Abstract
Cocaine use disorder (CUD) remains a significant public health challenge. Levo-tetrahydropalmatine (L-THP), a well-tolerated and non-addictive compound, shows promise for the management of CUD. Its pharmacologic profile includes blockade at dopamine and other monoamine receptors and attenuation of cocaine self-administration, reinstatement, and rewarding properties in rats. This study evaluated the safety of L-THP in human cocaine users and its influence on the safety and pharmacokinetics (PK) of cocaine. Twenty-four cocaine-using adult men were randomized to receive L-THP (30 mg BID orally) or placebo double-blind for 4 days, with an intranasal cocaine (40 mg) challenge on the fourth day. Safety and tolerability were evaluated using vital signs, EKG, clinical laboratory tests, and standardized self-report instruments. Peripheral venous blood was collected periodically and later assayed for L-THP and cocaine using highly sensitive and specific ultra-performance liquid chromatography-fluorescence detection (UPLC-FLD) methods. Twenty subjects completed the study, of whom 19 provided complete PK data. The short 3.5-day course of L-THP was safe and well tolerated and did not affect cocaine’s PK or its acute cardiovascular effects. The cocaine AUC0→∞ was 211.5 and 261.4 h*ng/ml, and the Cmax was 83.3 and 104.5 ng/ml for the L-THP and placebo groups respectively. In addition there were no significant difference in the number of side effects reported in each group (L-THP group: 22 [48%], Placebo group: 24 [52%]), or vital signs including, heart rate, blood pressure, complete blood count or EKG. These findings suggest that oral THP has promise for further development as a treatment for CUD.
Keywords: Addiction Medicine, Central Nervous System, Clinical Pharmacology, Clinical Trials, Drug-Drug Interactions, Pharmacokinetics and drug metabolism
Introduction
Cocaine use and addiction (cocaine use disorder [CUD] in DSM-5 terms)1 remain significant public health problems in many regions of the world. Cocaine is used annually by an estimated 17 million people world-wide, 0.4% of the global population 15–64 years old.2 In the US, there were an estimated 1.5 million cocaine users in 2013. An estimated 550,000 Americans had CUD in 2013; less than 600,000 received specialty treatment for a cocaine use problem.3 Cocaine use was mentioned in an estimated one-half million emergency department visits in 2011, about one-fifth of all drug/medication-related visits.4 Cocaine-related problems are likely to continue in the future, as an estimated 1,300 people initiate cocaine use in the US each day (500,000 in 2013).3 Despite this compelling need for effective treatment modalities for CUD and decades of intensive research, there is still no well proven, broadly effective treatment, and no medication is approved for the treatment of CUD by the US FDA or any national regulatory authority.5–9
There is one compound that shows particular promise as a new treatment for CUD. Levo-tetrahydropalmatine (L-THP), an alkaloid isolated from the Chinese plant Corydalis yanhusuo. Corydalis has historically been used in Chinese medicine for its analgesic and hypnotic properties, which have been attributed to L-THP.10 L-THP is a D1, D2, and D3 receptor antagonist in vitro,11,12 an antagonist at α1 adrenergic receptors and several 5-HT receptors,13 and an enhancer of GABAergic transmission.14
The unique pharmacologic profile of L-THP provides rationale for its potential to treat CUD. In particular, dopamine D3 receptor antagonists show promise in rodent models of cocaine addiction, i.e., they reduce cocaine self-administration, relapse to previously extinguished cocaine self-administration, and rewarding effects of cocaine.15–17 However, to date, no D3 antagonist compound has proceeded through human trials because of pharmacokinetic or toxicity problems.16 In addition, the anxiolytic properties of L-THP may alleviate drug withdrawal symptoms, making it easier for users to abstain. An in-depth review detailing the rationale for investigating L-THP for treating CUD has been previously published.18
Several animal studies support the therapeutic potential of L-THP. L-THP readily penetrates the blood brain barrier (BBB),19 reduces self-administration of cocaine, and prevents drug or stress-induced reinstatement of previously extinguished cocaine self-administration (an animal model of relapse) in rats.12,20–23
A Chinese phase I clinical trial involving L-THP (60 mg orally) in 12 healthy men found a mean Tmax of 1.25 ± 0.59 h, Cmax 0.19 ± 0.036 μg/mL, and elimination half-life 11.42 ± 2.43 h.24 A Chinese double-blind phase II clinical trial found that L-THP (30 mg bid for 4 weeks) in inpatient heroin users reduced craving and relapse to heroin use after discharge from the hospital.25
No study of L-THP has been performed in cocaine users. The goal of this phase I clinical trial was to evaluate the safety, tolerability and pharmacokinetics (PK) of oral L-THP in chronic cocaine users in the presence and absence of cocaine, with the aim of developing L-THP for phase II clinical trials to further evaluate its efficacy for the treatment of CUD.
Methods
Enrollment
The study was approved by the investigational review board (IRB) at the University of Maryland, Baltimore (UMB) and was registered at ClinicalTrials.gov (NCT01631383). All subjects gave written informed consent (when not acutely intoxicated) prior to starting study procedures. Participants were healthy, cocaine-using adults recruited from the community by media advertising and screened initially by telephone, then in person with a comprehensive psychological and medical evaluation. Inclusion criteria included men or non-pregnant/non-breastfeeding women age 18–50 years old with current CUD, self-reported cocaine use averaging at least weekly for the past 6 months, positive urine test for cocaine in the last month, HIV seronegative, EKG without clinically significant abnormality, normal blood pressure (90–140/50–90 mmHg), normal resting heart rate (60–90 bpm), ability to adhere to study restrictions and examination schedule, and contraception use during and for 2 weeks after the study by women with reproductive potential. Exclusion criteria included participation in any drug trial within 45 days prior to study entry, history of clinically significant adverse reaction or hypersensitivity to L-THP or cocaine, inability to communicate or cooperate with investigators, currently taking any prescribed psychoactive medication, current clinically significant medical problem that might interfere with safe study participation, current major depressive disorder, current schizophrenia, current bipolar disorder, or a score below 10/12 on the Evaluation to Sign Consent (ESC) questionnaire.26
Study Design
This study followed a randomized, double-blind, placebo-controlled design. Subjects participated while residing for 5 days/4 nights on a secure, medically monitored Brief Stay Unit (BSU) designed for phase I studies. Subjects were randomized to receive either L-THP (30 mg capsules Q12 h) or matching placebo for 7 doses (study Days 1–4), starting the morning of the day of admission (study Day 1). Ninety minutes following the final dose (morning of Day 4) subjects received a single 40 mg intranasal dose of cocaine. The L-THP dosing duration was chosen to have participants reach steady-state L-THP plasma concentration approximately 24 hours prior to cocaine challenge, based on an L-THP half-life of 11 hours.24 Subjects resided on the BSU from the morning of Day 1 until the evening of Day 5, then returned for a final blood collection and safety evaluation on Day 6 (Table 1).
