Abstract
Reactive oxygen species (ROS) and electric currents modulate regeneration; however, the interplay between biochemical and biophysical signals during regeneration remains poorly understood. We investigate the interactions between redox and bioelectric activities during tail regeneration in Xenopus laevis tadpoles. We show that inhibition of NADPH oxidase-mediated production of ROS, or scavenging or blocking their diffusion into cells, impairs regeneration and consistently regulates the dynamics of membrane potential, transepithelial potential (TEP) and electric current densities (JI) during regeneration. Depletion of ROS mimics the altered TEP and JI observed in the non-regenerative refractory period. Short-term application of hydrogen peroxide (H2O2) rescues (from depleted ROS) and induces (from the refractory period) regeneration, TEP increase and JI reversal. H2O2 is therefore necessary for and sufficient to induce regeneration and to regulate TEP and JI. Epistasis assays show that voltage-gated Na+ channels act downstream of H2O2 to modulate regeneration. Altogether, these results suggest a novel mechanism for regeneration via redox-bioelectric orchestration.
KEY WORDS: NADPH oxidases, Reactive oxygen species, Membrane potential, Transepithelial potential, Electric current density, Voltage-gated Na+ channels, Regeneration, Xenopus laevis
Highlighted article: In the regenerative Xenopus tailbud, reactive oxygen species regulate local injury-induced electric currents that in turn modulate regeneration.
INTRODUCTION
Understanding large-scale repair via regeneration is a fundamental issue in basic biology and regenerative medicine. Biochemical signals, such as Wnt and BMP pathways, have been extensively studied and have been shown to control regeneration (Alvarado and Tsonis, 2006; Stoick-Cooper et al., 2007). Reactive oxygen species (ROS), especially hydrogen peroxide (H2O2), are suggested to act as signaling cues in the wound microenvironment (Sen and Roy, 2008; Veal et al., 2007). Upon wounding, the Drosophila embryo (Moreira et al., 2010), zebrafish larva (Niethammer et al., 2009) and Xenopus tadpole (Love et al., 2013) produce a H2O2 gradient that precedes the oxidative burst. This gradient, in fact, attracts immune cells toward the wound edges. In addition, a redox sensor has been identified in the leading edge of neutrophils (Wittmann et al., 2012; Yoo et al., 2011, 2012) and topical application of low doses of H2O2 enhances wound closure in mice (Loo et al., 2012). Importantly, ROS are required for the regeneration of planarian head and tail (Pirotte et al., 2015), zebrafish caudal fin (Gauron et al., 2013) and heart (Han et al., 2014), Xenopus tadpole tail (Love et al., 2013), and gecko tail (Zhang et al., 2016).
In addition to ROS, biophysical signals such as the dynamic bioelectric activities measured during regeneration have been proposed to act as signaling cues (Levin, 2007; McCaig et al., 2005). It has been shown that transepithelial potential (TEP)-driven electric current densities (JI) correlate with and are required for regeneration in amphibians (Borgens et al., 1977b; Jenkins et al., 1996; Nawata, 2001; Reid et al., 2009). Electrical stimulation induces some regeneration in non-permissive adult frog and rat limbs (Becker, 1972; Borgens et al., 1977a; Leppik et al., 2015; Smith, 1967). Moreover, engineered devices (Golding et al., 2016) partially induce mouse digit tip regeneration (Hechavarria et al., 2010) and are in clinical trials to improve paraplegia (Shapiro et al., 2005). Re-emerging bioelectric studies are revealing transduction mechanisms. V-ATPase-mediated repolarization of membrane potential (Vm) and voltage-gated Na+ channel (VGSC or NaV) 1.2-mediated sodium influx are necessary for and sufficient to induce X. laevis tadpole tail regeneration (Adams et al., 2007; Tseng et al., 2010). V-ATPase proton efflux activity correlates with regeneration rate and ability in zebrafish caudal fin regeneration (Monteiro et al., 2014). H+/K+-ATPase-mediated Vm depolarization modulates planarian head regeneration (Beane et al., 2011). In addition, ion and Vm dynamics correlate with axolotl larvae tail regeneration (Özkucur et al., 2010).
Interestingly, redox and bioelectric activities control the expression and activity of signaling pathways, such as Wnt, FGF, BMP and Notch, in addition to cell behaviors, such as proliferation, apoptosis and innervation, which are required for regeneration (Adams et al., 2007; Gauron et al., 2013; Han et al., 2014; Love et al., 2013; Monteiro et al., 2014; Tseng et al., 2010). Redox (ROS) and bioelectric [Vm, TEP, electric fields (EF) and JI] states therefore affect regeneration. But how do these signals integrate? Do they act in parallel or in series during regeneration? Xenopus laevis tadpole tail regeneration provides an ideal model with which to address these questions (Love et al., 2013; Reid et al., 2009). The tail comprises epidermis, muscle, blood vessels and spinal cord, making it a prime model for biomedical research (Beck et al., 2009; Deuchar, 1975). In addition, the fluctuating regenerative abilities of tails through developmental stages offer unique opportunities to study regeneration-deficient tails, in the refractory period (Beck et al., 2003), using the same model organism.
RESULTS
Do ROS modulate regeneration via the regulation of Vm, TEP, EF and/or JI, all of which are known to be required for regeneration? At the cellular level, NADPH oxidases in the cell membrane catalyze electron transfer from cytoplasmic NADPH to extracellular molecular O2, generating ROS and depolarizing Vm (Demaurex and Petheö, 2005; Lambeth and Neish, 2014). This dual property – electrogenic and catalytic – of NADPH oxidases led us to hypothesize a two-way regulation: (1) the activity of NADPH oxidases per se depolarizes Vm; and (2) the ROS produced, especially H2O2, regulate TEP, EF and JI. To test this hypothesis, we performed descriptive spatiotemporal profiling and functional assays.
Injury-induced extracellular bioelectricity dynamically correlates with regeneration
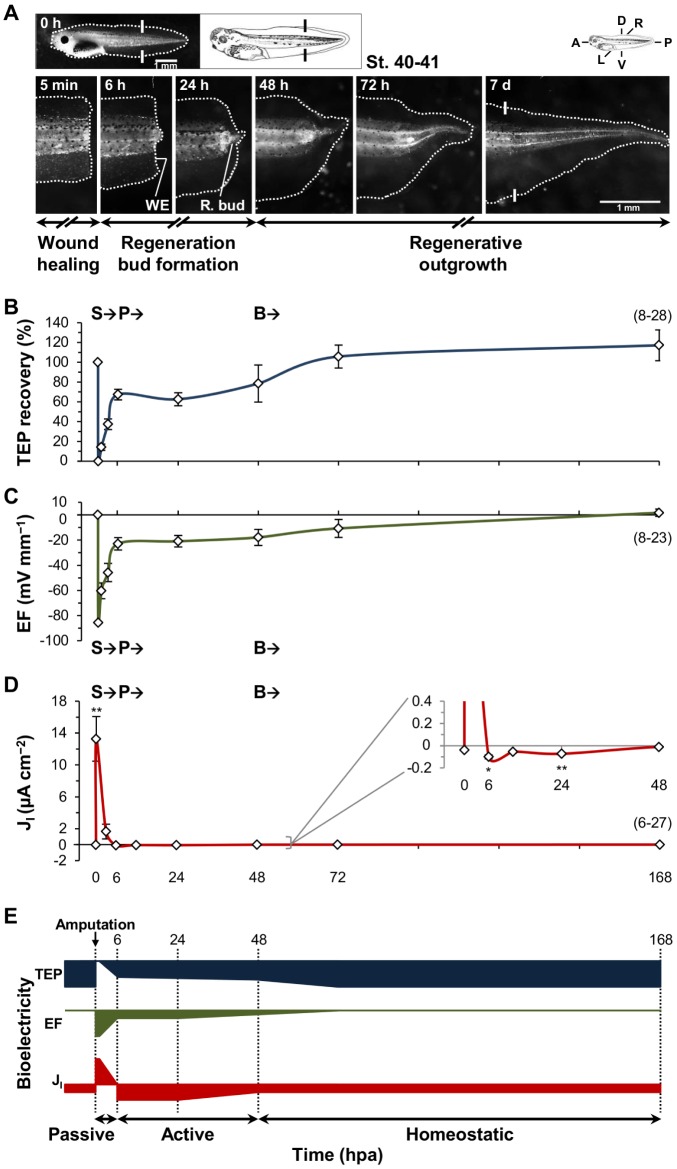
We first mapped the extracellular bioelectricity, TEP, EF and JI, during regeneration to select key spatiotemporal points at which to test the two-way regulation hypothesis. Regeneration can be divided into three phases, structurally completed in 7 days (Beck et al., 2009; Tseng et al., 2010): (1) wound healing [until ∼6 h post-amputation (hpa)] – wound epithelium covers amputation plane; (2) regeneration bud formation (∼24 hpa) – bud produces progenitor cells; and (3) regenerative outgrowth (∼48 hpa and after) – outgrowth and patterning reestablishes tail structure and function (Fig. 1A, Fig. S5).
Fig. 1.
Extracellular bioelectricity dynamically correlates with regeneration. (A) Regeneration time-lapse and phases of a representative tadpole tail amputated at stage 40-41. Major phenotypic structures annotated: wound epithelium (WE) and regeneration bud (R. bud). Photomicrographs of regenerating or regenerated tails are displayed in the same orientation as the whole organism showing anteroposterior (A/P), dorsoventral (D/V) and left-right (L/R) axes (top left scheme; applies to subsequent figures). White solid lines: amputation plane (black solid lines in schematic tadpole). Scale bars: 1 mm. (B) Temporal profile of TEP recovery in the regeneration bud in vehicle control (DMSO 0.1%). Calculated from data in Fig. S2C. (C) Temporal profile of lateral, trunk to bud, EF in vehicle control. Calculated from data in Fig. S2C. (D) Temporal profile of JI in the bud in vehicle control. Conventional current flow is used, thus positive values are net outward and negative are net inward currents (applies to subsequent figures). Profiles B-D are descriptively divided into three parts: S, slope; P, plateau; and B, baseline. (E) Diagrammatic representation integrating the temporal dynamics of TEP recovery, EFTrunk→Bud and JI during regeneration. Profiles are divided into three phases according to the bioelectric activity: passive, active and homeostatic. Magnitudes are not absolute but present the relative dynamics in temporal directions and magnitudes. Same x-axis labels for B-D; same x-axis titles for B-E. For B-D, n specimens indicated in brackets.
Before amputation, baseline trunk TEP was 28.64±2.27 mV (inside positive; n=9) and the tail tip had small inward currents of −0.04±0.005 µA cm−2 (n=7) (Fig. 1B, Fig. S2C). EF were assumed to be 0 mV mm−1, because over the measured distances (<1 mm), no consistent TEP gradients were detected. Upon amputation, a TEP short-circuit (to 0 mV) at the stump generated a strong lateral EFTrunk→Bud of −85.50 mV mm−1 (calculated using uncut TEP) and a low-resistance pathway leaking outward currents (the injury current) of 13.27±2.807 µA cm−2 (at 0.08 hpa; n=6, P=0.005) in the bud (Fig. 1B-D). Spatial profiles showed a consistent anteroposterior (A/P) gradient either directly (for TEP) or inversely (for EF) proportional to the distance from the amputation plane and evolving circuits (for JI and EF) during regeneration (Figs S2-S5). The spatial profile of JI was intended to unveil circuit loops (primarily dorsoventral) more than gradients (cases of TEP and Vm).
