Significance
Depression is a seriously disabling disorder, twice as common in women as in men. Lack of efficacy of existing pharmacotherapies in subsets of patients has led to an intensive search for new targets for antidepressant development, including receptors for neuropeptides such as galanin (GAL). In this study, we explore GAL and its three receptors, GAL1–3, comparing postmortem brain regions from depressed suicide patients and controls. Using quantitative PCR and bisulfite pyrosequencing, we report significant changes in the transcript and DNA methylation levels of GAL and galanin receptor 3 (GALR3) in the locus coeruleus and dorsal raphe nucleus, two regions important for mood regulation. Our findings suggest GAL3 involvement in depressive disorder, making it a possible drug target for this disease.
Keywords: epigenetics, human postmortem brain, neuropeptides, stress, transmitter coexistence
Abstract
Major depressive disorder (MDD) is a substantial burden to patients, families, and society, but many patients cannot be treated adequately. Rodent experiments suggest that the neuropeptide galanin (GAL) and its three G protein-coupled receptors, GAL1–3, are involved in mood regulation. To explore the translational potential of these results, we assessed the transcript levels (by quantitative PCR), DNA methylation status (by bisulfite pyrosequencing), and GAL peptide by RIA of the GAL system in postmortem brains from depressed persons who had committed suicide and controls. Transcripts for all four members were detected and showed marked regional variations, GAL and galanin receptor 1 (GALR1) being most abundant. Striking increases in GAL and GALR3 mRNA levels, especially in the noradrenergic locus coeruleus and the dorsal raphe nucleus, in parallel with decreased DNA methylation, were found in both male and female suicide subjects as compared with controls. In contrast, GAL and GALR3 transcript levels were decreased, GALR1 was increased, and DNA methylation was increased in the dorsolateral prefrontal cortex of male suicide subjects, however, there were no changes in the anterior cingulate cortex. Thus, GAL and its receptor GALR3 are differentially methylated and expressed in brains of MDD subjects in a region- and sex-specific manner. Such an epigenetic modification in GALR3, a hyperpolarizing receptor, might contribute to the dysregulation of noradrenergic and serotonergic neurons implicated in the pathogenesis of MDD. Thus, one may speculate that a GAL3 antagonist could have antidepressant properties by disinhibiting the firing of these neurons, resulting in increased release of noradrenaline and serotonin in forebrain areas involved in mood regulation.
Major depressive disorder (MDD) is a serious mental illness affecting up to 20% of the population at some point during their lives, women more frequently than men, and representing a major burden to patients, their families, and society (1, 2). MDD is thought to arise from the interaction of genetic and environmental factors, with stressful life events representing an important predisposing factor (3–5). Growing evidence suggests that epigenetic mechanisms mediate such interactions, namely through altered DNA methylation, thus leading to stable changes in brain function that may underlie psychopathology (6, 7).
Pharmacological management of depression currently involves drugs that often target the monoamine transporters, which include selective reuptake inhibitors for serotonin (5-hydroxytryptamine, 5-HT) (SSRIs), noradrenaline (NA) inhibitors (NRIs), or a combination of both (SNRIs) (8–10), as well as a number of other medications (11). However, the therapeutic efficacy of these antidepressants is hampered by a slow onset of action, a limited response rate, and considerable side effects (12, 13). These issues have led to intensive search for novel therapeutic approaches for MDD (14), including targeting receptors for neuropeptides (15–19), the most diverse family of brain messenger molecules (20).
In this context, the 29/30 amino acid neuropeptide galanin (GAL) (21), which is widely distributed in the rat (22–25) and human (26) brain, may be of special interest. In particular, it coexists with NA in the locus coeruleus in both rat (27–29) and human (26, 30–32) and in rat with 5-HT in the dorsal raphe nucleus (28, 33, 34).
GAL exerts its physiological actions via three subtypes of G protein-coupled receptors, GAL1–3 (35, 36). The distribution of these receptors has been mapped previously with ligand-binding autoradiography in rat (37, 38), monkey, and human (39–41) brain. More recently the receptor transcripts have been localized with in situ hybridization in rat brain (42–44) and in some regions of the human brain (32). It should be noted that GAL receptor subtypes can form dimers and heterodimers, a mechanism that can profoundly change GAL signaling (45).
Interestingly, certain differences between species exist in regions of potential importance for mood-related disorders. For example, galanin receptor 3 (GalR3) mRNA has a limited distribution in the rat brain (44), where it could not even be detected in the first publication on the cloning of this receptor (46). However, it appears to be expressed in human noradrenergic locus coeruleus (NA-locus coeruleus) neurons (32). In addition GAL itself has a different profile: it is expressed in 5-HT neurons in the dorsal raphe nucleus in the rat but not in humans, but its expression in the locus coeruleus is conserved in the species investigated to date.
Animal studies have provided strong evidence that the GAL system is involved in anxiety- and depression-like behavior (33, 47–55). A recent genetic association study supports a possible role of the GAL system in mood disorders, pointing to involvement of epigenetic processes and a strong association with high levels of stress (56). Moreover, there is an interaction between GAL and the 5-HT1A receptor (47), a receptor that plays an important role as autoreceptor in depression, as shown in experimental studies (57, 58) and in studies of suicide victims (59). In agreement with many animal experimental studies, PET imaging also has indicated a role for postsynaptic 5-HT1A receptors in depression (60).
The aim of the present study was to identify possible changes in the GAL system in MDD by analyzing postmortem brains from depressed subjects who committed suicide (hereafter “DS” subjects) and matched subjects without psychiatric symptoms (hereafter, simply “controls”). We used quantitative PCR (qPCR) to monitor transcript levels, bisulfite pyrosequencing to study DNA methylation (GAL and GALR1-3), and RIA to establish GAL peptide levels. All three analyses were carried out on the same samples from five different, relevant brain regions: dorsolateral prefrontal cortex [Brodmann area (BA) 8/9], anterior cingulate cortex (BA 24), locus coeruleus, dorsal raphe nucleus, and the medullary raphe nuclei.
Results
Cohort Demographics.
There were no significant differences between DS subjects and their matched controls in age (DS subjects vs. controls: 51.6 ± 15.4 vs. 57.5 ± 15.4 y, P = 0.11); postmortem interval (PMI) (DS subjects vs. controls: 43.56 ± 24.16 vs. 49.21 ± 34.44 h, P = 0.94); brain pH (DS subjects vs. controls: 6.61 ± 0.29 vs. 6.46 ± 0.34; P = 0.13); or RNA integrity number (RIN) (DS subjects vs. controls: 6.49 ± 1.70 vs. 6.68 ± 1.62; P < 0.49). The details of the demographic characteristics of DS subjects and controls for each of the regions analyzed are provided in Table 1, and detailed information on each individual subject is provided in Table S1.
Table 1.
Demographic characteristics of the cohort
| Samples | DLPFC | ACC | DRN | LC | MRN |
| Size | 40 | 40 | 42 | 42 | 47 |
| % male controls (n) | 25 (10) | 25 (10) | 26.1 (11) | 23.8 (10) | 25.5 (12) |
| % male DS subjects (n) | 25 (10) | 25 (10) | 24.1 (10) | 23.8 (10) | 21.2 (10) |
| % female controls (n) | 25 (10) | 25 (10) | 26.1 (11) | 28.5 (12) | 25.5 (12) |
| % female subjects DS (n) | 25 (10) | 25 (10) | 24.1 (10) | 23.5 (10) | 27.66 (13) |
| Age: mean years ± SD | 60.3 ± 14.49 | 60.9 ± 13.8 | 57.2 ± 16.8 | 60.9 ± 15.6 | 58.1 ± 15.62 |
| PMI: mean hours ± SD | 44.36 ± 28.91 | 46.28 ± 28.8 | 45.58 ± 28.33 | 47.67 ± 30.58 | 43.69 ± 26.02 |
| pH value: mean ± SD | 6.52 ± 0.35 | 6.55 ± 0.33 | 6.47 ± 0.34 | 6.55 ± 0.36 | 6.56 ± 0.35 |
| RIN value: mean ± SD | 6.99 ± 1.81 | 6.58 ± 0.27 | 6.49 ± 1.89 | 6.62 ± 1.35 | 6.24 ± 1.02 |
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DRN, dorsal raphe nucleus; LC, locus coeruleus; MRN, medullary raphe nucleus; PMI, postmortem interval; RIN, RNA integrity number.
Table S1.
Clinicopathological information for DS subjects and controls
| Sex | Age, y | Cause of death | PMI | pH | Axis 1 | Axis 1 dependence | Substance at death | Psychiatric medication last 3 mo |
| Male | 63 | Accident | 13 | 6.84 | Nil | Nil | Nil | Yes |
| Male | 81 | Accident | 98.7 | 6.80 | Nil | Nil | AD (SSRI), AP | Yes |
| Male | 41 | Natural | 24 | 6.00 | Nil | Nil | Nil | Nil |
| Male | 46 | Natural | 19.5 | 6.42 | Nil | Nil | Nil | Nil |
| Male | 64 | Natural | 70 | 5.65 | Nil | Nil | Nil | N/A |
| Male | 71 | Natural | 17 | 6.20 | Nil | Nil | Nil | Nil |
| Male | 43 | Natural | 27 | 6.70 | Nil | Nil | Cbd + Metab | Nil |
| Male | 51 | Accident | 15 | 6.83 | Nil | Nil | Eth | Nil |
| Male | 55 | Accident | 24 | 6.75 | Nil | Nil | Nil | Nil |
| Male | 40 | Accident | 24 | 6.32 | Nil | SD | Opt, BZ | N/A |
| Male | 42 | Accident | 63 | 6.75 | Nil | Nil | Nil | Nil |
| Male | 59 | Accident | 72.7 | 6.76 | Nil | Nil | Nil | N/A |
| Male | 26 | Accident | 12 | 6.75 | Nil | Nil | Eth, Cbd | Nil |
| Male | 42 | Natural | 20 | 6.62 | Nil | Nil | BZ | BZ |
| Male | 47 | Natural | 12 | 6.49 | Nil | Nil | Nil | Nil |
| Male | 54 | Natural | 25.2 | 6.61 | Nil | Nil | N/A | N/A |
| Male | 52 | Natural | 72.5 | 6.11 | Nil | Nil | Nil | Nil |
| Male | 55 | Natural | 27.5 | 5.80 | Nil | Nil | Nil | N/A |
| Male | 48 | Natural | 14 | 6.25 | Nil | Nil | Eth | Nil |
| Male | 57 | Natural | 115.3 | 6.34 | MDD | SD | BZ, AD (TCA)+Metb, Eth | N/A |
| Male | 40 | Suicide | 23 | 6.21 | MDD | SD | Nil | N/A |
| Male | 42 | Suicide | 21 | 6.40 | MDD | Nil | AD (TCA) | Classic AD, BZ |
| Male | 45 | Suicide | 20.5 | 6.57 | MDD | SD | Eth | N/A |
| Male | 68 | Suicide | 32 | 6.93 | dD, NOS | Nil | Nil | N/A |
| Male | 67 | Suicide | 56 | 6.85 | MDD | Nil | Nil | Nil |
| Male | 77 | Suicide | 26.7 | 6.30 | dD, NOS | Nil | N/A | Nil |
| Male | 64 | Suicide | 27.7 | 6.25 | dD, NOS | Nil | AD (SSRI) | AD (SSRI) |
| Male | 53 | Suicide | 29 | 6.30 | dD, NOS | SD | Nil | N/A |
| Male | 53 | Suicide | 14 | 6.64 | dD, NOS | Nil | N/A | N/A |
| Male | 48 | Suicide | 21.5 | 6.79 | MDD | SD | Eth, AD (SSRI), BZ | AD (SSRI) |
| Male | 39 | Suicide | 90 | 6.74 | dD, NOS | Nil | Eth | N/A |
| Male | 40 | Suicide | 20 | 6.33 | MDD | SD | Eth, BZ, Coc+Metb | AD (SSRI), BZ |
| Male | 42 | Suicide | 64 | 6.78 | MDD | Nil | Eth, DPH+Metb | AD (SSRI) |
| Male | 52 | Suicide | 86.5 | 6.20 | MDD | SD | Eth, Coc | Nil |
| Male | 48 | Suicide | 15 | 6.78 | MDD | SD | SD | Eth, AD (SNRI) |
| Female | 66 | Accident | 61 | 6.80 | Nil | Nil | N/A | Nil |
| Female | 76 | Accident | 26.5 | 6.50 | Nil | Nil | Nil | N/A |
| Female | 81 | Natural | 83 | 6.50 | Nil | Nil | Nil | BZ |
| Female | 72 | Natural | 17 | 6.10 | Nil | Nil | N/A | Nil |
| Female | 51 | Natural | 111.3 | 6.50 | Nil | Nil | AD (SSRI) | Classic AD |
| Female | 68 | Natural | 74.3 | 6.21 | Nil | Nil | Mor, AH | N/A |
| Female | 81 | Natural | 44.6 | 5.91 | Nil | Nil | BZ, DPH | Nil |
| Female | 49 | Accident | 67.2 | 6.81 | Nil | Nil | DPH | N/A |
| Female | 79 | Accident | 61.5 | 6.40 | Nil | Nil | Barb, BZ | Nil |
| Female | 82 | Natural | 106 | 7.00 | Nil | Nil | Nil | N/A |
| Female | 40 | Natural | 106.5 | 6.50 | dD, NOS | Nil | N/A | Nil |
| Female | 76 | Natural | 10.4 | 5.79 | Nil | Nil | N/A | Nil |
| Female | 70 | Natural | 37 | 5.7 | Nil | Nil | N/A | Nil |
| Female | 86 | Natural | 23.2 | 5.65 | Nil | Nil | N/A | Nil |
| Female | 75 | Natural | 10.7 | 5.68 | Nil | Nil | N/A | Nil |
| Female | 65 | Suicide | 64 | 6.31 | MDD | Nil | BZ, Cd | AD (SSRI, SARI, SNDRI & NaSSA), BZ |
| Female | 49 | Suicide | 59.5 | 7.50 | MDD | Nil | Opd, BZ, Opt | AD (SSRI), BZ |
| Female | 55 | Suicide | 26.2 | 6.50 | MDD | Nil | Eth | Antimanic |
| Female | 75 | Suicide | 97 | 6.50 | MDD | Nil | AD (SNRI), BZ | AD (SNRI), AP |
| Female | 85 | Suicide | 87 | 6.50 | MDD | Nil | βB, AD (SSRI) | AD (SSRI & SNRI) |
| Female | 80 | Suicide | 46.9 | 7.00 | dD, NOS | Nil | AD (SSRI) and metab | AD (SSRI) |
| Female | 59 | Suicide | 25.6 | 6.27 | MDD | Nil | AD (NaSSA) | AD (SSRI), BZ |
| Female | 25 | Suicide | 20 | 6.73 | dD, NOS | Nil | Nil | N/A |
| Female | 46 | Suicide | 15 | 6.53 | MDD | Nil | Eth, Barb, BZ | AD (SSRI & NaSSA), AP, BZ |
| Female | 40 | Suicide | 49.5 | 6.81 | MDD | Nil | AC, AD (SNRI), BZ | AD (TCA & SNRI), BZ, AC |
| Female | 25 | Suicide | 56 | 6.55 | MDD | Nil | N/A | AP |
| Female | 51 | Suicide | 36 | 6.86 | MDD | SD | Cd, Eth, Opt | AD (SSRI), BZ |
| Female | 44 | Suicide | 60 | 6.86 | MDD | SD | Eth | Nil |
| Female | 32 | Suicide | 41 | 6.89 | MDD | Nil | N/A | AD (SSRI), BZ |
| Female | 41 | Suicide | 54.2 | 6.70 | MDD | Nil | Nil | N/A |
| Female | 48 | Suicide | 36.7 | 6.50 | MDD | Nil | BZ, AD (Non-TCA & SNRI) | N/A |
AC, anticonvulsant; AD, antidepressant: AH, antihistamine; AP, antipsychotic; Barb, barbiturate; βB, β-blocker; BZ, benzodiazepine; Cbd, cannabinoid; Cd, codeine; Coc, cocaine; dD, depressive disorders; DPH, diphenhydramine; Eth, ethanol; MDD, major depressive disorder; Metab, metabolite; Mor, morphine; NaSSA, noradrenergic and specific serotonergic antidepressant; N/A, not available; NOS, not otherwise specified; Opd, opioid; Opt, opiate; SARI, serotonin antagonist and reuptake inhibitor; SD, substance dependence; SNDRI, serotonin-norepinephrine-dopamine reuptake inhibitor; SNRI, serotonin and noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Sample Anatomy.
