Abstract
Airway structure and function are key aspects of normal lung development, growth, and aging, as well as of lung responses to the environment and the pathophysiology of important diseases such as asthma, chronic obstructive pulmonary disease, and fibrosis. In this regard, the contributions of airway smooth muscle (ASM) are both functional, in the context of airway contractility and relaxation, as well as synthetic, involving production and modulation of extracellular components, modulation of the local immune environment, cellular contribution to airway structure, and, finally, interactions with other airway cell types such as epithelium, fibroblasts, and nerves. These ASM contributions are now found to be critical in airway hyperresponsiveness and remodeling that occur in lung diseases. This review emphasizes established and recent discoveries that underline the central role of ASM and sets the stage for future research toward understanding how ASM plays a central role by being both upstream and downstream in the many interactive processes that determine airway structure and function in health and disease.
Keywords: lung, asthma, inflammation, calcium, bronchoconstriction, bronchodilation, proliferation, extracellular matrix, hyperplasia, hypertrophy, remodeling, development
airway structure and function are important for respiratory and pulmonary physiology across all ages. Indeed, a number of normal and pathophysiological processes influence the initial embryonic formation and differentiation of cell types in conducting airways, as well as their function or dysfunction throughout life. Here, such processes are further modulated by interactions between cells, between cells and the environment, and, importantly, the mechanical forces of breathing. In these contexts, dysfunctional, typically excessive, narrowing of the conducting airways, and impaired relaxation occur in clinically important diseases such as asthma across age groups, in bronchitis, and in chronic obstructive pulmonary disease (COPD). Such functional changes may be accompanied or exacerbated by concomitant structural changes involving thickening of airway layers (particularly bronchial epithelium, also frequently dysfunctional) and airway smooth muscle (ASM) and by varying degrees of fibrosis. Etiologies for airway structural and functional changes vary with age, context, and exposure but include developmental abnormalities (e.g., genetic disorders, maternal and fetal insults), allergens and infectious agents, environmental exposures (e.g., cigarette smoke, toxins, and pollutants), and intrinsic factors such as age and sex (Fig. 1). Here, not only is it important to understand the complex molecular, genetic, proteomic, and physiological processes within a cell type (epithelial cells, ASM, fibroblasts, airway nerves, resident and circulating cells of the immune system, etc.) and their perturbation in disease, but also 1) how interactions between cell types contribute to mutual changes in cellular structure/function and to overall airway functionality or dysfunction; 2) the potential contribution of the extracellular matrix (ECM) that airway cells reside in and interact with; and 3) the role of mechanical forces exerted by breathing. Thus understanding the myriad of factors that drive normal airway structure and function from embryonic lung formation onward in cell- and context-specific fashions is understandably challenging (despite the many large-scale, high-throughput technologies) yet necessary toward appreciating how intrinsic and external forces drive induction, maintenance, exacerbation, and (where possible) alleviation and resolution of lung disease.
Fig. 1.
Airway development and growth across the ages in the context of disease. A multitude of intrinsic and extrinsic processes contribute to the structure and function of the bronchial airways at different life stages. In utero lung development involves intrinsic processes such as genetics, maternal and fetal steroids, mechanical forces induced by fetal breathing and external pressures, and, in the context of perinatal disease, immune and infectious processes. Postnatally, except for steroids, such processes can continue to contribute to airway growth or its disruption, especially in the context of prematurity and iatrogenic processes such as mechanical ventilation, infection, etc. With progressive development and aging, normal processes such as mechanical forces of breathing, cell-cell and cell-matrix interactions, aging and cellular senescence mechanisms, diet-induced changes, etc., contribute to airway growth, its maintenance or normal aging-related changes, modulated by sex steroids during different life stages. Exposure of the normal airway to insults such as allergens, microbes, or viruses or to environmental factors (pollutants, tobacco smoke) are overlaid on these normal processes to contribute to disease. Figure was generated using ScienceSlides graphics from Visiscience.
While the relative importance of different cell types in the airway can be argued, from a functional standpoint, ASM plays a critical role in regulating airway tone and contractility, which represent a balance between contractile vs. dilatory processes in response to local or circulating factors, i.e., processes and factors that contribute to the airway hyperreactivity (AHR) and impaired bronchodilation in diseases such as asthma. From a structural standpoint, extrinsic and intrinsic factors can contribute to ASM cell hyperplasia and/or hypertrophy, also resulting in reduced airway lumen. Furthermore, fibrotic processes can contribute to altered mechanical properties of the airway (stiffening) with implications for the work of breathing, and for the functional efficacy of ASM. All of these interconnected processes play variable roles in different lung diseases such as asthma, COPD, and pulmonary fibrosis. Accordingly, understanding the role of ASM per se in the airway becomes key. In this regard, the mechanisms that regulate the important aspect of ASM, i.e., contractility, in response of extrinsic stimuli of airway innervation, secreted factors from other airway cell types (epithelium, fibroblasts, immune cells, and vasculature), and/or circulating mediators still remain in the discovery phase and represent an exciting aspect of basic, translational, and even clinical lung research. Here, the idea that ASM is more than a passive recipient of external signals (regardless of source) and actively contributes to modulation of other cell types (and ASM itself), inflammation and its resolution, and the overall changes in airway structure and function in the context of disease makes for an exciting landscape for investigation.
In this review, I highlight, by no means in a comprehensive manner, recent themes and discoveries that have contributed to furthering the idea of ASM as more than a passive recipient and responder to external signals. I begin with novel findings relating to the unquestionable major role for ASM, viz. contractility involving intracellular Ca2+ concentration ([Ca2+]i) and Ca2+ sensitization, expounding on the role of G protein-coupled receptor (GPCR) based and non-GPCR-based signaling mechanisms. I next address emerging roles for ASM beyond contractility in airway disease via its contributions to remodeling: cell hypertrophy and proliferation, extracellular matrix formation, generation of mediators and growth factors. More complex and relatively underexplored topics are then addressed including epigenetic mechanisms, noncanonical roles of mitochondria, the increasing recognition of sex differences and sex steroids, and limited exploration of ASM in perinatal airway development and growth. These advances not only provide insight into ASM biology and disease pathophysiology but also point to novel approaches and targets for treatment of lung diseases.
Novel Regulatory Pathways in Intracellular Ca2+ and Contractility
The fundamental mechanisms involved in regulation of baseline and agonist-induced elevation of [Ca2+]i in ASM have been largely established (251, 260, 282, 335, 405, 417, 454, 470, 483, 497, 563). Briefly, bronchoconstrictor agonists, acting via Gq-coupled receptors elevate [Ca2+]i through several pathways that involve Ca2+ influx and sarcoplasmic reticulum (SR) Ca2+ release. Here, production of the second messengers inositol trisphosphate (IP3) and cyclic ADP ribose leads to intracellular Ca2+ release via SR channels that are activated by these second messengers (IP3-sensitive and ryanodine receptor channels, respectively) (Fig. 2).
Fig. 2.
Regulation intracellular Ca2+ ([Ca2+]i) in airway smooth muscle (ASM). While a number of mechanisms are well recognized, including agonist-induced production of the second messengers IP3 (via the enzyme phospholipase C; PLC) and cyclic ADP ribose (cADPR) via the ectoenzyme CD38, leading to sarcoplasmic reticulum Ca2+ release from IP3 receptor and ryanodine receptor channels, respectively, and Ca2+ influx through various types of channels, there are new regulatory pathways recently reported. For example, PLC can be upregulated by PKA and PKG but inhibited by flavanols and PDE4, while IP3 can regulate Ca2+ sparks and influx through voltage-gated Ca2+ influx channels (VGCC). Calcineurin (usually associated with downstream transcription) can regulate RyR channels and in turn be regulated by TRP influx channels. Influx of extracellular Ca2+ ([Ca2+]o) can occur through VGCCs, TRPC, and TRPV channels as well as reverse mode Na+/Ca2+ exchange (NCX). In terms of force sensitization involving RhoA, active RhoA levels can be regulated by RhoGAPs and RhoGEFs, an emerging area in ASM. CaCC, calcium-activated chloride channel; NAD, nicotinamide adenine dinucleotide; BKCa, big calcium-activated potassium channel; MLCP, myosin light chain phosphatase; IP3R, IP3 receptor; SERCA, sarcoendoplasmic reticulum calcium ATPase; SR, sarcoplasmic reticulum.
In terms of Ca2+ influx and efflux, a number of mechanisms have been described, although their roles under baseline (resting) conditions, in the context of agonist stimulation, and in pathophysiology are variable depending on the study (223, 243, 244, 246, 319; elegantly reviewed for example in Ref. 223). Key mechanisms include voltage-gated Ca2+ channels (VGCCs) (260, 405, 454, 483) including L-type and T-type channels, receptor-operated channels, nonselective cation channels particularly transient receptor potential channels (TRPs), Na+/Ca2+ exchange (9, 10, 224, 318, 424, 458), and, importantly, store-operated Ca2+ influx channels, the latter being activated upon SR Ca2+ depletion. Some recent developments regarding specific influx pathways are described further below (summarized in Fig. 2). The role of Na+/Ca2+ exchange (NCX), particularly in the context of its influx mode, is not clear for ASM. While the NCX protein is expressed (and even upregulated in airway inflammation for example), whether it operates largely in the efflux mode to extrude [Ca2+]i or whether it can bring in Ca2+ under certain conditions remains to be determined. Here, an interesting question may be what role extracellular Ca2+ ([Ca2+]o) plays in modulating influx (or even efflux) pathways in ASM. Unlike in cardiac muscle, where increasing [Ca2+]o is readily associated with increasing [Ca2+]i and contractility, this relationship is less well defined in ASM. Nonetheless, [Ca2+]o is certainly important for influx of Ca2+ via TRPs, reverse NCX, store-operated calcium entry (SOCE), and, as recently recognized, regulating the Ca2+-sensing receptor (CaSR; described in more detail below).
While this review is not specifically focused on exploring Ca2+ influx/efflux pathways, per se, in the context of recent discoveries regarding mechanisms regulating ASM contractility and of novel bronchodilators it is important to mention the contribution of membrane potential. For example, T-type VGCCs can increase force (223, 244), whereas blocking K+ channels (which normally induce membrane hyperpolarization and reduced [Ca2+]i) results in contraction (243). Furthermore, Cl− movement in ASM may also modulate contractility vs. relaxation (242), wherein elevated [Ca2+]i for example in response to agonist stimulation can activate small-conductance Cl− channels and further cause depolarization. Chloride may further be relevant to the potential role of γ-aminobutyric acid (GABA) in ASM (see below). In the context of bronchodilation, K+ channels are known to cause membrane hyperpolarization (69).
Beyond Ca2+, activation of the myosin light chain (MLC) kinase (MLCK)-MLC-actin/myosin cascade leads to contraction, while Ca2+ sensitization occurs through the RhoA and Rho kinase (ROCK) mechanism. Here, membrane potential itself can also regulate Ca2+ sensitization via ROCK (319). Accordingly, mechanisms that directly or indirectly modulate the Ca2+ and force regulatory cascades in ASM have the potential to enhance contractility (e.g., in the context of AHR in asthma), suppress it in the context of bronchodilation, or, conversely, impair bronchodilation itself. While this review cannot comprehensively cover the many such modulatory mechanisms known, some recent insights into Ca2+ regulation in ASM may be particularly relevant to understanding disease pathways.
Even with well-established pathways for Ca2+ and force regulation, there have been recent developments. For example, miRNAs and transcription factors have been shown to regulate the second messenger CD38 (138, 197, 259). Modulation of the enzyme phospholipase C (PLC) by several upstream mechanisms such as protein kinases A and G (PKA and PKG) (373), flavanols in the context of bronchodilation (68), and phosphodiesterase-4 (PDE4) in the context of quercetin effects (525) could contribute to altered Ca2+ regulation (Fig. 2). IP3 has been shown to activate Ca2+ influx in ASM (490) and regulate local Ca2+ sparks (323), while ryanodine receptor (RyR) sensitization results in abnormal Ca2+ handling (113). In terms of Ca2+ sensitization, ROCK appears to be important in mediating AHR in the context of environmental exposure and obesity (265, 298), while regulation of RhoA activation [via RhoGEFs (guanine nucleotide exchange factors) and RhoGAPs (GTPase activating proteins)] is an emerging (but thoroughly underexplored) area in ASM biology (46).
GPCRs.
Major bronchoconstricting agonists such as ACh, histamine, and endothelin-1 work through GPCRs, a superfamily of plasma membrane proteins that transduce extracellular signals not only to elevate (or decrease) [Ca2+]i but also to trigger other intracellular cascades important for cellular function. While common aspects of GPCR-mediated increase in [Ca2+]i are long recognized (as summarized above), some more recent aspects have also been noted, in the context of membrane potential. For example, muscarinic GPCRs can induce changes in membrane potential (40) while, conversely, membrane potential can modulate GPCR activity (41, 322, 333).
GPCRs (particularly those on ASM) continue to be the major drug targets for diseases such as asthma, allergy, and COPD, with the well-studied Gq-coupled pathway (which activates PLC-IP3, thus elevating Ca2+) being key. Conversely, the also-well-studied Gs-coupled pathway (which increases cAMP) is critical for bronchodilation, particularly through β2-adrenoceptor action. While Ca2+ can be indirectly increased through Gi-coupled pathway (which reduces cAMP), this aspect of GPCR signaling in ASM is less understood (49, 134, 398, 402, 403, 563).
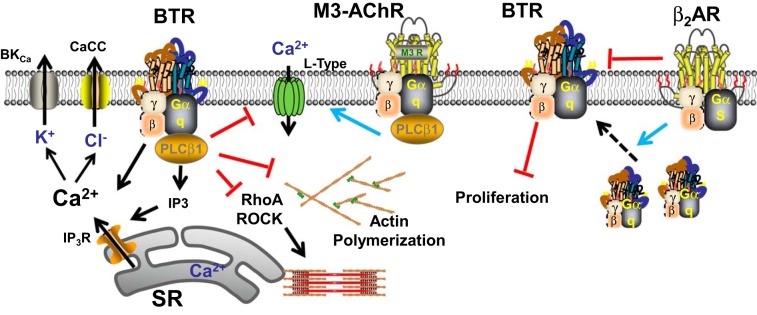
One novel bronchodilatory mechanism that continues to generate excitement (and intrigue) in the field is bitter taste receptor (BTR)-induced effects (Fig. 3). Originally identified as acting through the TAS2R family of GPCRs to elevate [Ca2+]i yet induce bronchodilation (135), studies have attempted to further explore this pathway and explain the dichotomy (13, 30, 76, 420, 472, 473, 548, 589, 591). Interest in the biology of BTRs in the airway is enhanced by recent findings of genetic variants in the context of infection, rhinosinusitis, and asthma (472, 561, 582). Given the diversity of [Ca2+]i regulatory pathways in ASM, there is potential for BTRs to activate or inhibit these depending on agonist, context, and perhaps species. Here, the point of interest may be the fact that TAS2Rs show low specificity and affinity to a wide range of bitter compounds, thus allowing for a diverse portfolio of signaling, depending on the agonist, concentration, whether the receptor desensitizes (437), and perhaps even restricted domains of signaling. Accordingly, studies have found that BTRs can induce membrane hyperpolarization via iberiotoxin-sensitive Ca2+-activated K channels (135, 420) and nonselective cation channels (591) and interact differently with specific bronchoconstrictors (420) (e.g., denatonium interacts with muscarinic receptors, while chloroquine is more broad). Furthermore, there is the emerging idea that BTRs can activate different Ca2+ signaling pathways in context-specific fashions. For example, one group has suggested that TAS2R stimulation activates Gβγ at baseline conditions to increase [Ca2+]i, while blocking activated VGCCs under conditions of bronchoconstrictor agonist stimulation to induce bronchodilation (589) (Fig. 3). More recently, another group has used the differential effects of specific TAS2R agonists to explore relationships between bitter tastants vs. bronchoconstrictor GPCR agonists in human ASM cells (76). They found that the heterogeneity in the inhibitory responses of TAS2R hinges on the bronchoconstrictor used [e.g., chloroquine inhibits histamine effects, but not endothelin, different from the findings of another group (420)], rather than the TAS2R subtype or even the Gq or Gi coupling of the bronchoconstrictor GPCR. Thus there could be a low-efficiency [Ca2+]i stimulation [also suggested by other groups (589)] and a higher efficiency inhibition of [Ca2+]i when specific constricting agonists are present. Separately, the bronchodilating effects of BTRs may rely on interactions with β2-adrenoceptor expression and signaling (13, 272, 548). For example, one study showed that TAS2R activation can induce relaxation even in the face of adrenoceptor desensitization (13) (making it an exciting alternative for bronchodilation in asthmatic subjects desensitized to β-agonists), while a more recent study suggests that β2-adrenoceptors may act as a double-edged sword: they increase cell surface expression of TAS2Rs (thus enhancing their function) but may also induce TAS2R desensitization when β-agonists are present (272), consistent with previously observed modest desensitization in human ASM and monkey airways (437). Whether the extent of desensitization depends on TAS2R subtype in ASM remains to be determined.
Fig. 3.
Bitter tastant receptor (BTR) in ASM. There is continuing interest and development regarding this G protein-coupled receptor (GPCR) based signaling mechanisms in ASM. Working through a Gq-coupled mechanism, TAS2R activation can increase SR Ca2+ release via IP3 receptor channels, but intriguingly causes bronchodilation. This latter effect could involve activation of Ca2+-activated K+ channels (BKCa) and chloride channels, but also inhibition of Ca2+ sensitization and actin polymerization. Recent findings suggest that BTRs can also counteract muscarinic receptor enhancement of Ca2+ influx via VGCCs, suggesting that under baseline conditions BTRs may enhance [Ca2+]i but, in the presence of bronchoconstrictor agonist, act to reduce [Ca2+]i. Furthermore, BTRs appear to have antimitogenic properties. Separately, BTRs can interact with β-adrenoceptor agonist signaling, although such agonists can on the one hand enhance plasma membrane insertion of BTRs but also desensitize BTRs. Thus BTRs continue to be intriguing in terms of signaling in ASM.
While the pathways of [Ca2+]i effects of BTRs are being teased out, the mechanisms of reduced contractility per se remain unresolved. A recent study suggests that effects on contractility may depend on TAS2R-bronchoconstrictor GPCR interactions, but the final pathways still lead to [Ca2+]i first and what downstream pathways are differentially influenced by TAS2R are not known. Inhibition of Ca2+ sensitivity (i.e., MLCK/MLC and actin/myosin) or sensitization (RhoA/ROCK) are obvious targets, but there is currently no information on these aspects. Interestingly, caffeine, which is also a TAS2R agonist, appears to influence actin polymerization in ASM (519). Furthermore, the relative roles of the different types of TAS2Rs in the airway in the context of normal function and contribution to diseases such as asthma or COPD (relevant to the eventual utility of bitter tastants in therapy) remain to be elucidated. Here, a single recent study suggests a novel role for BTRs as an antimitogen in ASM (473). The intriguing aspect here is that bitter tastants provide antimitogenic potential without activating the recently recognized pathways of TAS2R activation, but instead inhibit growth factor activated protein kinases, and induction of transcription factors such as AP-1 and STAT3 that are important in asthma.
In line with emerging mechanisms, there continues to be new discoveries in the role GABA, the major inhibitory neurotransmitter in mammalian central nervous system. To recall, GABA acts at both ligand-gated ionotropic GABAA receptors as well as G protein-linked metabotropic GABAB receptors. Clinically, the GABAB receptor agonist baclofen has been shown to worsen methacholine responses of asthmatic airways (136). In the airway, the epithelium appears to be a significant source of GABA (166). Functional GABAB receptors in ASM (166, 167, 388) and epithelium (364) have been shown. Although acting through Gi, baclofen and GABA increase IP3 and [Ca2+]i in the airway (363), an effect involving a Gβγ-mediated direct effect on PLCβ, and potentiation of Gq-mediated signaling. Conversely, GABAA receptors on ASM appear to be potent bronchodilators (122, 164, 165, 167, 168, 365, 581, 584). Here, one recent publication demonstrated the opportunity to selectively target GABAA receptors utilizing the fact that only α4 and α5 subunits are present in the receptors of human ASM (168). Such emerging data point to novel and nuanced approaches to selectively targeting the heterogeneity of receptor features in the ASM to induce bronchodilation. Indeed, it may not be just GABA but also glycine receptor (a Cl− channel) that is involved in bronchodilation (580), another mechanism that remains to be explored further.
It appears that GABA is not the only ligand typically associated with the central nervous system (CNS) that is also involved in ASM. The receptor for N-methyl-d-aspartate (NMDA), a glutamate analog, has been recently localized to human ASM cells (14) and is upregulated by cytokines (15). The rationale for exploring this unexpected pathway has been the association of increased plasma glutamate levels in airway inflammation, raising the question of whether glutamate receptors such as the NMDA receptor can contribute to AHR. NMDA receptor activation appears to increase [Ca2+]i in ASM cells, particularly involving the NR1 subunit. However, in the context of cytokine exposure, NMDA receptor activation appears to have dichotomous effects with increased [Ca2+]i in ASM per se, but bronchodilation in vivo, which may additionally involve the NOS pathway and thus neuronal or even epithelial modulation.
Finally, in the context of CNS-related ligands, dopaminergic signaling may also be relevant (75, 366, 367). “D1-like” receptors couple to Gs while “D2-like” receptors couple to Gi (361), and both types of dopamine receptors have been localized to ASM, although dopamine normally induces bronchodilation, particularly in asthmatic subjects.
An exciting and emerging GPCR is the CaSR. The CaSR is well-known for [Ca2+]o regulation [via effects at the parathyroid gland, kidney, and bone (8, 181, 522)], but it is also expressed in noncalciotropic tissues such as blood vessels, breast, and pancreas, where it can regulate [Ca2+]i, gene expression, ion channels, cell fate, and ECM (55, 64, 370, 434, 435). Altered CaSR expression is associated with inflammation, vascular calcification, and cancers (64, 370, 434, 435). However, beyond reports of CaSR in lung cancers (411, 552) or neuroendocrine tumors (308), there is surprisingly very little information on CaSR expression or function in the airway (111, 158, 360, 577). This paucity of information is increasingly being corrected, with recent publications on functional CaSR in the lung (65, 397, 433, 503, 518, 577) (although admittedly its role in the airway per se is still being investigated). CaSR appears to be important in developing lung although information in this aspect is minimal. CaSR is expressed in developing epithelium (158) and modulates branching morphogenesis at adult [Ca2+]o levels (∼1.2 mM), while higher fetal [Ca2+]o levels [∼1.7 mM (285)] may inhibit branching. What CaSR does in postnatal airways is unknown, although emerging data on its role in regulating human fetal lung development via the CFTR (65) is intriguing. In adults, CaSR is present in human airways (577), particularly in ASM, where it regulates [Ca2+]i and contractility (577). Furthermore, CaSR expression is increased in ASM of asthmatic subjects (577), making it a potential target for mechanistic exploration as well as a future therapeutic avenue. Here, the importance of CaSR may lie in the multiple endogenous modulators beyond [Ca2+]o, with CaSR responding to polyvalent cations (particularly spermine and eosinophil cationic protein), amino acids (e.g., l-arginine), ionic strength, and alkaline pH (434, 435) (Fig. 4). Depending on cell type and context, signaling pathways such as RhoA/ROCK, ERKs, PKC, and cAMP/PKA can all regulate CaSR expression and function (434, 435), making this receptor a multimodal sensor and effector for integrating multiple signals, many of which happen to be important in airway structure and function (Fig. 4). In this regard, emerging data on a pathophysiological role for CaSR in pulmonary artery smooth muscle (198, 400, 518, 566, 567) highlights the potential for this pathway in the airway as well: an area of unmet need. Here, the arginases may be particularly relevant, given that their enzymatic activity leads to shunting of l-arginine toward the ornithine pathway leading to production of the polycation spermine. Indeed, there is increasing interest in the role of arginases and polyamines in asthma (5, 61, 104, 172, 331, 467, 474) and even pulmonary hypertension (89, 95, 194, 254, 393, 566) (also see discussion on arginases further below). Certainly, other aspects of CaSR regulation (as above) remain to be explored in any cell type of the lung. Given the recent discovery of CaSR in asthmatic ASM, this not-so-novel GPCR may see an emergence in the context of lung disease.
