Abstract
The incidence of postoperative nausea and vomiting (PONV) can be as high as 80% in patients with risk factors (e.g., females, history of motion sickness). PONV delays postoperative recovery and costs several hundred million dollars annually. Cell-based assays show that halogenated ethers (e.g., isoflurane) activate 5-HT3 receptors, which are found on gastrointestinal vagal afferents and in the hindbrain - key pathways for producing nausea and vomiting. This project evaluated the role of the vagus and activation of the hindbrain in isoflurane-induced emesis in musk shrews – a small animal model with a vomiting reflex, which is lacking in rats and mice. Sham-operated and abdominal vagotomized shrews were exposed to 1 to 3% isoflurane to determine effects on emesis; vagotomy was confirmed by lack of vagal transport of the neuronal tracer Fluoro-Gold. In an additional study, shrews were exposed to isoflurane and hindbrain c-Fos was measured at 90 min after exposure using immunohistochemistry. There were no statistically significant effects of vagotomy on isoflurane-induced emesis compared to sham-operated controls. Isoflurane exposure produced a significant increase in c-Fos-positive cells in the nucleus of the solitary tract and vestibular nuclei but not in the area postrema or dorsal motor nucleus. These results indicate that the abdominal vagus plays no role in isoflurane-induced emesis and suggest that isoflurane activates emesis by action on the hindbrain, as shown by c-Fos labeling. Ultimately, knowledge of the mechanisms of inhalational anesthesia-induced PONV could lead to more targeted therapies to control PONV.
Keywords: isoflurane, NTS, anesthesiology, post-operative nausea and vomiting, perioperative care, post-anesthesia care unit
1. Introduction
Postoperative nausea and vomiting (PONV) is a significant healthcare concern. The incidence of PONV, defined as nausea and vomiting experienced in the post-anesthesia care unit (PACU), can be as high as 80% in patients with risk factors, such as female sex, younger age, non-smoking status, and history of motion sickness (Apfel et al., 2012). Patients report PONV as one of the most distressing symptoms after surgery (Gan et al., 2003). The lack of effective ways to treat PONV delays patient discharge from the PACU and is estimated to cost the healthcare system several hundred million dollars annually (Gan et al., 2003; Habib et al., 2006). While current therapies have existed for decades, systematic reviews predict only 28% of those affected by PONV find benefit from these approaches (Carlisle and Stevenson 2006).
PONV is primarily triggered by inhaled anesthetics and opioid analgesics (Apfel et al., 2012; Horn et al., 2014b). Inhaled anesthetics are known to cause PONV in the first two hours post-operatively (Apfel et al., 2002), coinciding with maximal symptomatology; however, little is known about the mechanism of inhalational anesthesia-induced PONV. Studies in cell-based assays indicate that halothane and isoflurane, inhaled anesthetics, enhance serotonin-sensitive 5-HT3 receptor function (Machu and Harrison 1994; Parker et al., 1996) and halothane-induced emesis in musk shrews is substantially reduced by pre-treatment with the 5-HT3 receptor antagonist ondansetron (Gardner and Perren 1998). Although it must be verified with each specific halogenated ether, these observations suggest that these agents produce emesis via a 5-HT3 mechanism. 5-HT3 receptors are known to play an integral role in emesis and are found peripherally on abdominal vagal afferents and centrally in the area postrema (AP) and nucleus of the solitary tract (NTS) (Barnes et al., 1990; Kilpatrick et al., 1990; Marazziti et al., 2001; Reynolds et al., 1995).;
The current studies were designed to test the effects of an ablation of the abdominal vagus on inhalational anesthesia-induced emesis and evaluate the activation of the hindbrain by isoflurane exposure. We used musk shrews (Suncus murinus) in these experiments because they have a vomiting reflex, which is lacking in mice and rats (Horn et al., 2013a); this species was also used in prior studies of inhalational anesthesia-induced emesis (Gardner and Perren 1998; Horn et al., 2012). Here we used isoflurane as the emetic stimulus because this agent produces emesis in musk shrews (Horn et al., 2012) and in humans, similar in intensity to other halogenated ethers (e.g., sevoflurane, Apfel et al., 2002). c-Fos protein, a measure of neuronal activation (Sharp et al., 1993), was used in the current project to assess activation of the hindbrain areas believed to play a role in producing emesis, including the NTS and AP (Miller and Leslie 1994a; Yamada et al., 2000).
2. Materials and methods
2.1. Animals
In total, 48 experimentally naïve female musk shrews were used (>35 days of age, 20–50 g body weight; n = 22, Study 1, n = 24, Study 2, and n = 2, c-Fos anti-body testing); shrews were offspring from breeding stock obtained from the Chinese University of Hong Kong (a strain originating from Taiwan; Wang, 1994). Female animals were used because female sex is reported as a risk factor for PONV in clinical studies (e.g., Apfel et al., 2012). Animals were housed individually and fed a mixture of 75% Purina Cat Chow Complete Formula and 25% Complete Gro-Fur mink food pellets (Milk Specialty, New Holstein, WI; Temple, 2004). Musk shrews were maintained on a 12-h light/12-h dark cycle (lights on at 0700 h), with free access to food and water. Experiments were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee, and animals were housed in an animal care facility accredited by the Association of Assessment and Accreditation of Laboratory Animal Care International.
2.2. Study 1: Effect of abdominal vagotomy on isoflurane-induced emesis
2.2.1. Abdominal vagotomy surgery
Similar to a prior study (Horn et al., 2014a), shrews received a sham-operation or abdominal vagotomy by random assignment (n = 11/group). Animals were anesthetized with isoflurane (2 to 3%) using an induction chamber followed by a nose cone during surgery. The ventral abdominal surface was shaved and the skin sterilized with betadine surgical scrub and 70% isopropyl alcohol. After a midline 1.5-cm laparotomy incision, the vagal trunks running along the esophagus were bluntly dissected and transected using a thermal cautery. The peritoneum was sutured (2–0 silk; Ethicon), and the skin was closed with surgical staples (7.5 × 1.75 mm, Michel). Sham operations were performed using a similar approach, except that the vagi were not manipulated or lesioned. Animals were weighed daily after surgery to assess body weight changes and were allowed to recover for 1 wk before behavioral testing. For 2 days after surgery, animals received the analgesic ketoprofen (Sigma-Aldrich; 2 mg/kg sc, twice daily).
