Deletion of the mouse HDL receptor SR-BI's three carboxy-terminal residues induces hypercholesterolemia and decreased receptor expression in liver and steroidogenic tissues, suggesting previously unrecognized carboxy terminus-binding adaptor protein in steroidogenic cells. SR-BIΔCT/apoE knockout mice fed a regular chow diet show severe coronary heart disease and premature death secondary to myocardial infarction.
Keywords: SR-BI, steroidogenic organs, PDZ domains, atherosclerosis, myocardial infarction
Abstract
The HDL receptor SR-BI mediates the transfer of cholesteryl esters from HDL to cells and controls HDL abundance and structure. Depending on the genetic background, loss of SR-BI causes hypercholesterolemia, anemia, reticulocytosis, splenomegaly, thrombocytopenia, female infertility, and fatal coronary heart disease (CHD). The carboxy terminus of SR-BI (505QEAKL509) must bind to the cytoplasmic adaptor PDZK1 for normal hepatic—but not steroidogenic cell—expression of SR-BI protein. To determine whether SR-BI's carboxy terminus is also required for normal protein levels in steroidogenic cells, we introduced into SR-BI's gene a 507Ala/STOP mutation that produces a truncated receptor (SR-BIΔCT). As expected, the dramatic reduction of hepatic receptor protein in SR-BIΔCT mice was similar to that in PDZK1 knockout (KO) mice. Unlike SR-BI KO females, SR-BIΔCT females were fertile. The severity of SR-BIΔCT mice's hypercholesterolemia was intermediate between those of SR-BI KO and PDZK1 KO mice. Substantially reduced levels of the receptor in adrenal cortical cells, ovarian cells, and testicular Leydig cells in SR-BIΔCT mice suggested that steroidogenic cells have an adaptor(s) functionally analogous to hepatic PDZK1. When SR-BIΔCT mice were crossed with apolipoprotein E KO mice (SR-BIΔCT/apoE KO), pathologies including hypercholesterolemia, macrocytic anemia, hepatic and splenic extramedullary hematopoiesis, massive splenomegaly, reticulocytosis, thrombocytopenia, and rapid-onset and fatal occlusive coronary arterial atherosclerosis and CHD (median age of death: 9 wk) were observed. These results provide new insights into the control of SR-BI in steroidogenic cells and establish SR-BIΔCT/apoE KO mice as a new animal model for the study of CHD.
NEW & NOTEWORTHY
Deletion of the mouse HDL receptor SR-BI's three carboxy-terminal residues induces hypercholesterolemia and decreased receptor expression in liver and steroidogenic tissues, suggesting previously unrecognized carboxy terminus-binding adaptor protein in steroidogenic cells. SR-BIΔCT/apoE knockout mice fed a regular chow diet show severe coronary heart disease and premature death secondary to myocardial infarction.
scavenger receptor class b type I (SR-BI) is a 509-amino acid cell surface receptor with a large extracellular loop and short intracytoplasmic amino and carboxy termini (8 and 45 amino acids long, respectively) (49) that is most highly expressed in the liver and in the steroidogenic cells of the adrenal gland, testes, and ovary (1). A minor mRNA splicing isoform, SR-BII, with a different carboxy terminus (39 residues replace 45 in SR-BI) has been described (68). As a high-density lipoprotein (HDL) receptor, SR-BI plays an important role in the transfer of cholesterol from HDL particles to cells (1, 49), controls HDL abundance and structure in both mice (50% C57BL/6-50% 129-S4 background) (50) and humans (59, 65, 79), serves as a signaling receptor to control endothelial nitric oxide synthase activity and vascular tone (77), and is a coreceptor for hepatitis C virus (8) and malaria (51, 69). Inactivation of murine SR-BI [SR-BI knockout (KO) mice] results in a 2.2-fold increase of plasma cholesterol in abnormally large HDL particles with an abnormally high ratio of unesterified to total cholesterol (UC:TC) (5, 50, 63). Analyses of SR-BI KO mice (50) and mice with hepatic overexpression of SR-BI (35, 73) have established that SR-BI can influence a variety of physiological and pathophysiological systems, including red blood cell (RBC) maturation and stability (25), platelet stability and function (13), biliary cholesterol secretion (35, 39), reverse cholesterol transport (83), steroidogenesis (37, 58), female fertility (41, 61, 72), deep vein thrombosis (6), and atherosclerosis/coronary heart disease (CHD) (4, 61).
The expression, localization, and function of SR-BI and many other membrane proteins can be regulated by cytoplasmic adaptor proteins (34, 74). A large group of such cytoplasmic proteins consists of the PDZ (PSD-95, Discs-large, ZO-1) domain protein family (64). PDZ domains are globular structures of 80–90 amino acids, usually interacting with the carboxy-terminal amino acids of their target protein using a well-defined binding pocket, although some PDZ domains can recognize internal peptide sequences (14, 22, 46) and some bind to anionic lipids in the cytoplasmic leaflets of cellular membranes (9, 62, 85). More than 100 PDZ domain-containing proteins have been described in humans, many of which contain multiple PDZ domains that allow them to function as scaffolds to bring together their target proteins/membranes for signal transduction and complex cellular functions (31, 64, 70). One of these multi-PDZ-domain adaptor proteins, PDZK1, is 519 amino acids long, contains four PDZ domains, and interacts with several membrane-associated proteins, mostly ion channels (31, 32).
One of PDZK1's target proteins is the HDL receptor SR-BI. PDZK1 was first shown to interact with the carboxy terminus of SR-BI by Ikemoto et al. (27) and was the first described tissue-specific adaptor protein of SR-BI (33). The carboxy terminus of SR-BI binds to either the first (PDZ1, high-affinity interaction) or the third (PDZ3, low-affinity interaction) PDZ domain of PDZK1 (27, 29, 30). The five carboxy-terminal residues of SR-BI (505QEAKL509) form hydrogen bonds and hydrophobic contacts with the canonical peptide binding pockets in these two PDZ domains (29, 30). The PDZ4 domain of PDZK1 also is required for full hepatic expression of SR-BI protein. PDZ4 appears to function by mediating PDZK1 binding directly to lipids in the inner leaflet of the plasma membrane rather than by canonical binding to the carboxy terminus of a target protein (62). Thus bidentate binding of PDZK1 to both SR-BI and membrane lipids is essential for normal hepatic SR-BI expression. This regulation influences the intracellular localization of SR-BI as well as the amount of SR-BI protein, presumably in part as a consequence of reduced protein stability. The results of these and other structure/function studies of the role of PDZK1 in regulating SR-BI are summarized in Reference 62.
In hepatocytes, PDZK1 posttranscriptionally controls the expression, localization, and function of SR-BI (33). In PDZK1 KO mice (129SvEv genetic background), hepatic SR-BI protein is reduced by 95% compared with wild-type (WT) control mice. As a consequence there is increased plasma cholesterol (1.7 fold) in the form of abnormally large HDL particles, a phenotype similar to, but not as severe as, that in SR-BI KO mice (33). Unlike SR-BI KO mice, PDZK1 KO mice do not exhibit an abnormally high UC:TC and the females are fertile (33). When PDZK1 KO mice are crossed with apolipoprotein E (apoE) KO mice, the atherogenic diet-fed double KO (dKO) mice (PDZK1/apoE dKO) exhibit increased atherosclerosis relative to apoE KO mice and can develop CHD that is substantially less severe than that of SR-BI/apoE dKO mice (34, 71).
In PDZK1 KO mice, there is a striking difference in the very low expression of SR-BI protein in hepatocytes (<5%) compared with the essentially WT level of SR-BI in steroidogenic cells (100%) (33). It is possible that distinctive features of hepatocytes (polarity, membrane trafficking, etc.) not shared by steroidogenic cells confer a requirement for SR-BI's carboxy terminus to bind to an adaptor protein. Alternatively, in steroidogenic cells there may be a distinct adaptor protein(s) that can bind to SR-BI's carboxy terminus and play a role analogous to that of PDZK1 in the liver in maintaining normal levels of SR-BI protein expression. To explore these possibilities, we have used knock-in technology in mice to insert in SR-BI's gene a Stop codon in place of the codon encoding 507Ala in SR-BI's carboxy terminus. The resultant truncated protein, SR-BIΔCT, is three residues (507AKL509) shorter than WT SR-BI. Those residues are part of SR-BI's five-residue PDZ domain-binding motif; thus SR-BIΔCT is not expected to bind to PDZ domain-containing (e.g., PDZK1) or other adaptors that recognize SR-BI's most carboxy-terminal residues. Analyses in transfected cell lines have shown that removal, or removal and replacement, of all or part of the PDZ domain-binding motif in SR-BI does not prevent the receptor's cell surface expression or alter its lipid transport activities (11, 12, 19, 54). As expected from previous studies (29, 30, 33, 54), homozygous knock-in mice expressing SR-BIΔCT exhibited a marked reduction of SR-BIΔCT protein expression in the liver. Strikingly, there was also a marked reduction in receptor expression in steroidogenic cells. Thus, as in the case of the liver, in steroidogenic cells normal SR-BI protein expression requires its three carboxy-terminal cytoplasmic residues, most likely because those residues are required for binding to a distinct cytoplasmic adaptor required to maintain normal receptor levels.
We compared a variety of characteristics of SR-BIΔCT mice to those of WT, SR-BI KO, and PDZK1 KO mice, including composition and size of the HDL, female fertility, RBC and platelet levels, cholesteryl ester (CE) stores in steroidogenic cells, and atherosclerosis and CHD susceptibility when combined with apoE deficiency. For some phenotypes, the SR-BIΔCT mice resembled WT mice (female fertility, RBC and platelets, CE stores). Other phenotypes of SR-BIΔCT mice resembled those of either SR-BI KO or PDZK1 KO mice (plasma lipoprotein composition and size, atherosclerosis, and CHD susceptibility). For example, when SR-BIΔCT mice were crossed with apoE KO mice, the SR-BIΔCT/apoE mice fed a standard chow diet exhibited early-onset, fatal atherosclerotic CHD and thus provide a novel model for the study of CHD.
MATERIALS AND METHODS
Generation of SR-BIΔCT Knock-In Mice
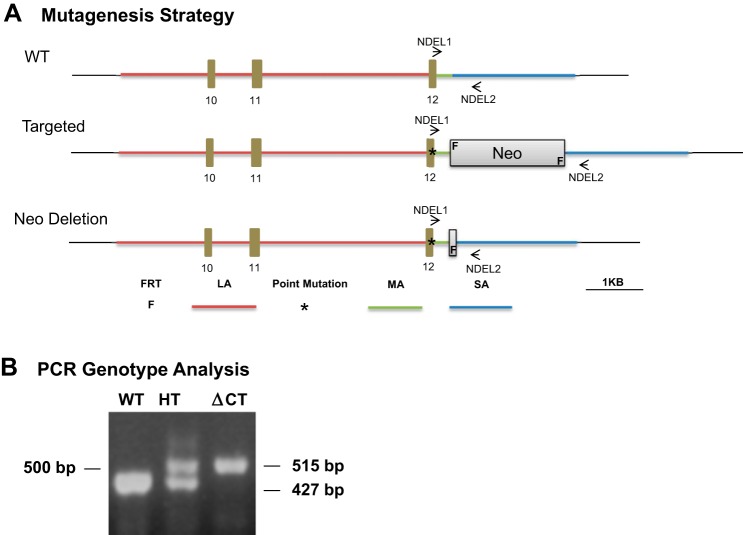
A SR-BIΔCT knock-in mouse containing a deletion of the last three carboxy-terminal amino acids of SR-BI was generated by Ingenious Targeting Laboratory (Fig. 1A). The mouse BAC clone RP23-21D2 (chromosome 5, 125587731–125789903) was used to build the targeting vector, which was constructed with an homologous-based recombination technique. The vector consisted of a 5.0-kb long arm, including exons 10, 11, and 12 of the SR-BI gene, and a 1.9-kb short arm. The SPEC cassette, containing the ΔCT-SR-BI mutation (replacement of 507Ala by a Stop codon), as well as the Neo cassette were generated by PCR and inserted into the BAC clone by bacterial homologous recombination (middle arm). A DNA fragment containing the long, middle (SPEC and Neo cassettes), and short arms was subcloned from the BAC clone into the targeting vector (total size, 13.05 kb) (Fig. 1A). Ten micrograms of the targeting vector was linearized with NotI before electroporation into iTL BA1 (129/SvEv × C57BL/6; 50:50) hybrid embryonic stem (ES) cells. After selection with G418, surviving clones were expanded for PCR analysis to identify homologous recombinant ES cell clones. Several clones were identified as positive and selected for further use. Confirmation of the mutation was performed by PCR and DNA sequencing. Further confirmation of the positive ES cell clones was performed by Southern blot. Homologous recombinant ES cells were microinjected into C57BL/6 blastocysts. Embryos were transferred into pseudopregnant mice. Resulting chimeras with high-percentage agouti coat color were mated to C57BL/6 FLP mice to remove the Neo cassette. Tail DNA was tested by PCR to determine mouse genotypes and the removal of the Neo cassette using the NDEL1 (CCTCTTCACCCCACCTACTCATAGC) and NDEL2 (GGACACTGAGAAGCAACTGGCCTAAC) oligonucleotide primers. The WT allele generated a band at 427 bp, while the SR-BIΔCT knock-in allele generated a band at 515 bp (Fig. 1B). Mice heterozygous for the mutation were mated to 129-Elite Mice (129S2/SvCrl) (Charles River). Heterozygous mice resulting from this mating were used to generate WT mice—used as background-matched controls—and homozygous knock-in mutants. Intercrosses of the heterozygous mice resulted in fertile homozygous knock-in mice (SR-BIΔCT).
