Abstract
Background
Each encounter of asymptomatic individuals with the healthcare system presents an opportunity for improvement of cardiovascular disease (CVD) awareness and sudden cardiac death (SCD) risk assessment. ECG sign deep terminal negativity of the P wave in V1 (DTNPV1) was shown to be associated with an increased risk of SCD in the general population.
Objective
To evaluate association of DTNPV1 with all-cause mortality and newly diagnosed atrial fibrillation (AFib) in the large tertiary healthcare system patient population.
Methods
Retrospective double cohort study compared two levels of exposure (automatically measured amplitude of P-prime (Pp) in V1): DTNPV1 (Pp from −100μV to −200μV) and ZeroPpV1 (Pp=0). An entire healthcare system (2010–2014) ECG database was screened. Medical records of children and patients with previously diagnosed AFib/atrial flutter (AFl), implanted pacemaker or cardioverter-defibrillator were excluded. DTNPV1 (n=3,413) and ZeroPpV1 (n=3,405) cohorts were matched by age and sex. Primary outcome was all-cause mortality. Secondary outcomes were newly diagnosed AFib/AFl. Median follow-up was 2.5 y.
Results
DTNPV1 was associated with all-cause mortality (HR 1.95(1.64–2.31); P<0.0001) and newly diagnosed AFib (HR 1.29(1.04–1.59); P=0.021) after adjustment for CVD, comorbidities, other ECG parameters, medications, and index ECG referral. Index ECG referral by a cardiologist was independently associated with 34% relative risk reduction of mortality (HR 0.66(0.52–0.84); P=0.001), as compared to ECG referral by a non-cardiologist.
Conclusion
DTNPV1 is independently associated with twice higher risk of all-cause death, as compared to patients without P prime in V1. Life-saving effect of the index ECG referral by a cardiologist requires further study.
Keywords: electrocardiogram, mortality, health system, patient education
Introduction
In the United States, up to 450,000 people per year die suddenly, an average of 1 death every 70 seconds[1]. Estimated 347,000 out-of-hospital sudden cardiac arrests (SCA) in adults occur annually in the United States[1]. Survival to discharge remains low (7–8%) in the last three decades[2], although a positive trend for improvement was observed recently[3]. It was previously shown that in more than half of sudden cardiac death (SCD) cases, SCA is the first manifestation of cardiovascular disease (CVD)[4]. However, recent findings of the OregonSUDS study[5] showed that about a half of SCA victims experienced non-specific symptoms within four weeks before SCA, which often recurred within 24 hours before SCA[5]. Importantly, patients’ awareness of CVD significantly improved survival of SCA[5]. Patients who were aware of their cardiovascular condition were more likely to call 911 before SCA, while those who had no previously diagnosed CVD ignored their symptoms[5]. Thus, timely diagnosed CVD and patient education on determined risk of SCD may save lives. Each encounter of asymptomatic individuals with the healthcare system presents an opportunity for improvement of CVD awareness and SCD risk assessment, which is currently underutilized.
The resting 12-lead electrocardiogram (ECG) is a commonly used diagnostic test. A large multicenter study (PRIMERI)[6] demonstrated the feasibility of automated screening of the entire healthcare system ECG database to identify individuals at increased risk of death. Our group recently showed that an easily recognizable ECG sign deep terminal negativity of the P wave in V1 (DTNPV1) represents an epiphenomenon of a fibrotic heart[7], and is independently associated with increased risk of all-cause and CVD mortality, as well as death due to ischemic heart disease[8]. Moreover, in a large community-dwelling prospective cohort study[9], DTNPV1 was independently associated with a 2.5-fold increased risk of SCD and a 5-fold increased risk of incident atrial fibrillation (AFib)[9]. However, the association of DTNPV1 with all-cause mortality and newly diagnosed AFib in an entire healthcare population has not been previously studied. We hypothesized that DTNPV1 is associated with increased all-cause mortality and newly diagnosed AF in a large tertiary health system patient population.
Methods
Study Population
We conducted a retrospective double-cohort study. An Institutional Committee on Human Research (Oregon Health and Science University [OHSU] Institutional Review Board) approved this study. Double-cohort study design allows comparison of two different levels of exposure (amplitude of P-prime in V1). One cohort was exposed to the potential risk factor (DTNPV1 cohort), and another cohort experienced no exposure (Zero PpV1 cohort). We screened all 12-lead ECGs recorded at the OHSU healthcare system over 4 years (August 3, 2010–July 7, 2014) and comprised cohorts according to inclusion and exclusion criteria.
DTNPV1 cohort included OHSU patients with DTNPV1 on index baseline ECG. DTNPV1 was defined as the presence of biphasic P wave (positive/negative) in V1 with the amplitude of the terminal negative phase >100 μV. Exclusion criteria were: (1) age < 18 y; (2) previously diagnosed AFib or atrial flutter (AFl); (3) implanted pacemaker or implantable cardioverter-defibrillator (ICD); (4) concomitant non-cardiac disease with high likelihood of death within 1 year of follow-up; (5) P-prime amplitude in lead V1 below −200 μV (to eliminate risk of ECG measurement errors).