2.2.2. Behavioral testing
Four emetic tests (0, 1, 2, 3% isoflurane; Henry Schein) were conducted with 1 wk between each test. Order of exposure was determined using a 4 × 4 Latin-square design (orders were 0>1>2>3%, 1>0>3>2%, 2>3>0>1%, and 3>2>1>0%). Isoflurane was provided from a calibrated isoflurane vaporizer (General Anesthetic Services Inc., South Park, PA, USA) with input of compressed 100% O2 flowing at 6 L/min. 0% isoflurane tests consisted of 100% O2. Gas was provided into a cylindrical induction chamber (10 × 8.5 cm, height and diameter of cylinder) and allowed to fill for 2 min prior to placing an animal inside (Horn et al., 2012). After 10 min of exposure in the induction chamber, shrews were transferred to the testing chamber (19 × 27 cm, width and length) for 30 min. Animal behavior was recorded using digital video cameras (Sony DCR-SR300 or HDR-XR550V, wide field lenses) positioned above the induction and testing chambers. While in the chambers, animals were observed and recorded for emetic episodes, abdominal contractions, body swaying, and sedation using keystrokes on a laptop computer running JWatcher software (http://www.jwatcher.ucla.edu; Blumstein and Daniel 2007; Horn et al., 2013b). Emetic episodes in musk shrews occur as a series of closely spaced retches, which end with an expulsion phase (a retch = an abdominal contraction with a rostral movement of the body while standing in place; an emetic episode = ~6 retches plus a larger expulsion movement with ~0.2 s between each event; Huang et al., 2011). Emetic episodes with and without vomiting (expulsion) were recorded. Sedation was recorded when the animal was not standing or locomoting. Swaying was recorded as a series of lateral movements of the center of the body when standing in place and abdominal contractions were isolated contractions that did not occur as part of an emetic episode (Horn et al., 2013b). Body weight, food intake and water consumption were measured daily using a digital platform balance (Ohaus).
2.2.3. Confirmation of abdominal vagotomy
Fluoro-Gold (Fluorochrome LLC, Denver, CO, USA), a retrograde neuronal tracer, was used to confirm the completeness of subdiaphragmatic vagotomy (Horn et al., 2014a; Powley et al., 1987). Animals were injected intraperitoneally with 0.2 ml of the tracer (1% solution in 0.15 NaCl). Five days after Fluoro-Gold injection, animals were euthanized via an overdose of Beuthanasia-D solution (0.03 ml, ip, containing 390 mg/ml pentobarbital sodium; Intervet/Merck). Following cessation of respiration and loss of the toe pinch reflex, animals were transcardially perfused with 0.15 M NaCl rinse (10 ml) and then 4% paraformaldehyde (in 0.1M phosphate-buffered saline, PBS, 20 ml; Sigma-Aldrich). Brains were removed, fixed overnight in 4% paraformaldehyde, and cryoprotected in 20% sucrose. Tissue was then frozen on dry ice and sectioned at 30 µm directly onto microscope slides (SuperFrost Plus; Fisher Scientific) using a cryostat (−20°C, Microm HM500). Slides were dried overnight, rinsed with Milli-Q water, 70% ethanol, 95% ethanol, 100% ethanol, and Histo-Clear (National Diagnostics, Atlanta, GA, USA), and coverslipped with DPX mountant (VWR International, Radnor, PA, USA).
2.3. Study 2: Effect of isoflurane on c-Fos labeling in the hindbrain
2.3.1. Behavioral testing and tissue collection
Shrews were randomly assigned to three groups, 0% (n = 8), 1% (n = 7), and 3% (n = 7) isoflurane; an additional two shrews were used in a hypertonic saline experiment to determine the appropriate concentration of the c-Fos anti-body. Animals were adapted to handling and the test chambers to reduce the effects of stress on c-Fos measurement (Sharp et al., 1991). For two consecutive days, animals were placed in the induction chamber for 10 min followed by exposure to the testing chamber for 30 min (no isoflurane exposure). On the test day, using the procedures from Study 1, shrews were exposed to gas mixtures for 10 min in the induction chamber followed by placement in the testing chamber, with all behavior video recorded from cameras placed above the chambers (emetic behaivors were not directly observed and documented during exposure). In this study, animals stayed in the testing chamber for 90 min before receiving an overdose of Beuthanasia-D (0.03 ml, ip) for euthanization; tissue was fixed and extracted as indicated in section 2.2.3. The 90 min delay was based on the peak times for c-Fos expression after exposure to a stimulus (Sharp et al., 1991).
2.3.2. Tissue preparation and c-Fos immunohistochemistry
Following cryopreservation in 25% sucrose/PBS, brains were frozen on dry ice and stored at −80°C until sectioning. Tissue was sectioned at 30 µm (−20°C, cryostat) and placed directly into Watson’s cryoprotectant (Watson et al., 1986) and stored at −20°C until processing. Similar to prior studies (De Jonghe and Horn 2009; Horn, 2009; Horn et al., 2007), tissue sections were processed free-floating for immunohistochemistry. At the beginning, sections were removed from cryoprotectant and rinsed in 0.1 M PB (phosphate buffer, 1 h), 0.5% sodium borohydride (in 0.1 M PB; 30 min), 0.1 M PB (20 min), 0.3% and H2O2 (in 0.1 M PB for 15 min). Tissue was then incubated for 21 h in 1:20K anti-c-Fos (sc-52, Lot#I2514, Santa Cruz Biotechnology, Dallas, TX, USA; in 1% normal donkey serum, NDS, 0.3% triton X-100, and 0.1 M PB). After removal from primary anti-body, sections were rinsed with 0.1 M PB (1 h) and incubated for 1 h in secondary anti-body (biotinylated donkey anti-rabbit IgG, Jackson ImmunoResearch, West Grove, PA, USA; diluted 1:500 in 0.1M PB containing 1% NDS and 0.3% triton X-100). This was followed by rinsing in 0.1 M PB (1 h) and incubation for 1.5 h in avidin-biotin complex (Elite kit, Vector Laboratories, Burlingame, CA, USA; 22.5 µl avidin + 22.5 µl biotin per 5 ml of 0.1 M PB containing 0.3% triton X-100). Tissue was rinsed in 0.1 M PB (30 min), 0.1M acetate-imidazole buffer (A/I; 20 min), and exposed to nickel-DAB solution for 10 min (5 mg/ml, 3,3’-diaminobenzidine, DAB, in A/I buffer, 0.25 g/ml nickel (II) sulfate, and 50 µl/ml 1% H2O2). Finally, sections were rinsed in A/I Buffer (20 min), 0.1 M PB (20 min), mounted to microscope slides (SuperFrost Plus), air dried overnight, rinsed in Milli-Q water, 70% ethanol, 95% ethanol, 100% ethanol, and Histo-clear, and coverslipped with DPX mountant. To determine the concentration of c-Fos anti-body (i.e., 1:20K) that would produce appropriate levels of nuclear staining, with minimal background labeling, an additional two animals were injected with hypertonic saline (9% NaCl at 2% body weight, ip) and euthanized at 90 min after injection Beuthanasia-D (0.03 ml, ip). The specificity of the c-Fos polyclonal anti-body was confirmed by pre-absorption of the primary anti-body solution with c-Fos protein (1:5K, sc-52P, Lot#F1914, Santa Cruz Biotechnology), which completely blocked all c-Fos labeling.
