Abstract
Minocycline is purported to have neuroprotective properties in experimental models of some human neurologic diseases, and has therefore been identified as a putative neuroprotectant for chemotherapy-induced cognitive impairment (CICI) in breast cancer patients. However, because its mechanism of action is believed to be mediated through anti-inflammatory, anti-apoptotic, and anti-oxidant pathways, co-administration of minocycline with chemotherapeutic agents has the potential to reduce the efficacy of anticancer drugs. The objective of this study is to evaluate the effect of minocycline on the activity of the AC chemotherapeutic regimen (Adriamycin [doxorubicin], Cytoxan [cyclophosphamide]) in in vitro and in vivo models of triple-negative breast cancer (TNBC). Clonogenic and methylthiazol tetrazolium (MTT) assays were used to assess survival and viability in two TNBC cell lines treated with increasing concentrations of AC in the presence or absence of minocycline. Biomarkers of apoptosis, cell stress, and DNA damage were evaluated by western blot. The in vivo effects of AC and minocycline, each alone and in combination, were assessed in a xenograft model of TNBC in female athymic nude mice by weekly tumor volume measurement, body and organ weight measurement, and histopathology. Apoptosis and proliferation were characterized by immunohistochemistry in the xenografts tumors. Brains from tumor-bearing mice were evaluated for microglial activation, glial scars, and the proportion of neural progenitor cells. Data from these in vitro and in vivo studies demonstrate that minocycline does not diminish the cytotoxic and tumor-suppressive effects of this chemotherapeutic drug combination in TNBC cells. Moreover, minocycline appeared to prevent the reduction in doublecortin-positive neural progenitor cells observed in AC-treated mice. We posit that minocycline may be useful clinically for its reported neuroprotective activity in breast cancer patients receiving AC without loss of chemotherapeutic efficacy.
Keywords: triple-negative breast cancer, minocycline, chemotherapy, chemotherapy-induced cognitive impairment, chemo fog
1. Introduction
Minocycline is a semi-synthetic second-generation tetracycline derivative that, in addition to its use as a broad-spectrum antibiotic, has demonstrated antioxidant, anti-inflammatory, and anti-apoptotic effects in the nervous system which confer neuroprotection (Domercq and Matute, 2004; Kim and Suh, 2009; Plane et al., 2010, Garrido-Mesa et al., 2013). Increased neuron survival, preserved neuronogenesis, and reduced neuroinflammation have been reported in cell culture and rodent studies of a multitude of human neurologic disease states, including hypoglycemia, ischemia, and neurodegenerative disorders (Yrjänheikki et al., 1998; Popovic et al., 2002; Wang et al., 2004; Switzer et al., 2011; Won et al., 2012). In the brain, minocycline exerts its effects in part through protection against cell death and decreased neuroinflammation. Protection against cell death occurs via caspase-independent factors, such as upregulation of pro-survival Bcl-2, and caspase-dependent factors, including decreased cytochrome c release from mitochondria and decreased caspase-1 and caspase-3 levels (Plane et al., 2010; Lee et al., 2011) as well as antioxidant activity through inhibition of cyclooxygenase-2 and inducible nitric oxide synthase and decreased p38 MAP kinase phosphorylation (Du et al., 2001; Plane et al., 2010). Anti-inflammatory effects of minocycline in the central nervous system are attributed to decreased microglial activation and decreased T cell migration, which are associated with lower levels of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-6, and matrix metalloproteases (Popovic et al., 2002; Plane et al., 2010). Mounting pre-clinical data in support of the neuroprotective effects of minocycline have led to phase II clinical trials for traumatic spinal cord injuries in which minocycline has shown beneficial outcomes in motor recovery in a subset of patients with cervical injury (Casha et al., 2012). Minocycline has also demonstrated protection against ototoxicity associated with cisplatin administration in an animal model which evaluated auditory hair cell degeneration and the auditory brainstem response (Lee et al., 2011).
Based on these purported neuroprotective properties, we have identified minocycline as a candidate therapeutic agent for the treatment of chemotherapy-induced cognitive impairment (CICI). This phenomenon, colloquially termed “chemo brain” or “chemo fog,” describes a state of prolonged cognitive dysfunction resulting from administration of a variety of common cytotoxic chemotherapeutic agents, including doxorubicin and cyclophosphamide (Christie et al., 2012), which may last years after cessation of chemotherapy (Winocur et al., 2012; Johnston, 2014). Cognitive decline following chemotherapy is reported in 17-78% of cancer patients (Seigers and Fardell, 2011; Winocur et al., 2012; Reuter-Lorenz and Cimprich, 2013). CICI is well-documented in women receiving chemotherapy for breast cancer (van Dam et al., 1998; Villanueva, 2012; Jim et al., 2012, Reuter-Lorenz and Cimprich, 2013) and has been recapitulated in numerous mouse and rat models (Gandal et al., 2008; Seigers and Fardell, 2011; Winocur et al., 2012). Because the median survival for breast cancer patients has improved significantly in recent decades, the patients' quality of life following chemotherapy must be carefully considered as part of survivor care (Raffa, 2011, Cheng et al., 2013). Our particular focus is on patients with triple-negative breast cancer (TNBC), in which tumors cells do not express estrogen receptor-alpha, progesterone receptor, or human epidermal growth factor receptor 2/neu (Foulkes et al., 2010; Holliday and Speirs, 2011). The absence of these cellular receptors leaves fewer therapeutic options, and thus these patients are more likely to receive adjuvant chemotherapeutic drug combinations like the AC regimen (Adriamycin [doxorubicin] and Cytoxan [cyclophosphamide]) (O'Toole et al., 2013).
Of significant concern, however, is that the antioxidant and anti-apoptotic effects of minocycline could diminish the cytotoxic effects of chemotherapy on tumor cells. Such concerns have provoked considerable debate about the use of supplemental antioxidants and whether they can alter the efficacy of chemo- and radiotherapy in cancer patients. Reviews of published clinical trial results reporting the concurrent use of antioxidants with chemotherapy have revealed conflicting data, with some suggesting no decrease in therapeutic efficacy and others indicating compromised tumor control (Block et al., 2008; Simone et al., 2007; Lawenda et al., 2008; Yasueda et al., 2016). Ultimately, it was concluded that the safety and efficacy of these combinations remains unclear and warrants further research. In the context of CICI, preclinical data indicate that the glutathione precursor, N-acetyl cysteine, can mitigate chemotherapy-related cognitive deficits during AC chemotherapy in tumor-free rats, supporting a role for antioxidants in the treatment of CICI (Konat et al., 2008). Moreover, minocycline has been reported to have antineoplastic properties in studies of ovarian carcinoma (Pourgholami et al., 2012) and in combination with antiangiogenic agents in de novo pancreatic islet cell tumorigenesis in mice (Parangi et al., 1996), which could potentially lead to a summational cytotoxic effect if co-administered with chemotherapeutic agents.
The over-arching goal of this study is to describe the effect of minocycline on TNBC cells in vitro and in vivo to determine whether it alters their susceptibility to common chemotherapeutic drugs, before minocycline can be considered as a potential neuroprotective agent to be administered concurrently to breast cancer patients receiving chemotherapy. In this study, the effects of minocycline on the activity of the AC chemotherapeutic drug combination were assessed in in vitro (MDA-MB-231 and MDA-MB-468 cells) and in vivo (MDA-MB-231 xenograft tumors) models of TNBC. In addition, the presence of neuroanatomic lesions consistent with the proposed pathophysiology of CICI and the effects of therapeutic intervention with minocycline on specific brain regions were also evaluated. To the authors' knowledge, this is the first reported study examining the effects of combined AC and minocycline therapy in xenograft tumors and the brains of tumor-bearing mice simultaneously.