The five brain regions analyzed encompass a heterogeneous collection of neurons. It is important to note that in the samples labeled locus coeruleus and especially in those labeled dorsal raphe and medullary raphe only a part of these neurons are monoaminergic (SI Materials and Methods, Brain Samples).
The Effects of Medication.
An inherent problem of autopsy analysis is the effect of medication, particularly when looking at a dynamic parameter, such as transcripts. For example, it has been reported that an antidepressant can exert epigenetic changes (61, 62). We have analyzed the data using ANOVA followed by Fisher’s least significant difference post hoc test and ANCOVA by treating antidepressants as a confounding factor to compare the effects in male and female DS subjects. The samples were divided into four groups based on the different medications, namely (i) SSRIs, (ii) SSRI + benzodiazepines, (iii) SSRI + others (including SNRIs, serotonin antagonist and reuptake inhibitors, serotonin-norepinephrine-dopamine reuptake inhibitors, tricyclic antidepressants, and noradrenergic and specific serotonergic antidepressants; details are given in Table S1), and (iv) none. Based on the analysis, we conclude that in our study the various psychiatric medications show no significant effect on the gene expression of GAL and GalR3 in the five regions analyzed. The longer imprints of DNA methylation would require analysis of the complete anamnesis.
Transcript Levels Vary Across Brain Regions.
The overall distribution and levels of the transcripts of the galanin system are summarized in Table 2. Briefly, transcripts for GAL and GAL1–3 are differentially expressed in the five brain regions, with the difference reaching statistical significance for all the markers studied (see Tables S2 and S3 for P values). GAL is expressed at high levels in the lower brainstem and at three- to-fourfold lower levels in the two cortical regions. The most prominent receptor transcript overall is GALR1, with up to eightfold differences in regional expression (dorsolateral prefrontal cortex > anterior cingulate cortex). The GALR2 mRNA level is high in the dorsal raphe nucleus and low in medullary raphe nuclei. GALR3 mRNA follows the pattern of GAL with high levels in the lower brainstem and low levels in cortex.
Table 2.
Raw cycle threshold (Ct) values ± SEM for samples from the regions analyzed in male and female controls and DS subjects
| Region | GAL | GalR1 | GalR2 | GalR3 |
| DLPFC | 27.1 ± 0.2 | 25.7 ± 0.2 | 31.4 ± 0.2 | 33.2 ± 0.1 |
| ACC | 27.5 ± 0.1 | 28.6 ± 0.1 | 31.6 ± 0.2 | 33.5 ± 0.2 |
| DRN | 26.2 ± 0.4 | 27 ± 0.4 | 29.3 ± 0.2 | 31.5 ± 0.4 |
| LC | 24.6 ± 0.5 | 26.4 ± 0.6 | 32.3 ± 0.1 | 31.4 ± 0.6 |
| MRN | 25.2 ± 0.4 | 26.9 ± 0.4 | 34.6 ± 0.2 | 31.9 ± 0.4 |
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DRN, dorsal raphe nucleus; LC, locus coeruleus; MRN, medullary raphe nucleus.
Table S2.
Raw Ct values ± SEM for the samples analyzed from male and female control and DS subjects
| Region | GAL | GalR1 | GalR2 | GalR3 |
| DLPFC | 27.1 ± 0.2 | 25.7 ± 0.2 | 31.4 ± 0.2 | 33.2 ± 0.1 |
| ACC | 27.5 ± 0.1 | 28.6 ± 0 | 31.6 ± 0.2 | 33.5 ± 0.2 |
| DRN | 26.2 ± 0.4 | 27 ± 0.4 | 29.3 ± 0.2 | 31.5 ± 0.4 |
| LC | 24.6 ± 0.5 | 26.4 ± 0.6 | 32.3 ± 0.1 | 31.4 ± 0.6 |
| MRN | 25.2 ± 0.4 | 26.9 ± 0 | 34.6 ± 0.2 | 31.9 ± 0.4 |
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DRN, dorsal raphe nucleus; LC, locus coeruleus; MRN, medullary raphe nucleus.
Table S3.
The matrix of pairwise comparisons probabilities shows ANOVA probability at the top and the result of Tukey’s honestly significant difference multiple comparisons
| Variable | DLPFC | ACC | DRN | LC | MRN |
| GAL (P < 0.001) | |||||
| DLPFC | 1.000 | ||||
| ACC | 0.748 | 1.000 | |||
| DRN | <0.001 | 0.015 | 1.000 | ||
| LC | <0.001 | <0.001 | <0.001 | 1.000 | |
| MRN | <0.001 | <0.001 | <0.001 | 0.556 | 1.000 |
