Abstract
Medicinal plants have been a main source of therapeutic agents from ancient time to cure diseases. Terminalia arjuna (Roxb.) Wight & Arn. (T. arjuna) is one of the most accepted and beneficial medicinal plants in indigenous system of medicine for the treatment of various critical diseases. This comprehensive review provides various aspects of its ethnomedical, phytochemical, pharmacognostical, pharmacological and clinical significance to different diseases particularly in cardiovascular conditions. This plant has a good safety outline when used in combination with other conventional drugs. This review highlights various medicinal properties of T. arjuna through different studies such as antioxidant, hypotensive, anti-atherogenic, anti-inflammatory, anti-carcinogenic, anti-mutagenic and gastro-productive effect.
Keywords: Terminalia arjuna, Medicinal property, Phytochemistry, Coronary artery disease, Triterpenoids
Graphical abstract
1. Introduction
Medicinal plants play an essential role in health care and are the major raw materials for both traditional and conventional medicine preparations; still most of the people choose herbal medicines than conventional medicines.1 They expanded attention due to their effectiveness, lack of current medical alternatives, increasing cost of modern medicines and cultural preferences.2, 3 Ethnobotanical studies are most important to expose the ancient times and current culture about plants in the world and reserving original knowledge of medicinal plants. The quantitative ethnobotanical studies were used to identify the plant uses as food,4 human health care medicines,5 veterinary medicine6 and economically important.7
Around the world, the traditional knowledge system has expanded chief importance in perspective with protection, sustainable growth and search for new utilization patterns of plant resources. Traditional medicine system includes the knowledge, skills and practices based on the presumptions, beliefs and experiences of folk communities to protect their health problems. Traditional herbal medicines are considered to be of huge importance among different rural or native communities in many developing countries.8 According to WHO, almost 80% of the world's population depending on traditional medicine and in India 60% of the people in rural areas use herbal medicines.1 During the last few years, use of herbal supplements increased from 2.5% to 12%.9 In recent years, there has also been an increasing demand for nanoparticles derived from medicinal plants like Terminalia family due to their applications in various fields of research like medicine, catalysis, energy and materials.10, 11, 12
In the earliest India, medicinal plants were used to prevent different critical diseases and they would be the best source to obtain a variety of drugs. The Indian traditional medicine is based on various systems such as Ayurveda, Siddha, Unanai, etc. In recent years there has been an increasing awareness about the importance of medicinal plants. Herbal drugs are easily accessible, secure, less pricey, efficient and have very rare side effects. The evaluation of new drugs, especially the phytochemical obtained materials has opened a vast area for research and helpful in making a transition from traditional to modern medicine in India. Medicinal plants contain some organic compounds which provide definite physiological action on the human body and these bioactive substances include tannins, alkaloids, carbohydrates, terpenoids, steroids, flavonoids, and phenols.13
Even though numerous medicinal plants have been explained in the Indian customary therapeutic system for treatment of several diseases, very few plant products are nowadays utilized in the modern medical system to treat most of the diseases, particularly; cardiovascular diseases (CVD), ulcers, diabetes, cough, excessive perspiration, asthma, tumor, inflammation and skin disorders. Among the plants, one of the medicinal plants indigenous to India is Terminalia arjuna (Roxb.) Wight and Arn., (T. arjuna) commonly known as ‘Arjuna’, which has been used as a cardiotonic in heart failure, ischemic, cardiomyopathy, atherosclerosis, myocardium necrosis and has been used for the treatment of different human diseases like blood diseases, anemia, venereal and viral disease; and to continue excellent healthiness. It is used in the treatment of fractures, ulcers, hepatic and showed hypocholesterolemic, antibacterial, antimicrobial, antitumoral, antioxidant, antiallergic and antifeedant, antifertility and anti-HIV activities.14, 15, 16 T. arjuna is reported that to possess strong hydrolipidemic properties. It is trusted that the saponin glycosides in T. arjuna may be responsible for its inotropic effects, while the flavonoids/phenolics may supply antioxidant activity as well as vascular amplification activity, in this manner authenticating the multiple activities of this plant for its cardio-protective function.17, 18, 19 The aim of this review is to summarize the information and knowledge about the T. arjuna and updating available research data on the aspects of botany, ethnopharmacology, phytochemistry and clinical studies.
2. Methods
Systematic literature searches were carried out and the available information on various plants traditionally used for cardiovascular disorders was collected via electronic search (using Pubmed, SciFinder, Scopus, Scirus, ScienceDirect, Google Scholar and Web of Science) and a library search for articles published in peer-reviewed journals and also locally available books.
3. Occurrences, botanical description and ethnopharmacology
T. arjuna is an ayurvedic plant with important medicinal value. It is commonly known as Arjuna, Indradru, Partha and Veeravriksha20 which is belongs to Combretaceae family comprising of nearly 200 species distributed around the world. Nearly 24 species of Terminalia have been reported from various parts of India, some selected species are T. arjuna, Terminalia bellirica, Terminalia bialata, Terminalia catappa, Terminalia elliptica, Terminalia porphyrocarpa, Terminalia mantaly etc. In India, T. arjuna is about 60–80 feet in height, buttressed trunk and horizontally spreading crown and drooping branches distributed in India, Burma, Mauritius and Sri Lanka.19, 21, 22 T. arjuna is distributed throughout sub Indo-Himalayan tracts of Uttar Pradesh, Punjab, Deccan, South Bihar, Orissa, West Bengal and Madhya Pradesh mainly along riverside, rivulets and ponds. It is known by its various vernacular names, the most commonly used ones are Arjuna (Common Name), Arjun (Hindi), Marudhu (Tamil and Malayalam), Tella Maddi (Telugu), Arjhan (Bengali), Sadaru (Marathi), Sadado (Gujarati), Neer matti (Kannada) and some traditional formulations prescribe in the name of Arjunarishta and Arjunaghrita.
Leaves of T. arjuna are simple, often crenulations, borne sub-opposite, shortly acute or obtuse at the apex, coriaceous and oblong or elliptic. Their upper face is pale or dark green and the lower face is pale brown. The tree bears white sessile bisexual flowers in short auxiliary spikes or in a terminal panicle arrangement. Fruits of T. arjuna are drupe, ovoid, fibrous-woody and smooth-skinned with five hard wings or angles which are oblique and curved upwards. Stem bark is simple, smooth and pinkish-gray in color in external view. An internal view, the bark is soft and reddish in color.23
4. Phytochemistry
The major constituents of T. arjuna in stem bark, root bark, fruits, leaves and seeds are well characterized (Table 1). The preliminary phytochemical analysis of existing compounds in T. arjuna was carried out according to various standard protocols as mentioned by Harbone54 in Table 2. As bark was considered to be the most important constituent from the medicinal point of view, initially reported that the bark had 34% ash content consisting entirely of pure calcium carbonate. Aqueous extract of T. arjuna is reported to have 23% calcium salts and 16% tannins. Organic extracts of T. arjuna bark were also prepared using the sequential methods with a number of organic solvents such as hexane, benzene, chloroform, acetone, dichloromethane, ethyl acetate, butanol, ethanol, methanol and ether, etc., to extract various phytochemical constituents. The chemical structures of available compounds were confirmed by various advanced techniques like HPLC, UPLC, LC-ESI-MS/MS analysis.26, 27, 55, 56 Polyphenols, flavonoids, tannins, triterpenoids, saponins, sterols and minerals are the major constituents of T. arjuna. Such amino acids like tryptophan, tyrosine, histidine and cysteine are also the main ingredients in T. arjuna.24, 29, 31, 57
Table 1.
Phytochemical constituents of various parts of Terminalia arjuna (Roxb.) Wight and Arn.
| Part used | Major chemical constituents | References |
|---|---|---|
| Stem bark | Triterpenoids | |
| Arjunin | Row et al24 | |
| Arjunic acid | ||
| Arjungenin | Honda et al25 Singh et al26, 27 |
|
| Terminic acid | Anjaneyulu and Prasad28 | |
| Terminoltin | Singh et al29 | |
| Arjunolic acid | Singh et al26, 27 | |
| Ursane triterpenoids | ||
| 2α,3β-dihydroyurs-12,18-oic acid 28-O-β-d-glucopyranosyl ester 2α,3β,23-trihydroxyurs-12,18-dien-28-oic acid 28-O-β-glucopyranosyl ester Qudranoside VIII Kajiichigoside F1 2α,3β,23-trihydroxyurs-23-trihydroxyurs-12,19-dien-28-oic acid 28-O-β-d-glucopyranosyl ester |
Wang et al30 | |
| Glycosides | ||
| Arjunetin | Row et al24, 31 Singh et al26, 27 |
|
| Arjunoside I, II | Honda et al25, 32 | |
| Arjunolone | Sharma et al33 | |
| Arjunolitin | Tripathi et al34 | |
| Arjunaphthanoloside | Ali et al35, 36 | |
| Arjunglucoside IV and V, Arjunasides A-E | Wang et al34, 37 | |
| Olean-3β, 22β-diol-12-en-28 β-D-glucopyranosie-oic acid | Patnaik et al38 | |
| Terminarjunoside I and II | Alam et al39 | |
| Terminoside A | Ahmad et al40 | |
| Termionic acid | ||
| Flavonoids and phenolics | ||
| Arjunone | Sharma et al33 | |
| Luteolin | Pettit et al41 | |
| Baicalein | Anonymous42 | |
| Ethyl gallate | ||
| Gallic acid | ||
| Kempferol | ||
| Oligomeric proanthocyanidins | ||
| Pelargonidin | ||
| Quercetin | ||
| (+)-catechin, (+)-gallocatechin and (−)-epigallocatechin | Saha and Pawar43 | |
| Gallic acid, ellagic acid and its derivatives such as 3-O-methyl-ellagic acid 4-O-β-d-xylopyranoside, 3-O-methyl ellagic acid 3-O-rhamnoside | ||
| 3-O-methyl ellagic acid 4′-O-α-l-rhamnophranoside (−)-epicatechin |
Wang et al30 | |
| Tannins | ||
| Pyrocatechols | Takahashi et al44 | |
| Punicallin | Lin et al45 | |
| Castalagin | Kuo et al46 | |
| Casuariin | ||
| Casuarinin | ||
| Punicalagin | ||
| Terchebulin | ||
| Terflavin C | ||
| Minerals and trace elements | ||
| Calcium, magnesium, aluminum, zinc, copper, silica | Dwivedi and Udupa47 | |
| Other compounds | ||
| β-Sitosterol | Anjaneyulu and Prasad28 | |
| Roots | Triterpenoids | |
| Arjunoside I-IV | Anjaneyulu and Prasad48, 49 | |
| Arjunolic acid | Anjaneyulu and Prasad28 | |
| Oleanolic acid | ||
| Terminic acid | ||
| 2α,19α-Dihydroxy-3Oxo-Olean-12-En28-Olic acid 28-O-β-d-glucopyranoside | Choubey and Srivastava50 | |
| Arjunic acid | Singh et al26, 27 | |
| Glycosides | ||
| Arjunetosie (3-O-β-d-glucopyranosyl-2α, 3β, 19α-trihydroxyolean-12-en-28-oic acid 28-O-β-d-glucopyranoside) | Upadhyay et al51 | |
| Fruits | Triterpenoids and flavonoids | |
| Arjunic acid, Arjunone, Arachidic stearate, Cerasidin, Ellagic acid, Fridelin, Gallic acid, Hentriacontane, Methyl oleaolate, Myristyl oleate, β-Sitisterol | Rastogi and Mehrotra52 | |
| Leaves and seeds | Flavonoids and glycosides | |
| Luteolin, 14,16-dianhydrogitoxigenin 3-β-d-xylopyranosyl-(1 > 2)-O-β-d-galactopyranoside | Pettit et al41 Yadava and Rathore53 |
Table 2.