2.4. Data Analysis
2.4.1 Behavioral Data
Behavioral data files containing emesis and other behavioral timestamps were processed using custom scripts written in R (version 3.2.2; https://www.r-project.org). To create these data files for Study 2, an experimenter (blind to the experimental condition) watched the videos and scored the data similarly to Study 1 using BORIS (Behavioral Observation Research Interactive Software; http://penelope.unito.it/boris). Custom R scripts extracted variables from JWatcher and BORIS files including total emetic episodes, duration of emesis (time between first and last episode; min), rate of emesis (rate/min), standard deviation of the emetic episode interval (min), total abdominal contractions, total swaying movements, and latency to first emetic episode (min). Study 2 did not divide emetic episodes into those with and without vomiting because this could not be determined from the view angle of the camera. Behavioral variables were analyzed using analysis of variance (ANOVA), and when statistically significant results were detected, Tukey’s tests were performed to compare group means (using the R “ez” package for ANOVA, https://cran.r-project.org/web/packages/ez, and R base Tukey test function). Latency data were analyzed using the R “survival” package for log-rank tests (https://cran.r-project.org/web/packages/survival). Pearson product-moment correlations (in R) were performed on the duration of anesthesia (sedation) and the numbers of emetic episodes, abdominal contractions, and swaying movements. p < 0.05 was used to determine statistical significance and group values in the body of the text are reported as the mean ± SEM.
2.4.2 Fluoro-Gold staining
The presence of Fluoro-Gold in the DMN was determined by fluorescent detection (Horn et al., 2014a). Images were collected using a Nikon epifluorescence microscope equipped with a wide-band UV filter and analyzed with ImageJ software (NIH; http://rsb.info.nih.gov/ij/) to assess the density of staining (gray level) in the left and right DMN relative to the adjacent NTS (higher ratio values, DMN/NTS, indicate more fluorescent staining in the DMN compared with the NTS). Data values were collected by an experimenter naïve to the surgical condition of the samples. A two-sample, two-tailed, t-test was used to compare groups using R (p < 0.05 was used to determine statistical significance and group values in the body of the text are reported as the mean ± SEM).
2.4.3 Quantification of c-Fos
c-Fos labeling was determined using a semi-automatic method in ImageJ software by two investigators blinded to the experimental condition of the tissue sections. Images were collected using a Nikon Eclipse E800 microscope and converted to 8-bit grayscale. A random selection of 10% of images was used to create templates for cell sizes for each brain region, using a manually selected threshold for cell detection, and exporting the minimum and maximum cell sizes and the minimum cell circularity measure. All images were processed in ImageJ by running automatic Otsu thresholding (http://imagej.nih.gov/ij/plugins/otsu-thresholding), watershedding (to split overlapping cells), outlining the region of interest, and using the above cell parameters, determined from the templates, to automatically quantify c-Fos cell numbers. c-Fos cell counts were computed for the AP, NTS, DMN, and vestibular nuclei (VeN). Cell counts were analyzed by ANOVA and planned comparisons using Least significance difference tests (LSD-tests, in R). p < 0.05 was used to determine statistical significance and group values in the body of the text are reported as the mean ± SEM.
3. Results
3.1. Study 1: Effect of abdominal vagotomy on isoflurane-induced emesis
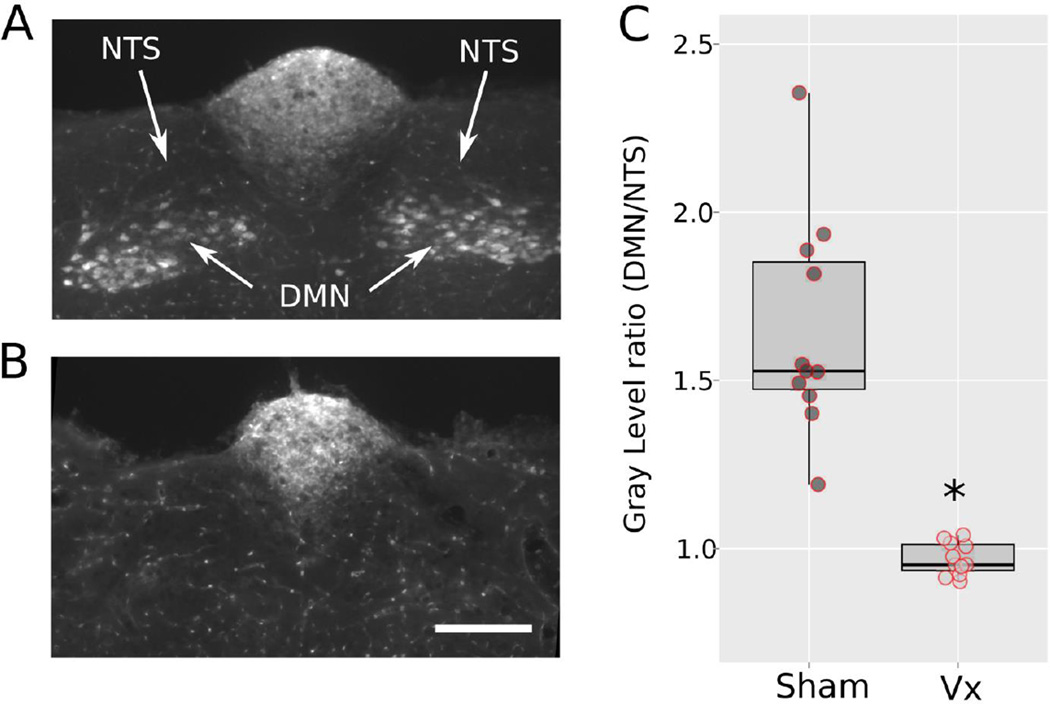
Subdiaphragmatic vagotomy eliminated fluorescent labeling of motor neurons in the DMN compared to the NTS (Fig. 1A and B show representative labeling from sham and vagotomized animals). The ratio of gray level in the DMN relative to the adjacent NTS was significantly higher in sham-operated compared to vagotomized shrews [1.6 ± 0.3 versus 1.0 ± 0.1; t(10) = 6.9, p < 0.00004 Fig. 1C]. There was no overlap in the distribution of these individual ratio labeling values between the two groups (Fig. 1C).
