Abstract
The protein kinase C (PKC) family consists of 10 related serine/threonine protein kinases some of which are critical regulators of cell proliferation, survival and cell death. While early studies relied on broad spectrum chemical activators or inhibitors of this family, the generation of isoform specific tools has greatly facilitated our understanding of the contribution of specific PKC isoforms to cell proliferation and apoptosis. These studies suggest that PKC-alpha, PKC-epsilon, and the atypical PKC’s, PKC-lambda/iota and PKC-zeta, preferentially function to promote cell proliferation and survival, while the novel isoform, PKC-delta is an important regulator of apoptosis. The essential role of this kinase family in both cell survival and apoptosis suggests that specific isoforms may function as molecular sensors, promoting cell survival or cell death depending on environmental cues. Given their central role in cell and tissue homeostasis, it is not surprising that the expression or activity of some of these kinases is altered in human diseases, particularly cancer.
Keywords: Protein kinase C, Phosphorylation, Apoptosis, Proliferation, Survival, Cancer, Review
2. INTRODUCTION
The PKC family consists of 10 serine/threonine protein kinases originally characterized by their dependency upon lipids for catalytic activity (1–4). The past 30 years of investigation has revealed roles for PKC in the regulation of a plethora of cellular processes. Early studies relied on activation of PKC by phorbol-12-myristate-13-acetate (PMA) and/or inhibition by pharmacological agents to demonstrate a role for PKC in the regulation of specific cell functions. More recently, the complexity and potential redundancy of the PKC signaling network has prompted the development of PKC isoform specific tools including dominant inhibitory kinases, mouse models in which specific PKC isoforms are disrupted, and PKC isoform specific antisense/siRNA to define isoform-specific functions of PKC. These tools have provided evidence that PKC isoforms regulate a variety of essential biological processes including cell migration, contraction, immunity, neural plasticity, proliferation, differentiation, apoptosis and metabolism (5). A remarkable finding is that the function of a specific PKC isoform can vary between different cell types, implying that the specification of responses may rely on interaction of PKC isoforms with anchoring proteins or other regulatory pathways in the cell. This review will focus on the biology of the PKC isoform family and discuss the role of specific isoforms in the regulation of cell proliferation and apoptosis. The implications of alterations in the activation/expression of this important kinase family in human cancer will also be considered.
2.1. The PKC superfamily
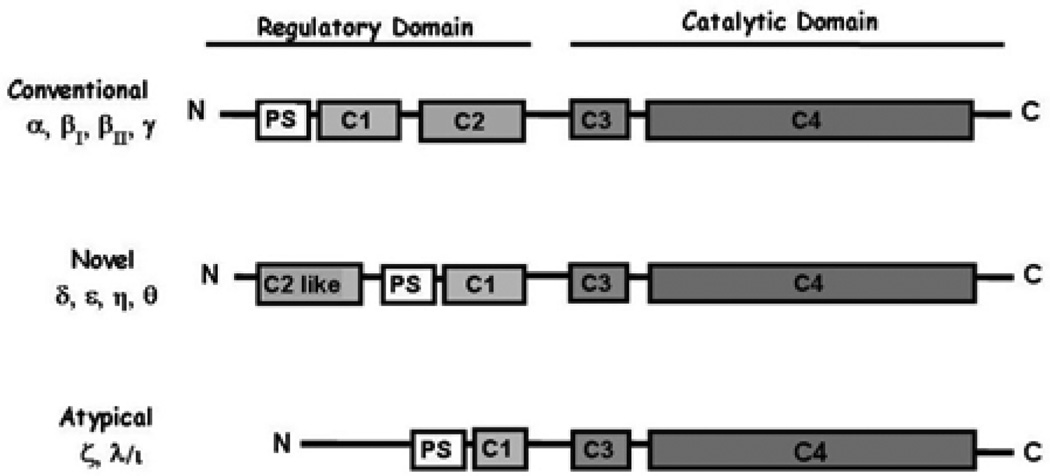
Protein kinase C was discovered by Yasutomi Nishizuka in 1977 when his group purified a cyclic nucleotide independent, Ca2+ and lipid-dependent, kinase from rat and bovine cerebellum (1, 2). Southern hybridization using low stringency probes later revealed a family of related isoforms (6). In mammals, the PKC superfamily is divided into conventional, novel and atypical subfamilies based on isoform structure and requirement for specific lipid and ion co-activators (3, 5) [see Figure 1]. PKC’s are also found in lower eukaryotes; C. elegans has four PKC’s that represent all classes of mammalian isoforms, while S. cerevisae has a single PKC (Pkc1) that resembles the mammalian atypical PKCs (7). The remarkable conservation of this kinase family from yeast to humans suggests that PKC plays a critical role in cellular signaling, and analysis of their role in simple systems has provided valuable insights into more complex mammalian systems.
Figure 1.
The PKC superfamily. PKC isoforms contain four structurally conserved domains (C1-C4). The N-terminal regulatory domain contains a pseudosubstrate binding site (PS), and the DAG (C1) and Ca++ C2, or “C2-like”) binding sites. The catalytic terminal domain contains the ATP binding domain (C3), the substrate binding site, and the kinase domain (C4). The regulatory and catalytic domains are separated by a flexible hinge region which is the site of cleavage of PKC-delta by caspase-3 in apoptotic cells.
2.2. Modes of regulation
The PKC superfamily, together with cAMP-dependent kinase (PKA) and cGMP- dependent kinase (PKG), comprises a related family of enzymes, the “AGC” kinases (8). The AGC kinases consist of a catalytic core whose activity is allosterically controlled by a regulatory domain. The lipid dependence of the PKC enzyme family has greatly facilitated the identification of upstream activators of these kinases. Physiologic regulators of PKC, including growth factors and hormones, induce the generation of diacylglycerol (DAG) and the release of intracellular Ca++. Sub-families of PKC are defined by their requirement for these activators. The conventional isoforms (PKC-alpha, -beta Ι, -beta ΙΙ and -gamma) require DAG and Ca++, the novel isoforms (PKC-delta, -epsilon, -eta and -theta) require DAG, but not Ca++, while the atypical isoforms (PKC-zeta and lambda/iota) require neither.
The conventional and novel PKC isoforms are structurally conserved, consisting of a N-terminal regulatory domain which contains a pseudosubstrate binding site, and the DAG and Ca++ cofactor binding C1 and C2 domains, respectively (8). The C-terminal domain is highly conserved between isoforms and contains the ATP binding domain, the substrate binding site, and the catalytic domain (Figure 1). Phosphorylation at three sites in C-terminal domain is required to generate a mature form of the kinase that can be activated by lipids (9–15). It should be appreciated that these are constitutive, and not stimulus regulated, phosphorylation events and thus are not thought to play a role in determining the specificity of isoform activity. The first of these phosphorylation events occurs at a conserved threonine in the activation loop; phosphorylation at this site appears to be essential for activity of most isoforms (12, 15). Several laboratories have identified the PIP3 regulated kinase, PDK-1, as the kinase responsible for PKC activation loop phosphorylation (10, 16). Phosphorylation at two additional C-terminal sites contributes to the stability of the kinase. These include an autophosphorylation site and a C-terminal hydrophobic site (12). Phosphorylation at these sites renders PKC protease and phosphatase resistant, and catalytically competent; however phosphorylated PKC is still in an inactive conformation in which the substrate binding pocket is occupied by the pseudosubstrate domain.
It is thought that most cellular PKC is fully phosphorylated and in an auto-inhibited conformation in the cytoplasm. Stimulus induced generation of DAG and Ca++ results in allosteric activation of the conventional and novel PKC isoforms via a number of well defined steps (4, 12). First, binding of DAG to the C1 domain increases the affinity of PKC for the membrane. In the case of isoforms that are also regulated by Ca++, Ca++ is thought to increase the affinity of the C1 domain for DAG. Second, interaction with acidic membrane lipids provides the energy necessary to dislodge the pseudosubstrate from the substrate binding pocket and activate the kinase. Activated PKC can then bind and phosphorylate substrates at the membrane. Notably, while membrane translocation is often used as a read out of PKC activation, “active” PKC is known to be targeted to other locations within the cell. This targeting may follow activation of the kinase at the membrane, or PKC may be activated at non-membrane sites perhaps by lipid-independent mechanisms (see discussion below). For instance, the PKC N-terminal regulatory domain contains binding sites for anchoring proteins; interaction with these anchoring proteins is hypothesized to target the activated kinase to specific subcellular sites and/or substrates. These include the Receptors for Activated C Kinase (RACK’s) as well as other PKC-interacting proteins (17, 18). Interaction of PKC with anchoring proteins may help to explain the substrate specificity of PKC isoforms, a perplexing question since multiple isoforms can be activated downstream of a given signal.
While lipid-dependent conventional and novel isoforms are thought to be largely activated by the “canonical” pathway described above, little is known about how the lipid-independent atypical PKC’s (PKC-zeta and PKC−lambda/iota) are activated. Recent studies suggest that the activity of these isoforms may be regulated through protein-protein interactions involving the PB-1 domain of the kinase. Interaction of PKC-lambda/iota with Prostate Apoptosis Response-6 (PAR-6), and PKC-zeta with Prostate Apoptosis Response-4 (PAR -4), both require the PB-1 domain of the kinase and these interactions have been shown to modulate kinase activity (19, 20). Notably, candidate drugs that disrupt interaction of the PB-1 domain of PKC-lambda with PAR-6 have been identified and shown to inhibit the transformed growth of lung cancer cell lines (19). It is likely that in response to some stimuli conventional and novel PKC isoforms may also be activated by alternative, lipid-independent pathways. This includes allosteric regulation via protein-protein interactions, tyrosine phosphorylation, and perhaps most dramatically, caspase cleavage of PKC-delta in apoptotic cells to generate a constitutively active kinase (21, 22). A common theme of these alternative activation mechanisms appears to be modification of interactions between the catalytic and regulatory domain, or in the case of caspase cleavage, removal of the regulatory domain, to allow exposure of the substrate binding site and possibly other C-terminal motifs.
