Abstract
The altered metabolism observed in cancer cells generally consists in increased glucose uptake and glycolytic activity. This is associated with an overexpression of glucose transporter proteins (GLUTs), which facilitate glucose uptake across the plasma membrane and play a crucial role in the survival of cancer cells. Therefore GLUTs are considered as suitable targets for the treatment of cancer. Herein we review some of the most relevant GLUT inhibitors that have been recently developed as prospective anticancer agents.
Introduction
Tumours consume huge amounts of glucose compared to healthy tissues, because they display a high rate of glycolysis. In order to do so, cancer cells generally take up sugars at a higher rate than normal cells. This peculiarity, which is also known as the Warburg effect,1 has led to the development of imaging techniques, such as PET scan, that track a radioactively labelled glucose analogue, 2-deoxy-2-(18F)fluoro-d-glucose (18F-FDG).2,3 Hence, this metabolic feature has been widely exploited for the detection of cancer tissues, whereas attempts to do the same for therapeutic purposes are still in their infancy.4-6
Glucose transporters (GLUTs), which are transmembrane proteins that facilitate the concentration-driven uptake of glucose inside the cell, are often overexpressed in cancer cells. Therefore, they may be either targeted by GLUT-inhibitors, in order to counteract the “tumor gluttony” mentioned above, or they may be exploited for the internalization of glycoconjugates anticancer agents.7 This review recapitulates some of the most significant examples of natural and synthetic inhibitors of the various isoforms of GLUTs, as well as of sugar-conjugated anticancer agents that exploit the Warburg effect in order to selectively target cancer cells.
Natural GLUT inhibitors
A large class of GLUT inhibitors is represented by flavonoids, polyphenolic compounds that are widely found in many fruits and vegetables as plant secondary metabolites, and most of them showed an appreciable activity on GLUTs.8
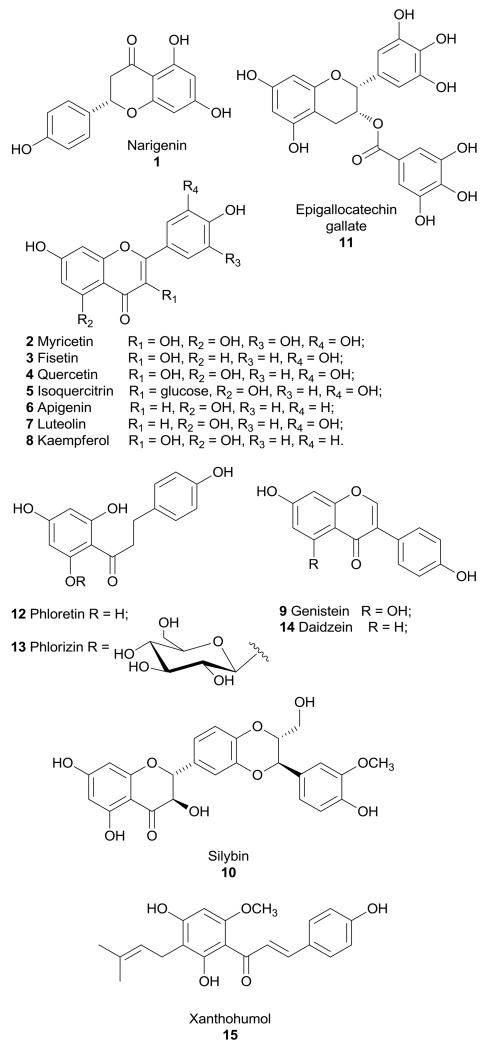
Naringenin (1, Figure 1) is a flavanone found in high concentration in grapefruit and orange, which shows a broad range of biological effects: it is a well-known antioxidant, it displays anticancer properties,9 it reduces the risk of atherosclerosis,10 it binds with good affinity and selectivity to estrogen receptor β,11 and it exerts a potent cardioprotective effect.12 In addition to these properties, it displayed a certain inhibitory effect on glucose uptake in myelocytic U937 cells (IC50 was about 50 µM).13 The same effect was confirmed in 3T3-L1 adipocytes, where naringenin blocked insulin-stimulated glucose uptake in a dose-dependent manner: it was calculated that a physiological dose of 6 µM of naringenin (for example by regular consumption of grapefruit juice) resulted in an inhibition of glucose uptake by approximately 20%.14 This effect was confirmed also in cultured cancer cells: a 100 µM concentration of naringenin inhibited glucose uptake by 50% and cell proliferation by 20% in MCF-7 breast cancer cells.15,16 These effects were exerted by inhibiting the activity of phosphoinositide 3-kinase (PI3K), a key regulator of insulin-induced GLUT4 translocation to the cellular membrane, and the mitogen-activated protein kinases (MAPK) pathway, which regulates cellular functions including proliferation. However, if naringenin impaired cancer cell proliferation by affecting glucose uptake or the two actions are independent is still not well understood. A controversial effect was reported in L6 skeletal muscle cells, in which naringenin stimulated glucose uptake independent of insulin and increased AMP-activated protein kinase (AMPK) activation and, as suggested by authors, these differences could be explained supposing a cell-specific effect of naringenin.17
Figure 1.
Structures of flavonoids that inhibit GLUTs.
Myricetin (2, Figure 1), fisetin (3, Figure 1), quercetin (4, Figure 1) and the glucoside of quercetin, isoquercitrin (5, Figure 1), share the same flavonol central scaffold and differ only for the presence of hydroxyl substituents or of a glucose moiety. These four flavonoids were found to inhibit the intestinal isoform GLUT2 by a noncompetitive mechanism, thus suggesting a potential use as antidiabetic and antiobesity agents. In particular, myricetin and quercetin were the most potent compounds in the inhibition of 2-deoxyglucose and fructose uptake, with IC50 values in the range of 12-17 μM.18 Quercetin proved to bind GLUT1 in the inward-facing conformation at a site that is accessible on the outer surface of the carrier.19
Moreover, myricetin, fisetin and quercetin, together with other flavonoids such as apigenin (6, Figure 1), luteolin (7, Figure 1), kaempferol (8, Figure 1), genistein (9, Figure 1) and silybin (10, Figure 1), proved to have an effect on glucose uptake in adipocytes involving a block of insulin-stimulated GLUT4 translocation to the plasma membrane.20 Strobel et al. reported a direct interaction of myricetin and quercetin with GLUT4, showing a competitive inhibition mechanism in adipocytes (Ki =33.5 and 16 µM, respectively).21 However, for this group of compounds no activities on the GLUT isoforms involved in cancer progression and development were described. In the same class of compounds, it is worth mentioning flavone apigenin (6), which inhibits GLUT1-mediated glucose uptake in human pancreatic cancer cells, in adenoid cystic carcinoma and in laryngeal carcinoma and it also inhibits GLUT1 expression, leading to a block of proliferation by apoptosis. The mechanism by which apigenin inhibits GLUT1 expression may involve the PI3K/Akt pathway, but this effect is not the only responsible of inhibition of glucose uptake.22-24
The flavan-3-ol epigallocatechin gallate (11, Figure 1), which is the main polyphenol present in green tea, and quercetin (4) markedly inhibited 3H-DG uptake in estrogen receptor (ER)-positive MCF7 and ER-negative MDA-MB-231 human breast cancer cell lines. Both compounds at a concentration of 100 µM were able to reduce lactate production in MCF7 cells and decreased MCF7 cell viability and proliferation.25
Kaempferol (8) was reported as a potent inhibitor of 3H-DG uptake in breast MCF-7 cells, with a IC50 value of 4 µM, showing a mixed-type competitive behavior, moreover it decreased GLUT1 protein levels. This compound produced a significant increase in the amount of extracellular lactate by inhibiting also the reuptake of lactate, which is mediated by monocarboxylate transporter 1 (MCT1), thus affecting the glycolytic metabolism of cancer cells at two different levels. This promising flavonol at a concentration of 100 µM reduced cell viability and proliferation rate. The same effects were mimicked by low glucose conditions and were reversed by high concentrations of glucose, suggesting that the cytotoxic effects are provoked by the block of glucose uptake.26
