Abstract
Purpose
Our previous work suggested that HER3 inhibition sensitizes HNSCC to EGFR inhibition with cetuximab. This study aimed to define the role of HER3 in cetuximab resistance and the anti-tumor mechanisms of EGFR/HER3 dual targeting in HNSCC.
Experimental Design
We treated cetuximab-resistant HNSCC UMSCC1-C and parental UMSCC1-P cell lines with anti-EGFR antibody cetuximab, anti-HER3 antibody MM-121, and their combination. We assessed activities of HER2, HER3 and downstream signaling pathways by Western blotting, and cell growth by sulforhodamine B (SRB) and colony formation assays. HER3-specific shRNA was used to confirm the role of HER3 in cetuximab response. The combined efficacy and alterations in biomarkers were evaluated in UMSCC1-C xenograft and patient-derived xenograft (PDX) models.
Results
Cetuximab treatment induced HER3 activation and HER2/HER3 dimerization in HNSCC cell lines. Combined treatment with cetuximab and MM-121 blocked EGFR and HER3 activities and inhibited PI3K/AKT and ERK signaling pathways and HNSCC cell growth more effectively than each antibody alone. HER3 knockdown reduced HER2 activation and re-sensitized cells to cetuximab. Cetuximab-resistant xenografts and PDX models revealed greater efficacy of dual EGFR and HER3 inhibition compared to single antibodies. In PDX tissue samples, cetuximab induced HER3 expression and MM-121 reduced AKT activity.
Conclusion
Clinically relevant PDX models demonstrate that dual targeting of EGFR and HER3 is superior to EGFR targeting alone in HNSCC. Our study illustrates the upregulation of HER3 by cetuximab as one mechanism underlying resistance to EGFR inhibition in HNSCC, supporting further clinical investigations using multiple targeting strategies in patients who have failed cetuximab-based therapy.
Keywords: HNSCC, EGFR, HER3, PDX, Cetuximab
Introduction
Targeting epidermal growth factor receptor (EGFR) in head and neck squamous cell carcinoma (HNSCC) is an attractive and rational strategy given that more than 90% of these tumors overexpress EGFR (1, 2). Cetuximab, a chimerized antibody against EGFR, remains the only Food and Drug Administration (FDA)-approved targeted agent for HNSCC since its approval in 2006. The addition of cetuximab to platinum and fluorouracil treatment resulted in improved overall survival of patients with recurrent/metastatic HNSCC, and this combination has been adopted as the current standard of care for this population (3). Despite this success, the overall response rate to cetuximab as a single agent does not exceed 13%, with a response duration of less than 2–3 months (4). Moreover, intrinsic and acquired resistance during EGFR therapy inevitably occurs (5, 6). Several mechanisms have been identified through which resistance to EGFR-targeted agents occurs in HNSCC. EGFR gene mutation and compensatory signaling from HER3 and other EGFR (ErbB) family members have been suggested to be associated with sensitivity to cetuximab therapy in HNSCC (7, 8).
HER3 (ErbB3) is a member of the human EGFR family which consists of four type 1 transmembrane tyrosine kinase receptors: HER1 (EGFR, ErbB1), HER2 (Neu, ErbB2), HER3 (ErbB3), and HER4 (ErbB4). Upregulation of HER3 is commonly observed in various malignancies including breast, colorectal carcinoma, HNSCC, gastric, ovarian, prostate, and bladder cancers and correlates with poorer survival (9–13). Upon binding of HRG1, the physiological HER3 receptor ligand, HER3 dimerizes with other ErbB family members, preferentially HER2. Dimerization results in transphosphorylation of HER3 on tyrosine residues contained within the cytoplasmic tail of the protein (14–16). Phosphorylation of these sites creates SH2 docking sites for SH2-containing proteins, and specifically PI3-kinase (17). HER3 is a potent activator of AKT as it possesses six tyrosine phosphorylation sites with YXXM motifs that serve as excellent binding sites of the p85 regulatory subunit of PI3K, resulting in subsequent activation of the downstream AKT pathway (18, 19). These six PI3K sites serve as a strong amplifier of HER3 signaling. Activation of this pathway further elicits several important biological processes including cell growth and survival (20). Ligand-independent HER2/HER3 interaction has also been reported in HER2-amplified cells (21). Since HER3 can dimerize with EGFR, HER2 even c-Met, it likely plays a central role in response to EGFR-targeted therapy. Identifying biomarkers that can predict the clinical activity of HER3 and EGFR-targeted therapy will be crucial in understanding the mechanism of resistance to anti-EGFR therapy in HNSCC.
This study aimed to elucidate the role of HER3 in cetuximab resistance in HNSCC, to investigate whether adding anti-HER3 treatment to cetuximab-based regimens can improve the treatment of HNSCC in models more relevant to the clinic, and to understand the underlying anti-tumor mechanisms of anti-EGFR/HER3 approaches. For that purpose, we addressed the role of HER3 in cetuximab resistance in three settings: HNSCC cell lines, a xenograft mouse model using a cetuximab resistant HNSCC cell line, and multiple patient derived xenograft (PDX) mouse models using tissues from HNSCC patients.
Materials and Methods
Cell lines and reagents
Cetuximab was obtained from ImClone (New York, NY) and MM-121/SAR256212 was provided by Merrimack Pharmaceuticals (Cambridge, MA) and Sanofi (Bridgewater, NJ). Human HNSCC cell line UMSCC1-P and its cetuximab resistant counterpart UMSCC1-C were provided by Dr. Paul Harari (University of Wisconsin School of Medicine and Public Health, Madison, WI). The genotypes of the two cell lines were confirmed using the STR method by the Emory Integrated Genomics Core. The genotype of these two cell lines is identical to the original UMSCC1 cell line published previously by Zhao et al (22). All cell lines were maintained in Dulbecco's Modified Eagle's Media (DMEM) with 5% FBS and 0.4 µg/mL hydrocortisone at 37°C, 10% CO2 (23).
Colony formation assay
Cells were plated in 6-well culture plates at the concentration of 200 per well. After 24h incubation, cells were treated with PBS, cetuximab, MM-121 or the combination of cetuximab and MM-121 (CM combination) for 10 days to form colonies as previously described (24). Medium was changed every three days. The colonies were then stained with 0.2% crystal violet with buffered formalin (Sigma). Colony numbers were manually counted using Image J software. Cell numbers ≥50 were considered as a colony.
Sulforhodamine B (SRB) assay
The SRB assay was used for cell growth determination. Cells were seeded in 96-well plates in medium containing PBS control, cetuximab, MM-121, and CM combination for 48 hours. Cells were fixed with 10% trichloroacetic acid (Sigma-Aldrich St. Louis, MO) after an additional 24, 48 hours of culture. Cells then were washed 5 times with distilled and de-ionized water. After air drying, cells were incubated in 50 µl SRB (Sigma-Aldrich St. Louis, MO) for 10 min. Cells were then washed with 1% acetic acid 5 times. After air drying, 10mM Tris solution (pH 10) was added to dissolve the bound dye. Cell growth was assessed by optical density (OD) determination at 510 nm using a microplate reader.