Table 1.
Schedule for L-THP/placebo oral dosing and Pharmacokinetic Peripheral Venous Blood Sampling
| Day | Time | Event |
|---|---|---|
| Day 1 | 07:25 | Baseline blood collection |
| 07:30 | 1st L-THP/placebo dose | |
| 08:00 | Blood | |
| 08:30 | Blood | |
| 09:00 | Blood | |
| 09:30 | Blood | |
| 10:00 | Blood | |
| 10:30 | Blood | |
| 11:30 | Blood | |
| 12:30 | Blood | |
| 13:30 | Blood | |
| 15:30 | Blood | |
| 19:25 | Blood | |
| 19:30 | 2nd L-THP/placebo dose | |
| 02:30 | Blood | |
| 05:30 | Blood | |
| Day 2 | 07:25 | Blood |
| 07:30 | 3rd L-THP/placebo dose | |
| 19:25 | Blood | |
| 19:30 | 4th L-THP/placebo dose | |
| Day3 | 07:25 | Blood |
| 07:30 | 5th L-THP/placebo dose | |
| 19:25 | Blood | |
| 19:30 | 6th L-THP/placebo dose | |
| Day 4 Cocaine Challenge Day |
07:00 | Baseline blood collection |
| 07:30 | 7th L-THP/placebo dose | |
| 9:00 | Blood | |
| 9:10 | Blood | |
| 9:20 | Blood | |
| 9:30 | Blood | |
| 9:40 | Blood | |
| 9:50 | Blood | |
| 10:00 | Blood | |
| 10:10 | Blood | |
| 10:20 | Blood | |
| 10:30 | Blood | |
| 10:40 | Blood | |
| 10:50 | Blood | |
| 11:00 | Blood | |
| 11:30 | Blood | |
| 12:00 | Blood | |
| 13:05 | Blood | |
| 14:00 | Blood | |
| 15:00 | Blood | |
| 16:00 | Blood | |
| 19:00 | Blood | |
| Day 5 | 07:00 | Blood |
| 13:30 | Blood | |
| Day 6 | 14:30 | Final blood |
Study medications
Bulk L-THP was purchased from Wuxi Gorunjie Technology Co., Ltd. (No. 97, Wuqiao West Road, Wuxi, Jiangsu, 214044 CN) and L-THP and matching placebo capsules prepared at the UMSOP in compliance with Chemistry, Manufacturing and Controls (CMC) standards.
Cocaine hydrochloride USP was obtained through the National Institute on Drug Abuse (NIDA) Drug Supply Program from Covidien, meeting the guidelines for CMC standards. For the cocaine challenge at 9 am on Day 4, 40 mg of cocaine powder was weighed and placed on a mirror in front of the seated subject. The subject divided the powder into 2 approximately equal lines using a spatula, then inhaled each line into a different nostril using a 65mm plastic straw. Subjects had up to 5 min to ingest each line (all took < 1 minute), and waited 4–6 minutes between lines to allow for checking of vital signs and 12-lead ECG with 3-minute rhythm strip.
Randomization and Blinding
The study biostatistician used the permuted block method of randomization to generate multiple sequences (blocks) of size four, with two participants each assigned to l-THP or placebo. When a new participant met study eligibility criteria and gave informed consent, the study coordinator requested a randomization from the study biostatistician, who responded by sending a code number to the unblinded study pharmacist identifying the next randomization in the sequence. The pharmacist prepared the appropriate dose of L-THP or placebo, and provided this to the study coordinator, in a package labeled “L-THP or Placebo, 30 mg capsules Q12 h.” Only the study biostatistician and the pharmacist were aware of the actual contents of the package: the participant and all other study personnel remained blinded.
L-THP and cocaine assays
Blood samples (5 mL) were obtained via an indwelling peripheral venous catheter before each L-THP dose, 2 hours before cocaine administration (Day 4), and then every 10 minutes for 2 hours, every 30 minutes for 2 hours, every hour for 3 hours, and then at 10, 22, 28.5, and 53.5 hours after cocaine administration. Table 1 shows blood sampling details. Samples were collected into Vacutainer® tubes (BD Diagnostics, Franklin Lakes, NJ) containing EDTA and NaF, centrifuged at 3000 rpm × 15 minutes, and the separated plasma transferred immediately into plastic Eppendorf™ tubes (Fisher scientific, Hampton, NH) for storage at −80°C until later assay within five days. Plasma concentrations of cocaine and L-THP were measured by the University of Maryland School of Pharmacy (UMSOP) Pharmacokinetics and Biopharmaceutics Laboratory (PBL) using a previously validated ultra-performance liquid chromatography-fluorescence detection (UPLC-FL) analysis method developed by our group.27 Assay limit of quantification (LOQ) was 2.5 ng/mL for both L-THP and cocaine.
Non-compartmental Analysis of L-THP and cocaine
PK parameters were initially determined using a model-independent approach, non-compartmental analysis (NCA), with estimation of AUCs by linear trapezoidal method using Phoenix WinNonlin 6.4 (Pharsight Corporation, Cary, NC) that was validated using Phoenix Validation Suite 2.2.7.0 (Pharsight Corporation, Cary, NC). PK analysis was performed using the actual times that individual blood samples were obtained and their actual measured analyte concentrations. PK parameters (geometric means) estimated included maximum observed plasma concentration (Cmax), time of Cmax (Tmax), terminal half-life (t1/2), area under the plasma concentration-time curve from time 0 up to the time of the last quantifiable sample estimated (AUC0–last), area under the plasma concentration time curve from time 0 up to infinity (AUC0–∞), and clearance following oral or intranasal administration (CL/F), where F is defined as bioavailability. Statistical comparisons of cocaine PK parameters with and without exposure to L-THP were conducted by two-sample t-tests, assuming unequal variance, with GraphPad Prism version 5.04 (GraphPad Software, Inc., La Jolla, CA). Statistical significance was set at two-tailed alpha = 0.05.