Preliminary tests for phytochemical analysis of the Terminalia arjuna (Roxb.) Wight and Arn. extract.
| Phytoconstituents | Test |
|---|---|
| Alkaloides | Dragendroff's test |
| Carbohydrates | Molisch's test |
| Flavonoids | Lead acetate test |
| Glycosides | Keller–Killiant test |
| Lactones | Legal's test |
| Phenolic compounds and tannins | 5% FeCl3 test |
| Proteins | Ninhydrin test |
| Phytosterols | Salkowski's test |
| Saponins | Foam test |
| Triterpenoids | Liebermann–Burchard's test |
4.1. Terpenoids, ursane triterpenoids and glycosides
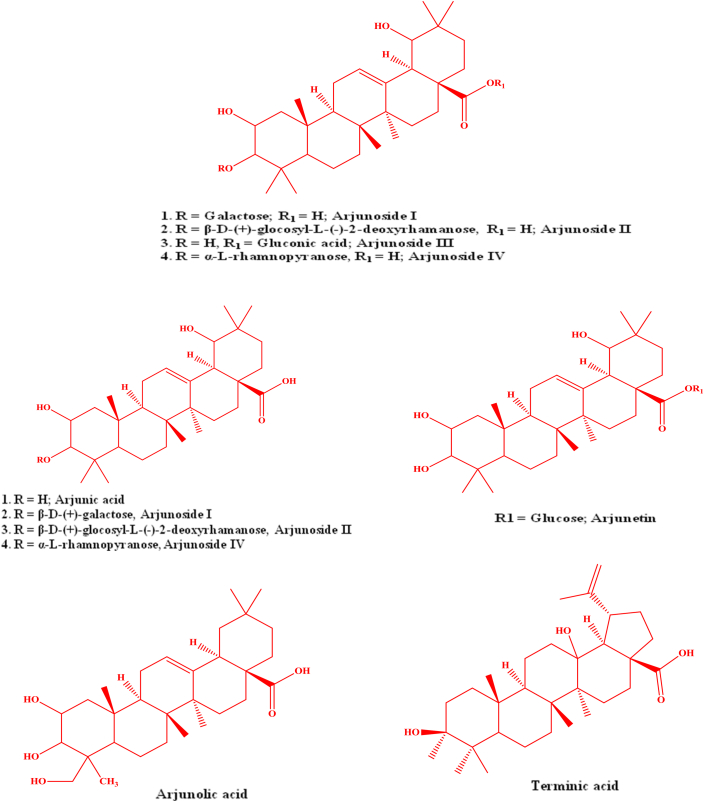
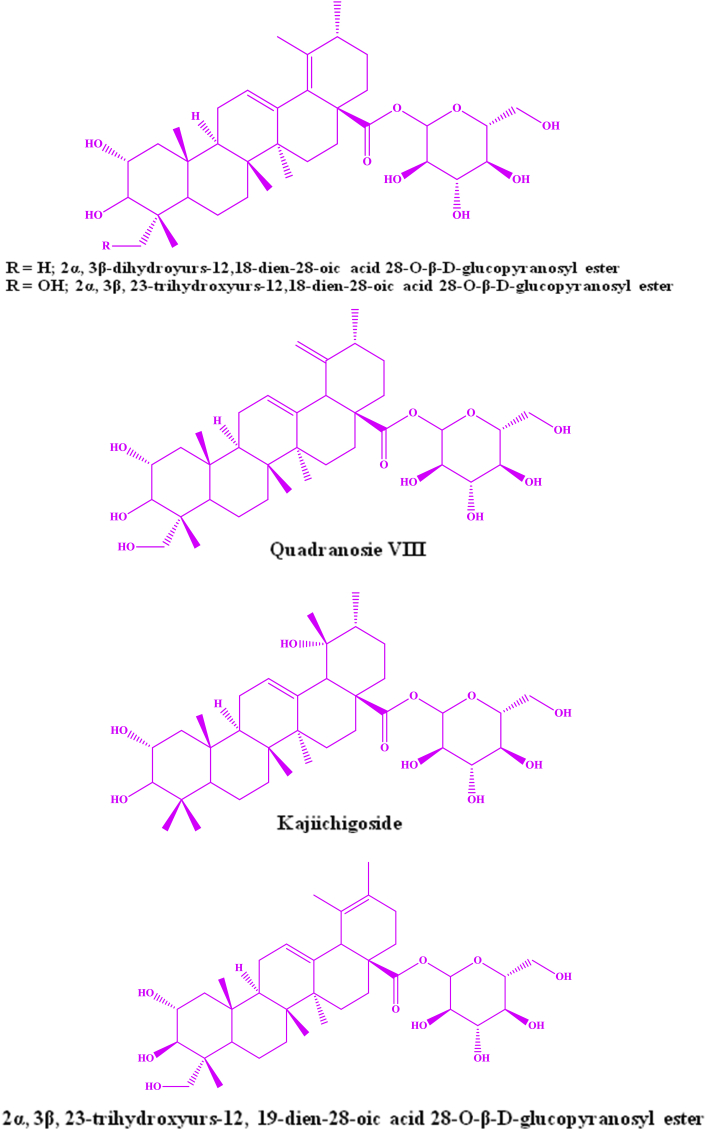
At first an oleanane triterpenoid named, arjunin, and a lactone, arjunetin were isolated from the benzene and ethanolic extracts of its bark respectively (Fig. 1). Honda et al25, 32 initially confirmed that the presence of arjunic acid and arjungenin and latterly reported that two more glucosides namely arjunglucoside I and II (Fig. 1) in the stem bark of T. arjuna. Anjaneyulu and Prasad48, 49 confirmed that the presence of arjunoside III and IV, terminic acid, and a triterpene carboxylic acid by ethyl acetate extraction of roots of T. arjuna (Fig. 1). Hexane extraction of stem of T. arjuna authenticated that the presence of terminic acid and β-sitosterol.28 Ali et al36 has isolated another oleanane type triterpane, terminoside A from the acetone fraction of the ethanolic extract of T. arjuna's stem bark. The structure of this new compound was established as olean-1α,3β,22β-triol-12-en-28-oic acid-3β-d-glucopyranoside. It was exhibited that terminoside A inhibits nitric oxide production and decreases inducible nitric oxide synthase levels in lipopolysaccharide stimulate macrophages.35, 36 Five ursane type triterpene glucosyl ester including new one, 2α, 3β-dihydroyurs-12,18-dien-28-oic acid 28-O-β-d-glucopyranosyl ester, and four known ursane triterpene glycosyl esters namely, 2α, 3β, 23-trihydroxyurs-12,18-dien-28-oic acid 28-O-β-d-glucopyranosyl ester, quadranosie VIII, kajiichigoside and 2α, 3β, 23-trihydroxyurs-12, 19-dien-28-oic acid 28-O-β-d-glucopyranosyl ester were isolated from bark of T. arjuna (Fig. 2).30 3-O-β-d-glucopyranosyl-2α, 3β, 19α-trihydroxyolean-12-en-28-oic acid, 28-O-β-d-glucopyranoside and 2α, 19α-dihydroxy-3-oxo-olean-12-en28-oic acid 28-O-β-D-gluco-pyranoside are isolated from bark of T. arjuna by Choubey and Srivastava50 and Upadhyay et al51 through spectrochemical analysis. Patnaik et al38 using chromatography technique isolated a triterpenoid glycoside from the bark of T. arjuna and identified it is an olean-3β, 22β-diol-12-en-28 β-d-glucopyranoside-oic acid. Alam et al39 were isolated two more glycosides namely Termiarjunoside I (olean-1α,3β,9α,22α-tetraol-12-en-28-oicacid-3β-d-glucopyranoside) and Termiarjunoside II (Olean-3α,5α, 25-triol-12-en-23,28-dioicacid-3β-d-glucophyranoside) from the ethanolic extract of TA bark. Arjunglucoside IV and V, Arjunasides A-E were isolated from the ethanolic extract of the stem bark of T. arjuna by Wang et al.30, 58
Fig. 1.
Structure of important terpenoids and glycosides isolated from Terminalia arjuna.
Fig. 2.
Structure of ursane triterpenoids isolated from Terminalia arjuna.