Fig. 4.

Calcium sensing receptor (CaSR), a novel signaling mechanism in ASM known for its activity in the parathyroid gland. Recent data suggest the involvement of CaSR in elevating [Ca2+]i and contractility in human ASM, especially in the context of inflammation which increases CaSR expression. Normally, CaSR is sensitive to extracellular Ca2+ ([Ca2+]o) and is modulated by polyamines (e.g., spermine, eosinophilic cationic protein), pH, and amino acids such as l-arginine. Intracellularly, CaSR activates the PLC/IP3 pathway, but also enhances PKC, RhoA/ROCK, ERK, and other cascades that can contribute to increased [Ca2+]i and proliferation. Conversely, CaSR can block cAMP and thus impair bronchodilation. The potential importance of CaSR lies in the existence and trialing for CaSR agonists (calcimimetics) vs. antagonists (calcilytics) for other diseases.
In the context of continued discoveries of GPCRs in bronchoconstriction vs. dilation, the complex roles of prostaglandins (PGs) in the airway (99, 215, 372, 447, 455, 586) are being teased out. Products of the COX pathway in arachidonic acid metabolism, the five primary PGs (PGD2, PGE3, PGF2, PGI2, and TXA2), all signal through distinct GPCRs. Complexity arises from the fact that some PGs counteract actions of others, or that the same PG may have opposing effects depending on the specific receptor involved, which is further context sensitive. Here, thromboxane (TXA2) is one of the most potent endogenous bronchoconstrictors across several species, exerting biological functions through interactions with the thromboxane-prostanoid (TP) receptor coupled to Gq, and thus the PLC/IP3/Ca2+ pathway (Fig. 5). Increased TXA2 levels are seen in asthmatic airways (555) and in COPD (125), while inhibition of TP receptors attenuates allergic bronchoconstriction while TP inhibition attenuates bronchoconstriction. Here, TP expression on ASM has been demonstrated (11, 79, 245), supporting the idea of direct action on ASM to induce bronchoconstriction. However, TXA2 effects appear more complex, involving indirect actions via neuronal stimulation leading to ACh release as well as mechanical stimuli (120, 121, 215, 247, 456). Furthermore, the relative role of ASM TP vs. neuronal TP appears to depend on the state of allergic inflammation, with ASM TP being more active under normal conditions (114), thus raising the question of whether targeting TP on ASM for bronchodilation will be sufficient. Nonetheless, TP may play other roles in smooth muscle in terms of actin polymerization (and thus tissue stiffening) (156, 157). Furthermore, interactions of TP with other GPCRs could be relevant to understanding whether specific constricting (263, 310) or dilating agents (420, 476, 510) (such as BTRs) may be more potent under certain conditions.
Fig. 5.

Eicosanoids in ASM. Both established and emerging data indicate a role for the thromboxane (TXA2) receptor (TP) to increase [Ca2+]i and enhance actin polymerization in ASM, leading to bronchoconstriction and airway stiffening. Prostaglandin E2 (PGE2) can activate 1 of 4 epoprostanoid receptors (EP1–EP4) with differing effects, where EP2 and EP4 reduce [Ca2+]i by activating cAMP, while EP1 serves to increase [Ca2+]i and EP3 to promote mitogenic activity.
In addition to TXA2, there is resurging interest in the potential beneficial effect of PGE2 and the complex role of epoprostanoid (EP) receptor subtypes in bronchoconstriction vs. dilation. The relevance of PGE2 goes beyond direct effects on the airway, with influences on responsiveness of the lung to infection (139, 195, 238), immune cell function (140, 569), epithelial and endothelial function (24, 186, 278, 316, 356), and even senescence (207). PGE2 is abundantly produced by both airway epithelium and ASM (97, 130, 569). Previous studies suggest endogenous PGE2 is bronchoprotective in human asthma (395). Epithelially derived PGE2 reduces vagal cholinergic contraction of ASM (38) with additional effects on the immune system (464). Conversely, such epithelial PGE2 can induce ASM to produce amphiregulin (for example) to feedback onto epithelial EGFR (127). PGE2 can inhibit ASM migration and proliferation when used in combination with β2-adrenergic receptor agonists (182, 568). Furthermore, lack of the microsomal PGE2 generating enzyme mPGEs1 results in defective clearance of bacteria (139). Accordingly, there should be substantial interest in PGE2 agonism. PGE2 signals through four distinct GPCRs, EP1-4 with each EP receptor having distinct G protein coupling and downstream signal, with complexity arising from the fact that these downstream pathways can be counteractive (99). EP1 increases Ca2+ while EP2 and EP4 increase cAMP to induce bronchodilation (Fig. 5). While this dichotomy should facilitate interest in inhibitors of EP1 with concurrent promotion of EP2 or EP4 activation to enhance bronchodilation in asthma (25, 45, 451), previous trials may not have been as successful due to the fact that tested drugs with broad EP agonism can also activate EP3, which causes ASM contraction by decreasing cAMP synthesis. Furthermore, EP3 activation mediates the cough induced by PGE2 (334). Such differential, counteractive roles of EP3 are also noted in the context of ASM migration (25). Accordingly, there is now interest in exploring EP2/4-specific agonism to limit the side-effect profile of PGE2. However, even here, it may be important to consider species differences in EP receptor subtype express and function that makes interpretation complex. For example, a recent study found that a traditional Chinese medicine herbal formula ASHMI dilates ACh-induced mouse airways via EP2/4 signaling (498), while another study found that in human bronchial rings it may be EP4 that is particularly significant for bronchodilation (45). This may be important in the context of recent findings of anti-inflammatory effects of EP4 (51).
There continues to be development in our understanding of novel roles for Wnt signaling in the airway, including in ASM (26, 248, 291, 292, 295, 431, 486, 495, 541, 579, 600), epithelium (175, 211, 291, 486, 496, 544, 549, 565, 588, 600), and fibroblasts (357, 376, 495, 535). Wnt proteins bind the extracellular domain of receptors of the Frizzled (Frz) family of GPCRs (332, 431), facilitated by coreceptors such as lipoprotein receptor-related protein (LRP)-5/6, Ryk, and ROR2. Wnt signaling can integrate inputs via canonical β-catenin-dependent and -independent pathways and noncanonical Wnt/Ca2+ pathways. The emerging relevance of Wnt signaling in ASM appears to be in remodeling, particularly in the context of TGF-β interactions and ECM production (291, 292, 431). However, TGF-β-independent effects may also be involved, as recently suggested by reports of eosinophil-induced enhancement of ASM Wnt/β-catenin expression and signaling (248). In fibroblasts, Wnt5B can increase IL-6 and CXCL8 secretion and thus indirectly influence airway remodeling (535). Furthermore, remodeling can be indirectly mediated via Wnt signaling in epithelial cells that produce ECM in the context of inflammation (588, 600). Noncanonical Wnt signaling (e.g., via Wnt5a) can involve effects on [Ca2+]i (292, 579). Thus Wnt signaling has the potential to substantially influence airway structure and function.
Non-GPCR mechanisms.
A large number of non-GPCR pathways have been explored in the context of airway contractility and relaxation and cannot be reasonably summarized here. Nonetheless, some specific pathways have gained recent interest.
Relevant to airway irritability, and the potential for airway innervation to influence contractility, the role of transient receptor channels (TRPs) has been explored, with particular emphasis on the TRPA1 and TRPV1 channels (88, 271, 315, 334), that are activated by PKC [e.g., following stimulation of GPCRs such as bradykinin or prostanoid receptors (334)]. However, there is now increasing evidence that ASM also expresses TRPA1 (88, 251, 374, 590), TRPV1 (362, 551, 593, 594), and, similar to the vasculature (31, 115, 371, 425, 539, 572), TRPV4 (252, 350, 511, 592). Here, TRPA1 has been shown to enhance ASM-derived IL-8 secretion (374), to enhance airway inflammation and AHR (374), as well as to mobilize [Ca2+]i (251) (Fig. 2), while conversely inhibiting ASM proliferation (590). In contrast, TRPV1 on ASM appears to promote proliferation (593, 594). However, TRPV4 appears to be involved in elevation of [Ca2+]i (592) and contractility (350) as well as release of procontractile factors (350, 511). The relevance of these emerging pathways lies in leveraging previous neuroscience work on TRPA and TRPV channels for benefit in airway disease. Further relevance lies in linking such TRP channels to other mechanisms for airway irritability, such as acid-sensing ion channels that are found in ASM as well as vascular smooth muscle (152, 185, 412), and the emerging family of proton-sensing GPCRs (e.g., OGR1 or GPR68) that respond to extracellular pH (237, 347, 384, 462, 524).
Although well recognized in the context of epithelial dysfunction in cystic fibrosis (106, 422), the CFTR appears to now be expressed in ASM and is functional and potentially contributory in airway disease (1, 65, 110, 352, 359, 543). While its expression profile and functionality are still under exploration, it appears that ASM CFTR contributes to SR Ca2+ function (110, 359), and, importantly, altered CFTR function can lead to AHR (7, 352, 543). Conversely, administrator of CFTR-targeting drugs appear to promote bronchodilation (1, 379). These exciting findings suggest an entirely novel area for investigation in the context of ASM mechanisms that contribute to AHR.
An interesting aspect of ASM signaling is the role of EGFR (305, 317, 375, 386, 463, 482, 570), typically associated with epithelial cells (62, 127, 193, 261, 597). Emerging data suggest that in addition to secreting amphiregulin (EGFR ligand) (127), ASM also express EGFR, which can be involved in signaling of procontractile agonists such as endothelin-1 (ET1) (317) and brain natriuretic peptide (BNP) (386) as well as airway inflammation (482).
An interesting pathway in the context of Ca2+ regulation that has been recently reported is calcineurin/NFAT (185, 201, 461, 491, 592, 595). Although calcineurin can mediate its effects via the transcription factor NFAT, a recent study reported that calcineurin can also modulate local Ca2+ signaling and contractility in ASM, specifically via RyR channels (461) (Fig. 2). Conversely, local Ca2+ signals created by TRPV4 can activate calcineurin to further increase ASM Ca2+ and proliferation (592). Furthermore, the Ca2+ influx channel TRPC3 can activate the calcineurin/NFAT pathway in modulating airway contractility (491). Beyond Ca2+, calcineurin also appears to be involved in modulation of actin polymerization and tension development in ASM (595). The relevance of calcineurin lies in the well-known importance of its inhibition in immunosuppressive therapies, and their potential application in airway disease.
Unexpected mechanisms modulating Ca2+ and contractility are increasingly being recognized. For example, there is much interest in the role of the glycosaminoglycan hyaluronan, a major component of the ECM, in modulating a number of aspects of airway disease (149, 170, 265, 279, 298, 301–304, 336, 391, 492, 502, 559). It appears that ASM itself produces hyaluronan, and increased expression occurs in the context of inflammation while steroids can reduce such expression (279, 301, 391), raising the question of what role(s) hyaluronan could play in the airway. Hyaluronan is involved in water homeostasis, cell-matrix signaling, cell proliferation and migration, as well as modulation of inflammation (170). Here, emerging data suggest that high- vs. low-molecular-weight hyaluronan may play differential roles in enhancing vs. alleviating disease processes (304). Exogenous hyaluronan can prevent inflammation and, importantly, blunt AHR, including reducing ASM Ca2+ and contractility in the context of injury (170, 304), thus making it an exciting and novel potential therapy.
Novel ASM Mechanisms in Airway Remodeling
Diseases such as asthma and COPD are well appreciated to involve extensive airway remodeling, although what constitutes remodeling per se is defined loosely or at least differently in different studies. Remodeling was recognized quite early as airway wall thickening and mucus plugging in severe asthma (233), with more recent studies better delineating, characterizing and quantifying structural changes with remodeling (57, 74, 82, 94, 147, 150, 170, 255, 277, 295, 390, 392, 414, 417, 431, 446, 471, 473, 479, 482, 529, 540, 556). Changes include thickened epithelium with mucus gland hypertrophy (likely contributing to the increased mucus plugging), subepithelial membrane thickening and fibrosis with altered ECM composition and deposition, and increased ASM mass reflected by both hyperplasia and hypertrophy (35, 42–44, 129, 146, 240, 431, 473, 540). An important aspect of remodeling is the substantial heterogeneity in the type and extent of changes that occur in airways of different sizes with large bronchi showing cellular hyperplasia for example, while other parts of the airway showing hypertrophy (42, 146). Furthermore, the models and systems used to explore remodeling differ with the use of in vitro models of animal or human ASM (or other cell types), and preclinical in vivo models (71, 220, 281, 290, 351, 500). While these models have certainly enhanced our understanding of remodeling and the role of ASM for example, the longitudinal and progressive aspects of remodeling continue to be limiting for our understanding of the underlying mechanisms, particularly at different stages of airway disease. Conversely, explorations using such models may help us understand whether aspects of AHR not responsiveness to current therapy represent remodeling (107, 173, 188, 293, 394, 396, 438, 441, 446, 562).
In terms of ASM hypertrophy, both mechanical and inflammatory pathways could be contributory. Mechanical stretch induces ASM hypertrophy via Wnt and GSK3β (295), Akt (43, 330, 369), and mTOR (598) [especially when stimulated by TGFβ or endothelin (180, 355, 596)]. Other hypertrophy mechanisms have also recently been reported such as SRF and Elk1 (94) (Fig. 6); these have not been examined in ASM per se, although substantial insight could be obtained from emerging data in pulmonary vasculature for signaling intermediates and transcription factors such as the CaSR (198, 397, 566), G6PD (93), Klf5 (312), and Akt1 (517).
Fig. 6.
Role of ASM in remodeling. In addition to an essential role in contractility and bronchodilation, ASM acts as recipient of extrinsic factors such as allergens, infection, environmental factors, cytokines, growth factors, and even the extracellular ASM. In turn, ASM demonstrates “synthetic” aspects, itself producing a range of ECM proteins, matrix metalloproteinases (MMPs) and inhibitors (TIMPs), pro- and anti-inflammatory cytokines, growth factors such as neurotrophins and VEGF, and a range of other proteins. The relevance of these activities lies in the hypertrophy vs. hyperplasia of ASM as well as fibrosis that contribute to airway remodeling in disease. Here, the factors (extrinsic or ASM-derived) that contribute to hypertrophy vs. hyperplasia can differ and could include mechanical forces, signaling cascades and emerging mechanisms such as miRNAs, mitochondria, endoplasmic reticulum (ER) stress, etc. Furthermore, some mechanisms can blunt hypertrophy and hyperplasia. See text for various abbreviations in figure.
A number of mechanisms underlying ASM hyperplasia have been reported (Fig. 6) and involve upregulation of cell proliferation (or inhibition of processes that prevent it), or alternatively inhibition of apoptosis. As with hypertrophy, mechanical stretch alone can induce proliferation, working through Wnt or altered matrix stiffness (478). Mechanical stress in airways can also induce EGF release from epithelial cells and indirectly contribute to remodeling (477). Beyond stress, a range of inflammatory stimuli are known to enhance ASM proliferation, including the classic Th1 and Th2 cytokines such as TNF-α, IL-4, IL-5, and IL-13, as well as TGFβ, TSLP, IL-6, and those of the Th17 family (35, 85, 129, 131, 138, 236, 270, 342, 407, 428, 429, 439, 536). Such stimuli activate “classic” pathways such as p38 and p42/44 MAP kinases, PI3/Akt, and NF-κB (18, 85, 117, 190, 401, 404, 439, 473, 491, 594).
In addition to inflammatory stimuli, several new triggers for proliferation have been noted. Even influx channels could be involved, as exemplified by TRPC1 (491) and TRPV1 (594), while, conversely, activation of TRPM8 and TRPA1 channels appear to inhibit proliferation (590). Regardless of their mechanosensitive role, Wnts can modulate ASM proliferation (248, 431, 486). Locally produced factors such as VEGF (338), neurotrophins (18, 128, 163), amphiregulin (305, 481), prostaglandins (536), and leukotrienes (353, 508, 515) could be involved. And finally some studies have reported a higher proliferation of asthmatic ASM cells at baseline (257, 530), although the underlying mechanisms are not completely understood. However, it should also be emphasized baseline higher ASM proliferation in asthmatic subjects is not conclusively proven (266, 550). Thus a plethora of mechanisms are potentially involved in increasing ASM mass in the context of remodeling, with their relative contribution likely depending on context.
What are less understood are the potential mechanisms that limit ongoing proliferation, especially in the context of inflammation (which also needs limitation). Therapeutic agents such as corticosteroids and β2-agonists can limit proliferation (48, 182). Recent studies have also identified pathways that can limit smooth muscle proliferation including the structural protein caveolin-1 (22, 189, 190), the channels TRPM8 and TRPA1 (590), bitter taste ligands (473), PPARγ ligands (141, 142, 160, 161, 177, 321, 432, 501, 511, 560), and vitamin D (102, 118, 329, 339, 583). Although the above discussion, albeit brief, highlights some pathways for limiting remodeling, there is surprisingly little information regarding how inflammation effects are suppressed under normal conditions. Obviously, drugs such as glucocorticoids can suppress NF-κB and other pathways, as well as activating anti-inflammatory cascades. However, in other lung cell types, including pulmonary artery smooth muscle, there is increasing evidence for the resolvins (particularly D1) (112, 219, 253, 387, 419, 476), reducing contractility, cell migration, and other features that are common to airway remodeling. Conversely, in the context of responsiveness to infectious and other stimuli mediated by Toll-like receptors (TLRs), activation of the inflammasome is thought to be important for homeostasis. The inflammasome is an intracellular multimeric protein complex regulating maturation and release of proinflammatory cytokines such as IL-1β and IL-8 in response to pathogens and endogenous danger signals. There is now emerging evidence that the inflammasome itself plays a role in respiratory diseases. However, there is currently little information regarding factors that activate (or not activate) the inflammasome in ASM (221, 311, 416, 489). Understanding the role of factors such as resolvins and the inflammasome represent novel areas for research in the context of ASM and airway disease.
In addition to fibroblasts, ASM is now also well recognized to generate a range of ECM proteins (35, 47, 52, 73, 128, 222, 291, 450, 562), which has bearing given that the increased fractional area of the matrix within the smooth muscle layer of fatal asthmatics (29). Furthermore the ECM network can passively and actively modulate cell proliferation and migration, and thus influence airway structure and function. Here, ASM cells of asthmatic subjects may not only produce more ECM, but also a different profile. The ECM proteins of particular relevance in the context of ASM include collagens, fibronectin, versican, tenascin, perlecan, decorin (59, 72, 226, 241, 256, 295, 502), and members of the MMP family (MMP-1, -2, -9, and -12) (98, 267, 286, 296, 382, 507) [although some proteins such as collagen IV and elastin decrease (72)]. Furthermore, there is increasing interest in the transmembrane integrins (particularly α5 and β1) that can mediate and modulate cell-cell and cell-ECM interactions (562). Here, the ECM can also regulate activity of local growth factors (e.g., neurotrophins, VEGF) and cytokines by cleavage and inactivation (148, 176), creating a complex network to regulate remodeling. Accordingly, altered ECM production, e.g., modulated by inflammatory mediators or growth factors produced either by ASM itself or other cells, can have interactive effects on airway structure and function. This has further significance in the context of whether and how therapies such as glucocorticoids can influence airway structure (i.e., remodeling) by modulating the local inflammatory environment, and thus in turn the ECM.
A number of signaling pathways in the context of inflammation and ECM have been explored and some are highlighted here (72, 286, 457, 532, 536). Here, ASM can produce a wide variety of pro- and anti-inflammatory factors (37, 225, 230, 286, 427, 428, 484, 488, 512), including IL-1β, IL-5, IL-6, IL-8, IL-17, PDGF, TNF-α, TLSP, and TGFβ, although their autocrine/paracrine function in the context of remodeling are still being explored. For example, IL-6 can induce ASM hyperplasia and further modulate immune cell function. TNF-α can mediate effects by enhancing IFNβ secretion (346) while TGFβ can regulate IL-6 release (358). There are several examples of such cytokines influencing ECM and remodeling. For example, IL-1β can interact with TNF-α to increases MMP-12 (564) and MMP-9 (313), which can promote cell migration and remodeling and can further modulate growth factor activity (148, 176). TGFβ enhances deposition of perlecan (235), while Wnt/β-catenin pathway can regulate TGFβ effects on ASM-derived ECM (27, 286). Conversely, decorin, an ECM proteoglycan, binds TGFβ and blunts ECM production (343). Downstream, glucocorticoids can inhibit TNF-α-induced ECM production by activating the NF-κB inhibitor TNAIP3 (457). These examples highlight ASM involvement in ECM and remodeling.
ASM can also produce chemokines such as RANTES, eotaxin, MCP-1, CXCL10 (119), CXCL8 (103, 127, 153, 197), and CXCR3 ligand (516), although their effects in the context of remodeling are likely indirect via recruitment of inflammatory cells. In addition, ASM is now increasingly shown to produce growth factors such as BDNF (20, 418) and VEGF (127, 439, 478) that can also affect remodeling by enhancing ECM production.
Beyond inflammatory mediators and growth factors, a number of emerging mechanisms have been reported in the context of ASM and airway remodeling. One example is vitamin D, which has been shown to suppress remodeling both in vitro and in vivo (33, 66, 67, 102, 118, 268). This is an intriguing concept given recent reports that calcitriol is not additionally beneficial in severe asthma (63, 83). However, it also important to note that the mechanisms by which vitamin D influences airway cells are still under investigation, with recent studies attempting to explore differential gene regulation by this vitamin (162). Surprisingly, other vitamins in the context of remodeling have barely been examined with a single report on vitamin E (205). Another emerging mechanism is thyroxine, which appears to enhance ASM proliferation (129) and in the context of airway development results in malformed airways when thyroxine levels are low (179). Similarly, insulin appears to enhance ASM proliferation and ECM formation (5, 485) and may be important in the context of metabolic syndrome and other conditions of elevated insulin levels (6, 486, 487) as well as lung development (349, 385). Another emerging topic is the role of sphingolipids in airway inflammation, AHR and remodeling particularly the effect of sphingosine-1-phosphate in promoting contractility, inflammation and remodeling via ASM (86, 87, 440, 442, 448, 449, 534). Finally, the potential role of transglutaminase has also been preliminarily examined (203) although it has been studied more in the context of allergy, asthma and epithelial function (381, 383, 494) and in pulmonary hypertension (399, 400). The relevance of these disparate mechanisms lies in understanding the complex inputs and outputs with ASM at the center in the context of remodeling, only making it more clear that remodeling will not be easy to target.
Epigenetic Mechanisms and ASM
There is now increasing interest in and evidence for epigenetic regulation of multiple aspects of ASM structure and function in the context of disease. Epigenetics involves altered patterns of gene expression resulting from chromosomal changes that do not involve alterations in the DNA sequence. Epigenetic changes alter accessibility of promoters and enhancer/silencer regions within chromatin to transcription factors and thus regulate global activation or local transcription for specific genes. Such changes are mediated by a number of mechanisms such as posttranscriptional modification of histone proteins [acetylation and deacetylation via histone acetyltransferases (HATs) and deacetylases (HDACs)], miRNAs and noncoding RNAs (ncRNAs), and DNA methylation. The combination of these many mechanisms results in complex, potentially interactive effects on gene expression. Accordingly, even with increasing data on epigenetic mechanisms in ASM in the context disease, it becomes difficult to assess whether inhibition or activation of specific epigenetic pathways will alleviate vs. worsen disease. Nonetheless, there is now substantial interest in therapeutic avenues such as targeting of miRNAs, HDAC inhibitors, etc., for asthma and COPD. However, it is important to stress that the evidence to date is not necessarily convergent at this stage.
Initial evidence for abnormal histone modifications in asthmatic ASM comes from limited studies showing that enhanced CXCL8/IL8 (103) and VEGF (101) secretion involve aberrant HAT binding and resultant histone H3 acetylation at their respective promoters. Conversely, trichostatin A (a class I and II HDAC inhibitor) blunts the inhibitory effect of IFNγ on TNF-α-induced CXCL8 secretion in human ASM (269). Along similar lines, TNF-α induces H4 acetylation of the eotaxin promoter (269, 469), while TNF-α and IFNγ induce H4 but not H3 acetylation at the CXCL10 promoter (100). These disparate pieces of evidence highlight the potential for histone-based epigenetic mechanisms in immunomodulatory roles for ASM.
