Abstract
Personalized medicine strives to deliver the ‘right drug at the right dose’ by considering inter-person variability, one of the causes for therapeutic failure in specialized populations of patients. Physiologically-based Pharmacokinetic (PBPK) modeling is a key tool in the advancement of personalized medicine to evaluate complex clinical scenarios, making use of physiological information as well as physicochemical data to simulate various physiological states to predict the distribution of pharmacokinetic responses. The increased dependency on PBPK models to address regulatory questions is aligned with the ability of PBPK models to minimize ethical and technical difficulties associated with pharmacokinetic and toxicology experiments for special patient populations. Subpopulation modeling can be achieved through an iterative and integrative approach using an adopt, adapt, develop, assess, amend, and deliver [(AAD)2] methodology. PBPK modeling has two valuable applications in personalized medicine: (1) determining the importance of certain subpopulations within a distribution of pharmacokinetic responses for a given drug formulation and (2) establishing the formulation design space needed to attain a targeted drug plasma concentration profile. This review article focuses on model development for physiological differences associated with sex (male vs. female), age (pediatric vs. young adults vs. elderly), disease state (healthy vs. unhealthy), and temporal variation (influence of biological rhythms), connecting them to drug product formulation development within the Quality by Design framework. Although PBPK modeling has come a long way, there is still a lengthy road before it can be fully accepted by pharmacologists, clinicians, and the broader industry.
Keywords: Personalized medicine, PBPK modeling, special populations, inter-patient variability, sex, gender, age, circadian
Graphical Abstract
Introduction
Inter-person variability can lead to therapeutic failure and adverse effects in individuals or specialized subpopulations of patients during clinical practice [1]. Historically, clinicians were tasked with identifying factors driving differences in patient response by conducting ad hoc post-trial analyses [2]. Currently, researchers are looking to capture this patient variability upfront through personalized medicine, striving to deliver the ‘right drug at the right dose’ by individualizing treatment [3] for the ‘right disease and at the right time’ [4]. While ‘right drug’ emphasizes the need for therapies specifically designed for subpopulations of patients, ‘right dose’ highlights the need to maintain the drug plasma concentration within the therapeutic window to optimize patient benefit and minimize risk [3]. At the individual level, these differences are attributed to a person’s clinical phenotype, collectively defined by the individual’s metabolome, proteome, transcriptome, and genome [3]. As pointed out in a review by Redekop and Mladsi [5], the term ‘personalized medicine’ encompasses a multitude of definitions. While much of the discussion focuses on genetic or genomic based therapies, a broader interpretation for personalized medicine is “the use of combined knowledge (genetic or otherwise) about a person to predict disease susceptibility, disease prognosis, or treatment response and thereby improve that person’s health [5]”. Although individualization may be achievable down to the ‘-omics’ level, significant advances in personalized medicine have been achieved at the population level where subgroups of patients are identified by commonalities in pharmacokinetic exposure and pharmacodynamic response to treatment.
Virtual or simulated clinical trials through physiologically-based pharmacokinetic (PBPK) population modeling of these complex clinical scenarios has yet to be fully explored. However, several reviews on the current state of PBPK modeling point to personalized medicine as a key application [6–8]. PBPK models have successfully been embedded throughout drug development to explore various patient risks factors such as drug-drug interactions (DDI) [9], first-in-human (FIH) drug dosing [10,11], formulation development for better drug absorption [12–16,7,17–24], food effect and gastrointestinal pH analysis [12,14,16,25], late stage development [11], and regulatory science [6,26,27]. Additional studies evaluated the extrapolation of PBPK models to other species [28], and explored sensitivity analysis to understand model robustness and uncertainty [29–33]. In the context of personalized medicine, the aim of the model would be to guide physicians to prescribe the right drug dosing regimen [34,35]. The assumption that a relationship exists between drug dose, physiology, and plasma concentration concurs with current clinical practices such as therapeutic drug monitoring (TDM) which aims at designing and administering a dosing regimen in a way that results in constant drug concentration within a target therapeutic window [36]. As such, model-based drug development would enable clinical trial design to be optimized in special populations in silico, providing a rationale for dose selection and aiding in assessment of patient risk and benefit [37].
This review provides an overview of the utility and implementation of PBPK models in the context of personalized medicine. Model parameterization to account for inter-patient variability in physiological state on the basis of sex, age, disease state, and biological rhythms will be highlighted. The impact of the physiological states will be discussed in the context of the Quality by Design framework through the application of PBPK modeling across the continuum of drug product development.
Evolution of Physiologically-based in silico Predictions
The development of high-throughput screening (HTS) and combinatorial chemistry in the 1990s revolutionized the drug discovery phase of the pharmaceutical industry, enabling the identification of an impressive number of biologically active chemical entities [38]. The remarkable breakthrough gave sudden access to a virtually unlimited quantity of potential drug candidates, but also exposed a new question: how can drug product development keep up with the newly fast and efficient pace of drug discovery? More specifically, how can the in vivo behavior of so many compounds be studied in a fast, efficient, and accurate fashion? Studies showed that the majority of attrition in drug candidates is due to poor pharmacokinetic behavior, making the determination of properties related to ADME (Absorption, Distribution, Metabolism, and Excretion) a crucial step in the process [38,28,39]. Different challenges must be addressed for each classwithin the Biopharmaceutical Classification System (BCS) for orally administered drugs [40]. These classes are defined as the following: Class I (high permeability, high solubility), Class II (high permeability, low solubility), Class III (low permeability, high solubility), and Class IV (low permeability, low solubility. As of late, this problem is becoming increasingly prominent with the rising level of poorly soluble BCS Class II drugs under development, as industry shifts towards manufacturing more chemically stable compounds that allow for once-daily dosing [6]. The conventional way to determine the pharmacokinetics of a chemical compound in vivo is to conduct preclinical studies in animals and, for the most promising drugs, clinical studies in humans. However, these experimental techniques are costly, resource-intensive, lengthy, and most of all not suited for screening large volumes of drug candidates. For example, the US National Toxicology Program conducted toxicity studies on only 600 compounds out of the 80,000+ chemicals in existence in commerce in the last 43 years, representing only a minuscule fraction of the total number of chemicals [41]. Until experimental techniques are able to catch up to the current rate of production, the bottleneck effect clearly prompts an alternative approach. The need for an accurate, efficient method of studying and understanding the behavior of these new drug candidates is apparent.
A potential solution lies in Physiologically-based Pharmacokinetic (PBPK) modeling, making use of physiological information as well as physicochemical data to portray the complex transport processes of a compound throughout the body and to simulate its in vivo performance. The model structure consists of organ and tissue compartments connected by a circuit of flowing blood, subdivided as arterial and venous blood pools. The properties of each compartment are described by a series of differential equations with physiological parameters to represent the system in an integrated and biologically meaningful manner.
PBPK modeling was first presented in 1937 in Teorell’s “Kinetics of Distribution of Substances Administered to the Body” [42]. However, the mathematical complexity of the model exceeded the knowledge and computational power at that time. Today, improvements in computing technology combined with the pressing need to study the pharmacokinetics and absorption of compounds quickly and efficiently enabled PBPK modeling to emerge as a powerful simulation tool to guide pharmaceutical development. The implementation of PBPK modeling fits well with current expectations from the United States Food and Drug Administration (US FDA) in the recent shift towards Quality by Design (QbD) process development. The Quality by Design paradigm aims to facilitate and advance the efficiency and quality of product development while mitigating potential risks associated with the process [43]. PBPK modeling provides a quantitative and mechanistic methodology that connects a drug’s Critical Quality Attributes (CQAs), which encompass a large variety of modeling parameters such as manufacturing criteria and drug-specific properties, to its in vivo behavior as characterized by its Quality Target Product Profile (QTPP). In recent years, studies hailing from academia as well as industry demonstrated how these models can be implemented within the Quality by Design paradigm [44,45] such that PBPK modeling developed intoa powerful and essential tool to increase mechanistic understanding and provide a holistic description of drug exposure in the body.
PBPK models draw data from in vitro experiments as well as in vivo preclinical and clinical data as opposed to traditional data-fitting pharmacokinetic models which follow a ‘'top down’ approach and to ‘bottom up’ approaches which advocate modeling entirely on a virtual basis. Kostewicz et al. classified PBPK modeling as a ‘'middle out’ approach, where the model is built and refined during the drug development process in an iterative manner as more in vitro and/or in vivo data become available [17]. Model parameters sourced from in silico estimations, in vitro experiments and preclinical data can be classified in two broad categories: drug-dependent and system-dependent. For orally administered therapies, which are the focus of this review, drug-dependent parameters can be further subdivided into intrinsic compound properties (molecular weight, diffusion coefficient, solubility over the range of gastrointestinal pH, and ionization) and formulation factors (dosage form and composition, particle size and shape, excipients, tablet dimensions, powder compression force, coating composition, and thickness). Depending on the complexity and flexibility of the model, system-dependent parameters (gastric emptying rate, gastrointestinal fluid pH, intestinal transit and mobility, secretion and reabsorption, intestinal blood flow, bile secretion rate, intake of food and fluids, etc.) can be adjusted to describe virtually any physiology or clinical condition [8]. As such, these properties can also be categorized into intrinsic physiological properties and physiological factors related specifically to the clinical scenario under study. This richness in information is precisely what gives PBPK models tremendous potential for robust simulations and suitability for development of personalized treatments, from the discovery phase through regulatory review. As knowledge of physiology expands and computer simulations improve, PBPK models become crucial in minimizing the ethical and technical difficulties associated with pharmacokinetic and toxicology experiments for special patient populations.
Industrial and Regulatory Perspective
Beginning in the 1990s, the US FDA encouraged the use of modeling and simulation to establish the best dosing strategy and characterize patient risk in a variety of complex clinical scenarios, minimizing the need for animal studies to predict human exposure [6] and enabling streamlined drug development and regulatory review [35,46]. Through model-based regulatory research and clinical trial simulations, traditional drug development and regulatory review is shifting from inefficient and empirical to quantitative and mechanistic, enabling a deeper understanding of the behavior of drugs within the body while offering an explanation for sources of variability in exposure and drug response [47]. The significant advances and improved accuracy in mathematical modeling of physiological processes [48–52,10] promoted PBPK modeling to an important tool within the regulatory review process [53]. The increased dependency on PBPK models to address regulatory questions coincides with the improved ability to simulate the impact of specific diseases states at the individual patient level as well as the population level, the advancement towards improved in silico prediction of physicochemical drug properties, and a deeper understanding of the exposure-response relationship by linking PBPK models with systems biology [6]. This effort has been realized through the exchange of knowledge amongst the US FDA, industry, academia, and patient advocacy groups [35]. Within the US FDA, the Office of Clinical Pharmacology (OCP) is especially important for assuring safety and effectiveness as part of the review process, with emphasis on understanding the balance between benefit and risk in regards to drug-body interactions, inter-patient variabilities, selection of optimal dose and dose regimen, and expansion of clinical knowledge for improved risk assessment [54]. Drug developers benefit extensively from early engagement with the OCP for implementation of these integrative approaches to maximize learning from early-phase clinical trials [55].
Of the 33 Investigational New Drug (IND) and New Drug Applications (NDA) PBPK submissions received by the Office of Clinical Pharmacology from 2008 to 2012, the primary focus was on understanding drug-drug interactions (61%), with lesser importance placed on pediatrics (18%), absorption (9%), hepatic impairment (6%), pharmacogenetics (3%), and drug-drug interactions with other factors (3%) [35]. Thus far, PBPK models enabled identification of critical quality attributes, selection of clinical specifications, support for biowaivers, establishment of in vitro-in vivo correlations, and assessment of risk, amongst other considerations included in the Chemistry, Manufacturing, and Control (CMC) section of regulatory submissions [46]. From a broader context, PBPK models play an extensive role in the design of clinical pharmacology studies, identification of additional studies to address gaps or residual risks, as well as an understanding of risk-benefit relationships for new entities [53]. Regulatory submissions follow the “predict-learn-confirm” paradigm such that discussion is oriented around the specific development and clinical questions to be addressed by the model [56].
These efforts have continued to gain support from the US FDA as evident at the 2014 workshop entitled “Application of Physiologically-based Pharmacokinetic (PBPK) Modeling to Support Dose Selection”, which assessed the current state of simulation in regulatory decision making and discussed best practices for dose selection in specific patient populations [11]. Dr. Janet Woodcock, director of the US FDA Center for Drug Evaluation and Research (CDER), emphasized the importance of using PBPK modeling to address challenges in safety and efficacy during drug product development. Similar support was echoed by Dr. Vikram Sinha, Director of the US FDA’s Division of Pharmacometrics, who identified PBPK as a viable option to answer the following regulatory question: “Since dose-response information of efficacy and safety in special populations is often lacking, what are the options for getting the dose ‘right’ for these groups?” [11]. Patient focus remains of utmost importance to regulatory agencies and as such these predictive tools guide drug development towards an efficacious and safe dosing strategy, leveraging a combination of preclinical, in vitro, in vivo, and in silico methods [35]. In general, industry has low confidence in the current state of PBPK models for special populations due to limited knowledge describing changes in gut and hepatic physiology, such as enzyme and transporter activity in these special groups [57]. Therefore, a deeper knowledge of these specialized physiologies is key for improving confidence in specialized PBPK models and reaching the ultimate goal of personalized medicine. Readers are directed to the review by Jones et al., which summarizes the confidence level, limitations and challenges from an industrial perspective for various PBPK modeling applications [57].