| GalR1 (P < 0.001) | |||||
| DLPFC | 1.000 | ||||
| ACC | <0.001 | 1.000 | |||
| DRN | <0.001 | <0.001 | 1.000 | ||
| LC | <0.001 | 0.193 | 0.081 | 1.000 | |
| MRN | <0.001 | 0.010 | 0.645 | 0.782 | 1.000 |
| GalR2 (P < 0.001) | |||||
| DLPFC | 1.000 | ||||
| ACC | 0.718 | 1.000 | |||
| DRN | <0.001 | <0.001 | 1.000 | ||
| LC | 0.015 | <0.001 | <0.001 | 1.000 | |
| MRN | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 |
| GalR3 (P < 0.001) | |||||
| DLPFC | 1.000 | ||||
| ACC | 0.230 | 1.000 | |||
| DRN | <0.001 | <0.001 | 1.000 | ||
| LC | <0.001 | <0.001 | 0.190 | 1.000 | |
| MRN | <0.001 | <0.001 | 0.154 | <0.001 | 1.000 |
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DRN, dorsal raphe nucleus; LC, locus coeruleus; MRN, medullary raphe nucleus.
Expression of GAL and Its Receptors Is Altered in the Brains of DS Subjects.
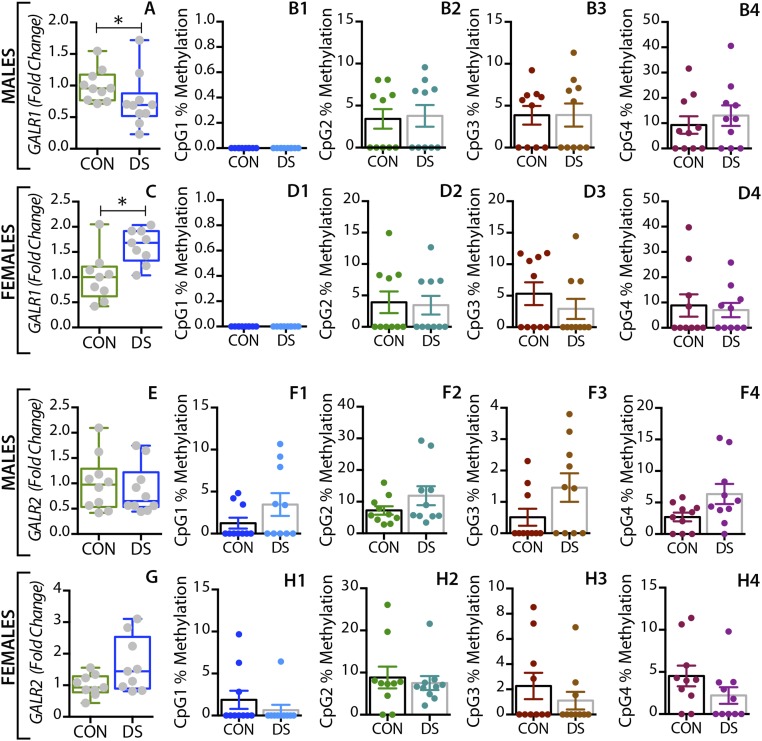
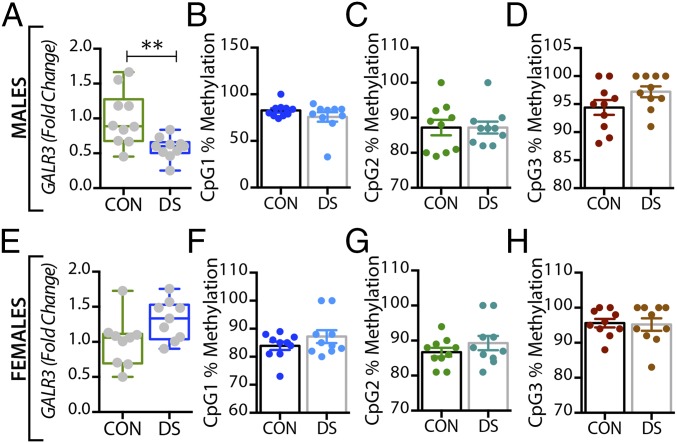
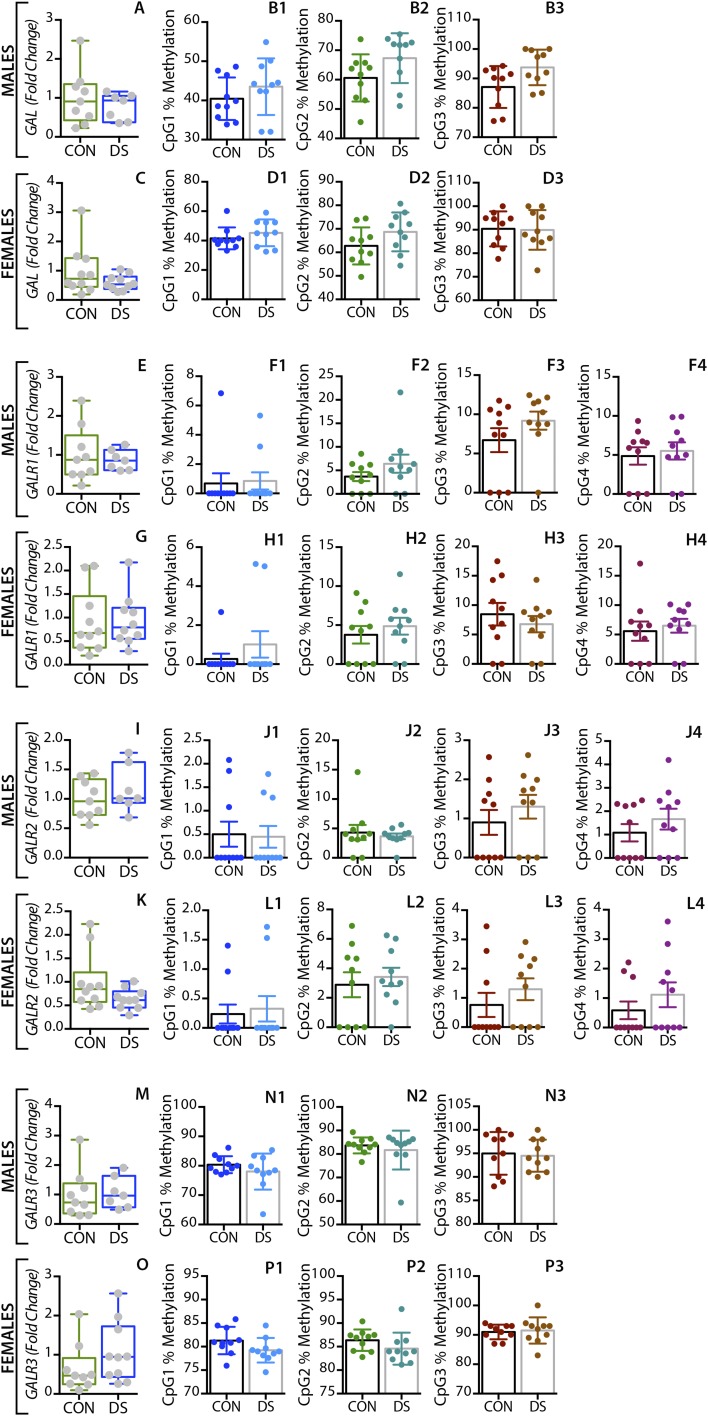
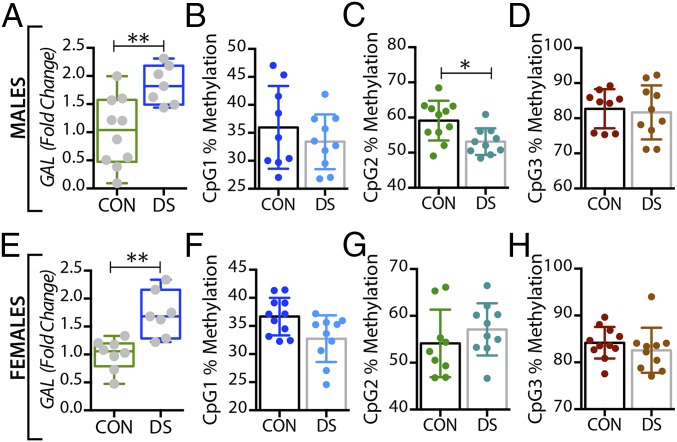
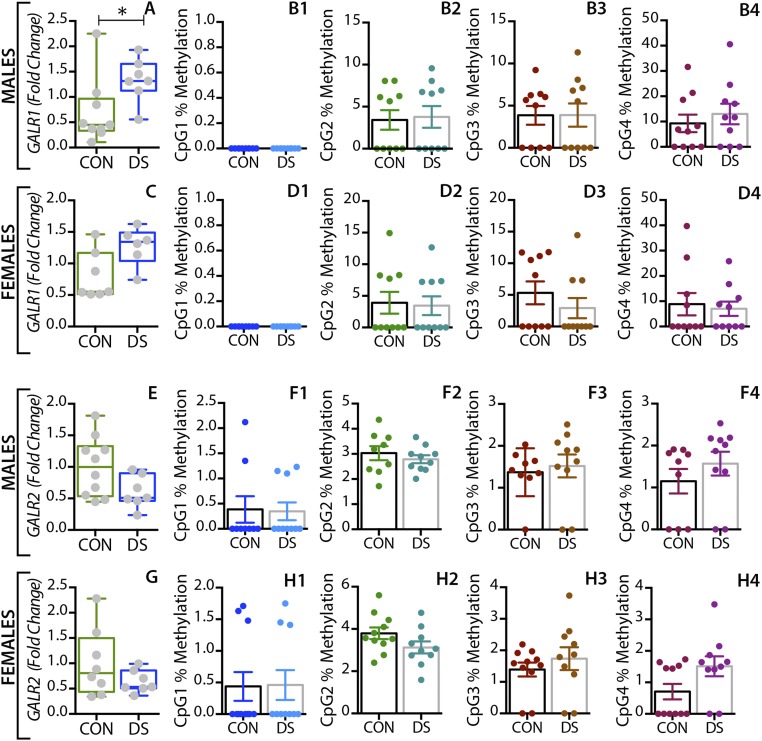
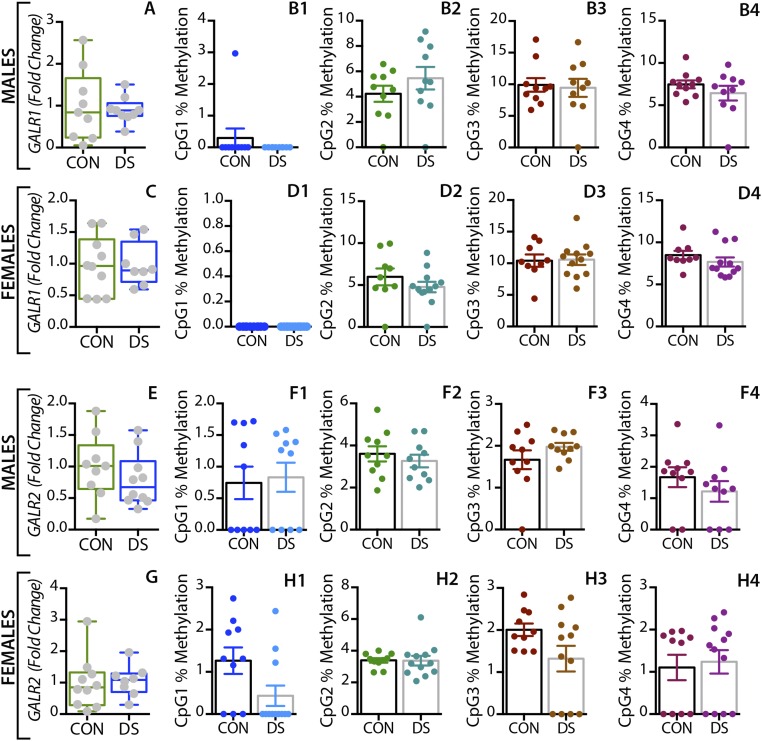
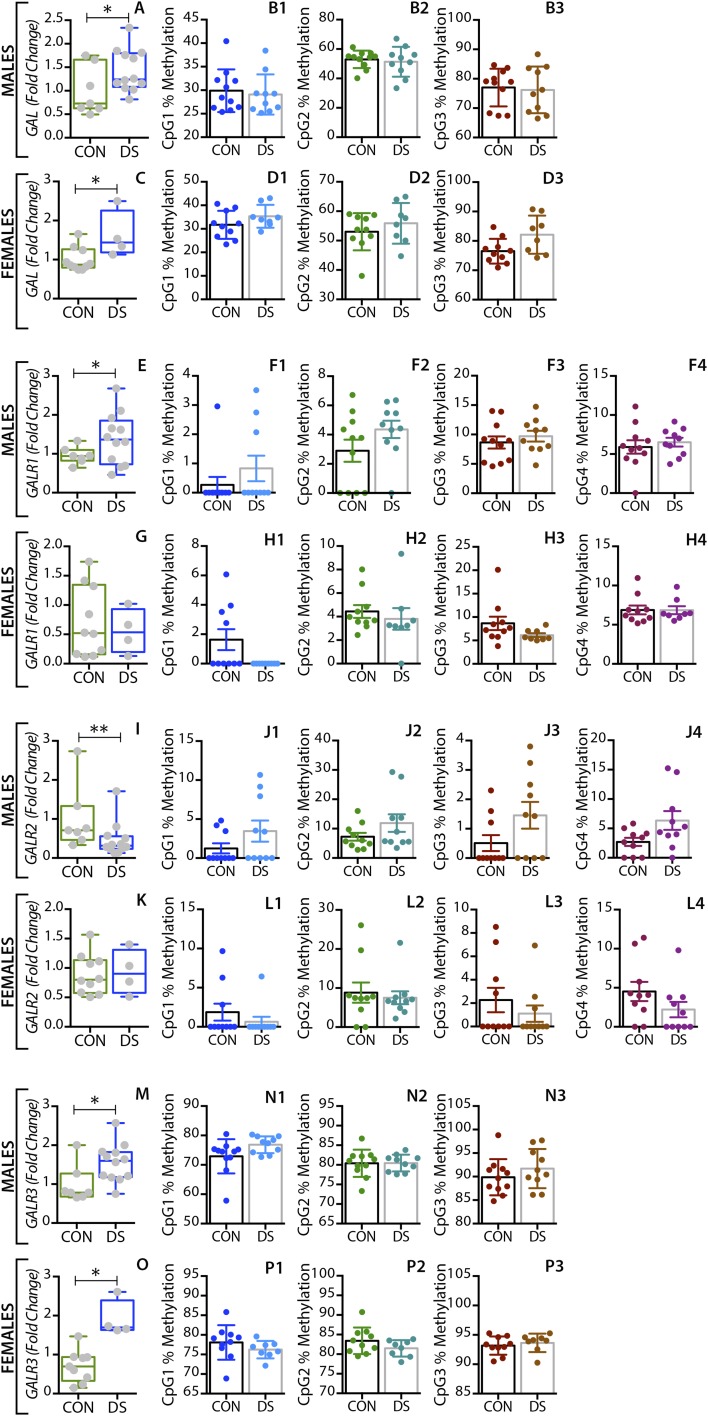
In the dorsolateral prefrontal cortex, GAL mRNA levels were significantly decreased in male DS subjects compared with controls (P < 0.01) (Fig. 1A) and were significantly increased in female DS subjects (P < 0.05) (Fig. 1E), a distinct sex difference. GALR1 mRNA levels were significantly increased in both male and female DS subjects (P < 0.05) (Fig. S1 A and C). GALR3 mRNA levels were significantly decreased in male (P < 0.01) (Fig. 2A) but not in female (Fig. 2E) DS subjects. However, there were no significant changes in GALR2 mRNA levels in either sex (Fig. S1 E and G). In the anterior cingulate cortex, no significant changes in GAL or its receptors were found (Fig. S2).
Fig. 1.
Alterations in GAL gene expression and DNA methylation in postmortem dorsolateral prefrontal cortex (BA 8/9) of male and female DS subjects. (A and E) Quantitative RT-PCR was used to examine the mRNA expression levels of GAL in the dorsolateral prefrontal cortex of male (A) and female (E) controls and DS subjects. The expression level of GAL was normalized to HPRT levels and expressed relative to their respective control levels. (B–D and F–H) Bisulfite pyrosequencing was used to quantify percentage of DNA methylation levels at individual CpG sites of the GAL gene in male (B–D) and female (F–H) controls and DS subjects. All data are presented as mean ± SEM; n = 10 per group. Significant differences between DS subjects and controls are indicated: *P < 0.05, **P < 0.01. CON, controls; DS, depressed suicide.
Fig. S1.
Alterations in GALR1 and GALR2 gene expression and DNA methylation in the dorsolateral prefrontal cortex of male and female DS subjects. Gene-expression levels of GALR1 and GALR2 in the dorsolateral prefrontal cortex of male (A and E) and female (C and G) controls and DS subjects. Percent DNA methylation levels at individual CpG sites of the GALR1 and GALR2 gene in male (B and F) and female (D and H) control and DS subjects. All data are presented as mean ± SEM; n = 10 per group. Significant differences between DS subjects and controls are indicated: *P < 0.05. CON, controls; DS, depressed suicide.
Fig. 2.
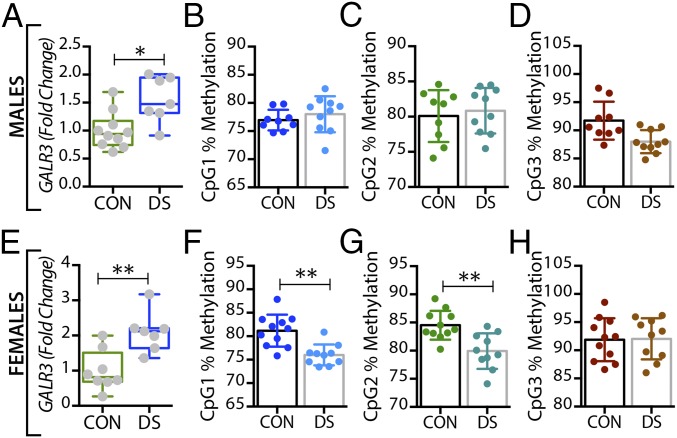
Alterations in GALR3 mRNA and DNA methylation in the dorsolateral prefrontal cortex (BA 8/9) of male and female DS subjects. (A and E) Gene-expression levels of GALR3 in the dorsolateral prefrontal cortex of male (A) and female (E) controls and DS subjects. (B–D and F–H) Percentage of DNA methylation levels at individual CpG sites of the GALR3 gene in male (B–D) and female (F–H) controls and DS subjects. All data are presented as mean ± SEM; n = 10 per group. Significant differences between DS subjects and controls are indicated: **P < 0.01. CON, controls; DS, depressed suicide.