In the context of ASM function, there are only limited data for histone modification. For example, HDACs are downregulated in diseases such as asthma and COPD (36, 444–446) and may contribute to corticosteroid insensitivity. Accordingly, histone modifications could influence contractility, remodeling as well as inflammation, and further make HDAC upregulation (not downregulation) a novel therapeutic strategy. Yet it is the nonselective HDAC inhibitor trichostatin A that has been found to blunt ASM contraction (34), while other studies show that trichostatin A can blunt airway inflammation (229, 443, 523). This only highlights the complexity of these epigenetic mechanisms in the airway.
In terms of ASM cell proliferation as an aspect of remodeling, there is little to no evidence for epigenetic regulation. A single study found that specific bromodomains that control histone acetylation could be altered in asthmatic ASM and influence proliferation (409). However, there is increasing evidence for histone acetylation in pulmonary artery smooth muscle proliferation (380, 571) that may provide insight into potential roles in ASM. Similarly, there is no information on epigenetic regulation of ASM-derived ECM formation, but again emerging data from the vasculature (84, 183, 306, 413, 554) may provide insight.
Compared with the limited data on histone modifications, there are considerably more studies on miRNA regulation of ASM. As with most cell types or conditions where miRNAs are explored, a multitude of candidate miRNAs have been identified as playing multiple roles in ASM, making it again difficult to evaluate their relative contributions to normal ASM structure/function vs. disease. Furthermore, given that the same miRNA may or may not have the same targets in ASM of different species, data correlation between in vitro work (typically done in human ASM) to in vivo studies in mouse models of asthma, or where specific miRNAs have been up/downregulated, becomes difficult. One initial study profiled miRNAs in airway biopsies of subjects with mild asthma and surprisingly found no major or consistent changes in miRNAs to suggest a role in this condition, or in the responsiveness to steroid therapy (558). However, a number of later studies have found several miRNAs regulating specific aspects of ASM, with some studies pointing to particular miRNAs with broader function (interpreted as having broader and perhaps substantial role in disease pathophysiology). For example, one study in human ASM showed that exposure to proinflammatory cytokines such as IL-1β, TNF-α, and IFNγ downregulated 11 miRNAs, particularly miR-25, miR-140, miR-188, and miR-320, with miR-25 having a broad role in modulating expression of a range of inflammatory mediators, ECM, and contractile proteins and the transcription factor KLF4 (289). While this study showed the typical downregulation of multiple “protective” miRNAs, another study (299) showed that miR-146, which is widely induced in inflammation, is also enhanced in ASM with IL-1β exposure but does not mediate IL-1β-induced cytokine release by ASM. On the other hand, another group also explored miR-146a and miR-146b in human ASM from asthmatic and nonasthmatic subjects treated with cytomix (IL-1β, TNF-α, IFNγ) found elevated miR-146a and miR-146b, particularly in asthmatic patients. However, only miR-146a (which negatively regulates COX-2 and IL-1β) was functionally important (108). These contrasting results highlight the difficulty of simply correlating miRNA expression with specific functions. Another study showed that miR-140-3p regulates the important enzyme CD38 (259), which could have a multitude of downstream effects including [Ca2+]i and proliferation (133, 138, 196). Interestingly, mechanical stretch induces miR-26a that indirectly acts to induce ASM hypertrophy by blunting GSK3β (368, 369). Yet ASM proliferation appears to be driven by multiple miRNAs, including miR-10a (232), miR-23b (91), miR-138 (325), miR-145 (324), miR-203 (314), miR-221 (408), and miR-708 (138). There is relatively less known regarding ECM modulation, with studies showing a role for miR-25 (289) and miR-145 (324) at least. While these emerging studies show the potential for multiple miRNAs in ASM itself, it would be relevant to compare and contrast similar emerging data from other cell types and conditions in the lung toward understanding the complex roles of miRNAs in airway disease. For example, in fibroblasts, let-7d (234), miR-96 (377), miR-146a (108, 354), and miR-210 (53) appear to be important in the context of fibrosis, whereas in alveoli and lung injury miR-21 (537) miR-29b (140), miR-153, and miR-155 (547, 574) and in developing lung miR-124 (549) and miR-489 (385) have been shown to be relevant. Overall, these many pieces of data point to the need for understanding miRNA regulation in ASM (and other cell types as well), but important questions remain to be addressed in this context: 1) how do conditions contributing to disease, including inflammatory mediators, growth factors, etc., influence miRNA profiles; 2) does disease severity matter; 3) how do miRNAs interact with each other; 4) what are the feedback effects of specific factors in regulating miRNAs per se; and, 5) finally, how should the in vitro data be verified in vivo, and are animal models appropriate? Even in the context of therapy, while targeting of miRNAs is now being attempted, specificity and access to particular cell types remain difficult to address, especially if miRNA function differs between cell types.
Mitochondria and ASM
There is now substantial evidence that mitochondria play substantial roles in the airway beyond production of ATP, including Ca2+ buffering (21, 109, 436) (which also helps regulate mitochondrial energy production to match demand), ER pathways, Ca2+ influx such as SOCE, cell proliferation, and survival. While these concepts have been recently studied to a greater extent in other cell types (particularly cancers), there is now emerging evidence in ASM as well (16, 17, 19, 21, 92, 116, 131, 132, 155, 178, 206, 216, 294, 344, 358, 509, 530, 557). Here, it appears that mitochondrial structure in the context of fission and fusion, mitochondrial biogenesis and mitochondrial destruction via autophagy (mitophagy), and reactive oxygen species (ROS) are all important aspects of the noncanonical contributions for mitochondria (21, 178). For example, depletion of mitochondrial DNA blunts [Ca2+]i responses in ASM (92). TGFβ enhances ASM mitochondrial ROS in promoting cytokine secretion (358). Such ROS are also involved in cigarette smoke effects on ASM mitochondria (19). Conversely, inflammation impairs mitochondrial Ca2+ buffering that leads to elevated cytosolic Ca2+ (131, 132). Such impairment can lead not only to elevated ROS, but also to elevated ER stress and the unfolded protein response (UPR) (131). While the role of both ER stress and UPR in ASM per se has been minimally investigated (131, 174, 264, 389, 599), the relevance of these pathways lies in their potential to influence protein expression and function in the context of contractility and remodeling in asthma: an emerging topic of interest (78, 154, 199, 227, 231, 274, 275, 307, 337, 480, 545). Indeed, recent studies have explored the possibility of chemical chaperones that influence the UPR in the context of allergic asthma (199, 227, 337), while other studies have found links between the ER protein ORMDL3 and the UPR (231, 545).
Insight into the relevance of mitochondria in airway disease also comes from emerging data in other lung cell types and conditions where mitochondrial dysfunction appears to play a role. For example, impaired mitochondrial biogenesis contributes to pulmonary hypertension (2, 341, 465, 578), while a range of insults including cigarette smoke, hyperoxia, infection, excessive ventilation, etc., result in impaired mitochondrial function and/or ER stress in alveolar cells (4, 12, 32, 60, 90, 123, 124, 126, 187, 208, 210, 288, 326–328, 415, 533). Mitophagy also appears to be play a role in pulmonary vascular disease (3, 209, 421). Conversely, protective mechanisms, particularly the sirtuins, may be important to consider but have barely been explored in the lung (2, 169, 264, 280, 341, 520, 573). Mitochondrial dysfunction may also be relevant in lung manifestations of metabolic diseases such as diabetes (5, 6, 486) and in aging (204, 553) and senescence (204, 258, 426): topics that have been barely explored. Thus the potential links between mitochondria, ER stress, and ASM structure/function represent a novel area of research in airway disease.
Sex Differences and Sex Steroids
With the NIH's ongoing emphasis on exploring intrinsic sex differences in all diseases, it is all the more imperative to understand concepts of sex differences and sex steroid signaling in lung diseases, given clinical evidence for peripubertal and perimenopausal changes in the incidence of asthma and increased asthma in elderly men (54, 184, 460, 468, 526, 538, 587). Here, studies have explored both intrinsic and extrinsic (steroid-mediated) effects (137, 297, 345, 452, 499, 513, 527, 528, 546). What makes this topic intriguing, yet difficult, is separating intrinsic differences between men and women (or boys vs. girls) in airway structure/function, particularly in disease, from the extrinsic effects of sex steroids (56, 348, 460, 528). Here, it could be argued that intrinsic differences are what they are, and while it is important to understand such differences in the context of age-dependent changes in the airway, the superimposed effects of sex steroids from fetal stages (both fetal and maternal steroids) onward through puberty and beyond make it relatively more important to understand the extrinsic effects of sex steroids. In this regard, estrogens, progesterone, and/or testosterone can be expected to work both genomically and nongenomically, but it is not clear that any or all of these sex steroid effects are consistent across cell types or even within a specific sex. Akin to their role in vascular smooth muscle, estrogens can rapidly (nongenomically) reduce [Ca2+]i in ASM, working via a variety of pathways (528), acting via ERα and ERβ (527). Whether such estrogen effects are maintained in asthma is not known, although unpublished data from our groups (Sathish et al., unpublished observations) suggest that inflammation and/or asthma lead to an altered balance of ERα vs. ERβ levels, with a greater influence of ERβ. In the context of remodeling, there are both intrinsic sex differences (513) (although the role of ASM per se is not known), as well as different effects of estrogens vs. testosterone (137, 499). Furthermore, ER variants appear to also be important in female asthmatic subjects (137). Whether such variants are also important in men, or whether ER profiles differ between the sexes, with age and pubertal status, etc., are all unknown. It is also important to consider whether and how progesterone influences ASM with limited data of effects on the airway function (200, 213, 239, 514). A similar question can be raised regarding whether testosterone, acting via androgen receptors, is bronchodilatory or protective as suggested by some studies (77, 80, 81, 283, 284, 410) and whether differences in receptor regulation between men vs. women play a role (284, 585). Overall, these many unanswered questions represent another important area for investigation in airway disease, particularly the role of ASM.
ASM in Airway Development
There is increasing evidence that factors early in airway development can influence not only normal lung growth, but also airway diseases throughout life such as such as asthma or COPD (39, 144, 475). While postnatal airway growth and its dysfunction can be studied in animal models as well as in babies and children, even if not as easily as in adults, our insight into processes regulating prenatal airway development has also been facilitated with approaches such as lineage studies, transgenic animals, and imaging techniques. Here, understanding bronchial airway growth may be particularly important given that the number of airways is fixed at birth, unlike continued postnatal development of alveoli.
The ASM likely plays a role in modulating developing airway structure and function. Even if the prenatal airway is not involved in ventilation per se, airway peristalsis has been shown to occur in vitro (249, 250) with cyclical Ca2+ waves within fetal ASM cells. Such processes may promote airway branching and elongation and underline a functional role for ASM. What mechanisms underlie airway peristalsis is not clear. In adult ASM (and in other smooth muscle cell types), baseline [Ca2+]i oscillations modulated by agonist stimulation have now been shown extensively (70, 223, 406, 425, 459, 521). Many of the [Ca2+]i regulatory pathways involved in adult ASM are also present in developing ASM (206) and could play a role. An intriguing additional mechanism may be the CaSR. Although CaSR is known to be expressed in developing epithelium (158) and modulates branching morphogenesis at adult [Ca2+]o levels (∼1.2 mM), the higher fetal [Ca2+]o levels [∼1.7 mM (285)] cause CaSR to inhibit branching. However, it is possible that CaSR is also expressed in fetal ASM and given the higher [Ca2+]o is more sensitive and thus elevates ASM [Ca2+]i, setting the stage of [Ca2+]i oscillations and peristalsis. Alternatively, oscillations in membrane potential and cyclical Ca2+ influx (again facilitated by higher [Ca2+]o) may be involved. The relevance of understanding these pathways lies in determining causes of lung hypoplasia, and particularly the effects of iatrogenic insults such as hyperoxia, mechanical stretch of CPAP, and mechanical ventilation in the context of premature birth and respiratory support of the immature neonate (217, 218). Here, the emerging role of mechanosensitive pathways in modulating expression of genes and proteins should be a model for understanding how mechanical forces within the developing airway induced by ASM cells as well as external forces on ASM influence airway growth. These processes could be modulated by growth factors and transcription factors in the context of normal lung development, such as FGF10 (191, 250, 466), neurotrophins (171, 423, 493, 575, 576), and Wnt (202).
Beyond Ca2+ and growth factors, an intriguing aspect of airway development in the context of subsequent disease in childhood or adults is epigenetic effects that could occur in utero to influence asthma (58, 144, 214, 228, 273) and even traverse generations, particularly in cigarette smoke effects, and contribute to COPD (287, 309, 506). Whether ASM per se is involved is not known but again makes for an exciting area for investigating its role in identifying developmental origins of adult disease.
Emerging Topics in ASM Biology
There are understandably a number of areas where the role of ASM is being or should be explored but cannot be justifiably summarized here. Indeed, one of the limitations of introducing or discussing these topics is that they have been addressed either in barely a few studies, done entirely in vitro (sometimes in cells from nonhuman species), in a very limited context, or are hypothesized based on studies in other cell types and thus insufficiently teased out to be able to draw conclusions regarding their role in ASM per se. Conversely, these topics represent emerging areas that could be relevant to understanding and targeting airway disease. Topics include 1) the role of TLRs in the context of infection effects at the level of ASM (143, 153, 193, 300, 340) and the related inflammasome (221, 416); 2) the role of mechanosensitive pathways that are being increasingly recognized in other diseases or aspects of lung growth and disease, including YAP/TAZ (320, 453), Wnt/β-catenin (105, 151, 212), and Piezo channels (28, 192, 430, 542); 3) the role of circadian rhythms and clock genes in the context of normal airway structure/function and asthma (145, 504, 505, 531); and 4) the role of senescence mechanisms that have been explored in other lung cell types and diseases (23, 50, 96, 159, 204, 207, 262, 276, 378) but are likely important in the context of the aging airway and asthma in the elderly.
An obvious consideration when exploring the role of ASM is the presence of surrounding cells and the ECM. Clearly, by virtue of its response to secreted factors by surrounding epithelial cells, fibroblasts, nerves, immune cells, and even to the ECM, as well as, conversely, being a source of factors that influence its surrounding, the concepts of cell-cell and cell-matrix interactions are emerging areas of interest. This is particularly relevant in the context of inflammation or other insults, where initial responses may involve immune and epithelial cells (for example) and the ASM may “simply” be a recipient, but with chronic disease, ASM may take on more complex roles including that of a synthetic and immunomodulatory cell and a source of pro- or anti-inflammatory factors. Furthermore, by contributing to remodeling including fibrosis, ASM can modulate the extent of proliferation and migration of other cell types, alter barrier function, and promote vascularity. Thus ASM may contribute to maintenance and exacerbation of airway disease even following the removal of the insults that initiate disease and therefore be a part of the disease phenotype that is resistant to current therapies that largely target inflammation. How this role of ASM differs between ages, sexes, initiating conditions for disease, and a host of other variables is not known but could be important to understanding and treating diseases such as asthma. Accordingly, it becomes important not only to understand mechanisms that contribute to ASM structure and function in health and disease, but also to develop appropriate, integrative biological, imaging, informatics, and other approaches (including those involving multiple cell types to facilitate study of cell-cell interactions) to analyze pathways and interactions and overall place ASM in the context of airway disease.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL088029, HL056470, and HL126451 (Y. S. Prakash).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.S.P. conceived and designed research, prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
REFERENCES
- 1.Adam RJ, Hisert KB, Dodd JD, Grogan B, Launspach JL, Barnes JK, Gallagher CG, Sieren JP, Gross TJ, Fischer AJ, Cavanaugh JE, Hoffman EA, Singh PK, Welsh MJ, McKone EF, Stoltz DA. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight 1: e86183, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afolayan AJ, Eis A, Alexander M, Michalkiewicz T, Teng RJ, Lakshminrusimha S, Konduri GG. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L40–L49, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afolayan AJ, Teng RJ, Eis A, Rana U, Broniowska KA, Corbett JA, Pritchard K, Konduri GG. Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. Am J Physiol Lung Cell Mol Physiol 306: L351–L360, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal AR, Yin F, Cadenas E. Metabolic shift in lung alveolar cell mitochondria following acrolein exposure. Am J Physiol Lung Cell Mol Physiol 305: L764–L773, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. Am J Respir Cell Mol Biol 44: 270–275, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A, Prakash YS. Obesity, metabolic syndrome, and airway disease: a bioenergetic problem? Immunol Allergy Clin North Am 34: 785–796, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcolado NG, Conrad DJ, Poroca D, Li M, Alshafie W, Chappe FG, Pelis RM, Anini Y, Xu Z, Hamidi S, Said SI, Chappe VM. Cystic fibrosis transmembrane conductance regulator dysfunction in VIP knockout mice. Am J Physiol Cell Physiol 307: C195–C207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfadda TI, Saleh AM, Houillier P, Geibel JP. Calcium-sensing receptor 20 years later. Am J Physiol Cell Physiol 307: C221–C231, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algara-Suarez P, Mejia-Elizondo R, Sims SM, Saavedra-Alanis VM, Espinosa-Tanguma R. The 13 isoform of Na+-Ca2+ exchanger expressed in guinea pig tracheal smooth muscle is less sensitive to KB-R7943. J Physiol Biochem 66: 117–125, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Algara-Suarez P, Romero-Mendez C, Chrones T, Sanchez-Armass S, Meza U, Sims SM, Espinosa-Tanguma R. Functional coupling between the Na+/Ca2+ exchanger and nonselective cation channels during histamine stimulation in guinea pig tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L191–L198, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Allen IC, Hartney JM, Coffman TM, Penn RB, Wess J, Koller BH. Thromboxane A2 induces airway constriction through an M3 muscarinic acetylcholine receptor-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 290: L526–L533, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Alli AA, Brewer EM, Montgomery DS, Ghant MS, Eaton DC, Brown LA, Helms MN. Chronic ethanol exposure alters the lung proteome and leads to mitochondrial dysfunction in alveolar type 2 cells. Am J Physiol Lung Cell Mol Physiol 306: L1026–L1035, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, Panettieri RA Jr, Liggett SB. TAS2R activation promotes airway smooth muscle relaxation despite β2-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol 303: L304–L311, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anaparti V, Ilarraza R, Orihara K, Stelmack GL, Ojo OO, Mahood TH, Unruh H, Halayko AJ, Moqbel R. NMDA receptors mediate contractile responses in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 308: L1253–L1264, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Anaparti VV, Pascoe CD, Jha A, Mahood TH, Ilarraza R, Unruh H, Moqbel R, Halayko AJ. Tumor necrosis factor regulates NMDA receptor mediated airway smooth muscle contractile function and airway responsiveness. Am J Physiol Lung Cell Mol Physiol 311: L467–L480, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Aravamudan B, Delmotte P, Thompson M, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Response to letter by Dr. Marc Hershenson (exposure of airway smooth muscle cells to cigarette smoke extract). Am J Physiol Lung Cell Mol Physiol 307: L346, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aravamudan B, Kiel A, Freeman M, Delmotte P, Thompson M, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 306: L840–L854, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med 16: 812–823, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aravamudan B, Thompson M, Sieck GC, Vassallo R, Pabelick CM, Prakash YS. Functional effects of cigarette smoke-induced changes in airway smooth muscle mitochondrial morphology. J Cell Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aravamudan B, Thompson MA, Pabelick CM, Prakash YS. Mechanisms of BDNF regulation in asthmatic airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 311: L270–L279, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aravamudan B, Thompson MA, Pabelick CM, Prakash YS. Mitochondria in lung diseases. Expert Rev Respir Med 7: 631–646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aravamudan B, VanOosten SK, Meuchel LW, Vohra P, Thompson M, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 knockout mice exhibit airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol 303: L669–L681, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Kawabata Y, Hano H, Nakayama K, Kuwano K. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L56–L69, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Aso H, Ito S, Mori A, Morioka M, Suganuma N, Kondo M, Imaizumi K, Hasegawa Y. Prostaglandin E2 enhances interleukin-8 production via EP4 receptor in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 302: L266–L273, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Aso H, Ito S, Mori A, Suganuma N, Morioka M, Takahara N, Kondo M, Hasegawa Y. Differential regulation of airway smooth muscle cell migration by E-prostanoid receptor subtypes. Am J Respir Cell Mol Biol 48: 322–329, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Baarsma HA, Konigshoff M, Gosens R. The WNT signaling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol Therapeut 138: 66–83, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Baarsma HA, Menzen MH, Halayko AJ, Meurs H, Kerstjens HA, Gosens R. β-Catenin signaling is required for TGF-β1-induced extracellular matrix production by airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 301: L956–L965, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Bagriantsev SN, Gracheva EO, Gallagher PG. Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem 289: 31673–31681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai TR, Cooper J, Koelmeyer T, Pare PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med 162: 663–669, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Bai Y, Krishnamoorthy N, Patel KR, Rosas I, Sanderson MJ, Ai X. Cryopreserved human precision-cut lung slices as a bioassay for live tissue banking. A viability study of bronchodilation with bitter-taste receptor agonists. Am J Respir Cell Mol Biol 54: 656–663, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballweg K, Mutze K, Konigshoff M, Eickelberg O, Meiners S. Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 307: L895–L907, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee A, Panettieri R Jr. Vitamin D modulates airway smooth muscle function in COPD. Curr Opin Pharmacol 12: 266–274, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee A, Trivedi CM, Damera G, Jiang M, Jester W, Hoshi T, Epstein JA, Panettieri RA Jr. Trichostatin A abrogates airway constriction, but not inflammation, in murine and human asthma models. Am J Respir Cell Mol Biol 46: 132–138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J 36: 1174–1184, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 131: 636–645, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev 242: 31–50, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Barnett K, Jacoby DB, Nadel JA, Lazarus SC. The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am Rev Respir Dis 138: 780–783, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Beers MF, Morrisey EE. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest 121: 2065–2073, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem 278: 22482–22491, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Ben Chaim Y, Bochnik S, Parnas I, Parnas H. Voltage affects the dissociation rate constant of the m2 muscarinic receptor. PLoS One 8: e74354, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 167: 1360–1368, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Bentley JK, Deng H, Linn MJ, Lei J, Dokshin GA, Fingar DC, Bitar KN, Henderson WR Jr, Hershenson MB. Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3β phosphorylation in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol 296: L176–L184, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bentley JK, Hershenson MB. Airway smooth muscle growth in asthma: proliferation, hypertrophy, and migration. Proc Am Thoracic Soc 5: 89–96, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leseche G, Longrois D, Norel X. PGE2 receptor (EP4) agonists: potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther 25: 115–118, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A, Burlingame AL, Matthay MA, Sheppard D. IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia. Am J Physiol Lung Cell Mol Physiol 303: L12–L19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bidan CM, Veldsink AC, Meurs H, Gosens R. Airway and extracellular matrix mechanics in COPD. Front Physiol 6: 346, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther 26: 112–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 4: 2, 2003. [PMC free article] [PubMed] [Google Scholar]