Temporal profiles presented exponential-like curves, positive for TEP recovery and for EF (Fig. 1B,C), and negative for JI (Fig. 1D), that correlated with regeneration. The dramatic TEP recovery (to 67.42±5.35%, n=26, P<0.0001 versus 1 hpa), decrease in EF (to −22.94±4.89 mV mm−1, n=23, P=0.0002 versus 1 hpa; 73% drop versus 0.08 hpa) and JI reversal (to −0.10±0.017 µA cm−2, n=27, P=0.005 versus 0.08 hpa; 99% shift) by 6 hpa (Fig. 1B-D) match the wound epithelium formation phase and possible bud initiation (Fig. 1A,E, Fig. S5). Spatially, JI reversals progressed in a centripetal way until 6 hpa, lastly in the bud (Fig. S4C), which is possibly due to centripetal healing. We descriptively termed this part of the curve as ‘slope’ (‘S’; Fig. 1B-D). Analogous TEP recovery is seen in wound healing assays in vitro (human skin) and in vivo (porcine skin) (Dubé et al., 2010). Similar drops in EF (75%) and JI reversal (in the spinal cord) by 6 hpa occur in newt limbs and X. laevis tadpole tail regeneration, respectively (McGinnis and Vanable, 1986; Reid et al., 2009). From 6 to 48 (especially to 24) hpa, TEP, EF and JI stabilization (P≫0.05 to all comparisons from 6 to 24 hpa; Fig. 1B-D) correlated with the regeneration bud formation phase (Fig. 1A,E, Fig. S5). We termed this part of the curve as ‘plateau’ (‘P’; Fig. 1B-D). From 48 hpa onwards, a final shift completed TEP recovery (Fig. 1B; bud versus trunk non-significant at 72 hpa, n=11, P=0.106; Fig. S2C), resolved EF (−10.74±7.12 mV mm−1 at 72 hpa, n=11; Fig. 1C) and returned JI to even lower than uncut baseline (−0.01±0.002 µA cm−2 at 48 hpa, n=11, P<0.0001; Fig. 1D). The very small JI from 48 hpa might be due to the increased surface area to volume ratio, which is due to the animal growth. We therefore termed this part of the curve as ‘baseline’ (‘B’; Fig. 1B-D) and it correlated with regenerative outgrowth phase (Fig. 1A,E, Fig. S5). Altogether, extracellular bioelectricity shows a dynamic correlation with regeneration and suggests that the bud at 6 hpa is a key spatiotemporal point to test the proposed hypothesis.
Injury-induced ROS production, availability and diffusion are necessary for regeneration
Next, we sought to clarify whether H2O2 is the main ROS involved in regeneration. We used drugs that target specific steps of the ROS ‘life cycle’ (see Fig. 6A): (1) production – by blocking NADPH oxidases with diphenyleneiodonium (DPI) (O'Donnell et al., 1993); (2) availability – by scavenging ROS with MCI-186 (MCI) (Otomo, 2003); and (3) diffusion – by preventing entry of H2O2 into cells through aquaporins with AgNO3 (Niemietz and Tyerman, 2002). As previously shown (Han et al., 2014; Love et al., 2013; Niethammer et al., 2009), we confirmed that these drugs decreased levels of intracellular ROS in the bud (Fig. S6).
Fig. 6.
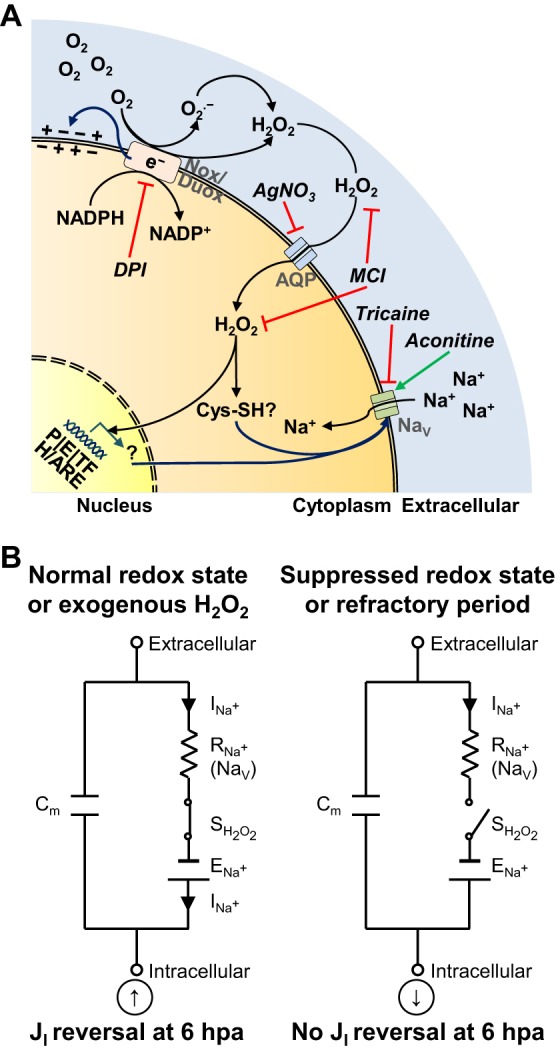
Biochemical and bioelectrical integration during early regeneration via H2O2. (A) Drug targets and H2O2 effects on bioelectricity at distinct steps during a putative H2O2 ‘life cycle’. From top to bottom, steps are production of H2O2 from NADPH oxidases, availability and diffusion of produced H2O2, and H2O2-mediated influences on bioelectric activities or parameters (Vm, TEP, EF, JI and NaV). A representative cell potentially from wound epithelium (overlaying bud) and/or mesenchymal bud of a tadpole amputated at stage 40-41. It is also possible that one cell produces H2O2 and another takes it up to regulate its bioelectric state. For simplicity, we present everything in the same hypothetical cell. e−, electrons; Nox/Duox, NADPH oxidase families (Nox1, Nox2, Nox4 and Nox5 produce superoxide anion (O2.−), whereas Duox1 and Duox2 produce H2O2 directly); AQP, aquaporin; NaV, voltage-gated Na+ channel; Cys-SH, cysteine (Cys) residues of proteins containing thiol (-SH) groups suitable for oxidation; P, promoters; E, enhancers; TF, transcription factors; H/ARE, hypoxia and antioxidant responsive elements; black arrows, ‘life cycle’ of H2O2, from upstream O2 to downstream effect; blue arrows, redox-mediated bioelectric outcome; green arrow, pharmacological activation; red lines, pharmacological inhibition. Drugs are in italic. (B) H2O2 is a switcher in the electrical equivalent circuit. Simplified circuit in a membrane patch of, potentially, a wound epithelium (overlaying bud) and/or mesenchymal bud cell of a tadpole amputated during the regenerative (stage 40-41) or refractory (stage 45-46) period. In the absence of H2O2 (switcher of NaV channels), putative Na+ current (charge carrier) does not cross the membrane through NaV channels and current reversal does not occur at 6 hpa. Suppressed redox state mimics the refractory period circuit. Exogenous H2O2 rescues (from depleted ROS) and induces (from the refractory period) JI reversal. Na+, charge carrier; Cm, capacitance of membrane; ENa+, electromotive force driving Na+; INa+, current of Na+; RNa+, resistance to Na+ flux (synonymous of NaV channels); SH2O2, switcher H2O2. Arrows inside circles indicate net inward (down arrow) or outward (up arrow) current measured extracellularly.
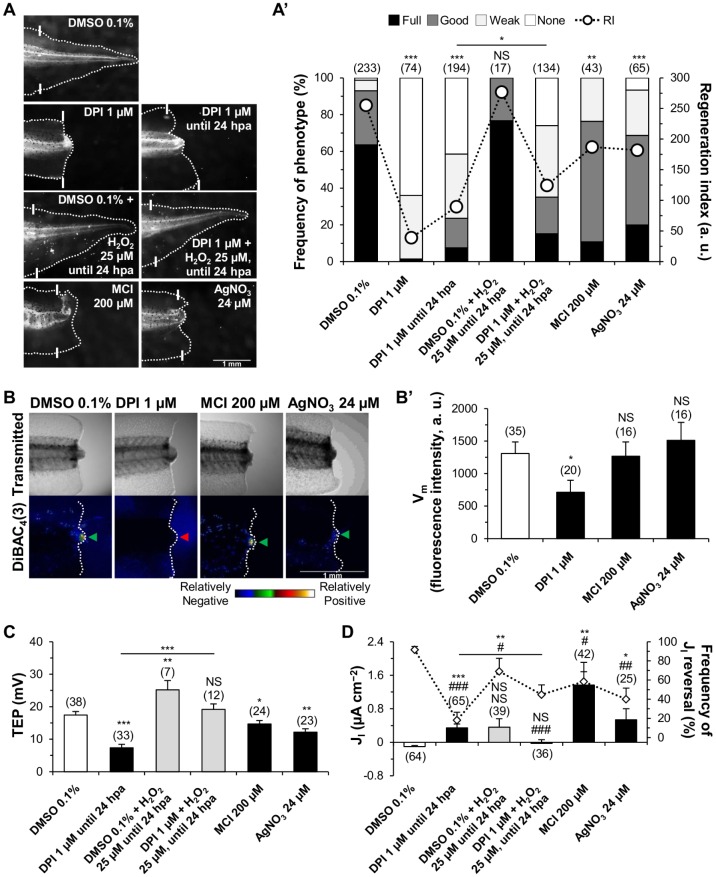
Similarly to another study (Love et al., 2013), DPI-treated tadpoles showed significantly inhibited regeneration, with virtually all tails presenting either a weak (35%, sixfold increase relative to vehicle control 6%) or none (64%, >50-fold increase) phenotype [regeneration index (RI) reduced from 255, n=233, to 39, n=74, P<0.0001; Fig. 2A,A′]. To establish NADPH oxidases as the main ROS source for regeneration, we used the alternative drugs apocynin (Simons et al., 1990) and VAS2870 (VAS) (ten Freyhaus et al., 2006). Besides being less penetrant than DPI, both drugs significantly impaired regeneration (Fig. S7A). Scavenging ROS with the antioxidant MCI significantly impaired regeneration with a 83% decrease in full (11%) and a fourfold increase in weak (24%) phenotypes (RI reduced from 255 to 187, n=43, P=0.005; Fig. 2A,A′). More penetrant effects of MCI have previously been shown if quantified at 3 days post-amputation (dpa), but not at 7 dpa (Love et al., 2013). Most H2O2 diffuses through aquaporins, such as AQP3 and AQP8 (Bienert and Chaumont, 2014; Miller et al., 2010). Highly specific small-molecule drugs for aquaporins are not yet available (Verkman et al., 2014) so we used AgNO3. AgNO3-treated tadpoles showed significantly impaired regeneration with a 69% reduction in the full phenotype (20%), and four- and fivefold increases in, respectively, the weak (25%) and the none (7%) phenotypes (RI reduced from 255 to 182, n=65, P<0.0001; Fig. 2A,A′). Penetrance on regeneration inhibition was therefore DPI≫AgNO3>MCI (Fig. 2A′). Altogether, H2O2 is probably the main ROS responsible for the impaired regeneration and it must diffuse into cells, likely via aquaporins, to be effective.