Fig. S2.
Alterations in GAL and GALR1-3 gene expression and DNA methylation in the anterior cingulate cortex of male and female DS subjects. Gene-expression levels of GAL and GALR1-3 in the the anterior cingulate cortex of male (A, E, I, M) and female (C, G, K, O) controls and DS subjects. Percent DNA methylation levels at individual CpG sites of the GAL and GALR1-3 genes in male (B, F, J, N) and female (D, H, L, P) control and DS subjects. All data are presented as mean ± SEM n = 10 per group. CON, controls; DS, depressed suicide.
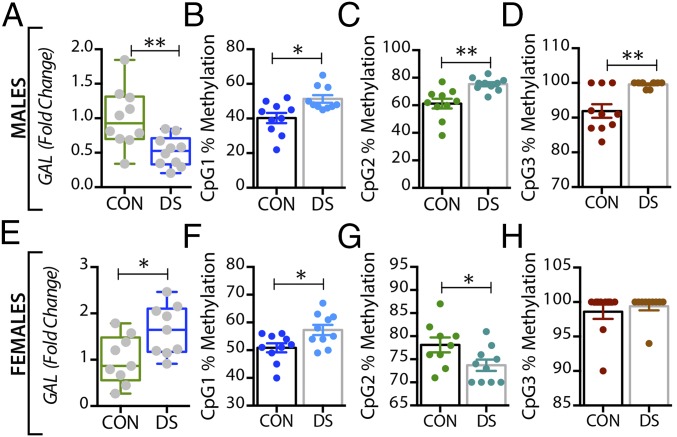
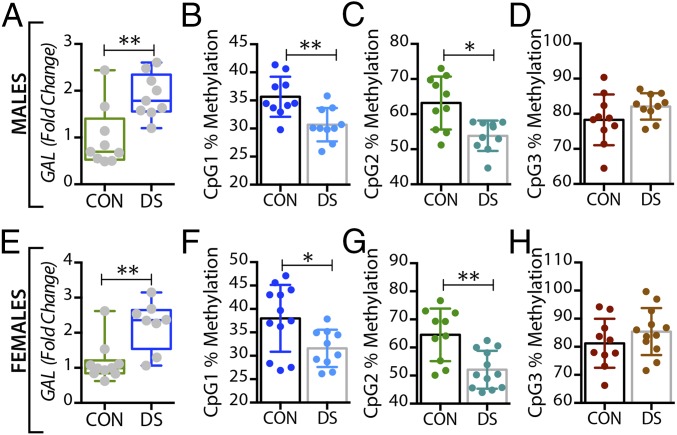
In the dorsal raphe nucleus and locus coeruleus, GAL mRNA levels were significantly increased in both male (P < 0.01) (Figs. 3A and 4A) and female (P < 0.01) (Figs. 3E and 4E) DS subjects. Interestingly, higher levels of tryptophan hydroxylase-2 (TPH2) mRNA and immunoreactive neurons have previously been shown in the dorsal raphe nucleus of suicides relative to normal controls (63, 64). GALR1 mRNA levels were significantly increased only in the dorsal raphe nucleus of male DS subjects (P < 0.05, Fig. S3 A and C) but were not increased in the locus coeruleus of these subjects (Fig. S4 A and C). The expression levels of GALR3 were significantly increased in both the dorsal raphe nucleus (Fig. 5 A and E) and locus coeruleus (Fig. 6 A and E) in both sexes, more robustly in females (female vs. males: P < 0.01 vs. P < 0.05). No significant changes were found in GALR2 mRNA levels in these two regions (Figs. S3 E and G and S4 E and G).
Fig. 3.
Alterations in GAL gene expression and DNA methylation in the dorsal raphe nucleus of male and female DS subjects. (A and E) Gene-expression levels of GAL in the dorsal raphe nucleus of male (A) and female (E) controls and DS subjects. (B–D and F–H) Percentage of DNA methylation levels at individual CpG sites of the GAL gene in male (B–D) and female (F–H) controls and DS subjects. All data are presented as mean ± SEM; males: n = 11 controls, 10 DS subjects; females: n = 11 controls, 10 DS subjects. Significant differences between DS subjects and controls are indicated: *P < 0.05, **P < 0.01. CON, controls; DS, depressed suicide.
Fig. 4.
Alterations in GAL gene expression and DNA methylation in the locus coeruleus of male and female DS subjects. (A and E) Gene-expression levels of GAL in the locus coeruleus of male (A) and female (E) controls and DS subjects. (B–D and F–H) Percentage of DNA methylation levels at individual CpG sites of the GAL gene in male (B–D) and female (F–H) controls and DS subjects. All data are presented as mean ± SEM; males: n = 10 controls, 10 DS subjects; females: n = 12 controls, 10 DS subjects. Significant differences between DS subjects and controls are indicated: *P < 0.05, **P < 0.01. CON, controls; DS, depressed suicide.
Fig. S3.
Alterations in GALR1 and GALR2 gene expression and DNA methylation in the dorsal raphe nucleus of male and female DS subjects. Gene-expression levels of GALR1 and GALR2 in the dorsal raphe nucleus of male (A and E) and female (C and G) controls and DS subjects. Percent DNA methylation levels at individual CpG sites of the GALR1 and GALR2 gene in male (B and F) and female (D and H) control and DS subjects. All data are presented as mean ± SEM, n = 10 per group. Significant differences between DS subjects and controls are indicated: *P < 0.05. CON, controls; DS, depressed suicide.
Fig. S4.
Alterations in GALR1 and GALR2 gene expression and DNA methylation in the locus coeruleus of male and female DS subjects. Gene-expression levels of GALR1 and GALR2 in the locus coeruleus of male (A and E) and female (C and G) controls and DS subjects. Percent DNA methylation levels at individual CpG sites of the GALR1 and GALR2 gene in male (B and F) and female (D and H) control and DS subjects. All data are presented as mean ± SEM; n = 10 per group. CON, controls; DS, depressed suicide.
Fig. 5.
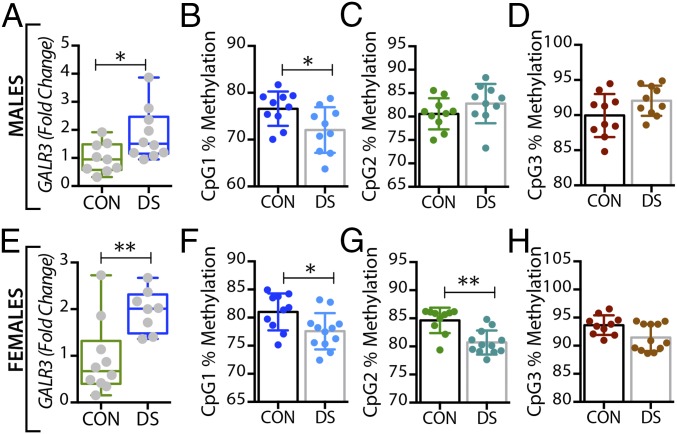
Alterations in GALR3 gene expression and DNA methylation in the dorsal raphe nucleus of male and female DS subjects. (A and E) Gene-expression levels of GALR3 in the dorsal raphe nucleus of male (A) and female (E) controls and DS subjects. (B–D and F–H) Percentage of DNA methylation levels at individual CpG sites of the GALR3 gene in male (B–D) and female (F–H) controls and DS subjects. All data are presented as mean ± SEM; males: n = 11 controls, 10 DS subjects; females: n = 11 controls, 10 DS subjects. Significant differences between DS subjects and controls are indicated: *P < 0.05, **P < 0.01. CON, controls; DS, depressed suicide.
Fig. 6.
Alterations in GALR3 gene expression and DNA methylation in the locus coeruleus of male and female DS subjects. (A and E) Gene expression levels of GALR3 in the locus coeruleus of male (A) and female (E) controls and DS subjects. (B–D and F–H) Percentage of DNA methylation levels at individual CpG sites of the GALR3 gene in male (B–D) and female (F–H) controls and DS subjects. All data are presented as mean ± SEM; males: n = 10 controls, 10 DS subjects; females: n = 12 controls, 10 DS subjects. Significant differences between DS subjects and controls are indicated: *P < 0.05, **P < 0.01. CON, controls; DS, depressed suicide.
In the medullary raphe nuclei, GAL mRNA levels were significantly increased in both male and female DS subjects (P < 0.05) (Fig. S5 A and C). In male DS subjects, the GALR1 expression levels were significantly increased (P < 0.05) (Fig. S5E), whereas GALR2 mRNA levels were significantly decreased (P < 0.01) (Fig. S5I). However, there were no significant changes in female DS subjects. On the other hand, the expression levels of GALR3 were significantly increased in both sexes (P < 0.05) (Fig. S5 M and O). All mRNA changes are summarized in Table 3.
Fig. S5.
Alterations in GAL and GALR1-3 gene expression and DNA methylation in the medullary raphe nuclei of male and female DS subjects. Gene expression levels of GAL and GALR1-3 in the medullary raphe nuclei of male (A, E, I, and M) and female (C, G, K, and O) controls and DS subjects. Percent DNA methylation levels at individual CpG sites of the GAL and GALR1-3 genes in male (B, F, J, and N) and female (D, H, L, and P) control and DS subjects. All data are presented as mean ± SEM; n = 10 per group. Significant differences between DS subjects and controls are indicated: *P < 0.05, **P < 0.01. CON, controls; DS, depressed suicide.
Table 3.
Overview of mRNA and DNA methylation changes
| GAL | GALR1 | GALR2 | GALR3 | ||||||||||||||||
| gDNA methylation | gDNA methylation | gDNA methylation | gDNA methylation | ||||||||||||||||
| Regions | Sex | mRNA | CpG1 | CpG2 | CpG3 | mRNA | CpG1 | CpG2 | CpG3 | CpG4 | mRNA | CpG1 | CpG2 | CpG3 | CpG4 | mRNA | CpG1 | CpG2 | CpG3 |
| DLPFC | M | ↓↓ | ↑ | ↑↑ | ↑↑ | ↑ | — | — | — | — | — | — | — | — | — | ↓↓ | — | — | — |
| F | ↑ | ↑ | ↓ | — | ↑ | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| ACC | M | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| F | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| DRN | M | ↑↑ | — | ↓ | ↑ | — | — | — | — | — | — | — | — | — | ↑ | — | — | — | |
| F | ↑↑ | — | — | — | — | — | — | — | — | — | — | — | — | — | ↑↑ | ↓↓ | ↓↓ | — | |
| LC | M | ↑↑ | ↓↓ | ↓ | — | — | — | — | — | — | — | — | — | — | — | ↑ | ↓ | — | |
| F | ↑↑ | ↓ | ↓↓ | — | — | — | — | — | — | — | — | — | — | — | ↑↑ | ↓ | ↓↓ | — | |
| MRN | M | ↑ | — | — | — | ↑ | — | — | — | — | ↓↓ | — | — | — | — | ↑ | — | — | — |
| F | ↑ | — | — | — | — | — | — | — | — | — | — | — | — | ↑ | — | — | — | ||
The arrows represent statistical significance; upward arrows signify increased in gene expression and methylation status, and downward arrows signify decreased gene expression and methylation status. A single arrow represents P < 0.05, and two arrows represent P < 0.01. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DRN, dorsal raphe nucleus; LC, locus coeruleus; MRN, medullary raphe nucleus.
The DNA Methylation Status of GAL and GALR3 Changes in the Brain of DS Patients.
In the dorsolateral prefrontal cortex, At the first three CpG sites analyzed (CpG1–3, Fig. 1B–D), the percentage of methylated DNA was significantly higher in male DS subjects than in controls (P < 0.05, P < 0.01, and P < 0.01, respectively). In female DS subjects, the methylation status was different among the CpG sites, with a significant increase at CpG1 and a significant decrease at CPG2 (P < 0.05) (Fig. 1 F and G, respectively), and with no changes at CpG3 (Fig. 1H). No significant changes were found for any of the receptors in this brain region (Fig. 2 B–H and Fig. S1) or for any of the four markers in the anterior cingulate cortex (Fig. S2).
In the dorsal raphe nucleus, a significant decrease was seen in the percentage of methylated DNA in the GAL promoter of male DS subjects, but only at the CpG1 site (P < 0.05) (Fig. 3B), with no changes in female DS subjects (Fig. 3 F–H). Interestingly, GALR3 was the only receptor that was significantly altered in the brains of DS subjects in a sex-dependent manner. Thus, only female DS subjects showed a significant decrease in the percentage of methylated DNA at CpG1 and CpG2 sites (P < 0.01) (Fig. 5 F and G, respectively).
In the locus coeruleus the percentage of methylated DNA in the GAL promoter at CpG1 and CPG2 was significantly decreased in DS subjects (male: P < 0.01 and P < 0.05; female: P < 0.05 and P < 0.01) (Fig. 4 B and C and F and G, respectively). There was a significant decrease in the percentage of methylated DNA at the CpG1 site of the GALR3 gene in DS subjects of both sexes (P < 0.05) (Fig. 6 B and F), whereas methylation at the CpG2 site was significantly decreased only in female DS subjects (P < 0.01) (Fig. 6G). No changes were found at the CpG3 site (Fig. 6 D and H).
