Abstract
Patients with pancreatic-insufficient cystic fibrosis (PI-CF) are at increased risk for developing diabetes. We determined β-cell secretory capacity and insulin secretory rates from glucose-potentiated arginine and mixed-meal tolerance tests (MMTTs), respectively, in pancreatic-sufficient cystic fibrosis (PS-CF), PI-CF, and normal control subjects, all with normal glucose tolerance, in order to identify early pathophysiologic defects. Acute islet cell secretory responses were determined under fasting, 230 mg/dL, and 340 mg/dL hyperglycemia clamp conditions. PI-CF subjects had lower acute insulin, C-peptide, and glucagon responses compared with PS-CF and normal control subjects, indicating reduced β-cell secretory capacity and α-cell function. Fasting proinsulin-to-C-peptide and proinsulin secretory ratios during glucose potentiation were higher in PI-CF, suggesting impaired proinsulin processing. In the first 30 min of the MMTT, insulin secretion was lower in PI-CF compared with PS-CF and normal control subjects, and glucagon-like peptide 1 and gastric inhibitory polypeptide were lower compared with PS-CF, and after 180 min, glucose was higher in PI-CF compared with normal control subjects. These findings indicate that despite “normal” glucose tolerance, adolescents and adults with PI-CF have impairments in functional islet mass and associated early-phase insulin secretion, which with decreased incretin responses likely leads to the early development of postprandial hyperglycemia in CF.
Introduction
Cystic fibrosis (CF) is a life-threatening autosomal recessive disorder in which the function of the cystic fibrosis transmembrane regulator (CFTR) is absent or severely reduced. More than 2,000 CFTR mutations have been reported, and alterations in CFTR function result in impaired bicarbonate and chloride transport across epithelial membranes that may result in impaired pancreatic exocrine secretion leading to pancreatic insufficiency (pancreatic-insufficient cystic fibrosis [PI-CF]). Additionally, impaired pulmonary secretion clearance leads to increased susceptibility to pulmonary infections, progressive decline in pulmonary function, and ultimately respiratory failure for many individuals with CF. Advances in CF nutrition and pulmonary care have resulted in improved median survival with which the development of cystic fibrosis–related diabetes (CFRD) has emerged as a major comorbidity affecting ∼40% of adults aged >30 years (1). CFRD is associated with worse clinical outcomes, including reduction in pulmonary function, worsening nutritional status, declining kidney function, and increased mortality (2).
Pancreatic insufficiency is associated with increased risk for developing CFRD (3), where pancreatogenic diabetes can result from pancreatic inflammation and the subsequent fibrosis and sclerosis disrupting pancreatic islet structure and function (4). Incretin secretion abnormalities arising from pancreatic insufficiency–related maldigestion are also posited to contribute to insulin secretion abnormalities (5). Although reduced functional β-cell mass is expected by the time diabetes is diagnosed, limited data are available to inform what early pathophysiologic mechanisms may be targeted therapeutically to prevent the development of CFRD.
To identify early defects affecting glucose homeostasis in CF, we recruited subjects with PI-CF and normal glucose tolerance according to the Cystic Fibrosis Foundation (CFF) criteria that are more stringent than current American Diabetes Association criteria in requiring a 1-h glucose <200 mg/dL during the standard 75-g oral glucose tolerance test (OGTT) (6,7). We hypothesized that participants with PI-CF, despite having normal glucose tolerance, would manifest impaired β-cell secretory capacity and insulin secretory rates (ISRs) as derived from glucose-potentiated arginine (GPA) and mixed-meal tolerance tests (MMTTs), respectively, compared with healthy control subjects without CF, findings that would support a primary islet defect as the earliest mechanism responsible for future risk of diabetes. To consider possible effects of diminished CFTR function on insulin and incretin secretion independent of pancreatic exocrine insufficiency, we also studied individuals with pancreatic-sufficient CF (PS-CF) to serve as disease control subjects.
Research Design and Methods
Subjects
Postpubertal adolescents and adults with a confirmed diagnosis of CF including positive sweat test or CFTR mutation analysis (8) were invited to participate. Subjects were recruited based on their pancreatic exocrine insufficiency status; pancreatic insufficiency was determined by clinical diagnosis including symptoms of malabsorption, treatment with pancreatic enzyme replacement therapy, and if ambiguous, confirmed by fecal elastase testing (9). Pancreatic sufficiency was defined by absence of malabsorption symptoms, absence of pancreatic enzyme replacement treatment, and previous fecal elastase levels >200 μg/g. Subjects did not undergo fecal elastase testing at the time of enrollment. Normal glucose tolerance (1-h glucose <200 mg/dL and 2-h glucose <140 mg/dL [6]) was documented by a standard 75-g OGTT within 3 months prior to study. Individuals with acute illness requiring a change in antibiotics or administration of oral or intravenous glucocorticoids within the previous 4 weeks, clinically symptomatic pancreatitis within the previous 12 months, prior lung or liver transplant, or significant kidney or liver dysfunction, as well as pregnant or nursing females were excluded.
Healthy individuals with normal glucose tolerance and of comparable sex, age, and BMI to CF participants served as control subjects. Control subjects for the GPA test (n = 11) were derived from a recently reported study (10), and MMTT and continuous glucose monitoring (CGM) control subjects (n = 10) were recruited prospectively with the CF participants.
The institutional review boards of the University of Pennsylvania (Penn) and Children's Hospital of Philadelphia (CHOP) approved the study, and all participants gave written informed consent and assent (when age appropriate) to participate. Study procedures were performed over two study visits conducted in the Penn or CHOP Clinical and Translational Research Center (CTRC) after a 12-h overnight fast.