Model Parameterization for Special Populations
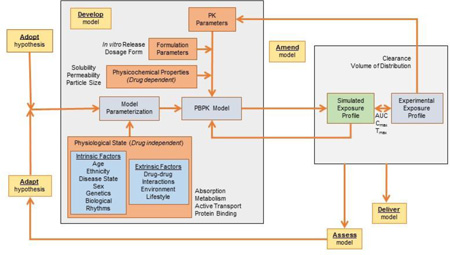
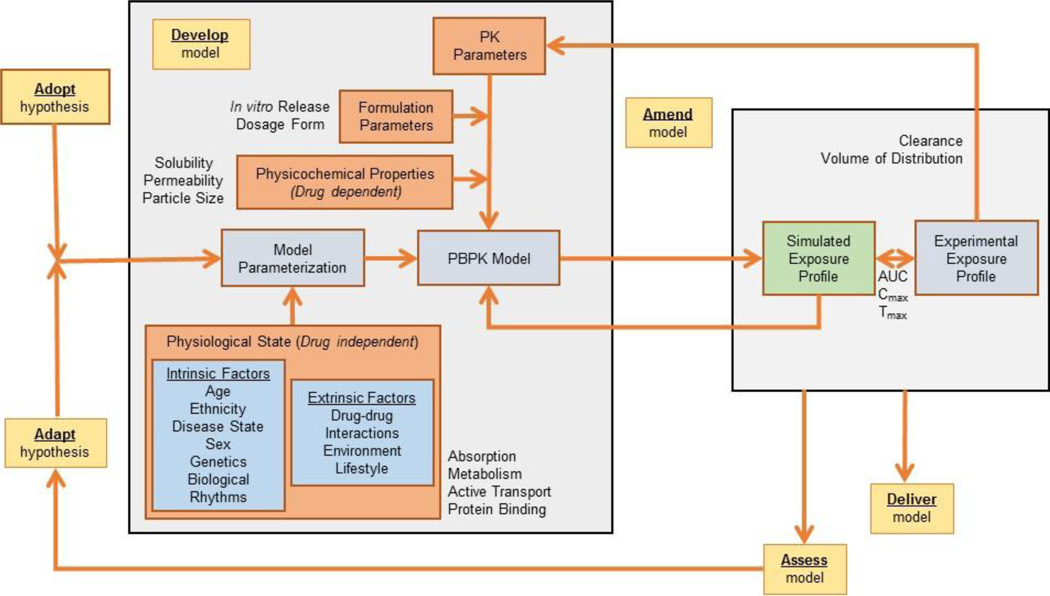
The literature contains several examples of PBPK models assessing phenotype, sex, age, and disease effects on absorption [7,8,58–61,50,17]. All the above examples paving the way towards reaching the ultimate goal of personalized or individualized medicine [62]. Physiologically-based predictions consider the effects of intrinsic factors such organ dysfunction, age, and genetics as well as extrinsic considerations such as drug-drug interactions, environmental effects, and lifestyle choices on absorption and drug disposition [53,63]. Under this context, PBPK modeling is highly complex, aiming to close the knowledge gap between limited experimental data associated with individualized physiology and the action or mechanism of a drug. Extending this approach to the population level, PBPK models consider physiology defined by similar intrinsic and/or extrinsic factors of patient subgroups or special populations to predict drug exposure [56]. Subpopulation modeling can be achieved through an iterative and integrative approach using the adopt, adapt, develop, assess, amend, and deliver [(AAD)2] computational methodology introduced in the framework of systems engineering [64]. Under this approach, an initial hypothesis is adopted and later adapted as knowledge of pathology, physiology, and underlying mechanisms become available. This hypothesis is the foundation from which an integrated in silico model is developed. Model predictions are assessed and the model is amended according to new data and/or model analyses, repeating this loop until the final model is delivered. Subpopulation modeling in the framework of the (AAD)2 methodology is shown in Fig 1. As can be seen, data from multiple scales (in silico estimations, in vitro experimental data and in vivo preclinical information) are integrated into the framework to support the prediction of drug exposure.
Fig 1.
Physiologically-based pharmacokinetics models for special populations within the AAD2 (adopt, adapt, development, assess, amend, deliver) computation methodology, an iterative and integrative approach to model development
Patient risk-benefit should be assessed under in silico conditions that are most representative of the complex clinical scenarios to be faced at later stages in development. Although limited knowledge of the drug’s efficacy and safety are available early in development, physiology is independent of the drug under study and can be incorporated into the model from the start. By capturing these intricacies as early as preclinical development, patient risk is realized sooner and future work can be tailored to address special considerations. However, significant challenges remain for PBPK modeling for special populations due to limited experience to draw conclusions for the effects of pregnancy, ethnicity, geriatrics, obesity, and disease states on exposure [11,65,53]. Therefore, modeling dedicated to address specific subpopulations is a critical interest within the US FDA’s Office of Clinical Pharmacology [55]. Conventional ‘top-down’ modeling has been implemented extensively using empirical data to quantify the effect of intrinsic and extrinsic patient factors on exposure (Cmax, AUC, bioavailability) and disposition (clearance, volume of distribution), but far fewer examples exist for ‘bottom-up’ or ‘middle-out’ modeling of subpopulations. Thus, the success of PBPK modeling in personalized medicine relies heavily on proper identification of patient-specific covariates that explain the observed pharmacokinetic parameters of individuals or subpopulations of individuals [66,67]. These patient characteristics are captured for special populations through model parameterization, which involves the identification of model input parameter values that describe various physiological states. PBPK modeling combines deterministic and non-deterministic components to enable simulation of individual pharmacokinetic exposure and intrinsic population variability. The deterministic component represents the biological and chemical systems of the body whereas the non-deterministic portion describes mathematically the uncertainty, variability, and covariance of the data and parameters in the deterministic model for a given population [68]. The extent of model calibration depends on the objective of the model and rigor needed to capture population dynamics. This review highlights the physiological impact of sex, age, disease state, and biological rhythms on PBPK model development, although several additional covariates exist beyond those discussed herein.
Sex
For the same dosing regimen, women may respond differently to therapies than males, both in regards to the effectiveness of a particular treatment as well as in the extent of observed adverse effects [69,70]. The occurrence of adverse events is 50 to75% higher in females than males [71]. Sex differences in responses are not always consistently reported in clinical studies given the influence of sex hormones during development, the menstrual cycle and oral contraceptive use [72]. Fluctuating levels of hormones contribute to a wider distribution in pharmacokinetic responses for females compared to males. Pregnancy and oral contraceptive use further affect patient response [72]. As a result, females were typically excluded from Phase I and II clinical trials by the US FDA prior to 1993 because of the risk to unborn children and the influence of the menstrual cycle [73]. However, the inclusion of women into clinical studies has become a key consideration in the design of more recent clinical studies given the high frequency of adverse events amongst female populations. The use of virtual or simulated clinical trials for proper dosage selection is especially favorable to sex-dependent pharmacokinetics, as this approach enables the evaluation of patient risk and benefit without engaging actual patients.
Females typically have reduced body weight relative to males. Thus, pharmacokinetic parameters (clearance, volume of distribution) and bioavailability are often normalized by body weight or body surface area to account for differences between male and female subjects. Many times sex-differences are minimized by this correction and deemed statistically insignificant [74]. However, corrections for anatomical differences are not always sufficient to eliminate sex differences completely in both animal and human studies [74–77]. Thus, physiological differences between sexes beyond body weight drive these variations in the rates and extent of absorption and drug distribution, revealing sex-specific bioavailability patterns. Signaling pathways are believed to be structurally similar between males and females, but differentially expressed and regulated by sex hormones [73]. This commonality between sexes enables the same modeling framework (i.e. set of differential equations) to be used for both females and males while requiring adjustment of input parameter values to account for sex-specific physiology.
Influence of Sex on Absorption and Disposition
Beyond body weight, there are several documented physiological differences between sexes, such as gastrointestinal pH, transit time, and volume, along the gastrointestinal tract that drive differences in the bioavailability of oral dosage forms [78,72,79,80]. Women secrete less gastric acid such that drugs with higher solubility in acidic conditions may have lower bioavailability in women than men [71]. Estrogen and progesterone prolong transit time through the gastrointestinal tract [73,81]. All of these differences can be captured in the PBPK model by defining female-specific parameter values for the absorption processes. In general, the influence of sex hormones can be separated into three considerations: (1) passive diffusion which is dependent on gastrointestinal tract physiology, (2) active transport which is affected by expression and activity of intestinal drug transporters such as P-glycoprotein, and (3) gut metabolism which is driven by expression and activity of gastric enzymes and cytochrome P450 isoforms (CYPs) located in enterocytes lining the gastrointestinal lumen [82]. Ultimately, these physiological differences drive the rate and extent of absorption from the gastrointestinal tract. Further sex differences are observed in transport protein expression due to regulation by sex hormones [72,83,60]. Similarly, the extent of plasma protein binding is also affected by sex hormones, driving wider distributions in female patient responses during menses [72,79,73]. These physiological processes are reflected in PBPK models through rate constants, scaling factors, and enzyme or transporter activity coefficients.
Women have a greater proportion of adipose tissue than men. As such, lipophilic drugs may accumulate and have a longer duration of action in women, requiring smaller doses to achieve the same therapeutic effect [71]. Male and female PBPK models would provide an opportunity to explore the sex-dependent interconnectivity between body habitus, drug/formulation properties, and drug exposure. Complex scenarios such as these can be evaluated through calibration of elimination processes for female-specific physiology, accounting for differences in the volume of distribution, ratio of adipose tissue to total body weight, and tissue-specific blood flows.
Sex-dependent Metabolism and Elimination
Metabolism also leads to variability between male and female oral bioavailability due to differing expression levels of hepatic enzymes [84]. This difference becomes an important consideration since drugs are often substrates for specific hepatic enzymes, to the point where certain treatments such as warfarin require different dosage depending on sex [71]. The isoforms of the cytochrome P450(CYPs) or Phase I enzymes in the liver and intestine have marked differences in expression and activity between sexes, further affected by the menstrual cycle, oral contraceptive use, and pregnancy as a result of regulation by sex hormones [85,86,81,72,80,69,79]. Many marketed drugs are substrates for CYP3A4, which is the primary isoform of cytochrome P450, making this enzyme a key contributor to sex-dependent metabolism and ultimately drug clearance. CYP metabolic activity is also affected by age and ethnicity which can lead to differences in clearance for drugs that are highly metabolized by CYPs [86]. Similarly, Phase II enzymes demonstrate sex-specific behavior [80,83,69]. Generally, conflicting findings are presented in the literature for sex-dependent expression and activity of enzymes during animal and clinical studies, which is likely due to the lack of control in regards to hormones levels and small number of women included in these studies. Conversely, PBPK modeling has great potential for predicting exposure in female subjects under targeted physiological conditions to tease out the collective effects of sex hormones on ADME processes and to identify risks associated with specific menstrual cycle phases, oral contraceptive use or even pregnancy. Given the influence of sex hormones on metabolism and elimination, the PBPK model must be carefully calibrated for metabolic enzyme activity and clearance under these distinct physiologies.
In addition to Phase I and Phase II enzymes, uptake and efflux transporters in the gastrointestinal tract and liver influence how much drug reaches systemic circulation. For a drug with high first-pass metabolism, sex-dependent enzyme and transporter activity by sex hormone regulation can lead to significant differences between males and females in bioavailability of oral drugs [82]. For the fraction of drug that escapes first pass metabolism, renal transporters have a key influence on drug clearance. For example, P-glycoprotein (P-gp) is a plasma membrane-bound transporter, which is present in drug-eliminating organs, mainly the liver and to a lesser extent in the intestine [85,87]. Many drugs are substrates to P-gp and thus an important consideration for drug product development is understanding its influence on bioavailability [60,88,85]. This protein has higher expression in males [72,83,60]. Similarly, multidrug-resistant protein transporters (MRPs), organic anion transporters (OATs), and organic cation transporters (OCTs) exhibit sex-specific expression due to differential regulation by sex hormones [82,89]. Incorporation of enzyme and transporter activity into PBPK models is complex for general populations with even greater difficulty for sex-specific mechanisms where little to no data are available. However, the model that describes enzyme and transporter activity is under constant refinement as more in vitro data become available. In vitro metabolism and transport studies can be conducted by determining the value of Michaelis-Menten constants to describe enzymatic activity in cells and extrapolate kinetics to whole organs [90]. If the enzymatic degradation pathways for the drug are well understood, then metabolism can be more accurately modeled based on sex-dependent expression between sexes. Lastly, excretion plays a role in personalized medicine due to the high variability in renal clearance between males and females. In general, renal clearance is known to be higher in men compared to women [71], decrease with age [6,91], correlate with body weight [92], and can be strongly impaired by certain diseases [93]. Thus, sex-dependent clearance values are essential for model calibration since it determines the shape of the latter portion of the plasma concentration profile as drug exits systemic circulation.
Challenges for Sex-specific PBPK Modeling
The development of sex-specific PBPK models is dependent on proper calibration of the model to account for the intrinsic differences in physiology between male and female populations across all ADME processes. However, quantitative differences between genders are rarely discussed in the literature and instead are often presented as a relative comparison (males > females, males < females, males = females). Thus, identifying and estimating sex-specific parameters is at the liberty of the researcher. Parameter sensitivity analysis will be an essential component to sex-specific PBPK model development to build confidence in model predictions, to anticipate the distribution of pharmacokinetic responses in male and female populations, and to establish whether sex-specific values are necessary for all model inputs. Parameter sensitivity analysis can be utilized to establish if the parameter range needed to induce a difference in drug exposure is physiologically relevant or not. Furthermore, the population simulator feature of PBPK software, such as GastroPlus™, can be leveraged to alter the variance associated with input parameters to reflect differences between males and females. To simulate the further spread in the distribution of pharmacokinetic responses due to hormonal fluctuations in females, a larger variance could be used to describe the female population compared to males.
Age
Patient age can strongly influence the way individuals respond to a pharmaceutical treatment due to changes in physiology and biochemical processes during early development and late in life. Furthermore, certain diseases and conditions are more prevalent in younger and elderly populations, which makes understanding age-dependent physiology especially important for proper dose selection at these extremes. Some drugs indicate that dose adjustment for age may not be necessary [94], whereas other drugs require adjustment in older patients [95]. Elderly patients represent a complex and nonhomogeneous population, which makes PBPK-based predictions of exposure difficult considering anagraphic age, frailty, nutritional status, comorbidities, interactions with concomitant drug administration, reduced clearance, lower drug-metabolizing capacity and decreased renal function [96,97]. Simply put, age-related changes in physiology are not consistent across all biochemical processes or organ functions and so some systems are compromised while others remain fully functional [97]. As such, elderly populations exhibit a higher variability in exposure due to differences in aging across individuals. Similarly, pediatric populations are associated with high variability in drug exposure due to differences in development rate. There are limited studies performed in infants and children due to ethical concerns, and so drug developers rely heavily on PBPK modeling for prediction of dose in infants and children. The US FDA strongly supports the integration of PBPK modeling into pediatric drug development for estimation of the starting dose for different age groups, prediction of environmental contaminant exposure, and optimization of clinical drug trial design [98]. However, uncertainty and skepticism regarding the robustness and reliability of predictions in pediatric patients to address efficacy and toxicity-related questions remain [99].
Parameterization of pediatric PBPK models often requires extrapolating from validated adult PK models [99,50] and to a lesser extent from animal studies [100,99]. The adaptation of an adult PBPK model for pediatric populations requires allometric scaling of model input parameters for body weight, height, and age [98]. This scaling method is simply a mathematical relationship accounting for age and anatomy, but does not consider physiological changes in developing children such as organ maturation, blood flow, body composition, and ontogeny of drug elimination and transport mechanisms [99]. Another approach which may be more physiologically-relevant is to utilize empirical regression analysis to account for age-dependent variation in model parameters, with increasing confidence in parameter estimations when expressions are fit directly to infant and children data [101]. Functions that describe the rate of organ development and maturation from pediatric physiology to adult physiology can be incorporated into PBPK models to improve confidence in predictions for developing patients [99,102]. Similar corrections to model parameter values can be made for elderly patients to account for slower biochemical processes due to aging and frailty. Physiologically-based parameterization rather than allometric estimation of parameters would lead to improved confidence in PBPK predictions for these highly variable populations. Thus, a strong understanding of ADME processes within pediatric and elderly patient populations is needed to support model development.
Influence of Age on Absorption and Disposition
Several physiological changes occur during human development that affect absorption from the gastrointestinal tract, suggesting that an age-dependent absorption model would improve predictions of drug disposition, particularly for infants and children [103]. These changes are reflected in the PBPK model by incorporating age-specific dependencies on gastric emptying and intestinal transit time, gastrointestinal volume and flow, effective surface area for absorption due to changes in the radius and length, gastrointestinal pH, and intestinal transporter and enzymes affecting first pass metabolism [99,104,95,103]. Gastric acid secretion and gastrointestinal mobility are known to decrease with age, but have limited clinical significance [95,97]. Thus, age-dependent parameterization for these factors is likely not needed for PBPK model development. As children reach maturity, physiology approaches that of adults. Thus, model parameters for adolescents can safely assume values used to describe adult physiology. In fact, the gastric emptying time, intestinal transit time, gastrointestinal tract pH, effects from bile salts, amongst other factors are set to adult values for pediatric age groups of 10–12 years and 13–17 years of age within the Simcyp® pediatric PK module . This assumption was made on the basis of several clinical studies and resulted in minimal error between simulated and observed plasma concentration profiles [105].