Safety and Tolerability Assessments
We measured a variety of safety outcomes to determine short-term tolerability of L-THP, using the following scales: Barnes Akathisia Scale (BAS) to assess objective and subjective components of akathisia;28 the Simpson-Angus Scale (SAS) to assess extrapyramidal symptoms;29 Epworth Sleepiness Scale (ESS)30 to assess sedation; Symptom Checklist–90 item Revised (SCL-90R)31 to assess psychological symptoms, analyzed in terms of Global Severity Index (GSI) and 4 subscales: Depression, Anxiety, Paranoid Ideation and Psychoticism; Side Effect Checklist (SEC), a 25-item, self-report scale assessing side effects associated with L-THP and non-specific side effects. Each item was rated on a 4-point Likert scale: “none”=1, “mild”=2, “moderate”=3 and “severe”=4.
Vital signs (systolic [SBP] and diastolic [DBP] blood pressure, heart rate [HR], respiratory rate) and 12-lead EKG were assessed by standard clinical technique on admission to the BSU (Day 1) prior to administration of any study medication (baseline for L-THP safety and tolerability analyses). EKG was performed again 3 and 12 hours after the initial L-THP dose on Day 1. Pulse and blood pressure were measured periodically during waking hours and at night for the duration of the residential stay.
Clinical laboratory tests (complete blood count [CBC], thyroid stimulating hormone [TSH], and blood chemistry panel were performed on admission to the BSU and then each morning (fasting) for the next three days (Days 2–4).
On the morning of Day 4, prior to last dose of L-THP and initiation of cocaine challenge, vital signs and EKG were assessed to determine baseline values for cocaine safety. Ninety minutes after L-THP or placebo administration, the participant self-administered cocaine by insufflation in the presence of an Advanced Cardiac Life Support (ACLS)-certified physician and nurse. Heart rate (EKG chest lead) was continuously monitored from approximately 5 minutes before to 2 hours after cocaine dosing or until values returned to within 20% of baseline values, whichever was later. Blood pressure and heart rate were monitored approximately every 15 minutes from 5 minutes prior to cocaine dosing until 2 hours after dosing, then every 30 minutes for 3 hours, and then every 2 hours for 6 hours. 12-lead EKGs were completed immediately following cocaine administration, then at 1, 2, 4 and 10 hours post administration. Clinical laboratory tests were performed at follow up (Day 6).
Sample size and power
For this Phase I study, sample size was determined by the need for adequate power to detect a potential drug-drug interaction between cocaine and L-THP which would dangerously elevate the acute cardiovascular (heart rate and/or blood pressure) response to cocaine administration, which was determined in consultation with the FDA to be the most serious safety question posed by the use of L-THP in cocaine-using participants. Had all 24 randomized participants completed the study, there would have been power=0.80 to detect a 0.2 standard deviation increase in peak change in HR, SBP or DBP (each tested at two-sided alpha=0.05); with only 20 participants completing the exposure to cocaine plus l-THP or placebo, there was power=0.80 to detect an 0.32 standard deviation increase in these measures.
Data Analysis
Baseline Characteristics and Safety/Tolerability Data
Baseline participant characteristics (Table 2) in the two medication groups were compared with Wilcoxon test for categorical variables and t-test for quantitative variables. The primary between-group comparison for cardiovascular, laboratory tests, SCL-90R, and ESS variables was the peak change from baseline for each subject, adjusted for baseline value using ANCOVA. Baseline was defined as the morning of admission (Day 1) prior to the first dose of L-THP or placebo. The endpoint for safety and tolerability data was the evening of Day 3 for completers or the last measurement before withdrawal. Between-group comparisons for movement assessments and side effects were based on the proportion of participants in each group showing an increase in severity from baseline, using Fishers exact test. For the BAS, an increase was considered at least a 2-point increase from a 0 baseline or at least a 1-point increase from a higher baseline. For the SAS, an increase was considered at least a 2-point increase in total score. For the Day 4 safety data relating to cocaine administration, we used the morning values prior to L-THP administration as the baseline, calculated maximal change from baseline, and compared the peak change between medication groups using Student’s t test.
Table 2.
Baseline demographic characteristics and substance use histories of study participants
| L-THP (N=11) | Placebo (N=12) | |
|---|---|---|
|
| ||
| Age (years) | 42.7 ± 7.3 | 45 ± 4.1 |
|
| ||
| Race (%): | ||
| AA: | 83.3 | 75.0 |
| White: | 8.3 | 25.0 |
| Mixed: | 8.3 | 0 |
|
| ||
| Weight (kg): | ||
| Day 1: | 78.6 ± 10.2 | 78.8 ± 11.7 |
| Day 4: | 79.2 ± 10.7 | 79.5 ± 11.8 |
|
| ||
| BMI (kg/m2): | ||
| Day 1: | 24.1 ± 3.2 | 25.1 ± 2.3 |
| Day 4: | 24.3 ± 3.7 | 25.3 ± 2.2 |
|
| ||
| *Frequency of cocaine use (%): | ||
| <Once a week | 0 | 8.3 |
| Once a week | 41.7 | 33.3 |
| >Once a week | 58.3 | 58.3 |
|
| ||
| *Amount of cocaine typically used weekly (%): | ||
| <$150/week | 36.4 | 41.7 |
| $150–$300/week | 36.4 | 50.0 |
| >$300/week | 27.2 | 8.3 |
|
| ||
| **Amount spent on cocaine use per week ($) | 431.3 ± 281.5 | 356.7 ± 267.9 |
|
| ||
| Current Cigarette Smoking (%) | 91.0 | 83.3 |
|
| ||
| *Frequency of Alcohol Use (%) | ||
| Not at all | 27.3 | 0 |
| <Once a week | 45.4 | 75.0 |
| Once a week | 27.3 | 12.5 |
| >Once a week | 0 | 12.5 |
|
| ||
| *Amount of alcohol use (%): | ||
| Never used | 27.3 | 8.3 |
| <10 drinking occasions/month | 45.5 | 75.0 |
| >10 drinking occasions/month | 27.3 | 16.7 |
|
| ||
| *Frequency of Marijuana Use (%) | ||
| Never used | 45.5 | 66.7 |
| <Once a week | 27.3 | 25.0 |
| Once a week | 18.2 | 8.3 |
| >Once a week | 9.0 | 0 |
|
| ||
| *Amount of Marijuana Used (%) | ||
| Never used | 45.5 | 66.7 |
| <Once a week | 27.3 | 25.0 |
| Once a week | 18.2 | 8.3 |
| >Once a week | 9.0 | 0 |
|
| ||
| *Frequency of Opiate Use (%) | ||
| Never used | 66.7 | 83.3 |
| Once or twice | 33.3 | 16.7 |
|
| ||
| *Amount of Opiate Use (%) | ||
| Never used | 81.8 | 66.7 |
| <$30/week | 18.2 | 33.3 |
Data presented as mean ± SD or %
AA = African American
In the 3 months prior to screening
In the month prior to screening
One participant reported using hallucinogens once in the 3 months prior to screening.