- 50.Birch J, Anderson RK, Correia-Melo C, Jurk D, Hewitt G, Marques FM, Green NJ, Moisey E, Birrell MA, Belvisi MG, Black F, Taylor JJ, Fisher AJ, De Soyza A, Passos JF. DNA damage response at telomeres contributes to lung aging and chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 309: L1124–L1137, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birrell MA, Maher SA, Dekkak B, Jones V, Wong S, Brook P, Belvisi MG. Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax 70: 740–747, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black JL, Roth M. Intrinsic asthma: is it intrinsic to the smooth muscle? Clin Exp Allergy 39: 962–965, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Bodempudi V, Hergert P, Smith K, Xia H, Herrera J, Peterson M, Khalil W, Kahm J, Bitterman PB, Henke CA. miR-210 promotes IPF fibroblast proliferation in response to hypoxia. Am J Physiol Lung Cell Mol Physiol 307: L283–L294, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol 13: 92–99, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borowiec AS, Bidaux G, Pigat N, Goffin V, Bernichtein S, Capiod T. Calcium channels, external calcium concentration and cell proliferation. Eur J Pharmacol 739: 19–25, 2014. [DOI] [PubMed] [Google Scholar]
- 56.Bosse Y. Endocrine regulation of airway contractility is overlooked. J Endocrinol 222: R61–R73, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Boushey H. Targets for asthma therapy. Allerg Immunol (Paris) 32: 336–341, 2000. [PubMed] [Google Scholar]
- 58.Bousquet J, Jacot W, Yssel H, Vignola AM, Humbert M. Epigenetic inheritance of fetal genes in allergic asthma. Allergy 59: 138–147, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Brandsma CA, Timens W, Jonker MR, Rutgers B, Noordhoek JA, Postma DS. Differential effects of fluticasone on extracellular matrix production by airway and parenchymal fibroblasts in severe COPD. Am J Physiol Lung Cell Mol Physiol 305: L582–L589, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Brar SS, Meyer JN, Bortner CD, Van Houten B, Martin WJ 2nd. Mitochondrial DNA-depleted A549 cells are resistant to bleomycin. Am J Physiol Lung Cell Mol Physiol 303: L413–L424, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bratt JM, Zeki AA, Last JA, Kenyon NJ. Competitive metabolism of l-arginine: arginase as a therapeutic target in asthma. J Biomed Res 25: 299–308, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brechbuhl HM, Li B, Smith RW, Reynolds SD. Epidermal growth factor receptor activity is necessary for mouse basal cell proliferation. Am J Physiol Lung Cell Mol Physiol 307: L800–L810, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brehm JM. Vitamin D and asthma—life after VIDA? Curr Allergy Asthma Rep 14: 461, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brennan SC, Thiem U, Roth S, Aggarwal A, Fetahu I, Tennakoon S, Gomes AR, Brandi ML, Bruggeman F, Mentaverri R, Riccardi D, Kallay E. Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta 1833: 1732–1744, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Brennan SC, Wilkinson WJ, Tseng HE, Finney B, Monk B, Dibble H, Quilliam S, Warburton D, Galietta LJ, Kemp PJ, Riccardi D. The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep 6: 21975, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Britt RD Jr, Faksh A, Vogel ER, Thompson MA, Chu V, Pandya HC, Amrani Y, Martin RJ, Pabelick CM, Prakash YS. Vitamin D attenuates cytokine-induced remodeling in human fetal airway smooth muscle cells. J Cell Physiol 230: 1189–1198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Britt RD Jr, Thompson MA, Freeman MR, Stewart AL, Pabelick CM, Prakash YS. Vitamin D reduces inflammation-induced contractility and remodeling of asthmatic human airway smooth muscle. Ann Am Thorac Soc 13, Suppl 1: S97–S98, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown A, Danielsson J, Townsend EA, Zhang Y, Perez-Zoghbi JF, Emala CW Sr, Gallos G. Attenuation of airway smooth muscle contractility via flavonol-mediated inhibition of phospholipase-Cβ. Am J Physiol Lung Cell Mol Physiol 310: L747–L758, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brueggemann LI, Kakad PP, Love RB, Solway J, Dowell ML, Cribbs LL, Byron KL. Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am J Physiol Lung Cell Mol Physiol 302: L120–L132, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brumen M, Fajmut A, Dobovisek A, Roux E. Mathematical modelling of Ca2+ oscillations in airway smooth muscle cells. J Biol Phys 31: 515–524, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bullone M, Lavoie JP. Asthma “of horses and men”–how can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol Immunol 66: 97–105, 2015. [DOI] [PubMed] [Google Scholar]
- 72.Burgess JK. The role of the extracellular matrix and specific growth factors in the regulation of inflammation and remodelling in asthma. Pharmacol Therapeut 122: 19–29, 2009. [DOI] [PubMed] [Google Scholar]
- 73.Burgess JK, Ceresa C, Johnson SR, Kanabar V, Moir LM, Nguyen TT, Oliver BG, Schuliga M, Ward J. Tissue and matrix influences on airway smooth muscle function. Pulm Pharmacol Ther 22: 379–387, 2009. [DOI] [PubMed] [Google Scholar]
- 74.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med 170: 683–690, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Cabezas GA, Velasco M. DA1 receptors modulation in rat isolated trachea. Am J Ther 17: 301–305, 2010. [DOI] [PubMed] [Google Scholar]
- 76.Camoretti-Mercado B, Pauer SH, Yong HM, Smith DC, Deshpande DA, An SS, Liggett SB. Pleiotropic effects of bitter taste receptors on [Ca2+]i mobilization, hyperpolarization, and relaxation of human airway smooth muscle cells. PLoS One 10: e0131582, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses 76: 585–588, 2011. [DOI] [PubMed] [Google Scholar]
- 78.Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet 19: 111–121, 2010. [DOI] [PubMed] [Google Scholar]
- 79.Capra V, Habib A, Accomazzo MR, Ravasi S, Citro S, Levy-Toledano S, Nicosia S, Rovati GE. Thromboxane prostanoid receptor in human airway smooth muscle cells: a relevant role in proliferation. Eur J Pharmacol 474: 149–159, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177: 621–630, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Card JW, Voltz JW, Ferguson CD, Carey MA, DeGraff LM, Peddada SD, Morgan DL, Zeldin DC. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. Am J Physiol Lung Cell Mol Physiol 292: L908–L914, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carter PM, Heinly TL, Yates SW, Lieberman PL. Asthma: the irreversible airways disease. J Investig Allergol Clin Immunol 7: 566–571, 1997. [PubMed] [Google Scholar]
- 83.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, Kazani SD, Moore WC, Moy J, Sorkness CA, Avila P, Bacharier LB, Bleecker E, Boushey HA, Chmiel J, Fitzpatrick AM, Gentile D, Hundal M, Israel E, Kraft M, Krishnan JA, LaForce C, Lazarus SC, Lemanske R, Lugogo N, Martin RJ, Mauger DT, Naureckas E, Peters SP, Phipatanakul W, Que LG, Sheshadri A, Smith L, Solway J, Sullivan-Vedder L, Sumino K, Wechsler ME, Wenzel S, White SR, Sutherland ER. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 311: 2083–2091, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 126: 321–334, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, Eidelman DH, Hamid Q. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J 26: 5152–5160, 2012. [DOI] [PubMed] [Google Scholar]
- 86.Che W, Manetsch M, Quante T, Rahman MM, Patel BS, Ge Q, Ammit AJ. Sphingosine 1-phosphate induces MKP-1 expression via p38 MAPK- and CREB-mediated pathways in airway smooth muscle cells. Biochim Biophys Acta 1823: 1658–1665, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Che W, Parmentier J, Seidel P, Manetsch M, Ramsay EE, Alkhouri H, Ge Q, Armour CL, Ammit AJ. Corticosteroids inhibit sphingosine 1-phosphate-induced interleukin-6 secretion from human airway smooth muscle via mitogen-activated protein kinase phosphatase 1-mediated repression of mitogen and stress-activated protein kinase 1. Am J Respir Cell Mol Biol 50: 358–368, 2014. [DOI] [PubMed] [Google Scholar]
- 88.Cheah EY, Burcham PC, Mann TS, Henry PJ. Acrolein relaxes mouse isolated tracheal smooth muscle via a TRPA1-dependent mechanism. Biochem Pharmacol 89: 148–156, 2014. [DOI] [PubMed] [Google Scholar]
- 89.Chen B, Xue J, Meng X, Slutzky JL, Calvert AE, Chicoine LG. Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am J Physiol Lung Cell Mol Physiol 307: L317–L325, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen HM, Yang CM, Chang JF, Wu CS, Sia KC, Lin WN. AdipoR-increased intracellular ROS promoted cPLA2 and COX-2 expression via activation of PKC and p300 in adiponectin-stimulated human alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 311: L255–L269, 2016. [DOI] [PubMed] [Google Scholar]
- 91.Chen M, Huang L, Zhang W, Shi J, Lin X, Lv Z, Liang R, Jiang S. MiR-23b controls TGF-β1 induced airway smooth muscle cell proliferation via TGFβR2/p-Smad3 signals. Mol Immunol 70: 84–93, 2016. [DOI] [PubMed] [Google Scholar]
- 92.Chen T, Zhu L, Wang T, Ye H, Huang K, Hu Q. Mitochondria depletion abolishes agonist-induced Ca2+ plateau in airway smooth muscle cells: potential role of H2O2. Am J Physiol Lung Cell Mol Physiol 298: L178–L188, 2010. [DOI] [PubMed] [Google Scholar]
- 93.Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L287–L300, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chevigny M, Guerin-Montpetit K, Vargas A, Lefebvre-Lavoie J, Lavoie JP. Contribution of SRF, Elk-1, and myocardin to airway smooth muscle remodeling in heaves, an asthma-like disease of horses. Am J Physiol Lung Cell Mol Physiol 309: L37–L45, 2015. [DOI] [PubMed] [Google Scholar]
- 95.Cho WK, Lee CM, Kang MJ, Huang Y, Giordano FJ, Lee PJ, Trow TK, Homer RJ, Sessa WC, Elias JA, Lee CG. IL-13 receptor α2-arginase 2 pathway mediates IL-13-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 304: L112–L124, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Christofidou-Solomidou M, Pietrofesa RA, Arguiri E, Schweitzer KS, Berdyshev EV, McCarthy M, Corbitt A, Alwood JS, Yu Y, Globus RK, Solomides CC, Ullrich RL, Petrache I. Space radiation-associated lung injury in a murine model. Am J Physiol Lung Cell Mol Physiol 308: L416–L428, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Churchill L, Chilton FH, Resau JH, Bascom R, Hubbard WC, Proud D. Cyclooxygenase metabolism of endogenous arachidonic acid by cultured human tracheal epithelial cells. Am Rev Respir Dis 140: 449–459, 1989. [DOI] [PubMed] [Google Scholar]
- 98.Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur Respir J 39: 197–209, 2012. [DOI] [PubMed] [Google Scholar]
- 99.Claar D, Hartert TV, Peebles RS Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med 9: 55–72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clarke DL, Clifford RL, Jindarat S, Proud D, Pang L, Belvisi M, Knox AJ. TNFα and IFNγ synergistically enhance transcriptional activation of CXCL10 in human airway smooth muscle cells via STAT-1, NF-κB, and the transcriptional coactivator CREB-binding protein. J Biol Chem 285: 29101–29110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clifford RL, John AE, Brightling CE, Knox AJ. Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma. J Immunol 189: 819–831, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clifford RL, Knox AJ. Vitamin D — a new treatment for airway remodelling in asthma? Br J Pharmacol 158: 1426–1428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clifford RL, Patel JK, John AE, Tatler AL, Mazengarb L, Brightling CE, Knox AJ. CXCL8 histone H3 acetylation is dysfunctional in airway smooth muscle in asthma: regulation by BET. Am J Physiol Lung Cell Mol Physiol 308: L962–L972, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cloots RH, Sankaranarayanan S, de Theije CC, Poynter ME, Terwindt E, van Dijk P, Hakvoort TB, Lamers WH, Kohler SE. Ablation of Arg1 in hematopoietic cells improves respiratory function of lung parenchyma, but not that of larger airways or inflammation in asthmatic mice. Am J Physiol Lung Cell Mol Physiol 305: L364–L376, 2013. [DOI] [PubMed] [Google Scholar]
- 105.Cohen JC, Larson JE, Killeen E, Love D, Takemaru K. CFTR and Wnt/β-catenin signaling in lung development. BMC Dev Biol 8: 70, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 307: L917–L923, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Comer BS, Ba M, Singer CA, Gerthoffer WT. Epigenetic targets for novel therapies of lung diseases. Pharmacol Therapeut 147: 91–110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Comer BS, Camoretti-Mercado B, Kogut PC, Halayko AJ, Solway J, Gerthoffer WT. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L727–L734, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta 1797: 607–618, 2010. [DOI] [PubMed] [Google Scholar]
- 110.Cook DP, Rector MV, Bouzek DC, Michalski AS, Gansemer ND, Reznikov LR, Li X, Stroik MR, Ostedgaard LS, Abou Alaiwa MH, Thompson MA, Prakash YS, Krishnan R, Meyerholz DK, Seow CY, Stoltz DA. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle. Implications for airway contractility. Am J Respir Crit Care Med 193: 417–426, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cortijo J, Milara J, Mata M, Donet E, Gavara N, Peel SE, Hall IP, Morcillo EJ. Nickel induces intracellular calcium mobilization and pathophysiological responses in human cultured airway epithelial cells. Chem Biol Interact 183: 25–33, 2010. [DOI] [PubMed] [Google Scholar]
- 112.Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am J Physiol Lung Cell Mol Physiol 309: L888–L901, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Croisier H, Tan X, Chen J, Sneyd J, Sanderson MJ, Brook BS. Ryanodine receptor sensitization results in abnormal calcium signaling in airway smooth muscle cells. Am J Respir Cell Mol Biol 53: 703–711, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cyphert JM, Allen IC, Church RJ, Latour AM, Snouwaert JN, Coffman TM, Koller BH. Allergic inflammation induces a persistent mechanistic switch in thromboxane-mediated airway constriction in the mouse. Am J Physiol Lung Cell Mol Physiol 302: L140–L151, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dahan D, Ducret T, Quignard JF, Marthan R, Savineau JP, Esteve E. Implication of the ryanodine receptor in TRPV4-induced calcium response in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 303: L824–L833, 2012. [DOI] [PubMed] [Google Scholar]
- 116.Dai J, Kuo KH, Leo JM, van Breemen C, Lee CH. Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium 37: 333–340, 2005. [DOI] [PubMed] [Google Scholar]
- 117.Damera G, Druey KM, Cooper PR, Krymskaya VP, Soberman RJ, Amrani Y, Hoshi T, Brightling CE, Panettieri RA Jr. An RGS4-mediated phenotypic switch of bronchial smooth muscle cells promotes fixed airway obstruction in asthma. PloS One 7: e28504, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA Jr. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol 158: 1429–1441, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Damera G, Tliba O, Panettieri RA Jr. Airway smooth muscle as an immunomodulatory cell. Pulm Pharmacol Ther 22: 353–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Daniel EE, Abela AP, Janssen LJ, O'Byrne PM. Effects of inflammatory mediators on canine airway neuromuscular function. Can J Physiol Pharmacol 70: 624–634, 1992. [DOI] [PubMed] [Google Scholar]
- 121.Daniel EE, O'Byrne P. Effect of inflammatory mediators on airway nerves and muscle. Am Rev Respir Dis 143: S3–S5, 1991. [DOI] [PubMed] [Google Scholar]
- 122.Danielsson J, Zaidi S, Kim B, Funayama H, Yim PD, Xu D, Worgall TS, Gallos G, Emala CW. Airway epithelial cell release of GABA is regulated by protein kinase A. Lung 194: 401–408, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Das KC. Thioredoxin-deficient mice, a novel phenotype sensitive to ambient air and hypersensitive to hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol 308: L429–L442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Datta A, Kim GA, Taylor JM, Gugino SF, Farrow KN, Schumacker PT, Berkelhamer SK. Mouse lung development and NOX1 induction during hyperoxia are developmentally regulated and mitochondrial ROS dependent. Am J Physiol Lung Cell Mol Physiol 309: L369–L377, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davi G, Basili S, Vieri M, Cipollone F, Santarone S, Alessandri C, Gazzaniga P, Cordova C, Violi F. Enhanced thromboxane biosynthesis in patients with chronic obstructive pulmonary disease. The Chronic Obstructive Bronchitis and Haemostasis Study Group. Am J Respir Crit Care Med 156: 1794–1799, 1997. [DOI] [PubMed] [Google Scholar]
- 126.de Vries M, Heijink IH, Gras R, den Boef LE, Reinders-Luinge M, Pouwels SD, Hylkema MN, van der Toorn M, Brouwer U, van Oosterhout AJ, Nawijn MC. Pim1 kinase protects airway epithelial cells from cigarette smoke-induced damage and airway inflammation. Am J Physiol Lung Cell Mol Physiol 307: L240–L251, 2014. [DOI] [PubMed] [Google Scholar]
- 127.Deacon K, Knox AJ. Human airway smooth muscle cells secrete amphiregulin via bradykinin/COX-2/PGE2, inducing COX-2, CXCL8, and VEGF expression in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 309: L237–L249, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc 6: 683–692, 2009. [DOI] [PubMed] [Google Scholar]
- 129.Dekkers BG, Naeimi S, Bos IS, Menzen MH, Halayko AJ, Hashjin GS, Meurs H. l-Thyroxine promotes a proliferative airway smooth muscle phenotype in the presence of TGF-β1. Am J Physiol Lung Cell Mol Physiol 308: L301–L306, 2015. [DOI] [PubMed] [Google Scholar]
- 130.Delamere F, Holland E, Patel S, Bennett J, Pavord I, Knox A. Production of PGE2 by bovine cultured airway smooth muscle cells and its inhibition by cyclo-oxygenase inhibitors. Br J Pharmacol 111: 983–988, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Delmotte P, Sieck GC. Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca2+ regulation in airway smooth muscle (ASM). Can J Physiol Pharmacol 93: 97–110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Delmotte P, Yang B, Thompson MA, Pabelick CM, Prakash YS, Sieck GC. Inflammation alters regional mitochondrial Ca2+ in human airway smooth muscle cells. Am J Physiol Cell Physiol 303: C244–C256, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deshpande DA, Dileepan M, Walseth TF, Subramanian S, Kannan MS. MicroRNA regulation of airway inflammation and airway smooth muscle function: relevance to asthma. Drug Dev Res 76: 286–295, 2015. [DOI] [PubMed] [Google Scholar]
- 134.Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal 18: 2105–2120, 2006. [DOI] [PubMed] [Google Scholar]
- 135.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dicpinigaitis PV. Effect of the GABA-agonist baclofen on bronchial responsiveness in asthmatics. Pulm Pharmacol Ther 12: 257–260, 1999. [DOI] [PubMed] [Google Scholar]
- 137.Dijkstra A, Howard TD, Vonk JM, Ampleford EJ, Lange LA, Bleecker ER, Meyers DA, Postma DS. Estrogen receptor 1 polymorphisms are associated with airway hyperresponsiveness and lung function decline, particularly in female subjects with asthma. J Allergy Clin Immunol 117: 604–611, 2006. [DOI] [PubMed] [Google Scholar]
- 138.Dileepan M, Jude JA, Rao SP, Walseth TF, Panettieri RA, Subramanian S, Kannan MS. MicroRNA-708 regulates CD38 expression through signaling pathways JNK MAP kinase and PTEN/AKT in human airway smooth muscle cells. Respir Res 15: 107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dolan JM, Weinberg JB, O'Brien E, Abashian A, Procario MC, Aronoff DM, Crofford LJ, Peters-Golden M, Ward L, Mancuso P. Increased lethality and defective pulmonary clearance of Streptococcus pneumoniae in microsomal prostaglandin E synthase-1-knockout mice. Am J Physiol Lung Cell Mol Physiol 310: L1111–L1120, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Domingo-Gonzalez R, Wilke CA, Huang SK, Laouar Y, Brown JP, Freeman CM, Curtis JL, Yanik GA, Moore BB. Transforming growth factor-β induces microRNA-29b to promote murine alveolar macrophage dysfunction after bone marrow transplantation. Am J Physiol Lung Cell Mol Physiol 308: L86–L95, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Donovan C, Bailey SR, Tran J, Haitsma G, Ibrahim ZA, Foster SR, Tang ML, Royce SG, Bourke JE. Rosiglitazone elicits in vitro relaxation in airways and precision cut lung slices from a mouse model of chronic allergic airways disease. Am J Physiol Lung Cell Mol Physiol 309: L1219–L1228, 2015. [DOI] [PubMed] [Google Scholar]
- 142.Donovan C, Tan X, Bourke JE. PPARγ ligands regulate noncontractile and contractile functions of airway smooth muscle: implications for asthma therapy. PPAR Res 2012: 809164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Drake MG, Kaufman EH, Fryer AD, Jacoby DB. The therapeutic potential of Toll-like receptor 7 stimulation in asthma. Inflamm Allergy Drug Targets 11: 484–491, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol 27: 5–14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Durrington HJ, Farrow SN, Loudon AS, Ray DW. The circadian clock and asthma. Thorax 69: 90–92, 2014. [DOI] [PubMed] [Google Scholar]
