Abstract
To build an understanding of the neurobiology underpinning arm recovery in people with severe arm impairment due to stroke, we conducted a pooled individual data systematic review to: 1) characterize brain biomarkers; 2) determine relationship(s) between biomarkers and motor outcome; and 3) establish relationship(s) between biomarkers and motor recovery. Three electronic databases were searched up to October 2, 2015. Eligible studies included adults with severe arm impairment after stroke. Descriptive statistics were calculated to characterize brain biomarkers, and pooling of individual patient data was performed using mixed-effects linear regression to examine relationships between brain biomarkers and motor outcome and recovery. Thirty-eight articles including individual data from 372 people with severe arm impairment were analysed. The majority of individuals were in the chronic (> 6 months) phase post stroke (51%) and had a subcortical stroke (49%). The presence of a motor evoked potential (indexed by transcranial magnetic stimulation) was the only biomarker related to better motor outcome (p = 0.02). There was no relationship between motor outcome and stroke volume (cm3), location (cortical, subcortical, mixed) or side (left vs. right), and corticospinal tract asymmetry index (extracted from diffusion weighted imaging). Only one study had longitudinal data, thus no data pooling was possible to address change over time (preventing our third objective). Based on the available evidence, motor evoked potentials at rest were the only biomarker that predicted motor outcome in individuals with severe arm impairment following stroke. Given that few biomarkers emerged, this review highlights the need to move beyond currently known biomarkers and identify new indices with sufficient variability and sensitivity to guide recovery models in individuals with severe motor impairments following stroke. PROSPERO: CRD42015026107.
Keywords: Stroke, Upper extremity, Biomarker, Neuroimaging, Neurophysiology
Highlights
-
•
The majority of studies investigated individuals in the chronic stage of stroke recovery.
-
•
Presence of a motor evoked potential at rest was related to a better motor outcome after stroke.
-
•
Lesion and white matter characteristics were not related to motor outcome in this individual patient data review.
1. Introduction
Stroke mortality rates have reduced in parallel with advancements in acute healthcare (Krueger et al., 2015). Though this is a positive development, the net result is a larger number of people surviving stroke with severe arm motor impairments (Krueger et al., 2015). This results in significant loss of participation, productivity, and engagement in meaningful activities (Barker and Brauer, 2005). While recovery of arm function after stroke is crucial for overall quality of life (Edwards et al., 2010), people with severe impairments have limited access to intensive rehabilitation efforts (Hayward and Brauer, 2015). There is evidence however, that some people with severe arm impairment can attain partial, and sometimes even complete recovery of arm function (Hayward et al., 2014). However, identifying individuals with potential for recovery after severe stroke is challenging. Thus, to build an understanding of how to identify and then promote the return of arm function in people with severe impairments, we need to understand the neurobiology underlying motor outcome.
Brain biomarkers may help to explain recovery trajectories and the neurobiology of motor outcomes after stroke. A biomarker is an indicator of disease state that can be used clinically to reflect underlying molecular/cellular processes that may be difficult to measure directly in humans, and could be used to predict recovery/treatment response (Bernhardt et al., 2016). Many biomarkers of brain structure and function have been discussed in the literature, including magnetic resonance imaging (MRI), resting (rsfMRI) or task-based functional MRI (fMRI), transcranial magnetic stimulation (TMS), positron emission tomography, and electroencephalography. Despite mixed opinions regarding the usefulness of different brain biomarkers (Krakauer and Hillis, 2014, Ward, 2015), people with severe impairment warrant investigation of brain biomarkers for two key reasons. Firstly, in individuals with severe motor impairment, both clinical scores (Prabhakaran et al., 2008, Stinear et al., 2012, Winters et al., 2015) and clinical opinion (Kwakkel et al., 2000) show limited capacity to predict functional recovery. This is likely due to floor effects of many clinical scoring approaches, which fail to capture the substantial variability between individuals with severe arm impairment (Campbell Stewart and Cramer, 2013). Secondly, the addition of a brain biomarker (e.g., indexed using TMS or MRI) can enhance our ability to identify subgroups beyond that possible using a clinical measure alone (Stinear et al., 2012). Thus, there is a need to define brain biomarkers that provide a sensitive index of brain structure and function, and help predict motor outcome and recovery potential in this cohort.
The use of brain biomarkers to determine a neurobiological basis for motor outcome of people with severe arm impairment is largely under-researched. A meta-analysis described the field as ‘skewed’, with preferential enrolment of people with minimal baseline behavioural impairments in the majority of included studies (Hodics et al., 2006). Improved understanding of who might recover from severe arm impairments, via the use of brain biomarkers, could inform the development of new treatment approaches for this cohort. It is therefore imperative that we expand our understanding of the interaction between brain biomarkers and motor outcome to guide the development of innovative, targeted, and individualized interventions for people with severe arm impairment.
This review sought to investigate brain biomarkers in people with severe arm impairment as a result of a stroke. In this cohort of individuals the objectives were to: 1) characterize brain biomarkers explored in the literature; 2) determine relationship/s between brain biomarkers and motor outcomes (controlling for select demographic variables of age, gender, and days post injury); and 3) establish relationship/s between biomarkers and motor recovery.
2. Methods
2.1. Search strategy and selection criteria
This study was registered on PROSPERO, October 26, 2015 (CRD42015026107). Electronic databases of MEDLINE OVID, EMBASE OVID and CINAHL EBSCO were searched up to October 2, 2015. Brain biomarkers targeted in our search strategy included magnetic MRI, fMRI, resting state fMRI, diffusion weighted imaging (DWI), electroencephalography (EEG), TMS, magnetoencephalography (MEG), magnetic resonance spectroscopy (MRS), positron emission tomography (PET). The full search strategy is available in Appendix A: Supplemental Data: Multimodal Component 2 for MEDLINE OVID, EMBASE OVID, and CINAHL EBSCO.
Eligible studies met the following criteria: a) adults (18 years and older) with a stroke (note: mixed samples were eligible if > 50% of the sample had a stroke (Cochrane Collaboration, 2011); and b) i) > 50% of individuals had arm impairment defined as severe in the study inclusion criteria, or ii) at least two individuals had severe arm impairment; and 3) had a brain biomarker investigation performed at the same time as motor outcome assessment. Determination of severe arm impairment was based on motor outcome cutoffs consistent with pronounced weakness or complete hemiplegia of the arm, making it impossible to elevate the arm against resistance, and nearly impossible to hold the arm against gravity or perform fine finger movements (Rehme et al., 2012), which was determined from individual outcome data and predefined cutoffs (see Table 1). Studies were excluded if they were: a) a review or case study; b) investigated a pharmacological intervention (e.g., botulinum toxin); or c) not available with the full text in English.
Table 1.
Referent cut-off values to define severe upper limb impairment and activity on commonly used scales.
| Outcome measure | Severity cut-off | Rationale for cut-off |
|---|---|---|
| Fugl Meyer Upper Limb Assessment | < 31 out of 66 | Consistent with a lack of dexterous hand function |
| Manual muscle testing | < 3 out of 5 | Consistent with a lack of movement against gravity |
| Motor Assessment Scale: upper arm function | < 3 out of 6 | Consistent with an inability to perform a straight-line reach against gravity |
| Action Research Arm Test | < 15 out of 60 | Consistent with a lack of dexterous hand function |
| Motricity Index, upper limb | < 20 out of 100 | Consistent with a lack of dexterous hand function |
| Frenchay Arm Test | < 2 out of 5 | Consistent with a lack of dexterous hand function |
| NIHSS upper limb item | 3 or 4 out of 4 | Consistent with a lack of movement against gravity |
| Mayo Clinic Strength | 3 or 4 out of 4 | Consistent with a lack of movement against gravity |
Note: NIHSS NIH Stroke Severity scale.