2. Materials and Methods
2.1. Cell lines and culture conditions
The TNBC cell lines MDA-MB-231 (HTB-26) and MDA-MB-468 (HTB-132) were obtained from American Type Culture Collection (Manassas, VA) and were cultured under sterile conditions in high-glucose Dulbecco's Modified Eagle Medium (DMEM, Life Technologies, Corp., Grand Island, NY) supplemented with 10% fetal bovine serum (Life Technologies) and 0.1% gentamicin and maintained in a 37°C, humidified incubator with 5% CO2. Media was changed every 48 hours and cells were passaged upon confluence.
2.2 Drugs
For in vitro experiments, the following stock solutions of drugs were prepared: minocycline HCl (Sigma-Aldrich, St. Louis, MO) at 25mM in sterile water, doxorubicin HCl (Sigma-Aldrich) at 25mM in sterile water, and cyclophosphamide monohydrate (Sigma-Aldrich) at 1M in sterile dimethyl sulfoxide (DMSO). For the in vivo experiment, minocycline for administration per os was completely dissolved in water (9mg/ml), physiologically balanced (pH 7.35) to eliminate the potential for chemical pharyngitis or esophagitis, and sterilized by filtration (0.22 μm). This minocycline working solution was stored at 4°C, protected from light, and prepared fresh every 48 hours. Pharmaceutical-grade doxorubicin (Bedford Laboratories, Bedford, OH) and cyclophosphamide (Baxter Healthcare Corp., Deerfield, IL) were reconstituted and diluted in 0.9% sodium chloride (USP, Hospira, Inc., Lake Forest, IL), and then combined for intravenous injection as a cocktail containing final concentrations of doxorubicin (A) and cyclophosphamide (C) at 1 and 20 mg/ml, respectively.
2.3 Cell viability
Cell viability was assessed by methylthiazol tetrazolium (MTT) assays using 3-(4,5-dimethylthiasol-2-yl)-2,5-diphenyltetrazolium bromide (Biomatik, Wilmington, DE). Cells were seeded at 3-4,000 cells per well in 96-well microtiter plates, incubated overnight, and then treated with minocycline alone or in combination with doxorubicin or AC. For assays involving minocycline co-treatment, cells were pretreated with minocycline for 24 hours, followed by 48hour exposure to the combination of minocycline with doxorubicin or AC. After drug treatment, cells were incubated with MTT solution for 60 minutes, the formazan crystals were solubilized with DMSO, and the absorbance was measured by spectrophotometry at 570nm wavelength. The IC50 values for doxorubicin and cyclophosphamide alone were determined by MTT in both the MDA-MB-231 and MDA-MB-468 cell lines (data not shown), and used to establish dose ranges for subsequent experiments.
2.4 Cell survival
Cell survival was evaluated by colony formation assays, which were performed by culturing 800 cells in 6-well plates, exposing cells to minocycline for 24 hours, followed by the minocycline-AC combination for 24 hours, then allowing the cells to proliferate for 12 days, with media changed every 72 hours. Colonies were fixed with 4% formaldehyde (Sigma-Aldrich) and stained with crystal violet (5mg/ml in 2% ethanol, Sigma-Aldrich), then counted.
2.5 Western blotting
Western blotting was performed by lysing cells with sodium dodecyl sulfate (SDS) lysis buffer (1% SDS, 50mmol/L Tris-HCl pH 8.0, 10mmol/L EDTA) plus protease inhibitor cocktail (Sigma-Aldrich) and PhoSTOP phosphatase inhibitor (Roche) and sonication. Protein concentrations were standardized with a Micro BCA Protein Assay kit (Pierce Chemical), and each sample was homogenized in SDS sample buffer. SDS-polyacrylamide gel electrophoresis was performed using a 12% resolving gel. Wet transfer to PVDF membrane (Bio-Rad) was followed by blocking with 5% non-fat milk in TBS-T (TBS, 0.1% Tween-20). Incubation with primary antibodies was performed at a 1:500 dilution overnight at 4°C. Membranes were washed with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies at a 1:5000 dilution at room temperature for 1hr. Signal detection was performed on x-ray film using a chemiluminescence system (Amersham). Mouse primary antibody sources are: β-actin (MP Biomedicals), Bcl-2 (Santa Cruz), caspase-3 (Imgenex), γH2AX Ser139 (Millipore). Rabbit primary antibody sources are: H2AX (Millipore), p-p38 (Cell Signaling), PARP (Cell Signaling), c-myc (Cell Signaling), p38 (Cell Signaling).
2.6 In vivo studies
Eight to 12 week old athymic nude female mice (NCr-nu/nu, strain 01B74) were obtained from the National Institutes of Health Animal Procurement Program and were group-housed under conditions of constant photoperiod (12 hours light: 12 hours dark) with ad libitum access to sterilized food and water. All experimental procedures using mice were done in accordance with protocols approved by The Ohio State University Institutional Animal Care and Use Committee. Xenograft tumors were established by suspending MDA-MB-231 cells in Matrigel (BD Biosciences) and injecting them subcutaneously into the right flank of anesthetized mice (isoflurane; 5×105 cells/0.1ml/mouse). Tumor volumes and body weights were measured once weekly. Tumor length and width were measured by SK using calipers, and volume was calculated by applying the equation for the volume of a prolate ellipsoid [0.52×(L×W2)]. Body weights of xenografted mice were adjusted for the tumor weight, as calculated by volume. Mice with a tumor volume between 50-75mm3 were eligible for recruitment to the study, and were randomized to four treatment groups: vehicle control, minocycline only, AC only, and AC with minocycline (n=6-7/group). All animals received pre-treatment with minocycline (90 mg/kg) or vehicle (sterile distilled water) once daily per os for 3 days (starting on Day -3) and continuing for an additional 21 days (Day 0 - 21). On Day 0, mice were treated with a single IV injection of either AC [doxorubicin (A) at 5 mg/kg; cyclophosphamide (C) at 100 mg/kg] or vehicle (sterile normal saline). Minocycline was administered to anesthetized animals using a sterile, flexible gavage needle. AC and vehicle were administered by tail vein injection to non-anesthetized animals in a restrainer by a laboratory animal health technician. The AC chemotherapy modeled a common clinical regimen in which patients are treated every 21 days (Stover and Winer, 2015). Animals were euthanized on Day 21 by CO2 asphyxiation followed by cervical dislocation. Each animal was subject to complete post-mortem examination. All tissues were preserved by immersion fixation in neutral buffered 10% formalin. Organ weights were recorded post-fixation and are tabulated as absolute organ weight, organ weight relative to body weight, and organ weight relative to brain weight.