There were no significant changes in the methylation status of GAL or its receptors in either the anterior cingulate cortex or the medullary raphe nuclei (Figs. S2 and S5). All methylation changes are summarized in Table 3. For the region including differentially methylated CpG sites in GAL and GALR3, a number of potential transcription factor-binding sites were identified using the JASPER database (Fig. S6C) (65, 66).
Fig. S6.
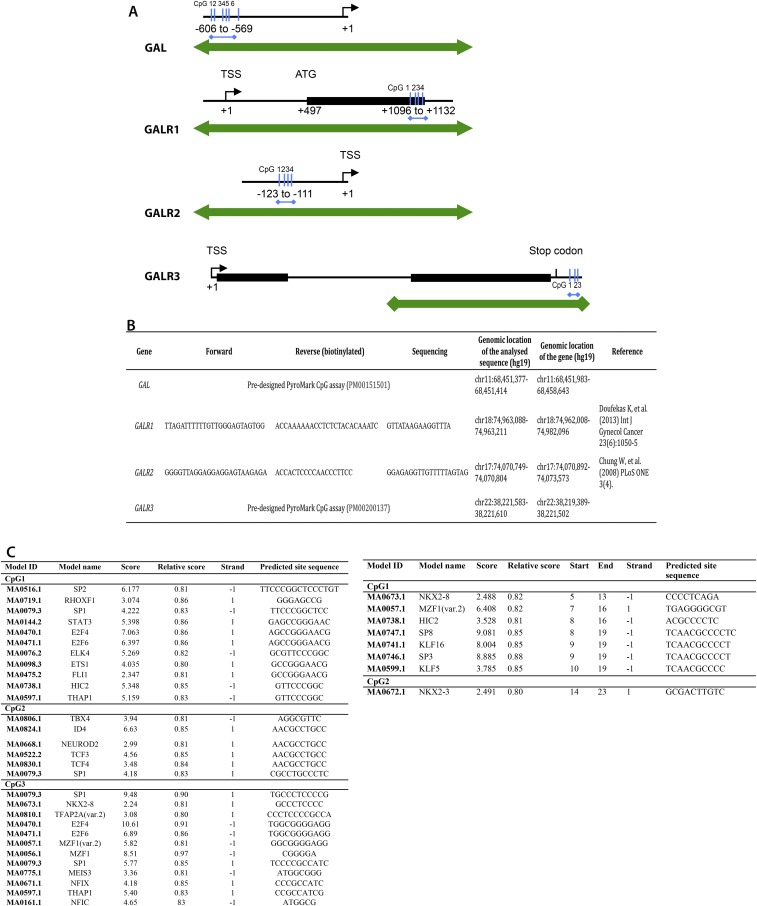
Genomic location of the regions analyzed by bisulfite pyrosequencing (A) and their respective primer sequences (B). Transcription factor binding sites analyzed by JASPER for GAL and GALR3 (C). Analyzed regions (blue arrows): GAL: promoter region between −606 and −569 bp upstream of the transcriptional start site (TSS); GALR1: exon 1 between 1,096 and 1,132 bp downstream of the TSS; GALR2: promoter region between −123 and −111 bp upstream of the TSS; GALR3 gene: between 83 and 107 bp downstream of the end of the gene. Double-headed green arrows depict CpG islands.
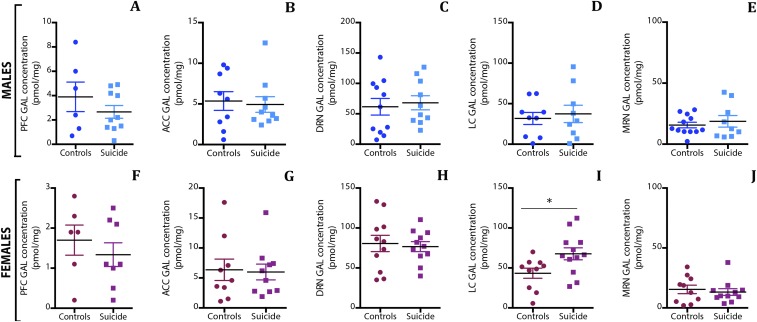
Increased Concentrations of GAL Protein in the Locus Coeruleus in Female DS Subjects.
The RIA monitors intracellular, vesicle-stored GAL concentrations and not extracellular peptide, which is rapidly degraded (67). The concentrations varied considerably among the regions analyzed, with low and intermediate levels in the forebrain and medullary raphe nuclei, respectively, and high and very high levels in locus coeruleus and dorsal raphe nucleus, respectively (Table 4). These results are reflected in the transcript levels. With regard to differences between DS subjects and controls, significant changes were only found in the female locus coeruleus (P = 0.025) (Fig. S7I), where the mean concentration was 56% higher in DS subjects than in controls (67.7 vs. 43.4 pmol/mg) (Table 4). In the dorsolateral prefrontal cortex there was an apparent difference between male and female controls, on the one hand, and male and female DS subjects, on the other. However, the differences in the mean and median concentrations of immunoreactive GAL did not reach statistical significance (P < 0.05) using either logarithmically transformed data in a parametric ANOVA or in a Kruskal–Wallis nonparametric test on nontransformed data.
Table 4.
Concentration of GAL (picomoles per milligram ± SEM) in the different regions analyzed by RIA
| Subject | DLPFC | ACC | DRN | LC | MRN |
| Male controls | 3.89 ± 1.21 | 5.35 ± 1.15 | 61.58 ± 13.74 | 31.80 ± 7.46 | 15.65 ± 2.32 |
| Male DS subjects | 2.68 ± 0.52 | 4.94 ± 0.96 | 68.14 ± 11.6 | 37.39 ± 10.71 | 18.73 ± 4.76 |
| Female controls | 1.70 ± 0.38 | 6.37 ± 1.79 | 80.62 ± 10.24 | 43.35 ± 6.33 | 15.45 ± 3.53 |
| Female DS subjects | 1.33 ± 0.29 | 5.97 ± 1.32 | 81.61 ± 5.19 | 67.68 ± 7.47 | 13.55 ± 2.95 |
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DRN, dorsal raphe nucleus; LC, locus coeruleus; MRN, medullary raphe nucleus.
Fig. S7.
RIA of GAL-like immunoreactivity (GAL-LI) in tissue extracts from male (A–E) and female (F–J) DS subjects and controls. The GAL concentration was measured in the five regions studied, and a significant increase was observed in the locus coeruleus of female DS subjects compared with controls. Data are shown as mean ± SEM. ACC, anterior cingulate cortex; DRN, dorsal raphe nucleus; LC, locus coeruleus; MRN, medullary raphe nucleus; PFC, prefrontal cortex. *P < 0.05.
SI Materials and Methods
Brain Samples.
Postmortem brain tissue was obtained by collaboration with the Quebec Coroner’s Office and the Suicide Section of the Douglas-Bell Canada Brain Bank (Douglas Mental Health University Institute, Montreal, Quebec, Canada). A total of 212 punched brain samples from five different regions of 61 individuals (controls and DS subjects) were included (Table 1 and Table S1). All individuals were of French-Canadian origin, a homogeneous population, and samples were matched for PMI (the interval between death and freezing of the brain), subject age at death, and tissue pH. Psychological autopsies were performed as described previously for both DS subjects and controls, and diagnoses were established by a panel of psychiatrists based on Diagnostic and Statistical Manual of Mental Disorders, edition 4 (DSM-IV) criteria. Subjects in the control group had died suddenly from accidental or natural causes. Samples were obtained from five different brain regions: dorsolateral prefrontal cortex Brodmann area (BA 8/9), anterior cingulate cortex (BA 24), dorsal raphe nucleus, locus coeruleus, and the medullary raphe nuclei.
Brains from DS subjects and controls underwent a process known as a “psychological autopsy” to retrieve phenotypic information. This proxy-based interview procedure is the accepted standard for obtaining diagnostic information postmortem. Briefly, a few months following death, subjects’ families were contacted, and the person best acquainted with the deceased was recruited to undergo a series of structured interviews. These interviews were supplemented with information from archival material obtained from hospitals, the coroner’s office, and social services. Following the interviews, clinical vignettes were produced and assessed by a panel of clinicians to generate DSM-IV diagnostic criteria. As detailed elsewhere (126), the psychological autopsies provide socio-demographic characteristics, social developmental history, DSM-IV axis I diagnostic information, and behavioral traits. We also obtain toxicological assessments and complete information on medication prescription.
The dorsal raphe nucleus and locus coeruleus were dissected using the following coordinates from a human brainstem atlas (127): dorsal raphe nucleus from Obex +32 to +39, locus coeruleus from Obex +24 to +31, and medullary raphe nuclei (obscurus and magnus raphe) from Obex 0 to +16. It should be noted that the samples labeled “locus coeruleus” and especially those labeled “dorsal raphe nucleus” include many different neuron populations that are not noradrenergic or serotonergic, respectively.
Ethical approval for this study was obtained from the Institutional Review Board of the Douglas Mental Health University Institute, and written informed consent was obtained from the family of each deceased subject before inclusion in the study. The Regional Ethical Board in Stockholm has granted The Karolinska Institutet group permission no. 2013/474-31/2 for processing postmortem brain samples.
RNA Isolation and Integrity Analysis.
Total RNA from each of the 212 samples was isolated using the RNeasy Plus Mini Kit (Qiagen). RNA quantity and quality were determined spectrophotometrically by using a ND1000 nanodrop (Saveen Werner). RNA integrity was checked using an Experion automated electrophoresis system (Bio-Rad). All samples that showed a RIN higher than 5 were considered good-quality RNA samples, and samples with a RIN higher than 8 were considered as perfect (128). Samples with very low RNA concentrations and integrity (RIN <4) were excluded from the RT-qPCR analysis (n = 27 of 212 punched samples). Total RNA was reverse transcribed to generate cDNA using a High-Capacity Reverse Transcription Kit (Life Technologies) according to the manufacturer’s instructions.
RT-qPCR.
RT-qPCR was performed as described previously (32) with some modifications. Briefly, 500 ng of RNA was reverse transcribed using the High-Capacity Reverse Transcription Kit (Life Technologies) with the cDNA subjected to 40 cycles of amplification and by using TaqMan gene-expression assays and TaqMan PCR Master Mix (Life Technologies) using the 7500 Fast Real-Time PCR System (Life Technologies). cDNA samples were loaded in duplicate, and the expression assays used four markers of the GAL system (GAL and the galanin receptor subtypes GALR1–3) in addition to the endogenous controls. Random samples were cloned into PCR II TOPO TA cloning vector (Life Technologies) and were sequenced at KIGene, Karolinska Institutet to confirm the specificity of the amplification reactions. A no-template control (NTC) reaction and an RT control reaction were used to check for unspecific amplification and amplification from gDNA, respectively. Relative fold changes were calculated by the comparative Ct method (2−ΔΔCT).
gDNA Extraction and PyroPCR.
gDNA from the 212 samples of the five regions was isolated using the DNeasy Blood and Tissue Kit (Qiagen). The quality and quantity of the isolated gDNA was checked spectrophotometrically, and 400 ng of gDNA from each sample was bisulfite-converted using the EZ DNA Methylation-Gold Kit (Zymo Research). One microliter of the converted DNA was subjected to 40 cycles of amplification using gene-specific primers and the pyroMark PCR kit (Qiagen). Details of the forward and biotinylated reverse primers are given in Fig. S6B. One-fifth of the PCR product was checked for specific amplification on 2% agarose gel by electrophoresis, and the remainder was used for bisulfite pyrosequencing.
Bisulfite Pyrosequencing.
Bisulfite pyrosequencing was performed using PyroMark Q96 ID (Qiagen) according to the manufacturer’s protocol and as previously described (129). Briefly, PCR products were mixed with binding buffer (Qiagen) and Sepharose beads (Sarstedt) with constant shaking using the Thermomixer Model 5350 Incubator shaker (Eppendorf) at 200 rpm for 10 min. In parallel, sequencing plates were loaded with sequencing primers for GAL and its receptors’ genes and annealing buffer. The vacuum workstation also was prepared with the buffers required for the washes. After mixing, vacuum was applied, and the PCR primers bound to the Sepharose beads were aspirated using the filter probes. The filter probes were flushed for 5 s in 70% ethanol, 10 s in denaturation buffer (Qiagen), and 10 s in wash buffer (Qiagen). With vacuum ON, the tool was raised beyond 90° vertical for 5 s; then, with vacuum OFF, the filter probes were lowered into the sequencing plate and agitated mildly to release the beads into the wells. The samples were heated at 80° for 2 min and then were cooled to room temperature for 5 min. Then the plate was processed for sequencing. The methylation percentage at each CpG site was analyzed using the PyroMark Q96 software (Qiagen).
RIA.
Human GAL was measured using antiserum G-026-01 (Phoenix Europe GmbH) raised in a rabbit against human GAL and cross-reacting 100% to porcine and rat GAL. It has no cross-reactivity to secretin, neuropeptide tyrosine (NPY), vasoactive intestinal polypeptide (VIP), peptide histidine-methionine (PHM-27), or GAL message-associated peptide [GMAP(1–41)]. HPLC-purified 125I-human GAL (Bachem) was used as the radioligand, and human GAL (Bachem) was used as the calibrator. Sac-Cel (IDS, Ltd.) was used to separate bound and free fractions. Tissue samples were weighed on a microscale and extracted in 1 mL boiling 1 mol/L acetic acid for 10 min before sonication and lyophilization. The pellet was resuspended in RIA buffer by sonication. The tissue concentrations of GAL were influenced significantly by brain-specific regions (P < 0.001) but not by sex (P = 0.157). The Shapiro–Wilks test found a non-Gaussian distribution in data from the anterior cingulate cortex but not from other brain regions. Therefore nontransformed data were used.
