Abstract
Background
Specific geriatric assessment tools may complement traditional perioperative risk stratification. Our aim was to evaluate whether self-reported mobility is predictive of postoperative outcomes in older patients undergoing elective noncardiac surgery.
Methods
Patients ≥69 years of age (n=197) underwent: 1) traditional risk assessments (American Society of Anesthesiologists [ASA] Physical Status Classification and Revised Cardiac Risk Index [RCRI]), 2) 5-point frailty evaluation, 3) self-reported mobility assessment using the Mobility Assessment Tool-short form (MAT-sf) (range: 30.21 [poor]-69.76 [excellent]), and 4) measurements of high-sensitivity C-reactive protein (hs-CRP). Outcomes were postoperative complications, time to discharge, and nursing home placement (NHP).
Results
In our sample (mean age = 75 ± 5 years, 51% female), 72% had intermediate- or high-risk surgery. Median time to discharge was 3 days (IQR: 1–4 days). Thirty patients (15%) developed postoperative complications, and 27 (13%) required NHP. After controlling for age, sex, body mass index, pain score, RCRI, ASA physical status, surgical risk, and hs-CRP, worse self-reported mobility (per 10 point decrease in MAT-sf, which is equivalent to 1 standard deviation) was associated with more postoperative complications (OR 1.69, 95% CI 1.05 – 2.73), later time to discharge (HR 0.81, 95% CI 0.68 – 0.96), and increased NHP (OR 2.01, 95% CI 1.13 – 3.56). Using the same model, intermediate frailty or frailty increased NHP (OR 3.11, 95% CI 1.02–9.54), but was not related to either postoperative complications or time to discharge.
Conclusions
Preoperative self-reported mobility using a novel and brief assessment may help identify elderly patients at risk for adverse postoperative events.
Introduction
One-third of inpatient surgeries in the U.S. are performed in older adults and this number is expected to increase by approximately 40% by 2020.1 The elderly have a greater risk of postoperative adverse outcomes, longer hospital stays, and greater need for long-term care, all of which are costly.2–4
Among the traditional risk assessment tools used today are the American Society of Anesthesiologists (ASA) Physical Status Classification and the Revised Cardiac Risk Index (RCRI). The ASA physical status score is known to predict postoperative morbidity, mortality,5–10 and hospital length of stay.11 The RCRI remains a well-accepted method to identify patients for whom preoperative noninvasive cardiac testing is justified and may change the plan of care.12,13 Nonetheless, the use of RCRI is limited by inherent difficulties in accurate weighting of multiple risk factors with variable degrees of cause-and-effect linkage. Older patients often exhibit declining reserves in physiologic and physical function in multiple organ systems14 that might be overlooked by traditional preoperative risk assessment measures.
The American College of Surgeons (ACS) recently created the National Surgical Quality Improvement Program (NSQIP) surgical risk calculator to be used to predict a multitude of postoperative outcomes.15 While it was developed using a large aggregate of multi-institutional data, the NSQIP calculator was not specifically validated in older surgical patients in whom geriatric syndromes are prevalent. One common geriatric syndrome is frailty, defined as a state of high vulnerability for adverse health outcomes, including disability, dependency, falls, need for long-term care, and mortality.16 A five-point frailty scoring system used to predict outcomes in older patients after noncardiac surgery appears to discriminate between low- and high-risk elderly surgical patients more effectively than traditional perioperative stratification tools such as the ASA physical status score and cardiac risk tools;17 however, adoption of frailty scoring in preoperative assessment practice has been slow.
Mobility is a powerful biomarker of the integrated health of multiple systems. Time to complete the Timed Up-and-Go (TUG) test, a performance-based assessment of mobility, accurately forecasts postoperative morbidity and mortality in older patients.18,19 However, such performance-based measures carry an inherent, albeit modest, risk of falls and injury and, more importantly, can be affected by transient states such as pain and acute illness, limiting their value in the preoperative setting.20
The novel Mobility Assessment Tool-short form (MAT-sf) presents patients with 10 computer-generated animations that depict mobility tasks in different environments. Unlike other self-report tools, the MAT-sf eliminates complex judgments and contextual factors by asking the participant about their ability to do the task depicted in the animation.21 It has been validated against measures of physical function, including the Pepper Assessment Tool for Disability, the Short Physical Performance Battery, and 400-meter walk test among older community dwellers.21 Therefore, we tested the hypothesis that self-reported mobility using the MAT-sf would predict postoperative complications, time to discharge, and nursing home placement (NHP) after adjusting for traditional risk assessment scores and a biomarker of inflammation. We also compared the MAT-sf to a frailty score previously used in the preoperative setting.
Materials and Methods
Study Participants
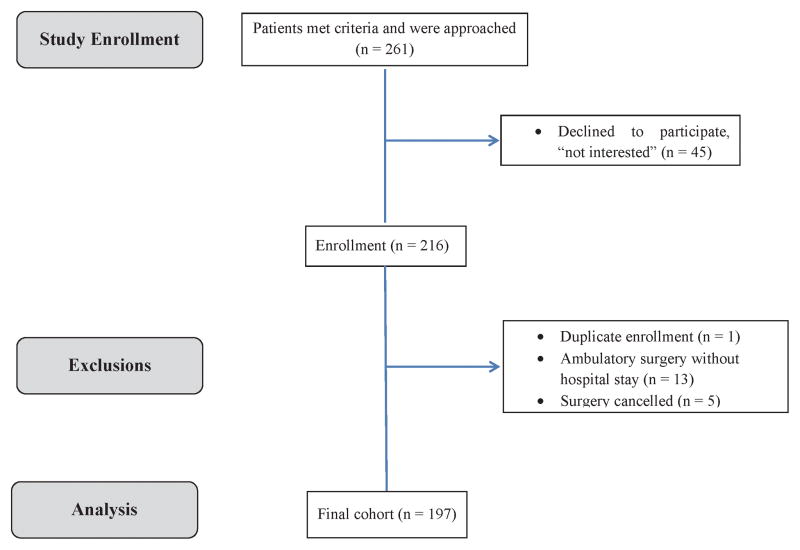
In this prospective cohort study, we recruited patients ≥69 years of age referred for preoperative assessment before elective noncardiac surgery at our institution from July 2012 to February 2014. Prospective participants were approached in the waiting room of the Preoperative Assessment Clinic (PAC) at Wake Forest Baptist Health, Winston-Salem, North Carolina, which serves >70 patients per day. We excluded those scheduled for emergency, cardiac, or outpatient procedures, as well as those who required assistive devices to walk 4 meters, had deficits in hearing or vision, or could not understand the MAT-sf questionnaires and directions. Of 261 eligible patients, 45 declined to participate (mean age 76; 68% female), leaving 216 who provided written informed consent (Institutional Review Board of Wake Forest School of Medicine, Winston-Salem, North Carolina - approval number 00019392l; 4/23/2012) before undergoing standardized assessments for frailty and mobility status by trained study personnel. Those with a duplicate enrollment, those who underwent ambulatory surgery without a hospital stay, and those whose surgery was cancelled were excluded from the analysis. The final cohort consisted of 197 individuals (Figure 1).
