymellein | Dunn et al. 1979 | |
| Trisdechloronornidulin | Steyn et al. 1981 | |
| A. corrugatus | Asperthecin | Frisvad 1985 |
| Emecorrugatin A & B | Fujimoto et al. 1998 | |
| Sterigmatocystin | Frisvad, 1985, Horie and Yamazaki, 1985, Fujimoto et al., 1998 | |
| Norsolorinic acid | Fujimoto et al. 1998 | |
| A. croceus | Kotanins, norsolorinic acid, orlandin, siderin, sterigmatocystin, versicolorins | Hubka et al. 2016 |
| A. desertorum | Desertorin A-C, 4,7-dihydroxy-5-methylcoumarin, 7-demethylsiderin | Nozawa et al., 1987a, Rizzacasa and Sargent, 1988, Mazzaferro et al., 2015 |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Emindol DA, Emindol DB, Emindol SA | Nozawa et al., 1987b, Nozawa et al., 1988a, Nozawa et al., 1989 | |
| Nidulol | Nozawa et al. 1987a | |
| Paxillin | Nozawa et al. 1987b | |
| Silvaticol | Nozawa et al. 1987a | |
| A. falconensis | 3,3′-Dihydroxy-5,5′-dimethyldiphenyl ether | Itabashi et al. 1993 |
| Falconensin A-N | Itabashi et al., 1992, Itabashi et al., 1993, Itabashi et al., 1996, Ogasawara and Kawai, 1998 | |
| Falconenson A-B | Ogasawara et al. 1997 | |
| Hopane-6α,7β,22-triol, hopane-7β,22-diol | Itabashi et al. 1996 | |
| Mitorubrin, monomethyldihydromitorubrin, monomethylmitorubrin | Ogasawara & Kawai 1998 | |
| Zeorin | Itabashi et al. 1996 | |
| A. foveolatus | Asperthecin | Frisvad 1985 |
| Dethiosecoemestrin, emestrin, emestrin B, secoemestrin C | Seya et al., 1986a, Seya et al., 1986b, Ooike et al., 1997 | |
| Secoemestrin D, emericellenes A-E | Xu et al. 2013, identity of producer was Emericella sp. | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Paxillin | Nozawa et al. 1989 | |
| Violaceic acid | Ooike et al. 1997 | |
| A. fruticulosus | Sterigmatocystin | Frisvad 1985 |
| A. latus | Asperthecin | Frisvad 1985 |
| Nidulalin A & B | Kawahara et al. 1994 | |
| Sterigmatocystin | El-Khady and Hafez, 1981, Frisvad, 1985, Horie and Yamazaki, 1985 | |
| A. multicolor | Asticolourin A-C; averufin, 5,6-dimethoxydihydrosterigmatocystin, 5,6-dimethoxysterigmatocystin, sterigmatocystin, versicolourin C | Rabie et al., 1984, Hamasaki et al., 1977, Hamasaki et al., 1980 (from IFO 8133, we could not confirm sterigmatocystin production by A. multicolor) |
| A. mulundensis | Dexoymulundocandin, mulundocandin | Roy et al., 1987, Mukhopadhyay et al., 1987, Mukhopadhyay et al., 1992 |
| A. navahoensis | Averufin, norsolorinic acid, 6,7,8-trihydroxy-3-methylisocoumarin | Yamazaki et al. 1988 |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Echinocandin B | de la Cruz et al. 2012 | |
| Sterigmatocystin | Frisvad 1985 | |
| A. nidulans | Aloe-emodin, chrysophanol, cichorine, 2-ω-dihydroxyemodin, 3-(2,6-dihydroxyphenyl)-4-hydroxy-6-methyl-1(3H)-isobenzofuranone, emodic acid, emodin, emodin anthrone, endocrocin, endocrocin anthrone, ω-hydroxyemodin | Ahmed et al., 1987, Sanchez et al., 2011, Schroeckh et al., 2009 |
| Arugosin A | See under A. rugulosus | |
| Arugosin H | Nielsen et al. 2011 | |
| Ascoquinone A & B, Interasco A-C | Brown & Salvo 1994 | |
| Asperfuranone, preasperfuranone | Chiang et al. 2009 | |
| Asperline | Argoudelis and Zieserl, 1966, Argoudelis et al., 1965, Hamasaki et al., 1983 | |
| Aspernidine A & B | Scherlach et al. 2010 | |
| Asperthecin, preasperthecin | Howard and Raistrick, 1955, Frisvad, 1985, Brown and Salvo, 1994, Szewczyk et al., 2008, Chiang et al., 2010, Yin et al., 2013 | |
| Asperugin A & B | Ballantine et al., 1965, Ballantine et al., 1967 | |
| Aspoquinolone A-D, aniquinazoline A-D, aflaquinolone A, aniduquinolone A-C, 6-dexoyaflaquinolone E, isoaflaquinolone E, 14-hydroxyaflaquinolone F | Scherlach and Hertweck, 2006, An et al., 2013a, An et al., 2013b, Neff et al., 2012. These quinolones and quinazolins were isolated from strains that may be misidentified, the strains are not available | |
| Aspyridone A & B, preaspyridone | Bergmann et al. 2007 | |
| Atrochrysone, atrochrysone carboxylic acid | Klejnstrup et al. 2012 | |
| Austinol, dehydroaustinol, austinolide, austinoneol, 11β-hydroxyisoaustinone, isoaustinone, (5′-R)-isoaustinone, neoaustinone; preaustinoid A3, A4, A5, protoaustinoid A | Fukuyama et al., 1980, Maebayashi et al., 1982, Sanchez et al., 2011, Lo et al., 2012 | |
| Averufin | Ishida et al. 1972 | |
| 3-Benzyl-4-phenyl-2,5-furandione, 3-Carboxy-2,4-diphenyl-but-2-enoic anhydride | Hamasaki et al. 1983 | |
| Citreoisocoumarin | Watanabe et al., 1998, Watanabe et al., 1999 | |
| Cordycepin | Kodama et al., 1979, Yoshino, 1979 | |
| Cordyol C, C-10-deoxygerfelin | Sanchez et al., 2010, Nahlik et al., 2010 | |
| 2′-Deoxycoforycin = co-viderabin = pentostatin, 3-deoxyadenosine | Kaczka et al., 1964, Woo and Dion, 1974, Kodama et al., 1979 | |
| Desferritriacetylfusigen | Middleton et al., 1978, de la Cruz et al., 2012 | |
| Diorcinol, orcinol, orsellinaldehyde, orsellinic acid, violeceol I & II | Sanchez et al., 2010, Nahlik et al., 2010 | |
| Echinocandin B (only in CBS 240.90) | de la Cruz et al. 2012 | |
| Emericellamide A-F | Oh et al., 2007, Chiang et al., 2008 | |
| Emericellin = varicoxanthone B | Ishida et al., 1975a, Ishida et al., 1975b, Sanchez et al., 2011 | |
| Emeridine A-B, emeriphenolicin A-D, aspernidine A-B, austin, austinol, dehydroaustin, acetoxydehydroaustin | Zhang et al. 2011, identity of producer is questionable | |
| Emerin | Ishida et al., 1972, Ishida et al., 1975b | |
| Epishamixanthone | Sanchez et al. 2011 | |
| F-9775A, F-9775B, paeciloxanthone | Sanchez et al. 2010 | |
| Ferricrocin | Eisendle et al. 2003 | |
| Ferrirhodin | Fidelis et al. 1990 | |
| Gerfelin | Sanchez et al., 2010, Nahlik et al., 2010, Klejnstrup et al., 2012 | |
| 8-Hydroxy-1-(hydroxymethyl)-3-methyl-9H-xanthen-9-one | Sanchez et al. 2011 | |
| Hydroxypreaspyridone | Bringmann et al. 2003 | |
| Koninginin A, E, trichodermatide B, citranfidiol, (4S,5R)-4-hydroxy-5-methylfuran-2-one, gingerglycolipid B, flavuside B, (2S)bis[9Z]-1-O,2-O-dilinoleoyl-3-O-[α-d-galactopyranosyl-(1″->6′)β-d-galactopyranosyli-glycerol, (2S)-bis[9Z,12Z]-1-O-,2-O-dilinoleoyl-3-O-β-d-galactopyranosylglycerol | Tarawneh et al. 2013, identity of the producing strain is dubious | |
| Lecanoric acid | Schroeckh et al. 2009 | |
| Methyl-(2E,6E)-10,11-dihydroxy-3,7,11-trimethyl-2,6-dodecadienoate, methyl (2E,6E)-10,11-epoxid-3,7,11-trimethyl-2,6-dodecadienoate, methyl-(2E,6E)-10-hydroxy-11-formyl-3,7,11-trimethyl-2,6-dodecadienoate, methyl (2,6,10)-3,7,11-trimethyl-2,6-dodecadienoate | Nielsen et al. 2013 | |
| Monodictyphenone | Sanchez et al. 2011 | |
| Nidulol | Aucamp & Holzapfel 1968 | |
| Nidulotoxin2 | Lafont et al., 1970, Lafont and Lafont, 1970 | |
| Nidurufin, versicolourin A-C | Aucamp & Holzapfel 1970 | |
| Penicillin G | Dulaney, 1947a, Dulaney, 1947b, Holt and MacDonald, 1968 | |
| Sanghaspirodin A-B | Scherlach et al. 2011 | |
| Sporogenic fatty acids | Mazur et al. 1990 | |
| Sterigmatocystin | Aucamp and Holzapfel, 1970, Pachler et al., 1976, Cox and Cole, 1977, El-Khady and Hafez, 1981, Frisvad, 1985, Horie and Yamazaki, 1985, Hajjar et al., 1989 | |
| Terrequinone A | Bok et al. 2006 | |
| 6,7,9-Trihydroxy-3-methylcyclohepta(c)-pyran-8(1H)-one = antibiotic C | Turner & Aldridge 1983 | |
| Triacetylfusarinine | Eisendle et al. 2003 | |
| YWA1 & 2 | Watanabe et al., 1999, Fujii et al., 2001 | |
| A. olivicola | Aflatoxin B1 | Zalar et al. 2008 |
| Emericellin (as arugosin E) | Zalar et al. 2008 | |
| Shamixanthone | Zalar et al. 2008 | |
| Siderin | Zalar et al. 2008 | |
| Sterigmatocystin | Zalar et al. 2008 | |
| Terrein | Zalar et al. 2008 | |
| Varitriol | Zalar et al. 2008 | |
| A. pachycristatus (also as A. nidulans var. roseus or Emericella rugulosa) | Echinocandins | Dreyfuss (1986) as Emericella nidulans, Klich et al., 2001, Cacho et al., 2012, Matsuzawa et al., 2012, Tóth et al., 2011, Tóth et al., 2012 |
| A. pluriseminatus | Desferritriacetylfusigen | de la Cruz et al. 2012 |
| A. purpureus | Emindol SA, emindol SB, emindol SC | Kawai et al., 1994, Hosoe et al., 2006 |
| Epurpurin A-C | Takahashi et al. 1996 | |
| Sterigmatocystin | Horie & Yamazaki 1985 | |
| Variecolactone | Takahashi et al. 1999 | |
| Variecolin, variecolol | Kawai et al., 1994, Takahashi et al., 1999, Hosoe et al., 2006 | |
| A. quadrilineatus | Asperthecin | Howard and Raistrick, 1955, Neelakantan et al., 1957, Birkinshaw and Gourlay, 1961, Frisvad, 1985 |
| Averufin, 7-methoxyaverufin, sterigmatocystin, versicolourin | Ahmad & Sultana 1985 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Echinocandin B & E | de la Cruz et al. 2012 | |
| Emericellin = variacoxanthone B | Kralj et al. 2006 (as Emericella nidulans var acristata) | |
| Emestrin, aurantioemestrin, dethiosecoemestrin | Ooike et al. 1997 | |
| Emindol DA | Kralj et al. 2006 | |
| Microperfuranone | Kralj et al., 2006, Yeh et al., 2012 | |
| Penicillin G | Dulaney, 1947b, Gill-Carey, 1949 | |
| Quadrilineatin | Birkinshaw et al. 1957 | |
| Sterigmatocystin | Rabie et al., 1977, El-Khady and Hafez, 1981, Frisvad, 1985, Horie and Yamazaki, 1985, de la Cruz et al., 2012 | |
| Violaceic acid | Ooike et al. 1997 | |
| A. filifer | Shamixanthones | Zalar et al. 2008 |
| Varitriol | Zalar et al. 2008 | |
| A. rugulosus | Arugosin A, B, C | Ballantine et al., 1970, Ballantine et al., 1973, Chexal et al., 1975a, Kralj et al., 2006, Nielsen et al., 2011 |
| Asperthecin | Howard and Raistrick, 1955, Frisvad, 1985 | |
| Aspertetronin A & B | Ballantine et al. 1969 | |
| Cyclo-l-isoleucyl-l-proline, cyclo-l-leucyl-l-proline, cyclo-l-valyl-l-proline | Trigos et al. 2005 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| 3,3′-Dihydroxy-5,5′-dimethyldiphenyl ether, 2,4-dihydroxy-6-(hydroxymethyl)-benzaldehyde, 2,4-dihydroxy-6-methylbenzaldehyde, 2,4-dihydroxy-6-(hydroxymethyl)-benzaldehyde | Ballantine et al. 1968 | |
| Echinocandin B, C & D | Nyfeler & Keller 1974 (as Emericella nidulans var. echinulata = A. spinulosporus = A. delacroxii); Traber et al., 1979, Dreyfuss, 1986, Hodges et al., 2000, Bills et al. 2008, de la Cruz et al., 2012, Yue et al., 2015 | |
| Epishamixanthone, shamixanthone | Ishida et al., 1976, Ishida et al., 1978, Fukuyama et al., 1978, Malmstrøm et al., 2002a, Malmstrøm et al., 2002b | |
| 14-Hydroxytajixanthone 25-O-acetate, 14-Methoxytajixanthone 25-O-acetate | Chexal et al. 1975b | |
| 14-Methoxytajixanthone | Figueroa et al., 2009, Moosophon et al., 2009 | |
| Orsellinaldehyde | Ballantine et al. 1968 | |
| Penicillin G | Dulaney et al. 1947b | |
| Ruguloxanthone | Moosophon et al. 2009 | |
| Sterigmatocystin | Rabie et al., 1977, El-Khady and Hafez, 1981, Frisvad, 1985, Horie and Yamazaki, 1985 | |
| A. spinulosporus = A. delacroxii | Asperthecin | Frisvad 1985 |
| Echinocandin B1 | Benz et al. 19741 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Sterigmatocystin | Frisvad 1985 | |
| A. stella-maris | Emericellin (as arugosin E) | Zalar et al. 2008 |
| Shamixanthone | Zalar et al. 2008 | |
| A. stellatus | Ajamxanthone, shamixanthone, tajixanthone, tajixanthone hydrate, tajixanthone methanoate, 19-O-methyl-22-methoxypre-shamixanthone, pre-shamixanthone, 15-acetyltajixanthone hydrate | Kamal et al., 1970c, Kamal et al., 1971a, Kamal et al., 1971b, Holker et al., 1974, Chexal et al., 1974, Chexal et al., 1975b, Malmstrøm et al., 2002a, Malmstrøm et al., 2002b, Pornpakakul et al., 2006, Fredimoses et al., 2014, Wu et al., 2015 |
| Altamashin2, aminin2, nasrin2, nazirin2 | Kamal et al. 1970b | |
| Andibenin A, B & C, andelesin A & B | Dunn et al., 1976, Dunn et al., 1978, Dunn et al., 1979, Simpson, 1979, Simpson et al., 1997 | |
| Anditomin | Bardshiri et al., 1980, Bartlett et al., 1981, Simpson, 1981, Simpson and Walkinshaw, 1981, Matsuda et al., 2014 | |
| Arugosin A, B | See under A. rugulosus | |
| Arugosin D | Chexal et al. 1975b | |
| Asperthecin | Frisvad 1985 | |
| Astellatol | Sadler and Simpson, 1989, Sadler and Simpson, 1992, Simpson, 1994 | |
| Astellolide A = parasiticolide A, astellolide B | Hamasaki et al., 1975, Gould et al., 1981 | |
| Asteltoxin | Kruger et al., 1979, Tadano et al., 1988 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Dihydroterrein | Malmstrøm et al., 2002a, Malmstrøm et al., 2002b | |
| Emericellin = variacoxanthone B, variacoxanthone A, C | Chexal et al., 1974, Chexal et al., 1975a, Sanchez et al., 2011 | |
| Emerixanthone A-D | Fredimoses et al. 2014 | |
| Emervaridione, varioxiranediol | Liangsakul et al. 2011 | |
| Epiisoshamixanthone | Kamal et al. 1971b | |
| 6-epiophiobolin C, G, K, N, ophiobolin C, G, H & K | Wei et al. 2004 | |
| Evariquinone | Bringmann et al. 2003 | |
| 2-Furanoic acid | Hicks and Feather, 1977, Bringmann et al., 2003 | |
| Islandicin | Ahmed et al. 1987 | |
| Isoemericellin | Bringmann et al. 2003 | |
| Kojic acid | Qureshi et al. 1968 | |
| 2-Methoxy-6-(3,4-dihydroxy-hepta-1,5-dienyl)benzyl alcohol | Dunn & Johnstone 1979 | |
| Najamxanthone, radixanthone, shahenxanthone | Kamal et al. 1972 | |
| Penicillin G | Dulaney 1947b | |
| Shimalactone A | Wei et al. 2005 | |
| Siderin | Chexal et al., 1975c, Dunn et al., 1979 | |
| Stellatic acid | Qureshi et al., 1980, Matsuda et al., 2015 | |
| Stellatin | Simpson 1978 | |
| Stromemycin | Hopmann et al., 2001, Bringmann et al., 2003 | |
| Terrein | Qureshi et al., 1968, Malmstrøm et al., 2002a, Malmstrøm et al., 2002b | |
| Variecoacetal A & B | Fujimoto et al. 2000 | |
| Variecolactone | Tezuka et al. 1998 | |
| Variecolin | Hensens et al., 1991, Yoganathan et al., 2004 | |
| Varioxirane, varixanthone, varitriol | Malmstrøm et al., 2002a, Malmstrøm et al., 2002b, Yue et al., 2015 | |
| Varioxiranol A-G | Wu et al. 2015 | |
| A. striatus | Asperthecin | Frisvad 1985 |
| Aurantioemestrin | Kawai et al. 1987 | |
| Cycloisoemericellin | Kawahara et al. 1988a | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Dithiosilvatin | Kawahara et al. 1987 | |
| Emericellin = variecoxanthone B | Nozawa et al., 1987c, Kawahara et al., 1988a | |
| Emestrin = striatin = EQ-1, dethiosecoemestrin, aurantioemestrin | Seya et al., 1985, Seya et al., 1986a, Seya et al., 1986b, Ooike et al., 1997 | |
| Emindol SA, emindol SB | Nozawa et al., 1987a, Nozawa et al., 1987b, Nozawa et al., 1988b | |
| 7-Hydroxyemodin | Bringmann et al. 2003 | |
| Paxillin, 1-O-acetylpaxillin | Nozawa et al., 1987b, Nozawa et al., 1989 | |
| Penicillin G | Dulaney 1947b | |
| Sterigmatocystin | Horie & Yamazaki 1985 | |
| Violaceic acid, violaceol I & II | Ooike et al. 1997 | |
| A. unguis | Agonodepside A & B | Cao et al. 2002 |
| Aspergillusether, aspergillusidone A-G, aspergillusphenol A & B, 2,4-dichlorounguinol | Sureram et al. 2012 | |
| 3-Chlorounguinol | Kawahara et al. 1988b | |
| Dechloronidulin | Dean et al., 1954, Dean et al., 1960, Beach and Richards, 1963 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| 3,5-Dibromo-2,4-dihydroxy-6-methyl benzoic acid methylester | Nicollier et al. 1978 | |
| Emeguisin A-C | Kawahara et al. 1988c | |
| 3-ethyl-5,7-dihydroxy-3,6-dimethylphthalide | Kawahara et al., 1988b, Kawahara et al., 1988c, Nielsen et al., 1999 | |
| Folipastatin | Hamano et al. 1992 | |
| Guisinol | Nielsen et al. 1999 | |
| Haiderin, khahilin2, sarwin2, shahidin2, yazidin2 | Kamal et al. 1970b | |
| Nidulin, nornidulin = ustin, dihydronornidulin = shirin | Dean et al., 1954, Dean et al., 1960, Beach and Richards, 1963, Kamal et al., 1970a, Kawahara et al., 1988b | |
| Penicillin G | Dulaney 1947b | |
| Rubinin | Kamal et al. 1972 | |
| Trisdechloronornidulin | Sierankiewicz & Gatenbeck 1972 | |
| Unguinol = yasminin = trisdechloronidulin | Stodola et al., 1972, Kawahara et al., 1988b | |
| Unguisin A-E | Malmstrøm, 1999, Malmstrøm et al., 2002a, Malmstrøm et al., 2002b, Liu and Shen, 2011 | |
| Unguispyrone | Sureram et al. 2012 | |
| A. venezuelensis | Aflatoxin B1, sterigmatocystin | Frisvad & Samson 2004 |
| A. violaceus | Aspermutarubrol = ethericin A = violaceol I | Shibata et al. 1978 (for A. sydowii), Taniguchi et al. 1978 (for A. sydowii), König et al., 1978, Yamazaki and Maybayashi, 1982b |
| Asperthecin | Frisvad 1985 | |
| Desferritriacetylfusigen | de la Cruz et al. 2012 | |
| Sterigmatocystin | El-Khady & Hafez 1981 | |
| Violaceol I & II, violaceic acid | Yamazaki and Maybayashi, 1982a, Yamazaki and Maybayashi, 1982b, Asami et al., 2012 |
1The strain of Emericella echinulata = A. spinulosporus = A. delacroxii of Benz et al. 1974 was claimed to be an A. rugulosus (Emericella rugulosa) by Dreyfuss 1986.
2Not structure elucidated.
The mycotoxin sterigmatocystin has not only been found in most species of sections Nidulantes, Aenei and Usti, but also in species of section Ochraceorosei (Table 7) (Frisvad, 1985, Horie and Yamazaki, 1985, Rank et al., 2011, Jurjevic et al., 2013). In Aspergillus section Nidulantes 35 species could produce sterigmatocystin, four additional species (A. multicolor, A. purpureus, A. stellatus and A. violaceus) have been reported to produce sterigmatocystin, but this could not be confirmed here, and two species remains to be examined for production of sterigmatocystin (A. omanensis and A. sulphureoviridis) (Table 7). Aflatoxin B1 is produced by four species: A. astellatus, A. miraensis, A. olivicola, and A. venezuelensis. This is the first report of aflatoxin production by A. miraensis. Other mycotoxins are also produced by a few species in section Nidulantes, such as verruculogen and fumitremorgins in A. caespitosus and asteltoxin produced by A. olivicola, A. qinqixianii, A. stellatus and A. filifer. Thus the most important mycotoxins in Aspergillus section Nidulantes are aflatoxins, sterigmatocystin, emestrin, fumitremorgins, asteltoxins, and paxillin while other extrolites are useful drugs or drug lead candidates such as echinocandins, mulundocandins, calbistrins, varitriols, variecolins and terrein, and some can be regarded as both mycotoxins and drug lead candidates, for example viridicatumtoxin. It is interesting to note that many of these compounds are also produced by other Aspergillus species in phylogenetically different subgenera, showing that species in section Nidulantes are quite closely related to these other species in many features (Frisvad & Larsen 2015).
Table 7.
Sterigmatocystin producers in Aspergillus subgenus Nidulantes (Frisvad, 1985, Frisvad and Samson, 2004, Frisvad et al., 2004, 2005, Horie and Yamazaki, 1985, Hubka et al., 2016, Jurjevic et al., 2013, Rabie et al., 1977, Rank et al., 2011, Varga et al. 2009, Varga et al., 2010a, Varga et al., 2010b).
| Species name |
|---|
| Section Aenei: |
| Aspergillus bicolor |
| A. discophorus |
| A. eburneocremeus |
| A. foeniculicola |
| A. spectabilis |
| Section Nidulantes: |
| A. askiburgiensis |
| A. spelunceus |
| A. amoenus |
| A. asperescens (reported here) |
| A. astellatus |
| A. aurantiobrunneus |
| A. aurantiopurpureus (reported here) |
| A. aureolatus (reported here) |
| A. corrugatus |
| A. creber |
| A. croceus |
| A. cvjetkovicii |
| A. falconensis |
| A. foveolatus |
| A. fruticulosus |
| A. fructus |
| A. jensenii |
| A. latus |
| A. miraensis (reported here) |
| A. multicolor (not confirmed) |
| A. navahoensis |
| A. nidulans |
| A. olivicola |
| A. pachycristatus |
| A. protuberus |
| A. purpureus (not confirmed) |
| A. puulaauensis |
| A. quadrilineatus |
| A. rugulosus |
| A. spinulosporus |
| A. stella-maris |
| A. stellatus (not confirmed) |
| A. striatus |
| A. subversicolor |
| A. tennesseensis |
| A. venenatus |
| A. venezuelensis |
| A. versicolor |
| A. violaceus (not confirmed) |
| Section Ochraceorosei: |
| A. ochraceoroseus |
| A. rambellii |
| Section Usti: |
| A. heterothallicus |
| A. ustus (trace; not confirmed in later studies) |
Discussion
Sectional classification in subgenus Nidulantes
Based on a multigene phylogeny, nine sections are proposed within subgenus Nidulantes. Eight of them were introduced in previous studies (Peterson, 2008, Peterson et al., 2008, Varga et al., 2010a, Varga et al., 2010b). Five species previously assigned in section Usti, namely A. californicus, A. cavernicola, A. egyptiacus, A. kassunensis and A. subsessilis form a monophyletic clade outside section Usti. The bootstrap support of this distinct clade is low (Hubka et al. 2016), but the species within this clade do share some phenotypic characters, most of them (except A. californicus) produce short conidiophores, which is not common in section Usti. Based on these observations, we propose Aspergillus section Cavernicolus to accommodate these species within subgenus Nidulantes. Section Aenei was included in section Nidulantes as Aspergillus aeneus clade (Hubka et al. 2016). However in our study, section Aenei locates outside section Nidulantes with full support, which agrees with Varga et al. (2010a). Phenotypically the homothallic species in section Aenei (A. discophorus, A. bicolor, A. spectabilis and A. foeniculicola) produce similar ascospores with A. nidulans clade, but none of them is able to grow at 40 °C. Based on these observations, section Aenei is kept to accommodate these species. The placement of A. funiculosus in a certain group is doubtful, Raper & Fennell (1965) accepted A. funiculosus as the only uniseriate species in section Sparsi (Aspergillus sparsus group), our phylogenetic results show that A. funiculosus is more closely to A. ochraceoroseus, however is not supported by bootstrap and Bayesian statistics as shown by Hubka et al. (2016), the belonging of this species needs further study.
Clade classification in section Nidulantes
Matsuzawa et al. (2012) performed the first multilocus analysis based on BenA, CaM and actin in the genus Emericella, eight clades were introduced for 37 species, clades I, II, III, IV, V and VI are equal with our A. nidulans clade, clades VII and VIII are equal with our A. stellatus clade, anamorphic species were not included in their analysis. Hubka et al. (2016) constructed a phylogenetic analysis for section Nidulantes, six statistically supported clades were designated, namely clades A. aeneus, A. spelunceus, A. versicolor, A. stellatus, A. nidulans and A. unguis, five of them are confirmed in our study, while Aspergillus aeneus clade is treated as section Aenei as discussed above. Besides these clades, additional two clades are introduced here, namely clades A. aurantiobrunneus and A. multicolor. The A. aurantiobrunneus clade contains A. aurantiobrunneus and A. purpureus. Hubka et al. (2016) included A. aurantiobrunneus in A. spelunceus clade although the grouping was not well supported (100/80/1 ML/MP/PP), A. purpureus was not included in their study. In our study, these two species form a full supported branch (1 pp, 100 % ML), morphologically they all produce globose and subglobose ascospores and grow restrictedly on all tested media. In contrast all species in A. spelunceus clade are anamorphic, and grow faster. Another newly introduced clade is A. multicolor clade, this clade contains A. multicolor, A. mulundensis and A. pluriseminatus, and is close related with A. nidulans clade. No species in this clade grow at 40 °C, A. pluriseminatus, the only homothallic species in this clade produces stellate ascospores, which show more similarity with species in A. stellatus clade.
Aspergillus section Nidulantes
= Emericella Berk., Intr. Crypt. Bot. (London): 340. 1857
= Theclospora Harkn., Bulletin of the California Academy of Sciences 1: 41. 1884
= Diplostephanus Langeron, Crypt. Fr. Exs.: 344. 1922
Anamorph present or absent, if present conidiophores more or less brown-pigmented, typically smooth but occasionally showing protuberances, usually sinuous; vesicles usually globose, subglobose or subclavate, biseriate, metulae and phialides usually about equal in length. Conidia globose to subglobose, ovate to ellipsoidal, echinulate or finely rough, less commonly smooth. Ascomata produced in most species, but lacking in others; emericella-like, cleistothecial, superficial, solitary or clustered, globose to subglobose, non-ostiolate, reddish brown, violet, dark brown or blackish, typically surrounded by a heavy to discontinuous layer of Hülle cells; Hülle cells hyaline, pale brown, orange brown or pink, globose, subglobose, pyriform or ovoid. Asci 8 spored, globose to subglobose or stellate, evanescent. Ascospores one-celled, orange, purplish, violet, reddish brown or brown, globose to subglobose or stellate, usually with equatorial crests, smooth or with different patterns of ornamentation, entire, dentate, defective or with filiform appendages.
Typus: Aspergillus nidulans (Eidam) G. Winter, Rabenh. Krypt.-Fl., ed. 2, 1: 62. 1884
Aspergillus nidulans clade
Description: Conidiophores (if present) typically smooth but occasionally showing surface protuberances, hyaline to yellowish brown; vesicles globose to subclavate, fertile over the upper half to two thirds; Conidia echinulate, globose to subglobose. Ascomata (if present), cleistothecial, superficial, reddish brown, violet or dark brown, globose to subglobose, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose, subglobose pyriform or ovoid. Asci 8 spored, globose to subglobose. Ascospores orange, reddish brown, brown or violet, in surface view globose to subglobose, spore bodies smooth or with verrucose, echinulate, reticulate or pitted ornamentation. Ascospore crests entire, dentate, defective or with irregular protuberance, inconspicuous in some species, mostly two in number, four crests are present in A. quadrilineatus. Most species grow optimally around 37 °C, do not grow at or above 50 °C, A. botswanensis, A. fruticulosus, A. latilabiatus and A. recurvatus do not grow at 45 °C (Table 5). Twenty-three species are accepted in this clade, 22 of them are homothallic, A. recurvatus is the only anamorphic species.
Accepted species:
Aspergillus botswanensis A.J. Chen, Frisvad & Samson, this study. [MB816095].
Aspergillus corrugatus Udagawa & Y. Horie, Mycotaxon 4: 535. 1976. [MB309216].
Aspergillus desertorum (Samson & Mouch) Samson, Visagie & Houbraken, Stud. Mycol. 78: 155. 2014. [MB809587].
Aspergillus falconensis Y. Horie et al., Trans. Mycol. Soc. Japan 30: 257. 1989. [MB127891].
Aspergillus foveolatus Y. Horie, Trans. Mycol. Soc. Japan 19: 313. 1978. [MB309221].
Aspergillus fruticulosus Raper & Fennell, Gen. Aspergillus: 506. 1965. [MB326630].
Aspergillus jaipurensis Samson, Visagie & Houbraken, Stud. Mycol. 78: 155. 2014. [MB809592].
Aspergillus latilabiatus A.J. Chen, Frisvad & Samson, this study. [MB816093].