The extent of plasma protein binding is an important consideration in pharmacokinetics as the fraction of unbound drug is considered the biologically or therapeutically active form of a drug, although the clinical significance remains unclear. Plasma protein binding is less in infants and children than adults [99]. Therefore, pediatric patients would likely have a higher exposure unless the dose is adjusted for the fraction of unbound protein, or the biologically active form of the drug, in addition to body habitus. In older adults, there is a similar decrease in the extent of protein binding by albumin and α1-acid glycoprotein [97]. Incorporation of age-dependent protein binding may improve confidence in pediatric simulations, particularly for drugs where the extent of protein binding has a significant effect on exposure and response. Factors that affect drug distribution, such as body fluid compartments and percentage of body fat, are also known to fluctuate with age [99]. Body size is reduced with age, while fat content increases [95,104,97]. Given that body habitus is closely related to the volume of distribution of a drug, drug disposition will be highly dependent on the physicochemical properties of the drug. As such, lipophilic drugs will have a higher volume of distribution in elderly patients due to increased adipose tissue whereas hydrophilic drugs will have a decreased volume of distribution [104]. The volume of distribution is a necessary parameter in PBPK models to ensure proper calibration of elimination processes and so age-dependent values will be needed to reflect drug distribution in pediatric and elderly populations. The interacting effects of body habitus and the physicochemical properties of drug on the volume of distribution highlight the importance of considering several patient factors concomitantly.
Age-dependent Metabolism and Elimination
Furthermore, the extent of metabolism and transporters activity has a strong age-dependency that can significantly impact exposure [99,50]. For example, many marketed drugs are known substrates for P-gp (P-glycoprotein), an efflux transporter that can have a significant effect on bioavailability. Activity of P-gp appears to be tissue specific, indicating increased and decreased activity with age depending on the nature of the study [97]. Similar to sex-specific PBPK modeling, this is an area of weakness for age-dependent predictions as limited data are available to mechanistically describe metabolic and transport processes in pediatric and elderly populations. The metabolic constants, Vmax and Km, of enzymes involved in drug metabolism are determined from isolated enzymes in vitro and then extrapolated to describe metabolic clearance from the whole liver [100]. This in vitro approach avoids the uncertainty associated with interspecies scaling, given that transporter activity is often species dependent and limited knowledge of extrapolation from neonatal animal activity to infants and children exists [100]. As in vitro methods continue to improve and a deeper understanding of metabolic and transporter activity is acquired, PBPK models will be able to better describe these biochemical processes in populations categorized by age. Unlike active transport, passive diffusion or permeability does not appear to change with aging.
Hepatic metabolism decreases with age due to reduced enzyme activity, blood flow and liver volume [104,97]. The activity of CYP1 and CYP2 enzymes are known to decrease with age, leading to reduced drug clearance. Similarly, the activity of CYP3A4, the most abundant isozyme of cytochrome P450, decreases with age [72,80,83,106,105] leading to reduced clearance for drugs metabolized by this enzyme. Ultimately, a reduction in metabolism leads to a risk of higher bioavailability in older populations [95]. This risk of higher exposure is further compounded by polypharmacy in elderly patients in which many individuals are undergoing concomitant therapies [97], representing a key application of PBPK modeling for elderly populations. Despite the significant reduction in Phase I enzyme activity, aging does not appear to alter the activity of Phase II metabolic pathways [95]. Thus, an understanding of the metabolic pathways or elimination routes for a particular drug are needed to ensure the PBPK model is properly calibrated for drug clearance. A confounding factor to be considered in PBPK models for elderly populations is sex-dependent susceptibility to physiological changes. The difference in clearance between men and women tends to be more pronounced in younger populations and less significant in elderly subjects [104], indicating a potentially significant age-by-sex interaction in patient physiology that must be incorporated into the PBPK model. The effects of age on renal clearance in elderly patients are well documented, with reductions in the glomerular filtrate rates in elder populations [100,95,83,104,99]. Similarly the glomerular filtrate rate, renal tubular absorption, and renal secretion are less in infants and children relative to adults [99]. These reductions may be attributed to age-dependent activity of renal transporters which show a decrease in activity from childhood to adolescence [107,95]. Again, these observations confirm the need to incorporate age-specific transporter activity into PBPK modeling framework for pediatric populations. However, the reduction of renal clearance in elderly patients may not be clinically relevant [108] and so uncertainty remains as to the level of scrutiny needed to describe elderly physiology for accurate predictions.
For pediatric populations, PBPK models are calibrated with clearance values scaled down from adults based on age-dependent weight, height, organ weights, and blood flows. The key assumptions in this approach are to assume that metabolic pathways contribute to drug clearance are the same in children as adults, well-stirred model conditions hold, and that enzyme metabolism follows first order kinetics such that the enzymes are not saturated in children [98]. Given that clearance is a key parameter for calibration of elimination process in PBPK models, validation of these assumptions through targeted in vitro or in vivo studies is needed to build confidence in predictions of exposure in pediatric populations. Until proven otherwise, the framework of PBPK models for pediatrics remains structurally similar to the adult model with model parameterization accounting for differences in absorption and drug disposition between age groups.
Disease
Often times drug therapies are administered to patients whose physiology is altered from the healthy state due to disease or morbidities. Animals, largely rats, are used to study the pathogenesis of human diseases and drug release under these altered physiological states [109]. However, the link between rats and humans in regards to pharmacokinetic behavior is not always clear under the disease state. Furthermore, healthy volunteers are commonly used in bioequivalence studies with the key assumption that pharmacokinetic behavior under healthy physiology is similar to the patho-physiologically altered conditions [109]. However, disease, disease state and factors related to disease treatment, such as therapeutic procedures and associated therapies, can have a significant effect on the pharmacokinetic behavior of a drug, requiring dose adaptation to account for modified physiology [110].For example, previous clinical studies revealed differences in the pharmacokinetics of ill, burn, and cancer patients [110]. The influence of disease and morbidities on pharmacokinetic outcomes can be evaluated by incorporating the modified population physiology into PBPK models. These models are often adapted from validated models for healthy populations. Age is a confounding factor in consideration of disease state as most clinical studies use young healthy adults [111]. Elderly populations are more vulnerable to disease and morbidities than their younger counterparts, leading to higher inter-patient variability in treatment response in aging adults [1,112].
PBPK models have been utilized to predict dose and frequency of drug administration for cancer drugs [113,114]. These models, together with oncology and systems biology computation models for tumor growth and cancer progress, form a complex multiscale framework that is powerful in the development of anticancer agents [115]. Anticancer therapies have very specific targets and so understanding tissue-specific drug concentration is important for ensuring that sufficient amount of drug reaches the tumor to induce a biological response. An understanding of how cancer alters patient physiology is needed to improve the accuracy of tissue-specific predictions. Physiological factors such as body fat, organ volume, blood flow, and protein binding, are reduced in cancer patients, whereas metabolic enzyme activity is not altered [116]. Cancer progression is complex and leads to large variabilities in physiological states of patients, confounded by age and comorbidities. Thus, the development of PBPK models for populations with cancer is difficult until more data become available to better characterize the altered physiological state. Prediction of drug exposure during infection and inflammation are also important considerations for disease state modeling, given that this altered state leads to downregulation of metabolizing enzymes such as cytochrome P450 enzymes in the liver and intestine due to elevated levels of pro-inflammatory cytokines [117]. PBPK models can be used to predict the impact of drugs that are primarily eliminated by these enzymes to quantify the risk of overexposure in patients with elevated cytokine levels, such as those with rheumatoid arthritis [117]. Proper dosing of antibacterial therapies is critical as under dosing can lead to further infection and overdosing can cause increased toxicity [110]. Given proper calibration for physiology, PBPK models are advantageous for risk-benefit analysis in the dose selection of these antibacterial therapies to ensure that therapeutic efficacy is achieved while minimizing adverse events. However, accurate predictions of pharmacokinetic exposure during inflammation or infection require an understanding of how these indications influence absorption and drug disposition.
Body habitus of obese patients leads to differences in drug disposition due to a higher amount of fat tissue, requiring adjustment of model input parameters beyond the simple corrections for total body weight. Given the high proportion of fat content, obese patients are likely to have different volumes of distribution of hydrophilic and lipophilic drugs due to variations in tissue-specific accumulation relative to healthy patients. Obesity is associated with glomerular hyperfiltration, but the effects of renal tubular secretion and renal tubular reabsorption are not known. Dosage adjustment is common to account for increased clearance observed in obese populations. However, scaling of renal and hepatic physiological parameters to account for body weight or body surface area is insufficient in obese patients and as such blood flow rate and organ weight to total body weight ratios are often optimized in PBPK models [118,119]. Simcyp® Population-based ADME simulator already has a built-in obese population [120], which can be further optimized as more data become available to describe the physiological impact of obesity on the human body [121]. Obesity can also be recognized as an inflammatory condition associated with elevated cytokine levels, leading to altered metabolic patterns [122–124]. Thus, treatment of obesity as a drug disposition problem is insufficient to accurately describe the physiological state of obese subjects. Incorporating the influence of obesity on metabolic enzyme and transporter activity is needed to improve PBPK predictions of drug exposure and to understand whether dose adjustment would be beneficial to the patient.
Influence of Disease State on ADME Processes
Dose selection for an individual patients is assigned based on the extent of drug clearance for that particular individual to avoid overexposure [110]. Renal impairment, which can significantly reduce clearance, is prevalent worldwide. Regulatory agencies emphasize the need for targeted pharmacokinetic studies to evaluate the risk of systemic overexposure and the potential for dose adjustment in patients with chronic kidney disease [125]. As such, disease state modeling heavily emphasizes understanding the impact of renal impairment [67]. Virtual patient populations with renal impairment (moderate or severe) and liver cirrhosis are currently available in Simcyp® Population-based ADME Simulator [120]. Renal impairment, hepatic impairment, and metabolic enzyme inhibition can lead to increased exposure and ultimately a risk of adverse effects due to a reduction in drug clearance under the disease state [111]. This risk may be amplified by concomitant drug therapies, which contribute to the large variability observed in pharmacokinetic responses for unhealthy populations [111]. Even for drugs which are not eliminated by the kidneys, renal impairment can indirectly affect the pharmacokinetics by altering metabolic and transport mechanisms [125]. Hepatic impairment leads to reduced organ blood flow, hepatocellular functionality and plasma protein binding which limit the ability of the liver to metabolize drugs [37]. Renal impairment is associated with reduced glomerular filtration rate, tubular secretion, and protein binding that collectively leads to reduced elimination. Renal disease can also affect the activity of intestinal and hepatic uptake transporters due to an increase in uremic toxins and chronic inflammation [37]. As such, renal impairment significantly affects drug disposition and is a critical consideration in dose selection to ensure proper drug exposure [126]. Incorporation of these insufficiencies into PBPK models leads to a more accurate prediction of inter-patient variability for the assessment of patient risk and benefit under the diseased state.
The combined effects of hepatic and renal impairment must be considered as both the liver and kidney contribute to the total drug clearance [125]. Drugs are often eliminated partly by urinary excretion and partly by hepatic metabolism [127]. Impairment of both the kidney and liver can have synergistic effect to reduce drug clearance further. For example, patients who have impaired CYP2D6 metabolism, the primary pathway for hepatic metabolism of priodopidine, a new drug for the treatment of Huntington’s disease, depended on renal excretion as the primary elimination pathway. Thus, renal impairment in these patients would result in a higher risk of overexposure than those with a fully functioning CYP2D6 pathway [127]. These impaired conditions are reflected in PBPK models through the model parameters that describe liver and kidney physiology as well as the model input parameters used to calibrate elimination processes for prediction of the entire exposure profile. Model parameterization under this disease state is achieved by extrapolating parameter values from the healthy state to describe the extent of impairment.
In addition to drug clearance, absorption can also be affected by disease and morbidities. Gastric dysfunction, often manifesting as delayed gastric emptying time, can impact in vivo drug release and ultimately bioavailability [109]. Altered intestinal metabolism has been associated with different disease states, such as renal dysfunction, liver cirrhosis and obesity [128]. These conditions modify intestinal enzyme and transporter activity which can lead to substantial differences in bioavailability. Critical illness, such as sepsis or multiple organ dysfunction, are associated with significant changes in passive diffusion through the apical tight junctions of epithelial cells lining the gut wall [129]. These physiological changes lead to increased permeability of the gut wall, resulting in faster absorption of a drug from the gastrointestinal tract. Thus, PBPK model under modified gastrointestinal physiology can be used to study the impact of these conditions on drug exposure. Additionally, gastrointestinal dysfunction may be linked with weight loss, a common comorbidity for patients with Parkinson’s disease [130]. A significant change in body habitus would alter the drug’s volume of distribution, and ultimately the rate of elimination. Given a deeper understanding of how Parkinson’s disease alters physiology, PBPK model may be applied to study the covariates, gastrointestinal impairment and body habitus, to quantify exposure risks in this subpopulation.
Circadian Rhythms
Daily biological or circadian rhythms are recognized as a key contributor to pharmacokinetic variability in clinical studies. As such, these pathological effects are minimized by controlling the time and frequency of administration. Circadian influences on efficacy have been demonstrated for anticancer, cardiovascular, respiratory, anti-ulcer, anti-inflammatory, immunosuppressive, and antiepileptic drugs [131–142]. To date, several therapies have been synchronized with biological rhythms to maximize patient benefit and minimize risk [143,131]. As an example, the synchronization of corticosteroid therapy with the circadian pattern of various cytokines and hormones influencing rheumatoid arthritis disease activity has been well documented [144,145]. In general, chronopharmacokinetics studies the inter-dependent relationship between disease symptoms, risk factors, pharmacologic sensitivity, and ADME processes such that the action (or release) of the drug fluctuates with the circadian rhythm of the morbidity [143]. In addition to influencing the dose-exposure relationship of a drug, circadian rhythms can affect the dose-response relationship and so time-of-day must be taken into consideration when modeling pharmacodynamics [146]. For example, evening dosing of amlodipine, a calcium channel blocker, showed that in addition to reducing elevated blood pressure, fluctuations in blood pressure were dampened following morning dosing [147]. Thus, time of administration for hypertension treatment is an important consideration for maximizing efficacy with further adjustment for sex due to differences in systolic and diastolic blood pressure patterns and heart rate.Circadian-driven variability is a result of fluctuations in several biochemical process controlling absorption and drug disposition, leading to changes in physiology. Gastric pH, acid secretion, motility, and gastric emptying demonstrate circadian rhythms which influence absorption from the gastrointestinal tract [131,147,148]. In practice, tablets administered at night time were shown to have longer gastric residence times and longer colon residence times than tablets administered during the day time due to patterns of bowel movement [17]. Blood flow, peripheral resistances to drug transport, and protein levels and binding also demonstrate fluctuations that alter distribution. Activity and rest periods relative to drug administration can also influence the distribution of the drug throughout the body [131]. Metabolism and elimination are altered by biological rhythms due to changes in perfusion, glomerular filtration rate, urine excretion rate, urine pH, and electrolyte balances [131,147,148]. Furthermore, circadian rhythms may modulate carrier-mediated transport activity significantly, leading to differences in absorption, intestinal metabolism, or even renal clearance [149,150,133]. Bioavailability of drugs that are P-glycoprotein substrates may have a dependence on circadian rhythms. Studies have shown that there is a link between circadian oscillation and time-dependent dose pharmacokinetic changes in rodents, but extrapolation from nocturnal rodents to diurnal humans is difficult [151]. PBPK modeling may be leveraged to study these circadian effects in humans by establishing time-dependent parameter values and running simulations over shorter time periods to reflect morning, afternoon or evening administration.