Figure 1.
Flow of 261 eligible participants who were approached in the preoperative clinic to cohort included in this study.
Study Assessments
For each patient, information regarding demographic characteristics, comorbidities, medications, pain scores, procedure type, and anesthesia time were gathered.
Mobility was assessed using the MAT-sf, which consists of 10 animated video clips of activities that span a wide range of functional capacity, including walking on level ground, a slow jog, walking outdoors on uneven terrain, walking up a ramp with and without using a handrail, stepping over hurdles, ascending and descending stairs with and without the use of a handrail, and climbing stairs while carrying bags. Items were selected based on individual response and information curves derived from Item Response Theory.21 Participants were asked to watch each animated video clip and then respond to a series of questions about their ability to perform each task (number of minutes, number of times, yes/no). The test was implemented using an iPad and scores were saved to an exportable, password-protected file. Scores on the MAT-sf range from 30.21 to 69.76, indicative of poor to excellent mobility in older adults. For example, participants with a mean age of 78.9 ±5.2 years in the LIFE Study20 scored a mean of 53.6 and SD of 8.0 on the MAT-sf. Their risk of major mobility disability after 3 years of follow-up was 22% for those with scores ≥60, 35% for those with scores 50–59, 52% for those with scores 40–49, and 66% for those with scores <40.
Frailty was assessed using the 5-point scale developed by Fried et al.,16 as modified by Makary and colleagues.17 Frailty was defined as the presence of 4 or more of the following 5 criteria: shrinking (unintentional weight loss of 10 or more pounds in the previous year); weakness measured by a hand-held dynamometer (e.g., men met the criteria for weakness if their body mass index (BMI) and grip strength were ≤24 kg/m2 and ≤29 kg; 24.1–26 kg/m2 and ≤30 kg; 26.1–28 kg/m2 and ≤31 kg; >28 kg/m2 and ≤32 kg, respectively; women met the criteria for weakness if their BMI and grip strength were 24 kg/m2 and ≤29 kg; 24.1–26 kg/m2 and ≤30 kg; 26.1–28 kg/m2 and ≤31 kg; >28 kg/m2 and ≤32 kg, respectively); exhaustion (measured by responses to questions about effort and motivation); reduced physical activity (determined by asking about leisure time physical activities); and slow walking speed based on the time to walk 15 feet (e.g., men met criteria if height and walk time were ≤173 cm and ≥7 seconds, or >173 cm and ≥6 seconds, respectively; women met criteria if height and walk time were ≤159 cm and ≥7 seconds, or >159 cm and ≥6 seconds, respectively). Intermediate frailty was defined as the presence of 2 or 3 of the 5 criteria. Patients with 0 or 1 of the 5 criteria were defined as non-frail.
We also obtained baseline blood samples from all participants to measure high sensitivity C-reactive protein (hs-CRP) as an indicator of inflammation.
Standard Preoperative Evaluations
All patients underwent a standard medical workup at the discretion of the PAC’s attending physician. This workup included the ASA Physical Status Classification and the RCRI.12
Study Outcomes
The primary outcomes of interest were postoperative complications within 30 days of the operation as defined by the ACS NSQIP,22 time to discharge (in days), and NHP. The postoperative surgical and medical complications included surgical site infection (superficial, deep, or organ-space), wound disruption, pneumonia, unplanned intubation, pulmonary embolism, on ventilator for more than 48 hours, progressive renal insufficiency or acute renal failure requiring dialysis, urinary tract infection, stroke, coma, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, bleeding requiring transfusion, deep venous thrombosis requiring therapy, or sepsis.22 In addition to the NSQIP list of medical complications, we included delirium, as defined by the Diagnostic and Statistical Manual of Mental Disorders-523 and atrial fibrillation. A complication was defined as present if it occurred during the initial hospital stay and was documented in the discharge summary. The presence of 1 or more complication was considered positive for postoperative complications.
Sample Size
Because the expected degree of relationship of mobility/disability with postoperative outcomes was not known, the sample size was based on data previously published in the literature on estimates of postoperative complications in older patients, according to Dzankic et al.24 A formal a priori sample size calculation was not conducted.
Statistical Analysis
Patients’ demographic variables, comorbidities, preoperative pain scores, hs-CRP and results of the frailty scoring and MAT-sf were expressed as mean ±SD, median with interquartile range (IQR), or percentages as appropriate. Percentages of postoperative complications and NHP among patients with two frailty categories (non-frail, intermediately frail or frail) and mobility (best, mid, and worst tertiles of MAT-sf score) were compared using Chi-Square tests. Student’s t-test was used to compare log-transformed hospital LOS between two frailty categories and analysis of variance (ANOVA) between mobility tertiles. Logistic regression models were used to estimate odds ratios of postoperative complications and NHP, and Cox proportional hazard regression models were used to compare time to discharge for frailty status and MAT-sf (as a continuous variable).