Aspergillus latus (Thom & Raper) A.J. Chen, Frisvad & Samson, comb. nov., this study. [MB816100].
Aspergillus navahoensis M. Chr. & States, Mycologia 74: 226. 1982. [MB110496].
Aspergillus nidulans (Eidam) G. Winter, Rabenh. Krypt.-Fl., ed. 2, 1: 62. 1884. [MB182069].
Aspergillus omanensis Y. Horie & Udagawa, Mycoscience 36: 391.1995. [MB414655].
Aspergillus pachycristatus Matsuzawa, Y. Horie & Yaguchi, Mycoscience 53: 439. 2012. [MB580944].
Aspergillus quadrilineatus Thom & Raper, Mycologia 31: 660. 1939. [MB275888].
Aspergillus recurvatus Raper & Fennell, Gen. Aspergillus: 529. 1965. [MB326653].
Aspergillus rugulosus Thom & Raper, Mycologia 31: 660. 1939. [MB277104].
Aspergillus savannensis A.J. Chen, Frisvad & Samson, this study. [MB816096].
Aspergillus aurantiopurpureus A.J. Chen, Frisvad & Samson, this study. [MB816087].
Aspergillus stercorarius A.J. Chen, Frisvad & Samson, this study. [MB816094].
Aspergillus striatus J.N. Rai, J.P. Tewari & Mukerji, Can. J. Bot. 42: 1521. 1964. [MB326659].
Aspergillus spinulosporus Hubka, S.W. Peterson & M. Kolařík, Plant Syst. Evol. 302: 1290. 2016. [MB816282].
Aspergillus sulphureoviridis A.J. Chen, Frisvad & Samson, this study. [MB816097].
Aspergillus violaceus Fennell & Raper, Mycologia 47: 75. 1955. [MB292863].
Aspergillus stellatus clade
Description: Conidiophores (if present) smooth, hyaline to yellowish brown; vesicles globose to subclavate, fertile over the upper half to two thirds; Conidia echinulate, globose to subglobose. Ascomata (if present), cleistothecial, superficial, reddish brown, violet or dark brown, globose to subglobose, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose, subglobose or ovoid. Asci 8 spored, subglobose to polygonal or stellate. Ascospores orange, reddish brown, brown or violet brown, globose, stellate or appendaged. Most species do not grow at 40 °C, three species (A. astellatus, A. miraensis and A. stella-maris) do not grow at 37 °C (Table 5). Twelve species are accepted in this clade, 11 of them are homothallic, A. caespitosus is the only anamorphic species.
Accepted species:
Aspergillus angustatus A.J. Chen, Frisvad & Samson, this study. [MB816090].
Aspergillus astellatus (Fennell & Raper) Houbraken, Visagie & Samson, Stud. Mycol. 78: 154. 2014. [MB809577].
Aspergillus caespitosus Raper & Thom, Mycologia 36: 563. 1944. [MB284298].
Aspergillus dromiae A.J. Chen, Frisvad & Samson, this study. [MB816089].
Aspergillus filifer Zalar, Frisvad & Samson, Mycologia 100: 787. 2008. [MB507357].
Aspergillus miraensis (Zhang, Chen & Guo) Hubka, S.W. Peterson & M. Kolařík, Plant Syst. Evol. 302: 1288. 2016. [MB816283].
Aspergillus olivicola Frisvad, Zalar & Samson, Mycologia 100: 781. 2008. [MB507362].
Aspergillus qinqixianii Y. Horie, Abliz & R.Y. Li, Mycoscience 41: 183. 2000. [MB464660].
Aspergillus stella-maris Zalar, Frisvad & Samson, Mycologia 100: 789. 2008. [MB507363].
Aspergillus stellatus Curzi, C.R. Accad. Lincei 19: 428. 1934. [MB254841].
Aspergillus undulatus H.Z. Kong & Z.T. Qi, Acta Mycol. Sin. 5: 211. 1986. [MB129004].
Aspergillus venezuelensis Frisvad & Samson, Syst. Appl. Microbiol. 27: 678. 2004. [MB368544].
Aspergillus versicolor clade
Description: Fide Jurjevic et al. (2012) conidiophores smooth to tuberose, hyaline to yellow or brownish; vesicles pyriform, spatulate or subglobose, fertile over half, two thirds or entire vesicle; Conidia smooth, spinulose or finely roughened, globose, subglobose or ellipsoidal. Hülle cells present in six species: A. cvjetkovicii, A. fructus, A. griseoaurantiacus, A. protuberus, A. puulaauensis and A. venenatus, hyaline, globose, subglobose, elliosoidal or pyriform. All species do not grow at 37 °C. Sixteen species are accepted, all of them are anamorphic species. (Jurjevic et al., 2012, Visagie et al., 2014, Tsang et al., 2016).
Accepted species:
Aspergillus amoenus M. Roberg, 1931, Hedwigia. 70: 138. 1931. [MB250654].
Aspergillus austroafricanus Jurjevic, S.W. Peterson & B. W. Horn, IMA Fungus 3: 67. 2012. [MB800597].
Aspergillus creber Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 69. 2012. [MB800598].
Aspergillus cvjetkovicii Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 69. 2012. [MB800599].
Aspergillus fructus Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 70. 2012. [MB800600].
Aspergillus griseoaurantiacus Visagie, Hirooka & Samson, Stud. Mycol. 78: 112. 2014. [MB809197].
Aspergillus hongkongensis Tsang et al. Diagn. Microbiol. Infect. Dis. 84: 130. 2016. [MB810279].
Aspergillus jensenii Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 70. 2012. [MB800601].
Aspergillus protuberus Munt.-Cvetk., Mikrobiologia. 5: 119. 1968. [MB326650].
Aspergillus puulaauensis Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 71. 2012. [MB800602].
Aspergillus subversicolor Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 72. 2012. [MB800603].
Aspergillus sydowii (Bainier & Sartory) Thom & Church, The Aspergilli: 147. 1926. [MB279636].
Aspergillus tabacinus Nakazawa, Y. Takeda, Simo & A. Watanabe, J. Agric. Chem. Soc. Japan 10: 177. 1934. [MB539544].
Aspergillus tennesseensis Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 73. 2012. [MB800604]
Aspergillus venenatus Jurjevic, S.W. Peterson & B.W. Horn, IMA Fungus 3: 73. 2012. [MB800605]
Aspergillus versicolor (Vuill.) Tirab., Annali Bot.7: 9. 1908. [MB172159].
Aspergillus spelunceus clade
Description: Conidiophores smooth, hyaline to yellowish brown; vesicles globose to subclavate, fertile over the upper half to whole surface; Conidia echinulate, globose, subglobose to ellipsoidal (in A. asperescens, conidia are smooth in young cultures but turn to rough in old cultures). Hülle cells present in three species: A. askiburgiensis, A. asperescens, and A. spelunceus, hyaline, globose, subglobose or ovoid. Most species do not grow at 37 °C (Hubka et al. 2016 reported several A. asperescens strains with restrict growth at 37 °C). Six species are accepted, all of them are anamorphic species.
Accepted species
Aspergillus askiburgiensis A. Nováková, Hubka, Frisvad, S.W. Peterson & M. Kolařík, Plant Syst. Evol. 302: 1285. 2016. [MB816280].
Aspergillus asperescens Stolk, Antonie van Leeuwenhoek 20: 303. 1954. [MB809583].
Aspergillus aureolatus Munt.-Cvetk. & Bata, Bull. Inst. Jard. Bot. Univ. Beograd 1: 196. 1964. [MB326614].
Aspergillus spelunceus Raper & Fennell [as ‘speluneus’], Gen. Aspergillus: 457. 1965. [MB326656].
Aspergillus varians Wehmer, Bot. Centralbl. 80: 460. 1899. [MB172782].
Aspergillus viridicatenatus A.J. Chen, Frisvad & Samson, this study [MB816088].
Aspergillus multicolor clade
Description: Conidiophores smooth, hyaline to yellowish brown; vesicles globose to subclavate, fertile over the two thirds; Conidia echinulate, globose to subglobose. Ascomata (only reported in A. pluriseminatus), superficial, globose, nonostiolate, blackish, produced very late, appearing after 2–3 months, surrounded by hyphae and Hülle cells; Hülle cells present in two species: A. pluriseminatus and A. multicolor, pale yellowish brown, orange brown to pink, globose, subglobose or ovoid. Ascospores violet-brown, stellate. Most species do not grow at 40 °C, A. pluriseminatus does not grow at 37 °C. Three species are accepted, A. pluriseminatus is homothallic, A. multicolor and A. mulundensis are anamorphic species.
Accepted species
Aspergillus unguis clade
Description: Conidiophores smooth, hyaline to yellowish brown; vesicles globose to subclavate, fertile over the upper half to one third; Conidia smooth to echinulate, globose to subglobose. Most species do not grow at 40 °C (Hubka et al. 2016 reported several A. unguis strains with restrict growth at 40 °C), two species: A. croceus and A. israelensis do not grow at 37 °C. Three species are accepted, A. croceus and A. israelensis are anamorphic, all A. unguis strains observed in this study are anamorphic, but A. unguis NRRL 2393 was reported to tardily produce ascospores (Fennell and Raper, 1955, Hubka et al., 2016).
Accepted species
Aspergillus aurantiobruneus clade
Description: Grow restrictedly on all tested media, anamorphic structures are hardly produced. Conidiophores smooth, hyaline to pale brown; vesicles globose to subclavate, fertile over two thirds to whole surface; Conidia echinulate, globose, subglobose, ellipsoidal to cylindrical. Ascomata cleistothecial, superficial, reddish brown, globose to subglobose, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose, subglobose or ovoid. Asci 8 spored, globose to subglobose. Ascospores light orange to brown, globose to subglobose. All species do not grow at 37 °C. Two homothallic species are accepted in this clade.
Accepted species
Phylogenetic species recognition
Based on a concatenated sequence analysis, 65 species are well resolved in section Nidulantes. ITS, the recommended official DNA barcode for fungi (Schoch et al. 2012), performs well in recognizing species in clades A. unguis, A. spelunceus and A. multicolor. However it is not variable sufficiently for recognizing species in other clades. BenA, CaM and RPB2 can identify 63 species respectively, with A. quadrilineatus sharing identical BenA with A. latus, A. qinqixianii and A. filifer sharing identical CaM and A. rugulosus and A. pachycristatus sharing identical RPB2 sequences. Matsuzawa et al. (2012) stated that A. nidulans (= E. nidulans), A. dentatus (= E. dentata), A. latus (= E. nidulans var. lata), A. sublatus (= E. sublata), A. montenegroi (= E. montenegroi), A. acristatus (= E. acristata), A. miyajii (= E. miyajii), A. quadrilineatus (= E. quadrilineata) and A. parvathecius (= E. parvathecia) were undistinguishable by phylogenetic analysis alone. These are confirmed in this study, to follow the genealogical concordance phylogenetic species recognition concept (GCPSR), several species are considered as synonyms here: A. dentatus is synonymised with A. nidulans; A. sublatus and A. montenegroi are synonymised with A. latus; A. acristatus, A. miyajii and A. parvathecius are synonymised with A. quadrilineatus as did Hubka et al. 2016. Overall species in A. nidulans clade are phylogenetically similar, both phylogenetic and morphological data are important to define the species boundary. Speices in other six clades are more divergent phylogenetically, the only exceptions are A. qinqixianii and A. filifer, they share identical CaM sequences, only small differences are present in BenA (99.7 % similarity, 344/345 bp), actin (98.9 % similarity, 366/370 bp) (Matsuzawa et al., 2012, Hubka et al., 2016) and RPB2 (99.7 % similarity, 912/914 bp).
Morphological species recognition
For homothallic species in section Nidulantes, the ascospore shape, ornamentation, color and size are of particular importance for differentiating species (Thom and Raper, 1939, Christensen and Raper, 1978, Horie, 1980, Christensen and States, 1982, Ismail et al., 1995, Zalar et al., 2008, Matsuzawa et al., 2012, Guarro et al., 2012). In A. nidulans clade, most species have unique ascospore morphology (Fig. 3, Fig. 4, Fig. 5). However in some species, ascospore morphology shows a range of diversity. For example most of A. nidulans strains have entire crests, but atypical dentate crests are presented in one strain (CBS 114.63), similarly in A. quadrilineatus, the crests in ascospores can be entire, defective or with irregular protuberance. In A. stellatus clade, species with stellate ascospores are morphologically very similar, molecular identification is recommended for distinguishing these species. Anamorphic structures are also important for species recognition, especially for anamorphic species. But the anamorphic structures can be affected by media and incubation conditions, here we follow the standardized method for laboratories working with Aspergillus (Samson et al. 2014), MEA is recommended for anamorphic description and OA is recommended for teleomorphic description in section Nidulantes.
Species descriptions
Aspergillus angustatus A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816090. Fig. 13.
Fig. 13.
Aspergillus angustatus CBS 273.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–D. Conidiophores and conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
Etymology: The name refers to the narrow vesicles of the aspergilla.
Diagnosis: Moderately dense or dense sporulation on CYA, MEA and YES, stellate ascospores and narrow vesicles measuring 8–12 μm.
Typus: Mali, Mangifera indica root, isolated by I.F.C. (holotype CBS H-22487, culture ex-type CBS 273.65 = DTO 319-H8).
ITS barcode: EU448283. (Alternative markers: BenA = AY339993; CaM = EU443984; RPB2 = KU867013).
Colony diam, 7 d (mm): CYA 37–38; CYA 37 °C 16–17; CYA 40 °C No growth; MEA 48–50; MEA 37 °C 1–2; OA 42–44; YES 52–53; CREA 13–15; CYAS 33–34; DG18 27–28.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white to light yellow; texture velvety to granular due to ascomata production; sporulation moderately dense, conidia en masse olive green; soluble pigments absent; exudates clear droplets; reverse dark brown at centre, light buff at edge; ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse dark green; soluble pigments absent; exudates clear to light yellow droplets; reverse dark brown at centre, cream yellow at edge; ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white to light yellow; texture granular due to ascomata production; sporulation moderately dense, conidia en masse olive green; soluble pigments absent; exudates absent; reverse dark brown at centre, cream yellow at edge; ascomata present after 1 wk. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse pale green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates clear droplets; reverse pale brownish green; ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown, globose to subglobose, 430–780 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose to ovoid, 17–35 μm. Asci 8 spored, stellate. Ascospores orange to reddish brown, in surface view stellate, 9–12 μm; spore bodies smooth, globose to subglobose, 3–4 × 3–3.5 μm; in side view broadly lenticular, with two stellate equatorial crests; undissected part of crests 0.5–1 μm broad, with 1.5–3 μm long extensions; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, pale brown, 200–400 × 4.5–6 μm; vesicles hyaline to pale brown, subglobose to subclavate, 8–12 μm wide, fertile over the upper half; metulae hyaline, 6–8 × 3–4.5 μm; phialides hyaline, flask-shaped, 7–8.5 × 2.5–3.5 μm. Conidia echinulate, globose to subglobose, 3–4.5 μm, green in mass.
Extrolites: asperthecin, a desertorin, emericellin, 2-ω-hydroxyemodin, shamixanthones.
Distinguishing characters: Aspergillus angustatus is morphologically and phylogenetically closely related to A. dromiae; however, A. angustatus sporulates well on CYA, MEA and YES, compared to the absent sporulation in A. dromiae. Furthermore, A. dromiae has wider vesicles (12–17 μm) than A. angustatus.
Aspergillus askiburgiensis A. Nováková, Hubka, Frisvad, S.W. Peterson & M. Kolařík, Plant Syst. Evol. 302: 1285. MycoBank MB816280.
Typus: Holotype PRM 924055; isotype 924056. Culture ex-type: CBS 134374 = CCF 4716 = CCF 4428 = NRRL 62818 = DSM 871 = IBT 33114 = IBT 32911.
ITS barcode: LN873939. (Alternative markers: BenA = LN873952; CaM = LN873965; RPB2 = LN873984).
Colony characters: Fide Hubka et al. (2016) colonies of both isolates assigned to A. askiburgiensis show numerous differences, and they are described separately. Colonies of CCF 4716T on CYA at 25 °C attained 24–35 mm diam in 14 days (19–20 mm in 7 days), velutinous, irregularly wrinkled with margin submerged 2–3 mm, pale orange yellow (ISCC–NBS No. 73) with olive-gray (113) marginal parts, sporulation visible, olive-gray, no exudate, dark orange yellow (72) soluble pigment, reverse strong brown (55) to dark brown (59) with strong orange yellow margin (68). Colonies of CCF 4085 attained 18–19 mm diam in 14 days (12–13 mm in 7 days), floccose, plane to irregularly wrinkled, moderate yellow (87) with greyish olive (110) to dark olive (108) tint in central part (sporulation), 1 mm broad marginal zone yellowish white (92), no exudate or small brownish orange (54) droplets, reverse moderate yellowish brown (77) with light orange yellow 2–3 mm margin, no soluble pigment. No growth on CYA at 37 °C. Colonies of CCF 4716T on MEA at 25 °C attained 23–28 mm diam in 14 days (15–18 mm in 7 days), plane to very slightly furrowed, velutinous, yellowish white (92) to pale yellow (89), no exudate, no soluble pigment, reverse deep orange yellow (69) with vivid yellow margin (82). Colonies of CCF 4085 attained 14–15 mm diam in 14 days (10–11 mm in 7 days), plane, with 2 mm broad colorless leather-like marginal zone, colony centre velutinous (good sporulation), moderate olive brown (95) to moderate olive (107), no exudate, no soluble pigment, reverse light greyish olive (109) with moderate yellow colony centre and colorless margin. Colonies of CCF 4716T on CZA at 25 °C attained 15–16 mm diam in 14 days (9–10 mm in 7 days), effuse, plane, yellowish white (92) with dark greyish yellowish brown (81) colored ring (sporulation) in the colony centre (6–8 mm diam), no exudate, brilliant yellow orange (67) soluble pigment, reverse deep yellowish brown (75) with dark yellowish brown (78) colony centre. Colonies of CCF 4085 attained 19–20 mm diam in 14 days (10–11 mm in 7 days), submerged, plane, moderate olive (107) to dark olive (108) sporulation, no exudate, no soluble pigment, reverse colorless. Colonies of CCF 4716T on CREA at 25 °C attained 15–17 mm diam in 14 days (10–11 mm in 7 days), effuse, yellowish white (92) to greyish greenish yellow (105), no acid production. Colonies of CCF 4085 attained 17–18 mm diam in 14 days (11–12 mm in 7 days), submerged, plane, good sporulation in colony centre, no exudate, no soluble pigment, no acid production.
Micromorphology: Fide Hubka et al. (2016) stipes on MEA brown, smooth, non-septate or occasionally with septum, most commonly 40–180 × 3–8.5 μm diam, diminutive conidiophores occasionally present; vesicles pyriform, subglobose, less frequently globose, 5.5–18.5 μm diam; biseriate; metulae cylindrical, 4–6 μm long, covering 1/2–3/4 of the vesicles; phialides ampulliform, 5–8 μm long; conidia subglobose or globose, green in mass, 2.5–4 (–4.5) μm diam, first almost smooth or finely roughened but later definitely spinulose. Hülle cells arranged in clusters, ellipsoidal or pyriform, 16–24 × 10–16.5 μm, or subglobose to globose, 11–20 μm diam, produced after 14 or more days of cultivation at 25 °C. Sexual state not observed.
Extrolites: Fide Hubka et al. (2016) sterigmatocystin, versicolorins, cf. monascorubramin.
Distinguishing characters: This species is closely related to A. spelunceus, A. asperescens and A. aureolatus, but A. spelunceus produces longer conidiophores (130–300 μm), A. asperescens produces larger ellipsoidal conidia (4–7 × 3–5 μm) and A. aureolatus is characterized by orange marginal zone of colonies.
Notes: Aspergillus askiburgiensis was described from European caves. For an illustration of the species, readers are referred to Hubka et al. (2016).
Aspergillus asperescens Stolk, Antonie van Leeuwenhoek 20: 303. 1954. MycoBank MB809583. Fig. 14.
Fig. 14.
Aspergillus asperescens CBS 110.51T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–F. Conidiophores. G. Conidia. Scale bars: B = 30 μm; C–E,G = 10 μm; F = 8 μm.
Typus: IMI 46813. Culture ex-type: CBS 110.51 = NRRL 2252 = NRRL 4770 = ATCC 11079 = DSM 871 = IMI 046813 = QM 1946 = WB 2252 = WB 4770 = WB 5038 = IBT 19363 = DTO 021-F4.
ITS barcode: EF652475. (Alternative markers: BenA = EF652299; CaM = EF652387; RPB2 = EF652211).
Colony diam, 7 d (mm): CYA 23–29; CYA 37 °C No growth; CYA 40 °C No growth; MEA 22–29; MEA 37 °C No growth; OA 23–27; YES 27–30; CREA 11–14; CYAS 17–20; DG18 10–15.
Colony characters: CYA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture velvety; sporulation moderately dense, conidia en masse olive; soluble pigments absent; exudates absent; reverse buff. MEA 25 °C, 7 d: Colonies moderately deep, plane to slightly sulcate; margins entire; mycelium white; texture velvety to floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse pale brown to brown. YES 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture velvety; sporulation dense, conidia en masse olive; soluble pigments absent; exudates absent; reverse pale brown. DG18 25 °C, 7 d: Colonies moderately deep, plane to slightly sulcate; margins entire; mycelium white; texture velvety to floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse yellow green at centre, cream white at edge. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse pale yellow green. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, yellowish brown, 200–400 × 6–8 μm; vesicles hyaline to pale yellowish brown, hemispherical to globose, 8–15 μm wide, fertile over the upper half; metulae hyaline to pale yellowish brown, 6–9 × 3–4 μm; phialides hyaline to pale yellowish brown, flask-shaped, 7.5–9 × 3–4 μm. Conidia in young cultures subglobose to ellipsoidal, smooth, 4–7 × 3–5 μm, in cultures older than two wks, rough conidia are formed.
Extrolites: a calbistrin, dehydroaustin, sterigmatocystin, versicolorins, violaceols.
Distinguishing characters: Aspergillus asperescens can be distinguished from other species by large, subglobose to ellipsoidal conidia that turn distinctly roughened with age.
Notes: Aspergillus asperescens was assigned in the A. nidulans series because of its yellow-green radiate conidial heads, brown conidiophores and Hülle cells (Stolk 1954). During our study, Hülle cells were not observed in the type culture; however, the characteristic asexual morphology and phylogeny prove its position in section Nidulantes.
Aspergillus astellatus (Fennell & Raper) Houbraken, Visagie & Samson, Stud. Mycol. 78: 154. 2014. MycoBank MB809577. Fig. 15.
Fig. 15.
Aspergillus astellatus CBS 261.93T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Aspergillus variecolor var. astellatus Fennell & Raper, Mycologia 47: 81. 1955. ≡ Aspergillus stellatus var. astellatus (Fennell & Raper) Subram., Curr. Sci. 41: 759. 1972. ≡ Emericella astellata (Fennell & Raper) Y. Horie, Trans. Mycol. Soc. Japan 21: 491. 1980.
Typus: IMI 061455. Culture ex-type: CBS 261.93 = CBS 134.55 = NRRL 2396 = ATCC 16817 = IMI 61455 = IMI 61455ii = NRRL A-1634 = QM 1910 = WB 2396 = IBT 21902 = IBT 22589 = DTO 010-I7.
ITS barcode: EF652446. (Alternative markers: BenA = EF652270; CaM = EF652358; RPB2 = EF652182).
Colony diam, 7 d (mm): CYA 12–16; CYA 37 °C No growth; CYA 40 °C No growth; MEA 25–27; MEA 37 °C No growth; OA 20–23; YES 20–23; CREA 3–5; CYAS 12–13; DG18 13–18.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane; margins entire to slightly irregular; mycelium brown; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse dark brown to black. MEA 25 °C, 7 d: Colonies moderately deep, plane to sulcate; margins entire; mycelium white to blue violet; texture granular due to ascomata production; sporulation absent to sparse; soluble pigments absent; exudates clear to light brown droplets; reverse dark brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium violet; texture floccose to granular due to ascomata production; sporulation absent to sparse; soluble pigments absent; exudates absent; reverse dark brown to dark gray. DG18 25 °C, 7 d: Colonies moderately deep, plane to slightly sulcate; margins entire; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse cream white to yellowish brown. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation absent; soluble pigments absent; exudates clear droplets; reverse light brown to greyish olive. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, violet to brown, globose to subglobose, 330–500 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 15–27 μm. Asci 8 spored, globose to subglobose. Ascospores reddish brown, in surface view globose, spore bodies smooth, 5.5–6 × 3.5–5 μm; in side view lenticular, with two equatorial crests measuring 2–3.5 μm wide; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, light brown, 80–200 × 3–4 μm; vesicles hyaline to pale brown, subclavate to subglobose, 5–7 μm wide, fertile over the upper half; metulae hyaline, 4.5–5.5 × 2–3 μm; phialides hyaline, flask-shaped, 4.5–5 × 2–4 μm. Conidia echinulate, globose to subglobose, 2.5–6 μm.
Extrolites: aflatoxin B1 and B2, asperthecin, 2-ω-hydroxyemodin, shamixanthones, sterigmatocystin and versicolorins.
Distinguishing characters: Aspergillus astellatus is characterized by ascospores with two wide undissected crests up to 3.5 μm wide. Phylogenetically it is close to A. venezuelensis and A. stella-maris, but the latter two produce stellate ascospores. All three species can produce sterigmatocystin and shamoxanthones. Only A. venezuelensis and A. astellatus produce aflatoxin B1, and A. venezuelensis and A. stella-maris produce emericellin (Table 6).
Aspergillus aurantiobrunneus (G.A. Atkins, Hindson & A.B. Russell) Raper & Fennell, Gen. Aspergillus: 511. 1965. MycoBank MB326612. Fig. 16.
Fig. 16.
Aspergillus aurantiobrunneus CBS 465.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–D. Conidiophores and conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella nidulans var. aurantio-brunnea G.A. Atkins, Hindson & A.B. Russell, Trans. Brit. Mycol. Soc. 41: 504. 1958. ≡ Emericella aurantiobrunnea (G.A. Atkins, Hindson & A.B. Russell) Malloch, Can. J. Bot. 50: 61. 1972. ≡ Aspergillus aurantiobrunneullus Ismail, Abdel-Sater & Zohri, Mycotaxon 53: 397. 1995.
Typus: IMI 074897. Culture ex-type: CBS 465.65 = NRRL 4545 = NRRL 2775 = IMI 074897 = LCP 84.2354 = ATCC 16821 = WB 4545 = DSL 48 = IMI 139821 = IBT 22880 = DTO 047-G7.
ITS barcode: EF652465. (Alternative markers: BenA = EF652289; CaM = EF652377; RPB2 = EF652201).
Colony diam, 7 d (mm): CYA 9–12; CYA 37 °C No growth; CYA 40 °C No growth; MEA 10–11; MEA 37 °C No growth; OA 9–10; YES 11–12; CREA No growth; CYAS 11–13; DG18 15–16.
Colony characters: CYA 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium white and orange; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse wood brown. MEA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown. YES 25 °C, 7 d: Colonies deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse cream yellow. DG18 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium white and orange; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse light yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse white. CREA 25 °C, 7 d: No growth.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown, globose to subglobose, 60–300 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose to ovoid, 14–25 μm. Asci 8 spored, globose to subglobose. Ascospores light orange, in surface view globose to subglobose, spore bodies smooth, 4–5 × 3.5–4.5 μm; in side view lenticular, with two equatorial crests measuring 0.8–1 μm. Conidiophores with smooth stipes, light brown, 50–200 × 3.5–4.5 μm; vesicles hyaline to pale brown, globose to subclavate, 7–12 μm wide, fertile over the two thirds to whole surface; metulae hyaline to pale brown, 4–6 × 2.5–3.5 μm; phialides hyaline to pale brown, flask-shaped, 6.5–7.5 × 2.5–3 μm. Conidia echinulate, globose to subglobose, 2.5–3.5 μm (Anamorphic structures were observed from YES).
Extrolites: emerin, an emindol, emericolin A-D, epurpurin A-C, eremophiline, stellatic acid, sterigmatocystin, variecoacetal A, B, variecolactone, variecolin, variecolol, versicolorins.
Distinguishing characters: Aspergillus aurantiobrunneus grows restrictedly on CYA, MEA, YES and OA, which differs from other morphologically similar species such as A. fruticulosus and A. pachycristatus. Phylogenetically it is close to A. purpureus, but A. purpureus produces larger ascospores (6–7 × 4.5–5 μm) and narrower ascospore crests (0.3–0.6 μm).
Aspergillus aurantiopurpureus A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816087. Fig. 17.
Fig. 17.
Aspergillus aurantiopurpureus CBS 140608T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
Etymology: Name refers to its ascospore colour, which is orange or reddish brown, later turns to violet.
Diagnosis: Yellow mycelium, smooth ascospores with crests measuring 0.8–1.2 μm wide, ascospores are first orange later turn to violet.
Typus: USA, New Mexico, Sevilette national wildlife refuge, kangaroo rat cheek pouch, 1989, isolated by L. Hawkins (holotype CBS H-22488, culture ex-type: CBS 140608 = IBT 12601 = DTO 060-A7).
ITS barcode: KU866588. (Alternative markers: BenA = KU866824; CaM = KU866711; RPB2 = KU866966).
Colony diam, 7 d (mm): CYA 32–35; CYA 37 °C 23–30; CYA 40 °C 28–30; MEA 38–41; MEA 37 °C 33–35; OA 25 °C 40–45; YES 42–49; CREA 6–7; CYAS 20–22; DG18 14–17.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium yellow; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse reddish brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow and white; texture floccose; sporulation sparse; soluble pigments absent; exudates clear droplets; reverse reddish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium light yellow and white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse orange red. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow to yellowish green; texture floccose; sporulation moderately dense; conidia en masse yellow green; soluble pigments absent; exudates absent; reverse dark brown. OA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white to light yellow; texture floccose; sporulation sparse; soluble pigments light brown; exudates clear droplets; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, enveloped by interwoven hyphae, blackish to dark brown, globose to subglobose, 200–320 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 11.5–20.5 μm. Asci 8 spored, globose to subglobose. Ascospores first orange to reddish brown, later turn to violet, in surface view globose, spore bodies smooth, 3.5–4.5 Χ 3–3.5 μm; in side view lenticular, with two equatorial crests measuring 0.8–1.2 μm wide; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, light brown, 130–260 × 3.5–5 μm; vesicles hyaline, subclavate to subglobose, 8–11.5 μm wide, fertile over the upper half; metulae hyaline, 5–6 × 2.5–4 μm; phialides hyaline, flask-shaped, 5–6.5 × 3–3.5 μm. Conidia echinulate, globose to subglobose, 3–3.5 μm, green in mass.
Extrolites: cyclopaldic acid, desertorins, falconensins, cf. falconensons, shamixanthones, sterigmatocystin.
Distinguishing characters: Phylogenetically it is close to A. navahoensis, but can be easily distinguished by wider pleated crests and ascospore colour.
Aspergillus aureolatus Munt.-Cvetk. & Bata, Bull. Inst. Jard. Bot. Univ. Beograd 1: 196. 1964. MycoBank MB326614. Fig. 18.
Fig. 18.
Aspergillus aureolatus CBS 190.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–G. Conidiophores. H. Conidia. Scale bars: B–C = 30 μm; D–H = 10 μm.
Typus: CBS H-6738. Culture ex-type: CBS 190.65 = NRRL 5126 = ATCC 16810 = IMI 136527 = IMI 136527ii = WB5126 = IBT 18471 = IBT 22670 = DTO 053-C1.
ITS barcode: EF652501. (Alternative markers: BenA = EF652325; CaM = EF652413; RPB2 = EF652237).