GPA Test
The GPA test followed established methodology for evaluation of β-cell secretory capacity and sensitivity to glucose (11–13). At 0700 h, one catheter was placed in an antecubital vein for infusions, and one catheter was placed in a distal forearm or hand vein for blood sampling, with the hand placed in a heating pad to promote arterialization of the venous blood. After at least 20 min of acclimatization to the catheters, baseline blood samples were taken at t = −5 and −1 min before injection of 5 g of 10% arginine over 1 min starting at t = 0. Additional blood samples were collected at t = 2, 3, 4, and 5 min after arginine injection. Beginning at t = 10 min, a hyperglycemic clamp technique (14) using a variable rate infusion of 20% dextrose was performed to achieve a plasma glucose concentration of ∼230 mg/dL. Blood samples were taken every 5 min to adjust the infusion rate and achieve the desired plasma glucose concentration. After 45 min of glucose infusion (at t = 55 min), a second 5-g arginine pulse was injected with identical blood sampling. The first administration of arginine has no effect on the subsequent response to arginine using this protocol (15). A subsequent 2-h period with no glucose infusion allowed plasma glucose to return to baseline. A second hyperglycemic clamp was then performed to achieve a plasma glucose concentration of ∼340 mg/dL. After 45 min of glucose infusion, a third 5-g arginine pulse was injected with identical blood sampling.
MMTT
The MMTT has previously been used to differentiate insulin and incretin secretory responses (16). At 0700 h, an antecubital or forearm vein catheter was placed for blood sampling. After at least 20 min of acclimatization to the catheter, baseline blood samples were taken at t = −10 and −1 min before ingestion of an 820 kcal breakfast over 15 min starting at t = 0. The carbohydrate, fat, and protein composition of the meal were 47, 40, and 13% of the total energy content (16). Subjects with pancreatic exocrine insufficiency took their prescribed dose of pancreatic enzyme replacement with the test meal. Additional blood samples were obtained at t = 10, 15, 20, 30, 60, 90, 120, 150, 180, 210, and 240 min from the start of the meal.
Biochemical Analysis
Plasma glucose was measured in duplicate by the glucose oxidase method using an automated glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH). Other samples were collected on ice into tubes containing EDTA and protease inhibitor cocktail (and for MMTT samples, DPP4 inhibitor; Sigma-Aldrich, St. Louis, MO), centrifuged at 4°C, separated, and frozen at −80°C for subsequent analysis. Plasma insulin, C-peptide, proinsulin, and glucagon were measured in duplicate by double-antibody radioimmunoassays (Millipore, Billerica, MA). Plasma free fatty acid levels were measured in duplicate using enzymatic colorimetrics (Wako Chemicals, Richmond, VA). Active glucagon-like peptide 1 (GLP-1) and total gastric inhibitory polypeptide (GIP) were measured in duplicate by ELISA (Millipore).
CGM
CGM (72 h) was performed as a dynamic assessment of glycemic control. The CGM system (CGMS iPro; Medtronic Minimed, Northridge, CA) measures interstitial glucose from a subcutaneously inserted sensor every 10 s and records an average value every 5 min. Interstitial CGM has previously been validated in CF (17). Subjects used a study glucometer (OneTouch Ultra; LifeScan, Milpitas, CA) to monitor blood glucose and calibrate the CGMS four times daily with no interval between readings exceeding 12 h. Subjects removed the sensor after 72 h. CGM data were excluded if <36 h in duration due to catheter dislodgement or if conducted with insufficient blood glucose calibration measurements.
Calculations and Statistical Analyses
GPA Test
Acute insulin, C-peptide, proinsulin, and glucagon responses to arginine (AIRarg, ACRarg, APRarg, and AGRarg, respectively) were calculated as the mean of the 2-, 3-, 4-, and 5-min values minus the mean of the baseline values (14). Acute responses during the 230 mg/dL glucose clamp enable determination of glucose potentiation of arginine-induced insulin (AIRpot), C-peptide (ACRpot), and proinsulin (APRpot) release, and glucose inhibition of arginine-induced glucagon (AGRinh) release. Acute responses during the 340 mg/dL glucose clamp allow for determination of the maximum arginine-induced insulin (AIRmax), C-peptide (ACRmax), and proinsulin (APRmax) release, i.e., β-cell secretory capacity, and of the minimum arginine-induced glucagon (AGRmin) release (11). The proinsulin-to-C-peptide ratio was calculated as the molar concentration of proinsulin divided by the molar concentration of C-peptide × 100 (18). Estimation of the proinsulin-to-C-peptide ratio within the secretory granules of the β-cell is most reliable after acute stimulation of secretion (19); therefore, we examined the proinsulin secretory ratio (PISR) in response to each injection of arginine from the respective acute proinsulin:C-peptide responses to arginine (12,18). Plasma glucose at which half-maximal insulin secretion is achieved (PG50) was calculated to assess β-cell sensitivity to glucose as previously described (11–13). Insulin sensitivity (M/I) was determined by dividing the mean glucose infusion rate required during the 230 mg/dL glucose clamp (M) by the mean prestimulus insulin level (I) between 40 and 45 min of the glucose infusion (12).
MMTT
Incremental areas under the curve (AUCs) for glucose, free fatty acids, ISRs, glucagon, GLP-1, and GIP were calculated with baseline values subtracted by the trapezoidal method using Origin software (Northampton, MA). The ISRs were calculated from C-peptide values and derived by parametric deconvolution of C-peptide kinetics using a two-compartment model (20), and insulin sensitivity (SI) was derived using the oral minimal model (21) in WinSAAM software 3.0.8 (University of Pennsylvania, New Bolton Center, Kennett Square, PA).
CGM
Interstitial glucose data were summarized to provide mean glucose, glucose SD, coefficient of variation (CV) and percent (%) time glucose >140 and >180 mg/dL. CV for glucose was calculated from the glucose SD divided by mean glucose.
Statistical Analyses
All data are expressed as median and interquartile range (IQR), unless otherwise noted. Comparison of results between the PS-CF, PI-CF, and normal control subjects was performed with the Kruskal-Wallis test, and when significant differences at P ≤ 0.05 were found, comparisons between groups were performed using the Mann-Whitney U test. Spearman rank correlation test was used to evaluate correlation between the OGTT and acute islet response measures. All analyses were performed using STATA 12 (StataCorp LP, College Station, TX). Significance was considered at P ≤ 0.05 (two-tailed).