From the initial search result, all duplicate references were excluded first using the Endnote “find duplicates” filter and second, by hand search of references. All reference titles and abstracts were screened according to the predetermined eligibility criteria. The full texts of remaining references were retrieved and reviewed for eligibility by two reviewers (KH/JS). Authors (first and/or primary contact) of studies were contacted to clarify study details when eligibility was unclear. If there was disagreement regarding inclusion of a study between KH/JS, resolution was first attempted through discussion and if not resolved, a third reviewer (LB) was involved to achieve consensus. If still not resolved, a further two reviewers (SP/KW see acknowledgments) were included to reach consensus. Reference lists of key eligible studies, along with key systematic and literature reviews yielded by the initial search strategy, were hand searched to identify potential studies not identified by the initial search strategy. Additional studies identified were subjected to the selection process as described above. Web of Science, a citation-tracking database, was also used to search for additional studies.
2.2. Data extraction
Using a predetermined data extraction form, two reviewers (KH/JS) extracted all data from the earliest time-point of collection in each study. This afforded the extraction of baseline data within an intervention study, which recognises that therapeutic interventions might selectively affect the physiology of the biomarker and therefore alter the interpretation of the influence of the biomarker on outcome or recovery (Burke and Cramer, 2013). Data extracted included: a) study information including authors, publication year, design, and definition of stroke severity; b) participant information including age, sex, and stroke hemisphere and location; c) brain biomarker information, including raw data, collection method, timing, and frequency of measurement; d) arm clinical index information, including outcome measure of impairment, activity or participation, along with timing, and frequency of measurement); e) group (i.e., means, standard deviations, p values, effect size) and individual data (i.e., raw scores) for biomarker, and clinical index; and f) miscellaneous data of potential importance. Studies of the same population were linked at data extraction to ensure that these data were only included once. Extracted data were entered and crosschecked from the original source by two authors independently (KH/JS). If queries or discrepancies regarding data extraction occurred, they were resolved through discussion. If consensus was not achieved, a third author (SP) extracted data. If still not resolved, two additional authors (LB/KW) performed data extraction. Coding of extracted data was completed prior to statistical analysis for four outcomes. Two authors independently coded data (KH/JS/SP/KW) and any discrepancies were resolved through discussion, and a third author (LB) as required. For full details of data extracted (b, c, d) and subsequent coding of variables extracted see Table 2.
Table 2.
Description for demographic and brain biomarkers of interest.
| Variable | Description of data extraction | Coding |
|---|---|---|
| Demographics | ||
| Age | In years at time of stroke | – |
| Days post stroke | Days, converted from months (*30) or years (*12*30) as required | 0–30 days; 31–90 days; 91–180 days, > 180 days |
| Sex | Male, Female | – |
| Brain biomarkers | ||
| Lesion volume | cm3 | – |
| Lesion side | Right, left | – |
| Lesion location | As reported in text; only extracted if reported from CT or MRI at time of study data collection | Cortical, subcortical, mixed (cortical + subcortical), non-specific haemorrhage |
| Motor evoked potential | At rest, and location of electromyographic collection | Motor evoked potential response: present or absent Location: hand, any muscle with an action on the fingers or thumb; forearm, any muscle with an action on the wrist or forearm; or upper arm, any muscle with an action at the elbow or shoulder |
| Corticospinal tract | Fractional anisotropy of segment, whole tract or region for the ipsilesional and contralesional hemisphere | Asymmetry index [contralesional − ipsilesional fractional anisotropy] / [contralesional + ipsilesional fractional anisotropy] |
2.3. Statistical analysis
To address each of the three objectives of the review the following analyses were undertaken.
Objective 1: To describe brain biomarkers and the sample of individuals with severe arm impairment, we used descriptive statistics (mean, SD, range).
Objective 2: To explore the relationship between a brain biomarker (predictor variable) and an index of arm motor outcome (dependent variable), we tested a series of mixed-effect regression models. All biomarkers were collected at the same time as the motor outcome, which needed to precede any planned interventions. As individuals were nested within different studies, the study ID was treated as a random-effect to control for the statistical dependence of the nested observations. Given that age, sex, and days post-injury may influence brain biomarkers these variables were treated as controlling variables in all statistical models. As such, for each brain biomarker, we first modelled motor outcome (dependent variable) as a function of biomarker (e.g., motor outcome ~ lesion location) and then added demographic factors in the next step (e.g., motor outcome ~ lesion location, age, sex, and days post-injury). All predictor variables were mean-centred prior to analysis. Cooks distances were calculated to determine if any data points had undue influence on the overall model parameter estimates (Nieuwenhuis et al., 2012). Undue influence was defined by data points that were > 4/number of groups in the grouping factor (Van der Meer et al., 2010). If this occurred, the data points were excluded from the final model. AIC, BIC and Wald's Test for statistical significance were used to assess model fit (Long, 2012). All analyses were conducted in software package R 3.2.3 (R Core Team, 2015) and used the “dplyr”, “lme4”, and “lmerTest” packages (Bates et al., 2015, Kuznetsova et al., 2015, Wickham and Francois, 2015). P-values for the individual coefficients in the statistical models were based on the Satterthwaite approximation (Kuznetsova et al., 2015). All models were fit using maximum likelihood estimation.
Objective 3: To determine the relationship between biomarkers and motor recovery in people with severe arm impairment, the same statistical approach as in objective 2 was adopted. The dependent variable, motor recovery, refers to the change in outcome between two timepoints (longitudinal). As such, to be included in this analysis there must have been two outcome scores separated by a period of time and no intervention. Biomarker information was extracted at all available timepoints. In addition to age, sex, and days post-injury, we also treated elapsed time between timepoints as a controlling variable in all statistical models. Here the dependent variable, motor recovery, was defined as change between timepoint 1 and timepoint 2.
For objectives 2 and 3 models were only developed when raw scores on a clinical index were available for ≥ 50% of individuals. This provided a large data set with consistent outcomes, mitigating the need for normalisation. For all statistical models, significance was set at p ≤ 0.05. If regression models could not be performed for a brain biomarker (e.g., due to insufficient individual data), results were reported descriptively.