4.2. Flavonoids and phenolics
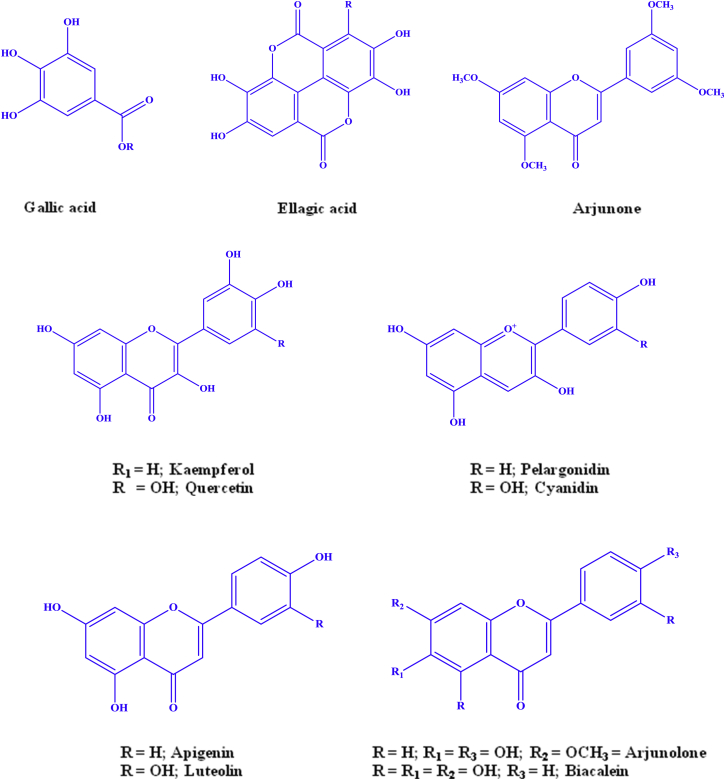
Bark of T. arjuna contains a very high level of flavonoids, namely arjunolone, flavones, luteolin, baicaleiin, quercetin, kempferol, and pelargonidin evaluated with other medicinal plants particularly having favorable effects on cardiovascular diseases. The compound luteolin has been isolated from the butanolic fraction of T. arjuna and it has been found to be antimutagenic and antibacterial activity. It inhibited gram negative pathogen growth with a minimum inhibitory concentration of 12.5 μg/disc. Aqueous extract of T. arjuna contains 70% polyphenols having a molecular weight greater than 3.5 kDa and they are confirmed by the HPLC and LC-MS. The aqueous extract contains flavon-3-ols, such as (+)-catechin, (+)-gallocatechin and (−)-epigallocatechin; gallic acid, ellagic acid and its derivatives such as 3-O-methyl ellagic acid 4-O-β-d-xylopyranoside and 3-O-methyl ellagic acid 3-O-rhamnoside (Fig. 3). Various studies support the fact that bioflavonoids inhibit LDL oxidation, endothelial activation and platelet aggregation.59, 60, 61, 62 Due to the presence of free radical scavenging action of the various phenolic contents in T. arjuna, it acts as strong anti-proliferative and anti-oxidant agent.63 There is an inversely relationship between the high intake of dietary flavonoids and the risk of coronary artery disease (CAD), so possible account for intake of high flavonoids content TA is beneficial effects in CAD.
Fig. 3.
Structure of important flavonoids isolated from Terminalia arjuna.
4.3. Tannins
Tannins are known to enhance the synthesis of nitric oxide and relax vascular segments pre-contracted with norepinephrine. In addition to a flavonoids variety of tannins have been isolated from the bark of T. arjuna. Around fifteen types of tannins and their related compounds were isolated from the bark of T. arjuna and their structures were elucidated with the help of spectral analysis. Hydrolyzable tannins are castalagin, casuariin, casuarinin, punicalagin, pyrocatechols, punicallin, terchebulin and terflavin C were isolated from the bark of T. arjuna.45 Tannins are considered to have wound healing, astringent, hypotensive, antioxidant and antimicrobial effects.64, 65
4.4. Minerals and amino acids
The bark of T. arjuna contains large amount of various minerals and trace elements such as magnesium (4000 μg/g), calcium (3133 μg/g), zinc (119 μg/g) and copper (19 μg/g).47 It contains some amino acids such as tryptophan, tyrosine, histidine and cysteine.26, 27, 57
5. Pharmacological and clinical studies
T. arjuna is a tree having an widespread medicinal potential in most of the diseases particularly cardiovascular disorders. Scientific investigations of T. arjuna extensively reported and discussed through various preclinical and clinical studies (Table 3, Table 4).
Table 3.
Pharmacological studies on Terminalia arjuna (Roxb.) Wight and Arn.
| Pharmacological activity | Model used and study design | Type of extract | Observations | References |
|---|---|---|---|---|
| Antioxidant, antiinflammatory, and immunomodulatory | CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes | Alcoholic and aqueous extract of T. arjuna at 35 μg/ml dose level | Alcoholic and aqueous extracts of T. arjuna showed significant inhibition activity of CYP3A4, CYP2D6 and CYP2C9 enzyme. Enzyme kinetic studies suggested that the extracts of T. arjuna showed rapidly reversible non-competitive inhibition of all three enzymes in human liver microsomes. |
Varghese et al66 |
| Antioxidant | Human polymorphonuclear (PMN) cells and hypochlorous acid from human neutrophils | Methanolic extract of T. arjuna | Arjungenin is the most active compound than others and had moderate inhibitory effect on the process of respiratory oxyburst and its IC50 value is shown 60 μg/ml. | Pawar and Bhutani67 |
| Antioxidant | Male Wistar albino rats (110–140 g) – (6–7 weeks old) | T. arjuna was administrated orally to Wistar rat at different doses (0.42 mg/kg to 6.8 mg/kg) for 6 days/week for 4 weeks | Chromic administration of butanolic fraction of alcoholic extract of T. arjuna bark has cardioprotective potential against Dox-induced cardiotoxicity. | Singh et al68 |
| Antioxidant and antimutagenic activity | Wistar rats (200–250 g) and Swiss albino mice (18–22 g) | Aqueous and ethanolic extraction of T. arjuna | The alcoholic extract of T. arjuna (ALTA) has shown potent antioxidant activity with EC50 of 2.491 ± 0.160, 50.110 ± 0.150 and 71.000 ± 0.025 in DPPH assay, superoxide radical scavenging activity and lipid peroxidation assay, respectively. In micronucleus test, EC50 of 2.410 ± 0.140, 40.500 ± 0.390 and 63.000 ± 0.360 in percentage of micronucleus in ALTA (100 and 200 mg/kg p.o) showed significant reduction in both polychromatic erythrocytes and normochromatic erythrocytes and also shown significant reduction in P/N ratio. |
Viswanatha et al69 |
| Anticarcinogenic and antimutagenic potential | In vitro and in vivo method | Aqueous extracts from 75 μg/ml to 200 μg/ml for lymphocyte culture for in vitro experiments Aqueous extracts from 50 mg/kg to 350 mg/kg body weight for in vivo experiments |
Used human lymphocyte culture and bone marrow cells of albino mice (8–10 weeks old and weight ranges between 25-35 g) The number of sister chromatid exchanges got reduced from a higher level of 15.0 ± 1.4 per cell to 7.7 ± 0.5 per cell with S9 mix at 48 h of treatment. The replication index was enhanced from 1.33 to 1.55 in vitro. In the in vivo experiments, effective reduction in clastogeny ranging from 15.22% to 54.82% from the mutagen treated positive control and the total frequencies in aberrant cells got reduced from 429 due to AFB1 to 141 due to 5th concentration of T. arjuna extracts at 32 h of exposure. |
Ahmad et al70 |
| Antioxidant, anti-inflammatory and immunomodulatory | Cell cultures of human monocytic (THP-1) and human aortic endothelial cells (HEACs) | T. arjuna alcoholic extract (TAAE) and T. arjuna Aqueous extract (TAWE) from steam bark at a dose of 1–50 μg/ml | TAAE and TAWE inhibited the lipid peroxidation and attenuated H2O2 mediated ROS generation in THP-1 cells by promoting catalase, glutathione peroxidase activities and by sustaining cellular reducing power. Marked effects of T. arjuna steam bark on cultured human monocytic and aortic endothelial cells provide the biochemical and molecular basis for therapeutic potential of T. arjuna steam bark against cardiovascular diseases (CVD). |
Kokkiripati et al56 |
| Antioxidant | Male albino Wistar rats (120–150 g body weight) were subjected to oxidative stress associated with in vitro ischemic reperfusion injury (IRI) | Two doses (500 and 750 mg/kg in 2% carboxy methyl cellulose (CMC)), 6 days per week for 12 weeks | T. arjuna augments endogenous antioxidant compounds of rat heart and also prevents oxidative stress associated with IRI of the heart. | Gauthaman et al71 |
| Antioxidant | Human neutrophils isolated from fresh, heparinized human blood by using Histoprep and suspended in HBSS medium containing gelatin. | Ethanolic extraction of T. arjuna containing arjunic acid, arjungenin, arjunetin and arjunglucoside II | Arjungenin and its glucoside extracted from T. arjuna and are exhibited a significant free radical scavenging activity on the superoxide release from PMN cells. Arjungenin exhibited great inhibitor action on the hypochlorous acid productin from human neutrophils. |
Pawar and Bhutani67 |
| Antioxidant | Male Wistar albino rats, weighing between 250 and 300 g; treated with STZ at a dose of 65 mg/kg | Therapeutic treatment through 50% ethanolic extract of T. arjuna at a dose of 500 mg/kg and rosuvastatin (20 mg/kg) for 30 days orally after 8 weeks of STZ treatment | T. arjuna bark extract improved cardiovascular autonomic neuropathy in rats having uncontrolled diabetes through maintaining endogenous antioxidant enzyme activities and decreasing cytokine levels. | Khaliq et al72 |
| Antioxidant | Male Swiss albino mice treated with NaF at a dose of 600 mg/L for 1 week. | Ethanolic extract of T. arjuna at a dose of 50 mg/kg of body weight and with vitamin C at a dose of 100 mg/kg body weight for 1 week | Ethanolic extract of T. arjuna protects murine hearts from NaF-induced oxidative stress via its antioxidant properties. | Sinha et al73 |
| Antioxidant | Wistar rats weight between 200-240 g. | Ethanolic extract of T. arjuna at a dose of 500 mg/kg for 15 days was administrated orally | Prophylactic and therapeutic treatment with T. arjuna improved cardiac functions and baroreflex sensitivity. It is attenuated hypertrophy and fibrosis of the LV. T. arjuna significantly reduced oxidative stress and inflammatory cytokine level in CHF rats |
Parveen et al74 |
| Antioxidant | Poloxamer (PX)-407 induced hyperlipidemic albino Wistar rats | Three fractions diethyl ether, ethyl acetate and ethanol of T. arjuna exerted hypolipidemic and antioxidative effects at two different doses levels (175 and 350 mg/kg body weight) | Hypolipidemic and antioxidant effects of T. arjuna fractions were noticed as ethanol > diethyl ether > ethyl acetate. Ethanolic fraction of T. arjuna possesses the potent properties of antioxidant and hypolipidemic than other fractions and has therapeutic potential for the prevention of coronary arterial disease. |