Dihydrochalcone phloretin (12, Figure 1) is found primarily in apples and pears and it can also be produced when its glycoside phlorizin (13, Figure 1) is orally consumed and subsequently nearly entirely converted into phloretin by hydrolytic enzymes in the small intestine. Phloretin strongly inhibits GLUT1 in a competitive manner.27-29 Both flavonoids, phloretin and phlorizin, were able to reduce tumour cell growth in vitro and in vivo and their cytotoxic effects were attributed to the inhibition of GLUT1.30,31 The block of glucose uptake elicited by phloretin also leads to sensitization of cancer cells to the chemotherapeutic agent daunorubicin, inducing apoptosis in a synergistic manner under hypoxic conditions.32 Phloretin induces apoptosis in HepG2 cells overexpressing GLUT2, which was reversed by glucose pretreatment. The same effect was induced by GLUT2 siRNA knockdown, suggesting that the cytotoxic action of phloretin involves the inhibition of GLUT2. Phloretin was administered in HepG2 xenografts and a reduction of final tumor weight and a severe decrease in 18F-FDG uptake by PET studies were observed without apparent toxicity, thus confirming the efficacy in vivo of this flavonoid in hepatoma cells.33
Isoflavone genistein (9) is commonly known as a tyrosine kinase inhibitor, but it was also reported as a competitive GLUT1 inhibitor (Ki of 7 µM) in human myeloid HL-60 cells, CHO-1 cells overexpressing GLUT1, and human erythrocytes, as well as a GLUT4 inhibitor in T3-L1 adipocytes (IC50 of 20 µM), although it cannot be considered as a specific inhibitor since it affects many other cellular activities.34,35 Kinetic studies on GLUT1 showed that genistein binds the transporter in the outward-facing conformation in a site that partially overlaps the external binding site of glucose.19
Phloretin, apigenin, and the two isoflavones daidzein (14, Figure 1) and genistein (9) were tested in androgen-sensitive (LNCaP) and -insensitive (PC-3) prostate cancer cells: GLUT protein levels are higher in androgen-insensitive PC-3 cells and therefore the four flavonoids inhibited cell growth more efficiently in LNCaP cells, where they modulate GLUT1 and GLUT4 expression and regulate glucose uptake in a quite complex manner, depending on androgenic signaling and incubation time. The direct interaction of these four compounds with GLUTs was supported by docking studies, which were performed in a model of the xylose transporter XylE crystallized from E. coli: they bind in a region of the receptor located near the active site present in both GLUT1 and GLUT4, thus hindering the glucose binding.36 The GLUT-inhibitory activity of daidzein, which differs from genistein since it lacks one hydroxyl group, is still controversial due to contrasting data, 34,37 although Cheong et al. observed an antihyperglycemic effect produced by daidzein, through an enhanced glucose uptake caused by GLUT4 translocation to plasma membrane in muscle cells and a subsequent suppression of the rises in blood glucose levels in vivo.38
The flavonoid silybin (10, known also as silibinin) is the major active constituent of the extract milk thistle (Silybum marianum) and it has been used for the treatment of liver diseases. Silybin, together with its oxidized form 2,3-dehydrosilybin, are competitive inhibitors of GLUT4 (Ki = 60 and 114 µM, respectively, in 3T3-L1 adipocytes), blocking glucose uptake and impairing cell viability in cancer cell lines.39 A phase I trial of silybin for the treatment of prostate cancer was completed in 2008, however this compound did not show an adequate tissue penetration despite the high blood concentrations observed in patients.40-42 A phase I study of a phosphatidylcholine derivative of silybin for the treatment of advanced hepatocellular carcinoma was prematurely stopped, due to the occurrence of adverse effects, consisting of an increase in liver function abnormalities and inflammatory biomarkers that led to the death of enrolled subjects.43
Xanthohumol (15, Figure 1) is a prenylated chalcone which occurs only in the hop plant and possesses a wide spectrum of anticancer activities. It proved to be a noncompetitive inhibitor of 3H-deoxy-d-glucose in a human first-trimester EVT cell line (HTR-8/SVneo cells) with an IC50 value of 3.55 µM. Moreover, it decreased cell viability, proliferation and culture growth, and these effects were mimicked by low extracellular glucose conditions and reversed by high extracellular glucose conditions. However, a specific effect of xanthohumol on GLUT1 was not determined, although this transporter is highly expressed in this specific cell line and it is responsible for the glucose uptake in placenta during pregnancy.44
Unfortunately, although this wide class of flavonoids exerts some relevant activity on GLUTs, their unspecific panels of biological activities make these compounds unsuitable to be developed as GLUT inhibitors for cancer treatment and it is difficult to discern if their beneficial antiproliferative activities on cancer cells derive from the block of glucose uptake or to their well-known antioxidant properties.
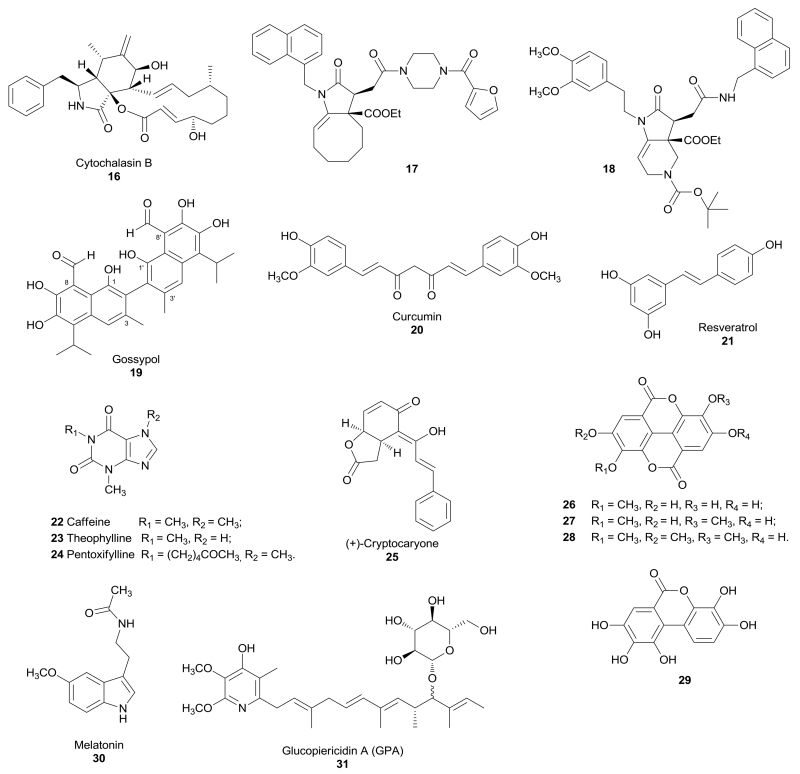
Cytochalasin B (16, Figure 2) is a macrocyclic mycotoxin known as a GLUT inhibitor in human erythrocytes,45,46 and it proved to bind GLUT1 and GLUT4 in skeletal muscle.47 Cytochalasin B in association with an OXPHOS inhibitor, such as antimycin A or leucascandrolide A, led to a rapid and synergistic intracellular ATP depletion in the highly glycolytic A549 lung carcinoma cells. A couple of derivatives of cytochalasin B, compounds 17 and 18 (Figure 2) that are noncompetitive inhibitors of GLUT1 (Ki = 1.2 and 0.8 µM for derivatives 17 and 18, respectively), proved to be able to suppress ATP synthesis when tested in combination with a blocker of mitochondrial function in A549 cells (IC50 = 10 and 3 µM for derivatives 17 and 18, respectively) and in CHO-K1 cells (IC50 = 2 and 1 µM for derivatives 17 and 18, respectively), but also in glioma and prostate cancer cells, and they reduced lactate production in the same cell lines. The two derivatives lack any off-target effect, such as depolymerization of the actin cytoskeleton, which is one of the most serious side effect of the parent compound cytochalasin B.48
Figure 2.
Structures of natural compounds that inhibit GLUTs.