Zero PpV1 cohort included OHSU patients without P-prime wave in V1 on index baseline ECG, randomly selected from the entire OHSU ECG MUSE (GE Marquette, Milwaukee, WI) database in the same time period (August 3, 2010–July 7, 2014). Exclusion criteria were the same as for DTNPV1 cohort. To minimize confounding effect of age and sex, we randomly matched cohorts by age and sex 1:1.
Electrocardiogram Analysis: Deep Terminal Negativity of P-prime in V1
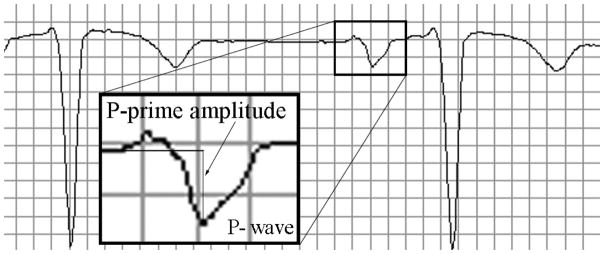
Standard 12-lead ECGs were automatically analyzed by the 12SL algorithm (GE Marquette, Milwaukee, WI). The amplitude of the terminal negative phase of P wave in V1 was measured automatically. We defined the presence of DTNPV1 if the amplitude of the terminal negative phase of a biphasic P wave in lead V1 exceeded 100 μV (Figure 1), but did not exceed 200 μV (to ensure exclusion of measurements errors). For reference, 100 μV = 0.1 mV = 1 mm (1 small box). In addition, heart rate, QTc interval, and PR interval were measured automatically by the 12SL algorithm, as well.
Figure 1.
A representative example of the deep terminal negativity of P wave in V1. Median beat PpV1 of −180μV.
Definition of clinical characteristics
Baseline demographic and clinical characteristics were obtained from the OHSU Epic electronic medical record (EMR), active problem list. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) was used to capture specific clinical conditions. As missing and incorrectly coded data have been previously reported in studies of the databases research quality[10, 11], we used the recommended combination of ICD-9-CM codes and clinical data[12–14], which were captured during the diagnosis and treatment process[12, 13]. To enhance the ICD-9-CM code data, we added historical patients’ medications lists, and referring provider identity of the index ECG.
Each patient’s entire medication history available in the EMR was reviewed to evaluate if the patient was ever prescribed medications of any kind by at OHSU. Historical patient medication lists were then assessed for presence or absence of 68 individual cardiovascular medications - both generic and brand name(s) classified as either (1) currently taking, (2) discontinued, or (3) never prescribed, and grouped by drug class.
Effect of patient management approach at the time of index ECG
In order to evaluate potential effects of the management approach at the time of index ECG, we assessed whether index ECG was referred by a cardiologist or non-cardiologist. In order to determine whether index ECG was referred/requested by a cardiology team member, a list of all personnel employed by the OHSU Cardiology Department/Knight Cardiovascular Institute at any point from January 1st, 2010–December 31st, 2014 was compared against the referring provider listed in the index ECG order form, as recorded in the MUSE database. Referring provider names were searched both backwards and forwards (firstName lastName, lastName firstName) and checked for common spelling errors. Patients with an index ECG referral returning a match to any Cardiology Department employee (including Cardiology Fellows) were defined as having an index ECG referral by a cardiologist. The absence of a match was defined as a referral by a non-cardiologist and blank referral fields or fields without enough information to determine provider identity were defined as unknown. In addition, we identified ECGs that were referred by any Intensive Care Unit (ICU), Trauma, or emergency department (ED), as well as ECGs that were referred from the Intensive Cardiac Care Unit (CCU).
Definition of the presence of manifested or subclinical cardiovascular disease
Whether or not a patient had CVD or its risk factors (subclinical CVD) was defined based on the presence of at least one of the following conditions: (1) history of, or newly diagnosed CVD, or newly (during follow-up) implanted ICD/pacemaker (by ICD-9-CM codes), (2) index ECG referral by cardiologist, (3) current use of medications for treatment or prevention of CVD (ACEi/ARBs, BB, CCA, nitrates/hydralazine, diuretics, statins, antiarrhythmics, anticoagulants [including heparin], antiplatelets [including aspirin]).
Follow-up and Primary Study Outcome
Cohorts were followed retrospectively via the review of the OHSU Epic EMR. All-cause mortality served as the primary outcome. Deaths reported in the EMR or identified by searching the Social Security Death Master File were considered if they occurred by February 26, 2015. Newly diagnosed AFib and newly diagnosed AFl served as the secondary outcomes. Newly diagnosed AFib and AFl were identified via review of Epic EMR as newly appearing diagnoses during the retrospective follow-up period.