Overcoming Circadian Influence through Formulation and Dosing
Synchronization of drug concentration to rhythms in disease or morbidity activity is achieved by carefully timing administration of formulated tablets or through special drug delivery systems with controlled release profiles [152,153]. In this context, formulation effects are an important consideration for chronopharmacokinetics. For example, lipophilic drugs are likely to absorb faster following morning administration relative to evening. Similarly, pharmacokinetic exposure is influenced by the extent of plasma binding which fluctuates differently for acidic or basic drugs [131]. Biological rhythms are likely more important for controlled-release formulations that result in sustained, rapid or pulsatile release depending on time of administration relative to biological cycles [154]. Controlled release for oral dosage forms is achieved through various drug delivery mechanisms including layered systems, enteric coatings, and press coated systems [143]. Drug release from such systems can be predicted through PBPK modeling, considering the physicochemical properties of the drug, the in vivo release profile, and physiological state of virtual patients. Thus, PBPK modeling is a critical tool in chronopharmacokinetics to determine the influence of circadian rhythms on dose-exposure-response relationships by treating model input variables with time-dependent values in accordance with physiological changes associated with internal biological rhythms or environmental influences (light). These models can tease out the effects of drug or formulation properties on release in accordance to changes in the ADME processes. Peng et al. successfully demonstrated how PBPK modeling could be utilized to predict the plasma concentration of melatonin, a compound with strong circadian rhythms [155]. This goal was achieved by de-lumping tissues (salivary and pineal gland) into individual compartments on the basis of strong circadian effects due to light. The model demonstrated how delivery of exogenous melatonin as a controlled release formulation could mimic the endogenous rhythms [155].
Other Biological Rhythms
Biological rhythms exist with phases that extend beyond 24 hrs. A key example of such influence is the menstrual cycle which has a significant impact on pharmacokinetics in women. This 28-day biological cycle is associated with hormone surges that prolong gastrointestinal transit time, influence plasma protein binding, and alter metabolizing enzyme activity, amongst other factors, leading to significant differences in bioavailability [73,81,69,156,86,157]. Thus, PBPK modeling provides a way to study variability in pharmacokinetic responses due to sex hormone fluctuations by changing physiological input parameters in accordance with the menstrual cycle. Similarly, circannual rhythms of disease-related symptoms have been documented for chronic immune and inflammatory conditions, particularly for rheumatoid arthritis in which patients are seasonally affected [145]. Again, PBPK models can be utilized to study these intrinsic or externally influenced rhythms by establishing the link between pathology and physiological with fluctuations in ADME model parameters throughout the seasons. In the broader context, biological rhythms due to intrinsic and extrinsic (or environmental) influences are achieved through time-dependent model parameterization, that is, model parameters are no longer treated as constant values for a given population. To date, this application of PBPK modeling is an underdeveloped area that requires a better understanding of rhythmic influences for accurate predictions of temporal variations.
Formulation Considerations for Special Populations
The extent and rate of absorption of an orally administered drug is thought to have a greater impact on bioavailability than post-absorptive processes, such as drug distribution and clearance [158]. Drug absorption depends on physicochemical properties of the drug, characteristics of the formulation, and interplay with the underlying physiological properties of the gastrointestinal tract [17]. In particular, three major factors that determine the rate and extent of intestinal absorption are the dissolution rate, solubility, and intestinal permeability [16,159]. Absorption is highly dependent on gastrointestinal physiology, which contributes significantly to the observed distribution in bioavailability for a population. The previous sections described model parameterization, independent of the drug under study, to establish population-specific gastrointestinal physiology on the basis of sex, age, disease and temporal variations due to biological rhythms. Necessary physiological input data included enzymatic constants for metabolism, passive diffusion clearance, transport clearances for influx or efflux at the basolateral membrane, transport parameters related to the apical membrane for secretion of the eliminating organ, and estimates of tissue to plasma or blood partitioning coefficients for weak bases and acids [160]. Built in physiological parameters, such as gastrointestinal transit time, pH, absorptive surface area, bile salt concentrations in each compartment, pore size and density, compartment dimensions, and fluid content are assigned default values that can be modified by the user to reflect specific physiologies [17]. Given an understanding of population-specific physiology, absorption can be optimized by altering drug-dependent factors to ensure the desired bioavailability is achieved. PBPK modeling is a critical tool to link the influences of drug and formulation input properties with physiology to achieve personalized medicine. Several drug and formulation dependent factors greatly affect exposure, including intrinsic drug properties and derived formulation properties, such as disintegration of the dosage form and drug dissolution. Key input data related to formulation are the drug aqueous solubility-pH relationship, permeability, particle size distribution, and targeted release profile (immediate, controlled, delayed). PBPK modeling can be used to evaluate primary and higher level interactions between these properties under specific physiological states, exploring the link between formulation and exposure in special populations.
Influence of Solubility and Lipophilicity on Absorption
The in vivo release rate of a drug is contingent on solubility, which is often a function of gastrointestinal pH due to changes in the degree of drug ionization [161]. In general, drug release in gastrointestinal fluid is better for charged species while permeability through cell membranes favor neutral species [159]. Poor compound solubility at physiological pH is a major hurdle for formulators. In addition to the physical properties of the drug (i.e. particle size) or tablet (i.e. shape) [162], solubility can also be affected by polymorphism, hydration state, and the nature of excipients [162,21,159]. Crystalline forms tend to be more soluble than amorphous forms, and anhydrous forms are more soluble than hydrated states. Many drug candidates have high molecular weight, high hydrophobicity, and many hydrogen bonds, leading to poor solubility in water [163]. The degree of ionization (polarity) of a drug depends on its acid dissociation constant (pKa), which is the pH at which the concentrations of ionized and non-ionized drug forms are equal. If the pKa of a drug is equal to the physiological pH, then 50% of the drug is ionized and 50% is non-ionized. In an acidic environment like the stomach, weakly acidic drugs are present in non-ionic forms while weakly basic drugs are ionized, leading to higher absorption for weakly acidic drugs. In the intestine, weakly basic drugs are present in non-ionic forms while weakly acidic drugs are ionized, leading to higher absorption for weakly basic drugs [164]. Variations in gastrointestinal pH will, therefore, affect the rate of absorption and contribute to the observed distribution of pharmacokinetic responses for a population.
Considering sex as an example, studies showed that women typically exhibit higher pH levels in the stomach compared to men [78,71]. As such, varying in vivo release rates can be expected for drugs with pH-sensitive solubility. Since all formulations cannot realistically be tested in vivo, in vitro dissolution testing serves as a surrogate technique to understand and compare how formulation and drug properties influence solubility. In turn, in vitro dissolution profiles become key input to PBPK models to account for formulation effects. Emphasis is placed on customizing the dissolution method to emulate the physiology of interest to better capture the anticipated in vivo behavior. For a sex-dependent dissolution study, the composition of the female-specific biorelevant dissolution medium must be formulated with higher pH compared to the male-specific biorelevant dissolution medium, as done in a study by Magallanes et al. [165]. Therefore, these biorelevant dissolution profiles can be used as PBPK model input to evaluate the clinical significance of physiological differences, to establish the extent of sex-dependent differences in absorption, and to enable detection of population-specific risks for a given dosing regimen.
A drug’s lipophilicity is highly informative for determining ADME behavior and overall suitability of drug candidates. The degree of lipophilicity is affected by intermolecular interactions which are largely dependent on the molecular structure, including positional isomerism, stereoisomerism, ionization, and molecular size [166]. Lipophilicity affects a drug’s solubility, permeability through membranes, potency, selectivity, promiscuity, metabolism, and ultimately the PK/PD/toxicological profiles [167,168]. Most lipophilic or non-ionized compounds are believed to diffuse rapidly across the cellular membranes of the intestinal epithelium, which is the main barrier for oral drug absorption [169]. Lipophilicity is reflected in the PBPK model by the drug’s partition coefficient (log P), which is the logarithmic ratio of drug concentrations in polar (water) and non-polar (octanol) phases. In general, a higher log P value is associated with improved drug distribution and clearance, where lower log P is associated with improved solubility, and reduced CYP inhibition [168]. Extremely lipophilic compounds are associated with low metabolic clearance, in vitro receptor promiscuity (undesirable interaction of drug with off-target proteins), and in vivo toxicity [167,168]. Continuing with the sex differences as an example, the distribution of a drug can vary considerably in males and females depending on its hydrophilicity and lipophilicity. Lipophilic compounds tend to distribute to and collect more in adipose tissue, leading to reduced circulation to other tissues. Since women typically have more adipose tissue than men, they tend to express lower plasma concentration and longer duration of action than men for lipophilic drugs such as benzodiazepines [71]. By contrast, a hydrophilic drug will exhibit a higher plasma concentration in females compared to males because males have a larger volume of body water than females and thus an increased volume of distribution. Similarly, age can contribute to different drug exposure due to the increase in adipose tissue in elderly populations. This fact must be kept in mind during the formulation stage of drug product development as the same dose for a highly hydrophilic or lipophilic compound can lead to very different drug exposure between sexes or in elderly patients.
Formulation Strategies for the Biopharmaceutical Classification System
Different formulation challenges are associated with the four classes of the Biopharmaceutical Classification System (BCS). BCS Class I drugs are characterized by high solubility that is independent of pH within the range of 1 to 8, which includes the physiological pH of the stomach, duodenum, and upper small intestine [170]. Therefore, the in vivo release of a BCS Class I drug from an immediate release formulation is expected to be rapid and not affected by physiological pH. With minimal absorption in the stomach, gastric emptying is likely to control the rate of absorption in the intestine rather than formulation effects unless a modified release profile is targeted [171,40]. Considering physiological influences of sex, age, and disease on gastric emptying, differences in absorption rate are anticipated for BSC Class I drugs across special populations. PBPK modeling could be leveraged to identify the clinical significance of these differences and the need, if any, for dose adjustment.
For a BSC Class II drug, solubility is low and likely to be pH dependent within the physiological range [172,173]. Class II drugs can be separated further into subclasses dependent on the drug’s pKa: weakly acidic (pKa ≤ 5, Class IIa), weakly basic (pKa ≥ 6, Class IIb), and neutral (Class IIc). Class IIa drugs are soluble at intestinal pH, whereas Class IIb are soluble in the stomach and may precipitate at intestinal pH. The solubility of Class IIc drugs is low at all physiological pH. Across all BCS Class II subclasses, the rate-limiting step to absorption will be the in vivo release. Thus, the interaction between gastrointestinal physiology and formulation will greatly affect the in vivo release profile. Sensitivity to formulation was previously shown for naproxen and ibuprofen, both BCS Class II drugs that have been extensively studied [174–176]. As such, subtle differences in gastrointestinal physiology may lead to significant differences in the release rate for the same formulation between patient subgroups. Drug developers counteract low solubility by formulating BCS Class II drugs in solubilized forms, as salts, or with reduced particle size to increase the rate of absorption [177,178]. Additional formulation strategies to improve solubility include precipitation inhibitors, metastable forms, solid dispersion, complexation, and lipid technologies [179]. PBPK modeling could be implemented to evaluate the sensitivity of special populations to these changes in solubility, determining the influence of specific gastrointestinal physiological conditions on the spectrum of pharmacokinetic responses, and the inherent sensitivity of exposure to inter-patient variability. Thus, PBPK modeling can prove useful for confirming that formulation design is actually capable of achieving the desired release rate and bioavailability in special populations by linking formulation physicochemical properties with the physiology under question.
The rate-limiting step to absorption of BCS Class III drugs is permeability, which presents a different set of challenges for formulators [40]. For this class of drugs, sufficient solubilized drug will be present in the gastrointestinal tract lumen, but active transport will be needed to overcome the intrinsic properties of the drug that contribute to poor permeability. Therefore, BCS Class III drugs are formulated to improve active transport [180]. These drug are usually poorly metabolized and eliminated primarily unchanged by renal and/or biliary routes. Given the extensive differences in transporter activity and clearance across physiologies, formulating BCS Class III drugs to overcome these challenges will be especially important for special populations. Formulation approaches to improve permeability include prodrugs, permeation enhancers, and ion pairing [179]. The ability of these changes to improve permeability in different physiological states can be evaluated using PBPK modeling. Finally, BCS Class IV drugs are generally associated with poor bioavailability due to low permeability and solubility [40]. Formulation must address both solubility and permeability challenges of which PBPK modeling can prove useful in understanding the impact of physicochemical properties. Formulation Design under the Quality by Design Paradigm
Kesisoglou and Mitra demonstrated how PBPK modeling could be implemented in the rational design of drug product under the Quality by Design (QbD) paradigm with the goal of linking the product critical quality attributes (CQAs) to the quality target product profile (QTPP) [44]. The first study evaluated the effects of gastric pH on absorption of a BCS Class II/IV compound relative to the QTPP, prior to first in human studies. Higher gastric pH reduced bioavailability, requiring the drug be formulated in such a way that mitigates pH sensitivity [44]. Since gastric pH is a common source of difference between special populations, this case study provides an example of how a drug may be formulated to accommodate these differences. In another study, the optimal drug release rate of a BCS Class II drug from a controlled release tablet was identified to maintain a specified plasma concentration 24 hours after administration and ensure Cmax did not exceed a given value. An immediate release formulation led to high Cmax, adverse side effects, and low concentration after 24 hrs whereas controlled release formulations were able to meet this criteria [44]. This example can be used to understand how controlled-release formulations may be adjusted to maintain the desired drug plasma concentration within the therapeutic window of a given population. The third case study assessed the impact of drug substance particle size and distribution of a low solubility compound on bioavailability during late stage formulation development. At low dose, minimal impact to bioavailability was observed whereas bioavailability was reduced at a higher dose with increasing particle size [44]. The sensitivity to particle size may vary across special populations due to differences in gastrointestinal physiology and so PBPK model can be leveraged to elucidate the contribution that particle size has to population variability. A fourth study explores the impact of drug form (salt vs. free base) in Caucasian and Japanese patients. Bioavailability decreased for larger free base fractions compared to the salt form. Simulations showed that higher stomach pH, common in Japanese populations, led to a decrease in absorption [44]. These case studies are excellent examples to demonstrate how PBPK modeling can be used to assess formulation risk and rationalize development decisions under the Quality by Design paradigm. Case studies such as these can easily be extended to other physiological states to address similar questions for special populations, enabling a better understanding of the anticipated variability and pharmacokinetic response spectrum in a clinical trial.