Subramaniam et al75 |
| Antioxidant | Male Wistar rats treated with isoprenaline to produce LVH | Aqueous extract of T. arjuna bark was evaluated at 63, 125 and 250 mg/kg given orally for antifibrotic and antioxidant effects in male Wistar rats given selective β-adrenoceptor agonist isoprenaline (5 mg/kg) for 28 days Captopril has given orally 50 mg/kg per day, an inhibitor of angiotensin-converting enzyme used as a standard cardioprotective drug |
Aqueous extract of T. arjuna significantly prevented isoprenaline-induced increase in oxidative stress and decline in endogenous antioxidant level and also prevented fibrosis. | Kumar et al76 |
| Antioxidant and antimicrobial activity | DPPH methods and Agar well diffusion method | Methanol extracts | Methanolic extracts has great free radical scavenging properties. It contains liberal amount of flavonoid compounds. It is exhibited good antimicrobial activity against two gram negative bacteria (E. coli and K pneumonia). |
Mandal et al77 |
| Antimicrobial activity | Five bacteria namely Staphylococcus aureus (Gram Positive) Acinetobacter sp., Proteus mirabilis, Escherichia coli and Pseudomonas aeruginosa (Gram negative) were used |
Methanol, ethanol, acetone aqueous extracts from the leaves and bark of T. arjuna | Acetone leaf extract was found to be best against S. aureus.Organic extract showed almost equal inhibition of all tested Gram negative bacteria except P. aeruginosa. Aqueous extract of T. arjuna bark exhibited good activity against S. aureus. |
Aneja et al78 |
| Antimicrobial activity | NZW albino rabbits subjected to 15 min coronary artery ligation followed by 60 min of reperfusion injury | Pretreatment of bark powder of 500–750 mg/kg/day for 12 weeks before ischemic-reperfusion injury | Chronic oral administration of the bark of T. arjuna in rabbit causes augmentation of myocardial endogenous antioxidants along with induction of HSP 72. It is offered further protection against oxidative stress associated with myocardial ischemic reperfusion injury. |
Gauthaman et al79 |
| Anticarcinogenic potential | Adult ventricular myocytes isolated from hearts of adult male Sprague-Dawley rats (250–300 g) | Ethanolic and aqueous extract of T. arjuna at a dose of 0.05–100 μg/ml | Aqueous extract of T. arjuna induced cardiotonic action via enhancing sarcoplasmic reticular function, an unique action minimizing the occurrence of arrhythmias, makes aqueous extract of T. arjuna a promising and relatively safe cardiotonic beneficial to the health heart and the treatment for chronic heart diseases. | Oberoi et al80 |
| DNA damage protecting and free radical scavenging | DNA stand breakage assay and comet assay analysis by using of pBR 322 plasmid and rat adrenal PC-12 cells | Ethanolic extracts and its fractions | Ethanolic extracts and its fractions of T. arjuna bark protected H2O2 induced DNA damage. Maximum inhibition of DPPH, hydroxyl, ABTS, nitric oxide radicals and metal chelation was observed in ethyl acetate fraction. T. arjuna extracts ameliorate various impairments associated with DNA damage and free radical formation. |
Phani Kumar et al16 |
| Gastro-productive effect | Diclofenac sodium (DIC) induced gastric ulcer in experimental rats (male albino rats of Wistar – (150–200 g weight) | Methanolic extract of T. arjuna | A significant reduction in lesion index was observed in ulcer induced animals treated with T. arjuna (DIC + TA) compared to ulcerated rats (DIC). A significant increase was observed in pH, NP-SH, GSH, enzymatic antioxidants, protein bound carbohydrate complexes, adherent mucus content, nucleic acid with a significant decrease in volume of gastric juice, free and total acidity, pepsin concentration, acid output, LPO levels and MPO activities in DIC + TA rats compared to DIC rats. |
Devi et al81 |
Table 4.
Clinical studies on Terminalia arjuna (Roxb.) Wight and Arn.
| Highlights of the study | Clinical conditions | Drug formulation and dosage | Clinical outcome | References |
|---|---|---|---|---|
| Idiopathic and ischemic cause | 93 patients with dilated cardiomyopathy (DCMP) of idiopathic and ischemic cause | T. arjuna capsules (500 mg at 8 hourly) | Patients with dilated cardiomyopathy with or without heart failure and reduced left ventricular ejection fraction due to either idiopathic or ischemic cause receiving combined standard therapy, and herbal medication showed significant improvement in systolic and diastolic functions as well as functional capacity in comparison to those receiving only standard therapy or only herbal medications | Bhawani et al82 |
| Heart failure | 12 patients with refractory chronic congestive heart failure | Aqueous extract from bark of T. arjuna was controlled 8 h at a dose of 500 mg | Adjuvant T. arjuna therapy in selected patients with refractory congestive heart failure, mostly related to idiopathic dilated cardiomyopathy, appeared safe and caused long lasting improvement in symptoms and signs of heart failure along with improvement in left ventricular ejection phase indices with definite improvement in quality of life | Bharani et al83 |
| Anti-ischemic effects | 40 patients with acute myocardial infarction with ischemic mitral regurgitation | Double-blind study with 500 mg thrice daily for 3 months along with conventional therapy | Reduction in mitral regurgitation jet area Improvement in E/A ratio |
Dwivedi et al84 |
| Anti-ischemic effects | 58 males with chronic stable angina (NYHA class II–III) with evidence of provocable ischemia | T. arjuna (500 mg 8 h), isosorbide mononitrate (40 mg/daily) or a matching placebo for one week each, separated by a wash-out period of three days in a randomized, doubled blind crossover design | Significant decrease in the frequency of angina and need for isosorbide dinitrate Significant improvement in the treadmill exercise. The total duration of exercise increased |
Bharani et al85 |
| Hypertension | 36 hypertensive patients (stage III) with increased LV mass | Ayurvedic formulation of T. arjuna, known as ‘Arjuna Kwath’ (25 ml twice a day) | A significant decrease in both SBP and DBP (P < 0.001) in both the groups LV mass index was only significantly reduced in the atenolol-plus-‘Arjuna Kwatha’ group as compared to atenolol |
Rao et al86 |
| Antioxidant, lowering effects of lipid and lipoprotein | 100 patients with stable CAD | In a placebo-controlled double-blind study, 500 mg of T. arjuna along twice a day in addition to receive the conventional treatment | A significant decrease in hyperlipidemia as well as in various inflammatory cytokines such as hsCRP, IL-18 (P,0.001), IL-6 and TNF-α (P < 0.05) was observed at 3 months in patients | Kapoor et al19 |
| Antioxidant activity | 30 patients with coronary artery disease | 500 mg bark powder of T. arjuna combined with conventional drugs | 16% reduction in LDL cholesterol 15% decrease in cholesterol 11% decrease in triglycerides Marginal decrease in nitrite levels |
Khalil87 |
| Antioxidant activity | 105 patients with stable coronary heart disease (CHD) | T. arjuna bark powder at a dose of 500 mg once daily for 30 days was compared with a known antioxidant, vitamin E (400 units once daily) | Significant reduction in lipids (total cholesterol, LDL-cholesterol) Lowering of lipid peroxide in T. arjuna group |
Gupta et al88 |
| Effect on endothelial dysfunction | Asymptomatic 18 health chronic smokers and 18 non-smokers | Double-blind, placebo-controlled, crossover design. 500 mg aqueous extract of T. arjuna bark powder administrated thrice daily | Improvement in brachial artery flow mediated dilation | Bharani et al89 |
5.1. Pharmacological studies
Cardioprotective potential of T. arjuna stem bark on the molecular basis was evaluated by Kokkiripati et al,56 using cell cultures of human monocytic (THP-1) and human aortic endothelial cells (HAECs). Inhibitory effect of alcoholic (TAAE) and aqueous (TAWE) extracts of T. arjuna stem bark was assessed on human 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, lipoprotein lipase (LpL) and lipid peroxidation in rat (Wistar) liver and heart homogenates. TAAE and TAWE inhibited the lipid peroxidation and HMG-CoA reductase. Both the extracts attenuated H2O2 mediated ROS generation in THP-1 cells by promoting catalase (CAT), glutathione peroxidase (GPx) activities, and by sustaining cellular reducing power. TAAE was highly effective in satisfying proinflammatory gene transcripts in THP-1 cells and HAECs, whereas the response to TAWE depended on the type of transcript and cell type. Both extracts decreased the levels of typical inflammatory marker proteins, viz. LPS induced tumor necrosis factor (TNF)-α secreted by THP-1 cells and TNF-α induced cell surface adhesion molecules on HAECs, namely vascular cell adhesion molecule-1 (VCAM-1) and E-selectin. The marked effects on cultured human monocytic and aortic endothelial cells (HAEC) provide the biochemical and molecular basis for the therapeutic potential of T. arjuna stem bark against cardiovascular diseases (CVD).