Western blot analysis
Lysates of cell lines and xenograft tissues were generated using lysis buffer as previously reported (25). The lysate was centrifuged at 16,000 g at 4°C for 15 min. 40 micrograms of total protein for each sample were separated by 8–10% SDS-PAGE and transferred onto a Westran S membrane (Whatman Inc. Floham Park, NJ). Desired proteins were probed with corresponding antibodies. Rabbit anti-human AKT, ERK, pAKT, pERK, EGFR, pEGFR, HER2 pHER2, HER3 and pHER3 antibodies (1:1000 dilutions) were purchased from Cell Signaling (Danvers, MA), mouse anti-human β-actin (1:10000 dilution) from Sigma (St. Louis, MO), anti-human EGFR and HER3 antibodies from Santa Cruz (Santa Cruz, CA), and anti-human pEGFR antibody from Millipore (Temcula CA). HRP-conjugated secondary anti-mouse and anti-rabbit IgG (H+L) was obtained from Promega (Madison, WI). Bound antibody was detected using the SuperSignal West Pico Chemoluminescence system (Pierce, Inc., Rockford, IL). Image J software was used for blot quantification. Protein densitometry was determined by Image J software.
HER3 knock-down
To knock down HER3 in UMSCC1-C cells, we used pLKO.1 puro vector (Addgene, Cambridge, MA). On-line software from www.ambion.com was used to locate 3 potential siRNA sequences. Three pairs of shRNA were designed following the protocol provided by lentiweb.com. Basically, 3 pairs of oligonucleotides each containing the shRNA sequence and hairpin sequence plus Age I and EcoR I sites were synthesized and cloned into the pLKO.1 lentiviral vector. Only one of the three constructed targeting sequences ‘TGGTAGAGTAGAGAATTCATT’ showed a significant knock-down effect after stable cell lines were made with puromycin selection. Western blot was carried out to confirm the HER3 knock-down efficiency in these purified cells. HER3 knock-down cells were named UMSCC1-C/H cells. A vector control cell line, SCC1-C/pLKO1, was simultaneously generated.
Immunohistochemistry (IHC) staining and analysis
In brief, patient tumor tissues were harvested, fixed in 10% buffered formalin and embedded in paraffin. The sections were incubated with a primary antibody: anti-EGFR (1:100 dilution; Cell Signaling), anti-HER3 (1:100 dilution), followed by secondary antibody and diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA) staining. Nuclei were counterstained with hematoxylin OS (Vector Laboratories). Immunoglobulin G was used as a negative control. The quantifications were determined by at least 2 individuals blindly and independently. Briefly, the staining was scored for the proportion of stained tumor cells and for staining intensity; each on a four-point scale: 1: <5%, 2: 5–20%, 3: 21–50%, 4: 51–100%, and 1=no staining, 2=low, 3=moderate, and 4=high, respectively. IHC scores = (proportion × intensity) as previously described (26). The highest score is 16. Scoring was done at 200× magnification.
Immunoprecipitation (IP)
Cells cultured in 10cm dishes were collected and lysed with cold CHAP buffer with protease/phosphatase inhibitors. 2000µg total protein in 300 µl lysate buffer was incubated with anti-HER3 antibody (1:100) overnight at 4°C. 50 µl of immunoprecipitation beads (Pierce ProteinA/G Agarose, ThermoScientific, Waltham, MA) was directly dispensed into each lysate and further incubated for 2hrs. Immunoprecipitation beads were then spun down and washed 3 times with CHAP buffer. The pellet was then suspended in SDS-PAGE loading buffer.
In vivo xenograft treatment study
The animal experimental protocol was approved by the Institutional Animal Care and Use Committees of Emory University. In brief, 2×106 UMSCC1-C1 cells were injected subcutaneously into nude mice (athymic nu/nu, Taconic, NY) aged 4 to 6 weeks. Mice were randomly divided into 5 groups after tumor formation: PBS control, cetuximab 100µg/dose, MM-121 300µg/dose (MM-121.LD), and combination with low dose MM-121 (comb. LD) and combination with high dose MM-121 600µg/dose (comb. HD) (n=7 for each treatment group). Doses were chosen based on previous studies (24, 27). Drugs were given by intraperitoneal injection (I.P.) twice a week. Tumor volume and bodyweight were measured three times a week. Tumor volume was calculated using the formula: V= π/6×larger diameter × (smaller diameter)2 as reported previously (28).
PDX animal models
Briefly, fresh tumor tissue from patients with HNSCC consented at the Midtown Hospital; Emory University (Atlanta, GA) in accordance with the protocol approved by the Emory Institutional Review Board was collected. Tumor section was cut into 3 mm ×3 mm×3 mm small pieces which were then implanted into the hind flanks of NOD SCID mice (Charles River Laboratories International, Inc, Wilmington, MA). Upon reaching 1,500 mm3, tumors were passed to a second colony of athymic nu/nu nude mice (Harlan, Indianapolis, IN). The third generation of 24 nude mice was divided into 4 groups for drug treatment: PBS control, cetuximab 100µg/dose, MM-121 300µg/dose (MM-121.LD), and combination with low dose MM-121 (comb. LD).
Statistical analysis
Comparison of means from multiple treatment groups was carried out using one-way ANOVA or Kruskal-Wallis test to determine the significance of tumor growth inhibition among treatment groups. A Bonferroni correction was introduced to correct for multiple comparisons. The pairwise comparison was used to compare mean tumor volumes of cell growth inhibition between the different groups over time. All p values were two-sided and p values less than 0.05 were considered significant.
Results
Cetuximab induces HER3 expression and activation in HNSCC cell lines
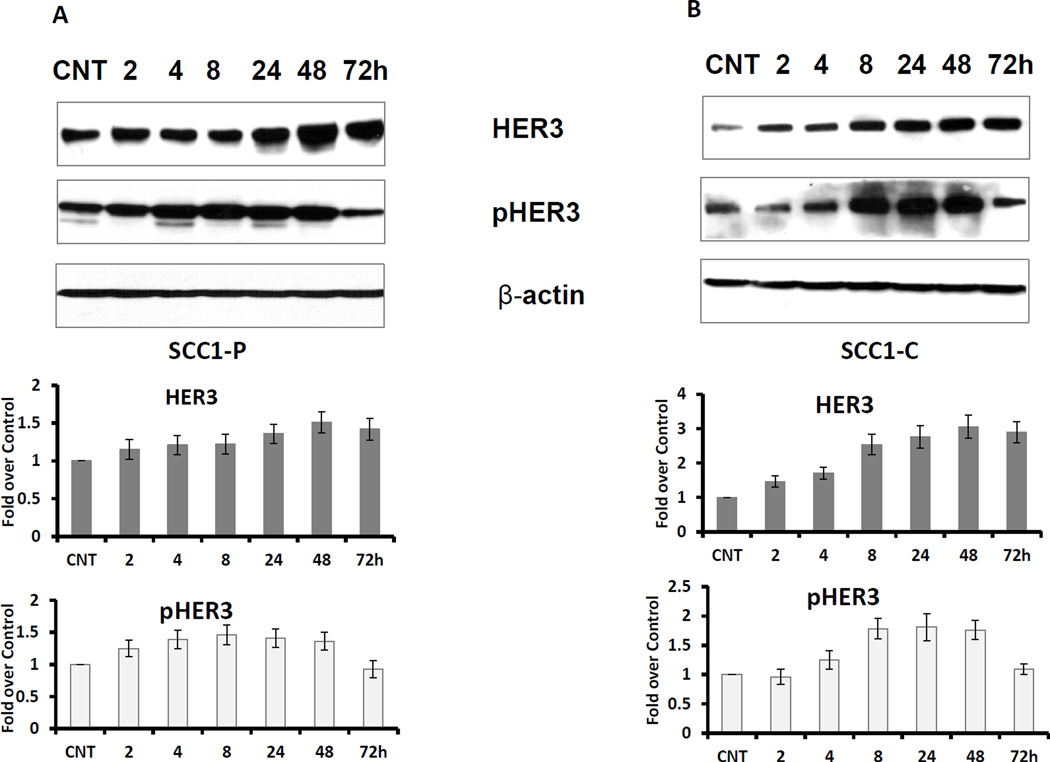
The compensatory overexpression of other HER family members has been implicated as one of the mechanisms that drive cetuximab resistance (7). To determine whether HER3 expression is affected by cetuximab, we treated both the cetuximab resistant UMSCC1-C cell line and its sensitive parental cell line UMSCC1-P with cetuximab. Ligand binding of HER3 causes a change in conformation that allows for dimerization, phosphorylation, and activation of HER3. Phosphorylation of HER3 at specific sites such as Y1289 and Y1222 by its heterodimerization partner, such as HER2, implicates activation of this protein. As shown in Figure 1 A and B, both HER3 expression and activation (indicated by the level of pHER3) were elevated in a time dependent manner. Induction and activation of HER3 by cetuximab was stronger in UMSCC1-C than UMSCC1-P cells.