3. PKC ISOFORMS THAT REGULATE CELL SURVIVAL
The tumor promoting properties of phorbol esters have been known for many years and well documented in animal models of human cancer. The discovery of PKC as the phorbol ester “receptor” lead to a heightened interest in the contribution of these kinases to tumorigenesis and tumor progression. In fact, as discussed below, altered expression of many PKC isoforms has been documented in human cancer, suggesting that these family members may function as oncogenes or tumor suppressors. Here we will discuss those PKC isoforms that are associated primarily with a pro-survival phenotype. However it should be appreciated that given the impact of cellular context on phenotype, attempts at categorizing specific PKC isoforms as pro-survival or pro-apoptotic will most certainly be inaccurate at times.
3.1. PKC-alpha
Numerous studies suggest that PKC−alpha promotes cell survival and that loss of PKC-alpha activity sensitizes cells to apoptotic agents. Whether PKC-alpha enhances cell survival by promoting cell proliferation is controversial. On one hand, overexpression of PKC-alpha increases proliferation in many cell types, including mouse fibroblasts, MCF-7 breast cancer cells and U87 malignant glioma cells, and depletion of PKC-alpha reduces the proliferation of lung cancer cells (23–26). On the other hand, some studies, including in vivo tumor studies (see below), suggest that PKC-alpha can negatively regulate cell proliferation and that loss of PKC-alpha function results in increased proliferation (27, 28).
Both the positive and negative effects of PKC-alpha on cell proliferation appear to be mediated via effects on the cell cycle machinery. For instance, PKC-alpha promotes cell cycle progression by increasing transcription of cyclin D1 in 3T3 fibroblasts and through up regulation of the CDK inhibitor, p21, in glioma cells (25, 29). However, PKC-alpha can also suppress proliferation in hepatoma cells, intestinal epithelial cells and pancreatic cancer cells through down regulation of cyclin D1 and/or up regulation of the CDK inhibitors, p21 and p27 (30–34). Intestinal epithelial cells have provided a useful model to investigate the signaling pathways downstream of PKC-alpha in suppression of proliferation. Studies in these cells suggest that PKC-alpha -dependent activation of the Ras/Raf/MAPK and/or Akt pathways may account for many of the effects of PKC-alpha on cell cycle regulators, including cyclin D1, p21 and p27 (31, 35). PKC-alpha can suppress cyclin D1 translation through Akt-dependent activation of the translational repressor, 4E-BP1 (31). PKC-alpha can also regulate the Akt pathway via direct phosphorylation of Akt at serine 473, and through activation of the serine/threonine protein kinase Raf-1 (36–39).
There is abundant evidence that PKC-alpha may support survival by suppressing apoptosis. In glioma cells, salivary epithelial cells, and melanoma and bladder carcinoma cell lines, depletion or inhibition of PKC-alpha induces apoptosis (23, 40–42). Likewise, Heregulin-induced apoptosis is enhanced by down regulation of PKC-alpha (43). Our studies in salivary epithelial cells indicate that the absence of PKC-alpha induces a PKC-delta-dependent apoptotic program (40). However, treatment of LNCaP prostate cancer cells with a synthetic DAG analog that activates PKC-alpha, but not PKC-delta, was shown to induce apoptosis (44, 45). Apoptosis induced by loss of PKC−alpha maybe secondary to loss of a proliferative signal, or may occur through a direct effect on the apoptotic machinery. In support of the later possibility, proteins involved in the execution of apoptosis have been identified as targets of PKC-alpha. Overexpression of PKC-alpha suppresses apoptosis in human pre-B REH cells and increases Bcl-2 phosphorylation at serine 70 which stabilizes and increases the anti-apoptotic function of Bcl-2 (46–48). Conversely, depletion of PKC-alpha in COS cells induces apoptosis and down regulation of Bcl-2 expression (42).
How can the discordant effects of PKC-alpha on proliferation, cell cycle progression and apoptosis be reconciled? Studies indicate that these divergent outcomes may be mediated at least in part by PKC-alpha-induced increases in the expression of p21. Although known primarily as a cell cycle inhibitor, p21 can also promote cell cycle progression and inhibit apoptosis, presumably through its interaction with distinct regulatory complexes (49, 50). A recent study by Oliva et al indicates that activation of PKC-alpha can also induce senescence of non-small cell lung cancer cells through increased expression of p21 (51). In non-dividing cells, PKC-alpha mediated increases in p21 may contribute to cell cycle inhibition and maintenance of the senescent state. However in the context of a proliferative cell, p21 may drive proliferation through interaction with a set of cell cycle regulators distinct from those that regulate senescence. The dual role of PKC-alpha in promoting and suppressing the cell cycle suggests that the cellular context may play a significant role in determining the outcome of PKC-alpha activation.
3.2. PKC-epsilon
PKC-epsilon plays a well established role in cell proliferation (52). Overexpression of PKC-epsilon in NIH 3T3 cells or Rat 6 fibroblasts results in increased growth rate, loss of contact inhibition and tumors in nude mice (53). Furthermore, overexpression and activation of PKC-epsilon in mouse epidermis results in squamous cell carcinomas, consistent with its proposed oncogenic function (54, 55). Downstream effectors of PKC-epsilon associated with proliferation and cell transformation include the Ras/Raf/MAPK pathway and Signal Transducer and Activator of Transcription 3 (STAT 3) (54, 55). Activation of the Ras/Raf/MAPK pathway is necessary for transformation by PKC-epsilon , as expression of a dominant negative Raf-1 can reverse the PKC-epsilon mediated transformation of Rat 6 fibroblasts and colonic epithelial cells (52, 56–58).
Suppression of apoptosis may contribute to PKC-epsilon-mediated tumorigenesis (59–63). Early in vitro studies showed that PKC-epsilon is required for PMA mediated protection of U937 cells from TNF-α or calphostin C induced apoptosis (64). Subsequent studies have demonstrated an anti-apoptotic role for PKC-epsilon in most, but not all, cell models investigated. In some cases PKC-epsilon may function down stream of growth factors to suppress apoptosis. In endothelial cells, activation of PKC-epsilon downstream of VEGF can suppress apoptosis associated with vascular injury (62). In small cell lung cancer cells, PKC-epsilon is required for suppression of apoptosis downstream of FGF-2 activation (61). Likewise, overexpression of PKC-epsilon generally protects against Fas and TRAIL-induced cell death (59, 60, 65). Caspase cleavage of PKC-epsilon also occurs in some cells undergoing apoptosis; in glioma cells caspase cleavage of PKC-epsilon contributes to the TRAIL-induced apoptotic program (66–68).
PKC-epsilon also protects against apoptosis induced by DNA damaging agents, and has been implicated in the resistance of tumor cells to anti-cancer drugs. Elevated PKC-epsilon levels are observed in chemoresistance non-small cell lung cancer cell lines and in chemo-resistant ovarian cells (63, 69). Consistent with these observations, overexpression of PKC-epsilon can promote survival of lung cancer cells and increase their resistance to chemotherapeutic drugs (63). In breast cancer cells PKC-epsilon controls Akt activation, and this appears to contribute to the suppression of apoptosis observed in breast cancer cells in which PKC-epsilon is overexpressed (59). PKC-epsilon may also directly inhibit apoptosis through up regulation of the anti-apoptotic protein, Bcl-2, or downregulation of pro-apoptotic proteins such as Bid (60, 62).
3.3. PKC-lambda/iota and PKC-zeta
The atypical PKC isoforms, PKC-iota (and the mouse homolog, PKC-lambda) and PKC-zeta are critical for cell survival signaling, presumably due to their role as downstream effectors of PI-3 kinase (70). Most of the effects of PKC-iota and PKC-zeta can be attributed to activation of the pro-survival NF-κB signaling pathway and suppression of apoptosis (20, 71, 72). Many studies indicate a correlation between the expression or activation of PKC-iota and/or PKC-zeta and sensitivity to apoptosis. PKC-zeta suppresses Fas-induced apoptosis in Jurkat cells, and PKC-zeta inhibition increases apoptosis in leukemia cells exposed to etoposide and TNF−α (73, 74). Conversely, overexpression of PKC-zeta suppresses apoptosis induced by chemotherapeutic agents in U937 cells; topoisomerase II was identified as a downstream target of PKC-zeta in these studies (75). Due to the high homology between the PKC-iota and PKC-zeta isoforms, tools to distinguish their individual functions have been limited. However recent studies in knock-out mice indicate distinct roles for these isoforms, as PKC-iota, but not PKC-zeta, is critical for mouse development (20). Studies by Murray and Fields suggest that PKC-iota may be the more important of these isoforms in the context of suppression of apoptosis. Their studies show that PKC-iota, but not PKC-zeta, protects leukemia cells from chemotherapeutic agents and that Bcr-Abl mediated resistance to apoptosis is mediated by PKC-iota (19, 76, 77).