Results
Subject Characteristics
Nine PS-CF, 11 PI-CF, 11 control subjects for the GPA test, and 10 control subjects for the MMTT participated (Table 1). Sex distribution, age, and BMI were comparable across the CF and control groups. The majority of subjects with PI-CF (6 of 11), but no subjects with PS-CF, were homozygous for the F508del CFTR mutation (Supplementary Table 1). Pulmonary function, as represented by percent-predicted forced expiratory volume in 1 s (FEV1% predicted), was not statistically different between subjects with PS-CF and subjects with PI-CF (91% [26–119] vs. 80% [54–99]).
Table 1.
Subject characteristics
| GPA control subjects (n = 11) | MMTT control subjects (n = 10) | PS-CF (n = 9) | PI-CF (n = 11) | Overall P value |
|||
|---|---|---|---|---|---|---|---|
| Control subjects vs. PI-CF | Control subjects vs. PS-CF | PI-CF vs. PS-CF | |||||
| Demographics | |||||||
| Sex (female) | 5 (45) | 5 (50) | 6 (67) | 5 (45) | 0.80 | ||
| Age (years) | 25 (21–38) | 25 (20–41) | 31 (16–56) | 19 (16–50) | 0.13 | ||
| BMI (kg/m2) | 24 (21–29) | 24 (18–27) | 24 (19–33) | 22 (17–31) | 0.64 | ||
| HbA1c (%)# | N/D | 5.0 (4.7–5.5) | 5.2 (3.8–6.0) | 5.5 (5.3–6.2) | 0.004 | ||
| 0.0006* | 0.34* | 0.06* | |||||
| OGTT profile | |||||||
| Fasting glucose (mg/dL) | 82 (74–107) | 85 (68–94) | 87 (74–102) | 92 (73–98) | 0.18 | ||
| 1-h glucose (mg/dL) | 122 (70–199) | 114 (60–157) | 118 (67–165) | 162 (127–193) | 0.01 | ||
| 0.001&* | 0.35* | 0.009* | |||||
| 2-h glucose (mg/dL) | 95 (60–128) | 94 (74–111) | 104 (46–115) | 97 (66–134) | 0.90 | ||
Data are medians and ranges (min–max) for continuous variables and number and (percentage) for categorical variables.
*Between-group comparisons performed when overall P value was significant at P ≤ 0.05;
#to convert to mmol/mol, multiply by 10.93 and subtract 23.50;
&significant differences between PI-CF and MMTT control subjects.
Participants with PI-CF had higher HbA1c (P < 0.001 vs. control subjects) and OGTT 1-h glucose (P < 0.05 vs. PS-CF and control subjects) even though all subjects had CFF-defined normal glucose tolerance (Table 1). Compared with control subjects, PI-CF also had higher CGM mean glucose, glucose SD, glucose CV, and percentage of time glucose >140 and >180 mg/dL (Table 2).
Table 2.
CGM profile
| CGMS measure | Control subjects (n = 6) | PS-CF (n = 7) | PI-CF (n = 10) | Overall P value |
||
|---|---|---|---|---|---|---|
| Control subjects vs. PI-CF | Control subjects vs. PS-CF | PS-CF vs. PI-CF | ||||
| Mean glucose (mg/dL) | 92 (88–96) | 112 (96–122) | 111 (104–121) | 0.009 | ||
| 0.002* | 0.03* | 0.70* | ||||
| Glucose SD (mg/dL) | 14 (13–15) | 14 (11–21) | 22 (18–28) | 0.001 | ||
| 0.004* | 0.7* | 0.06* | ||||
| Glucose CV | 0.14 (0.13–0.17) | 0.13 (0.11–0.19) | 0.19 (0.17–0.21) | 0.06 | ||
| % time >140 mg/dL | 0 (0–0) | 5 (0–15) | 10 (7–13) | 0.006 | ||
| 0.001* | 0.09* | 0.22* | ||||
| % time >180 mg/dL | 0 (0–0) | 0 (0–1) | 2 (0–4) | 0.06 | ||
Data are medians and IQRs.
*Between-group comparisons performed when overall P value was significant at P ≤ 0.05.
Glucose, Insulin, C-Peptide, and Glucagon During the GPA Test
Fasting glucose prior to GPA testing was normal for all participants (93 mg/dL [min–max: 89–97]) and was not significantly different between groups (data not shown). Prestimulus glucose during the 230 mg/dL clamp was 226 mg/dL (223–233) and during the 340 mg/dL clamp was 325 mg/dL (318–337), neither was different across groups.
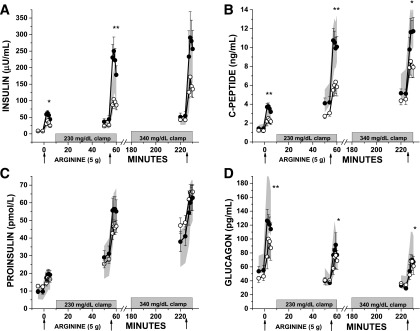
Fasting islet cell hormone concentrations were comparable among the groups (Table 3). For insulin responses (Fig. 1A and Table 3), AIRarg was greater in PS-CF compared with PI-CF and control subjects (P < 0.05 for both), and AIRpot was lower in PI-CF compared with PS-CF and control subjects (P < 0.01 for both). Similarly for the C-peptide responses (Fig. 1B and Table 3), PS-CF had higher ACRarg compared with PI-CF and control subjects (P ≤ 0.05 for both). Subjects with PI-CF demonstrated lower ACRpot and ACRmax compared with PS-CF (P < 0.05 for both) and control subjects (P < 0.05 for both). For the glucagon responses, AGRarg, AGRinh, and AGRmin were lower in PI-CF than PS-CF (P ≤ 0.05 for all) and control subjects (P < 0.05 for all) (Fig. 1D and Table 3).
Table 3.