Fig 1. Cetuximab induces HER3 expression and activation in HNSCC cell lines.
Cetuximab sensitive UMSCC1-P cells (A) and counterpart resistant UMSCC1-C cells (B) were cultured in medium with 5% FBS. The cells were treated with 2µg/ml cetuximab for the indicated times. CNT indicates the control sample. Both HER3 expression and activation (pHER3) levels were elevated by cetuximab treatment in a time dependent manner (Figure represents 1 of 3 experiments). The average fold increase was determined from 3 individual experiments with standard deviations.
HER2/HER3 dimerization is increased upon cetuximab treatment in UMSCC1-C cells
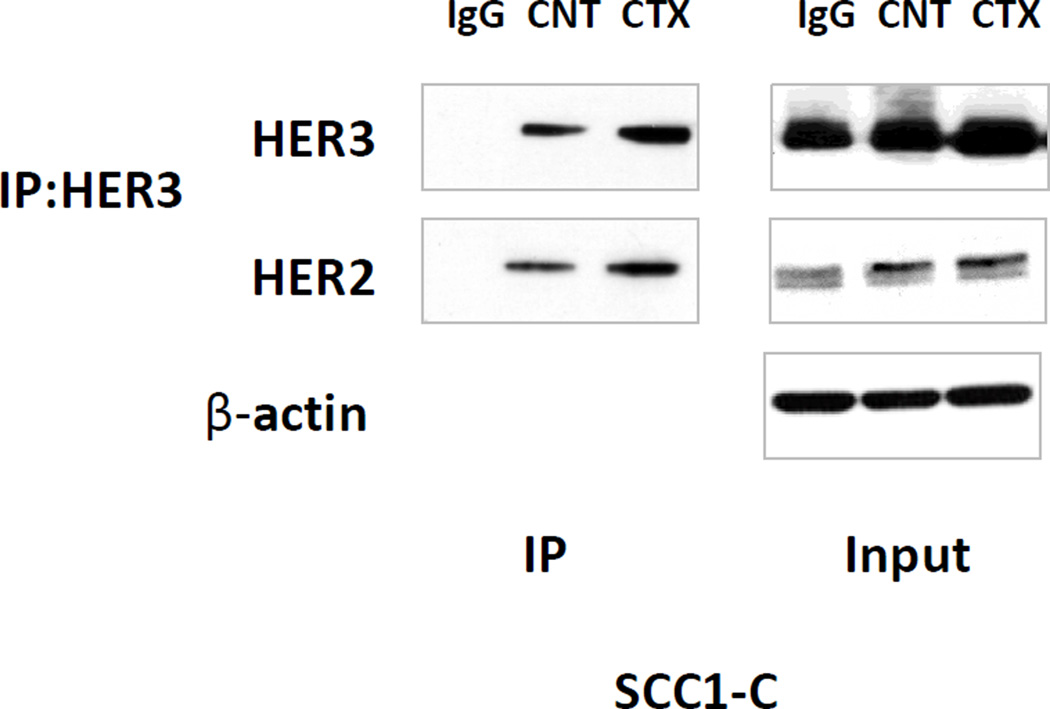
HER2/HER3 heterodimerization plays an important role in cancer progression (29–31). To examine whether cetuximab treatment has any effect on HER2/HER3 dimerization, we conducted an IP study. UMSCC1-C cells were incubated in the indicated cell medium with 5% FBS and 2µg/ml cetuximab for 24 hr. HER3 was immunoprecipitated with anti-HER3 antibody from the cell lysate. The immunoprecipitate was fractionated on SDS-PAGE followed by immunoblotting with anti HER2 and HER3. As Figure 2 shows, cetuximab treatment increased not only HER3 expression, but also HER2 and HER3 association in UMSCC1-C cells as illustrated by the greater level of HER2 detected in immunoblot analysis. The increase in HER2/HER3 dimerization by cetuximab treatment was also observed in MDA686TU cells (Supplementary Figure S1)
Fig 2. HER2/HER3 dimerization is increased upon cetuximab treatment.
UMSCC1-C cells were exposed to 2µg/ml cetuximab for 24 hr. HER3 was immunoprecipitated from the cell lysate with anti-HER3 antibody. The immunoprecipitate was fractionated on SDS-PAGE followed by immunoblotting with anti-HER2 and HER3 antibodies. As the image shows, cetuximab treatment increased HER3 expression in UMSCC1-C cells, at the same time HER2 and HER3 association was increased as more HER2 was detected by immunoblot in cetuximab treated cells compared with control cells and IgG control. β-actin was used as loading control (figure represents 1 of 3 experiments).
Inhibition of HER3 resensitizes resistant cell line UMSCC1-C to cetuximab
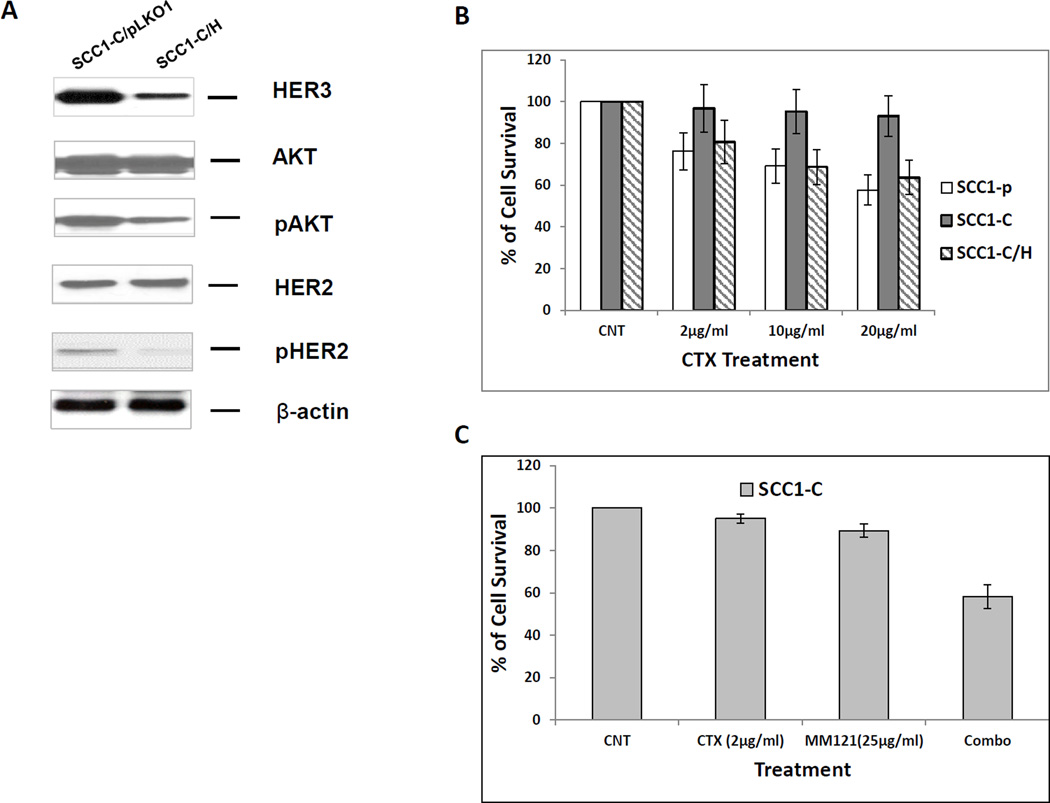
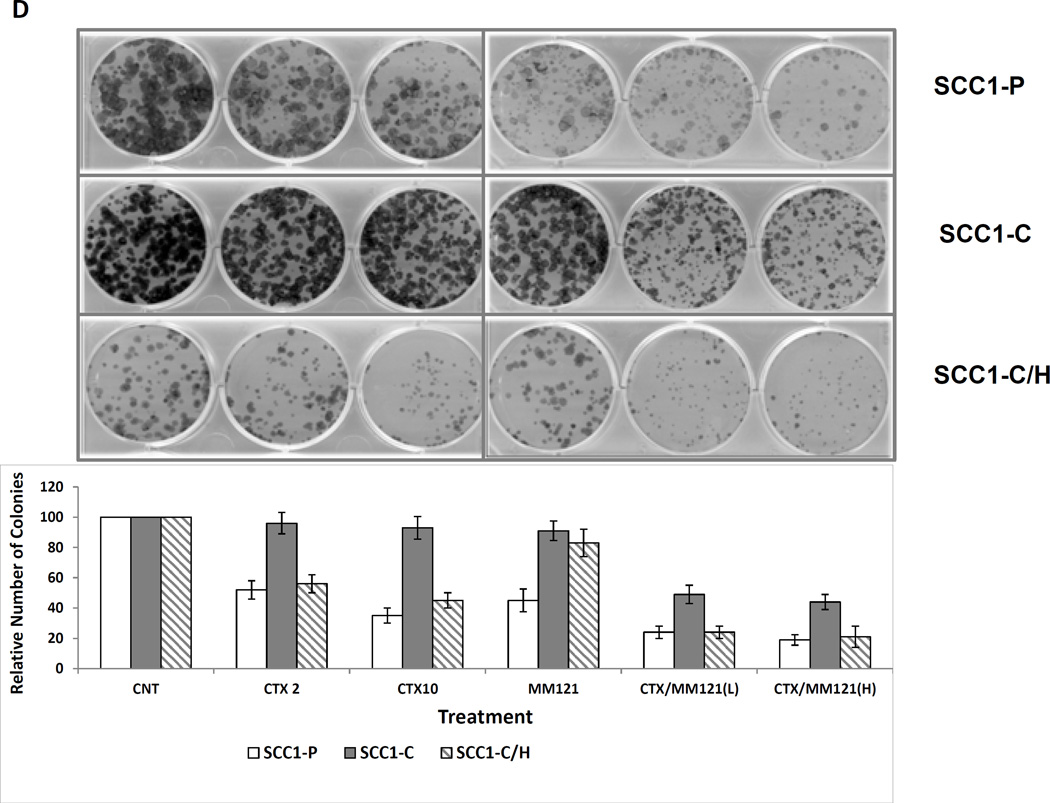