No significant difference between medication groups for any variable (Wilcoxon test for categorical variables, t-test for continuous variables)
All statistical comparisons used a two-tailed alpha = 0.05 and were conducted with SAS version 9.3 (SAS Institute, Cary, NC).
Results
Subjects
A total of 151 candidates were screened by telephone, 55 of whom were preliminarily qualified and screened in person (Figure 1). Of the 55 candidates screened, 27 failed to meet the eligibility criteria and 5 were excluded for other reasons. A total of 23 unique individuals were enrolled in the study, between January and December 2013. One subject, Subject L (L-THP group), was removed from the study on Day 3, but later reenrolled as Subject D (L-THP group). The two study periods for this individual were treated as two separate subjects for purposes of analyzing l-THP safety and PK data, bringing the total number of subjects randomized to study medication to 24 (Figure 1). Subjects’ baseline demographic and substance use histories are presented in Table 2.
Figure 1.
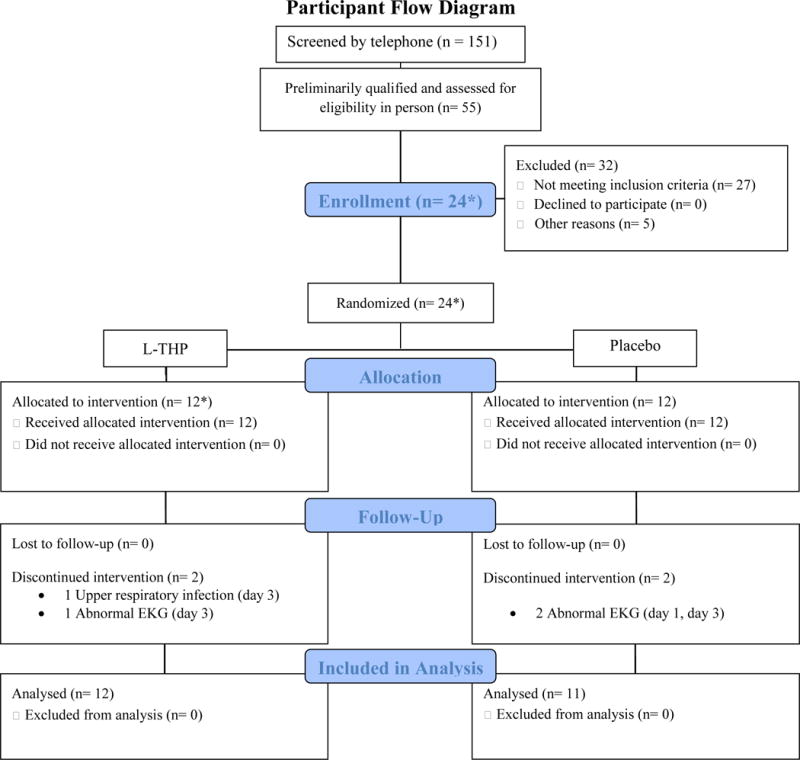
Flowchart of subjects included in PK analysis.
* 1 participant initially enrolled in l-THP group was withdrawn on study day 3 due to an upper respiratory infection and was later reenrolled, randomly reassigned to the l-THP group, and completed the study. This participant has been counted twice, resulting in n = 24 randomized and allocated to treatment.
Of the 24 subjects enrolled, 20 completed the study per protocol and four were withdrawn prior to cocaine administration. One subject, X (placebo group), was withdrawn on Day 1 (EKG changes) and three subjects were withdrawn on Day 3 (prior to receiving cocaine): E (EKG changes) and L (upper respiratory infection), both L-THP group, and M (EKG changes), placebo group. All withdrawals were deemed unrelated to the study drug.
Of the 20 subjects who completed the protocol, samples from 19 subjects (9 subjects in the L-THP group; 10 subjects in the Placebo group) were available for complete PK analysis. Samples from Subject B (L-THP group) were available on Days 1–3; samples on Days 4–6 could not be analyzed due to coagulation. There were no significant differences between medication groups in participant baseline characteristics (Table 2) or in the number of participants not completing the study. Figure 1 shows a flow chart of subject enrollment and completion.
Non-compartmental PK analysis
L-THP
Plasma concentrations of L-THP rose rapidly after the first dose, with a geometric mean Cmax of 42.8 ng/mL and median Tmax of 1.5 h (Table 3). Data from three subjects (B, E, and L) were included in calculating the geometric mean of Cmax and the median Tmax but were excluded from other PK analyses due to missing data after Day 3: blood coagulated (B) or no samples collected due to withdrawal from the study (E and L). The accumulation ratio of L-THP was calculated to be 1.2 using AUC0–12.
Table 3.