Statistical Analysis
Statistical analyses were performed using STATA 14 (StataCorp LP, College Station, TX). Continuous variables were reported as mean ±standard deviation (SD) after verification of normal distribution. To compare characteristics of two cohorts with different level of exposure (P-prime in V1 amplitude: zero PpV1 vs. DTNPV1), the Pearson chi-square test was used to compare categorical variables, and two-sample t-test was used to compare continuous variables. Unadjusted Kaplan-Meier survivor function was plotted for primary and secondary outcomes. The unadjusted log-rank statistic was computed to test the equality of survival functions across two cohorts. Cox proportional hazards models quantified the association between exposure and primary outcome (all-cause mortality). Three Cox regression models were constructed. Model 1 was minimally adjusted by age and sex, and quality control metric (prescription of any medication at any time in the medical history). Model 2 was, in addition, adjusted by prevalent coronary heart disease (CHD), heart failure (HF), history of myocardial infarction (MI), coronary artery bypass grafting (CABG), newly (during follow-up) implanted pacemaker or ICD, history of comorbidities (diabetes, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), chronic liver disease), and ECG parameters (heart rate, PR and QTc intervals). In order to evaluate the potential effect of ever diagnosed and treated CVD or its risk factors, model 3 was, in addition, adjusted for the historical (current or discontinued) use of medications (angiotensin-converting-enzyme inhibitors (ACEi)/angiotensin II receptor blockers (ARBs), beta-blockers (BB), antiarrhythmics, calcium channel blockers (CCBs), diuretics, antiplatelets (including aspirin), anticoagulants (including heparin), lipid-lowering drugs, and nitrates/hydralazine), and index ECG referral from cardiologist.
In addition, we constructed Model 4 to evaluate joint association of (1) DTNPV1, (2) index ECG referral as a proxy for management approach at the time of index ECG, and (3) presence of manifested or subclinical CVD as defined above, with all-cause mortality. Model 4 was adjusted for age, sex, and the use of any medication at any time in the medical record history (a proxy for OHSU healthcare system utilization).
Due to the retrospective nature of the study, we were not able to accurately determine the date of incident AFib/AFl. Thus, logistic regression analysis was performed to study the association between exposure and secondary outcomes. Three logistic regression models were constructed for each secondary outcome, adjusted for the same covariates as in the described above Cox models. A P-value of <0.05 was considered statistically significant.
To test the robustness of our findings, we conducted sensitivity analysis and repeated all described above analyses after exclusion of patients with index ECG recorded in the ED, and any ICU, including CCU.
Results
Study population
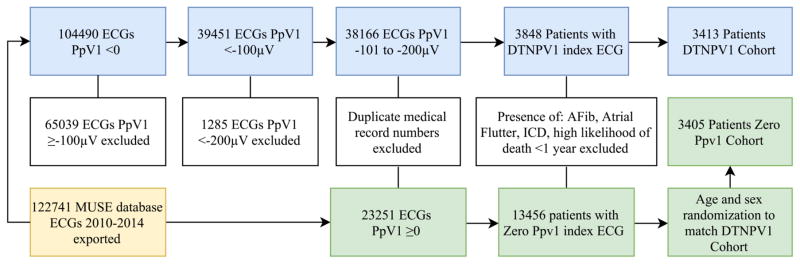
Study flow chart is presented in Figure 2. All ECGs acquired and stored at the OHSU healthcare system (including outpatient clinics) between August 3, 2010–July 7, 2014, from patients aged 18 to 100 years were reviewed in the MUSE ECG database. After exclusion of ineligible ECGs, 3,413 unique patient records comprised the DTNPV1 cohort, and 3,405 age- and sex-matched unique patient records comprised the zero PpV1 cohort.
Figure 2.
Study flow chart.
The study population (Table 1) was represented by middle-age adults with a relatively low prevalence of diagnosed CVD and comorbidities. The quality of the collected clinical data was satisfactory, as shown by a high percentage (>90%) of OHSU healthcare system utilization, demonstrated by prescription of at least one medication over the entire medical record history. At least one-third of patients were taking cardiovascular medications at some point in time in their medical history.