Triterpenoids are essentially responsible for cardiovascular properties. Alcoholic and aqueous bark extracts of T. arjuna, arjunic acid, arjunetin and arjungenin were evaluated for their potential to inhibit CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes by Varghese et al.66 They have demonstrated that alcoholic and aqueous bark extract of T. arjuna showed effective inhibition of all three enzymes in human liver microsomes with IC50 values less than 35 μg/ml. Enzyme kinetics studies suggested that the extracts of T. arjuna showed rapidly reversible non-competitive inhibition of all three enzymes in human liver microsomes. They suggest strongly that T. arjuna extracts significantly inhibit the activity of CYP3A4, CYP2D6 and CYP2C9 enzymes. Ahmad et al70 investigated and highlighted the anticarcinogenic and antimutagenic potential of extracts of T. arjuna. They have used human lymphocyte culture and bone marrow cells of albino mice as assay system. The parameters of studied were included chromosomal aberrations (CA), sister chromatid exchanges (SCEs) and cell growth kinetics (RI) both in the presence and in the absence of exogenous metabolic activation system for in vitro experiment, whereas total aberrant cells and the total frequencies of aberrations were taken for in vivo study. The role of T. arjuna extracts in reducing metaphase aberrations due to aflatoxin B1 (AFB1) is quite significant, the reduction varying from 23.49%, 42.47%, and 59.65% down to 12.32%, 28.00%, and 36.88% respectively at the highest dose T. arjuna for the three different durations viz., 24, 48 and 72 h. Similarly the number of sister chromatid exchanges got reduced from a higher level of 15.00 ± 1.40 per cell to 7.70 ± 0.50 per cell with liver microsomal metabolic activation system mix at 48 h of treatment. The replication index was enhanced from 1.33 to 1.55 in the in vitro experiment. Similar trends were noticed in the in vivo experiments that are effective reductions in clastogeny ranging from 15.22% to 54.82% from the mutagen treated positive control and the total frequencies in aberrant cells got reduced from 429 due to AFB1 to 141 due to 5th concentration of T. arjuna extracts at 32 h of exposure. Arjungenin and its glucoside are extracted from T. arjuna and exhibited a moderate free radical scavenging activity on the superoxide release from PMN cells. Arjungenin also exhibited greater inhibitory action on the hypochlorous acid production from human neutrophils.67 Viswanatha et al69 investigated the antioxidant and antimutagenic activities of alcoholic extract of TA bark. The alcoholic extract of the stem bark of T. arjuna (ALTA) has shown potent antioxidant activity with EC50 in DPPH assay, superoxide radical scavenging activity and lipid peroxidation assay. In micronucleus test ALTA showed significant reduction in percentage of micronucleus in both polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE) and also shown a significant reduction in P/N ratio. Singh et al68 investigated the effects of butanolic fraction of T. arjuna bark on Doxorubicin (Dox) induced cardiotoxicity using in vivo study with male Wistar rats and they found that T. arjuna bark has protective effects against Dox-induced cardiotoxicity and may have potential as a cardioprotective agent.
Dried pulverized bark of T. arjuna was administered orally to Wistar albino rats (120–150 g) in two doses (500 and 750 mg/kg in 2% carboxy methyl cellulose (CMC)), 6 days per week for 12 weeks. The determination of baseline changes in cardiac endogenous antioxidant compounds [superoxide dismutase (SOD), reduced glutathione (GSH) and catalase (CAT)] or the hearts were subjected to oxidative stress associated with in vitro ischemic-reperfusion injury (IRI). Significant rise in myocardial thiobarbituric acid reactive substance (TBARS) and loss of SOD, GSH and CAT occurred in the vehicle-treated hearts subjected to in vitro IRI. Hearts of rats were significantly protected from oxidative stress, when subjected to in vitro IRI. The crude bark of TA augments endogenous antioxidant compounds of rat heart and also prevented oxidative stress associated with IRI of the heart.71 Vascular complications are a leading cause of mortality and morbidity in diabetic patients. Therapeutic potential of T. arjuna bark extract was examined in improving myocardial function in streptozotocin (STZ) induced diabetic rats. After 8 weeks of STZ administration, rats showed a decline in left ventricular pressure (LVP), maximal rate of rise and fall in LVP (LV [dP/dt] max and LV [dP/dt] min), cardiac contractility index (LV [dP/dt] max/LVP), and a rise in LV end-diastolic pressure. Altered lipid profile, oxidative stress, and increased levels of endothelin 1 (ET-1), tumor necrosis factor-α (TNF-α), and interleukin 6 (IL-6) along with histological changes in heart and pancreas were observed in diabetic rats. T. arjuna significantly attenuated cardiac dysfunction and myocardial injury in diabetic rats. It also reduced oxidative stress, ET-1, and inflammatory cytokine levels.72 Sinha et al73 has investigated the antioxidative properties of an ethanol extract of the bark of T. arjuna (TAEE) against sodium fluoride (NaF)-induced oxidative stress in the murine heart. NaF intoxication significantly altered all the indices related to the prooxidant–antioxidant status of the heart. In addition, the ferric reducing/antioxidant power assay revealed that TAEE enhanced the cardiac intracellular antioxidant activity. Finally, they concluded that TAEE protects murine hearts from NaF-induced oxidative stress, probably via its antioxidant properties.
Parveen et al74 examined the protective effect of T. arjuna bark extract on left ventricular (LV) and baroreflex function in chronic heart failure and to elucidate the possible mechanistic clues in its cardioprotective action. Fifteen days after isoproterenol administration, rats exhibited cardiac dysfunction, hypertrophy, and LV remodeling along with reduced baroreflex sensitivity. Prophylactic and therapeutic treatment with T. arjuna improved cardiac functions and baroreflex sensitivity. It has also attenuated hypertrophy and fibrosis of the LV. T. arjuna exerts beneficial effect on LV functions, myocardial remodeling, and autonomic control in chronic heart failure possibly through maintaining endogenous antioxidant enzyme activities, inhibiting lipid peroxidation and cytokine levels. Diethyl ether, ethyl acetate and ethanol extractions of T. arjuna exerted hypolipidemic and antioxidative effects at two different dose levels of 175 and 350 mg/kg body weight in Poloxamer (PX)-407 induced hyperlipidemic albino Wistar rats. The results suggested that the ethanolic fraction of T. arjuna possesses the potent properties of being an antioxidant and hypolipidemic than other fractions.75 Kumar et al76 evaluated the effects of T. arjuna bark extract on myocardial fibrosis and oxidative stress induced by chronic β-adrenoceptor stimulation. Because myocardial fibrosis and oxidative stress accompany a number of cardiac disorders such as hypertrophic cardiomyopathy, hypertensive heart disease and cardiac failure. Aqueous extract of T. arjuna bark was evaluated at 63, 125 and 250 mg/kg given orally for antifibrotic and antioxidant effects in rats given the selective β-adrenoceptor agonist isoprenaline for 28 days. The T. arjuna bark extract significantly prevented the isoprenaline-induced increase in oxidative stress and decline in endogenous antioxidant level and also prevented fibrosis. Gauthaman et al79 studied that oral administration of T. arjuna for 12 weeks in rabbits caused augmentation of myocardial antioxidants; superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) along with induction of heat shock protein72 (HSP72). In vivo ischemic-reperfusion injury induced oxidative stress, tissue injury of heart and hemodynamic effects were prevented in the T. arjuna treated rabbit hearts.
Alcoholic extract of T. arjuna bark and its extracts were evaluated for DNA protection, protein oxidation and free radical scavenging activity. Ethanolic extract of T. arjuna bark (TAA) and its fractions, including dichloromethane (TAD), ethyl acetate (TAE), butanol (TAB) and water (TAW) has significant antioxidant activity and potential to prevent protein oxidation, DNA damage protection by pBR 322 DNA and SCGE assay. The potent antioxidative activity and DNA protection ability of T. arjuna bark extracts might be endorsed with phenolic/flavonoid compounds. A significant correlation was also observed between free radical scavenging activity, in vitro DNA damage activity and the total phenolic/flavonoid content.16 Physicochemical property and inotropic effect of the aqueous extract of T. arjuna bark (TAAqE) were investigated by Oberoi et al80 on adult rat ventricular myocytes in comparison with extracts prepared sequentially with organic extracts. They found that TAAqE decoctions exerted positive inotropy, accelerated myocyte relaxation and increased caffeine-induced contraction concentration dependently. TAAqE-induced cardiotonic action via enhancing SR function, a unique action minimizing the occurrence of arrhythmias, makes TAAqE a promising and relatively safe cardiotonic beneficial to the healthy heart and the treatment for chronic heart disease.
Mandal et al77 investigated antioxidative and antimicrobial properties of methanolic extract of T. arjuna bark. The antimicrobial activity showed that higher inhibition against Gram negative bacteria than gram positive bacteria and showed a promising antioxidant activity, as absorption of DPPH radicals decreased in DPPH free radical scavenging assay. Methanol extract from bark of T. arjuna exhibited medicinal as well as physiological activities. Methanol, ethanol, acetone, aqueous both hot and cold extracts from the leaves and bark of T. arjuna were tested for their antimicrobial activity against Staphylococcus aureus, Acinetobacter sp., Proteus mirabilis, Escherichia coli, Pseudomonas aeruginosa and Candida albicans, pathogens causing ear infections. Three organic solvents evaluated, acetonic leaf extract was found to be best against S. aureus. Organic bark extract showed almost equal inhibition of all tested Gram negative bacteria except P. aeruginosa. Aqueous extract of T. arjuna bark exhibited good activity against S. aureus.78 Devi et al81 evaluated the effect of methanolic extract of T. arjuna (100 mg/kg to 50 mg/kg body weight) on diclofenac sodium (80 mg/kg bodyweight in water, orally) induced gastric ulcer in rats. The gastroprotective effect of T. arjuna was assessed from volume of gastric juice, pH, free and total acidity, pepsin concentration, acid output in gastric juice, the levels of non-protein sulfhydryls (NP-SH), lipid peroxide (LPO), reduced glutathione (GSH), and activities of enzymic antioxidants-super oxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and myeloperoxidase (MPO) in gastric mucosa. The levels of DNA, protein bound carbohydrate complexes-hexose, hexosamine, sialic acid, fucose in gastric mucosa and gastric juice and the levels of RNA in gastric mucosa were assessed. The stomach tissues were used for adherent mucus content and also for the histological examination. A significant reduction in lesion index was observed in ulcer induced animals treated with T. arjuna (DIC + TA) compared to ulcerated rats (DIC). A significant increase was observed at pH, NP-SH, GSH, enzymic antioxidants, protein bound carbohydrate complexes, adherent mucus content, nucleic acids with a significant decrease in volume of gastric juice, free and total acidity, pepsin concentration, acid output, LPO levels and MPO activities in DIC + TA rats compared to DIC rats. It is proved that T. arjuna could act as a gastroprotective agent probably due to its free radical scavenging activity and cytoprotective nature.