Plasma Pharmacokinetic Parameters of L-THP and Cocaine
| Parametera | L-THP n=9 |
Cocaine | |||
|---|---|---|---|---|---|
| Total n=19 |
L-THP n=9 |
Placebo n=10 |
p-value (L-THP vs Placebo) |
||
| Cmax (ng/mL) | 42.8 (62.8)c | 93.8 (47.2) | 83.3 (56.3) | 104.5 (37.3) | 0.46 |
| Tmax (h)b | 1.5c | 0.8 | 0.8 | 0.8 | NA |
| t½ (h) | 13.3 (84.9) | 1.7 (49.4) | 1.5 (64.0) | 1.8 (33.8) | 0.74 |
| CL/F (L/h) | 75.7 (115.2) | 169 (38.8) | 189 (50.7) | 153 (23.4) | 0.14 |
| AUC0→∞ (h*ng/mL) | 396.1 (115.2) | 236.4 (38.8) | 211.5 (50.7) | 261.4 (23.4) | 0.43 |
| AUC0→last (h*ng/mL) | 310.0 (113.8) | 224.6 (40.3) | 200.7 (53.3) | 248.5 (23.8) | 0.47 |
Data are presented as geometric mean (geometric CV%) with the exception of Tmax
Tmax is presented as median
Three subjects who discontinued on Day 3 were included in this parameter estimation, resulting in n=12
NA: Not available
Cocaine
There were no significant differences in the PK parameters of cocaine (40 mg intranasal) administered after 3.5 days of L-THP (L-THP group) or of placebo (placebo group) (Table 3). The mean plasma cocaine concentration versus time plots were similar for both groups (Figure 2).
Figure 2.
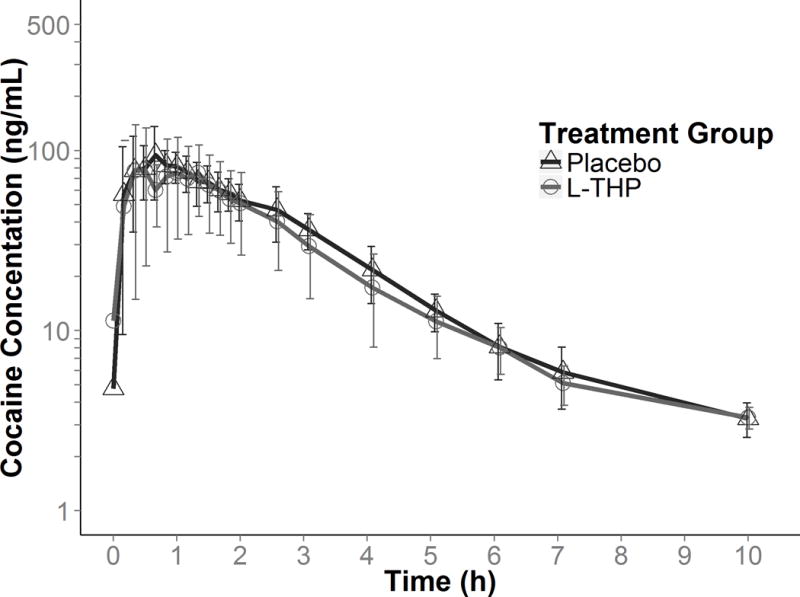
Mean (error bars presented as standard deviation) plasma cocaine concentrations by study group (placebo; dark grey triangles, L-THP; light grey circles).
No subject had measurable plasma cocaine concentration by Day 5; therefore, only data from Day 4 were included for cocaine PK analysis. At follow-up on Day 6, Subjects H and N had unexpectedly high plasma cocaine concentrations, assumed to be the result of cocaine self-administration after discharge on Day 5 (excluded from PK analysis). Cocaine was detected in the plasma of four subjects on Day 4 prior to cocaine administration: 3 subjects (D, G. V) had very low concentrations (<6 ng/mL); one subject (J) had a concentration of 26.2 ng/mL, which was verified by re-assay. Plasma cocaine present at baseline was assumed due to cocaine self-administered in the community prior to admission. A re-analysis excluding all four subjects with cocaine present before cocaine administration on Day 4 also indicated no significant differences in cocaine PK parameters between the L-THP and placebo groups.
Safety of L-THP (Days 1–3)
Seventeen (71%) participants reported a total of 46 side effects after exposure to study medication: 9 on L-THP (22 [48%] side effects) and 8 on placebo (24 [52%] side effects) (Table S1). All side-effects were non-serious and resolved completely without sequelae before participants left the study.
Sleepiness and Psychological Symptoms
There were no significant differences between L-THP and placebo groups in the mean (±SD) maximum change from baseline of the Epworth Sleepiness Scale (ESS) scores or the Symptom Checklist-90-R Global Severity Index or the anxiety, depression, paranoia, and psychoticism subscales (Table 4).
Table 4.
Symptom Checklist 90R and Epworth Sleepiness Scale Scores in Adult Male Cocaine Users
| L-THP—30 mg bid (N=12*) | Placebo (N=11**) | p-value | |
|---|---|---|---|
|
| |||
| Global Severity Index | |||
| Baseline | 46.6 ± 11.6 | 42.9 ± 15.0 | |
| Maximum change from baseline | −11.0 ± 7.8 | −8.8 ± 10.3 | p= 0.25 |
|
| |||
| Anxiety subscale | |||
| Baseline | 43.8 ± 9.4 | 43.0 ± 10.8 | |
| Maximum change from baseline | −4.6 ± 9.7 | −7.2 ± 8.3 | p= 0.63 |
|
| |||
| Depression subscale | |||
| Baseline | 48.8 ± 11.0 | 47.6 ± 15.9 | |
| Maximum change from baseline | −11.6 ± 9.8 | −10.4 ± 9.1 | p= 0.93 |
|
| |||
| Paranoia subscale | |||
| Baseline | 49.7 ± 9.3 | 46.7 ± 11.2 | |
| Maximum change from baseline | −10.3 ± 7.7 | −8.7 ± 9.9 | p= 0.57 |
|
| |||
| Psychoticism subscale | |||
| Baseline | 48.3 ± 10.3 | 47.6 ± 12.8 | |
| Maximum change from baseline | −7.7 ± 7.5 | −4.8 ± 10.0 | p= 0.46 |
|
| |||
| Epworth Sleepiness Scale | |||
| Baseline | 6.3 ± 3.8 | 8.4 ± 4.5 | |
| Maximum change from baseline | 0.9 ± 5.8 | −2.9 ± 2.6 | p = 0.07 |
Data presented as mean ± SD
includes one participant who contributed two sets of data—once as non-completer who withdrew on day 3, once as completer
Subject X was not included in the analysis as this subject discontinued on Day 1
Group differences at baseline and endpoint evaluated by Wilcoxon t test with 1 df.