Fig. 2.
Electrogenic property of NADPH oxidases is necessary for Vm depolarization and catalytic property for regeneration, TEP increase and JI reversal. (A,A′) Loss and rescue of regeneration by ROS and H2O2 modulation. (A) Representative 7 dpa tails from vehicle-control (DMSO 0.1%) and pharmacologically treated tadpoles. White lines: amputation plane. (A′) Qualitative and quantitative analyses of regeneration efficiency for the different conditions tested. RI, regeneration index. (B,B′) Activity of NADPH oxidases per se depolarizes Vm. (B) Representative tails under transmitted light (top panels) and fluorescence imaging (bottom panels) of membrane potential-sensitive dye DiBAC4(3) in vehicle control and after pharmacological treatment at 6 hpa. Green arrowhead, Vm depolarization (relatively positive) in the bud; red arrowhead, no Vm depolarization in the bud. (B′) Semi-quantitative analysis of Vm for the different conditions tested. (C,D) Pharmacological loss and rescue of regeneration are correlated with variations of TEP (C) and JI (D). In D, frequencies of current reversals (mean percentage of inward JI, diamonds) also correlated with pharmacological treatment. *P<0.05 versus JI magnitude; #P<0.05 versus JI reversals; **,##P<0.01; ***,###P<0.001. a.u., arbitrary units. Scale bars: 1 mm; n values are indicated in brackets.
Love et al. have shown that ROS production for the first 3 days is enough to impair regeneration (Love et al., 2013). We tested the role for ROS earlier, in wound epithelium and regeneration bud form, because both are limiting factors of regeneration (Fig. S7B-C′). We started by testing DPI until 6 and 24 hpa. These experiments still robustly inhibited regeneration (until 6 hpa, RI reduced from 246, n=94, to 160, n=52, P<0.0001; until 24 hpa, RI reduced to 89, n=50, P<0.0001; Fig. S7B). VAS treatment confirmed this early requirement (Fig. S7A). Next, we extended the temporal screening and found that ROS are also necessary for the last phase of regeneration (48 hpa onwards), although not for morphogenesis (RI) but for growth (area) (Fig. S7B-B″). This is congruent with impaired proliferation produced by inhibition of NADPH oxidases (Gauron et al., 2013; Han et al., 2014; Love et al., 2013). Finally, by increasing DPI dose, we found that ROS are immediately necessary for regeneration (Fig. S7C,C′). Altogether, ROS presented multi-phase pleiotropic effects during regeneration and NADPH oxidase-mediated ROS production is, to our knowledge, potentially the earliest biochemical limiting factor of regeneration.
NADPH oxidase-driven electron flow depolarizes Vm in the regeneration bud at 6 hpa
Cellular bioelectricity has previously been characterized in the same X. laevis model (Adams et al., 2007) and in axolotl larvae (Özkucur et al., 2010). These studies showed a robust Vm depolarization in the bud at 6 hpa that should repolarize by 24 hpa for complete regeneration (Adams et al., 2007). However, the origin of depolarization is unknown. Vm depolarization (Adams et al., 2007) and H2O2 concentration (Love et al., 2013) appear to overlap spatiotemporally. Activity of NADPH oxidases generates an electron flow that depolarizes Vm in immune cells that can be used as a direct quantification of holoenzyme activity (Demaurex and Petheö, 2005).
To test whether the activity of NADPH oxidases induced the Vm depolarization, we blocked them with DPI and imaged Vm with the membrane potential-sensitive dye DiBAC4(3) (Fig. S8A). DPI-treated tails at 6 hpa were, as in vehicle controls, still depolarized in the bud in relation to the rest of the tail (bud≫shoulder>trunk; Fig. S8A′), but had a significantly decreased depolarization (46% reduction, n=20, P=0.033; Fig. 2B,B′). Consequently, the spatial A/P gradient was also reduced (Fig. S8A′).
To exclude a possible effect of ROS/H2O2 in Vm, we next scavenged and prevented them from diffusing into cells as before. MCI- and AgNO3-treated tails showed no Vm variation in the bud at 6 hpa (n=16, P>0.05 to both; Fig. 2B,B′). Therefore, Vm depolarization in the bud at 6 hpa is originated by the activity of NADPH oxidases per se.
H2O2 regulates TEP increase and JI reversal in the regeneration bud
Next, we tested whether H2O2 regulates TEP, EF and JI. To achieve this, we blocked ROS/H2O2 production, availability and diffusion as before and measured TEP and JI in the bud at 6 hpa. DPI-treated tails showed a significantly decreased TEP (58% reduction, from 17.42±1.11, n=38, to 7.33±1.08 mV, n=33, P<0.0001; Fig. 2C). DPI reversed the direction of some TEPs to negative inside (e.g. 12% of total at 6 hpa). MCI- and AgNO3-treated tadpoles also had significantly decreased TEPs (MCI, 16% reduction, n=24, P=0.046; AgNO3, 30% reduction, n=23, P=0.002; Fig. 2C).
Drug-treated shoulder and trunk (amputated or uncut) TEP shifted in the same way as bud TEP (Figs S8B, S12C,D), suggesting a systemic baseline shift and effect on housekeeping translocators. As a consequence, spatial A/P gradients remained relatively unaffected (Fig. S8B), leading to constant TEP recoveries and long-range EFTrunk→Bud (Fig. S12E). However, short-range EFShoulder→Bud presented increased magnitudes (Fig. S16A), potentially powered by JI direction (below). For these reasons, we mainly compared absolute TEP in bud.
The TEP decrease, caused by depleted H2O2, anticipated an effect of H2O2 on JI. Almost all (91.60±3.38%) vehicle-control bud currents reversed at 6 hpa, giving a net inward JI (−0.10±0.025 µA cm−2, n=64). Only 18.05±8.32% reversed in DPI-treated tadpoles, maintaining a significant outward JI (0.34±0.096 µA cm−2, n=65, P<0.0001; Fig. 2D). Indeed, steady outward JI were measured for days (Fig. S9A). Further analysis showed the reversibility of DPI-prevented reversal (Fig. S9B). MCI and AgNO3 treatments also prevented reversal at 6 hpa with significant outward JI (MCI, n=42, P<0.01, 58.33±10.41% reversal; AgNO3, n=25, P=0.026, 40.00±11.55% reversal; Fig. 2D). Unlike DPI, JI in MCI- and AgNO3-treated tails reversed by 24 hpa (Fig. S8C), mimicking the refractory period (Fig. S11C).
Spatially, drug-sustained outward current at 6 hpa precluded an evolving electrical circuit (Figs S8C, S9C) and probably contributed to maintaining EF direction (cathode in the bud) and to increasing EF magnitude (Fig. S16A). Importantly, the penetrance of the drugs on TEP decrease and JI reversal was equal to impaired regeneration (DPI≫AgNO3>MCI; Fig. 2A′,C,D). Altogether, the TEP increase and JI reversal are regulated by H2O2 that diffuses into cells, likely via aquaporins.
Exogenous H2O2 rescues regeneration, TEP increase and JI reversal
After demonstrating that H2O2 is required for regeneration and regulates TEP and JI, we performed stimulation assays. We first attempted to rescue DPI-impaired regeneration, TEP and JI using exogenous H2O2. To impose a suitable spatiotemporal redox state was challenging due to diverse cell responses (physiological and biochemical) to disparate ROS levels. Briefly, high levels might produce oxidative stress, whereas low levels produce more signaling effects (Lambeth and Neish, 2014). Long-term exposure to H2O2 could turn counterproductive by setting too much of a steady state in the very dynamic process of regeneration. In addition, regeneration-specific events are more likely to be present within 24 hpa. Therefore, we focused on application of H2O2 in low doses (µM range) and for a short exposure (first 24 h).
H2O2 until 24 hpa significantly rescued DPI-impaired regeneration, TEP increase and JI reversal (Fig. 2A,A′,C,D). H2O2 increased twofold the frequency of full phenotypes compared with DPI-treated tails (from 7 to 15%) and decreased 37% the frequency of none phenotypes (from 41 to 26%). Overall, regeneration was significantly rescued (RI from 90, n=194, to 124, n=134, P=0.016; Fig. 2A,A′). Long-term (7 days) exposure to H2O2 did not rescue regeneration (Fig. S13), pointing to the importance of redox state dynamics (Loo et al., 2012).
TEP magnitude in the bud at 6 hpa was entirely rescued to values slightly higher than vehicle control [dimethyl sulfoxide (DMSO): 17.42±1.11 mV, n=38; DPI+H2O2: 19.15±1.72 mV, n=12; P=0.435; Fig. 2C]. H2O2 significantly rescued 161% of TEP (from 7.33±1.08, n=33, to 19.15±1.72 mV, n=12, P<0.0001; Fig. 2C) and enhanced 45% of TEP (from 17.42±1.11 to 25.20±2.84 mV, n=7, P=0.009; Fig. 2C). As for DPI, MCI and AgNO3, shoulder and trunk (amputated or uncut) TEP shifted in the same way as bud TEP (Figs S8B, S12C-E).
JI magnitude and JI reversal in the bud at 6 hpa were dramatically rescued to values closer to vehicle control (DMSO: −0.10±0.025 µA cm−2, 91.60±3.38% reversal, n=64; DPI+H2O2: −0.02±0.086 µA cm−2, 44.76±10.05% reversal, n=36; P=0.395; Fig. 2D). H2O2 significantly rescued 105% of JI magnitude to inward current (from 0.34±0.096, n=65, to −0.02±0.086 µA cm−2, n=36, P=0.005; Fig. 2D). Importantly, current reversals more than doubled (from 18.05±8.32 to 44.76±10.05%, P=0.029; Fig. 2D). Unlike the changes induced in TEP, H2O2 neither enhanced JI magnitude nor JI reversal frequency in vehicle control. Nonetheless, most currents still reversed by 6 hpa (69.05±13.52%), resulting in a large variance (0.36±0.204 µA cm−2, n=39, P=0.114; Fig. 2D). Altogether, short-term exposure to low-dose H2O2 rescued DPI-impaired regeneration, TEP increase and JI reversal. In addition, these results suggest that the electrogenicity of NADPH oxidases may not affect TEP and JI.
Exogenous H2O2 induces regeneration, TEP increase and JI reversal in the refractory period
The X. laevis non-regenerative refractory period provides a unique advantage among regeneration models. We explored it to test whether we could induce regeneration, TEP and JI using exogenous H2O2. First, we verified whether the refractory period has impaired bioelectricity. Remarkably, TEP decrease and postponed JI reversal in the bud mimicked the effects of depleted ROS (Fig. S11). Blocked JI reversal has previously been shown in the spinal cord (Reid et al., 2009). Application of H2O2 until 24 hpa increased the frequency of full phenotypes by 2.4-fold (from 13 to 31%) and decreased the frequency of none phenotypes by 62% (from 39 to 15%). Overall, regeneration was significantly induced (RI from 110, n=55, to 174, n=51, P=0.004; Fig. 3A,A′). As for rescue, long-term (7 days) exposure to H2O2 did not induce regeneration (Fig. S13).