For these models, three sets of analyses were performed: 1) unadjusted model: only frailty or MAT-sf included in the model; 2) traditional covariate-adjusted model plus hs-CRP: potential risk factors including age, sex, BMI, pain score, RCRI, ASA status, surgical risk, and hs-CRP were included in the model with either frailty or MAT-sf, but not both; and 3) fully adjusted model: similar to traditional covariate-adjusted plus hs-CRP model but with both frailty and MAT-sf included in the model to evaluate the improvement in model performance after adding the MAT-sf. The increase in the area under the curve (AUC) was calculated based on logistic regression models, where the AUC, as determined by the trapezoidal rule, was estimated by the concordance index. The receiver operating characteristics (ROC) comparisons were performed using a contrast matrix approach that enables the testing of differences of the areas under empirical ROC curves, as described in DeLong et al.25
For the univariate analysis, the MAT-sf was examined as tertiles and for the multivariate analyses it was treated as a continuous variable. All statistical analyses were done with SAS 9.4 (Cary, NC) and a 2-tailed test with p<0.05 was considered statistically significant. The criterion for rejection of the null hypothesis was not adjusted for multiple comparisons.
Results
Participant Characteristics
A total of 197 patients undergoing elective non-cardiac surgical procedures were included. Our sample had a mean age of 75 years, was 51% female, predominantly white, and 72% were overweight or obese based on BMI (Table 1). Given that obesity is a proinflammatory state characterized by the release of inflammatory compounds including hs-CRP from visceral adipose tissue, it was not surprising to find a greater proportion of overweight and obese patients, e.g., 65 and 69%, respectively, with hs-CRP levels ≥ 1mg/L, when compared to patients with BMIs < 25 kg/m2; wherein 50% had elevated levels. With regard to traditional risk measures, 24% and 69% of the patients were deemed to be ASA physical status 2 and 3, respectively, and the majority fell within a RCRI class of 1 or 2. Sixty-seven patients (34%) were intermediately frail, while one patient (0.5%) was frail. Only 18% of the surgeries were considered low risk, with the remainder being either intermediate or high risk. The median anesthesia time was 198 minutes and the median MAT-sf score was 53 (IQR = 46.4–61.1).
Table 1.
Characteristics of 197 Older Patients Undergoing Preoperative Assessment for Noncardiac Surgery
| Characteristics | |
|---|---|
| Age, mean (SD) | 75.2 (5.0) |
| Female, n (%) | 101 (51) |
| Race, n (%) | |
| White | 179 (91) |
| African American | 15 (8) |
| Other | 3 (1.5) |
| Body mass index (BMI), kg/m2 (mean, SD) | 27.8 (5.6) |
| BMI category, n (%) | |
| BMI <20 | 7 (4) |
| 20≤BMI<25 | 49 (25) |
| 25≤BMI<30 | 72 (37) |
| 30≤BMI | 68 (35) |
| Pain score, median (IQR) | 3 (0–6) |
| ASA Physical Status classification, n (%) | |
| 1 | 0 (0) |
| 2 | 47 (24) |
| 3 | 136 (69) |
| 4 | 14 (7) |
| Revised Cardiac Risk Index classification, n (%) | |
| 1 | 117 (59) |
| 2 | 56 (28) |
| 3 | 15 (8) |
| ≥4 | 9 (5) |
| Surgical risks, n (%) | |
| Low risk | 35 (18) |
| Intermediate to high risk | 162 (82) |
| Procedure completed, n (%) | |
| Vascular | 3 (2) |
| Knee or hip replacement surgery | 64 (33) |
| Intraperitoneal surgery | 19 (10) |
| Spinal surgery | 40 (21) |
| Urologic/gynecologic surgery | 32 (16) |
| Head and neck surgery | 14 (7) |
| Carotid endarterectomy | 5 (3) |
| Other | 18 (9) |
| Anesthesia time, median (IQR) | 198 (150–277) |
| 5-Point Frailty Score, n (%) | |
| Not-frail | 130 (66) |
| Intermediately frail | 66 (34) |
| Frail | 1 (0.5) |
| High sensitivity C-reactive protein ≥1mg/l, n (%) | 120 (62.5) |
| Mobility Assessment Test-short form, median (IQR) | 53 (46.4–61.1) |
Values are presented as mean ± standard deviation (SD), median (interquartile range), or (n) number of subjects (percentage of study population), as appropriate. ASA = American Society of Anesthesiologists; IQR = interquartile range
Univariate Analysis Results
Intermediate frailty or frailty was a significant risk factor for later discharge and NHP (Table 2). The median time to discharge for non-frail patients was 2 (IQR, 1–4) days, while for intermediately frail or frail patients it was 3 (IQR, 1–5) days. Correspondingly, 8% of non-frail patients were discharged to a nursing home while the rate for those considered to be either intermediately frail or frail was 25%. Interestingly, being intermediately frail or frail was unrelated to postoperative complications. In contrast, mobility limitation as characterized by worst (range: 30.2–47.70) and mid tertiles (range: 47.73–57.16) of the MAT-sf, as compared to the best tertile (range: 57.95–67.76), was a significant risk factor for all three postoperative outcomes (Appendix 1) including postoperative complications, later time to discharge, and NHP.
Table 2.
Univariate Association between Frailty and Self-reported Mobility Using the Mobility Assessment Tool-short form (MAT-sf) and Postoperative Complications, Time to Discharge, and Nursing Home Placement
| n | Postoperative Complications (%) | P-value | Time to Discharge Days, (median (IQR)) | P-value | Nursing Home Placement | P-value | |
|---|---|---|---|---|---|---|---|
| Frailty | |||||||
| Non-frail | 130 | 16 (12.3%) | 0.1120 | 2 (1–4) | 0.04 | 10 (7.7%) | <0.01 |
| Intermediately frail/frail | 67 | 14 (20.9%) | 3 (1–5) | 17 (25.4%) | |||
| Mobility* | |||||||
| Best tertile | 66 | 3 (4.5%) | <0.01 | 2 (1–4) | <0.01 | 2 (3.0%) | <0.01 |
| Mid tertile | 66 | 12 (18.2%) | 2 (1–4) | 9 (13.6%) | |||
| Worst tertile | 65 | 15 (23.1%) | 3 (2–6) | 16 (24.6%) | |||
Results for ‘postoperative complications’ and ‘nursing home placement’ are presented as number (n) of patients (percentage of frailty category or mobility tertile) (chi square test for association). Results for ‘time to discharge’ are presented as median days (interquartile range) (Student’s t-test was used to compare log-transformed time to discharge between the two frailty categories and analysis of variance between mobility tertiles).