Gossypol (19, Figure 2) is a natural polyphenolic binaphthyl disesquiterpene obtained from the cotton plant and it acts as an inhibitor of several dehydrogenases / protein kinases, as well as other various activities on many other targets. Pérez et al. identified gossypol as a GLUT1 inhibitor in human HL-60 cells, CHO cells, and human erythrocytes, with a mixed noncompetitive mechanism (Ki = 20 µM), by interacting with the transporter on the intracellular portion.49
Curcumin (20, Figure 2) derives from the rhizome of Curcuma longa and it is well known for its anti-inflammatory, anti-cancer and antioxidant properties. In order to investigate the exact mechanism of anti-cancer activity of curcumin, this natural compound was tested in lung cancer A549 cells and it showed an antiproliferative effect. Curcumin decreased cellular invasion and the expression of GLUT1, matrix metalloproteinase MT1-MMP and MMP2, which are considered as biomarkers of metastatic invasion. The anti-invasive effect of curcumin was impaired in GLUT1 over-expressing A549 cells. This result was confirmed in nude mice bearing tumors originated from A549 cells, in which over-expression of GLUT1 caused an increased resistance to the anti-metastatic effect of curcumin.50
Resveratrol (21, Figure 2) is a polyphenolic compound found in grape skins and peanuts, which is produced in response to injury or when the plant is attacked by pathogens, such as bacteria or fungi. Resveratrol is known as an anticancer, antioxidant, antinflammatory, antidiabetic and antiobesity agent, but it was reported to display a certain effect on glucose uptake and metabolism. In 2001 a study performed in myelocytic cell lines (U937 and HL-60) revealed that most glucose uptake in these cells was glucose-independent and was inhibited by resveratrol involving a direct action on GLUT1 and GLUT3 with Ki values of 89 and 85 µM, respectively.51 Lately, the behaviour of resveratrol in these two cell lines and in human red cells was better analyzed, revealing a mixed noncompetitive mechanism. In fact, resveratrol binds to an endofacial site of GLUT1, displacing the binding of cytochalasin B form the transporter.52 Conversely, resveratrol produced an increase in glucose uptake in skeletal muscle cells. However, this effect did not derive from the direct interaction of this compound with a GLUT transporter, rather it involved sirtuin-dependent AMPK activation which leads to stimulation of the activity of GLUT4.53
Methylxanthines, such as natural compounds caffeine (1,3,7-trimethylxanthine, 22, Figure 2) and theophylline (1,3-dimethylxanthin, 23, Figure 2), and synthetic derivative pentoxifylline [1-(5-oxohexyl)-3,7-dimethylxanthine, 24, Figure 2], were found to influence glucose uptake, but they also possess a variety of other physiological effects. They inhibit glucose uptake in human red blood cells by displacing previously-bound inhibitor cytochalasin B, thus confirming their direct interaction with the transporter. Methylxanthines bind a common site on the extracellular surface of GLUT1, which is close to, but distinct from, the glucose binding site.54
(+)-Cryptocaryone (25, Figure 2) is a natural compound isolated from tropical plants belonging to genus Cryptocarya (Lauraceae). Importantly, it proved to inhibit glucose uptake of about 40% at a concentration of 30 µM in human lung cancer H1299 cells.55 This inhibitory effect was considered to be responsible of the cytotoxic effect of this compound in human colon cancer HT-29 cells (IC50 = 0.32 µM) and also in murine leukemia P-388 cells (IC50 = 0.04 µM).56 In a separate study it was further investigated the mechanism of the antiproliferative activity in human prostate cancer PC-3 cells (IC50 = 1.6 µM) and it seemed induced by apoptosis through the activation of caspase-8 and 3.57
Four ellagic acid derivatives 26-29 (Figure 2) were isolated from Lagerstroemia speciosa, a tree originating from tropical countries. All of them were tested for their ability to inhibit the uptake of 2-deoxy-d-[3H]-glucose in 3T3-L1 adipocytes and proved to block the glucose transport with various potencies, however their exact mechanism of action was not further investigated.58
Melatonin (30, Figure 2) is a natural compound found in animal and plants. In humans it is secreted by the pineal gland and regulates the circadian rhythms of physiological functions, but it also participates to many other biological effects through the activation of its receptors. Many studies demonstrated that melatonin may influence insulin secretion and glucose homeostasis. The transport of melatonin inside cells is facilitated thanks to the amphipathic nature of its structure, but Hevia et al. demonstrated that GLUT1 was responsible of melatonin uptake into cancer cells and, in particular, melatonin binds the glucose binding site of the receptor. Differences in glucose concentrations and the presence of GLUT inhibitors affect the binding of melatonin. The presence of melatonin was observed inside human blood erythrocytes, which possess GLUT1 transporters, and its uptake was prevented by the inhibitor cytochalasin B. On the other hand, melatonin was not found in liposomes devoid of GLUT1. In prostate cancer LNCaP cells, it was observed a parallel trend for GLUT1 expression, intracellular concentration of melatonin and inhibition of cell proliferation. In order to confirm the antiproliferative effect of this compound, which is mediated by GLUT1, an in vivo study was performed on a mouse model for prostate cancer. This study revealed a longer survival of animals supplemented with glucose and melatonin than that of those supplemented only with glucose, thus confirming that melatonin attenuated the glucose-induced tumour growth.59
Kitagawa et al. identified glucopiericidin A (GPA, 31, Figure 2), extracted from a microbial broth of Lechevalieria sp., in a screening performed to find inhibitors of epidermial growth factor (EGF)-induced filopodia protrusion in human epidermal carcinoma A431 cells. In a metabolomic analysis this natural compound proved to decrease concentrations of pyruvate and lactate, as well as the lactate/pyruvate ratio. The glycolytic target of 31 was identified by observing that GPA significantly reduced [3H]-2DG uptake in cells, with an IC50 value of 4.9 nM, a value similar to that observed in the inhibition of filopodia protrusion. Therefore, since GLUT1 is responsible for glucose uptake in A431 cells, it was hypothesized that GPA inhibited GLUT1 function. GPA was tested also in Swiss 3T3-L1 adipocytes and demonstrated to inhibit the increased uptake of glucose in insulin-stimulated cells.60
It is noteworthy to mention the anticancer activity exerted by the extracts of the tropical tree usually known as Graviola (Annona muricata), which are composed mainly of long chained fatty acid derivatives (Annonaceous acetogenins). These natural extracts reduced the viability of pancreatic cancer cells and the expression levels of the GLUT1, GLUT4, as well as those of other glycolytic enzymes such as hexokinase II and lactate dehydrogenase A. Thus the impairment of the glycolytic metabolism is supposed to be responsible of the cytotoxic action exerted by these extracts in tumour cells. These effects probably led to the observed reduced glucose uptake, rather than a direct inhibition of the GLUTs by Graviola extracts.61
Synthetic GLUT inhibitors
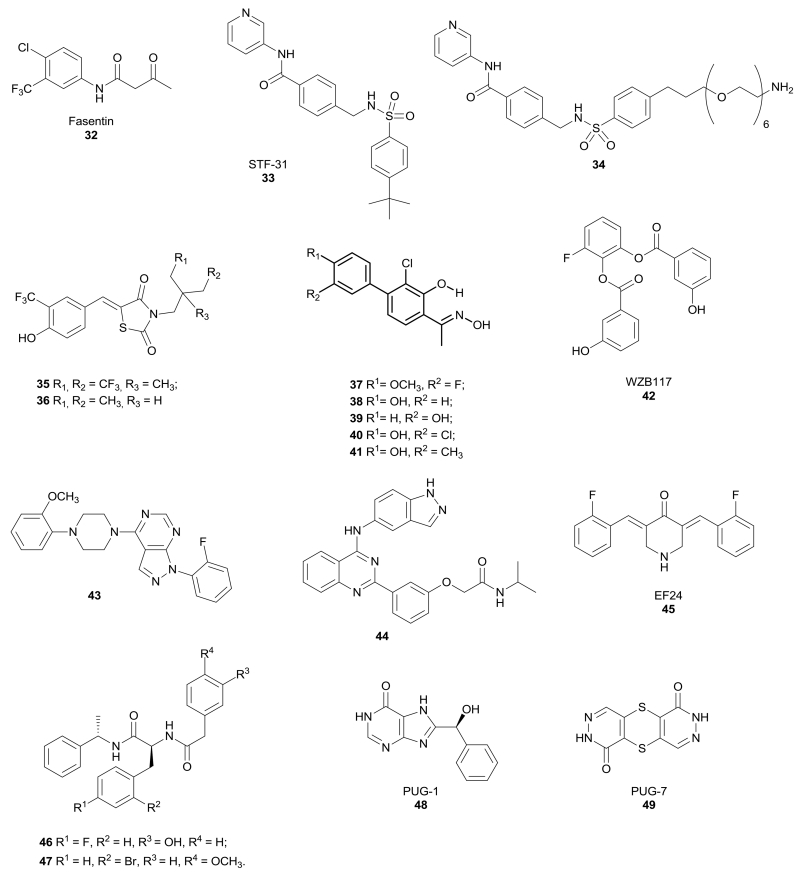
Enolic anilide Fasentin 32 (Figure 3) was identified as a GLUT1/GLUT4 inhibitor by a cell-based high-throughput screening to identify small molecules able to restore sensitivity of tumour cells to tumour necrosis factor (TNF) family death receptors and ligands.62 Fasentin partially inhibits glucose uptake at the same concentration at which it sensitizes cells to FAS, a death receptor belonging to the TNF family that is present on the surface of cells and leads to apoptosis. This suggests that fasentin acts by promoting intracellular glucose deprivation in leukemia U937 cells and prostate PPC-1 cells. Docking studies by using a GLUT1 homology model revealed that fasentin binds to the intramembrane channel of the protein. Moreover, its preferential inhibition of GLUT4 was assessed by observing that fasentin inhibits glucose uptake more potently in L6 myoblasts cells, which overexpress GLUT4.63
Figure 3.
Structures of synthetic compounds that inhibit GLUTs.
In 2011 the research group of Prof. Giaccia discovered a compound able to target GLUT1 and efficiently kill renal cell carcinoma, a tumour in which the von Hippel-Lindau (VHL) tumour suppressor gene is inactivated (VHL-deficient RCC4 cells), thus making it strongly dependent on aerobic glycolysis and consequently on GLUT1 activity. In fact, GLUT1 resulted to be overexpressed in these cells. Amido-sulfondamido derivative STF-31 (33, Figure 3) impaired glucose uptake and, therefore, it induced necrotic cell death in the VHL-mutated cells by disrupting their main source of energy. Modeling studies predicted the binding of STF-31 within the central channel of GLUT1, by establishing strong interactions with Arg126 and Trp412, which are essential residues for glucose transport.64 A STF-31 derivative (34, Figure 3) was immobilized to a column with a polyethylene glycol amino-alkyl linker introduced at the para position of the phenyl sulfonamide terminal moiety and it was used to confirm the specific binding of STF-31 to the GLUT1 isoform compared to GLUT2 and GLUT3. The immobilized drug only bound to GLUT1 from cell lysates of VHL-deficient RCC4 cells. This derivative retained activity, as demonstrated in a growth inhibition assays in the same cells, where it displayed an IC50 value of 7.9 µM. Similarly to its parent compound, 34 also maintained a good level of selectivity for VHL-negative vs. VHL-positive cells (selectivity ratio > 5). The maintained activity was due to a steric tolerance at the 4-position of the phenyl sulfonamide moiety, which is oriented toward the intracellular entrance of the channel.