Fig. 3.
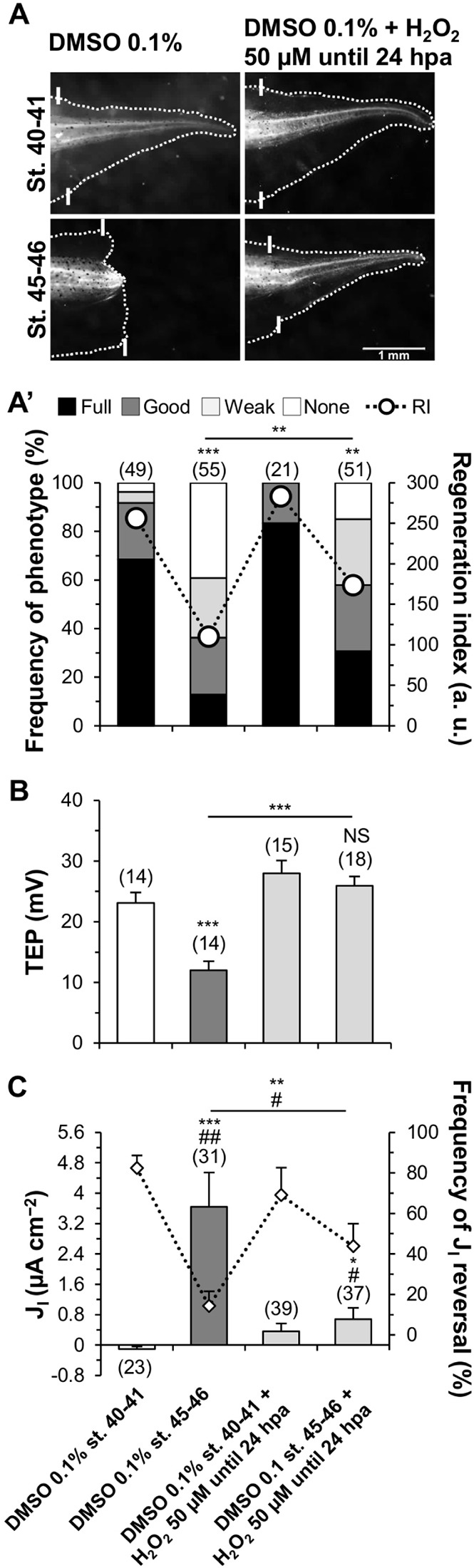
Exogenous H2O2 is sufficient to induce regeneration, TEP increase and JI reversal during the refractory period. (A,A′) Induction of regeneration by exogenous H2O2. (A) Representative 7 dpa tails from tadpoles amputated during the regenerative (stage 40-41) or refractory period (stage 45-46). Vehicle-control (DMSO 0.1%) and H2O2-treated (50 µM until 24 hpa) results are shown. White solid lines, amputation plane. Scale bar: 1 mm. (A′) Qualitative and quantitative analyses of regeneration efficiency for the different conditions tested. RI, regeneration index; a.u., arbitrary units. (B,C) Exogenous H2O2-mediated induction of regeneration is correlated with variations of TEP (B) and JI (C). In C, frequencies of current reversals (mean percentage of inward JI, diamonds) also correlated with pharmacological treatment. *P<0.05 versus JI magnitude; #P<0.05 versus JI reversals; **,##P<0.01; ***,###P<0.001. Horizontal axis labels in C also apply to A′,B. n values are indicated in brackets.
The magnitude of the TEP in the bud at 6 hpa increased to values slightly higher than those found during the regenerative period (DMSO stage 40-41: 23.10±1.74 mV, n=14; DMSO stage 45-46+H2O2: 25.92±1.54 mV, n=18; P=0.235; Fig. 3B). H2O2 raised the TEP by 115% (from 12.04±1.45, n=14, to 25.92±1.54 mV, n=18, P<0.0001; Fig. 3B). In addition, H2O2 shoulder and trunk (amputated or uncut) TEP shifted in the same way as bud TEP (Figs S10A and S12C-E).
JI magnitude and JI reversal in the bud at 6 hpa were dramatically induced to values closer to those found during the regenerative period (DMSO stage 40-41: −0.10±0.065 µA cm−2, 82.34±6.27% reversal, n=23; DMSO stage 45-46+H2O2: 0.68±0.296 µA cm−2, 43.81±11.18% reversal, n=37; P=0.013; Fig. 3C, Fig. S10B). H2O2 significantly decrease JI magnitude in 81% to a still outward current (from 3.64±0.897, n=31, to 0.68±0.296 µA cm−2, n=37, P=0.003; Fig. 3C). Importantly, current reversals increased threefold (from 14.29±7.19 to 43.81±11.18%, P=0.044; Fig. 3C).
We then attempted to induce ectopic tail formation during the regenerative period. Upon dorsal incisions, H2O2 treatment for 24 h slightly induced significant ectopic (abnormal or complete) tail formation (up to a frequency of 19%) in a dose-independent way (Fig. S15). Altogether, short-term exposure to low-dose H2O2 is sufficient to induce regeneration, TEP increase and JI reversal during the refractory period, in addition to inducing ectopic tail formation in the regenerative period.
Regeneration efficiency correlates linearly with TEP magnitude and JI reversals
Following the parallel responses of regeneration, TEP and JI to the different conditions used, we tested whether bioelectricity predicts regeneration efficiency. Plotting all conditions together showed a significant linear correlation of regeneration efficiency with TEP magnitude (r=0.74, P=0.036; Fig. S14) and with the frequency of JI reversals (r=0.86, P=0.006; Fig. 4).
Fig. 4.
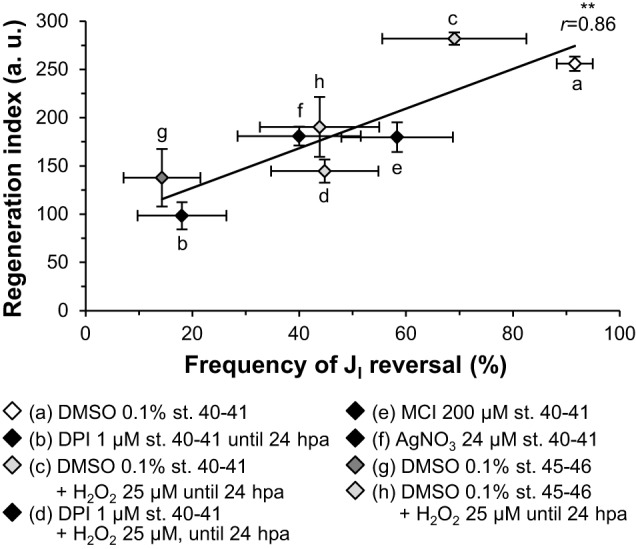
Regeneration efficiency is directly proportional to JI reversals. Compilation of the different conditions tested. Data of regeneration index are from Figs 2A′, 3A′ and data of frequency of JI reversal are from Figs 2D, 3C. Most crossed data are matched siblings. Solid line, linear regression (r2=0.75); r, correlation coefficient.
Voltage-gated Na+ channels are required for H2O2-modulated regeneration
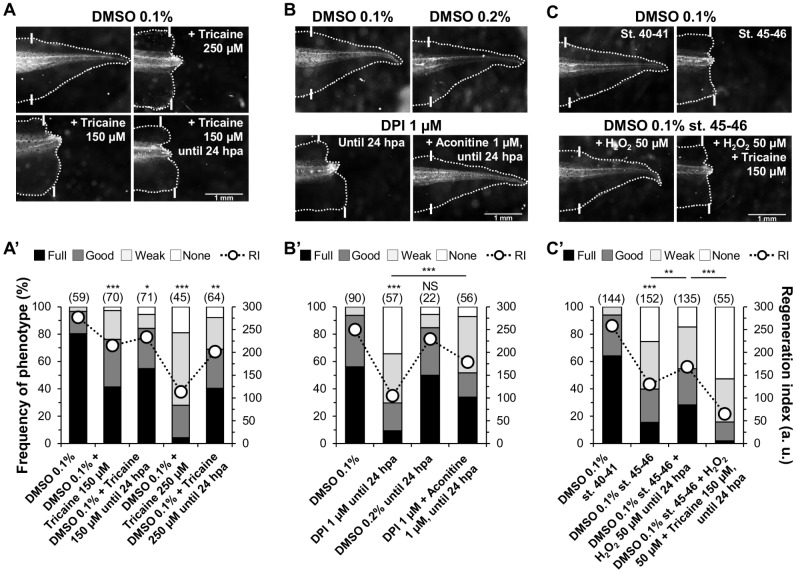
To unveil the mechanisms by which H2O2 modulates regeneration, we searched for ion translocators that ideally affect regeneration and bioelectricity. NaV1.2 is required for X. laevis tadpole tail regeneration via Na+ influx control (Tseng et al., 2010). Therefore, we tested whether NaV channels act downstream of H2O2 using complementary epistasis assays. First, we verified that the inhibition of NaV channels impairs regeneration. As expected (Tseng et al., 2010), the NaV inhibitor tricaine (Frazier and Narahashi, 1975) significantly impaired regeneration (250 µM: RI reduced from 277, n=59, to 113, n=45, P<0.0001; 250 µM until 24 hpa: RI reduced to 202, n=64, P=0.008). A lower dose (150 µM) had less penetrant effects (Fig. 5A,A′). Next, we designed the epistasis assays: (1) rescue DPI-impaired regeneration using the NaV activator aconitine; and (2) loss H2O2-induced regeneration in the refractory period using the NaV inhibitor tricaine.
Fig. 5.
H2O2 modulates regeneration via voltage-gated Na+ (NaV) channels. (A,A′) NaV channel inhibition with tricaine impaired regeneration. (A) Representative 7 dpa tails treated with vehicle (DMSO 0.1%) or tricaine (250 or 150 µM) from amputation or until 24 hpa. (A′) Qualitative and quantitative analyses of regeneration efficiency for the different conditions tested. (B,B′) NaV channel activation with aconitine rescued DPI-impaired regeneration. (B) Representative 7 dpa tails treated with vehicle (DMSO 0.1% and DMSO 0.2%) or DPI (1 µM), alone or with aconitine (1 µM), until 24 hpa. (B′) Qualitative and quantitative analyses of regeneration efficiency for the different conditions tested. (C,C′) NaV channel inhibition abolished H2O2-induced regeneration in the refractory period. (C) Representative 7 dpa tails amputated from tadpoles during the regenerative (stage 40-41) or refractory (stage 45-46) periods. Tails were treated until 24 hpa with vehicle, with H2O2 (50 µM) alone, or with H2O2 (50 µM) plus tricaine (150 µM). (C′) Qualitative and quantitative analyses of regeneration efficiency for the different conditions tested. Primary vertical axis title in A′ also applies to B′,C′; secondary vertical axis title in C′ also applies to A′,B′. RI, regeneration index; a.u., arbitrary units. White solid lines, amputation plane. Scale bars, 1 mm; n values are indicated in brackets.