Fasting islet cell hormones, acute responses, proinsulin-to-C-peptide ratio, and PISRs during the GPA test
| Control subjects (n = 11) | PS-CF (n = 9) | PI-CF (n = 11) | Overall P value |
|||
|---|---|---|---|---|---|---|
| Control subjects vs. PI-CF | Control subjects vs. PS-CF | PS-CF vs. PI-CF | ||||
| Fasting insulin (μU/mL) | 7.4 (6.8–10.4) | 8.8 (6.6–9.1) | 9.1 (6.2–10.4) | 0.85 | ||
| AIRarg (μU/mL) | 19.2 (13.7–46.3) | 41.3 (36.4–49.8) | 19.6 (13.9–31.3) | 0.01 | ||
| 0.82* | 0.03* | 0.003* | ||||
| AIRpot (μU/mL) | 99.8 (82.6–128.7) | 162.3 (125.2–227.2) | 56.1 (42.9–94.7) | 0.002 | ||
| 0.006* | 0.17* | 0.002* | ||||
| AIRmax (μU/mL) | 120.4 (91.7–190.2) | 116.7 (83.7–233.8) | 75.6 (52.4–143.0) | 0.13 | ||
| Fasting C-peptide (ng/mL) | 1.3 (1.00–1.8) | 1.6 (1.0–1.8) | 1.2 (0.8–1.2) | 0.70 | ||
| ACRarg (ng/mL) | 1.2 (0.9–2.2) | 2.0 (1.6–2.6) | 0.8 (0.5–1.2) | 0.004 | ||
| 0.07* | 0.05* | 0.002* | ||||
| ACRpot (ng/mL) | 4.9 (4.3–5.5) | 6.7 (4.4–7.2) | 2.2 (1.5–3.4) | 0.003 | ||
| 0.004* | 0.52* | 0.003* | ||||
| ACRmax (ng/mL) | 6.0 (3.8–6.9) | 5.5 (3.6–7.0) | 3.2 (1.5–4.4) | 0.03 | ||
| 0.02* | 0.90* | 0.04* | ||||
| Fasting glucagon (pg/mL) | 62 (53–74) | 47 (43–60) | 48 (25–62) | 0.09 | ||
| AGRarg (pg/mL) | 73 (39–94) | 62 (51–74) | 29 (17–38) | 0.008 | ||
| 0.02* | 0.73* | 0.003* | ||||
| AGRinh (pg/mL) | 42 (33–53) | 33 (28–51) | 16 (15–30) | 0.015 | ||
| 0.007* | 0.67* | 0.003* | ||||
| AGRmin (pg/mL) | 33 (27–51) | 37 (19–43) | 20 (8–29) | 0.03 | ||
| 0.01* | 0.79* | 0.05* | ||||
| Fasting proinsulin (pmol/L) | 8.6 (2.4–22.8) | 10.8 (2.0–13.1) | 11.7 (8.6–21.6) | 0.18 | ||
| APRarg (pmol/L) | 5.3 (3.2–9.6) | 7.1 (6.0–11.9) | 2.7 (0.8–6.4) | 0.07 | ||
| APRpot (pmol/L) | 23.1 (18.2–30.9) | 29.9 (17.7–33.5) | 18.7 (17.6–22.4) | 0.15 | ||
| APRmax (pmol/L) | 22.2 (15.2–26.7) | 19.0 (13.1–19.5) | 16.8 (13.1–19.5) | 0.20 | ||
| Fasting proinsulin-to-C-peptide ratio (%) | 2.3 (1.2–2.5) | 1.9 (1.7–2.4) | 3.4 (2.9–4.0) | 0.04 | ||
| 0.01* | 0.9* | 0.09* | ||||
| PISR, fasting (%) | 1.3 (1.0–1.6) | 1.1 (1.0–1.6) | 1.1 (0.3–1.7) | 0.90 | ||
| PISR, 230 mg/dL (%) | 1.3 (1.2–1.6) | 1.4 (0.8–1.6) | 2.5 (1.3–3.4) | 0.04 | ||
| 0.03* | 0.62* | 0.03* | ||||
| PISR, 340 mg/dL (%) | 1.2 (1.0–1.3) | 0.85 (0.8–1.3) | 1.4 (1.3–3.0) | 0.09 | ||
Data are medians and IQRs.
*Between-group comparisons performed when overall P value was significant at P ≤ 0.05.
Figure 1.
Islet cell hormone levels (A: insulin; B: C-peptide; C: proinsulin; D: glucagon) in response to bolus administration of arginine (arrows) under fasting, ∼230 mg/dL, and ∼340 mg/dL hyperglycemic clamp conditions in PS-CF (closed circles), PI-CF (open circles), and healthy control subjects (normal range given as the 95% CI and shown as gray shaded area). CF subject data are represented at mean ± SE. *P < 0.05; **P < 0.01.
Data were reanalyzed after exclusion of subjects with CF who were receiving ivacaftor for clinical indications (PS-CF, n = 2; PI-CF, n = 1) (Supplementary Fig. 1). AIRarg and ACRarg were now similar in PS-CF and control subjects, and ACRmax was no longer significantly different in PI-CF compared with PS-CF (P = 0.08), although remained significantly less than control subjects (P < 0.05).
Proinsulin and PISRs
The fasting proinsulin-to-C-peptide ratio was higher in PI-CF (P = 0.01 vs. control subjects) (Table 3). Proinsulin responses, APRarg, APRpot, and APRmax were not different across the PS-CF, PI-CF, and control groups (Fig. 1C and Table 3). However, because insulin and C-peptide secretory responses were decreased in PI-CF, the PISR was higher in PI-CF compared with PS-CF and control subjects during ∼230 mg/dL hyperglycemic clamp conditions (P < 0.05 for both) (Table 3). These differences persisted after exclusion of subjects with CF treated with ivacaftor.
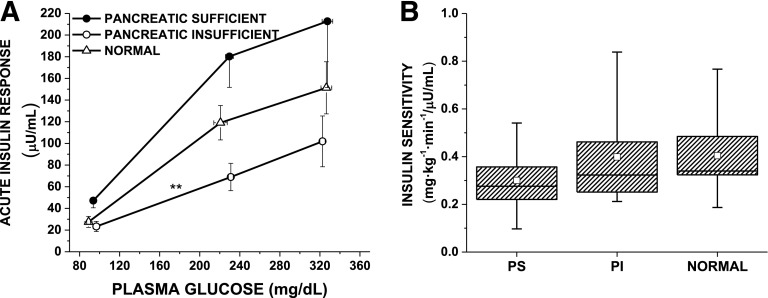
β-Cell Sensitivity to Glucose and Insulin Sensitivity During the GPA Test
PG50, a measure of β-cell sensitivity to glucose, was not different among PS-CF, PI-CF, and control participants (125 [101–142] vs. 158 [131–193] vs. 148 [128–175] mg/dL) (Fig. 2A). The glucose infusion rate (M), second-phase insulin level (I), and the resulting estimates of insulin sensitivity (M/I) (Fig. 2B) from the 230 mg/dL clamp were not different across the groups.