Fig. 1.
Study 1: Quantitative verification of vagotomy using retrograde labeling of the dorsal motor nucleus (DMN) produced by intraperitoneal Fluoro-Gold injection. (A) Representative labeling in a sham-operated animal. NTS, nucleus of the solitary tract. The fluorescently labeled area in the top middle is the area postrema, which has a reduced blood-brain barrier and indicated a successful intraperitoneal injection of Fluoro-Gold. (B) Representative labeling in an abdominal vagotomized animal. The scale bar = 200 µm. (C) Box and whisker plot of the ratio of labeling in the DMN compared to the NTS (bar = median value, upper and lower values of the box = 1st and 3rd quartiles, whiskers = 1.5 × the inter-quartile range). A scatter plot of raw values is also shown. * = t-test, p < 0.00005.
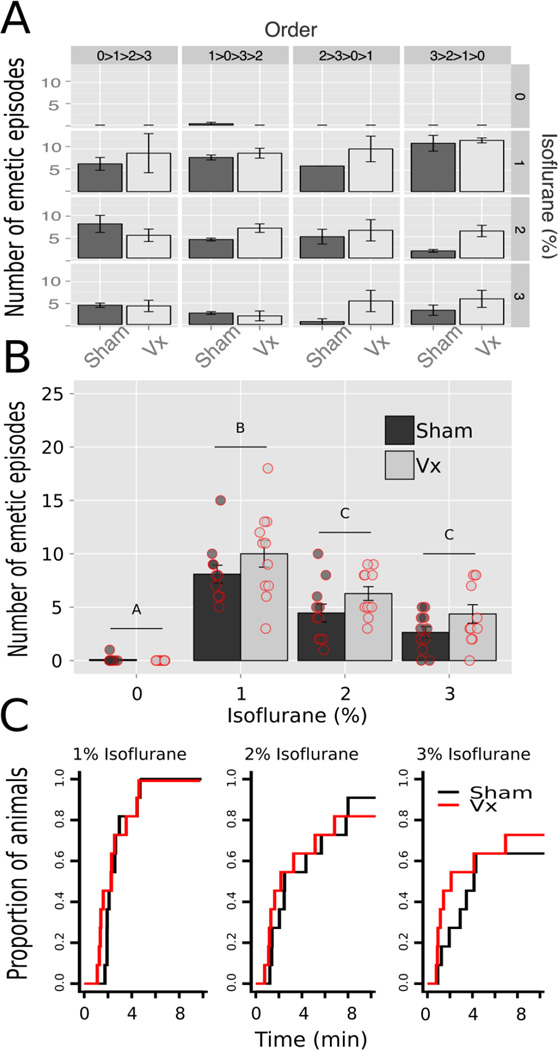
Behavioral data were analyzed with three-way ANOVA using the variables of Condition (sham and. vagotomy), Exposure (0, 1, 2, and 3% isoflurane), and Order (0>1>2>3, 1>0>3>2, 2>3>0>1, and 3>2>1>0 %; Latin-square order of testing). There were no statistically significant effects of surgical condition on total number of emetic episodes (interaction or main effects; ANOVA, ps > 0.5); however, there was a significant interaction effect of Exposure × Order [F(9,42) = 2.4, p = 0.03] but mean comparisons did not yield statistically significant effects across each isoflurane Exposure (Tukey’s tests, ps > 0.05; Fig. 2A). The Condition × Exposure means are plotted in Fig. 2B, which shows the decrement in total emetic responses comparing 1% with 2 and 3% isoflurane (Tukey’s tests, ps < 0.05; Fig. 2B). Latencies to the first emetic responses were also not statistically different between surgical conditions but 3% isoflurane compared to 1% isoflurane produced a lower proportion of responders (log-rank test, p < 0.03; Fig. 2C). There were no statistically significant effects of surgical Condition on other behavioral measures, including only emetic episodes with vomiting, only episodes without vomiting, duration of emesis, swaying movements, and abdominal contractions (ANOVAs, ps > 0.05).
Fig. 2.
Study 1: Isoflurane-induced emesis in sham operated and abdominal vagotomized (Vx) musk shrews. (A) Total number of emetic episodes in each Latin-square order of stimulus presentation; a repeated measures design (see Methods section). One concentration of isoflurane was presented each week. Values represent the mean ± SEM. (B) Total number of emetic episodes after removing the effects of Latin-square order. There was a statistically significant main effect of isoflurane (ANOVA; see Results section). Isoflurane conditions with a different letter above the bar are statistically different (Tukey’s test, p < 0.05). A scatter plot of raw data is also shown. (C) Cumulative proportion of animals showing the latency to the first emetic episode.
Duration and recovery from anesthesia was associated with emesis. In sham animals, a longer duration of anesthesia was correlated with fewer total emetic episodes (Pearson, r = −0.62, p < 0.0005) and more abdominal contractions (Pearson, r = 0.40, p < 0.05). Similarly, in vagotomized shrews, the duration of anesthesia was also negatively correlated with the total number of emetic episodes (Pearson, r = −0.48, p < 0.01). There were no statistically significant differences between surgical conditions on daily body weight, food intake, or water consumption (ANOVAs, ps > 0.05).
