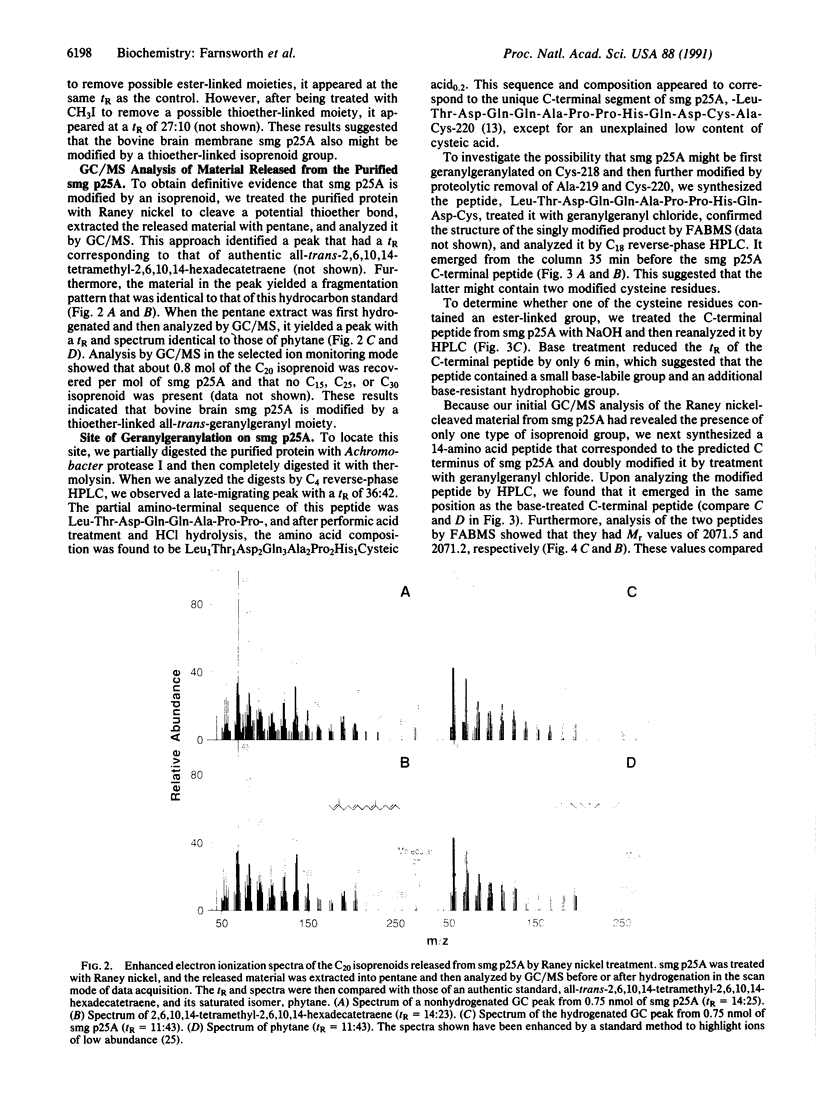
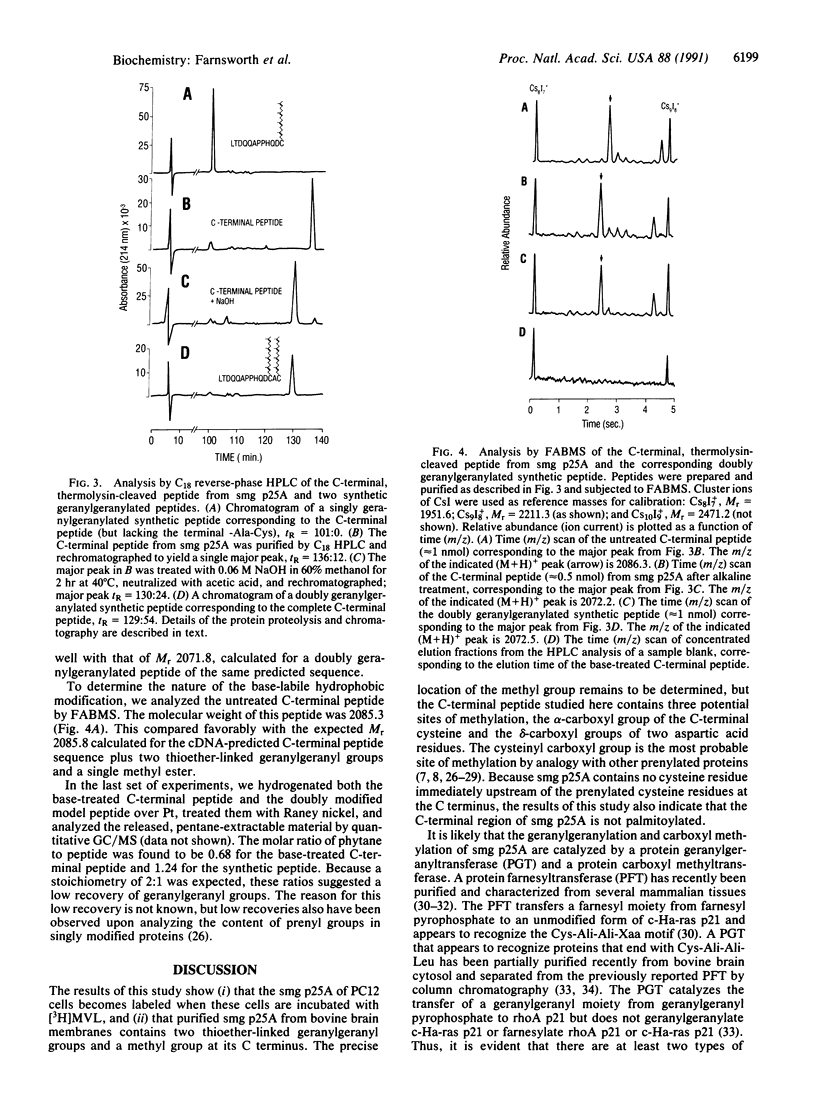
Abstract
smg p25A, also known as the rab3A protein, is a small GTP-binding protein that has been implicated in intracellular vesicle transport and the secretion of neurotransmitters. It has been shown to bind reversibly to membranes, though its cDNA-predicted sequence contains no obvious membrane-binding domains. However, smg p25A does contain a cDNA-predicted C-terminal Cys-Ala-Cys sequence at positions 218 through 220, which suggests that it may be posttranslationally modified. In the present study we used two different approaches to investigate this possibility. First, we incubated pheochromocytoma cells with [3H]mevalonolactone, examined the proteins that became labeled by two-dimensional gel electrophoresis, and demonstrated that two of these proteins exactly corresponded to smg p25A. Second, we purified smg p25A from bovine brain membranes and analyzed both the full-length protein and a proteolytically derived C-terminal peptide by a combination of high performance liquid chromatography and mass spectrometry. This approach revealed that the protein's C-terminal region is methyl-esterified and contains two geranylgeranyl groups linked via thioether bonds to Cys-218 and Cys-220. Since smg p25A is one of several small GTP-binding proteins that share a C-terminal Cys-Xaa-Cys consensus sequence (where Xaa is an unspecified amino acid), our results suggest that these proteins may be similarly geranylgeranylated and methyl-esterified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Araki S., Kaibuchi K., Sasaki T., Hata Y., Takai Y. Role of the C-terminal region of smg p25A in its interaction with membranes and the GDP/GTP exchange protein. Mol Cell Biol. 1991 Mar;11(3):1438–1447. doi: 10.1128/mcb.11.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S., Kikuchi A., Hata Y., Isomura M., Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990 Aug 5;265(22):13007–13015. [PubMed] [Google Scholar]