Colony diam, 7 d (mm): CYA 19–25; CYA 37 °C No growth; CYA 40 °C No growth; MEA 17–24; MEA 37 °C No growth; OA 16–19; YES 21–25; CREA 11–16; CYAS 18–21; DG18 11–17.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane; margins slightly irregular, surrounded by an orange halo; mycelium white; texture velvety; sporulation dense, conidia en masse greyish green to olive green; soluble pigments absent; exudates absent; reverse orange to reddish brown. MEA 25 °C, 7 d: Colonies deep, slightly sulcate; margins slightly irregular, surrounded by an orange halo; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green to blue green; soluble pigments absent; exudates clear droplets; reverse orange to reddish brown. YES 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular, surrounded by an golden to orange halo; mycelium white; texture velvety; sporulation dense, conidia en masse greyish green; soluble pigments absent; exudates absent; reverse orange. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium yellow; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse orange. OA 25 °C, 7 d: Colonies low, plane; margins slightly irregular, surrounded by an orange halo; mycelium white; texture velvety; sporulation dense, conidia en masse dark green; soluble pigments light brown; exudates clear droplets; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, yellowish brown, 80–200 × 4–5.5 μm, reduced conidial heads are formed, sometimes clusters of sterigmata appear along the ascending conidiophores; typical vesicles hyaline to pale yellowish brown, globose, 9–12 μm wide, fertile over the upper half to two thirds; metulae hyaline to pale green, 5–8.5 × 2–4 μm; phialides hyaline to pale green, flask-shaped, 5–7 × 2.5–3 μm. Conidia globose to subglobose, echinulate, 3.5–5 μm, green in mass.
Extrolites: austalides (tentatively identified), a desertorin, an emerin, sterigmatocystin, versicolorins.
Distinguishing characters: The striking orange halo surrounding the colony and globose vesicles can distinguish Aspergillus aureolatus from related non-ascosporic species.
Aspergillus botswanensis A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816095. Fig. 19.
Fig. 19.
Aspergillus botswanensis CBS 314.89T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B. Ascomata. C. Asci. D,F–H. Ascospores. E. Hülle cells. Scale bars: B = 1000 μm; C–E = 10 μm; F–H = 2 μm.
Etymology: Name refers to its origin, isolated from forest soil from Botswana.
Diagnosis: Brown ascospores ornamented with tuberculate to irregular reticulate ornamentation.
Typus: Botswana, Okavango Delta, Island Forest Area, at base of Diospyros mespiliformis (ebony tree), forest soil, 1986, collected by D. Pearce (holotype CBS H-22494, culture ex-type CBS 314.89 = DTO 047-I4).
ITS barcode: KU866572. (Alternative markers: BenA = KU866812; CaM = KU866695; RPB2 = KU866949).
Colony diam, 7 d (mm): CYA 8–10; CYA 37 °C 45–46; CYA 40 °C 38–39; MEA 33–34; MEA 37 °C >60; OA 30–31; YES 32–33; CREA 2–5; CYAS 8–10; DG18 18–21.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow to white; texture floccose; sporulation absent; soluble pigments absent; exudates clear droplets; reverse orange brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium light yellow and white; texture floccose; sporulation absent; soluble pigments absent; exudates clear droplets; reverse orange brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white; texture velvety; sporulation absent; soluble pigments absent; exudates absent; reverse buff yellow. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture grantular due to ascomata production; sporulation absent; soluble pigments absent; exudates absent; reverse pale olive. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, enveloped by interwoven mycelium, dark brown, globose, 90–180 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 12–16.5 μm. Asci 8 spored, globose to ovoid. Ascospores brown, in surface view globose to subglobose; spore bodies first tuberculate, later the extended protrusion melting into irregular reticulate ornamentation, 5–6 × 3.5–5 μm; in side view broadly lenticular, with two low equatorial crests, 0.4–0.8 wide. Anamorph absent.
Extrolites: asperthecin, desertorins, emericellin, an emindol, 2-ω-hydroxyemodin, paxillin, terrequinone A.
Distinguishing characters: The ascospores of Aspergillus botswanensis resemble those of A. violaceus, but A. violaceus produces constantly violet ascospores with regular reticulate ornamentation. Phylogenetically, A. botswanensis is close to A. desertorum, A. stercorarius and A. savannensis, but can be differed by it unique ascospore ornamentation.
Aspergillus caespitosus Raper & Thom, Mycologia 36: 563. 1944. MycoBank MB284298. Fig. 20.
Fig. 20.
Aspergillus caespitosus CBS 103.45T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C,F. Conidophores. D,E. Terminal or intercalary cells resembling Hülle cells. G. Conidia. Scale bars: B–C,E,G = 10 μm; F = 8 μm; D = 1000 μm.
Typus: IMI 16034ii. Culture ex-type: CBS 103.45 = NRRL 1929 = ATCC 11256 = IMI 16034 = MUCL 13587 = NCTC 6972 = NCTC 6973 = QM 7399 = WB 1929 = IBT 10624 = DTO 053-D1.
ITS barcode: EF652428. (Alternative markers: BenA = EF652252; CaM = EF652340; RPB2 = EF652164).
Colony diam, 7 d (mm): CYA 42–46; CYA 37 °C 7–30; CYA 40 °C No growth; MEA 46–54; MEA 37 °C 12–34; OA 45–55; YES 51–60; CREA 9–12; CYAS 25–35; DG18 32–35.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture velvety; sporulation sparse to moderately dense, conidia en masse graysih green; soluble pigments absent; exudates clear droplets; reverse vinaceous buff to grey olivaceous. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate to sulcate; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse yellow green to olive green; soluble pigments absent; exudates absent or clear droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture velvety; sporulation moderately dense, conidia en masse yellow green to blue green; dark brown soluble pigments present after 2 wks; exudates absent; reverse olive to yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse dark green; soluble pigments absent; exudates absent; reverse pale yellow green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent to pale yellow; exudates absent; reverse greenish yellow to yellow. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, pale brown, 200–300 × 3–6 μm; vesicles hyaline to pale brown, hemisphere to subclavate, 10–15 μm wide, fertile over the upper half; metulae hyaline to pale brown, 5–8 × 3–3.5 μm; phialides hyaline, flask-shaped, 6.5–8 Χ 3–4.5 μm. Conidia globose, echinulate, 3–4 μm, green in mass.
Extrolites: asperlicine, emodin, fischerin, fumitremorgin B, 2-ω-hydroxyemodin, 6-methoxymellein, mollicin (tentatively identified), secalonic acid D, TR-2, verruculogen.
Distinguishing characters: Aspergillus caespitosus is close to A. asperescens and A. unguis, but can be distinguished from A. asperescens by its globose conidia; from A. unguis by its longer conidiophores and wider vesicles. These three species share no extrolites, and can be easily distinguished chemically. A. caespitosus is the only species in section Nidulantes that produces fumitremorgins.
Notes: Abundant, thick walled, irregularly globose, ovoid or elliptical Hülle cells were mentioned in the original descriptions (Raper & Thom 1944); however, they are not confirmed in this study. Only some degenerated terminal or intercalary cells resembling Hülle cells are observed on CYA plates, measuring 7–16 × 5–10 μm.
Aspergillus corrugatus Udagawa & Y. Horie, Mycotaxon 4: 535. 1976. MycoBank MB309216. Fig. 21.
Fig. 21.
Aspergillus corrugatus CBS 191.77T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella corrugata Udagawa & Y. Horie, Mycotaxon 4: 535. 1976.
Typus: NHL 2763. Culture ex-type: CBS 191.77 = NHL 2763 = IMI 212201 = IBT 22829 = DTO 047-I9.
ITS barcode: KU866574. (Alternative markers: BenA = KU866814; CaM = KU866696; RPB2 = KU866951).
Colony diam, 7 d (mm): CYA 48–49; CYA 37 °C 53–54; CYA 40 °C 48–49; MEA 43–44; MEA 37 °C >60; OA 40–42; YES >60; CREA 10–13; CYAS 23–27; DG18 14–15.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white to light yellow; texture floccose; sporulation absent; soluble pigments light brown; exudates absent; reverse reddish brown to brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium salmon at centre, white at edge; texture floccose; sporulation sparse; soluble pigments absent; exudates clear droplets; reverse reddish brown to brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white to light yellow; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white to buff; texture floccose; sporulation moderately dense, conidia en masse greyish green; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation sparse; soluble pigments light brown; exudates absent; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown to brown, globose to subglobose, 200–360 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 16–23 μm. Asci 8 spored, globose to ovate. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies with irregularly wrinkled ornamentation, globose to subglobose, 3.5–4.5 × 3.5–4 μm; in side view lenticular, with two pleated equatorial crests measuring 0.5–1 μm. Conidiophores with smooth stipes, yellowish brown, 40–120 × 3.5–5.5 μm; vesicles pale brown, subclavate, 8–10 μm wide, fertile over the upper half to two thirds; metulae hyaline, pale green to brown, 5–8 × 2.5–4 μm; phialides hyaline to pale green, flask-shaped, 6–8 × 2.5–3.5 μm. Conidia echinulate, globose to subglobose, 2.5–3.5 μm.
Extrolites: asperthecin, asperugins, an austalide (tentatively identified), emecorrugatin, gregatins, shamixanthone, sterigmatocystin, versicolorins, violaceols.
Distinguishing characters: Aspergillus corrugatus is close to A. foveolatus, A. rugulosus and A. spinulosporus, but differs in its ascospore ornamentation. The convex walls are irregularly wrinkled in A. corrugatus, in contrast to the finely pitted convex walls in A. foveolatus, rugulose walls in A. rugulosus and echinulate walls in A. spinulosporus.
Aspergillus croceus Hubka, A. Nováková, Frisvad, S.W. Peterson & M. Kolařík, Plant Syst. Evol. 302: 1291. 2016. MycoBank MB816281.
Typus: Holotype PRM 924053; isotype 924054. Culture ex-type: CBS 134396 = CCF 4405 = NRRL 62495 = IBT 33602.
ITS barcode: LN873931. (Alternative markers: BenA = LN873944; CaM = LN873957; RPB2 = LN873976).
Colony characters: Fide Hubka et al. (2016) colonies on CYA at 25 °C attain 20–37 mm diam in 14 days (11–16 mm in 7 days), velutinous, plane, delicately furrowed to wrinkled with floccose vivid orange (No. 48) colony centre, colony margin plane, 2–3 mm wide, strong brown (No. 55), sporulation color greyish olive (110), greyish olive green (No. 127) to dark olive (No. 108), Hülle cells visible in some strains after 14 days, no exudate, no soluble pigment, reverse dark reddish brown (No. 44) with paler 1-mm-wide colony margin. No growth on CYA at 37 °C. Colonies on MEA at 25 °C attain 17–32 mm diam in 14 days (9–13 mm in 7 days), velutinous, plane to delicately furrowed with paler floccose central part, 3–5 mm in diam, deep orange (No. 69) with paler colony margin, brilliant yellow (83)-colored margins in some strains, sporadic sporulation in greyish olive (No. 110) to greyish olive green (No. 127), but in some strains more intense sporulation in greyish green (No. 150), no exudate, no soluble pigment, reverse brownish orange (No. 54) to strong brown (No. 55) with strong orange (No. 50) 1–2 mm margin. Colonies on CZA at 25 °C attain 19–28 mm diam in 14 days (7–12 mm in 7 days), velutinous to floccose, plane, mycelium light brownish gray (No. 63), brownish gray (No. 64) to strong reddish brown (No. 40) with medium brown (No. 58) to strong brown (No. 55) higher colony centre (12 mm) with sporulation (zone up to 12 mm in diam) in greyish olive green (No. 127), in some strains whitish mycelial overgrowth in the colony centre, no exudate or small droplets of dark brown (No. 59) exudate, soluble pigment medium pink (No. 5) to medium red (No. 15), reverse vivid deep red (No. 13) to dark reddish brown (No. 44) with deep red (No. 13) margin. Colonies on CREA at 25 °C attain 9–20 mm diam in 14 days (8–12 mm in 7 days), plane, submerged orange-colored mycelium, sparse sporulation, no acid production.
Micromorphology: Fide Hubka et al. (2016) stipes on MEA light brown to brown, rough-walled, warty to crustose, non-septate or occasionally with septum (sometimes separating vesicle and stipe), most commonly 90–200 × 3.5–5 μm diam, occasionally longer; vesicles pyriform, spathulate or clavate, 7–15 μm diam; metulae cylindrical, 7.5–10.5 μm long, covering 1/3–1/2 of vesicle; phialides ampulliform (6.5–) 7–9 (–9.5) μm long; conidia globose or subglobose, green in mass, 2–3 (–3.5) μm diam, smooth to finely roughened. Hülle cells arranged in clusters, globose, subglobose or pyriform, 8–18.5 × 8–11 (–17) μm, produced after 14 or more days cultivation at 25 °C. Sexual state not observed.
Extrolites: Fide Hubka et al. (2016) kotanins, norsolorinic acid, orlandin, siderin, sterigmatocystin, versicolorins.
Distinguishing characters: This species is closely related to A. unguis and A. israelensis, but the latter two produce narrower vesicles, (8–10 μm) in A. unguis and (7–10 μm) in A. israelensis.
Notes: For an illustration of the species, readers are referred to Hubka et al. (2016).
Aspergillus desertorum (Samson & Mouch) Samson, Visagie & Houbraken, Stud. Mycol. 78: 155. 2014. MycoBank MB809587. Fig. 22.
Fig. 22.
Aspergillus desertorum CBS 653.73T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B. Ascomata. C, E–G. Ascospores. D. Hülle cells. Scale bars: B = 30 μm; C–D = 10 μm; E–G = 2 μm.
≡ Emericella desertorum Samson & Mouch., Antonie van Leeuwenhoek 40: 121. 1974.
Typus: CBS H-7045. Culture ex-type: CBS 653.73 = NRRL 5921 = IMI 343076 = IBT 21899 = DTO 048-A1.
ITS barcode: EF652505. (Alternative markers: BenA = EF652329; CaM = EF652417; RPB2 = EF652241).
Colony diam, 7 d (mm): CYA 20–35; CYA 37 °C 29–53; CYA 40 °C 29–47; MEA 32–40; MEA 37 °C 43–60; OA 30–36; YES 41–50; CREA 0–2; CYAS 0–23; DG18 0–17.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium saffron and white; texture floccose; sporulation absent; soluble pigments absent; exudates light brown droplets; reverse yellowish brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates clear droplets; reverse orange to reddish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture velvety; sporulation absent; soluble pigments absent; exudates absent; reverse light brown. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation absent; soluble pigments light yellow; exudates light brown droplets; reverse greyish yellow. Violet ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, violet to brown reddish, globose to subglobose, 100–300 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose, ovoid or pyriform, 10–25 μm. Asci 8 spored, globose to subglobose. Ascospores reddish brown, in surface view globose to subglobose, spore bodies tuberculate, 6.5–7.5 × 6–7.5 μm; in side view broadly lenticular, with two low equatorial crests measuring 0.5 μm wide; Anamorph absent.
Extrolites: asperthecin, calbistrins, desertorin A, B & C, emindols, nidulol, paxillin, silvaticol, terrequinone A.
Distinguishing characters: Aspergillus desertorum is characterized by large ascospores, which are ornamented with two low crests. Its ascospores resemble those of A. purpureus and A. stercorarius, but A. purpureus produces smooth ascospores and grows slower on all tested media, A. stercorarius produces smooth and smaller ascospores (4.5–6 × 3.5–4.5 μm).
Aspergillus dromiae A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816089. Fig. 23.
Fig. 23.
Aspergillus dromiae CBS 140633T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
Etymology: Name refers to its origin, isolated from Dromia erythropus (crab, Crustacea).
Diagnosis: Stellate ascospores, echinulate conidia measuring 3.5–4.5 μm, vesicles measuring 12–17 μm.
Typus: Venezuela, Mochima Bay, Morro of Garapáta, Dromia erythropus (crab, Crustacea), isolated by J.C. Frisvad (holotype CBS H-22489, culture ex-type CBS 140633 = IBT 25166 = DTO 059-H5).
ITS barcode: KU866580. (Alternative markers: BenA = KU866885; CaM = KU866703; RPB2 = KU866958).
Colony diam, 7 d (mm): CYA 39–40; CYA 37 °C 10–11; CYA 40 °C No growth; MEA 45–47; MEA 37 °C 1–2; OA 40–45; YES 45–50; CREA 10–11; CYAS 33–34; DG18 18–27.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium greyish olive; texture floccose; sporulation absent; soluble pigments absent; exudates clear to light brown droplets; reverse dark olive; ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium light yellow and white; texture granular at centre due to ascomata production; sporulation absent; soluble pigments absent; exudates clear droplets; reverse dark brown at centre, yellowish brown at edge; ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and light yellow; texture floccose to granular due to ascomata production; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown; ascomata present after 1 wk. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse greenish yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture granular due to ascomata production; sporulation absent; soluble pigments absent; exudates clear droplets; reverse pale yellow green; ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown to dark brown, globose to subglobose, 450–800 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 16–31 μm. Asci 8 spored, stellate. Ascospores orange to reddish brown, in surface view stellate, 11–15 μm; spore bodies smooth, globose to subglobose, 3–4.5 × 3.5–4.5 μm; in side view broadly lenticular, with two stellate equatorial crests; undissected part of crests 1–1.5 μm broad, with 2–3 μm long extensions; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, pale brown, 300–410 × 4.5–6.5 μm; vesicles hyaline to pale brown, subclavate to subglobose, 12–17 μm wide, fertile over the upper half; metulae hyaline, 6–8 × 3–4.5 μm; phialides hyaline, flask-shaped, 6.5–10 × 3.5–4.5 μm. Conidia echinulate, globose to subglobose, 3.5–4.5 μm. (Anamorphic structures were observed from DG18).
Extrolites: a desertorin, emericellin, 2-ω-hydroxyemodin, shamixanthones.
Distinguishing characters: Aspergillus dromiae resembles A. stella-maris and A. miraensis, however A. stella-maris produces wider (3.5–7 μm), septate conidiophores, while A. miraensis produces smaller conidia (2–3.5 μm).
Aspergillus falconensis Y. Horie et al., Trans. Mycol. Soc. Japan 30: 257. 1989. MycoBank MB127891. Fig. 24.
Fig. 24.
Aspergillus falconensis CBS 271.91T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella falconensis Y. Horie et al., Trans. Mycol. Soc. Japan 30: 257. 1989.
Typus: CBM 10001. Culture ex-type: CBS 271.91 = IFM 4997 = NHL 2999 = ATCC 76117 = IBT 14808 = DTO 048-A2.
ITS barcode: KU866575. (Alternative markers: BenA = KU866815; CaM = KU866697; RPB2 = KU866952).
Colony diam, 7 d (mm): CYA 30–40; CYA 37 °C 34–52; CYA 40 °C 30–45; MEA 34–45; MEA 37 °C 43–60; OA 33–48; YES 47–>60; CREA 2–18; CYAS 2–20; DG18 2–24.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium light yellow and white; texture floccose; sporulation absent; soluble pigments absent; exudates light brown droplets; reverse reddish brown to brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow and white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse reddish brown to brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium light yellow and white; texture velvety; sporulation sparse; soluble pigments absent; exudates absent; reverse yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense; conidia en masse greyish green; soluble pigments absent; exudates absent; reverse light yellow. OA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow and white; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments yellowish brown; exudates clear droplets; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown to dark brown, globose to subglobose, 300–700 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 14–25 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, 4–6 × 3–3.5 μm; in side view lenticular, with two equatorial crests measuring 1–2 μm wide; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, light brown, 75–240 × 4–6.5 μm; vesicles hyaline to pale brown, globose to subclavate, 8–10 μm wide, fertile over the upper half; metulae hyaline, 6–10 × 2–3.5 μm; phialides hyaline, flask-shaped, 6–9 × 2–4 μm. Conidia echinulate, globose to subglobose, 2.5–4 μm, green in mass. (Anamorphic structures were observed from YES).
Extrolites: asperthecin, an austalide (tentatively identified), austinol, desertorins, falconensins, falconensons, shamixanthones, sterigmatocystin, versicolorins, violaceols, viridicatumtoxin.
Distinguishing characters: Aspergillus falconensis is characterized by ascospores with two conspicuously pleated crests up to 2 μm wide, which distinguish it from closely related A. fruticulosus and A. navahoensis.
Aspergillus filifer Zalar, Frisvad & Samson, Mycologia 100: 787. 2008. MycoBank MB507357. Fig. 25.
Fig. 25.
Aspergillus filifer CBS 113636T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella filifera Zalar, Frisvad & Samson, Mycologia 100: 787. 2008.
= Emericella appendiculata Y. Horie & D.M. Li, Mycoscience 39: 161. 1998. = Aspergillus appendiculatus Y. Horie & D.M. Li, Mycoscience 39: 161. 1998. nom. illeg. [Art. 52.1; McNeill et al. (2012)] = Aspergillus chinensis Samson, Visagie & Houbraken, Stud. Mycol. 78: 155. 2014.
Typus: CBS H-19886. Culture ex-type: CBS 113636 = IBT 23443 = DTO 011-A5.
ITS barcode: EU448277. (Alternative markers: BenA = EF428372; CaM = EU443973; RPB2 = KU866932).
Colony diam, 7 d (mm): CYA 32–40; CYA 37 °C 24–30; CYA 40 °C No growth; MEA 35–42; MEA 37 °C 23–30; OA 28–34; YES 40–48; CREA 6–13; CYAS 15–24; DG18 20–27.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white and pale olive; texture floccose; sporulation absent; soluble pigments absent; exudates clear to light brown droplets; reverse greyish brown at centre, buff at edge; Ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, plane to slightly sulcate; margins entire; mycelium white and light yellow; texture floccose; sporulation absent; soluble pigments absent; exudates clear to light brown droplets; reverse dark brown at centre, yellowish brown at edge. Ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep to deep, sulcate; margins entire; mycelium white and light yellow; texture velvety; sporulation sparse to moderately dense, conidia en masse yellow green; soluble pigments absent; exudates clear droplets; reverse light brown to cream yellow. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse pale yellow green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture granular due to ascomata production; sporulation absent; soluble pigments absent; exudates clear droplets; reverse pale brownish green. Ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, greyish green to brown, globose to subglobose 220–660 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 13–24 μm. Asci 8 spored, stellate, globose to subglobose. Ascospores brown, in surface view globose to subglobose, spore bodies 3.5–4.5 × 3–4 μm; in side view broadly lenticular, with two equatorial crests measuring 0.5–1.2 μm wide; Crest bearing hyaline, filiform appendages, measuring 3–6 μm long with swollen tips. Convex surface tuberculate. Conidiophores with smooth stipes, yellowish brown, 120–250 × 3–5 μm; vesicles hyaline to pale yellowish brown, subclavate to subglobose, 7–13 μm wide, fertile over the upper half to two thirds; metulae hyaline to pale yellowish brown, 7–10 × 3–5 μm; phialides hyaline to pale yellowish brown, flask-shaped, 7–11 × 2–4 μm. Conidia echinulate, globose to subglobose, 3–4 μm.
Extrolites: asperthecin, asperugins, asteltoxin, dihydroterrein, 2-ω-hydroemodin, emericellin, shamixanthones, terrein, a varitriol.
Distinguishing characters: Appendaged ascospores ornamented with capitate swellings. Morphologically this species is close to A. undulatus and A. qinqixianii, but can be easily distinguished from A. undulatus by filiform appendages and from A. qinqixianii by capitate swellings on convex surface of ascospores.
Notes: Phylogenetically and morphologically Aspergillus appendiculatus (= Aspergillus chinensis) (Samson et al. 2014) is identical with A. filifer, and is considered a synonym of A. filifer as did Matsuzawa et al. 2012 and Hubka et al. 2016.
Aspergillus foveolatus Y. Horie, Trans. Mycol. Soc. Japan 19: 313. 1978. MycoBank MB309221. Fig. 26.
Fig. 26.
Aspergillus foveolatus CBS 279.81T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella foveolata Y. Horie, Trans. Mycol. Soc. Japan 19: 313. 1978.
Typus: IFM 4547. Culture ex-type: CBS 279.81 = IFM 4547 = NHL 2839 = NBRC 30559 = IFO 30559 = IBT 22847 = DTO 320-D2.
ITS barcode: KX423658. (Alternative markers: BenA = KX423622; CaM = KX423635; RPB2 = KU867034).
Colony diam, 7 d (mm): CYA 50–51; CYA 37 °C >60; CYA 40 °C >60; MEA 50–52; MEA 37 °C >60; OA 35–40; YES >60; CREA 19–20; CYAS 39–40; DG18 21–24.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse pale green; soluble pigments absent; exudates light brown droplets; reverse brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse greyish green to dark green; soluble pigments absent; exudates clear droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse pale green; soluble pigments absent; exudates clear droplets; reverse brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse pale green; soluble pigments absent; exudates absent; reverse light olive. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse greyish green to dark green; soluble pigments light brown; exudates absent; reverse cream white. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, dark brown, globose to subglobose, 100–280 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 7–21.5 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies finely pitted, 4–5 × 3.5–4.5 μm; in side view lenticular, with two equatorial crests measuring 0.5–1 μm wide. Conidiophores with smooth stipes, light brown to brown, 40–200 × 4.5–6.5 μm; vesicles light brown, subglobose to subclavate, 12–15 μm wide, fertile over the upper half to two thirds; metulae hyaline, 5–7 × 2–4 μm; phialides hyaline, flask-shaped, 6–8 × 2–3 μm. Conidia echinulate, globose to subglobose, 3–4.5 μm.
Extrolites: asperthecin, asperugins, 2-ω-hydroxyemodin, emericellin, emestrin, paxillin, shamixanthones, sterigmatocystin, versicolorins, violaceols.
Distinguishing characters: Aspergillus foveolatus can be easily recognized by pitted ascospores.
Aspergillus fruticulosus Raper & Fennell, Gen. Aspergillus: 506. 1965. MycoBank MB326630. Fig. 27.
Fig. 27.
Aspergillus fruticulosus CBS 486.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella fruticulosa (Raper & Fennell) Malloch & Cain, Can. J. Bot. 50: 61. 1972. ≡ Aspergillus fruticans Samson & W. Gams, Adv. Pen. Asp. Syst.: 40. 1985.
Typus: IMI 139279. Culture ex-type: CBS 486.65 = NRRL 4903 = ATCC 16823 = IMI 139279 = O-1077 = QM 8033 = WB 4903 = IBT 33973 = DTO 047-H8.
ITS barcode: EF652483. (Alternative markers: BenA = EF652307; CaM = EF652395; RPB2 = EF652219).
Colony diam, 7 d (mm): CYA 24–25; CYA 37 °C 35–36; CYA 40 °C 30–31; MEA 35–36; MEA 37 °C 50–51; OA 30–31; YES 46–47; CREA 3–5; CYAS 13–14; DG18 25–26.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white and light yellow; texture floccose; sporulation moderately dense, conidia en masse pale green; soluble pigments absent; exudates light brown droplets; reverse dark brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation moderately dense; conidia en masse blue green; soluble pigments absent; exudates clear droplets; reverse reddish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and light yellow; texture velvety; sporulation moderately dense, conidia en masse blue green; soluble pigments absent; exudates absent; reverse brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense; conidia en masse pale green; soluble pigments absent; exudates absent; reverse light yellow at centre, olive green at edge. OA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow; texture floccose; sporulation moderately dense, conidia en masse blue green; soluble pigments yellowish brown; exudates clear droplets; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, blackish to dark brown, globose to subglobose, 230–500 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 10–20 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, 4.5–5.5 × 3–5 μm; in side view lenticular, with two equatorial crests measuring 0.8–1 μm wide; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, light brown, 40–200 × 4–6 μm; vesicles hyaline to pale brown, subglobose to subclavate, 8–12 μm wide, fertile over the upper half to two thirds; metulae hyaline, 5–6 × 3–4.5 μm; phialides hyaline, flask-shaped, 6–9 × 2–3.5 μm. Conidia echinulate, globose to subglobose, 3.5–4 μm, green in mass.
Extrolites: asperthecin, 2-ω-hydroxyemodin, falconensins, falconensons, sterigmatocystin, versicolorins, violaceols.
Distinguishing characters: Aspergillus fruticulosus is close to A. falconensis morphologically and phylogenetically, but the ascospore crests of A. falconensis (1–2 μm) are wider than in A. fruticulosus (0.8–1 μm).
Aspergillus israelensis A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816091. Fig. 28.
Fig. 28.
Aspergillus israelensis CBS 140627T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–F. Conidiophores. G. Conidia. Scale bars: B = 30 μm; C–E,G = 10 μm; F = 8 μm.
Etymology: Name refers to its origin, isolated from evaporation pond, Ein Bokek, Dead Sea, Israel.
Diagnosis: Slow growth on CYA, MEA, OA and YES, narrow conidiophore stipes (3.5–4.5 μm), vesicles (7–10 μm) and globose, tuberculate conidia measuring 2.5–3.5 μm.
Typus: Israel, Dead Sea, Ein Bokek, evaporation pond, 2002, isolated by L. Butinar (holotype CBS H-22491, culture ex-type: CBS 140627 = IBT 24293 = DTO 325-E2).
ITS barcode: KU866677. (Alternative markers: BenA = KU866915; CaM = KU866797; RPB2 = KU867062).
Colony diam, 7 d (mm): CYA 10–19; CYA 37 °C No growth; CYA 40 °C No growth; MEA 15–20; MEA 37 °C No growth; OA 14–15; YES 14–22; CREA 8–9; CYAS 12–16; DG18 10–13.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white, wood brown at edge; texture velvety; sporulation moderately dense, conidia en masse greyish green; soluble pigments absent; exudates absent; reverse dark brown. MEA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose at centre, velvety at edge; sporulation dense, conidia en masse dark green; soluble pigments absent; exudates absent; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium pale buff; texture velvety; sporulation moderately dense, conidia en masse pale green; soluble pigments absent; exudates absent; reverse light brown to yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and light buff; texture floccose; sporulation moderately dense, conidia en masse pale green; soluble pigments absent; exudates absent; reverse deep olive buff. OA 25 °C, 7 d: Colonies low, plane; margins entire; texture velvety to floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent to light brown; exudates absent; reverse light greyish olive. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, pale brown, 90–160 × 3.5–4.5 μm; vesicles hyaline to pale brown, hemisphere to subclavate, 7–10 μm wide, fertile over the upper half; metulae hyaline to pale brown, 5–8 × 2.5–3.5 μm; phialides hyaline, flask-shaped, 6–8 × 2–2.5 μm. Conidia globose, tuberculate, 2.5–3.5 μm, green in mass.
Extrolites: an emindol (and many extrolites, of unknown chemical constitution, only found in this species).
Distinguishing characters: Compared to other non-ascosporic species, A. israelensis grows slower on most of the media (CYA, MEA, OA and YES), it resembles A. unguis and A. asperescens, but A. unguis produces echinulate conidia; A. asperescens produces subglobose to ellipsoidal conidia.
Aspergillus jaipurensis Samson, Visagie & Houbraken, Stud. Mycol. 78: 155. 2014. MycoBank MB809592. Fig. 29.
Fig. 29.
Aspergillus jaipurensis CBS 952.97T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella indica Stchigel & Guarro, Mycol. Res. 103: 1059. 1999. (non Aspergillus indicus B.S. Mehrotra & Agnihotri, Mycologia 54: 403. 1963)
Typus: IMI 378525. Culture ex-type: CBS 952.97 = IMT 378525 = FMR 6232 = IBT 23715 = DTO 320-A9.
ITS barcode: KU866623. (Alternative markers: BenA = AY339988; CaM = KU866761; RPB2 = KU867024).