Fig. 1.
Generation of knock-in mutation into exon 12 of the SR-BI (Scarb1) gene encoding the truncated receptor SR-BIΔCT (A) and genotyping of SR-BIΔCT mice by PCR analysis (B). A, top: organization of a portion of the wild-type (WT) SR-BI (Scarb1) allele showing the corresponding positions in the targeting vector of the long arm (LA, red line), including exons 10–12, the middle arm (MA, green), and the short arm (SA, blue), as well as the sites for the NDEL1 and NDEL2 primers used for genotyping. Middle: the initially targeted allele including the 507Ala/STOP mutation (*) and the Neo cassette with FRT sites (F). Bottom: organization of a portion of the final, Neo cassette deleted, SR-BIΔCT mutant allele. B: PCR analysis using the NDEL1 and NDEL2 primers of genomic DNA from WT, heterozygous knock-in (HT), and homozygous knock-in (ΔCT) mutant mice. The WT allele generates a 427-bp band, while the SR-BIΔCT mutant allele generates a 515-bp band.
Animals
All procedures were performed in accordance with protocols reviewed and approved by the Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology Committees on Animal Care. WT and SR-BIΔCT mice [both on a mixed C57BL/6 × 129S2/SvCrl (37.5:62.5) genetic background, see above] were maintained on a normal chow diet. Six- to ten-week-old male and female mice were used for experiments. For atherosclerosis studies, apoE-deficient mice (C57BL/6 background) (82) were purchased from Jackson Laboratories (Bar Harbor, ME), mated with SR-BIΔCT mice, and maintained on a standard chow diet. Genotypes were determined by PCR with established protocols (see above and Jackson Laboratories web site). After the initial breeding, apoE KO mice heterozygous for the SR-BIΔCT mutation and apoE KO were mated to generate apoE KO and SR-BIΔCT/apoE KO mice with the same proportion of C57/B6 and 129 backgrounds (68.75:31.25, respectively) and were used for experiments. We compare the results described here with results previously reported in mice with the following genetic backgrounds: SR-BI KO [mixed C57BL/6 × 129S2/SvCrl (50:50)]; PDZK1 KO [129SvEv (100)]; and SR-BI/apoE dKO [mixed C57BL/6 × 129S2/SvCrl (75:25) genetic background].
Blood and Tissue Sampling, Processing, and Analysis
Plasma, liver, spleen, adrenal glands, testes, and ovaries were collected and processed as previously described (33, 50). Total and unesterified plasma cholesterol levels and fast protein liquid chromatography (FPLC) cholesterol profiles that separate plasma lipoproteins by size were obtained as previously described (16). The presence of apoA1 and apoE in individual chromatographic fractions was determined by immunoblotting (see below).
Hearts were excised after a short in vivo perfusion with PBS, weighed, and frozen in OCT compound. Transverse or sagittal frozen sections (5 μm) were stained with Oil Red O-hematoxylin to assess the presence of atherosclerotic lesions in the aortic root and coronary vessels as previously described (71). Cardiac fibrosis was evaluated on cryosections (5 μm) stained with Masson's trichrome (71).
Erythrocyte and Platelet Analysis
Blood was collected by cardiac puncture into EDTA tubes (Microvette* 100 from Sarstedt). Hematocrit, erythrocyte measurements, including mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), and platelet counts were determined with an automated Hemavet HV950 analyzer (Drew Scientific) by the Department of Comparative Medicine at MIT. Peripheral smears were performed to assess RBC morphology, stained with Wright-Giemsa, and examined with standard light and differential interference contrast microscopy. Reticulocyte counts were performed manually after brief staining in 0.5% methylene blue.
RNA Extraction and Gene Expression Evaluation by qPCR
Total RNA was isolated from liver and adrenal glands harvested from 6- to 8-wk-old male and female WT and SR-BIΔCT mice (8 WT and 6 SR-BIΔCT liver, 14 WT and 14 SR-BIΔCT adrenal glands) with an RNeasy mini kit (Qiagen). Corresponding cDNA was generated by reverse transcription with SuperScript III (Invitrogen) using random primers. Quantitative real-time PCR (qPCR) was performed with SYBR Green 1 (Qiagen). Multigene transcriptional profiling, a form of qPCR, was used to determine mRNA copy numbers. The number of mRNA copies was calculated by normalization to 18S rRNA abundance and expressed as n copies/106 copies of 18S (53). The DNA primers used were TGGAACGGACTCAGCAAGATC and GTCATGAAGGGTGCCCACAT for SR-BI + SR-BII and GCGCAGCCAGGGTCCTGAA and TGGCTGGTCTGACCAAGCTA for SR-BII only.
Immunoblotting
Protein samples (∼30 μg, as determined with a Bio-Rad DC protein assay) from total tissue lysates of both male and female mice (7 livers, 7 adrenal glands, and 3 ovaries per group) were fractionated by 5–20% gradient SDS-PAGE, transferred to nitrocellulose membranes, and incubated with a rabbit polyclonal anti-SR-BI antibody (mSR-BI495-112) raised against a carboxy-terminal peptide of the protein, the anti-SR-BI KKB-1 antibody (19), a rabbit polyclonal antibody that recognizes both SR-BI and SR-BII (16), or a specific rabbit anti-SR-BII (68) antibody (Novus Biologicals) (all used at 1:500 dilution), followed by an anti-rabbit IgG conjugated to horseradish peroxidase (Invitrogen, 1:10,000), and visualized by ECL chemiluminescence (GE Healthcare). Immunoblotting using a polyclonal anti-ε-COP antibody (1:5,000) (20) was used to control for small variations in loading. The relative amounts of proteins were determined quantitatively with FluorChemQ System Quantitative Western Blot Imaging. Western blot analyses of the FPLC fractions were performed with rabbit anti-apoAI (1:2,000) (Biodesign) and rabbit anti-apoE (1:400) (Novus) antibodies and anti-rabbit secondary antibody. Longer exposures for chemiluminescence detection of SR-BI/II were used for liver samples than for adrenal and ovarian samples because of the substantially lower receptor levels in the liver.
Cholesterol Measurements in Adrenal Glands
Adrenal glands obtained from WT and SR-BIΔCT mice (6 males and 6 females in each group) were homogenized in 0.75 ml of lysis buffer (0.25 M sucrose, 50 mM Tris, pH 7.4) with a Dounce homogenizer. The homogenate obtained was mixed with 3 ml of chloroform-methanol (1:2 vol/vol), vortexed, and incubated on a shaker at room temperature for 1 h. One milliliter of chloroform and one milliliter of distilled water were added and vortexed. The extract was centrifuged at 1,000 rpm for 10 min at 4°C. The lower organic phase was separated and dried. Dried lipids were resuspended in 0.2 ml of 0.1% Triton X-100 and sonicated. Total cholesterol was measured with a kit from Wako Diagnostics according to the manufacturer's protocol. Protein amounts were determined after drying the aqueous phase, which was reconstituted in 0.3 N NaOH and 0.1% Triton X-100.
Immunoperoxidase Analysis
Livers, adrenals, ovaries, and testes (tissue samples from 5 or 6 male or female mice per group) were harvested, fixed in 4% paraformaldehyde in PBS for 4 h, transferred overnight into 30% sucrose in PBS, and frozen, and 5-μm cryosections (2 or 3 sections per slide) were generated and stained with the anti-SR-BI KKB-1 antibody and biotinylated anti-rabbit IgG, visualized by immunoperoxidase staining, and counterstained with Harris modified hematoxylin, as described previously (33).
Hemodynamic Studies
Cardiac function was evaluated in 7-wk-old mice with left ventricular pressure-volume loop measurements as previously described (3, 10). Maximum and minimum left ventricular volumes, cardiac output, ejection fraction, change in pressure with time (dP/dt), and stroke work were determined.
Statistical Analysis
Data are shown as means ± SE. Statistically significant differences were determined by either pairwise comparisons of values using the unpaired t-test or one-way ANOVA with Tukey post hoc testing. Mean values for experimental groups are considered statistically significantly different at P < 0.05 for both types of tests.
RESULTS
Role of Carboxy Terminus of SR-BI in Regulation of SR-BI Protein Expression and Function
To determine the impact of the absence of the three carboxy-terminal amino acids of SR-BI on its regulation in the liver and in steroidogenic organs and the influence of the truncated protein on SR-BI-dependent physiology and pathophysiology, we used knock-in technology to modify the endogenous mouse SR-BI gene so that the SR-BI protein would contain a deletion (ΔCT) of the last three carboxy-terminal (CT) amino acids (residues 507AKL509) by replacing the codon for 507Ala in exon 12 with a Stop codon (Fig. 1A). The ΔCT mutation was designed to prevent the interaction of the carboxy terminus of SR-BI with cytoplasmic adaptors that recognize the carboxy-terminal residues, such as the PDZ1 and PDZ3 domains in PDZK1 (29, 30). Mice homozygous for this deletion mutation, designated SR-BIΔCT, were generated and have been observed for 1 yr. They develop normally and show no alteration in gross morphology, weight, and size compared with WT mice. These mice and their WT controls used for the experiments reported here are on a mixed C57BL/6:129S2/SvCrl (37.5:62.5) genetic background.
Effects of SR-BIΔCT Mutation on SR-BI mRNA and Protein Expression in Liver
Expression levels in the livers of WT and SR-BIΔCT mice of the mRNA for SR-BI and its minor splice isoform SR-BII were evaluated by qPCR. We used oligonucleotide primers either that recognized both SR-BI and its minor splice isoform SR-BII or that were specific for the SR-BII isoform only (see materials and methods). Steady-state levels of receptor protein were measured by quantitative immunoblot analysis using three specific antibodies: an anti-carboxy terminus antipeptide antibody (anti-SR-BI495-112) (1, 16) that recognizes SR-BI but not SR-BII, whose carboxy terminus differs from that of SR-BI (68); a commercial anti-carboxy terminus antipeptide antibody (Novus) that recognizes SR-BII but not SR-BI; and the polyclonal antibody KKB-1 (19) that recognizes the extracellular loops of both SR-BI and SR-BII (33, 68). The KKB-1 antibody is the only one of these three expected to recognize SR-BIΔCT.
The qPCR analysis established that the truncated receptor's mRNA copy number (expressed as n copies/106 copies of 18S rRNA; see materials and methods) in SR-BIΔCT liver was similar to that of full-length SR-BI in WT liver (WT: 48.0 ± 2.8; ΔCT: 40.5 ± 0.9; ∼16% reduction, P = 0.04). This small difference might be due to reduced transcription, stability, or both. The copy number of the mRNA for the minor splice variant SR-BII was low in WT liver (6.3 ± 0.4) and unexpectedly increased by 2.5-fold in SR-BIΔCT liver (15.9 ± 0.9, P < 0.0001).