2.7 Histopathology and immunohistochemistry
Selected tissues were subject to a standard process of histopathologic evaluation (Crissman 2004), and routine H&E staining was performed by the Comparative Pathology and Mouse Phenotyping Shared Resource (CPMPSR). Tissues for toxicologic evaluation included: lung, heart, liver, kidney, spleen, bone marrow, small intestine, and brain. Brains were examined in 6 standard coronal sections per mouse (Bolon et al., 2013) as guided by CPMPSR technical staff. Due to collection technique, intact olfactory bulbs were not uniformly available for all mice being evaluated, and as such, were not considered in our analysis. Immunohistochemistry (IHC) was performed by CPMPSR using the same blocks. Five animals per treatment group closest to the group mean tumor volume were selected for immunohistochemical analysis. Primary antibodies for brain IHC are as follows: Iba1 (Abcam, 1:800), doublecortin (Abcam, 1:500), GFAP (Dako, 1:5000). Following primary antibody incubation, specimens were incubated with biotinylated secondary antibody and DAB chromagen, then counterstained with hematoxylin. One xenograft cross-section per mouse was processed for histology, and IHC was performed by CPMPSR using the same blocks. Primary antibodies for tumor IHC are as follows: cleaved caspase 3 (Cell Signaling, 1:180), Ki67 (Abcam, 1:200). Following primary antibody incubation, specimens were incubated with biotinylated secondary antibody and DAB chromagen, then counterstained with hematoxylin. All histologic analyses were performed by LH, and the features of selected tissues were peer-reviewed by a CPMPSR comparative pathologist. These assessments were performed using coding, where the pathologists were blinded to experimental conditions.
2.8 Digital immunohistochemical quantification
Blinded quantification of immunoreactivity was performed by LH with ImageScope Image Analysis Toolbox (Leica Biosystems). In the tumors, staining was distributed evenly throughout the tissues, so a subsampling technique was employed. Cleaved caspase 3 positivity in each tumor section was calculated by applying the positive pixel count algorithm to quantify strongly positive-stained pixels per number of total pixels in 5 high-power fields (400× magnification) per tumor section. Ki67 positivity in each tumor section was calculated by applying the nuclear algorithm to quantify the number of positively labeled nuclei per total number of nuclei in 5 high-power (400× magnification) fields per tumor section. Mean values per tumor and per treatment group were calculated. In brain sections, the relative level of doublecortin (DCX) in the hippocampus was calculated by applying the positive pixel count algorithm to quantify positive-stained pixels per number of total pixels in the dentate gyrus of each brain section. Mean values per brain and per treatment group were calculated.
2.9 Data analysis
Randomization for animal studies was performed using a free, web-based random sequence generator (www.random.org). All data are represented as the group mean +/- 1 standard deviation. Statistical significance in each experiment was tested by determining variance by F-test, then performing a Student's t-test with the alpha level set at 0.05 a priori.
3. Results
3.1 Minocycline cannot protect TNBC cells from the cytotoxic effect of AC in vitro
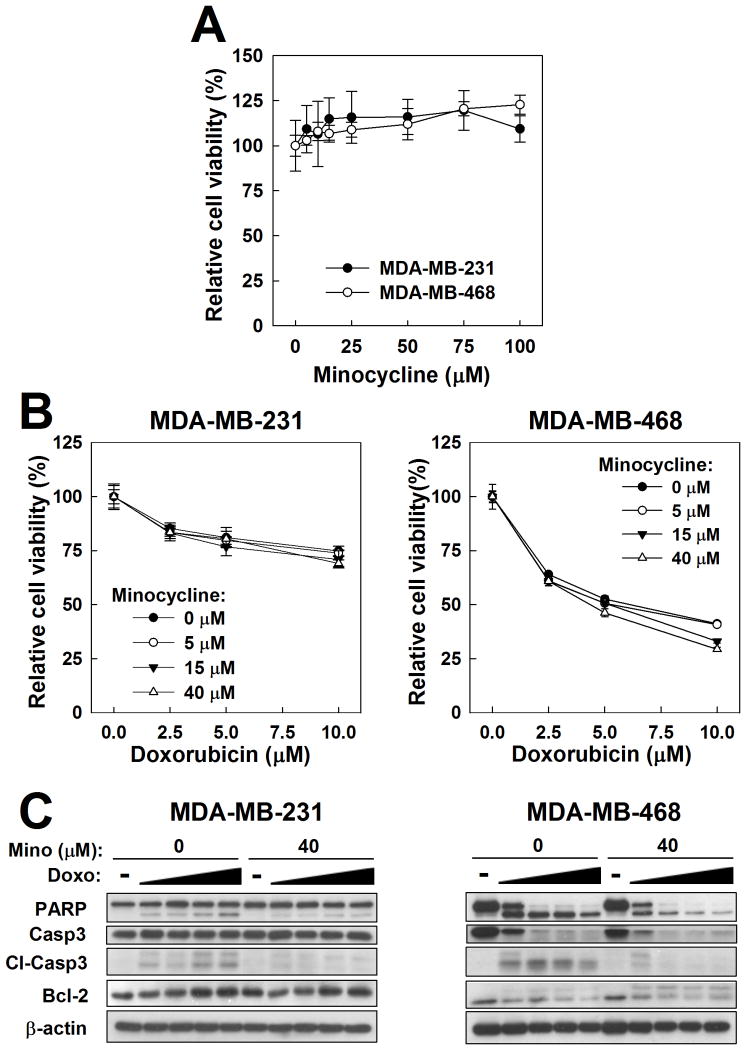
The effect of minocycline alone on TNBC cell viability was assessed by MTT assay in MDA-MB-231 and MDA-MB-468 cells. After 48-hour treatment, relative cell viability in either cell line was not diminished by minocycline, even at concentrations of up to 100μM (Fig. 1A). In MDA-MB-468 cells, however, a statistically significant increase in cell viability was observed at the highest concentrations tested (75 and 100μM; t-test, p<0.05, vs 0μM). The clinical relevance of the effects of these minocycline concentrations, however, is questionable as they are at least an order-of-magnitude greater than the plasma Cmax achieved with various clinical oral formulations of minocycline (Agwuh and MacGowan, 2006; Araujo et al., 2001; Setiawati et al., 2009; Abramowicz, 2006; Gupta et al., 2006).
Figure 1.
Effects of minocycline alone and in combination with doxorubicin in MDA-MB-231 and MDA-MB-468 TNBC cells. A and B. Cell viability was assessed by MTT assay after treatment with minocycline alone (A) and in combination with doxorubicin (B). C. Evaluation of the effect of minocycline (Mino) on apoptosis-related biomarkers in doxorubicin (Doxo)-treated TNBC cells by western blot. Concentrations of doxorubicin used were 0, 1, 2.5, 5 and 10 μM. Cl-Casp3, cleaved caspase-3. In both B and C, cells were pretreated with minocycline for 24 hours, followed by 48-hour exposure to the doxorubicin-minocycline combination.
For initial assessment of the effects of minocycline on chemotherapy-induced cytotoxicity, TNBC cells were co-treated with minocycline and doxorubicin, an anthracycline that can induce growth arrest and apoptosis in cancer cells through disruption of DNA repair and generation of reactive oxygen species (Gewirtz, 1999). As shown in Fig. 1B, minocycline at concentrations of up to 40μM had no significant effect on the viability of doxorubicin-treated MDA-MB-231 or MDA-MB-468 cells. Western blot analysis, however, revealed that minocycline at 40μM decreased doxorubicin-induced cleavage of caspase-3 and PARP (Fig. 1C), reminiscent of its effects in cisplatin-treated HEI-OC1 auditory cells (Lee et al., 2011). While change in Bcl-2 abundance was not detected in MDA-MB-231 cells, MDA-MB-468 cells exhibited an increase in a 30kDa Bcl-2 immunopositive band in the presence of minocycline that may represent phosphorylated Bcl-2 or another isoform of the Bcl-2 protein (Engel et al., 2004).