Colony diam, 7 d (mm): CYA 25–33; CYA 37 °C 38–43; CYA 40 °C 34–36; MEA 32–36; MEA 37 °C 55–>60; OA 31–34; YES 48–51; CREA 5–6; CYAS 14–15; DG18 14–20.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium light brown at centre, white at edge; texture velvety; sporulation sparse; soluble pigments absent; exudates light brown droplets; reverse wood brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates light brown droplets; reverse wood brown to yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse light brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse pale green to yellow green; soluble pigments absent; exudates absent; reverse light brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, violet to dark brown, globose to subglobose, 150–500 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish, globose to ovoid, 14–25 μm. Asci 8 spored, globose to subglobose. Ascospores purplish red, in surface view globose to subglobose, spore bodies smooth, 6–7.5 × 5.5–6 μm; in side view lenticular, with two pleated equatorial crests measuring 0.8–1 μm; crests ornamented with longitudinal, 0.2 μm wide pleats. Conidiophores with smooth stipes, pale yellowish brown, 30–100 × 4–6 μm; vesicles hyaline to pale brown, subclavate to subglobose, 7–9 μm wide, fertile over the upper half; metulae hyaline to pale brown, 5–6.5 × 2.5–3.5 μm; phialides hyaline to pale brown, flask-shaped, 5–7 × 2–3.5 μm. Conidia verrucose to tuberculate, globose to subglobose, 4–6.5 × 3–4.5 μm. (Anamorphic structures were observed from OA).
Extrolites: asperugins, an austalide (tentatively identified), emestrin, emindols, shamixanthones, violaceols.
Distinguishing characters: Aspergillus jaipurensis is characterized by large, purplish red ascospores, which can easily distinguish it from other species in section Nidulantes.
Notes: In combination with the recent adoption of the one fungus one name concept, Emericella indica was transferred to Aspergillus. Since the name A. indicus is already occupied, the new name A. jaipurensis was proposed (Samson et al. 2014), which was named after the city Jaipur in India, the origin of the type strain.
Aspergillus latilabiatus A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816093. Fig. 30.
Fig. 30.
Aspergillus latilabiatus CBS 426.93T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Ascomata. D,F–H. Ascospores. E. Hülle cells. Scale bars: B = 30 μm; C–E = 10 μm; F–H = 2 μm.
Etymology: The name refers to the lip shaped crests of ascospores.
Diagnosis: Brown, smooth ascospores with two thick equatorial crests.
Typus: Algeria, Kerzaz, sheep dung, 1993, isolated by M. Locquin-Linard (holotype CBS H-22514, culture ex-type: CBS 426.93 = IBT 33959 = DTO 320-B2).
ITS barcode: KU866624. (Alternative markers: BenA = KU866864; CaM = KU866762; RPB2 = KU867025).
Colony diam, 7 d (mm): CYA 22–23; CYA 37 °C 33–37; CYA 40 °C 25–28; MEA 27–28; MEA 37 °C 41–42; OA 22–23; YES 27–28; CREA No growth; CYAS 16–17; DG18 1–4.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium white; texture floccose to velvety; sporulation absent; soluble pigments absent; exudates absent; reverse brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown to reddish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse light brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown to brown. CREA 25 °C, 7 d: No growth.
Micromorphology: Ascomata cleistothecial, superficial, blackish to dark brown, globose, 100–160 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 13–24 μm. Asci 8 spored, globose to subglobose. Ascospores brown, in surface view globose to subglobose, spore bodies smooth, 5.5–7 × 4.5–6 μm; in side view lenticular, with two equatorial crests measuring 0.5–1 μm wide; crests smooth. Anamorph absent.
Extrolites: asperthecin, emericellin, emerins, a versicolorin.
Distinguishing characters: Aspergillus latilabiatus is close to A. navahoensis and A. purpureus, but A. navahoensis produces reddish brown, smaller ascospores (3.5–4.5 × 3–3.5 μm); A. purpureus grows slower on CYA, MEA, YES and OA.
Aspergillus latus (Thom & Raper) A.J. Chen, Frisvad & Samson, comb. nov. MycoBank MB816100. Fig. 31.
Fig. 31.
Aspergillus latus CBS 492.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
Basionym: Aspergillus nidulans var. latus Thom & Raper, Mycologia 31: 660. 1939.
≡ Emericella nidulans var. lata (Thom & Raper) Subram., Curr. Sci. 41: 758 1972.
= Aspergillus sublatus Y. Horie, Trans. Mycol. Soc. Japan 20: 481. 1979. = Emericella sublata Y. Horie, Trans. Mycol. Soc. Japan 20: 481. 1979.
=Aspergillus montenegroi Y. Horie, Miyaji & Nishim., Mycoscience 37: 137. 1996 = Emericella montenegroi Y. Horie, Miyaji & Nishim., Mycoscience 37: 137. 1996.
Typus: CBS H-7051. Culture ex-type: CBS 492.65 = ATCC 16848 = IMI 074181 = NRRL 200 = QM 7425 = WB 200 = IBT 22844 = DTO 047-H2.
ITS barcode: KF465768. (Alternative markers: BenA = AB248334; CaM = KU866693; RPB2 = KU866946).
Colony diam, 7 d (mm): CYA 43–52; CYA 37 °C >60; CYA 40 °C 54– >60; MEA 38–51; MEA 37 °C >60; OA 33–46; YES 58– >60; CREA 12–46; CYAS 25–46; DG18 13–31.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire or slightly irregular; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse dark brown to yellowish brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse yellow green to olive green; soluble pigments absent; exudates light brown droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and light yellow; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse light yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and pale yellow; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation sparse to moderately dense, conidia en masse yellow green; soluble pigments light brown; exudates clear droplets; reverse light yellowish brown. Ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, dark violet to reddish brown, globose to subglobose, 150–400 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 14–30 μm. Asci 8 spored, globose to subglobose. Ascospores light orange, orange or reddish brown, in surface view globose to subglobose, spore bodies smooth, incompletely reticulate or ribbed, globose to subglobose, 3.5–5 × 3–5 μm; in side view lenticular, with two pleated equatorial crests measuring 1–1.5 μm. Conidiophores with smooth stipes, pale brown, 150–300 × 4.5–5.6 μm; vesicles pale brown, subglobose to subclavate, 10–12.5 μm wide, fertile over the upper half; metulae hyaline to pale green, 3.5–8 × 3–4 μm; phialides hyaline to pale green, flask-shaped, 6.5–11 × 2–3.5 μm. Conidia echinulate, globose to subglobose, 2.5–5 μm, green in mass.
Extrolites: asperthecin, asperugins, an austalide (tentatively identified), emericellin, an emindol, shamixanthones, sterigmatocystin, versicolorins, a violaceol.
Distinguishing characters: Aspergillus latus resembles A. nidulans, A. quadrilineatus and A. spinulosporus, but can be distinguished by two wider crests and smooth convex.
Notes: Horie (1979) described Aspergillus sublatus based on its relatively smaller ascospores compared with A. latus, but we observed similar ascospores in the type culture: 4–4.5 × 3–5 μm in CBS 140630 (ex-type of A. sublatus Fig. 4I,J); 3.5–5 × 3–5 μm in CBS 492.65 (ex-type of A. latus Fig. 4G,H). Aspergillus montenegroi produces similar ascospores measuring 3.6–4.8 × 3.2–3.6 μm, some of the ascospores have smooth convex, while some have incompletely reticulate or ribbed ornamentation on convex walls (Horie et al. 1996b). Aspergillus sublatus and A. montenegroi are phylogenetically identical to A. latus, and are considered synonyms.
In Matsuzawa et al. (2012), the ex-type of A. latus clustered with A. quadrilineatus based on BenA, but clustered with A. nidulans based on CaM and actin, we re-sequenced this strain, and it shows consistent position with A. quadrilineatus based on BenA, CaM and RPB2.
Aspergillus miraensis (Zhang, Chen & Guo) Hubka, S.W. Peterson & M. Kolařík, Plant Syst. Evol. 302: 1288. 2016. Mycobank MB816283. Fig. 32.
Fig. 32.
Aspergillus miraensis CBS 140625T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella miraensis Zhang, Chen & Guo, Mycotaxon 125: 132. 2013.
Typus: CGMCC 3.14984. Culture ex-type: CBS 140625 = CGMCC 3.14984 = IBT 33946 = IBT 36278 = DTO 323-B2.
ITS barcode: KU866642 (Alternative markers: BenA =KC342577; CaM = KU866780; RPB2 = KU867045).
Colony diam, 7 d (mm): CYA 47–48; CYA 37 °C No growth; CYA 40 °C No growth; MEA >60; MEA 37 °C No growth; OA >60; YES >60; CREA 13–20; CYAS 42–45; DG18 33–34.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium light yellow and white; texture granular due to ascomata production; sporulation moderately dense, conidia en masse olive green; soluble pigments absent; exudates absent; reverse light yellow with radiate dark brown; large amount of ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate to sulcate; margins entire; mycelium white and saffron; texture granular due to ascomata production; sporulation moderately dense, conidia en masse olive green; soluble pigments absent; exudates clear droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies deep, sulcate; margins entire; mycelium saffron and white; texture floccose; sporulation moderately dense, conidia en masse greyish green; soluble pigments absent; exudates absent; reverse cream yellow. Ascomata present after 1 wk. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse light yellow to yellowish green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture granular due to ascomata production; sporulation sparse, conidia en masse yellow green; soluble pigments absent; exudates clear droplets; reverse greyish olive. Ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown, globose to subglobose, 320–600 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 14–22 μm. Asci 8 spored, stellate to subglobose. Ascospores orange to reddish brown (violet in original description of Zhang et al. 2013), in surface view stellate, 8–10 μm; spore bodies smooth, (verrucose in original description of Zhang et al. 2013) globose to subglobose, 2–4 × 2–3 μm; in side view broadly lenticular, with two stellate equatorial crests; undissected part of crests 0.7–1 μm broad, with 1.5–2.5 μm long extentions; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, light brown, 300–500 Χ 5–6 μm; vesicles hyaline to pale green, subclavate to subglobose, 12–15 μm wide, fertile over the upper half to two thirds; metulae hyaline to pale green, 5–8 × 3–4 μm; phialides hyaline to pale green, flask-shaped, 6–8 × 2–3.5 μm. Conidia echinulate, globose to subglobose, 2–3.5 μm.
Extrolites: aflatoxin B1, asperthecin, 2-ω-hydroxyemodin, a desertorin, emericellin, shamixanthones, sterigmatocystin.
Distinguishing characters: Aspergillus miraensis is close to A. stellatus and A. stella-maris, but can be distinguished by smaller ascospores and conidia. In addition, A. miraensis grows faster on CYA, MEA, YES and OA plates.
Aspergillus multicolor Sappa, Allionia 2: 87. 1954. MycoBank MB292849. Fig. 33.
Fig. 33.
Aspergillus multicolor CBS 133.54T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–E. Conidiophores and Conidia. F,G. Hülle cells. Scale bars: B = 30 μm; C–F = 10 μm; G = 1000 μm.
Typus: IMI 69875. Culture ex-type: CBS 133.54 = NRRL 4775 = ATCC 16804 = IFO 8133 = IMI 69857 = LSHBBB .356 = QM 1952 = WB 4281 = WB 4775 = DTO 053-C9.
ITS barcode: EF652477. (Alternative markers: BenA = EF652301; CaM = EF652389; RPB2 = EF652213).
Colony diam, 7 d (mm): CYA 39–40; CYA 37 °C 7–8; CYA 40 °C No growth; MEA 46–47; MEA 37 °C 5–6; OA 44–45; YES 55–56; CREA 8–12; CYAS 25–26; DG18 21–22.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium pink to purple drab; texture velvety; sporulation moderately dense, conidia en masse dull green to olive green; soluble pigments absent; exudates light brown droplets; reverse greyish olive. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium pink to purple drab; texture velvety; sporulation moderately dense, conidia en masse dull green to olive green; soluble pigments absent; exudates light brown droplets; reverse buffy brown. YES 25 °C, 7 d: Colonies deep, sulcate; margins entire; mycelium pink to purple drab; texture velvety; sporulation absent; soluble pigments absent; exudates light brown droplets; reverse greyish olive. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety to floccose; sporulation dense, conidia en masse yellow green to olive green; soluble pigments absent; exudates absent; reverse light brown at centre, cream yellow at edge. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium pink to purple drab; texture velvety; sporulation limited at centre, conidia en masse blue green; soluble pigments light brown; exudates clear droplets; reverse deep olive buff. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, pale brown, 300–350 × 5–7 μm; vesicles hyaline, globose to subclavate, 16–20 μm wide, fertile over the two thirds; metulae hyaline, 6–10 × 3–4 μm; phialides hyaline, flask-shaped, 8–9 × 2.5–3 μm. Conidia globose to subglobose, echinulate, 3.5–5.5 μm. Hülle cells pale pink, globose to ovoid, 12–20 μm.
Extrolites: asticolourin A, B, C, violaceols.
Distinguishing characters: The pink to purple drab mycelium and pink Hülle cells can easily distinguish A. multicolor from other related species. Brown Hülle cells were mentioned in Raper & Fennell (1965).
Aspergillus mulundensis Bills & Frisvad, J Antibiot. 69: 143. 2016. MycoBank MB813062. Fig. 34.
Fig. 34.
Aspergillus mulundensis CBS 140610T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–F. Conidiophores. G. Conidia. Scale bars: B = 30 μm; C–E,G = 10 μm; F = 8 μm.
≡ Aspergillus sydowii var. mulundensis Roy J Antibiot. 40:275. 1987. nomen nudum
Typus: DSMZ 5745. Culture ex-type: CBS 140610 = DSMZ 5745 = IBT 33104 = DTO 316-C9.
ITS barcode: KU866604. (Alternative markers: BenA = KU866833; CaM = KU866729; RPB2 = KU866989).
Colony diam, 7 d (mm): CYA 22–23; CYA 37 °C 4–8; CYA 40 °C No growth; MEA 24–25; MEA 37 °C 5–6; OA 30–31; YES 36–37; CREA 6–11; CYAS 13–15; DG18 15–17.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white and buff; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse yellowish brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium white and light yellow; texture velvety; sporulation moderately dense, conidia en masse blue green; soluble pigments absent; exudates light brown droplets; reverse reddish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and light yellow; texture velvety; sporulation absent; soluble pigments absent; exudates absent; reverse saffron. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse pale green to blue green; soluble pigments light brown; exudates clear droplets; reverse light brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, hyaline, 33–70 × 2.5–4.5 μm; vesicles hyaline, globose to subclavate, 5.5–15 μm wide, fertile over the two thirds; metulae hyaline, 5–7 × 2.5–4.5 μm; phialides hyaline, flask-shaped, 6–7.5 × 2.5–4 μm. Conidia globose to subglobose, echinulate, 2.5–3.5 μm. Hülle cells (18.7–31.2 × 25–37.5 μm) were reported in the first publication of the nomen nudum Aspergillus sydowii var. mulundensis (Roy et al. 1987).
Extrolites: deoxymulundocandin, dibenzofurans (asticolourins?), mulundocandin, emericellamides, sclerotiorins or similar azaphilones with the same UV spectra as sclerotiorins (sclerotiorins tentatively identified).
Distinguishing characters: Phylogenetically Aspergillus mulundensis is close to A. multicolor, but A. multicolour produces longer conidiophores (300–350 μm), larger vesicles (16–20 μm) and conidia (3.5–5.5 μm). Morphologically A. mulundensis resembles A. aurantiobrunneus, but A. mulundensis grows faster on all tested media.
Aspergillus navahoensis M. Chr. & States, Mycologia 74: 226. 1982. MycoBank MB110496. Fig. 35.
Fig. 35.
Aspergillus navahoensis CBS 351.81T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella navahoensis M. Chr. & States, Mycologia 74: 226. 1982.
Typus: NY SD-5. Culture ex-type: CBS 351.81 = NRRL 13002 = ATCC 44663 = IMI 259971 = IMI 304939 = IBT 10950 = LCP 84.2561 = DTO 047-H7.
ITS barcode: EF652424. (Alternative markers: BenA = EF652248; CaM = EF652336; RPB2 = EF652160).
Colony diam, 7 d (mm): CYA 34–35; CYA 37 °C 30–33; CYA 40 °C 26–27; MEA 24–25; MEA 37 °C 35–38; OA 40–42; YES 34–35; CREA 2–8; CYAS 18–19; DG18 15–16.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium luteous; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins slightly irregular; mycelium light yellow and white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse dark brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium light yellow; texture velvety; sporulation moderately dense, conidia en masse yellow green; soluble pigments light brown; exudates absent; reverse dark brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium luteous; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse yellowish brown to brown. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium light yellow and white; texture floccose; sporulation moderately dense, conidia en masse pale green; soluble pigments light brown; exudates light brown droplets; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, blackish to dark brown, globose, 140–400 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 13–23 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, 3.5–4.5 × 3–3.5 μm; in side view lenticular, with two equatorial crests measuring 0.7–1 μm wide, 0.4 μm thick; crests smooth. Conidiophores with smooth stipes, light brown, 35–150 × 2.5–3 μm; vesicles hyaline to pale brown, subclavate to globose, 6–8 μm wide, fertile over the upper half to two thirds; metulae hyaline, 6–9.5 × 3–4.5 μm; phialides hyaline, flask-shaped, 6–8 × 2.5–3 μm. Conidia echinulate, globose to subglobose, 3.5–4.5 μm, green in mass. (Anamorphic structures were observed from OA).
Extrolites: asperthecin, falconensins, cf. falconensons, gregatins, sterigmatocystin, 6,7,8-hydroxy-3-methylisocoumarin, versicolorins, violaceols.
Distinguishing characters: Aspergillus navahoensis is close to A. fruticulosus, A. nidulans and A. pachycristatus, but can be easily distinguished by smooth, thick crests of ascospores, longer metulae and narrower vesicles.
Aspergillus nidulans (Eidam) G. Winter, Rabenh. Krypt.-Fl., ed. 2, 1: 62. 1884. MycoBank MB182069. Fig. 36, Fig. 37.
Fig. 36.
Aspergillus nidulans CBS 589.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
Fig. 37.
Aspergillus nidulans CBS 114.63 (ex-type of A. dentatus). A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Sterigmatocystis nidulans Eidam, Beitr. Biol. Pflanzen 3: 393.1883.
≡ Emericella nidulans (Eidam) Vuill., C. R. hebd. Seanc. Acad. Sci. Paris 184: 137. 1927. ≡ Aspergillus nidulellus Samson & W. Gams, Adv. Pen. Asp. Syst.: 44. 1985.
=Aspergillus nidulans (Eidam) Wint. var. dentatus D.K. Sandhu & R.S. Sandhu, Mycologia 55: 297. 1963. = Emericella dentata var. dentata (D.K. Sandhu & R.S. Sandhu) Subram., Curr. Sci. 41: 758. 1972. = Emericella dentata (D.K. Sandhu & R.S. Sandhu) Y. Horie, Trans. Mycol. Soc. Japan 21: 491.1980. = Aspergillus dentatulus Ismail, Abdel-Sater & Zohri, Mycotaxon 53: 397. 1995.
Typus: IMI 86806. Culture ex-type: CBS 589.65 = NRRL 187 = ATCC 10074 = IHEM 3563 = IMI 126691 = IMI 86806 = QM 1985 = Thom 4640.5 = WB 187 = DTO 047-H9.
ITS barcode: EF652427. (Alternative markers: BenA = EF652251; CaM = EF652339; RPB2 = EF652163).
Colony diam, 7 d (mm): CYA 30–39; CYA 37 °C 47–58; CYA 40 °C 49–55; MEA 41–52; MEA 37 °C >60; OA 36–52; YES >60; CREA 5–10; CYAS 14–40; DG18 22–38.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation moderately dense, conidia en masse olive green; purple red soluble pigment produced after 2 wks; exudates clear droplets; reverse dark reddish brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation dense, conidia en masse greyish green; soluble pigments absent; exudates clear droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse pale green to yellow green; soluble pigments absent; exudates absent; reverse brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse greyish green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates clear droplets; reverse buffy brown, ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, violet to dark brown, globose to subglobose, 150–420 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 12–20 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, globose to subglobose, 3.5–5 × 3–4.5 μm; in side view lenticular, with two pleated equatorial crests measuring 0.5–1 μm, crests entire or dentate. Conidiophores with smooth stipes, yellowish brown, 70–220 × 5–8 μm; vesicles pale brown, globose to subclavate, 8–14.5 μm wide, fertile over the upper half to two thirds; metulae hyaline, pale green to pale brown, 5–8 × 2.5–4.5 μm; phialides hyaline to pale green, flask-shaped, 6–8 × 2.5–3.5 μm. Conidia echinulate, globose to subglobose, 3–4 μm.
Extrolites: asperthecin, asperugins, austinol, cordycepin, dehydroaustinol, 2-ω-hydroxyemodin, diorcinol, emericellin, shamixanthones, sterigmatocystin, versicolorins, violaceols. Asperugins were only detected on CYA with 5 % NaCl. Many further extrolites have been found in this species (Nielsen et al. 2011) using different combinations of media, after biological interaction etc (Table 6).
Distinguishing characters: Aspergillus nidulans resembles A. quadrilineatus, but differs in two crests in contrast to four crests on the ascospores of A. quadrilineatus.
Notes: Aspergillus nidulans var. dentatus (CBS 114.63), isolated from diseased human fingernails, showed almost full phenotypic agreement with A. nidulans except for its dentate equatorial crests (Sandhu & Sandhu 1963), our observation confirms the original description (Fig. 3, Fig. 37). Aspergillus dentatus shares identical sequences (ITS, BenA, CaM and RPB2) and extrolites with A. nidulans, therefore is considered a synonym here.
Aspergillus olivicola Frisvad, Zalar & Samson, Mycologia 100: 781. 2008. MycoBank MB507362. Fig. 38.
Fig. 38.
Aspergillus olivicola CBS 119.37T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella olivicola Frisvad, Zalar & Samson, Mycologia 100: 788. 2008.
Typus: CBS H-19888. Culture ex-type: CBS 119.37 = IBT 21903 = IBT 26499 = DTO 011-A8 = DTO 002-I2.
ITS barcode: EU448268. (Alternative markers: BenA = AY339996; CaM = EU443986; RPB2 = KU866923).
Colony diam, 7 d (mm): CYA 30–36; CYA 37 °C 0–14; CYA 40 °C No growth; MEA 33–42; MEA 37 °C 5–10; OA 30–35; YES 24–39; CREA 12–18; CYAS 27–30; DG18 20–25.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane to slightly sulcate; margins entire; mycelium gray to greyish violet; texture floccose; sporulation absent to moderately dense, conidia en masse olive green; soluble pigments absent; exudates absent; reverse dark violet to dark brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate to sulcate; margins entire; mycelium white; texture granular due to ascomata production; sporulation moderately dense, conidia en masse pale green; soluble pigments absent; exudates clear droplets; reverse yellowish brown to cream brown with brown dots, large amount of ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep, plane to slightly sulcate; margins entire; mycelium smoke gray; texture floccose; sporulation sparse to moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse dark brown at centre, cream white at edge. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture granular due to ascomata production; sporulation sparse; soluble pigments absent; exudates clear droplets; reverse white. Ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, greenish brown, globose to subglobose, 400–770 μm, surrounded by numerous Hülle cells; Hülle cells brown, globose to ovoid, 15–28 μm. Asci 8 spored, stellate to subglobose. Ascospores orange to reddish brown, in surface view stellate, 7.5–11 μm; spore bodies smooth, globose to subglobose, 3–4.5 × 3–4 μm; in side view broadly lenticular, with two stellate equatorial crests; undissected part of crests 0.4–0.7 μm broad, with 1–3 μm long extentions; crests ornamented with longitudinal, 0.3–0.5 μm wide pleats. Conidiophores with smooth stipes, yellowish brown, 150–340 × 4–5.5 μm; vesicles hyaline to pale brown, subglobose to subclavate, 8–15 μm wide, fertile over the upper half to two thirds; metulae hyaline, 7.5–10.5 × 2–3.5 μm; phialides hyaline to pale brown, flask-shaped, 7.5–12.5 × 1.5–3 μm. Conidia coarsely echinulate to tuberculate, globose to subglobose, 2–3.5 μm.
Extrolites: aflatoxin B1 and B2, asperugins, asteltoxin, desertorins, emericellin, shamixanthones, sterigmatocystin, terrein, varitriols.
Distinguishing characters: Thin-walled Hülle cells, relatively long metulae (7.5–10.5 × 2–3.5 μm) and phialides (7.5–12.5 × 1.5–3 μm), coarsely echinulate conidia, these characters distinguish A. olivicola from other stellate ascospored species.
Aspergillus omanensis Y. Horie & Udagawa, Mycoscience 36: 391. 1995. MycoBank MB414655.
≡ Emericella omanensis Y. Horie & Udagawa, Mycoscience 36: 391. 1995.
Typus: CBM FA-700. Culture ex-type: CBM FA-700 = IFM 54275.
ITS barcode: n.a. (Alternative markers: BenA = AB248347; CaM = AB524047; RPB2 = n.a.).
Colony characters: Fide Horie & Udagawa (1995) colonies on Czapek's solution agar growing restrictedly, attaining a diameter of 25–26 mm in 14 days at 25 °C, more or less floccose, plane, consisting of a thin mycelial felt, producing scattered cleistothecia, Yellowish White (3A2 after Korner & Wanscher 1978) to pale Orange (6A3); conidial heads limited in number, not affecting the colony colour; reverse Brownish Orange (7C4) to Brown (7E6). Colonies on MEA spreading broadly, attaining a diameter of 56–57 mm in 14 days at 25 °C, more or less floccose, plane, consisting of a thin mycelial felt, granular in appearance due to the production of abundant cleistothecia with Hülle cells, overgrown by loose network of aerial hyphae and numerous conidial heads, Greenish Gray (1C2) to Greyish Green (1D3); reverse Greyish Orange (5B3) to Brownish Orange (5C4).
Micromorphology: Fide Horie & Udagawa (1995), cleistothecia superficial, scattered or aggregated in a thin layer, globose to subglobose, 180–370 μm in diam, surrounded by a hyaline to pale yellowish brown layer of scattered hyphae bearing numerous globose to subglobose thick-walled Hülle cells measuring 10–35 μm in diam; peridium brown to dark brown, thin, of texture intricate, 2–3-layered; outermost layer consisting of hyphal cells measuring 3–17 μm wide. Asci irregularly disposed, 8-spored, globose to subglobose or ovoid, 11–13.5 × 10–11 μm, evanescent. Ascospores at first hyaline to pale reddish brown, becoming brownish red, broadly lenticular, 4.5–5.5 × 4–4.5 μm including crests, with two conspicuously pleated equatorial crests measuring about 1 μm wide, with a tuberculate or verruculose convex wall. Conidial heads greyish green, short columnar to columnar, 70–190 μm long and 40–70 μm wide. Conidiophores arising mostly from aerial hyphae; stipes short, more or less sinuous, 50–120 × 4–7 μm, orange gray to brownish orange, smooth-walled; vesicles subglobose to subclavate, orange gray, 10–14 μm in diam, fertile over the upper half. Aspergilla biseriate; metulae greyish white to pale greyish green, 4–7 × 2–3 μm; phialides greyish white to pale greyish green, 5–8 × 2–4 μm. Conidia globose to subglobose, 4–5.5 μm in diam, verruculose.
Extrolites: Strain not available.
Distinguishing characters: Molecular analysis shows A. omanensis as a unique species. Fide Horie & Udagawa (1995) its morphology resembles that of A. spinulosporus and A. desertorum, but can be distinguished from A. spinulosporus by tuberculate or verruculose ornamentation on ascospore convex walls; and from A. desertorum by much smaller ascospores. Unfortunately, the type strain is unavailable and cannot be investigated during our study.
Aspergillus pachycristatus Matsuzawa, Y. Horie & Yaguchi, Mycoscience 53: 439. 2012. MycoBank MB580944. Fig. 39.
Fig. 39.
Aspergillus pachycristatus NRRL 11440. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella pachycristata Matsuzawa, Y. Horie & Yaguchi, Mycoscience 53: 439. 2012.
Typus: IFM 55265. Culture ex-type: IFM 55265 = NBRC 104790.
ITS barcode: n.a. (Alternative markers: BenA = AB375875; CaM = AB524062; RPB2 = n.a.).
Colony diam, 7 d (mm): CYA 13–14; CYA 37 °C 45–56; CYA 40 °C 42–54; MEA 15–16; MEA 37 °C >60; OA 25–32; YES 17–19; CREA 3–4; CYAS 10–12; DG18 7–10.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium white and rosy buff; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse dark reddish brown. MEA 25 °C, 7 d: Colonies morderately deep, slightly sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates light brown to purple droplets, large amount of purple droplets present on MEA at 37 °C after 1 wk; reverse dark reddish brown. Ascomata present after 2 wks. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white and light yellow; texture floccose; sporulation moderately dense, conidia en masse greyish green; soluble pigments absent; exudates absent; reverse reddish brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium buff; texture floccose; sporulation moderately dense, conidia en masse greyish green; soluble pigments absent; exudates absent; reverse ochraceous buff. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture floccose to velvety; sporulation moderately dense, conidia en masse yellow green; soluble pigments light brown; exudates absent; reverse cream white to light brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, blackish to dark brown, globose to subglobose, 200–500 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 11–21 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, 4–5 × 3.5–4 μm; in side view lenticular, with two equatorial crests measuring 0.7–1 μm wide, 0.4 μm thick; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, light brown, 150–260 × 5–6 μm; vesicles hyaline to pale brown, subclavate, 8–12 μm wide, fertile over the upper half to two thirds; metulae hyaline, 5.5–7.5 × 2.5–4 μm; phialides hyaline, flask-shaped, 6–9 × 2.5–3.5 μm. Conidia echinulate, globose to subglobose, 3–4 μm. (Anamorphic structures were observed from OA).
Extrolites: asperugins, echinocandins, emecorrugatin, sterigmatocystin, versicolorins, violaceols.
Distinguishing characters: Morphologically, Aspergillus pachycristatus resembles A. nidulans, but the ascospore crests are thicker in A. pachycristatus. The smooth ascospore convex can distinguish it from phylogenetically related A. rugulosus.
Aspergillus pluriseminatus (Stchigel & Guarro) Samson, Visagie & Houbraken, Stud. Mycol. 78: 155. 2014. MycoBank MB809595.
≡ Emericella pluriseminata Stchigel & Guarro, Mycologia 89: 937. 1997.
Typus: FMR 5588; isotype IMI 370867. Culture ex-type: CBS 100523 = FMR 5588 = IMI 370867 = DTO 011-H1.
ITS barcode: KU866566. (Alternative markers: BenA = AY339989; CaM = EU443988; RPB2 = KU866937).
Colony diam, 7 d (mm): CYA 20–25; CYA 37 °C No growth; CYA 40 °C No growth; MEA 31–32; MEA 37 °C No growth; OA 12–22; YES 28–30; CREA No growth; CYAS 1–2; DG18 1–2.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium pale pink to pale yellow at centre, white at edge; texture velvety; sporulation absent; soluble pigments absent; exudates clear droplets; reverse reddish brown. MEA 25 °C, 7 d: Colonies sulcate, plane; margins entire; mycelium white; texture velvety; sporulation absent; soluble pigments absent; exudates light brown droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium pale pink to pale yellow at centre, white at edge; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white to yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse reddish brown to yellowish brown. CREA 25 °C, 7 d: No growth.