3.2. Study 2: Effect of isoflurane on c-Fos expression in the hindbrain
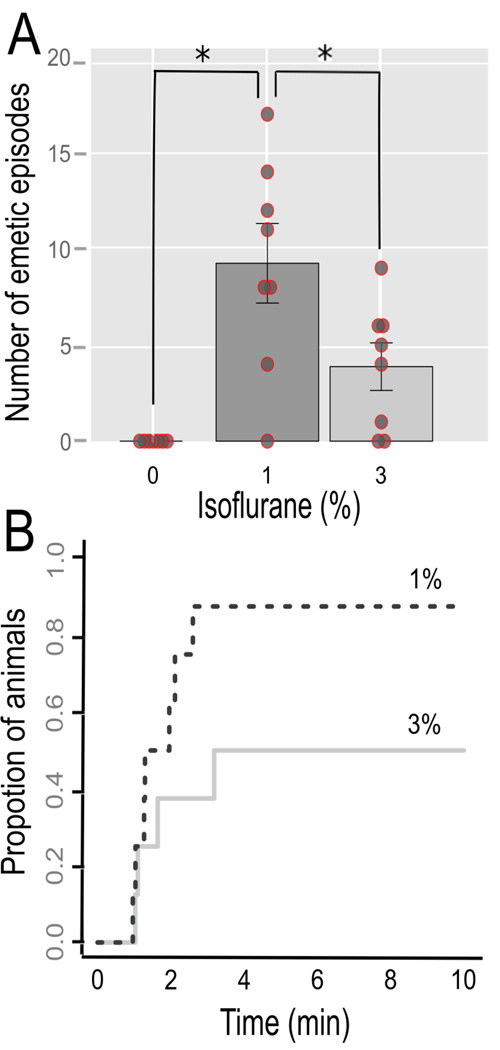
Shrews exposed to 1% isoflurane compared to 3% isoflurane experienced more emetic episodes [9.2 ± 1.9 vs. 3.9 ± 3.3; Tukey’s test, p < 0.03; F(2,20) = 11.0, p < 0.0005, ANOVA; Fig. 3A]. The median latencies to the first emetic episodes were 1.3 min for 1% isoflurane and 2.4 min for 3 % isoflurane (Fig. 3B; p > 0.05, log-rank test). Two animals in the 3% and one animal in the 1% isoflurane condition did not show emesis. There were no statistically significant effects of isoflurane exposure on swaying movements or abdominal contractions (ANOVAs, ps > 0.05).
Fig. 3.
Study 2: Isoflurane-induced emesis. (A) Total number of emetic episodes in each condition (0, 1, and 3% isoflurane exposure. Values represent the mean ± SEM. * = Tukey’s test, p < 0.05. A scatter plot of raw data is also shown. (B) Cumulative proportion of animals showing the latency to the first emetic episode in the 1 and 3% isoflurance conditions.
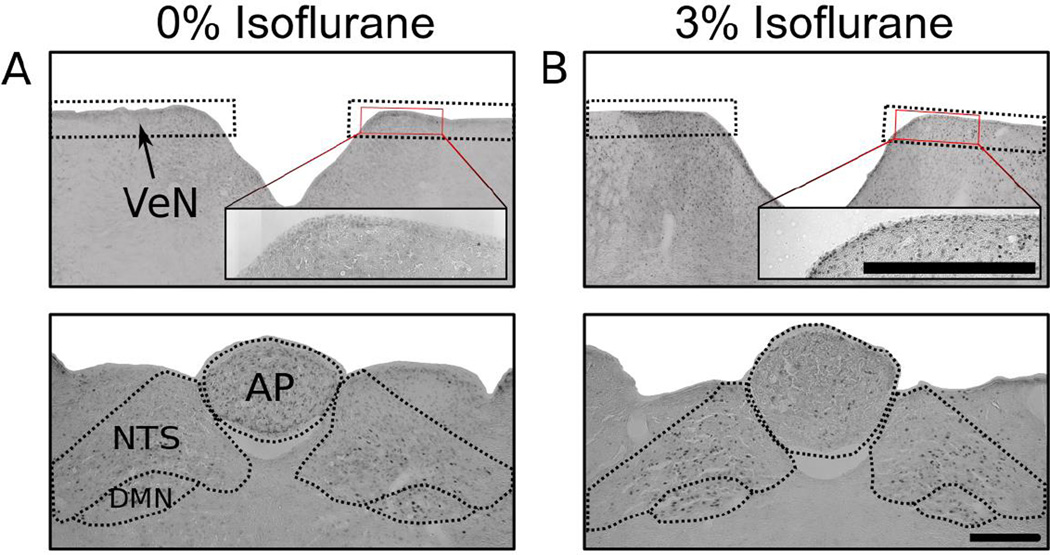
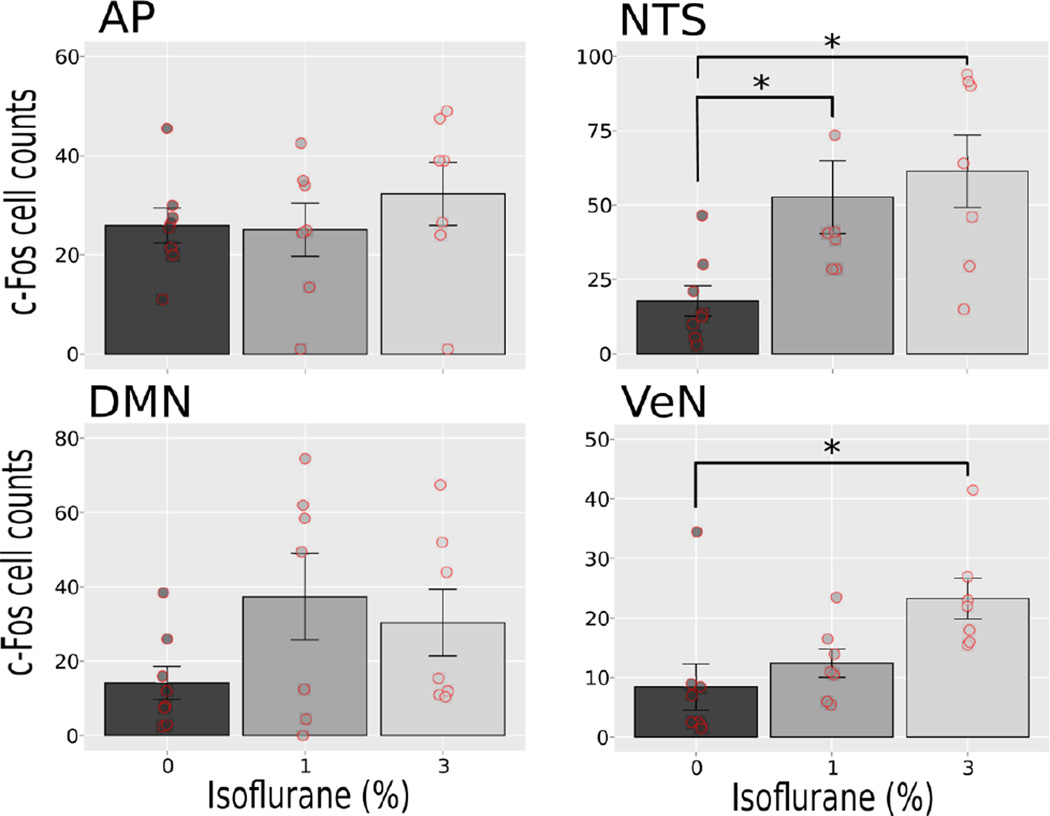
Fig. 4 shows representative hindbrain sections. Isoflurane exposure failed to produce a statistically significant increase in c-Fos cell counts in the AP and DMN (one-way ANOVAs, ps > 0.05; Fig. 5). Both 1% and 3% isoflurane exposure increased c-Fos-positive cells in the NTS [LSD-tests, ps < 0.05, Fig. 5; F(2,19) = 5.5, p < 0.05], and 3% isoflurane produced an increase in the VeN relative to 0% control [LSD-test, p < 0.05, Fig. 5; F(2,19) = 5.2, p < 0.02].