Treatment with aconitine (Ameri, 1998) until 24 hpa significantly rescued DPI-impaired regeneration (Fig. 5B,B′). Aconitine increased 3.6-fold the frequency of tails with full phenotypes in relation to DPI-treated tails (from 9 to 34%) and decreased by ∼80% the frequency of none phenotypes (from 34 to 7%). Overall, regeneration was significantly rescued (RI from 105, n=57, to 179, n=56, P<0.0001; Fig. 5B,B′). Interestingly, aconitine (in the µM range) was lethal in the absence of DPI, pointing to antagonistic competition.
Treatment with tricaine until 24 hpa significantly impaired H2O2-induced regeneration (Fig. 5C,C′). Tricaine virtually eliminated full phenotypes in relation to H2O2-treated tails (from 30 to 2%) and increased 3.4-fold the frequency of none phenotypes (from 17 to 57%). Overall, regeneration was significantly lost (RI from 168, n=135, to 65, n=55, P<0.0001; Fig. 5C,C′). Therefore, NaV channels act downstream of H2O2 and mediate H2O2-modulated regeneration – a new mechanism.
DISCUSSION
Bioelectric and, more recently, redox activities have been shown, independently, to modulate regeneration in widespread models: planaria (Beane et al., 2011; Pirotte et al., 2015), zebrafish (Gauron et al., 2013; Monteiro et al., 2014) and amphibians (Love et al., 2013; Reid et al., 2009). Seeking a biochemical and biophysical integration, we aimed to determine whether and how these pervasive activities interact during regeneration.
Redox-modulated regeneration is mediated by early bioelectric activities
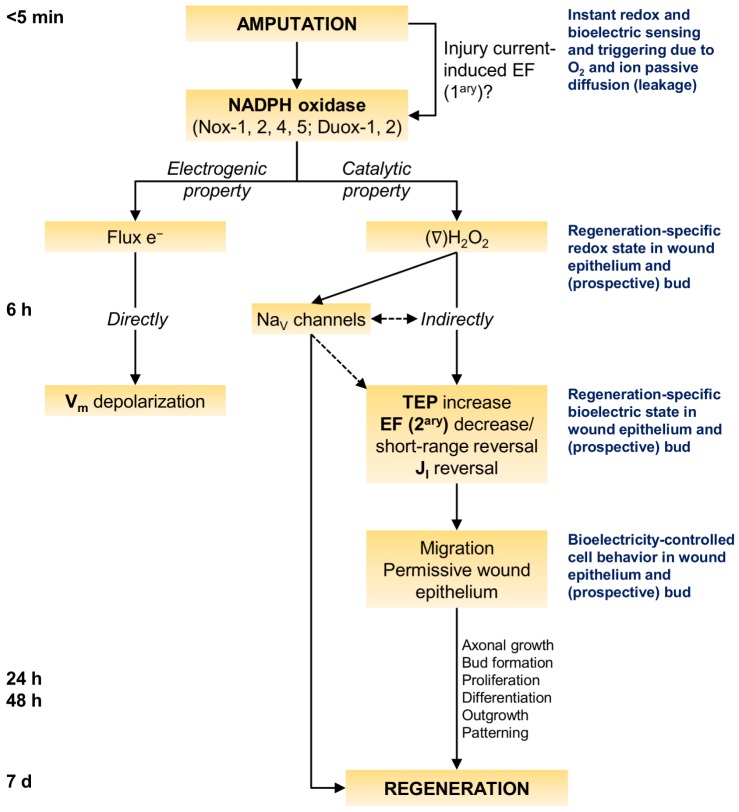
Do ROS modulate regeneration via regulation of bioelectric activities that are known to be required for regeneration? Our results support the two-way regulation hypothesis proposed to address this question. First we mapped the spatiotemporal dynamics of extracellular bioelectricity (Fig. 1, Figs S2-S5) and the temporal requirement for NADPH oxidase-mediated ROS production (Fig. 2A,A′, Fig. S7). These profiles identified the bud at 6 hpa as key to testing the proposed hypothesis, owing to its bioelectric state. Next, we blocked NADPH oxidases and imaged Vm. We found that Vm depolarization resulted from NADPH oxidase activity per se (Fig. 2B,B′). This supported the first part of the two-way regulation hypothesis, arguing that the electrogenic property of NADPH oxidases is the mechanism by which Vm depolarization occurs. As depolarization is not required for regeneration (Adams et al., 2007), we then focused on the extracellular bioelectricity. Drug-depleted ROS levels decreased TEP and prevented or postponed JI reversal, maintaining a steady outward current at 6 hpa (Fig. 2C,D). This mimicked the altered TEP and JI measured during the refractory period (Fig. S11). Exogenous H2O2 rescued and induced regeneration, TEP and JI (Figs 2, 3). It also induced ectopic tail formation (Fig. S15). Regeneration efficiency was directly proportional to TEP increase and JI reversals (Fig. 4, Fig. S14). These results supported the second part of the two-way regulation hypothesis, arguing that H2O2 regulates TEP, EF and JI. Ion translocators are known to mediate bioelectricity (McCaig et al., 2005), thus H2O2 is likely to be epistatic to translocators. Indeed, epistasis assays revealed that NaV channels act downstream of H2O2 (Fig. 5). Altogether, the convergent results from disparate methods provide considerable consilience to conclude that redox-modulated regeneration is mediated by early bioelectric activities (Figs 6, 7).
Fig. 7.
Stepwise model of redox and bioelectric integration during regeneration. NADPH oxidase-mediated electron flow and H2O2 production independently regulate different bioelectric activities. This dual property – electrogenic and catalytic – of NADPH oxidases explicitly represents the proposed two-way regulation hypothesis. The focus is on the early timing of 6 hpa, when the hallmark H2O2-switched current reversal occurs in regenerative tadpoles. A redox-bioelectric feedback module may be present, where bioelectric signals (instant primary EF) might activate redox signals (NADPH oxidases and generated H2O2), which in turn regulate bioelectricity (Vm, TEP, secondary EF and JI) to modulate regeneration. Bioelectrically controlled cell migration and subsequent permissive wound epithelium is suggested as a mechanism by which, ultimately, H2O2 may control regeneration. e−, electrons; ∇, gradient.
Integrating bioelectric activities in the context of regeneration
Endogenous electric activities arise due to energetically expensive resting electrochemical potentials, primarily Vm and TEP. Can these biological batteries be, in part, dormant sensors and effectors ready to detect and to react to ubiquitous injuries? In fact, the idea that endogenous batteries play a role in wound healing (primarily guiding closure) is not entirely new (Barker et al., 1982; Borgens, 1984; Lykken, 1971). For an integrative view, we propose three phases of extracellular bioelectric contributions in regeneration: passive, active and homeostatic (Fig. 1E, Fig. S5).
Passive (until 6 hpa)
Barrier breaking disrupts the in/out discontinuum so ions leak down their electrochemical gradients, resulting in injury current and lateral EF (primary) with the cathode at the amputation plane. The trunk TEP remains unchanged with amputation, thus the A/P gradient is resolved at this position. Thereby, the ‘bioelectric microenvironment’ – spatial range of physiologically meaningful bioelectricity – capable of influencing regeneration spans ∼335 µm anterior to the amputation plane. Importantly, this microenvironment matches maximum mobilization distances, ∼400 µm, of cells for wound closure in urodeles (Lash, 1955).
Active (6-48 hpa, stronger until 24 hpa)
Presumptive barrier restoration re-establishes the discontinuum so ions diffuse with or without energy costs through the wound epithelium. Leaky epithelia are not highly efficient ion-flux barriers due to paracellular movement. Thus, the timing from passive to active activity is difficult to determine precisely. Rather, according to the TEP increase and JI reversal frequency, a transition should occur from 1 to 6 hpa. Epithelial resistance plus JI reversal leads to an exponential build-up of TEP, provoking an equivalent drop in EF. The current reversal possibly reversed short-range EF (secondary) locally in the bud (to anode). The plateau part in both TEP and JI suggests that inward current magnitude should go beyond TEP recovery.
Homeostatic (from 48 hpa)
Ion fluxes should now be mainly for housekeeping functions, as TEP and JI return to uncut baseline, resolving EF. Uncut and complete regeneration tails have an inside-positive TEP and a small inward JI, in agreement with the salt uptake (osmoregulatory) function of amphibian skin (Koefoed-Johnsen and Ussing, 1958). Remarkably, TEP recovers to the new stage-specific level, not to the level before amputation (Fig. S2C), implying systemic control synchronizing development and regeneration. Bioelectric and regeneration phases dynamically correlate (Figs 1A,E, 4, Fig. S5), suggesting a control of regeneration by bioelectric events.
Is NADPH oxidase-driven Vm depolarization an injury-specific by-product of ROS production?
We showed that the characteristic Vm depolarization in the bud at 6 hpa mechanistically mimics the well-known NADPH oxidase-driven depolarization in immune cells during an oxidative burst (Bankers-Fulbright et al., 2003). This makes it attractive to speculate that Vm depolarization is a by-product of ROS production, which is reinforced by the non-requirement of Vm depolarization for regeneration. Vm should be repolarized by H+ efflux at 24 hpa to allow regeneration (Adams et al., 2007). We noticed that, irrespective of the drugs used, bud repolarization occurred by 24 hpa (Fig. S8A′), showing that redox state modulates regeneration in a Vm-independent way (Fig. 7). Similarly, robust Vm depolarization around the wound was measured in the caudal fin inter-ray wound model in zebrafish (F.F., unpublished). Importantly, Gauron et al. showed increased ROS levels produced by NADPH oxidases around wounds of the same model (Gauron et al., 2013). Thus, NADPH oxidase-driven Vm depolarization might be a universal injury-specific by-product of ROS production. However, depolarization is not excluded from having functions (Tseng et al., 2010) not addressed in the present study. In the past two decades, non-injury related Vm polarizations are being unveiled as being key in diverse developmental and patterning processes (Levin, 2014). Future work may unveil whether the activities of NADPH oxidases also have a role in these contexts, when ROS production is involved.