5.2. Clinical studies
Recently, Kapoor et al19 investigated the therapeutic potential of T. arjuna on the inflammatory markers in subjects with stable coronary artery disease (CAD). In a placebo-controlled, randomized double-blind study, 116 patients with stable CAD who were on standard cardiac medications for more than three months were enrolled and received either placebo or 500 mg of T. arjuna from Himalayan Herbal Healthcare, Bangalore, India twice a day in addition to receiving the conventional treatment. A significant decrease in serum triglycerides as well as in various inflammatory cytokines such as hsCRP, IL-18 (P < 0.001), IL-6 and TNF-α (P < 0.05) was observed at 3 months in patients who were on drug treatment as compared to the placebo. The effects were maintained till 6 months follow-up and showed a further reduction in hyperlipidemia and inflammatory markers with time. An observational study was conducted to find out the effects of T. arjuna in patients with dilated cardiomyopathy (DCMP) of idiopathic and ischemic cause. Ninety three patients with DCMP receiving standard therapy and/or bark extract of T. arjuna 500 mg 8 hourly were enrolled. Three groups as standard therapy (ST, Group 1), T. arjuna therapy (TA, Group 2) and standard therapy with T. arjuna (ST + TA, Group 3) were formed. At the end of the study period, patients of group 3 showed significant improvement in percentage of left ventricular ejection fraction (LVEF%) (7 ± 1.6, P < 0.00001) compared to group 1 and 2 (P < 0.00001, P < 0.0001). Reductions in Left ventricular end systolic and diastolic diameters and volumes were most significant in group 3 (8.3 ± 4.7, P < 0.0001 and 3.1 ± 5.7, P < 0.001) and (11 ± 26, 9 ± 21 P < 0.01) respectively in comparison to other groups. Pulmonary artery pressure reduced significantly in group 1 and 3 (P < 0.0001). A similar reduction in diastolic score and mitral regurgitation (P < 0.01 and P < 0.0001) was observed in groups 1 and 3. From the results, dilated cardiomyopathy with reduced LVEF due to either idiopathic or ischemic cause receiving standard therapy with T. arjuna showed significant improvement in left ventricular parameters as well as functional capacity.82
Bharani et al83 investigated the salutary effect of T. arjuna in patients with severe refractory heart failure. Twelve patients with refractory chronic congestive heart failure (Class IV NYHA), related to idiopathic dilated cardiomyopathy (10 patients); previous myocardial infarction (one patient) and peripartum cardiomyopathy (one patient), received T. arjuna, as bark extract (500 mg 8 hourly) or matching placebo for 2 weeks each, separated by 2 week washout period, in a double blind crossover design as an adjuvant to maximally tolerable conventional therapy (Phase I). On long term evaluation in an open design (Phase II), wherein Phase I participants continued T. arjuna in fixed dosage (500 mg 8-hourly) in addition to flexible diuretic, vasodilator and digitalis dosage for 20–28 months (mean 24 months) on outpatient basis, patients showed continued improvement in symptoms, signs, effort tolerance and NYHA Class, with improvement in quality of life. Dwivedi et al84 were conducted a study to evaluate the role of T. arjuna in ischemic mitral regurgitation (IMR) following acute myocardial infarction (AMI). 40 patients with fresh AMI showing IMR were randomly divided into 2 groups of 20 each. Two groups were observed between one and three months therapy with T. arjuna at a dose of 500 mg twice a day and showed significant decreases in IMR, improvement in E/A ratio and considerable reduction in angina frequency. Bharani et al85 conducted a study on the efficacy of T. arjuna in chronic stable angina. Fifty eight males with chronic stable angina (NYHA class II–III) with evidence of provocable ischemia on treadmill exercise test received TA (500 mg 8 hourly), isosorbide (40 mg/daily) or a matching placebo for one week each, separated by a washout period of at least three days in a randomized, double-blind, crossover design. They underwent clinical, biochemical and treadmill exercise evaluation at the end of each therapy, which were compared during the three therapy periods. T. arjuna therapy was associated with a significant decrease in the frequency of angina and the need for isosorbide dinitrate. T. arjuna bark extract, 500 mg 8 hourly, given to patients with stable angina with provocable ischemia on treadmill exercise, led to improvement in clinical and treadmill exercise parameters as compared to placebo therapy. These benefits were similar to those observed with isosorbide mononitrate (40 mg/day) therapy and the extract was well tolerated.
The effect of an Ayurvedic formulation of T. arjuna, known as ‘Arjuna Kwatha’ was assessed by Rao et al86 in 36 hypertensive patients at stage III with increased LV mass. The patients were divided into two groups, one group received atenolol (50 mg twice daily) and the other group ‘Arjuna Kwatha’ (25 ml twice daily) along with atenolol for 6 months. A significant decrease was observed in both SBP and DBP (P < 0.001) in both the groups. However, LV mass index was only significantly reduced in the atenolol-plus-‘Arjuna Kwatha’ group as compared to atenolol alone (P < 0.001), due to negative chronotropic and inotropic effects of the herbal preparation. Khalil87 reported that the administration of T. arjuna bark powder along with statins for 3 months to 30 patients with coronary artery disease resulted in a 16% in LDL-cholesterol, 15% decrease in total cholesterol and 11% in triglycerides, confirming its immense potential to correct dyslipidemia in conjunction with statins. Gupta et al88 evaluated the antioxidant and hypocholesterolaemic effects of T. arjuna tree bark and to compare it with a known antioxidant, vitamin E, also performed a randomized controlled trial. One hundred and five successive patients with coronary heart disease (CHD) were recruited and divided into 3 groups of 35 each in this study. Group I received placebo capsules; Group II vitamin E capsules 400 units/day; and Group III received finely pulverized T. arjuna tree bark-powder (500 mg) in capsules daily. Lipids and lipid peroxide levels were determined at 30 days follow-up. No significant changes in total, HDL, LDL cholesterol and triglycerides levels were seen in Groups I and II. In Group III, there was a significant decrease in total cholesterol (−9.7 ± 12.7%), and LDL cholesterol (−15.8 ± 25.6%) (paired t-test P < 0.01). Lipid peroxide levels decreased significantly in both the treatment groups (P < 0.01). This decrease was more in vitamin E group (−36.4 ± 17.7%) as compared to the T. arjuna group (−29.3 ± 18.9%). T. arjuna tree bark powder has significant antioxidant action that is comparable to vitamin E and also has a significant hypocholesterolaemic effect. A study was conducted by Bharani et al89 to determine the improvement of endothelial dysfunction in smokers. Eighteen healthy male smokers (age 28.16 ± 9.45 years) and an equal number of age-matched, non-smoker controls participated in the study. The smokers were given T. arjuna (500 mg, 8 h) or matching placebo randomly in a double blind crossover design for two weeks each, followed by repetition of brachial artery reactivity studies to determine various parameters including flow-mediated dilation after each period. The flow-mediated dilation showed significant improvement from baseline values after T. arjuna therapy.
6. Toxicity and side effects
T. arjuna has been used in the dose between 1 to 2 g per day in different clinical studies and found that this is an optimum dose in the patients particularly CAD. These doses have lesser side effect like headache, mild gastritis and constipation. There were no reports in the regards of hematological, hepatic, metabolic and renal toxicity after more than two years of its administration.83 Recently Bhawani et al82 reported that there was no significant variation in the body and organ weights between the control and the treated group of 93 patients with dilated cardiomyopathy (DCMP) of idiopathic and ischemic cause was observed after 28 days of treatment under the treatment of T. arjuna capsules (500 mg at 8 h). Hematological analysis and biochemical parameters revealed no toxic effects of the extract. Pathologically, neither gross abnormalities nor histopathological changes were observed and there was no mortality recorded in 28 days. Yaidikar et al90 reported that pretreatment with arjunolic acid from the T. arjuna bark effectively prevented the cerebral I/R induced oxidative damage by virtue of its antioxidant potential and supplementation of arjunolic acid may be beneficial in stroke prone population. Arjunolic acid from T. arjuna attenuated sodium nitrite-induced cardiac damage in rats and restored the normal balance between pro- and anti-inflammatory cytokines. Moreover, arjunolic acid protected cardiac tissues from both extrinsic and intrinsic cell death pathways.91 Parmar et al92 were observed a decrease in serum concentration of thyroid hormones as well as an increase in the hepatic LPO with higher doses of T. arjuna. There is a vital need for well controlled multicentric clinical trials in a larger setup of subjects with a standardized product for exploring the true therapeutic potential of T. arjuna.
7. Conclusion
On the basis of the available literature evidences, T. arjuna is widely used for treatment of cardiovascular diseases, including heart diseases and related chest pain, high blood pressure and high cholesterol. It is also used for earaches and diseases of the urinary tract. The effectiveness T. arjuna as an anti-ischemic agent and as a potent antioxidant preventing LDL, reperfusion ischemic injury to the heart and its potential to reduce atherogenic lipid levels have been sufficiently demonstrated in different experimental and clinical studies. However, continuous research progress of using T. arjuna is very much needed in the regards of exact molecular mechanism, drug administration, drug-drug interactions and toxicological studies.