To determine if the reduction of HER3 expression has any effect on cetuximab sensitivity of the resistant cell line UMSCC1-C, we knocked down HER3 in UMSCC1-C cells with the pLKO.1 system to generate a stable cell line, UMSCC1-C/H, in which HER3 expression was reduced by more than 85% (Figure 3A). Interestingly, as HER3 level was knocked down, the levels of activated HER2 (pHER2) and AKT (pAKT) were also largely reduced as compared with the control UMSCC1-C cells (Figure 3A). SRB assay was performed to determine the sensitivity to cetuximab in parental UMSCC1-P, UMSCC1-C and HER3 knock-down UMSCC1-C/H cells. As shown in Figure 3B, the sensitivity of UMSCC1-C/H cells to cetuximab was recovered. Our previous study indicated a synergistic inhibitory effect of cetuximab and MM-121 on HNSCC cell lines TU212 and UMSCC47. In this study, SRB assay and colony formation assay were carried out to compare the growth inhibitory effect of MM-121 and its combination with cetuximab in UMSCC1-P, UMSCC1-C, and UMSCC1-C/H cells. In UMSCC1-C cells, treatment with cetuximab alone did not inhibit cell growth. However, MM-121 re-sensitized UMSCC1-C to cetuximab, as shown by the significant inhibition of cell growth by the combined treatment (Figure 3C). The MM-121 and cetuximab combination showed significantly greater inhibition of colony formation in comparison with either single drug in all three cell lines (Figure 3D). Consistent with the observations using the SRB assay, knock-down of HER3 in UMSCC1-C/H cells restored cell sensitivity to cetuximab, as indicated by the significant reduction in colony number in UMSCC1-C/H cells when treated with cetuximab as compared with UMSCC1-C cells (Figure 3D).
Fig 3. Inhibition of HER3 re-sensitizes resistant UMSCC1-C cell line to cetuximab.
(A) HER was knocked down in UMSCC1-C/H cells. HER2 and AKT activities were also reduced, as demonstrated by a decrease in both pHER2 and pAKT levels. (B) SRB assay shows that cetuximab reduces the growth rate of sensitive parental UMSCC1-P cells at the indicated concentrations after treatment for 48 h. No growth inhibition was observed in UMSCC1-C cells. Knock down of HER3 by shRNA re-sensitized UMSCC1-C cells to cetuximab inhibition. (C) Combination of cetuximab (2µg/ml) and MM-121 (25µg/ml)(combo) more potently inhibited UMSCC1-C growth in SRB assay. (D) In a colony formation assay, cetuximab inhibited colony formation of UMSCC1-P cells but not UMSCC1-C cells at the indicated concentration. When HER3 was knocked down by shRNA, cetuximab inhibition of colony formation was restored. Inhibition of HER3 by its antibody MM-121 (25 µg/ml) also resensitized UMSCC1-C to cetuximab treatment. (CTX2: cetuximab 2µg/ml, CTX10: cetuximab 10µg/ml, CTX/MM-121(L): combination of cetuximab 2µg/ml and MM-121, CTX/MM-121(H): combination of cetuximab 10µg/ml and MM-121). Image represents 3 individual experiments.