MAT-sf cutoff tertiles (mean ± SD): Best tertile (59.45 ± 4.68); Middle tertile (48.59 ± 2.31); Worst tertile (37.95 ± 7.80).
IQR = interquartile range; MAT-sf = Mobility Assessment Tool-short form.
Multivariate Analysis Results
In the unadjusted model, intermediate frailty or frailty was a predictor of NHP, while worse mobility, defined by lower MAT-sf scores, significantly predicted higher postoperative complications, later time to discharge, and higher need for NHP (Table 3). After adjusting for traditional covariates including age, sex, BMI, pain score, RCRI, ASA physical status, and surgical risk, plus hs-CRP, intermediate frailty or frailty significantly predicted NHP but failed to associate with postoperative complications or time to discharge. In multivariate analyses with the same covariates, mobility based on the MAT-sf was a significant predictor of postoperative complications, time to discharge, and NHP. When both intermediate frailty or frailty and self-reported mobility were added to the model, intermediate frailty or frailty continued to be significant for only NHP (OR 3.11, 95% CI 1.02–9.54). Poor self-reported mobility, as reflected by lower scores on the MAT-sf, remained significant in predicting more postoperative complications (OR 1.69 0 for every 10 point decrease in MAT-sf, which is equivalent to 1 standard deviation, 95% CI 1.05–2.73), later time to discharge (HR 0.81 for every 10 point decrease in MAT-sf, 95% CI0.68–0.96), and an increased risk of being discharged to a nursing home (OR 2.01 for every 10 point decrease in MAT-sf, 95% CI 1.13–3.56) (Table 3).
Table 3.
Association between frailty and self-reported mobility using the Mobility Assessment Tool-short form (MAT-sf) postoperative complications, time to discharge, and nursing home placement after multivariate adjustment
| Predictor | Postoperative Complications | Time to Discharge | Nursing Home Placement | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | HR | 95% CI | P-value | OR | 95% CI | P-value | |
| Unadjusted* | |||||||||
| Frailty | |||||||||
| Intermediately Frail/Frail | 1.88 | 0.86–4.14 | 0.12 | 0.79 | 0.58–1.06 | 0.12 | 4.08 | 1.75–9.53 | <0.01 |
| MAT-sf§ | 1.96 | 1.31–2.92 | <0.01 | 0.81 | 0.71–0.93 | <0.01 | 2.60 | 1.62–4.17 | <0.01 |
|
| |||||||||
| Traditional Covariate-Adjusted + C-Reactive Protein (CRP)† | |||||||||
| Frailty | |||||||||
| Intermediately Frail/Frail | 1.40 | 0.41–2.66 | 0.93 | 1.00 | 0.71–1.41 | 0.98 | 4.18 | 1.41–12.40 | 0.01 |
| MAT-sf§ | 1.64 | 1.03–2.59 | 0.04 | 0.82 | 0.69–0.96 | 0.02 | 2.22 | 1.28–3.87 | <0.01 |
|
| |||||||||
| Fully adjusted‡ | |||||||||
| Frailty | |||||||||
| Intermediately Frail/Frail | 0.78 | 0.29–2.11 | 0.62 | 1.13 | 0.79–1.60 | 0.51 | 3.11 | 1.02–9.54 | 0.05 |
| MAT-sf§ | 1.69 | 1.05–2.73 | 0.03 | 0.81 | 0.68–0.96 | 0.01 | 2.01 | 1.13–3.56 | 0.02 |
Values represent odds ratios (OR) or hazard ratios (HR) and 95% CI. Logistic regression models were used to estimate odds ratios of postoperative complications and nursing home placement, and Cox proportional hazard regression models were used to compare time to discharge for frailty status and MAT-sf (as a continuous variable).
Intermediately Frail/Frail or MAT-sf as the only predictor in the model.
Odds ratios or hazard ratios were for every 10-point decrease of MAT-sf, which is equivalent to 1 standard deviation (SD).
Adjusted for traditional covariates, including age, sex, body mass index, pain score, Revised Cardiac Risk Index, ASA Physical
Classification status and surgical risk, plus CRP, but only either frailty or MAT-sf as the predictor in the model.
All covariates in the traditional + CRP model, plus both frailty and MAT-sf were in the model.
ASA = American Society of Anesthesiologists; CRP = high-sensitivity C-reactive protein; MAT-sf = Mobility Assessment Tool-short form
Besides the MAT-sf, no other variables were predictive of postoperative complications. In the fully adjusted model to predict hospital discharge (early hospital discharge), ASA physical status ≥3 (HR= 1.65, 95% CI 1.11–2.46), intermediate-risk or high-risk surgery (HR=2.83, 95% CI 1.84–4.36), and hs-CRP ≥1 mg/dL (HR=1.40, 95% CI 1.02–1.92)) were also significant. Age (OR=1.15, 95% CI 1.05–1.27), and preoperative pain scores (OR=0.83, 95% CI 0.70–0.99) were significant predictors of NHP.
Mobility and Predictive Power
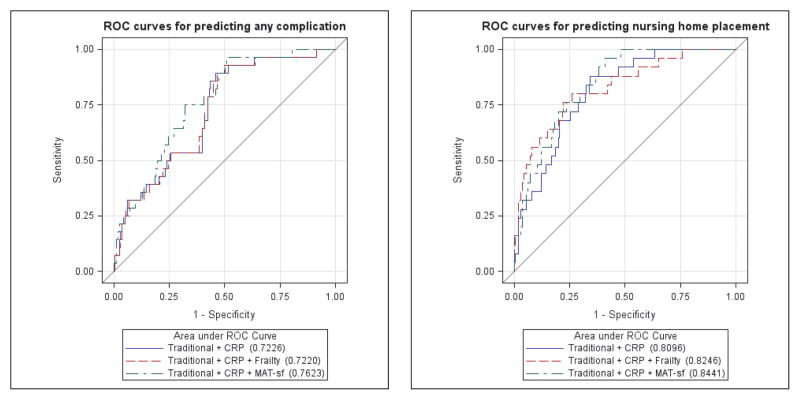
We performed receiver operator characteristic (ROC) analysis to assess the ability of the models to predict postoperative complications and NHP. The ROC curves for the models predicting any complication based on frailty status or MAT-sf are presented in Figure 2A. The corresponding area under the curves (AUCs) for intermediate frailty or frailty and MAT-sf were 0.7220 and 0.7623, respectively, with the difference of 0.0403 not statistically significant (P=0.77). The ROC curves for the models predicting NHP based on intermediate frailty or frailty and MAT-sf are presented in Figure 2B. The corresponding AUCs were 0.8246 and 0.8441, respectively, with the difference of 0.0195 not statistically significant (P=0.53).