Endpoint = value at evening of Day 3 or last measurement before withdrawal
Vital Signs and Laboratory Values
There were no significant differences between L-THP and placebo groups in mean maximum change from baseline for heart rate, systolic and diastolic blood pressure, individual tests of complete blood count, clinical chemistry panel, or EKG QTcB interval (Table 5). All values for each variable were within the expected “normal” range.
Table 5.
Vital Signs and Clinical Laboratory Test Values in 23 Adult Cocaine-Using Men at baseline and following 3.5 days of L-THP (30 mg po BID)or placebo treatment
| L-THP (N=12*) | Placebo (N=11**) | p-value | |
|---|---|---|---|
|
| |||
| Systolic Blood Pressure (mmHg) | |||
| Baseline | 127.8 ± 14.3 | 120.1 ± 15.4 | |
| Maximum change from baseline | −6.8 ± 26.3 | 4.9 ± 29.5 | 0.36 |
|
| |||
| Diastolic Blood Pressure (mmHg) | |||
| Baseline | 76.7 ± 8.3 | 78.6 ± 8.6 | |
| Maximum change from baseline | −5.0 ± 17.1 | −2.7 ± 18.9 | 0.88 |
|
| |||
| Pulse (bpm) | |||
| Baseline | 72.3 ± 10.2 | 73.0 ± 13.5 | |
| Maximum change from baseline | 2.6 ± 21.9 | 4.7 ± 23.5 | 0.95 |
|
| |||
| QTcB interval (msec) | |||
| Baseline | 413.1 ± 24.6 | 415.7 ± 25.5 | |
| Maximum change from baseline | 6.3 ± 33.6 | 1.1 ± 27.1 | 0.58 |
|
| |||
| Red Blood Cells (M/mm3) | |||
| Baseline | 4.6 ± 0.3 | 4.7 ± 0.3 | |
| Maximum change from baseline | −0.0 ± 0.4 | −0.0 ± 0.3 | 1.00 |
|
| |||
| White Blood Cells (K/mm3) | |||
| Baseline | 6.4 ± 2.1 | 7.6 ± 2.8 | |
| Maximum change from baseline | 0.6 ± 1.9 | 0.1 ± 2.8 | 1.00 |
|
| |||
| Platelets (K/mm3) | |||
| Baseline | 287.3 ± 63.2 | 263.5 ± 44.7 | |
| Maximum change from baseline | −6.4 ± 50.0 | −3.6 ± 23.8 | 0.85 |
|
| |||
| Hemoglobin (g/dL) | |||
| Baseline | 13.0 ± 1.5 | 13.8 ± 1.5 | |
| Maximum change from baseline | 0.0 ± 1.0 | −0.1 ± 1.0 | 0.98 |
|
| |||
| Hematocrit (%) | |||
| Baseline | 39.0 ± 3.4 | 41.0 ± 3.2 | |
| Maximum change from baseline | 0.9 ± 3.1 | 0.5 ± 2.8 | 0.85 |
|
| |||
| Total Cholesterol (mg/dL) | |||
| Baseline | 153.2 ± 37.5 | 174.6 ± 33.4 | |
| Maximum change from baseline | 1.9 ± 24.0 | 2.7 ± 20.6 | 0.88 |
|
| |||
| High Density Lipoprotein (HDL) (mg/dL) | |||
| Baseline | 60.7 ± 16.3 | 55.2 ± 15.2 | |
| Maximum change from baseline | −4.7 ± 9.1 | −1.5 ± 11.1 | 0.69 |
|
| |||
| Low Density Lipoprotein (LDL) (mg/dL) | |||
| Baseline | 76.1 ± 38.2 | 97.8 ± 33.4 | |
| Maximum change from baseline | 2.0 ± 19.8 | −8.6 ± 22.5 | 0.30 |
|
| |||
| Triglycerides (mg/dL) | |||
| Baseline | 81.3 ± 36.6 | 108.3 ± 42.0 | |
| Maximum change from baseline | 31.6 ± 52.0 | 53.0 ± 87.3 | 0.52 |
|
| |||
| Fasting Blood Glucose (mg/dL) | |||
| Baseline | 91.2 ± 7.1 | 97.6 ± 10.8 | |
| Maximum change from baseline | 15.8 ± 16.4 | 5.1 ± 28.3 | 0.25 |
|
| |||
| Thyroid Stimulating Hormone (uIU/ml) (TSH) | |||
| Baseline | 1.7 ± 1.3 | 1.7 ± 0.9 | |
| Maximum change from baseline | −0.0 ± 1.0 | −0.4 ± 1.1 | 0.50 |
|
| |||
| Albumin (g/dL) | |||
| Baseline | 4.1 ± 0.2 | 4.1 ± 0.2 | |
| Maximum change from baseline | −0.1 ± 0.4 | −0.2 ± 0.5 | 0.46 |
|
| |||
| ALT (U/L) | |||
| Baseline | 19.2 ± 7.1 | 18.8 ± 5.9 | |
| Maximum change from baseline | 5.5 ± 10.2 | 3.6 ± 4.6 | 0.69 |
|
| |||
| AST (U/L) | |||
| Baseline | 21.4 ± 9.1 | 19.3 ± 4.0 | |
| Maximum change from baseline | 1.6 ± 15.4 | −0.3 ± 4.6 | 0.60 |
|
| |||
| Bilirubin (mg/dL) | |||
| Baseline | 0.4 ± 0.2 | 0.3 ± 0.1 | |
| Maximum change from baseline | −0.2 ± 0.3 | 0.1 ± 0.1 | 0.64 |
|
| |||
| Blood Urea Nitrogen (BUN) (mg/dL) | |||
| Baseline | 13.3 ± 3.6 | 13.5 ± 3.8 | |
| Maximum change from baseline | 14.1 ± 3.4 | 15.2 ± 2.9 | 0.90 |
|
| |||
| Creatinine (mg/dL) | |||
| Baseline | 1.0 ± 0.2 | 1.1 ± 0.2 | |
| Maximum change from baseline | 1.3 ± 3.7 | 1.6 ± 4.6 | 0.90 |
|
| |||
| Calcium (mg/dL) | |||
| Baseline | 9.2 ± 0.4 | 9.2 ± 0.4 | |
| Maximum change from baseline | 0.1 ± 0.6 | −0.2 ± 0.7 | 0.37 |
|
| |||
| Chloride (mEq/L) | |||
| Baseline | 103.7 ± 2.4 | 102.8 ± 3.3 | |
| Maximum change from baseline | 2.4 ± 2.4 | 2.7 ± 3.3 | 0.52 |
|
| |||
| Potassium (mEq/L) | |||
| Baseline | 4.1 ± 0.4 | 4.0 ± 0.4 | |
| Maximum change from baseline | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.71 |
|
| |||
| Sodium (mEq/L) | |||
| Baseline | 139.4 ± 2.6 | 138.8 ± 1.5 | |
| Maximum change from baseline | 0.3 ± 3.6 | 0.5 ± 3.2 | 0.85 |
includes one participant who contributed two sets of data; once as a non-completer withdrawn on Day 3 and once as a completer
Subject X was not included in the analysis as this subject discontinued on Day 1
QTcB = QTc interval with Bazett’s correction
Safety of Cocaine Challenge (Day 4)
All 20 subjects completed the cocaine challenge; no participant had any clinically significant changes in vital signs or ECG parameters after ingestion of the first 20 mg or the cumulative 40 mg dose. There were no significant differences in change from baseline after cocaine challenge of these safety parameters between the l-THP and placebo groups (Table S2). No side effects were attributed to the cocaine challenge.