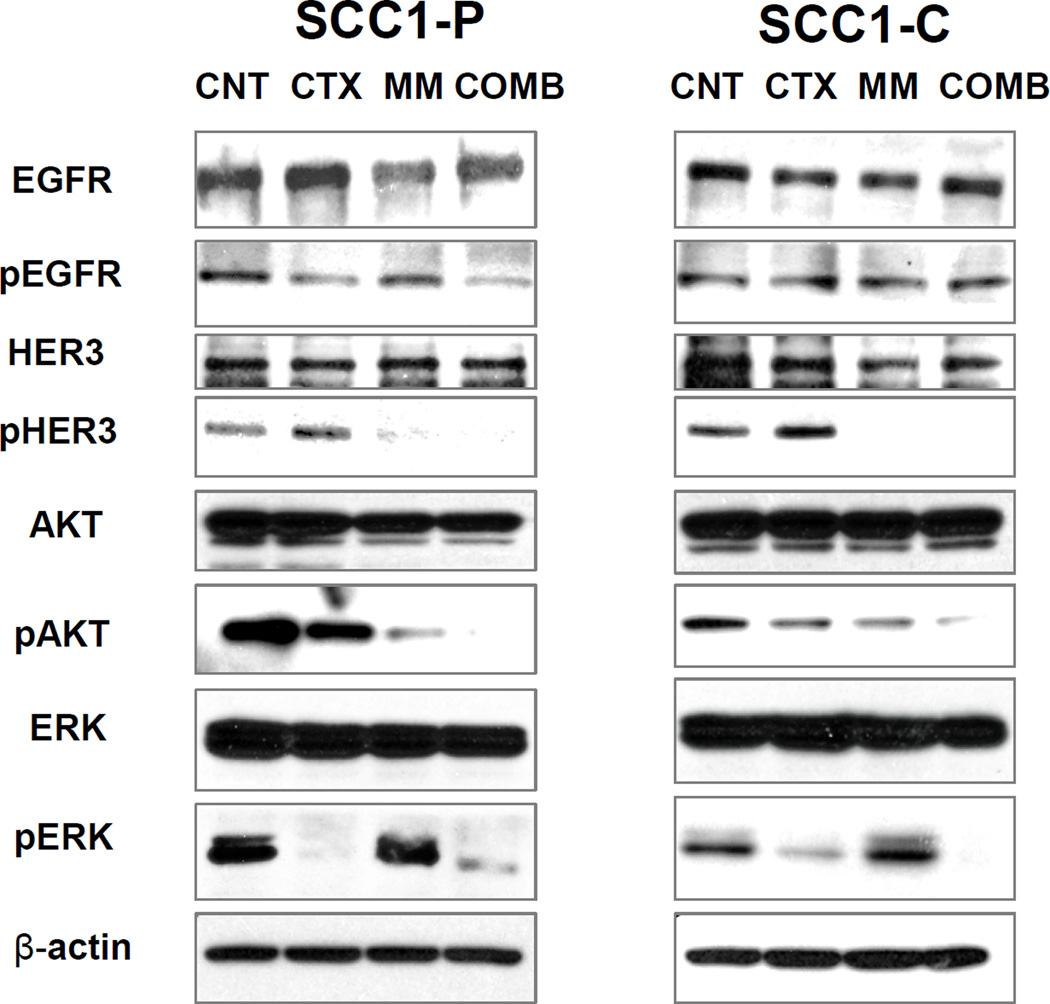
Combination of cetuximab and MM-121 inhibits both PI3K/AKT and ERK signaling pathways
HER family receptors activate a number of important signal transduction pathways including PI3K/AKT and ERK pathways, which are critical for cell growth. To identify the mechanism underlying the restoration of cetuximab sensitivity in the resistant cells, we treated both UMSCC1-P and UMSCC1-C cells with cetuximab, MM-121 and the combination and conducted a western blot assay to determine alterations in the PI3K/AKT and ERK pathways resulting from cetuximab and MM-121 treatment. As shown in Figure 4, the combination of cetuximab and MM-121 more potently inhibited both ERK and AKT pathway compared to each single agent. Inhibition of HER3 by MM-121 affected mainly the pAKT level while only moderate ERK alteration was observed.
Fig 4. Combination of cetuximab and MM-121 inhibits both PI3K/AKT and ERK signaling pathways.
UMSCC1-P (A) and UMSCC1-C (B) cells were treated with 2µg/mL cetuximab, 125µg/mL MM-121, and the combination, respectively. As shown in (A) and (B), after 48 hours of treatment, AKT and ERK activation was simultaneously ablated by the combination compared to single drugs and the control. The inhibition of pAKT was greater by MM-121 than cetuximab in both cell lines (figure represents 1 of 3 experiments).
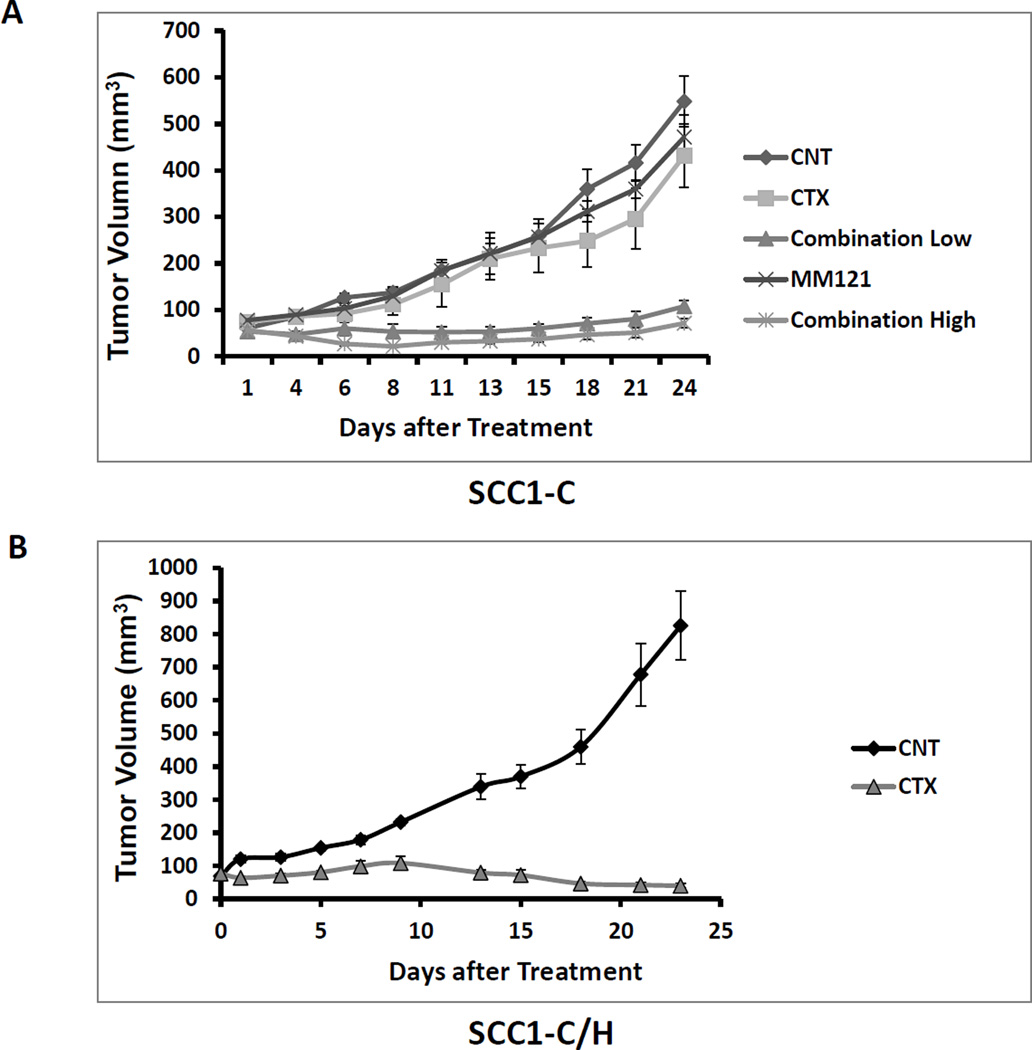
Inhibition of HER3 resensitizes resistant HNSCC tumor to cetuximab in xenograft models
To expand our findings into the in vivo setting, a xenograft model using the cetuximab resistant UMSCC1-C cell line in nude mice was established as previously described (7, 24). Mice were randomly assigned to five treatment groups: PBS control, cetuximab (C), MM-121 (M) and combination of cetuximab and MM-121 (CM high and low). Mice were treated twice a week through intraperitoneal injection (IP). Consistent with our in vitro observations, CM combination showed the greatest tumor growth inhibition of UMSCC1-C xenografts. As shown in the tumor volume measurement in Figure 5A, neither cetuximab nor MM-121 significantly reduced tumor growth compared to the PBS control. However, the group treated with CM combination showed significantly suppressed tumor growth as compared with the PBS control (p < 0.001), cetuximab (p < 0.001) and MM-121 (p < 0.001) alone for both high and low dose combination. Furthermore, in UMSCC1-C/H cells where HER3 expression is knocked down, the xenografted tumor was resensitized to cetuximab, such that cetuximab alone was sufficient to significantly inhibit UMSCC1-C/H tumor growth as compared with the control (Figure 5B) (p<0.001).