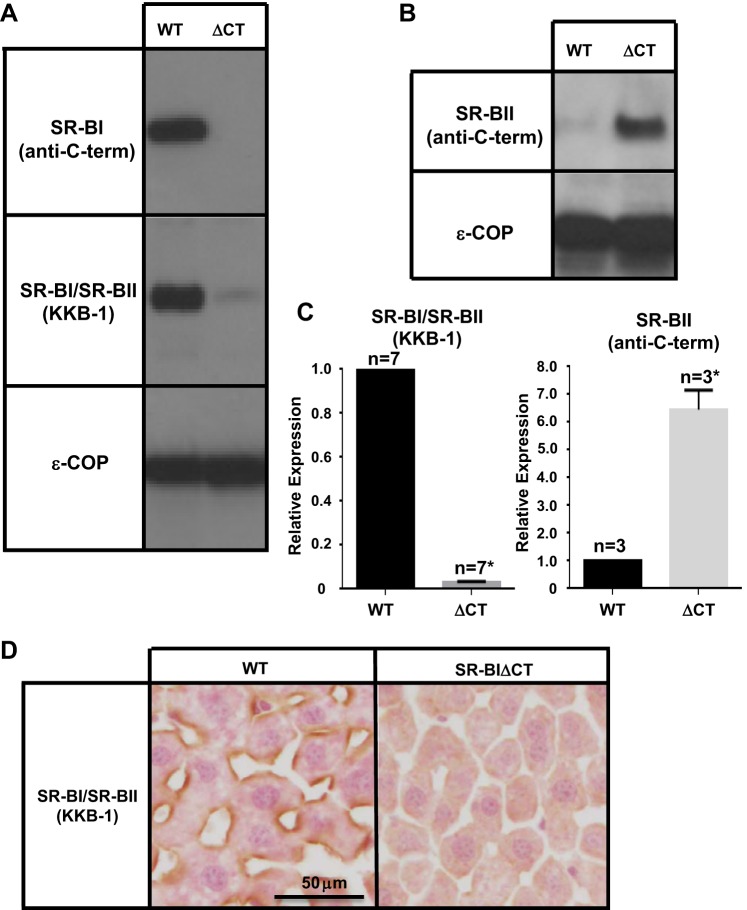
As demonstrated in Fig. 2A, top, the anti-carboxy terminus anti-SR-BI495-112 antibody (anti-C-term) could readily detect a band corresponding to SR-BI (∼82 kDa) in WT liver (and adrenal gland and ovary, see below) but not in tissues from SR-BIΔCT mice carrying the carboxy-terminal, three-residue deletion. An antibody to the cytosolic COPI coat subunit ε-COP was used as a loading control (Fig. 2A, bottom). The absence of signal in the SR-BIΔCT tissues is likely due to the inability of the anti-carboxy terminus antibody to recognize the SR-BIΔCT protein or the complete absence of this protein. With the KKB-1 antibody, which does not require an intact carboxy terminus to detect SR-BI or SR-BII, a strong signal could be seen in the immunoblot of WT livers (Fig. 2A, middle left), but there was a >95% reduction in the signal in livers from SR-BIΔCT mice [the relative expression -SR-BIΔCT/WT- was 0.03 ± 0.003 (P < 0.0001); Fig. 2C, left]. These results were confirmed by immunohistochemical localization of receptors using the KKB-1 antibody. There was robust staining of the sinusoidal membranes of hepatocytes in WT liver but no significant staining in SR-BIΔCT liver (Fig. 2D). Thus there was a dramatic reduction in the amount of hepatic SR-BIΔCT protein in SR-BIΔCT mice relative to the amount of full-length SR-BI protein in WT livers. This observation was expected because the carboxy terminus of SR-BIΔCT does not bind to PDZK1 [as predicted by isothermal titration calorimetry, which demonstrated that SR-BI 500KGTVLQE506 peptide missing the three carboxy-terminal amino acids 507AKL509 did not bind to PDZK1 recombinant protein (data not shown)] and normally high levels of hepatic SR-BI protein expression require SR-BI's binding to PDZK1 (29, 33).
Fig. 2.
Effects of the SR-BIΔCT mutation on the hepatic expression of SR-BI and SR-BII proteins. A and B: immunoblotting analyses of liver lysates (∼30 μg protein) from male WT and SR-BIΔCT (ΔCT) 6- to 10-wk-old mice. Results in female mice were virtually identical. Bands were visualized by chemiluminescence. A: proteins were detected with either a SR-BI-specific anti-carboxy-terminal peptide antibody [SR-BI (anti-C-term)] or a rabbit polyclonal antibody that recognizes the extracellular domains of both SR-BI and its minor splice isoform SR-BII [SR-BI/SR-BII (KKB-1), ∼82 kDa] or polyclonal rabbit anti-ε-COP (∼34 kDa) that was used as a loading control. B: immunoblotting analysis of the expression of SR-BII using a rabbit polyclonal, SR-BII-specific anti-carboxy-terminal peptide antibody [SR-BII (anti-C-term)] and anti-ε-COP. C: quantitative analyses of immunoblots was used to determine the relative expression of SR-BI/SR-BII (KKB-1) and SR-BII in the livers of WT and SR-BIΔCT (ΔCT) mice. *Significantly different [P < 0.0001 for SR-BI/SR-BII(KKB-1) and P = 0.001 for SR-BII]. D: livers from male WT and SR-BIΔCT mice were fixed, frozen, and sectioned, and the sections were stained with the polyclonal anti-SR-BI/SR-BII KKB-1 antibody and a biotinylated anti-rabbit IgG secondary antibody and visualized by immunoperoxidase staining. Magnification ×600.
A small amount of the signal in the WT livers, detected with the KKB-1 antibody (Fig. 2A, middle left), is likely to be contributed by the minor isoform SR-BII. Analysis of PDZK1 KO mice established that the level of hepatic SR-BII protein, whose carboxy terminus differs from that of SR-BI and is not expected to bind to PDZ domains, is PDZK1 independent (33). Indeed, when a SR-BII-specific antipeptide antibody was used for immunoblotting (Fig. 2B) the relatively weak signal for SR-BII in WT liver was increased 6.5-fold in SR-BIΔCT livers [relative expression SR-BIΔCT/WT = 6.5 ± 0.7 (P = 0.001); Fig. 2C, right]. The increased SR-BII protein expression might have been due, at least in part, to the 2.5-fold increase in SR-BII mRNA, increased translation, increased protein stability, or some combination of these. Although there was an increase in the minor splice form of the receptor protein, SR-BII, in SR-BIΔCT compared with WT livers, the overall hepatic levels of all isoforms of this receptor detected by the KKB-1 antibody were dramatically lower in SR-BIΔCT mice compared with WT mice (Fig. 2A, middle left). It is likely that the reduced steady-state levels of receptor, primarily the truncated SR-BI isoform, were a consequence of its inability to bind to the adaptor protein PDZK1. Thus the dramatic reduction in receptor protein in the liver in SR-BIΔCT mice mirrors the loss of hepatic full-length SR-BI in PDZK1 KO mice (33).
Effects of SR-BIΔCT Mutation on SR-BI mRNA and Protein Expression in Steroidogenic Tissues
We next addressed the question of whether or not there was a requirement for the three carboxy-terminal residues of SR-BI for its normal protein expression in steroidogenic cells. Such a requirement would suggest that an adaptor protein functionally analogous to hepatic PDZK1 would be present in steroidogenic cells. First, we focused on receptor mRNA expression in adrenal glands, where SR-BI is highly expressed in the cortex (1, 37, 48).
The copy number of all isoforms of the receptor's mRNA was reduced by 27% in SR-BIΔCT adrenal glands compared with WT adrenal glands (WT: 799.4 ± 56.1; SR-BIΔCT: 587.1 ± 55.8, P = 0.01). The ∼15-fold greater number of mRNAs (SR-BI + SR-BII) in the adrenal gland relative to the liver of WT mice is consistent with previous reports of substantially higher SR-BI mRNA and protein expressed in the adrenal gland (1, 33, 37). The copy number of the SR-BII isoform's mRNA increased 4.4-fold (WT: 45.2 ± 3.9; SR-BIΔCT: 197.9 ± 40.4, P = 0.0009).
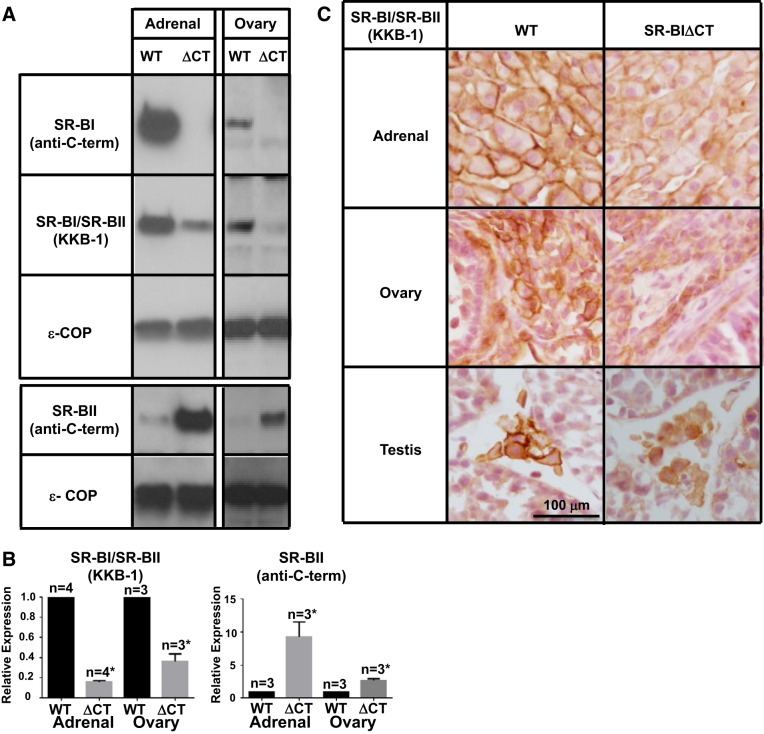
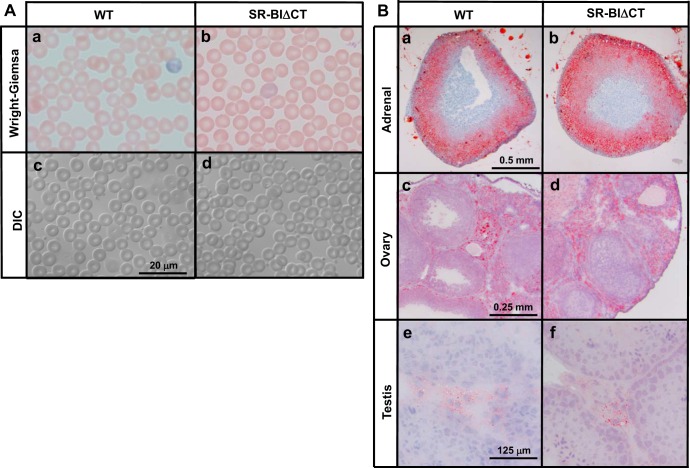
We used immunoblotting with the KKB-1 antibody, which recognizes both SR-BI and SR-BII and does not require an intact carboxy terminus to detect the receptors, to assess the steady-state receptor protein levels in the adrenal glands and ovaries. Fig. 3A (2nd panel) shows that, as previously reported (1, 33, 37), in the tissues from the WT mice there was an intense receptor band in the adrenal glands and a weaker one in the ovaries. In contrast, in the tissues from SR-BIΔCT mice compared with those from WT there was an 84% reduction in the intensity of SR-BI/SR-BII protein in adrenal glands [relative expression of SR-BIΔCT/WT = 0.16 ± 0.01 (P < 0.0001); Fig. 3B, left] and a ∼64% reduction in the ovaries [relative expression SR-BIΔCT/WT = 0.36 ± 0.07 (P = 0.0009); Fig. 3B, left]. These results were confirmed by immunohistochemical imaging of receptors using the KKB-1 antibody. There was robust staining of the plasma membranes of adrenal cortical cells (Fig. 3C, top left) and ovarian stromal cells (Fig. 3C, middle left) in WT mice, but the staining was clearly less intense in SR-BIΔCT mice (Fig. 3C, top and middle right), particularly at the cell surfaces. Although we did not perform qPCR or immunoblotting analyses of the testes, immunohistochemical analysis (Fig. 3C, bottom) indicated that in the steroidogenic Leydig cells of SR-BIΔCT mice relative to those in WT mice there was a reduction in SR-BI/SR-BII protein, particularly at the cell surfaces.
Fig. 3.
Effects of the SR-BIΔCT mutation on the expression of SR-BI and SR-BII proteins in steroidogenic tissues. A: immunoblotting analyses of adrenal gland and ovary lysates (∼30 μg protein) from WT and SR-BIΔCT 6- to 10-wk-old mice. Bands were visualized by chemiluminescence but with exposure times significantly shorter than those used in Fig 2A because of the higher protein expression in these steroidogenic tissues than in the liver. Proteins were detected with an SR-BI-specific [SR-BI (anti-C-term)] antibody, an anti-SR-BI/SR-BII (KKB-1) antibody, or an SR-BII-specific [SR-BII (anti-C-term)] antibody, as described in Fig 2. An anti-ε-COP antibody was used as a loading control. B: relative expression of SR-BI/SR-BII (KKB-1) and SR-BII in WT and SR-BIΔCT (ΔCT) mouse adrenal gland and ovary. *Significantly different [P < 0.0001 in adrenal glands and P = 0.0009 in ovaries for SR-BI/SR-BII (KKB-1) and P = 0.02 in adrenal glands and P = 0.003 in ovaries for SR-BII]. C: adrenal glands, ovaries, and testes from WT and SR-BIΔCT mice were fixed, frozen, and sectioned, and the sections were stained with the polyclonal anti-SR-BI/SR-BII KKB-1 antibody and a biotinylated anti-rabbit IgG secondary antibody and visualized by immunoperoxidase staining. Results for male and female mouse adrenal glands were identical. Magnification ×300.
A small amount of the receptor protein in SR-BIΔCT adrenal glands and ovaries detected with the KKB-1 antibody (Fig. 3A, 2nd panel) is likely to have been contributed by the minor isoform SR-BII. When a SR-BII-specific antipeptide antibody was used for immunoblotting (Fig. 3A, 4th panel) the relatively weak intensities for SR-BII in both WT tissues were increased in SR-BIΔCT tissues, 9.2 ± 2.2-fold (P = 0.02) in adrenal glands and 2.7 ± 0.3-fold (P = 0.003) in the ovaries (Fig. 3A, 4th panel). The increased SR-BII protein expression might have been due, at least in part, to the increase in SR-BII mRNA, increased translation or increased protein stability, or some combination of these. Despite the increase in SR-BII protein in these steroidogenic tissues in SR-BIΔCT compared with WT mice, the overall levels of both isoforms of this receptor detected by the KKB-1 antibody were substantially lower (84%) in SR-BIΔCT mice compared with WT mice (Fig. 3A, 2nd panel). These results differed dramatically from those in PDZK1 KO mice, in which there is essentially no loss of SR-BI protein expression in steroidogenic tissues compared with WT mice (33). On the basis of these results, we propose that the steroidogenic cells in the adrenal glands and ovaries, and possibly the testes, express an adaptor protein(s) that is functionally similar to PDZK1 in hepatocytes in that it recognizes the carboxy-terminal three residues of SR-BI and is required for expression of normal SR-BI protein levels in these cells.