Figure 2.
A) Area under the receiver operating characteristic curve for postoperative complications; and B) nursing home placement in models including traditional covariates plus CRP and with either frailty or MAT-sf scores. Traditional covariates included age, sex, body mass index, pain score, Revised Cardiac Risk Index, ASA Physical Status Classification status, and surgical risk. ASA = American Society of Anesthesiologists; CRP = high-sensitivity C-reactive protein; MAT-sf = Mobility Assessment Tool-short form.
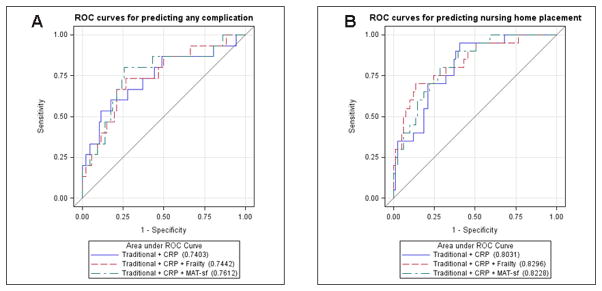
A sensitivity analysis was performed to assess the importance of orthopedic- or pain-related limitations on postoperative complications, time to discharge and NHP in the 104 patients who underwent an orthopedic procedure, including hip, knee or spine surgery. In the univariate analysis, being intermediately frail or frail significantly associated with all three outcomes whereas worse self-reported mobility associated with postoperative complications and time to discharge (Appendix 2). The results of multivariate analyses were similar to Table 3. Specifically, in the unadjusted multivariate analysis, both frailty and mobility status among orthopedic patients significantly associated with all three outcomes. After adjusting for traditional covariates and hs-CRP, intermediate frailty or frailty remained significant for time to discharge (HR 0.61, 95% CI 0.37 – 1.01, P=0.05) and NHP (OR 3.66, 95% 0.98 – 13.67, P=0.05) while MAT-sf-based mobility remained significant for only postoperative complications (OR 0.52, 95% CI 0.28 – 1.00, P<0.05). In the fully adjusted model, these associations were no longer significant, possibly due to a smaller sample size (Appendix 3). Among the orthopedic-only patients, ROCs were performed for predicting postoperative complications and NHP. The corresponding AUCs were similar to the AUCs involving all patients (Appendix 4).
Discussion
We evaluated traditional risk factors and geriatric-specific assessments, including mobility and frailty, prior to elective noncardiac surgery in a cohort of patients ≥69 years of age and found that self-reported mobility assessed by the MAT-sf was the only measure that consistently predicted early postoperative complications, time to discharge, and NHP. In keeping with previous studies,17,26,27 intermediate frailty (or frailty) was associated with NHP. However, there was no relationship between frailty scores and postoperative complications or time to discharge.
Preoperative assessment of participant characteristics that can assist in the evaluation of risk for adverse postoperative outcomes is important to patients, their families, and their surgeons. This information is even more important for the elderly, in whom postoperative complications are more likely. While the ASA physical status is very good at separating the perfectly healthy (ASA physical status 1) and the deathly ill (ASA physical status 5) from the rest of the surgical population and even the less than healthy (ASA physical status 2) from the critically ill (ASA physical status 4), it is less accurate for determining risk for patients with ASA physical status 3 (a common classification of those 65 years and older) because criteria for evaluation are comprehensive and may not always be limited to specific end-organ impairments. Other traditional, preoperative risk assessment measures focus on single organ systems as a predictor of perioperative outcomes, such as the cardiac and pulmonary systems.28,29 However, elderly patients develop geriatric-specific syndromes that involve multiple organ systems; declining function across multiple systems may be a significant risk factor for adverse postoperative outcomes.17,30 Evaluation of multiple systems takes time and adds cost making comprehensive assessment difficult to implement in clinical practice. It is the authors’ contention that the time requirement to conduct frailty assessments, using even the 5-domain tool17 might be the main barrier to the inclusion of frailty in preoperative assessments. Although the actual times devoted to frailty and MAT-sf assessments were not recorded, on average, 10–15 minutes were required for the evaluation of frailty and ≤5 minutes for self-reported mobility using the MAT-sf. Therefore, identifying an efficient index to evaluate the health of multiple systems using a simple preoperative assessment could have a significant benefit to patient care and medical expenditures.
The ability to move freely in the daily environment, as assessed by a mobility tool such as the MAT-sf, requires integration and coordination between sensory feedback, neural control, and the musculoskeletal system. Mobility has also been associated with cognitive abilities, including those used in planning and monitoring performance (executive function).31,32 Mobility limitations are associated with higher rates of disability, NHP, and mortality.33–35 Therefore, it is a logical target for preoperative evaluation of older adults when postoperative events are outcomes of interest. In the perioperative setting, the TUG test and gait speed have been most commonly studied. In elderly patients undergoing colorectal and cardiac surgery, slower TUG times were associated with increased postoperative complications and one-year mortality.18 In elderly patients undergoing cardiac surgery, slow gait speed (≥6 s to walk 5-meter) predicted the composite outcome of in-hospital mortality or major morbidity.19 However, performance-based tests of mobility may not be practical in a preoperative clinic setting, particularly when attempting to evaluate older adults with orthopedic conditions, comorbidities that limit physical function, and illness.