Therefore, the introduction of the long chain linker in this position did not interfere with the activity of the resulting compound and, at the same time, provided a suitable connection point to conjugate the compound with a resin to be used for affinity chromatography for target identification.65 Further in vivo experiments were performed with a more soluble STF-31 analogue, whose structure has not been disclosed yet: it delayed tumour growth and produced a marked decrease in tumour glucose uptake, as demonstrated by FDG-PET, without provoking toxicity in the treated animals.
A series of thiazolidinedione derivatives were developed as GLUT inhibitors thanks to the observation that a peroxisome proliferator-activated receptor γ (PPAR γ) agonist exerted part of its action through the inhibition of glucose uptake.66 Compounds 35 and 36 (Figure 3) proved to block glucose uptake by LNCaP prostate cancer cells. In particular, compound 35 showed an improved activity compared to compound 36 (IC50 = 2.5 µM for 35 versus 6 µM for 36) and the same trend was observed in the suppression of cell viability by inducing apoptosis, without affecting prostate and mammary epithelial cells. The specific action of these compounds on GLUT1 was confirmed by assessing that LNCaP cell line mostly expressed GLUT1. Moreover, these cancer cells were transfected with plasmids encoding specific GLUT isoforms and the two compounds proved to inhibit glucose uptake preferentially in GLUT1-overexpressing LNCaP cells, with IC50 values very close to those measured in the original cell line (IC50 = 2 µM for 35 versus 5 µM for 36). Instead, weaker inhibition levels were found in cells transfected with GLUT3, GLUT4 and GLUT9, thus confirming that GLUT1 is the predominant isoform targeted by these compounds. Docking simulations revealed a binding site for compound 35 which is different from that of glucose. In fact, compound 35 seems to occupy the central part of the intermembrane channel and bind the transporter mostly by electrostatic and π-π stacking interactions. Further detailed studies were performed on compound 36 and it proved to be able to restore the sensitivity to gemcitabine in drug-resistant pancreatic cancer cells, by enhancing gemcitabine-induced DNA damage and inhibiting cell survival. Compound 36 abrogated the DNA damage response by inhibiting the activation of some factors involved in DNA reparation, thus increasing the efficacy of gemcitabine. The synergism with gemcitabine was demonstrated also in vivo: the co-administration of the two drugs reduced tumour growth to a greater extent than the treatment with the two compounds alone, without any evident signs of toxicity in treated mice.67
A series of methyl-ketoxime derivatives based on a salicylic scaffold, possessing a chlorine atom in position 3 of the central ring and a substituted phenyl ring in position para to the oxime group, proved to be active as GLUT1 inhibitors. These compounds (37-41, Figure 3) display a high similarity with many other GLUT1 inhibitors reported in literature, since most of them have similarly-spaced phenol-type hydroxy groups.68,69 This class of compounds was originally designed as estrogen receptor (ER) ligands, but luckily 4-aryl-substituted salicylketoximes, such as compounds 37-41, did not show any relevant biding affinity to estrogen receptors with the single exception of compound 38 which displayed a Ki value of 0.4 µM for ERβ.70 All the compounds were screened for inhibition of glucose transport through GLUT1 by a glucose uptake assay and were also subjected to an antiproliferative assay in a human non-small cell lung cancer cell line. They produced a significant reduction of glucose uptake, (IC50 values in the range 10-15 µM), and they showed also good cytotoxic activities (IC50 values in the range 18-46 µM). In order to confirm the mechanism of action of the four most selective GLUT1 inhibitors, the fluorescent glucose analogue 2-NBDG was used to visualize and quantify the inhibition of glucose uptake in cancer cells by fluorescence microscopy. These assays confirmed that ketoximes 37 and 39-41 exerted a more potent effect than that of the reference inhibitor phloretin. Modeling studies were performed in a homology model of the human GLUT1 receptor, built on the basis of the available X-ray structure of XylE, an Escherichia coli transporter homologue of GLUT1, and they identified three possible binding cavities; in particular, the intracellular cavity was identified as the most probable binding site of these compounds.
A library of polyphenolic esters was found to inhibit glucose transport and, in particular, compound WZB117 (42, Figure 3) proved to be an efficient inhibitor of glucose transport (93% inhibition at 30 µM), as well as cancer cell growth (41% inhibition at 30 µM, IC50 = 10 µM) in H1299 lung cancer cells. Although the polyphenolic ester structure of this compound constitutes a chemical liability since these groups can readily undergo hydrolytic transformations, the activities of these compounds both on glucose transport and on cancer cells were demonstrated to be due to the unaltered parent ester compounds, since their hydrolysis products were completely inactive.71,72 The mechanism of action of WZB117 was specific on GLUT1 isoform, since it inhibited glucose transport in human red blood cells, which express uniquely GLUT1. Modeling studies suggest the central channel region of the transporter as a possible binding site of these molecules, highlighting some interactions with important residues of the protein. These inhibitors caused a reduction of intracellular ATP and extracellular lactate concentration, due to a decreased glycolytic flux, and this finally led to cell-cycle arrest and necrosis. The antiproliferative activity of WZB117 was further confirmed on a series of cancer cell lines, such as non-small cell lung cancer A549 and breast cancer MCF7 cells, without affecting their non-tumorigenic counterparts, NL20 and MCF12A respectively, with a more pronounced effect under hypoxic conditions. A daily intraperitoneal injection of this inhibitor at 10 mg/kg in an A549 xenograft model of human lung cancer induced a reduction of the tumour volume of more than 70% compared to the control group, while producing only mild and temporary hyperglycemia in the animal.73
Very recently, an extensive series of 1H-pyrazolo[3,4-d]pyrimidines were discovered as GLUT1 inhibitors by a cell-based HTS screening at Bayer Pharma AG, in which the colorectal adenocarcinoma DLD1 cells were co-incubated with both the potential GLUT1 inhibitors and the oxidative phosphorylation inhibitor rotenone, in order to directly correlate the ATP production detected in cells with the amount of internalized glucose.74 Further cell-based studies revealed the competitive behavior of these compounds and their selectivity for GLUT1 over the other two main isoforms, GLUT2 and GLUT3. The representative compound 43 reported in Figure 3 possess some structural motives that resulted to be essential by SAR studies, such as: a) the ortho-methoxy group of the aryl ring connected to the piperazine; b) the central piperazine ring; c) the pyrazolo-pyrimidine scaffold; d) the presence of an electron-withdrawing group, like a fluorine atom, in the ortho position of the phenyl ring connected to the pyrazole. All these structural moieties led to an optimal balance between GLUT1 inhibition potency (IC50 = 0.007 µM) and excellent or moderate selectivity over GLUT2 (IC50 = 1.1 µM) and GLUT3 (IC50 = 0.04 µM). This compound showed a good permeability in Caco-2 cells and a suitable metabolic stability in pharmacokinetic assays performed in human liver microsomes and rat hepatocytes. The promising in vivo PK profile of this GLUT1 inhibitor revealed a low blood clearance and a good oral bioavailability (67%), indicating a complete absorption, together with a high volume of distribution and an intermediate terminal half-life.