3. Results
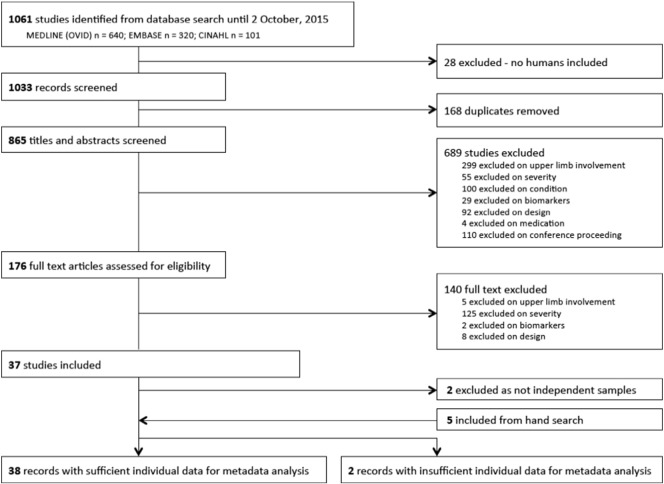
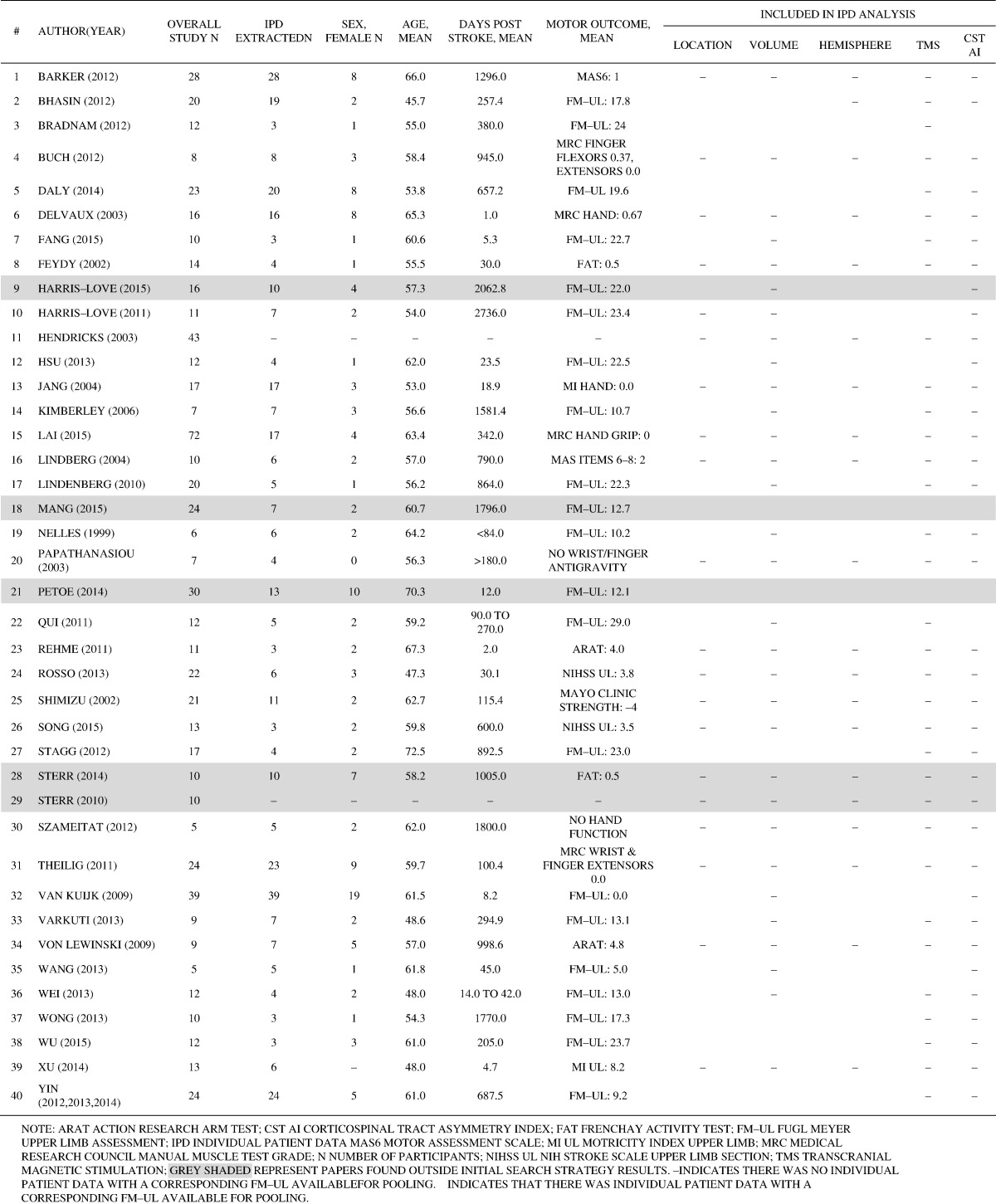
From the initial search strategy, 37 articles were included, with 35 independent study samples. Fig. 1 outlines the flow of studies from search strategy through to inclusion. There were no instances of disagreement regarding potential inclusion that could not be resolved through discussion of the primary reviewers (KH/JS). Of the studies included, there were 329 individuals across 35 studies that had severe arm impairment out of a total of 506 participants (65%). An additional five studies were identified from hand searches, which comprised 104 individuals with severe arm impairment out of a total 178 participants (58%). Therefore, 40 studies published from 1999 to 2015 were included, of which 433 (out of total study n = 684, 63%) had severe arm impairment (See Table 3).
Fig. 1.
Flow of studies from search results through to data extraction.
Table 3.
Table of included study characteristics.
We attempted to extract individual data from all 40 studies (see Supplemental A). Twelve authors were contacted to provide additional individual brain biomarker and demographic data, of which six authors (50%) returned additional data. As such, we had 372 (out of 433, 86%) individuals with individual patient data for various biomarkers of interest across 38 studies. Data extraction was consistent across the two independent reviewers, with only four data points of difference in data extraction for which consensus was achieved through discussion.
3.1. Objective 1: characterize brain biomarkers
Individual patient data was documented for stroke lesion characteristics of volume (n = 117, 31% of individuals), location as identified by a CT or MRI (n = 316, 85% of individuals), and hemisphere (n = 336, 90% of individuals); presence or absence of a resting motor evoked potential (MEP) using TMS (n = 195, 52% of individuals); and corticospinal tract asymmetry index (n = 37, 10% of individuals) (See Table 4). For fMRI, only one study had individual data available (n = 17, 5% of individuals).
Table 4.
Demographic, brain biomarker and motor outcome characteristics.
| Variable | na | Mean (SD) | Range |
|---|---|---|---|
| Demographics | |||
| Age (yrs) | 355 | 58.6 (12.8) | 17.0; 86.0 |
| Days post stroke | 351 | 564.8 (903.8) | 0.0; 5040.0 |
| Sex | |||
| Male | 220 | – | – |
| Female | 129 | – | – |
| Brain biomarkers | |||
| Lesion volume (cm3) | 117 | 84.9 (236.6) | 0.1; 2371.0 |
| Lesion side | |||
| Right | 171 | – | – |
| Left | 165 | – | – |
| Lesion location | |||
| Cortical | 23 | – | – |
| Subcortical | 155 | – | – |
| Mixed | 133 | – | – |
| Nonspecific haemorrhage | 5 | – | – |
| Corticospinal tract asymmetry index | 37 | 0.19 (0.21) | − 0.45, 1.0 |
| Motor outcome measures | |||
| Fugl Meyer Arm | 206 | 13.0 (10.0) | 0.0, 30.0 |
| Action Research Arm Test | 13 | 8.7 (8.9) | 0.0, 29.0 |
| Motricity Index, Arm | 28 | 3.8 (9.3) | 0.0, 39.0 |
| Frenchay Activity Test | 14 | 0.5 (0.6) | 0.0, 1.5 |
| NIH Stroke Scale | 9 | 3.7 (0.5) | 3.0, 4.0 |
Total number of individuals was 372.
3.1.1. Lesion characteristics
The mean stroke lesion volume was 85 cm3 (SD 237 cm3), with lesion size < 40 cm3 in the majority of individuals (n = 72, 62% of individuals). Of those individuals with specified lesion locations, the majority of individuals had a subcortical (n = 155, 49%), or mixed subcortical and cortical stroke (n = 133, 42% of individuals), with few having a cortical only stroke (n = 23, 7% of individuals) or other e.g., non-specific haemorrhage (n = 5, 2% of individuals). There were comparable numbers of individuals with left (n = 165, 49% of individuals) and right (n = 171, 51% of individuals) hemisphere stroke lesions.