Table 1.
Comparison of demographic, clinical, and ECG characteristics of the two cohorts
| Both cohorts | Cohort zero PpV1 N = 3,405 |
Cohort DTNPV1 N = 3,413 |
P-value | |
|---|---|---|---|---|
| Age (SD), y | 58.3(16.2) | 58.0(16.2) | 58.5(16.1) | 0.203 |
| Female, n (%) | 3,046(44.7) | 1,520(44.6) | 1,526(44.7) | 0.953 |
| Referred by cardiologist, n(%) | 1,143(16.8) | 623(18.3) | 520(15.2) | <0.0001 |
| Ordered in Intensive Care Unit or ED, n(%) | 119(1.8) | 46(1.4) | 73(2.4) | 0.013 |
| Ordered in Intensive Cardiac Care Unit, n(%) | 33(0.5) | 11(0.3) | 22(0.6) | 0.056 |
|
| ||||
| Coronary heart disease, n(%) | 984(14.4) | 364(10.7) | 620(18.2) | <0.0001 |
| History of myocardial infarction, n(%) | 361(5.3) | 126(3.7) | 235(6.9) | <0.0001 |
| History of CABG, n(%) | 136(2.0) | 44(1.3) | 92(2.7) | <0.0001 |
| Heart failure, n(%) | 900(13.2) | 273(8.0) | 627(18.4) | <0.0001 |
| New Implantable Cardioverter Defibrillator, n(%) | 223(3.3) | 68(2.0) | 155(4.5) | <0.0001 |
| New Pacemaker, n(%) | 168(2.5) | 77(2.3) | 91(2.7) | 0.281 |
| Chronic obstructive pulmonary disease, n(%) | 397(5.8) | 156(4.6) | 241(7.1) | <0.0001 |
| Chronic liver disease, n(%) | 246(3.6) | 123(3.6) | 123(3.6) | 0.985 |
| Diabetes mellitus, n(%) | 1,176(17.3) | 478(14.0) | 697(20.4) | <0.0001 |
| Chronic kidney disease, n(%) | 80(1.2) | 23(0.7) | 57(1.7) | <0.0001 |
|
| ||||
| Ever prescribed any medication at OHSU, n(%) | 6,174(90.6) | 3,000(88.1) | 3,174(93.0) | <0.0001 |
| ACE inhibitors/ARBs, n(%) | 2,040(29.9) | 894(26.3) | 1,146(33.6) | <0.0001 |
| Antiarrhythmic drugs, n(%) | 215(3.2) | 89(2.6) | 126(3.7) | 0.011 |
| Anticoagulants, n(%) | 831(12.2) | 412(12.1) | 419(12.3) | 0.824 |
| Anti-platelets/ASA, n(%) | 2,695(39.5) | 1,158(34.0) | 1,537(45.0) | <0.0001 |
| Beta blockers, n(%) | 2,491(36.5) | 1,073(29.8) | 1,524(43.2) | <0.0001 |
| Calcium channel blockers, n(%) | 1,077(15.8) | 457(13.4) | 620(18.2) | <0.0001 |
| Diuretics, n(%) | 1,885(27.7) | 827(24.3) | 1,058(31.0) | <0.0001 |
| Nitrates/hydralazine, n (%) | 395(5.8) | 139(4.1) | 256(7.5) | <0.0001 |
| Statins, n(%) | 2,349(34.5) | 1,045(30.7) | 1,304(38.2) | <0.0001 |
| Any CV condition/risk factor/meds/referral, n(%) | 4,803(70.5) | 2,215(65.1) | 2,588(75.8) | <0.0001 |
|
| ||||
| P-prime V1 amplitude, μV(SD) | - | 0 | −119.5(19.6) | <0.0001 |
| Heart rate, bpm(SD) | 81.7(21.6) | 78.6(22.7) | 84.7(20.0) | <0.0001 |
| PR interval, n (SD) | 147.0(52.4) | 135.0(64.3) | 159.0(32.7) | <0.0001 |
| QTc interval, n (SD) | 448.1(39.0) | 441.8(39.5) | 454.5(37.4) | <0.0001 |
SD=standard deviation; ED=Emergency Department; ACE=angiotensin-converting-enzyme; ARBs = angiotensin II receptor blockers; ASA=acetylsalicylic acid.
As expected, individuals with DTNPV1 were more likely to have history of cardiac conditions (CHD, MI, HF) and its risk factors, used cardiovascular medications, had higher heart rate, longer QTc and PR intervals on index ECG, and were more likely to have a newly implanted ICD or pacemaker during follow-up, as compared to individuals in the zero PpV1 cohort (Table 1). Interestingly, the index ECG in the DTNPV1 cohort was less likely to be requested by a cardiologist, as compared to the index ECG in Zero PpV1 cohort.
To evaluate the potential effect of patient management at the time of index ECG recording, we categorized patients based on the index ECG referral. For most patients (42%), index ECG was referred by non-cardiologists (Table 2), whereas cardiology team members requested index ECG for only 17% of patients, on average. Unfortunately, ECG referral was unknown for 41% of participants. Patients with an ECG referred by cardiology providers were more likely to have CVD history and were currently taking or previously had taken (discontinued) CVD medications (Table 2).
Table 2.