Micromorphology: Fide Stchigel & Guarro (1997) ascomata superficial, globose, nonostiolate, blackish, with green iridescence, 80–250 μm diam, produced very late, appearing after 2–3 months, surrounded by a felt of hyphae and Hülle cells and supported by masses of scattered hyphae and Hülle cells; Hülle cells pale yellowish to orange-brown, globose to irregularly shaped, thick-walled, 10–22 μm diam. Peridium 4–12 μm thick, pale to yellowish-brown, semi-translucent to translucent, textura intricate to epidermoidea, 3–7 layered, cells of the outer layer measuring 3–14 μm diam. Asci 16 spored (8 spored according to Zalar et al. 2008), globose to broadly ellipsoidal, with several broad wall protrusions, 22–35 μm diam, evanescent. Ascospores one-celled, at first hyaline, becoming violet-brown, lenticular, 7–9 × 6–7 μm (crest not included), with two conspicuously pleated, stellate and striate equatorial crests, 4–8 μm wide; convex surface tuberculate under SEM. Anamorph absent.
Extrolites: Dibenzofurans (asticolourins?), sclerotiorins (tentatively identified), violaceols.
Distinguishing characters: The large, violet stellate ascospores with tuberculate convex surface can distinguish Aspergillus pluriseminatus from other related species.
Notes: According to Stchigel & Guarro (1997), ascomata of A. pluriseminatus are produced very late, and only produced on PCA. Unfortunately, we could not find ascomata on several kinds of media including PCA after 3 months. Zalar et al. (2008) observed 8 spored instead of 16 spored asci in A. pluriseminatus.
Aspergillus purpureus Samson & Mouch., Antonie van Leeuwenhoek 41: 350. 1975. MycoBank MB309237. Fig. 40.
Fig. 40.
Aspergillus purpureus CBS 754.74T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B. Ascomata. C. Asci. D,F–H. Ascospores. E. Hülle cells. Scale bars: B = 1000 μm; C–E = 10 μm; F–H = 2 μm.
≡ Emericella purpurea Samson & Mouch., Antonie van Leeuwenhoek 41: 350. 1975.
Typus: CBS 754.74. Culture ex-type: CBS 754.74 = NRRL 6133 = IMI 334937 = LCP 82.3323 = DTO 047-H5.
ITS barcode: EF652506. (Alternative markers: BenA = EF652330; CaM = EF652418; RPB2 = EF652242).
Colony diam, 7 d (mm): CYA 5–7; CYA 37 °C No growth; CYA 40 °C No growth; MEA 7–10; MEA 37 °C No growth; OA 5–7; YES 7–9; CREA 1–2; CYAS No growth; DG18 7–8.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse rosy buff; MEA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse reddish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse dark brown at centre, cream yellow at edge. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse white. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, blackish to dark brown, globose to subglobose, 90–200 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 8–20 μm. Asci 8 spored, globose to subglobose. Ascospores brown, in surface view globose to subglobose, spore bodies smooth, 6–7 × 4.5–5 μm; in side view lenticular, with two low crests measuring 0.3–0.6 μm wide. Fide Samson & Mouchacca (1975) conidial structures mostly absent on Czapek or MEA, but sometimes produced in old slant cultures on the glass surface; on Czapek agar with 20 % or more sucrose conidiophores are produced after one month. Conidial heads white, radiate, biseriate. Conidiophores hyaline, 40–50 × 2.5–5 μm. Vesicles ellipsoidal to clavate, 6–8 μm in diam. Metulae cylindrical, 3.5–6 × 2.5–3.5 μm bearing 2 to 3 phialides each. Phialides flask-shaped with short but distinct neck, 6–8 × 2.5–3 μm. Conidia ellipsoidal to cylindrical, hyaline, smooth, 3.5–5.5 × 1.5–2 μm.
Extrolites: calbistrins, emerin, emindol PA, epurpurin A–C, norsolorinic acid, shamixanthones, variecolins, versicolorins.
Distinguishing characters: Aspergillus purpureus can be distinguished from other related species by large brown ascospores and the restricted growth on tested media.
Notes: Hyaline conidiophores, smooth-walled cylindrical conidia (3.5–5.5 × 1.5–2 μm) were recorded in the original description (Samson & Mouchacca 1975), but are not confirmed in this study.
Aspergillus qinqixianii Y. Horie, Abliz & R.Y. Li, Mycoscience 41: 183. 2000. MycoBank MB464660. Fig. 41.
Fig. 41.
Aspergillus qinqixianii CBS 128788T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella qinqixianii Y. Horie, Abliz & R.Y. Li, Mycoscience 41: 183. 2000.
Typus: CBM FA-866. Culture ex-type: CBS 128788 = IFM 55020 = CMB-FA-866 = DTO 098-H6.
ITS barcode: KU866600. (Alternative markers: BenA = AB524360; CaM = AB524051; RPB2 = KU866980).
Colony diam, 7 d (mm): CYA 40–42; CYA 37 °C 23–30; CYA 40 °C No growth; MEA 45–46; MEA 37 °C 26–28; OA 35–38; YES 54–55; CREA 16–17; CYAS 25–34; DG18 21–25.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white and gray; texture floccose; sporulation sparse; soluble pigments absent; exudates clear to light brown droplets; reverse dark olive green; Ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation sparse; soluble pigments absent; exudates clear to light brown droplets; reverse dark brown at centre, yellowish brown at edge. Ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse cream yellow to dark brown. Ascomata present after 1 wk. DG18 25 °C, 7 d: Colonies moderately deep; plane; margins entire; mycelium buff at centre, white at edge; texture floccose; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse pale yellow green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture granular due to ascomata production; sporulation moderately dense; conidia en masse yellow green; soluble pigments absent; exudates clear to light brown droplets; reverse pale olive. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, greyish green to brown, globose to subglobose, 200–510 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 16–24 μm. Asci 8 spored, stellate. Ascospores brown, in surface view globose to subglobose, spore bodies smooth, 3.5–4.5 × 3–4 μm; in side view broadly lenticular, with two equatorial crests measuring 0.5 μm wide; Crest bearing hyaline, filiform appendages, measuring 3–7 μm long with swollen tips. Conidiophores with smooth stipes, yellowish brown, 120–280 × 3–5 μm; vesicles hyaline to pale yellowish brown, subglobose to subclavate, 7–12 μm wide, fertile over the upper half; metulae hyaline to pale yellowish brown, 4–8 × 3–5 μm; phialides hyaline to pale yellowish brown, flask-shaped, 7–8 × 2–4 μm. Conidia echinulate, globose to subglobose, 3–4 μm.
Extrolites: Asteltoxin, asperthecin, emericellin, 2-ω-hydroxyemodin, shamixanthones, terrein (CBS 128789 in addition produced curvularin and dehydrocurvularin).
Distinguishing characters: Aspergillus qinqixianii is close to A. filifer, they share identical CaM, but can be distinguished by small differences in BenA and RPB2. Morphologically these two species can be easily differentiated by the ornamentation on convex surface, the ascospores of A. qinqixianii have smooth ascospore convex in contrast with tuberculate convex in A. filifer.
Aspergillus quadrilineatus Thom & Raper, Mycologia 31: 660. 1939. MycoBank MB275888. Fig. 42.
Fig. 42.
Aspergillus quadrilineatus CBS 591.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. I. Ascospores of CBS 119.55, ex-type of A. acristatus. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H,I = 2 μm.
≡ Emericella quadrilineata (Thom & Raper) C.R. Benj., Mycologia 47: 680. 1955. ≡ Aspergillus tetrazonus Samson & W. Gams, Adv Pen. Asp. Syst., 48. 1985.
= Aspergillus nidulans var. acristatus Fennell & Raper, Mycologia 47: 79. 1955. = Emericella nidulans (Eidam) Vuill. var. acristata (Fennell & Raper) Subram. Curr. Sci. 41: 758. 1972. (later homonym). = Emericella acristata (Fennell & Raper) Y. Horie, Trans. Mycol. Soc. Japan 21: 491. 1980 = Aspergillus acristatulus Ismail, Abdel-Sater & Zohri, Mycotaxon 53: 396. 1995.
= Aspergillus parvathecius Raper & Fennell, Gen. Aspergillus: 509. 1965. = Emericella parvathecia (Raper & Fennell) Malloch & Cain, Can. J. Bot. 50: 62. 1972. = Aspergillus microthecius Samson & W. Gams, Adv. Pen. Asp. Syst.: 43. 1985.
= Aspergillus floriformis Samson and Mouch., Antonie van Leeuwenhoek 40: 343, 1975.
= Aspergillus miyajii Y. Horie, Mycoscience 37: 323. 1996. ≡ Emericella miyajii Y. Horie, Mycoscience 37: 323. 1996.
Typus: IMI 089351. Culture ex-type: CBS 591.65 = NRRL 201 = ATCC 16816 = IMI 089351ii = IMI 89351 = LSHBA 546 = QM 7465 = Thom 4138.N8 = WB 201 = DTO 048-A9.
ITS barcode: EF652433. (Alternative markers: BenA = EF652257; CaM = EF652345; RPB2 = EF652169).
Colony diam, 7 d (mm): CYA 26–46; CYA 37 °C >60; CYA 40 °C >60; MEA 31–47; MEA 37 °C >60; OA 41–48; YES >60; CREA 8–11; CYAS 18–33; DG18 22–28.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium buff and white; texture floccose; sporulation sparse; light brown soluble pigments produced after 2 wks; exudates clear droplets; reverse dark brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium buff and white; texture floccose; sporulation sparse; soluble pigments absent; exudates clear droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium buffy brown fading into white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse dark brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium buff; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse light brown. OA 25 °C, 7 d: Colonies morderately deep, plane; margins entire; mycelium buff and white; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments light brown; exudates clear droplets; reverse yellowish brown, ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, developing throughout the colony, reddish brown, globose to subglobose, 100–700 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 10–24 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, globose to subglobose, 4–4.5 × 3–4.5 μm; in side view lenticular, with two plaited equatorial crests about 0.5-1 μm in width paralleled by a secondary narrower pair which are sometimes indistinct, crests are entire, defective or with irregular protuberance. Conidiophores with smooth stipes, pale brownish, 50–150 × 4–5.5 μm; vesicles pale brown, globose, 10–13 μm wide, fertile over the upper half to two thirds; metulae hyaline, 5–7 × 2–4.5 μm; phialides hyaline, flask-shaped, 5–7 × 2–4 μm. Conidia echinulate, globose to subglobose, 3–4 μm.
Extrolites: asperthecin, asperugins, echinocandins, emestrin, emericellin, emindols, quadrilineatin, shamixanthone, sterigmatocystin, violaceols.
Distinguishing characters: Aspergillus quadrilineatus is close to A. nidulans and A. latus, but can be distinguished by four crests.
Notes: Phylogenetically Aspergillus acristatus, A. floriformis, A. parvathecius and A. miyajii are identical with A. quadrilineatus and are considered as synonyms as did Hubka et al. (2016). Morphologically these species have minor differences in ascospore crests. Aspergillus acristatus was introduced as a crest-free variety of A. nidulans (Fig. 42I), Fennell and Raper (1955) suggested a close relationship between A. acristatus and A. quadrilineatus, because a number of A. quadrilineatus strains also show reduced four crests. Aspergillus floriformis was described as a anamorphic species, only Hülle cells were mentioned in the original description (Samson & Mouchacca 1975), but the ex-type (CBS 937.73) of A. floriformis is now degenerated and does not produce any anamorphic or teleomorphic structures. Also the ex-type (CBS 493.65) of A. parvathecius which was described with ascospores does not produce the teleomorph. Hubka et al. (2016) speculated that both A. parvathecius and A. miyajii represent atypical A. quadrilineatus strains characterized by smaller ascomata with delayed maturation in the first and ascospores with aberrant development and shape in the latter. We agree with their opinion, one strain (CBS 853.96) collected from Spain further confirms the diversity of ascospore phenotype in A. quadrilineatus, the ascospore crests in this isolate are irregularly protuberate (Fig. 3G, H), phylogenetically it is identical in ITS, CaM and BenA with other A. quadrilineatus strains, but shows seven bp differences in RPB2 (99.2 % similarity, 907/914 bp).
Aspergillus recurvatus Raper & Fennell, Gen. Aspergillus: 529. 1965. MycoBank MB326653. Fig. 43.
Fig. 43.
Aspergillus recurvatus CBS 496.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–E. Conidiophores and Conidia. F,G. Hülle cells. Scale bars: B = 30 μm; C–F = 10 μm; G = 1000 μm.
Typus: IMI 36528. Culture ex-type: CBS 496.65 = NRRL 4902 = ATCC 16809 = IMI 136528 = O-566 = QM 7972 = WB 4902 = IBT 23271 = DTO 053-C8.
ITS barcode: EF652482. (Alternative markers: BenA = EF652306; CaM = EF652394; RPB2 = EF652218).
Colony diam, 7 d (mm): CYA 12–34; CYA 37 °C 21–45; CYA 40 °C 25–29; MEA 19–21; MEA 37 °C 27–52; OA 34–36; YES 22–50; CREA 2–12; CYAS 8–14; DG18 9–15.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium lemon yellow; texture velvety; sporulation absent; soluble pigments absent; exudates light brown droplets; reverse dark brown. MEA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium light yellow and white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse reddish brown. YES 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium light yellow and white; texture floccose to velvety; sporulation absent; soluble pigments absent; exudates absent; reverse ochraceous buff. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium lemon yellow; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium light yellow and white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse light yellow. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, light brown, 40–150 × 3.5–4.5 μm; vesicles hyaline to pale brown, subclavate, 8–10 μm wide, fertile over the upper half to two thirds; metulae hyaline, 5–6 × 2.5–3.5 μm; phialides hyaline to yellowish green, flask-shaped, 4.5–5.5 × 2–3 μm. Conidia olive green, echinulate, globose to subglobose, 3–4.5 μm. Hülle cells hyaline, globose to ovoid, 18–30 μm.
Extrolites: an austalide (tentatively identified), falconensins, falconensons, violaceols.
Distinguishing characters: This species is characterized by yellow mycelium, which resembles A. aurantiopurpureus, but A. aurantiopurpureus produces longer conidiophores (130–260 × 3.5–5 μm), smaller conidia (3–3.5 μm) and is able to produce ascospores.
Aspergillus rugulosus Thom & Raper, Mycologia 31: 660. 1939. MycoBank MB277104. Fig. 44.
Fig. 44.
Aspergillus rugulosus CBS 133.60T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella rugulosa (Thom & Raper) C.R. Benj., Mycologia 47: 680. 1955. ≡ Aspergillus rugulovalvus Samson & W. Gams, Adv. Penicillium Aspergillus Syst.: 49. 1985.
= Emericella cleistominuta B.S. Mehrotra & R. Prasad, Trans. Br. Mycol. Soc. 52: 333. 1969. = Aspergillus cleistominutus B.S. Mehrotra & R. Prasad, Trans. Br. Mycol. Soc. 52: 333. 1969.
= Emericella rugulosa var. lazulina Horie, Miyaji & Nishimura, Mycoscience 37: 140. 1996.
Typus: IMI 136775. Culture ex-type: CBS 133.60 = NRRL 206 = ATCC 16820 = IMI 136775 = QM 1987 = Thom 4138.T11 = WB 206 = IBT 22820 = DTO 321-H1.
ITS barcode: EF652434. (Alternative markers: BenA = EF652258; CaM = EF652346; RPB2 = EF652170).
Colony diam, 7 d (mm): CYA 14–16; CYA 37 °C 35–58; CYA 40 °C 35–55; MEA 15–22; MEA 37 °C >60; OA 35–60; YES 22–30; CREA 3–8; CYAS 10–15; DG18 4–10.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse reddish brown. MEA 25 °C, 7 d: Colonies deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation absent to moderately dense, conidia en masse if present, olive green; soluble pigments absent; exudates absent to brown droplets; reverse reddish brown to yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse brown to light brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation absent to moderately dense, conidia en masse if present, yellow green; soluble pigments absent; exudates absent; reverse olive brown. OA 25 °C, 7 d: Colonies low to morderately deep, plane; margins entire; mycelium olive buff and white; texture floccose; sporulation sparse to moderately dense, conidia en masse olive green; soluble pigments light brown; exudates absent to light brown droplets; reverse light brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown to dark brown, globose to subglobose, 220–350 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 14–24 μm. Asci 8 spored, globose to subglobose. Ascospores orange, greyish violet, reddish purple or brownish red, in surface view globose to subglobose, spore bodies conspicuously rugulose, globose to subglobose, 4–4.5 × 3.5–4 μm; in side view lenticular, with two plaited equatorial crests with sinuate and entire margins measuring 0.5–0.6 μm wide. Conidiophores with smooth stipes, pale brown, 50–200 × 5–6 μm; vesicles pale brown, hemisphere to subclavate, 8–12 μm wide, fertile over the upper half to two thirds; metulae hyaline to pale brown, 7–8 × 3–3.5 μm; phialides hyaline to pale brown, flask-shaped, 6–7 × 2.5–3 μm. Conidia echinulate, globose to subglobose, 3–4 μm, green in mass.
Extrolites: asperugins, echinocandins, emecorrugatin, emericellin, emestrin, sterigmatocystin, versicolorins, violaceols.
Distinguishing characters: Aspergillus rugulosus can be easily distinguished from other species by rugulose ornamentation on convex surface of ascospores.
Notes: The ascospore colour in A. rugulosus varies from greyish red to dark greyish red or reddish purple (Benjamin, 1955, Raper and Fennell, 1965). In this study, the type strain CBS 133.60 produces orange red ascospores, which turn to reddish purple after months. Emericella rugulosa var. lazulina was described based on its greyish magenta to greyish violet ascospores (Horie et al. 1996b), since it is identical in morphology (except the ascospores colour) and phylogeny with A. rugulosus, we treat it as a synonym as did Hubka et al. (2016). According to Mehrotra & Prasad (1969) Emericella cleistominuta differed from A. rugulosus in producing much smaller ascomata (15–50 μm). However, we observed ascomata measuring 200–300 μm in E. cleistominuta, the ascospores of these two species are identical too (Fig. 5E–H). Based on morphological and molecular results, E. cleistominuta is treated as a synonym.
Aspergillus savannensis A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816096. Fig. 45.
Fig. 45.
Aspergillus savannensis CBS 140607T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
Etymology: Name refers to its origin, isolated from A1 horizon soil, Halile Rest Camp south of Dolemile Hill, savanna.
Diagnosis: Moderately dense to dense sporulation on CYA, MEA, YES, OA and DG18, reddish brown, smooth ascospores, green conidia measuring 3.5–5 μm.
Typus: Namibia, south of Dolomite Hill (savanna), in Halili Rest Camp, A1 horizon soil, 2001, isolated by M. Christensen (holotype CBS H-22495, culture ex-type: CBS 140607 = IBT 23422 = DTO 059-H6).
ITS barcode: KU866581. (Alternative markers: BenA = KU866818; CaM = KU866704; RPB2 = KU866959).
Colony diam, 7 d (mm): CYA 33–35; CYA 37 °C 55–56; CYA 40 °C 55–56; MEA 45–48; MEA 37 °C >60; OA 47–48; YES 64–65; CREA 6–7; CYAS 37–38; DG18 37–38.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium smoke gray; texture velvety; sporulation moderately dense, conidia en masse olive; soluble pigments absent; exudates light brown droplets; reverse dark reddish brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose at centre, velvety at edge; sporulation dense, conidia en masse dark green; soluble pigments absent; exudates clear droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture velvety; sporulation moderately dense, conidia en masse pale green to yellow green; soluble pigments absent; exudates absent; reverse buff. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green to dark green; soluble pigments absent; exudates absent; reverse pale green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse dark green; soluble pigments absent; exudates clear droplets; reverse pale yellow green. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, dark brown, globose to subglobose, 65–120 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 11–16.5 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, 4–5 × 3.5–4; in side view lenticular, with two equatorial crests measuring 0.5–1 μm. Conidiophores with smooth stipes, pale brown, 85–190 × 5–7 μm; vesicles pale brown, globose to subclavate, 8–15.5 μm wide, fertile over the upper half to two thirds; metulae hyaline, 4.5–8 × 3.5–4.5 μm; phialides hyaline, flask-shaped, 7.5–9 × 3–4 μm. Conidia echinulate, globose to subglobose, 3.5–5 μm, green in mass.
Extrolites: asperthecin, desertorins, emerins, epurpurins, paspaline, paspalinine, paxillin.
Distinguishing characters: Phylogenetically Aspergillus savannensis clusters with A. desertorum, A. botswanensis and A. stercorarius, but the latter three species do not produce anamorph on any media, while A. savannensis sporulates well on CYA, MEA, YES, OA and DG18.
Aspergillus spelunceus Raper & Fennell [as ‘speluneus’], Gen. Aspergillus: 457. 1965. MycoBank MB326656. Fig. 46.
Fig. 46.
Aspergillus spelunceus CBS 497.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–F. Conidiophores. G. Conidia. Scale bars: B = 30 μm; C–G = 10 μm.
Typus: IMI 211389. Culture ex-type: CBS 497.65 = NRRL 4989 = ATCC 16838 = IMI 211389 = NRRL A-3676 = QM 8898 = WB 4989 = IBT 33967 = DTO 053-C4.
ITS barcode: EF652490. (Alternative markers: BenA = EF652314; CaM = EF652226; RPB2 =EF652402).
Colony diam, 7 d (mm): CYA 18–19; CYA 37 °C No growth; CYA 40 °C No growth; MEA 22–23; MEA 37 °C No growth; OA 20–21; YES 18–19; CREA 11–12; CYAS 13–14; DG18 13–14.
Colony characters: CYA 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium white and buff; texture floccose; sporulation sparse to moderately dense, conidia en masse pale green; soluble pigments absent; exudates absent; reverse dark brown at centre, buff at edge. MEA 25 °C, 7 d: Colonies deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation sparse to moderately dense, conidia en masse blue green; soluble pigments absent; exudates absent; reverse coral red at centre, yellowish brown at edge. YES 25 °C, 7 d: Colonies deep, slightly sulcate; margins entire; mycelium white; sporulation sparse; soluble pigments absent; exudates absent; reverse reddish brown. DG18 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse pale green to blue green; soluble pigments absent; exudates absent; reverse cream white. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, yellowish brown, 130–300 × 4–6 μm; vesicles coloured as the conidiophores, globose to subglobose, 7–11 μm wide, fertile over the two thirds to whole surface; metulae hyaline, 4–6.5 × 2.5–3.5 μm; phialides hyaline, flask-shaped, 5.5–7.5 × 2–2.5 μm. Conidia globose to subglobose, tuberculate, 2.5–3.5 μm, blue green in mass.
Extrolites: cyclopenol, sterigmatocystin, versicolorins, viridicatin, viridicatol.
Distinguishing characters: Aspergillus spelunceus is close to A. aureolatus morphologically and phylogenetically, but can be distinguished by its smaller, more roughened conidia.
Note: According to Emmons, Hülle cells were observed in the original isolation cultures grown on an agar medium containing 1 % neopeptone and 2 % glucose as nutrient. Raper & Fennell (1965) observed limited, degenerated terminal or intercalary cells that resemble Hülle cells. During our study, Hülle cells were not observed, the capacity to produce Hülle cells seems to have disappeared with continued laboratory cultivation.
Aspergillus spinulosporus Hubka, S.W. Peterson & M. Kolařík, Plant Syst. Evol. 302: 1290. MycoBank MB816282. Fig. 47.
Fig. 47.
Aspergillus spinulosporus CBS 120.55T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Aspergillus nidulans var. echinulatus Fennell & Raper, Mycologia 47: 79. 1955. ≡ Emericella nidulans var. echinulata (Fennell & Raper) Godeas, Mycopathol. Mycol. Appl. 46: 193. 1972. ≡ Emericella echinulata (Fennell & Raper) Y. Horie, Trans. Mycol. Soc. Japan 21: 492. 1980. Non Aspergillus echinulatus (Delacr.) Thom & Church, The Aspergilli: 107. 1926. ≡ Aspergillus delacroxii Samson, Visagie & Houbraken, Stud. Mycol. 78: 155. 2014.
Typus: IMI 061454, Culture ex-type CBS 120.55 = NRRL 2395 = ATCC 16825 = IMI 061454 = LCP 84.2557 = QM 1909 = WB 2395 = IBT 22841 = DTO 047-G9.
ITS barcode: EF652445. (Alternative markers: BenA = AY573553; CaM = EF652357; RPB2 = EF652181).
Colony diam, 7 d (mm): CYA 33–38; CYA 37 °C >60; CYA 40 °C >60; MEA 44–50; MEA 37 °C >60; OA 42–48; YES 55–62; CREA 15–26; CYAS 22–36; DG18 5–19.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium saffron and white; texture floccose; sporulation absent; soluble pigments absent; exudates brown droplets; reverse deep wood brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates brown droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium saffron and white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium saffron and white; texture velvety; sporulation sparse; soluble pigments light brown; exudates absent; reverse light yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, dark brown, globose to subglobose, 200–550 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 15–30 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies echinulate, globose to subglobose, 3.5–4.5 × 3–4.5 μm; in side view lenticular, with two pleated equatorial crests measuring 0.8–1 μm. Conidiophores with smooth stipes, yellowish brown, 70–120 × 5–6 μm; vesicles yellowish brown, subclavate, 9–11 μm wide, fertile over the upper half; metulae pale brown to pale green, 6–8 × 3–4 μm; phialides hyaline to pale green, flask-shaped, 6–8.5 × 2–3 μm. Conidia echinulate, globose to subglobose, 3–4 μm, green in mass.
Extrolites: asperthecin, asperugins, shamixanthone, sterigmatocystins, versicolorins, violaceols.
Distinguishing characters: Aspergillus spinulosporus can be easily distinguished by echinulate convex surface of ascospores.
Notes: This species was introduced as A. nidulans var. echinulatus (Fennell & Raper 1955). Molecular data show it as a unique species, which is also proved by its special ascospore ornamentation. Since the name A. echinulatus is already occupied, the new name A. delacroxii was proposed (Samson et al. 2014). Hubka et al. (2016) treated “A. delacroxii ” as a correctable orthographical error and proposed a new name A. spinulosporus. In our study we also concur with this.
Aspergillus stella-maris Zalar, Frisvad & Samson, Mycologia 100: 789. 2008. MycoBank MB507363. Fig. 48.
Fig. 48.
Aspergillus stella–maris CBS 113638T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 200 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella stella-maris Zalar, Frisvad & Samson, Mycologia 100: 789. 2008.
Typus: CBS H-19887. Culture ex-type: CBS 113638 = IBT 23439 = DTO 011-A2.
ITS barcode: EU448269. (Alternative markers: BenA = KU866886; CaM = EU443978; RPB2 = KU866929).
Colony diam, 7 d (mm): CYA 35–39; CYA 37 °C No growth; CYA 40 °C No growth; MEA 38–40; MEA 37 °C No growth; OA 33–35; YES 42–49; CREA 8–11; CYAS 29–32; DG18 23–25.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and buff; texture granular due to ascomata production; sporulation sparse to moderately dense, conidia en masse olive green to dark green; soluble pigments absent; exudates clear droplets; reverse buff with radiate brown. Ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate to sulcate; margins entire; mycelium white and buff; texture granular due to ascomata production; sporulation moderately dense, conidia en masse olive green to dark green; soluble pigments absent; exudates clear droplets; reverse dark brown at centre, yellowish brown at edge. Ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and buff; texture velvety; sporulation dense, conidia en masse olive green; soluble pigments absent; exudates absent; reverse dark brown at centre, cream white at edge. Ascomata present after 1 wk. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse dark green at centre, olive buff at edge. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture velvety to granular; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates clear to light brown droplets; reverse pale greyish green. Ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown, globose to subglobose, 370–770 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 16–22 μm. Asci 8 spored, stellate. Ascospores orange to reddish brown, in surface view stellate, 13–16 μm; spore bodies smooth, globose to subglobose, 3–4.5 × 2.5–4.5 μm; in side view broadly lenticular, with two stellate equatorial; undissected part of crests 1–1.5 μm broad, with 3–4.5 μm long extentions; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, yellowish brown, 300–800 × 3.5–7 μm; vesicles hyaline to pale green, globose to subclavate, 9–20 μm wide, fertile over the upper two thirds; metulae hyaline to pale green, 5–9 × 3–4 μm; phialides hyaline to green, flask-shaped, 6–9 × 2–3.5 μm. Conidia smooth to finely echinulate, globose to subglobose, 3–4 μm, green in mass.
Extrolites: emericellin, shamixanthones, sterigmatocystin, versicolorins.
Distinguishing characters: Until now stellate ascospores were described for A. pluriseminatus, A. venezuelensis, A. miraensis, A. stellatus, A. olivicola, A. dromiae and A. angustatus. Among these species, A. stella-maris is close to A. miraensis and A. stellatus in vesicle shape, but can be distinguished by septate conidiophores and larger ascospores and conidia.
Aspergillus stellatus Curzi, C.R. Accad. Lincei 19: 428. 1934. MycoBank MB254841. Fig. 49.
Fig. 49.
Aspergillus stellatus CBS 598.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
= Emericella variecolor Berk. & Broome, Intr. crypt. bot. (London): 340.1857. = Aspergillus variecolor (Berk. & Broome) Thom & Raper, Mycologia 31: 663. 1939. = Aspergillus stellifer Samson & W. Gams, Adv. Pen. Asp. Syst.: 52. 1985.
Typus: Bowenpilly near Secundarabad, s. coll., (K). Culture ex-epitype: CBS 598.65 = NRRL 1858 = ATCC 16819 = IMI 136778 = QM 6835 = WB 1858 = IBT 32730 = DTO 327-F3.
ITS barcode: EF652426. (Alternative markers: BenA = EF652250; CaM = EF652338; RPB2 = EF652162).
Colony diam, 7 d (mm): CYA 26–35; CYA 37 °C 21–34; CYA 40 °C No growth; MEA 32–46; MEA 37 °C 18–35; OA 22–35; YES 38–53; CREA 7–10; CYAS 19–30; DG18 11–22.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and buff; texture velvety; sporulation moderately dense, conidia en masse olive green; soluble pigments absent; exudates clear droplets; reverse dark olive fading into buff. Ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture velvety; sporulation dense, conidia en masse yellow green to dark green; soluble pigments absent; exudates clear droplets; reverse yellowish brown with brown ring. Ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture velvety to granular due to ascomata production; sporulation dense, conidia en masse dark green; soluble pigments absent; exudates absent; reverse brown at centre, yellowish brown at edge. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture velvety; sporulation dense; conidia en masse yellow green; soluble pigments absent; exudates absent; reverse pale yellow green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates clear droplets; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, violet to reddish brown, globose to subglobose, 300–600 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 11.5–25.5 μm. Asci 8 spored, subglobose to polygonal or stellate. Ascospores orange to reddish brown, in surface view stellate, 10–14 μm; spore bodies smooth, globose to subglobose, 3.5–4 × 3–4 μm; in side view broadly lenticular, with two stellate equatorial crests; undissected part of crests 0.5–1 μm broad, with 2.5–4 μm long extentions; crests ornamented with longitudinal, 0.3–0.4 μm wide pleats. Conidiophores with smooth stipes, yellowish brown, 320–610 × 4.5–6.5 μm; vesicles hyaline to pale yellowish brown, globose to subclavate, 13.5–18.5 μm wide, fertile over the upper half to two thirds; metulae hyaline, 4–7.5 × 3.5–4 μm; phialides hyaline, flask-shaped, 6–8.5 × 2.5–3.5 μm. Conidia echinulate, globose to subglobose, 2.5–3 μm.
Extrolites: asperthecin, asperugins, astellolide, asteltoxin, austinol, a desertorin, emericellin, 2-ω-hydroxyemodin, Mer-NF8054X, shamixanthones, varitriol, violaceols.
Distinguishing characters: The stellate ascospores of Aspergillus stellatus resemble those of A. stella-maris and A. dromiae, but it differs from A. dromiae by smaller conidia, differs from A. stella-maris by non-septate conidiophores.
Aspergillus stercorarius A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816094. Fig. 50.
Fig. 50.
Aspergillus stercorarius CBS 428.93T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B. Ascomata. C. Asci. D,F–H. Ascospores. E. Hülle cells. Scale bars: B = 1000 μm; C–E = 10 μm; F–H = 2 μm.