Some quinazoline-based compounds were patented as GLUT1 inhibitors, but their target selectivity was low since most of them are also activators of AMPK, and/or inhibitors of protein kinase 2 (CK2) and Rho kinase (ROCK2). For example, compound 44 reported in Figure 3 is active on all the four targets and, in particular, it produced an inhibition of glucose consumption greater than 90% at 10 µM in fibrosarcoma HT1080 cells. This inhibitor was administered to mice fed with a high fat diet provoking an increase in fatty acid oxidation and blood glucose level, probably indicating a reduced cellular uptake of glucose.75
The structurally simple curcumin analogue EF24 (45, Figure 3) exerted antiproliferative and anti-angiogenic effects on a wide panel of cancer cells and it induced tumour regression in nude mice.76 Moreover, EF24 induced cell cycle arrest and apoptosis by means of a redox-dependent mechanism in MDA-MB-231 human breast and DU-145 human prostate cancer cells,77 and inhibited HIF-1.78 In 2013, it was demonstrated that EF24 exerted an anticancer effect also on ovarian cancer by targeting GLUT1. As a matter of fact, a 3 µM concentration of this molecule suppressed the proliferation and the invasivity of three ovarian cancer cells (SKOV-3, A2780 and OVCAR-3 cells), and it also influenced the metabolism by reducing glucose uptake, the rate of glycolysis and lactate production. These effects seem to be mediated by GLUT1 downregulation. The in vivo efficacy of EF24 was demonstrated in a xenograft tumour model, in which treatment with this compound decreased the growth of tumours and the number of intraperitoneal and lung metastases of SKOV-3 shControl cells more evidently than those produced by tumours of SKOV-3 shGLUT1 cells, thus suggesting that the anticancer activity of EF24 is mediated by GLUT1.79
Kapoor et al. identified a couple of new GLUT1 inhibitors based on a phenylalanine amide scaffold by a cell-based ultrahigh-throughput screening and these compounds were co-crystallized with human GLUT1 in the inward-open conformation (compounds 46 and 47, Figure 3). They potently inhibited glucose transport with IC50 values in the nanomolar range. In particular, compound 47 was more efficient in block the glucose uptake through GLUT1 (IC50 = 140 nM) than compound 46 (IC50 = 267 nM), but it was also more active in isoform GLUT4 (IC50 = 90 nM) compared to compound 46 (IC50 = 195 nM). Both the compounds were less potent on transporters GLUT2 and GLUT3. An X-ray study revealed that the binding sites of the newly discovered inhibitors, as well as that of cytochalasin B, overlap each other in the glucose site of the central cavity, despite the different chemical structures of these molecules. Amidic inhibitor 46 showed π-π interactions between its terminal phenyl and phenol rings and Trp388, as well as between the central fluoro-substituted phenyl ring and Phe379. Moreover, both the inhibitors 46 and 47 established hydrophobic interactions with Asn411 and Trp412. A determinant role was identified for Trp88 because it is conserved in all the GLUT family and its binding with the substrate glucose probably induces the closure of the central cavity of the transporter. Therefore, interactions of inhibitors with this residue may hinder the movement of the loop that closes the cavity and, therefore, block the flux of glucose through the transporter.80
Although a crystal structure of human GLUT1 in an inward open conformation was recently deposited,81 Ung and co-workers built a homology model of GLUT1 in the partially occluded outward open conformation, based on the X-ray structure of the E. coli xylose transporter, XylE. The occluded XylE structure is a suitable template thanks to its high resolution and limited dimensions, thus it can help to understand the interactions involved in substrate binding. This study revealed the presence of a hydrophobic pocket adjacent to the polar substrate binding site, which partially overlaps with the sugar-biding site. After the construction and the validation of the homology model by site-directed mutagenesis studies, a virtual screening of commercially available compounds was performed, focusing on the top-ranking compounds that interact in the glucose-biding site and in the newly discovered hydrophobic pocket. Among the eight best compounds, the xanthine derivative PUG-1 (48, Figure 3) proved to be a potent inhibitor of glucose transport, showing an IC50 value of 450 nM in the [3H]-2-deoxy-d-glucose uptake assay in the Chinese hamster ovary CHO cell line overexpressing GLUT1, and a high ligand efficiency value. PUG-1 was predicted to establish many hydrogen bonds in the sugar binding site as well as occupying the hydrophobic pocket. Moreover, it seems to interact with transmembrane helix 10, which strongly influences the size of the sugar binding site by shifting it in the outward open conformation and, therefore, blocking the channel portion between the substrate binding site and the cytosol. A pyridazinone-based compound PUG-7 (49, Figure 3) also resulted as a hit from the same virtual screening in spite of displaying a chemical structure which is quite different from those of the other GLUT1 inhibitors found. However, its inhibitory potency in the glucose uptake assay was lower (IC50 value of 11.8 µM) than that of PUG-1 probably because, due to its compact structure, it does not reach the hydrophobic pocket.82
Sugar-conjugate compounds interacting with GLUTs
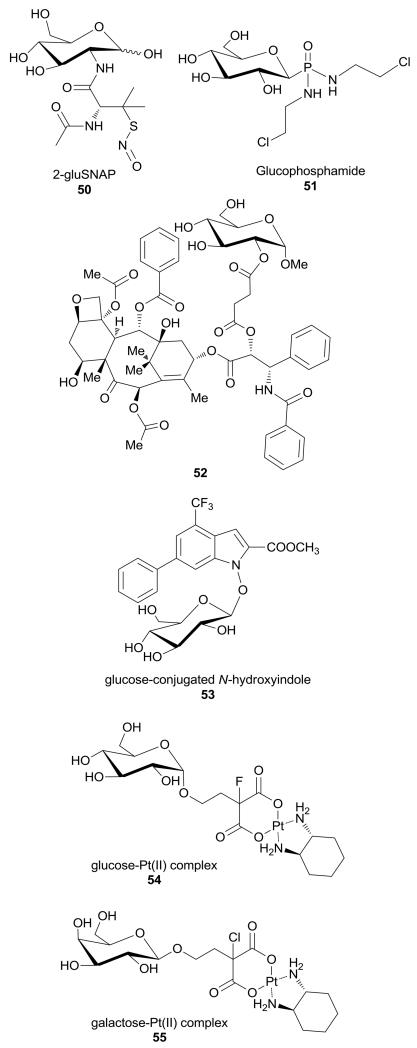
The conjugation of anticancer agents with glucose or different sugar portions is a widely exploited technique to design therapeutic agents, in order to improve their uptake into highly glycolytic cancer cells overexpressing GLUTs.7 Some of these agents take advantage of the sugar-conjugation to be internalized through GLUT receptors into the cancer cells without inhibiting GLUTs themselves. The glucose-conjugated S-nitroso-N-acetyl-penicillamine 2-Glu-SNAP 50 (Figure 4) was initially designed as a nitric oxide (NO) releasing compound, in which the carbohydrate unit linked through its 2-position has the function of enhancing its pharmacokinetic properties.83 This molecule exerted a marked cytotoxic effect in both cisplatin-sensitive (A2780S) and insensitive (A2780cP) human ovarian cancer cell lines, with IC50 values of 4.2 and 380 nM, respectively, and the different sensibility to 2-Glu-SNAP was explained considering the higher expression of GLUT1 in A2780S cells, although no better explanations were given about the possible mechanism of 2-Glu-SNAP cytotoxicity. 2-Glu-SNAP was 5000-fold more effective than the NO-donating moiety (SNAP) alone in A2780S cells, suggesting that the glucose moiety is responsible of the improved uptake into cancer cells.84 2-Glu-SNAP also proved to inhibit proliferation preferentially in GLUT1-overexpressing glioblastoma cell line T98G, while a GLUT1-deficient osteoblastoma cell line and its mitochondria-deficient variant rhoo cell line were only minimally affected, thus confirming the previous hypothesis.85
Figure 4.
Structures of sugar-conjugated compounds entering the cell by GLUTs.
Glucophosphamide 51 (Figure 4) is a DNA alkylating agent in which the isophosphoramide mustard, the cytotoxic metabolite of this drug, is covalently linked to glucose. Its anticancer potency was reduced if cells were co-treated with GLUT1 inhibitors, suggesting that its entry into the cells is mediated by GLUT1.86 It possesses a favorable safety profile and an evident anti-tumour activity in many resistant and advanced carcinomas overexpressing GLUT1, and many clinical trials demonstrated its efficacy and relative selectivity for cancer cells.87,88 Recently, two phase 2 studies of the efficacy and safety of glucophosphamide in previously treated advanced soft tissue sarcoma and in ovarian cancer were terminated (https://www.clinicaltrials.gov/ct2/search last access in February 2nd 2016), although they displayed a certain degree of adverse events associated to its administration. Now glucophosphamide is being studied in a phase 3 study in comparison with fluorouracil (5-FU) in patients with metastatic pancreatic adenocarcinoma previously treated with gemcitabine.