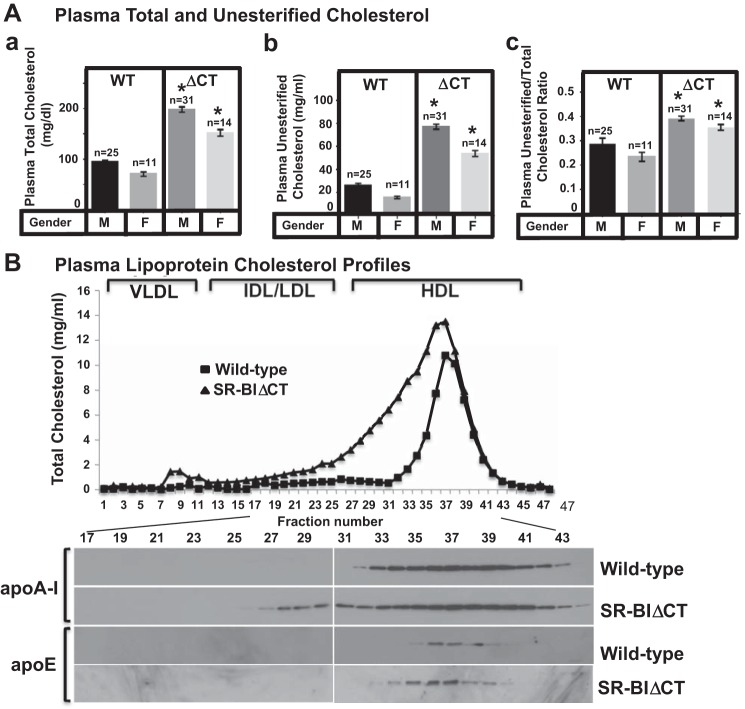
Fig. 4.
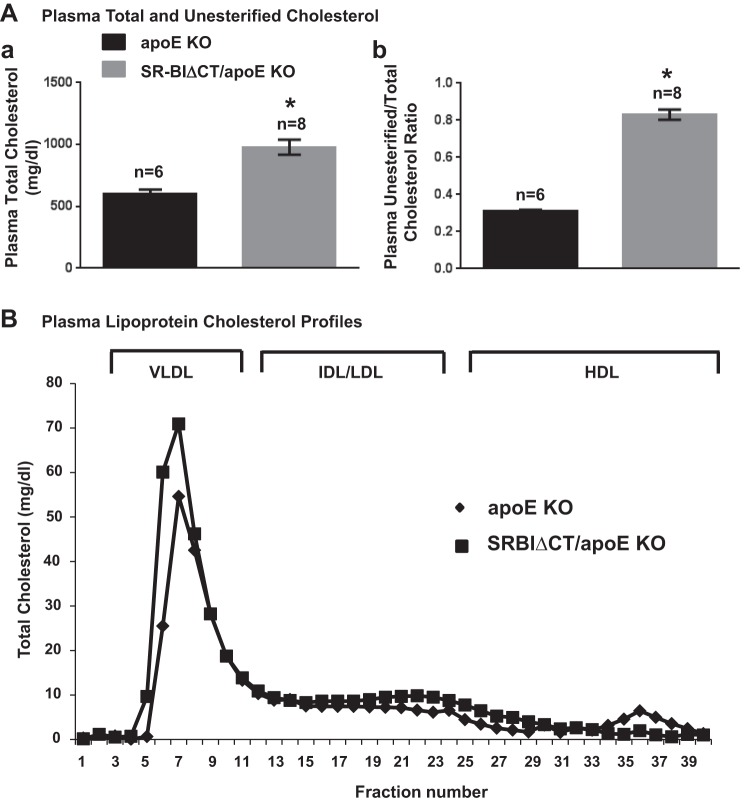
Effects of the SR-BIΔCT (ΔCT) mutation on plasma total (TC) and unesterified (UC) cholesterol levels and the UC-to-TC ratio (UC:TC; A) and on plasma lipoprotein size distribution profiles (cholesterol and apolipoproteins; B). Plasma samples were harvested from male (M) and female (F) WT and SR-BIΔCT (ΔCT) 6- to 10-wk-old mice. A: plasma total (a) and unesterified (b) cholesterol levels and UC:TC (c) were determined in individual samples by enzymatic assay; mean (±SE) values from the indicated numbers of animals (n) are shown. *Significantly different (P < 0.0001) between ΔCT mice and the corresponding WT control mice of the same sex. B: pooled plasma samples from 3 males of each genotype were size-fractionated by FPLC, and the total cholesterol content of each fraction was determined by an enzymatic assay (top). The chromatograms are representative of multiple individually determined profiles. Approximate elution positions of native VLDL, IDL/LDL, and HDL particles are indicated by brackets and were determined as previously described (50). The FPLC fractions were analyzed by immunoblotting (bottom) to determine the distribution of the apolipoproteins apoA-I and apoE.
Functional Consequences of SR-BIΔCT Mutation in SR-BIΔCT KI Mice
Plasma cholesterol and lipoproteins in SR-BIΔCT mice.
Figure 4A shows a comparison of the plasma levels of total (unesterified plus esterified, Fig. 4Aa) and unesterified cholesterol (Fig. 4Ab) in male and female WT and SR-BIΔCT (ΔCT) mice. There was a significant 2.1-fold increase in total plasma cholesterol (Fig. 4Aa) in both male and female SR-BIΔCT mice compared with WT mice (male WT: 94.6 ± 3.7 mg/dl, male SR-BIΔCT: 198.6 ± 5.3 mg/dl, female WT: 71.0 ± 4.3 mg/dl, female SR-BIΔCT: 152.4 ± 6.4 mg/dl, P < 0.0001). Total plasma cholesterol levels were significantly different (P = 0.008) between male and female WT mice. There was also an approximately threefold increase in unesterified cholesterol (Fig. 4Ab) in both male and female SR-BIΔCT mice compared with WT mice (male WT: 26.6 ± 1.6 mg/dl, male SR-BIΔCT: 77.4 ± 2.2 mg/dl, female WT: 16.1 ± 1.0 mg/dl, female SR-BIΔCT: 54.1 ± 2.5 mg/dl, P < 0.0001). A distinctive feature of the plasma of SR-BI KO mice compared with WT and PDZK1 KO mice is a marked, ∼65%, increase in UC:TC [SR-BI KO, 0.515 ± 0.027; WT and PDZK1 KO, ∼0.23–0.31; UC:TC can vary depending on sex and genetic background (present study and Refs. 5, 33, 63)]. The mixed genetic backgrounds of the mice analyzed in this report and of those to which they are compared are provided in Animals. The increased UC:TC appears to be responsible for some of the pathophysiology exhibited in SR-BI KO mice (abnormal RBCs and platelets, female infertility) (25, 41, 61, 72). There was an increase in UC:TC in SR-BIΔCT mice (Fig. 4Ac), although the elevation was not as great as that in SR-BI KO mice [SR-BIΔCT (male/female) 0.39 ± 0.01/0.36 ± 0.01; WT (male/female) 0.28 ± 0.02/0.23 ± 0.02, P < 0.0001 for both sexes].
FPLC size fractionation of plasma lipoproteins from WT mice shows that most of the plasma cholesterol (unesterified and esterified) is carried in HDL-size particles that contain the major HDL apolipoprotein apoA-1 as well as some apoE, both detected by immunoblotting (Fig. 4B) (36, 50, 67, 76). In SR-BIΔCT mice the large apoA-1- and apoE-containing HDL peak is partially shifted to the left in the lipoprotein cholesterol profile (Fig. 4B), indicating a larger and more heterogeneous population of HDL particles. Thus the dramatic reduction in hepatic SR-BI in SR-BIΔCT mice resulted in plasma cholesterol and lipoprotein phenotypes that were similar to, but not quite as severe as, those in SR-BI KO mice (50). It is not clear why these abnormal phenotypes in SR-BIΔCT mice were more severe than those in PDZK1 KO mice (e.g., increased UC:TC in SR-BIΔCT mice), although disruption of normal SR-BI activity in other organs in the SR-BIΔCT mice (e.g., intestines, steroidogenic tissues, etc.) may play a role.
Fertility of SR-BIΔCT mice.
It is noteworthy that, despite the modestly elevated UC:TC in female and male SR-BIΔCT mice, these mutant mice were fertile and their litter sizes (5.6 pups/litter, n = 12) were comparable to those of WT mice (5.2 pups/litter, n = 16).In contrast, SR-BI KO female (but not male) mice, which have a very high UC:TC in their plasma, are infertile because of excess unesterified cholesterol deposition in and premature activation of their eggs (41, 61, 72). PDZK1 KO mice are fertile (33).
Red blood cells and platelets in SR-BIΔCT mice.
Table 1 and Fig. 5A show that the hematologic characteristics of SR-BIΔCT mice are similar to those of WT mice (RBC morphology—assessed with standard and differential interference contrast microscopy, MCH, % reticulocytes, and platelet count), with a slightly elevated hematocrit (55.9 ± 3.2% vs. 45.8 ± 2.3%, P = 0.04) and 21% increase in RBC volume (MCV; P = 0.001). As was the case with female fertility, the RBC and platelet phenotypes of SR-BIΔCT mice (UC:TC of 0.36 ± 0.01) were essentially normal, whereas those of SR-BI KO mice exhibited some abnormalities that are likely a consequence of their abnormally high UC:TC (0.515 ± 0.027) (13, 25). Although the SR-BIΔCT mutation alone apparently did not markedly alter the RBCs and platelets, it did have effects on these blood cells when combined with deletion of the apoE gene, as this combination dramatically increased UC:TC (described below).
Table 1.
Hematologic data for wild-type and SR-BIΔCT mice
| Genotype | Hematocrit, % | MCV, fl | Reticulocytes, % | MCH concentration, g/dl | MCH, pg | Platelets, ×106/ml |
|---|---|---|---|---|---|---|
| WT (n = 5) | 45.8 ± 2.3 | 55.9 ± 2.4 | 2.7 ± 0.3 | 27.3 ± 1.4 | 15.8 ± 0.1 | 687.8 ± 44.1 |
| SR-BIΔCT (n = 6) | 55.9 ± 3.2 | 67.8 ± 1.0 | 2.8 ± 0.5 | 24.8 ± 0.2 | 16.8 ± 0.2 | 608.4 ± 51.0 |
| P value | 0.04 | 0.001 | >0.05 | >0.05 | 0.005 | >0.05 |
Fig. 5.
Red blood cell morphology (A) and Oil Red O (neutral lipid) staining of steroidogenic tissues (B) in WT and SR-BIΔCT mice. A: blood samples from 6- to 10-wk-old WT (a and c) and SR-BIΔCT (b and d) mice were stained with Wright-Giemsa and visualized by standard light microscopy (a and b) or differential interference contrast (DIC) optics (c and d). Magnification ×1,000. B: adrenal, ovarian, and testicular tissues from WT and SR-BIΔCT mice were frozen, and frozen sections (5 μm) were stained with Oil Red O-hematoxylin. Neutral lipids (e.g., cholesteryl esters) stain red. Representative sections are shown, and results for male and female mouse adrenal glands were essentially identical. Magnification ×25 adrenal gland, ×50 ovary, and ×250 testis.
Cholesteryl ester stores in adrenal glands and ovaries of SR-BIΔCT mice.
In WT mice, CEs are stored in cytoplasmic lipid droplets in steroidogenic cells to provide cholesterol as feedstock for steroidogenesis, and SR-BI plays an important role in maintaining these CE stores (50, 61; reviewed in 49). The CE stores may be detected by chemical analysis (e.g., extraction and quantitative analysis), staining of tissue sections with the neutral lipid staining dye Oil Red O, or visual inspection in the case of adrenal glands (24, 33, 49, 50, 61). In SR-BIΔCT mice, Oil Red O staining (Fig. 5B) and visual inspection indicated no substantial reduction in the cellular neutral lipid content in the adrenal cortex, the ovarian stroma, and testicular Leydig cells compared with WT control mice. These qualitative histochemical results were confirmed quantitatively in adrenal glands when we measured the total cholesterol content of adrenal glands from male and female (n = 6 in each group) WT and SR-BIΔCT mice. In female mice, there was no statistically significant difference (P > 0.05) in adrenal gland total cholesterol content between WT and SR-BIΔCT mice (98.67 ± 6.33 and 109.6 ± 6.88 μg cholesterol/mg protein, respectively). The total cholesterol content of the adrenal glands from SR-BIΔCT male mice (99.23 ± 4.44 μg cholesterol/mg protein) was similar to that of their female counterparts (P > 0.05). As previously described (57, 66), we observed a significant difference in adrenal gland total cholesterol between male and female WT mice (53.55 ± 3.90 and 98.67 ± 6.33 μg cholesterol/mg protein, respectively, P < 0.0001). The mechanism(s) responsible for this sexual dimorphism in WT mice remains uncertain.