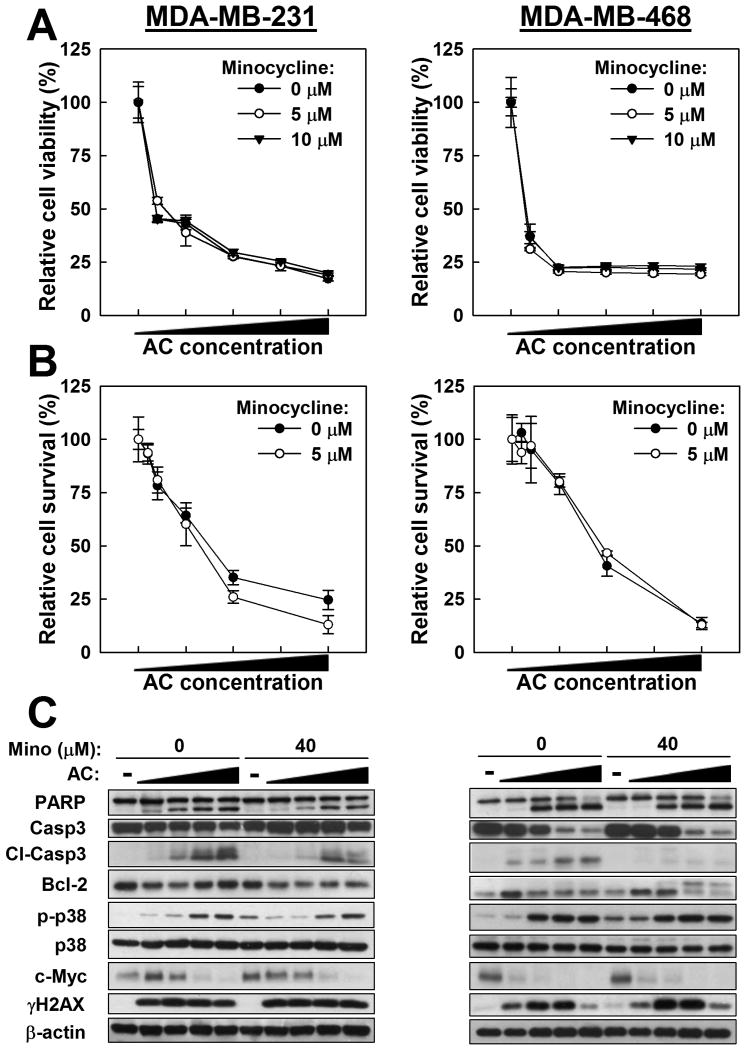
To evaluate the effects of minocycline on the activity of a common doxorubicin-based adjuvant therapy regimen, MDA-MB-231 and MDA-MB-468 cells were co-treated with minocycline and the AC combination. Similar to the findings above, minocycline showed no protective effect against AC-induced inhibition of cell viability or clonogenicity as determined by MTT (Fig. 2A) and colony formation (Fig. 2B) assays, respectively, but still reduced the abundance of AC-induced cleaved caspase-3, as determined by western blot (Fig. 2C). However, the apparent anti-apoptotic effect of minocycline on PARP cleavage that was observed in cells treated with minocycline plus doxorubicin as a single chemotherapeutic agent was lost (Fig. 2C). Moreover, no changes to AC-induced p38 phosphorylation, c-myc downregulation, or H2AX phosphorylation were observed with the addition of minocycline to AC treatment (Fig. 2C).
Figure 2.
Effects of minocycline on the cytotoxicity of AC chemotherapy in MDA-MB-231 and MDA-MB-468 TNBC cells. A. Cell viability was assessed by MTT assay after minocycline co-treatment with AC (doxorubicin/cyclophosphamide [μM/mM]: 0/0, 1/2.5, 2.5/5, 5/10, 7.5/15, and 10/20). B. Cell survival was assessed by colony formation assay after minocycline co-treatment with AC (nM/μM: 0/0, 0.5/0.5, 1/1, 2.5/2.5, 5/5, 10/10). C. Evaluation of the effect of minocycline (Mino) on biomarkers in AC-treated TNBC cells by western blot. Cells were treated with AC at 0/0 0.5/1, 1/2.5, 2.5/5, 5/10 μM/mM. For panels A and C, cells were pretreated with minocycline for 24 hours, followed by 48-hour exposure to the AC-minocycline combination. For B, cells were treated with minocycline for 24 hours, and then the AC-minocycline combination for 24 hours, followed by a 12-day growth period.
3.2 Minocycline cannot protect TNBC xenograft tumors from the growth suppressive effect of AC in vivo
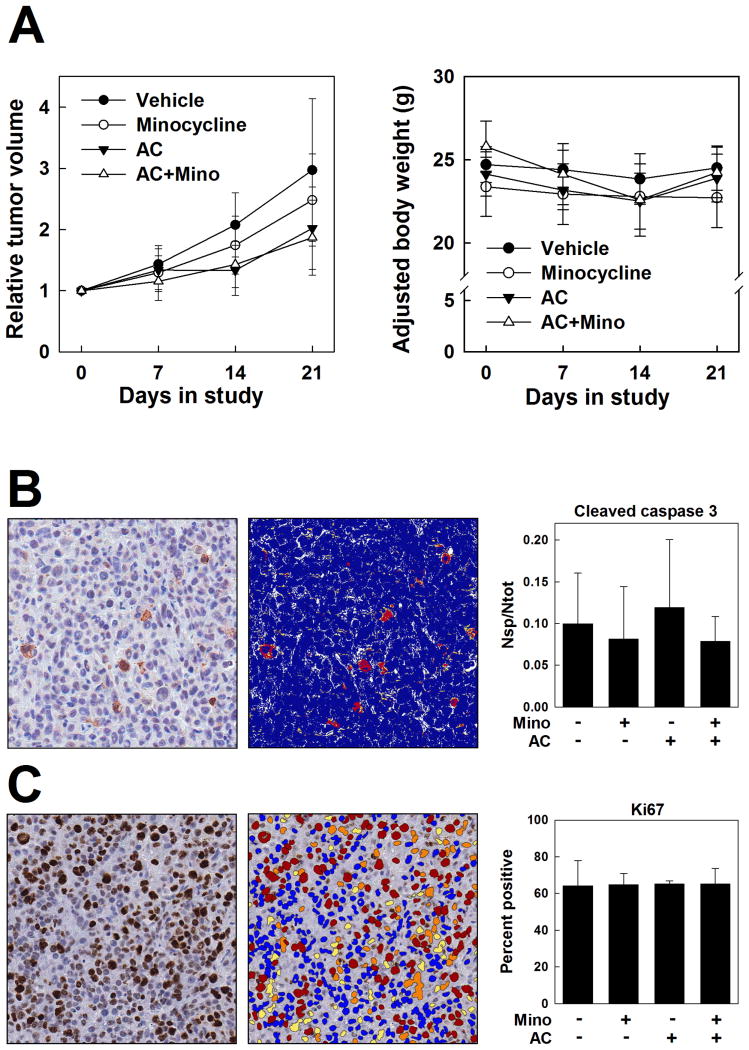
To investigate the effects of minocycline on AC activity in vivo, athymic nude mice bearing subcutaneous MDA-MB-231 xenograft tumors were treated with minocycline at 90mg/kg or vehicle per os once daily for 3 days and continuing for an additional 21 days following a single administration of AC or vehicle by IV injection. As shown in Fig. 3A, treatment with minocycline alone did not significantly affect tumor growth compared to the vehicle–treated control, and, more importantly, minocycline co-treatment did not alter the tumor-suppressive activity of AC treatment. Moreover, mean adjusted body weights of the drug-treated groups over this time period were not significantly different from those of the vehicle-treated control group (Fig. 3A). Both the AC and the AC plus minocycline treatment groups experienced body weight loss which was most pronounced at day 14, but recovered by day 21, following a pattern observed for a similar AC-based chemotherapeutic regimen in mice (Franci et al., 2013). Postmortem toxicologic evaluation revealed a consistent, significant decrease in splenic absolute weight and relative weight normalized to body or brain weight in the combined AC plus minocycline treatment group (Table 1). Histologic lesions were not appreciated by light microscopic examination of the spleen or bone marrow. No evidence of drug-related systemic toxicity was identified on histopathology at the study endpoint (data not shown).