Comparison of demographic, clinical, and ECG characteristics by the ECG referral
| Non-cardiologist N=2,856 |
Unknown N=2,819 |
Cardiologist N=1,143 |
ANOVAP-value | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Use of medication | Any time | currently | Any time | currently | Any time | currently | Any time | currently |
| Age (SD), y | 59.5(15.3) | 56.3(16.9) | 59.2(16.1) | <0.0001 | ||||
| Female, n(%) | 1,345(47.1) | 1,211(43.0) | 490(42.9) | 0.003 | ||||
| Ordered in Intensive Care Unit or ED, n(%) | 14(0.5) | 70(2.5) | 35(3.1) | <0.0001 | ||||
|
| ||||||||
| Coronary heart disease, n(%) | 382(13.4) | 378(13.4) | 224(19.6) | <0.0001 | ||||
| History of myocardial infarction, n(%) | 129(4.5) | 148(5.3) | 84(7.4) | 0.001 | ||||
| History of CABG, n(%) | 48(1.7) | 51(1.8) | 37(3.2) | 0.004 | ||||
| Heart failure, n(%) | 309(10.8) | 377(13.4) | 214(18.7) | <0.0001 | ||||
| New Implantable Cardioverter Defibrillator, n(%) | 76(2.7) | 77(2.7) | 70(6.1) | <0.0001 | ||||
| New Pacemaker, n(%) | 57(2.0) | 56(2.0) | 55(4.8) | <0.0001 | ||||
| Chronic obstructive pulmonary disease, n(%) | 147(5.2) | 186(6.6) | 64(5.6) | 0.062 | ||||
| Chronic liver disease, n(%) | 97(3.4) | 122(4.3) | 27(2.4) | 0.008 | ||||
| Diabetes mellitus, n(%) | 481(16.8) | 503(17.8) | 192(16.8) | 0.551 | ||||
| Chronic kidney disease, n(%) | 44(1.5) | 25(0.9) | 11(1.0) | 0.056 | ||||
|
| ||||||||
| Ever prescribed any medication at OHSU, n(%) | 2,642(92.5) | 2,462(87.3) | 1,070(93.6) | <0.0001 | ||||
| ACE inhibitors/ARBs, n(%) | 871(30.5) | 475(16.6) | 783(27.8) | 446(15.8) | 386(33.8) | 227(19.9) | 0.001 | 0.008 |
| Antiarrhythmic drugs, n(%) | 79(2.8) | 39(1.4) | 72(2.6) | 41(1.5) | 64(5.6) | 38(3.3) | <0.0001 | |
| Anticoagulants, n(%) | 346(12.1) | 204(7.1) | 302(10.7) | 174(6.2) | 183(16.0) | 111(9.7) | <0.0001 | |
| Anti-platelets/ASA, n(%) | 1,120(39.2) | 584(20.5) | 1,017(36.1) | 559(19.8) | 558(48.8) | 331(29.0) | <0.0001 | |
| Beta blockers, n(%) | 1,045(36.6) | 594(20.8) | 958(34.0) | 559(19.8) | 488(42.7) | 306(26.8) | <0.0001 | |
| Calcium channel blockers, n(%) | 481(16.8) | 249(8.7) | 424(15.0) | 228(8.1) | 172(15.1) | 92(8.1) | 0.133 | 0.639 |
| Diuretics, n(%) | 789(27.6) | 442(15.5) | 727(25.8) | 422(15.0) | 369(32.3) | 221(19.3) | <0.0001 | 0.002 |
| Nitrates/hydralazine, n(%) | 143(5.0) | 90(3.2) | 165(5.9) | 88(3.1) | 87(7.6) | 44(3.9) | 0.006 | 0.463 |
| Statins, n(%) | 988(34.6) | 509(17.8) | 879(31.2) | 484(17.2) | 482(42.2) | 285(24.9) | <0.0001 | |
|
| ||||||||
| P-prime V1 amplitude, μV(SD) | −118.6(19.2) | −120.0(19.6) | −120.3(20.5) | 0.092 | ||||
| Heart rate, bpm(SD) | 79.4(21.5) | 85.6(22.0) | 77.6(19.0) | <0.0001 | ||||
| PR interval, n(SD) | 146.3(53.6) | 146.6(48.8) | 149.7(57.8) | 0.171 | ||||
| QTc interval, n(SD) | 445.1(38.6) | 450.2(38.2) | 450.6(41.4) | <0.0001 | ||||
All-cause mortality
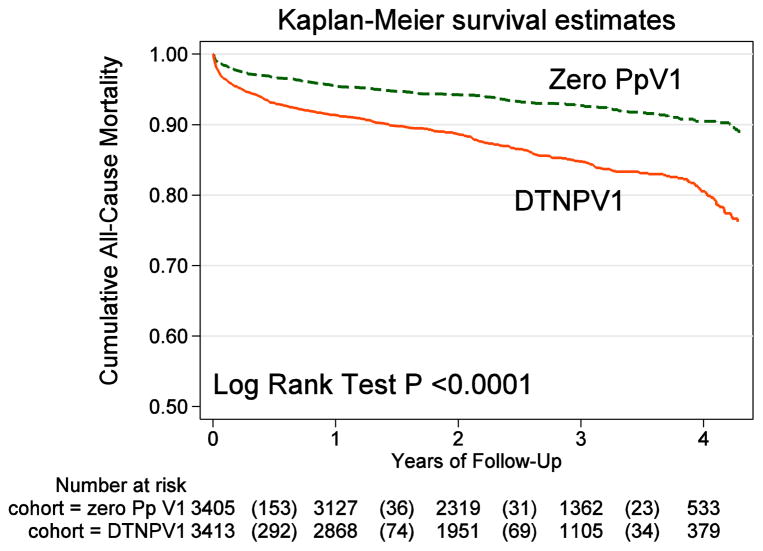
During a median follow-up time of 2.5 years (mean 2.5±1.2 years), a total of 750 deaths occurred (incidence 44.4 (95%CI 41.3–47.7) per 1,000 person-years. Mortality was more than twice higher in DTNPV1 cohort (incidence rate 62.4(95%CI 57.1–68.1) per 1,000 person-years, as compared to Zero PpV1 cohort (incidence rate 28.7(95%CI 25.5–32.5) per 1,000 person-years; P<0.0001. Unadjusted Kaplan-Meier analysis (Figure 3) showed significantly higher mortality in DTNPV1 cohort.
Figure 3.
Unadjusted Kaplan-Meier curves for the cumulative probability of all-cause death in patients with the deep terminal negativity of P-prime in lead V1 (DTNPV1), and those without P-prime in lead V1 (Zero PpV1).