3.1.2. Motor evoked potential
The most common location for evaluating MEP was in the hand (n = 127, 65%; e.g., first dorsal interossei), followed by upper arm (n = 45, 23%; e.g., triceps brachii), and forearm (n = 23, 12%; e.g., extensor carpi radialis). Irrespective of time point post stroke, the majority of individuals had an absent MEP (n = 133, 68%) post stroke.
3.1.3. Corticospinal tract integrity
This was extracted from raw data defining a segment or the whole tract. The mean asymmetry index of the corticospinal tract across all studies (n = 28) irrespective of time post stroke was 0.19 (SD 0.21).
3.1.4. Functional magnetic resonance imaging
Sensorimotor cortex activation during passive movements was explored in five studies (n = 50), one of which had individual data (n = 17, 5% of individuals) (Jang et al., 2004).
3.1.5. Potential confounding demographic variables
Age at stroke onset was available for 355 individuals (95% of the sample), sex for 349 individuals (94%), and days post stroke for 351 individuals (91%). As shown in Table 4, there was considerable variation in age (17 to 86 years) and days post stroke (0 to 5040 days) in our sample.
3.2. Objective 2: determine relationship(s) between biomarkers and motor outcome
Fugl Meyer Arm (FM-UL) assessment was chosen as the outcome measure for statistical modelling because it was the most commonly reported (n = 206, 55% of individuals; Table 4). Brain biomarkers were not consistently measured across all included studies, which made it difficult to include multiple brain biomarker measures in a single model, and impossible to compare across different models, as they were based on different data. As such, models are reported for motor outcome as a function of brain biomarker, followed by a model controlling for demographic variables of interest.
3.2.1. Stroke lesion location
There was no significant relationship between FM-UL and lesion location (Table 5). Furthermore, controlling for age, sex, and days since stroke did not significantly improve the fit of the model (Wald Test between models p = 0.28).
Table 5.
Model comparisons for the effect of lesion location on motor outcome, Fugl-Meyer Arm score.
| Best fitting model parameters (based on Wald Test) | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 15.56 | 1.83 | 8.50 | < 0.001 |
| Lesion Contrast1 | − 0.07 | 1.29 | − 0.06 | 0.96 |
| Lesion Contrast2 | 0.50 | 0.46 | 1.08 | 0.28 |
Note. The best fitting model included only the contrast coded predictors of lesion location (AIC = 1245.5; BIC = 1261.6) with 187 individuals across 19 different studies. Adding the factors of Sex, Age, and Days Since Stroke (DSS) did not significantly improve the fit of the model (AIC = 1247.7, BIC = 1273.5, Wald Test p = 0.28). Sex was coded as female = 0·5, male = − 0·5; age was centred around overall mean age = 58·56; and days since stroke (DSS) was square root transformed (rtDSS) and then centred around the root-transformed mean. This led to an approximately normal distribution of residuals and homoscedasticity of the residuals. Orthogonal contrasts for lesion location as cortical, mixed, and sub-cortical stroke as 1, 0, − 1, respectively for Contrast 1 (Contrast 2 = − 1, 2, − 1). AIC Akaike information criterion. BIC Bayesian information criterion. AIC Akaike information criterion. BIC Bayesian information criterion.
3.2.2. Stroke lesion volume
There was no statistically significant relationship between lesion volume (square root transformed and mean centred) and FM-UL (Table 6). Controlling for participant age, sex, and days since stroke did significantly improve the fit of the model: a statistically significant negative relationship between days since stroke and FM-UL score (p = 0.01), and significantly lower FM-UL scores for female compared to male individuals (p = 0.03) were observed. However, analysis of the Cook's distances for each model, demonstrated that data-points from Yin had undue influence on the parameter estimates for sex and days since stroke. Thus, when the models were re-analysed with Yin excluded, there were no statistically significant effects of lesion volume, sex, age, or days since stroke.
Table 6.
Model comparisons for the effects of lesion volume on motor outcome, Fugl-Meyer Arm score.
| Estimate | SE | t-Value | p-Value | |
|---|---|---|---|---|
| Best fitting model parameters (based on Wald Test) | ||||
| Intercept | 18.21 | 1.81 | 10.07 | < 0.001 |
| Lesion rtVolume | 0.002 | 0.003 | 0.85 | 0.40 |
| Sex | − 3.50 | 1.56 | − 2.25 | 0.03 |
| Age | 0.03 | 0.06 | 0.47 | 0.64 |
| rtDSS | − 0.19 | 0.07 | − 2.62 | 0.01 |
| Best fitting model parameters (excluding Yin, 2012) | ||||
| Intercept | 19.30 | 1.39 | 13.86 | < 0.001 |
| Lesion rtVolume | 0.003 | 0.002 | 1.11 | 0.27 |
| Sex | − 2.24 | 1.54 | − 1.45 | 0.15 |
| Age | 0.03 | 0.06 | 0.44 | 0.66 |
| rtDSS | − 0.12 | 0.07 | − 1.63 | 0.11 |
Note. The best fitting model included lesion volume, Sex, Age, Days Since Stroke (DSS; AIC = 605.4, BIC = 622.9, Wald Test p < 0.01) and was based on 89 individuals from 9 different studies. The fit of this model was a significant improvement beyond lesion volume alone (AIC = 611.8, BIC = 621.7). Sex was coded as female = 0·5, male = − 0·5; age was centred around overall mean age = 58·56; and DSS and lesion volume were square root transformed (rtDSS, rtVolume) and then centred around the root-transformed mean. This led to an approximately normal distribution of residuals and homoscedasticity of the residuals. Data from Yin (2012) exerted significant leverage on the model, and thus the model was rerun with these data excluded (AIC = 426.6; 441.8; based on 65 individuals from 8 different studies). Cook's distance for Yin (2012) was 0·51, which is greater than the ‘4/number of groups in the grouping factor’ recommended cut-off (i.e., 4/9 = 0.44; (Van der Meer et al., 2010) and thus was excluded in the final model. AIC Akaike information criterion. BIC Bayesian information criterion.
3.2.3. Stroke lesion hemisphere
There was no statistically significant relationship between lesion hemisphere and FM-UL (Table 7). Controlling for participant age, sex, and days since stroke did not significantly improve the fit of the model, and there were no statistically significant relationships between any of these factors and FM-UL.
Table 7.
Model comparisons for the effects of lesion hemisphere on motor outcome, Fugl-Meyer Arm score.
| Best fitting model parameters (based on Wald Test) | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 16.54 | 1.68 | 9.86 | < 0.001 |
| Lesion hemisphere | 0.35 | 0.99 | 0.36 | 0.72 |
Note. The best fitting model included only the contrast coded predictor of lesion hemisphere (AIC = 1192.8; BIC = 1205.5) with 179 individuals across 20 different studies. Adding the factors of Sex, Age, and Days Since Stroke (DSS) did not significantly improve the fit of the model (AIC = 1196.5, BIC = 1218.8, Wald Test p = 0.53). Hemisphere was coded right = 0·5, left = − 0·5; sex was coded as female = 0·5, male = − 0·5; age was centred around overall mean age = 58·56; and DSS was square root transformed and then centred around the root-transformed mean. This led to an approximately normal distribution of residuals and homoscedasticity of the residuals. AIC Akaike information criterion. BIC Bayesian information criterion.