Figure 2.
A: AIRs to arginine as a function of the prestimulus plasma glucose concentration in PS-CF (closed circles), PI-CF (open circles), and healthy control subjects (normal, open triangles). Data are given as mean ± SE. The glucose potentiation slope (GPS), calculated as the difference in the AIR at fasted and ∼230 mg/dL glucose levels divided by the difference in plasma glucose, is impaired in PI-CF vs. both PS-CF and normal (0.3 ± 0.2 vs. 1.0 ± 0.6 and 0.7 ± 0.4; **P = 0.002). β-Cell sensitivity to glucose is determined as the PG50 using the y intercept (b) of the GPS to solve the equation AIRmax/2 = GPS × PG50 + b and was not different across groups. B: Box plot of insulin sensitivity (M/I) by study group, given as median and IQR (box) and mean (open squares) and range (error bars), is similar across PS-CF, PI-CF, and normal control subjects.
Correlations Between OGTT Glucose and Acute Islet Responses on GPA Test
The OGTT 1-h glucose was negatively correlated with AIRpot (ρ = −0.43, P = 0.02), ACRarg (ρ = −0.37, P = 0.04), ACRpot (ρ = −0.41, P = 0.03), and ACRmax (ρ = −0.40, P = 0.03) in all subjects. In subjects with PI-CF, the OGTT 1-h glucose was negatively correlated with AIRpot (ρ = −0.65, P = 0.03), ACRmax (ρ = −0.67, P = 0.02), and APRpot (ρ = −0.61, P = 0.05) and trended toward negative correlation with AIRarg, AIRmax, ACRarg, and ACRpot (P < 0.1 for all). Similar correlations were not seen between either fasting glucose or 2-h glucose and the acute β-cell responses. Acute glucagon responses did not significantly correlate with OGTT glucose.
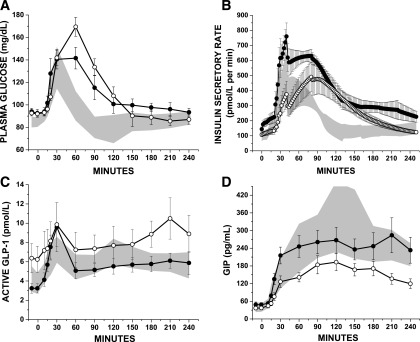
Insulin and Incretin Secretion During the MMTT
During the first 30 min after meal ingestion, glucose (AUCglu) (Fig. 3A and Table 4) was not different across groups. However, insulin secretion (AUCISR) was lower in PI-CF compared with PS-CF and control subjects (P < 0.05 for both) (Fig. 3B and Table 4), and the AUCISR-to-AUCglu ratio was lower in PI-CF compared with PS-CF and control subjects (P < 0.01 for both) (Table 4). GLP-1 (AUCGLP) and GIP (AUCGIP) were lower in PI-CF compared with PS-CF (P < 0.05 for both) (Fig. 3C and D and Table 4).
Figure 3.
Glucose (A), ISR (B), GLP-1 (C), and GIP (D) levels during the 4-h MMTT in PS-CF (closed circles), PI-CF (open circles), and healthy control subjects (normal range given as the 95% CI and shown as gray shaded area). CF subject data are given as mean ± SE.
Table 4.
MMTT responses during the first 30 and 180 min postingestion
| MMTT control subjects (n = 10) | PS-CF (n = 8) | PI-CF (n = 11)# | Overall P value |
|||
|---|---|---|---|---|---|---|
| Control subjects vs. PI-CF | Control subjects vs. PS-CF | PS-CF vs. PI-CF | ||||
| 30 min | ||||||
| AUCglu (mg ⋅ min/dL) | 540 (276–729) | 447 (272–521) | 462 (268–509) | 0.60 | ||
| AUCISR (pmol/L) | 3,041 (1,459–4,721) | 2,857 (1,950–4,201) | 1,033 (200–1,677) | 0.03* | 0.03 | 0.02* |
| 0.80* | ||||||
| AUCISR/AUCglu (mU ⋅ min/mg) | 0.09 (0.07–0.12) | 0.16 (0.10–0.17) | 0.05 (0.03–0.05) | 0.007* | 0.004 | 0.007* |
| 0.12* | ||||||
| AUCGLP-1 (pmol ⋅ min/L) | 32 (21–81) | 91 (52–121) | 16 (10–71) | 0.32* | 0.04 | 0.02* |
| 0.05* | ||||||
| AUCGIP (pg ⋅ min/mL) | 1,367 (734–1,838) | 1,900 (1,048–2,354) | 747 (355–1,195) | 0.11* | 0.04 | 0.02* |
| 0.34* | ||||||
| 180 min | ||||||
| AUCglu (mg ⋅ min/dL) | 506 (−145 to 2,958) | 3,501 (2,707–5,123) | 5,075 (2,836–7,989) | 0.004* | 0.007 | 0.46* |
| 0.02* | ||||||
| AUCISR (pmol/L) | 25,917 (23,659–28,693) | 53,375 (47,607–58,495) | 26,156 (23,176–49,651) | 0.60* | 0.006 | 0.08* |
| 0.0004* | ||||||
| AUCISR/AUCglu (mU ⋅ min/mg) | 0.13 (−0.93 to 0.19) | 0.26 (0.15–0.35) | 0.13 (0.08–0.16) | 0.13 | ||
| AUCGLP-1 (pmol ⋅ min/L) | 395 (278–589) | 577 (230–738) | 216 (103–494) | 0.27 | ||
| AUCGIP (pg ⋅ min/mL) | 34,702 (28,526–40,934) | 30,586 (23,471–45,227) | 18,721 (11,502–28,675) | 0.006* | 0.01 | 0.03* |
| 0.42* | ||||||
Data are medians and IQRs. Glu, glucose.