One of the more interesting aspects of the atypical PKC’s is their dynamic regulation by agents which induce cell death. In some contexts, transient activation of these isoforms is observed in response to apoptotic agents. PKC-iota/PKC-zeta are activated by caspase cleavage which results in its degradation through the ubiquitin-proteasome pathway (78, 79). Likewise, atypical PKC expression is increased prior to cell death induced by UV, ceramide, and in cytokine-mediated cartilage destruction (80–82). PKC-zeta activity in apoptotic cells is negatively regulated by it’s interaction with the pro-apoptotic protein, PAR-4, which suppresses NF-κB activation (71, 83–85). Sequestration of PKC-zeta and PKC-lambda by PAR-4 is one mechanism by which PAR-4 induces apoptosis. PAR-4 can also directly interact with and suppress the enzymatic activity of PKC-zeta and PKC-lambda in apoptotic cells (86). PKC-zeta may also be inhibited by interaction with p38, as seen in apoptotic chondrocytes (87). Recently it has been shown that p38 interacts with the regulatory domain of PKC-zeta and suppresses PKC-zeta activity by blocking autophosphorylation of the kinase (88). Conversely, PKC-zeta and PKC-lambda have been shown to bind to, and inactivate, the pro-survival kinase, Akt, in response to ceramide and growth factors, and to phosphorylate and inhibit the pro-apoptotic protein, Bad (89–92). The cumulative data suggests that through regulation of pro-survival pathways, the atypical PKC’s may act as a switch between cell survival and cell death.
4. PKC ISOFORMS THAT REGULATE APOPTOSIS
Both PKC-delta and PKC-theta can be cleaved by caspase-3 to generate a constitutively activated kinase, which, when introduced into cells, can induce apoptosis (93, 94). Based on this observation these kinases are often grouped together as “pro-apoptotic”. However, there is only limited evidence that PKC-theta plays a significant role in apoptosis. PKC-theta appears instead to be critical for immune cell function and T cell survival, and PKC-theta null mice display severe defects in T-cell signaling and activation (95). In contrast, the function of PKC-delta in apoptotic cells has been well established.
PKC-delta is a ubiquitously expressed isoform and studies in PKC-delta-/- mice have identified diverse roles for this signaling molecule in control of proliferation, immunity, apoptosis and cell migration (96). While PKC-delta is clearly an important regulator of apoptosis, it also has been shown to suppress cell cycle progression and negatively regulate proliferation. Studies from Santiago-Walker, et al, suggest that the anti-proliferative effects of PKC-delta may contribute to induction of apoptosis (97). In these studies, the overexpression of PKC-delta stimulated progression through the G1 phase of the cell cycle, but induced S phase arrest followed by apoptosis (97). In contrast, some studies, particularly in transformed cells, support a role for PKC-delta in promoting cell cycle progression and proliferation (98). In this context PKC-delta may act downstream of growth factors such as IGF-1 to promote proliferation (99, 100). Here we will discuss data that supports a role for PKC-delta as an early regulator of apoptosis, and mechanisms which regulate pro-apoptotic signal transduction by this ubiquitously expressed kinase.
4.1. Regulation of apoptosis by PKC-delta
PKC-delta is activated by numerous apoptotic stimuli and is required for apoptosis induced by genotoxins (22, 101), oxidative stress (102) and death receptors (103). Treatment with the PKC-delta selective inhibitor, rottlerin (104), expression of kinase inactive PKC-delta (PKC-delta KD) (101) or the introduction of a PKC-delta specific RACK inhibitory peptide (105), have all been shown to inhibit apoptosis. We have used inhibitors of PKC-delta to probe the function of PKC−delta in the apoptotic pathway. Our studies show that expression of PKC-delta KD inhibits both downstream apoptotic events such as caspase activation and DNA fragmentation, as well as upstream apoptotic events such as loss of mitochondrial membrane potential (101, 104). These studies from our lab, together with numerous studies from other labs, point to an essential role for PKC-delta as a regulator of early events in the apoptotic pathway (101, 104, 106, 107). In support of this conclusion, smooth muscle cells and primary salivary epithelial cells derived from PKC-delta -/- mice are defective in mitochondria-dependent apoptosis (107, 108). Analysis of primary parotid cells from PKC-delta -/- mice shows a decrease in cytochrome c release, PARP cleavage and caspase-3 activation in response to etoposide, however, apoptosis can be completely restored by reintroduction of wild type PKC-delta (107). Loss of PKC-delta also protects parotid glands against γ-irradiation induced apoptosis in vivo (107). Although apoptosis is suppressed, activation of p53 is normal in primary parotid cells from wild type and PKC-delta -/- mice, suggesting that PKC-delta is activated downstream of cellular damage signals (107).
Substrates of PKC-delta identified in apoptotic cells include transcription factors, protein kinases, structural proteins, DNA repair and checkpoint molecules and Bcl-2 family members. The majority of PKC-delta’s substrates in apoptotic cells are nuclear proteins. PKC-delta interacts with and phosphorylates DNA-dependent protein kinase (DNA-PK) in cells exposed to genotoxins (109, 110). Phosphorylation of DNA-PK inhibits DNA binding, suggesting that PKC-delta mediated phosphorylation inactivates the DNA double strand break repair function of this protein (109). PKC-delta can also phosphorylate the checkpoint protein Rad9; phosphorylated Rad9 can then bind and sequester Bcl-2, inducing cell death (110). Other reports suggest that PKC-delta may directly target and inactivate the apoptosis machinery. PKC-delta has been shown to phosphorylate and activate capsase-3 (111), to phosphorylate and target the anti-apoptotic protein Mcl-1 for degradation (112), and to suppress phosphorylation of the pro-apoptotic protein, Bad (113). Finally, PKC-delta may regulate transcription through activation of p53. In one study, downregulation of PKC-delta inhibited basal transcription of p53, while other studies report PKC-delta dependent accumulation of the p53 protein in apoptotic cells (114–116).
PKC-delta has been shown to interface with downstream signaling cascades to regulate the apoptotic machinery. Our laboratory has shown that PKC-delta and Signal Transducer and Activator of Transcription 1 (STAT1) can interact in etoposide treated cells and that STAT1 is a downstream target of PKC-delta in the apoptotic pathway (115). In addition, there is accumulating evidence that the PI3-kinase/AKT pathway, the ERK, JNK and p38 pathways may be downstream targets of PKC-delta in apoptotic cells. In cardiomyocytes exposed to ischemia, Akt is dephosphorylated and inactivated in a PKC-delta dependent manner. Inactivation of Akt results in dephosphorylation and release of the pro-apoptotic protein, Bad, and initiation of cell death (113). PKC-delta enhances radiation-induced apoptosis via ERK1/2 activation and suppression of radiation-induced G2-M arrest. PKC-delta activates the JNK pathway in irradiation and Ara-c induced apoptosis, possibly through phosphorylation and activation of MEKK1 (117). Primary cells derived from PKC-delta -/- mice are deficient in JNK activation, and JNK activation in vivo in response to x-irradiation is reduced in these mice (107). In contrast, binding of HSP25 to PKC-delta inhibits cell death, suggesting that the pro-apoptotic function of PKC-delta may be negatively regulated through interaction with other proteins (118).
4.2. Activation of PKC-delta by apoptotic signals
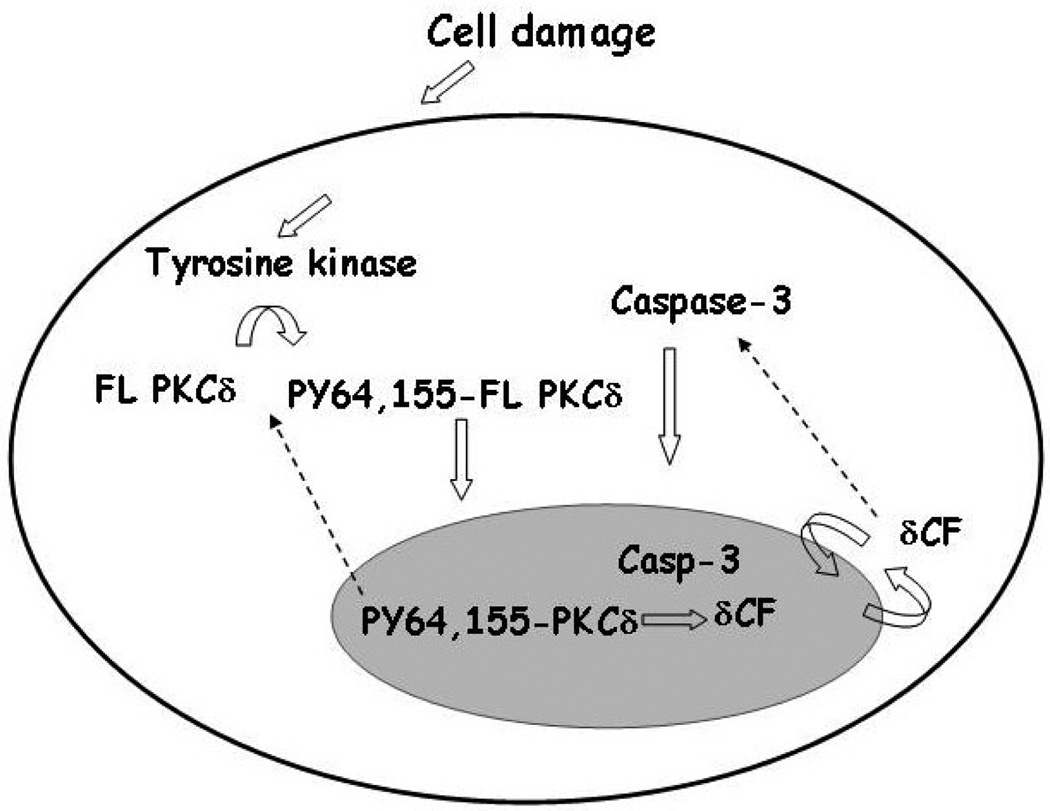
PKC-delta is ubiquitously expressed and regulates diverse cell functions, hence, under normal conditions the proapoptotic function of PKC-delta must be tightly regulated in order to assure cell survival. We have defined multiple events that regulate the pro-apoptotic function of PKC-delta and have shown that these events are temporally coordinated in apoptotic cells (see Figure 2). The first event appears to be transduction of a “death” signal to PKC-delta by a damage sensing pathway. Our studies suggest that this occurs through phosphorylation of PKC-delta on specific tyrosine residues (119). Activated PKC-delta then transiently accumulates in the nucleus (120). If caspase-3 is also translocated to the nucleus, nuclear PKC-delta is cleaved by caspase to generate the PKC-delta catalytic fragment (delta-CF), a constitutively activated, pro-apoptotic form of PKC-delta that accumulates in the nucleus (120). Nuclear accumulation of PKC-delta appears to be both necessary and sufficient to commit a cell to apoptosis, whereas cytoplasmic retention of PKC-delta in the cytoplasm assures cell survival (120).