Electric current reversal: a potential hallmark of regeneration switched on by H2O2
Reid et al. showed a correlation between electric current reversals (from outward to inward) and X. laevis tadpole tail regeneration. Ion substitution assays revealed that Na+ contributed to approximately two-thirds of the reversed current. However, neither ion nor translocator modulations were able to actually maintain the outward current (Reid et al., 2009). Remarkably, using non-ionic modulation, we either prevented or postponed current reversal in the bud at 6 hpa, mimicking the refractory period. This led to a dramatic inhibition of regeneration. We showed that H2O2 diffuses into the cell and stimulates the reversal. Thus, in an electrical equivalent circuit, we propose that H2O2 is a switcher that permits Na+-carried current to diffuse into the stump (Fig. 6B). In the same model, Tseng et al. unveiled part of the molecular basis for Na+ influx, the channel NaV1.2 (Tseng et al., 2010). We found that H2O2 acts upstream of the NaV channel family. Future work is necessary to reveal the mechanism of this activation, which can be indirect (e.g. redox-sensitive pathways or gene transcription of channels proteins/regulators) or direct (e.g. oxidation of channels cysteine residues, if available) (Fig. 6A). NaV1.2 is present by 18 hpa in the bud, accumulating cations there (Tseng et al., 2010). The mismatch with our measured reversal at 6 hpa might imply other NaV channel(s) in action, possibly in the wound epithelium. This would complete the path of Na+ from outside to bud, as barrier restoration prevents most ion leakage and reduces paracellular flux. In principle, however, some Na+ transporter(s) should be present in the wound epithelium to move the ions against the electrochemical gradient. The sharp TEP increase until 6 hpa supports this and might suggest the ubiquitous Na+/K+-ATPase as a candidate (Dubé et al., 2010). Thus, it is possible that H2O2 (or other ROS) modulates additional ion translocators (Ma, 2011; Matalon et al., 2003). Exogenous H2O2 rescued and induced regeneration and induced ectopic tails, suggesting that H2O2 provides indispensable morphogenetic information. H2O2 robustly rescued and induced TEP increase and JI reversal at 6 hpa. Therefore, we propose that H2O2-switched current reversal is a hallmark of regeneration. As a limiting and stimulating factor of regeneration, this hallmark can be used as a diagnostic characteristic of regeneration and as a marker and prognosis of its efficiency (Fig. 4). Furthermore, it can be used to promote regeneration in regenerative-deficient animals.
JI reversal prior to blastema formation was also seen in newt limbs (unquantified frequency, apparently occasional due to net outward current) (Borgens et al., 1977b) and tails (Nawata, 2001), and in X. laevis tadpole limb regeneration (G.L., unpublished). Reversal in newt tails was assumed to be a return to baseline (Nawata, 2001), despite the higher magnitude than before amputation. Surface potential measurements revealed stump potential reversal (from positive to negative) prior to blastema formation in urodels, but not in non-permissive anuran limb regeneration (Becker, 1961; Rose and Rose, 1974), showing a correlation with regeneration. However, another study found potential reversal in both taxa (Lassalle, 1979). We measured instead transepithelial potentials that, apart from sporadic reversals, had no overall mean reversal. Short-range EFShoulder→Bud presented more reversal frequency (21%) than did long-range EFTrunk→Bud (9%) at 6 hpa (increasing thereafter; Fig. S3C), indicating the predominance of local reversal (in the bud). Indeed, in newt limbs, the surface potential reversal occurred only a short distance from the amputation plane (Rose and Rose, 1974). The small and soft bud hindered the spatial resolution required to fully characterize local reversals. Unambiguous current reversal supports the short-range potential and EF reversals in the bud. Altogether, our results showed a close correlation between reversals, bud formation and regeneration (Figs 4, 6, 7).
How can the H2O2-switched current reversal mechanistically impact regeneration? Cell migration precedes proliferation (Adams et al., 2007) and is essential for wound closure in the first phase of regeneration (Beck et al., 2009). Thus, the early and vectorial JI reversal suggests migration as a prime candidate. However, other cell behaviors are not excluded. Cumulative evidence shows that endogenous or exogenous EF act as guiding cues for migration during wound healing (McCaig et al., 2005; Zhao et al., 1996, 2006). Importantly, most cells, including keratinocytes and neurons, migrate or grow towards the cathode (Song, 2004; Sun et al., 2013). ROS-depleted and refractory period tails at 6 hpa had a sustained outward JI that led to lower bud TEP and higher short-range EF, establishing a long-term cathode at amputation plane (Figs 2C, 3B, Figs S11, S16A). Therefore, we postulate that a non-reversed JI by 6 hpa might lead to cell overmigration. In short, cells are deluded into behaving as if the wound was still open (Fig. S16B). Wound epithelium from the refractory period is thicker than regenerative X. laevis tadpole tails (Beck et al., 2003; Reid et al., 2009; Tseng et al., 2010). Mature skin flapped or grafted to amputated newt limbs or children's fingertips blocks regeneration (Illingworth, 1974; Mescher, 1976). Interestingly, these skin-like structures have altered currents (Altizer et al., 2002; Reid et al., 2009) (Fig. S11) and might disrupt epithelial-mesenchymal crosstalk (Lee et al., 2009), both of which are important for regeneration. Thereby, cell overmigration might produce a non-permissive refractory-like wound epithelium. This might explain why JI reversal by 24 hpa in MCI- and AgNO3-treated tails (Fig. S8C) was not sufficient for regeneration (Fig. 2A,A′). Although we cannot exclude direct ROS-induced chemotaxis (Hurd et al., 2012; Pan et al., 2011), ROS-modulated electrotaxis might be the major cue (Zhao, 2009). This rationale could apply to axonal sprouting and to axonal growth (electrotropism). Neuronal presence is a long-recognized limiting and stimulating factor in regeneration (Kumar and Brockes, 2012; Sidman and Singer, 1951). Importantly, applied currents enhance nerve growth and regeneration in adult frogs (Borgens et al., 1979). Observed JI reversal per se or the establishment of a steady inward current might be independent cues. Reversal can be a transitory electric pole discretely sensed by cells. Therefore, JI reversal may fine-tune (mitigate) migration of cells and axonal growth, setting the conditions for or triggering bud initiation around 6 hpa and subsequent proliferation and de novo tail formation. Indeed, a permissive epithelium neuronally supplied (Thornton, 1954) is the basis of the accessory limb formation in axolotls (Endo et al., 2004). H2O2-induced ectopic tails might thus result from bioelectric effects on neuronal tissue.
Towards a comprehensive model of redox and bioelectric integration during regeneration
We aimed to conceptualize a comprehensive model that integrates redox and bioelectric states during regeneration (Fig. 7). We showed that NADPH oxidase-mediated ROS production is pleiotropic (early on it affects morphogenesis; later on it affects growth) and is required immediately upon amputation. Thus, an elusive ultra-fast signal needs to trigger the activity of NADPH oxidases. Probably the fastest injury-induced signals are bioelectric in nature. Exogenous EF activate NADPH oxidases in cells in vitro (Chatterjee et al., 2012; Li et al., 2013). Thus, we propose that endogenous EF activate NADPH oxidases (Fig. 7). Another candidate is Ca2+ signaling. Wounding induces fast Ca2+ influx and Ca2+ flashes in X. laevis oocytes and Drosophila embryo, respectively (Luxardi et al., 2014; Razzell et al., 2013). Importantly, those flashes activate a NADPH oxidase (Duox). Excitingly, this points to a feedback module, where bioelectric signals (primary EF and/or Ca2+) might activate redox signals (NADPH oxidases and generated H2O2) that in turn regulate bioelectricity (Vm, TEP, secondary EF and JI) to modulate regeneration (Fig. 7). This might be a starting point from which to test whether bioelectricity-modulated redox activities also occur during regeneration. By 6 hpa, the electrogenic property of NADPH oxidases depolarizes Vm and the catalytic property stimulates TEP recovery and switches on JI reversal (Figs 6B, 7). H2O2-modulated regeneration is then Vm independent. However, both paths might interact. For example, preventing Vm repolarization downregulates NaV1.2 expression (Tseng et al., 2010). H2O2 diffusion into cells revealed for the first time, to our knowledge, that aquaporins are potential new targets to modulate epimorphic regeneration (Fig. 6A). H2O2-switched current reversal, potentially via NaV channels, may fine-tune cell migration and axonal growth that is essential for regeneration (Figs 6, 7).
Although the evidence supporting H2O2 is compelling, the role of other ROS in controlling bioelectric and regenerative outcomes should not be excluded (Li et al., 2013). Moreover, the possible importance of a H2O2 extracellular gradient is not excluded by this study. Indeed, it might be translated into a gradient of bioelectric activity that can be a blueprint for positional information and axes patterning (Jaffe, 1981; Tseng and Levin, 2013). Engineered solutions for controlled release of H2O2 (Garland et al., 2014) may prove useful for future work, especially in non-aquatic models.
In conclusion, this study unveils the interplay between biochemical and biophysical signals during regeneration, suggesting a novel mechanism that mediates regeneration: early H2O2-switched JI reversal, potentially via NaV channels. It also highlights the opportunities for interdisciplinary integration of apparently disparate states (Serena et al., 2009; Tandon et al., 2014) that may reserve promising advances for translational medicine.
MATERIALS AND METHODS
Animals and surgery
Animal procedures and pharmacological treatments were approved by local Institutional Animal Care and Use Committee (protocols 16822 and 18601). Xenopus laevis (Daudin, 1802) tadpoles acquired from Xenopus Express (www.xenopus.com) were staged (Nieuwkoop and Faber, 1967), sorted and transferred to fresh Marc's modified Ringer (MMR) 0.1× culture medium, composed of (in mM): NaCl 10, CaCl2·2H2O 0.2, KCl 0.2, MgCl2·6H2O 0.1 and HEPES 0.5 (pH 7.1-7.2) (Sigma-Aldrich). Surgery was executed as previously described (Adams et al., 2007; Reid et al., 2009). For further details, see Supplementary Materials and Methods.
Tail regeneration assay
Tail regeneration assays were performed as previously described (Adams et al., 2007; Tseng et al., 2010). For further details, see Supplementary Materials and Methods.
Ectopic tail induction
Ectopic tails were induced by a dorsal incision severing down to the spinal cord (inclusive; Fig. S15A) (Sugiura et al., 2009). For further details, see Supplementary Materials and Methods.
Pharmacological modulations
Drugs and respective vehicle reconstituted in MMR 0.1× were applied via immersion (bath) at the doses and exposures specified. Most drugs were stocked in DMSO (Sigma-Aldrich, cat. no. D2650) at −20°C: DPI (Sigma-Aldrich, cat. no. D2926) 1 and 10 mM; MCI (Cayman chemical, cat. no. 13320) 200 mM; AgNO3 (Sigma-Aldrich, cat. no. 209139) 24 mM; apocynin (Sigma-Aldrich, cat. no. W508454) 200 mM; VAS (EMD Millipore, cat. no. 492000) 1.4 mM; and aconitine (Alamone Labs, cat. no. A-150) 1 mM. Vehicle (DMSO) had non-significant effects on readouts (Fig. S1). H2O2 (EMD Millipore, cat. no. HX0635) was kept as a 1 M stock in deionized water at 4°C. Occasionally, stocks of 25 and 50 mM were kept for short periods at −20°C. Tricaine was kept as a 1 mM stock in MMR 0.1× (pH readjusted to 7.2) at −20°C. Working solutions were freshly prepared. For further details, see Supplementary Materials and Methods.
ROS and Vm fluorescence imaging
Intracellular relative ROS and Vm were measured non-invasively by fluorescence imaging of vital dyes as previously described (Adams et al., 2007; Owusu-Ansah et al., 2008). For further details, see Supplementary Materials and Methods.
Glass microelectrode measurement
Transepithelial potential was measured invasively by glass microelectrode impalement through the epithelial layers as previously described (Luxardi et al., 2014; McCaig and Robinson, 1982). For further details, see Supplementary Materials and Methods.
Vibrating probe measurement
Extracellular net electric current density was measured non-invasively with a locked-in one dimensional vibrating probe, prepared and used as previously described (Reid et al., 2007, 2009). For further details, see Supplementary Materials and Methods.