Figure 3.
Effects of minocycline on the tumor-suppressive activity of AC chemotherapy in the MDA-MB-231 TNBC xenograft model. Upon recruitment to the study, tumor-bearing mice were treated with minocycline (90mg/kg, per os, once daily) or vehicle for 24 days (Day -3 to Day 21). On Day 0, mice were treated with AC (A [doxorubicin], 5mg/kg; C [cyclophosphamide], 100mg/kg; IV) or vehicle. A. Relative tumor volumes and body weights in vehicle control, minocycline, AC, and AC plus minocycline treatment groups. B and C. Immunohistochemistry for cleaved caspase 3 (B) and Ki67 (C) in formalin-fixed paraffin-embedded MDA-MB-231 xenograft tumors. Representative immunostained sections (left panels; counterstained with hematoxylin) and corresponding mockup images for digital analysis (middle) from the vehicle treatment group are shown. Right, quantification of cleaved caspase 3 staining expressed as the number of strong positive pixels per total pixels analyzed (Nsp/Ntot) and Ki67 staining expressed as percent positive nuclei per total nuclei analyzed in tumors from the four treatment groups (n=5). Positivity is represented in the mock-up images as a heat map wherein strongly positive pixels are red, moderately positive pixels are orange, and weakly positive pixels are yellow; negative pixels are blue, and unquantified pixels in an area of analysis are white. Magnification, 40×.
Table 1. Post-fixation absolute and relative organ weightsa of mice treated with vehicle, minocycline, doxorubicin-cyclophosphamide (AC), or the combination of AC plus minocycline.
| Organ | Vehicle | Minocycline | AC | AC + Minocycline |
|---|---|---|---|---|
| Liver | ||||
| Absolute (mg) | 1190.55 ± 79.39 | 1073.20 ± 172.58 | 1165.40 ± 135.20 | 1126.81 ± 119.18 |
| Relativebody | 4.86 ± 0.33 | 4.70 ± 0.52 | 4.82 ± 0.43 | 4.62 ± 0.62 |
| Relativebrain | 239.06 ± 14.90 | 215.75 ± 25.13 | 234.66 ± 23.22 | 229.25 ± 24.80 |
|
| ||||
| Kidneys | ||||
| Absolute (mg) | 366.06 ± 26.54 | 354.53 ± 40.81 | 370.31 ± 63.48 | 494.65 ± 18.86† |
| Relativebody | 1.49 ± 0.08 | 1.56 ± 0.12 | 1.53 ± 0.20 | 1.52 ± 0.20 |
| Relativebrain | 73.45 ± 4.03 | 71.48 ± 6.69 | 74.57 ± 12.15 | 75.21 ± 7.43 |
|
| ||||
| Heart | ||||
| Absolute (mg) | 155.54 ± 21.40 | 147.54 ± 22.41 | 160.35 ± 23.68 | 163.80 ± 23.04 |
| Relativebody | 0.64 ± 0.09 | 0.65 ± 0.08 | 0.66 ± 0.09 | 0.67 ± 0.11 |
| Relativebrain | 31.18 ± 3.74 | 29.74 ± 3.86 | 32.32 ± 4.60 | 33.33 ± 4.85 |
|
| ||||
| Spleen | ||||
| Absolute (mg) | 149.96 ± 15.97 | 119.04 ± 30.28* | 121.15 ± 16.23 | 102.76 ± 20.57* |
| Relativebody | 0.61 ± 0.10 | 0.52 ± 0.10 | 0.50 ± 0.10* | 0.42 ± 0.10* |
| Relativebrain | 30.02 ± 5.33 | 24.16 ± 5.06* | 24.50 ± 6.09 | 20.83 ± 4.90* |
values represent means ± SD (n=6-7); Relativebody, organ weight/body weight × 100; Relativebrain, organ weight/brain weight × 100; paired organs were weighed together.
increased weight, p<0.05 vs Vehicle control.
decreased weight, p<0.05 vs Vehicle control.
Intratumoral biomarkers of apoptosis (cleaved caspase 3) and proliferation (Ki67) were evaluated by immunohistochemistry in tumors collected at the study endpoint. No significant differences among the treatment groups for either marker were identified by routine light microscopy, and this was corroborated by quantitative analysis. Cleaved caspase 3 immunopositivity, defined as the number of strongly positive pixels per total number of pixels, was 9.98% in the vehicle-treated group, 8.13% in the minocycline group, 11.95% in the AC group, and 7.86% in the AC plus minocycline group (Fig. 3B). These differences, although not statistically significant, resembled the trend in cleaved caspase 3 expression observed in our in vitro findings (Fig. 2C), hinting at a partially protective effect of minocycline against AC-driven caspase 3 activation, albeit an effect that was not reflected in diminished tumor control. Ki67 immunopositivity, defined as the number of positive nuclei per total number of nuclei, was 64.20% in the vehicle treated group, 64.85% in the minocycline group, 65.18% in the AC group, and 65.32% in the AC plus minocycline group (Fig. 3C).
3.3 Effect of minocycline on CNS in AC-treated, TNBC tumor-bearing mice
To evaluate the presence of neuroanatomic lesions consistent with the proposed pathophysiology of CICI, the brains of the tumor-bearing mice from the in vivo study described above were examined by H&E and IHC for evidence of microglial activation (Iba1) and astroglial scars (GFAP) as potential indicators of neuroinflammation, and by IHC for doublecortin (DCX) reactivity, a marker of immature neural progenitor cells which would be expected to be sensitive to neurotoxicity caused by AC treatment. Evaluations of neuroinflammation and neurogenic markers are common endpoints in studies of cellular changes associated with chemotherapy-related neurotoxicity and/or cognitive deficits in rodents (reviewed in Seigers and Fardell, 2011) and frequently employ the same cell markers used in our study. For instance, activated microglia have been evaluated in chemotherapy-treated mice using the markers ED1 (Christie et al., 2012; Acharya et al., 2015) and Iba1 (Seigers et al., 2010; Seigers et al., 2016), and GFAP has been used as a marker of astroglial reaction in rodent models of chemotherapy-related neurotoxicity and/or cognitive deficits (Dietrich et al., 2006; Fardell et al., 2014; Acharya et al., 2015). DCX has been used as a marker of neural progenitor cells in the hippocampus, in combination with markers of proliferation or apoptosis, to assess the effects of chemotherapeutic agents on neurogenesis (Dietrich et al., 2006; Yang et al., 2010; Mustafa et al., 2008; Christie et al., 2012; Seigers et al., 2016).