Figure 2.
Regulation of the proapoptotic function of PKC-delta. Under normal growth conditions PKC-delta is retained in the cytoplasm by a mechanism that is dependent upon the regulatory domain. Apoptotic signals result in tyrosine phosphorylation of the regulatory domain and allow nuclear accumulation of PKC-delta. Active capsase-3 likewise accumulates in the nucleus resulting in cleavage of PKC-delta and generation of delta-CF. Delta-CF localizes constitutively in the nucleus where it may regulate proteins involved in the cell damage response.
4.2.1. Tyrosine phosphorylation of PKC-delta
Studies from our lab and others suggest that phosphorylation of PKC-delta on tyrosine may be a mechanism to dictate stimulus specific functions of PKC-delta (119, 121, 122). Functionally important tyrosine residues in the context of apoptosis include in PKC-delta include Y64 and Y187 in glioma cells treated with etoposide (121) and Y311, Y332 and Y512 in response to H2O2 (122). We have shown that tyrosine phosphorylation of PKC-delta in response to apoptotic agents occurs very rapidly and that tyrosines 64 and 155 in the regulatory domain must be phosphorylated for nuclear translocation of PKC-delta to occur (119). Non-receptor tyrosine kinases, specifically c-Abl, Src and Lyn have been implicated in the phosphorylation of PKC-delta in response to apoptotic agents (95, 123, 124).
4.2.2. Nuclear localization of PKC-delta
Many PKC-delta substrates in apoptotic cells are nuclear proteins, consistent with the nuclear translocation of PKC-delta in response to many apoptotic agents (125, 126). We have defined a nuclear localization sequence in the catalytic domain of PKC-delta and have shown that nuclear localization is required for regulation of apoptosis by PKC-delta (125). Our studies suggest that once tyrosine phosphorylated PKC-delta rapidly accumulates in the nucleus (119). Hence, tyrosine phosphorylation in the regulatory domain may facilitate the binding of nuclear import receptors to the PKC-delta nuclear localization signal. This suggests that the regulatory domain of PKC-delta functions in part to retain PKC-delta in the cytoplasm in the absence of an apoptotic signal.
4.2.3. Caspase-cleavage of PKC-delta
Cleavage of PKC-delta by caspase occurs in the hinge domain of the protein between the regulatory and catalytic domains and results in release of a constitutively active catalytic fragment (delta-CF). We have shown that cleavage of PKC-delta by caspase occurs in the nucleus of apoptotic cells and is dependent on the coordinated import of activated caspase-3 into the nucleus (120). Importantly, while PKC-delta transiently accumulates in the nucleus in response to genotoxins, delta-CF when generated is largely or entirely nuclear at the early stages of apoptosis (120). Thus caspase cleavage facilitates the sustained nuclear accumulation of PKC-delta, which appears to be critical for commitment to apoptosis in some cells (120). The delta-CF may have additional functions in apoptotic cells, particularly in the later stages of apoptosis when it is also found in the cytoplasm (120). For instance, Sitailo et al have shown that expression of delta-CF results in activation of the pro-apoptotic protein, Bax, and cytochrome c release in keratinocytes (127). Taken together, our studies suggest that regulating the subcellular locale of PKC-delta is critical for cell survival, and that caspase cleavage of PKC-delta, which allows its nuclear accumulation, signals an irreversible commitment to apoptosis.
5. PKC AND DISEASE: A POTENTIAL ROLE IN HUMAN CANCER
The ability of phorbol esters to promote tumors has been known for many years and well documented in animal models of human cancer. The discovery of PKC as the major phorbol ester “receptor” in the cell has lead to intensive investigation into the potential contribution of these kinases to human cancer (128, 129). Not surprisingly, the PKC isoforms most commonly associated with increased proliferation and/or survival, PKC-alpha and PKC-epsilon, are those most commonly overexpressed in human cancer, and represent potential oncogenes. To this group should be added PKC-iota, as increased expression of PKC-iota has recently been shown to correlate with tumor stage in non-small cell lung cancer, and depletion of PKC-iota was shown to reverse the transformed phenotype of these cells (30). In contrast, the observation that loss of PKC-delta is associated with cell transformation in some cells, together with the preponderance of data suggesting an anti-proliferative/pro-apoptotic role for PKC-delta, has lead to the suggestion the PKC-delta may function as a tumor suppressor (98, 130). Although this hypothesis has not been tested in vivo, PKC-delta expression is reduced in human squamous cell carcinomas, and PKC-delta expression decreases with increasing tumor grade in human endometrial carcinomas (131, 132).
Overexpression of PKC-alpha is seen in a variety of human tumors, suggesting that it may contribute to tumor progression (133–135). In particular, in human breast cancer cells PKC-alpha has been shown to regulate HER2/neu expression and contribute to HER2/neumediated cell invasion (43, 133). PKC-alpha antisense oligonucleotides have been shown in some instances to decrease proliferation and sensitize cells to apoptosis, and PKC-alpha antisense oligonucleotides reduce tumor growth in mouse xenographs (136–138). However, clinical trials using this approach for the treatment of human tumors using have been disappointing (139, 140). Given the overlapping roles of PKC-alpha in both promoting and suppressing cell cycle progression, its functional analysis in vivo is likely to be complex. In this regard, recent studies using mouse models in which PKC-alpha is inhibited or genetically “knocked-out” indicate that, perhaps contrary to expectation, that the loss of PKC-alpha can accelerate colon cancer and result in increased B cell proliferation and leukemia (27, 28).
Clinical studies suggest that increased PKC-epsilon expression is strongly associated with tumor progression in many human cancers. PKC-epsilon has been documented as a biomarker of aggressive breast cancer, and high PKC-epsilon expression correlates with poor prognosis and positive Her2/neu status (141). The PKC-epsilon gene is also amplified in 28% of thyroid cancers and a chimeric/truncated version of PKC-epsilon has been cloned from human thyroid cancer cells (142). Likewise, PKC-epsilon expression is increased in gliomas, and depletion of PKC-epsilon can trigger apoptosis in glioma cell lines possibly through an effect on Akt mediated survival (65, 143). Overexpression of PKC-epsilon is commonly seen in human prostate tumors, and is associated with conversion from an androgen dependent to androgen independent state (144, 145). PKC-epsilon may also contribute to the resistance of prostate cancer cells to apoptosis through interaction with the pro-apoptotic protein, Bax (146). Interestingly, increased association of PKC-epsilon with Bax correlates with the increased resistance to apoptosis in prostate cancer cells (146). In addition to its pro-proliferative properties, PKC-epsilon may regulate the invasion and metastasis of cancer cells (147).
With few exceptions, proof of a definitive role for specific PKC isoforms in cancer cell growth, invasion and survival has been elusive. This presumably reflects both the paucity of in vivo models available, as well as the multiple functions many of these kinases appear to have in vivo. However, there is compelling evidence that PKC isoform expressions is altered in human tumors, suggesting that a subset of these kinases may function as tumor suppressors or oncogenes, or cooperate with other genes to promote human tumors (148).
6. CONCLUSIONS
Abundant evidence implicates PKC isoforms as critical regulators of cell proliferation and apoptosis. PKC kinases regulate cell survival and apoptosis both “upstream” via growth factor signaling, as well as “downstream” via regulation of transcription factors and/or posttranslational modification of proteins. As might be expected, alterations in the expression and/or activity of specific members of this family are associated with the pathogenesis of some human diseases. In most cases, the contribution of PKC isoforms to human disease appears to be related to their role as regulators of cell proliferation and apoptosis. Thus, understanding the molecular mechanisms by which specific isoforms regulate cellular signaling pathways may enable the identification of new therapeutic targets to treat human disease.