Implementation of PBPK Modeling for Personalized Medicine
A calibrated PBPK model for adesired physiology offers great potential for streamlining formulation design and understanding patient pharmacokinetic responses. Among other applications, the mechanistic aspect of PBPK modeling enables its utilization for personalized medicine in two valuable approaches: (1) determining the level of importance certain populations have within a distribution of pharmacokinetic responses for a given drug formulation and (2) establishing the formulation design space needed to attain a targeted drug plasma concentration profile. The first application leverages the predictive capabilities of PBPK modeling and its ability to adapt physiological parameters to represent specific populations to gain a better understanding of how various sections of the pharmacokinetic response distribution can be classified according to subpopulation effects. The second application adopts a reverse approach where a deconvolution technique is used to determine the formulation needed to match a desired plasma concentration profile for a special population. The latter can be particularly valuable when designing a product for a distinct group of patients, such as therapies for pediatric care.
Determining the Impact of Population Variability for a Given Formulation
Clinical studies are essential to maximizing knowledge about a drug’s possible mechanisms in the human body. In order to be truly effective, clinical design should ensure that participants represent the entire population in an unbiased manner by including patients from diversified profiles in terms of age, sex, ethnicity, extent of disease, and other special population classifications. The entire populationcan be incorporated in a PBPK model by modifying the physiological parameters that manifest variability between different special populations. For example, GastroPlus™ allows the generation of virtual clinical trials for overall or generic randomized populations as well as user-defined subpopulations [183]. Randomized population models can be conducted using default parameters from human GastroPlus™ physiology through adaptation of the coefficient of variation (CV %) with a larger coefficient of variation correlating to wider distribution of input parameter values. Special population simulations are more complex since user-defined physiological and pharmacokinetic parameters are generally required. Thus, a significant portion of model development and validation is spent on model parameterization. These parameters can be especially difficult to source from publications for newer or less established drugs, leading to greater uncertainty in predictions. In some cases, the difficult search for physiological parameters can be circumvented by making use of Simcyp®. Simcyp® contains a subpopulation virtual trial feature and is widely used for subpopulation modeling since it partially predefines physiological and pharmacokinetic settings for gender and some ethnicities (European Caucasian and Japanese), as well as a specific interface for pediatric applications [184]. Several studies demonstrated the use and accuracy of these special population resources [44,185,186].
Overall population simulations for drug exposure can exhibit a wide distribution of pharmacokinetic responses for a given drug formulation. Repeating the simulation for the same formulation, but considering a different subpopulation physiology can help match and explain which subpopulations contribute to certain regions of the exposure spectrum for the larger generic population PBPK models could be used to correlate certain areas of the response spectrum with patient features such as age, sex, and disease state‥ For example, if an overall population simulation for a highly lipophilic drug shows a wide range of plasma concentration profiles, lower exposure may be affiliated with women while the higher exposure may be associated with men as more drug would be distributed to and accumulate in the increased adipose tissue of women.
Establishing the Formulation Design Space to Attain a Target Exposure As opposed to the first application which proposes a forward method involving the prediction of in vivo exposurefor a defined formulation, an alternative method of applying PBPK modeling is to reverse the process and use models to predict how a formulation should be manufactured to achieve a target in vivo profile. If a target bioavailability, Cmax, and Tmax are known for a given drug, a PBPK model can be used to determine the formulation design space which will yield the desired pharmacokinetic profile. This aspect of PBPK modeling has the potential to greatly optimize and improve the efficiency of drug design so that fewer formulations would need to be tested in clinical trials. In terms of personalized medicine, this concept can prove to be particularly useful in pediatrics, where differences in physiology between children and adults are significant enough that dosing for some drugs cannot simply be scaled according to body weight but ethical reasons often complicate the ease of clinical trials. A PBPK model suited to a child’s physiology could be used as a way to predict the ideal formulation design space for a target pharmacokinetic profile.
Starting from a desired or observed plasma concentration profile, deconvolution can be used to calculate the corresponding in vivo drug release profile directly from the target plasma concentration profile [183,187]. The in vivo release profile provides insight into the mechanism of absorption (i.e. zero order, first order, etc) and elucidates in vivo effects of formulation on solubility and permeability [188]. From this target in vivo release profile, a manufacturing design space can be established to describe how a tablet must be produced in order to obtain the desired in vivo performance. This goal is achieved in several steps. First an in vitro-in vivo correlation (IVIVC) is needed to link the in vivo release profile to in vitro dissolution data. A point-to-point relationship is needed to predict the entirety of the in vitro dissolution profile for future formulations and thus a Level A IVIVR will be required [173,189]. Once the target in vitro dissolution profile is determined, experimental studies can be completed to determine the formulation and process conditions that are needed to achieve the desired rate. If an in silico approach is preferred, available software, such as the Optimization Module in DDDPlus™, may be used to translate the obtained release profile into manufacturing parameters such as tablet porosity, compression force, and particle size [190]. When put into perspective with personalized medicine, this reverse methodology can be applied for any subpopulation given an exposure profile (either simulated or experimental) for certain subpopulations, leveraging mathematical deconvolution and established IVIV correlations to relate in vivo release back to in vitro dissolution for the populations of interest.
Challenges and Opportunities
Although PBPK modeling has come a long way due to the advancements in technology and computing power, the emergence of user-friendly software, and improvements in in vitro methodologies, there is still a lengthy road before acceptance by pharmacologists and clinicians as a tool with the ability to replace clinical experiments completely. Currently, in silico models cannot predict bioavailability as well as in vitro tests, but can still be used for qualitative analysis. One study showed 84% successful prediction for in vitro tests for apparent permeability and intrinsic clearance compared to 53% accuracy for in silico models within ±20% acceptance interval [191]. Much of the hesitancy towards physiologically-based modeling originates from doubt regarding the degree of accuracy and reliability of its input. This skepticism is understandable since a PBPK model is only as accurate as the quality of its input parameters. Therefore, if the incorporated data contain errors, inconsistencies or miscalculations, the resulting simulations may not truly emulate in vivo conditions and performance. Inaccuracies in input data may arise from a variety of reasons such as the high inherent variability of biological systems, imperfect experimental instrumentation, misinterpretation of collected data, or a fundamental misunderstanding of the drug compound [39,38]. Additionally, the severe lack of detailed databases for physiological parameters, particularly for specialized subpopulations, requires that much of the input data are drawn from several sources, which further increases the possibility of inconsistent information [50].
Incorrect model input may also stem from the significant gap in knowledge surrounding certain sections of the human physiology/anatomy. Literature reviews exposed limited understanding in the lower small intestine and the colon [17], issues in accurately estimating human drug clearance [28,10,6], and a lack of comprehensive knowledge regarding drug metabolism and transport. While PBPK simulations typically show adequate correlations between expected and observed plasma concentration profiles for drugs with passive transport mechanisms, poorer correlations are often obtained for actively transported drugs [38]. The complexity of the transport phenomena occurring throughout the body translates to an equally intricate model composed of a large number of mathematical equations with a multitude of parameters. These gaps in knowledge, particularly with respect to metabolism and transporters, prove to be especially limiting in the field of personalized medicine, since these parameters are precisely what cause differences in physiology and thus variable responses between special populations [50]. Other poorly characterized physiological processes, such as drug absorption in newborns and infants, could also hinder the development of a drug adapted to the pediatric population [50].
Often times, pharmacokinetic properties used for calibration of the model are obtained from allometric scaling of animal studies or extrapolated from general populations other than the clinical scenario under evaluation. Scaling of these parameters demonstrates the complexity of prediction for humans, which is even more complicated in PBPK modeling given the increasing number of physiologically-based constants to describe absorption and the vast differences between species. Thus, interspecies allometric scaling and single species methods, such as linear scaling or direct proportionality, represent another variability in model input [192–194]. Conducting a sensitivity analysis to establish the influence of certain parameters on the predicted drug exposure can aid in understanding the strength of the model [31,195,196,33]. To date, no perfect PBPK model exists. However, as knowledge about ADME processes improves and as input parameters become more closely linked with biologically-representative in vitro data, PBPK models will become the key to effective and efficient drug manufacturing for the benefit of the patient.
Another challenge involves linking pharmacokinetics with pharmacodynamics. Much emphasis is placed on the prediction of exposure from PBPK models. However, this understanding needs to be extended to determine the exposure-response relationship, which is equally important to understanding the impact that a particular dose has on the efficacy of a drug. For example, methylprednisolone, a drug metabolized primarily by CYP3A isoenzymes, demonstrated sex-specific pharmacokinetics in regards to clearance and AUC, consistent with greater expression of CYP3A in women and increased metabolism compared to men [70]. However, women were shown to be much more sensitive to the effects of this drug. The reduced pharmacokinetic exposure balanced the increased response sensitivity so that the same dose had similar impact on cortisol secretion between sexes [70]. This study identifies yet another challenge for personalized medicine, indicating that the physiological state not only affects absorption and drug disposition, but also the mechanistic action of the drug and ultimately patient pharmacodynamics. To truly achieve personalized medicine for special populations, the interconnectivity between drug dose, exposure and response must be recognized.
Despite several challenges associated with PBPK modeling for special populations, in silico prediction presents several opportunities for the advancement of personalized medicine. A recent paper by Kesisoglou et al. evaluated five case studies to understand the impact of biopharmaceutics on formulation strategies across the drug development continuum from first in human (FIH) to late-stage development: (1) prediction of absorption prior to FIH studies; (2) optimization of formulation and dissolution method post-FIH data; (3) early exploration of a modified-release formulation; (4) addressing bridging questions for late-stage formulation changes; and (5) prediction of pharmacokinetics in the fed state for a BCS Class I drug with fasted state data [24]. These case studies can be applied to various physiological states to establish and explain the anticipated spectrum of pharmacokinetic responses. PBPK modeling for special populations can assess risk prior to FIH studies, enable formulation development and optimization for subgroups of patients, and evaluate whether dose adjustment is needed for certain subgroups of patients. When leveraged throughout drug product development, PBPK models can address several key patient-centric regulatory considerations to maximize therapeutic effectiveness and safety. PBPK models enable enhanced specificity of diagnostic and therapeutic technologies, differentiation of responders from non-responders, reduced inter-patient variability, and fewer adverse events [4]. A key opportunity for PBPK modeling is the evaluation of complex clinical scenarios associated with ethical concerns when testing in actual patients. One such consideration is pregnancy, which alters the physiology and pathology of women significantly, a special population that remains to be fully studied [197]. The full potential of PBPK models in translational research and formulation development for personalized medicines has yet to be realized.
Perspective and Outlook
Personalized medicine remains a very current and pressing challenge, despite a strong interest from researchers and physicians for decades. As knowledge in the field advances, the need for physiologically-suited therapies is more and more apparent. Important physiological and pharmacokinetic differences exist between patients according to sex, age, ethnicity, disease state, and pregnancy. This review article focused on the physiological differences associated with sex (male vs. female), age (pediatric vs. young adults vs. elderly), disease state (healthy vs. unhealthy), and temporal fluctuations (time of day, month, year), connecting them to drug product formulation development through PBPK modeling within the Quality by Design framework. Inter-patient and inter-subpopulation variability can be difficult to represent in clinical studies without very large numbers of patients and can be further complicated by technical and ethical obstacles for certain subpopulations such as pediatrics. For these reasons, clinical studies often omit or include limited numbers of patients whose physiological backgrounds may lead to higher variations in data. These complexities hinder the progress of personalized medicine which prompts for an alternative approach to lessen the financial and ethical burden of experimental trials. A solution lies in PBPK modeling, which offers a mechanistic approach to create virtual population trials with user-defined patient physiology from which oral bioavailability can be predicted in silico. These models leverage the interplay between drug specific characteristics and human physiology, portrayed by the model input parameters, to emulate specific physiological or pathological conditions and can aid in the development of a formulation for that particular physiology.
PBPK models operate under the AAD2 methodology which enables the tuning and refining of their inputs and structure to obtain biologically significant and accurate representations of the drug and population of study. Some improvements are still needed before PBPK models can be used as standalone evidence, particularly with respects to specific knowledge gaps in human anatomy. However, recent advancements in technology like the development of user-friendly PBPK software and improvement of in vitro methods, have clearly enhanced the capabilities of PBPK modeling as a tool for effective and efficient drug manufacturing for the benefit of the patient. Rowland et al. summarized this objective by stating that “One day, when sufficient information is available in a patient, clinicians will be able to link that person to his or her virtual twin within a PBPK model to provide safe, effective, individualized dosage” [6]. This concept lies at the heart of personalized medicine which emphasizes the goal of delivering the “right drug at the right dose at the right time”.