Fig. 4.
Study 2: Representative hindbrain images for c-Fos quantification. (A) 0% isoflurane. (B) 3% isoflurane. Top row: Vestibular nuclei (VeN) and Bottom row: Dorsal vagal complex (AP, NTS, DMN).
Fig. 5.
Study 2: Quantification of c-Fos-positive cells at 0, 1 and 3% isoflurane exposures. Brain areas include the area postrema (AP), nucleus of the solitary tract (NTS), dorsal motor nucleus (DMN), and the vestibular nuclei (VeN). Values are means ± SEM, with scatter plots of the raw values. * = p < 0.05, LSD-tests.
4. Discussion
The current results indicate a narrowing of the list of potential neural pathway mechanisms that drive isoflurane-induced emesis. Study 1 demonstrated that vagotomized shrews, confirmed by Fluoro-Gold tracing, fail to differ in emetic responses to isoflurane exposure compared to sham-operated controls, indicating that the vagus plays no role in isoflurane-induced emesis. Furthermore, Study 2, using c-Fos immunohistochemistry, suggests that isoflurane is activating the critical “final common pathway” for emesis, i.e., the NTS (Horn, 2008). Conversely, isoflurane exposure failed to increase c-Fos cell counts in AP, a brain area often referenced as “the chemoreceptor trigger zone” (Miller et al., 1994a). The current data suggest that isoflurane produces emesis by direct action on the hindbrain.
The hindbrain contains the essential motor circuit for producing the emetic reflex (Miller et al., 1994b; Smith et al., 2002), and four neural pathways are known to project to this circuit (Horn, 2008; Horn, 2014): (1) vagal afferents from the gastrointestinal tract, sensitive to mechanical and chemical perturbations, e.g., food poisoning (Hu et al., 2007); (2) input to the VeN from the middle ear, e.g., motion sickness (Yates et al., 1998); (3) AP, an area with a low blood-brain barrier, sensitive to circulating toxins (Miller et al., 1994a); and (4) descending input from the forebrain, potentially responsible for psychogenic or learned emetic responses (Muraoka et al., 1990; Roscoe et al., 2011). An additional possibility, arguably not “normal” sensory activation of of the emetic circuit, would be stimulus actions on the internal components of the emetic system. For example, this could occur with tumor growth in the brainstem or an epileptic seizure (Mann et al., 1998; Pietrafusa et al., 2015). Based on the results, it is possible that isoflurane could also affect the emetic system by an impact on one or more internal brain connections associated with emesis. Our c-Fos cell counts after isoflurane exposure suggest that the NTS and VeN are activated (Fig. 4); notably, the VeN is known to project to the NTS (Sugiyama et al., 2011; Yates et al., 1994); however, an effect on one or more forebrain sites cannot be ruled out. Motion sickness therapies, like scopolamine (a non-selective muscarinic antagonist), significantly decrease the risk of PONV (Apfel et al., 2010), evidence that the mechanism of PONV is not solely mediated by 5-HT3 receptors. The effects of isoflurane on the VeN may also be due to compromised balance of the animal. Activation of the NTS (or VeN), as determined by c-Fos cell counts, was not closely related to the amount of emesis. Although isoflurane exposure increased c-Fos in the NTS and VeN in an ascending fashion with increasing isoflurane concentration (Fig. 4), 3% isoflurane produced fewer emetic episodes and a longer latency to emesis than exposure to 1% isoflurane (Fig. 2B and 2C). We also reported this effect on emesis in our prior study (Horn et al., 2012), a result that also corresponds to the greater number of emetic episodes occurring at the beginning of exposure with 1% isoflurane in the induction chamber compared to 3% isoflurane. This effect could be caused by a suppressive effect of high concentrations of isoflurane on emetic motor output.
The strengths of the current work include the use of a sensitive emetic test species and methological approaches. Typical preclinical models, such as rats and mice, lack an emetic reflex and hence are not suitable for emesis research (Horn et al., 2013a). Among the animal models for emesis research (ferret, dog, cat, pig, and shrew), the musk shrew has the most well characterized emetic response to inhalational anesthesia (Gardner and Perren 1998; Horn et al., 2012). Surprisingly, the ferret, a primary model in anti-emetic drug development, does not vomit to isoflurane exposure (Horn et al., 2012). Furthermore, our studies included a wide range of parameters, beyond the typical measure of emetic episodes, which could have potentially detected the effects of vagotomy, including abdominal contractions, swaying movement, food intake, water consumption, and body weight change.