Abbreviations
- PKC
protein kinase C
- DAG
diacylglycerol
- RACK’s
Receptors for Activated C Kinase
- STAT 3
Signal Transducer and Activator of Transcription 3
- PAR-4
Prostate Apoptosis Response-4
- PAR-6
Prostate Apoptosis Response-6
- PKC-delta KD
kinase inactive PKC-delta
- STAT1
Signal Transducer and Activator of Transcription 1
- delta-CF
PKC-delta catalytic fragment
REFERENCES
- 1.Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- 2.Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- 3.Newton A. Regulation of the ABC kinases by phosphorylation : protein kinase C as a paradigm. Biochemical J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2352–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 6.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988;263:6927–6932. [PubMed] [Google Scholar]
- 7.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold MG, Barford D, Komander D. Lining the pockets of kinases and phosphatases. Curr Opin Struct Biol. 2006;16:693–701. doi: 10.1016/j.sbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Bornancin F, Parker PJ. Phosphorylation of protein kinase C-alpha on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase- resistant state. J Biol Chem. 1997;272:3544–3549. doi: 10.1074/jbc.272.6.3544. [DOI] [PubMed] [Google Scholar]
- 10.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-Kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 11.Parekh D, Ziegler W, Yonezawa K, Hara K, Parker PJ. Mammalian TOR controls one of two kinase pathways acting upon nPKC-delta and nPKC-epsilon. J Biol Chem. 1999;274:34758–34764. doi: 10.1074/jbc.274.49.34758. [DOI] [PubMed] [Google Scholar]
- 12.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Zhang J, Bottaro DP, Li W, Pierce JH. Identification of serine 643 of protein kinase C-delta as an important autophosphorylation site for its enzymatic activity. J Biol Chem. 1997;272:24550–24555. doi: 10.1074/jbc.272.39.24550. [DOI] [PubMed] [Google Scholar]
- 14.Stempka L, Schnolzer M, Radke S, Rincke G, Marks F, Gschwendt M. Requirements of protein kinase C-delta for catalytic function. Role of glutamic acid 500 and autophosphorylation of serine 643. J Biol Chem. 1999;274:8886–8892. doi: 10.1074/jbc.274.13.8886. [DOI] [PubMed] [Google Scholar]
- 15.Stempka L, Girodr A, Muller H-J, Rincke G, Marks F, Gschwendt M, Bossemeyer D. Phosphorylation of protein kinase C-delta at threonine 505 is not a prerequisite for enzymatic Activity. Expression of rat PKC-delta and an alanine 505 mutant in bacteria in a functional form. J Biol Chem. 1997;272:6805–6811. doi: 10.1074/jbc.272.10.6805. [DOI] [PubMed] [Google Scholar]
- 16.Dutil EM, Toker A, Newton A. Regulation of conventional protein kinase C isozymes by phophoinositide-dependent kinase 1 (PDK-1) Curr Biol. 1998;8:1366–1375. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 17.Jaken S, Parker P. Protein kinase C binding partners. BioEssays. 2000;22:245–254. doi: 10.1002/(SICI)1521-1878(200003)22:3<245::AID-BIES6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.Poole AW, Pula G, Hers I, Crosby D, Jones ML. PKC-interacting proteins: from function to pharmacology. Trends Pharm Sci. 2004;25:528–535. doi: 10.1016/j.tips.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Fields AP, Regala RP. Protein kinase Ciota: Human oncogene, prognostic marker and therapeutic target: Pharmacol Res. 2007;55:487–497. doi: 10.1016/j.phrs.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscat J, Rennert P, Diaz-Meco MT. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ. 2005;13:702–711. doi: 10.1038/sj.cdd.4401823. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg S. Distinctive activation mechanisms and functions for protein kinase C-delta. Biochem J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C- delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandil R, Ashkenazi E, Blass M, Kronfeld I, Kazimirsky G, Rosenthal G, Umansky F, Lorenzo PS, Blumberg PM, Brodie C. Protein kinase C-alpha and protein kinase C-delta play opposite roles in the proliferation and apoptosis of glioma cells. Cancer Res. 2001;61:4612–4619. [PubMed] [Google Scholar]
- 24.Ways DK, Kukoly CA, deVente J, Hooker JL, Bryant WO, Posekany KJ, Fletcher DJ, Cook PP, Parker PJ. MCF-7 breast cancer cells transfected with protein kinase C-alpha exhibit altered expression of other protein kinase C isoforms and display a more aggressive neoplastic phenotype. J Clin Invest. 1995;95:1906–1915. doi: 10.1172/JCI117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soh J-W, Weinstein IB. Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem. 2003;278:34709–34716. doi: 10.1074/jbc.M302016200. [DOI] [PubMed] [Google Scholar]
- 26.Yin J, Gao J, Shao R, Tian W, Wang J, Wan Y. siRNA agents inhibit oncogene expression and attenuate human tumor cell growth. J Exp Ther Oncol. 2003;3:194–204. doi: 10.1046/j.1359-4117.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa R, Soh JW, Michie AM. Subversion of Protein kinase C alpha signaling in hematopoietic progenitor cells results in the generation of a B-cell chronic lymphocytic leukemia-like population in vivo. Cancer Res. 2006;66:527–534. doi: 10.1158/0008-5472.CAN-05-0841. [DOI] [PubMed] [Google Scholar]
- 28.Oster H, Leitges M. Protein Kinase C alpha but not PKC-zeta suppresses intestinal tumor formation in ApcMin/+ Mice. Cancer Res. 2006;66:6955–6963. doi: 10.1158/0008-5472.CAN-06-0268. [DOI] [PubMed] [Google Scholar]
- 29.Besson A, Yong VW. Involvement of p21 waf1/Cip1 in protein kinase-alpha induced cell cycle progression. Mol Cell Biol. 2000;20:4580–4590. doi: 10.1128/mcb.20.13.4580-4590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hizli AA, Black AR, Pysz MA, Black JD. Protein kinase C-alpha signaling inhibits cyclin D1 translation in intestinal epithelial cells. J Biol Chem. 2006;281:14596–14603. doi: 10.1074/jbc.M601959200. [DOI] [PubMed] [Google Scholar]
- 31.Guan L, Song K, Pysz MA, Curry KJ, Hizli AA, Danielpour D, Black AR, Black JD. Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/Akt-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial Cells. J Biol Chem. 2007;282:14213–14225. doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- 32.Wu T, Hsieh Y-H, Hsieh Y-S, Liu J. Reduction of PKC-alpha decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J Cell Biochem. 2008;103:9–20. doi: 10.1002/jcb.21378. [DOI] [PubMed] [Google Scholar]
- 33.Frey MR, Clark JA, Leontieva O, Uronis JM, Black AR, Black JD. Protein kinase C signaling mediates a program of cell cycle withdrawal in the intestinal epithelium. J Cell Biol. 2000;151:763–778. doi: 10.1083/jcb.151.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Detjen K, Brembeck F, Welzel M, Kaiser A, Haller H, Wiedenmann B, Rosewicz S. Activation of protein kinase Calpha inhibits growth of pancreatic cancer cells via p21 (cip)-mediated G (1) arrest. J Cell Sci. 2000;113:3025–3035. doi: 10.1242/jcs.113.17.3025. [DOI] [PubMed] [Google Scholar]
- 35.Clark JA, Black AR, Leontieva OV, Frey MR, Pysz MA, Kunneva L, Woloszynska-Read A, Roy D, Black JD. Involvement of the ERK signaling cascade in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J Biol Chem. 2004;279:9233–9247. doi: 10.1074/jbc.M312268200. [DOI] [PubMed] [Google Scholar]
- 36.Kolch W, Heidecker G, Kocks G, Hummel R, Vahldl H, Mischak N, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Sampat K, Hu N, Zakari J, Yuspa SH. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J Biol Chem. 2006;281:3237–3243. doi: 10.1074/jbc.M512167200. [DOI] [PubMed] [Google Scholar]
- 38.Partovian C, Simons M. Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase C-alpha in endothelial cells. Cell Signal. 2004;16:951–957. doi: 10.1016/j.cellsig.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Zhang J, Flechner L, Hyun T, Yam A, Franke T, Pierce J. Protein kinase C-alpha overexpression stimulates Akt activity and suppresses apoptosis induced by interleukin 3 withdrawal. Oncogene. 1999;18:6564–6572. doi: 10.1038/sj.onc.1203065. [DOI] [PubMed] [Google Scholar]
- 40.Matassa AA, Kalkofen RL, Carpenter L, Biden TJ, Reyland ME. Inhibition of PKC-alpha induces a PKC-delta-dependent apoptotic program in salivary epithelial cells. Cell Death Differ. 2003;10:269–277. doi: 10.1038/sj.cdd.4401149. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen K, Skrede M, Crucianir V, Mikalsen S-O, Slipicevic A, Florenes VA. Phorbol ester phorbol-12-myristate-13-acetate promotes anchorage-independent growth and survival of melanomas through MEK-independent activation of ERK1/2. Biochem Biophys Res Commun. 2005;329:266–274. doi: 10.1016/j.bbrc.2005.01.143. [DOI] [PubMed] [Google Scholar]