Fig 5. Combination of cetuximab and MM-121 shows strong antitumor activity in HNSCC cetuximab resistant tumor xenograft animal model.
(A) In xenograft models using cetuximab resistant UMSCC1-C cells, mice were randomly assigned to five treatment groups: PBS control, cetuximab (100µg/dose), MM-121 (300ug/dose), Comb.LD (cetuximab 100µg/dose/MM-121 300ug/dose), and Comb.HD (cetuximab 100µg/dose/MM-121 600ug/dose) and were treated twice a week through intraperitoneal injection. Consistent with our in vitro observations, the CM combination showed the greatest tumor growth inhibition in UMSCC1-C xenografts. Neither cetuximab nor MM-121 alone significantly reduced the tumor growth compared to PBS control. However, the groups treated with both high and low doses of CM combination showed significantly suppressed tumor growth as compared with those treated with PBS control, cetuximab and MM-121 alone. (p<0.001, n=6). (B) Mice carrying UMSCC1-C/H cell xenografts were randomly assigned to two treatment groups: PBS control, cetuximab (100µg/dose), Treatment with cetuximab significantly inhibited tumor growth (p<0.001, n=6).
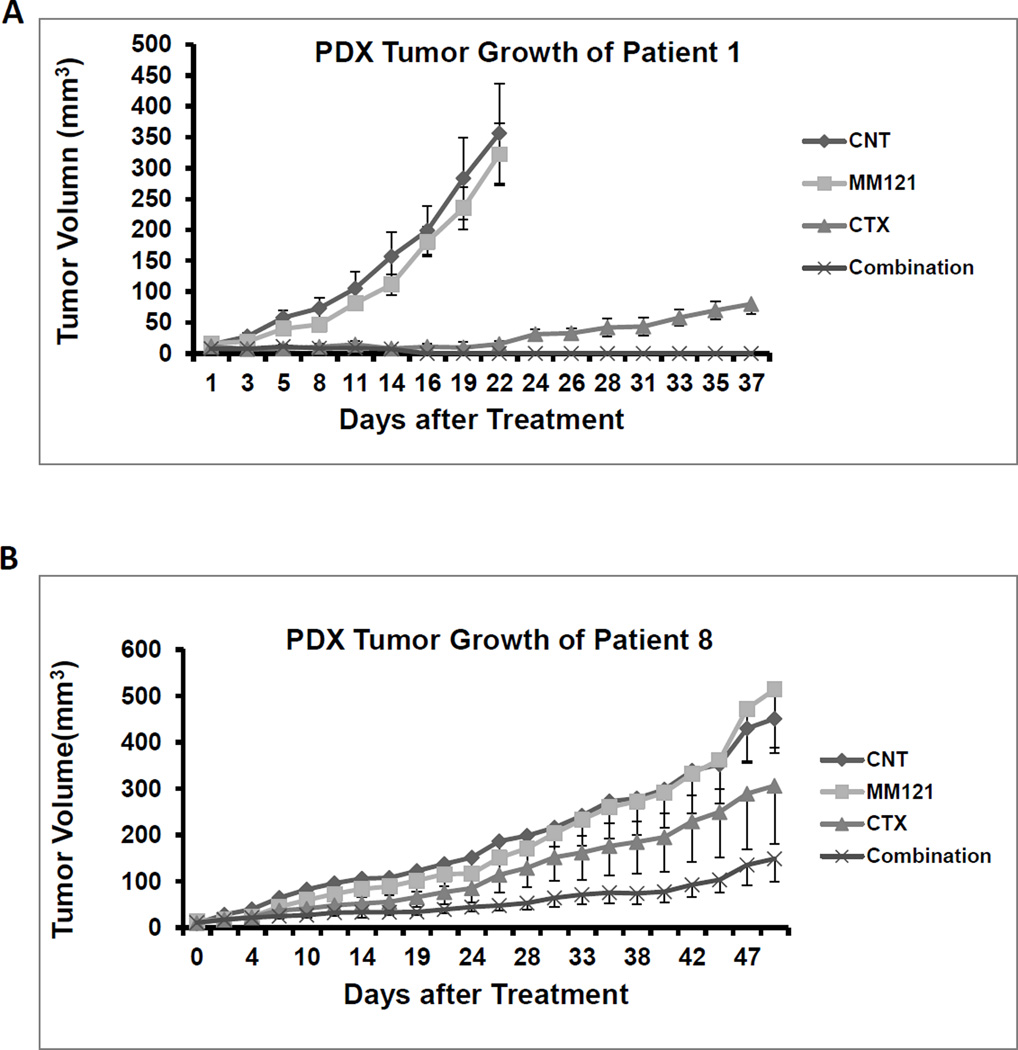
Combination of MM-121 and cetuximab shows strong antitumor activity in multiple PDX models
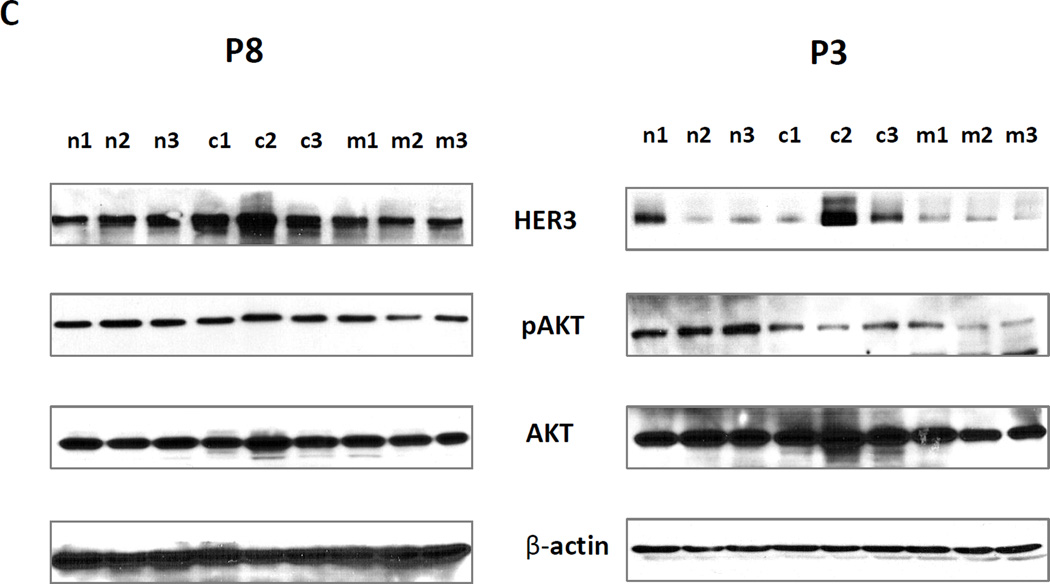
Tumor tissues from six patients with HNSCC were used to generate PDX models. The clinical characteristics of each patient are shown in Table S1. EGFR and HER3 expression levels in each patient tumor sample are shown in Supplementary Figure S2 A and B. For each PDX model, six tumor bearing mice were randomly assigned to each group treated with PBS, cetuximab (100ug/dose), MM-121 (300µg/dose) and the combination (100µg/dose cetuximab /300µg/dose MM-121). In PDX models derived from Patient 1 (FOM/tongue), Patient 2 (pyriform), Patient 4 (larynx, recurrent tumor with prior cetuximab treated), and Patient 5 (retromolar trigone), both cetuximab and the combination significantly inhibited tumor growth in nude mice (p<0.001 for both treatment) (Figure 6A, Supplementary Figure S3 A, B and C, and Table S1). There was no significant difference in tumor growth inhibition between single cetuximab and the combination treatment. However, the PDX tumors derived from Patient 1 grew back after stopping the treatment with cetuximab alone, while no tumor relapse was detected in the combination group (Figure 6A). Moreover, only the combined treatment could completely block the tumors growth in most of the mice even in recurrent patient (patient 4) PDX (Supplementary Figure S3). In PDX models derived from Patient 8 (supraglottis), only the combination treatment significantly inhibited tumor growth (p<0.004) (Figure 6B). We have identified a KRAS mutation by RNA-seq in this patient sample (data not shown). The combined treatment was also more effective than either single agent (p<0.032 and p<0.035 respectively). In PDX models derived from Patient 3 (FOM), both cetuximab and the combination significantly inhibited tumor growth in nude mice (p<0.001 for both treatment) (Supplementary Figures S4 A and B). However, the combination was significantly more effective than either of the single agents (p<0.01 for both). Interestingly, Western blot analyses of PDX tissues from Patient 3 and Patient 8 indicated induction of HER3 expression by cetuximab and reduction of AKT activity by MM-121 (Figure 6C). This is consistent with our observations in in vitro cell lines.