Acknowledgments
CH is supported by a US Department of Education GAANN grant to the Department of Biomedical Engineering at Rutgers University (P200A150131). MS is supported by a Bristol-Myers Squibb Doctoral Fellowship. IPA acknowledges support from NIH Grant GM 24211
Footnotes
Conflict of Interest
None
References
- 1.Noetzli M, Eap CB. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer's disease. Clin Pharmacokinet. 2013;52(4):225–241. doi: 10.1007/s40262-013-0038-9. [DOI] [PubMed] [Google Scholar]
- 2.Schork NJ. Personalized medicine: Time for one-person trials. Nature. 2015;520(7549):609–611. doi: 10.1038/520609a. [DOI] [PubMed] [Google Scholar]
- 3.Lesko LJ, Schmidt S. Individualization of drug therapy: history, present state, and opportunities for the future. Clin Pharmacol Ther. 2012;92(4):458–466. doi: 10.1038/clpt.2012.113. [DOI] [PubMed] [Google Scholar]
- 4.Waldman SA, Terzic A. Patient-centric clinical pharmacology advances the path to personalized medicine. Biomark Med. 2011;5(6):697–700. doi: 10.2217/bmm.11.78. [DOI] [PubMed] [Google Scholar]
- 5.Redekop WK, Mladsi D. The faces of personalized medicine: a framework for understanding its meaning and scope. Value Health. 2013;16(6 Suppl):S4–S9. doi: 10.1016/j.jval.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. doi: 10.1146/annurev-pharmtox-010510-100540. [DOI] [PubMed] [Google Scholar]
- 7.Chetty M, Rose RH, Abduljalil K, Patel N, Lu G, Cain T, Jamei M, Rostami-Hodjegan A. Applications of linking PBPK and PD models to predict the impact of genotypic variability, formulation differences, differences in target binding capacity and target site drug concentrations on drug responses and variability. Front Pharmacol. 2014;5:258. doi: 10.3389/fphar.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, Tucker G. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11(2):225–237. doi: 10.1208/s12248-009-9099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenel M, Bouzom F, Aarons L, Ogungbenro K. Drug-drug interaction predictions with PBPK models and optimal multiresponse sampling time designs: application to midazolam and a phase I compound. Part 1: comparison of uniresponse and multiresponse designs using PopDes. J Pharmacokinet Pharmacodyn. 2008;35(6):635–659. doi: 10.1007/s10928-008-9104-6. [DOI] [PubMed] [Google Scholar]
- 10.Jones HM, Mayawala K, Poulin P. Dose selection based on physiologically based pharmacokinetic (PBPK) approaches. AAPS J. 2013;15(2):377–387. doi: 10.1208/s12248-012-9446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner C, Zhao P, Pan Y, Hsu V, Grillo J, Huang SM, Sinha V. Application of Physiologically Based Pharmacokinetic (PBPK) Modeling to Support Dose Selection: Report of an FDA Public Workshop on PBPK. CPT Pharmacometrics Syst Pharmacol. 2015;4(4):226–230. doi: 10.1002/psp4.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musib L, Choo E, Deng Y, Eppler S, Rooney I, Chan IT, Dresser MJ. Absolute bioavailability and effect of formulation change, food, or elevated pH with rabeprazole on cobimetinib absorption in healthy subjects. Mol Pharm. 2013;10(11):4046–4054. doi: 10.1021/mp400383x. [DOI] [PubMed] [Google Scholar]
- 13.Shono Y, Jantratid E, Kesisoglou F, Reppas C, Dressman JB. Forecasting in vivo oral absorption and food effect of micronized and nanosized aprepitant formulations in humans. Eur J Pharm Biopharm. 2010;76(1):95–104. doi: 10.1016/j.ejpb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Pandey P, Hamey R, Bindra DS, Huang Z, Mathias N, Eley T, Crison J, Yan B, Perrone R, Vemavarapu C. From bench to humans: formulation development of a poorly water soluble drug to mitigate food effect. AAPS PharmSciTech. 2014;15(2):407–416. doi: 10.1208/s12249-013-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivares-Morales A, Kamiyama Y, Darwich AS, Aarons L, Rostami-Hodjegan A. Analysis of the impact of controlled release formulations on oral drug absorption, gut wall metabolism and relative bioavailability of CYP3A substrates using a physiologically-based pharmacokinetic model. Eur J Pharm Sci. 2015;67:32–44. doi: 10.1016/j.ejps.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Willmann S, Thelen K, Lippert J. Integration of dissolution into physiologically-based pharmacokinetic models III: PK-Sim(R) J Pharm Pharmacol. 2012;64(7):997–1007. doi: 10.1111/j.2042-7158.2012.01534.x. [DOI] [PubMed] [Google Scholar]
- 17.Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, Jamei M, Lloyd R, Pepin X, Rostami-Hodjegan A, Sjogren E, Tannergren C, Turner DB, Wagner C, Weitschies W, Dressman J. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:300–321. doi: 10.1016/j.ejps.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Parrott N, Hainzl D, Scheubel E, Krimmer S, Boetsch C, Guerini E, Martin-Facklam M. Physiologically based absorption modelling to predict the impact of drug properties on pharmacokinetics of bitopertin. AAPS J. 2014;16(5):1077–1084. doi: 10.1208/s12248-014-9639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kambayashi A, Blume H, Dressman JB. Predicting the oral pharmacokinetic profiles of multiple-unit (pellet) dosage forms using a modeling and simulation approach coupled with biorelevant dissolution testing: case example diclofenac sodium. Eur J Pharm Biopharm. 2014;87(2):236–243. doi: 10.1016/j.ejpb.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Mathias NR, Crison J. The use of modeling tools to drive efficient oral product design. AAPS J. 2012;14(3):591–600. doi: 10.1208/s12248-012-9372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesisoglou F, Wu Y. Understanding the effect of API properties on bioavailability through absorption modeling. AAPS J. 2008;10(4):516–525. doi: 10.1208/s12248-008-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kambayashi A, Blume H, Dressman J. Understanding the in vivo performance of enteric coated tablets using an in vitro-in silico-in vivo approach: case example diclofenac. Eur J Pharm Biopharm. 2013;85(3 Pt B):1337–1347. doi: 10.1016/j.ejpb.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Kesisoglou F, Balakrishnan A, Manser K. Utility of PBPK Absorption Modeling to Guide Modified Release Formulation Development of Gaboxadol, a Highly Soluble Compound with Region-Dependent Absorption. J Pharm Sci. 2015 doi: 10.1002/jps.24674. [DOI] [PubMed] [Google Scholar]
- 24.Kesisoglou F, Chung J, van Asperen J, Heimbach T. Physiologically Based Absorption Modeling to Impact Biopharmaceutics and Formulation Strategies in Drug Development-Industry Case Studies. J Pharm Sci. 2016 doi: 10.1016/j.xphs.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Shono Y, Jantratid E, Janssen N, Kesisoglou F, Mao Y, Vertzoni M, Reppas C, Dressman JB. Prediction of food effects on the absorption of celecoxib based on biorelevant dissolution testing coupled with physiologically based pharmacokinetic modeling. Eur J Pharm Biopharm. 2009;73(1):107–114. doi: 10.1016/j.ejpb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Certara. Physiologically Based Modeling in Development Decisions and Regulatory Interactions. 2014 [Google Scholar]
- 27.Lionberger RA. Regulatory Applications of Modelling and Simulations at FDA. Paper presented at the Physiologically Based Pharmacokinetic (PBPK) Modeling in Drug Development and Evaluation; Alexandria, VA. April 6.2009. [Google Scholar]
- 28.Luttringer O, Theil FP, Poulin P, Schmitt-Hoffmann AH, Guentert TW, Lave T. Physiologically based pharmacokinetic (PBPK) modeling of disposition of epiroprim in humans. J Pharm Sci. 2003;92(10):1990–2007. doi: 10.1002/jps.10461. [DOI] [PubMed] [Google Scholar]
- 29.Berlin M, Ruff A, Kesisoglou F, Xu W, Wang MH, Dressman JB. Advances and challenges in PBPK modeling--Analysis of factors contributing to the oral absorption of atazanavir, a poorly soluble weak base. Eur J Pharm Biopharm. 2015;93:267–280. doi: 10.1016/j.ejpb.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhury MM, Kim DH, Ahn JK. A Physiologically Based Pharmacokinetic Model for Absorption and Distribution of Imatinib in Human Body. Bull Korean Chemistry Society. 2011;32(11) [Google Scholar]
- 31.McNally K, Cotton R, Loizou GD. A Workflow for Global Sensitivity Analysis of PBPK Models. Front Pharmacol. 2011;2:31. doi: 10.3389/fphar.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans MV, Andersen ME. Sensitivity analysis of a physiological model for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): assessing the impact of specific model parameters on sequestration in liver and fat in the rat. Toxicol Sci. 2000;54(1):71–80. doi: 10.1093/toxsci/54.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Gueorguieva I, Nestorov IA, Rowland M. Reducing whole body physiologically based pharmacokinetic models using global sensitivity analysis: diazepam case study. J Pharmacokinet Pharmacodyn. 2006;33(1):1–27. doi: 10.1007/s10928-005-0004-8. [DOI] [PubMed] [Google Scholar]
- 34.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 35.Huang SM, Abernethy DR, Wang Y, Zhao P, Zineh I. The utility of modeling and simulation in drug development and regulatory review. J Pharm Sci. 2013;102(9):2912–2923. doi: 10.1002/jps.23570. [DOI] [PubMed] [Google Scholar]
- 36.Kang JS, Lee MH. Overview of therapeutic drug monitoring. Korean J Intern Med. 2009;24(1):1–10. doi: 10.3904/kjim.2009.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strougo A, Yassen A, Krauwinkel W, Danhof M, Freijer J. A semiphysiological population model for prediction of the pharmacokinetics of drugs under liver and renal disease conditions. Drug Metab Dispos. 2011;39(7):1278–1287. doi: 10.1124/dmd.110.037838. [DOI] [PubMed] [Google Scholar]
- 38.Johansson F, Paterson R. Physiologically Based in Silico Models for Prediction of Oral Drug Absorption. 21:486–509. [Google Scholar]
- 39.Nestorov I. Whole body pharmacokinetic models. Clin Pharmacokinet. 2003;42(10):883–908. doi: 10.2165/00003088-200342100-00002. [DOI] [PubMed] [Google Scholar]
- 40.Kaur H, Singh J. In vitro in vivo correlation (IVIVC) 2015 Feb 3; [Google Scholar]
- 41.Yang R, Mayeno A, Liao K, Raeardon K, Reisfeld B. Integration of PBPK and reaction Network Modelling: Predictive Xenobiotic Metabolomics. Altex. 2005;6(2):373–379. [Google Scholar]
- 42.Teorell T. Kinetics of distribution of substances administered to the body. Archives Internationales de Pharmacodynamie et de Therapie. 1937;57:205–240. [Google Scholar]
- 43.Winkle HN. Evolution of the Global Regulatory Environment: A Practical Approach to Change -Implementing Quality by Design. PDA/FDA Joint Regulatory Conference.2007. [Google Scholar]
- 44.Kesisoglou F, Mitra A. Application of Absorption Modeling in Rational Design of Drug Product Under Quality-by-Design Paradigm. AAPS J. 2015;17(5):1224–1236. doi: 10.1208/s12248-015-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing Quality by Design in drug development. AAPS J. 2011;13(1):59–71. doi: 10.1208/s12248-010-9250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang W, Kim S, Zhang X, Lionberger RA, Davit BM, Conner DP, Yu LX. The role of predictive biopharmaceutical modeling and simulation in drug development and regulatory evaluation. Int J Pharm. 2011;418(2):151–160. doi: 10.1016/j.ijpharm.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Peck CC. Quantitative clinical pharmacology is transforming drug regulation. J Pharmacokinet Pharmacodyn. 2010;37(6):617–628. doi: 10.1007/s10928-010-9171-3. [DOI] [PubMed] [Google Scholar]
- 48.Graf JF, Scholz BJ, Zavodszky MI. BioDMET: a physiologically based pharmacokinetic simulation tool for assessing proposed solutions to complex biological problems. J Pharmacokinet Pharmacodyn. 2012;39(1):37–54. doi: 10.1007/s10928-011-9229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grass GM, Sinko PJ. Physiologically-based pharmacokinetic simulation modelling. Adv Drug Deliv Rev. 2002;54(3):433–451. doi: 10.1016/s0169-409x(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 50.Khalil F, Laer S. Physiologically based pharmacokinetic modeling: methodology, applications, and limitations with a focus on its role in pediatric drug development. J Biomed Biotechnol. 2011;2011:907461. doi: 10.1155/2011/907461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dressman JB, Thelen K, Willmann S. An update on computational oral absorption simulation. Expert Opin Drug Metab Toxicol. 2011;7(11):1345–1364. doi: 10.1517/17425255.2011.617743. [DOI] [PubMed] [Google Scholar]
- 52.Paixao P, Gouveia LF, Morais JA. Prediction of the human oral bioavailability by using in vitro and in silico drug related parameters in a physiologically based absorption model. Int J Pharm. 2012;429(1–2):84–98. doi: 10.1016/j.ijpharm.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, Song P, Brar SS, Madabushi R, Wu TC, Booth BP, Rahman NA, Reynolds KS, Gil Berglund E, Lesko LJ, Huang SM. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89(2):259–267. doi: 10.1038/clpt.2010.298. [DOI] [PubMed] [Google Scholar]
- 54.Office of Clinical Pharmacology (OCP) [Accessed June 15 2016];2015 http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106189.htm.