- 42.Whelan DHR, Parker PJ. Loss of protein kinase C function induces an apoptotic response. Oncogene. 1998;16:1939–1944. doi: 10.1038/sj.onc.1201725. [DOI] [PubMed] [Google Scholar]
- 43.Le X, Marcelli M, McWatters A, Nan B, Mills G, O'Brian C, BastRC J. Heregulin-induced apoptosis is mediated by down-regulation of Bcl-2 and activation of caspase-7 and is potentiated by impairment of protein kinase C-alpha activity. Oncogene. 2001;20:8258–8269. doi: 10.1038/sj.onc.1205039. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Bermejo ML, Leskow FC, Fujii T, Wang Q, Blumberg PM, Ohba M, Kuroki T, Han KC, Lee J, Marquez VE, Kazanietz MG. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKC-alpha. J Biol Chem. 2002;277:645–655. doi: 10.1074/jbc.M107639200. [DOI] [PubMed] [Google Scholar]
- 45.Gavrielides M, Frijhoff A, Conti C, MG K. Protein kinase C and prostate carcinogenesis: targeting the cell cycle and apoptotic mechanisms. Curr Drug Targets. 2004;5:431–443. doi: 10.2174/1389450043345380. [DOI] [PubMed] [Google Scholar]
- 46.Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase C-alpha Bcl-2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 47.Jiffar T, Kurinna S, Suck G, Carlson-Bremer D, Ricciardi MR, Konopleva M, Andreeff M, Ruvolo PP. PKC-alpha mediates chemoresistance in acute lymphoblastic leukemia through effects on Bcl2 phosphorylation. Leukemia. 2004;18:505–512. doi: 10.1038/sj.leu.2403275. [DOI] [PubMed] [Google Scholar]
- 48.Deng X, Ito T, Carr B, Mumby M, May WS., Jr Reversible phosphorylation of Bcl2 following interleukin 3 or bryostatin 1 is mediated by direct interaction with protein phosphatase 2A. J Biol Chem. 1998;273:34157–34163. doi: 10.1074/jbc.273.51.34157. [DOI] [PubMed] [Google Scholar]
- 49.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 50.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 51.Oliva JL, Caino MC, Senderowicz AM, Kazanietz MG. S-phase-specific activation of PKC-alpha induces senescence in non-small cell lung cancer cells. J Biol Chem. 2008;283:5466–5476. doi: 10.1074/jbc.M707576200. [DOI] [PubMed] [Google Scholar]
- 52.Cacace AM, Ueffing M, Philipp A, Han EK, Kolch W, Weinstein IB. PKC-epsilon functions as an oncogene by enhancing activation of the Raf kinase. Oncogene. 1996;13:2517–2626. [PubMed] [Google Scholar]
- 53.Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 54.Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase C epsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3 Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- 55.Aziz MH, Moammir H, Manoharan H, Sand J, Verma A. Proteion kinase C epsilon interacts with Stat3 and regulates its activation that is essential for the development of skin cancer. Mol Carcinog. 2007;46:646–653. doi: 10.1002/mc.20356. [DOI] [PubMed] [Google Scholar]
- 56.Perletti GP, Folini M, Lin HC, Mischak H, Piccinini F, Tashjian AHJ. Overexpression of protein kinase C-epsilon is oncogenic in rat colonic epithelial cells. Oncogene. 1996;12:847–854. [PubMed] [Google Scholar]
- 57.Perletti GP, Concari P, Brusaferri S, Marras E, Piccinini F, Tashjian AH. Protein kinase C-epsilon is oncogenic in colon epithelial cells by interaction with the ras signal transduction pathway. Oncogene. 1998;16:3345–3348. doi: 10.1038/sj.onc.1201871. [DOI] [PubMed] [Google Scholar]
- 58.Piiper A, Elez R, You S-J, Kronenberger B, Loitsch S, Roche S, Zeuzem S. Cholecystokinin stimulates extracellular signal-regulated kinase through activation of the epidermal growth factor receptor, Yes, and protein kinase C. SIGNAL AMPLIFICATION AT THE LEVEL OF Raf BY ACTIVATION OF PROTEIN KINASE C-epsilon. J Biol Chem. 2003;278:7065–7072. doi: 10.1074/jbc.M211234200. [DOI] [PubMed] [Google Scholar]
- 59.Lu D, Huang J, Basu A. Protein kinase C epsilon activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-alpha-induced cell death. J Biol Chem. 2006;281:22799–22807. doi: 10.1074/jbc.M603390200. [DOI] [PubMed] [Google Scholar]
- 60.Sivaprasad U, Shankar E, Basu A. Downregulation of Bid is associated with PKC-epsilon mediated TRAIL resistance. Cell Death Differ. 2006;14:851–860. doi: 10.1038/sj.cdd.4402077. [DOI] [PubMed] [Google Scholar]
- 61.Pardo OE, Wellbrock P, Khanzada U, Aubert M, Arozarena I, Davidson S, Bowen F, Parker P, Filonenko V, Gout I, Sebire N, Marais R, Downward J, Seckl M. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKC, B-Raf and S6K2. EMBO J. 2006;25:3078–3088. doi: 10.1038/sj.emboj.7601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinberg R, Harari OA, Lidington EA, Boyle JJ, Nohadani M, Samarel AM, Ohba M, Haskard DO, Mason JC. A protein kinase Cepsilon-anti-apoptotic kinase signaling complex protects human vascular endothelial cells against apoptosis through induction of Bcl-2. J Biol Chem. 2007;282:32288–32297. doi: 10.1074/jbc.M704001200. [DOI] [PubMed] [Google Scholar]
- 63.Ding L, Wang H, Lang W, Xiao L. Protein Kinase C-epsilon Promotes Survival of Lung cancer cells by suppressing apoptosis through dysregulation of the mitochondrial caspase pathway. J Biol Chem. 2002;277:35305–35313. doi: 10.1074/jbc.M201460200. [DOI] [PubMed] [Google Scholar]
- 64.Mayne GC, Murray AW. Evidence that protein kinase Cepsilon mediates phorbol ester inhibition of calphostin C- and tumor necrosis factor-alpha-induced apoptosis in U937 histiocytic lymphoma cells. J Biol Chem. 1998;273:24115–24121. doi: 10.1074/jbc.273.37.24115. [DOI] [PubMed] [Google Scholar]
- 65.Okhrimenko H, Lu W, Xiang C, Hamburger N, Kazimirsky G, Brodie C. Protein kinase C-epsilon regulates the apoptosis and survival of glioma cells. Cancer Res. 2005;65:7301–7309. doi: 10.1158/0008-5472.CAN-05-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shinohara H, Kayagaki N, Yagita H, Oyaizu N, Ohba M, Kuroki T, Brodie Y. A protective role of PKC-epsilon against TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in glioma cells. Biochem Biophys Res Commun. 2001;284:1162–1167. doi: 10.1006/bbrc.2001.5104. [DOI] [PubMed] [Google Scholar]
- 67.Koriyama H, Kouchi Z, Umeda T, Saido TC, Momoi T, Ishiura S, Suzuki K. Proteolytic activation of protein kinase C delta and epsilon by Caspase-3 in U937 cells during chemotherapeutic agent-induced apoptosis. Cel Signal. 1999;11:831–838. doi: 10.1016/s0898-6568(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 68.Basu A, Lu D, Sun B, Moor AN, Akkaraju GR, Huang J. Proteolytic activation of Protein Kinase C-epsilon by caspase-mediated processing and transduction of antiapoptotic signals. J Biol Chem. 2002;277:41850–41856. doi: 10.1074/jbc.M205997200. [DOI] [PubMed] [Google Scholar]
- 69.Castrillo A, Pennington DJ, Otto F, Parker PJ, Owen MJ, Bosca L. Protein Kinase C epsilon is required for macrophage activation and defense against bacterial infection. J Exp Med. 2001;194:1231–1242. doi: 10.1084/jem.194.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S, Mizuno K, Hirai S, Kazlauskas A, Ohno S. EGF of PDGF receptors activate atypical PKC-lambda through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 71.Diaz-Meco MT, Berra E, Municio MM, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera MT, Alcami J, Paya CV. A dominant negative protein kinase C-zeta subspecies blocks NF-kappa B activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berra E, Diaz-Meco MT, Lozano J, Frutos S, Municio MM, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Filomenko R, Poirson-Bichat F, Billerey C, Belon J-P, Garrido C, Solary E, Bettaieb A. Atypical protein kinase C zeta as a target for chemosensitization of tumor cells. Cancer Res. 2002;62:1815–1821. [PubMed] [Google Scholar]
- 74.Leroy I, de Thonel A, Laurent G, Quillet-Mary A. Protein kinase C zeta associates with death inducing signaling complex and regulates Fas ligand-induced apoptosis. Cell Signal. 2005;17:1149–1157. doi: 10.1016/j.cellsig.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 75.Plo I, Hernandez H, Kohlhagen G, Lautier D, Pommier Y, Laurent G. Overexpression of the atypical protein kinase C zeta reduces topoisomerase II catalytic activity, cleavable complexes formation, and drug-induced cytotoxicity in monocytic U937 leukemia cells. J Biol Chem. 2002;277:31407–31415. doi: 10.1074/jbc.M204654200. [DOI] [PubMed] [Google Scholar]
- 76.Jamieson L, Carpenter L, Biden TJ, Fields AP. Protein Kinase C-iota activity is necessary for Bcr-Abl-mediated resistance to drug-induced apoptosis. J Biol Chem. 1999;274:3927–3930. doi: 10.1074/jbc.274.7.3927. [DOI] [PubMed] [Google Scholar]