Fig 6. Combination of anti-HER3 antibody MM-121 and cetuximab more potently inhibits tumor growth in HNSCC patient derived tumor xenografts than single treatment with only MM-121 or cetuximab alone.
(A) In PDXs derived from Patient 1 tumor tissue, both cetuximab (100µg/dose) and the combination (cetuximab 100µg/dose and MM-121 300ug/dose) significantly inhibited tumor growth in nude mice (p<0.001 for both treatments). There was no significant difference between cetuximab alone and the combination. However, tumors treated with cetuximab alone grew back while no tumor relapse could be detected in the combination group. (B) In PDXs derived from Patient 8, only the combination treatment significantly inhibited tumor growth (p<0.004). Combination treatment was also more effective than either single agent (p<0.032 respectively). (C) As shown by immunoblotting, HER3 expression was induced in cetuximab treated PDX tumor samples, while pAKT was reduced by MM-121 treatment. No samples from the combination-treated PDXs were available for testing as the tumors were too small to isolate sufficient protein sample. n1–3: PDX tumor samples from 3 different PDXs in the control group. c1–3: three different tumor samples from the cetuximab treated group. m1–3: three different tumor samples from the MM-121 treated group. Image represents 3 repeated experiments.
Discussion
The clinical benefit of anti-EGFR therapy in HNSCC is limited by de novo and acquired resistance. Novel strategies to overcome this resistance are therefore highly justified. The HER3 protein is reportedly expressed in 32–87% HNSCC patient tumors (depending on the study) and is positively correlated with invasion and metastasis (32–34). Hyperactivation of HER3 has previously been reported to negatively correlate with response to anti-EGFR therapy (35). Moreover, HER3 activation represents a critical step by which HNSCC cells escape from cetuximab inhibition (7). Dual inhibition of both EGFR and HER3 is hence an attractive clinical strategy for treating HNSCC. Harari’s group observed a strong activation of HER3 in established cetuximab resistant cell lines. They also found that MEHD7945A, a monoclonal antibody that targets both EGFR and HER3, was more effective than a combination of cetuximab and anti-HER3 antibody at inhibiting both EGFR/HER3 signaling and tumor growth (36). Nakagawa et al have shown that the HER3 ligand heregulin is associated with both de novo and acquired resistance to cetuximab (37). They also found that patritumab, an antibody specific for HER3, is able to overcome such resistance. Our recent research identified that high heregulin mRNA and high HER3 protein levels are independent prognostic factors for poor overall survival in patients with oropharyngeal squamous cell carcinoma (OPSCC) (38). Our current study further demonstrated both in vitro and in vivo that inhibiting HER3 could resensitize cetuximab-resistant HNSCC cells to this agent.
In addition, we have previously shown that the combination of cetuximab and MM-121 significantly inhibited HNSCC tumor cell growth both in vitro and in vivo through inhibition of AKT, ERK, and S6 signaling pathways, suggesting that a multitargeting approach to the EGFR family of signaling receptors may provide potential clinical benefit for patients with HNSCC (24). In the current study, we demonstrated that HER3 expression and activity is upregulated after cetuximab treatment, suggesting that HER3 may hold the key to cetuximab resistance. Previous studies have shown that HER3 activity plays an important role in AKT activation (15, 39) and is induced by cetuximab, possibly due to the inhibition of AKT activity which in turn induces HER3 expression through FOXO (40). The inhibitory effect of cetuximab on AKT activation is, however, reduced as the elevated HER3 activates AKT, which prevents its complete inhibition. Our data demonstrated that either the combination of cetuximab and MM-121 or the use of shRNA against HER3 more potently reduced AKT activity and exerted greater cell growth inhibition in cetuximab-resistant cells than cetuximab alone, which is consistent with the findings from other groups (7, 41). More importantly, knock-down of HER3 with shRNA resulted in reduction of activated HER2, suggesting that HER2/HER3 heterodimerization probably contributes to cetuximab resistance. This notion is supported by our immunoprecipitation results demonstrating an increase in HER3 expression level following cetuximab exposure with greater HER3/HER2 heterodimerization, which is consistent with previous studies demonstrating increased HER2/HER3 dimerization leading to the activation of both HER2 and HER3 and their downstream signaling pathway in cancer (29, 42).
Antibody-dependent cell-mediated cytotoxicity (ADCC) plays an important role in antibody-based cancer therapy. ADCC has been reported for cetuximab (43, 44). In our study, MM-121 itself cannot trigger ADCC, because of its IgG2 isotype (45). Although the influence of MM121 on cetuximab ADCC in nude mice is unknown, it is unlikely that ADCC is the main reason for the resensitization of the resistant cancer cell line to cetuximab. However, the effect of MM121 on ADCC of cetuximab deserves further investigation.
In our xenograft models using the cetuximab-resistant UMSCC1-C cell line, neither cetuximab nor MM-121 alone significantly reduced tumor growth compared to PBS. The combination group, however, had significantly suppressed tumor growth compared to the single agent control. This result further supports the role of HER3 in cetuximab resistance. To our knowledge, this is the first time to explore dual inhibition of EGFR and HER3 in xenograft tumor models derived from patient-derived tumor tissue (PDTT). At low passage, it is believed that PDTT will conserve the original tumor characteristics such as heterogeneous histology, clinical biomolecular signature, malignant phenotypes and genotypes, tumor architecture and tumor vasculature (46–48). Patient-derived tumor grafts are believed to offer relevant predictive insights into clinical outcomes when evaluating the efficacy of novel cancer therapies. PDX models are biologically stable in terms of global gene expression patterns, mutational status, metastatic potential, drug response and tumor structure when passaged in short generations of mice. These characteristics might provide significant improvements over established cell-line xenograft models (49). We conducted PDX animal studies using tissues derived from 6 patients. Our study demonstrated the improved efficacy of the combination compared to single antibodies. Meanwhile immunoblotting from the available tumor samples revealed an elevated level of HER3 following cetuximab treatment, and a reduction in AKT activity following exposure to anti-HER3 in vivo. This further solidifies our in vitro observations. The lack of significant clinical activity using prior dual EGFR and HER inhibitors in HNSCC probably stems from the fact that these studies did not focus on a cetuximab-resistant patient population; we believe based on our results that future clinical investigations ought to focus on patients with prior exposure to EGFR inhibitors and perhaps with a more specific biomarker profile.