Conflict of interest statement
We declare that we have no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.WHO . 2002. World Health Organization, Traditional Medicine Strategy Report, Document WHO/EDM/TRH/2002.1. [Google Scholar]
- 2.Heinrich M. Ethnobotany and its role in drug development. Phytother Res. 2000;14:479–488. doi: 10.1002/1099-1573(200011)14:7<479::aid-ptr958>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Tabuti J.R.S., Lye K.A., Dhillion S.S. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J Ethnopharmacol. 2003;88:19–44. doi: 10.1016/s0378-8741(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 4.Pieroni A. Evaluation of the cultural significance of wild food botanicals traditionally consumed in Northwestern Tuscany, Italy. J Ethnobiol. 2001;21:89–104. [Google Scholar]
- 5.Kim H., Song M.J. Ethnomedicinal practices for treating liver disorder of local communities in the southern regions of Korea. J Evid Based Complement Altern Med. 2013 doi: 10.1155/2013/869176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyay B., Singh K.P., Kumar A. Ethnoveterinary uses and informants consensus factor of medicinal plants of Sariska region, Rajasthan, India. J Ethnopharmacol. 2011;133:14–25. doi: 10.1016/j.jep.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 7.Reyes-Garcia V., Huanca T., Vadez V., Leonard W., Wilkie D. Cultural, practical, and economic value of wild plants: a quantitative study in the Bolivian, Amazon. Econ Bot. 2006;60:62–74. [Google Scholar]
- 8.Gosh A. Herbal folk remedies of Bantura & Medinipur districts, West Bengal (India) Indian J Tradit Knowl. 2003;2:393–396. [Google Scholar]
- 9.Stickel F., Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. doi: 10.1016/j.dld.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Gopinath K., Venkatesh K.S., Ilangovan R., Sankaranarayanan K., Arumugam A. Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind Crop Prod. 2013;50:737–742. [Google Scholar]
- 11.Yallappa S., Manjanna J., Sindhe M.A., Satyanarayan N.D., Pramod S.N., Nagaraja K. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim Acta A. 2013;110:108–115. doi: 10.1016/j.saa.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Edison T.J.I., Sethuraman M.G. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012;47:1351–1357. [Google Scholar]
- 13.Sharma J., Gairola S., Gaur R.D., Painuli R.M. The treatment of jaundice with medicinal plants in indigenous communities of the Sub-Himalayan region of Uttarakhand, India. J Ethnopharmacol. 2012;143:262–291. doi: 10.1016/j.jep.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Ram A., Lauria P., Gupta R., Kumar P., Sharma V.N. Hypocholesterolaemic effects of Terminalia arjuna tree bark. J Ethnopharmacol. 1997;55:165–169. doi: 10.1016/s0378-8741(96)01493-6. [DOI] [PubMed] [Google Scholar]
- 15.Bachaya H.A., Iqbal Z., Khan M.N., Jabbar A., Gilani A.H., Din I.U. In vitro and in vivo anthelmintic activity of Terminalia arjuna bark. Int J Agric Biol. 2009;11:273–278. [Google Scholar]
- 16.Phani Kumar G., Navya K., Ramya E.M., Venkataramana M., Anand T., Anilakumar K.R. DNA damage protecting and free radical scavenging properties of Terminalia arjuna bark in PC-12 cells and plasmid DNA. Free Radic Antioxid. 2013;3:35–39. [Google Scholar]
- 17.Dwivedi S. Terminalia arjuna Wight &Arn.- a useful drug for cardiovascular disorders. J Ethnopharmacol. 2007;114:114–129. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Maulik S.K., Talwar K.K. Therapeutic potential of Terminalia arjuna in cardiovascular disorders. Am J Cardiovasc Drugs. 2012;12:157–163. doi: 10.2165/11598990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor D., Vijayvergiya R., Dhawan V. Terminalia arjuna in coronary artery disease: ethnopharmacology, pre-clinical, clinical & safety evaluation. J Ethnopharmacol. 2014;155:1029–1045. doi: 10.1016/j.jep.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P.C., Yelne M.B., Dennis T.J. CCRAS (The Central Council for Research in Ayurvedic Sciences); New Delhi: 2005. Database on Medicinal Plants Used in Ayurveda. [Google Scholar]
- 21.Chopra R.N., Chopra I.C., Handa K.L., Kapur L.D. Terminalia arjuna W&A (Combretaceae) In: Chopra R.N., Chopra I.C., Handa K.L., Kapur L.D., editors. Chopra's Indigenous Drugs of India. 1st ed. UNDhur & Sons; Calcutta, India: 1958. pp. 421–424. [Google Scholar]
- 22.Nadkarni A.K. 1st ed. Popular Prakashan; Mumbai, India: 1976. Indian Materia Medica. [Google Scholar]
- 23.Ali M. 1st ed. CBS Publishers; New Delhi: 1994. Text Book of Pharmacognosy. [Google Scholar]
- 24.Row L.R., Murty P.S., SubbaRao G.S.R., Sastry C.S.P., Rao K.V.J. Chemical examination of Terminalia arjuna: Part-XII: isolation and structure determination of arjunic acid, a new trihydroxytriterpene carboxylic acid from Terminalia arjuna bark. Indian J Chem. 1970;8:716–721. [Google Scholar]
- 25.Honda T., Murae T., Tsuyuki T., Takahashi T., Sawai M. Arjungenin, arjunglucoside I and arjunglucoside II, a new triterpene and new triterpene-glucosides from Terminalia arjuna. Bull Chem Soc Jpn. 1976;49:3213–3218. [Google Scholar]
- 26.Singh D.V., Verma R.K., Gupta M.M., Kumar S. Quantitative determination of oleane derivatives in Terminalia arjuna by high performance thin layer chromatography. Phytochem Anal. 2002;13:207–210. doi: 10.1002/pca.643. [DOI] [PubMed] [Google Scholar]
- 27.Singh D.V., Verma R.K., Singh S.C., Gupta M.M. RP-LC determinationof oleane derivatives in Terminalia arjuna. J Pharm Biomed Anal. 2002;28:447–452. doi: 10.1016/s0731-7085(01)00590-8. [DOI] [PubMed] [Google Scholar]
- 28.Anjaneyulu A.S.R., Prasad A.V.R. Structure of terminic acid, a dihydroxy-triterpene carboxylic acid from Terminalia arjuna. Phytochemistry. 1983;22:993–998. [Google Scholar]
- 29.Singh B., Singh V.P., Pandey V.B., Rucker G. A new triterpeneglycoside from Terminalia arjuna. Planta Med. 1995;61:576–577. doi: 10.1055/s-2006-959380. [DOI] [PubMed] [Google Scholar]
- 30.Wang W., Ali Z., Shen Y., Li X., Khan I.A. Ursane triterpenoids from the bark of Terminalia arjuna. Fitoterapia. 2010;81:480–484. doi: 10.1016/j.fitote.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Row L.R., Murty P.S., Subba Rao G.S.R., Sastry C.S.P., Rao K.V.J. Chemical examination of Terminalia arjuna: Part-XII: Isolation and structure determination of arjunetin from Terminalia arjuna bark. Indian J Chem. 1970;8:772–775. [Google Scholar]
- 32.Honda T., Murae T., Tsuyuki T., Takahashi T. The structure of arjungen: a new sapogenin from Terminalia arjuna. Chem Pharm Bull. 1976;24:178–180. [Google Scholar]
- 33.Sharma P.N., Shoeb A., Kapil R.S., Popli S.P. Arjunolone: a new flavones from stem bark of Terminalia arjuna. Indian J Chem. 1982;21B:263–264. [Google Scholar]
- 34.Tripathi V.K., Pandey V.B., Udupa K.N., Rucker G. Arjunolitin, a triterpene glycoside from Terminalia arjuna. Phytochemistry. 1992;31:349–351. [Google Scholar]
- 35.Ali A., Kaur G., Hayat K., Ali M., Ather M. A novel naphthanolglycoside from Terminalia arjuna with antioxidant and nitricoxide inhibitory activities. Pharmazie. 2003;58:932–934. [PubMed] [Google Scholar]
- 36.Ali A., Kaur G., Hamid H. Terminoside A, a new triterpineglycoside from the bark of Terminalia arjuna inhibits nitricoxide production in murine macrophages. J Asian Nat Prod Res. 2003;5:137–142. doi: 10.1080/1028602031000066834. [DOI] [PubMed] [Google Scholar]
- 37.Wang W., Ali Z., Li X.C., Shen Y., Khan I.A. Triterpenoids from two Terminalia species. Planta Med. 2010;76:1751–1754. doi: 10.1055/s-0030-1249809. [DOI] [PubMed] [Google Scholar]
- 38.Patnaik T., Dey R.K., Gouda P. Isolation of triterpenoidglycoside from bark of Terminalia arjuna using chromatographic technique and investigation of pharmacological behavior upon muscle tissues. E-J Chem. 2007;4:474–479. [Google Scholar]
- 39.Alam M.S., Kaur G., Ali A., Hamid H., Ali M., Athar M. Two new bioactive oleanane triterpene glycoside from Terminalia arjuna. Nat Prod Res. 2008;22:1279–1288. doi: 10.1080/14786410701766380. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad M.U., Mullah K.B., Norin T., Ulla J.K. Terminoic acid, a new trihydroxytriterpene carboxylic acid from bark of Terminalia arjuna. Indian J Chem. 1983;22:738–740. [Google Scholar]
- 41.Pettit G.R., Hoard M.S., Doubek D.L. Antineoplastic agents 338. The cancer cell growth inhibitory. Constituents of Terminalia arjuna (Combretaceae) J Ethnopharmacol. 1996;53:57–63. doi: 10.1016/S0378-8741(96)01421-3. [DOI] [PubMed] [Google Scholar]
- 42.Anonymous Terminalia arjuna. Altern Med Rev. 1999;4:436–437. [PubMed] [Google Scholar]
- 43.Saha A., Pawar V.M., Jayaraman S. Characterization of polyphenols in Terminalia arjuna bark extract. Indian J Pharm Sci. 2012;74:339–347. doi: 10.4103/0250-474X.107067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi S.H., Tanaka H., Hano Y., ItoK T., Nomura T., Shigenobu K. Hypotensive effects in rats of hydrophilic extract from Terminalia arjuna containing tannin-related compounds. Phytother Res. 1997;11:424–427. [Google Scholar]
- 45.Lin T.C., Chien S.C., Chen H.F., Hsu F.L. Tannins and related compounds from Combretaceae plants. Chin Pharm J. 2001;52:1–26. [Google Scholar]
- 46.Kuo P.L., Hsu Y.L., Lin T.C., Lin L.T., Chang J.K., Lin C.C. Casuarinin from the bark of Terminalia arjuna induces apoptosis and cell cycle arrest in human breast adenocarcinoma MCF-7cells. Planta Med. 2005;71:237–243. doi: 10.1055/s-2005-837823. [DOI] [PubMed] [Google Scholar]
- 47.Dwivedi S., Udupa N. Terminalia arjuna: pharmacognosy, phytochemistry, pharmacology and clinical use: a review. Fitoterpia. 1989;60:413–420. [Google Scholar]
- 48.Anjaneyulu A.S.R., Prasad A.V.R. Chemical examination of the roots of Terminalia arjuna characterization of two new triterpenoidglycoside. Indian J Chem. 1982;21:530–533. [Google Scholar]