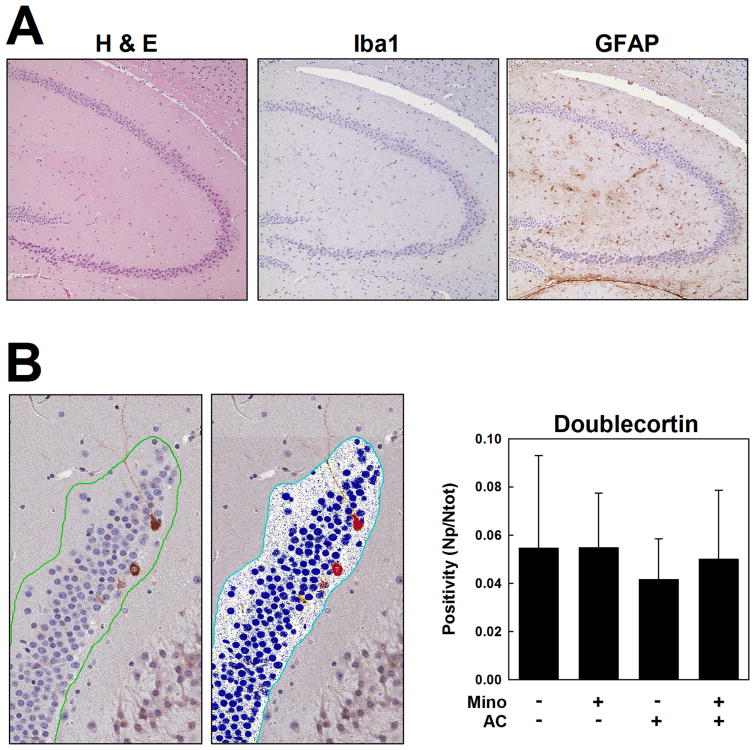
In our study, six standard coronal sections per mouse were evaluated with particular attention to the hippocampus, and no evidence of microglial activation or glial scars was found (Fig. 4A). Differences among treatment groups in DCX immunopositivity were first identified in subgranular zone cells of the dentate gyrus by subjective light microscopic assessment, so digitization and quantification were performed. AC-treated mice showed a 23.8% decrease in DCX positivity compared to vehicle controls, whereas mice receiving minocycline with AC showed only an 8.4% decrease in DCX-positive cells compared to the vehicle-treated group. These changes, however, did not achieve statistical significance. Mice in the vehicle control and minocycline only groups exhibited approximately equivalent levels of DCX positivity (Fig. 4B).
Figure 4.
Neuroanatomic evaluation in MDA-MB-231 xenograft tumor-bearing mice. A. Representative partial sections of hippocampus and dentate gyrus are shown for H&E, Iba1, and GFAP stains from the AC treatment group. B. Doublecortin immunohistochemistry (left) and a corresponding mockup image for digital analysis (middle) of a representative section of dentate gyrus from the vehicle treatment group. Right, quantification of doublecortin staining expressed as positivity (the number of positive pixels per total pixels analyzed) in brains from the four treatment groups (n=5). Positivity is represented in the mock-up image as a heat map wherein strongly positive pixels are red, moderately positive pixels are orange, and weakly positive pixels are yellow; negative pixels are blue, and unquantified pixels in an area of analysis are white. Magnification, 36×.
4. Discussion
Together, our data demonstrate that minocycline may provide protection against AC-induced neurological effects without compromising its chemotherapeutic efficacy. Specifically, minocycline did not protect TNBC cells from the cytotoxic and tumor-suppressive effects of AC chemotherapy, nor did it appear to have any inherent anti-cancer activity in TNBC cells. In MDA-MB-231 and MDA-MB-468 cells treated with doxorubicin or AC, an anti-apoptotic effect of minocycline was consistently apparent by western blot, as evidenced by decreased caspase-3 cleavage, thus illustrating the anti-apoptotic potential of minocycline. This effect on AC-induced caspase-3 activation was also observed by IHC, although not at a statistically significant level, in TNBC xenograft tumors from mice treated with minocycline and a single cycle of AC chemotherapy. However, assays of cell viability and clonogenic survival, as well as the evaluation of other biomarkers of apoptosis (PARP), cell stress (phospho-p38), and DNA damage (γH2AX), indicated that this decreased caspase-3 cleavage was insufficient to rescue TNBC cells from the combined cytotoxicity of AC chemotherapy. Moreover, minocycline did not alter the tumor-suppressive efficacy of AC chemotherapy in TNBC xenograft tumor-bearing mice, and it appeared to be relatively well-tolerated over the course of 24 days of once daily oral administration. Taken together, these findings support the evaluation of minocycline in clinical trials as a neuroprotective agent for the amelioration of CICI in women receiving AC chemotherapy for breast cancer.
We cannot account for the decreases in absolute and relative splenic weights observed in the combined AC plus minocycline treatment group. Histologic lesions such as reduced erythropoiesis or myelopoiesis were not appreciated by light microscopic examination of the spleen or bone marrow, nor did the mice develop clinical symptoms of myelosuppression. It is established that hematopoietic organs, such as the spleen, can exhibit a high degree of variability in weight, and any changes therein should be interpreted in light of histopathologic findings (Sellers et al., 2007). This finding may support the need for increased vigilance in the clinical setting in monitoring the hemogram and leukogram of patients on an AC plus minocycline regimen.
The presence of an increased proportion of DCX-positive cells in the hippocampi of tumor-bearing, AC-treated mice that received minocycline, relative to those that did not, suggests the possibility for increased neural plasticity vis-à-vis neural regeneration from this progenitor cell pool. Progenitor cells in the adult mammalian brain are indeed a susceptible population (Kim et al., 2008; Monje and Dietrich, 2012) that could be inadvertently targeted by cytotoxic agents, leading to deleterious effects on cognition (Dubois et al., 2014) due to cell death and altered dendritic spine connectivity (Zhao et al., 2006). In a recent study, neural stem cell transplantation was proven sufficient to reverse “chemobrain” in a mouse model of CICI (Acharya et al., 2015). Thus, we posit that preservation of neural progenitor cell populations associated with minocycline treatment could have a similarly beneficial effect; further studies are warranted to determine whether the neuroprotection provided by minocycline is functionally significant. In addition, without a time course study, it is not possible to differentiate whether our findings represent a true increase in the number of neural progenitors, or possibly arrest of pre-existing progenitors in a less differentiated state. Furthermore, if minocycline is capable of increasing a stem-like or progenitor cell pool in the brain, it would be worthy of investigation to determine whether the same phenomenon occurs in tumors, where this effect may be undesirable.
Our study found no evidence of microglial activation or astroglial scarring in the brains of tumor-bearing mice 21 days after a single dose of AC, as evaluated by Iba1 and GFAP immunostaining, respectively. It is possible that neuroanatomic changes in microglia and astroglia may not be microscopically detectable following only one cycle of AC therapy (i.e. insufficient cumulative total dose), or that the cognitive ramifications of AC are manifest in functional aberrations that may be better characterized by cytokine profiling, behavioral endpoints, or functional in vivo assays like functional (f)MRI in conjunction with histopathology and immunohistochemistry. Recent evidence in rodents supports the contention that chemotherapy-induced cognitive deficits can be present without appreciable, or with only modest, changes to neurobiological parameters. In tumor-free mice, the one-time administration of cytotoxic chemotherapeutic agents at doses that caused deficits in various cognitive measures (Seigers et al., 2015) had no effect, in the same model, on neurogenesis and microglial activation in the hippocampus at 3 or 16 weeks after treatment, and had modest effects on microgial activation in the prefrontal cortex at 3, but not 16, weeks post-treatment (Seigers et al., 2016). This study echoes our own in the doses used, in the timing of agent administration and endpoint evaluation, and in the results generated. Thus, it is tempting to speculate that the mice in our study would have similarly exhibited cognitive deficits.