*Between-group comparisons performed when overall P value was significant at P ≤ 0.05;
#n = 9 for AUCISR and AUCISR/AUCglu.
Over the 180 min, AUCglu was higher in PI-CF compared with control subjects (P < 0.01) (Fig. 3A and Table 4) and AUCGIP was lower (P < 0.01) (Fig. 3D and Table 4). The oral minimal model–derived SI was lower in PS-CF compared with control subjects (5.4 [3.9–7.3] vs. 14.1 [8.7–19.3] × 10−4 [(μU/mL)−1 ⋅ min−1]; P = 0.005) but was not different for PI-CF compared with PS-CF or control subjects (9.8 [4.6–12.7] × 10−4 [(μU/mL)−1 ⋅ min−1]; P = 0.16 and 0.13, respectively).
Discussion
This is the first study to assess β-cell secretory capacity and ISRs in PI-CF individuals with normal glucose tolerance and comparable healthy and disease control subjects. Our data suggest that adolescents and adults with PI-CF, despite exhibiting “normal” glucose tolerance as defined by standard measures of glucose homeostasis, have impaired insulin and incretin secretion. The decreased β-cell secretory capacity and incretin secretion abnormalities likely explain impaired early-phase insulin response and postprandial hyperglycemia after meal ingestion, but their direct contribution to declines in CF outcomes has yet to be determined.
Although all subjects included in this study met CFF criteria (6) for normal glucose tolerance (1-h glucose <200 and 2-h glucose <140 mg/dL), the 1-h glucose was significantly higher within the “normal” range in the PI-CF group. Despite returning to normal glucose within 2-h post-OGTT, this same group also had both the highest postprandial glucose during the MMTT and higher glucose and glucose variability during CGM under everyday life conditions. A higher 1-h glucose may be clinically relevant, as glucose concentrations as low as 155 mg/dL are associated with increased risk of developing type 2 diabetes (22,23), and in CF, a 1-h glucose as low as 160 mg/dL during an OGTT is associated with a four to fivefold increased risk for developing CFRD over the subsequent 5 years (24). The correlation between acute insulin and C-peptide responses and 1-h glucose seen here suggests that this measure may be useful for identifying PI-CF patients with impaired β-cell secretory capacity, who as a consequence are at higher risk for the development of diabetes.
Although CFRD is associated with worse nutritional status and pulmonary function, declines in these CF outcomes occur within the several years prior to CFRD development (2). These declines suggest that the early insulin secretion defects demonstrated here are indeed clinically relevant. Potentially compounding insulin deficiency is impaired suppression of protein catabolism that has been reported at least in the setting of CFRD (25). Indeed lean body mass correlates with pulmonary function in CF, higher 1-h glucose is associated with worse lung function (24), and a lower 1-h insulin is associated with lower BMI% (26).
Little is known about the function of the endocrine pancreas in CF prior to the development of glucose intolerance. Neither the onset of nor the mechanisms underlying the earliest insulin secretion abnormalities can be gleaned from an OGTT. Therefore, the more sophisticated and sensitive techniques of GPA and deconvolution of C-peptide were used to identify differences in β-cell secretory capacity and ISRs in adolescents and young adults with “normal” glucose tolerance based on CFF criteria. β-Cell secretory capacity derived from glucose potentiation of arginine-induced insulin secretion represents the best in vivo estimate of functional β-cell mass (27). Here, we report data that indicate a reduced functional β-cell mass is a key early defect responsible for the future risk of diabetes in PI-CF. Previous studies have shown that subjects with PI-CF demonstrate impaired β-, α-, and pancreatic polypeptide cell function compared with PS-CF and normal control subjects (28–30) and that the β-cell defect is worse in the setting of overtly impaired or diabetic glucose tolerance (30–32). These earlier reports, however, did not exclude PI-CF subjects with indeterminate glucose tolerance (1-h ≥200 mg/dL) and used surrogate measures of insulin secretion and secretory capacity, and so have not addressed the initial mechanisms preceding the development of impaired glucose tolerance in CF.
The confirmation of decreased β-cell secretory capacity with normal β-cell sensitivity to glucose (PG50) supports the hypothesis that the impaired insulin responses are at least partly a consequence of reduced β-cell mass, rather than a defect in the functional response of the β-cell. Decreased β-cell mass may be explained by islet loss in PI-CF due to extension of pancreatic exocrine fibrosis (33–35), disruption and loss of pancreatic vascularity (4), and/or related effects on pancreatic islet progenitor cells that preclude the development of a “normal” islet mass. Previous work supports a close correlation of pancreatic insufficiency and the development of CFRD (36). Such hypothesized “collateral damage” effects are anticipated to affect non–β-islet cells as well, and indeed, here we report lower glucagon responses in participants with PI-CF but preserved glucagon responses in PS-CF; these data are consistent with generalized islet loss present in those with pancreatic insufficiency. That the reduced functional β-cell mass in PI-CF is experiencing a relative increased demand for insulin secretion is supported by the increased fasting proinsulin-to-C-peptide and PISRs during hyperglycemia in PI-CF. Increased β-cell secretory demand results in the recruitment of immature secretory granules containing an abundance of incompletely processed proinsulin; however, the contribution of defective CFTR functioning on posttranslational processing of proinsulin may also be possible.
A direct role for CFTR in islet function has been demonstrated in the ferret and pig models of CF (37,38). In mice, attenuation of glucose-stimulated insulin secretion is present in F508del CFTR mice compared with wild-type mice, and VX-809 (lumacaftor), a corrector of F508del trafficking, can reverse this dampened response (39). Moreover, CFTR may be important for β-cell recovery from injury (40), as likely occurs during the fibrosis of the pancreas and development of pancreatic insufficiency. Reported improvement in glycemic status affecting patients with at least 1 of 10 rare mutations treated with the CFTR modulator, ivacaftor, are prompting further human investigations of the underlying mechanisms of possible CFTR effects in the islet (41,42). Three subjects (two with PS-CF and one with PI-CF) in the current study were receiving ivacaftor therapy for approved clinical indications. Such treatment may have affected insulin secretion results (Supplemental Fig. 1), but excluding these subjects from the analyses did not affect the significance of the impaired insulin and incretin secretion comparisons for the PI-CF group.