Etymology: The name refers to the dung (from Uromastix acanthinurus) habitat.
Diagnosis: Brown, smooth ascospores measuring 4.5–6 × 3.5–4.5 μm, with two low equatorial crests measuring 0.3–0.4 μm wide.
Typus: Algeria, Sahara, Uromastix acanthinurus dung, 1993, isolated by M. Locquin-Linard (holotype CBS H-22496, culture ex-type: CBS 428.93 = IBT 28024 = DTO 320-B3).
ITS barcode: KU866625. (Alternative markers: BenA = KU866865; CaM = KU866763; RPB2 = KU867026).
Colony diam, 7 d (mm): CYA 30–40; CYA 37 °C 53–56; CYA 40 °C 47–48; MEA 42–46; MEA 37 °C >60; OA 35–42; YES 52–60; CREA 5–7; CYAS 26–27; DG18 15–16.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white and buff; texture velvety; sporulation absent; soluble pigments absent; exudates light brown droplets; reverse dark brown fading into yellowish brown. Ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation absent; soluble pigments absent; exudates clear droplets; reverse orange brown fading into yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and light yellow; texture velvety; sporulation absent; soluble pigments absent; exudates clear droplets; reverse yellowish brown. DG18 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent, exudates absent; reverse light yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and light yellow; texture floccose; sporulation absent; light yellow to light orange pigments; exudates clear droplets; reverse luteous, ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, purple to dark brown, globose to subglobose, 70–150 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 8–14.5 μm. Asci 8 spored, globose to subglobose. Ascospores brown, in surface view globose to subglobose, spore bodies smooth, 4.5–6 × 3.5–4.5 μm; in side view lenticular, with two low equatorial crests measuring 0.3–0.4 μm wide. Anamorph absent.
Extrolites: cf. asperthecin, calbistrins, desertorins, emindols, paspaline, paspalinine, paxillin, terrequinone A.
Distinguishing characters: Aspergillus stercorarius is close to A. latilabiatus and A. desertorum, but differs in smaller, smooth ascospores.
Aspergillus striatus J.N. Rai, J.P. Tewari & Mukerji, Can. J. Bot. 42: 1521. 1964. MycoBank MB326659. Fig. 51.
Fig. 51.
Aspergillus striatus CBS 592.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B. Ascomata. C,E–G. Ascospores. D. Hülle cells. Scale bars: B = 1000 μm; C–D = 10 μm; E–G = 2 μm.
≡ Emericella striata (J.N. Rai, J.P. Tewari & Mukerji) Malloch & Cain, Can. J. Bot. 50: 62. 1972. ≡ Aspergillus striatulus Samson & W. Gams, Adv. Pen. Asp. Syst.: 50. 1985.
Typus: IMI 96679. Culture ex-type: CBS 283.67 = CBS 592.65 = NRRL 4699 = ATCC 16815 = IMI 96679 = QM 8901 = WB 4699 = DTO 320-D3.
ITS barcode: EF652470. (Alternative markers: BenA = EF652294; CaM = EF652382; RPB2 = EF652206).
Colony diam, 7 d (mm): CYA 38–41; CYA 37 °C 47–60; CYA 40 °C 48–55; MEA 38–45; MEA 37 °C >60; OA 32–35; YES 50–57; CREA 3–5; CYAS 12–22; DG18 11–15.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and saffron; texture velvety; sporulation absent; soluble pigments absent; exudates clear droplets; reverse brown fading into saffron. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium saffron at centre, white at edge; texture floccose; sporulation absent; soluble pigments absent; exudates clear droplets; reverse yellowish brown to reddish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white and light yellow; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium saffron at centre, white at edge; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white to yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and saffron; texture floccose; sporulation absent; soluble pigments yellowish brown to light brown; exudates clear droplets; reverse yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, blackish to dark brown, globose to subglobose, 180–500 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose to ovoid, 14–23 μm. Asci 8 spored, globose to ovoid. Ascospores orange, in surface view globose to subglobose; spore bodies roughened, convex surface bearing simple or anastomosing thickenings arranged in more or less concentric rings, 6–7 × 5–5.5 μm; in side view broadly lenticular. Anamorph absent.
Extrolites: asperthecin, emericellin, emestrin, emindol SA, paxillin, shamixanthones, sterigmatocystin, versicolorins, violaceols.
Distinguishing characters: Morphologically this species is close to A. rugulosus and A. violaceus, but differs in orange ascospores with fingerprint like ornamentation.
Aspergillus sulphureoviridis A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816097. Fig. 52.
Fig. 52.
Aspergillus sulphureoviridis CBS 140626T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
Etymology: Name refers to its bluish green conidia mass.
Diagnosis: Large ascospores measuring 4.5–5.5 × 3.5–4.5 μm and bluish green conidia measuring 3.5–5 μm.
Typus: Denmark, factory, indoor air, 1999, isolated by J.C. Frisvad (holotype CBS H-22497, culture ex-type CBS 140626 = IBT 21868 = DTO 325-D1).
ITS barcode: KU866673. (Alternative markers: BenA = KU866911; CaM = KU866793; RPB2 = KU867058).
Colony diam, 7 d (mm): CYA 30–31; CYA 37 °C 55–56; CYA 40 °C 40–41; MEA 39–41; MEA 37 °C >60; OA 42–43; YES 43–45; CREA 12–13; CYAS 28–29; DG18 28–29.
Colony characters: CYA 25 °C, 7 d: Colonies deep, sulcate; margins slightly irregular; mycelium white and saffron; texture floccose; sporulation sparse; soluble pigments absent; exudates clear droplets; reverse dark brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse reddish brown fading into orange and yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse buff. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates absent; reverse yellow. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white and buff; texture floccose; sporulation sparse, conidia en masse pale green; soluble pigments light brown; exudates clear droplets; reverse buff. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, reddish brown to brown, globose to subglobose, 350–600 μm, surrounded by numerous Hülle cells; Hülle cells hyaline, globose to ovoid, 10–22.5 μm. Asci 8 spored, globose to subglobose. Ascospores orange to reddish brown, in surface view globose to subglobose, spore bodies smooth, 4.5–5.5 × 3.5–4.5 μm; in side view lenticular, with two equatorial crests measuring 0.8–1.2 μm. Conidiophores with smooth stipes, pale brown, 30–80 × 3–5 μm; vesicles pale brown, subglobose to subclavate, 7–10 μm wide, fertile over the upper half to two thirds; metulae hyaline, 6.5–8.5 × 2.5–3.5 μm; phialides hyaline, flask-shaped, 6.5–7.5 × 3–4 μm. Conidia tuberculate, globose to subglobose, 3.5–5 μm, bluish green in mass.
Distinguishing characters: The ornamentation on the ascospore crests (holes measuring 0.2–0.5 μm) resembles those of A. foveolatus and A. aurantiobrunneus, but A. foveolatus produces pitted ascospores and A. aurantiobrunneus produces smaller conidia (2.5–3.5 μm).
Aspergillus undulatus H.Z. Kong & Z.T. Qi, Acta Mycol. Sin. 5: 211. 1986. MycoBank MB129004. Fig. 53.
Fig. 53.
Aspergillus undulatus CBS 261.88T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella undulata H.Z. Kong & Z.T. Qi, Acta Mycol. Sin. 5: 211. 1986.
Typus: HMAS 47644. Culture ex-type: CBS 261.88 = AS 3.4510 = IBT 28027 = DTO 011-H1.
ITS barcode: EU448275. (Alternative markers: BenA = EF428363; CaM = EU443989; RPB2 = KU866928).
Colony diam, 7 d (mm): CYA 13–14; CYA 37 °C 10–15; CYA 40 °C No growth; MEA 25–26; MEA 37 °C 14–18; OA 33–34; YES 29–30; CREA 3–5; CYAS 17–18; DG18 15–17.
Colony characters: CYA 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium saffron and white; texture granular due to abundant ascomata production; sporulation absent; soluble pigments absent; exudates absent; reverse dark brown at centre, fading into light brown. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture granular due to abundant ascomata production; sporulation sparse; soluble pigments absent; exudates clear droplets; reverse dark brown. YES 25 °C, 7 d: Colonies moderately deep, plane; margins slightly irregular; mycelium white; texture granular due to abundant ascomata production; sporulation absent; soluble pigments absent; exudates absent; reverse dark brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins slightly irregular; mycelium white; texture velvety to floccose; sporulation dense, conidia en masse pale green; soluble pigments absent; exudates absent; reverse yellow green. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white at centre, light yellow at edge; texture granular due to abundant ascomata production; sporulation absent; soluble pigments light brown; exudates clear droplets; reverse dull greenish yellow. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, dark reddish brown, globose, 300–500 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale brown, globose to ovoid, 14–26 μm . Asci 8 spored, globose to subglobose. Ascospores brown, in surface view globose to subglobose, spore bodies tuberculate, 4–4.5 × 3.5–4 μm; in side view lenticular, with two waved equatorial crests, low part measuring 0.3–0.7 μm, high part measuring 0.8–1.3 μm. Conidiophores with smooth stipes, hyaline, 80–150 × 3–4.5 μm; vesicles hyaline to pale brown, hemisphere to subclavate, 7–11 μm wide, fertile over the upper half to two thirds; metulae hyaline, 5.5–7.5 × 2.5–3 μm; phialides hyaline to pale brown, flask-shaped, 6.5–7.5 × 2–2.5 μm. Conidia echinulate, globose to subglobose, 3–4 μm.
Extrolites: a gregatin, a varitriol.
Distinguishing characters: The wave-crested ascospores with tuberculate convex can easily distinguish Aspergillus undulatus from other species.
Aspergillus unguis (Emile-Weill & L. Gaudin) Thom & Raper, Mycologia 31: 667. 1939. MycoBank MB255264. Fig. 54.
Fig. 54.
Aspergillus unguis CBS 132.55T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–F. Conidiophores. G. Conidia. Scale bars: B = 30 μm; C–E,G = 10 μm; F = 8 μm.
≡ Sterigmatocystis unguis Emile-Weill & L. Gaudin, Arch. Med. Exp. Anat. Pathol. 28:
463. 1918. ≡ Emericella unguis Malloch & Cain, Can. J. Bot. 50: 62. 1972.
Typus: IMI 136526. Culture ex-type: CBS 132.55 = NRRL 2393 = ATCC 16812 = IMI 136526 = NRRL A-2391 = NRRLA-445 = QM 25B = WB 2393 = DTO 047-I5.
ITS barcode: EF652443. (Alternative markers: BenA = EF652267; CaM = EF652355; RPB2 = EF652179).
Colony diam, 7 d (mm): CYA 22–35; CYA 37 °C 19–27; CYA 40 °C no growth or 1–2; MEA 23–35; MEA 37 °C 22–25; OA 30–35; YES 34–45; CREA 10–17; CYAS 32–35; DG18 18–22.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane to slightly sulcate; margins entire; mycelium white and light yellow; texture floccose to velvety; sporulation sparse to moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse vinaceous buff. MEA 25 °C, 7 d: Colonies moderately deep to deep, plane to slightly sulcate; margins entire; mycelium white; texture floccose to velvety; sporulation moderately dense, conidia en masse greyish green; soluble pigments absent; exudates absent or clear droplets; reverse brown fading into yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, plane to sulcate; margins entire; mycelium white; texture velvety to floccose; sporulation sparse to moderately dense, conidia en masse greyish olive to olive green; soluble pigments absent; exudates absent; reverse light brown to vinaceous buff. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation sparse to moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse vinaceous buff. OA 25 °C, 7 d: Colonies low, plane; margins entire; texture velvety to floccose; sporulation sparse to moderately dense, conidia en masse dark green; soluble pigments absent; exudates absent; reverse pale green. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, pale brown, 50–100 × 3–5 μm; vesicles hyaline to pale brown, subclavate, 8–10 μm wide, fertile over the upper half; metulae hyaline to pale brown, 5–7 × 2.5–3.5 μm; phialides hyaline, flask-shaped, 5–9 × 2–2.5 μm. Conidia globose to subglobose, echinulate, 2.5–4 μm.
Extrolites: asperugins, nidulin, nornidulin, unguisin, unguisinol, ustilagionoidin C.
Distinguishing characters: Aspergillus unguis is close to A. asperescens and A. aureolatus, but A. asperescens produces longer conidiophores (200–400 × 6–8 μm) and large, ellipsoidal conidia (4–7 × 3–5 μm); A. aureolatus is characterized by orange marginal zone of colonies. In addition A. asperescens and A. aureolatus cannot grow at 37 °C, while A. unguis grows well at this temperature.
Aspergillus varians Wehmer, Bot. Centralbl. 80: 460. 1899. MycoBank: MB172782. Fig. 55.
Fig. 55.
Aspergillus varians CBS 505.65T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–F. Conidiophores. G. Conidia. Scale bars: B = 30 μm; C–G = 10 μm.
Typus: IMI 172297. Culture ex-type: CBS 505.65 = NRRL 4793 = ATCC 16836 = IFO
4114 = IMI 172297 = WB 4793 = IBT 22568 = DTO 073-B5.
ITS barcode: EF652479. (Alternative markers: BenA = EF652303; CaM = EF652391; RPB2 = EF652215).
Colony diam, 7 d (mm): CYA 20–30; CYA 37 °C No growth; CYA 40 °C No growth; MEA 21–22; MEA 37 °C No growth; OA 25–26; YES 25–26; CREA 28–30; CYAS 24–25; DG18 14–15.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white and gray; texture floccose; sporulation moderately dense, conidia en masse greyish green; soluble pigments absent; exudates absent; reverse orange at centre, dark brown at edge. MEA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse glaucous; soluble pigments absent; exudates absent; reverse orange. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium yellow at centre, white at edge; texture floccose to velvety; sporulation moderately dense, conidia en masse dull green to greyish green; soluble pigments absent; exudates absent; reverse light yellow to light brown. DG18 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse yellow ocher. OA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium white; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse pale green. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, hyaline to pale brown, 600–1200 × 7–12 μm; vesicles hyaline, hemispherical to subclavate, 20–30 μm wide, fertile over the upper half to two thirds; metulae hyaline, 7–10 × 3.5–4.5 μm; phialides hyaline, flask-shaped, 8–12 × 3–4 μm. Conidia subglobose to ellipsoidal, smooth, 4–6 × 3.5–4 μm.
Extrolites: 2-ω-hydroxyemodin, emerin, epurpurin A, B & C, shamixanthones, versicolorins.
Distinguishing characters: The long conidiophores (600–1200 μm) and wide vesicles (20–30 μm) can easily distinguish Aspergillus varians from other related species.
Aspergillus venezuelensis Frisvad & Samson, Syst. Appl. Microbiol. 27: 678. 2004. MycoBank MB368544. Fig. 56.
Fig. 56.
Aspergillus venezuelensis CBS 868.97T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B,C. Conidiophores. D. Conidia. E. Ascomata. F. Hülle cells. G,H. Ascospores. Scale bars: B = 30 μm; C,D,F,G = 10 μm; E = 1000 μm; H = 2 μm.
≡ Emericella venezuelensis Frisvad & Samson, Syst. Appl. Microbiol. 27: 678 2004.
Typus: CBS 868.97. Culture ex-type: CBS 868.97 = IBT 20956 = DTO 011-A4.
ITS barcode: AJ874119. (Alternative markers: BenA = AY339998; CaM = EU443977; RPB2 = KU866931).
Colony diam, 7 d (mm): CYA 37–38; CYA 37 °C 3–5; CYA 40 °C No growth; MEA 42–43; MEA 37 °C No growth; OA 31–35; YES 49–50; CREA 18–19; CYAS 37–38; DG18 23–26.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, plane; margins entire; mycelium light yellow at centre, white at edge; texture floccose at centre, velvety at edge; sporulation absent; soluble pigments absent; exudates clear droplets; reverse light yellow fading into cream white; ascomata present after 1 wk. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium light yellow at centre, white at edge; texture floccose at centre, velvety at edge; sporulation absent; soluble pigments absent; exudates clear droplets; reverse dark brown at centre, yellowish brown at edge; ascomata present after 1 wk. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose to velvety; sporulation absent; soluble pigments absent; exudates absent; reverse cream white; ascomata present after 1 wk. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium light yellow at centre, white at edge; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse citron yellow; ascomata present after 1 wk. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium light yellow at centre, white at edge; texture floccose at centre, velvety at edge; sporulation absent; soluble pigments absent; exudates clear droplets; reverse cream white; ascomata present after 1 wk. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, superficial, violet to brown, globose to subglobose, 400–1000 μm, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 12–21.5 μm. Asci 8 spored, stellate. Ascospores orange to reddish brown, in surface view stellate, 12.5–19.5 μm; spore bodies covered with triangular flaps, globose to subglobose, 4–5 × 3.5–4.5 μm; in side view broadly lenticular, with two stellate equatorial; undissected part of crests 1–1.2 μm broad, with 2.5–4 μm long extentions; crests ornamented with longitudinal, 0.3–0.5 μm wide pleats. Conidiophores with smooth stipes, light yellowish brown, 65–130 × 2–3 μm; vesicles hyaline to pale yellowish brown, subclavate, 5.5–7 μm wide, fertile over the upper half to two thirds; metulae hyaline, 4–5 × 2.5–3.5 μm; phialides hyaline, flask-shaped, 6–7 × 2.5–3.5 μm. Conidia echinulate, globose to subglobose, 2.5–4 μm (Anamorphic structures were observed from CYA).
Extrolites: aflatoxin B1, B2, a desertorin, emericellin, an emerin, shamixanthones, sterigmatocystin, versicolorins.
Distinguishing characters: Triangular flaps on the ascospore convex surface can distinguish this species from other stellate ascospored species.
Notes: The anamorph of A. venezuelensis occurs quite late (after 1 month) on unconventional media such as CREA, CYA + 40 % sucrose, while absent on conventional growth media (CYA, MEA, OA) (Frisvad & Samson 2004). During our study, sparse conidiophores are present on CYA after 2 months. The presented conidiophores show typical characters of section Nidulantes, but the vesicles are smaller (5.5–7 μm) compared with original description (7–10 μm).
Aspergillus violaceus Fennell & Raper, Mycologia 47: 75. 1955. MycoBank MB292863. Fig. 57.
Fig. 57.
Aspergillus violaceus CBS 138.55T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B. Conidiophores. C. Conidia. D. Hülle cells. E. Ascomata. F–H. Ascospores. Scale bars: B–D,F = 10 μm; E = 1000 μm; G–H = 2 μm.
≡ Emericella violacea (Fennell & Raper) Malloch & Cain, Can. J. Bot. 50: 62. 1972. ≡ Aspergillus violaceobrunneus Samson & W. Gams, Adv. Pen. Asp. Syst.: 53. 1985.
= Emericella similis Y. Horie et al., Trans. Mycol. Soc. Japan 31: 425. 1990. = Aspergillus similis (Y. Horie et al.) Samson, Visagie & Houbraken, Stud. Mycol. 78: 157. 2014.
Typus: IMI 61449. Culture ex-type: CBS 138.55 = NRRL 2240 = ATCC 16813 = CECT2587 = IFO 8106 = IMI 061449ii = IMI 61449 = LCP 82.3318 = NRRL A-3156 = QM 1905 = UC4511 = WB 2240 = DTO 048-B2.
ITS barcode: EF652438. (Alternative markers: BenA = EF652262; CaM = EF652350; RPB2 = EF652174).
Colony diam, 7 d (mm): CYA 23–33; CYA 37 °C 45–56; CYA 40 °C 41–45; MEA 22–41; MEA 37 °C 58– >60; OA 30–32; YES 38–53; CREA 5–13; CYAS 18–25; DG18 3–16.
Colony characters: CYA 25 °C, 7 d: Colonies moderately deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse brown. MEA 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, sulcate; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse cream white to olive brown. OA 25 °C, 7 d: Colonies low, plane; margins entire; mycelium white; texture velvety; sporulation absent; soluble pigments light olive to light brown; exudates clear droplets; reverse light yellowish brown. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata cleistothecial, blackish to dark brown, globose, 25–50 μm in CBS 138.55T, up to 190 μm in CBS 293.93, surrounded by numerous Hülle cells; Hülle cells hyaline to pale yellowish brown, globose to ovoid, 6–26 μm. Asci 8 spored, globose to ovoid. Ascospores violet, in surface view globose to subglobose; spore bodies roughened, with reticulate intertwined ornamentation, 4–6.5 × 3–5 μm; in side view broadly lenticular, with two low equatorial crest, less than 0.3 μm wide. Fide Fennell & Raper (1955) the conidial structures on hay-infusion agar scattered, small and commonly fractional, not affecting the colony appearance. Conidiophores arising primarily from aerial hyphae, smooth-walled, very short, 30–50 μm in length by 3–4 μm in diameter, somewhat sinuous, thin-walled, hyaline or nearly so, terminating in rounded and somewhat enlarged vesicular areas mostly 5–6 μm, metualae few in number, borne on the upper part of the vesicle only, variable in dimensions, mostly 6–7.5 × 3–3.5 μm; phialides about 5–6 × 2–2.5 μm, flask-shaped, bearing conidia in short chains; conidia globose or nearly so, light green in colour, smooth or delicately roughened, mostly 2.8–3.3 μm in diameter.
Extrolites: asperugin, emestrin, violaceols, emericellin, paxillin.
Distinguishing characters: Violet ascospores with reticulate intertwined ornamentation.
Notes: According to Fennell & Raper (1955), limited and generally minute conidial heads were produced on hay-infusion agar. During our observation, atypical conidiophores and much bigger conidia (3.5–4.5 μm) are produced quite late (after 2 months) on CYA. Aspergillus similis (ex-type CBS 293.93) is undifferentiated from A. violaceus in ascospore morphology (Fig. 5S, T) and multi-gene phylogeny, thus is considered a synonym of A. violaceus.
Aspergillus viridicatenatus A.J. Chen, Frisvad & Samson, sp. nov. MycoBank MB816088. Fig. 58.
Fig. 58.
Aspergillus viridicatenatus CBS 140629T. A. Colonies: top row left to right, obverse CYA, obverse MEA, YES and OA; bottom row left to right, reverse CYA, reverse MEA, DG18 and CREA. B–F. Conidiophores. G. Conidia. Scale bars: B = 30 μm; C–G = 10 μm.
Etymology: Name refers to its long green conidial chains.
Diagnosis: Subglobose, ellipsoidal to cylindrical conidia measuring 3–5 × 2.5–4 μm, teleomorph not observed.
Typus: Denmark, root of Gymnadenia conopsea, 2011, isolated by J.C. Frisvad (holotype CBS H-22498, culture ex-type CBS 140629 = IBT 31492 = DTO 325-F4).
ITS barcode: KU866682. (Alternative markers: BenA = KX423621; CaM = KU866802; RPB2 = KU867067).
Colony diam, 7 d (mm): CYA 15–16; CYA 37 °C No growth; CYA 40 °C No growth; MEA 21–23; MEA 37 °C No growth; OA 19–20; YES 20–21; CREA 10–11; CYAS 12–13; DG18 29–30.
Colony characters: CYA 25 °C, 7 d: Colonies deep, plane; margins entire; mycelium buff; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse buff yellow. MEA 25 °C, 7 d: Colonies deep, sulcate; margins slightly irregular; mycelium white; texture floccose; sporulation sparse; soluble pigments absent; exudates dark brown droplets; reverse yellowish brown. YES 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium brown; texture floccose; sporulation moderately dense, conidia en masse yellow green; soluble pigments absent; exudates absent; reverse light yellow to light brown. DG18 25 °C, 7 d: Colonies moderately deep, slightly sulcate; margins entire; mycelium light yellow and white; texture floccose; sporulation absent; soluble pigments absent; exudates absent; reverse yellowish brown. OA 25 °C, 7 d: Colonies low, plane; margins entire; texture velvety to floccose; sporulation moderately dense, conidia en masse yellow green to dark green; soluble pigments absent; exudates absent; reverse pale green. CREA 25 °C, 7 d: Acid production absent.
Micromorphology: Ascomata not observed. Conidiophores with smooth stipes, yellowish brown to brown, 120–270 × 5–6 μm; vesicles hyaline to pale brown, globose to subglobose, 10–15 μm wide, fertile over the two thirds; metulae hyaline to pale brown, 6–9 × 2.5–3.5 μm; phialides hyaline to pale green, flask-shaped, 6–9.5 × 2.5–3.5 μm. Conidia subglobose, ellipsoidal to cylindrical, echinulate, 3–5 × 2.5–4 μm, green in mass.
Extrolites: An unidentified extrolite in common with Penicillium bialowiezense, which has a UV spectrum with absorption maxima at 220 nm, 312 nm and 324 nm, was present. The extrolite with this UV spectrum has not been found in any other Aspergillus species yet.
Distinguishing characters: Aspergillus viridicatenatus is close to A. aureolatus and A. spelunceus, but can be distinguished by its ellipsoidal to cylindrical conidia.
Aspergillus section Cavernicolus A.J. Chen, Frisvad & Samson, sect. nov. MycoBank MB816113.
Typus: Aspergillus cavernicola Lörinczi, Contrt. Bot. Cluj: 341. 1969.
=Aspergillus amylovorus Panas. ex Samson, Stud. Mycol. 18: 28. 1979 =Aspergillus amylovorus Panas., Mycologia 56: 58. 1964
Description: Conidial heads radiate to columnar, conidiophores biseriate, smooth, uncoloured or in brown shades. Vesicles globose to subglobose. Conidia smooth to rough. Hülle cells regularly present.
Five species previously assigned to section Usti, namely A. amylovorus, A. californicus, A. cavernicola, A. egyptiacus, A. kassunensis and A. subsessilis are included in this new section mainly based on multigene phylogeny (Fig. 1). Phylogenetically A. amylovorus is identical with A. cavernicola, although A. amylovorus was published at 1964, the name was not validated until 1979 (Samson 1979), thus A. cavernicola has priority based on its publication date, A. amylovorus is considered as a synonym.
Doubtful species list
Aspergillus sub-unguis Wadhwani, Dudeja & Srivastava Curr. Sci. 53: 443, 1984 (Wadhwani et al. 1984). – Typus IMI 254637. ITS barcode: n.a. (Alternative markers: BenA = n.a.; CaM = n.a.; RPB2 = n.a.). Type culture is not available anymore and its taxonomic position cannot be determined.
Acknowledgements
This project was supported by the Alfred P. Sloan Foundation Program on the Microbiology of the Built Environment, by National Natural Science Foundation of China No. 81473345 and by the Hungarian Research Fund (OTKA K115690). We thank Yuguang Zhou (CGMCC, China) for providing cultures from China. Uwe Braun is acknowledged for his advice on the new species names.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Supplementary material related to this article can be found at http://dx.doi.org/10.1016/j.simyco.2016.10.001.
Appendix A. Supplementary material
The following are the Supplementary material related to this article:
Phylogenetic tree of section Nidulantes inferred from ITS.
Phylogenetic tree of section Nidulantes inferred from BenA.
Phylogenetic tree of section Nidulantes inferred from CaM.
Phylogenetic tree of section Nidulantes inferred from RPB2.
References
- Ahmad V.U., Sultana V. A new metabolite from Aspergillus quadrilineatus. Zeitschrift für Naturforschung Section B – A Journal of Chemical Sciences. 1985;40:319–320. [Google Scholar]
- Ahmed S.A., Bardshire E., McIntyre C.R. Biosynthetic studies on tajixanthone and shamixanthone, polyketide hemiterpenoid metabolites of Aspergillus variecolor. Australian Journal of Chemistry. 1992;45:249–274. [Google Scholar]
- Ahmed S.A., Bardshiri E., Simpson T.J. A convenient synthesis of isotopically labelled anthraquinones, chrysophanol, islandicin, and emodin. Incorporation of [methyl-2H3] chrysophanol into tajixanthone in Aspergillus variecolor. Journal of the Chemical Society, Chemical Communications. 1987;1987:883–884. [Google Scholar]
- An C.Y., Li X.M., Li C.S. Aniquinazolines A-D, four new quinazolinone alkaloids from marine-derived endophytic fungus Aspergillus nidulans. Marine Drugs. 2013;11:2682–2694. doi: 10.3390/md11072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C.Y., Li X.M., Luo H. 4-Phenyl-3,4-dihydroquinolone derivatives from Aspergillus nidulans MA-143, an endophytic fungus isolated from the mangrove plant Rhizophora stylosa. Journal of Natural Products. 2013;76:1896–1901. doi: 10.1021/np4004646. [DOI] [PubMed] [Google Scholar]
- Arabatzis M., Kambouris M., Kyprianou M. Polyphasic identification and susceptibility to seven antifungals of 102 Aspergillus isolates recovered from immunocompromised hosts in Greece. Antimicrobial Agents and Chemotherapy. 2011;55:3025–3030. doi: 10.1128/AAC.01491-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argoudelis A.D., Coats J.H., Herr R.R. Isolation and characterisation of a new antibiotic, U-13,933. Antimicrobial Agents & Chemotherapy. 1965;5:801–803. [PubMed] [Google Scholar]
- Argoudelis A.D., Zieserl J.F. The structure of U-13,933, a new antibiotic. Tetrahedron Letters. 1966;7:1969–1973. doi: 10.1016/s0040-4039(00)76280-0. [DOI] [PubMed] [Google Scholar]
- Asami Y., Jang J.H., Oh H. Violaceols function as actin inhibitors inducing cell shape elongation in fibroblast cells. Bioscience Biotechnology and Biochemistry. 2012;76:1431–1437. doi: 10.1271/bbb.120074. [DOI] [PubMed] [Google Scholar]
- Aucamp P.J., Holzapfel C.W. Constitution of nidulol (5-hydroxy-7-methoxy-6-methylphthalide), a metabolic product of Aspergillus nidulans (Eidam) Wint. Journal of the South African Chemical Institute. 1968;21:26–32. [Google Scholar]
- Aucamp P.J., Holzapfel C.W. Polyhydroxyanthraquinones from Aspergillus versicolor, Aspergillus nidulans and Bipolaris sp., their significance in relation to biogenetic theories on aflatoxin B1. Journal of the South African Chemical Institute. 1970;23:40–56. [Google Scholar]
- Ballantine J.A., Ferrito V., Hassall C.H. The biosynthesis of phenols. Part XXIV. Arugosin C, a metabolite of a mutant strain of Aspergillus rugulosus. Journal of the Chemical Society Perkin Transactions I. 1973;1973:1825–1830. doi: 10.1039/p19730001825. [DOI] [PubMed] [Google Scholar]
- Ballantine J.A., Ferrito V., Hassall C.H. Aspertetronin A and B, two novel tetronic acid derivatives produced by a blocked mutant of Aspergillus rugulosus. Journal of the Chemical Society C. 1969;1969:56–61. [Google Scholar]
- Ballantine J.A., Francis D.J., Hassall D.H. The biosynthesis of phenols. Part. XXI. The molecular structure of arugosin, a metabolite of a wild-type strain of Aspergillus rugulosus. Journal of the Chemical Society C. 1970;1970:1175–1182. doi: 10.1039/j39700001175. [DOI] [PubMed] [Google Scholar]
- Ballantine J.A., Hassall C.H., Jones G. The biosyntheis of phenols. Part. IX. Asperugin, a metabolic product of Aspergillus rugulosus. Journal of the Chemical Society. 1965;1965:4672–4678. doi: 10.1039/jr9650004672. [DOI] [PubMed] [Google Scholar]
- Ballantine J.A., Hassall C.H., Jones B.D. The biosynthesis of phenols. XVII. Some phenolic metabolites of mutant strains of Aspergillus rugulosus. Phytochemistry. 1968;7:1529–1534. [Google Scholar]
- Ballantine J.A., Hassall C.H., Jones B.D. The biosynthesis of phenols. XII. Asperugin B, a metabolite of Aspergillus rugulosus. Phytochemistry. 1967;6:1157–1159. [Google Scholar]
- Bardshiri E., Simpson T.J., Walkinshaw M.D. 1980. IUPAC 12th Int. Symp. Chem. Nat. Prod., Tenerife. 1980, Abstr. A8. [Google Scholar]
- Bartlett A.J., Holker J.S.E., O'Brian E., Simpson T.J. Biosynthesis of the meroterpenoid metabolite andibenin A: Aromatic precursors. Journal of the Chemical Society Chemical Communications. 1981;1981:1198–1200. [Google Scholar]
- Baylet R., Lacoste P., Camain R. Aspergillus nidulans agent of mycetomas human Africa. Bulletin de la Societe de pathologia Exotique. 1968;61:359–365. [PubMed] [Google Scholar]
- Beach W.F., Richards J.H. The structure and biosynthesis of nidulin. Journal of Organic Chemistry. 1963;28:2746–2751. [Google Scholar]
- Benjamin C.R. Ascocarps of Aspergillus and Penicillium. Mycologia. 1955;47:669–687. [Google Scholar]
- Benz F., Knusel F., Nuesch J. Echinocandin B, a new polypeptide antibiotic from Aspergillus nidulans var. echinulatus. Helvetica Chimica Acta. 1974;57:2459–2477. doi: 10.1002/hlca.19740570818. [DOI] [PubMed] [Google Scholar]
- Bergmann S., Schumann J., Scherlach K. Genomics-driven discovery of PKS–NRPS hybrid metabolites from Aspergillus nidulans. Nature Chemical Biology. 2007;3:213–217. doi: 10.1038/nchembio869. [DOI] [PubMed] [Google Scholar]
- Bills G.F., Fillola A., Jimenez M.R. Enhancement of antibiotic and secondary metabolite detection from filamentous fungi by growth nutritional arrays. Journal of Applied Microbiology. 2008;104:1644–1658. doi: 10.1111/j.1365-2672.2008.03735.x. [DOI] [PubMed] [Google Scholar]
- Bills G.F., Li Y., Chen L. New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibols. Natural Product Reports. 2014;31:1348–1375. doi: 10.1039/c4np00046c. [DOI] [PubMed] [Google Scholar]
- Bills G.F., Yue Q., Chen L. Aspergillus mulundensis sp. nov., a new species for the fungus producing the antifungal echinocandin lipopeptides, mulundocandins. Journal of Antibiotics. 2016;69:141–148. doi: 10.1038/ja.2015.105. [DOI] [PubMed] [Google Scholar]
- Birkinshaw J.H., Chaplen P., Lahoz-Oliver R. Studies in the biochemistry of microorganisms. 102. Quadrilineatin (1:2-diformyl-5-hydroxy-3-methoxy-4-methylbenzene), a metabolic product of Aspergillus quadrilineatus Thom and Raper. Biochemical Journal. 1957;67:155–164. doi: 10.1042/bj0670155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkinshaw J.H., Gourlay R. Studies in the biochemistry of microorganisms. 109. The structure of asperthecin. Biochemical Journal. 1961;81:618–622. doi: 10.1042/bj0810618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckner LD, Kastner RE (1981). Method for producing the A-30912 antibiotics. U.S patent 4,288,549, Sept. 8.