- 146.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis 148: 720–726, 1993. [DOI] [PubMed] [Google Scholar]
- 147.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 104: 1001–1006, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res 85: 2813–2823, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Eurlings IM, Dentener MA, Mercken EM, de Cabo R, Bracke KR, Vernooy JH, Wouters EF, Reynaert NL. A comparative study of matrix remodeling in chronic models for COPD; mechanistic insights into the role of TNF-α. Am J Physiol Lung Cell Mol Physiol 307: L557–L565, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fahy JV. Remodeling of the airway epithelium in asthma. Am J Respir Crit Care Med 164: S46–S51, 2001. [DOI] [PubMed] [Google Scholar]
- 151.Faisy C, Pinto FM, Le Guen M, Naline E, Grassin Delyle S, Sage E, Candenas ML, Devillier P. Airway response to acute mechanical stress in a human bronchial model of stretch. Crit Care 15: R208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Faisy C, Planquette B, Naline E, Risse PA, Frossard N, Fagon JY, Advenier C, Devillier P. Acid-induced modulation of airway basal tone and contractility: role of acid-sensing ion channels (ASICs) and TRPV1 receptor. Life Sci 81: 1094–1102, 2007. [DOI] [PubMed] [Google Scholar]
- 153.Faksh A, Britt RD Jr, Vogel ER, Thompson MA, Pandya HC, Martin RJ, Pabelick CM, Prakash YS. TLR3 activation increases chemokine expression in human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 310: L202–L211, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fatani SH. Biomarkers of oxidative stress in acute and chronic bronchial asthma. J Asthma 51: 578–584, 2014. [DOI] [PubMed] [Google Scholar]
- 155.Fayon M, Andrieux A, Bara I, Rebola M, Labbe A, Marthan R, Berger P. An age-wise comparison of human airway smooth muscle proliferative capacity. PLoS One 10: e0122446, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fediuk J, Gutsol A, Nolette N, Dakshinamurti S. Thromboxane-induced actin polymerization in hypoxic pulmonary artery is independent of Rho. Am J Physiol Lung Cell Mol Physiol 302: L13–L26, 2012. [DOI] [PubMed] [Google Scholar]
- 157.Fediuk J, Sikarwar AS, Nolette N, Dakshinamurti S. Thromboxane-induced actin polymerization in hypoxic neonatal pulmonary arterial myocytes involves Cdc42 signaling. Am J Physiol Lung Cell Mol Physiol 307: L877–L887, 2014. [DOI] [PubMed] [Google Scholar]
- 158.Finney BA, del Moral PM, Wilkinson WJ, Cayzac S, Cole M, Warburton D, Kemp PJ, Riccardi D. Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. J Physiol 586: 6007–6019, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fischer BM, Wong JK, Degan S, Kummarapurugu AB, Zheng S, Haridass P, Voynow JA. Increased expression of senescence markers in cystic fibrosis airways. Am J Physiol Lung Cell Mol Physiol 304: L394–L400, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fogli S, Pellegrini S, Adinolfi B, Mariotti V, Melissari E, Betti L, Fabbrini L, Giannaccini G, Lucacchini A, Bardelli C, Stefanelli F, Brunelleschi S, Breschi MC. Rosiglitazone reverses salbutamol-induced β2-adrenoceptor tolerance in airway smooth muscle. Br J Pharmacol 162: 378–391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fogli S, Stefanelli F, Picchianti L, Del Re M, Mey V, Bardelli C, Danesi R, Breschi MC. Synergistic interaction between PPAR ligands and salbutamol on human bronchial smooth muscle cell proliferation. Br J Pharmacol 168: 266–275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Foong RE, Bosco A, Troy NM, Gorman S, Hart PH, Kicic A, Zosky GR. Identification of genes differentially regulated by vitamin D deficiency that alter lung pathophysiology and inflammation in allergic airways disease. Am J Physiol Lung Cell Mol Physiol 311: L653–L663, 2016. [DOI] [PubMed] [Google Scholar]
- 163.Freund-Michel V, Bertrand C, Frossard N. TrkA signalling pathways in human airway smooth muscle cell proliferation. Cell Signal 18: 621–627, 2006. [DOI] [PubMed] [Google Scholar]
- 164.Gallos G, Gleason NR, Virag L, Zhang Y, Mizuta K, Whittington RA, Emala CW. Endogenous γ-aminobutyric acid modulates tonic guinea pig airway tone and propofol-induced airway smooth muscle relaxation. Anesthesiology 110: 748–758, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gallos G, Gleason NR, Zhang Y, Pak SW, Sonett JR, Yang J, Emala CW. Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. Am J Physiol Lung Cell Mol Physiol 295: L1040–L1047, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gallos G, Townsend E, Yim P, Virag L, Zhang Y, Xu D, Bacchetta M, Emala CW. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am J Physiol Lung Cell Mol Physiol 304: L191–L197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, Gerthoffer WT, Emala CW Sr. Targeting the restricted α-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol 302: L248–L256, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW Sr. Selective targeting of the α5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am J Physiol Lung Cell Mol Physiol 308: L931–L942, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J. Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am J Physiol Lung Cell Mol Physiol 308: L847–L853, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garantziotis S, Brezina M, Castelnuovo P, Drago L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am J Physiol Lung Cell Mol Physiol 310: L785–L795, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Garcia-Suarez O, Perez-Pinera P, Laura R, Germana A, Esteban I, Cabo R, Silos-Santiago I, Cobo JL, Vega JA. TrkB is necessary for the normal development of the lung. Respir Physiol Neurobiol 167: 281–291, 2009. [DOI] [PubMed] [Google Scholar]
- 172.Gaston B. The biochemistry of asthma. Biochim Biophys Acta 1810: 1017–1024, 2011. [DOI] [PubMed] [Google Scholar]
- 173.Gerthoffer WT, Solway J, Camoretti-Mercado B. Emerging targets for novel therapy of asthma. Curr Opin Pharmacol 13: 324–330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ghavami S, Sharma P, Yeganeh B, Ojo OO, Jha A, Mutawe MM, Kashani HH, Los MJ, Klonisch T, Unruh H, Halayko AJ. Airway mesenchymal cell death by mevalonate cascade inhibition: integration of autophagy, unfolded protein response and apoptosis focusing on Bcl2 family proteins. Biochim Biophys Acta 1843: 1259–1271, 2014. [DOI] [PubMed] [Google Scholar]
- 175.Ghosh MC, Gorantla V, Makena PS, Luellen C, Sinclair SE, Schwingshackl A, Waters CM. Insulin-like growth factor-I stimulates differentiation of ATII cells to ATI-like cells through activation of Wnt5a. Am J Physiol Lung Cell Mol Physiol 305: L222–L228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Giblin SP, Midwood KS. Tenascin-C: Form versus function. Cell Adh Migr 9: 48–82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gien J, Tseng N, Seedorf G, Roe G, Abman SH. Peroxisome proliferator activated receptor-γ-Rho-kinase interactions contribute to vascular remodeling after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L299–L308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Girodet PO, Allard B, Thumerel M, Begueret H, Dupin I, Ousova O, Lassalle R, Maurat E, Ozier A, Trian T, Marthan R, Berger P. Bronchial smooth muscle remodeling in nonsevere asthma. Am J Respir Crit Care Med 193: 627–633, 2016. [DOI] [PubMed] [Google Scholar]
- 179.Godbole MM, Rao G, Paul BN, Mohan V, Singh P, Khare D, Babu S, Nath A, Singh PK, Tiwari S. Prenatal iodine deficiency results in structurally and functionally immature lungs in neonatal rats. Am J Physiol Lung Cell Mol Physiol 302: L1037–L1043, 2012. [DOI] [PubMed] [Google Scholar]
- 180.Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor-β induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 34: 247–254, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goltzman D, Hendy GN. The calcium-sensing receptor in bone—mechanistic and therapeutic insights. Nat Rev Endocrinol 11: 298–307, 2015. [DOI] [PubMed] [Google Scholar]
- 182.Goncharova EA, Goncharov DA, Zhao H, Penn RB, Krymskaya VP, Panettieri RA Jr. β2-Adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am J Respir Cell Mol Biol 46: 48–54, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S, Chen YF. Hypoxia induces downregulation of PPAR-γ in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-β signaling. Am J Physiol Lung Cell Mol Physiol 301: L899–L907, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gonzalez-Arenas A, Agramonte-Hevia J. Sex steroid hormone effects in normal and pathologic conditions in lung physiology. Mini Rev Medicinal Chem 12: 1055–1062, 2012. [DOI] [PubMed] [Google Scholar]
- 185.Gonzalez Bosc LV, Plomaritas DR, Herbert LM, Giermakowska W, Browning C, Jernigan NL. ASIC1-mediated calcium entry stimulates NFATc3 nuclear translocation via PICK1 coupling in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 311: L48–L58, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T, Dard N, Planes C, Matthay MA, Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 306: L975–L985, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gopallawa I, Uhal BD. Angiotensin-(1–7)/mas inhibits apoptosis in alveolar epithelial cells through upregulation of MAP kinase phosphatase-2. Am J Physiol Lung Cell Mol Physiol 310: L240–L248, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gosens R, Grainge C. Bronchoconstriction and airway biology: potential impact and therapeutic opportunities. Chest 147: 798–803, 2015. [DOI] [PubMed] [Google Scholar]
- 189.Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med 8: 741–753, 2008. [DOI] [PubMed] [Google Scholar]
- 190.Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006. [DOI] [PubMed] [Google Scholar]
- 191.Goss AM, Tian Y, Cheng L, Yang J, Zhou D, Cohen ED, Morrisey EE. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol 356: 541–552, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gottlieb PA, Sachs F. Piezo1: properties of a cation selective mechanical channel. Channels (Austin) 6: 214–219, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther 140: 290–305, 2013. [DOI] [PubMed] [Google Scholar]
- 194.Grasemann H, Dhaliwal R, Ivanovska J, Kantores C, McNamara PJ, Scott JA, Belik J, Jankov RP. Arginase inhibition prevents bleomycin-induced pulmonary hypertension, vascular remodeling, and collagen deposition in neonatal rat lungs. Am J Physiol Lung Cell Mol Physiol 308: L503–L510, 2015. [DOI] [PubMed] [Google Scholar]
- 195.Gregory DJ, Kobzik L. Influenza lung injury: mechanisms and therapeutic opportunities. Am J Physiol Lung Cell Mol Physiol 309: L1041–L1046, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guedes AG, Deshpande DA, Dileepan M, Walseth TF, Panettieri RA Jr, Subramanian S, Kannan MS. CD38 and airway hyper-responsiveness: studies on human airway smooth muscle cells and mouse models. Can J Physiol Pharmacol 93: 145–153, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guedes AG, Jude JA, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. Airway responsiveness in CD38-deficient mice in allergic airway disease: studies with bone marrow chimeras. Am J Physiol Lung Cell Mol Physiol 308: L485–L493, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guo Q, Huang JA, Yamamura A, Yamamura H, Zimnicka AM, Fernandez R, Yuan JX. Inhibition of the Ca2+-sensing receptor rescues pulmonary hypertension in rats and mice. Hypertens Res 37: 116–124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Guo Q, Li H, Liu J, Xu L, Yang L, Sun Z, Zhou B. Tunicamycin aggravates endoplasmic reticulum stress and airway inflammation via PERK-ATF4-CHOP signaling in a murine model of neutrophilic asthma. J Asthma: 0, 2016. [DOI] [PubMed] [Google Scholar]
- 200.Haggerty CL, Ness RB, Kelsey S, Waterer GW. The impact of estrogen and progesterone on asthma. Annal Allergy Asthma Immunol 90: 284–291, 2003. [DOI] [PubMed] [Google Scholar]
- 201.Hai CM. Airway smooth muscle cell as therapeutic target of inflammation. Curr Med Chem 14: 67–76, 2007. [DOI] [PubMed] [Google Scholar]
- 202.Haley KJ, Lasky-Su J, Manoli SE, Smith LA, Shahsafaei A, Weiss ST, Tantisira K. RUNX transcription factors: association with pediatric asthma and modulated by maternal smoking. Am J Physiol Lung Cell Mol Physiol 301: L693–L701, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hallstrand TS, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WR. Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS One 5: e8583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hara H, Araya J, Ito S, Kobayashi K, Takasaka N, Yoshii Y, Wakui H, Kojima J, Shimizu K, Numata T, Kawaishi M, Kamiya N, Odaka M, Morikawa T, Kaneko Y, Nakayama K, Kuwano K. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol 305: L737–L746, 2013. [DOI] [PubMed] [Google Scholar]
- 205.Harada T, Yamasaki A, Chikumi H, Hashimoto K, Okazaki R, Takata M, Fukushima T, Watanabe M, Kurai J, Halayko AJ, Shimizu E. γ-Tocotrienol reduces human airway smooth muscle cell proliferation and migration. Pulm Pharmacol Ther 32: 45–52, 2015. [DOI] [PubMed] [Google Scholar]
- 206.Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L711–L719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hashimoto Y, Sugiura H, Togo S, Koarai A, Abe K, Yamada M, Ichikawa T, Kikuchi T, Numakura T, Onodera K, Tanaka R, Sato K, Yanagisawa S, Okazaki T, Tamada T, Hoshikawa Y, Okada Y, Ichinose M. 27-Hydroxycholesterol accelerates cellular senescence in human lung resident cells. Am J Physiol Lung Cell Mol Physiol 310: L1028–L1041, 2016. [DOI] [PubMed] [Google Scholar]
- 208.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC. Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol Lung Cell Mol Physiol 304: L287–L297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol 35: 1166–1178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hawkins A, Guttentag SH, Deterding R, Funkhouser WK, Goralski JL, Chatterjee S, Mulugeta S, Beers MF. A non-BRICHOS SFTPC mutant (SP-CI73T) linked to interstitial lung disease promotes a late block in macroautophagy disrupting cellular proteostasis and mitophagy. Am J Physiol Lung Cell Mol Physiol 308: L33–L47, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Heijink IH, de Bruin HG, Dennebos R, Jonker MR, Noordhoek JA, Brandsma CA, van den Berge M, Postma DS. Cigarette smoke-induced epithelial expression of WNT-5B: implications for COPD. Eur Respir J 48: 504–515, 2016. [DOI] [PubMed] [Google Scholar]
- 212.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem 286: 17435–17444, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy 33: 1457–1463, 2003. [DOI] [PubMed] [Google Scholar]
- 214.Henderson AJ, Warner JO. Fetal origins of asthma. Semin Fetal Neonatal Med 17: 82–91, 2012. [DOI] [PubMed] [Google Scholar]
- 215.Hernandez JM, Janssen LJ. Revisiting the usefulness of thromboxane-A2 modulation in the treatment of bronchoconstriction in asthma. Can J Physiol Pharmacol 93: 111–117, 2015. [DOI] [PubMed] [Google Scholar]
- 216.Hershenson MB, Chung Y. Exposure of airway smooth muscle cells to cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol 307: L345, 2014. [DOI] [PubMed] [Google Scholar]
- 217.Hillman NH, Nitsos I, Berry C, Pillow JJ, Kallapur SG, Jobe AH. Positive end-expiratory pressure and surfactant decrease lung injury during initiation of ventilation in fetal sheep. Am J Physiol Lung Cell Mol Physiol 301: L712–L720, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hillman NH, Polglase GR, Pillow JJ, Saito M, Kallapur SG, Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol 300: L232–L241, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hiram R, Rizcallah E, Marouan S, Sirois C, Sirois M, Morin C, Fortin S, Rousseau E. Resolvin E1 normalizes contractility, Ca2+ sensitivity and smooth muscle cell migration rate in TNF-α- and IL-6-pretreated human pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 309: L776–L788, 2015. [DOI] [PubMed] [Google Scholar]
- 220.Hirota JA, Hackett TL, Inman MD, Knight DA. Modeling asthma in mice: what have we learned about the airway epithelium? Am J Respir Cell Mol Biol 44: 431–438, 2011. [DOI] [PubMed] [Google Scholar]
- 221.Hirota JA, Im H, Rahman MM, Rumzhum NN, Manetsch M, Pascoe CD, Bunge K, Alkhouri H, Oliver BG, Ammit AJ. The nucleotide-binding domain and leucine-rich repeat protein-3 inflammasome is not activated in airway smooth muscle upon toll-like receptor-2 ligation. Am J Respir Cell Mol Biol 49: 517–524, 2013. [DOI] [PubMed] [Google Scholar]
- 222.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 144: 1026–1032, 2013. [DOI] [PubMed] [Google Scholar]
- 223.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 30: 114–133, 2007. [DOI] [PubMed] [Google Scholar]
- 224.Hirota S, Janssen LJ. Store-refilling involves both L-type calcium channels and reverse-mode sodium-calcium exchange in airway smooth muscle. Eur Respir J 30: 269–278, 2007. [DOI] [PubMed] [Google Scholar]
- 225.Hirst SJ. Regulation of airway smooth muscle cell immunomodulatory function: role in asthma. Respir Physiol Neurobiol 137: 309–326, 2003. [DOI] [PubMed] [Google Scholar]
- 226.Hocking DC. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest 122: 275S–278S, 2002. [DOI] [PubMed] [Google Scholar]
- 227.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME, Anathy V. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir Res 14: 141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, Schwartz DA. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest 118: 3462–3469, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 229.Hou X, Wan H, Ai X, Shi Y, Ni Y, Tang W, Shi G. Histone deacetylase inhibitor regulates the balance of Th17/Treg in allergic asthma. Clin Respir J 10: 371–379, 2016. [DOI] [PubMed] [Google Scholar]
- 230.Howarth PH, Knox AJ, Amrani Y, Tliba O, Panettieri RA Jr, Johnson M. Synthetic responses in airway smooth muscle. J Allergy Clin Immunol 114: S32–S50, 2004. [DOI] [PubMed] [Google Scholar]
- 231.Hsu KJ, Turvey SE. Functional analysis of the impact of ORMDL3 expression on inflammation and activation of the unfolded protein response in human airway epithelial cells. Allergy Asthma Clin Immunol 9: 4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hu R, Pan W, Fedulov AV, Jester W, Jones MR, Weiss ST, Panettieri RA Jr, Tantisira K, Lu Q. MicroRNA-10a controls airway smooth muscle cell proliferation via direct targeting of the PI3 kinase pathway. FASEB J 28: 2347–2357, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Huber HL, Koessler KK. The pathology of bronchial asthma. Arch Intern Med 30: 689–760, 1922. [Google Scholar]
- 234.Huleihel L, Ben-Yehudah A, Milosevic J, Yu G, Pandit K, Sakamoto K, Yousef H, LeJeune M, Coon TA, Redinger CJ, Chensny L, Manor E, Schatten G, Kaminski N. Let-7d microRNA affects mesenchymal phenotypic properties of lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 306: L534–L542, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ichimaru Y, Krimmer DI, Burgess JK, Black JL, Oliver BG. TGF-β enhances deposition of perlecan from COPD airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L325–L333, 2012. [DOI] [PubMed] [Google Scholar]
- 236.Ichimaru Y, Krimmer DI, Burgess JK, Black JL, Oliver BG. TGF-β enhances deposition of perlecan from COPD airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L325–L333, 2012. [DOI] [PubMed] [Google Scholar]
- 237.Ichimonji I, Tomura H, Mogi C, Sato K, Aoki H, Hisada T, Dobashi K, Ishizuka T, Mori M, Okajima F. Extracellular acidification stimulates IL-6 production and Ca2+ mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L567–L577, 2010. [DOI] [PubMed] [Google Scholar]
- 238.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am J Physiol Lung Cell Mol Physiol 308: L1178–L1188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol 46: 446–453, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.James A, Mauad T, Abramson M, Green F. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med 186: 568, 2012. [DOI] [PubMed] [Google Scholar]
- 241.James ML, Ross AC, Nicola T, Steele C, Ambalavanan N. VARA attenuates hyperoxia-induced impaired alveolar development and lung function in newborn mice. Am J Physiol Lung Cell Mol Physiol 304: L803–L812, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Janssen LJ. Airway smooth muscle electrophysiology in a state of flux? Am J Physiol Lung Cell Mol Physiol 302: L730–L732, 2012. [DOI] [PubMed] [Google Scholar]
- 243.Janssen LJ. Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol 282: L1161–L1178, 2002. [DOI] [PubMed] [Google Scholar]
- 244.Janssen LJ. T-type and L-type Ca2+ currents in canine bronchial smooth muscle: characterization and physiological roles. Am J Physiol Cell Physiol 272: C1757–C1765, 1997. [DOI] [PubMed] [Google Scholar]
- 245.Janssen LJ, Daniel EE. Pre- and postjunctional effects of a thromboxane mimetic in canine bronchi. Am J Physiol Lung Cell Mol Physiol 261: L271–L276, 1991. [DOI] [PubMed] [Google Scholar]
- 246.Janssen LJ, Hague C, Nana R. Ionic mechanisms underlying electrical slow waves in canine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 275: L516–L523, 1998. [DOI] [PubMed] [Google Scholar]
- 247.Janssen LJ, McGrogan I, Wattie J, O'Byrne PM, Daniel EE. Myogenic and neurogenic mechanisms and arachidonate metabolites in bronchial muscle response to allergen. Am J Physiol Lung Cell Mol Physiol 273: L1118–L1125, 1997. [DOI] [PubMed] [Google Scholar]
- 248.Januskevicius A, Vaitkiene S, Gosens R, Janulaityte I, Hoppenot D, Sakalauskas R, Malakauskas K. Eosinophils enhance WNT-5a and TGF-β1 genes expression in airway smooth muscle cells and promote their proliferation by increased extracellular matrix proteins production in asthma. BMC Pulm Med 16: 94, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jesudason EC. Airway smooth muscle: an architect of the lung? Thorax 64: 541–545, 2009. [DOI] [PubMed] [Google Scholar]
- 250.Jesudason EC, Keshet E, Warburton D. Entrained pulmonary clocks: epithelium and vasculature keeping pace. Am J Physiol Lung Cell Mol Physiol 299: L453–L454, 2010. [DOI] [PubMed] [Google Scholar]
- 251.Jha A, Sharma P, Anaparti V, Ryu MH, Halayko AJ. A role for transient receptor potential ankyrin 1 cation channel (TRPA1) in airway hyper-responsiveness? Can J Physiol Pharmacol 93: 171–176, 2015. [DOI] [PubMed] [Google Scholar]
- 252.Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287: L272–L278, 2004. [DOI] [PubMed] [Google Scholar]
- 253.Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol 305: L844–L855, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jin Y, Chen B, Tipple TE, Nelin LD. Arginase II is a target of miR-17-5p and regulates miR-17-5p expression in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 307: L197–L204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Johnson JR, Folestad E, Rowley JE, Noll EM, Walker SA, Lloyd CM, Rankin SM, Pietras K, Eriksson U, Fuxe J. Pericytes contribute to airway remodeling in a mouse model of chronic allergic asthma. Am J Physiol Lung Cell Mol Physiol 308: L658–L671, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Johnson PR. Role of human airway smooth muscle in altered extracellular matrix production in asthma. Clin Exp Pharmacol Physiol 28: 233–236, 2001. [DOI] [PubMed] [Google Scholar]
- 257.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med 164: 474–477, 2001. [DOI] [PubMed] [Google Scholar]
- 258.Jose C, Melser S, Benard G, Rossignol R. Mitoplasticity: adaptation biology of the mitochondrion to the cellular redox state in physiology and carcinogenesis. Antioxid Redox Signal 18: 808–849, 2013. [DOI] [PubMed] [Google Scholar]
- 259.Jude JA, Dileepan M, Subramanian S, Solway J, Panettieri RA Jr,Walseth TF, Kannan MS. miR-140-3p regulation of TNF-α-induced CD38 expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L460–L468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jude JA, Wylam ME, Walseth TF, Kannan MS. Calcium signaling in airway smooth muscle. Proc Am Thor Soc 5: 15–22, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kalinowski A, Ueki I, Min-Oo G, Ballon-Landa E, Knoff D, Galen B, Lanier LL, Nadel JA, Koff JL. EGFR activation suppresses respiratory virus-induced IRF1-dependent CXCL10 production. Am J Physiol Lung Cell Mol Physiol 307: L186–L196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kanaji N, Basma H, Nelson A, Farid M, Sato T, Nakanishi M, Wang X, Michalski J, Li Y, Gunji Y, Feghali-Bostwick C, Liu X, Rennard SI. Fibroblasts that resist cigarette smoke-induced senescence acquire profibrotic phenotypes. Am J Physiol Lung Cell Mol Physiol 307: L364–L373, 2014. [DOI] [PubMed] [Google Scholar]