Discussion
Our goal was to characterize the safety/tolerability and PK profile of oral L-THP and intranasal cocaine and their interaction in otherwise healthy cocaine-using adult men. To the best of our knowledge, this is the first study to characterize the safety and PK profile of L-THP in cocaine users and to assess the L-THP-cocaine interaction.
Exposure to L-THP for 3.5 days had no significant effect on the PK profile of or acute cardiovascular response to a 40 mg intranasal cocaine challenge (Table 3, Figure 2), indicating that short-term L-THP administration does not significantly alter the PK or acute cardiovascular effects of cocaine. The PK profile of oral L-THP in our study sample of largely African-American adult cocaine-using men was similar to that previously reported in Chinese adults, either healthy24 or with opiate abuse,25 suggesting that a similar PK profile would most probably be found in the heterogeneous US population. As our cocaine-using subjects had normal liver function and normal levels of serum proteins, it is unlikely that variations in L-THP binding contributed to the observed inter-subject variability in L-THP PK parameters. Comparable pharmacokinetic parameters for L-THP were observed for clearance (75.7 and 59.9 L/h), terminal half-life (13.3 and 11.4), AUC0→∞ (396.1 and 1000 h*ng/ml), Tmax (1.5 and 1.25 hours), and Cmax (42.8 and 190 ng/ml) between cocaine users (30 mg L-THP tablet) and healthy adults (60 mg L-THP disintegrating tablet), respectively.24 Taken together, these findings suggest the safety of L-THP as a potential treatment for CUD, and support further research towards this end.
We found two distinct peaks of cocaine concentration following intranasal administration, as previously reported.32 This is presumably due to some of the intranasal dose being swallowed, resulting in a delayed absorption across the gastric mucosa, in addition to the immediate absorption across the nasal mucosa. This difference may be due to inconsistencies in insufflation technique among subjects. Six (32%) subjects exhibited the second peak associated with gastrointestinal absorption of the cocaine dose. It is likely that subjects who absorbed lesser amounts of cocaine through the nasal mucosa exhibited the second peak.
Limitations of this study include evaluation of single doses of L-THP and cocaine (precluding full evaluation of the potential interaction), and homogeneity (100% men, 84% cigarette smokers, 70% African-American), and small size (19 subjects, of whom only 9 received L-THP) of the study sample, which reduced external validity and the ability to evaluate PK associations with subject characteristics. Strengths of this study include the randomized, double-blind design and detailed characterization of PK parameters.
Our study found that the safety profile of cocaine was not affected by short-term L-THP administration. There was an expected increase in blood pressure and heart rate following cocaine administration that was not influenced by L-THP (Table S2). Furthermore, L-THP did not affect the QTc interval. Although hepatotoxicity has been associated with L-THP and L-THP-containing supplements,33,34 this study showed no difference in liver transaminase levels between participants receiving L-THP and those receiving placebo (Table 5). However, we cannot rule out the possibility of hepatotoxicity from L-THP administration longer than 3.5 days, as would be the case for CUD treatment. L-THP has been reported to have sedating effects,18 but we did not observe any significant differences between the L-THP and placebo groups in Epworth Sleepiness Scale scores, suggesting that the dose of L-THP used was not significantly sedating.
Conclusion
In conclusion, our study found that oral L-THP was safe and well-tolerated in adult male cocaine users and did not affect the PK of cocaine. This provides strong evidence of the safety of L-THP and highlights its potential for further assessment in Phase II clinical trials to evaluate its efficacy for treatment of CUD.
Supplementary Material
Acknowledgments
The authors acknowledge the Center for Translational Medicine (CTM), UMBSOP, for providing access to Phoenix platform and the research and clinical staff of the Brief Stay Unit and Treatment Research Program of MPRC, UMSOM.
Funding
This work was supported by The National Institute on Drug Abuse (NIDA) [grant number 1DP1DA031401].