Disruption of the enteroinsular axis, which is important in postprandial glucose homeostasis, is hypothesized to contribute to the delayed insulin response and postprandial hyperglycemia in CF. Active and total levels of postprandial GLP-1 and GIP have been studied in PI-CF with conflicting results (5,32,43), although pancreatic enzyme supplementation improves secretion of these incretins in PI-CF (44). All subjects with PI-CF took pancreatic enzymes before the MMTT, yet our subjects with PI-CF exhibited lower early incretin responses than those with PS-CF, and over 180 min, the AUCGIP was impaired in our PI-CF group. These data are consistent with earlier work in young adults (5). Thus, defects in the enteroinsular axis likely contribute to postprandial hyperglycemia in CF.
Insulin resistance in the absence of systemic glucocorticoid treatment or illness is not a prominent feature during the development of CFRD (45), and assessment of peripheral insulin sensitivity (M/I during GPA testing and SI during the MMTT) in our PI-CF individuals with normal glucose tolerance did not reveal insulin sensitivity that was different than control subjects. As all subjects were free of a recent pulmonary exacerbation and not receiving glucocorticoids, decreased insulin sensitivity is unlikely to have confounded our insulin secretion measures. Nevertheless, periods of previous illness and glucocorticoid exposure may have contributed to increased β-cell demand prior to the time of study that may have affected the β-cell secretory capacity. Curiously, the PS-CF group had increased β-cell secretory responses under fasting conditions and higher postprandial glucose and insulin secretion than healthy control subjects of comparable age, sex, and BMI; these findings are suggestive of impaired insulin sensitivity with compensatory increased insulin secretion. Exclusion of the one obese subject with a BMI of 33 kg/m2 attenuated the difference in acute insulin and C-peptide responses between the PS-CF group and control subjects, but did not affect the differences between the PS-CF and PI-CF groups. Whereas insulin sensitivity estimated from the oral minimal model was lower in the PS-CF participants, a previous study utilizing the more sensitive hyperinsulinemic-euglycemic clamp did not identify insulin resistance in PS-CF (46).
In conclusion, patients with PI-CF who demonstrate strictly normal glucose tolerance defined by standard OGTT criteria manifest decreased insulin and incretin secretion, disproportionately increased proinsulin secretion during hyperglycemia, and higher postprandial glucose excursions than PS-CF and otherwise healthy individuals without CF. Given these defects in glucose regulation and in insulin and incretin secretion, introduction of interventions that enhance incretin effects or reduce β-cell demand early in the development of glucose abnormalities, for example when the 1-h glucose during an OGTT becomes “elevated,” may be beneficial in the prevention of or delay in progression to CFRD.
Supplementary Material
Article Information
Acknowledgments. The authors are indebted to the CF subjects for their participation, to the nursing and dietary staff of the Penn and CHOP CTRCs for their subject care and technical assistance, to Dr. Heather Collins (University of Pennsylvania Diabetes Research Center) for performance of the radioimmunoassays, to Samir Sayed (Children’s Hospital of Philadelphia’s Translational Core Laboratory) for performance of the ELISAs, and to Huong-Lan Nguyen (Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity, and Metabolism) for laboratory assistance.
Funding. This work was supported by the Cystic Fibrosis Foundation (to A.K. and M.R.R.), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK97830 to A.K. and M.R.R., K23DK107937 to S.S., P30DK19525 [University of Pennsylvania Diabetes Research Center], and T32DK007314 [University of Pennsylvania Training Grant in Diabetes, Endocrine and Metabolic Diseases]), the National Center for Advancing Translational Sciences (UL1TR000003 [Penn-CHOP Clinical and Translational Research Centers]), the Joanne and Raymond Welsh Research Fellowship (to L.G.), and the Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity, and Metabolism.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.S. and L.G. participated in the conduct of the study, researched and analyzed data, and wrote the manuscript. D.D.D.L. and D.H. contributed to the design, researched data, and revised the manuscript critically for important intellectual content. C.K., N.K.R., S.C.N., A.J.P., and S.M. participated in the conduct of the study, researched data, and reviewed and edited the manuscript. D.S. researched and analyzed data and reviewed and edited the manuscript. M.C. researched data and reviewed and edited the manuscript. R.C.R., A.K., and M.R.R. designed and conducted the study, researched and analyzed data, and wrote the manuscript. A.K. and M.R.R. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0394/-/DC1.
See accompanying article, p. 20.