Statistical analysis
Regeneration efficiencies were compared using the Fisher's exact test. Frequencies (in JI reversals and ectopic tails) were compared using the Mann-Whitney U-test. Correlations were tested with the Pearson's correlation coefficient r. Linear regressions (coefficient of determination r2) were applied to fit data to a statistical model. All remaining comparisons were analyzed using the unpaired Student's t-test.
Data are presented as mean±s.e.m. Sample size (n, biological replicates) used in each experiment are stated in figures and text. At least two independent batches of tadpoles were used per readout. Differences were significant if P<0.05 and the levels of significance are: NS, non-significant; *P<0.05; **P<0.01 and ***P<0.001. Means and standard errors were calculated and plotted using Excel, and statistics were performed using GraphPad Prism 5 (GraphPad Software). For further details, see Supplementary Materials and Methods.
Acknowledgements
We apologize for not being able to cite all the relevant studies due to space or context constraints. We are grateful to Dr Andreia Gomes (Departamento de Biologia, CBMA, Universidade do Minho, Portugal) and to Dr VijayKrishna Raghunathan [Department of Surgical and Radiological Sciences, University of California (UC) Davis; The Ocular Surface Institute, College of Optometry, University of Houston, TX, USA] for helpful discussion. We thank Dr Paul Fitzgerald (Department of Cell Biology and Human Anatomy, UC Davis) and Dr Andrew Ishida [Department of Neurobiology, Physiology, and Behavior (NPB), UC Davis] for providing access to confocal microscopes. We are grateful to Dr Tyler Stradleighand (Department of NPB, UC Davis) and to Dr Qizhi Gong (Department of Cell Biology and Human Anatomy, UC Davis) for training and suggestions in confocal microscopy. We thank Dr Yao-Hui Sun for providing zebrafish and all other lab members (past and present) for helpful discussion.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
F.F. and M.Z. conceived the study. F.F., G.L. and M.Z. designed the experiments. F.F. performed the experiments and analyzed the results. G.L. and B.R. provided technical training and guidance, and helped to interpret some results. F.F., G.L. and M.Z. outlined the manuscript. F.F. wrote the manuscript. All authors read, commented and agreed with the final version of the manuscript.
Funding
This work was supported by the UC Davis Principal Investigator Bridge Program and the National Institutes of Health (EB015737), and, in part, by an unrestricted grant from Research to Prevent Blindness from UC Davis Ophthalmology. F.F. was supported by Fundação para a Ciência e a Tecnologia (SFRH/BD/87256/2012). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.142034.supplemental
References
- Adams D. S., Masi A. and Levin M. (2007). H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 134, 1323-1335. 10.1242/dev.02812 [DOI] [PubMed] [Google Scholar]
- Altizer A. M., Stewart S. G., Albertson B. K. and Borgens R. B. (2002). Skin flaps inhibit both the current of injury at the amputation surface and regeneration of that limb in newts. J. Exp. Zool. 293, 467-477. 10.1002/jez.10141 [DOI] [PubMed] [Google Scholar]
- Alvarado A. S. and Tsonis P. A. (2006). Bridging the regeneration gap: genetic insights from diverse animal models. Nat. Rev. Genet. 7, 873-884. 10.1038/nrg1923 [DOI] [PubMed] [Google Scholar]
- Ameri A. (1998). The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 56, 211-235. 10.1016/S0301-0082(98)00037-9 [DOI] [PubMed] [Google Scholar]
- Bankers-Fulbright J. L., Gleich G. J., Kephart G. M., Kita H. and O'Grady S. M. (2003). Regulation of eosinophil membrane depolarization during NADPH oxidase activation. J. Cell Sci. 116, 3221-3226. 10.1242/jcs.00627 [DOI] [PubMed] [Google Scholar]
- Barker A. T., Jaffe L. F. and Vanable J. W. (1982). The glabrous epidermis of cavies contains a powerful battery. Am. J. Physiol. 242, R358-R366. [DOI] [PubMed] [Google Scholar]
- Beane W. S., Morokuma J., Adams D. S. and Levin M. (2011). A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem. Biol. 18, 77-89. 10.1016/j.chembiol.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. W., Christen B. and Slack J. M. W. (2003). Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev. Cell 5, 429-439. 10.1016/S1534-5807(03)00233-8 [DOI] [PubMed] [Google Scholar]
- Beck C. W., Izpisúa Belmonte J. C. and Christen B. (2009). Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev. Dyn. 238, 1226-1248. 10.1002/dvdy.21890 [DOI] [PubMed] [Google Scholar]
- Becker R. O. (1961). The bioelectric factors regeneration in amphibian-limb regeneration. J. Bone Jt. Surg. 43, 643-656. [PubMed] [Google Scholar]
- Becker R. O. (1972). Stimulation of partial limb regeneration in rats. Nature 235, 109-111. 10.1038/235109a0 [DOI] [PubMed] [Google Scholar]
- Bienert G. P. and Chaumont F. (2014). Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596-1604. 10.1016/j.bbagen.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Borgens R. B. (1984). Are limb development and limb regeneration both initiated by an integumentary wounding? A hypothesis. Differentiation 28, 87-93. 10.1111/j.1432-0436.1984.tb00270.x [DOI] [PubMed] [Google Scholar]
- Borgens R. B., Vanable J. W. and Jaffe L. F. (1977a). Bioelectricity and regeneration. I. Initiation of frog limb regeneration by minute currents. J. Exp. Zool. 200, 403-416. 10.1002/jez.1402000310 [DOI] [PubMed] [Google Scholar]
- Borgens R. B., Vanable J. W. and Jaffe L. F. (1977b). Bioelectricity and regeneration: large currents leave the stumps of regenerating newt limbs. Proc. Natl. Acad. Sci. USA 74, 4528-4532. 10.1073/pnas.74.10.4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens R. B., Vanable J. W. and Jaffe L. F. (1979). Small artificial currents enhance Xenopus limb regeneration. J. Exp. Zool. 207, 217-226. 10.1002/jez.1402070206 [DOI] [Google Scholar]
- Chatterjee S., Browning E. A., Hong N., DeBolt K., Sorokina E. M., Liu W., Birnbaum M. J. and Fisher A. B. (2012). Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. AJP Hear. Circ. Physiol. 302, H105-H114. 10.1152/ajpheart.00298.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N. and Petheö G. L. (2005). Electron and proton transport by NADPH oxidases. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 2315-2325. 10.1098/rstb.2005.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchar E. M. (1975). Regeneration of the tail bud in Xenopus embryos. J. Exp. Zool. 192, 381-389. 10.1002/jez.1401920311 [DOI] [PubMed] [Google Scholar]
- Dubé J., Rochette-Drouin O., Lévesque P., Gauvin R., Roberge C. J., Auger F. A., Goulet D., Bourdages M., Plante M., Germain L. et al. (2010). Restoration of the transepithelial potential within tissue-engineered human skin in vitro and during the wound healing process in vivo. Tissue Eng. Part A 16, 3055-3063. 10.1089/ten.tea.2010.0030 [DOI] [PubMed] [Google Scholar]
- Endo T., Bryant S. V. and Gardiner D. M. (2004). A stepwise model system for limb regeneration. Dev. Biol. 270, 135-145. 10.1016/j.ydbio.2004.02.016 [DOI] [PubMed] [Google Scholar]
- Frazier D. T. and Narahashi T. (1975). Tricaine (MS-222): effects on ionic conductances of squid axon membranes. Eur. J. Pharmacol. 33, 313-317. 10.1016/0014-2999(75)90175-2 [DOI] [PubMed] [Google Scholar]
- Garland S. P., Wang R. Y., Raghunathan V. K., Lam K. S., Murphy C. J., Russell P., Sun G. and Pan T. (2014). Photopatternable and photoactive hydrogel for on-demand generation of hydrogen peroxide in cell culture. Biomaterials 35, 1762-1770. 10.1016/j.biomaterials.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauron C., Rampon C., Bouzaffour M., Ipendey E., Teillon J., Volovitch M. and Vriz S. (2013). Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci. Rep. 3, 2084 10.1038/srep02084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding A., Guay J. A., Herrera-Rincon C., Levin M. and Kaplan D. L. (2016). A tunable silk hydrogel device for studying limb regeneration in adult Xenopus Laevis. PLoS ONE 11, e0155618 10.1371/journal.pone.0155618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Zhou X.-H., Chang N., Xiao C.-L., Yan S., Ren H., Yang X.-Z., Zhang M.-L., Wu Q., Tang B. et al. (2014). Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res. 24, 1091-1107. 10.1038/cr.2014.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechavarria D., Dewilde A., Braunhut S., Levin M. and Kaplan D. L. (2010). BioDome regenerative sleeve for biochemical and biophysical stimulation of tissue regeneration. Med. Eng. Phys. 32, 1065-1073. 10.1016/j.medengphy.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd T. R., DeGennaro M. and Lehmann R. (2012). Redox regulation of cell migration and adhesion. Trends Cell Biol. 22, 107-115. 10.1016/j.tcb.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth C. M. (1974). Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 9, 853-858. 10.1016/S0022-3468(74)80220-4 [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. (1981). The role of ionic currents in establishing developmental pattern. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 295, 553-566. 10.1098/rstb.1981.0160 [DOI] [PubMed] [Google Scholar]
- Jenkins L. S., Duerstock B. S. and Borgens R. B. (1996). Reduction of the current of injury leaving the amputation inhibits limb regeneration in the red spotted newt. Dev. Biol. 178, 251-262. 10.1006/dbio.1996.0216 [DOI] [PubMed] [Google Scholar]
- Koefoed-Johnsen V. and Ussing H. H. (1958). The nature of the frog skin potential. Acta Physiol. Scand. 42, 298-308. 10.1111/j.1748-1716.1958.tb01563.x [DOI] [PubMed] [Google Scholar]
- Kumar A. and Brockes J. P. (2012). Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 35, 691-699. 10.1016/j.tins.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. and Neish A. S. (2014). Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol. 9, 119-145. 10.1146/annurev-pathol-012513-104651 [DOI] [PubMed] [Google Scholar]
- Lash J. W. (1955). Studies on wound closure in urodeles. J. Exp. Zool. 128, 13-28. 10.1002/jez.1401280103 [DOI] [Google Scholar]
- Lassalle B. (1979). Surface potentials and the control of amphibian limb regeneration. J. Embryol. Exp. Morphol. 53, 213-223. [PubMed] [Google Scholar]