Discussion
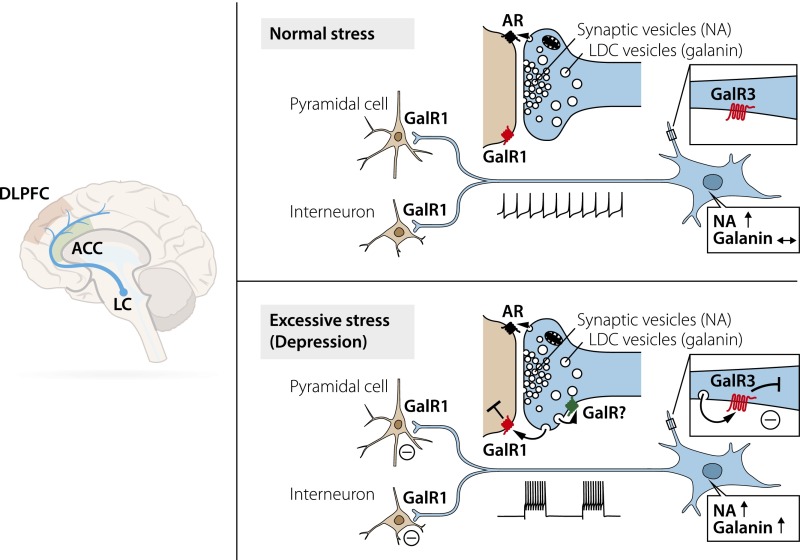
The present study provides a comprehensive set of results suggesting that the GAL system has an important role in MDD. The analysis of postmortem brain samples from DS subjects and matched controls reveals significant increases in transcription levels for GAL and GALR3 with parallel decreases in DNA methylation in the locus coeruleus in DS subjects of both sexes. A similar profile was seen in the dorsal raphe nucleus samples, although the methylation changes were found mainly for the female GALR3 transcript. In contrast, in the forebrain GAL mRNA levels were decreased in the male but were increased in the female, dorsolateral prefrontal cortex, with a wide increase in methylation in the male GAL gene. Some ways that these changes in the locus coeruleus might influence the development of MDD are illustrated in Fig. 7.
Fig. 7.
The locus coeruleus (LC) pathway to cortex and its involvement in stress. Many neurons in the locus coeruleus coexpress NA and GAL. In rats, under normal circumstances these neurons fire spontaneously in a slow and regular fashion (124), but in response to stress they react with increased activity and burst firing (100). NA is stored primarily in synaptic vesicles and is released at low activity and more so after stress (98, 99), acting on adrenoreceptors (ARs). GAL is stored only in large, dense core (LDC) vesicles and is released extrasynaptically (125) in response to increased/burst firing (106) from these vesicles in nerve endings in the forebrain. In humans (this study), GAL may, e.g., activate inhibitory postsynaptic GAL1 receptors (GalR1) on neurons in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC). Presumably, release also can occur from dendrites/soma in the locus coeruleus, activating inhibitory GAL3 autoreceptors (GalR3). It is hypothesized that under excessive/chronic stress GAL induces a long-lasting inhibition of NA neurons and thus of NA release in forebrain regions such as the dorsolateral prefrontal cortex and anterior cingulate cortex, contributing to the development of MDD. Not included are changes in GAL, GALR1, and GALR3 mRNA levels in the dorsolateral prefrontal cortex.
In addition, the present study reports markedly different transcript levels for the four GAL family members with individual, regional distribution patterns. GAL and GALR1 mRNAs are the most abundant transcripts, GALR1 mRNA being expressed at eightfold higher levels in dorsolateral prefrontal cortex than in anterior cingulate cortex (Table 2). Finally, the RIA shows major differences in GAL peptide levels among the brain regions analyzed, paralleling transcript levels and evidencing translation of the transcript. However, a sex difference could be detected, but only in the locus coeruleus, where levels were higher in females.
These results are discussed in relation to our previous in situ hybridization analysis describing the cellular localization of the galanin system in the lower brainstem (locus coeruleus and dorsal raphe nucleus) of the normal human postmortem brain (32). This approach can suggest which neuron populations express the transcripts and are the site of the methylations, here reported based on qPCR and bisulfite pyrosequencing, i.e., biochemical analyses (see below). For a comparison of the results in Le Maître, et al. (32) and the present study, see SI Discussion, Comparison of in Situ Hybridization and RT-qPCR Results.
GAL and MDD: Genome-Wide Association Studies.
Two recent large genome-wide association studies (GWAS) (68, 69) have analyzed in-depth cohorts of MDD patients and controls. The first study, investigating 2,431 cases and 3,673 controls, found a suggestive association of GAL with MDD using a gene-based test, which retained low-association P values in two additional independent cohorts (68). However, in the second GWAS involving 9,240 MDD cases and 9,519 controls (at that time the largest GWAS conducted), no SNP achieved genome-wide significance in either the MDD discovery or the replication phase (69).
In addition, two recent huge GWAS analyzing depressive symptoms (70) and self-reported depression (71), with tens of thousands participants, could not support a role of the galanin system genes within their top results. However, these GWAS did not control for the well-known environmental risk factors of depression (72). In fact, in the study by Juhasz, et al. (56) galanin system gene variants altered the development of depression only in people who also were exposed to strong stress, and no significant effect of GAL and its receptors could be seen without taking stress into account (SI Discussion, GAL and MDD: GWAS).
GAL and MDD: Candidate Gene and Gene-Environment Approach.
A recent candidate gene analysis based on self-reported questionnaires from 2,361 individuals (∼70% females; ∼30% males) measuring lifetime depression, depressive and anxiety symptoms, and life stressors showed that gene variants for GAL and all three of its receptors are associated with increased risk of lifetime and current depression and anxiety, but only after childhood adversity or recent negative life events, suggesting that the galanin system genes alter the development of depression through epigenetic mechanisms (56). In agreement with this study, the present report reveals correlations, mainly for GAL and GALR3, between transcript levels and promoter methylation in several brain regions of both sexes (SI Discussion, GAL and MDD: Candidate Gene Approach).
DNA Methylation.
Methylation changes for the galanin system have been described previously, mainly in studies on various types of cancer, and were associated with tumor suppression (73, 74). Here we report that DNA methylation of members of the galanin system also may play a role in MDD. Analyzing three or four selected CpG sites, we observed significant changes in the degree of methylation at several sites in the dorsolateral prefrontal cortex, dorsal raphe nucleus, and locus coeruleus of DS subjects compared with controls. The changes in receptor methylation were associated with GALR3 in the locus coeruleus and dorsal raphe nucleus, mainly in female subjects. With some exceptions, there was an inverse correlation between methylation and transcript levels, in agreement with the view that methylation represses gene transcription (75) (SI Discussion, DNA Methylation).
Transcription Factor-Binding Analysis.
In many cases, DNA methylation of specific CpGs influences transcription factor binding. Using the JASPER database (65, 66), we identified a number of potential transcription factor-binding sites in the regions including the differentially methylated CpG sites in GAL and GALR3. Two of the transcription factors, specificity protein 1 (SP1) and NEUROD2, regulate GAL expression in mouse (76, 77) and potentially also in human (78). Further, SP1 binding is known to be affected by DNA methylation (79). Both transcription factors potentially bind to CpG2, which was hypermethylated in male and hypomethylated in female DS subjects, corresponding to decreased and increased mRNA expression, respectively. An additional SP1-binding site was identified in the region including CpG1. For GALR3, the transcription factors binding to the identified sites have not been shown to be involved in the regulation of this gene. Taken together, these findings strengthen the notion that the observed epigenetic and gene-expression changes are connected and open the way for mechanistic studies to understand the regulation of these genes in depth.
Locus Coeruleus and Dorsal Raphe Nucleus.
The locus coeruleus and dorsal raphe nucleus regions in the rat lower brainstem harbor NA and 5-HT cell bodies, respectively (80) and are key nodes in the mood circuitry. Both the GAL and GALR3 transcripts in the locus coeruleus samples are expressed mainly in the noradrenergic neurons (32); thus they are likely to be the sites for the regulation of transcripts and the methylation shown in the present study.
The dorsal raphe nucleus, where 50–70% of all neurons in rodents are nonserotonergic (81–83), can be discussed in a similar way: The monitored GAL and GALR1 mRNAs may not be present in 5-HT neurons, but GALR3 likely is present. However, both GAL and GALR1 transcripts have been observed in the close vicinity of the 5-HT neurons (32). Thus the changes in GAL mRNA and methylation likely occur in nonserotonin cells. Interestingly, the up-regulation of GALR1 mRNA in the ventral periaqueductal gray is in agreement with two recent experimental studies on male rats that showed an increase in GALR1 mRNA levels (likely not in 5-HT neurons) after mild, blast-induced traumatic brain injury (84) and chronic mild stress (85). For a discussion of medullary raphe nuclei, see SI Discussion, Medullary Raphe Nuclei. However, we note here that in this region there is an increase in GAL and GALR3 transcript levels in males and females and in GALR1 transcript levels in males only, as well as a robust decrease in GALR2 mRNA in males only; in fact, this is the only region showing a change in this receptor transcript. No effects on DNA methylation were recorded.
Prefrontal Cortex.
A number of symptoms and diagnostic markers of MDD have been associated with prefrontal cortex, including decreased gray matter volume, hypermetabolism, and spine morphology (86). We observed distinct decreases in GAL and GALR3 mRNA levels in the dorsolateral prefrontal cortex of DS males, with increased methylation at three sites of the GAL gene, the reverse of the effects seen in locus coeruleus and dorsal raphe nucleus. In contrast, GAL mRNA is up-regulated in this region in female DS subjects; this up-regulation is the only sex difference for the GAL transcript seen in the qPCR analysis and requires further analysis. However, no changes were detected in another, closely related cortical area, the anterior cingulate cortex, which also has been consistently implicated in depression and suicide (87). Interestingly, although the average levels for GAL, GALR2, and GALR3 transcripts are approximately similar in the cortical regions, GALR1 mRNA levels are around eightfold higher in dorsolateral prefrontal cortex than in anterior cingulate cortex.
The findings in the prefrontal cortex are difficult to interpret, because we have no information on the cells expressing GAL system members in human dorsolateral prefrontal and anterior cingulate cortices. In principle, however, the mechanistic effects of GAL release should be equally mediated by GAL1 and GAL3, because both exhibit a similar (nanomolar) affinity for GAL (88). Therefore the differences will depend on which neuron subtype expresses the receptor and on the coupling to intracellular signaling cascades.
In general, peptides in the cortical region are detected in interneurons (89), but many pyramidal neurons express the transcript for cholecystokinin (90) and the peptide itself (91). Quantitative autoradiographic 125I-GAL–binding studies showed a strong cortical binding in the human brain (39, 41), in sharp contrast with the rat brain, which lacks 125I-GAL binding in dorsal cortical and hippocampal areas (37, 38). However, 125I-GAL(1-15ol) binds to these regions (92), and GAL(1-15) hyperpolarizes hippocampal C3 neurons (93). Investigation of these findings is continuing (SI Discussion, Dorsolateral Prefrontal Cortex) (94).
As for the mechanism(s) involved, one may speculate that the increased GAL mRNA levels in the locus coeruleus result in up-regulated GAL synthesis, enhanced centrifugal, axonal GAL transport, and increased GAL release in dorsolateral prefrontal cortex (Fig. 7). Here, in a possibly desensitizing process, GAL may down-regulate GALR3 expression in cortical projections and/or interneurons.
Functional Significance.
It has been suggested that epigenetic mechanisms, such as DNA methylation, may be involved in psychiatric disorders (95, 96) and play an important role in stress and depression (7, 97). Here CpG methylation, catalyzed by DNA methyltransferases, in general represses gene transcription (75). We focus the discussion on the NA/locus coeruleus system and the prefrontal cortex. The role of NA vs. GAL has been discussed by Kuteeva, et al. (54).
Animal Studies in the Locus Coeruleus.
Early experimental evidence suggested that locus coeruleus neurons in rats are activated by stress, resulting in increased NA synthesis and NA release in the forebrain (98–100). Stress also increases GAL expression in this nucleus (101, 102). In general terms neuropeptides are released following increased/burst firing (103, 104), also in the forebrain (105, 106). Thus, stress and increased/burst firing likely causes GAL release from nerve terminals in the forebrain as well as from soma/dendrites of the locus coeruleus neurons. Peptide release from soma/dendrites was first shown for oxytocin/vasopressin in magnocellular neurons in the hypothalamic paraventricular nucleus (107) and may be relevant for GAL in the locus coeruleus also (108).
Rat locus coeruleus neurons express GALR1 mRNA (42, 43), and, as evidenced by electrophysiological studies (109), this receptor is likely functional in this site, mediating hyperpolarization (46, 110). Thus, somato-dendritically released GAL may inhibit firing via a GAL1 autoreceptor, hypothetically to prevent overexcitation (54). In agreement, results from a study on a transgenic mouse overexpressing GAL under the dopamine β-hydroxylase promoter with a fivefold increase in GAL mRNA in the locus coeruleus (111) suggest that GAL is important for modulating anxiety states driven by high noradrenergic signaling (112).