- Balch W. E. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990 Dec;15(12):473–477. doi: 10.1016/0968-0004(90)90301-q. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Burstein E. S., Linko-Stentz K., Lu Z. J., Macara I. G. Regulation of the GTPase activity of the ras-like protein p25rab3A. Evidence for a rab3A-specific GAP. J Biol Chem. 1991 Feb 15;266(5):2689–2692. [PubMed] [Google Scholar]
- Buss J. E., Quilliam L. A., Kato K., Casey P. J., Solski P. A., Wong G., Clark R., McCormick F., Bokoch G. M., Der C. J. The COOH-terminal domain of the Rap1A (Krev-1) protein is isoprenylated and supports transformation by an H-Ras:Rap1A chimeric protein. Mol Cell Biol. 1991 Mar;11(3):1523–1530. doi: 10.1128/mcb.11.3.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989 Dec 5;264(34):20422–20429. [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., Cochrane C. G., Bourne J. R., Solski P. A., Buss J. E., Der C. J. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M., Farnsworth C. C., Yoshida Y., Gelb M. H., Glomset J. A., Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8960–8964. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Yamashita T., Kawata M., Yamamoto K., Ikeda K., Tanimoto T., Takai Y. Purification and characterization of a novel GTP-binding protein with a molecular weight of 24,000 from bovine brain membranes. J Biol Chem. 1988 Feb 25;263(6):2897–2904. [PubMed] [Google Scholar]
- Manne V., Roberts D., Tobin A., O'Rourke E., De Virgilio M., Meyers C., Ahmed N., Kurz B., Resh M., Kung H. F. Identification and preliminary characterization of protein-cysteine farnesyltransferase. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7541–7545. doi: 10.1073/pnas.87.19.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Araki S., Hata Y., Kondo J., Teranishi Y., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for smg p25A, a ras p21-like GTP-binding protein. Mol Cell Biol. 1990 Aug;10(8):4116–4122. doi: 10.1128/mcb.10.8.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Kim S., Ueda T., Kikuchi A., Yorifuji H., Hirokawa N., Takai Y. Localization and subcellular distribution of smg p25A, a ras p21-like GTP-binding protein, in rat brain. J Biol Chem. 1990 Jul 15;265(20):11872–11879. [PubMed] [Google Scholar]