- 49.Anjaneyulu A.S.R., Prasad A.V.R. Chemical examination of roots of Terminalia arjuna-the structure of arjunoside III andarjunoside IV, two new triterpenoid glycosides. Phytochemistry. 1982;21:2057–2060. [Google Scholar]
- 50.Choubey B.K., Srivastava S.K. Antifungal agents from Terminalia arjuna. Indian J Chem. 2001;40B:354–356. [Google Scholar]
- 51.Upadhyay R.K., Pandey M.B., Jha R.N., Singh V.P., Pandey V.B. Triterpene glycoside from Terminalia arjuna. J Asian Nat Prod Res. 2001;3:207–212. doi: 10.1080/10286020108041392. [DOI] [PubMed] [Google Scholar]
- 52.Rastogi R.P., Mehrotra B.N. vol. 3. CSIR; New Delhi: 1993. (Compendium of Indian Medicinal Plants). [Google Scholar]
- 53.Yadava R.N., Rathore K. A new cardenolide from the seeds of Terminalia arjuna (W and A) J Asian Nat Prod Res. 2000;2:97–101. doi: 10.1080/10286020008039898. [DOI] [PubMed] [Google Scholar]
- 54.Harbone J.B. 3rd ed. ChapmanandHall; London: 1998. Phytochemical Methods; pp. 117–119. [Google Scholar]
- 55.Chitlange S.S., Kulkarni P.S., Patil D., Patwardhan B., Nanda R.K. High-performance liquid chromatographic fingerprint for quality control of Terminalia arjuna containing Ayurvedic churna formulation. J AOAC Int. 2009;92:1016–1020. [PubMed] [Google Scholar]
- 56.Kokkiripati P.K., Kamsala R.V., Bashyam L. Stem-bark of Terminalia arjuna attenuates human monocytic (THP-1) and aortic endothelial cell activation. J Ethnopharmacol. 2013;146:456–464. doi: 10.1016/j.jep.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 57.Kandil F.E., Nassar M.I. A tannin anti-cancer promotor from Terminalia arjuna. Phytochemistry. 1998;47:1567–1568. doi: 10.1016/s0031-9422(97)01078-9. [DOI] [PubMed] [Google Scholar]
- 58.Wang W., Ali Z., Li X.C., Shen Y., Khan I.A. 18,19-Secooleananetype triterpeneglycosylesters from the bark of Terminalia arjuna. Planta Med. 2010;76:903–908. doi: 10.1055/s-0029-1240841. [DOI] [PubMed] [Google Scholar]
- 59.Fuhrman B., Aviram M. Antiatherogenecity of nutritional compounds. I Drugs. 2001;4:82–92. [PubMed] [Google Scholar]
- 60.Carluccio M.A., Sicuella L., Ancora M.A. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 61.Ruff J.C. Wine and polyphenols related to platelet aggregation and atherothrombosis. Drugs Exp Clin Res. 2003;25:125–131. [PubMed] [Google Scholar]
- 62.Martikainen J.A., Ottelin A.-M., Kiviniemi V., Gylling H. Plant stanol esters are potentially cost-effective in the prevention of coronary heart disease in men: Bayesian modeling approach. Eur J Cardiovasc Prev Rehabil. 2007;14:265–272. doi: 10.1097/01.hjr.0000216550.74258.12. [DOI] [PubMed] [Google Scholar]
- 63.Bajpai M., Pande A., Tewari S.K., Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005;56:287–291. doi: 10.1080/09637480500146606. [DOI] [PubMed] [Google Scholar]
- 64.Kolodziej H., Kiderlen A.F. Anti-leishmanial activity and immunemodulatory effects of tannins and related compounds on Leishmania parasitised RAW264.7 cells. Phytochemistry. 2005;66:2056–2071. doi: 10.1016/j.phytochem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhari M., Mengi S. Evaluation of phytoconstituents of Terminalia arjuna for wound healing activity in rats. Phytother Res. 2006;20:799–805. doi: 10.1002/ptr.1857. [DOI] [PubMed] [Google Scholar]
- 66.Varghese A., Savai J., Pandita N., Gaud R. In vitro modulatory effects of Terminalia arjuna, arjunic acid, arjunetin and arjungenin on CYP3A4, CYP2D6 and CYP2C9 enzyme activity in human liver microsomes. Toxicol Rep. 2015;2:806–816. doi: 10.1016/j.toxrep.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pawar R.S., Bhutani K.K. Effect of oleananetriterpenoids from Terminalia arjuna: a cardioprotective drug on the process of respiratory oxyburst. Phytomedicine. 2005;12:391–393. doi: 10.1016/j.phymed.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Singh G., Singh A.T., Abrahama A. Protective effects of Terminalia arjuna against doxorubicin-induced cardiotoxicity. J Ethnopharmacol. 2008;117:123–129. doi: 10.1016/j.jep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 69.Viswanatha G.L., Vaidya S., Ramesh C., Krishnadas N., Rangappa S. Antioxidant and antimutagenic activities of bark extract of Terminalia arjuna. Asian Pac J Trop Med. 2010;3:965–970. [Google Scholar]
- 70.Ahmad M.S., Ahmad S., Gautam B.J., Arshad M., Afzal M. Terminalia arjuna, a herbal remedy against environmental carcinogenicity: an in vitro and in vivo study. Egypt J Med Hum Genet. 2014;15:61–67. [Google Scholar]
- 71.Gauthaman K., Maulik M., Kumari R., Manchanda S.C., Dinda A.K., Maulik S.K. Effect of chronic treatment with bark of Terminalia arjuna: a study on the isolated ischemic-reperfused rat heart. J Ethnopharmacol. 2001;75:197–201. doi: 10.1016/s0378-8741(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 72.Khaliq F., Parveen A., Singh S., Gondal R., Hussain M.E., Fahim M. Improvement in myocardial function by Terminalia arjuna in streptozotocin- induced diabetic rats: possible mechanisms. J Cardiovasc Pharmacol Ther. 2013;18:481–489. doi: 10.1177/1074248413488831. [DOI] [PubMed] [Google Scholar]
- 73.Sinha M., Manna P., Sil P.C. Terminalia arjuna protects mouse hearts against sodium fluoride-induced oxidative stress. J Med Food. 2008;11:733–740. doi: 10.1089/jmf.2007.0130. [DOI] [PubMed] [Google Scholar]
- 74.Parveen A., Babbar R., Agarwal S., Kotwani A., Fahim M. Terminalia arjuna enhances baroreflex sensitivity and myocardial function in isoproterenol-induced chronic heart failure in rats. J Cardiovasc Pharmacol Ther. 2012;17:199–207. doi: 10.1177/1074248411416816. [DOI] [PubMed] [Google Scholar]
- 75.Subramaniam S., Ramachandran S., Uthrapathi S., Gnamanickam V.R., Dubey G.P. Anti-hyperlipidemic and antioxidant potential of different fractions of Terminalia arjuna (Roxb.) bark against PX-407 induced hyperlipidemia. Indian J Exp Biol. 2011;49:282–288. [PubMed] [Google Scholar]
- 76.Kumar S., Enjamoori R., Jaiswal A., Ray R., Seth S., Maulik S.K. Catecholamine-induced myocardial fibrosis and oxidative stress is attenuated by Terminalia arjuna (Roxb.) J Pharm Pharmacol. 2009;61:1529–1536. doi: 10.1211/jpp/61.11.0013. [DOI] [PubMed] [Google Scholar]
- 77.Mandal S., Patra A., Samanta A. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac J Trop Biomed. 2013;3:960–966. doi: 10.1016/S2221-1691(13)60186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aneja K.R., Sharma C., Joshi R. Antimicrobial activity of Terminalia arjuna Wight & Arn.: an ethnomedicinal plant against pathogens causing ear infection. Braz J Otorhinolaryngol. 2012;78:68–74. doi: 10.1590/S1808-86942012000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gauthaman K., Banerjee S.K., Dinda A.K., Ghosh C.C., Maulik S.K. Terminalia arjuna (Roxb.) protects rabbit heart against ischemic-reperfusion injury: role of antioxidant enzymes and heat-shock protein. J Ethnopharmacol. 2005;96:403–409. doi: 10.1016/j.jep.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 80.Oberoi L., Akiyama T., Lee K.H., Liu S.J. The aqueous extract, not organic extracts, of Terminalia arjuna bark exerts cardiotonic effect on adult ventricular myocytes. Phytomedicine. 2011;18:259–266. doi: 10.1016/j.phymed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Devi R.S., Narayan S., Vani G., Devi C.S.S. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcer. Chem Biol Interact. 2007;167:71–83. doi: 10.1016/j.cbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Bhawani G., Kumar A., Murthy K.S.N., Kumari N., Ganapati Swami Ch. A retrospective study of effect of Terminalia arjuna and evidence based standard therapy on echocardiographic parameters in patients of dilated cardiomyopathy. J Pharm Res. 2013;6:493–498. [Google Scholar]
- 83.Bharani A., Ganguli A., Bhargava K.D. Salutary effect of Terminalia arjuna in patients with severe refractory heart failure. Int J Cardiol. 1995;49:191–199. doi: 10.1016/0167-5273(95)02320-v. [DOI] [PubMed] [Google Scholar]
- 84.Dwivedi S., Aggarwal A., Agarwal M.P., Rajpal S. Role of Terminalia arjuna in ischemic mitral regurgitation. Int J Cardiol. 2005;100:507–508. doi: 10.1016/j.ijcard.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 85.Bharani A., Ganguli A., Mathur L.K., Jamira Y., Raman P.G. Efficacy of Terminalia arjuna in chronic stable angina. Indian Heart J. 2002;54:441–444. [PubMed] [Google Scholar]
- 86.Rao B.C.S., Singh R.H., Tripathi K. Effect of Terminalia arjuna (W&A) on regression of LVH in hypertensives: a clinical study. J Res Ayurveda Siddha. 2001;22:216–227. [Google Scholar]
- 87.Khalil S. National Board of Examination; New Delhi, India: 2005. Effect of Statin Versus Terminalia arjuna on Acute Myocardial Infarction. (DNB thesis) [Google Scholar]
- 88.Gupta R., Singhal S., Goyle A., Sharma V.N. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree-bark powder: a randomised placebo-controlled trial. J Assoc Physicians India. 2001;49:231–235. [PubMed] [Google Scholar]
- 89.Bharani A., Ahirwal K., Jain N. Terminalia arjuna reverses impaired endothelial function in chronic smokers. Indian Heart J. 2004;56:123–128. [PubMed] [Google Scholar]
- 90.Yaidikar L., Thakur S. Arjunolic acid, a pentacyclic triterpenoidal saponin of Terminalia arjuna bark protects neurons from oxidative stress associated damage in focal cerebral ischemia and reperfusion. Pharmacol Rep. 2015;67:890–895. doi: 10.1016/j.pharep.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Al-Gayyar M.M.H., Al Youssef A., Sherif I.O., Shams M.E.E., Abbas A. Protective effects of arjunolic acid against cardiac toxicity induced by oral sodium nitrite: effects on cytokine balance and apoptosis. Life Sci. 2014;111:18–26. doi: 10.1016/j.lfs.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Parmar H.S., Panda S., Jatwa R., Kar A. Cardio-protective role of Terminalia arjuna bark extract is possibly mediated through alterations in thyroid hormones. Pharmazie. 2006;61:793–795. [PubMed] [Google Scholar]