The Human Locus Coeruleus.
In trying to translate the animal experimental scenario to humans and MDD, one could argue that (i) GAL3 seems to have replaced GAR1 in human locus coeruleus neurons (32); (ii) hyperpolarization is one transduction mechanism for GAL1 and also may be the transduction mechanism for GAL3 (46, 88, 110); and (iii) GAL and GALR3 transcripts are increased in the locus coeruleus of DS subjects’ brains. It may be speculated that chronic stress and, as a consequence, constant and persistent inhibition of the NA-locus coeruleus neurons by GAL may result in reduced NA levels in the forebrain regions involved in mood control, e.g., the dorsolateral prefrontal cortex and anterior cingulate cortex; reduced NA levels are a characteristic feature of mood-related disorders. Together with a genetic vulnerability (lack of resilience), this reduction in NA levels may contribute to the symptoms encountered in such disorders (Fig. 7).
Prefrontal Cortex.
In the prefrontal cortex, reciprocal interactions between the dorsolateral prefrontal cortex and anterior cingulate cortex, as well as between the anterior cingulate cortex and amygdala, have been described, whereby MDD subjects show reduced functional connectivity between the dorsolateral prefrontal cortex and anterior cingulate cortex (87). The transcript levels of all four GAL members are very similar in the two regions, except that (i) GALR1 mRNA levels are eightfold higher in the dorsolateral prefrontal cortex than in the anterior cingulate cortex and (ii) the differences between MDD and controls are mainly for GAL and are found only in male dorsolateral prefrontal cortex, indicating that any prefrontal cortical GALergic mechanisms in MDD are associated with GAL and GALR3 in this region and sex (SI Discussion, Dorsolateral Prefrontal Cortex). Of note, the apparent differences in GAL concentrations in this region between normal male and female controls did not reach significance but may still be taken as support for sex having an influence on information processing in this cortical region.
Treatment of MDD with Neuropeptides.
Neuropeptide receptors may be promising targets for drug development in general because, as mentioned previously, they are primarily released upon high-frequency/burst firing (103, 104) that may occur in a selectively challenged, pathologically afflicted system (16, 113). Therefore, the antagonist will block only an overactivated signaling pathway, not affecting other silent or moderately active (no peptide release) systems producing the same ligand, and thus resulting in fewer side effects.
Treatment of depression with neuropeptide antagonists has been considered previously (15–19, 114). In fact, an initial report on the administration of a substance P/neurokinin 1 antagonist showed a significant efficacy vs. placebo, without side effects (115). This result could not be reproduced in an expanded phase 3 study (15, 116); however, clinical work on neurokinin antagonists is ongoing (117, 118).
Treatment of MDD with GAL Ligands.
Both GAL and GAL3 are up-regulated in the locus coeruleus and dorsal raphe nucleus of MDD patients, possibly resulting in attenuated NA and 5-HT release in the forebrain. Treatment with a GAL3 antagonist could, by disinhibition, have antidepressant activity, restoring NA and 5-HT levels in these brain regions, just as SNRIs do. Moreover, it may be speculated that the well-known delay in the onset of SSRIs’ effect caused by the activation of somatic, inhibitory 5-HT1A receptors (8, 58) may not occur with GAL3 antagonists. Also, GAL3 signaling in some other brain regions, e.g., the dorsolateral prefrontal cortex, likely will not be affected, because our study shows that GAL and GALR3 mRNA are unchanged (in females) or even down-regulated (in males), possibly excluding GAL system-related side effects. Moreover, the regionally selective effect of a GAL3 antagonist should compare favorably with SSRIs, SNRIs, and NRIs, which increase extracellular monoamine levels in all regions that monoamine neurons innervate, i.e., essentially throughout the entire nervous system. In fact, small-molecule, blood–brain barrier-penetrating GalR3 antagonists have been generated (119, 120). The extent to which such an antagonist would act via mechanisms different from those of SNRIs and whether the currently used antidepressant drugs act via the GAL system has been discussed (121).
Limitations.
Several limitations are associated with the present study. They are discussed in some detail in SI Discussion, Limitations and encompass (i) concerns about GAL3, including its transduction mechanism(s) [this receptor has emerged as a complex receptor lacking well-defined signaling properties, contrasting GAL1 and GAL2 (36); so far there is no evidence that GAL3 is involved in receptor di- or heterodimerization (45)]; (ii) the lack of information about the protein, because only the transcripts for the receptors have been studied; (iii) the modest, albeit significant, changes in transcript mRNA levels; (iv) the relatively low number of postmortem brains analyzed; (v) the incomplete knowledge (in some instances) of the identity of the neurons expressing the transcripts (and thus of the site where methylation occurs); for example, the study by Le Maître, et al. (32) was not systematic, and galanin–5-HT coexistence in regions not analyzed cannot be excluded; and (vi) the general low sensitivity of histochemical techniques; this low sensitivity may have been an issue when Le Maître, et al. (32) could not detect the GAL1 transcript in the locus coeruleus with in situ hybridization, whereas the present qPCR analysis showed high levels of this transcript in this region; (vii) the superficial investigation of methylation sites and their functional significance; and (viii) the influence of treatment with various drugs (Table S1), which requires further investigation (also see ref. 121).
Concluding Remarks.
The present results, based on the analysis of five human brain regions, suggest the GAL system is involved in MDD. Interestingly, exactly 40 y ago Asberg and colleagues (122) reported that significantly lower concentrations of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the cerebrospinal fluid are associated with a very high rate of serious suicidal attempts, opening the way for the development of SSRIs aimed at elevating extraneuronal 5-HT levels. This class of drugs then replaced the monoamine oxidase inhibitors and the tricyclic antidepressants.
It is important to note that many overlapping transmitter systems exist and, in a way similar to the GAL family, may be involved in MDD psychopathology. The available detailed knowledge of the cellular organization and transmitter receptor architecture of the human prefrontal cortex, two subregions of which are studied here, provides a broad perspective on the present work and also shows how much research still needs to be carried out (123).
SI Discussion
Comparison of in Situ Hybridization and RT-qPCR Results.
mRNA levels were monitored with RT-qPCR in the present study and by in situ hybridization and RT-qPCR in the study by Le Maître, et al. (32). With regard to in situ hybridization, it is clear that, at least in rodents, the GAL system, especially the receptors and certainly GALR2 and -3, represent low-abundance transcripts: In the Allen Brain Atlas study (on mouse) no results were reported for GALR3, and only incomplete results for GALR2 mRNA (82). In agreement, the histochemical analysis of human brain requires high-quality tissue samples, i.e., short PMIs: We could see a signal for GALR1 and -3 only in samples with a PMI lower than 4 h (32).
With regard to RT-qPCR, it is obvious that the raw Ct values (cycle numbers) are lower in the present study than in the study by Le Maître, et al. (32); i.e., the sensitivity of the present study is higher. We believe this higher sensitivity results from several modifications of the procedure. Namely, RT-qPCR was carried out on a 7500 fast real-time PCR system (Applied Biosystems); also, the PCR plates used in this study had a higher refractive index, and therefore the raw Ct values of the markers were lower in general. The kit used for reverse transcription also was different (SI Materials and Methods). Furthermore, this study used a macrodissection procedure for the brainstem regions of dorsal raphe nucleus and locus coeruleus rather than the microdissections used by Le Maître, et al. (32).
The forebrain was analyzed in both papers. However, the extent to the areas described as “frontal cortex” and “cingulate cortex” by Le Maître, et al. (32) really are comparable to “dorsolateral prefrontal cortex, BA 8/9” and “anterior cingulate cortex, BA 24” in the present study cannot be determined at this point. Even if the forebrain Ct values are much lower overall in the present study, the ranking between the receptors remains the same, with large (up to 32-fold) differences: GALR1 > GALR2 > GALR3.
However, a difficult-to-explain difference is the low expression of GALR1 in dorsal raphe nucleus and locus coeruleus in the earlier study compared with considerably higher levels found in the present study. One possible explanation is the method of dissection, with the present study encompassing a larger region than the dorsal raphe nucleus and locus coeruleus isolated by microdissection from frozen sections (32). It is possible that the locus coeruleus and dorsal raphe nucleus proper and the locus coeruleus have lower GALR1 and -2 but higher GALR3 mRNA levels than the surrounding region included in the present study.
Comparing the RT-qPCR and in situ hybridization results is complex. In the in situ hybridization analysis we never succeeded in generating a working probe for GALR2, although one of the cloned probes was positive after sequencing. The reason for this lack of success could be inefficient hybridization of the GalR2 riboprobe or levels that were too low to be recorded and fell below the detection limit of in situ hybridization. The GALR1 probe worked, both in the dorsal raphe nucleus and locus coeruleus sections. However, there was no evidence for the coexistence of NA and 5-HT neurons. With the low GALR1 mRNA signal in the earlier study, we accepted the fairly weak in situ hybridization signal, but, considering the robust mRNA levels now obtained, it seems surprising that we did not see a stronger signal. However, the GALR1 riboprobe may not have hybridized optimally, in contrast with the GALR3 probe (32).
GAL and MDD: GWAS.
Two recent large GWASs (68, 69) have analyzed in-depth cohorts of MDD patients and controls. The first, investigating 2,431 cases and 3,673 controls, obtained a suggestive association of GAL with MDD using a gene-based test that retained low-association P values in two additional independent cohorts (68). The most associated SNP found in GAL (rs2156464, P = 2.7 × 10−5) is located in the haplotype block that has been identified as being associated with panic disorder (130). This haplotype block is in close proximity to the GAL CpG island, suggesting that epigenetic mechanisms may play a role in both disorders.
The second GWAS, the largest conducted so far, involved 9,240 MDD cases and 9,519 controls. No SNP achieved genome-wide significance in either the MDD discovery or the replication phase (69) although in the galanin system genes some SNPs (e.g., GAL rs2156464 P = 0.022) showed nominally significant associations with MDD. Thus, the interesting findings reported by Wray, et al. (68) could not be supported by further evidence.
In addition, two recent huge GWAS studies, with tens of thousands participants, analyzing depressive symptoms (70) and self-reported depression (71) could not reinforce the role of the galanin system genes within their top results. However, self-reported depression showed GWAS-level associations with genes encoding transcription factors that are important in brain development (131), whereas variants regulating gene expression in the central nervous system were most abundant in the analysis of depressive symptoms (70). These latter results emphasize that dynamic changes in gene expression (frequently governed by environmental factors) have a major role in the development of depression. However, these GWAS studies were not controlled for the well-known environmental risk factors of depression (72).
GAL and MDD: Candidate Gene Approach.
A recent candidate gene analysis based on self-reported questionnaires from 2,361 individuals (∼70% females and ∼30% males) (56), measuring lifetime depression, depressive and anxiety symptoms, and life stressors, showed that gene variants for GAL and all three of its receptors are associated with increased risk of lifetime and current depression and anxiety following childhood adversity or recent negative life events. Seven of the 12 haplotype tag SNPs investigated were statistically associated with one or more of the three clinical phenotypes, and six of these SNPs acted through interaction with either childhood adversity or recent negative life events. In line with these results, a system-level statistical analysis considering the whole galanin system demonstrated that genetic variations in the GAL and GALR1–3 genes were more relevant in those who experienced severe life stresses than in those who did not, again emphasizing the importance of potential epigenetic mechanisms. Specifically, in the case of early childhood adversity, GALR1 was highly relevant, whereas experiencing recent negative life events increased the relevance of GAL and GALR1–3 genes, especially GALR2 and GALR3. The investigated haplotype tag SNPs tagged several functional variants within the galanin system that influence the transcriptional or translational activity of the genes, including transcription-binding sites (e.g., GAL rs3136540, rs3136541; GALR1 rs11662010; GALR2 rs8836; and GALR3 rs2017022), miRNA-binding sites (e.g., GAL rs3136541; GALR1 rs11665337; and GALR2 rs8836), and CpG islands (e.g., GALR1 rs5375) demonstrated by the SNP Function Prediction tool (snpinfo.niehs.nih.gov/snpinfo/snpfunc.php). In agreement with this previous study, the present report reveals often robust correlations, in several brain regions and in both sexes, between transcript levels and promoter methylation, mainly for GAL and GALR3.
It is now established that a functional polymorphism in the promoter region of the serotonin transporter, 5-HTTLPR, is associated with anxiety-related traits and susceptibility for depression (71, 132). Our subsequent analysis suggests that this effect may be mediated in part by the GAL system and that the effect of this system on stress-induced depressive systems is at least as strong as that of the SERT functional polymorphism (56).
DNA Methylation.
Changes in methylation for the galanin system have been described previously, mainly in studies on various types of cancer and in association with tumor suppression. For example, hypermethylation (inactivation) of the GAL and/or GALR1 and GALR2 genes in head and neck (73, 133, 134) and endometrial (74) cancer is related to poor survival, indicating the importance of these galanin genes for survival and a potential biomarker function. Moreover, mice lacking de novo DNA methyltransferase (Dnmt3a) have a metabolic syndrome-like phenotype and a highly up-regulated expression of GAL (and tyrosine hydroxylase) in the hypothalamic paraventricular nucleus (135).