The anticancer drug paclitaxel is widely used for the treatment of breast, ovarian, and lung carcinomas, but its low water solubility has severely reduced its clinical application. Among the many adopted strategies to improve its pharmacokinetic properties, the glycoconjugation of paclitaxel led to a derivative in which the C-2’ hydroxyl group of the drug was linked to a 1-methyl glucose molecule via a short succinic acid linker (52, Figure 4).89 This new prodrug was designed to enhance the solubility, as well as to selectively deliver it to cancer versus normal cells by a preferential uptake via GLUTs. The resulting compound, whose transport was mediated at least in part by GLUT1 as confirmed by fluorescence microscopy studies, showed improved pharmaceutical properties and a comparable cytotoxicity against MCF-7 breast cancer cells without toxicity on normal cells, but it showed a reduced toxicity on most of the tested cancer cell lines compared to the parent compound.90
Calvaresi et al. discovered a glucose-conjugated methyl ester N-hydroxyindole 53 (Figure 4), which inhibits hLDH5 with a Ki value of 37.8 µM. This inhibitor proved to be able to cross the cytoplasmatic membrane very efficiently by means of GLUT1, which allowed its preferred uptake by cancer cells, as demonstrated by confocal laser scanning microscopy studies by using a fluorescent bioprobe that competes for cellular entry through GLUT transporters. Unfortunately, the conjugation with glucose made this hLDH5 inhibitor weaker than the N-OH analogue aglycone (Ki = 5.1 µM) on the isolated enzyme. Furthermore, the glycoconjugate N-hydroxyindole-based compound efficiently reduced lactate production in HeLa cells and compromised cell proliferation in several cancer cell lines.91
The platinum antitumour drug oxaliplatin is a commonly used chemotherapeutic agent applied in clinics especially for colorectal cancer, however its multiple side effects severely limited its benefits. The conjugation with sugar portions was introduced as a strategy to improve the tumour-targeting ability of the drug and also to enhance its water solubility, thus it can be excreted intact by the kidneys and avoid systemic toxicity. The glucosylated (trans-R,R-cyclohexane-1,2-diamine)-malonatoplatinum(II) derivative 54 (Figure 4) showed an increased cytotoxicity compared to oxaliplatin in all the tested human carcinoma cell lines. Its potency was prevented when human colon cancer (HT29) and breast cancer (MCF7) cells, which overexpress GLUTs, were treated with GLUT inhibitor phlorizin, thus confirming that the uptake and the antiproliferative activity of this compound are mediated by GLUT transporters.92
A further development of this platinum conjugate was obtained with the synthesis of galactose-platinum(II) complex 55 (Figure 4), in which platinum(II) is chelated by the same ligand trans-R,R-cyclohenxane-1,2-diamine. This compound was recently evaluated in human cancer cell lines and against two different xenograft tumour models. The results observed in human colon cancer HT29 cells by using GLUT inhibitor quercetin highlighted that, in the presence of the GLUT inhibitor the antiproliferative activity of galactose-platinum complex was highly reduced, suggesting that the uptake of the complex was regulated by GLUT. These results were confirmed by further competition assays by using fluorescent glucose bioprobe 2-NBDG: a 20 µM concentration of the Pt-complex resulted in a 30% decrease in 2-NBDG uptake, compared to HT29 untreated cells.93
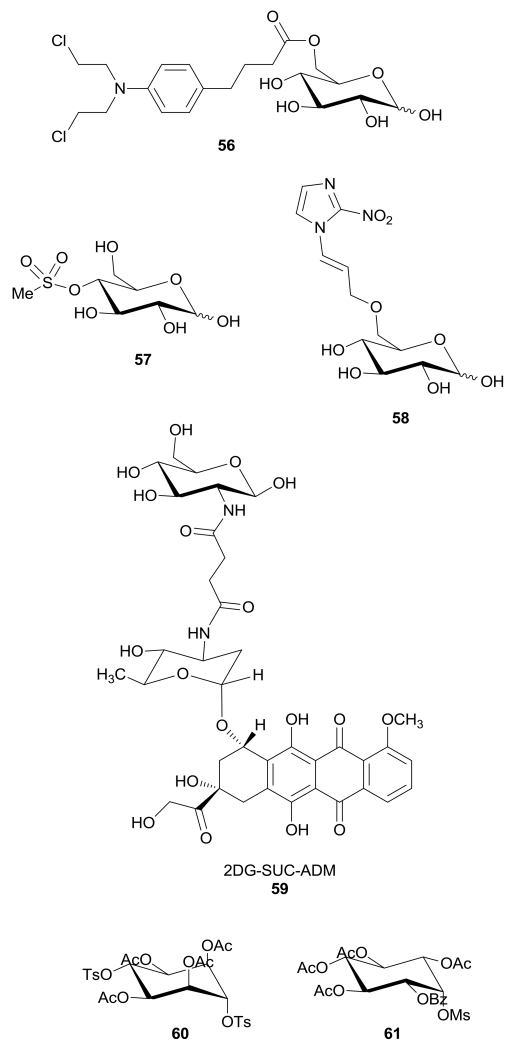
Some glyco-conjugated molecules reach their intracellular target by using GLUT-mediated transport, but at the same time they also directly inhibit or interact with GLUTs. An example is represented by the glucose-chlorambucil derivative 56 (Figure 5), where the alkylating portion is linked to the hydroxy group in position 6 of the glucose molecule to facilitate its delivery to the tumor. This derivative inhibits the d-[14C]glucose uptake by GLUT1 in human erythrocytes by a reversible and competitive mechanism (IC50 = 65 µM), thus excluding any alkylating mechanism on the transporter protein. The presence of the ester moiety and of the free anomeric group seems to be essential for its activity on GLUT, since both the amidic analogue and the derivative bearing a methoxy group at the anomeric position were less active.94
Figure 5.
Structures of sugar-conjugated compounds that inhibit GLUTs.
In 1997 a series of glycoconjugate analogues of the alkylating antineoplastic agent busulfan were synthesized, with the aim of improving selectivity and brain penetration to overcome the problem of poor uptake into the central nervous system of chemotherapeutic agents against cerebral tumours. In particular, the 4-O-methylsulfonyl-d-glucose 57 (Figure 5) proved to inhibit d-[14C]glucose uptake into the human erythrocyte by GLUT1 transporter, but its potency as GLUT inhibitor was very low (IC50 = 32 mM).95
Kumar et al. reported a 2-nitroimidazolyl−glucose conjugate coupled at C6-O of glucose via a propyl linker (58, Figure 5). This glucoazomycin was developed as a hypoxia-selective radiosensitizer and the sugar moiety was introduced with the aim of reducing the toxicity of the classical 2-nitroimidazoles by improving cancer cell selective delivery. Compound 58 was shown to bind the transporter and to compete with d-[14C]glucose uptake in Xenopus oocytes expressing GLUT1, with a weak ED50 of approximately 0.5 mM. This compound also proved to be an effective radiosensitizer in multiple cancer cell lines using radiation doses up to 18 Gy.96
The anthracycline antitumor antibiotic doxorubicin conjugated via a succinic spacer with a 2-amino-2-deoxy-glucose portion 59 (2DG-SUC-ADM, Figure 5) was designed to enhance the selectivity of doxorubicin against cancer cells and to reduce its toxicity to healthy cells. Its uptake resulted to be mostly mediated by GLUT1, since it was significantly inhibited by 2-deoxyglucose or quercetin in the human liver carcinoma HepG2 cells. The GLUT1-mediated transport inside cells explained the specificity of 2DG-SUC-ADM: it exerted an antiproliferative effect in cancer cells but, unlike doxorubicin alone, it was not cytotoxic for human embryonic lung fibroblast HELF cells. In fact, the uptake of free doxorubicin mainly occurs through molecular diffusion, whereas the uptake of 2DG-SUC-ADM is mostly mediated by GLUT1. Similarly, in vivo studies demonstrated that this conjugate significantly decreased systemic toxicity and enhanced the antitumor efficacy compared with free aglycone doxorubicin.97
A cell-based screening led to the discovery of two synthetic carbasugars 60 and 61 (Figure 5) that inhibited glycolysis by blocking glucose uptake in a dose-dependent manner, without interfering with other two glycolytic enzymes, such as hexokinase and pyruvate kinase, although in this case the authors did not mention any possible direct inhibition of GLUTs.98
Conclusions
Glucose transporters are currently being considered as some of the most relevant glycolytic effectors, which may be profitably targeted by innovative therapeutic interventions aimed at treating tumours. Their several-fold overexpression in cancer cells, when compared to normal cells, should guarantee a safe therapeutic window and, therefore, a selective cytotoxic effect of compounds that are able to interact with these transporters. In fact, the high efficiency of the glucose uptake by cancer cells has been successfully exploited for the selective delivery of various types of anticancer agents. Furthermore, the remarkable addiction displayed by cancer cells for sugars has led to the development of various classes of GLUT-inhibitors as prospective novel and selective anticancer drugs. Nevertheless, a certain degree of side effects may be expected, especially those occurring in organs characterized by high glucose-consumption rates, such as the brain. In any case, it is known that in starvation ketone bodies produced by the liver can replace glucose as fuel for the brain, whereas highly glycolytic cancer cells should not be able to fully utilize them. Therefore, a combined administration of glucose-interfering agents with either a ketogenic diet or dietary supplements such as triheptanoin (which is currently being tested for the treatment of GLUT1 deficiency99), should improve the safety profile of these compounds.
Finally, the recent publication of X-ray structures of these transporter will be of invaluable value in the near future for the refinement of the molecular modeling approaches dedicated to the discovery of more potent and selective inhibitors.
Supplementary Material
Acknowledgements
Support from the US National Institutes of Health (R01M098453) and intramural funds from the University of Pisa are gratefully acknowledged.
Footnotes
The authors declare no competing interests.