The MAT-sf is a quick, reliable, cost-efficient clinical tool for identifying older adults at risk for future major mobility disability36 and early postoperative adverse events. The MAT-sf provides animated videos of complex, real-world tasks such as stepping over objects and walking on uneven paths outdoors, and in contrast to objective tests such as the TUG and 5-meter walk test, there is no need for dedicated space, and concerns surrounding patient safety are eliminated. In fact, while sitting in the waiting room of the preoperative assessment clinic, patients can provide an assessment of their ability to perform each mobility challenge on a laptop computer or an iPad in 5 minutes or less. As reading comprehension for the MAT-sf was designed at an 8-grade level, only minimal assistance with the computer or iPad is needed. Indeed, the electronic format reduces the chance for human error and eliminates the need for extensive assessor training.36 Low preoperative MAT-sf scores (e.g., ≤50) could be used to alert patients, their families, caregivers and physicians of the potential for needing postoperative activities of daily living and instrumental activities of daily living assistance, falls prevention, home modifications, or home health care following elective surgery. Even though the receiver operating characteristic curves show that the MAT-sf does as well as frailty in predicting postoperative complications and NHP, it is the authors’ contention that the MAT-sf is more practical to perform, and thus more appealing to be used in a busy preoperative clinic setting than frailty assessments.
Our study has several limitations. First, this was a prospective cohort study of a convenience sample from a single institution. It is possible that this resulted in selection bias. Moreover, these participants might not generalize to the broader preoperative population. Most of our patients were Caucasian, and racial disparities in predictors of mobility have been recognized.37 Second, we assessed postoperative outcomes up to 30 days post-surgery, so we cannot make any inferences about long-term postoperative outcomes. However, 30 days is the time frame most often used in other postoperative outcome studies, since 30-day readmission is a key indicator of healthcare outcomes.38 Third, we did not measure cognitive function preoperatively, and we could not control for cognition when we analyzed these data. Indeed, preoperative cognitive impairment is a risk factor for poor postoperative outcomes.39,40 Given that walking speed is affected by cognition, especially executive function,41,42 it would be interesting to determine in future studies whether the MAT-sf captures both mobility and underlying cognitive impairment that affects postoperative outcomes. Fourth, we enrolled patients who were undergoing various surgeries, including orthopedic procedures for conditions that have the capacity to cause mobility- and chronic pain –related limitations and other procedures for conditions that do not overtly impair mobility. The impact of preoperative mobility limitation, enhanced recovery protocols and/or standardization of care on postoperative outcomes might be different in these two surgical groups. However, in our sensitivity analysis involving orthopedic-only patients, neither self-reported mobility nor frailty status was associated with postoperative outcomes (fully adjusted model), suggesting that mobility-related limitations linked to joint, spine and/or bone surgery probably did not skew our overall findings. Moreover, since the study wasn’t designed for the current analysis and our analysis was exploratory, further investigations using the MAT-sf score as an indicator of physical status and a risk factor in the assessment of postoperative outcome are needed. Finally, there was only one patient who was frail, perhaps related to the fact that our patients were there for an elective surgery and because we excluded those patients who required assistive devices to walk 4 meters. More work is needed to assess the efficacy of the MAT-sf in predicting postoperative outcomes in frail patients. Still, the MAT-sf provides a tool to discriminate among older elective surgical patients who are not frail.
Conclusions
In this single center cohort study, we provide conditional evidence that preoperative self-reported mobility, as measured by the MAT-sf, predicts early postoperative complications, hospital LOS, and discharge to a nursing home in older patients scheduled for elective noncardiac surgery. In addition to validating these findings in a larger, multi-center investigation, future studies are warranted to test whether preoperative strength and balance training might limit adverse postoperative outcomes and reduce LOS in older patients with self-reported measures of limitations in mobility.
What we already know about this topic
Preoperative assessment of patient characteristics that can assist in the evaluation of risk for adverse postoperative outcomes is especially important for the elderly, in whom postoperative complications are more likely.
Traditional risk assessments, such as American Society of Anesthesiologists (ASA) Physical Status Classification, Revised Cardiac Risk Index (RCRI), and 5-point frailty evaluation, may be too comprehensive, too focused on single organ systems, or too impractical.
What this article tells us that is new
Preoperative self-reported mobility, as measured by the quick, reliable, and cost-effective mobility assessment tool-short form (MAT-sf), predicted early postoperative complications, hospital length of stay, and discharge to a nursing home in older patients scheduled for elective noncardiac surgery.
Acknowledgments
This study was funded in part by the Anesthesia Patient Safety Foundation (LG), the Translational Science Center at Wake Forest University, the Center for Integrative Medicine (LG), and the Claude D. Pepper Center Older Americans Independence Center (P30 AG21332) (SK), Wake Forest School of Medicine, Winston-Salem, NC.
Appendix 1. MAT-sf Tertile
| MAT-sf Tertile | ||||
|---|---|---|---|---|
| Complications | Worst | Middle | Best | Total |
| n | n | n | n | |
| Pneumonia | 0 | 1 | 0 | 1 |
| Infection | 3 | 6 | 2 | 11 |
| Ileus | 2 | 1 | 2 | 5 |
| Renal Insufficiency | 1 | 0 | 1 | 2 |
| Thromboembolism | 2 | 0 | 0 | 2 |
| Myocardial infarction | 0 | 2 | 0 | 2 |
| Congestive heart failure | 2 | 0 | 0 | 2 |
| Atrial fibrillation | 3 | 1 | 0 | 4 |
| Delirium | 7 | 4 | 0 | 11 |
| Total | 20 | 15 | 5 | 40 |
Data represent the number (n) of patients within each tertile of the MAT-sf who had a documented postoperative complication. Greater postoperative delirium was found among patients with mobility limitation, as characterized by worst (range: 30.2–47.7) and mid tertiles (range: 57.95–67.76) of the MAT-sf, as compared to those in the best tertile of the MAT-sf (range: 57.95–67.76) (P = 0.03).
MAT-sf = Mobility Assessment Tool-short form.