In minimally adjusted Cox regression analysis, DTNPV1 was associated with a two-fold increase in the risk of mortality (Table 3). Adjustment for CVD history, comorbidities, other index ECG parameters, use of medications, and index ECG referral very slightly attenuated the association of DTNPV1 with primary outcome (Models 2–3, Table 3).
Table 3.
Association of DTNPV1, prevalent cardiovascular disease, and index ECG referral with all-cause mortality in Cox regression analyses
| Predictor | Model | HR(95%CI) | P-value |
|---|---|---|---|
| DTNPV1 | 1 | 2.12(1.82–2.48) | <0.0001 |
| DTNPV1 | 2 | 1.97(1.66–2.33) | <0.0001 |
| DTNPV1 | 3 | 1.95(1.64–2.31) | <0.0001 |
|
| |||
| DTNPV1 | 4 | 2.04(1.75–2.38) | <0.0001 |
| Cardiovascular disease | 4 | 1.22(1.03–1.43) | 0.018 |
| ECG referred by non-cardiologist | 4 | Reference | |
| Unknown ECG referral | 4 | 1.05(0.90–1.23) | 0.535 |
| ECG referred by cardiologist | 4 | 0.66(0.52–0.84) | 0.001 |
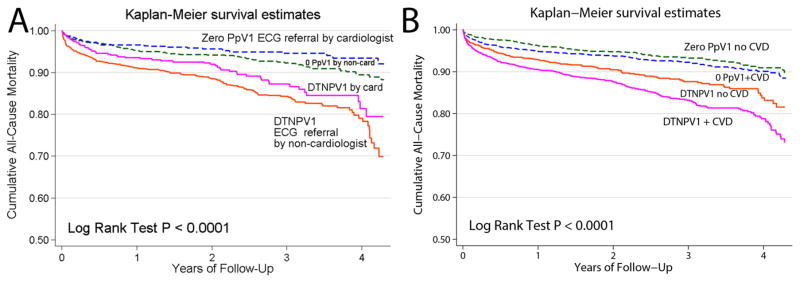
Interestingly, index ECG referral was independently associated with the primary outcome (Figure 4): survival of patients was significantly better if index ECG was referred by a cardiologist, as compared with non-cardiologist (Figure 4A). The presence of manifested or subclinical CVD was associated with independent opposite effect: mortality was higher in patients with CVD (Figure 4B). All three predictors (DTNPV1, presence of manifested or subclinical CVD, and index ECG referral by a cardiologist) had an independent association with all-cause mortality in fully adjusted Cox regression model 4 (Table 3). Index ECG referral by a cardiologist was associated with 34% relative risk reduction, as compared to ECG referral by non-cardiologist. Stratified by cohort fully adjusted analyses (Model 4) association of index ECG referral was similar in both cohorts: in DTNPV1 (HR 0.69(0.51–0.93); P=0.015), and in ZeroPpV1 (HR 0.63(95%CI 0.42–0.94); P=0.025). However, manifested or subclinical CVD was associated with increased mortality only in DTNPV1 cohort (HR 1.34(95%CI 1.09–1.65); P=0.006), but not in the ZeroPpV1 cohort (HR 1.02(95%CI 1.02–1.03): P=0.905).
Figure 4.
Unadjusted Kaplan-Meier curves for the cumulative probability of all-cause death in 4 categories of patients: (A) with DTNPV1 and index ECG referred by cardiologist vs. non-cardiologist, and those with Zero PpV1 and index ECG referred by cardiologist vs. non- cardiologist. (B) with DTNPV1 vs. those with Zero PpV1, and either diagnosed or non-diagnosed with cardiovascular disease (CVD), defined as the presence at least one of the following: (1) current use of cardiovascular medications, (2) index ECG referred by a cardiologist, (3) ICD-9-CM code.
New atrial fibrillation and/or atrial flutter
In adjusted logistic regression analyses, DTNPV1 was significantly associated with newly diagnosed Afib and Afl (Table 4). Referral of index ECG was not associated with secondary outcomes in adjusted logistic regression analyses.
Table 4.
Association of DTNPV1 with atrial fibrillation and atrial flutter in logistic regression
| Atrial fibrillation | Atrial flutter | |||
|---|---|---|---|---|
|
| ||||
| OR(95%CI) | P-value | OR(95%CI) | P-value | |
| Model 1 | 0.86(0.73–1.00) | 0.056 | 1.51(1.11–2.05) | 0.009 |
| Model 2 | 1.28(1.05–1.55) | 0.016 | 1.44(1.02–2.04) | 0.040 |
| Model 3 | 1.29(1.04–1.59) | 0.021 | 1.50(1.04–2.15) | 0.029 |
Sensitivity analyses
Association of DTNPV1 with all-cause mortality and Afib strengthened after exclusion of patients with index ECG recorded in the CCU, ICU, and the ED (Supplemental Tables 1 and 2).