References
- 1.Warburg O. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann K, Benz MR, Krause BJ, Pomykala KL, Buck AK, Czernin J. Q. J. Nucl. Med. Mol. Imag. 2011;55:620–632. [PubMed] [Google Scholar]
- 3.Ben-Haim S, Ell P. J. Nucl. Med. 2009;50:88–99. doi: 10.2967/jnumed.108.054205. [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Chen X, Wang L, Chen S. Oncotarget. 2016 DOI: 10.18632/oncotarget.7676. [Google Scholar]
- 5.Granchi C, Fancelli D, Minutolo F. Bioorg. Med. Chem. Lett. 2014;24:4915–4925. doi: 10.1016/j.bmcl.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Granchi C, Minutolo F. ChemMedChem. 2012;7:1318–1350. doi: 10.1002/cmdc.201200176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvaresi EC, Hergenrother PJ. Chem. Sci. 2013;4:2319–2333. doi: 10.1039/C3SC22205E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin HJ, Kornmann F, Fuhrmann GF. Chem. Biol. Interact. 2003;146:225–235. doi: 10.1016/j.cbi.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Guthrie N, Carroll KK. Adv. Exp. Med. Biol. 1998;439:227–236. doi: 10.1007/978-1-4615-5335-9_16. [DOI] [PubMed] [Google Scholar]
- 10.Salvamani S, Gunasekaran B, Shaharuddin NA, Ahmad SA, Shukor MY. Biomed. Res. Int. 2014;2014:480258. doi: 10.1155/2014/480258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA. Med. Res. Rev. 2011;31:364–442. doi: 10.1002/med.20186. [DOI] [PubMed] [Google Scholar]
- 12.Akhlaghi M, Bandy B. J. Mol. Cell. Cardiol. 2009;46:309–317. doi: 10.1016/j.yjmcc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Park JB. Biochem. Biophys. Res. Commun. 1999;260:568–574. doi: 10.1006/bbrc.1999.0890. [DOI] [PubMed] [Google Scholar]
- 14.Harmon AW, Patel YM. Biochem. Biophys. Res. Commun. 2003;305:229–234. doi: 10.1016/s0006-291x(03)00720-4. [DOI] [PubMed] [Google Scholar]
- 15.So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Nutr. Cancer. 1996;26:167–181. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- 16.Harmon AW, Patel YM. Breast Cancer Res. Treat. 2004;85:103–110. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- 17.Zygmunt K, Faubert B, MacNeil J, Tsiani E. Biochem. Biophys. Res. Commun. 2010;398:178–183. doi: 10.1016/j.bbrc.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 18.Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M, Levine M. FASEB J. 2007;21:366–377. doi: 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- 19.Pérez A, Ojeda P, Ojeda L, Salas M, Rivas CI, Vera JC, Reyes AM. Biochemistry. 2011;50:8834–8845. doi: 10.1021/bi200748b. [DOI] [PubMed] [Google Scholar]
- 20.Nomura M, Takahashi T, Nagata N, Tsutsumi K, Kobayashi S, Akiba T, Yokogawa K, Moritani S, Miyamoto K. Biol. Pharm. Bull. 2008;31:1403–1409. doi: 10.1248/bpb.31.1403. [DOI] [PubMed] [Google Scholar]
- 21.Strobel P, Allard C, Perez-Acle T, Calderon R, Aldunate R, Leighton F. Biochem. J. 2005;386(Pt 3):471–478. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melstrom LG, Salabat MR, Ding XZ, Milam BM, Strouch M, Pelling JC, Bentrem DJ. Pancreas. 2008;37:426–431. doi: 10.1097/MPA.0b013e3181735ccb. [DOI] [PubMed] [Google Scholar]
- 23.Fang J, Bao YY, Zhou SH, Fan J. Mol. Med. Rep. 2015;12:6461–6466. doi: 10.3892/mmr.2015.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao YY, Zhou SH, Lu ZJ, Fan J, Huang YP. Oncol. Rep. 2015;34:1805–1814. doi: 10.3892/or.2015.4158. [DOI] [PubMed] [Google Scholar]
- 25.Moreira L, Araújo I, Costa T, Correia-Branco A, Faria A, Martel F, Keating E. Exp. Cell Res. 2013;319:1784–1795. doi: 10.1016/j.yexcr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Azevedo C, Correia-Branco A, Araújo JR, Guimarães JT, Keating E, Martel F. Nutr. Cancer. 2015;67:504–513. doi: 10.1080/01635581.2015.1002625. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg T, Wilbrandt W. Helv. Physiol. Pharmacol. Acta. 1957;15:168–176. [PubMed] [Google Scholar]
- 28.Tsujihara K, Hongu M, Saito K, Inamasu M, Arakawa K, Oku A, Matsumoto M. Chem. Pharm. Bull. 1996;44:1174–1180. doi: 10.1248/cpb.44.1174. [DOI] [PubMed] [Google Scholar]
- 29.Lefevre PG, Marshall JK. J. Biol. Chem. 1959;234:3022–3026. [PubMed] [Google Scholar]
- 30.Kobori M, Shinmoto H, Tsushida T, Shinohara K. Cancer Lett. 1997;119:207–212. doi: 10.1016/s0304-3835(97)00271-1. [DOI] [PubMed] [Google Scholar]
- 31.Nelson JA, Falk RE. Anticancer Res. 1993;13:2287–2292. [PubMed] [Google Scholar]
- 32.Cao X, Fang XL, Gibbs S, Huang Y, Dai Z, Wen P, Zheng X, Sadee W, Sun D. Cancer Chemother. Pharmacol. 2007;59:495–505. doi: 10.1007/s00280-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 33.Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, Lee CH, Liu RS, Lin SY. Int. J. Cancer. 2009;124:2210–2219. doi: 10.1002/ijc.24189. [DOI] [PubMed] [Google Scholar]
- 34.Vera JC, Reyes AM, Cárcamo JG, Velásquez FV, Rivas CI, Zhang RH, Strobel P, Iribarren R, Scher HI, Slebe JC, Golde DW. J. Biol. Chem. 1996;271:8719–8724. doi: 10.1074/jbc.271.15.8719. [DOI] [PubMed] [Google Scholar]
- 35.Bazuine M, van den Broek PJ, Maassen JA. Biochem. Biophys. Res. Commun. 2005;326:511–514. doi: 10.1016/j.bbrc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Menendez P, Hevia D, Rodriguez-Garcia A, Mayo JC, Sainz RM. Endocrinology. 2014;155:3238–3250. doi: 10.1210/en.2014-1260. [DOI] [PubMed] [Google Scholar]
- 37.Vera JC, Reyes AM, Velásquez FV, Rivas CI, Zhang RH, Strobel P, Slebe JC, Núñez-Alarcón J, Golde DW. Biochemistry. 2001;40:777–790. doi: 10.1021/bi001660j. [DOI] [PubMed] [Google Scholar]
- 38.Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, Yagasaki K. J. Nutr. Biochem. 2014;25:136–143. doi: 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Zhan T, Digel M, Küch EM, Stremmel W, Füllekrug J. J. Cell. Biochem. 2011;112:849–859. doi: 10.1002/jcb.22984. [DOI] [PubMed] [Google Scholar]
- 40.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. Invest. New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 41.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DCF, Agarwal C, Agarwal R. Cancer Res. 2002;62:3063–3069. [PubMed] [Google Scholar]
- 42.Flaig TW, Glodé M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, Su LJ, Li Y, Harrison G, Agarwal R, Crawford ED, Lucia MS, Pollak M. Prostate. 2010;70:848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 43.Siegel AB, Narayan R, Rodriguez R, Goyal A, Jacobson JS, Kelly K, Ladas E, Lunghofer PJ, Hansen RJ, Gustafson DL, Flaig TW, Tsai WY, Wu DP, Lee V, Greenlee H. Integr. Cancer Ther. 2014;13:46–53. doi: 10.1177/1534735413490798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correia-Branco A, Azevedo CF, Araújo JR, Guimarães JT, Faria A, Keating E, Martel F. Mol. Hum. Reprod. 2015;21:803–815. doi: 10.1093/molehr/gav043. [DOI] [PubMed] [Google Scholar]
- 45.Jung CY, Rampal AL. J. Biol. Chem. 1977;252:5456–5463. [PubMed] [Google Scholar]
- 46.Devés R, Krupka RM. Biochim. Biophys. Acta. 1978;510:339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- 47.Klip A, Paquet MR. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- 48.Ulanovskaya OA, Cui J, Kron SJ, Kozmin SA. Chem. Biol. 2011;18:222–230. doi: 10.1016/j.chembiol.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez A, Ojeda P, Valenzuela X, Ortega M, Sánchez C, Ojeda L, Castro M, Cárcamo JG, Rauch MC, Concha II, Rivas CI, Vera JC, Reyes AM. Am. J. Physiol. Cell. Physiol. 2009;297:C86–93. doi: 10.1152/ajpcell.00501.2008. [DOI] [PubMed] [Google Scholar]
- 50.Liao H, Wang Z, Deng Z, Ren H, Li X. Int. J. Clin. Exp. Med. 2015;8:8948–8957. [PMC free article] [PubMed] [Google Scholar]
- 51.Park JB. J. Nat. Prod. 2001;64:381–384. doi: 10.1021/np000411t. [DOI] [PubMed] [Google Scholar]
- 52.Salas M, Obando P, Ojeda L, Ojeda P, Pérez A, Vargas-Uribe M, Rivas CI, Vera JC, Reyes AM. Am. J. Physiol. Cell. Physiol. 2013;305:C90–99. doi: 10.1152/ajpcell.00387.2012. [DOI] [PubMed] [Google Scholar]
- 53.Breen DM, Sanli T, Giacca A, Tsiani E. Biochem. Biophys. Res. Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 54.Ojeda P, Pérez A, Ojeda L, Vargas-Uribe M, Rivas CI, Salas M, Vera JC, Reyes AM. Am. J. Physiol. Cell Physiol. 2012;303:C530–C539. doi: 10.1152/ajpcell.00145.2012. [DOI] [PubMed] [Google Scholar]
- 55.Ren Y, Yuan C, Qian Y, Chai HB, Chen X, Goetz M, Kinghorn AD. J. Nat. Prod. 2014;77:550–556. doi: 10.1021/np400809w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurniadewi F, Juliawaty LD, Syah YM, Achmad SA, Hakim EH, Koyama K, Kinoshita K, Takahashi K. J. Nat. Med. 2010;64:121–125. doi: 10.1007/s11418-009-0368-y. [DOI] [PubMed] [Google Scholar]
- 57.Chen YC, Kung FL, Tsai IL, Chou TH, Chen IS, Guh JH. J. Urol. 2010;183:2409–2418. doi: 10.1016/j.juro.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 58.Bai N, He K, Roller M, Zheng B, Chen X, Shao Z, Peng T, Zheng Q. J. Agric. Food Chem. 2008;56:11668–11674. doi: 10.1021/jf802152z. [DOI] [PubMed] [Google Scholar]
- 59.Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM. J. Pineal. Res. 2015;58:234–250. doi: 10.1111/jpi.12210. [DOI] [PubMed] [Google Scholar]
- 60.Kitagawa M, Ikeda S, Tashiro E, Soga T, Imoto M. Chem. Biol. 2010;17:989–998. doi: 10.1016/j.chembiol.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED, Johansson SL, Singh PK, Ganti AK, Batra SK. Cancer Lett. 2012;323:29–40. doi: 10.1016/j.canlet.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schimmer AD, Thomas MP, Hurren R, Gronda M, Pellecchia M, Pond GR, Konopleva M, Gurfinkel D, Mawji IA, Brown E, Reed JC. Cancer Res. 2006;66:2367–2375. doi: 10.1158/0008-5472.CAN-05-1061. [DOI] [PubMed] [Google Scholar]
- 63.Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, Klip A, Batey RA, Schimmer AD. Mol. Cancer Ther. 2008;7:3546–3555. doi: 10.1158/1535-7163.MCT-08-0569. [DOI] [PubMed] [Google Scholar]
- 64.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Sci. Transl. Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonnet M, Flanagan JU, Chan DA, Giaccia AJ, Hay MP. Bioorg. Med. Chem. 2014;22:711–720. doi: 10.1016/j.bmc.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sutphin P, Chan D, Turcotte S, Denny WA, Hay MP, Giddens AC, Bonnet M, Giaccia AJ. WO2011011514.