There are several interpretive limitations of the current results. First, the surgical vagotomy performed disrupts both the sensory and motor components of the vagus, making it impossible to resolve the role of only the sensory input (of particular interest in this study) versus motor output. Additionally, the time period over which Study 1 was conducted could have allowed for neuronal plasticity in vagotomized animals; for example, the spinal afferents supplying the gastrointestinal system could have become sensitive to emetic stimulus after vagotomy, an effect that occurs in the context of chemotherapy-induced emesis in the ferret (Andrews et al., 1990). Second, we detected an effect of testing order in Study 1, which could complicate the analysis; however, surgical condition was not a statistically significant factor on the effect of order. Finally, while c-Fos can measure neuronal activation, it is limited in temporal resolution, cannot measure inhibition of neurons, and does not show universal stimulus-driven increases in all regions of the central nervous system. There is also a limitation in counting cells in areas like the VeN, which is difficult to delineate without additional histological counter-staining; this study’s designation for counting the VeN was based on similar work in the musk shrew (Ito et al., 2003), but we were conservative in our counting of cells in the VeN by using a uniform “rectangle” to define a region of interest and avoid the potential for sampling the rostral NTS, which closely borders the VeN in the musk shrew (Ito et al., 2003). Finally, c-Fos assessment could be limited in this context because of isoflurane itself; this agent could suppress neuronal activity, as reported (Jinks et al., 2002), and the current data produced less activation of the NTS and DMN than is typical for emetic stimuli (Andrews et al., 2000; De Jonghe and Horn 2009; Ito et al., 2003). Despite these limitations in the use of c-Fos immunohistochemistry, this method could provide more information in future studies by using double-labeling of c-Fos positive cells for additional antigens (e.g., peptides) to determine cell phenotypes.
Future studies to determine the precise mechanisms of inhalational anesthesia-induced vomiting will require further investigation into the NTS, VeN and forebrain mechanisms. This may be accomplished by chemically ablating specific neuronal populations in these brain areas using saporin-conjugated neurochemicals (e.g., Baskin et al., 2010; Freiria-Oliveira et al., 2015). Newer methods could also be applied based on the expression of light activated opsins into specific cell types (Zhang et al., 2010). These cell types could be driven to excitation or inhibition depending on the opsins chosen. Examples of these reversal techniques exist in rodent studies, including non-transgenic animals (Iyer et al., 2014). Ultimately, defining the neural pathways that contribute to inhalational anesthesia-induced emesis could lead to the development of more specific and effective pharmacological agents for the control of PONV.
Highlights.
A small animal model with a vomiting reflex, musk shrew, was used to investigate inhalational anesthesia-induced emesis.
Isoflurane exposure increased emesis in the musk shrew but vagotomy did not affect this response
Isoflurane exposure produced activation of the vestibular nuclei and the nucleus of the solitary tract, indicated by an increase in c-Fos-positive cells
These results indicate that the abdominal vagus plays no role in isoflurane-induced emesis and suggest that isoflurane activates emesis by action on the hindbrain.
Acknowledgments
The authors wish to thank Kelly Meyers and Celine Gouilloux for help with data collection, David Rosenberg for assistance with computerized analysis, and the University of Pittsburgh, Division of Laboratory Animal Research, especially Megan Lambert and Dr. Joseph Newsome for excellent care of the musk shrew colony at the University of Pittsburgh Cancer Institute (UPCI). This work was supported by an NIH grant to the University of Pittsburgh Cancer Institute, P30 CA047904 (Cancer Center Support Grant). RGG was supported by the University of Pittsburgh School of Medicine Dean’s Summer Research Program for medical students. This project used the UPCI Animal Facility and was supported in part by award P30CA047904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
CCH designed the research; RGG, CS, YR, and MGS performed the research; RGG, CCH, CS, YR, and MGS analyzed data; RGG and CCH wrote the manuscript; RGG, CCH, CS, YR, and MGS edited the manuscript.
Conflict of interests
The authors declare no conflicts of interest.
References
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Okada F, Woods AJ, Hagiwara H, Kakaimoto S, Toyoda M, Matsuki N. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br J Pharmacol. 2000;130:1247–1254. doi: 10.1038/sj.bjp.0703428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, Zhang K, Cakmakkaya OS. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–753. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, Heineck R, Greim CA, Roewer N. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88:659–668. doi: 10.1093/bja/88.5.659. [DOI] [PubMed] [Google Scholar]
- Apfel CC, Zhang K, George E, Shi S, Jalota L, Hornuss C, Fero KE, Heidrich F, Pergolizzi JV, Cakmakkaya OS, Kranke P. Transdermal scopolamine for the prevention of postoperative nausea and vomiting: a systematic review and meta-analysis. Clin Ther. 2010;32:1987–2002. doi: 10.1016/j.clinthera.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Naylor IL, Naylor RJ, Rudd JA. Topographical distribution of 5-HT3 receptor recognition sites in the ferret brain stem. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:17–21. doi: 10.1007/BF00178966. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, Simhan HN, Moralejo DH, Blevins JE. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology. 2010;151:4207–4213. doi: 10.1210/en.2010-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC. Quantifying behavior the JWatcher way Sinauer Associates. x. Sunderland, Mass: 2007. p. 211. [Google Scholar]
- Carlisle JB, Stevenson CA. Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2006:CD004125. doi: 10.1002/14651858.CD004125.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus) Am J Physiol Regul Integr Comp Physiol. 2009;296:R902–R911. doi: 10.1152/ajpregu.90952.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiria-Oliveira AH, Blanch GT, Pedrino GR, Cravo SL, Murphy D, Menani JV, Colombari DS. Catecholaminergic neurons in the comissural region of the nucleus of the solitary tract modulate hyperosmolality-induced responses. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1082–R1091. doi: 10.1152/ajpregu.00432.2014. [DOI] [PubMed] [Google Scholar]
- Gan TJ, Ing RJ, de LDG, Wright D, El-Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid intraoperative awareness? J Clin Anesth. 2003;15:108–112. doi: 10.1016/s0952-8180(02)00507-x. [DOI] [PubMed] [Google Scholar]