- 55.Sinha V, Zhao P, Huang SM, Zineh I. Physiologically based pharmacokinetic modeling: from regulatory science to regulatory policy. Clin Pharmacol Ther. 2014;95(5):478–480. doi: 10.1038/clpt.2014.46. [DOI] [PubMed] [Google Scholar]
- 56.Zhao P, Rowland M, Huang SM. Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther. 2012;92(1):17–20. doi: 10.1038/clpt.2012.68. [DOI] [PubMed] [Google Scholar]
- 57.Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, Snoeys J, Upreti VV, Zheng M, Hall SD. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3):247–262. doi: 10.1002/cpt.37. [DOI] [PubMed] [Google Scholar]
- 58.Dickschen K, Willmann S, Thelen K, Lippert J, Hempel G, Eissing T. Physiologically-based pharmacokinetic modeling of tamoxifen and its metabolites in women of different CYP2D6 phenotypes provides new insight into the tamoxifen mass balance. Front Pharmacol 2012 May 21 3. 2012 doi: 10.3389/fphar.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Almukainzi M, Jamali F, Aghazadeh-Habashi A, Löbenberg R. Disease specific modeling: Simulation of the pharmacokinetics of meloxicam and ibuprofen in disease state vs. healthy conditions. European Journal of Pharmaceutics and Biopharmaceutics. 2016;100:77–84. doi: 10.1016/j.ejpb.2015.12.004. doi: http://dx.doi.org/10.1016/j.ejpb.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Carrasco-Portugal MdC, Flores-Murrieta FJ. Gender Differences in the Pharmacokinetics of Oral Drugs. Pharmacology & Pharmacy. 2011;02(01):31–41. [Google Scholar]
- 61.Khalil F, Laer S. Physiologically based pharmacokinetic models in the prediction of oral drug exposure over the entire pediatric age range-sotalol as a model drug. AAPS J. 2014;16(2):226–239. doi: 10.1208/s12248-013-9555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wening K, Breitkreutz J. Oral drug delivery in personalized medicine: unmet needs and novel approaches. Int J Pharm. 2011;404(1–2):1–9. doi: 10.1016/j.ijpharm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Abbiati RA, Manca D. A modeling tool for the personalization of pharmacokinetic predictions. Computers & Chemical Engineering. 2016;91:28–37. [Google Scholar]
- 64.Androulakis IP. Systems engineering meets quantitative systems pharmacology: from low-level targets to engaging the host defenses. Wiley Interdiscip Rev Syst Biol Med. 2015;7(3):101–112. doi: 10.1002/wsbm.1294. [DOI] [PubMed] [Google Scholar]
- 65.Tucker G, DeSilva B, Dressman J, Ito M, Kumamoto T, Mager D, Mahler HC, Maitland-van der Zee AH, Pauletti GM, Sasaki H, Shah V, Tang D, Ward M. Current Challenges and Potential Opportunities for the Pharmaceutical Sciences to Make Global Impact: An FIP Perspective. J Pharm Sci. 2016 doi: 10.1016/j.xphs.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Price PS, Conolly RB, Chaisson CF, Gross EA, Young JS, Mathis ET, Tedder DR. Modeling Interindividual Variation in Physiological Factors Used in PBPK Models of Humans. Critical Reviews in Toxicology. 2010;33(5):469–503. [PubMed] [Google Scholar]
- 67.Jamei M. Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Current Pharmacology Reports. 2016;1(3):161–169. doi: 10.1007/s40495-016-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barton HA, Chiu WA, Setzer RW, Andersen ME, Bailer AJ, Bois FY, Dewoskin RS, Hays S, Johanson G, Jones N, Loizou G, Macphail RC, Portier CJ, Spendiff M, Tan YM. Characterizing uncertainty and variability in physiologically based pharmacokinetic models: state of the science and needs for research and implementation. Toxicol Sci. 2007;99(2):395–402. doi: 10.1093/toxsci/kfm100. [DOI] [PubMed] [Google Scholar]
- 69.Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55(2):81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Fletcher CV, Acosta EP, Strykowski JM. Gender differences in human pharmacokinetics and pharmacodynamics. Journal of Adolescent Health. 1994;15:619–629. doi: 10.1016/s1054-139x(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 71.Whitley H, Lindsey W. Sex-based differences in drug activity. Am Fam Physician. 2009;80(11):1254–1258. [PubMed] [Google Scholar]
- 72.Damoiseaux VA, Proost JH, Jiawan VC, Melgert BN. Sex differences in the pharmacokinetics of antidepressants: influence of female sex hormones and oral contraceptives. Clin Pharmacokinet. 2014;53(6):509–519. doi: 10.1007/s40262-014-0145-2. [DOI] [PubMed] [Google Scholar]
- 73.Handbook of Experimental Pharmacology. Heidelberg: Springer; Regitz-Zagrosek V Sex and Gender Differences in Pharmacology. [Google Scholar]
- 74.Chen ML, Lee SC, Ng MJ, Schuirmann DJ, Lesko LJ, Williams RL. Pharmacokinetic analysis of bioequivalence trials: implications for sex-related issues in clinical pharmacology and biopharmaceutics. Clin Pharmacol Ther. 2000;68(5):510–521. doi: 10.1067/mcp.2000.111184. [DOI] [PubMed] [Google Scholar]
- 75.Greenblatt DJ, Harmatz JS, Roth T, Singh NN, Moline ML, Harris SC, Kapil RP. Comparison of pharmacokinetic profiles of zolpidem buffered sublingual tablet and zolpidem oral immediate-release tablet: results from a single-center, single-dose, randomized, open-label crossover study in healthy adults. Clin Ther. 2013;35(5):604–611. doi: 10.1016/j.clinthera.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Xu H, Gan J, Liu X, Wu R, Jin Y, Li M, Yuan B. Gender-dependent pharmacokinetics of lignans in rats after single and multiple oral administration of Schisandra chinensis extract. J Ethnopharmacol. 2013;147(1):224–231. doi: 10.1016/j.jep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Hu L, Jin Y, Li YG, Borel A. Population pharmacokinetic/pharmacodynamic assessment of pharmacological effect of a selective estrogen receptor β agonist on total testosterone in healthy men. Clinical Pharmacology in Drug Development. 2015;4(4):305–314. doi: 10.1002/cpdd.184. [DOI] [PubMed] [Google Scholar]
- 78.Freire AC, Basit AW, Choudhary R, Piong CW, Merchant HA. Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. Int J Pharm. 2011;415(1–2):15–28. doi: 10.1016/j.ijpharm.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 79.Marazziti D, Baroni S, Picchetti M, Piccinni A, Carlini M, Vatteroni E, Falaschi V, Lombardi A, Dell'Osso L. Pharmacokinetics and pharmacodynamics of psychotropic drugs: effect of sex. CNS Spectr. 2013;18(3):118–127. doi: 10.1017/S1092852912001010. [DOI] [PubMed] [Google Scholar]
- 80.Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol. 2011;2011:187103. doi: 10.1155/2011/187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kashuba AD, Nafziger AN. Physiological changes during the menstrual cycle and their effect on the pharmacokinetics and pharmacodynamics of drugs. Clin Pharmacokinet. 1998;34(3):203–218. doi: 10.2165/00003088-199834030-00003. [DOI] [PubMed] [Google Scholar]
- 82.Sheth AN, Lahiri CD, Ofotokun I. Sex Differences in Metabolism and Pharmacokinetics. 2015:75–102. [Google Scholar]
- 83.Anderson GD. Chapter 1 Gender Differences in Pharmacological Response. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- 84.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cummins CL, Wu CY, Benet LZ. Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P-glycoprotein. Clin Pharmacol Ther. 2002;72(5):474–489. doi: 10.1067/mcp.2002.128388. [DOI] [PubMed] [Google Scholar]
- 86.Anderson GD. Sex and racial differences in pharmacological response: where is the evidence? pharmacogenetics, pharmacokinetics, and pharmacodynamics. Journal of Women's Health. 2005:19–29. doi: 10.1089/jwh.2005.14.19. [DOI] [PubMed] [Google Scholar]
- 87.Benet LZ, Cummins CL, Wu CY. Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int J Pharm. 2004;277(1–2):3–9. doi: 10.1016/j.ijpharm.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Ibarra M, Vazquez M, Fagiolino P, Derendorf H. Sex related differences on valproic acid pharmacokinetics after oral single dose. J Pharmacokinet Pharmacodyn. 2013;40(4):479–486. doi: 10.1007/s10928-013-9323-3. [DOI] [PubMed] [Google Scholar]
- 89.Worley RR, Fisher J. Application of physiologically-based pharmacokinetic modeling to explore the role of kidney transporters in renal reabsorption of perfluorooctanoic acid in the rat. Toxicol Appl Pharmacol. 2015;289(3):428–441. doi: 10.1016/j.taap.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heikkinen AT, Baneyx G, Caruso A, Parrott N. Application of PBPK modeling to predict human intestinal metabolism of CYP3A substrates -an evaluation and case study using GastroPlus. Eur J Pharm Sci. 2012;47(2):375–386. doi: 10.1016/j.ejps.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 91.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105(Suppl 1):i16–i23. doi: 10.1093/bja/aeq312. [DOI] [PubMed] [Google Scholar]
- 93.Feng B, LaPerle JL, Chang G, Varma MV. Renal clearance in drug discovery and development: molecular descriptors, drug transporters and disease state. Expert Opin Drug Metab Toxicol. 2010;6(8):939–952. doi: 10.1517/17425255.2010.482930. [DOI] [PubMed] [Google Scholar]
- 94.Frost CE, Song Y, Shenker A, Wang J, Barrett YC, Schuster A, Harris SI, LaCreta F. Effects of age and sex on the single-dose pharmacokinetics and pharmacodynamics of apixaban. Clin Pharmacokinet. 2015;54(6):651–662. doi: 10.1007/s40262-014-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwartz JB. The Current State of Knowledge on Age, Sex, and Their Interactions on Clinical Pharmacology. Clinical Pharmacology & Therapeutics. 2007;82(1):87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 96.Italiano D, Perucca E. Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age: an update. Clin Pharmacokinet. 2013;52(8):627–645. doi: 10.1007/s40262-013-0067-4. [DOI] [PubMed] [Google Scholar]
- 97.Reeve E, Wiese MD, Mangoni AA. Alterations in drug disposition in older adults. Expert Opin Drug Metab Toxicol. 2015;11(4):491–508. doi: 10.1517/17425255.2015.1004310. [DOI] [PubMed] [Google Scholar]
- 98.Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455–464. doi: 10.1208/s12248-013-9451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–49. doi: 10.1038/clpt.2012.64. [DOI] [PubMed] [Google Scholar]
- 100.Yoon M, Clewell HJ., 3rd Addressing Early Life Sensitivity Using Physiologically Based Pharmacokinetic Modeling and In Vitro to In Vivo Extrapolation. Toxicol Res. 2016;32(1):15–20. doi: 10.5487/TR.2016.32.1.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang F, Tong X, McCarver DG, Hines RN, Beard DA. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2006;33(4):485–518. doi: 10.1007/s10928-006-9018-0. [DOI] [PubMed] [Google Scholar]
- 102.Huang SM. Advisory Committee for Pharmaceutical Science and Clinical Pharmacology Meeting; National Harbor, MD. March 12.2012. [Google Scholar]
- 103.Villiger A, Stillhart C, Parrott N, Kuentz M. Using physiologically based pharmacokinetic (PBPK) modeling to gain insights into the effect of physiological factors on oral absorption in pediatric populations. The AAPS Journal. 2016;18(4):933–947. doi: 10.1208/s12248-016-9896-z. [DOI] [PubMed] [Google Scholar]
- 104.Cotreau M, von Moltke L, Greenblatt D. The influence of age and sex on the clearance of Cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 105.Johnson TN, Zhou D, Bui KH. Development of physiologically based pharmacokinetic model to evaluate the relative systemic exposure to quetiapine after administration of IR and XR formulations to adults, children and adolescents. Biopharm Drug Dispos. 2014;35(6):341–352. doi: 10.1002/bdd.1899. [DOI] [PubMed] [Google Scholar]
- 106.Nicolas JM, Espie P, Molimard M. Gender and interindividual variability in pharmacokinetics. Drug Metab Rev. 2009;41(3):408–421. doi: 10.1080/10837450902891485. [DOI] [PubMed] [Google Scholar]
- 107.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–529. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 108.Kubitza D, Becka M, Roth A, Mueck W. The influence of age and gender on the pharmacokinetics and pharmacodynamics of rivaroxaban--an oral, direct Factor Xa inhibitor. J Clin Pharmacol. 2013;53(3):249–255. doi: 10.1002/jcph.5. [DOI] [PubMed] [Google Scholar]
- 109.Almukainzi M, Jamali F, Aghazadeh-Habashi A, Lobenberg R. Disease specific modeling: Simulation of the pharmacokinetics of meloxicam and ibuprofen in disease state vs. healthy conditions. Eur J Pharm Biopharm. 2016;100:77–84. doi: 10.1016/j.ejpb.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 110.Zhao W, Zhang D, Storme T, Baruchel A, Decleves X, Jacqz-Aigrain E. Population pharmacokinetics and dosing optimization of teicoplanin in children with malignant haematological disease. Br J Clin Pharmacol. 2015;80(5):1197–1207. doi: 10.1111/bcp.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grillo JA, Zhao P, Bullock JM, Booth BP, Lu M, Robie-Suh K, Berglund EG, Pang KS, Rahman A, Zhang L, Lesko LJ, Huang SM. Utility of a physiologically-based pharmacokinetic (PBPK) modeling appraoch to quantitatively predict a complex drug-drug-disease interaction scenario for rivaroxaban during the drug review process: implications for clinical practice. Biopharm Drug Dispos. 33:99–110. doi: 10.1002/bdd.1771. (20012) [DOI] [PubMed] [Google Scholar]
- 112.Thompson CM, Johns DO, Sonawane B, Barton HA, Hattis D, Tardif R, Krishnan K. Database for physiologically based pharmacokinetic (PBPK) modeling: physiological data for healthy and health-impaired elderly. J Toxicol Environ Health B Crit Rev. 2009;12(1):1–24. doi: 10.1080/10937400802545060. [DOI] [PubMed] [Google Scholar]
- 113.Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004;40(6):837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 114.Vizirianakis IS, Mystridis GA, Avgoustakis K, Fatouros DG, Spanakis M. Enabling personalized cancer medicine decisions: The challenging pharmacological approach of PBPK models for nanomedicine and pharmacogenomics (Review) Oncol Rep. 2016;35(4):1891–1904. doi: 10.3892/or.2016.4575. [DOI] [PubMed] [Google Scholar]
- 115.Barbolosi D, Ciccolini J, Lacarelle B, Barlesi F, Andre N. Computational oncology -mathematical modelling of drug regimens for precision medicine. Nat Rev Clin Oncol. 2016;13(4):242–254. doi: 10.1038/nrclinonc.2015.204. [DOI] [PubMed] [Google Scholar]
- 116.Cheeti S, Budha NR, Rajan S, Dresser MJ, Jin JY. A physiologically based pharmacokinetic (PBPK) approach to evaluate pharmacokinetics in patients with cancer. Biopharm Drug Dispos. 2013;34(3):141–154. doi: 10.1002/bdd.1830. [DOI] [PubMed] [Google Scholar]
- 117.Machavaram KK, Almond LM, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, Wong S, Joshi A, Kenny JR. A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94(2):260–268. doi: 10.1038/clpt.2013.79. [DOI] [PubMed] [Google Scholar]
- 118.Pai MP. Estimating the glomerular filtration rate in obese adult patients for drug dosing. Adv Chronic Kidney Dis. 2010;17(5):e53–e62. doi: 10.1053/j.ackd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 119.Levitt DG, Schnider TW. Human physiologically based pharmacokinetic model for propofol. BMC Anesthesiol. 2005;5(1):4. doi: 10.1186/1471-2253-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp(R) Population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5(2):211–223. doi: 10.1517/17425250802691074. [DOI] [PubMed] [Google Scholar]
- 121.Ghobadi C, Johnson TN, Aarabi M, Almond LM, Allabi AC, Rowland-Yeo K, Jamei M, Rostami-Hodjegan A. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin Pharmacokinet. 2011;50(12):809–822. doi: 10.2165/11594420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 122.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 124.Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006;113(10):1141–1147. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 125.Zhao P, Vieira Mde L, Grillo JA, Song P, Wu TC, Zheng JH, Arya V, Berglund EG, Atkinson AJ, Jr, Sugiyama Y, Pang KS, Reynolds KS, Abernethy DR, Zhang L, Lesko LJ, Huang SM. Evaluation of exposure change of nonrenally eliminated drugs in patients with chronic kidney disease using physiologically based pharmacokinetic modeling and simulation. J Clin Pharmacol. 2012;52(1 Suppl):91S–108S. doi: 10.1177/0091270011415528. [DOI] [PubMed] [Google Scholar]
- 126.Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, Lee TC, Timmins P, Graham GG, Furlong TJ, Greenfield JR, Williams KM, Day RO. Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet. 2013;52(5):373–384. doi: 10.1007/s40262-013-0046-9. [DOI] [PubMed] [Google Scholar]