- 66.Wang D, Chu PC, Yang CN, Yan R, Chuang YC, Kulp SK, Chen CS. J. Med. Chem. 2012;55:3827–3836. doi: 10.1021/jm300015m. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Lai IL, Chou CC, Lai PT, Fang CS, Shirley LA, Yan R, Mo X, Bloomston M, Kulp SK, Bekaii-Saab T, Chen CS. Carcinogenesis. 2014;35:2203–2213. doi: 10.1093/carcin/bgu124. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Tuccinardi T, Granchi C, Iegre J, Paterni I, Bertini S, Macchia M, Martinelli A, Qian Y, Chen X, Minutolo F. Bioorg. Med. Chem. Lett. 2013;23:6923–6927. doi: 10.1016/j.bmcl.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 69.Granchi C, Qian Y, Lee HY, Paterni I, Pasero C, Iegre J, Carlson KE, Tuccinardi T, Chen X, Katzenellenbogen JA, Hergenrother PJ, Minutolo F. ChemMedChem. 2015;10:1892–1900. doi: 10.1002/cmdc.201500320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minutolo F, Bertini S, Granchi C, Marchitiello T, Prota G, Rapposelli S, Tuccinardi T, Martinelli A, Gunther JR, Carlson KE, Katzenellenbogen JA, Macchia M. J. Med. Chem. 2009;52:858–867. doi: 10.1021/jm801458t. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Liu Y, Chen X, Bergmeier SC. Bioorg. Med. Chem. Lett. 2010;20:2191–2194. doi: 10.1016/j.bmcl.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Zhang W, Cao Y, Liu Y, Bergmeier S, Chen X. Cancer Lett. 2010;298:176–185. doi: 10.1016/j.canlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. Mol. Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 74.Siebeneicher H, Bauser M, Buchmann B, Heisler I, Müller T, Neuhaus R, Rehwinkel H, Telser J, Zorn L. Bioorg. Med. Chem. Lett. 2016;26:1732–1737. doi: 10.1016/j.bmcl.2016.02.050. [DOI] [PubMed] [Google Scholar]; Heisler I, Mueller T, Golz S, Telser J, Rehwinkel H, Siebeneicher H, Buchmann B, Zorn L, Eis K, Koppitz M, Lindner N, Heroult M, Neuhaus R. WO2013182612.
- 75.Kim E, Cole B, Sweetnam P, Wong E. WO2012040499
- 76.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Bioorg. Med. Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Thomas SL, Zhong D, Zhou W, Malik S, Liotta D, Snyder JP, Hamel E, Giannakakou P. Cell Cycle. 2008;7:2409–2417. doi: 10.4161/cc.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang D, Wang Y, Dong L, Huang Y, Yuan J, Ben W, Yang Y, Ning N, Lu M, Guan Y. Cancer Sci. 2013;104:1690–1696. doi: 10.1111/cas.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kapoor K, Finer-Moore JS, Pedersen BP, Caboni L, Waight A, Hillig RC, Bringmann P, Heisler I, Müller T, Siebeneicher H, Stroud RM. Proc. Natl. Acad. Sci. U S A. 2016;113:4711–4716. doi: 10.1073/pnas.1603735113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N. Nature. 2014;510:121–125. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 82.Ung PM, Song W, Cheng L, Zhao X, Hu H, Chen L, Schlessinger A. ACS Chem. Biol. 2016 doi: 10.1021/acschembio.6b00304. DOI: 10.1021/acschembio.6b00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramirez J, Yu LB, Li J, Braunschweiger PG, Wang PG. Bioorg. Med. Chem. Lett. 1996;6:2575–2580. [Google Scholar]
- 84.Cantuaria G, Magalhaes A, Angioli R, Mendez L, Mirhashemi R, Wang J, Wang P, Penalver M, Averette H, Braunschweiger P. Cancer. 2000;88:381–388. [PubMed] [Google Scholar]
- 85.Subbarayan PR, Wang PG, Lampidis TJ, Ardalan B, Braunschweiger P. J. Chemother. 2008;20:106–111. doi: 10.1179/joc.2008.20.1.106. [DOI] [PubMed] [Google Scholar]
- 86.Pohl J, Bertram B, Hilgard P, Nowrousian MR, Stüben J, Wiessler M. Cancer Chemother. Pharmacol. 1995;35:364–370. doi: 10.1007/s002800050248. [DOI] [PubMed] [Google Scholar]
- 87.Briasoulis E, Judson I, Pavlidis N, Beale P, Wanders J, Groot Y, Veerman G, Schuessler M, Niebch G, Siamopoulos K, Tzamakou E, Rammou D, Wolf L, Walker R, Hanauske A. J. Clin. Oncol. 2000;18:3535–3544. doi: 10.1200/JCO.2000.18.20.3535. [DOI] [PubMed] [Google Scholar]
- 88.Shimizu T, Okamoto I, Tamura K, Satoh T, Miyazaki M, Akashi Y, Ozaki T, Fukuoka M, Nakagawa K. Cancer Chemother. Pharmacol. 2010;65:243–250. doi: 10.1007/s00280-009-1028-3. [DOI] [PubMed] [Google Scholar]
- 89.Liu DZ, Sinchaikul S, Reddy PV, Chang MY, Chen ST. Bioorg. Med. Chem. Lett. 2007;17:617–620. doi: 10.1016/j.bmcl.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Lin YS, Tungpradit R, Sinchaikul S, An FM, Liu DZ, Phutrakul S, Chen ST. J. Med. Chem. 2008;51:7428–7441. doi: 10.1021/jm8006257. [DOI] [PubMed] [Google Scholar]
- 91.Calvaresi EC, Granchi C, Tuccinardi T, Di Bussolo V, Huigens RW, 3rd, Lee HY, Palchaudhuri R, Macchia M, Martinelli A, Minutolo F, Hergenrother PJ. ChemBioChem. 2013;14:2263–2267. doi: 10.1002/cbic.201300562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu P, Lu Y, Gao X, Liu R, Zhang-Negrerie D, Shi Y, Wang Y, Wang S, Gao Q. Chem. Commun. (Camb) 2013;49:2421–2423. doi: 10.1039/c3cc38589b. [DOI] [PubMed] [Google Scholar]
- 93.Wu M, Li H, Liu R, Gao X, Zhang M, Liu P, Fu Z, Yang J, Zhang-Negrerie D, Gao Q. Eur. J. Med. Chem. 2016;110:32–42. doi: 10.1016/j.ejmech.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 94.Halmos T, Santarromana M, Antonakis K, Scherman D. Eur. J. Pharmacol. 1996;318:477–484. doi: 10.1016/s0014-2999(96)00796-0. [DOI] [PubMed] [Google Scholar]
- 95.Halmos T, Santarromana M, Antonakis K, Scherman D. Carbohydr Res. 1997;299:15–21. doi: 10.1016/s0008-6215(96)00328-x. [DOI] [PubMed] [Google Scholar]
- 96.Kumar P, Shustov G, Liang H, Khlebnikov V, Zheng W, Yang XH, Cheeseman C, Wiebe LI. J. Med. Chem. 2012;55:6033–6046. doi: 10.1021/jm2017336. [DOI] [PubMed] [Google Scholar]
- 97.Cao J, Cui S, Li S, Du C, Tian J, Wan S, Qian Z, Gu Y, Chen WR, Wang G. Cancer Res. 2013;73:1362–1373. doi: 10.1158/0008-5472.CAN-12-2072. [DOI] [PubMed] [Google Scholar]
- 98.Kitagawa M, Misawa M, Ogawa S, Tashiro E, Imoto M. Biosci. Biotechnol. Biochem. 2011;75:367–369. doi: 10.1271/bbb.100693. [DOI] [PubMed] [Google Scholar]
- 99.Mochel F, Hainque E, Gras D, Adanyeguh IM, Caillet S, Héron B, Roubertie A, Kaphan E, Valabregue R, Rinaldi D, Vuillaumier S, Schiffmann R, Ottolenghi C, Hogrel JY, Servais L, Roze E. J. Neurol. Neurosurg. Psychiatry. 2016;87:550–553. doi: 10.1136/jnnp-2015-311475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.