3.2.4. Motor evoked potential
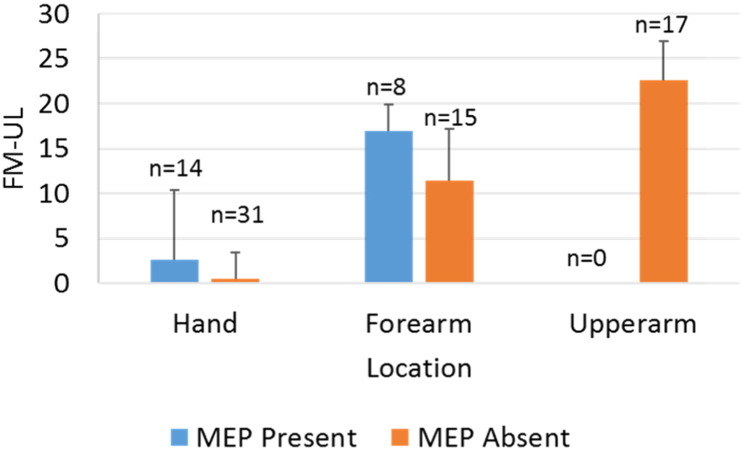
The first model predicted FM-UL as a function of presence or absence of a MEP post stroke for that individual. Individuals who had a MEP present had significantly higher FM-UL scores (p = 0.02). However, as researchers used different sites to record MEP, it is important to include location of MEP recording as a factor. We added a contrast coded MEP recording location predictor in the second model. Individuals who had a MEP present had statistically higher FM-UL scores compared to those who did not have a MEP (p = 0.002). Furthermore, there was a significant interaction of presence of a MEP and recording location (p = 0.01). Specifically, the difference in FM-UL between individuals who had a MEP and those who did not, was larger in studies that indexed MEP in the forearm (Fig. 2).
Fig. 2.
Fugl Meyer Arm Assessment scores as a function of whether or not a motor evoked potential could be elicited and recording location. ‘n’ denotes the number of individuals contributing to the means and SDs at each point.
Due to the limited number of observations with complete MEP and recording location data (68 individuals from 5 studies) for the dependent measure of interest (FM-UL), we were cautious not to overfit our models by controlling for age, sex, and days since stroke. We did, however, analyse Cook's distances for these models. This showed that data-points from Hsu (2013) had undue influence on the parameter estimates, especially for the intercept and the effect of recording location. Thus, the models were re-analysed excluding Hsu (2013). Importantly, the significant interaction of MEP absence/presence by recording location persisted even with Hsu (2013) removed (Table 8).
Table 8.
Model comparisons for the effects of motor evoked potential (MEP) response on motor outcome, Fugl-Meyer Arm score.
| Estimate | SE | t-Value | p-Value | |
|---|---|---|---|---|
| Best fitting model parameters (based on Wald Test) | ||||
| Intercept | 11.18 | 3.87 | 2.89 | 0.05 |
| MEP | 3.52 | 1.06 | 3.32 | < 0.01 |
| Location | 1.88 | 3.89 | 0.48 | 0.63 |
| MEP ∗ location | − 5.59 | 2.12 | − 2.64 | 0.01 |
| Best fitting model parameters (excluding Hsu, 2013) | ||||
| Intercept | 7.58 | 0.96 | 7.94 | < 0.01 |
| MEP | 3.24 | 1.12 | 2.90 | < 0.01 |
| Location | − 11.72 | 1.92 | − 6.14 | < 0.01 |
| MEP ∗ location | − 5.84 | 2.24 | − 2.61 | 0.01 |
Note. The best fitting model included MEP presence, recording location, and the interaction of these terms (AIC = 404.5, BIC = 417.8, Wald Test p = 0.04) and was based on 68 individuals from 5 different studies. In this model, upper arm recording locations were excluded, as there were no MEPs elicited in the upper arm. The fit of this model was a significant improvement beyond MEP presence alone (AIC = 406.9; BIC = 415.8). MEP was coded 0·5 = MEP positive, − 0·5 = MEP negative; recording location was coded hand = 0·5, forearm = − 0·5. This led to an approximately normal distribution of residuals and homoscedasticity of the residuals. Data from Hsu (2013) exerted significant leverage on the model, and thus the model was rerun with these data excluded (AIC = 369.5; BIC = 382.5; based on 64 individuals from 4 different studies). Cook's distance for Hsu (2013) was 17·57, greater than the ‘4/number of groups in the grouping factor’ recommended cut-off (i.e., 4/5 = 0.2; (Van der Meer et al., 2010) and thus was excluded in the final model. AIC Akaike information criterion. BIC Bayesian information criterion. Italics indicates significant effect (p ≤ 0.05).
3.2.5. Corticospinal tract integrity
There was no statistically significant relationship between corticospinal tract asymmetry index post stroke and FM-UL (p = 0.45; Table 9). Given the small amount of available data for corticospinal tract integrity (28 individuals from 4 studies), we were cautious not to overfit the models by controlling for age, sex, and days since stroke (which had thus far proven to be poor predictors of FM-UL in individuals with severe stroke).
Table 9.
Model comparisons for the effects of corticospinal tract asymmetry index on motor outcome, Fugl-Meyer Arm score.
| Parameters of the best fitting model (by Wald Test) | Estimate | SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 13.20 | 1.65 | 8.01 | < 0.001 |
| Corticospinal tract asymmetry index | 0.44 | 8.64 | 0.05 | 0.96 |
| MEP | 5.70 | 2.77 | 2.06 | 0.05 |
Note. The best fitting model included MEP presence and the Corticospinal tract asymmetry index (AIC = 137.7, BIC = 142.6, Wald Test p = 0.05) and was based on 20 individuals from 2 different studies. The fit of this model was significantly better than Corticospinal tract asymmetry alone (AIC = 139.51; BIC 143.49). Corticospinal tract asymmetry index was centred around the group mean of 0·19. Motor Evoked Potential (MEP) was coded 0·5 = MEP positive, − 0·5 = MEP negative. There was an approximately normal distribution of residuals and homoscedasticity of the residuals. AIC Akaike information criterion. BIC Bayesian information criterion. Italics indicates significant effect (p ≤ 0.05).
3.2.6. Combining brain biomarkers of structural integrity
We were interested in the predictive utility of combining measures of MEP presence/absence and corticospinal tract integrity. Thus, in an exploratory model we included the main-effects of MEP and corticospinal tract integrity. Controlling for the presence of an MEP, there was not a significant relationship between corticospinal tract integrity and FM-UL (p = 0.96), but controlling for CST integrity, there was still evidence that individuals who had a MEP also had higher FM-UL scores compared to individuals who did not have a MEP (p = 0.05; Table 9). It should be noted, however, that these results combine data from only 20 different individuals in two separate studies (Mang et al., 2015, Petoe et al., 2014) (Fig. 3).
Fig. 3.
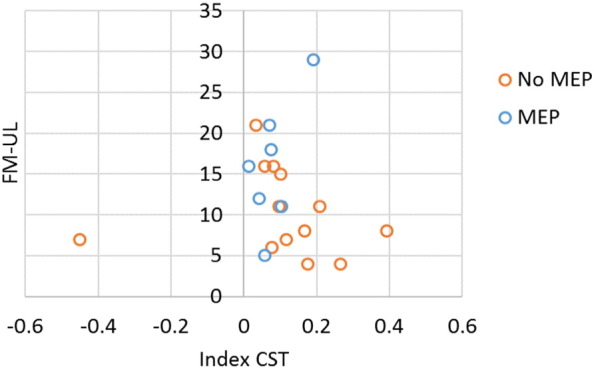
Fugl Meyer Arm assessment as a function of the asymmetry index of corticospinal tract asymmetry index and whether or not a motor evoked potential could be elicited for that participant. At this level of stratification, we only have 20 individuals from two studies (Petoe et al., and Mang et al.).