- 263.Kandhi S, Qin J, Froogh G, Jiang H, Luo M, Wolin MS, Huang A, Sun D. EET-dependent potentiation of pulmonary arterial pressure: sex-different regulation of soluble epoxide hydrolase. Am J Physiol Lung Cell Mol Physiol 309: L1478–L1486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kaphalia L, Kalita M, Kaphalia BS, Calhoun WJ. Effects of acute ethanol exposure on cytokine production by primary airway smooth muscle cells. Toxicol Appl Pharmacol 292: 85–93, 2016. [DOI] [PubMed] [Google Scholar]
- 265.Kasahara DI, Mathews JA, Park CY, Cho Y, Hunt G, Wurmbrand AP, Liao JK, Shore SA. ROCK insufficiency attenuates ozone-induced airway hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol 309: L736–L746, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kaur D, Hollins F, Saunders R, Woodman L, Sutcliffe A, Cruse G, Bradding P, Brightling C. Airway smooth muscle proliferation and survival is not modulated by mast cells. Clin Exp Allergy 40: 279–288, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kelly EA, Jarjour NN. Role of matrix metalloproteinases in asthma. Curr Opin Pulm Med 9: 28–33, 2003. [DOI] [PubMed] [Google Scholar]
- 268.Kerley CP, Elnazir B, Faul J, Cormican L. Vitamin D as an adjunctive therapy in asthma. Part 1: a review of potential mechanisms. Pulm Pharmacol Ther 32: 60–74, 2015. [DOI] [PubMed] [Google Scholar]
- 269.Keslacy S, Tliba O, Baidouri H, Amrani Y. Inhibition of tumor necrosis factor-α-inducible inflammatory genes by interferon-γ is associated with altered nuclear factor-κB transactivation and enhanced histone deacetylase activity. Mol Pharmacol 71: 609–618, 2007. [DOI] [PubMed] [Google Scholar]
- 270.Khan MA. Inflammation signals airway smooth muscle cell proliferation in asthma pathogenesis. Multidiscip Respir Med 8: 11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kichko TI, Kobal G, Reeh PW. Cigarette smoke has sensory effects through nicotinic and TRPA1 but not TRPV1 receptors on the isolated mouse trachea and larynx. Am J Physiol Lung Cell Mol Physiol 309: L812–L820, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kim D, Pauer SH, Yong HM, An SS, Liggett SB. β2-Adrenergic receptors chaperone trapped bitter taste receptor 14 to the cell surface as a heterodimer and exert unidirectional desensitization of taste receptor function. J Biol Chem 291: 17616–17628 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res 10: 49, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kim SR, Kim DI, Kang MR, Lee KS, Park SY, Jeong JS, Lee YC. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor κB activation. J Allergy Clin Immunol 132: 1397–1408, 2013. [DOI] [PubMed] [Google Scholar]
- 275.Kim SR, Lee YC. Endoplasmic reticulum stress and the related signaling networks in severe asthma. Allergy Asthma Immunol Res 7: 106–117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kim SY, Lee JH, Kim HJ, Park MK, Huh JW, Ro JY, Oh YM, Lee SD, Lee YS. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol 302: L891–L908, 2012. [DOI] [PubMed] [Google Scholar]
- 277.Kistemaker LE, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci 36: 164–171, 2015. [DOI] [PubMed] [Google Scholar]
- 278.Kizub IV, Lakhkar A, Dhagia V, Joshi SR, Jiang H, Wolin MS, Falck JR, Koduru SR, Errabelli R, Jacobs ER, Schwartzman ML, Gupte SA. Involvement of gap junctions between smooth muscle cells in sustained hypoxic pulmonary vasoconstriction development: a potential role for 15-HETE and 20-HETE. Am J Physiol Lung Cell Mol Physiol 310: L772–L783, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, Papakonstantinou E, Roth M. Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur Respir J 34: 616–628, 2009. [DOI] [PubMed] [Google Scholar]
- 280.Knobloch J, Wahl C, Feldmann M, Jungck D, Strauch J, Stoelben E, Koch A. Resveratrol attenuates the release of inflammatory cytokines from human bronchial smooth muscle cells exposed to lipoteichoic acid in chronic obstructive pulmonary disease. Basic Clin Pharmacol Toxicol 114: 202–209, 2014. [DOI] [PubMed] [Google Scholar]
- 281.Konigshoff M, Uhl F, Gosens R. From molecule to man: integrating molecular biology with whole organ physiology in studying respiratory disease. Pulm Pharmacol Ther 24: 466–470, 2011. [DOI] [PubMed] [Google Scholar]
- 282.Koopmans T, Anaparti V, Castro-Piedras I, Yarova P, Irechukwu N, Nelson C, Perez-Zoghbi J, Tan X, Ward JP, Wright DB. Ca2+ handling and sensitivity in airway smooth muscle: emerging concepts for mechanistic understanding and therapeutic targeting. Pulm Pharmacol Ther 29: 108–120, 2014. [DOI] [PubMed] [Google Scholar]
- 283.Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol 149: 1083–1091, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Sexual dimorphism in airway responsiveness to sex hormones in rabbits. Am J Physiol Lung Cell Mol Physiol 293: L516, 2007. [DOI] [PubMed] [Google Scholar]
- 285.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18: 832–872, 1997. [DOI] [PubMed] [Google Scholar]
- 286.Koziol-White CJ, Panettieri RA Jr. Airway smooth muscle and immunomodulation in acute exacerbations of airway disease. Immunol Rev 242: 178–185, 2011. [DOI] [PubMed] [Google Scholar]
- 287.Krauss-Etschmann S, Meyer KF, Dehmel S, Hylkema MN. Inter- and transgenerational epigenetic inheritance: evidence in asthma and COPD? Clin Epigenetics 7: 53, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kroon AA, Delriccio V, Tseu I, Kavanagh BP, Post M. Mechanical ventilation-induced apoptosis in newborn rat lung is mediated via FasL/Fas pathway. Am J Physiol Lung Cell Mol Physiol 305: L795–L804, 2013. [DOI] [PubMed] [Google Scholar]
- 289.Kuhn AR, Schlauch K, Lao R, Halayko AJ, Gerthoffer WT, Singer CA. MicroRNA expression in human airway smooth muscle cells: role of miR-25 in regulation of airway smooth muscle phenotype. Am J Respir Cell Mol Biol 42: 506–513, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kumar RK, Foster PS. Are mouse models of asthma appropriate for investigating the pathogenesis of airway hyper-responsiveness? Front Physiol 3: 312, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kumawat K, Koopmans T, Gosens R. β-Catenin as a regulator and therapeutic target for asthmatic airway remodeling. Expert Opin Ther Targets 18: 1023–1034, 2014. [DOI] [PubMed] [Google Scholar]
- 292.Kumawat K, Menzen MH, Bos IS, Baarsma HA, Borger P, Roth M, Tamm M, Halayko AJ, Simoons M, Prins A, Postma DS, Schmidt M, Gosens R. Noncanonical WNT-5A signaling regulates TGF-β-induced extracellular matrix production by airway smooth muscle cells. FASEB J 27: 1631–1643, 2013. [DOI] [PubMed] [Google Scholar]
- 293.Kume H, Fukunaga K, Oguma T. Research and development of bronchodilators for asthma and COPD with a focus on G protein/KCa channel linkage and β2-adrenergic intrinsic efficacy. Pharmacol Therapeut 156: 75–89, 2015. [DOI] [PubMed] [Google Scholar]
- 294.Kuo KH, Herrera AM, Seow CY. Ultrastructure of airway smooth muscle. Respir Physiol Neurobiol 137: 197–208, 2003. [DOI] [PubMed] [Google Scholar]
- 295.Kwak HJ, Park DW, Seo JY, Moon JY, Kim TH, Sohn JW, Shin DH, Yoon HJ, Park SS, Kim SH. The Wnt/β-catenin signaling pathway regulates the development of airway remodeling in patients with asthma. Exp Mol Medi 47: e198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lagente V, Boichot E. Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J Mol Cell Cardiol 48: 440–444, 2010. [DOI] [PubMed] [Google Scholar]
- 297.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185: 965–980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lambert JA, Song W. Ozone-induced airway hyperresponsiveness: roles of ROCK isoforms. Am J Physiol Lung Cell Mol Physiol 309: L1394–L1397, 2015. [DOI] [PubMed] [Google Scholar]
- 299.Larner-Svensson HM, Williams AE, Tsitsiou E, Perry MM, Jiang X, Chung KF, Lindsay MA. Pharmacological studies of the mechanism and function of interleukin-1β-induced miRNA-146a expression in primary human airway smooth muscle. Respir Res 11: 68, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Larsson OJ, Manson ML, Starkhammar M, Fuchs B, Adner M, Kumlien Georen S, Cardell LO. The TLR7 agonist imiquimod induces bronchodilation via a nonneuronal TLR7-independent mechanism: a possible role for quinoline in airway dilation. Am J Physiol Lung Cell Mol Physiol 310: L1121–L1129, 2016. [DOI] [PubMed] [Google Scholar]
- 301.Lauer ME, Cheng G, Swaidani S, Aronica MA, Weigel PH, Hascall VC. Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J Biol Chem 288: 423–431, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lauer ME, Fulop C, Mukhopadhyay D, Comhair S, Erzurum SC, Hascall VC. Airway smooth muscle cells synthesize hyaluronan cable structures independent of inter-α-inhibitor heavy chain attachment. J Biol Chem 284: 5313–5323, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lauer ME, Majors AK, Comhair S, Ruple LM, Matuska B, Subramanian A, Farver C, Dworski R, Grandon D, Laskowski D, Dweik RA, Erzurum SC, Hascall VC, Aronica MA. Hyaluronan and its heavy chain modification in asthma severity and experimental asthma exacerbation. J Biol Chem 290: 23124–23134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, Holtzman MJ, Whitsett JA, Hershey GK. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol 300: L414–L421, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lee CW, Lin CC, Lin WN, Liang KC, Luo SF, Wu CB, Wang SW, Yang CM. TNF-α induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-κB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L799–L812, 2007. [DOI] [PubMed] [Google Scholar]
- 307.Lee KS, Jeong JS, Kim SR, Cho SH, Kolliputi N, Ko YH, Lee KB, Park SC, Park HJ, Lee YC. Phosphoinositide 3-kinase-δ regulates fungus-induced allergic lung inflammation through endoplasmic reticulum stress. Thorax 71: 52–63, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lembrechts R, Brouns I, Schnorbusch K, Pintelon I, Kemp PJ, Timmermans JP, Riccardi D, Adriaensen D. Functional expression of the multimodal extracellular calcium-sensing receptor in pulmonary neuroendocrine cells. J Cell Sci 126: 4490–4501, 2013. [DOI] [PubMed] [Google Scholar]
- 309.Leslie FM. Multigenerational epigenetic effects of nicotine on lung function. BMC Med 11: 27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Leuchte HH, Prechtl C, Callegari J, Meis T, Haziraj S, Bevec D, Behr J. Augmentation of the effects of vasoactive intestinal peptide aerosol on pulmonary hypertension via coapplication of a neutral endopeptidase 24.11 inhibitor. Am J Physiol Lung Cell Mol Physiol 308: L563–L568, 2015. [DOI] [PubMed] [Google Scholar]
- 311.Li P, Li YL, Li ZY, Wu YN, Zhang CC, A X, Wang CX, Shi HT, Hui MZ, Xie B, Ahmed M, Du J. Cross talk between vascular smooth muscle cells and monocytes through interleukin-1β/interleukin-18 signaling promotes vein graft thickening. Arterioscler Thromb Vasc Biol 34: 2001–2011, 2014. [DOI] [PubMed] [Google Scholar]
- 312.Li X, He Y, Xu Y, Huang X, Liu J, Xie M, Liu X. KLF5 mediates vascular remodeling via HIF-1α in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L299–L310, 2016. [DOI] [PubMed] [Google Scholar]
- 313.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM. Interleukin-1β induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-κB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol 211: 759–770, 2007. [DOI] [PubMed] [Google Scholar]
- 314.Liao G, Panettieri RA, Tang DD. MicroRNA-203 negatively regulates c-Abl, ERK1/2 phosphorylation, and proliferation in smooth muscle cells. Physiol Rep 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lieu TM, Myers AC, Meeker S, Undem BJ. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 302: L941–L948, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lin CC, Lee IT, Wu WL, Lin WN, Yang CM. Adenosine triphosphate regulates NADPH oxidase activity leading to hydrogen peroxide production and COX-2/PGE2 expression in A549 cells. Am J Physiol Lung Cell Mol Physiol 303: L401–L412, 2012. [DOI] [PubMed] [Google Scholar]
- 317.Lin CC, Lin WN, Hou WC, Hsiao LD, Yang CM. Endothelin-1 induces VCAM-1 expression-mediated inflammation via receptor tyrosine kinases and Elk/p300 in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L211–L225, 2015. [DOI] [PubMed] [Google Scholar]
- 318.Liu B, Peel SE, Fox J, Hall IP. Reverse mode Na+/Ca2+ exchange mediated by STIM1 contributes to Ca2+ influx in airway smooth muscle following agonist stimulation. Respir Res 11: 168, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Liu C, Zuo J, Pertens E, Helli PB, Janssen LJ. Regulation of Rho/ROCK signaling in airway smooth muscle by membrane potential and [Ca2+]i. Am J Physiol Lung Cell Mol Physiol 289: L574–L582, 2005. [DOI] [PubMed] [Google Scholar]
- 320.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Liu J, Sakurai R, Rehan VK. PPAR-γ agonist rosiglitazone reverses perinatal nicotine exposure-induced asthma in rat offspring. Am J Physiol Lung Cell Mol Physiol 308: L788–L796, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci USA 106: 11418–11423, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Liu QH, Zheng YM, Wang YX. Two distinct signaling pathways for regulation of spontaneous local Ca2+ release by phospholipase C in airway smooth muscle cells. Pflügers Arch 453: 531–541, 2007. [DOI] [PubMed] [Google Scholar]
- 324.Liu Y, Sun X, Wu Y, Fang P, Shi H, Xu J, Li M. Effects of miRNA-145 on airway smooth muscle cells function. Mol Cell Biochem 409: 135–143, 2015. [DOI] [PubMed] [Google Scholar]
- 325.Liu Y, Yang K, Sun X, Fang P, Shi H, Xu J, Xie M, Li M. MiR-138 suppresses airway smooth muscle cell proliferation through the PI3K/AKT signaling pathway by targeting PDK1. Exp Lung Res 41: 363–369, 2015. [DOI] [PubMed] [Google Scholar]
- 326.Lottes RG, Newton DA, Spyropoulos DD, Baatz JE. Alveolar type II cells maintain bioenergetic homeostasis in hypoxia through metabolic and molecular adaptation. Am J Physiol Lung Cell Mol Physiol 306: L947–L955, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lottes RG, Newton DA, Spyropoulos DD, Baatz JE. Lactate as substrate for mitochondrial respiration in alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 308: L953–L961, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lu Q, Sakhatskyy P, Newton J, Shamirian P, Hsiao V, Curren S, Gabino Miranda GA, Pedroza M, Blackburn MR, Rounds S. Sustained adenosine exposure causes lung endothelial apoptosis: a possible contributor to cigarette smoke-induced endothelial apoptosis and lung injury. Am J Physiol Lung Cell Mol Physiol 304: L361–L370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lykkedegn S, Sorensen GL, Beck-Nielsen SS, Christesen HT. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol 308: L587–L602, 2015. [DOI] [PubMed] [Google Scholar]
- 330.Ma L, Brown M, Kogut P, Serban K, Li X, McConville J, Chen B, Bentley JK, Hershenson MB, Dulin N, Solway J, Camoretti-Mercado B. Akt activation induces hypertrophy without contractile phenotypic maturation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 300: L701–L709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Maarsingh H, Zaagsma J, Meurs H. Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br J Pharmacol 158: 652–664, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci 29: 421–429, 2008. [DOI] [PubMed] [Google Scholar]
- 334.Maher SA, Dubuis ED, Belvisi MG. G-protein coupled receptors regulating cough. Curr Opin Pharmacol 11: 248–253, 2011. [DOI] [PubMed] [Google Scholar]
- 335.Mahn K, Ojo OO, Chadwick G, Aaronson PI, Ward JP, Lee TH. Ca2+ homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 65: 547–552, 2010. [DOI] [PubMed] [Google Scholar]
- 336.Maile R, Jones S, Pan Y, Zhou H, Jaspers I, Peden DB, Cairns BA, Noah TL. Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. Am J Physiol Lung Cell Mol Physiol 308: L855–L860, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Makhija L, Krishnan V, Rehman R, Chakraborty S, Maity S, Mabalirajan U, Chakraborty K, Ghosh B, Agrawal A. Chemical chaperones mitigate experimental asthma by attenuating endoplasmic reticulum stress. Am J Respir Cell Mol Biol 50: 923–931, 2014. [DOI] [PubMed] [Google Scholar]
- 338.Makinde T, Murphy RF, Agrawal DK. Immunomodulatory role of vascular endothelial growth factor and angiopoietin-1 in airway remodeling. Curr Mol Med 6: 831–841, 2006. [DOI] [PubMed] [Google Scholar]
- 339.Mandell E, Seedorf G, Gien J, Abman SH. Vitamin D treatment improves survival and infant lung structure after intra-amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 306: L420–L428, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Manetsch M, Seidel P, Heintz U, Che W, Hughes JM, Ge Q, Sukkar MB, Ammit AJ. TLR2 ligand engagement upregulates airway smooth muscle TNFα-induced cytokine production. Am J Physiol Lung Cell Mol Physiol 302: L838–L845, 2012. [DOI] [PubMed] [Google Scholar]
- 341.Mannam P, Shinn AS, Srivastava A, Neamu RF, Walker WE, Bohanon M, Merkel J, Kang MJ, Dela Cruz CS, Ahasic AM, Pisani MA, Trentalange M, West AP, Shadel GS, Elias JA, Lee PJ. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol 306: L604–L619, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol 31: 3–10, 2013. [PubMed] [Google Scholar]
- 343.Marchica CL, Pinelli V, Borges M, Zummer J, Narayanan V, Iozzo RV, Ludwig MS. A role for decorin in a murine model of allergen-induced asthma. Am J Physiol Lung Cell Mol Physiol 300: L863–L873, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Marhl M, Noble D, Roux E. Modeling of molecular and cellular mechanisms involved in Ca2+ signal encoding in airway myocytes. Cell Biochem Biophys 46: 285–302, 2006. [DOI] [PubMed] [Google Scholar]
- 345.Martin YN, Manlove L, Dong J, Carey WA, Thompson MA, Pabelick CM, Pandya HC, Martin RJ, Wigle DA, Prakash YS. Hyperoxia-induced changes in estradiol metabolism in postnatal airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 308: L141–L146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Matera MG, Calzetta L, Cazzola M. TNF-α inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm Pharmacol Ther 23: 121–128, 2010. [DOI] [PubMed] [Google Scholar]
- 347.Matsuzaki S, Ishizuka T, Yamada H, Kamide Y, Hisada T, Ichimonji I, Aoki H, Yatomi M, Komachi M, Tsurumaki H, Ono A, Koga Y, Dobashi K, Mogi C, Sato K, Tomura H, Mori M, Okajima F. Extracellular acidification induces connective tissue growth factor production through proton-sensing receptor OGR1 in human airway smooth muscle cells. Biochem Biophys Res Commun 413: 499–503, 2011. [DOI] [PubMed] [Google Scholar]
- 348.Matteis M, Polverino F, Spaziano G, Roviezzo F, Santoriello C, Sullo N, Bucci MR, Rossi F, Polverino M, Owen CA, D'Agostino B. Effects of sex hormones on bronchial reactivity during the menstrual cycle. BMC Pulm Med 14: 108, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mayor RS, Finch KE, Zehr J, Morselli E, Neinast MD, Frank AP, Hahner LD, Wang J, Rakheja D, Palmer BF, Rosenfeld CR, Savani RC, Clegg DJ. Maternal high-fat diet is associated with impaired fetal lung development. Am J Physiol Lung Cell Mol Physiol 309: L360–L368, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.McAlexander MA, Luttmann MA, Hunsberger GE, Undem BJ. Transient receptor potential vanilloid 4 activation constricts the human bronchus via the release of cysteinyl leukotrienes. J Pharmacol Exp Therapeut 349: 118–125, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.McAnulty RJ. Models and approaches to understand the role of airway remodelling in disease. Pulm Pharmacol Ther 24: 478–486, 2011. [DOI] [PubMed] [Google Scholar]
- 352.McCuaig S, Martin JG. How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: implications for airway hyper-responsiveness and asthma in cystic fibrosis. Lancet Respir Med 1: 137–147, 2013. [DOI] [PubMed] [Google Scholar]
- 353.McGovern T, Risse PA, Tsuchiya K, Hassan M, Frigola G, Martin JG. LTD4 induces HB-EGF-dependent CXCL8 release through EGFR activation in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 299: L808–L815, 2010. [DOI] [PubMed] [Google Scholar]
- 354.McMillan DH, Woeller CF, Thatcher TH, Spinelli SL, Maggirwar SB, Sime PJ, Phipps RP. Attenuation of inflammatory mediator production by the NF-κB member RelB is mediated by microRNA-146a in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 304: L774–L781, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.McWhinnie R, Pechkovsky DV, Zhou D, Lane D, Halayko AJ, Knight DA, Bai TR. Endothelin-1 induces hypertrophy and inhibits apoptosis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L278–L286, 2007. [DOI] [PubMed] [Google Scholar]
- 356.Melboucy-Belkhir S, Pradere P, Tadbiri S, Habib S, Bacrot A, Brayer S, Mari B, Besnard V, Mailleux A, Guenther A, Castier Y, Mal H, Crestani B, Plantier L. Forkhead Box F1 represses cell growth and inhibits COL1 and ARPC2 expression in lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol 307: L838–L847, 2014. [DOI] [PubMed] [Google Scholar]
- 357.Meuten T, Hickey A, Franklin K, Grossi B, Tobias J, Newman DR, Jennings SH, Correa M, Sannes PL. WNT7B in fibroblastic foci of idiopathic pulmonary fibrosis. Respir Res 13: 62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L295–L304, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Michoud MC, Robert R, Hassan M, Moynihan B, Haston C, Govindaraju V, Ferraro P, Hanrahan JW, Martin JG. Role of the cystic fibrosis transmembrane conductance channel in human airway smooth muscle. Am J Respir Cell Mol Biol 40: 217–222, 2009. [DOI] [PubMed] [Google Scholar]
- 360.Milara J, Mata M, Serrano A, Peiro T, Morcillo EJ, Cortijo J. Extracellular calcium-sensing receptor mediates human bronchial epithelial wound repair. Biochem Pharmacol 80: 236–246, 2010. [DOI] [PubMed] [Google Scholar]
- 361.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998. [DOI] [PubMed] [Google Scholar]
- 362.Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, Compton SJ, Morice AH. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res 31: 295–306, 2005. [DOI] [PubMed] [Google Scholar]
- 363.Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA Jr, Emala CW. Gi-coupled γ-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol 45: 1232–1238, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW. Functional expression of GABAB receptors in airway epithelium. Am J Respir Cell Mol Biol 39: 296–304, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA Jr, Yang J, Emala CW Sr. GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 294: L1206–L1216, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Mizuta K, Zhang Y, Xu D, Masaki E, Panettieri RA Jr, Emala CW. The dopamine D2 receptor is expressed and sensitizes adenylyl cyclase activity in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L316–L324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Mizuta K, Zhang Y, Xu D, Mizuta F, D'Ovidio F, Masaki E, Emala CW. The dopamine D1 receptor is expressed and facilitates relaxation in airway smooth muscle. Resp Res 14: 89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Mohamed JS, Hajira A, Li Z, Paulin D, Boriek AM. Desmin regulates airway smooth muscle hypertrophy through early growth-responsive protein-1 and microRNA-26a. J Biol Chem 286: 43394–43404, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3β. J Biol Chem 285: 29336–29347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Molostvov G, Bland R, Zehnder D. Expression and role of the calcium-sensing receptor in the blood vessel wall. Curr Pharmaceut Biotechnol 10: 282–288, 2009. [DOI] [PubMed] [Google Scholar]
- 371.Morty RE, Kuebler WM. TRPV4: an exciting new target to promote alveolocapillary barrier function. Am J Physiol Lung Cell Mol Physiol 307: L817–L821, 2014. [DOI] [PubMed] [Google Scholar]
- 372.Nagai H. Prostaglandin as a target molecule for pharmacotherapy of allergic inflammatory diseases. Allergol Int 57: 187–196, 2008. [DOI] [PubMed] [Google Scholar]
- 373.Nalli AD, Kumar DP, Al-Shboul O, Mahavadi S, Kuemmerle JF, Grider JR, Murthy KS. Regulation of Gβγi-dependent PLC-β3 activity in smooth muscle: inhibitory phosphorylation of PLC-β3 by PKA and PKG and stimulatory phosphorylation of Gαi-GTPase-activating protein RGS2 by PKG. Cell Biochem Biophys 70: 867–880, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, Svitacheva N, McGarvey L, Patacchini R, Damholt AB, Geppetti P, Materazzi S. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One 7: e42454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Nasuhara Y, Munakata M, Sato A, Amishima M, Homma Y, Kawakami Y. Mechanisms of epidermal growth factor-induced contraction of guinea pig airways. Eur J Pharmacol 296: 161–168, 1996. [DOI] [PubMed] [Google Scholar]