Although there was a reduction of cell surface HDL receptor protein in the adrenal glands, ovaries, and testes of SR-BIΔCT KI mice compared with WT mice (Fig. 3), the residual levels of receptor apparently were sufficient to maintain nearly normal CE stores. While clearly less than those in WT adrenal glands, those residual receptor levels presumably were adequate to maintain the stores under nonstressed conditions. Cai et al. (7) and Hoekstra et al. (23) have shown that adrenal insufficiency develops in stressed SR-BI KO mice. Future studies will be required to assess the influence of stress on adrenal cortical CE stores and adrenal function in the SR-BIΔCT mice.
Influence of SR-BIΔCT mutation on atherosclerosis and coronary heart disease.
The apoE KO mouse is a standard model used to study aortic root and aortic atherosclerosis but typically does not develop robust coronary arterial atherosclerosis or CHD during the first 4 mo of life (44).
To assess the effects of the SR-BIΔCT mutation on atherosclerosis, CHD, and cardiac physiology, we crossed the SR-BIΔCT mice with apoE KO mice to obtain two mouse populations with matching genetic backgrounds [C57BL/6:129S2/SvCrl (68.75:31.25)]: SR-BIΔCT/apoE KO mice and their apoE KO controls. Both were maintained on a standard lab chow diet for all of the studies described below.
Analysis of the plasma from these mice showed that there was a significant 1.6-fold increase in total plasma cholesterol in SR-BIΔCT/apoE KO mice (there was no difference between males and females; therefore the data were combined) compared with apoE KO mice (SR-BIΔCT/apoE KO: 978 ± 61 mg/dl, apoE KO: 603 ± 32 mg/dl, P = 0.0004) (Fig. 6Aa). In addition, there was a 276% increase in UC:TC in SR-BIΔCT/apoE KO compared with apoE KO mice (SR-BIΔCT/apoE KO: 0.83 ± 0.02, apoE KO: 0.30 ± 0.01, P < 0.0001) (Fig. 6Ab). The very high UC:TC in SR-BIΔCT/apoE KO was similar to that in SR-BI/apoE dKO mice (0.81 ± 0.01) (5). Fractionation of plasma lipoproteins by FPLC (Fig. 6B) showed that the size distributions of lipoproteins for these mice were similar to each other, with much of the cholesterol in large VLDL-size particles. Compared with those in apoE KO mice, in SR-BIΔCT/apoE KO mice the VLDL-size and intermediate-density lipoprotein (IDL)/LDL-size particles carried more cholesterol and the HDL-size particles carried substantially less cholesterol.
Fig. 6.
Effects of the SR-BIΔCT (ΔCT) mutation in apoE KO mice on plasma total (TC) and unesterified (UC) cholesterol levels and UC:TC (A) and on the plasma lipoprotein cholesterol size distribution profile (B). A: plasma samples were harvested from apoE KO and SR-BIΔCT/apoE KO 6- to 8-wk-old mice. No significant differences were observed between males and females. Total plasma and unesterified cholesterol levels were determined in individual samples by enzymatic assay; mean (±SE error) values from the indicated numbers of animals (n) are shown for each genotype. *P < 0.0001 for SR-BIΔCT/apoE KO compared with apoE KO mice. B: pooled plasma samples from 3 male mice were size-fractionated by FPLC, and the total cholesterol content of each fraction was determined by an enzymatic assay. The shapes of the chromatograms are representative of multiple individually determined profiles. Approximate elution positions of native VLDL, IDL/LDL, and HDL particles are indicated by brackets and were determined as previously described (50).
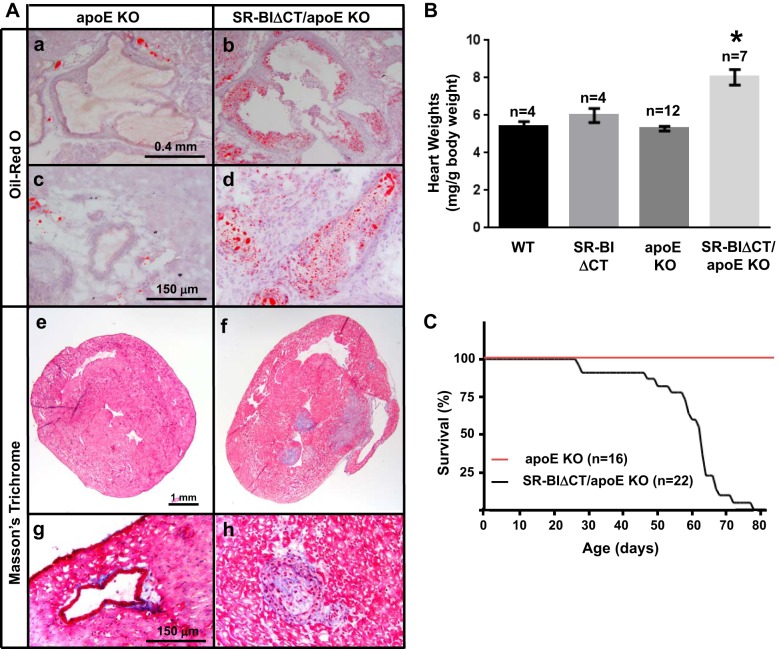
The elevated hypercholesterolemia with markedly increased plasma UC:TC in SR-BIΔCT/apoE KO mice raised the possibility that, as is the case with SR-BI/apoE dKO mice (5, 61), these mice might exhibit early-onset atherosclerosis. Figure 7A shows that Oil Red O-hematoxylin staining of heart sections of the SR-BIΔCT/apoE KO mice exhibited substantial lipid-rich (Oil Red O-positive staining) aortic root atherosclerosis (9 wk of age, Fig. 7Ab) before development of atherosclerosis in apoE KO mice (9 wk of age, Fig. 7Aa). Furthermore, unlike the case with apoE KO mice, examination of heart sections from 9-wk-old SR-BIΔCT/apoE KO, stained with either Oil Red O-hematoxylin (Fig. 7Ad) or Masson's trichrome (Fig. 7Ah) exhibited partial or complete, lipid-rich atherosclerotic occlusions in coronary arteries. No coronary arterial atherosclerotic lesions were observed in apoE KO controls (Fig. 7, Ac and Ag). As one would expect given the occlusive coronary arterial atherosclerosis, trichrome staining of myocardial sections from SR-BIΔCT/apoE KO mice (9 wk of age) showed evidence of myocardial infarction (MI; Fig. 7Af), while no fibrosis was observed in the corresponding apoE KO mice (Fig. 7Ae).
Fig. 7.
Effects of the SR-BIΔCT mutation in chow diet-fed apoE KO mice on aortic root (A, a and b) and coronary atherosclerosis (A, c and d and g and h), cardiac fibrosis (A, e and f), heart-to-body weight ratio (B), and survival (C). Hearts were harvested from 6- to 8-wk old standard chow diet-fed apoE KO (A, a, c, e, and g) and SR-BIΔCT/apoE KO (A, b, d, f, and h) mice as described in materials and methods; representative images are shown. a–d: Oil Red O -stained aortic root (a and b) and coronary artery (c and d) lesions (magnifications ×20 and ×100). e–h: Masson's trichrome-stained cross section of myocardium at low magnification (e and f, magnification ×10) and higher magnification (g and h, magnification ×100). Fibrotic tissue is stained blue and normal myocardium red. A patent coronary arteriole is seen in g (apoE KO), whereas a totally occluded arteriole is seen in h (SR-BIΔCT/apoE KO). B: heart-to-body weight ratios are expressed as milligrams of heart weight/gram of body weight. *P < 0.0001, for SR-BIΔCT/apoE KO compared with apoE KO hearts. P = 0.24 for WT compared with SR-BIΔCT hearts. C: Kaplan-Meier survival curves for chow-fed apoE KO and SR-BIΔCT/apoE KO (median age of death of 63 days) mice.
There were three pathological phenotypes in SR-BIΔCT/apoE KO mice not observed in apoE KO mice that were likely consequences of the occlusive coronary arterial atherosclerosis and MI. The first is cardiomegaly (Fig. 7B). The hearts in SR-BIΔCT/apoE KO mice (8.24 ± 0.67 mg/g body wt) were abnormally large compared with those of apoE KO mice (5.21 ± 0.14 mg/g body wt, P < 0.0001). There was no statistical difference in the heart-to-body weight ratios of apoE KO, WT (5.40 ± 0.26 mg/g body wt), and SR-BIΔCT (5.98 ± 0.37 mg/g body wt) mice (P = 0.08). Second, the SR-BIΔCT/apoE KO hearts exhibited severe cardiac dysfunction/heart failure. We performed hemodynamic studies using the left ventricular pressure-volume loop method on two groups of mice that were analyzed separately because they had different genetic backgrounds: group 1: WT and SR-BIΔCT mice [mixed C57BL/6:129S2/SvCrl (37.5:62.5) genetic background] and group 2: apoE KO and SR-BIΔCT/apoE KO mice [mixed C57BL/6:129S2/SvCrl (68.75:31.25) genetic background] Comparison of WT and SR-BIΔCT mice (group 1) established that there were no significant differences in maximum and minimum left ventricular volumes, cardiac output, ejection fraction, dP/dt, and stroke work (Table 2). Thus the SR-BIΔCT mutation alone did not alter these baseline characteristics of heart function. Compared with the control apoE KO hearts, the SR-BIΔCT/apoE KO hearts exhibited significantly lower cardiac output (reduced to 71% of control), ejection fraction (58%), and stroke work (68%), and significantly higher maximum and minimum left ventricular volumes (143% and 183% of control, respectively) (Table 3). The SR-BIΔCT/apoE KO mice also exhibited significantly lower +dP/dt (60% of control) and −dP/dt (66% of control), suggesting both systolic and diastolic dysfunction (Table 3). The ejection fractions determined by pressure-volume loop measurement were highly reproducible; however, they were somewhat lower than previously reported for mice (10, 45). We believe that the lower values are probably a consequence of our using in the present study mice significantly younger (7 wk old) than those used previously (typically 14–16 wk old). We analyzed cardiac function in very young mice because the SR-BIΔCT/apoE KO mice died prematurely (Fig. 7C), presumably because of heart failure. The SR-BIΔCT/apoE KO mice began to die at 28 days of age, half were dead by 63 days of age, and all had died by 79 days of age, with the large majority (65%) dying between the 59th and 69th days (8.4 and 9.9 wk) of age. Thus SR-BIΔCT/apoE KO mice resemble SR-BI/apoE dKO mice in that the chow-fed animals exhibit severe CHD, spontaneously developing occlusive coronary arterial atherosclerosis, MI, heart dysfunction, and premature death (4). The mean age of death for SR-BI/apoE dKO mice is 42 days (4), ∼21 days earlier than that of SR-BIΔCT/apoE KO mice.
Table 2.
Left ventricular pressure-volume loop measurements for wild-type and SR-BIΔCT mice
| Maximum Left Ventricular Volume, μl | Minimum Left Ventricular Volume, μl | Cardiac Output, μl/min | Ejection Fraction, % | Stroke Work, mmHg·μl | dP/dtmax, mmHg/s | dP/dtmin, mmHg/s | |
|---|---|---|---|---|---|---|---|
| WT (n = 7) | 45.9 ± 2.4 | 27.2 ± 1.5 | 5.9 ± 0.3 | 31.7 ± 1.3 | 1,703 ± 96 | 8,438 ± 653 | −7,661 ± 630 |
| SR-BIΔCT (n = 6) | 45.3 ± 1.4 | 27.3 ± 0.8 | 5.4 ± 0.3 | 28.8 ± 1.5 | 1,718 ± 116 | 8,638 ± 645 | −8,199 ± 593 |
| P value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
dP/dtmax and dP/dtmin indicate maximal and minimal rate of changes in pressure over time and measure systolic contractility and diastolic relaxation, respectively.
Table 3.
Left ventricular pressure-volume loop measurements for apoE KO and SR-BIΔCT/apoE KO mice
| Maximum Left Ventricular Volume, μl | Minimum Left Ventricular Volume, μl | Cardiac Output, μl/min | Ejection Fraction, % | Stroke Work, mmHg·μl | +dP/dt, mmHg/s | −dP/dt, mmHg/s | |
|---|---|---|---|---|---|---|---|
| apoE KO (n = 6) | 41.5 ± 1.9 | 22.3 ± 1.1 | 6.6 ± 0.5 | 36.5 ± 1.5 | 1,947 ± 137 | 10,737 ± 454 | −8,084 ± 400 |
| SR-BIΔCT/apoE KO (n = 8) | 59.5 ± 6.8 | 40.9 ± 4.6 | 4.7 ± 0.3 | 21.0 ± 1.6 | 1,331 ± 120 | 6,404 ± 654 | −5,318 ± 574 |
| P value | 0.04 | 0.005 | 0.005 | <0.0001 | 0.006 | 0.0003 | 0.0032 |
Hematologic abnormalities in SR-BIΔCT/apoE KO mice.