References
- 1.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran A, Becker D, Casella SJ, et al.; CFRD Consensus Conference Committee . Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care 2010;33:2677–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681–687 [DOI] [PubMed] [Google Scholar]
- 4.Gudipaty, L, Rickels MR. Pancreatogenic (Type 3c) Diabetes. 2015. Pancreapedia: Exocrine Pancreas Knowledge Base. DOI: 10.3998/panc.2015.35
- 5.Kuo P, Stevens JE, Russo A, et al. . Gastric emptying, incretin hormone secretion, and postprandial glycemia in cystic fibrosis--effects of pancreatic enzyme supplementation. J Clin Endocrinol Metab 2011;96:E851–E855 [DOI] [PubMed] [Google Scholar]
- 6.Moran A, Brunzell C, Cohen RC, et al.; CFRD Guidelines Committee . Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med 2004;21:691–696 [DOI] [PubMed] [Google Scholar]
- 8.Farrell PM, Rosenstein BJ, White TB, et al.; Cystic Fibrosis Foundation . Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008;153:S4–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut 1996;39:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickels MR, Goeser ES, Fuller C, et al. . Loss-of-function mutations in ABCA1 and enhanced β-cell secretory capacity in young adults. Diabetes 2015;64:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest 1992;89:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guldstrand M, Ahrén B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab 2003;284:E557–E565 [DOI] [PubMed] [Google Scholar]
- 13.Gudipaty L, Rosenfeld NK, Fuller CS, Gallop R, Schutta MH, Rickels MR. Effect of exenatide, sitagliptin, or glimepiride on β-cell secretory capacity in early type 2 diabetes. Diabetes Care 2014;37:2451–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward WK, Halter JB, Beard JC, Porte D Jr. Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol 1984;246:E405–E411 [DOI] [PubMed] [Google Scholar]
- 15.Larsson H, Ahrén B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 1998;41:772–777 [DOI] [PubMed] [Google Scholar]
- 16.Vollmer K, Holst JJ, Baller B, et al. . Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57:678–687 [DOI] [PubMed] [Google Scholar]
- 17.Dobson L, Sheldon CD, Hattersley AT. Validation of interstitial fluid continuous glucose monitoring in cystic fibrosis. Diabetes Care 2003;26:1940–1941 [DOI] [PubMed] [Google Scholar]
- 18.Loopstra-Masters RC, Haffner SM, Lorenzo C, Wagenknecht LE, Hanley AJ. Proinsulin-to-C-peptide ratio versus proinsulin-to-insulin ratio in the prediction of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetologia 2011;54:3047–3054 [DOI] [PubMed] [Google Scholar]
- 19.Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest 1975;55:1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polonsky KS, Given BD, Hirsch L, et al. . Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest 1988;81:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643 [DOI] [PubMed] [Google Scholar]
- 22.Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Succurro E, Marini MA, Arturi F, et al. . Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 2009;207:245–249 [DOI] [PubMed] [Google Scholar]
- 24.Sheikh S, Putt ME, Forde KA, Rubenstein RC, Kelly A. Elevation of one hour plasma glucose during oral glucose tolerance testing. Pediatr Pulmonol 2015;50:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran A, Milla C, Ducret R, Nair KS. Protein metabolism in clinically stable adult cystic fibrosis patients with abnormal glucose tolerance. Diabetes 2001;50:1336–1343 [DOI] [PubMed] [Google Scholar]
- 26.Coriati A, Ziai S, Lavoie A, Berthiaume Y, Rabasa-Lhoret R. The 1-h oral glucose tolerance test glucose and insulin values are associated with markers of clinical deterioration in cystic fibrosis. Acta Diabetol 2016;53:359–366 [DOI] [PubMed] [Google Scholar]
- 27.Robertson RP, Bogachus LD, Oseid E, et al. . Assessment of β-cell mass and α- and β-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015;64:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohan V, Alagappan V, Snehalatha C, Ramachandran A, Thiruvengadam KV, Viswanathan M. Insulin and C-peptide responses to glucose load in cystic fibrosis. Diabete Metab 1985;11:376–379 [PubMed] [Google Scholar]
- 29.Moran A, Diem P, Klein DJ, Levitt MD, Robertson RP. Pancreatic endocrine function in cystic fibrosis. J Pediatr 1991;118:715–723 [DOI] [PubMed] [Google Scholar]
- 30.Lanng S, Thorsteinsson B, Røder ME, et al. . Pancreas and gut hormone responses to oral glucose and intravenous glucagon in cystic fibrosis patients with normal, impaired, and diabetic glucose tolerance. Acta Endocrinol (Copenh) 1993;128:207–214 [DOI] [PubMed] [Google Scholar]
- 31.Elder DA, Wooldridge JL, Dolan LM, D’Alessio DA. Glucose tolerance, insulin secretion, and insulin sensitivity in children and adolescents with cystic fibrosis and no prior history of diabetes. J Pediatr 2007;151:653–658 [DOI] [PubMed] [Google Scholar]
- 32.Anzeneder L, Kircher F, Feghelm N, Fischer R, Seissler J. Kinetics of insulin secretion and glucose intolerance in adult patients with cystic fibrosis. Horm Metab Res 2011;43:355–360 [DOI] [PubMed] [Google Scholar]
- 33.Iannucci A, Mukai K, Johnson D, Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol 1984;15:278–284 [DOI] [PubMed] [Google Scholar]
- 34.Abdul-Karim FW, Dahms BB, Velasco ME, Rodman HM. Islets of Langerhans in adolescents and adults with cystic fibrosis. A quantitative study. Arch Pathol Lab Med 1986;110:602–606 [PubMed] [Google Scholar]
- 35.Soejima K, Landing BH. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: morphometric and immunocytologic studies. Pediatr Pathol 1986;6:25–46 [DOI] [PubMed] [Google Scholar]
- 36.Adler AI, Shine BS, Chamnan P, Haworth CS, Bilton D. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care 2008;31:1789–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivier AK, Yi Y, Sun X, et al. . Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 2012;122:3755–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uc A, Olivier AK, Griffin MA, et al. . Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo JH, Chen H, Ruan YC, et al. . Glucose-induced electrical activities and insulin secretion in pancreatic islet β-cells are modulated by CFTR. Nat Commun 2014;5:4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stalvey MS, Muller C, Schatz DA, et al. . Cystic fibrosis transmembrane conductance regulator deficiency exacerbates islet cell dysfunction after beta-cell injury. Diabetes 2006;55:1939–1945 [DOI] [PubMed] [Google Scholar]
- 41.Bellin MD, Laguna T, Leschyshyn J, et al. . Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes 2013;14:417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes D Jr, McCoy KS, Sheikh SI. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. Am J Respir Crit Care Med 2014;190:590–591 [DOI] [PubMed] [Google Scholar]
- 43.Ross SA, Morrison D, McArthur RG. Hypersecretion of gastric inhibitory polypeptide in nondiabetic children with cystic fibrosis. Pediatrics 1981;67:252–254 [PubMed] [Google Scholar]
- 44.Perano SJ, Couper JJ, Horowitz M, et al. . Pancreatic enzyme supplementation improves the incretin hormone response and attenuates postprandial glycemia in adolescents with cystic fibrosis: a randomized crossover trial. J Clin Endocrinol Metab 2014;99:2486–2493 [DOI] [PubMed] [Google Scholar]
- 45.Mohan K, Miller H, Dyce P, et al. . Mechanisms of glucose intolerance in cystic fibrosis. Diabet Med 2009;26:582–588 [DOI] [PubMed] [Google Scholar]
- 46.Moran A, Pyzdrowski KL, Weinreb J, et al. . Insulin sensitivity in cystic fibrosis. Diabetes 1994;43:1020–1026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.