Taken together, our results demonstrate for the first time that dual targeting of EGFR and HER3 is more effective than EGFR targeting alone in HNSCC using a PDX model. Since this model is highly clinically predictive, our results pave the way for further clinical investigation focusing on pan-HER inhibition in a carefully selected HNSCC patient population, specifically those who have progressed after cetuximab-based therapy.
Supplementary Material
Translational Relevance.
The clinical benefit of anti-EGFR therapy using cetuximab in HNSCC is limited by de novo or acquired resistance. Novel strategies to overcome this resistance are therefore highly justified. Activation of HER3 has previously been reported to negatively correlate with response to anti-EGFR therapy. In this study, we demonstrated that dual targeting of EGFR and HER3 was superior to EGFR targeting alone in clinically relevant HNSCC PDX models; both a cetuximab resistant cell line and PDX models consistently demonstrated upregulation of HER3 and HER2/HER3 dimer by cetuximab as one mechanism underlying resistance to EGFR inhibition in HNSCC. Our results support further clinical investigations using multiple targeting strategies in patients who have failed cetuximab-based therapy.
Acknowledgments
The authors thank Dr. Anthea Hammond for her editing of the manuscript.
Financial support: The present study was supported by grants from National Institute of Health (R21 CA182662, PIs Saba NF and Chen ZG) and WCI Gregory Family Fund to Drs. Saba NF and Chen ZG.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Ongkeko WM, Altuna X, Weisman RA, Wang-Rodriguez J. Expression of protein tyrosine kinases in head and neck squamous cell carcinomas. Am J Clin Pathol. 2005;124:71–76. doi: 10.1309/BTLN5WTMJ3PCNRRC. [DOI] [PubMed] [Google Scholar]
- 2.Bei R, Budillon A, Masuelli L, Cereda V, Vitolo D, et al. Frequent overexpression of multiple ErbB receptors by head and neck squamous cell carcinoma contrasts with rare antibody immunity in patients. J Pathol. 2004;204:317–325. doi: 10.1002/path.1642. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 4.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 5.Shaib W, Kono S, Saba N. Antiepidermal growth factor receptor therapy in squamous cell carcinoma of the head and neck. J Oncol. 2012;2012:521215. doi: 10.1155/2012/521215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlacich G, Coffey RJ. Resistance to EGFR-targeted therapy: a family affair. Cancer Cell. 2011;20:423–425. doi: 10.1016/j.ccr.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Saba NF, Chen GZ, Shin DM. Targeting HER (ERBB) signaling in head and neck cancer: An essential update. Mol Aspects Med. 2015;45:74–86. doi: 10.1016/j.mam.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slamon DJ, Clark GM. Amplification of c-erbB-2 and aggressive human breast tumors? Science. 1988;240:1795–1798. doi: 10.1126/science.3289120. [DOI] [PubMed] [Google Scholar]
- 11.Maurer CA, Friess H, Kretschmann B, Zimmermann A, Stauffer A, et al. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol. 1998;29:771–777. doi: 10.1016/s0046-8177(98)90444-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 13.Beji A, Horst D, Engel J, Kirchner T, Ullrich A. Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer. Clin Cancer Res. 2012;18:956–968. doi: 10.1158/1078-0432.CCR-11-1186. [DOI] [PubMed] [Google Scholar]
- 14.Soltoff SP, Carraway KL, 3rd, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedi P, Pierce JH, di Fiore PP, Kraus MH. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol. 1994;14:492–500. doi: 10.1128/mcb.14.1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Suenaga A, Takada N, Hatakeyama M, Ichikawa M, Yu X, et al. Novel mechanism of interaction of p85 subunit of phosphatidylinositol 3-kinase and ErbB3 receptor-derived phosphotyrosyl peptides. J Biol Chem. 2005;280:1321–1326. doi: 10.1074/jbc.M410436200. [DOI] [PubMed] [Google Scholar]
- 19.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 20.Sergina NV, Rausch M, Wang D, Blair J, Hann B, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res. 2011;17:7248–7264. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32:417–426. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang N, Wang D, Hu Z, Shin HJ, Qian G, et al. Combination of anti-HER3 antibody MM-121/SAR256212 and cetuximab inhibits tumor growth in preclinical models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2014;13:1826–1836. doi: 10.1158/1535-7163.MCT-13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Muller S, Amin AR, Huang D, Su L, et al. The pivotal role of integrin beta1 in metastasis of head and neck squamous cell carcinoma. Clin Cancer Res. 2012;18:4589–4599. doi: 10.1158/1078-0432.CCR-11-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Souza G, Carey TE, William WN, Jr, Nguyen ML, Ko EC, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603–610. doi: 10.1097/QAI.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70:2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Yun S, Batuwangala TD, Steward M, Holmes SD, et al. A dual-targeting antibody against EGFR-VEGF for lung and head and neck cancer treatment. Int J Cancer. 2012;131:956–969. doi: 10.1002/ijc.26427. [DOI] [PubMed] [Google Scholar]
- 29.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 31.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia W, Lau YK, Zhang HZ, Xiao FY, Johnston DA, et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. 1999;5:4164–4174. [PubMed] [Google Scholar]
- 33.Takikita M, Xie R, Chung JY, Cho H, Ylaya K, et al. Membranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinoma. J Transl Med. 2011;9:126. doi: 10.1186/1479-5876-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bei R, Pompa G, Vitolo D, Moriconi E, Ciocci L, et al. Co-localization of multiple ErbB receptors in stratified epithelium of oral squamous cell carcinoma. J Pathol. 2001;195:343–348. doi: 10.1002/path.965. [DOI] [PubMed] [Google Scholar]
- 35.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer G, Haber L, Crocker LM, Shia S, Shao L, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011;20:472–486. doi: 10.1016/j.ccr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami H, Okamoto I, Yonesaka K, Okamoto K, Shibata K, et al. The anti-HER3 antibody patritumab abrogates cetuximab resistance mediated by heregulin in colorectal cancer cells. Oncotarget. 2014;5:11847–11856. doi: 10.18632/oncotarget.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian G, Jiang N, Wang D, Newman S, Kim S, et al. Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. Cancer. 2015;121:3600–3611. doi: 10.1002/cncr.29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 40.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S, Li C, Armstrong EA, Peet CR, Saker J, et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2013;73:824–833. doi: 10.1158/0008-5472.CAN-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A. 2010;107:7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincenzi B. Hot topic: biology in anticancer treatment. Curr Cancer Drug Targets. 2010;10:1–2. doi: 10.2174/156800910790980214. [DOI] [PubMed] [Google Scholar]
- 44.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang J, Wang S, Lyu H, Cai B, Yang X, et al. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer. 2013;12:134. doi: 10.1186/1476-4598-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanz L, Cuesta AM, Salas C, Corbacho C, Bellas C, et al. Differential transplantability of human endothelial cells in colorectal cancer and renal cell carcinoma primary xenografts. Lab Invest. 2009;89:91–97. doi: 10.1038/labinvest.2008.108. [DOI] [PubMed] [Google Scholar]
- 48.Gray DR, Huss WJ, Yau JM, Durham LE, Werdin ES, et al. Short-term human prostate primary xenografts: an in vivo model of human prostate cancer vasculature and angiogenesis. Cancer Res. 2004;64:1712–1721. doi: 10.1158/0008-5472.can-03-2700. [DOI] [PubMed] [Google Scholar]
- 49.Vandamme TF. Use of rodents as models of human diseases. J Pharm Bioallied Sci. 2014;6:2–9. doi: 10.4103/0975-7406.124301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.