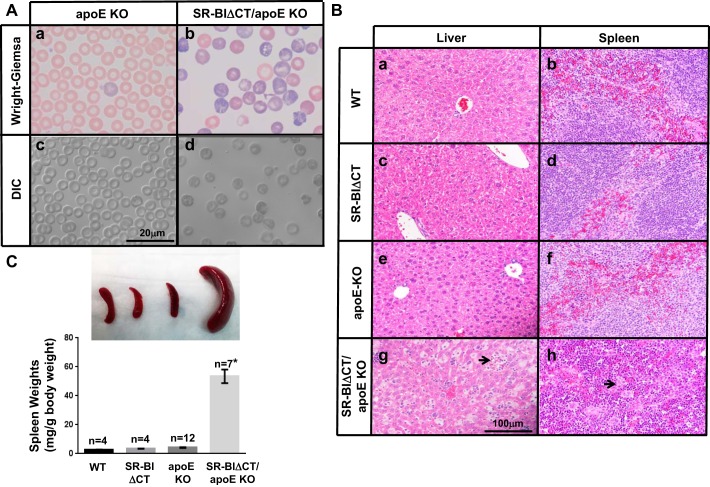
We also examined the consequences of combining the SR-BIΔCT mutation with apoE deficiency on RBCs and platelets, because the RBC and platelet abnormalities in SR-BI KO mice are exacerbated in SR-BI/apoE dKO mice (13, 25). The results of our hematologic analyses are shown in Table 4 and Fig. 8A. Indeed, SR-BIΔCT/apoE KO mice developed anemia (a hematocrit of 29.0 ± 2.4% that was 66% of that in apoE KO controls, P = 0.0014). Their RBCs showed a dramatic increase in size (MCV) compared with apoE KO (P < 0.0001), with abnormal morphology, including macrocytosis, fragmentation, and presence of intracellular inclusions (Fig. 8A, b and d), and there was a 31.0 ± 6.3% reticulocytosis (vs. 2.3 ± 0.8% in apoE KO mice, P = 0.0012). As a result and as shown in the histological sections in Fig. 8B, both liver and spleen in SR-BIΔCT/apoE KO mice (Fig. 8B, g and h)—but not WT (Fig. 8B, a and b), SR-BIΔCT (Fig. 8B, c and d), or apoE KO (Fig. 8B, e and f) mice—exhibited extramedullary hematopoiesis, with expansion of the red pulp and virtual disappearance of the white pulp in the spleen. As a consequence there was a dramatic increase in the size of the spleen (11.7 fold) in SR-BIΔCT/apoE KO compared with apoE KO mice (52.6 ± 6.9 vs. 4.5 ± 0.3 mg/g body wt, P < 0.0001) (Fig. 8C). An additional hematologic abnormality in SR-BIΔCT/apoE KO mice was thrombocytopenia, with a platelet count decreased by 43% compared with control apoE KO mice (P = 0.0004) (Table 4). It seems likely that the substantial elevation of UC:TC in SR-BIΔCT/apoE KO mice was responsible, at least in part, for their RBC and platelet abnormalities.
Table 4.
Hematologic data for apoE KO and SR-BIΔCT/apoE KO mice
| Genotype | Hematocrit, % | MCV, fl | Reticulocytes,% | MCH concentration, g/dl | MCH, pg | Platelets, ×106/ml |
|---|---|---|---|---|---|---|
| apoE KO (n = 6) | 43.7 ± 2.2 | 49.6 ± 1.7 | 2.3 ± 0.8 | 31.9 ± 0.4 | 15.8 ± 0.4 | 777.5 ± 55.8 |
| SR-BIΔCT/apoE KO (n = 6) | 29.0 ± 2.4 | 86.4 ± 3.2 | 31.0 ± 6.3 | 27.2 ± 0.7 | 23.5 ± 0.6 | 444.7 ± 31.0 |
| P value | 0.001 | <0.0001 | 0.001 | 0.0003 | <0.0001 | 0.0004 |
Fig. 8.
Morphological evaluation of red blood cells (A), liver (B), and spleen (B and C) from WT, SR-BIΔCT, apoE KO, and SR-BIΔCT/apoE KO mice. A: blood samples from apoE KO (a and c) and SR-BIΔCT/apoE KO (b and d) 6- to 8-wk-old mice were stained with Wright-Giemsa and visualized by standard light microscopy (a and b) or differential interference contrast (DIC) optics (c and d). B: livers (a, c, e, and g) and spleens (b, d, f, and h) from 6- to 8-wk-old mice of the indicated genotypes were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Extramedullary hematopoiesis is observed in g and h (arrows). C: representative photographs of spleens from mice of the indicated genotypes. Spleen-to-body weight ratios from mice of the indicated genotypes (n = 4–12) are expressed as milligrams of spleen weight/gram of body weight. *P < 0.0001 for SR-BIΔCT/apoE KO compared with apoE KO spleens. P = 0.27 for WT compared with SR-BIΔCT spleens.
DISCUSSION
PDZK1 is a “tissue-specific” adaptor of SR-BI in that in PDZK1 KO mice relative to WT mice the expression of SR-BI is decreased by 95% in the liver and by 50% in intestinal mucosa but is essentially unchanged in steroidogenic cells (33). We hypothesized that the PDZK1 independence of SR-BI in steroidogenic cells might be a consequence of one or more alternative adapters in those cells that would be functionally analogous to hepatic PDZK1 in that it would bind the carboxy terminus of SR-BI and would be required to maintain normal SR-BI protein levels. To test this hypothesis, we used homologous recombination “knock-in” technology to introduce a 507Ala/STOP mutation in the SR-BI gene (scarb1) resulting in the deletion of the last three amino acid residues (507AKL509) at the carboxy terminus of SR-BI (SR-BIΔCT). This mutation would not alter the structure of the alternatively spliced minor isoform SR-BII. We call the animals carrying these homozygous knock-in truncation mutations SR-BIΔCT mice. The mixed genetic backgrounds of the mice generated in this study and the genetic backgrounds of previously described mice to which comparisons are made are provided in Animals.
According to well-established principles of PDZ domain binding to target peptides (55) and previous studies of mutations in SR-BI, the 507AKL509 deletion in SR-BIΔCT will abrogate all receptor activity dependent on carboxy terminus binding to PDZ domains in PDZK1 but will not alter the surface expression or intrinsic lipid transport activities of SR-BI. The PDZK1-dependent activities include normal SR-BI protein expression in the liver (33), HDL-mediated regulation of endothelial cell physiology [endothelial nitric oxide synthase activity, cell migration, reendothelialization after injury (84)], and hepatitis C virus infectivity (8). Intrinsic lipid transport activities include selective lipid (e.g., CE) uptake (1, 42, 56), cellular efflux of unesterified cholesterol (28), and altered accessibility of plasma membrane cholesterol (56). For example, deletion of SR-BI's carboxy-terminal L509 abrogates its binding to PDZK1 and consequent regulation of endothelial nitric oxide synthase activity via a PDZK1-dependent signaling pathway in endothelial cells (2) but does not impair HDL binding and selective lipid uptake in cultured cells (54). Replacement of either the cytoplasmic carboxy-terminal 45 or 42 residues of SR-BI with the unrelated cytoplasmic carboxy-terminal 6 or 14 residues of CD36, another class B scavenger receptor, does not alter HDL binding or lipid transport (11, 12, 19). Indeed, truncation of SR-BI's carboxy-terminal 42 residues without replacement also does not alter HDL binding or lipid transport (11).
In the present study we found that the carboxy terminus of SR-BI is required for maintaining normal receptor levels not only in the liver (<5% of normal in SR-BIΔCT mice) but also in the adrenal gland, ovary, and testes (e.g., ∼84% and ∼64% reductions in adrenal gland and ovary, respectively). It is possible that metabolic regulation (52), e.g., cholesterol stores-mediated suppression of receptor expression (57, 66), may have contributed to reduced receptor protein levels in the steroidogenic cells in SR-BIΔCT mice. However, most of the metabolic (cholesterol stores) and hormonal (ACTH) regulation of SR-BI in steroidogenic cells is thought to occur by controlling mRNA levels (e.g., transcriptional control) (52, 57, 66), and there was only a very small change in receptor mRNA in the SR-BIΔCT mice compared with WT control mice. Thus we conclude that, as is the case in the liver, the reduction in receptor protein levels in SR-BIΔCT mice is primarily a posttranslational process. We propose that, as is the case in the liver with the adaptor protein PDZK1, it is likely that there is an adaptor(s) in steroidogenic cells that is distinct from PDZK1, possibly contains one or more PDZ domains, recognizes SR-BI's carboxy terminus, and mediates stable SR-BI protein expression. The residual levels of receptor protein in the adrenal glands, ovaries, and testes in SR-BIΔCT mice were apparently sufficient to maintain nearly normal CE stores in these chow diet-fed mice under standard housing conditions. In contrast, in SR-BI KO mice that express no SR-BI protein, there is a striking reduction in the CE stores in the adrenal glands (24, 49, 50) and ovarian corpora lutea (61).
SR-BI is expressed in many tissues at varying levels, and our results with steroidogenic cells raise the possibility that adaptors that recognize its carboxy terminus may play roles in other nonhepatic and nonsteroidogenic cells as well. For example, in intestines there is a partial dependence of SR-BI expression on PDZK1 (33)—it is possible that an additional carboxy-terminal adaptor(s) together with PDZK1 in intestines participates in mediating normal levels of SR-BI expression. Future analyses of receptor expression in other tissues of SR-BIΔCT mice will help address this issue. There is precedent for the activities of a lipoprotein receptor depending on different cytoplasmic adaptors in different types of cells. LDL receptors use at least two different adaptors for clathrin-mediated endocytosis, ARH and Dab2. Both adaptors bind to the receptor's NPXY internalization motif and to phospholipids (18). ARH is required for normal LDL receptor-mediated endocytosis in hepatocytes and lymphocytes but is not essential for endocytosis in fibroblasts (18, 21, 86). Apparently both ARH and Dab2 can mediate LDL receptor endocytosis in fibroblasts, with endocytosis dramatically reduced in fibroblasts lacking ARH when Dab2 expression is additionally suppressed by small interfering RNA (15, 78). The identity of the putative adaptor(s) for SR-BI in steroidogenic cells is unknown. Azhar and colleagues (26, 52) have reported that two PDZ domain-containing proteins, NHERF1 and NHERF2, have the ability to interact with SR-BI, apparently even when its carboxy terminus is blocked by an epitope tag, and might be involved with the negative regulation of SR-BI in steroidogenic cells. As the putative adaptor proposed here is expected to positively regulate SR-BI (i.e., reduced SR-BI when the interaction is blocked by the carboxy-terminal deletion), neither NHERF1 nor NHERF2 is likely to be the putative adaptor proposed here. Future studies will be required to identify the putative adaptor for SR-BI in steroidogenic cells.
In addition to examining receptor protein levels in the livers and steroidogenic tissues of SR-BIΔCT mice, we observed a number of abnormal phenotypes of SR-BIΔCT mice that were intermediate between those of PDZK1 KO mice and SR-BI KO mice. As the SR-BIΔCT, PDZK1 KO, and SR-BI KO mice have different genetic backgrounds (see materials and methods), caution should be exercised when interpreting some of the phenotypic differences among these mutant mice. The PDZK1 KO mice exhibit limited, tissue-specific reduction or loss of SR-BI activity (33), whereas SR-BI KO mice are completely SR-BI/SR-BII negative (50). SR-BI KO, SR-BIΔCT, and PDZK1 KO mice are all hypercholesterolemic (2.2-, 2.1-, and 1.7-fold plasma cholesterol levels above controls, respectively), with varying amounts of abnormally large HDL particles (32, 33, 50). The absence (SR-BI KO) or dramatic reduction (≤5% in SR-BIΔCT and PDZK1 KO mice) of the hepatic receptor accounts for these striking alterations in plasma HDL (73). SR-BI KO and SR-BIΔCT mice, but not PDZK1 KO mice, have abnormally high UC:TC: 0.515, ∼0.375, and 0.25, respectively. We do not understand the mechanism underlying the abnormally high UC:TC in SR-BI KO and SR-BIΔCT mice [reduced susceptibility of HDL to lecithin:cholesterol acyl transferase-mediated cholesterol esterification may be involved (38, 60, 75)]. However, our results raise the possibility that reduced levels of receptor activity in extrahepatic tissues in SR-BI KO and SR-BIΔCT mice that do not occur in PDZK1 KO mice may contribute to this phenotype.