- 376.Newman DR, Sills WS, Hanrahan K, Ziegler A, Tidd KM, Cook E, Sannes PL. Expression of WNT5A in idiopathic pulmonary fibrosis and its control by TGF-β and WNT7B in human lung fibroblasts. J Histochem Cytochem 64: 99–111, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Nho RS, Im J, Ho YY, Hergert P. MicroRNA-96 inhibits FoxO3a function in IPF fibroblasts on type I collagen matrix. Am J Physiol Lung Cell Mol Physiol 307: L632–L642, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Nijmeh H, Balasubramaniam V, Burns N, Ahmad A, Stenmark KR, Gerasimovskaya EV. High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization. Am J Physiol Lung Cell Mol Physiol 306: L661–L671, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Norez C, Jayle C, Becq F, Vandebrouck C. Bronchorelaxation of the human bronchi by CFTR activators. Pulm Pharmacol Ther 27: 38–43, 2014. [DOI] [PubMed] [Google Scholar]
- 380.Nozik-Grayck E, Woods C, Stearman RS, Venkataraman S, Ferguson BS, Swain K, Bowler RP, Geraci MW, Ihida-Stansbury K, Stenmark KR, McKinsey TA, Domann FE. Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 311: L124–L134, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Oh K, Seo MW, Lee GY, Byoun OJ, Kang HR, Cho SH, Lee DS. Airway epithelial cells initiate the allergen response through transglutaminase 2 by inducing IL-33 expression and a subsequent Th2 response. Respir Res 14: 35, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Target Inflamm Allergy 4: 177–181, 2005. [DOI] [PubMed] [Google Scholar]
- 383.Ohlmeier S, Nieminen P, Gao J, Kanerva T, Ronty M, Toljamo T, Bergmann U, Mazur W, Pulkkinen V. Lung tissue proteomics identifies elevated transglutaminase 2 levels in stable chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 310: L1155–L1165, 2016. [DOI] [PubMed] [Google Scholar]
- 384.Okajima F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell Signal 25: 2263–2271, 2013. [DOI] [PubMed] [Google Scholar]
- 385.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos PV, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Orlandi A, Calzetta L, Doldo E, Tarquini C, Matera MG, Passeri D. Brain natriuretic peptide modulates calcium homeostasis and epidermal growth factor receptor gene signalling in asthmatic airways smooth muscle cells. Pulm Pharmacol Ther 31: 51–54, 2015. [DOI] [PubMed] [Google Scholar]
- 387.Orr SK, Colas RA, Dalli J, Chiang N, Serhan CN. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am J Physiol Lung Cell Mol Physiol 308: L904–L911, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Osawa Y, Xu D, Sternberg D, Sonett JR, D'Armiento J, Panettieri RA, Emala CW. Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L923–L931, 2006. [DOI] [PubMed] [Google Scholar]
- 389.Osorio F, Lambrecht B, Janssens S. The UPR and lung disease. Semin Immunopathol 35: 293–306, 2013. [DOI] [PubMed] [Google Scholar]
- 390.Panettieri RA., Jr Cellular and molecular mechanisms regulating airway smooth muscle proliferation and cell adhesion molecule expression. Am J Respir Crit Care Med 158: S133–S140, 1998. [DOI] [PubMed] [Google Scholar]
- 391.Papakonstantinou E, Klagas I, Karakiulakis G, Hostettler K, S'Ng CT, Kotoula V, Savic S, Tamm M, Roth M. Steroids and β2-agonists regulate hyaluronan metabolism in asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol 47: 759–767, 2012. [DOI] [PubMed] [Google Scholar]
- 392.Park JA, Fredberg JJ, Drazen JM. Putting the squeeze on airway epithelia. Physiology 30: 293–303, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α. Am J Physiol Lung Cell Mol Physiol 306: L383–L391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Pauwels B, Jonstam K, Bachert C. Emerging biologics for the treatment of chronic rhinosinusitis. Expert Rev Clin Immunol 11: 349–361, 2015. [DOI] [PubMed] [Google Scholar]
- 395.Pavord ID, Tattersfield AE. Bronchoprotective role for endogenous prostaglandin E2. Lancet 345: 436–438, 1995. [DOI] [PubMed] [Google Scholar]
- 396.Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discovery 11: 958–972, 2012. [DOI] [PubMed] [Google Scholar]
- 397.Peng X, Li HX, Shao HJ, Li GW, Sun J, Xi YH, Li HZ, Wang XY, Wang LN, Bai SZ, Zhang WH, Zhang L, Yang GD, Wu LY, Wang R, Xu CQ. Involvement of calcium-sensing receptors in hypoxia-induced vascular remodeling and pulmonary hypertension by promoting phenotypic modulation of small pulmonary arteries. Mol Cell Biochem 396: 87–98, 2014. [DOI] [PubMed] [Google Scholar]
- 398.Penn RB, Bond RA, Walker JK. GPCRs and arrestins in airways: implications for asthma. Hand Exp Pharmacol 219: 387–403, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Penumatsa KC, Fanburg BL. Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L309–L315, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Penumatsa KC, Toksoz D, Warburton RR, Hilmer AJ, Liu T, Khosla C, Comhair SA, Fanburg BL. Role of hypoxia-induced transglutaminase 2 in pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 307: L576–L585, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Pera T, Gosens R, Lesterhuis AH, Sami R, van der Toorn M, Zaagsma J, Meurs H. Cigarette smoke and lipopolysaccharide induce a proliferative airway smooth muscle phenotype. Respir Res 11: 48, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Pera T, Penn RB. Bronchoprotection and bronchorelaxation in asthma: New targets, and new ways to target the old ones. Pharmacol Ther 164: 82–96, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Pera T, Penn RB. Crosstalk between β-2-adrenoceptor and muscarinic acetylcholine receptors in the airway. Curr Opin Pharmacol 16: 72–81, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Pera T, Sami R, Zaagsma J, Meurs H. TAK1 plays a major role in growth factor-induced phenotypic modulation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L822–L828, 2011. [DOI] [PubMed] [Google Scholar]
- 405.Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther 22: 388–397, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 125: 535–553, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M, Finkelman FD. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med 208: 853–867, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol 50: 7–17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Perry MM, Durham AL, Austin PJ, Adcock IM, Chung KF. BET bromodomains regulate transforming growth factor-β-induced proliferation and cytokine release in asthmatic airway smooth muscle. J Biol Chem 290: 9111–9121, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Perusquia M, Flores-Soto E, Sommer B, Campuzano-Gonzalez E, Martinez-Villa I, Martinez-Banderas AI, Montano LM. Testosterone-induced relaxation involves L-type and store-operated Ca2+ channels blockade, and PGE 2 in guinea pig airway smooth muscle. Pflügers Arch 467: 767–777, 2015. [DOI] [PubMed] [Google Scholar]
- 411.Peters U, Chatterjee N, Yeager M, Chanock SJ, Schoen RE, McGlynn KA, Church TR, Weissfeld JL, Schatzkin A, Hayes RB. Association of genetic variants in the calcium-sensing receptor with risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev 13: 2181–2186, 2004. [PubMed] [Google Scholar]
- 412.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, Jernigan NL. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L419–L430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Pons D, de Vries FR, van den Elsen PJ, Heijmans BT, Quax PH, Jukema JW. Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. Eur Heart J 30: 266–277, 2009. [DOI] [PubMed] [Google Scholar]
- 414.Postma DS, Reddel HK, ten Hacken NH, van den Berge M. Asthma and chronic obstructive pulmonary disease: similarities and differences. Clin Chest Med 35: 143–156, 2014. [DOI] [PubMed] [Google Scholar]
- 415.Pouwels SD, Zijlstra GJ, van der Toorn M, Hesse L, Gras R, Ten Hacken NH, Krysko DV, Vandenabeele P, de Vries M, van Oosterhout AJ, Heijink IH, Nawijn MC. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol 310: L377–L386, 2016. [DOI] [PubMed] [Google Scholar]
- 416.Prabhala P, Bunge K, Rahman MM, Ge Q, Clark AR, Ammit AJ. Temporal regulation of cytokine mRNA expression by tristetraprolin: dynamic control by p38 MAPK and MKP-1. Am J Physiol Lung Cell Mol Physiol 308: L973–L980, 2015. [DOI] [PubMed] [Google Scholar]
- 417.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol 305: L912–L933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Prakash YS, Thompson MA, Pabelick CM. Brain-derived neurotrophic factor in TNF-α modulation of Ca2+ in human airway smooth muscle. Am J Respir Cell Mol Biol 41: 603–611, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308: L229–L252, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Pulkkinen V, Manson ML, Safholm J, Adner M, Dahlen SE. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol 303: L956–L966, 2012. [DOI] [PubMed] [Google Scholar]
- 421.Qipshidze N, Tyagi N, Metreveli N, Lominadze D, Tyagi SC. Autophagy mechanism of right ventricular remodeling in murine model of pulmonary artery constriction. Am J Physiol Heart Circ Physiol 302: H688–H696, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol 305: L530–L541, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci 31: 15407–15415, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Rahman M, Inman M, Kiss L, Janssen LJ. Reverse-mode NCX current in mouse airway smooth muscle: Na+ and voltage dependence, contributions to Ca2+ influx and contraction, and altered expression in a model of allergen-induced hyperresponsiveness. Acta Physiol 205: 279–291, 2012. [DOI] [PubMed] [Google Scholar]
- 425.Rahman M, Mukherjee S, Sheng W, Nilius B, Janssen LJ. Electrophysiological characterization of voltage-dependent calcium currents and TRPV4 currents in human pulmonary fibroblasts. Am J Physiol Lung Cell Mol Physiol 310: L603–L614, 2016. [DOI] [PubMed] [Google Scholar]
- 426.Reddy PH. Mitochondrial dysfunction and oxidative stress in asthma: implications for mitochondria-targeted antioxidant therapeutics. Pharmaceuticals (Basel) 4: 429–456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Redhu NS, Gounni AS. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin Exp Allergy 42: 994–1005, 2012. [DOI] [PubMed] [Google Scholar]
- 428.Redhu NS, Saleh A, Halayko AJ, Ali AS, Gounni AS. Essential role of NF-κB and AP-1 transcription factors in TNF-α-induced TSLP expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L479–L485, 2011. [DOI] [PubMed] [Google Scholar]
- 429.Redhu NS, Shan L, Movassagh H, Gounni AS. Thymic stromal lymphopoietin induces migration in human airway smooth muscle cells. Sci Rep 3: 2301, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honore E. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep 13: 1161–1171, 2015. [DOI] [PubMed] [Google Scholar]
- 431.Reuter S, Beckert H, Taube C. Take the Wnt out of the inflammatory sails: modulatory effects of Wnt in airway diseases. Lab Invest 96: 177–185, 2016. [DOI] [PubMed] [Google Scholar]
- 432.Rhee CK, Lee SY, Kang JY, Kim SJ, Kwon SS, Kim YK, Park SH. Effect of peroxisome proliferator-activated receptor-γ on airway smooth muscle thickening in a murine model of chronic asthma. Int Arch Allergy Immunol 148: 289–296, 2009. [DOI] [PubMed] [Google Scholar]
- 433.Riccardi D, Brennan SC, Chang W. The extracellular calcium-sensing receptor, CaSR, in fetal development. Best Pract Res Clin Endocrinol Metab 27: 443–453, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 298: F485–F499, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol 74: 271–297, 2012. [DOI] [PubMed] [Google Scholar]
- 436.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578, 2012. [DOI] [PubMed] [Google Scholar]
- 437.Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol 45: 1069–1074, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Robinson CB, Leonard J, Panettieri RA Jr. Drug development for severe asthma: what are the metrics? Pharmacol Therapeut 135: 176–181, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Robinson MB, Deshpande DA, Chou J, Cui W, Smith S, Langefeld C, Hastie AT, Bleecker ER, Hawkins GA. IL-6 trans-signaling increases expression of airways disease genes in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 309: L129–L138, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J 17: 1789–1799, 2003. [DOI] [PubMed] [Google Scholar]
- 441.Roth M. Airway and lung remodelling in chronic pulmonary obstructive disease: a role for muscarinic receptor antagonists? Drugs 75: 1–8, 2015. [DOI] [PubMed] [Google Scholar]
- 442.Roviezzo F, D'Agostino B, Brancaleone V, De Gruttola L, Bucci M, De Dominicis G, Orlotti D, D'Aiuto E, De Palma R, Rossi F, Sorrentino R, Cirino G. Systemic administration of sphingosine-1-phosphate increases bronchial hyperresponsiveness in the mouse. Am J Respir Cell Mol Biol 42: 572–577, 2010. [DOI] [PubMed] [Google Scholar]
- 443.Royce SG, Dang W, Yuan G, Tran J, El-Osta A, Karagiannis TC, Tang ML. Effects of the histone deacetylase inhibitor, trichostatin A, in a chronic allergic airways disease model in mice. Arch Immunol Ther Exp (Warsz) 60: 295–306, 2012. [DOI] [PubMed] [Google Scholar]
- 444.Royce SG, Karagiannis TC. Histone deacetylases and their inhibitors: new implications for asthma and chronic respiratory conditions. Curr Opin Allergy Clin Immunol 14: 44–48, 2014. [DOI] [PubMed] [Google Scholar]
- 445.Royce SG, Karagiannis TC. Histone deacetylases and their role in asthma. J Asthma 49: 121–128, 2012. [DOI] [PubMed] [Google Scholar]
- 446.Royce SG, Moodley Y, Samuel CS. Novel therapeutic strategies for lung disorders associated with airway remodelling and fibrosis. Pharmacol Therapeut 141: 250–260, 2014. [DOI] [PubMed] [Google Scholar]
- 447.Rumzhum NN, Ammit AJ. Cyclooxygenase 2: its regulation, role and impact in airway inflammation. Clin Exp Allergy 46: 397–410, 2016. [DOI] [PubMed] [Google Scholar]
- 448.Rumzhum NN, Rahman MM, Oliver BG, Ammit AJ. Effect of sphingosine 1-phosphate on cyclo-oxygenase-2 expression, prostaglandin E2 secretion, and β2-adrenergic receptor desensitization. Am J Respir Cell Mol Biol 54: 128–135, 2016. [DOI] [PubMed] [Google Scholar]
- 449.Ryan JJ, Spiegel S. The role of sphingosine-1-phosphate and its receptors in asthma. Drug News Perspect 21: 89–96, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Rydell-Tormanen K, Risse PA, Kanabar V, Bagchi R, Czubryt MP, Johnson JR. Smooth muscle in tissue remodeling and hyper-reactivity: airways and arteries. Pulm Pharmacol Ther 26: 13–23, 2013. [DOI] [PubMed] [Google Scholar]
- 451.Safholm J, Dahlen SE, Delin I, Maxey K, Stark K, Cardell LO, Adner M. PGE2 maintains the tone of the guinea pig trachea through a balance between activation of contractile EP1 receptors and relaxant EP2 receptors. Br J Pharmacol 168: 794–806, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Saint-Criq V, Harvey BJ. Estrogen and the cystic fibrosis gender gap. Steroids 81: 4–8, 2014. [DOI] [PubMed] [Google Scholar]
- 453.Saito A, Nagase T. Hippo and TGF-β interplay in the lung field. Am J Physiol Lung Cell Mol Physiol 309: L756–L767, 2015. [DOI] [PubMed] [Google Scholar]
- 454.Sanderson MJ, Delmotte P, Bai Y,Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thor Soc 5: 23–31, 2008. [DOI] [PubMed] [Google Scholar]
- 455.Santini G, Mores N, Malerba M, Mondino C, Macis G, Montuschi P. Investigational prostaglandin D2 receptor antagonists for airway inflammation. Expert Opin Invest Drug 25: 639–652, 2016. [DOI] [PubMed] [Google Scholar]
- 456.Saroea HG, Inman MD, O'Byrne PM. U46619-induced bronchoconstriction in asthmatic subjects is mediated by acetylcholine release. Am J Respir Crit Care Med 151: 321–324, 1995. [DOI] [PubMed] [Google Scholar]
- 457.Sasse SK, Altonsy MO, Kadiyala V, Cao G, Panettieri RA Jr, Gerber AN. Glucocorticoid and TNF signaling converge at A20 (TNFAIP3) to repress airway smooth muscle cytokine expression. Am J Physiol Lung Cell Mol Physiol 311: L421–L432, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Sathish V, Delmotte PF, Thompson MA, Pabelick CM, Sieck GC, Prakash YS. Sodium-calcium exchange in intracellular calcium handling of human airway smooth muscle. PLoS One 6: e23662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Sathish V, Leblebici F, Kip SN, Thompson MA, Pabelick CM, Prakash YS, Sieck GC. Regulation of sarcoplasmic reticulum Ca2+ reuptake in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 294: L787–L796, 2008. [DOI] [PubMed] [Google Scholar]
- 460.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther 150: 94–108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Savoia CP, Liu QH, Zheng YM, Yadav V, Zhang Z, Wu LG, Wang YX. Calcineurin upregulates local Ca2+ signaling through ryanodine receptor-1 in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 307: L781–L790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Saxena H, Deshpande DA, Tiegs BC, Yan H, Battafarano RJ, Burrows WM, Damera G, Panettieri RA, Dubose TD Jr, An SS, Penn RB. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol 166: 981–990, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Schaafsma D, Gosens R, Bos IS, Meurs H, Zaagsma J, Nelemans SA. Role of contractile prostaglandins and Rho-kinase in growth factor-induced airway smooth muscle contraction. Respir Res 6: 85, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Schmidt LM, Belvisi MG, Bode KA, Bauer J, Schmidt C, Suchy MT, Tsikas D, Scheuerer J, Lasitschka F, Grone HJ, Dalpke AH. Bronchial epithelial cell-derived prostaglandin E2 dampens the reactivity of dendritic cells. J Immunol 186: 2095–2105, 2011. [DOI] [PubMed] [Google Scholar]
- 465.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 306: L962–L974, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Scott CL, Walker DJ, Cwiklinski E, Tait C, Tee AR, Land SC. Control of HIF-1α and vascular signaling in fetal lung involves cross talk between mTORC1 and the FGF-10/FGFR2b/Spry2 airway branching periodicity clock. Am J Physiol Lung Cell Mol Physiol 299: L455–L471, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Scott JA, Grasemann H. Arginine metabolism in asthma. Immunol Allergy Clin North Am 34: 767–775, 2014. [DOI] [PubMed] [Google Scholar]
- 468.Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab 21: 729–738, 2010. [DOI] [PubMed] [Google Scholar]
- 469.Seidel P, Roth M, Ge Q, Merfort I, S'Ng CT, Ammit AJ. IκBα glutathionylation and reduced histone H3 phosphorylation inhibit eotaxin and RANTES. Eur Respir J 38: 1444–1452, 2011. [DOI] [PubMed] [Google Scholar]
- 470.Seow CY. Passive stiffness of airway smooth muscle: the next target for improving airway distensibility and treatment for asthma? Pulm Pharmacol Ther 26: 37–41, 2013. [DOI] [PubMed] [Google Scholar]
- 471.Setlakwe EL, Lemos KR, Lavoie-Lamoureux A, Duguay JD, Lavoie JP. Airway collagen and elastic fiber content correlates with lung function in equine heaves. Am J Physiol Lung Cell Mol Physiol 307: L252–L260, 2014. [DOI] [PubMed] [Google Scholar]
- 472.Shaik FA, Singh N, Arakawa M, Duan K, Bhullar RP, Chelikani P. Bitter taste receptors: extraoral roles in pathophysiology. Int J Biochem Cell Biol 77: 197–204, 2016. [DOI] [PubMed] [Google Scholar]
- 473.Sharma P, Panebra A, Pera T, Tiegs BC, Hershfeld A, Kenyon LC, Deshpande DA. Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 310: L365–L376, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Shaw OM, Hurst RD, Harper JL. Boysenberry ingestion supports fibrolytic macrophages with the capacity to ameliorate chronic lung remodeling. Am J Physiol Lung Cell Mol Physiol 311: L628–L638, 2016. [DOI] [PubMed] [Google Scholar]
- 475.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest 132: 651–656, 2007. [DOI] [PubMed] [Google Scholar]
- 476.Shinohara M, Kibi M, Riley IR, Chiang N, Dalli J, Kraft BD, Piantadosi CA, Choi AM, Serhan CN. Cell-cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1. Am J Physiol Lung Cell Mol Physiol 307: L746–L757, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Shiomi T, Tschumperlin DJ, Park JA, Sunnarborg SW, Horiuchi K, Blobel CP, Drazen JM. TNF-α-converting enzyme/a disintegrin and metalloprotease-17 mediates mechanotransduction in murine tracheal epithelial cells. Am J Respir Cell Mol Biol 45: 376–385, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash YS, Kong H. Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 308: L1125–L1135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Shore SA. Modeling airway remodeling: the winner by a nose? Am J Respir Crit Care Med 168: 910–911, 2003. [DOI] [PubMed] [Google Scholar]
- 480.Siddesha JM, Nakada EM, Mihavics BR, Hoffman SM, Rattu GK, Chamberlain N, Cahoon JM, Lahue KG, Daphtary N, Aliyeva M, Chapman DG, Desai DH, Poynter ME, Anathy V. Effect of a chemical chaperone, tauroursodeoxycholic acid, on HDM-induced allergic airway disease. Am J Physiol Lung Cell Mol Physiol 310: L1243–L1259, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Siddiqui S, Novali M, Tsuchiya K, Hirota N, Geller BJ, McGovern TK, Risse PA, Jo T, Zeroual MA, Martin JG. The modulation of large airway smooth muscle phenotype and effects of epidermal growth factor receptor inhibition in the repeatedly allergen-challenged rat. Am J Physiol Lung Cell Mol Physiol 304: L853–L862, 2013. [DOI] [PubMed] [Google Scholar]
- 482.Siddiqui S, Novali M, Tsuchiya K, Hirota N, Geller BJ, McGovern TK, Risse PA, Jo T, Zeroual MA, Martin JG. The modulation of large airway smooth muscle phenotype and effects of epidermal growth factor receptor inhibition in the repeatedly allergen-challenged rat. Am J Physiol Lung Cell Mol Physiol 304: L853–L862, 2013. [DOI] [PubMed] [Google Scholar]
- 483.Siddiqui S, Redhu NS, Ojo OO, Liu B, Irechukwu N, Billington C, Janssen L, Moir LM. Emerging airway smooth muscle targets to treat asthma. Pulm Pharmacol Ther 26: 132–144, 2013. [DOI] [PubMed] [Google Scholar]
- 484.Singer CA. T-bet is induced by interferon-γ to mediate chemokine secretion and migration in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L633–L641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, Thompson M, Freeman M, Mabalirajan U, Gosens R, Ghosh B, Pabelick C, Linneberg A, Prakash YS, Agrawal A. Author response to letter to editor: Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol 311: L183–L184, 2016. [DOI] [PubMed] [Google Scholar]
- 486.Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, Thompson M, Freeman M, Mabalirajan U, Gosens R, Ghosh B, Pabelick C, Linneberg A, Prakash YS, Agrawal A. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol 310: L837–L845, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Singh S, Prakash YS, Linneberg A, Agrawal A. Insulin and the lung: connecting asthma and metabolic syndrome. J Allergy (Cairo) 2013: 627384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol 185: 3035–3040, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Song C, He L, Zhang J, Ma H, Yuan X, Hu G, Tao L, Meng J. Fluorofenidone attenuates pulmonary inflammation and fibrosis via inhibiting the activation of NALP3 inflammasome and IL-1β/IL-1R1/MyD88/NF-κB pathway. J Cell Mol Med 20: 2064–2077, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Song T, Hao Q, Zheng YM, Liu QH, Wang YX. Inositol 1,4,5-trisphosphate activates TRPC3 channels to cause extracellular Ca2+ influx in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L1455–L1466, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Song T, Zheng YM, Vincent PA, Cai D, Rosenberg P, Wang YX. Canonical transient receptor potential 3 channels activate NF-κB to mediate allergic airway disease via PKC-α/IκB-α and calcineurin/IκB-β pathways. FASEB J 30: 214–229, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Song W, Yu Z, Doran SF, Ambalavanan N, Steele C, Garantziotis S, Matalon S. Respiratory syncytial virus infection increases chlorine-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol 309: L205–L210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Sopi RB, Martin RJ, Haxhiu MA, Dreshaj IA, Yao Q, Jafri A, Zaidi SI. Role of brain-derived neurotrophic factor in hyperoxia-induced enhancement of contractility and impairment of relaxation in lung parenchyma. Am J Physiol Lung Cell Mol Physiol 295: L348–L355, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Soveg F, Abdala-Valencia H, Campbell J, Morales-Nebreda L, Mutlu GM,Cook-Mills JM. Regulation of allergic lung inflammation by endothelial cell transglutaminase 2. Am J Physiol Lung Cell Mol Physiol 309: L573–L583, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Spanjer AI, Baarsma HA, Oostenbrink LM, Jansen SR, Kuipers CC, Lindner M, Postma DS, Meurs H, Heijink IH, Gosens R, Konigshoff M. TGF-β-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J 30: 1823–1835, 2016. [DOI] [PubMed] [Google Scholar]