Appendix 2. Univariate association between frailty and self-reported mobility using the MAT-sf and postoperative complications, time to discharge, and nursing home placement among orthopedic-only patients (n= 104)
| Postoperative | Time to Discharge | Nursing Home | |||||
|---|---|---|---|---|---|---|---|
| n | Complications (%) | P-value | (days, mean (SD)) | P-value | Placement (%) | P-value | |
| Frailty | |||||||
| Non-frail | 63 | 6 (9.5%) | 0.0400 | 2.9 (1.6) | <0.01 | 7 (11.11%) | <0.01 |
| Intermediately frail/frail | 41 | 10 (24.4%) | 5.2 (6.6) | 14 (34.15%) | |||
| Mobility* | |||||||
| Best tertile | 35 | 3 (8.6%) | 0.0485 | 2.6 (1.9) | <0.01 | 2 (5.7%) | 0.1147 |
| Mid tertile | 35 | 7 (20%) | 4.3 (6.8) | 6 (17.1%) | |||
| Worst tertile | 34 | 11 (32.4%) | 4.4 (2.8) | 8 (23.5%) | |||
Results for ‘postoperative complications’ and ‘nursing home placement’ are presented as number (n) of patients (percentage of frailty category or mobility tertile) (chi square test for association). Results for ‘time to discharge’ are presented as mean days (SD) (Student’s t-test was used to compare log-transformed time to discharge between the two frailty categories and analysis of variance (ANOVA) between mobility tertiles).
MAT-sf cutoff tertiles (mean ± SD): Best tertile (59.45 ± 4.68); Middle tertile (48.59 ± 2.31); Worst tertile (37.95 ± 7.80).
MAT-sf = Mobility Assessment Tool-short form.
Appendix 3. Association between frailty and self-reported mobility using the MAT-sf postoperative complications, time to discharge, and nursing home placement after multivariate adjustment among orthopedic-only patients (n = 104)
| Predictor | Postoperative Complications | Time to Discharge | Nursing Home Placement | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | HR | 95% CI | P-value | OR | 95% CI | P-value | |
| Unadjusted* | |||||||||
| Frailty | |||||||||
| Intermediately Frail/Frail | 3.07 | 1.02–9.23 | 0.05 | 0.56 | 0.37–0.85 | <0.01 | 4.15 | 1.50–11.47 | <0.01 |
| MAT-sf† | 2.13 | 1.17–3.87 | 0.01 | 0.77 | 0.63–0.94 | <0.01 | 1.92 | 1.13–3.25 | 0.02 |
| Traditional Covariate-adjusted + CRP‡ | |||||||||
| Frailty | |||||||||
| Intermediately Frail/Frail | 2.44 | 0.64–9.27 | 0.19 | 0.61 | 0.37–1.01 | 0.05 | 3.66 | 0.98–13.67 | 0.05 |
| MAT-sf† | 1.91 | 1.00–3.64 | 0.05 | 0.82 | 0.66–1.01 | 0.06 | 1.55 | 0.87–2.78 | 0.14 |
| Full Adjusted§ | |||||||||
| Frailty | |||||||||
| Intermediately Frail/Frail | 1.96 | 0.49–7.85 | 0.34 | 0.67 | 0.40–1.12 | 0.13 | 3.22 | 0.85–12.19 | 0.08 |
| MAT-sf† | 1.82 | 0.94–3.52 | 0.08 | 0.85 | 0.68–1.05 | 0.12 | 1.45 | 0.79–2.67 | 0.24 |
Values represent odds ratios (OR) or hazard ratios (HR) and 95% CI. Logistic regression models were used to estimate odds ratios of postoperative complications and nursing home placement, and Cox proportional hazard regression models were used to compare time to discharge for frailty status and MAT-sf (as a continuous variable).
Intermediately Frail/Frail or Mobility Assessment Test-short form (MAT-sf) as the only predictor in the model.
Odds ratios or hazard ratios were for every 10-point decrease of MAT-sf, which is equivalent to 1 standard deviation (SD).
Adjusted for traditional covariates, including age, sex, body mass index, pain score, Revised Cardiac Risk Index, ASA Physical Status classification and surgical risk, plus CRP, but only either frailty or MAT-sf as the predictor in the model.
All covariates in the traditional + CRP model, plus both frailty and MAT-sf were in the model.
ASA = American Society of Anesthesiologists; CRP = high-sensitivity C-reactive protein; MAT-sf = Mobility Assessment Tool-short form
Appendix 4
A) Area under the receiver operating characteristic curve in orthopedic-only patients (n=104) for postoperative complications and B) nursing home placement in models including traditional covariates plus CRP and with either frailty or MAT-sf scores. Traditional covariates included age, sex, body mass index, pain score, Revised Cardiac Risk Index, ASA Physical Status Classification status, and surgical risk. ASA = American Society of Anesthesiologists; CRP = high-sensitivity C-reactive protein; MAT-sf = Mobility Assessment Tool-short form.
Footnotes
Competing Interests
The authors declare no competing interests.
Presented in part at the 8th Annual Perioperative Medicine Summit, March 7–9, 2013, Miami, Florida; the American Geriatrics Society Annual Meeting, May 2–5, 2013, Grapevine, Texas; the American Society of Anesthesiologists Annual Meeting, October 12–16, 2013, San Francisco, California; and the Gerontological Society of America’s Annual Meeting, November 18–21, 2015, Orlando, Florida.