- Bok J.W., Hoffmeister D., Maggio-Hall L.A. Genomic mining for Aspergillus natural products. Chemistry & Biology. 2006;13:31–37. doi: 10.1016/j.chembiol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bringmann G., Lang G., Steffens S. Evariquinone, isoemericellin, and stromenomycin from a sponge derived strain of the fungus Emericella variecolor. Phytochemistry. 2003;63:437–443. doi: 10.1016/s0031-9422(03)00189-4. [DOI] [PubMed] [Google Scholar]
- Brown D.W., Salvo J.J. Isolation and characterization of sexual spore pigments from Aspergillus nidulans. Applied and Environmental Microbiology. 1994;60:979–983. doi: 10.1128/aem.60.3.979-983.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacho R.A., Jiang W., Chooi Y.-H. Identification and characterization of the echinocandin B biosynthetic genme cluster from Emericella rugulosa NRRL 11440. Journal of the American Chemical Society. 2012;134:16781–16790. doi: 10.1021/ja307220z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Lee A.S.Y., Huang Y. Agonodepsides A and B: Two new depsides from a filamentous fungus F7524. Journal of Natural Products. 2002;65:1037–1038. doi: 10.1021/np010626i. [DOI] [PubMed] [Google Scholar]
- Chexal K.K., Fouweather C., Holker J.S.E. Biosynthesis of fungal metabolites. 7. Production and biosynthesis of 4,7-dimethoxy-5-methylcoumarin in Aspergillus variecolor. Journal of the Chemical Society Perkin Transactions I. 1975;1975:554–556. [PubMed] [Google Scholar]
- Chexal K.K., Fouweather C., Holker J.S.E. The biosynthesis of fungal metabolites. Part III. Structure of shamixanthone and tajixanthone, metabolites of Aspergillus variecolor. Journal of the Chemical Society Perkin Transactions I. 1974;1974:1584–1593. [Google Scholar]
- Chexal K.K., Holker J.S.E., Simpson T.J. Biosynthesis of fungal metabolites. 6. Structure and biosynthesis of some minor metabolites from variant strains of Aspergillus variecolor. Journal of the Chemical Society Perkin Transactions I. 1975;1975:549–554. [PubMed] [Google Scholar]
- Chexal K.K., Holker J.S.E., Simpson T.J. Biosynthesis of fungal metabolites. Part V. Structure of variecoxanthones A, B, and C, metabolites of Aspergillus variecolor. Conversion of variecoxanthone A into (±)-de-C-prenylepishamixanthone. Journal of the Chemical Society Perkin Transactions I. 1975;1975:543–548. [PubMed] [Google Scholar]
- Chiang Y., Szewscyk E., Davidson A.D. A gene cluster containing two fungal polyketide synthases encodes the biosynthetic pathway for a polyketide, asperfuranone, in Aspergillus nidulans. Journal of the American Chemical Society. 2009;131:2965–2970. doi: 10.1021/ja8088185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y.M., Oakley B.R., Keller N.P. Unraveling polyketide synthesis in members of the genus Aspergillus. Applied Microbiology and Biotechnology. 2010;86:1719–1736. doi: 10.1007/s00253-010-2525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y.M., Szewczyk E., Nayak T. Molecular genetic mining of the Aspergillus secondary metabolome: discovery of the Emericellamide biosynthetic pathway. Chemistry & Biology. 2008;15:527–532. doi: 10.1016/j.chembiol.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M., Raper K.B. Synoptic key to Aspergillus nidulans group species and related Emericella. Transactions of the British Mycological Society. 1978;71:177–191. [Google Scholar]
- Christensen M., States J.S. Aspergillus nidulans group: Aspergillus navahoensis, and a revised synoptic key. Mycologia. 1982;74:226–235. [Google Scholar]
- Cole R.J., Cox R.H. Academic Press; London: 1981. Handbook of toxic fungal metabolites. [Google Scholar]
- Cox R.H., Cole R.J. Carbon-13 nuclear resonance studies of fungal metabolites, aflatoxixns, and sterigmatocystins. Journal of Organic Chemistry. 1977;42:112–114. doi: 10.1021/jo00421a022. [DOI] [PubMed] [Google Scholar]
- de la Cruz M., Martin J., Gonzales-Menedez V. Chemical and physical modulation of antibiotic activity in Emericella species. Chemistry and Biodiversity. 2012;9:1095–1113. doi: 10.1002/cbdv.201100362. [DOI] [PubMed] [Google Scholar]
- Dean F.M., Deohra D.S., Erni A.D.T. The structure of nidulin, a metabolite of Aspergillus nidulans. Journal of the Chemical Society. 1960;1960:4829–4837. [Google Scholar]
- Dean F.M., Roberts J.C., Robertson A. The chemistry of fungi. Part XXII. Nidulin and nornidulin (“ustin”): chlorine-containing metabolic products of Aspergillus nidulans. Journal of the Chemical Society. 1954;1954:1432–1439. [Google Scholar]
- Dean R.A., Timberlake W.E. Production of cell wall-degrading enzymes by Aspergillus nidulans: a model system for fungal pathogenesis of plants. The Plant Cell. 1989;1:265–273. doi: 10.1105/tpc.1.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doby J.M., Kombila-Favry M. Presence of sexual forms (Cleistothecia and Hülle cells) of Aspergillus nidulans associated with Aspergillus fumigatus in aspergillosis of human maxillary sinus. Mycopathologia. 1978;64:157–163. doi: 10.1007/BF00576367. [DOI] [PubMed] [Google Scholar]
- Dreyfuss M.M. Neue Erkentnisse aus einem pharmakologischen Pilz-Screening. Sydowia. 1986;39:22–36. [Google Scholar]
- Dulaney E.L. Some aspects of penicillin production by Aspergillus nidulans. Mycologia. 1947;39:570–581. [PubMed] [Google Scholar]
- Dulaney E.L. Penicillin production by the Aspergillus nidulans group. Mycologia. 1947;39:582–586. [PubMed] [Google Scholar]
- Dunn A.W., Johnstone R.A.W. Fungal metabolites. Part 8. Isolation of 2-methoxy-6-(3,4-dihydroxy-hepta-1,5-dienyl)benzyl alcohol. Journal of the Chemical Society Perkin Transactions 1. 1979;1979:2122–2123. [Google Scholar]
- Dunn A.W., Johnstone R.A.W., King T.J. Fungal metabolites. 7. Structures of C-25 compounds from Aspergillus variecolor. Journal of the Chemical Society Perkin Transactions 1. 1979;1979:2113–2117. [Google Scholar]
- Dunn A.W., Johnstone R.A.W., Sklarz B. Isolation of C25 polyisoprenoids from Aspergillus sp.: crystal structure of andibenin. Journal of the Chemical Society Chemical Communications. 1976;1976:270–271. [Google Scholar]
- Dunn A.W., Johnstone R.A.W., Sklarz B. Andilesin, a C25 polyisoprenoid from Aspergillus sp. X-ray crystallographic and spectroscopic determination of the structure. Journal of the Chemical Society Chemical Communications. 1978;1978:533–534. [Google Scholar]
- Eisendle M., Oberegger H., Zadra I. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding 1-ornithineN 5-monooxygenase (sidA) and a non-ribosomal peptide synthase (sidC) Molecular Microbiology. 2003;49:345–375. doi: 10.1046/j.1365-2958.2003.03586.x. [DOI] [PubMed] [Google Scholar]
- El-Khady I.A., Hafez S.I.I.A. Production of sterigmatocystin by some species and varieties of Aspergillus nidulans group. Cryptogamie Mycologie. 1981;2:239–244. [Google Scholar]
- Fennell D.I., Raper K.B. New species and varieties of Aspergillus. Mycologia. 1955;47:68–89. [Google Scholar]
- Fidelis K., Hossain M.B., Jalal M.A.F. Structure and molecular mechanics of ferrirhodin. Acta Crystallographica C. 1990;46:1612–1617. doi: 10.1107/s0108270189013624. [DOI] [PubMed] [Google Scholar]
- Figueroa M., Gonzalez M.D., Rodriguez-Sotres R. Calmodulin inhibitors from the fungus Emericella sp. Bioorganic & Medicinal Chemistry. 2009;17:2167–2174. doi: 10.1016/j.bmc.2008.10.079. [DOI] [PubMed] [Google Scholar]
- de Fontbrune F.S., Denis B., Meunier M. Iterative breakthrough invasive aspergillosis due to TR(34)/298H azole-resistant Aspergillus fumigatus and Emericella sublata in a single hematopoietic stem cell transplant patient. Transplant Infectious Disease. 2014;16:687–691. doi: 10.1111/tid.12231. [DOI] [PubMed] [Google Scholar]
- Fredimoses M., Zhou X., Lin X. New prenylxanthones from the deep -sea derived fungus Emericella sp. SCSIO 05240. Marine Drugs. 2014;12:3190–3202. doi: 10.3390/md12063190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad J.C. Secondary metabolites as an aid to Emericella classification. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus systematics. Vol. 102. Plenum Press; New York: 1985. pp. 437–443. (NATO ASI Series. Ser. A.: Life Sciences). [Google Scholar]
- Frisvad J.C., Larsen T.O. Chemodiversity in the genus Aspergillus. Applied Microbiology and Biotechnology. 2015;99:7859–7877. doi: 10.1007/s00253-015-6839-z. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Samson R.A. Emericella venezuelensis, a new species with stellate ascospores producing sterigmatocystin and aflatoxin B1. Systematic and Applied Microbiology. 2004;27:672–680. doi: 10.1078/0723202042369910. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Samson R.A., Smedsgaard J. Emericella astellata, a new producer of aflatoxin B1. Systematic and Applied Microbiology. 2004;38:440–445. doi: 10.1111/j.1472-765X.2004.01520.x. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Skouboe P., Samson R.A. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Systematic and Applied Microbiology. 2005;28:442–453. doi: 10.1016/j.syapm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Thrane U. Standardized High-Performance Liquid Chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone indices and UV-VIS spectra (diode-array detection) Journal of Chromatography. 1987;404:195–214. doi: 10.1016/s0021-9673(01)86850-3. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C., Thrane U. Liquid column chromatography of mycotoxins. In: Betina V., editor. Chromatography of mycotoxins: techniques and applications. Vol. 54. Elsevier; Amsterdam: 1993. pp. 253–372. (Journal of Chromatography Library). [Google Scholar]
- Fujii I., Watanabe A., Sankawa U. Identification of Claisen cyclase domain in fungal polyketide synthase WA, a naphthopyrone synthase of Aspergillus nidulans. Chemistry & Biology. 2001;8:189–197. doi: 10.1016/s1074-5521(00)90068-1. [DOI] [PubMed] [Google Scholar]
- Fujimoto H., Nakamura E., Okuyama E. Immunomodulatory constituents from an ascomycete, Emericella aurantio-brunnea. Chemical and Pharmaceutical Bulletin. 2000;48:1436–1441. doi: 10.1248/cpb.48.1436. [DOI] [PubMed] [Google Scholar]
- Fujimoto H., Yamamoto K., Arisawa M. New toxic metabolites from an ascomycete, Emericella corrugata. Mycotoxins. 1998;46:29–34. [Google Scholar]
- Fukuyama K., Hamada K., Tsukihara T. Determination of absolute configuration of epishamixanthone, a metabolite of Aspergillus rugulosus, by anomalous scattering of light atoms. Bulletin of the Chemical Society of Japan. 1978;51:37–44. [Google Scholar]
- Fukuyama K., Katsube Y., Ishido H. The absolute configuration of desacetylaustin isolated from Emerciella nidulans var. dentata. Chemical and Pharmaceutical. Bulletin. 1980;28:2270–2271. [Google Scholar]
- Galaghan J.E., Calvo S.E., Cuomo C. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- Gams W., Christensen M., Onions A.H.S. Infrageneric taxa of Aspergillus. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus systematics. Vol. 102. Plenum Press; New York: 1985. pp. 55–62. (NATO ASI Series. Ser. A.: Life Sciences). [Google Scholar]
- Gill-Carey D. Antibiotics from Aspergilli. British Journal of Experimental Pathology. 1949;30:114–118. [PMC free article] [PubMed] [Google Scholar]
- Gould R.O., Simpson T.J., Walkinshaw M.D. Isolation and X-ray crystal structures of astellolides A and astellolides B. sesquiterpenoid metabolites of Aspergillus variecolor. Tetrahedron Letters. 1981;22:1047–1050. [Google Scholar]
- Guarro J., Gené J., Stchigel A.M. CBS-KNAW Fungal Biodiversity Centre, Centraalbureau voor Schimmelcultures; Utrecht: 2012. Atlas of soil Ascomycetes; pp. 169–183. CBS Biodiversity Series 10. [Google Scholar]
- Hajjar J.D., Bennett J.W., Bhatnagar D. Sterigmatocystin production by laboratory strains of Aspergillus nidulans. Mycological Research. 1989;93:548–551. [Google Scholar]
- Hamano K., Kinoshita-Okami M., Hemmi A. Folipastatin, a new depsidone compound from Aspergillus unguis as an inhibitor of phospholipase A2. Taxonomy, fermentation, isolation, structure determination and biological properties. Journal of Antibiotics. 1992;45:1195–1201. doi: 10.7164/antibiotics.45.1195. [DOI] [PubMed] [Google Scholar]
- Hamasaki T., Kuwano H., Isono K. A new metabolite, parasiticolide A, from Aspergillus parasiticus. Agricultural and Biological Chemistry. 1975;39:749–751. [Google Scholar]
- Hamasaki T., Nakagomi T., Hatsuda Y. 5,6-dimethoxysterigmatocystin, a new metabolite from Aspergillus multicolor. Tetrahedron Letters. 1977;18:2765–2766. [Google Scholar]
- Hamasaki T., Nakagomi T., Hatsuda Y. 5,6-dimethoxysterigmatocystin and related metabolites from Aspergillus multicolor. Agricultural and Biological Chemistry. 1980;44:1149–1155. [Google Scholar]
- Hamasaki T., Nakajima H., Yokota T. A new metabolite, 3-carboxy-2,4-diphenyl-but-2-enoic anhydride, produced by Aspergillus nidulans. Agricultural and Biological Chemistry. 1983;47:891–892. [Google Scholar]
- Henriet S.S.V., Verweij P.E., Warris A. Aspergillus nidulans and chronic granulomatous disease: A unique host-pathogen interaction. Journal of Infectious Diseases. 2012;206:1128–1137. doi: 10.1093/infdis/jis473. [DOI] [PubMed] [Google Scholar]
- Hensens O.D., Zink D., Williamson J.M. Variecolin, a sesterpenoid of novel skeleton from Aspergillus variecolor MF138. Journal of Organic Chemistry. 1991;56:3399–3403. [Google Scholar]
- Herbert N., Arst J.R. Integrator gene in Aspergillus nidulans. Nature. 1976;262:231–234. doi: 10.1038/262231a0. [DOI] [PubMed] [Google Scholar]
- Hicks K.B., Feather M.S. The acid-catalyzed dehydration of 1-benzylamino-1-deoxy-d-threo-pentulose, 1-dibenzylamino-1-deoxy-d-fructuronic acid, and d-glucuronic acid. Carbohydrate Research. 1977;54:209–215. [Google Scholar]
- Hodges R.L., Kelkar H.S., Xuei X. Characterization of an echinocandin B-producing strain blocked for sterigmatocystin biosynthesis reveals a translocation in the stcW gene of the aflatoxin pathway. Journal of Industrial Microbiology and Biotechnology. 2000;25:333–341. doi: 10.1038/sj.jim.7000076. [DOI] [PubMed] [Google Scholar]
- Holker J.S.E., Lapper R.D., Simpson T.J. The biosynthesis of fungal metabolites. Part. IV. Tajixanthone: 13C nuclear magnetic resonance spectrum and feedings with [1-13C]- and [2-13C]-acetate. Journal of the Chemical Society Perkin Transactions. I. 1974;1974:2135–2140. [Google Scholar]
- Holt G., MacDonald K.D. Penicillin production and its mode of inheritance in Aspergillus nidulans. Antonie van Leeuwenhoek. 1968;34:409–416. doi: 10.1007/BF02046463. [DOI] [PubMed] [Google Scholar]
- de Hoog G.S., Guarro G., Gené J. 2nd ed. Centraalbureau voor Schimmelcultures (CBS); Utrecht, the Netherlands: 2000. Atlas of clinical fungi. [Google Scholar]
- Hopmann C, Knauf MA, Weithmann K, et al. (2001). Aventis Pharma Deutchland. G.m.p.H., Germany, 2001. PCT International Patent Application No. WO 01/44264 A2.
- Horie Y. A new species of Emericella from Indian herbal drugs. Transactions of the Mycological Society of Japan. 1978;19:313–317. [Google Scholar]
- Horie Y. New or interesting Emericella from herbal drugs. Transactions of the Mycological Society of Japan. 1979;20:481–491. [Google Scholar]
- Horie Y. Ascospore ornamentation and its application to the taxonomic re-evaluation in Emericella. Transactions of the Mycological Society of Japan. 1980;21:483–493. [Google Scholar]
- Horie Y., Abliz P., Hui Y. Emericella qinqixianii, a new species from desert soil in China. Mycoscience. 2000;41:183–187. [Google Scholar]
- Horie Y., Fukiharu T., Nishimura K. New and interesting species of Emericella from Chinese soil. Mycoscience. 1996;37:323–329. [Google Scholar]
- Horie Y., Li D.M., Fukiharu T. Emericella appendiculata, a new species from Chinese soil. Mycoscience. 1998;39:161–165. [Google Scholar]
- Horie Y., Miyaji M., Nishimura K. Emericella falconensis, a new species from Venezuelan soil. Transactions of the Mycological Society of Japan. 1989;30:257–263. [Google Scholar]
- Horie Y., Miyaji M., Nishimura K. New and interesting species of Emericella from Brazilian soil. Mycoscience. 1996;37:137–144. [Google Scholar]
- Horie Y., Udagawa S. Emericella omanensis, a new species from Oman soil. Mycoscience. 1995;36:391–394. [Google Scholar]
- Horie Y., Udagawa S., Abdullah J.K. Emericella similis, a new species from Iraqi soil. Transactions of the Mycological Society of Japan. 1990;31:425–430. [Google Scholar]
- Horie Y., Yamazaki M. Production of carcinogenic mycotoxins, sterigmatocystin and allied compounds, by Emericella species. Transactions of the Mycological Society of Japan. 1985;26:411–419. [Google Scholar]
- Hosoe T., Itabashi T., Kobayashi N. Three new types of indoloditerpenes, emindole PA-PC, from Emericella purpurea. Revision of the structure of emindole PA. Chemical and Pharmaceutical Bulletin. 2006;54:185–187. doi: 10.1248/cpb.54.185. [DOI] [PubMed] [Google Scholar]
- Houbraken J., de Vries R.P., Samson R.A. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Advances in Applied Microbiology. 2014;86:199–249. doi: 10.1016/B978-0-12-800262-9.00004-4. [DOI] [PubMed] [Google Scholar]
- Houbraken J., Due M., Varga J. Polyphasic taxonomy of Aspergillus section Usti. Studies in Mycology. 2007;59:107–128. doi: 10.3114/sim.2007.59.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J., Samson R.A. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology. 2011;70:1–51. doi: 10.3114/sim.2011.70.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B.H., Raistrick H. Studies in the biochemistry of microorganisms. 94. The colouring matters of species in the Aspergillus nidulans group. Part I. Asperthecin, a crystalline colouring matter of Aspergillus quadrilineatus Thom & Raper. Biochemical Journal. 1955;59:475–484. doi: 10.1042/bj0590475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubka V., Nováková A., Peterson S.W. A reappraisal of Aspergillus section Nidulantes with descriptions of two new sterigmatocystin-producing species. Plant Systematics and Evolution. 2016;302:1267–1299. [Google Scholar]
- Ishida M., Hamasaki T., Hatsuda Y. A new metabolite from Aspergillus nidulans. Agricultural and Biological Chemistry. 1972;36:1847–1848. [Google Scholar]
- Ishida M., Hamasaki T., Hatsuda Y. The structure of two new metabolites, emerin and emericellin, from Aspergillus nidulans. Agricultural and Biological Chemistry. 1975;39:2181–2184. [Google Scholar]
- Ishida M., Hamasaki T., Hatsuda Y. Biosynthesis of shamixanthone. Agricultural and Biological Chemistry. 1978;42:465–466. [Google Scholar]
- Ishida M., Hamasaki T., Hatsuda Y. Epishamixanthone, a new metabolite from Aspergillus rugulosus. Agricultural and Biological Chemistry. 1976;40:1051–1052. [Google Scholar]
- Ishida M., Hamasaki Y., Hatsuda Y. Emericellin, a new metabolite from Aspergillus nidulans. Agricultural and Biological Chemistry. 1975;39:291–292. [Google Scholar]
- Ismail M.A., Abdelsater M.A., Zohri A.A. A synoptic key to species of the Aspergillus nidulellus Emericella assemblage common to Egypt. Mycotaxon. 1995;53:391–405. [Google Scholar]
- Itabashi T., Nozawa K., Miyaji M. Falconensins A, B, C, and D, new compounds related to azaphilone, from Emericella falconensis. Chemical and Pharmaceutical Bulletin. 1992;40:3142–3144. [Google Scholar]
- Itabashi T., Nozawa K., Nakajima S. A new azaphilone, falconensin H, from Emericella falconensis. Chemical and Pharmaceutical Bulletin. 1993;41:2040–2041. [Google Scholar]
- Itabashi T., Ogasawara N., Nozawa K. Isolation and structures of new azaphilone derivatives, falconensins E-G, from Emericella falconensis and absolute configuration of falconensins A-G. Chemical and Pharmaceutical Bulletin. 1996;44:2213–2217. [Google Scholar]
- Joshi K.R., Mathur D.R., Sharma J.C. Mycetoma caused by Aspergillus nidulans in India. Journal of Tropical Medicine and Hygiene. 1985;88:41–44. [PubMed] [Google Scholar]
- Jurjevic Z., Peterson S.W., Horn B.W. Aspergillus section Versicolores: nine new species and multiloocus DNA sequence based phylogeny. IMA Fungus. 2012;3:59–79. doi: 10.5598/imafungus.2012.03.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurjevic Z., Peterson S.W., Solfrizzo M. Sterigmatocystin production by nine newly described Aspergillus species in section Versicolores grown on two different media. Mycotoxin Research. 2013;29:141–145. doi: 10.1007/s12550-013-0160-4. [DOI] [PubMed] [Google Scholar]
- Kaczka E.A., Dulaney E.L., Gitterman C.O. Isolation and inhibitory effects on KB cell cultures of 3′-deoxyadenosine from Aspergillus nidulans (Eidam) Wint. Biochemical and. Biophysical Research Communications. 1964;14:452–455. doi: 10.1016/0006-291x(64)90085-3. [DOI] [PubMed] [Google Scholar]
- Kamal A., Haider Y., Khan Y.A. Studies in the biochemistry of microorganisms. Part X. Isolation, structure and stereochemistry of yasminin and other metabolic products of Aspergillus unguis Emile-Weil and Gaudin. Pakistan Journal of Scientific and Industrial Research. 1970;13:244–250. [Google Scholar]
- Kamal A., Haider Y., Qureshi A.A. Studies in the biochemistry of microorganisms XII. Isolation and structures of haiderin, rubinin, shirin and nasrin metabolic products of Aspergillus unguis Emile-Weil and Gaudin. Pakistan Journal of Scientific and Industrial Research. 1970;13:364–372. [Google Scholar]
- Kamal A., Husain S.A., Noorani R. Biochemistry of microorganisms. XI. Isolation of tajixanthone, shamixanthone, ajamxanthone, shahenxanthone, najamxanthone, radixanthone and mannitol from mycelium of Aspergillus stellatus. Pakistan Journal of Scientific and Industrial Research. 1970;13:251–255. [Google Scholar]
- Kamal A., Husain S.A., Qureshi A.A. Biochemistry of microorganisms. XXV. Structure and stereochemistry of shamixanthone and ajamxanthone, metabolic products of Aspergillus stellatus. Pakistan Journal of Scientific and Industrial Research. 1971;14:104–112. [Google Scholar]
- Kamal A., Husain S.A., Qureshi A.A. Biochemistry of microorganisms. XXIV. Structure and stereochemistry of tajixanthone, a metabolic product of Aspergillus stellatus. Pakistan Journal of Scientific and Industrial Research. 1971;14:90–103. [Google Scholar]
- Kamal A., Husain S.A., Qureshi A.A. Biochemistry of microorganisms. XXVI. Structure of shamixanthone and najamxanthone, metabolic products of Aspergillus stellatus. Pakistan Journal of Scientific and Industrial Research. 1972;15:49–57. [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara N., Nakajima S., Satoh Y. Studies on fungal products. XVIII. Isolation and structures of a new fungal depsidone related to nidulin and a new phthalide from Emericella unguis. Chemical and Pharmaceutical Bulletin. 1988;36:1970–1975. [Google Scholar]
- Kawahara N., Nozawa K., Nakajima S. Studies on fungal products. Part 13. Isolation and structures of dithiosilvatin and silvathione. Novel dioxopiperazine derivatives from Aspergillus silvaticus. Journal of the Chemical Society Perkin Transactions I. 1987;1987:2099–2101. [Google Scholar]
- Kawahara N., Nozawa K., Nakajima S. Studies on fungal products. XV. Isolation and structure determination of arugosin E from Aspergillus stellatus and cycloisoemericellin from Emericella striata. Journal of the Chemical Society Perkin Transactions I. 1988;1988:907–911. [Google Scholar]
- Kawahara N., Nozawa K., Nakajima S. Isolation and structures of novel fungal depsidones, emeguisins, A, B, and C, from Emericella unguis. Journal of the Chemical Society Perkin Transactions I. 1988;1988:2611–2614. [Google Scholar]
- Kawahara N., Sekita S., Satake M. Structures of new dihydroxanthone derivative, nidulalin A, and a new benzophenone derivative, nidulalin B, from Emericella nidulans. Chemical and Pharmaceutical Bulletin. 1994;42:1720–1723. [Google Scholar]
- Kawai K., Nozawa K., Nakajima S. Structure of a new type of indoloterpenoid from Emericella purpurea. Journal of the Chemical Society Perkin Transactions I. 1994;1994:1673–1674. [Google Scholar]
- Kawai K., Nozawa K., Seya H. Studies on fungal products. XII. Structure of aurantioemestrin from Emericella striata. Heterocycles. 1987;26:475–479. [Google Scholar]
- Kildgaard S., Mansson M., Dosen I. Accurate dereplication of bioactive secondary metabolites from marine-derived fungi by UHPLC-DAD-QTOFMS and MS/HRMS library. Marine Drugs. 2014;12:3681–3705. doi: 10.3390/md12063681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.M., Reid-Yu S.A., Wang W. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klejnstrup M.L., Frandsen R.J.N., Holm D.K. Genetics of polyketide metabolism in Aspergillus nidulans. Metabolites. 2012;2:100–133. doi: 10.3390/metabo2010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klich M., Mendoza C., Mullaney E. A new sterigmatocystin producing Emericella variant from agricultural desert soils. Systematic and Applied Microbiology. 2001;24:131–138. doi: 10.1078/0723-2020-00007. [DOI] [PubMed] [Google Scholar]
- Klich M.A. Morphological studies of Aspergillus section Versicolores and related species. Mycologia. 1993;85:100–107. [Google Scholar]
- Klitgaard A., Iversen A., Andersen M.R. Aggressive dereplication using UHPLC-DAD-QTOF – screening extracts for up to 3000 fungal secondary metabolites. Analytical and Bioanalytical Chemistry. 2014;406:1933–1943. doi: 10.1007/s00216-013-7582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K., Kusakabe H., Machida H. Isolation of 2'-deoxycoformycin and cordycepin from wheat bran culture of Aspergillus nidulans Y 176-2. Agricultural and Biological Chemistry. 1979;43:2375–2377. [Google Scholar]
- Kong H.Z., Qi Z.T. A new species of Emericella. Acta Mycologica Sinica. 1986;5:211–214. [Google Scholar]
- Korner C., Wanscher J.H. 3rd ed. Eyre Methuen; London: 1978. Methuen handbook of colour. [Google Scholar]
- König W.A., Pfaff K.P., Loeffler W. Ethericin A: Isolierung, Charakterisierung und Strukturaufklärung eines neues, antibiotisch wirksamen Diphenylethers. Liebigs Annalen der Chemie. 1978;1978:1289–1296. [Google Scholar]
- Kralj A., Kehraus S., Krick A. Arugosins G and H: Prenylated polyketides from the marine-derived fungus Emericella nidulans var. acristata. Journal of Natural Products. 2006;69:995–1000. doi: 10.1021/np050454f. [DOI] [PubMed] [Google Scholar]
- Kritmitzas A., Pyrri I., Kouvelis V.N. A phylogenetic analysis of Greek isolates of Aspergillus based on morphology and nuclear and mitochondrial gene sequences. Biomedical Research International. 2013;2013:260395. doi: 10.1155/2013/260395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger G.J., Steyn P.S., Vleggaar R. X-ray crystal structure of asteltoxin, a novel mycotoxin from Aspergillus stellatus Curzi. Journal of the Chemical Society Chemical Communications. 1979;1979:441–442. [Google Scholar]
- Lafont P., Lafont J. Production de nidulotoxine par des Aspergillus appartenant à différentes espèces. Experientia. 1970;26:807–808. doi: 10.1007/BF02232565. [DOI] [PubMed] [Google Scholar]
- Lafont P., Lafont L., Frayssinet C. La nidulotoxine: toxine d'Aspergillus nidulans. Experientia. 1970;26:61–62. doi: 10.1007/BF01900392. [DOI] [PubMed] [Google Scholar]
- Liangsakul J., Pornpakakul S., Sangvichien E. Emervaridione and varioxiranediol, two new metabolites from the endophytic fungus, Emericella variecolor. Tetrahedron Letters. 2011;52:6427–6430. [Google Scholar]
- Liu S., Shen Y. A new cyclic peptide from the marine fungal strain Aspergillus sp. AF119. Chemistry of Natural Compounds. 2011;47:786–788. [Google Scholar]
- Lo H.C., Entwistle R., Guo C.J. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids austinol and dehydroaustinol in Aspergillus nidulans. Journal of the American Chemical Society. 2012;134:4709–4720. doi: 10.1021/ja209809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maebayashi Y., Okuyama E., Yamazaki M. Structure of ED-1 isolated from Emericella dentata. Chemical and Pharmaceutical Bulletin. 1982;30:1911–1912. [Google Scholar]
- Malmstrøm J. Unguisin A and B: new cyclic peptides from the marine-derived fungus Emericella unguis. Journal of Natural Products. 1999;62:787–789. doi: 10.1021/np980539z. [DOI] [PubMed] [Google Scholar]