The abnormally high UC:TC in SR-BI KO mice (0.515) has been linked to a number of abnormalities, including reticulocytosis (11.9%) (25), thrombocytopenia (13), and female infertility (41, 61, 72), all of which appear to arise, at least in part, because of abnormally high levels of unesterified cholesterol accumulating in the membranes of RBCs, platelets, and eggs, respectively. These abnormalities, which are not present in PDZK1 KO mice, were not observed in SR-BIΔCT mice. It seems likely that the relatively modest increase in UC:TC in standard chow-fed SR-BIΔCT mice (∼0.375) compared with WT and PDZK1 KO mice did not result in accumulation of grossly pathogenic levels of unesterified cholesterol in susceptible cells. Subjecting the SR-BIΔCT mice to additional stress [e.g., additional genetic abnormalities (see below) or possibly an atherogenic diet] might increase UC:TC and induce associated pathology.
An additional group of intermediate, pathological phenotypes in SR-BIΔCT mice—relative to SR-BI KO and PDZK1 KO mice—were observed when the mice were crossed with apoE KO mice, a standard model for aortic (but not coronary arterial) atherosclerosis (81). ApoE KO mice, which are hypercholesterolemic but have an essentially normal UC:TC (∼0.29), do not exhibit anemia, reticulocytosis, thrombocytopenia, or female infertility. In SR-BI/apoE dKO mice fed a standard, low-fat, chow diet, the total cholesterol is 2.2-fold higher than in apoE KO mice and 4.6-fold higher than in SR-BI KO mice and UC:TC is dramatically elevated to 0.81 (5, 61). The SR-BI/apoE dKO mice exhibit anemia (hematocrit ∼66% of control), severe reticulocytosis (100%), marked thrombocytopenia (11.9% of control), and splenomegaly (unpublished observation). Analyses by Fuller et al. (17) of atherogenic diet-fed SR-BI/LDLR dKO mice (UC:TC as great as 0.81) are generally consistent with the studies on chow-fed SR-BI/apoE dKO mice, namely, loss of SR-BI can result in anemia, enlarged RBCs, and splenomegaly, although statistically significant thrombocytopenia was not observed in SR-BI/LDLR dKO mice (also see Ref. 40). SR-BI/apoE dKO mice fed a standard chow diet rapidly develop severe occlusive coronary arterial atherosclerosis, MI, heart dysfunction, and premature death [death between 5 and 8 wk of age; median age of death is 6 wk (4, 61)]. PDZK1/apoE dKO mice exhibit far less severe atherosclerosis-related phenotypes. When PDZK1/apoE dKO mice are fed a moderately atherogenic (“Western”) diet for 3 mo, they develop more aortic root atherosclerosis than apoE KO control mice but no occlusive coronary arterial atherosclerosis, MI, or very early death (34). When the PDZK1/apoE dKO mice are fed a more severe atherogenic (Paigen or HFC 15.8% fat, 1.25% cholesterol, 0.5% sodium cholate) diet for 3 mo, they not only exhibit more aortic root atherosclerosis than the apoE KO mice but also develop some occlusive coronary arterial atherosclerosis and cardiac fibrosis but do not exhibit premature death (71).
In SR-BIΔCT/apoE KO mice fed a standard chow diet, the total cholesterol was 1.6-fold higher than in apoE KO control mice and ∼5.7-fold higher than in SR-BIΔCT mice and UC:TC was 0.83. The SR-BIΔCT/apoE KO mice exhibited severe macrocytic anemia (hematocrit ∼66% of control) with abnormal RBC morphology, resulting in hepatic and splenic extramedullary hematopoiesis, massive splenomegaly, and marked reticulocytosis (31%) as well as thrombocytopenia (57% of control). In addition, SR-BIΔCT/apoE KO mice rapidly developed extensive aortic root and severe occlusive coronary arterial atherosclerosis, MI, heart dysfunction and failure (assessed by pressure-volume loop method), and premature death (median age of death was 9 wk; 65% died between 8.4 and 9.9 wk of age). As noted above, our results raise the possibility that reduced levels of receptor activity in extrahepatic tissues in SR-BI/apoE dKO and SR-BIΔCT/apoE KO mice that do not occur in PDZK1/apoE dKO mice may have contributed to severe, lethal CHD.
The SR-BIΔCT/apoE KO mice provide a new addition to the limited collection of mouse models of atherosclerotic CHD (e.g., see Refs. 4, 80). Some of these models involve administration of an atherogenic diet, and others do not. In addition to SR-BIΔCT/apoE KO, SR-BI KO/apoE dKO, SR-BI KO/LDLR dKO (17), and PDZK1 KO/apoE dKO mice (71), there is a fifth SR-BI-related atherosclerotic CHD mouse model, HypoE mice (SR-BI KO/ApoeR61h/h) (80). In addition to homozygous null mutations in the SR-BI gene, HypoE mice have a severe, but not absolute, deficiency of apoE due to a modification of the apoE gene (ApoeR61h/h). ApoeR61h/h mice (47) express a mutant murine apoE (Thr61→Arg61) at substantially lower plasma concentrations (2–5%) than apoE in control WT mice. When HypoE mice are fed an atherogenic diet (e.g., Paigen/HFC), but not a standard chow diet, they develop atherosclerotic CHD, MI, and heart dysfunction and die prematurely (50% mortality ∼40 days after initiation of a Paigen diet). The rate of disease progression is environmentally titratable (e.g., severity of the atherogenic diet, substitution of chow diet after short exposure to atherogenic diet, social isolation) (43). CHD progression in SR-BI KO/LDLR dKO is also titratable in that it is proportional to the severity of the atherogenic diet (17). It seems likely that a modified HypoE mouse, namely, SR-BIΔCT/ApoeR61h/h, in which SR-BIΔCT replaces the SR-BI KO, likely would also be an environmentally titratable atherosclerotic CHD model. Because of the fertility of SR-BIΔCT females, SR-BIΔCT/ApoeR61h/h mice likely would be an easier and less expensive CHD model than SR-BIΔCT/apoE KO, SR-BI KO/apoE dKO, SR-BI/LDLR dKO, and HypoE mice.
In conclusion, the new SR-BIΔCT mouse is likely to be a powerful mouse model that will provide a better understanding of steroid hormone production and the mode of regulation of SR-BI in steroidogenic organs. It also provides the research community with a new, convenient mouse model, the SR-BIΔCT/apoE KO mouse, that closely recapitulates the findings observed in human cardiovascular disease.
GRANTS
This work was supported by National Institutes of Health Grants HL-077780 (O. Kocher) and HL-127174 (M. Krieger) and Pre-Doctoral Training Grant T32 GM-007287 (L. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.P., Q.K., G.A.P., A.Y., M.L.P., L.W., C.C., P.M.K., and O.K. performed experiments; R.P., Q.K., G.A.P., A.Y., M.L.P., L.W., C.C., P.M.K., M.K., and O.K. approved final version of manuscript; G.A.P., A.Y., M.L.P., L.W., P.M.K., M.K., and O.K. analyzed data; G.A.P., A.Y., P.M.K., M.K., and O.K. interpreted results of experiments; G.A.P., A.Y., C.C., P.M.K., M.K., and O.K. edited and revised manuscript; P.M.K., M.K., and O.K. conceived and designed research; M.K. and O.K. prepared figures; M.K. and O.K. drafted manuscript.
ACKNOWLEDGMENTS
We thank Robert Farese, Jr. and Tobias Walther for generously making their facilities available and supporting C. Chitraju and Laura Liscum for help in performing preliminary experiments.
REFERENCES
- 1.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271: 518–520, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest 115: 969–977, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae S, Siu P, Choudhury S, Ke Q, Choi J, Koh Y, Kang P. Delayed activation of caspase-independent apoptosis during heart failure in transgenic mice overexpressing caspase inhibitor CrmA. Am J Physiol Heart Circ Physiol 299: H1374–H1381, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res 90: 270–276, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Braun A, Zhang S, Miettinen H, Ebrahim S, Holm T, Vasile E, Post MJ, Yoerger D, Picard M, Krieger J, Andrews N, Simons M, Krieger M. Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor SR-BI/apolipoprotein E double knockout mouse. Proc Natl Acad Sci USA 100: 7283–7288, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brill A, Yesilaltay A, De Meyer S, Kisucka J, Fuchs T, Kocher O, Krieger M, Wagner D. Extrahepatic high-density lipoprotein receptor SR-BI and apoA-I protect against deep vein thrombosis in mice. Arterioscler Thromb Vasc Biol 32: 1841–1847, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai L, Ji A, de Beer F, Tannock L, van der Westhuyzen D. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest 118: 364–375, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catanese M, Loureiro J, Jones C, Dorner M, von Hahn T, Rice C. Different requirements for scavenger receptor class B type I in hepatitis C virus cell-free versus cell-to-cell transmission. J Virol 87: 8282–8293, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Sheng R, Källberg M, Silkov A, Tun M, Bhardwaj N, Kurilova S, Hall R, Honig B, Lu H, Cho W. Genome-wide functional annotation of dual-specificity protein- and lipid-binding modules that regulate protein interactions. Mol Cell 46: 226–237, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhury S, Bae S, Ke Q, Lee J, Kim J, Kang P. Mitochondria to nucleus translocation of AIF in mice lacking Hsp70 during ischemia/reperfusion. Basic Res Cardiol 106: 397–407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connelly M, de la Llera-Moya M, Monzo P, Yancey P, Drazul D, Stoudt G, Fournier N, Klein S, Rothblat G, Williams D. Analysis of chimeric receptors shows that multiple distinct functional activities of scavenger receptor, class B, type I (SR-BI), are localized to the extracellular receptor domain. Biochemistry 40: 5249–5259, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Connelly M, Klein S, Azhar S, Abumrad N, Williams D. Comparison of class B scavenger receptors, CD36 and scavenger receptor BI (SR-BI), shows that both receptors mediate high density lipoprotein-cholesteryl ester selective uptake but SR-BI exhibits a unique enhancement of cholesteryl ester uptake. J Biol Chem 274: 41–47, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Dole V, Matuskova J, Vasile E, Yesilaltay A, Bergmeier A, Bernimoulin M, Wagner D, Krieger M. Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 28: 1111–1116, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell 85: 1067–1076, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Eden E, Sun X, Patel D, Soutar A. Adaptor protein Disabled-2 modulates low density lipoprotein receptor synthesis in fibroblasts from patients with autosomal recessive hypercholesterolaemia. Hum Mol Genet 16: 2751–2759, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Fenske S, Yesilaltay A, Pal R, Daniels K, Rigotti A, Krieger M, Kocher O. Overexpression of the PDZ1 domain of PDZK1 blocks the activity of hepatic scavenger receptor, class B, type I by altering its abundance and cellular localization. J Biol Chem 283: 22097–22104, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller M, Dadoo O, Serkis V, Abutouk D, MacDonald M, Dhingani N, Macri J, Igdoura S, Trigatti B. The effects of diet on occlusive coronary artery atherosclerosis and myocardial infarction in scavenger receptor class B, type 1/low-density lipoprotein receptor double knockout mice. Arterioscler Thromb Vasc Biol 34: 2394–2403, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Garcia CK, Wilund K, Arca M, Zuliani G, Fellin R, Maioli M, Calandra S, Bertolini S, Cossu F, Grishin N, Barnes R, Cohen JC, Hobbs HH. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 292: 1394–1398, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Gu X, Kozarsky K, Krieger M. Scavenger receptor class B, type I-mediated [3H]cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor. J Biol Chem 275: 29993–30001, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Guo Q, Penman M, Trigatti B, Krieger M. A single point mutation in epsilon-COP results in temperature-sensitive, lethal defects in membrane transport in a Chinese hamster ovary cell mutant. J Biol Chem 271: 11191–11196, 1996. [DOI] [PubMed] [Google Scholar]
- 21.He G, Gupta S, Yi M, Michaely P, Hobbs HH, Cohen JC. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin and AP-2. J Biol Chem 277: 44044–44049, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Hillier B, Christopherson K, Prehoda K, Bredt D, Lim W. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science 284: 812–815, 1999. [PubMed] [Google Scholar]