References
- 1.Etzioni DA, Liu JH, O’Connell JB, Maggard MA, Ko CY. Elderly patients in surgical workloads: a population-based analysis. Am Surg. 2003;69:961–5. [PubMed] [Google Scholar]
- 2.Buldu I, Tepeler A, Karatag T, Bodakci MN, Hatipoglu NK, Penbegul N, Akman T, Istanbulluoglu O, Armagan A. Does aging affect the outcome of percutaneous nephrolithotomy? Urolithiasis. 2015;43:183–7. doi: 10.1007/s00240-014-0742-4. [DOI] [PubMed] [Google Scholar]
- 3.Chung F, Mezei G. Adverse outcomes in ambulatory anesthesia. Can J Anaesth. 1999;46:R18–34. doi: 10.1007/BF03013179. [DOI] [PubMed] [Google Scholar]
- 4.Sieber FE, Barnett SR. Preventing postoperative complications in the elderly. Anesthesiol Clin. 2011;29:83–97. doi: 10.1016/j.anclin.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiret L, Hatton F, Desmonts JM, Vourc'h G. Prediction of outcome of anaesthesia in patients over 40 years: a multifactorial risk index. Stat Med. 1988;7:947–54. doi: 10.1002/sim.4780070906. [DOI] [PubMed] [Google Scholar]
- 6.Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217–22. doi: 10.1093/bja/77.2.217. [DOI] [PubMed] [Google Scholar]
- 7.Sauvanet A, Mariette C, Thomas P, Lozac'h P, Segol P, Tiret E, Delpero JR, Collet D, Leborgne J, Pradère B, Bourgeon A, Triboulet JP. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201:253–62. doi: 10.1016/j.jamcollsurg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Zakriya KJ, Christmas C, Wenz JF, Sr, Franckowiak S, Anderson R, Sieber FE. Preoperative factors associated with postoperative change in confusion assessment method score in hip fracture patients. Anesth Analg. 2002;94:1628–32. doi: 10.1097/00000539-200206000-00050. [DOI] [PubMed] [Google Scholar]
- 9.Prause G, Offner A, Ratzenhofer-Komenda B, Vicenzi M, Smolle J, Smolle-Jüttner F. Comparison of two preoperative indices to predict perioperative mortality in non-cardiac thoracic surgery. Eur J Cardiothorac Surg. 1997;11:670–5. doi: 10.1016/s1010-7940(97)01150-0. [DOI] [PubMed] [Google Scholar]
- 10.Rauh MA, Krackow KA. In-hospital deaths following elective total joint arthroplasty. Orthopedics. 2004;27:407–11. doi: 10.3928/0147-7447-20040401-18. [DOI] [PubMed] [Google Scholar]
- 11.Carey MS, Victory R, Stitt L, Tsang N. Factors that influence length of stay for in-patient gynaecology surgery: is the Case Mix Group (CMG) or type of procedure more important? J Obstet Gynaecol Can. 2006;28:149–55. doi: 10.1016/s1701-2163(16)32057-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 13.Eagle KA, Vaishnava P, Froehlich JB. Perioperative cardiovascular care for patients undergoing noncardiac surgical intervention. JAMA Intern Med. 2015;175:835–9. doi: 10.1001/jamainternmed.2015.0150. [DOI] [PubMed] [Google Scholar]
- 14.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. 2014;311:2110–20. doi: 10.1001/jama.2014.4573. [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–42. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 17.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TN, Wu DS, Sauaia A, Dunn CL, Stevens-Lapsley JE, Moss M, Stiegmann GV, Gajdos C, Cleveland JC, Jr, Inouye SK. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg. 2013;258:582–8. doi: 10.1097/SLA.0b013e3182a4e96c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–76. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 20.Rejeski WJ, Marsh AP, Anton S, Chen SH, Church T, Gill TM, Guralnik JM, Glynn NW, King AC, Rushing J, Ip EH LIFE Research Group. The MAT-sf: clinical relevance and validity. J Gerontol A Biol Sci Med Sci. 2013;68:1567–74. doi: 10.1093/gerona/glt068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rejeski WJ, Ip EH, Marsh AP, Barnard RT. Development and validation of a video-animated tool for assessing mobility. J Gerontol A Biol Sci Med Sci. 2010;65:664–71. doi: 10.1093/gerona/glq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, Chong V, Fabri PJ, Gibbs JO, Grover F, Hammermeister K, Irvin G, 3rd, McDonald G, Passaro E, Jr, Phillips L, Scamman F, Spencer J, Stremple JF. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 24.Dzankic S, Pastor D, Gonzalez C, Leung JM. The Prevalence and predictive value of abnormal preoperative laboratory tests in elderly surgical patients. Anesth Analg. 2001;93:301–308. doi: 10.1097/00000539-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 26.Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, Sharp TJ, Buckley MJ, Moss M. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213:37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganapathi AM, Englum BR, Hanna JM, Schechter MA, Gaca JG, Hurwitz LM, Hughes GC. Frailty and risk in proximal aortic surgery. J Thorac Cardiovasc Surg. 2014;147:186–91. doi: 10.1016/j.jtcvs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64:e77–137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 29.Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, Weiss K, Owens DK, Aronson M, Barry P, Casey DE, Jr, Cross JT, Jr, Fitterman N, Sherif KD, Weiss KB Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575–80. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 30.Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, Sharp TJ, Moss M. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 31.Ble A, Volpato S, Zuliani G, Guralnik JM, Bandinelli S, Lauretani F, Bartali B, Maraldi C, Fellin R, Ferrucci L. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–5. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 32.Holtzer R, Wang C, Lipton R, Verghese J. The protective effects of executive functions and episodic memory on gait speed decline in aging defined in the context of cognitive reserve. J Am Geriatr Soc. 2012;60:2093–8. doi: 10.1111/j.1532-5415.2012.04193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310:1168–77. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 34.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 35.Rantakokko M, Mänty M, Rantanen T. Mobility decline in old age. Exerc Sport Sci Rev. 2013;41:19–25. doi: 10.1097/JES.0b013e3182556f1e. [DOI] [PubMed] [Google Scholar]
- 36.Rejeski WJ, Rushing J, Guralnik JM, Ip EH, King AC, Manini TM, Marsh AP, McDermott MM, Fielding RA, Newman AB, Tudor-Locke C, Gill TM LIFE Study Group. The MAT-sf: identifying risk for major mobility disability. J Gerontol A Biol Sci Med Sci. 2015;70:641–6. doi: 10.1093/gerona/glv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allman RM, Baker PS, Maisiak RM, Sims RV, Roseman JM. Racial similarities and differences in predictors of mobility change over eighteen months. J Gen Intern Med. 2004;19:1118–26. doi: 10.1111/j.1525-1497.2004.30239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 39.Ansaloni L, Catena F, Chattat R, Fortuna D, Franceschi C, Mascitti P, Melotti RM. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010;97:273–80. doi: 10.1002/bjs.6843. [DOI] [PubMed] [Google Scholar]
- 40.Robinson TN, Wu DS, Pointer LF, Dunn CL, Moss M. Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg. 2012;215:12–7. doi: 10.1016/j.jamcollsurg.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eggermont LH, Gavett BE, Volkers KM, Blankevoort CG, Scherder EJ, Jefferson AL, Steinberg E, Nair A, Green RC, Stern RA. Lower-extremity function in cognitively healthy aging, mild cognitive impairment, and Alzheimer's disease. Arch Phys Med Rehabil. 2010;91:584–8. doi: 10.1016/j.apmr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coppin AK, Shumway-Cook A, Saczynski JS, Patel KV, Ble A, Ferrucci L, Guralnik JM. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–2. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]