CICI is a complex cognitive phenomenon that poses significant challenges for recapitulation in an animal model. For example, the cognitive decline that some breast cancer patients begin to experience even before beginning chemotherapy has been attributed, at least in part, to the psycho-emotional implications of a cancer diagnosis, including depression, fatigue, and anxiety (Kaiser and Dietrich, 2014; Churchill et al., 2015). Importantly, structural changes in the brains of breast cancer patients have been detected by MRI prior to chemotherapy, along with decreased cognitive performance and altered brain function (Menning et al., 2015). While it would be fascinating to see whether histopathologic lesions are present in the brains of human breast cancer patients before beginning chemotherapy, the availability of tissue from such a patient population through biopsy or accidental death is improbable. Rodent models may provide insight in this regard as ample evidence suggests that peripheral tumor-associated inflammatory factors, independent of chemotherapy, can alter neurobiological pathways and affect behavior and cognition (reviewed in Schrepf et al., 2015). While this appears to be inconsistent with our finding that vehicle-treated tumor-bearing mice showed no neuroanatomic abnormalities by light microscopy, this discrepancy may reflect differences in tumor models. The studies reviewed by Schrepf et al. (2015) used syngeneic and spontaneous tumor models involving immunocompetent rodents, which might be expected to elicit an inflammatory microenvironment that is markedly different from that present in an immunocompromised mouse system like the one used in our study.
As our ability to investigate brain biology deepens through technologies like PET scanning, diffusion tensor imaging, fMRI, and improved neuropsychological assessments (Reuter-Lorenz and Cimprich, 2013; Bernstein et al., 2014), much-needed longitudinal and prospective studies thoroughly evaluating cognitive function pre- and post-chemotherapy and risk factors in breast cancer patients are beginning to surface (Shilling et al., 2006; Schagen et al., 2006; Ahles and Saykin, 2007; Pomykala et al., 2013; Andreotti et al., 2016). We anticipate that patient selection would be of utmost importance in choosing when to prescribe minocycline as a neuroprotectant in patients receiving AC chemotherapy for breast cancer. The concept of antimicrobial stewardship must not be ignored. Patient selection criteria might include patients who are more susceptible to neurotoxicity by xenobiotics due to efflux transporter mutations, or possibly patients who begin to show pre-treatment abnormalities in brain imaging following their cancer diagnosis. We anticipate that treatment of CICI symptoms in cancer patients would also involve a multidisciplinary approach, as non-pharmacologic interventions such as cognitive and behavioral rehabilitation therapy are also of value (Sleight, 2016).
Survivor care is a critical aspect of holistic cancer treatment and will be increasingly important moving forward, as breast cancer patients are surviving longer than ever before (Fallowfield and Jenkins, 2015). Because treatment for TNBC, an aggressive form of breast cancer, is still largely dependent on cytotoxic drugs, like the AC regimen, management of untoward side-effects, such as CICI, is paramount. We propose that minocycline should be considered as a potential neuroprotective agent to be administered to breast cancer patients receiving AC chemotherapy and that our data support moving such a therapeutic approach towards clinical trials.
Acknowledgments
This work was supported by funding from the National Institutes of Health (5T32 OD010429-12 to L.E.H. and CA112250 to C.S.C.), the Comparative Pathology and Mouse Phenotyping Shared Resource at The Ohio State University (supported in part by National Cancer Institute grant P30 CA016058), the Center for Clinical and Translational Science at The Ohio State University (Clinical and Translational Science Award UL1TR000090), and the Stefanie Spielman Fund for Breast Cancer Research (to C.S.C.). The authors are grateful to Dr. Brad Bolon (GEMpath, Inc., Longmont CO) for valuable consultation on neuropathological evaluation of mice.
Abbreviations
- AC
Adriamycin-Cytoxan
- CICI
chemotherapy-induced cognitive impairment
- DCX
doublecortin
- IHC
immunohistochemistry
- MTT
methylthiazol tetrazolium
- TNBC
triple-negative breast cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowicz M, editor. Extended-release minocycline (Solodyn) for acne. Med Letter Drugs Ther. 2006;48(1248):95–96. [PubMed] [Google Scholar]
- Acharya MM, Martirosian V, Chmielewski NN, Hanna N, Tran KK, Liao AC, et al. Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction. Cancer Res. 2015;75(4):676–686. doi: 10.1158/0008-5472.CAN-14-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother. 2006;58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, Root JC, Schagen SB, McDonald BC, Saykin AJ, Atkinson TM, et al. Reliable change in neuropsychological assessment of breast cancer survivors. Psycho-Oncology. 2016;25(1):43–50. doi: 10.1002/pon.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo MVF, Ifa DR, Ribeiro W, Moraes ME, Moraes MO, de Nucci G. Determination of minocycline in human plasma by high-performance liquid chromatography coupled to tandem mass spectrometry: application to bioequivalence study. J Chromatogr B. 2001;755:1–7. doi: 10.1016/s0378-4347(00)00472-2. [DOI] [PubMed] [Google Scholar]
- Bernstein LJ, Catton PA, Tannock IF. Intra-individual variability in women with breast cancer. J Int Neuropsychol Soc. 2014;20:380–390. doi: 10.1017/S1355617714000125. [DOI] [PubMed] [Google Scholar]
- Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Cancer. 2008;123(6):1227–1239. doi: 10.1002/ijc.23754. [DOI] [PubMed] [Google Scholar]
- Bolon B, Garman RH, Pardo ID, Jensen K, Sills RC, Roulois A, et al. STP position paper: Recommended practices for sampling and processing the nervous system (brain, spinal cord, nerve, and eye) during nonclinical general toxicity studies. Toxicol Pathol. 2013;41(7):1028–1048. doi: 10.1177/0192623312474865. [DOI] [PubMed] [Google Scholar]
- Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224–1236. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- Cheng H, Yang Z, Dong B, Chen C, Zhang M, Huang Z, et al. Chemotherapy-induced prospective memory impairment in patients with breast cancer. Psychooncology. 2013;22(10):2391–2395. doi: 10.1002/pon.3291. [DOI] [PubMed] [Google Scholar]
- Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18(7):1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- Churchill NW, Cimprich B, Askren MK, Reuter-Lorenz PA, Jung MS, Peltier S, et al. Scale-free brain dynamics under physical and psychological distress: pre-treatment effects in women diagnosed with breast cancer. Hum Brain Mapp. 2015;36:1077–1092. doi: 10.1002/hbm.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, Riley JH, et al. Best Practices Guideline: Toxicologic Histopathology. Toxicol Pathol. 2004;32(1):126–131. doi: 10.1080/01926230490268756. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Pröschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci. 2004;25(12):609–612. doi: 10.1016/j.tips.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. PNAS. 2001;98(25):14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Lapinte N, Villier V, Lecointre C, Roy V, Tonon M-C, et al. Chemotherapy-induced long-term alteration of executive functions and hippocampal cell proliferation: role of glucose as adjuvant. Neuropharmacology. 2014;79:234–248. doi: 10.1016/j.neuropharm.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Engel D, Peshock R, Armstrong RC, Sivasubramanian N, Mann DL. Cardiac myocyte apoptosis provokes adverse cardiac remodeling in transgenic mice with targeted TNF overexpression. Am J Physiol Heart Circ Physiol. 2004;287:H1303–H1311. doi: 10.1152/ajpheart.00053.2004. [DOI] [PubMed] [Google Scholar]