- Gardner C, Perren M. Inhibition of anaesthetic-induced emesis by a NK1 or 5-HT3 receptor antagonist in the house musk shrew, Suncus murinus. Neuropharmacology. 1998;37:1643–1644. doi: 10.1016/s0028-3908(98)00133-6. [DOI] [PubMed] [Google Scholar]
- Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Curr Med Res Opin. 2006;22:1093–1099. doi: 10.1185/030079906X104830. [DOI] [PubMed] [Google Scholar]
- Horn CC. Why is the neurobiology of nausea and vomiting so important? Appetite. 2008;50:430–434. doi: 10.1016/j.appet.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC. Brain Fos expression induced by the chemotherapy agent cisplatin in the rat is partially dependent on an intact abdominal vagus. Auton Neurosci. 2009;148:76–82. doi: 10.1016/j.autneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC. The medical implications of gastrointestinal vagal afferent pathways in nausea and vomiting. Curr Pharm Des. 2014;20:2703–2712. doi: 10.2174/13816128113199990568. [DOI] [PubMed] [Google Scholar]
- Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci. 2007;132:44–51. doi: 10.1016/j.autneu.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PL. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 2013a;8:e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Meyers K, Lim A, Dye M, Pak D, Rinaman L, Yates BJ. Delineation of vagal emetic pathways: intragastric copper sulfate-induced emesis and viral tract tracing in musk shrews. Am J Physiol Regul Integr Comp Physiol. 2014a;306:R341–R351. doi: 10.1152/ajpregu.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Meyers K, Pak D, Nagy A, Apfel CC, Williams BA. Post-anesthesia vomiting: impact of isoflurane and morphine on ferrets and musk shrews. Physiol Behav. 2012;106:562–568. doi: 10.1016/j.physbeh.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Wallisch WJ, Homanics GE, Williams JP. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol. 2014b;722:55–66. doi: 10.1016/j.ejphar.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Wang H, Estival L, Meyers K, Magnusson MS. Novel dynamic measures of emetic behavior in musk shrews. Auton Neurosci. 2013b;179:60–67. doi: 10.1016/j.autneu.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DL, Zhu G, Mori F, Omoe K, Okada M, Wakabayashi K, Kaneko S, Shinagawa K, Nakane A. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell Microbiol. 2007;9:2267–2277. doi: 10.1111/j.1462-5822.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- Huang D, Meyers K, Henry S, De la Torre F, Horn CC. Computerized detection and analysis of cancer chemotherapy-induced emesis in a small animal model, musk shrew. J Neurosci Methods. 2011;197:249–258. doi: 10.1016/j.jneumeth.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Induction of Fos protein in neurons in the medulla oblongata after motion- and X-irradiation-induced emesis in musk shrews (Suncus murinus) Auton Neurosci. 2003;107:1–8. doi: 10.1016/S1566-0702(03)00026-2. [DOI] [PubMed] [Google Scholar]
- Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol. 2014;32:274–278. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Antognini JF, Martin JT, Jung S-W, Carstens E, Atherley R. Isoflurane, but not halothane, depresses c-fos expression in rat spinal cord at concentrations that suppress reflex movement after supramaximal noxious stimulation. Anesth Analg. 2002;95:1622–1628. doi: 10.1097/00000539-200212000-00028. table of contents. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Butler A, Hagan RM, Jones BJ, Tyers MB. [3H] GR67330, a very high affinity ligand for 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:22–30. doi: 10.1007/BF00178967. [DOI] [PubMed] [Google Scholar]
- Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. J Pharmacol Exp Ther. 1994;271:898–905. [PubMed] [Google Scholar]
- Mann SD, Danesh BJ, Kamm MA. Intractable vomiting due to a brainstem lesion in the absence of neurological signs or raised intracranial pressure. Gut. 1998;42:875–877. doi: 10.1136/gut.42.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Betti L, Giannaccini G, Rossi A, Masala I, Baroni S, Cassano GB, Lucacchini A. Distribution of [3H]GR65630 binding in human brain postmortem. Neurochem Res. 2001;26:187–190. doi: 10.1023/a:1010939530412. [DOI] [PubMed] [Google Scholar]
- Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994a;15:301–320. doi: 10.1006/frne.1994.1012. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res. 1994b;647:255–264. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- Muraoka M, Mine K, Matsumoto K, Nakai Y, Nakagawa T. Psychogenic vomiting: the relation between patterns of vomiting and psychiatric diagnoses. Gut. 1990;31:526–528. doi: 10.1136/gut.31.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RM, Bentley KR, Barnes NM. Allosteric modulation of 5-HT3 receptors: focus on alcohols and anaesthetic agents. Trends Pharmacol Sci. 1996;17:95–99. doi: 10.1016/0165-6147(96)10003-1. [DOI] [PubMed] [Google Scholar]
- Pietrafusa N, de Palma L, De Benedictis A, Trivisano M, Marras CE, Vigevano F, Specchio N. Ictal vomiting as a sign of temporal lobe epilepsy confirmed by stereo-EEG and surgical outcome. Epilepsy Behav. 2015;53:112–116. doi: 10.1016/j.yebeh.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol. 1987;253:R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- Reynolds DJM, Andrews PLR, Davis CJ. Serotonin and the Scientific Basis of Anti-Emetic Therapy Oxford. Philadelphia: 1995. [Google Scholar]
- Roscoe JA, Morrow GR, Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2011;19:1533–1538. doi: 10.1007/s00520-010-0980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci. 1991;11:2321–2331. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Swanson RA. Metabolic mapping with cellular resolution: c-fos vs. 2-deoxyglucose. Crit Rev Neurobiol. 1993;7:205–228. [PubMed] [Google Scholar]
- Smith JE, Paton JF, Andrews PL. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Exp Physiol. 2002;87:563–574. doi: 10.1113/eph8702424. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki T, DeStefino VJ, Yates BJ. Integrative responses of neurons in nucleus tractus solitarius to visceral afferent stimulation and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1380–R1390. doi: 10.1152/ajpregu.00361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. Ilar j. 2004;45:25–34. doi: 10.1093/ilar.45.1.25. [DOI] [PubMed] [Google Scholar]
- Wang C. Introduction: A new experimental animal, Suncus murinus. In: Saito H, Wang C, Chen C, editors. Proceeding of ROC-Japan Symposium on Suncus murinus: New experimental animal, its speciality and usefulness. (Chia Nan, Taiwan ROC), Tainan, Taiwan, R.O.C.: Chia Nan Junior College of Pharmacy Press; 1994. [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Tsukamoto G, Kobashi M, Sasaki A, Matsumura T. Abdominal vagi mediate c-Fos expression induced by X-ray irradiation in the nucleus tractus solitarii of the rat. Auton Neurosci. 2000;83:29–36. doi: 10.1016/S0165-1838(00)00105-3. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Grelot L, Kerman IA, Balaban CD, Jakus J, Miller AD. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol. 1994;267:R974–R983. doi: 10.1152/ajpregu.1994.267.4.R974. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]