3.2.7. Functional magnetic resonance imaging
Only one study had individual fMRI data (Jang et al., 2004) and as a result we were unable to pool data. In this study there was a non-significant between group differences that demonstrated that people who activated the contralateral sensorimotor cortex post stroke showed better motor recovery than those who did not activate the contralesional sensorimotor cortex during passive movement.
3.3. Objective 3: establish relationship(s) between biomarkers and motor recovery
This question could not be answered using individual data in this review. One study reported assessing MEP longitudinally without the effect of an intervention (van Kuijk et al., 2009). This study only contributed baseline data; thus, we were unable to perform any analyses. This study did report that presence or absence of a MEP in abductor digiti minimi had similar predictive value for long-term hand motor recovery (26-weeks, FM-Hand > 3 out of 14 points) as compared with clinical assessments (FM-UL) at 1- and 3-weeks post stroke.
4. Discussion
This individual data review sought to investigate brain biomarkers in people with severe arm impairment that were associated with motor outcome after, or recovery from, stroke. To our knowledge this is the largest review of individuals with severe arm impairment after stroke to explore this topic by pooling individual patient data. In the available data, the presence of a MEP (collected using TMS) was the only biomarker related to a better motor outcome, indexed by FM-UL; this effect became stronger when MEP recording location was an analysis factor. Interestingly, there was no relationship between motor outcome and stroke lesion characteristics including volume (cm3), location (cortical, subcortical, mixed) or side (left vs. right), nor corticospinal tract asymmetry (collected using diffusion-weighted imaging). Furthermore, there was insufficient research identified by our search strategy to explore motor recovery.
4.1. Motor evoked potential presence was associated with better motor outcomes
Our analysis demonstrated that it is important to index the integrity of the motor system using TMS in individuals with severe arm impairment after stroke. Assessment of whether a MEP is present or absent at rest provides insights into the physiology of neural circuits underlying motor impairment. The absence of a MEP indicates that the physiological integrity of the corticospinal tract is significantly damaged, preventing electrical potentials reaching the muscle of interest to enable a motor response (Byrnes et al., 1999, Turton et al., 1996). The results of the current study, which pooled individual patient data on people with severe impairment only, are consistent with previous research that included the full spectrum of severity, that is people with mild through to severe impairment (Pizzi et al., 2009, Stinear et al., 2012). This suggests that MEP presence is still sensitive to differences in function even in this restricted range of the FM-UL (≤ 30 out of 66).
When we investigated MEP by location of electromyographic collection (forearm vs. hand, upper arm) there were two interesting findings that emerged. Firstly, there were no MEPs present proximally (i.e., triceps brachii). This is perhaps not surprising given that distal muscles have been reported to more readily evoke a response relative to proximal muscles in healthy individuals, which is thought to be due to the larger cortical representation and lower activation thresholds (Byrnes et al., 1999).
Secondly, when controlling for the location of MEP response (forearm vs. hand, upper arm excluded), the difference in impairment between individuals who had a MEP present compared with those who did not, was larger in studies that measured at the forearm. That is, studies in which the MEP was reported at the forearm had individuals who were less impaired than studies in which the MEP was assessed at the hand. This finding cannot be directly interpreted as evidence of a relationship between MEP presence, recording location, and impairment, as no studies directly compared MEP presence at the forearm and hand location within the same individuals. It is possible, for instance, that this result reflects a difference in MEP recording sites adopted in the primary studies rather than a relationship between MEP response and impairment itself. This highlights a gap that should be addressed by future research – what is the optimal location for MEP data collection in individuals with severe impairment? We can hypothesise that the forearm may be ideal based on cortical representation, as isolated muscles of the hand have a larger and more topographically defined cortical representation, whereas muscles of the forearm have a smaller, more diffuse cortical representation compared to the forearm (Hlustik et al., 2001). Alternatively, it may reflect natural recovery and return of movement after stroke, which has been found to be more likely for gross movements (e.g., wrist extension) as compared to fine movements (e.g., isolated finger extension) in people with severe impairment (Barker et al., 2008).
Taken together, although MEP presence appears to be a good predictor of impairment status at the group level, there is considerable individual variation within those groups that needs to be explained (which can be seen in Fig. 2 and the standard errors for the effect of MEP in Table 8).
4.2. Lack of relationship between other brain biomarkers and motor outcome
Surprisingly, there was no relationship between arm impairment and stroke lesion size, location and hemisphere, and corticospinal tract integrity as measured by diffusion weighted imaging. This may be explained by several factors. First, a large factor influencing these null findings might be range restriction in the variables investigated e.g., FM-UL (≤ 30/66), coding of the lesion location (i.e., cortical, cortical + subcortical, subcortical) and white matter integrity (i.e., asymmetry index for the corticospinal tract usually ascribed as > 0.15–0.25). Range restriction creates a problem because the statistical strength of the relationship (e.g., between Fugl Meyer and lesion volume) might be large when we consider the full spectrum of impairment, yet, when we constrain ourselves to the most severely impaired individuals, this relationship might be much smaller or non-existent. While this may indicate a limitation in the way we defined our range, it also suggests that these biomarkers may not be ideal when attempting to differentiate the most severely impaired individuals.
Second, it is possible that we have not identified the ideal clinical index that best captures the relationship between motor outcome and brain biomarkers. In this review, the majority of studies indexed an individual's motor impairment according to the FM-UL. While this measure has limitations (e.g., based on reflexive and normal patterns of movement), it is widely used in research to rate level of impairment and has strong psychometric properties (Duncan et al., 1983, Hseuh and Hseih, 2002, Platz et al., 2005). Yet, it is possible that an alternate measure of impairment that focuses solely on muscle strength (e.g., manual muscle test of Shoulder Abduction and Finger Extension, SAFE (Stinear et al., 2012)) or a measure of motor capacity (e.g., Action Research Arm Test (Nordin et al., 2014) or Wolf Motor Function Test (Wolf et al., 2001)) may be better to use when relating to brain biomarkers.
Next, it is possible that we are not exploring the ideal regions of interest in the severely damaged brain. For example, the lack of a relationship between corticospinal tract asymmetry index and motor outcome might appear contradictory to previous work (Feng et al., 2015, Stinear et al., 2012). However, past studies had mixed samples that were dominated by individuals with mild to moderate arm impairment after stroke (Mang et al., 2015, Petoe et al., 2014). Our analyses suggest that for those with severe arm impairment the integrity of the corticospinal tract as measured by diffusion imaging (i.e., asymmetry index) is not reliably related to motor outcome. This may be due to significant damage and subsequent lack of signal that can be derived from this tract when investigating a group of individuals with severe impairment. The lack of significant brain biomarkers derived from neuroimaging in the current literature provides the impetus to explore alternative areas of the brain that may generate a more sensitive biomarker/s.
Our data suggest that the field may not be considering the most informative neuroimaging modalities in people with severe arm impairment. Limited studies were identified that explored resting- or task-based fMRI in people with severe impairment, and satisfied our inclusion criteria. Given the inherent challenge for people with little movement to perform tasks during fMRI it was reasonable to expect that we would identify few studies. It was surprising that no studies included rsfMRI, as a benefit of this imaging technique is people with no to little movement can undergo the imaging protocol. As this modality is relatively new and used less often in the field, its inclusion in few studies may reflect this issue. Alternatively, our inclusion criteria may have influenced inclusion of rsfMRI studies, with predominantly mixed samples (mild through to severe) ineligible. This does highlight an area for future work, as it will be important to determine if rsfMRI is a biomarker of motor outcome and recovery in this cohort.