- Fallowfield L, Jenkins V. Psychosocial/survivorship issues in breast cancer: are we doing better? J Natl Cancer Inst. 2015;107(1):dju335. doi: 10.1093/jnci/dju335. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Zhang J, De Souza R, Vardy J, Johnston I, Allen C, et al. The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology (Berl) 2014;231(5):841–852. doi: 10.1007/s00213-013-3301-8. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS. Current concepts: triple-negative breast cancer. N Eng J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Franci C, Zhou J, Jiang Z, Modrusan Z, Good Z, Jackson E, et al. Biomarkers of residual disease, disseminated tumor cells, and metastases in the MMTV-PyMT breast cancer model. PLoS One. 2013;8(3):e58183. doi: 10.1371/journal.pone.0058183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157(1):95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gover MD, Abramovits W, Perlmutter A. Solodyn (Minocycline HCl, USP) extended release tablets. SKINmed. 2006;5:291–292. doi: 10.1111/j.1540-9740.2006.05991.x. [DOI] [PubMed] [Google Scholar]
- Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13(4):215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN. Chemotherapy-induced cognitive deficits, white matter pathologies and cytokines. Brain Behav Immun. 2014;35:21–22. doi: 10.1016/j.bbi.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Dietrich J. Challenges in research on the neural basis of chemobrain. Transl Neurosci. 2014;5(3):222–225. [Google Scholar]
- Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196(2):168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Lee H-J, Kim JC, Kang SS, Bae C-S, Shin T, et al. Transient Impairment of Hippocampus-dependent Learning and Memory in Relatively Low-Dose of Acute Radiation Syndrome is Associated with Inhibition of Hippocampal Neurogenesis. J Radiat Res. 2008;49(5):517–526. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- Konat GW, Kraszpulski M, James I, Zhang HT, Abraham J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab Brain Dis. 2008;23(3):325–333. doi: 10.1007/s11011-008-9100-y. [DOI] [PubMed] [Google Scholar]
- Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100(11):733–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- Lee C-K, Shin J-I, Cho Y-S. Protective effect of minocycline against cisplatin-induced ototoxicity. Clin Exp Otorhinolaryngol. 2011;4(2):77–82. doi: 10.3342/ceo.2011.4.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menning S, de Ruiter MB, Veltman DJ, Koppelmans V, Kirschbaum C, Boogerd W, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment-the role of fatigue. Neuroimage Clin. 2015;7:547–554. doi: 10.1016/j.nicl.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227(2):376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S, Walker A, Bennett G, Wigmore PM. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28(2):323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- O'Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, et al. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013;66(6):530–542. doi: 10.1136/jclinpath-2012-201361. [DOI] [PubMed] [Google Scholar]
- Parangi S, O'Reilly M, Christofori G, Holmgren L, Grosfeld J, Golkman J, et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. PNAS. 1996;93:2002–2007. doi: 10.1073/pnas.93.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol. 2010;67(12):1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, Mallam S, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant therapy for breast cancer. Brain Imaging Behav. 2013;7:511–523. doi: 10.1007/s11682-013-9243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51(2):215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- Pourgholami MH, Mekkawy AH, Badar S, Morris DL. Minocycline inhibits growth of epithelial ovarian cancer. Gynecol Oncol. 2012;125(2):433–440. doi: 10.1016/j.ygyno.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Raffa RB. A proposed mechanism for chemotherapy-related cognitive impairment (‘chemo-fog’) J Clin Pharm Ther. 2011;36(3):257–259. doi: 10.1111/j.1365-2710.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137(1):33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98(23):1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- Schrepf A, Lutgendorf SK, Pyter LM. Pre-treatment effects of peripheral tumors on brain and behavior: neuroinflammatory mechanisms in humans and rodents. Brain Behav Immun. 2015;49:1–17. doi: 10.1016/j.bbi.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35(3):729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Seigers R, Loos M, Van Tellingen O, Boogerd W, Smit AB, Schagen SB. Cognitive impact of cytotoxic agents in mice. Psychopharmacology (Berl) 2015;232(1):17–37. doi: 10.1007/s00213-014-3636-9. [DOI] [PubMed] [Google Scholar]
- Seigers R, Loos M, Van Tellingen O, Boogerd W, Smit AB, Schagen SB. Neurobiological changes by cytotoxic agents in mice. Behav Brain Res. 2016;299:19–26. doi: 10.1016/j.bbr.2015.10.057. [DOI] [PubMed] [Google Scholar]
- Seigers R, Timmermans J, van der Horn HJ, de Vries EFJ, Dierckx RA, Visser L, et al. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res. 2010;207(2):265–272. doi: 10.1016/j.bbr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, et al. Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol. 2007;35(5):751–755. doi: 10.1080/01926230701595300. [DOI] [PubMed] [Google Scholar]
- Setiawati E, Purnomo A, Deniati SH, Yunaidi DA, Handayani LR, Harinato G, et al. Bioequivalence study of two minocycline capsule formulations in healthy volunteers. Arzneimittelforschung. 2009;59(10):532–536. doi: 10.1055/s-0031-1296438. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog--methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Res Treat. 2006;95(2):125–129. doi: 10.1007/s10549-005-9055-1. [DOI] [PubMed] [Google Scholar]
- Simone CB, Simone NL, Simone V, Simone CB. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 2. Altern Ther Health Med. 2007;13(2):40–47. [PubMed] [Google Scholar]
- Sleight A. Coping with cancer-related cognitive dysfunction: a scoping review of the literature. Disabil Rehabil. 2016;38(4):400–408. doi: 10.3109/09638288.2015.1038364. [DOI] [PubMed] [Google Scholar]
- Stover DG, Winer EP. Tailoring adjuvant chemotherapy regimens for patients with triple negative breast cancer. Breast. 2015;24(Suppl 2):S132–S135. doi: 10.1016/j.breast.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Switzer JA, Hess DC, Ergul A, Waller JL, Machado LS, Portik-Dobos V, et al. Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke. 2011;42(9):2633–2635. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam FSAM, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose therapy. J Natl Cancer Inst. 1998;90(3):210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- Villanueva MT. Breast cancer: chemo fog becomes clearer. Nat Rev Clin Oncol. 2012;9(11):609. doi: 10.1038/nrclinonc.2012.164. [DOI] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279(19):19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- Winocur G, Henkelman M, Wojtowicz JM, Zhang H, Binns MA, Tannock IF. The effects of chemotherapy on cognitive function in a mouse model: a prospective study. Clin Cancer Res. 2012;18(11):3112–3121. doi: 10.1158/1078-0432.CCR-12-0060. [DOI] [PubMed] [Google Scholar]
- Won SJ, Kim JH, Yoo BY, Sohn M, Kauppinen TM, Park M-S, et al. Prevention of hypoglycemia-induced neuronal death by minocycline. J Neuroinflammation. 2012;9:225. doi: 10.1186/1742-2094-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93(4):487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Yasueda A, Urushima H, Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr Cancer Ther. 2016;15(1):17–39. doi: 10.1177/1534735415610427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. PNAS. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming G-L, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]