- 77.Murray NR, Fields AP. Atypical protein kinase C-iota protects human leukemia cells against drug-induced apoptosis. J Biol Chem. 1997;272:27521–27524. doi: 10.1074/jbc.272.44.27521. [DOI] [PubMed] [Google Scholar]
- 78.Garin G, Abe J-i, Mohan A, Lu W, Yan C, Newby AC, Rhaman A, Berk BC. Flow antagonizes TNF-alpha signaling in endothelial cells by inhibiting caspase-dependent PKC-zeta processing. Circ Res. 2007;101:97–105. doi: 10.1161/CIRCRESAHA.107.148270. [DOI] [PubMed] [Google Scholar]
- 79.Smith L, Chen L, Reyland ME, DeVries TA, Talanian RV, Omura S, Smith JB. Activation of atypical protein kinase C-zeta by caspase processing and degradation by the ubiquitin-proteasome system. J Biol Chem. 2000;275:40620–40627. doi: 10.1074/jbc.M908517199. [DOI] [PubMed] [Google Scholar]
- 80.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase C-zeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 81.Berra E, Municio M, Sanz L, Frutos S, Diaz-Meco M, Moscat J. Positioning atypical protein kinase C isoforms in the UV-induced apoptotic signaling cascade. Mol Cell Biol. 1997;17:4346–4354. doi: 10.1128/mcb.17.8.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LaVallie ER, Chockalingam PS, Collins-Racie LA, Freeman BA, Keohan CC, Leitges M, Dorner AJ, Morris EA, Majumdar MK, Arai M. Protein Kinase C-zeta is up-regulated in osteoarthritic cartilage and is required for activation of NF-kappaB by tumor necrosis factor and interleukin-1 in articular chondrocytes. J Biol Chem. 2006;281:24124–24137. doi: 10.1074/jbc.M601905200. [DOI] [PubMed] [Google Scholar]
- 83.Diaz J, Oltersdorf T, Horne W, McConnell M, Wilson G, Weeks S, Garcia T, Fritz LC. A common binding site mediates heterodimerization and homodimerization of Bcl-2 family members. J Biol Chem. 1997;272:11350–11355. doi: 10.1074/jbc.272.17.11350. [DOI] [PubMed] [Google Scholar]
- 84.Diaz-Meco MT, Lallena M-J, Monjas A, Frutos S, Moscat J. Inactivation of the inhibitory kappa B protein kinase/nuclear factor kappaB pathway by Par-4 expression potentiates tumor necrosis factor alpha -induced apoptosis. J Biol Chem. 1999;274:19606–19612. doi: 10.1074/jbc.274.28.19606. [DOI] [PubMed] [Google Scholar]
- 85.Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. Targeted disruption of the zeta PKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 86.Diaz-Meco M, Municio M, Frutos S, Sanchez P, Lazano J, Sanz L, Moscat J. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 87.Kim S-J, Kim H-G, Oh C-D, Hwang S-G, Song W-K, Yoo Y-J, Kang S-S, Chun J-S. p38 kinase-dependent and -independent inhibition of protein kinase C-zeta and -alpha regulates nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes. J Biol Chem. 2002;277:30375–30381. doi: 10.1074/jbc.M205193200. [DOI] [PubMed] [Google Scholar]
- 88.Kim J-S, Park Z-Y, Yoo Y-J, Yu S-S, Chun J-S. p38 kinase mediates nitric oxide-induced apoptosis of chondrocytes through the inhibition of protein kinase C-zeta by blocking autophosphorylation. J Biol Chem. 2005;12:201–212. doi: 10.1038/sj.cdd.4401511. [DOI] [PubMed] [Google Scholar]
- 89.Xin M, Gao F, May WS, Flagg T, Deng X. Protein Kinase Czeta abrogates the proapoptotic function of bax through phosphorylation. J Biol Chem. 2007;282:21268–21277. doi: 10.1074/jbc.M701613200. [DOI] [PubMed] [Google Scholar]
- 90.Mao M, Fang X, Lu Y, Lapushin R, Bast R, Mills G. Inhibition of growth-factor-induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem J. 2000;352:475–482. [PMC free article] [PubMed] [Google Scholar]
- 91.Doornbos RP, Theelen M, van der Hoeven PCJ, van Blitterswijk WJ, Verkleij AJ, van Bergenen Henegouwen PMP. Protein kinase C-zeta is a negative regulator of protein kinase B activity. J Biol Chem. 1999;274:8589–8596. doi: 10.1074/jbc.274.13.8589. [DOI] [PubMed] [Google Scholar]
- 92.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKC-zeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Datta R, Kojima H, Yoshida K, Kufe D. Caspase-3-mediated cleavage of protein kinase C-theta in induction of apoptosis. J Biol Chem. 1997;272:20317–20320. doi: 10.1074/jbc.272.33.20317. [DOI] [PubMed] [Google Scholar]
- 94.Schultz A, Jönsson J-I, Larsson C. The regulatory domain of protein kinase C localises to the Golgi complex and induces apoptosis in neuroblastoma and Jurkat cells. Cell Death Diff. 2003;10:662–675. doi: 10.1038/sj.cdd.4401235. [DOI] [PubMed] [Google Scholar]
- 95.Sun X, Wu F, Datta R, Kharbanda S, Kufe D. Interaction between Protein Kinase C-delta and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J Biol Chem. 2000;275:7470–7473. doi: 10.1074/jbc.275.11.7470. [DOI] [PubMed] [Google Scholar]
- 96.Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama K, Humphries M, Reyland M, DA F. Suppression of cell migration by protein kinase C-delta. Oncogene. 2005;24:3067–3072. doi: 10.1038/sj.onc.1208465. [DOI] [PubMed] [Google Scholar]
- 97.Santiago-Walker AE, Fikaris AJ, Kao GD, Brown EJ, Kazanietz MG, Meinkoth JL. Protein kinase C-delta stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J Biol Chem. 2005;280:32107–32114. doi: 10.1074/jbc.M504432200. [DOI] [PubMed] [Google Scholar]
- 98.Jackson DN, Foster DA. The enigmatic protein kinase C-delta: complex roles in cell proliferation and survival. FASEB J. 2004;18:627–636. doi: 10.1096/fj.03-0979rev. [DOI] [PubMed] [Google Scholar]
- 99.Datta K, Nambudripad R, Pal S, Zhou M, Cohen HT, Mukhopadhyay D. Inhibition of insulin-like growth factor-I-mediated cell signaling by the von Hippel-Lindau gene product in renal cancer. J Biol Chem. 2000;275:20700–20706. doi: 10.1074/jbc.M909970199. [DOI] [PubMed] [Google Scholar]
- 100.Li W, Jiang Y-X, Zhang J, Soon L, Flechner L, Kapoor V, Pierce JH, Wang L-H. Protein kinase C-delta is an important signaling molecule in insulin-like growth factor I receptor-mediated cell transformation. Mol Cell Biol. 1998;18:5888–5898. doi: 10.1128/mcb.18.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matassa A, Carpenter L, Biden T, Humphries M, Reyland M. PKC-delta is required for mitochondrial dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276:29719–29728. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- 102.Majumder P, Mishra N, Sun X, Bharti A, Kharbanda S, Saxena S, Kufe D. Targeting of protein kinase C-delta to mitochondria in the oxidative stress response. Cell Death Differ. 2001;12:465–470. [PubMed] [Google Scholar]
- 103.Khwaja A, Tatton L. Caspase-mediated proteolysis and activation of protein kinase C-delta plays a central role in neutrophil apoptosis. Blood. 1999;94:291–301. [PubMed] [Google Scholar]
- 104.Reyland M, Anderson S, Matassa A, Barzen K, Quissell D. Protein kinase C-delta is essential for etoposide-induced apoptosis in salivary acinar cells. J Biol Chem. 1999;274:11915–11923. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- 105.Schechtman D, Mochly-Rosen D. Isozyme-specific inhibitors and activators of protein kinase C. Methods Enzymol. 2002;345:470–489. doi: 10.1016/s0076-6879(02)45039-2. [DOI] [PubMed] [Google Scholar]
- 106.Reyland ME. PKC and Apoptosis. In: Srivastava R, editor. Apoptosis, Cell Signaling, and Human Diseas. Molecular Mechanisms. Totowa, N.J.: Humana Press Inc; 2007. [Google Scholar]
- 107.Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the protein kinase C-delta null mouse in vivo. J Biol Chem. 2006;281:9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- 108.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase C delta-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bharti A, Kraeft S-K, Gounder M, Pandey P, Jin S, Yuan Z-M, Lees-Miller SP, Weichselbaum R, Weaver D, Chen LB, Kufe D, Kharbanda S. Inactivation of DNA-dependent protein kinase by protein kinase C-delta: Implications for apoptosis. Mol Cell Biol. 1998;18:6719–6728. doi: 10.1128/mcb.18.11.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoshida K, Wang H, Miki Y, Kufe D. Protein kinase C delta is responsible for constitutive and DNA damage induced phosphorylation of Rad9. EMBO J. 2003;22:1431–1441. doi: 10.1093/emboj/cdg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voss OH, Kim S, Wewers MD, Doseff AI. Regulation of monocyte apoptosis by the protein kinase C delta-dependent phosphorylation of caspase-3. J Biol Chem. 2005;280:17371–17379. doi: 10.1074/jbc.M412449200. [DOI] [PubMed] [Google Scholar]