- Mizoguchi A., Kim S., Ueda T., Takai Y. Tissue distribution of smg p25A, a ras p21-like GTP-binding protein, studied by use of a specific monoclonal antibody. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1438–1445. doi: 10.1016/0006-291x(89)90835-8. [DOI] [PubMed] [Google Scholar]
- Molenaar C. M., Prange R., Gallwitz D. A carboxyl-terminal cysteine residue is required for palmitic acid binding and biological activity of the ras-related yeast YPT1 protein. EMBO J. 1988 Apr;7(4):971–976. doi: 10.1002/j.1460-2075.1988.tb02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y., Goldstein J. L., Seabra M. C., Casey P. J., Brown M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990 Jul 13;62(1):81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- Sakagami Y., Yoshida M., Isogai A., Suzuki A. Peptidal Sex Hormones Inducing Conjugation Tube Formation in Compatible Mating-Type Cells of Tremella mesenterica. Science. 1981 Jun 26;212(4502):1525–1527. doi: 10.1126/science.212.4502.1525. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kikuchi A., Araki S., Hata Y., Isomura M., Kuroda S., Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990 Feb 5;265(4):2333–2337. [PubMed] [Google Scholar]
- Schaber M. D., O'Hara M. B., Garsky V. M., Mosser S. C., Bergstrom J. D., Moores S. L., Marshall M. S., Friedman P. A., Dixon R. A., Gibbs J. B. Polyisoprenylation of Ras in vitro by a farnesyl-protein transferase. J Biol Chem. 1990 Sep 5;265(25):14701–14704. [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Evans T., Howald W. N., Gelb M. H., Glomset J. A., Clarke S., Fung B. K. Membrane-binding domain of the small G protein G25K contains an S-(all-trans-geranylgeranyl)cysteine methyl ester at its carboxyl terminus. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):286–290. doi: 10.1073/pnas.88.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Goodwin G. W., Ghomashchi F., Glomset J. A., Gelb M. H. A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5302–5306. doi: 10.1073/pnas.88.12.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Kawata M., Katayama M., Horiuchi H., Kita Y., Takai Y. A geranylgeranyltransferase for rhoA p21 distinct from the farnesyltransferase for ras p21S. Biochem Biophys Res Commun. 1991 Mar 15;175(2):720–728. doi: 10.1016/0006-291x(91)91625-m. [DOI] [PubMed] [Google Scholar]