- 127.Rabinovich-Guilatt L, Siegler KE, Schultz A, Halabi A, Rembratt A, Spiegelstein O. The effect of mild and moderate renal impairment on the pharmacokinetics of pridopidine, a new drug for Huntington's disease. Br J Clin Pharmacol. 2016;81(2):246–255. doi: 10.1111/bcp.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.CY Chow E, Sandy Pang K. Why We Need Proper PBPK Models to Examine Intestine and Liver Oral Drug Absorption. Current Drug Metabolism. 2013;14(1):57–79. [PubMed] [Google Scholar]
- 129.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–223. doi: 10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. The Lancet Neurology. 2003;2(2):107–116. doi: 10.1016/s1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- 131.Baraldo M. The influence of circadian rhythms on the kinetics of drugs in humans. Expert Opin Drug Metab Toxicol. 2008;4(2):175–192. doi: 10.1517/17425255.4.2.175. [DOI] [PubMed] [Google Scholar]
- 132.Binkhorst L, Kloth JS, de Wit AS, de Bruijn P, Lam MH, Chaves I, Burger H, van Alphen RJ, Hamberg P, van Schaik RH, Jager A, Koch BC, Wiemer EA, van Gelder T, van der Horst GT, Mathijssen RH. Circadian variation in tamoxifen pharmacokinetics in mice and breast cancer patients. Breast Cancer Res Treat. 2015;152(1):119–128. doi: 10.1007/s10549-015-3452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dallmann R, Okyar A, Levi F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol Med. 2016;22(5):430–445. doi: 10.1016/j.molmed.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 134.Rao RT, DuBois DC, Almon RR, Jusko WJ, Androulakis IP. Mathematical Modeling of the Circadian Dynamics of the Neuroendocrine-Immune Network in Experimentally Induced Arthritis. Am J Physiol Endocrinol Metab:ajpendo 00006 02016. 2016 doi: 10.1152/ajpendo.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ovacik MA, Sukumaran S, Almon RR, DuBois DC, Jusko WJ, Androulakis IP. Circadian signatures in rat liver: from gene expression to pathways. BMC Bioinformatics. 2010;11:540. doi: 10.1186/1471-2105-11-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Almon RR, Yang E, Lai W, Androulakis IP, Ghimbovschi S, Hoffman EP, Jusko WJ, Dubois DC. Relationships between circadian rhythms and modulation of gene expression by glucocorticoids in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295(4):R1031–R1047. doi: 10.1152/ajpregu.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Almon RR, Yang E, Lai W, Androulakis IP, DuBois DC, Jusko WJ. Circadian variations in rat liver gene expression: relationships to drug actions. J Pharmacol Exp Ther. 2008;326(3):700–716. doi: 10.1124/jpet.108.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pierre K, Schlesinger N, Androulakis IP. The role of the hypothalamic-pituitary-adrenal axis in modulating seasonal changes in immunity. Physiol Genomics:physiolgenomics 00006 02016. 2016 doi: 10.1152/physiolgenomics.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mavroudis PD, Corbett SA, Calvano SE, Androulakis IP. Circadian characteristics of permissive and suppressive effects of cortisol and their role in homeostasis and the acute inflammatory response. Math Biosci. 2015;260:54–64. doi: 10.1016/j.mbs.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mavroudis PD, Corbett SA, Calvano SE, Androulakis IP. Mathematical modeling of lightmediated HPA axis activity and downstream implications on the entrainment of peripheral clock genes. Physiol Genomics. 2014;46(20):766–778. doi: 10.1152/physiolgenomics.00026.2014. [DOI] [PubMed] [Google Scholar]
- 141.Mavroudis PD, Scheff JD, Calvano SE, Androulakis IP. Systems biology of circadianimmune interactions. J Innate Immun. 2013;5(2):153–162. doi: 10.1159/000342427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Entrainment of peripheral clock genes by cortisol. Physiol Genomics. 2012;44(11):607–621. doi: 10.1152/physiolgenomics.00001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sajan J, Cinu T, Chacko A, LItty J, Jaseeda T. Chronotherapeutics and chronotherapeutic drug delivery systems. Tropical Journal of Pharmaceutical Research. 2009;8(5):467–475. [Google Scholar]
- 144.Haus E, Sackett-Lundeen L, Smolensky MH. Rheumatoid arthritis: circadian rhythms in disease activity, signs and symptoms, and rationale for chronotherapy with corticosteroids and other medications. Bull NYU Hosp Jt Dis. 2012;70(Suppl 1):3–10. [PubMed] [Google Scholar]
- 145.Cutolo M, Straub RH. Circadian rhythms in arthritis: hormonal effects on the immune/inflammatory reaction. Autoimmun Rev. 2008;7(3):223–228. doi: 10.1016/j.autrev.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 146.Lemmer B. Chronopharmacokinetics: Implications for Drug Treatment. Journal of Pharmacy and Pharmacology. 1999;51(8):887–890. doi: 10.1211/0022357991773294. [DOI] [PubMed] [Google Scholar]
- 147.Lemmer B. The importance of biological rhythms in drug treatment of hypertension and sex-dependent modifications. ChronoPhysiology and Therapy. 2012:9. [Google Scholar]
- 148.Erkekoglu P, Baydar T. Chronopharmacokinetics of drugs in toxicological aspects: A short review for pharmacy practitioners. J Res Pharm Pract. 2012;1(1):3–9. doi: 10.4103/2279-042X.99670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okyar A, Dressler C, Hanafy A, Baktir G, Lemmer B, Spahn-Langguth H. Circadian variations in exsorptive transport: in situ intestinal perfusion data and in vivo relevance. Chronobiol Int. 2012;29(4):443–453. doi: 10.3109/07420528.2012.668996. [DOI] [PubMed] [Google Scholar]
- 150.Iwasaki M, Koyanagi S, Suzuki N, Katamune C, Matsunaga N, Watanabe N, Takahashi M, Izumi T, Ohdo S. Circadian modulation in the intestinal absorption of P-glycoprotein substrates in monkeys. Mol Pharmacol. 2015;88(1):29–37. doi: 10.1124/mol.114.096735. [DOI] [PubMed] [Google Scholar]
- 151.Ohdo S. Circadian Modulation in the Intestinal Absorption of P-Glycoprotein Substrates in Monkeys. Molecular Pharmacology. 2015;88:29–37. doi: 10.1124/mol.114.096735. [DOI] [PubMed] [Google Scholar]
- 152.Adil MS, HM A, M I, K MA, Haadi A, K MA, Nematullah M. Chronoterapuetics: targeting the disease at its ideal time. The Pharma Innovation Journal. 2014;2(12) [Google Scholar]
- 153.Smolensky MH, Peppas NA. Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Deliv Rev. 2007;59(9–10):828–851. doi: 10.1016/j.addr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 154.Liu Q, Gong Y, Shi Y, Jiang L, Zheng C, Ge L, Liu J, Zhu J. A novel multi-unit tablet for treating circadian rhythm diseases. AAPS PharmSciTech. 2013;14(2):861–869. doi: 10.1208/s12249-013-9975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Peng HT, Bouak F, Vartanian O, Cheung B. A physiologically based pharmacokinetics model for melatonin--effects of light and routes of administration. Int J Pharm. 2013;458(1):156–168. doi: 10.1016/j.ijpharm.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 156.Tuck CH, Holleran S, Berglund L. Hormonal Regulation of Lipoprotein(a) Levels: Effects of Estrogen Replacement Therapy on Lipoprotein(a) and Acute Phase Reactants in Postmenopausal Women. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(9):1822–1829. doi: 10.1161/01.atv.17.9.1822. [DOI] [PubMed] [Google Scholar]
- 157.Bisdee J, Garlick P, James W. Metabolic changes during the menstrual cycle. British Journal of Nutrition. 1989;61:641–650. doi: 10.1079/bjn19890151. [DOI] [PubMed] [Google Scholar]
- 158.Mark Berlin AR, Kesisoglou Filippos, Xu Wei, Wang Michael Hong, Dressman Jennifer B. Advances and challenges in PBPK modeling --Analysis of factors contributing to the oral absorption of atazanavir, a poorly soluble weak base. European Journal of Pharmaceutics and Biopharmaceutics. 2015;93:267–280. doi: 10.1016/j.ejpb.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 159.Wikipedia Foundation I. Wikipedia, The Free Encyclopedia. 2016 https://en.wikipedia.org/. 2016.
- 160.Chen A, Yarmush ML, Maguire T. Physiologically based pharmacokinetic models: integration of in silico approaches with micro cell culture analogues. Curr Drug Metab. 2012;13(6):863–880. doi: 10.2174/138920012800840419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jinno J, Oh D, Crison JR, Amidon GL. Dissolution of ionizable water-insoluble drugs: the combined effect of pH and surfactant. J Pharm Sci. 2000;89(2):268–274. doi: 10.1002/(SICI)1520-6017(200002)89:2<268::AID-JPS14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 162.RSC Dissolution and solubility. STEM Learning Ltd; [Google Scholar]
- 163.Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, Yamashita S. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm Res. 2006;23(6):1144–1156. doi: 10.1007/s11095-006-0162-4. [DOI] [PubMed] [Google Scholar]
- 164.Jahid D. Pharmacokinetics. 2013 Oct 26; [Google Scholar]
- 165.Magallanes L, Fotaki N, Bertola V, Barindelli A, Vazquez M, Fagiolio P. Biorevelevant In Vitro Dissolution Testing In Vivo Absorption of Furosemide after Oral Administration. AAPS. 2015 [Google Scholar]
- 166.Testa B, Crivori P, Reist M, Carrupt P. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspective in Drug Discovery and Design. 2000;19:179–211. [Google Scholar]
- 167.Hopkins AL. Drug discovery: Predicting promiscuity. Nature. 2009;462(7270):167–168. doi: 10.1038/462167a. [DOI] [PubMed] [Google Scholar]
- 168.Arnott JA, Kumar R, Planey SL. Lipophilicity Indices for Drug Development. Journal of Applied Biopharmaceutics and Pharmacokinetics. 2013;1:31–38. [Google Scholar]
- 169.Wils P, Warnery A, Phung-Ba V, Legrain S, Scherman D. High lipophilicity decreases drug transport across intestinal epithelial cells. J Pharmacol Exp Ther. 1994;269(2):654–658. [PubMed] [Google Scholar]
- 170.Shah VP, Amidon GL. Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 2014;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]; 1995--backstory of BCS. AAPS J. 16(5):894–898. doi: 10.1208/s12248-014-9620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gray V, Kelly G, Xia M, Butler C, Thomas S, Mayock S. The science of USP 1 and 2 dissolution: present challenges and future relevance. Pharm Res. 2009;26(6):1289–1302. doi: 10.1007/s11095-008-9822-x. [DOI] [PubMed] [Google Scholar]
- 172.Mirza T, Bykadi SA, Ellison CD, Yang Y, Davit BM, Khan MA. Use of in vitro-in vivo correlation to predict the pharmacokinetics of several products containing a BCS class 1 drug in extended release matrices. Pharm Res. 2013;30(1):179–190. doi: 10.1007/s11095-012-0861-y. [DOI] [PubMed] [Google Scholar]
- 173.Gonzalez-Garcia I, Mangas-Sanjuan V, Merino-Sanjuan M, Bermejo M. In vitro-in vivo correlations: general concepts, methodologies and regulatory applications. Drug Dev Ind Pharm. 2015;41(12):1935–1947. doi: 10.3109/03639045.2015.1054833. [DOI] [PubMed] [Google Scholar]
- 174.Khan GM, Jiabi Z. Formulation and in vitro evaluation of ibuprofen-carbopol® 974P-NF controlled release matrix tablets III: influence of co-excipients on release rate of the drug. Journal of Controlled Release. 1998;54(2):185–190. doi: 10.1016/s0168-3659(97)00225-3. [DOI] [PubMed] [Google Scholar]
- 175.Shoaib MH, Tazeen J, Merchant HA, Yousuf RI. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm SCi. 2006;19(2):119–124. [PubMed] [Google Scholar]
- 176.Yasmin D, Rahman R, Akter M. Formulation development of directly compressed Naproxen SR tablet using Kollidon SR and Avicel PH 102 polymer. International Current Pharmaceutical Journal. 2013;2(6):112–114. [Google Scholar]
- 177.Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–287. doi: 10.1016/j.ejps.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 178.Serajuddin AT. Salt formation to improve drug solubility. Adv Drug Deliv Rev. 2007;59(7):603–616. doi: 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 179.Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–791. doi: 10.1007/s11095-007-9511-1. [DOI] [PubMed] [Google Scholar]
- 180.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 181.Koren G, Nordeng H, MacLeod S. Gender differences in drug bioequivalence: time to rethink practices. Clin Pharmacol Ther. 2013;93(3):260–262. doi: 10.1038/clpt.2012.233. [DOI] [PubMed] [Google Scholar]
- 182.Ibarra M, Magallanes L, Lorier M, Vazquez M, Fagiolino P. Sex-by-formulation interaction assessed through a bioequivalence study of efavirenz tablets. Eur J Pharm Sci. 2016;85:106–111. doi: 10.1016/j.ejps.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 183.GastroPlus User Manual -Simulation Software for Drug Discovery and Development. Version 9.0 edn. Simulations Plus. 2015 [Google Scholar]
- 184. [Accessed June 20 2016];Certara Simyp: Physiologically-Based Pharmacokinetic Modeling and Simulation. [Google Scholar]
- 185.Wang HY, Chen X, Jiang J, Shi J, Hu P. Evaluating a physiologically based pharmacokinetic model for predicting the pharmacokinetics of midazolam in Chinese after oral administration. Acta Pharmacol Sin. 2016;37(2):276–284. doi: 10.1038/aps.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Barter ZE, Tucker GT, Rowland-Yeo K. Differences in cytochrome p450-mediated pharmacokinetics between chinese and caucasian populations predicted by mechanistic physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2013;52(12):1085–1100. doi: 10.1007/s40262-013-0089-y. [DOI] [PubMed] [Google Scholar]
- 187.Certara, editor. Patel N Use of Mechanistic Population-Based PBPK Models in the Establishment and Application of IVIVCs. UK: Simcyp; 2012. [Google Scholar]
- 188.Mahmood AH, Liu X, Grice JE, Medley GA, Roberts MS. Using deconvolution to understand the mechanism for variable plasma concentration-time profiles after intramuscular injection. Int J Pharm. 2015;481(1–2):71–78. doi: 10.1016/j.ijpharm.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 189.Kesisoglou F, Xia B, Agrawal NG. Comparison of Deconvolution-Based and Absorption Modeling IVIVC for Extended Release Formulations of a BCS III Drug Development Candidate. AAPS J. 2015;17(6):1492–1500. doi: 10.1208/s12248-015-9816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wray S, Fox NC. Stem cell therapy for Alzheimer's disease: hope or hype? The Lancet Neurology. 2016;15(2):133–135. doi: 10.1016/S1474-4422(15)00382-8. [DOI] [PubMed] [Google Scholar]
- 191.Paulo Paixao LFG, Jose AGMorais. Prediction of Human Oral Bioavailability by Using In Vitro In Silico Drug Related Parameters in a Physiologically Based Absorption Model. International Journal of Pharmaceutics. 2012;429:84–98. doi: 10.1016/j.ijpharm.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 192.Mordenti J. Pharmacokinetic scale-up: accurate prediction of human pharmacokinetic profiles from animal data. JOurnal of Pharmaceutical Sciences. 1985;74(10):1097–1099. doi: 10.1002/jps.2600741017. [DOI] [PubMed] [Google Scholar]
- 193.Lombardo F, Waters NJ, Argikar UA, Dennehy MK, Zhan J, Gunduz M, Harriman SP, Berellini G, Liric Rajlic I, Obach RS. Comprehensive assessment of human pharmacokinetic prediction based on in vivo animal pharmacokinetic data, part 2: clearance. J Clin Pharmacol. 2013;53(2):178–191. doi: 10.1177/0091270012440282. [DOI] [PubMed] [Google Scholar]
- 194.Lombardo F, Waters NJ, Argikar UA, Dennehy MK, Zhan J, Gunduz M, Harriman SP, Berellini G, Rajlic IL, Obach RS. Comprehensive assessment of human pharmacokinetic prediction based on in vivo animal pharmacokinetic data, part 1: volume of distribution at steady state. J Clin Pharmacol. 2013;53(2):167–177. doi: 10.1177/0091270012440281. [DOI] [PubMed] [Google Scholar]
- 195.Gueorguieva II, Nestorov IA, Rowland M. Fuzzy simulation of pharmacokinetic models: case study of whole body physiologically based model of diazepam. J Pharmacokinet Pharmacodyn. 2004;31(3):185–213. doi: 10.1023/b:jopa.0000039564.35602.78. [DOI] [PubMed] [Google Scholar]
- 196.Gueorguieva I, Nestorov I, Rowland M. Reducing PBPK Models Using Global Sensitivity Approach and Benefit/Cost Criterion. Paper presented at the Population Approach Group; Europe, Paris, France. 2002. [Google Scholar]
- 197.Haas DM, Hebert MF, Soldin OP, Flockhart DA, Madadi P, Nocon JJ, Chambers CD, Hankins GD, Clark S, Wisner KL, Li L, Renbarger JL, Learman LA. Pharmacotherapy and pregnancy: highlights from the Second International Conference for Individualized Pharmacotherapy in Pregnancy. Clin Transl Sci. 2009;2(6):439–443. doi: 10.1111/j.1752-8062.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]