- 112.Sitailo LA, Tibudan SS, Denning MF. The protein kinase C-delta catalytic fragment targets Mcl-1 for degradation to trigger apoptosis. J Biol Chem. 2006;281:29703–29710. doi: 10.1074/jbc.M607351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: A mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 114.Niwa K, Inanami O, Yamamori T, Ohta T, Hamasu T, Karino T, Kuwabara M. Roles of protein kinase C-delta in the accumulation of p53 and the induction of apoptosis in H2O2-treated bovine endothelial cells. Free Radic Res. 2002;36:1147–1153. doi: 10.1080/1071576021000016409. [DOI] [PubMed] [Google Scholar]
- 115.DeVries TA, Kalkofen RL, Matassa AA, Reyland ME. Protein kinase C-delta regulates apoptosis via activation of STAT1. J Biol Chem. 2004;279:45603–45612. doi: 10.1074/jbc.M407448200. [DOI] [PubMed] [Google Scholar]
- 116.Ren J, Datta R, Shioya H, Li Y, Oki E, Biedermann V, Bharti A, Kufe D. p73 beta is regulated by the protein kinase C delta catalytic fragment generated in the apoptotic response to DNA damage. J Biol Chem. 2002;277:33758–33765. doi: 10.1074/jbc.M110667200. [DOI] [PubMed] [Google Scholar]
- 117.Yoshida K, Miki Y, Kufe D. Activation of SAPK/JNK signaling by Protein Kinase C-delta in response to DNA damage. J Biol Chem. 2002;277:48372–48378. doi: 10.1074/jbc.M205485200. [DOI] [PubMed] [Google Scholar]
- 118.Lee Y-J, Lee D-H, Cho C-K, Bae S, Jhon G-J, Lee S-J, Soh J-W, Lee Y-S. HSP25 Inhibits Protein kinase Cdelta-mediated cell death through direct interaction. J Biol Chem. 2005;280:18108–18119. doi: 10.1074/jbc.M501131200. [DOI] [PubMed] [Google Scholar]
- 119.Humphries M, Ohm A, Schaack J, Adwan T, Reyland M. Tyrosine phosphorylation regulates nuclear localization of PKCdelta. Oncogene. 2008 doi: 10.1038/sj.onc.1210967. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME. Induction of apoptosis is driven by nuclear retention of PKCdelta. J Biol Chem. 2007;282:22307–22314. doi: 10.1074/jbc.M703661200. [DOI] [PubMed] [Google Scholar]
- 121.Blass M, Kronfeld I, Kazimirsky G, Blumberg P, Brodie C. Tyrosine phosphorylation of protein kinase C-delta is essential for its apoptotic effect in response to etoposide. Mol Cell Biol. 2002;22:182–195. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Konishi H, Tanaka M, Takemura Y, Hiderori H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kharbanda S, Pandey P, Jin S, Inoue S, Bharti A, Yuan Z-M, Weichselbaum R, Weaver D, Kufe D. Functional interaction between DNA-PK and c-Abl in response to DNA damage. Nature. 1997;386:732–735. doi: 10.1038/386732a0. [DOI] [PubMed] [Google Scholar]
- 124.Zang Q, Lu Z, Curto M, Barile N, Shalloway D, Foster DA. Association between v-Src and protein kinase C-delta in v-Src-transformed fibroblasts. J Biol Chem. 1997;272:13275–13280. doi: 10.1074/jbc.272.20.13275. [DOI] [PubMed] [Google Scholar]
- 125.DeVries TA, Neville MC, Reyland ME. Nuclear import of PKC-delta is required for apoptosis: Identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuan Z-M, Utsugisawa T, Ishiko T, Nakada S, Huang Y, Kharbanda S, Weichselbaum R, Kufe D. Activation of protein kinase C-delta by the c-Abl tyrosine kinase in response to ionizing radiation. Oncogene. 1998;16:1643–1648. doi: 10.1038/sj.onc.1201698. [DOI] [PubMed] [Google Scholar]
- 127.Sitailo LA, Tibudan SS, Denning MF. Bax activation and induction of apoptosis in human keratinocytes by the protein kinase C delta catalytic domain. J Investig Dermatol. 2004;123:434–443. doi: 10.1111/j.0022-202X.2004.23403.x. [DOI] [PubMed] [Google Scholar]
- 128.Castanga M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- 129.Kikkawa U, Takai Y, Tanaka Y, Miyake R, Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983;258:11442–11445. [PubMed] [Google Scholar]
- 130.Lu Z, Hornia Y-W, Jiang A, Zang Q, Ohno S, Foster D. Tumor promotion by depleting cells of protein kinase C-delta. Mol Cell Biol. 1997;17:3418–3428. doi: 10.1128/mcb.17.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP. Analysis of protein kinase C delta expression in endometrial tumors. Human Pathology. 2008;39:21–29. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.D'Costa A, Robinson J, Maududi T, Chaturvedi V, Nickoloff B, MF D. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene. 2006;25:375–386. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- 133.Tan M, Li P, Sun M, Yin G, Yu D. Upregulation and activation of PKC by ErbB2 through Src promotes breast cancer cell invasion that can be blocked by combined treatment with PKC and Src inhibitors. Oncogene. 2006;25:3286–3295. doi: 10.1038/sj.onc.1209361. [DOI] [PubMed] [Google Scholar]
- 134.Michie AM, Nakagawa R. The link between PKC-alpha regulation and cellular transformation. Immunol Lett. 2005;96:155–162. doi: 10.1016/j.imlet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 135.Lahna M, Köhlerd G, Sundella K, Sub C, Lib S, Paterson B, Bumolc T. Protein kinase C -alpha expression in breast and ovarian cancer. Oncology. 2004;67:1–10. doi: 10.1159/000080279. [DOI] [PubMed] [Google Scholar]
- 136.Jiang X-H, Tu S-P, Cui J-T, Lin MCM, Xia HHX, Wong WM, Chan AO-O, Yuen MF, Jiang S-H, Lam S-K, Kung H-F, Soh JW, Weinstein IB, Wong BC-Y. Antisense targeting protein kinase C-alpha and -beta1 inhibits gastric carcinogenesis. Cancer Res. 2004;64:5787–5794. doi: 10.1158/0008-5472.CAN-03-1172. [DOI] [PubMed] [Google Scholar]
- 137.Shen L, Dean N, Glazer R. Induction of p53- dependent, insulin-like growth factor-binding protein-3-mediated apoptosis in glioblastoma multiforme cells by a protein kinase C-alpha antisense oligonucleotide. Mol Pharmacol. 1999;55:396–402. doi: 10.1124/mol.55.2.396. [DOI] [PubMed] [Google Scholar]
- 138.Lahn M, Sundell K, Moore S. Targeting protein kinase C-alpha in cancer with the phosphorothioate antisense oligonucleotide aprinocarsen. Ann NY Acad Sci. 2003;1002:263–270. doi: 10.1196/annals.1281.029. [DOI] [PubMed] [Google Scholar]
- 139.Grossman S, Alavi J, Supko J, Carson K, Priet R, Dorr F, Grundy J, Holmlund J. Efficacy and toxicity of the antisense oligonucleotide aprinocarsen directed against protein kinase C- alpha delivered as a 21-day continuous intravenous infusion in patients with recurrent high-grade astrocytomas. Neuro-Oncology. 2005;1:32–40. doi: 10.1215/S1152851703000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vansteenkiste J, Canon J-L, Riska H, Pirker R, Peterson P, John W, Mali P, Lahn M. Randomized phase II evaluation of aprinocarsen in combination with gemcitabine and cisplatin for patients with advanced/metastatic non-small cell lung cancer. Invest New Drugs. 2005;23:263–269. doi: 10.1007/s10637-005-6736-x. [DOI] [PubMed] [Google Scholar]
- 141.Pan Q, Bao LW, Kleer CG, Sabel MS, Griffith KA, Teknos TN, Merajver SD. Protein kinase C-epsilon is a predictive biomarker of aggressive breast cancer and a validated target for RNA interference anticancer therapy. Cancer Res. 2005;65:8366–8371. doi: 10.1158/0008-5472.CAN-05-0553. [DOI] [PubMed] [Google Scholar]
- 142.Knauf AJ, Elisei R, Mochly-Rosen D, Liron T, Chen X-N, Gonsky R, Korenberg JR, Fagin JA. Involvement of protein kinase Cε in thyroid cell death. A truncated chimeric PKC-epsilon cloned from a thyroid cancer cell line protects thyroid cells from apoptosis. J Biol Chem. 1999;274:23414–23425. doi: 10.1074/jbc.274.33.23414. [DOI] [PubMed] [Google Scholar]
- 143.Sharif T, Sharif M. Overexpression of protein kinase C-epsilon in astroglial brain tumor derived cell lines and primary tumor samples. Int J Oncol. 1999;15:237–243. [PubMed] [Google Scholar]
- 144.Cornford P, Evans J, Dodson A, Parsons K, Woolfenden A, Neoptolemos J, Foster CS. Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am J Pathol. 1999;154:137–144. doi: 10.1016/S0002-9440(10)65260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu D, Foreman TL, Gregory CW, McJilton MA, Wescott GG, Ford OH, Alvey RF, Mohler JL, Terrian DM. Protein kinase C-epsilon has the potential to advance the recurrence of human prostate cancer. Cancer Res. 2002;62:2423–2429. [PubMed] [Google Scholar]
- 146.McJilton MA, Van. Sikes C, Wescott GG, Wu D, Foreman TL, Gregory CW, Weidner DA, Ford OH, Lasater AM, Mohler JL, Terrian DM. Protein kinase C-epsilon interacts with Bax and promotes survival of human prostate cancer cells. Oncogene. 2003;22:7958–7968. doi: 10.1038/sj.onc.1206795. [DOI] [PubMed] [Google Scholar]
- 147.Tachado S, Mayhew M, Wescott G, Foreman T, Goodwin C, McJilton M, Terrian D. Regulation of tumor invasion and metastasis in protein kinase C-epsilon-transformed NIH3T3 fibroblasts. J Cell Biochem. 2002;85:785–797. doi: 10.1002/jcb.10164. [DOI] [PubMed] [Google Scholar]
- 148.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]