- Malmstrøm J., Christophersen C., Barrero A.F. Bioactive metabolites from a marine-derived strain of the fungus Emericella variecolor. Journal of Natural Products. 2002;65:364–367. doi: 10.1021/np0103214. [DOI] [PubMed] [Google Scholar]
- Malmstrøm J., Ryager A., Anthoni U. Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry. 2002;60:869–872. doi: 10.1016/s0031-9422(02)00150-4. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Mitsuhashi T., Quan Z. Molecular basis for stellatic acid biosynthesis: a genome mining approach for discovery of sesterpene synthases. Organic Letters. 2015;17:4644–4647. doi: 10.1021/acs.orglett.5b02404. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Wakimoto T., Mori T. Complete biosynthetic pathway of anditomin: nature's sophisticated route to a complex fungal meroterpenoid. Journal of the American Chemical Society. 2014;136:15326–15336. doi: 10.1021/ja508127q. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T., Tanaka R., Horie Y. The correlation among molecular phylogenetics, morphological data, and growth temperature of the genus Emericella, and a new species. Mycoscience. 2012;53:433–445. [Google Scholar]
- Mazur P., Meyers H.V., Nakanishi K. Structural elucidation of sporogenic fatty acid metabolites from Aspergillus nidulans. Tetrahedron Letters. 1990;31:3837–3840. [Google Scholar]
- Mazzaferro L.S., Hüttel W., Fries A. Cytochrome P450-catalyzed region- and stereoselective phenol coupling of fungal natural products. Journal of the American Chemical Society. 2015;137:1228–12295. doi: 10.1021/jacs.5b06776. [DOI] [PubMed] [Google Scholar]
- McNeill J., Barrie F.R., Buck W.R. Regnum Vegetabile 154, Koeltz Scientific Books; Königstein: 2012. International code of nomenclature for algae, fungi, and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress, Melbourne, Australia, July 2011. [Google Scholar]
- Mehrotra B.S., Prasad R. Aspergillus dimorphicus and Emericella cleistominuta spp. nov. from Indian soils. Transactions of the British Mycological Society. 1969;52:331–336. [Google Scholar]
- Middleton A.J., Cole D.S., MacDonald K.D. A hydroxamic acid from Aspergillus nidulans with antibiotic activity against Proteus species. Journal of Antibiotics. 1978;31:1110–1115. doi: 10.7164/antibiotics.31.1110. [DOI] [PubMed] [Google Scholar]
- Mizuba S., Lee K., Jiu J. Three antimicrobial metabolites from Aspergillus caespitosus. Canadian Journal of Microbiology. 1975;21:1781–1787. doi: 10.1139/m75-259. [DOI] [PubMed] [Google Scholar]
- Moosophon P., Kanokmedhakul S., Kanokmedhakul K. Prenylxanthones and a bicyclo[3.3.1]nona-2,6-diene derivative from the fungus Emericella rugulosa. Journal of Natural Products. 2009;72:1442–1446. doi: 10.1021/np800805f. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay T., Ganguli P.N., Fehlhaber H.W. Mulundocandin, a new lipopeptide antibiotic. 2. Structure elucidation. Journal of Antibiotics. 1987;40:281–289. doi: 10.7164/antibiotics.40.281. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay T., Roy K., Bhat R.G. Deoxymulundocandin – a new echinocandin type antifungal antibiotic. Journal of Antibiotics. 1992;45:618–623. doi: 10.7164/antibiotics.45.618. [DOI] [PubMed] [Google Scholar]
- Nahlik K., Dumkow M., Bayram Ö. The COP9 signalosome mediates transcriptional and metabolic response to hormones, oxidative stress production and cell wall rearrangement during fungal development. Molecular Microbiology. 2010;78:964–979. doi: 10.1111/j.1365-2958.2010.07384.x. [DOI] [PubMed] [Google Scholar]
- Neelakantan S.A., Pocker A., Raistrick H. Studies in the biochemistry of microorganisms. The colouring matters of species in the Aspergillus nidulans group. Part II. Further observations on the structure of asperthecin. Biochemical Journal. 1957;66:234–237. doi: 10.1042/bj0660234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff S.A., Lee S.U., Asami Y. Aflaquinolones A–G: Secondary metabolites from marine and fungicolous isolates of Aspergillus spp. Journal of Natural Products. 2012;75:464–472. doi: 10.1021/np200958r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicollier G., Robetez M., Tabacchi R. Synthesis of evernin. Helvetica Chimica Acta. 1978;61:2899–2904. [Google Scholar]
- Nielsen J., Nielsen P.H., Frisvad J.C. Fungal depside, guisinol, from a marine derived strain of Emericella unguis. Phytochemistry. 1999;50:263–265. [Google Scholar]
- Nielsen M.L., Nielsen J.B., Rank C. A genome-wide polyketide synthase deletion library uncovers novel genetic links to polyketides and meroterpenoids in Aspergillus nidulans. FEMS Microbiology Letters. 2011;321:157–166. doi: 10.1111/j.1574-6968.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- Nielsen M.T., Klejnstrup M.L., Rohlfs M. Aspergillus nidulans synthesize insect juvenile hormones upon expression of a heterologous regulatory protein and in response to grazing by Drosophila melanogaster larvae. PLoS One. 2013;8:e73369. doi: 10.1371/journal.pone.0073369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K., Horie Y., Udagawa S. Isolation of new tremorgenic indoloditerpene, 1′-O-acetylpaxilline, from Emericella striata and distribution of paxilline in Emericella spp. Chemical and Pharmaceutical Bulletin. 1989;37:1387–1389. doi: 10.1248/cpb.37.1387. [DOI] [PubMed] [Google Scholar]
- Nozawa K., Nakajima S., Kawai K. Studies on fungal products. Part 17. Isolation and structures of novel indoloterpenes, emindoles DA and DB, from Emericella desertorum: X-ray molecular structure of emindole DA acetate. Journal of the Chemical Society Perkin Transactions I. 1988;1988:1689–1694. [Google Scholar]
- Nozawa K., Seya S., Nakajima S. Studies on fungal products. 10. Isolation and structures of novel bicoumarins, desertorin A, desertorin B, and desertorin C, from Emericella desertorum. Journal of the Chemical Society Perkin Transactions I. 1987;1987:1735–1738. [Google Scholar]
- Nozawa K., Udagawa S., Nakajima S. Structure of two stereoisomers of a new type of indoloterpene related to the tremorgenic mycotoxin paxilline, from Emericella desertorum and Emericella striata. Journal of the Chemical Society Perkin Transactions I. 1987;1987:1157–1159. [Google Scholar]
- Nozawa K., Udagawa S., Nakajima S. Studies on fungal products. XIV. Emestrin B, a new epitrithiodioxopiperazine, from Emericella striata. Chemical and Pharmaceutical Bulletin. 1987;35:3460–3463. doi: 10.1248/cpb.34.2411. [DOI] [PubMed] [Google Scholar]
- Nozawa K., Yuyama M., Nakajima S. Studies on fungal products. Part 19. Isolation and structure of a novel indoloditerpene, emindole SA, from Emericella striata. Journal of the Chemical Society Perkin Transactions I. 1988;1988:2155–2160. [Google Scholar]
- Nyfeler R., Keller-Schierlein W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: isolation and structural components. Helvetica Chimica Acta. 1974;57:2459–2477. doi: 10.1002/hlca.19740570818. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Kawai K. Hydrogenated azaphilones from Emericella falconensis and E. fructiculosa. Phytochemistry. 1998;47:1131–1135. [Google Scholar]
- Ogasawara N., Mizuno R., Kawai K. Structures of a new type of yellow pigments, falconensones A and B, from Emericella falconensis. Journal of the Chemical Society Perkin Transactions 1. 1997;1997:2527–2530. [Google Scholar]
- Oh D.C., Kauffman C.A., Jensen P.R. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. Journal of Natural Products. 2007;70:515–520. doi: 10.1021/np060381f. [DOI] [PubMed] [Google Scholar]
- Ooike M., Nozawa K., Kawai K. An epitetrathiooxopiperazine related to emestrin from Emericella foveolata. Phytochemistry. 1997;46:123–126. [Google Scholar]
- Pachler K.G.R., Steyn P.S., Vleggaar R. Carbon-13 nuclear magnetic resonance assignments and biosynthesis of aflatoxin B1 and sterigmatocystin. Journal of the Chemical Society Perkin Transactions 1. 1976;1976:1182–1189. doi: 10.1039/p19760001182. [DOI] [PubMed] [Google Scholar]
- Peintner U., Rainer J. A blue ascospore colour variant of Emericella nidulans. Mycotaxon. 1999;70:445–451. [Google Scholar]
- Peterson S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia. 2008;100:205–226. doi: 10.3852/mycologia.100.2.205. [DOI] [PubMed] [Google Scholar]
- Peterson S.W., Varga J., Frisvad J.C. Phylogeny and subgeneric taxonomy of Aspergillus. In: Varga J., Samson R.A., editors. Aspergillus in the genomic era. Wageningen Academic Publishers; Wageningen: 2008. pp. 33–56. [Google Scholar]
- Polacheck I., Nagler A., Okon E. Aspergillus quadrilineatus, a new causative agent of fungal sinusitis. Journal of Clinical Microbiology. 1992;30:3290–3293. doi: 10.1128/jcm.30.12.3290-3293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo G., Gloor E.T., Forbes E. Analysis of mitotic recombination in Aspergillus nidulans. Journal of Genetics. 1954;52:226–237. [Google Scholar]
- Pornpakakul S., Liangsakul J., Ngamrojanavanich N. Cytotoxic acitivity of four xanthones from Emericella variecolor, an endophytic fungus isolated from Croton oblongifolius. Archives of Pharmacological Research. 2006;29:140–144. doi: 10.1007/BF02974275. [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Qureshi I.H., Husain A.S., Noorani R. Stellatic acid: a new class of sesterterpenoids; X-ray crystal structure. Tetrahedron Letters. 1980;21:1961–1962. [Google Scholar]
- Qureshi I.H., Kamal A., Noorani R. Biochemistry of microorganisms. VII. Terrein and kojic acid; metabolic products of Aspergillus stellatus. Pakistan Journal of Scientific and Industrial Research. 1968;11:367–369. [Google Scholar]
- Rabie C.J., Simpson T.J., Steyn P.S. Structure and absolute configuration of the asticolorins, toxic metabolites from Aspergillus multicolor. Journal of the Chemical Society Chemical Communications. 1984;1984:764–765. [Google Scholar]
- Rabie C.J., Steyn M., van Schalkwyk G.C. New species of Aspergillus producing sterigmatocystin. Applied and Environmental Microbiology. 1977;33:1023–1025. doi: 10.1128/aem.33.5.1023-1025.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank C., Nielsen K.F., Larsen T.O. Distribution of sterigmatocystin in filamentous fungi. Fungal Biology. 2011;115:406–420. doi: 10.1016/j.funbio.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Raper K.B., Fennell D.I. Williams & Wilkins; Baltimore, MD: 1965. The genus Aspergillus. [Google Scholar]
- Raper K.B., Thom C. New Aspergilli from soil. Mycologia. 1944;36:555–557. [Google Scholar]
- Rizzacasa M.A., Sargent M.V. The synthesis of desertorin C, a bicoumarin from the fungus Emericella desertorum. Journal of the Chemical Society Perkin Transactions 1. 1988;1988:2425–2428. [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes version 3.0: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Roy K., Mukhopadhyay T., Reddy G.C. Mulundocandin, a new lipopeptide antibiotic. I. Taxonomy, fermentation, isolation and characterization. The Journal of Antibiotics. 1987;40:275–280. doi: 10.7164/antibiotics.40.275. [DOI] [PubMed] [Google Scholar]
- Sabino R., Verissimo C., Parada H. Molecular screening of 246 Portugese Aspergillus isolates among different clinical and environmental sources. Medical Mycology. 2014;52:517–527. doi: 10.1093/mmy/myu006. [DOI] [PubMed] [Google Scholar]
- Sadler I.H., Simpson T.J. The determination by NMR methods of the structure and stereochemistry of astellatol, a new and unusual sesterpene. Journal of the Chemical Society Chemical Communications. 1989;1989:1602–1604. [Google Scholar]
- Sadler I.H., Simpson T.J. Determination by NMR methods of the structure and stereochemistry of astellatol, a new unusual sesterpene. Magnetic Resonance in Chemistry. 1992;30:S18–S23. [Google Scholar]
- Saito T., Itabashi T., Wakana D. Isolation and structure elucidation of new phthalide and phthalane derivatives, isoalted as antimicrobial agents from Emericella sp. IFM57991. Journal of Antibiotics. 2016;69:89–96. doi: 10.1038/ja.2015.85. [DOI] [PubMed] [Google Scholar]
- Samson R.A. A compilation of the Aspergillus described since 1965. Studies in Mycology. 1979;18:1–38. [Google Scholar]
- Samson R.A., Houbraken J., Frisvad J.C. CBS-KNAW Fungal Biodiversity Centre; Utrecht: 2010. Food and indoor fungi. [Google Scholar]
- Samson R.A., Mouchacca J. Additional notes on species of Aspergillus, Eurotium and Emericella from Egyptian desert soil. Antonie van Leeuwenhoek. 1975;41:343–351. doi: 10.1007/BF02565069. [DOI] [PubMed] [Google Scholar]
- Samson R.A., Varga J., Meijer M. New species in Aspergillus section Usti. Studies in Mycology. 2011;69:81–97. doi: 10.3114/sim.2011.69.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R.A., Visagie C.M., Houbraken J. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.F., Chiang Y.M., Szewczyk E. Molecular genetic analysis of the orsellinic/F9775 gene cluster of Aspergillus nidulans. Molecular BioSystems. 2010;6:587–593. doi: 10.1039/b904541d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.F., Entwistle R., Hung J.H. Genome-based deletion analysis reveals the prenyl xanthone biosynthesis pathway in Aspergillus nidulans. Journal of the American Chemical Society. 2011;133:4010–4017. doi: 10.1021/ja1096682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu D.K., Sandhu R.S. A new variety of Aspergillus nidulans. Mycologia. 1963;55:297–299. [Google Scholar]
- Scherlach K., Hertweck C. Discovery of aspoquinolones A-D, prenylated quinoline-2-one alkaloids from Aspergillus nidulans, motivated by genome mining. Organic & Biomolecular Chemistry. 2006;4:3517–3520. doi: 10.1039/b607011f. [DOI] [PubMed] [Google Scholar]
- Scherlach K., Sarkar A., Schroeckh V. Two induced fungal polyketide pathways converge into antiproliferative spiroanthrones. ChemBioChem. 2011;12:1836–1839. doi: 10.1002/cbic.201100132. [DOI] [PubMed] [Google Scholar]
- Scherlach K., Schuemann J., Dahse H.-M. Aspernidine A and B, prenylated isoindolinone alkaloids from the model fungus Aspergilus nidulans. Journal of Antibiotics. 2010;63:375–377. doi: 10.1038/ja.2010.46. [DOI] [PubMed] [Google Scholar]
- Schoch C., Seifert K.A., Huhndorf S. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoustra S.E., Debets A.J.M., Slakhorst M. Reducing the cost of resistance; experimental evolution in the filamentous fungus Aspergillus nidulans. Journal of Evolutionary Biology. 2006;19:1115–1127. doi: 10.1111/j.1420-9101.2006.01102.x. [DOI] [PubMed] [Google Scholar]
- Schroeckh V., Scherlach K., Nützmann H.W. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proceedings of the National Academy of Sciences of Unites States of America. 2009;106:14558–14563. doi: 10.1073/pnas.0901870106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H.W., Cole R.J., Hein H. Tremorgenic mycotoxins from Aspergillus caespitosus. Applied Microbiology. 1975;29:857–858. doi: 10.1128/am.29.6.857-858.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.H., DeCarlo E.S., Kwon-Chung K.J. Aspergillus nidulans infection in chronic granulomatous disease. Medicine. 1998;77:345–354. doi: 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Seya H., Nakajima S., Kawai K. Structure and absolute configuration of emestrin, a new macrocyclic epidithiodioxopiperazine from Emericella striata. Journal of the Chemical Society Chemical Communications. 1985;1985:657–658. [Google Scholar]
- Seya H., Nozawa K., Nakajima S. Studies on fungal products. Part 8. Isolation and structure of emestrin, a novel antifungal macrocyclic epidithiooxopiperazine from Emericella striata. X-ray molecular structure of emestrin. Journal of the Chemical Society Perkin Transactions 1. 1986;1986:109–116. [Google Scholar]
- Seya H., Nozawa K., Udagawa S. Studies on fungal products. IX. Dethiosecoemestrin, a new metabolite related to emestrin, from Emericella striata. Chemical and Pharmaceutical Bulletin. 1986;34:2411–2416. doi: 10.1248/cpb.34.2411. [DOI] [PubMed] [Google Scholar]
- Shibata K., Kamikawa T., Kaneda M. A new metabolite, aspermutarubrol, from Aspergillus sydowii. Chemical Letters. 1978;1978:797–798. [Google Scholar]
- Sierankiewicz J., Gatenbeck S. A new depsidone from Aspergillus nidulans. Acta Chemica Scandinavica. 1972;26:455–458. doi: 10.3891/acta.chem.scand.26-0455. [DOI] [PubMed] [Google Scholar]
- Simpson T.J. Use of long range proton-carbon-13 couplings in structure determination: stellatin, a novel dihydroisocoumarin from Aspergillus variecolor. Journal of the Chemical Society Chemical Communications. 1978;1978:627–628. [Google Scholar]
- Simpson T.J. Studies on fungal metabolites. 1. Structures of andibenins A and andibenins C, and andilesins A, andilesins B, and andilesins C, meroterpenoids from Aspergillus variecolor. Journal of the Chemical Society Perkin Transactions 1. 1979;1979:2118–2121. [Google Scholar]
- Simpson T.J. Biosynthesis if highly modified meroterpenoids in Aspergillus variecolor. Incorporation of 13C-labelled acetates and methionione into anditomin and andilesin C. Tetrahedron Letters. 1981;22:3785–3788. [Google Scholar]
- Simpson T.J. Biosynthesis of astellatol, novel rearranged sesterterpenoid metabolites of Aspergillus variecolor. Journal of the Chemical Society Perkin Transactions I. 1994;1994:3055–3056. [Google Scholar]
- Simpson T.J., Ahmed S.A., McIntyre C.R. Biosynthesis of polyketide-terpenoid (meroterpenoid) metabolites andebenin B and andilesin A in Aspergillus variecolor. Tetrahedron. 1997;53:4013–4034. [Google Scholar]
- Simpson T.J., Stenzel D.J., Bartlett A.J. Studies on fungal metabolites. 3. 13C N.M.R. spectral and structural studies on austin and new related meroterpenoids from Aspergillus ustus, Aspergillus variecolor, and Penicillium diversum. Journal of the Chemical Society Perkin Transactions I. 1982;1982:2687–2692. [Google Scholar]
- Simpson T.J., Walkinshaw M.D. Anditomin, a new C25 metabolite from Aspergillus variecolor. Journal of the Chemical Society Chemical Communications. 1981;1981:914–915. [Google Scholar]
- Smedsgaard J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography A. 1997;760:264–270. doi: 10.1016/s0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML Web-Servers. Systematic Biology. 2008;75:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stchigel A.M., Guarro J. A new species of Emericella from Indian soil. Mycologia. 1997;89:937–941. [Google Scholar]
- Steyn P.S., Vleggaar R., Rabie C.J. Alkaloids from Aspergillus caespitosus. Phytochemistry. 1981;20:538–539. [Google Scholar]
- Stodola F.H., Vesonder R.F., Fennell D.I. A new depsidone from Aspergillus unguis. Phytochemistry. 1972;11:2107–2108. [Google Scholar]
- Stolk A.C. Aspergillus asperescens n. sp. Antonie van Leeuwenhoek. 1954;20:299–304. doi: 10.1007/BF02543732. [DOI] [PubMed] [Google Scholar]
- Sureram S., Wiyakrutta S., Ngamrojanavanich N. Depsaidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Medica. 2012;78:582–588. doi: 10.1055/s-0031-1298228. [DOI] [PubMed] [Google Scholar]
- Szewczyk E., Chiang Y.M., Oakley C.E. Identification and characterization of the asperthecin gene cluster of Aspergillus nidulans. Applied and Environmental Microbiology. 2008;74:7607–7612. doi: 10.1128/AEM.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadano K., Yamada H., Idogaki Y. Stereoselective synthesis of the bis (tetrahydrofuran) moiety (C-1 to C-9) of (+)-asteltoxin, a novel mycotoxin from Aspergillus stellatus. Tetrahedron Letters. 1988;29:655–658. [Google Scholar]
- Takahashi H., Hosoe T., Nozawa K. Two new sesterpenes from the ascomycetous fungus Emericella purpurea. Journal of Natural Products. 1999;62:1712–1713. [Google Scholar]
- Takahashi H., Nozawa K., Kawai K. Isolation and structures of dicyanide derivatives, epurpurins A to C, from Emericella purpurea. Chemical and Pharmaceutical Bulletin. 1996;44:2227–2230. [Google Scholar]
- Taniguchi M., Kaneda N., Shibata K. Isolation and biological activity of aspermutarubrol, a self-growth inhibitor of Aspergillus sydowii. Agricultural and Biological Chemistry. 1978;42:1629–1630. [Google Scholar]
- Tarawneh A.H., Leon F., Radwan M.M. Secondary metabolites from the fungus Emericella nidulans. Natural Product Communications. 2013;8:1285–1288. [PMC free article] [PubMed] [Google Scholar]
- Tezuka Y, Takahashi A, Nozawa K, et al. (1998). Japanese Patent, JP 10045662.
- Thom C., Church M.B. Williams & Wilkins; Baltimore, MD: 1926. The Aspergilli. [Google Scholar]
- Thom C., Raper K.B. The Aspergillus nidulans group. Mycologia. 1939;31:653–669. [Google Scholar]
- Thom C., Raper K.B. Williams & Wilkins; Maryland, MD: 1945. A manual of the Aspergilli. [Google Scholar]
- Todd R.B., Davis M.A., Hynes M.J. Genetic manipulation of Aspergillus nidulans: meiotic progeny for genetic analysis and strain construction. Nature Protocols. 2007;2:811–821. doi: 10.1038/nprot.2007.112. [DOI] [PubMed] [Google Scholar]
- Tóth V., Nagy C.T., Miskei M. Polyphasic characterization of “Aspergillus nidulans var. roseus” ATCC 58397. Folia Microbiologica. 2011;56:381–388. doi: 10.1007/s12223-011-0059-4. [DOI] [PubMed] [Google Scholar]
- Tóth V., Nagy C.T., Pócsi I. The echinocandin B producer fungus Aspergillus nidulans var. roseus ATCC 58397 does not possess innate resistance against its lipopeptide antimycotic. Applied Microbiology and Biotechnology. 2012;95:113–122. doi: 10.1007/s00253-012-4027-y. [DOI] [PubMed] [Google Scholar]
- Traber R., Keller-Juslén C., Loosli H.R. Cyclopeptide antibiotics from Aspergillus species – structure of echinocandin C and echinocandin D. Helvetica Chimica Acta. 1979;62:1252–1267. [Google Scholar]
- Trigos Á., Martínez L.C., López-Reyes E.E. Diketopiperazines from cultures of the fungus Emericella rugulosa. Micologia Aplicada International. 2005;17:1–4. [Google Scholar]
- Tsang C.C., Hui T.W.S., Lee K.C. Genetic diversity of Aspergillus species isolated from onychomycosis and Aspergillus hongkongensis sp. nov., with implications to antifungal susceptibility testing. Diagnostic Microbiology and Infectious Disease. 2016;84:125–134. doi: 10.1016/j.diagmicrobio.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Turner W.B. Academic Press; London: 1971. Fungal metabolites. [Google Scholar]
- Turner W.B., Aldridge D.C. Academic Press; London: 1983. Fungal metabolites II. [Google Scholar]
- Varga J., Frisvad J.C., Samson R.A. A reappraisal of fungi producing aflatoxin. World Mycotoxin Journal. 2009;2:263–277. [Google Scholar]
- Varga J., Frisvad J.C., Samson R.A. Aspergillus sect. Aenei sect. nov., a new section of the genus for A. karnatakaensis sp. nov. and some allied fungi. IMA Fungus. 2010;1:197–205. doi: 10.5598/imafungus.2010.01.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J., Frisvad J.C., Samson R.A. Polyphasic taxonomy of Aspergillus section Sparsi. IMA Fungus. 2010;1:187–195. doi: 10.5598/imafungus.2010.01.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie C.M., Hirooka Y., Tanney J.B. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Studies in Mycology. 2014;78:63–139. doi: 10.1016/j.simyco.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij P.E., Varga J., Houbraken J. Emericella quadrilineata as cause of invasive aspergillosis. Emerging Infectious Diseases. 2008;14:566–572. doi: 10.3201/eid1404.071157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwani K., Dudeja S.K., Srivastava M.P. Aspergillus sub-unguis spec. nov.: a new member of A. nidulans group. Current Science. 1984;53:443–444. [Google Scholar]
- Watanabe A., Fujii I., Sankawa U. Re-identification of Aspergillus nidulans wA gene to code for a polyketide synthase of naphthopyrone. Tetrahedron Letters. 1999;40:91–94. [Google Scholar]
- Watanabe A., Ono Y., Fujii I. Product identification of polyketide synthase coded by Aspergillus nidulans wA gene. Tetrahedron Letters. 1998;39:7733–7736. [Google Scholar]
- Wei H., Itoh T., Kinoshita M. Cytotoxic sesterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolor GF10. Tetrahedron. 2004;60:6015–6019. [Google Scholar]
- Wei H., Itoh T., Konoshita M. Shimalactone A, a novel polyketide, from marine-derived fungus Emericella variecolor GF10. Tetrahedron. 2005;61:8054–8058. [Google Scholar]
- Woo P.W.K., Dion H.W. A novel adenosine and Ara-A deaminase inhibitor, (R)-3-(2-deoxy-β-d-erythro-pento-furanosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3] diazepin-9-ol. Journal of Heterocyclic Chemistry. 1974;11:641–643. [Google Scholar]
- Wu Q., Wu C., Long H. Varioxiranol A-G and 19-O-methyl-22-methoxypre-shamixanthone, PKS and hybrid PKS-derived metabolites from a sponge-associated Emericella variecolor fungus. Journal of Natural Products. 2015;78:2461–2470. doi: 10.1021/acs.jnatprod.5b00578. [DOI] [PubMed] [Google Scholar]
- Xu Y.M., Espinosa-Artiles P., Liu M.X. Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine, and emericellenes A–E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astralagus lentiginosus. Journal of Natural Products. 2013;76:2330–2336. doi: 10.1021/np400762k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Maybayashi Y. The isolation and structure determination of violaceic acid, a new biphenyl ether type metabolite from Emericella violacea. Chemical and Pharmaceutical Bulletin. 1982;30:509–513. [Google Scholar]
- Yamazaki M., Maybayashi Y. Structure determination of violaceol–I and –II, new fungal metabolites from a strain of Emericella violacea. Chemical and Pharmaceutical Bulletin. 1982;30:514–518. [Google Scholar]
- Yamazaki M., Satoh Y., Maebayashi Y. Monoamine oxidase inhibitors from a fungus, Emericella navahoensis. Chemical and Pharmaceutical Bulletin. 1988;36:670–675. doi: 10.1248/cpb.36.670. [DOI] [PubMed] [Google Scholar]
- Yeh H.H., Chiang Y.M., Entwistle R. Molecular genetic analysis reveals that a nonribosomal peptide synthetase-like (NRPS-like) gene in Aspergillus nidulans is responsible for microperfuranone biosynthesis. Applied Microbiology and Biotechnology. 2012;96:739–748. doi: 10.1007/s00253-012-4098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W.B., Reinke A.W., Szilágyi M. bZIP transcription factors affecting secondary metabolism, sexual development and stress responses in Aspergillus nidulans. Microbiology. 2013;159:77–88. doi: 10.1099/mic.0.063370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganathan K., Rossant C., Glover R.P. Inhibition of the human chemokine receptor CCR5 by variecolin and variecolol and isolation of four new variecolin analogues, emericolins A–D from Emericella aurantiobrunnea. Journal of Natural Products. 2004;67:1681–1684. doi: 10.1021/np049844c. [DOI] [PubMed] [Google Scholar]
- Yoshino H. Isolation of 2'-deoxycoformycin and cordycepin from wheat bran culture of Aspergillus nidulans Y 176-2. Agricultural and Biological Chemistry. 1979;43:2375–2377. [Google Scholar]
- Yu J., Mu X., Li R. Invasive pulmonary aspergillosis due to Emericella nidulans var. echinulata, succesfully cured by voriconazol and micafungin. Journal of Clinical Microbiology. 2013;51:1327–1329. doi: 10.1128/JCM.02487-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q., Chen L., Zhang X.L. Evolution of chemical diversity in echinocandin lipopeptide antifungal metabolites. Eukaryotic Cell. 2015;14:698–718. doi: 10.1128/EC.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalar P., Frisvad J.C., Gunde-Cimerman N. Four new species of Emericella from the Mediterranean region of Europe. Mycologia. 2008;100:779–795. doi: 10.3852/08-078. [DOI] [PubMed] [Google Scholar]
- Zhang G., Sun S., Zhu T. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry. 2011;72:1436–1442. doi: 10.1016/j.phytochem.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Zhang L.C., Chen J., Guo W.H. A new species of Emericella from China. Mycotaxon. 2013;125:131–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of section Nidulantes inferred from ITS.
Phylogenetic tree of section Nidulantes inferred from BenA.
Phylogenetic tree of section Nidulantes inferred from CaM.
Phylogenetic tree of section Nidulantes inferred from RPB2.