Discussion
This large retrospective double cohort study conveyed two major findings. First, the study validated the strong independent association of DTNPV1 with both all-cause mortality and AFib/AFl in the entire population of a large tertiary healthcare system. In addition, the study revealed an independent association of the index ECG referral with all-cause death. Index ECG referral by the cardiology providers was associated with 34% relative risk reduction of mortality, as compared to the index ECG referral by non-cardiology providers. Life-saving effect of index ECG referral by cardiologists was independent of an association of manifested or subclinical CVD and its medical management with increased risk of death; it was likely due to the effect of patients’ education and increased CVD awareness. Taken together, findings of our study call for the next step: randomized controlled trial (RCT) to test the efficacy of patient education for prevention of SCD.
Association of DTNPV1 with mortality and atrial fibrillation
DTNPV1 is a simplified ECG metric[7–9], originating from well-established ECG risk marker called P-terminal force, first developed by Morris et al[15] in 1964. DTNPV1 is particularly useful in that no calculations are necessary to determine its presence (Figure 1). DTNPV1, being easily visualized on ECG without requiring calculations can thus be a good surrogate for P-terminal force and, in consequence, be a simple marker to identify individuals in the general population at risk of poor outcomes[8, 9]. Importantly, we previously showed a strong association of DTNPV1 specifically with competing risk of SCD[9]. In the general adult population associated with DTNPV1, risk of SCD exceeded the risk of non-fatal CHD, HF, and stroke; only risk of AF exceeded the risk of SCD[9]. Mechanistically, DTNPV1 is an intermediate marker on the pathway linking cardiac fibrosis[7] with atrial and ventricular arrhythmias and SCD[16, 17]. Observed in this study strength of association of DTNPV1 with all-cause mortality was similar to previously reported[9] after similar adjustment: risk of death was approximately twice higher in DTNPV1 cohort. In our OHSU health system population, all-cause mortality (44.4 per 1000 person-years) was at least 2.5 fold higher than all-cause mortality in the Atherosclerosis Risk in Communities (ARIC) cohort (17.2 per 1000 person-years)[9]. Assuming proportional increase of SCD rate, we can estimate that approximately 38% of all-cause deaths (n~281) in this study were SCDs.
The strength of association of DTNPV1 with newly diagnosed AFib was weaker in this study, as compared to the previously reported[9] association of DTNPV1 with incident AFib. Such difference could be explained by differences in AFib secondary outcome data acquisition. As this study was a retrospective cohort, many incident AFib cases were likely missing.
Life-saving effect of patient education
Recent results of the OregonSUDS study showed that at least one-third of SCA victims have non-specific symptoms within hours, days and even weeks before SCA[5]. Unfortunately, SCA victims and their healthcare providers often ignore these symptoms. SCA victims who are aware that they have CVD are more likely to seek medical care after symptom onset and have significantly better survival after SCA. Therefore, diagnosing CVD and increasing awareness of the link between CVD and SCA is likely to improve SCA outcomes. Each contact a patient has with the healthcare system theoretically provides an opportunity for CVD screening/diagnosis and education. Feasibility of screening the entire healthcare system’s ECG databases has been previously demonstrated[6] and was validated in our study for DTNPV1 ECG marker. ECG is an inexpensive and widely available method to identify patients at increased risk of all-cause mortality.
Our study showed that the patients who had their index ECG referred by a cardiology team member had significantly better survival: relative risk of death was reduced by 34%, as compared to patients with index ECG referred by a non-cardiologist. This effect was independent of the effect of manifested or subclinical CVD diagnosis and treatment (Figure 4), and was within the range of estimated rate of SCD in this population (38%). Relative risk reduction was also independent of the disease substrate, reflected by P-prime in V1: effect size was similar in both DTNPV1 and ZeroPpV1 cohorts. Therefore, we speculate that life-saving effect of index ECG referral was likely due to the effect of patients’ education and increased CVD awareness. Life-saving effect of cardiac arrhythmia awareness has been recently demonstrated[18]. Risk of death nearly doubled in AFib patients who were unaware of having AFib[18]. Unfortunately, CVD awareness in the US is low[19]. The VIRGO study recently demonstrated that at best only half of acute myocardial infarction patients reported being aware of having CVD or its risk factors before their acute event hospitalization[19]. However, it is important to emphasize that this retrospective study was not specifically designed to measure the effect of patients’ education, and therefore, finding of the life-saving effect of the index ECG referral by a cardiologist must be considered hypothesis-generating, requiring further evaluation in a prospectively designed RCT.
The fact that presumed effect of patients’ education had a similar effect in both cohorts raised a question whether ECG screening and identification of individuals at increased risk of death might be useful, or, instead, education should be delivered to every individual, regardless of their risk level. This hypothesis should be tested in RCT as well. We speculate that the observed association of the life-saving effect of index ECG referral by a cardiologist (as compared to a non-cardiologist) suggests that perception of education intervention might depend on whether or not individual considers himself/herself to be at risk. Education might be ignored, unless conceivable risk of CVD (or SCD) is determined, and conveyed to the specific individual. Evaluation and communication of SCD risk to the patient could be an important part of a patient’s education. This hypothesis should be tested in an RCT.
Limitations
This was a retrospective study, and therefore, unmeasured confounders could influence results of the study. In addition, all-cause mortality (rather than cause-specific mortality) served as a primary outcome. To overcome these limitations, results of the study should be validated in another prospective study. Missing and incorrectly coded data have been previously reported in studies of the databases research quality[10, 11]. To minimize such limitations, we used the combination of the ICD-9-CM codes and clinical data[12–14] (historical patients’ medications lists, and referral of the index ECG data). High utilization of OHSU Healthcare (91% of patients were on at least one medication) confirmed good quality data of the OHSU EMR database[12–14].