- 23.Hoekstra M, Meurs I, Koenders M, Out R, Hildebrand R, Kruijt J, Van Eck M, Van Berkel T. Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J Lipid Res 49: 738–745, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hoekstra M, Ye D, Hildebrand R, Zhao Y, Lammers B, Stitzinger M, Kuiper J, Van Berkel T, Van Eck M. Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J Lipid Res 50: 1039–1046, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm T, Braun A, Trigatti B, Brugnara C, Sakamoto M, Krieger M, Andrews N. Failure of red blood cell maturation in mice with defects in the high-density lipoprotein receptor SR-BI. Blood 99: 1817–1824, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Hu Z, Hu J, Zhang Z, Shen W, Yun C, Berlot C, Kraemer F, Azhar S. Regulation of expression and function of scavenger receptor class B, type I (SR-BI) by Na+/H+ exchanger regulatory factors (NHERFs). J Biol Chem 288: 11416–11435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikemoto M, Arai H, Feng D, Tanaka K, Aoki J, Dohmae N, Takio K, Adachi H, Tsujimoto M, Inoue K. Identification of a PDZ-domain-containing protein that interacts with the scavenger receptor class B type I. Proc Natl Acad Sci USA 97: 6538–6543, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian B, De la Llera-Moya M, Ji Y, Wang N, Phillips M, Swaney J, Tall A, Rothblat G. Scavenger receptor class B type I as a mediator of cellular cholesterol efflux to lipoproteins and phospholipid acceptors. J Biol Chem 273: 5599–5606, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Kocher O, Birrane G, Tsukamoto K, Fenske S, Yesilaltay A, Pal R, Daniels K, Ladias J, Krieger M. In vitro and in vivo analysis of the binding of the C terminus of the HDL receptor scavenger receptor class B, type I (SR-BI), to the PDZ1 domain of its adaptor protein PDZK1. J Biol Chem 285: 34999–35010, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kocher O, Birrane G, Yesilaltay A, Shechter S, Pal R, Daniels K, Krieger M. Identification of the PDZ3 domain of the adaptor protein PDZK1 as a second, physiologically functional binding site for the C terminus of the high density lipoprotein receptor scavenger receptor class B type I. J Biol Chem 286: 25171–25186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kocher O, Krieger M. Role of the adaptor protein PDZK1 in controlling the HDL receptor SR-BI. Curr Opin Lipidol 20: 236–241, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocher O, Pal R, Roberts M, Cirovic C, Gilchrist A. Targeted disruption of the PDZK1 gene by homologous recombination. Mol Cell Biol 23: 1175–1180, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocher O, Yesilaltay A, Cirovic C, Pal R, Rigotti A, Krieger M. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J Biol Chem 278: 52820–52825, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Kocher O, Yesilaltay A, Shen C, Zhang S, Daniels K, Pal R, Chen J, Krieger M. Influence of PDZK1 on lipoprotein metabolism and atherosclerosis. Biochim Biophys Acta 1782: 310–316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozarsky K, Donahee M, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature 387: 414–417, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Kozarsky K, Jooss K, Donahee M, Strauss Jr, Wilson J. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat Genet 13: 54–62, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Landschulz KT, Pathak R, Rigotti A, Krieger M, Hobbs HH. Regulation of scavenger receptor, class B type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest 98: 984–995, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma K, Forte T, Otvos J, Chan L. Differential additive effects of endothelial lipase and scavenger receptor-class B type I on high-density lipoprotein metabolism in knockout mouse models. Arterioscler Thromb Vasc Biol 25: 149–154, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Mardones P, Quinones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen H, Trigatti B, Krieger M, VanPatten S, Cohen DE, Rigotti A. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res 42: 170–180, 2001. [PubMed] [Google Scholar]
- 40.Meurs I, Hoekstra M, van Wanrooij E, Hildebrand R, Kuiper J, Kuipers F, Hardeman M, Van Berkel T, Van Eck M. HDL cholesterol levels are an important factor for determining the lifespan of erythrocytes. Exp Hematol 33: 1309–1319, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Miettinen H, Rayburn H, Krieger M. Abnormal lipoprotein metabolism in HDL receptor (SR-BI)-deficient mice and reversible female fertility. J Clin Invest 108: 1717–1722, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison J, Silvestre M, Pittman R. Cholesteryl ester transfer between high density lipoprotein and phospholipid bilayers. J Biol Chem 269: 13911–13918, 1994. [PubMed] [Google Scholar]
- 43.Nakagawa-Toyama Y, Zhang S, Krieger M. Dietary manipulation and social isolation alter disease progression in a murine model of coronary heart disease. PLoS One 7: e47965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakashima Y, Plump A, Raines E, Breslow J, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 14: 133–140, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Park S, Yoon J, Bae S, Park M, Kang C, Ke Q, Lee D, Kang P. Therapeutic use of H2O2-responsive anti-oxidant polymer nanoparticles for doxorubicin-induced cardiomyopathy. Biomaterials 35: 5944–5953, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Pawson T, Nash P. Assembly of cell regulatory systems through protein interaction domains. Science 300: 445–452, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Raffai R, Weisgraber K. Hypomorphic apolipoprotein E mice: a new model of conditional gene repair to examine apolipoprotein E-mediated metabolism. J Biol Chem 277: 11064–11068, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Rigotti A, Edelman ER, Seifert P, Iqbal SN, DeMattos RB, Temel RE, Krieger M, Williams DL. Regulation by adrenocorticotropic hormone of the in vivo expression of scavenger receptor class B type I (SR-BI), a high density lipoprotein receptor, in steroidogenic cells of the murine adrenal gland. J Biol Chem 271: 33545–33549, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Rigotti A, Miettinen H, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev 24: 357–387, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA 94: 12610–12615, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigues C, Hannus M, Prudêncio M, Martin C, Gonçalves L, Portugal S, Epiphanio S, Akinc A, Hadwiger P, Jahn-Hofmann K, Röhl I, van Gemert G, Franetich J, Luty A, Sauerwein R, Mazier D, Koteliansky V, Vornlocher H, Echeverri C, Mota M. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe 4: 271–282, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Shen W, Azhar S, Kraemer FB. ACTH regulation of adrenal SR-B1. Front Endocrinol (Lausanne) 7: 42, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shih S, Smith L. Quantitative multi-gene transcriptional profiling using real-time PCR with a master template. Exp Mol Pathol 79: 14–22, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Silver DL. A carboxyl-terminal PDZ-interacting domain of scavenger receptor B, type I is essential for cell surface expression in liver. J Biol Chem 277: 34042–34047, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275: 73–77, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Stein Y, Dabach Y, Hollander G, Halperin G, Stein O. Metabolism of HDL-cholesteryl ester in the rat, studied with a non-hydrolyzable analog, cholesteryl linoleyl ether. Biochim Biophys Acta 752: 98–105, 1983. [DOI] [PubMed] [Google Scholar]
- 57.Sun Y, Wang N, Tall A. Regulation of adrenal scavenger receptor-BI expression by ACTH and cellular cholesterol pools. J Lipid Res 40: 1799–1805, 1999. [PubMed] [Google Scholar]
- 58.Temel R, Trigatti B, DeMattos R, Azhar S, Krieger M, Williams D. Scavenger receptor class B, type I (SR-BI) is the major route for the delivery of high density lipoprotein cholesterol to the steroidogenic pathway in cultured mouse adrenocortical cells. Proc Natl Acad Sci USA 94: 13600–13605, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Cho YS, Go MJ, Kim YJ, Lee JY, Park T, Kim K, Sim X, Ong RT, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Zhao JH, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O'Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Hovingh GK, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466: 707–713, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thacker S, Rousset X, Esmail S, Zarzour A, Jin X, Collins H, Sampson M, Stonik J, Demosky S, Malide D, Freeman L, Vaisman B, Kruth H, Adelman S, Remaley A. Increased plasma cholesterol esterification by LCAT reduces diet-induced atherosclerosis in SR-BI knockout mice. J Lipid Res 56: 1282–1295, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA 96: 9322–9327, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsukamoto K, Wales T, Daniels K, Pal R, Sheng R, Cho W, Stafford W, Engen J, Krieger M, Kocher O. Noncanonical role of the PDZ4 domain of the adaptor protein PDZK1 in the regulation of the hepatic high density lipoprotein receptor scavenger receptor class B, type I (SR-BI). J Biol Chem 288: 19845–19860, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Eck M, Twisk J, Hoekstra M, Van Rij B, Van der Lans C, Bos I, Kruijt J, Kuipers F, Van Berkel T. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem 278: 23699–23705, 2003. [DOI] [PubMed] [Google Scholar]
- 64.van Ham M, Hendriks W. PDZ domains—glue and guide. Mol Biol Rep 30: 69–82, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Vergeer M, Korporaal S, Franssen R, Meurs I, Out R, Hovingh G, Hoekstra M, Sierts J, Dallinga-Thie G, Motazacker M, Holleboom A, Van Berkel T, Kastelein J, Van Eck M, Kuivenhoven J. Genetic variant of the scavenger receptor BI in humans. N Engl J Med 364: 136–145, 2011. [DOI] [PubMed] [Google Scholar]
- 66.Wang N, Weng W, Breslow J, Tall A. Scavenger receptor BI (SR-BI) is up-regulated in adrenal gland in Apolipoprotein A-I and hepatic lipase knock-out mice as a response to depletion of cholesterol stores. J Biol Chem 271: 21001–21004, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Warden C, Hedrick C, Qiao J, Castellani L, Lusis A. Atherosclerosis in transgenic mice overexpressing apolipoprotein A-II. Science 261: 469–472, 1993. [DOI] [PubMed] [Google Scholar]
- 68.Webb NR, Connell PM, Graf GA, Smart EJ, De Villiers WJ, De Beer FC, Van der Westhuyzen DR. SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J Biol Chem 273: 15241–15248, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Yalaoui S, Huby T, Franetich J, Gego A, Rametti A, Moreau M, Collet X, Siau A, van Gemert G, Sauerwein R, Luty A, Vaillant J, Hannoun L, Chapman J, Mazier D, Froissard P. Scavenger receptor BI boosts hepatocyte permissiveness to Plasmodium infection. Cell Host Microbe 4: 283–292, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Ye F, Zhang M. Structures and target recognition modes of PDZ domains: recurring themes and emerging pictures. Biochem J 455: 1–14, 2013. [DOI] [PubMed] [Google Scholar]
- 71.Yesilaltay A, Daniels K, Pal R, Krieger M, Kocher O. Loss of PDZK1 causes coronary artery occlusion and myocardial infarction in Paigen diet-fed apolipoprotein E deficient mice. PLoS One 4: e8103, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yesilaltay A, Dokshin G, Busso D, Wang L, Galiani D, Chavarria T, Vasile E, Quilaqueo L, Orellana J, Walzer D, Shalgi R, Dekel N, Albertini D, Rigotti A, Page D, Krieger M. Excess cholesterol induces mouse egg activation and may cause female infertility. Proc Natl Acad Sci USA 111: E4972–E4980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yesilaltay A, Kocher O, Pal R, Leiva A, Quinon es V, Rigotti A, Krieger M. PDZK1 is required for maintaining hepatic scavenger receptor, class B, type I (SR-BI) steady state levels but not its surface localization or function. J Biol Chem 281: 28975–28980, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Yesilaltay A, Kocher O, Rigotti A, Krieger M. Regulation of SR-BI-mediated high-density lipoprotein metabolism by the tissue-specific adaptor protein PDZK1. Curr Opin Lipidol 16: 147–152, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Yesilaltay A, Morales M, Amigo L, Zanlungo S, Rigotti A, Karackattu S, Donahee M, Kozarsky K, Krieger M. Effects of hepatic expression of the high-density lipoprotein receptor SR-BI on lipoprotein metabolism and female infertility. Endocrinology 147: 1577–1588, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Yokode M, Hammer R, Ishibashi S, Brown M, Goldstein J. Diet-induced hypercholesterolemia in mice: prevention by overexpression of LDL receptors. Science 250: 1273–1275, 1990. [DOI] [PubMed] [Google Scholar]
- 77.Yuhanna I, Zhu Y, Cox B, Hahner L, Osborne-Lawrence S, Lu P, Marcel Y, Anderson R, Mendelsohn M, Hobbs H, Shaul P. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 7: 853–857, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Yun M, Keshvara L, Park C, Zhang Y, Dickerson J, Zheng J, Rock C, Curran T, Park H. Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem 278: 36572–36581, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Zanoni P, Khetarpal S, Larach D, Hancock-Cerutti W, Millar J, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema J, De Craen A, Deloukas P, Sattar N, Ford I, Packard C, Majumder A, Alam D, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard B, Nielsen S, Tybjærg-Hansen A, Schmidt R, Kuulasmaa K, Liu D, Perola M, Blankenberg S, Salomaa V, Männistö S, Amouyel P, Arveiler D, Ferrieres J, Müller-Nurasyid M, Ferrario M, Kee F, Willer C, Samani N, Schunkert H, Butterworth A, Howson J, Peloso G, Stitziel N, Danesh J, Kathiresan S, Rader D, CHD Exome+ Consortium, CARDIoGRAM Exome Consortium, Global Lipids Genetics Consortium. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science 351: 1166–1171, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S, Picard M, Vasile E, Zhu Y, Raffai R, Weisgraber K, Krieger M. Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient hypomorphic apolipoprotein ER61 mice. Circulation 111: 3457–3464, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Zhang S, Reddick R, Burkey B, Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest 94: 937–945, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S, Reddick R, Piedrahita J, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258: 468–471, 1992. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Zanotti I, Reilly M, Glick J, Rothblat G, Rader D. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 108: 661–663, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Zhu W, Saddar S, Seetharam D, Chambliss K, Longoria C, Silver DL, Yuhanna IS, Shaul PW, Mineo C. The scavenger receptor class B type I adaptor protein PDZK1 maintains endothelial monolayer integrity. Circ Res 102: 480–487, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Zimmermann P. The prevalence and significance of PDZ domain-phosphoinositide interactions. Biochim Biophys Acta 1761: 947–956, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Zuliani G, Arca M, Signore A, Bader G, Fazio S, Chianelli M, Bellosta S, Campagna F, Montali A, Maioli M, Pacifico A, Ricci G, Fellin R. Characterization of a new form of inherited hypercholesterolemia: familial recessive hypercholesterolemia. Arterioscler Thromb Vasc Biol 19: 802–809, 1999. [DOI] [PubMed] [Google Scholar]