Footnotes
Conflict of Interest/Disclosure
The authors declare no conflict of interest
References
- 1.Diagnostic and Statistical Manual of Mental Disorders:: DSM-5. American Psychiatric Association; 2013. [Google Scholar]
- 2.United Nations Office on Drugs and Crime. (United Nations publication, Sales No. E.15.XI.6).World Drug Report 2015. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health : Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. (NSDUH Series H-48, HHS Publication No. (SMA) 14-4863). [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network (DAWN), 2011: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. Jan 01, (HHS Publication No. (SMA) 13-4760, DAWN Series D-39). [Google Scholar]
- 5.Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC medicine. 2011;9:119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haile CN, Mahoney JJ, Newton TF, De La Garza R. Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacology & therapeutics. 2012;134:260–277. doi: 10.1016/j.pharmthera.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penberthy JK, Ait-Daoud N, Vaughan M, Fanning T. Review of treatment for cocaine dependence. Current drug abuse reviews. 2010;3:49–62. doi: 10.2174/1874473711003010049. [DOI] [PubMed] [Google Scholar]
- 8.Mariani JJ, Levin FR. Psychostimulant Treatment of Cocaine Dependence. Psychiatric Clinics of North America. 2012;35:425–439. doi: 10.1016/j.psc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorelick D. Pharmacologic interventions for cocaine, methamphetamine, and other stimulant addiction. In: Ries R, DFiellin D, Miller S, editors. Principles of Addiction Medicine. 5th. 2014. pp. 796–810. [Google Scholar]
- 10.Chu H, Jin G, Friedman E, Zhen X. Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cellular and molecular neurobiology. 2008;28:491–499. doi: 10.1007/s10571-007-9179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu SX, Yu LP, Han YR, Chen Y, Jin GZ. Effects of tetrahydroprotoberberines on dopamine receptor subtypes in brain. Zhongguo yao li xue bao = Acta pharmacologica Sinica. 1989;10:104–110. [PubMed] [Google Scholar]
- 12.Mantsch JR, Wisniewski S, Vranjkovic O, et al. Levo-tetrahydropalmatine attenuates cocaine self-administration under a progressive-ratio schedule and cocaine discrimination in rats. Pharmacology, biochemistry, and behavior. 2010;97:310–316. doi: 10.1016/j.pbb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Yang Z, Li R, et al. Responses of dopaminergic, serotonergic and noradrenergic networks to acute levo-tetrahydropalmatine administration in naïve rats detected at 9.4 T. Magnetic resonance imaging. 2012;30:261–270. doi: 10.1016/j.mri.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henkes H, Franz M, Kendall O, et al. Evaluation of the anxiolytic properties of tetrahydropalmatine, a Corydalis yanhusuo compound, in the male Sprague-Dawley rat. AANA journal. 2011;79:S75–80. [PubMed] [Google Scholar]
- 15.Galaj E, Manuszak M, Babic S, Ananthan S, Ranaldi R. The selective dopamine D3 receptor antagonist, SR 21502, reduces cue-induced reinstatement of heroin seeking and heroin conditioned place preference in rats. Drug Alcohol Depend. 2015 Nov 1;156:228–233. doi: 10.1016/j.drugalcdep.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song R, Bi GH, Zhang HY, et al. Blockade of D3 receptors by YQA14 inhibits cocaine’s rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology. 2014 Feb;77:398–405. doi: 10.1016/j.neuropharm.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galaj E, Ananthan S, Saliba M, Ranaldi R. The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats. Psychopharmacology (Berl) 2014 Feb;231(3):501–510. doi: 10.1007/s00213-013-3254-y. [DOI] [PubMed] [Google Scholar]
- 18.Wang JB, Mantsch JR. l-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future medicinal chemistry. 2012;4:177–186. doi: 10.4155/fmc.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong Z, Fan G, Le J, Chai Y, Yin X, Wu Y. Brain pharmacokinetics and tissue distribution of tetrahydropalmatine enantiomers in rats after oral administration of the racemate. Biopharmaceutics & drug disposition. 2006;27:111–117. doi: 10.1002/bdd.489. [DOI] [PubMed] [Google Scholar]
- 20.Mantsch JR, Li S-J, Risinger R, et al. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology. 2007;192:581–591. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- 21.Jin GZ. Progress in studies of the pharmacology of l-tetrahydropalmatine and l-stepholidine. Yao xue xue bao = Acta pharmaceutica Sinica. 1987;22:472–480. [PubMed] [Google Scholar]
- 22.Figueroa-Guzman Y, Mueller C, Vranjkovic O, et al. Oral administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. Drug and alcohol dependence. 2011;116:72–79. doi: 10.1016/j.drugalcdep.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi Z-X, Yang Z, Li S-J, et al. Levo-tetrahydropalmatine inhibits cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology. 2007;53:771–782. doi: 10.1016/j.neuropharm.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao-Wu L, Shuo Z, Hai-Qing G, Xiu-Mei Z. Determination of L-tetrahydropalmatine in human plasma by HPLC and pharmacokinetics of its disintegrating tablets in healthy Chinese. European journal of drug metabolism and pharmacokinetics. 2011;36:257–262. doi: 10.1007/s13318-011-0045-x. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Shao Y-c, Li S-j, et al. Medication of l-tetrahydropalmatine significantly ameliorates opiate craving and increases the abstinence rate in heroin users: a pilot study. Acta pharmacologica Sinica. 2008;29:781–788. doi: 10.1111/j.1745-7254.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeRenzo EG, Conley RR, Love R. Assessment of capacity to give consent to research participation: state-of-the-art and beyond. J Health Care Law Policy. 1998;1(1):66–87. [PubMed] [Google Scholar]
- 27.Yu M, Hassan HE, Ibrahim A, Bauer KS, Kelly DL, Wang JB. Simultaneous determination of L-tetrahydropalmatine and cocaine in human plasma by simple UPLC-FLD method: application in clinical studies. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2014;965:39–44. doi: 10.1016/j.jchromb.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989 May;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 29.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 30.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 31.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- 32.Fattinger K, Benowitz NL, Jones RT, Verotta D. Nasal mucosal versus gastrointestinal absorption of nasally administered cocaine. European journal of clinical pharmacology. 2000;56:305–310. doi: 10.1007/s002280000147. [DOI] [PubMed] [Google Scholar]
- 33.Woolf GM. Acute Hepatitis Associated with the Chinese Herbal Product Jin Bu Huan. Annals of Internal Medicine. 1994;121:729. doi: 10.7326/0003-4819-121-10-199411150-00001. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Zhou J, Wang S, et al. Shotgun approach based comparative proteomic analysis of levo-tetrahydropalmatine-induced apoptosis in hepatocytes. Toxicology letters. 2010;194:8–15. doi: 10.1016/j.toxlet.2010.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