Finally, given the complexity of relationships between biomarkers, it may be that a multimodal approach is required that combines several biomarkers together e.g., combining lesion size, location or motor evoked potential response with damage to the corticospinal tract (Feng et al., 2015, Stinear et al., 2012). However, a lack of common data elements and methods adopted across studies made such a multimodal assessment impossible in this review.
4.3. Describing the sample of people with severe arm impairment
The majority of individuals (52%) whose data were included in this review were over 6-months post stroke, while 8% were between 1- and 6-months post stroke and only one study performed longitudinal assessments. This is concerning as it appears we know very little about recovery trajectories after severe stroke during the time frame that is considered optimal for spontaneous (Byblow et al., 2015, Prabhakaran et al., 2008, Winters et al., 2015) and functional recovery (Houwink et al., 2013, Kwakkel et al., 2003). Given that people with severe arm impairment often do not follow the spontaneous biological recovery model (Prabhakaran et al., 2008), more research is required in the first 6 months after stroke to understand the neurobiology of recovery. Recent work suggested that MEP presence identifies those who will follow the spontaneous biological recovery model (Byblow et al., 2015). Given that this work has predominantly focused on individuals with mild to moderate impairment, it remains to be determined if MEP presence is sufficient, or if other biomarkers interplay, to explain why the severe cohort do not closely follow the model of spontaneous biological recovery. Individual differences in severely impaired individuals in the present data suggest that although MEP presence/absence is very informative, there is considerable variation left to be explained.
4.4. Strengths and limitations
The present pooled review of individual patient data captured a dataset of individuals with severe arm impairment after stroke and undertook an individual patient data analysis process (e.g., nested study statistics, looked for outlier studies using Cook's test, investigated the fit of the model using Wald Test). This allowed us to provide reliable reports of the available evidence and enabled extraction and analysis of data from individuals with severe impairment in isolation from other individuals who had mild to moderate impairments. Furthermore, pooling of individual data enabled an investigation beyond the mean of a single study. Previous studies have demonstrated that pooled individual data can depict a different story to aggregate group data (Jones et al., 2009). In the current review, it was critical to pool individual data from multiple studies, effectively creating a larger sample size with statistical power to explore biomarkers in people with severe arm impairment. Other strengths of the individual data pooled in this review were consistent reporting of potential confounders that enabled us to control for the effect of age, sex, and days post stroke; a common definition for categorising severe impairment (i.e., coding and cut-points) using raw scores; and representation from data collected in US, Canada, New Zealand and European countries. Taken together, the current meta-analysis represents an important step forward in the field of neurorehabilitation research that can inform future studies using individual participant data.
While we pooled a large amount of individual data on an under-researched population of individuals, there are some limitations. Firstly, for some biomarkers our sample size was limited e.g., corticospinal tract asymmetry. A larger sample in an isolated cohort of people with severe upper limb impairment will be necessary to confirm our finding. We could not control for deficits in cognition and sensation, as these were generally not reported in the studies we reviewed; yet, it is plausible that both factors influence motor outcome. Secondly, we were limited in our ability to investigate the contribution of multiple biomarkers to the assessment of motor outcome and recovery. As we recognise that no isolated biomarker is likely to explain all the variance in motor outcome, there is a need to build a larger data set around this question in people with severe impairment. This would allow investigation of more complex, multi-variable relationships. Similarly, we could not perform analyses for hand dominance or motor function due the limited data available. These are important future lines of research to investigate convergence of impairment findings and highlight the need to identify a common data element for functional outcomes in future studies. We could not obtain individual data from all eligible studies, as some people did not return individual data, which may have influenced our findings. Finally, there were some biomarkers that have a theoretically sound rationale for use in people with severe impairment such as rsfMRI, but no studies were found. This reflects a gap between potential benefit and research uptake.
5. Conclusion
Taken together the findings of this review suggest that future work needs to include brain biomarkers to enable us to build an understanding of the neurobiology of recovery after severe stroke. Based on the evidence included in this pooled review of individual patient data, it appeared that a MEP at rest is the only current biomarker that is associated with outcome in individuals with severe arm impairment following stroke. Review of existing data suggests that data from the forearm location has the strongest relationship with motor outcomes in this cohort. Yet, given that few biomarkers emerged, our review also shows that we need to move beyond currently known biomarkers and look to identify new indices that have sufficient variability and sensitivity to guide recovery models in people with severe impairment.
Conflict of interest
None.
Ethical approval
NA.
Acknowledgements
KSH was supported by the Heart and Stroke Foundation of Canada, Michael Smith Foundation for Health Research (MSFHR) British Columbia Canada, and the National Health and Medical Research Council (NHMRC) of Australia (1088449); SP was supported by the Canadian Institutes of Health Research; JB was supported by the NHMRC of Australia (1058635); NAL was supported by the NHMRC of Australia (1112158); LAB was supported by the Canada Research Chairs (CI-SCH-01796) and MSFHR British Columbia Canada.
Research affiliates (KSH, JB) of the Florey Institute of Neuroscience and Mental Health acknowledge the strong support from the Victorian Government, and in particular funding from the Operational Infrastructure Support Grant.
Authors acknowledge assistance with data coding from Ms. Katie P Wadden (KW), Graduate Research Assistant, University of British Columbia.
This study was completed under funding received from the Jakeway Foundation (KSH and LB). The Jakeway Foundation had no role in writing or decision to submit the publication. LB (corresponding author) had full access to all data and takes full responsibility for the decision to submit for publication.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.09.015.
Appendix A. Supplementary data
PROSPERO Registration.
Search strategy details.