- 496.Spanjer AI, Menzen MH, Dijkstra AE, van den Berge M, Boezen HM, Nickle DC, Sin DD, Bosse Y, Brandsma CA, Timens W, Postma DS, Meurs H, Heijink IH, Gosens R. A pro-inflammatory role for the Frizzled-8 receptor in chronic bronchitis. Thorax 71: 312–322, 2016. [DOI] [PubMed] [Google Scholar]
- 497.Spinelli AM, Trebak M. Orai channel-mediated Ca2+ signals in vascular and airway smooth muscle. Am J Physiol Cell Physiol 310: C402–C413, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Srivastava K, Sampson HA, Emala CW Sr, Li XM. The anti-asthma herbal medicine ASHMI acutely inhibits airway smooth muscle contraction via prostaglandin E2 activation of EP2/EP4 receptors. Am J Physiol Lung Cell Mol Physiol 305: L1002–L1010, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Stamatiou R, Paraskeva E, Papagianni M, Molyvdas PA, Hatziefthimiou A. The mitogenic effect of testosterone and 17β-estradiol on airway smooth muscle cells. Steroids 76: 400–408, 2011. [DOI] [PubMed] [Google Scholar]
- 500.Starkey MR, Jarnicki AG, Essilfie AT, Gellatly SL, Kim RY, Brown AC, Foster PS, Horvat JC, Hansbro PM. Murine models of infectious exacerbations of airway inflammation. Curr Opin Pharmacol 13: 337–344, 2013. [DOI] [PubMed] [Google Scholar]
- 501.Stephen J, Delvecchio C, Spitale N, Giesler A, Radford K, Bilan P, Cox PG, Capone JP, Nair P. PPAR ligands decrease human airway smooth muscle cell migration and extracellular matrix synthesis. Eur Respir J 41: 425–432, 2013. [DOI] [PubMed] [Google Scholar]
- 502.Stober VP, Szczesniak C, Childress Q, Heise RL, Bortner C, Hollingsworth JW, Neuringer IP, Palmer SM, Garantziotis S. Bronchial epithelial injury in the context of alloimmunity promotes lymphocytic bronchiolitis through hyaluronan expression. Am J Physiol Lung Cell Mol Physiol 306: L1045–L1055, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Sun J, Murphy E. Calcium-sensing receptor: a sensor and mediator of ischemic preconditioning in the heart. Am J Physiol Heart Circ Physiol 299: H1309–H1317, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Sundar IK, Yao H, Sellix MT, Rahman I. Circadian clock-coupled lung cellular and molecular functions in chronic airway diseases. Am J Respir Cell Mol Biol 53: 285–290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Sundar IK, Yao H, Sellix MT, Rahman I. Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol 309: L1056–L1075, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Suter MA, Abramovici AR, Griffin E, Branch DW, Lane RH, Mastrobattista J, Rehan VK, Aagaard K. In utero nicotine exposure epigenetically alters fetal chromatin structure and differentially regulates transcription of the glucocorticoid receptor in a rat model. Birth Defects Res A Clin Mol Teratol 103: 583–588, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Suzuki R, Miyazaki Y, Takagi K, Torii K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: implications for therapy. Treat Respir Med 3: 17–27, 2004. [DOI] [PubMed] [Google Scholar]
- 508.Suzuki Y, Asano K, Shiraishi Y, Oguma T, Shiomi T, Fukunaga K, Nakajima T, Niimi K, Yamaguchi K, Ishizaka A. Human bronchial smooth muscle cell proliferation via thromboxane A2 receptor. Prostagland Leukotri Essent Fatty Acid 71: 375–382, 2004. [DOI] [PubMed] [Google Scholar]
- 509.Syyong HT, Pascoe CD, Zhang J, Arsenault BA, Solomon D, Elliott WM, Hackett TL, Walker DC, Pare PD, Seow CY. Ultrastructure of human tracheal smooth muscle from subjects with asthma and nonasthmatic subjects. Standardized methods for comparison. Am J Respir Cell Mol Biol 52: 304–314, 2015. [DOI] [PubMed] [Google Scholar]
- 510.Tabet Y, Sirois M, Sirois C, Rizcallah E, Rousseau E. Relationship between bradykinin-induced relaxation and endogenous epoxyeicosanoid synthesis in human bronchi. Am J Physiol Lung Cell Mol Physiol 304: L562–L569, 2013. [DOI] [PubMed] [Google Scholar]
- 511.Takahara N, Ito S, Furuya K, Naruse K, Aso H, Kondo M, Sokabe M, Hasegawa Y. Real-time imaging of ATP release induced by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol 51: 772–782, 2014. [DOI] [PubMed] [Google Scholar]
- 512.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int 61: 3–17, 2012. [DOI] [PubMed] [Google Scholar]
- 513.Tam A, Churg A, Wright JL, Zhou S, Kirby M, Coxson HO, Lam S, Man SF, Sin DD. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 193: 825–834, 2016. [DOI] [PubMed] [Google Scholar]
- 514.Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Women Health 11: 24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Tamaoka M, Hassan M, McGovern T, Ramos-Barbon D, Jo T, Yoshizawa Y, Tolloczko B, Hamid Q, Martin JG. The epidermal growth factor receptor mediates allergic airway remodelling in the rat. Eur Respir J 32: 1213–1223, 2008. [DOI] [PubMed] [Google Scholar]
- 516.Tan X, Alrashdan YA, Alkhouri H, Oliver BG, Armour CL, Hughes JM. Airway smooth muscle CXCR3 ligand production: regulation by JAK-STAT1 and intracellular Ca2+. Am J Physiol Lung Cell Mol Physiol 304: L790–L802, 2013. [DOI] [PubMed] [Google Scholar]
- 517.Tang H, Chen J, Fraidenburg DR, Song S, Sysol JR, Drennan AR, Offermanns S, Ye RD, Bonini MG, Minshall RD, Garcia JG, Machado RF, Makino A, Yuan JX. Deficiency of Akt1, but not Akt2, attenuates the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L208–L220, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Tang H, Yamamura A, Yamamura H, Song S, Fraidenburg DR, Chen J, Gu Y, Pohl NM, Zhou T, Jimenez-Perez L, Ayon RJ, Desai AA, Goltzman D, Rischard F, Khalpey Z, Black SM, Garcia JG, Makino A, Yuan JX. Pathogenic role of calcium-sensing receptors in the development and progression of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L846–L859, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Tazzeo T, Bates G, Roman HN, Lauzon AM, Khasnis MD, Eto M, Janssen LJ. Caffeine relaxes smooth muscle through actin depolymerization. Am J Physiol Lung Cell Mol Physiol 303: L334–L342, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Thangjam GS, Birmpas C, Barabutis N, Gregory BW, Clemens MA, Newton JR, Fulton D, Catravas JD. Hsp90 inhibition suppresses NF-κB transcriptional activation via Sirt-2 in human lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 310: L964–L974, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Thompson MA, Prakash YS, Pabelick CM. Arachidonate-regulated Ca2+ influx in human airway smooth muscle. Am J Respir Cell Mol Biol 51: 68–76, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Toka HR, Pollak MR, Houillier P. Calcium sensing in the renal tubule. Physiology 30: 317–326, 2015. [DOI] [PubMed] [Google Scholar]
- 523.Toki S, Goleniewska K, Reiss S, Zhou W, Newcomb DC, Bloodworth MH, Stier MT, Boyd KL, Polosukhin VV, Subramaniam S, Peebles RS Jr. The histone deacetylase inhibitor trichostatin A suppresses murine innate allergic inflammation by blocking group 2 innate lymphoid cell (ILC2) activation. Thorax 71: 633–645, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Tomura H, Wang JQ, Komachi M, Damirin A, Mogi C, Tobo M, Kon J, Misawa N, Sato K, Okajima F. Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem 280: 34458–34464, 2005. [DOI] [PubMed] [Google Scholar]
- 525.Townsend EA, Emala CW Sr. Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am J Physiol Lung Cell Mol Physiol 305: L396–L403, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 33: 1–47, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol 303: L923–L928, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 298: L521–L530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Trejo Bittar HE, Yousem SA, Wenzel SE. Pathobiology of severe asthma. Annu Rev Pathol 10: 511–545, 2015. [DOI] [PubMed] [Google Scholar]
- 530.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, Berger P. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med 204: 3173–3181, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Truong KK, Lam MT, Grandner MA, Sassoon CS, Malhotra A. Timing matters: circadian rhythm in sepsis, obstructive lung disease, obstructive sleep apnea, and cancer. Ann Am Thorac Soc 13: 1144–1154, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Tsuchiya K, Siddiqui S, Risse PA, Hirota N, Martin JG. The presence of LPS in OVA inhalations affects airway inflammation and AHR but not remodeling in a rodent model of asthma. Am J Physiol Lung Cell Mol Physiol 303: L54–L63, 2012. [DOI] [PubMed] [Google Scholar]
- 533.Uhal BD, Nguyen H, Dang M, Gopallawa I, Jiang J, Dang V, Ono S, Morimoto K. Abrogation of ER stress-induced apoptosis of alveolar epithelial cells by angiotensin 1–7. Am J Physiol Lung Cell Mol Physiol 305: L33–L41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med 178: 1100–1114, 2008. [DOI] [PubMed] [Google Scholar]
- 535.van Dijk EM, Menzen MH, Spanjer AI, Middag LD, Brandsma CA, Gosens R. Noncanonical WNT-5B signaling induces inflammatory responses in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 310: L1166–L1176, 2016. [DOI] [PubMed] [Google Scholar]
- 536.Van Ly D, Burgess JK, Brock TG, Lee TH, Black JL, Oliver BG. Prostaglandins but not leukotrienes alter extracellular matrix protein deposition and cytokine release in primary human airway smooth muscle cells and fibroblasts. Am J Physiol Lung Cell Mol Physiol 303: L239–L250, 2012. [DOI] [PubMed] [Google Scholar]
- 537.Vaporidi K, Vergadi E, Kaniaris E, Hatziapostolou M, Lagoudaki E, Georgopoulos D, Zapol WM, Bloch KD, Iliopoulos D. Pulmonary microRNA profiling in a mouse model of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 303: L199–L207, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Verma MK, Miki Y, Sasano H. Sex steroid receptors in human lung diseases. J Steroid Biochem Mol Biol 127: 216–222, 2011. [DOI] [PubMed] [Google Scholar]
- 539.Villalta PC, Rocic P, Townsley MI. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am J Physiol Lung Cell Mol Physiol 307: L652–L659, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Vogel ER, VanOosten SK, Holman MA, Hohbein DD, Thompson MA, Vassallo R, Pandya HC, Prakash YS, Pabelick CM. Cigarette smoke enhances proliferation and extracellular matrix deposition by human fetal airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L978–L986, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflügers Arch 467: 95–99, 2015. [DOI] [PubMed] [Google Scholar]
- 543.Wallace HL, Southern KW, Connell MG, Wray S, Burdyga T. Abnormal tracheal smooth muscle function in the CF mouse. Physiol Rep 1: e00138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Wang G, Wang R, Strulovici-Barel Y, Salit J, Staudt MR, Ahmed J, Tilley AE, Yee-Levin J, Hollmann C, Harvey BG, Kaner RJ, Mezey JG Sridhar S, Pillai SG, Hilton H, Wolff G, Bitter H, Visvanathan S, Fine JS, Stevenson CS, Crystal RG. Persistence of smoking-induced dysregulation of miRNA expression in the small airway epithelium despite smoking cessation. PLoS One 10: e0120824, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Wang S, Robinet P, Smith JD, Gulshan K. ORMDL orosomucoid-like proteins are degraded by free-cholesterol-loading-induced autophagy. Proc Natl Acad Sci USA 112: 3728–3733, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Wang SY, Freeman MR, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Sex steroids influence brain-derived neurotropic factor secretion from human airway smooth muscle cells. J Cell Physiol 231: 1586–1592, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Wang W, Liu Z, Su J, Chen WS, Wang XW, Bai SX, Zhang JZ, Yu SQ. Macrophage MicroRNA-155 promotes lipopolysaccharide-induced acute lung injury in mice and rats. Am J Physiol Lung Cell Mol Physiol 311: L494–L506, 2016. [DOI] [PubMed] [Google Scholar]
- 548.Wang WC, Pauer SH, Smith DC, Dixon MA, Disimile DJ, Panebra A, An SS, Camoretti-Mercado B, Liggett SB. Targeted transgenesis identifies Gαs as the bottleneck in β2-adrenergic receptor cell signaling and physiological function in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L775–L780, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Wang Y, Huang C, Chintagari NR, Xi D, Weng T, Liu L. miR-124 regulates fetal pulmonary epithelial cell maturation. Am J Physiol Lung Cell Mol Physiol 309: L400–L413, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Ward JE, Harris T, Bamford T, Mast A, Pain MC, Robertson C, Smallwood D, Tran T, Wilson J, Stewart AG. Proliferation is not increased in airway myofibroblasts isolated from asthmatics. Eur Respir J 32: 362–371, 2008. [DOI] [PubMed] [Google Scholar]
- 551.Watanabe N, Horie S, Spina D, Michael GJ, Page CP, Priestley JV. Immunohistochemical localization of transient receptor potential vanilloid subtype 1 in the trachea of ovalbumin-sensitized Guinea pigs. Intl Archiv Allerg Immunol 146, Suppl 1: 28–32, 2008. [DOI] [PubMed] [Google Scholar]
- 552.Wen L, Sun L, Xi Y, Chen X, Xing Y, Sun W, Meng Q, Cai L. Expression of calcium sensing receptor and E-cadherin correlated with survival of lung adenocarcinoma. Thorac Cancer 6: 754–760, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Wen X, Zhou J, Zhang D, Li J, Wang Q, Feng N, Zhu H, Song Y, Li H, Bai C. Denatonium inhibits growth and induces apoptosis of airway epithelial cells through mitochondrial signaling pathways. Respir Res 16: 13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Weng X, Cheng X, Wu X, Xu H, Fang M, Xu Y. Sin3B mediates collagen type I gene repression by interferon γ in vascular smooth muscle cells. Biochem Biophys Res Commun 447: 263–270, 2014. [DOI] [PubMed] [Google Scholar]
- 555.Wenzel SE, Westcott JY, Smith HR, Larsen GL. Spectrum of prostanoid release after bronchoalveolar allergen challenge in atopic asthmatics and in control groups. An alteration in the ratio of bronchoconstrictive to bronchoprotective mediators. Am Rev Respir Dis 139: 450–457, 1989. [DOI] [PubMed] [Google Scholar]
- 556.West AR, Syyong HT, Siddiqui S, Pascoe CD, Murphy TM, Maarsingh H, Deng L, Maksym GN, Bosse Y. Airway contractility and remodeling: links to asthma symptoms. Pulm Pharmacol Ther 26: 3–12, 2013. [DOI] [PubMed] [Google Scholar]
- 557.Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, Bittner A, Rao N, Murphy MP, Kirkham PA, Chung KF, Adcock IM. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 136: 769–780, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF, Lindsay MA. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One 4: e5889, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Williams AS, Mathews JA, Kasahara DI, Wurmbrand AP, Chen L, Shore SA. Innate and ozone-induced airway hyperresponsiveness in obese mice: role of TNF-α. Am J Physiol Lung Cell Mol Physiol 308: L1168–L1177, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Wolf D, Tseng N, Seedorf G, Roe G, Abman SH, Gien J. Endothelin-1 decreases endothelial PPARγ signaling and impairs angiogenesis after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L361–L371, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Workman AD, Palmer JN, Adappa ND, Cohen NA. The role of bitter and sweet taste receptors in upper airway immunity. Curr Allergy Asthma Rep 15: 72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Wright DB, Meurs H, Dekkers BG. Integrins: therapeutic targets in airway hyperresponsiveness and remodelling? Trends Pharmacol Sci 35: 567–574, 2014. [DOI] [PubMed] [Google Scholar]
- 563.Wright DB, Tripathi S, Sikarwar A, Santosh KT, Perez-Zoghbi J, Ojo OO, Irechukwu N, Ward JP, Schaafsma D. Regulation of GPCR-mediated smooth muscle contraction: implications for asthma and pulmonary hypertension. Pulm Pharmacol Ther 26: 121–131, 2013. [DOI] [PubMed] [Google Scholar]
- 564.Xie S, Issa R, Sukkar MB, Oltmanns U, Bhavsar PK, Papi A, Caramori G, Adcock I, Chung KF. Induction and regulation of matrix metalloproteinase-12 in human airway smooth muscle cells. Respir Res 6: 148, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Xie W, Lynch TJ, Liu X, Tyler SR, Yu S, Zhou X, Luo M, Kusner DM, Sun X, Yi Y, Zhang Y, Goodheart MJ, Parekh KR, Wells JM, Xue HH, Pevny LH, Engelhardt JF. Sox2 modulates Lef-1 expression during airway submucosal gland development. Am J Physiol Lung Cell Mol Physiol 306: L645–L660, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA, Fernandez RA, Zeifman A, Makino A, Dong H, Yuan JX. Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res 111: 469–481, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Yamamura A, Yagi S, Ohara N, Tsukamoto K. Calcilytics enhance sildenafil-induced antiproliferation in idiopathic pulmonary arterial hypertension. Eur J Pharmacol 784: 15–21, 2016. [DOI] [PubMed] [Google Scholar]
- 568.Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, Pascual RM, Panettieri RA, Penn RB. Anti-mitogenic effects of β-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J 25: 389–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Yang CM, Lee IT, Chi PL, Cheng SE, Hsiao LD, Hsu CK. TNF-α induces cytosolic phospholipase A2 expression via Jak2/PDGFR-dependent Elk-1/p300 activation in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 306: L543–L551, 2014. [DOI] [PubMed] [Google Scholar]
- 570.Yang CM, Lin CC, Lee IT, Hsu CK, Tai YC, Hsieh HL, Chi PL, Hsiao LD. c-Src-dependent transactivation of EGFR mediates CORM-2-induced HO-1 expression in human tracheal smooth muscle cells. J Cell Physiol 230: 2351–2361, 2015. [DOI] [PubMed] [Google Scholar]
- 571.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 302: L521–L529, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L555–L568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Yao H, Sundar IK, Ahmad T, Lerner C, Gerloff J, Friedman AE, Phipps RP, Sime PJ, McBurney MW, Guarente L, Rahman I. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 306: L816–L828, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Yao M, Wang X, Tang Y, Zhang W, Cui B, Liu Q, Xing L. Dicer mediating the expression of miR-143 and miR-155 regulates hexokinase II associated cellular response to hypoxia. Am J Physiol Lung Cell Mol Physiol 307: L829–L837, 2014. [DOI] [PubMed] [Google Scholar]
- 575.Yao Q, Haxhiu MA, Zaidi SI, Liu S, Jafri A, Martin RJ. Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. Am J Physiol Lung Cell Mol Physiol 289: L307–L314, 2005. [DOI] [PubMed] [Google Scholar]
- 576.Yao Q, Zaidi SI, Haxhiu MA, Martin RJ. Neonatal lung and airway injury: a role for neurotrophins. Semin Perinatol 30: 156–162, 2006. [DOI] [PubMed] [Google Scholar]
- 577.Yarova PL, Stewart AL, Sathish V, Britt RD Jr, Thompson MA, AP PL, Freeman M, Aravamudan B, Kita H, Brennan SC, Schepelmann M, Davies T, Yung S, Cholisoh Z, Kidd EJ, Ford WR, Broadley KJ, Rietdorf K, Chang W, Bin Khayat ME, Ward DT, Corrigan CJ, JP TW, Kemp PJ, Pabelick CM, Prakash YS, Riccardi D.. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med 7: 284ra260, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Ye JX, Wang SS, Ge M, Wang DJ. Suppression of endothelial PGC-1α is associated with hypoxia-induced endothelial dysfunction and provides a new therapeutic target in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 310: L1233–L1242, 2016. [DOI] [PubMed] [Google Scholar]
- 579.Yeganeh B, Mukherjee S, Moir LM, Kumawat K, Kashani HH, Bagchi RA, Baarsma HA, Gosens R, Ghavami S. Novel non-canonical TGF-β signaling networks: emerging roles in airway smooth muscle phenotype and function. Pulm Pharmacol Ther 26: 50–63, 2013. [DOI] [PubMed] [Google Scholar]
- 580.Yim PD, Gallos G, Xu D, Zhang Y, Emala CW. Novel expression of a functional glycine receptor chloride channel that attenuates contraction in airway smooth muscle. FASEB J 25: 1706–1717, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Yocum GT, Gallos G, Zhang Y, Jahan R, Stephen MR, Varagic Z, Puthenkalam R, Ernst M, Cook JM, Emala CW. Targeting the γ-aminobutyric acid A receptor α4 subunit in airway smooth muscle to alleviate bronchoconstriction. Am J Respir Cell Mol Biol 54: 546–553, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Yoon SY, Shin ES, Park SY, Kim S, Kwon HS, Cho YS, Moon HB, Kim TB. Association between polymorphisms in bitter taste receptor genes and clinical features in Korean asthmatics. Respiration 91: 141–150, 2016. [DOI] [PubMed] [Google Scholar]
- 583.Yurt M, Liu J, Sakurai R, Gong M, Husain SM, Siddiqui MA, Husain M, Villarreal P, Akcay F, Torday JS, Rehan VK. Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. Am J Physiol Lung Cell Mol Physiol 307: L859–L867, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Zaidi S, Gallos G, Yim PD, Xu D, Sonett JR, Panettieri RA Jr, Gerthoffer W, Emala CW. Functional expression of γ-amino butyric acid transporter 2 in human and guinea pig airway epithelium and smooth muscle. Am J Respir Cell Mol Biol 45: 332–339, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Zarazua A, Gonzalez-Arenas A, Ramirez-Velez G, Bazan-Perkins B, Guerra-Araiza C,Campos-Lara MG. Sexual dimorphism in the regulation of estrogen, progesterone, and androgen receptors by sex steroids in the rat airway smooth muscle cells. Int J Endocrinol 2016: 8423192, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Zaslona Z, Peters-Golden M. Prostanoids in asthma and COPD: actions, dysregulation, and therapeutic opportunities. Chest 148: 1300–1306, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep 15: 28, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, Lau LF, Kahn M, Downey GP. Role of β-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 304: L415–L427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol 11: e1001501, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Zhang L, An X, Wang Q, He M. Activation of cold-sensitive channels TRPM8 and TRPA1 inhibits the proliferative airway smooth muscle cell phenotype. Lung 194: 595–603, 2016. [DOI] [PubMed] [Google Scholar]
- 591.Zhang T, Luo XJ, Sai WB, Yu MF, Li WE, Ma YF, Chen W, Zhai K, Qin G, Guo D, Zheng YM, Wang YX, Shen JH, Ji G, Liu QH. Non-selective cation channels mediate chloroquine-induced relaxation in precontracted mouse airway smooth muscle. PLoS One 9: e101578, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Zhao L, Sullivan MN, Chase M, Gonzales AL, Earley S. Calcineurin/nuclear factor of activated T cells-coupled vanilliod transient receptor potential channel 4 Ca2+ sparklets stimulate airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol 50: 1064–1075, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Zhao L, Zhang X, Kuang H, Wu J, Guo Y, Ma L. Effect of TRPV1 channel on the proliferation and apoptosis in asthmatic rat airway smooth muscle cells. Exp Lung Res 39: 283–294, 2013. [DOI] [PubMed] [Google Scholar]
- 594.Zhao LM, Kuang HY, Zhang LX, Wu JZ, Chen XL, Zhang XY, Ma LJ. Effect of TRPV1 channel on proliferation and apoptosis of airway smooth muscle cells of rats. J Huazhong Univ Sci Technol Med Sci 34: 504–509, 2014. [DOI] [PubMed] [Google Scholar]
- 595.Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem 283: 36522–36531, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Zhou D, Zheng X, Wang L, Stelmack G, Halayko AJ, Dorscheid D, Bai TR. Expression and effects of cardiotrophin-1 (CT-1) in human airway smooth muscle cells. Br J Pharmacol 140: 1237–1244, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Zhou JS, Zhao Y, Zhou HB, Wang Y, Wu YF, Li ZY, Xuan NX, Zhang C, Hua W, Ying SM, Li W, Shen HH, Chen ZH. Autophagy plays an essential role in cigarette smoke-induced expression of MUC5AC in airway epithelium. Am J Physiol Lung Cell Mol Physiol 310: L1042–L1052, 2016. [DOI] [PubMed] [Google Scholar]
- 598.Zhou L, Goldsmith AM, Bentley JK, Jia Y, Rodriguez ML, Abe MK, Fingar DC, Hershenson MB. 4E-binding protein phosphorylation and eukaryotic initiation factor-4E release are required for airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 33: 195–202, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Zhou X, Lin P, Yamazaki D, Park KH, Komazaki S, Chen SR, Takeshima H, Ma J. Trimeric intracellular cation channels and sarcoplasmic/endoplasmic reticulum calcium homeostasis. Circ Res 114: 706–716, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Zou W, Zou Y, Zhao Z, Li B, Ran P. Nicotine-induced epithelial-mesenchymal transition via Wnt/β-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 304: L199–L209, 2013. [DOI] [PubMed] [Google Scholar]