Conclusion
In this large retrospective double cohort study from an entire health system ECG database, DTNPV1, an easy to visually detect ECG marker, was independently associated with twice higher risk of all-cause death, as compared to patients without negative deflection of P prime in V1. Life-saving effect of the index ECG referral by a cardiologist requires further study. RCT to test the effect of patients’ education for SCD prevention is warranted.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported in part by R01HL118277 (LGT)
This work was supported in part by the National Institutes of Health, R01HL118277 to LGT.
Footnotes
The authors report no relationships that could be construed as a conflict of interest.
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 3.Daya MR, Schmicker RH, Zive DM, Rea TD, Nichol G, Buick JE, et al. Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–15. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–52. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 5.Marijon E, Uy-Evanado A, Dumas F, Karam N, Reinier K, Teodorescu C, et al. Warning Symptoms Are Associated With Survival From Sudden Cardiac ArrestWarning Symptoms and Sudden Cardiac Arrest. Annals of Internal Medicine. 2016;164:23–9. doi: 10.7326/M14-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauss DG, Mewton N, Verrier RL, Nearing B, Killian T, Marchlinsky FE, et al. Screening Entire Health System ECG Databases to Identify Patients with Arrhythmogenic Myocardial Substrate at Increased Risk of Death. Circulation. 2011;124:2. doi: 10.1161/CIRCEP.113.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiffany Win T, Ambale Venkatesh B, Volpe GJ, Mewton N, Rizzi P, Sharma RK, et al. Associations of electrocardiographic P-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The PRIMERI Study. Heart Rhythm. 2015;12:155–62. doi: 10.1016/j.hrthm.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tereshchenko LG, Shah AJ, Li Y, Soliman EZ. Electrocardiographic deep terminal negativity of the P wave in V1 and risk of mortality: the National Health and Nutrition Examination Survey III. J Cardiovasc Electrophysiol. 2014;25:1242–8. doi: 10.1111/jce.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tereshchenko LG, Henrikson CA, Sotoodehnia N, Arking DE, Agarwal SK, Siscovick DS, et al. Electrocardiographic deep terminal negativity of the P wave in V(1) and risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2014;3:e001387. doi: 10.1161/JAHA.114.001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hsia DC, Krushat WM, Fagan AB, Tebbutt JA, Kusserow RP. Accuracy of diagnostic coding for Medicare patients under the prospective-payment system. N Engl J Med. 1988;318:352–5. doi: 10.1056/NEJM198802113180604. [DOI] [PubMed] [Google Scholar]
- 12.Pine M, Jordan HS, Elixhauser A, Fry DE, Hoaglin DC, Jones B, et al. Enhancement of claims data to improve risk adjustment of hospital mortality. JAMA. 2007;297:71–6. doi: 10.1001/jama.297.1.71. [DOI] [PubMed] [Google Scholar]
- 13.Pine M, Jordan HS, Elixhauser A, Fry DE, Hoaglin DC, Jones B, et al. Modifying ICD-9-CM coding of secondary diagnoses to improve risk-adjustment of inpatient mortality rates. Med Decis Making. 2009;29:69–81. doi: 10.1177/0272989X08323297. [DOI] [PubMed] [Google Scholar]
- 14.Pine M, Sonneborn M, Schindler J, Stanek M, Maeda JL, Hanlon C. Harnessing the power of enhanced data for healthcare quality improvement: lessons from a Minnesota Hospital Association Pilot Project. J Healthc Manag. 2012;57:406–18. discussion 19–20. [PubMed] [Google Scholar]
- 15.MORRIS JJ, Jr, Estes EH, Jr, WHALEN RE, THOMPSON HK, Jr, McINTOSH HD. P-WAVE ANALYSIS IN VALVULAR HEART DISEASE. Circulation. 1964;29:242–52. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 16.de JS, van Veen TA, van Rijen HV, de Bakker JM. Fibrosis and cardiac arrhythmias. J CardiovascPharmacol. 2011;57:630–8. doi: 10.1097/FJC.0b013e318207a35f. [DOI] [PubMed] [Google Scholar]
- 17.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, et al. Association Between Extracellular Matrix Expansion Quantified by Cardiovascular Magnetic Resonance and Short-Term Mortality. Circulation. 2012;126:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neal WT, Efird JT, Judd SE, McClure LA, Howard VJ, Howard G, et al. Impact of Awareness and Patterns of Nonhospitalized Atrial Fibrillation on the Risk of Mortality: The Reasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Clin Cardiol. 2016;39:103–10. doi: 10.1002/clc.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leifheit-Limson EC, D'Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, et al. Sex Differences in Cardiac Risk Factors, Perceived Risk, and Health Care Provider Discussion of Risk and Risk Modification Among Young Patients With Acute Myocardial Infarction: The VIRGO Study. J Am Coll Cardiol. 2015;66:1949–57. doi: 10.1016/j.jacc.2015.08.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.