References
- Barker R.N., Brauer S.G. Upper limb recovery after stroke: The stroke survivors' perspective. Disabil. Rehabil. 2005;27:1213–1223. doi: 10.1080/09638280500075717. [DOI] [PubMed] [Google Scholar]
- Barker R.N., Brauer S.G., Carson R.G. Training of reaching in stroke survivors with severe and chronic upper limb paresis: A randomised clinical trial. Stroke. 2008;39:1800–1807. doi: 10.1161/STROKEAHA.107.498485. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Bernhardt J., Borschmann K., Boyd L.A., Carmichael S.T., Corbett D., Cramer S.C., Hoffman T., Kwakkel G., Savitz S., Saposnik G., Walker M., Ward N.S. Moving rehabilitation research forward: Developing consensus statements for rehabilitation and recovery research. Int. J. Stroke. 2016 doi: 10.1177/1747493016643851. (in press) [DOI] [PubMed] [Google Scholar]
- Burke E., Cramer S.C. Biomarkers and predictors of restorative therapy effects after stroke. Curr. Neurol. Neurosci. Rep. 2013;13:329–339. doi: 10.1007/s11910-012-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byblow W.D., Stinear C.M., Barber P.A., Petoe M.A., Ackerley S.J. Proportional recovery after stroke depends on corticomotor integrity. Ann. Neurol. 2015;78:848–859. doi: 10.1002/ana.24472. [DOI] [PubMed] [Google Scholar]
- Byrnes M.L., Thickbroom G.W., Phillips B.A., Wilson S.A., Mastaglia F.L. Physiological studies of the corticomotor projection to the hand after subcortical stroke. Clin. Neurophysiol. 1999;110:487–498. doi: 10.1016/s1388-2457(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Campbell Stewart J., Cramer S.C. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44:1111–1116. doi: 10.1161/STROKEAHA.111.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins J.P.T., Green S., editors. Version 5.1.0. 2011. [Google Scholar]
- Duncan P.W., Propst M., Nelson S.G. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys. Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- Edwards J.D., Koehoorn M., Boyd L.A., Levy A.R. Is health-related quality of life improving after stroke? A comparison of health utilities indices among Canadians with stroke between 1996 and 2005. Stroke. 2010;41:996–1000. doi: 10.1161/STROKEAHA.109.576678. [DOI] [PubMed] [Google Scholar]
- Feng W., Wang J., Chhatbar P.Y., Doughty C., Landsittel D., Lioutas V.-A., Kautz S., Schaug G. Corticospinal tract lesion load - A potential imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015;78:860–870. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward K.S., Brauer S.G. Dose of arm activity training during acute and subacute rehabilitation post stroke: A systematic review of the literature. Clin. Rehabil. 2015;29:1234–1243. doi: 10.1177/0269215514565395. [DOI] [PubMed] [Google Scholar]
- Hayward K.S., Kuys S.S., Barker R.N., Brauer S.G. Can stroke survivors with severe upper arm disability achieve a clinically important change in arm function during inpatient rehabilitation? A multicentre, prospective, observational study. NeuroRehabilitation. 2014;35:773–779. doi: 10.3233/NRE-141096. [DOI] [PubMed] [Google Scholar]
- Hlustik P., Solodkin A., Gullapalli R.P., Noll D.C., Small S.L. Somatotopy in human primary motor and somatosensory hand representations revisited. Cereb. Cortex. 2001;11:312–321. doi: 10.1093/cercor/11.4.312. [DOI] [PubMed] [Google Scholar]
- Hodics T., Cohen L.G., Cramer S.C. Functional imaging of intervention effects in stroke motor rehabilitation. Arch. Phys. Med. Rehabil. 2006;87:36–42. doi: 10.1016/j.apmr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Houwink A., Nijland R., Guerts A.C., Kwakkel G. Functional recovery of the paretic upper limb after stroke: Who regains hand capacity? Arch. Phys. Med. Rehabil. 2013;94:839–844. doi: 10.1016/j.apmr.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Hseuh I.P., Hseih C.L. Responsiveness of two upper extremity function instruments for stroke inpatients receiving rehabilitation. Clin. Rehabil. 2002;16:617–624. doi: 10.1191/0269215502cr530oa. [DOI] [PubMed] [Google Scholar]
- Jang S.H., Kim Y.-H., Chang Y., Han B.S., Byun W.M., Chang C.H. The predictive value of cortical activation by passive movement for motor recovery in stroke patients. Restor. Neurol. Neurosci. 2004;22:59–63. [PubMed] [Google Scholar]
- Jones A.P., Riley R.D., Williamson P.R., Anne Whitehead A. Meta-analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clin. Trials. 2009;6:16–27. doi: 10.1177/1740774508100984. [DOI] [PubMed] [Google Scholar]
- Krakauer J.W., Hillis A.E. The future of stroke treatment: bringing evaluation of behavior back to stroke neurology. JAMA Neurol. 2014;71:1473–1474. doi: 10.1001/jamaneurol.2014.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger H., Koot J., Hall R.E., O'Callaghan C., Bayley M., Corbett D. Prevalence of individuals experiencing the effects of stroke in Canada: Trends and projections. Stroke. 2015;46:2226–2231. doi: 10.1161/STROKEAHA.115.009616. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A., Bruun Brockhoff P., Bojesen Christensen R.H. lmerTest: Tests in Linear Mixed Effects Models. R package version 2.0-29. 2015. https://CRAN.R-project.org/package=lmerTest
- Kwakkel G., van Dijk G.M., Wagenaar R.C. Accuracy of physical and occupational therapists' early predictions of recovery after severe middle cerebral artery stroke. Clin. Rehabil. 2000;14:28–41. doi: 10.1191/026921500675130139. [DOI] [PubMed] [Google Scholar]
- Kwakkel G., Kollen B.J., Van der Grond J., Prevo A.J. Probability of regaining dexterity in the flaccid upper limb: The impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- Long J.D. Sage Publishing; 2012. Longitudinal data analysis for the behavioural sciences using R. [Google Scholar]
- Mang C.S., Borich M.R., Brodie S.M., Brown K.E., Snow N.J., Wadden K.P., Boyd L.A. Diffusion imaging and transcranial magnetic stimulation assessment of transcallosal pathways in chronic stroke. Clin. Neurophysiol. 2015;10:1951–1971. doi: 10.1016/j.clinph.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis R., te Grotenhuis M., Pelzer B. influence.ME: Tools for detecting influential data in mixed effect models. R J. 2012;4:38–47. [Google Scholar]
- Nordin A., Alt Murphy M., Danielsson A. Intra-rater and inter-rater reliability at the item level of the Action Research Arm Test for patients with stroke. J. Rehabil. Med. 2014;46:738–745. doi: 10.2340/16501977-1831. [DOI] [PubMed] [Google Scholar]
- Petoe M.A., Byblow W.D., de Vries E.J., Krishnamurthy V., Zhong C.S., Barber P.A., Stinear C.M. A template-based procedure for determining white matter integrity in the internal capsule early after stroke. Neuroimage Clin. 2014;4:695–700. doi: 10.1016/j.nicl.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi A., Carrai R., Falsini C., Martini M., Verdesca S., Grippo A. Prognostic value of motor evoked potentials in motor function recovery of upper limb after stroke. J. Rehabil. Med. 2009;41:654–660. doi: 10.2340/16501977-0389. [DOI] [PubMed] [Google Scholar]
- Platz T., Pinkowski C., van Wijck F., Kim I.H., di Bella P., Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin. Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- Prabhakaran S., Zarahn E., Riley C., Speizer A., Chong J.Y., Lazar R.M., Marshall R.S., Krakauer J.W. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil. Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- Rehme A.K., Eickhoff S.B., Rottschy C., Fink G.R., Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59:2771–2782. doi: 10.1016/j.neuroimage.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Stinear C.M., Barber P.A., Petoe M., Anwar S., Byblow W.D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;135:2527–2535. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- Turton A., Wroe S., Trepte N., Fraser C., Lemon R.N. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr. Clin. Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Van der Meer T., te Grotenhuis M., Pelzer B. Influential cases in multilevel modelling. A methodological comment. Am. Sociol. Rev. 2010;75:173–178. [Google Scholar]
- van Kuijk A.A., Pasman J.W., Hendricks H.T., Zwarts M.J., Geurts A.C.H. Predicting hand motor recovery in severe stroke: the role of motor evoked potentials in relation to early clinical assessment. Neurorehabil. Neural Repair. 2009;23:45–51. doi: 10.1177/1545968308317578. [DOI] [PubMed] [Google Scholar]
- Ward N.S. Does neuroimaging help to deliver better recovery of movement after stroke? Curr. Opin. Neurol. 2015;28:323–329. doi: 10.1097/WCO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- Wickham H., Francois R. dplyr: A Grammar of Data Manipulation. R package version 0.4.3. 2015. https://CRAN.R-project.org/package=dplyr
- Winters C., van Wegen E.E., Daffertshofer A., Kwakkel G. Generalizability of the Proportional Recovery Model for the Upper Extremity After an Ischemic Stroke. Neurorehabil. Neural Repair. 2015;29:614–622. doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]
- Wolf S.L., Catlin P.A., Ellis M., Archer A.L., Morgan B., Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PROSPERO Registration.
Search strategy details.