- Lee Y., Hami D., De Val S., Kagermeier-Schenk B., Wills A. A., Black B. L., Weidinger G. and Poss K. D. (2009). Maintenance of blastemal proliferation by functionally diverse epidermis in regenerating zebrafish fins. Dev. Biol. 331, 270-280. 10.1016/j.ydbio.2009.05.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik L. P., Froemel D., Slavici A., Ovadia Z. N., Hudak L., Henrich D., Marzi I. and Barker J. H. (2015). Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci. Rep. 5, 18353 10.1038/srep18353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. (2007). Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 17, 261-270. 10.1016/j.tcb.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Levin M. (2014). Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 25, 3835-3850. 10.1091/mbc.E13-12-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Chen T., Hu S., Lin J., Hu R. and Feng H. (2013). Superoxide mediates direct current electric field-induced directional migration of glioma cells through the activation of AKT and ERK. PLoS ONE 8, e61195 10.1371/journal.pone.0061195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo A. E. K., Wong Y. T., Ho R., Wasser M., Du T., Ng W. T. and Halliwell B. (2012). Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLoS ONE 7, e49215 10.1371/journal.pone.0049215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love N. R., Chen Y., Ishibashi S., Kritsiligkou P., Lea R., Koh Y., Gallop J. L., Dorey K. and Amaya E. (2013). Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 15, 222-228. 10.1038/ncb2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxardi G., Reid B., Maillard P. and Zhao M. (2014). Single cell wound generates electric current circuit and cell membrane potential variations that requires calcium influx. Integr. Biol. 6, 662-672. 10.1039/C4IB00041B [DOI] [PubMed] [Google Scholar]
- Lykken D. T. (1971). Square-wave analysis of skin impedance. Psychophysiology 7, 262-275. 10.1111/j.1469-8986.1970.tb02232.x [DOI] [PubMed] [Google Scholar]
- Ma H.-P. (2011). Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J. Biol. Chem. 286, 32444-32453. 10.1074/jbc.M111.254102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S., Hardiman K. M., Jain L., Eaton D. C., Kotlikoff M., Eu J. P., Sun J., Meissner G., Stamler J. S., Hardiman K. M. et al. (2003). Regulation of ion channel structure and function by reactive oxygen-nitrogen species. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L1184-L1189. 10.1152/ajplung.00281.2003 [DOI] [PubMed] [Google Scholar]
- McCaig C. D. and Robinson K. R. (1982). The ontogeny of the transepidermal potential difference in frog embryos. Dev. Biol. 90, 335-339. 10.1016/0012-1606(82)90382-7 [DOI] [PubMed] [Google Scholar]
- McCaig C. D., Rajnicek A. M., Song B. and Zhao M. (2005). Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 85, 943-978. 10.1152/physrev.00020.2004 [DOI] [PubMed] [Google Scholar]
- McGinnis M. E. and Vanable J. W. (1986). Electrical fields in Notophthalmus viridescens limb stumps. Dev. Biol. 116, 184-193. 10.1016/0012-1606(86)90055-2 [DOI] [Google Scholar]
- Mescher A. L. (1976). Effects on adult newt limb regeneration of partial and complete skin flaps over the amputation surface. J. Exp. Zool. 195, 117-127. 10.1002/jez.1401950111 [DOI] [PubMed] [Google Scholar]
- Miller E. W., Dickinson B. C. and Chang C. J. (2010). Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 107, 15681-15686. 10.1073/pnas.1005776107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J., Aires R., Becker J. D., Jacinto A., Certal A. C. and Rodríguez-León J. (2014). V-ATPase proton pumping activity is required for adult zebrafish appendage regeneration. PLoS ONE 9, e92594 10.1371/journal.pone.0092594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S., Stramer B., Evans I., Wood W. and Martin P. (2010). Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 20, 464-470. 10.1016/j.cub.2010.01.047 [DOI] [PubMed] [Google Scholar]
- Nawata T. (2001). Wound currents following amputation of tail tip in the Japanese Newt, Cynops pyrrhogaster. Zoolog. Sci. 18, 11-15. 10.2108/zsj.18.11 [DOI] [Google Scholar]
- Niemietz C. M. and Tyerman S. D. (2002). New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 531, 443-447. 10.1016/S0014-5793(02)03581-0 [DOI] [PubMed] [Google Scholar]
- Niethammer P., Grabher C., Look A. T. and Mitchison T. J. (2009). A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996-999. 10.1038/nature08119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P. D. and Faber J. (1967). Normal Table of Xenopus laevis (Daudin). Amsterdam: North-Holland. [Google Scholar]
- O'Donnell V. B., Tew D. G., Jones O. T. G. and England P. J. (1993). Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 290, 41-49. 10.1042/bj2900041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo E. (2003). Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc. Dis. 15, 222-229. 10.1159/000069318 [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E., Yavari A., Mandal S. and Banerjee U. (2008). Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 40, 356-361. 10.1038/ng.2007.50 [DOI] [PubMed] [Google Scholar]
- Özkucur N., Epperlein H.-H. and Funk R. H. W. (2010). Ion imaging during axolotl tail regeneration in vivo. Dev. Dyn. 239, 2048-2057. 10.1002/dvdy.22323 [DOI] [PubMed] [Google Scholar]
- Pan Q., Qiu W.-Y., Huo Y.-N., Yao Y.-F. and Lou M. F. (2011). Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing. Invest. Ophthalmol. Vis. Sci. 52, 1723-1734. 10.1167/iovs.10-5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotte N., Stevens A.-S., Fraguas S., Plusquin M., Van Roten A., Van Belleghem F., Paesen R., Ameloot M., Cebrià F., Artois T. et al. (2015). Reactive oxygen species in planarian regeneration: an upstream necessity for correct patterning and brain formation. Oxid. Med. Cell. Longev. 2015, 1-19. 10.1155/2015/392476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzell W., Evans I. R., Martin P. and Wood W. (2013). Calcium flashes orchestrate the wound inflammatory response through duox activation and hydrogen peroxide release. Curr. Biol. 23, 424-429. 10.1016/j.cub.2013.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B., Nuccitelli R. and Zhao M. (2007). Non-invasive measurement of bioelectric currents with a vibrating probe. Nat. Protoc. 2, 661-669. 10.1038/nprot.2007.91 [DOI] [PubMed] [Google Scholar]
- Reid B., Song B. and Zhao M. (2009). Electric currents in Xenopus tadpole tail regeneration. Dev. Biol. 335, 198-207. 10.1016/j.ydbio.2009.08.028 [DOI] [PubMed] [Google Scholar]
- Rose M. S. and Rose F. C. (1974). Electrical studies on normally regenerating, on X-rayed, and on denervated limb stumps of Triturus. Growth 38, 363-380. [PubMed] [Google Scholar]
- Sen C. K. and Roy S. (2008). Redox signals in wound healing. Biochim. Biophys. Acta 1780, 1348-1361. 10.1016/j.bbagen.2008.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serena E., Figallo E., Tandon N., Cannizzaro C., Gerecht S., Elvassore N. and Vunjak-Novakovic G. (2009). Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp. Cell Res. 315, 3611-3619. 10.1016/j.yexcr.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S., Borgens R., Pascuzzi R., Roos K., Groff M., Purvines S., Rodgers R. B., Hagy S. and Nelson P. (2005). Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J. Neurosurg. Spine 2, 3-10. 10.3171/spi.2005.2.1.0003 [DOI] [PubMed] [Google Scholar]
- Sidman R. L. and Singer M. (1951). Stimulation of forelimb regeneration in the newt, Triturus viridescens, by a sensory nerve supply isolated from the central nervous system. Am. J. Physiol. 165, 257-260. [DOI] [PubMed] [Google Scholar]
- Simons J. M., ‘t Hart B. A., Ip Vai Ching T. R. A. M., Van Dijk H. and Labadie R. P. (1990). Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic. Biol. Med. 8, 251-258. 10.1016/0891-5849(90)90070-Y [DOI] [PubMed] [Google Scholar]
- Smith S. D. (1967). Induction of partial limb regeneration in Rana pipiens by galvanic stimulation. Anat. Rec. 158, 89-97. 10.1002/ar.1091580110 [DOI] [PubMed] [Google Scholar]
- Song B., Zhao M., Forrester J. and McCaig C. (2004). Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 117, 4681-4690. 10.1242/jcs.01341 [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper C. L., Moon R. T. and Weidinger G. (2007). Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 21, 1292-1315. 10.1101/gad.1540507 [DOI] [PubMed] [Google Scholar]
- Sugiura T., Tazaki A., Ueno N., Watanabe K. and Mochii M. (2009). Xenopus Wnt-5a induces an ectopic larval tail at injured site, suggesting a crucial role for noncanonical Wnt signal in tail regeneration. Mech. Dev. 126, 56-67. 10.1016/j.mod.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Sun Y., Do H., Gao J., Zhao R., Zhao M. and Mogilner A. (2013). Keratocyte fragments and cells utilize competing pathways to move in opposite directions in an electric field. Curr. Biol. 23, 569-574. 10.1016/j.cub.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N., Cimetta E., Villasante A., Kupferstein N., Southall M. D., Fassih A., Xie J., Sun Y. and Vunjak-Novakovic G. (2014). Galvanic microparticles increase migration of human dermal fibroblasts in a wound-healing model via reactive oxygen species pathway. Exp. Cell Res. 320, 79-91. 10.1016/j.yexcr.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Freyhaus H., Huntgeburth M., Wingler K., Schnitker J., Bäumer A. T., Vantler M., Bekhite M. M., Wartenberg M., Sauer H. and Rosenkranz S. (2006). Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 71, 331-341. 10.1016/j.cardiores.2006.01.022 [DOI] [PubMed] [Google Scholar]
- Thornton C. S. (1954). The relation of epidermal innervation to limb regeneration in Amblystoma larvae. J. Exp. Zool. 127, 577-601. 10.1002/jez.1401270307 [DOI] [Google Scholar]
- Tseng A.-S. and Levin M. (2013). Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun. Integr. Biol. 6, 1-8. 10.4161/cib.22595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A.-S., Beane W. S., Lemire J. M., Masi A. and Levin M. (2010). Induction of vertebrate regeneration by a transient sodium current. J. Neurosci. 30, 13192-13200. 10.1523/JNEUROSCI.3315-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal E. A., Day A. M. and Morgan B. A. (2007). Hydrogen peroxide sensing and signaling. Mol. Cell 26, 1-14. 10.1016/j.molcel.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Anderson M. O. and Papadopoulos M. C. (2014). Aquaporins: important but elusive drug targets. Nat. Rev. Drug Discov. 13, 259-277. 10.1038/nrd4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C., Chockley P., Singh S. K., Pase L., Lieschke G. J. and Grabher C. (2012). Hydrogen peroxide in inflammation: messenger, guide, and assassin. Adv. Hematol. 2012, 541471 10.1155/2012/541471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. K., Starnes T. W., Deng Q. and Huttenlocher A. (2011). Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480, 109-112. 10.1038/nature10632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. K., Freisinger C. M., LeBert D. C. and Huttenlocher A. (2012). Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J. Cell Biol. 199, 225-234. 10.1083/jcb.201203154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang Y., Man L., Zhu Z., Bai X., Wei S., Liu Y., Liu M., Wang X., Gu X. et al. (2016). Reactive oxygen species generated from skeletal muscles are required for gecko tail regeneration. Sci. Rep. 6, 1-11. 10.1038/srep20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. (2009). Electrical fields in wound healing—an overriding signal that directs cell migration. Semin. Cell Dev. Biol. 20, 674-682. 10.1016/j.semcdb.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Zhao M., Agius-Fernandez A., Forrester J. V. and McCaig C. D. (1996). Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J. Cell Sci. 109, 1405-1414. [DOI] [PubMed] [Google Scholar]
- Zhao M., Song B., Pu J., Wada T., Reid B., Tai G., Wang F., Guo A., Walczysko P., Gu Y. et al. (2006). Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442, 457-460. 10.1038/nature04925 [DOI] [PubMed] [Google Scholar]