Here we report that, in agreement, methylation of members of the GAL system also may play a role in MDD. Thus, analyzing four selected CpG sites, we observed significant changes in the degree of methylation at several sites in the galanin promoter in the dorsolateral prefrontal cortex, dorsal raphe nucleus, and locus coeruleus of DS subjects compared with controls. For the receptors, methylation changes compared with controls were found in the promoter of GALR3 only in the dorsal raphe nucleus and locus coeruleus, mainly in female subjects. With some exceptions, there was an inverse correlation between methylation and transcript levels. The effects observed are mostly robust. These results are in agreement with methodological studies on the methylation state of DNA extracted from human post mortem brains of patients, showing that there is no correlation between DNA methylation state and pH and suggesting that DNA methylation is stable (136). Moreover, recent experiments on animal models report that microRNA is very resistant, whereas RNA degradation is transcript specific, and that housekeeping genes are more robust than genes with low expression (137).
Medullary Raphe Nuclei.
A remark on the GAL system in the medullary raphe nuclei: We included this brain region as a control, because in the rat many 5-HT neurons in the medullary raphe and adjacent nuclei: nucleus raphe pallidus, nucleus raphe obscurus, nucleus raphe magnus (B1–3 cell clusters) (80) also express GAL (28), but we considered them unlikely to be important for mood regulation. Of note, in this brain region nothing is known about GAL-receptor mRNAs in the rat or about the GAL cell bodies and receptors in humans. Perhaps surprisingly, we found increased GAL and GALR3 mRNA levels in both males and females, and in males we found an increase in GALR1 and a decrease in GALR2 transcript levels; these are the only change in GALR2 seen at any brain region studied. These alterations in transcripts suggest that GAL and GalR3 may be regulated in a system not directly related to MDD. Whether these two molecules are expressed in 5-HT neurons remains to be analyzed. Nevertheless, these receptors, in addition to those associated with NA neurons in the locus coeruleus and with 5-HT neurons in the dorsal raphe nucleus, would be affected by treatment with GAL3 antagonists.
Dorsolateral Prefrontal Cortex.
Interestingly, binding the dorsolateral prefrontal cortex was seen with autoradiography in the rat using 125I-Gal(1–15ol) as ligand (92), a finding that has led to further exploration of this N-terminal fragment in hippocampal and mood function. Xu, et al. (93) reported a yet-to-be-cloned GAL(1–15)-selective hyperpolarizing receptor on CA3 neurons in the dorsal hippocampus. Strong depression-related and anxiogenic-like effects were observed after intrahippocampal administration of GAL(1–15), likely involving GAL1–GAL2 heterocomplexes (138) and possibly acting via enhancement of the antidepressant effect induced by 8-OH-DPAT via 5-HT1A receptors (94). However, a distinct difference is that there are no changes at all in methylation in any of the receptors in cortical regions.
Limitations.
We call attention to the following limitations:
-
i)
The results highlight, in particular, GAL3, which is a complex receptor (36) not found in all mammals (139). Its signaling properties are still not well defined; although GALR3-transfected cell lines have been generated (140, 141, 142), these cells could not, thus far, be used for stable signaling experiments (36) Still, GAL3 presumably acts via a pertussis toxin-sensitive Gi/o-type G protein, which in turn regulates inwardly rectifying K+ channels (88), similar to GAL1 receptors (88). This lack of information contrasts sharply with our profound knowledge about GAL1 and GAL2 (36).
-
ii)
A further obstacle is that the main thrust of the analysis is on transcripts, thus making statements about the translational products, i.e., the proteins, uncertain. It is difficult to analyze the receptor proteins, because generating reliable antibodies to GAL receptors has been problematic (143).
-
iii)
The effects on transcript levels are not dramatic (up to twofold), but modest effects are also seen in animal stress models: In a rat chronic social stress model, a 75%increase in transcript level was seen in the locus coeruleus after 13 d (101); a similar increase was seen 24 h after a saline injection in rats (54); after 8 wk of chronic mild stress there was no increase at all in mice (85); a 90% increase was recorded 2 h after a mild blast traumatic injury in rats (84). These modest changes could be compared with the dramatic and acute up-regulation of GAL in dorsal root ganglia after peripheral nerve injury, where protein levels increase by 120× after 3 d with a corresponding increase in mRNA levels (144).
-
iv)
The number of subjects is still limited. Of note, here we look at actual brain tissue-specific epigenetic changes, whereas the genetic analysis is based on stable DNA variants from blood samples.
-
v)
The results in Le Maître, et al. (32) indicate that 5-HT and GAL in human dorsal raphe nucleus are not synthesized in the same neurons. However, the previous study was not systematic, i.e., encompassing all levels of the serotonergic and pontine raphe complex, and the possibility that the two messengers coexist in a subdivision(s) of this complex in DS subjects and/or control brains cannot be excluded.
-
vi)
The apparent absence of a molecule in histochemical studies should be interpreted with caution in general, and even more so when human postmortem tissue is involved. Therefore, the comparison between the results in Le Maître, et al. (32) and the present biochemical findings is capricious. For example, the failure to detect GALR1 transcript in locus coeruleus neurons with in situ hybridization, despite the high mRNA levels shown here in the locus coeruleus sample, warrants further investigation.
-
vii)
In studies of this type it may be difficult to draw any conclusions about the relationship between gene expression and DNA methylation. However, mechanistic in vitro studies are needed to delineate a causal relationship between the two. In addition, interpretation of methylation status for GalR1 and GalR2 should be carried out also, because methylation changes might occur at other CpG sites within the genome that have not been studied here.
Materials and Methods
Brain Samples.
Postmortem brain tissue was obtained from the Douglas-Bell Canada Brain Bank. A total of 212 punched samples from five different brain regions was included (Table 1). Ethical approval for this study was obtained from the Institutional Review Board of the Douglas Mental Health University Institute, with written informed consent from the families. The Regional Ethical Board in Stockholm has granted The Karolinska Institutet group permission no. 2013/474-31/2 for processing postmortem brain samples (SI Materials and Methods, Brain Samples and Table S1).
RNA Isolation and Integrity Analysis.
Total RNA was isolated using the RNeasy Plus Mini Kit. RNA quantity and quality were determined spectrophotometrically by using a ND1000 nanodrop. RNA integrity was checked using Experion automated electrophoresis system (Bio-Rad Laboratories). Total RNA was reverse transcribed to generate cDNA using a High-Capacity Reverse Transcription Kit (Life Technologies) (SI Materials and Methods).
RT-qPCR.
qPCR was performed as described previously (SI Materials and Methods) (32).
Genomic DNA Extraction and PyroMark PCR.
Genomic DNA (gDNA) was isolated by using the DNeasy Blood and Tissue Kit (Qiagen), checked spectrophotometrically, and bisulfite-converted by using the EZ DNA Methylation-Gold Kit (Zymo Research). Converted DNA was subject to 40 cycles of amplification by using gene-specific primers and the pyroPCR PyroMark (Qiagen) kit (SI Materials and Methods).
Bisulfite Pyrosequencing.
Bisulfite pyrosequencing was performed using PyroMark Q96 ID (Qiagen) according to the manufacturer’s protocol and as previously described (122). Primer sequences are listed in Fig. S6B, and the genomic locations of the CpGs analyzed are shown in Fig. S6A; also see SI Materials and Methods.
RIA.
Human GAL was measured using antiserum G-026-01 (Phoenix Europe GmbH) raised in a rabbit against human GAL (SI Materials and Methods).
Statistical Analysis.
Statistical analysis was performed with GraphPad Prism 6 (GraphPad Software), StatView (SAS Institute Inc.), and Systat 11 (Systat Software, Inc.). RIN, age, PMI, and tissue pH values for the five brain regions in controls and DS subjects were analyzed by multivariate ANOVA and two-tailed t test for independent groups. The Shapiro–Wilks test was used to test for Gaussian distribution. Significant outliers for qPCR fold change and percent methylation were analyzed by the ROUT (robust regression followed by outlier identification) method and were excluded if P < 0.05. qPCR data were analyzed by using the nonparametric Mann–Whitney U test. Differences in DNA methylation between controls and suicides were analyzed using the Mann–Whitney U test. P values below 0.05 were considered significant, and P values between 0.05 and 0.1 were considered to represent a trend.
Acknowledgments
We thank Drs. Tamas Bartfai (Stockholm University and Oxford University), Jean-Pierre Changeux (Institut Pasteur), Wayne C. Drevets (Janssen Research and Development), Eric Kandel (Columbia University), Diego Pizzagalli (McLean Hospital), and Sol Snyder (Johns Hopkins University) for valuable comments and suggestions. This project presently is supported by Swedish Research Council Grant 04X-2887 (to T.G.M.H.); the Swedish Brain Foundation (T.G.M.H.); and by grants from Karolinska Institutet (to T.G.M.H.) and the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (Formas) (to J.R.). Earlier phases of this project were supported by a National Association for Research on Schizophrenia and Depression Distinguished Investigator Award (2009) (to T.G.M.H.), the European Union Framework 6 Integrated Project New-Mood LSHM-CT-2004-503474 (2004–2008) (to G.B., G.J., and T.G.M.H.), AFA Insurance (2008) (T.G.M.H.), and by a 5-y unrestricted Bristol-Myers-Squibb Grant in Neuroscience (to T.G.M.H.). Of particular importance were generous grants over a longer period from the Marianne and Marcus Wallenberg Foundation (1998–2009) and from the Knut and Alice Wallenberg Foundation (to T.G.M.H.). N.M. is a Canadian Institute of Health Research New Investigator and Fonds de la Recherche en Santé du Québec (FRQ-S) Chercheur-boursier. The Douglas–Bell Canada Brain Bank is supported by the Réseau Québécois sur le Suicide, les Troubles de l’Humeur et les Troubles Associés (FRQ-S) and by a Platform Support Grant from Brain Canada (to N.M.). Support was provided by the Magyar Tudományos Akadémia (MTA)–Semmelweis Egyetem (SE)–Nemzeti Agykutatási Program (NAP) B alprogram Genetic Brain Imaging Migraine Research Group through Kutatási és Technológiai Innovációs Alap (KTIA) (Grant KTIA_NAP_13-2-2015-0001, to G.J.); by the NAP A-SE Research Group (Grant KTIA_NAP_13-1-2013-0001, to G.B.) and (Grant KTIA_13_NAP-A-II/14, to G.B.); and by the MTA-SE Neuropsychopharmacology and Neurochemistry Research Group (G.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617824113/-/DCSupplemental.
References
- 1.Kessler RC, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Wittchen HU, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 3.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 4.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2-3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labonté B, et al. Genome-wide methylation changes in the brains of suicide completers. Am J Psychiatry. 2013;170(5):511–520. doi: 10.1176/appi.ajp.2012.12050627. [DOI] [PubMed] [Google Scholar]
- 7.Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol. 2013;53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15(7):220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 9.Millan MJ. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110(2):135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Gardier AM, Malagié I, Trillat AC, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: Recent findings from in vivo microdialysis studies. Fundam Clin Pharmacol. 1996;10(1):16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 11.Millan MJ, Goodwin GM, Meyer-Lindenberg A, Ove Ögren S. Learning from the past and looking to the future: Emerging perspectives for improving the treatment of psychiatric disorders. Eur Neuropsychopharmacol. 2015;25(5):599–656. doi: 10.1016/j.euroneuro.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi MH, et al. STAR*D Study Team Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery SA. Why do we need new and better antidepressants? Int Clin Psychopharmacol. 2006;21(Suppl 1):S1–S10. doi: 10.1097/01.yic.0000199455.39552.1c. [DOI] [PubMed] [Google Scholar]
- 14.Artigas F. Developments in the field of antidepressants, where do we go now? Eur Neuropsychopharmacol. 2015;25(5):657–670. doi: 10.1016/j.euroneuro.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: The end of the beginning? Nat Rev Drug Discov. 2012;11(6):462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- 16.Hökfelt T, Bartfai T, Bloom F. Neuropeptides: Opportunities for drug discovery. Lancet Neurol. 2003;2(8):463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 17.Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24(11):580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Maubach KA, Rupniak NM, Kramer MS, Hill RG. Novel strategies for pharmacotherapy of depression. Curr Opin Chem Biol. 1999;3(4):481–488. doi: 10.1016/S1367-5931(99)80070-2. [DOI] [PubMed] [Google Scholar]
- 19.Nemeroff CB, Vale WW. The neurobiology of depression: Inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 20.Burbach JP. Neuropeptides from concept to online database. Eur J Pharmacol. 2010;626(1):27–48. doi: 10.1016/j.ejphar.2009.10.015. www.neuropeptides.nl [DOI] [PubMed] [Google Scholar]
- 21.Tatemoto K, Rökaeus A, Jörnvall H, McDonald TJ, Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 22.Rökaeus A, et al. A galanin-like peptide in the central nervous system and intestine of the rat. Neurosci Lett. 1984;47(2):161–166. doi: 10.1016/0304-3940(84)90423-3. [DOI] [PubMed] [Google Scholar]
- 23.Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6(3):509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 24.Skofitsch G, Jacobowitz DM. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986;7(4):609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 25.Melander T, Hökfelt T, Rökaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986;248(4):475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 26.Kordower JH, Le HK, Mufson EJ. Galanin immunoreactivity in the primate central nervous system. J Comp Neurol. 1992;319(4):479–500. doi: 10.1002/cne.903190403. [DOI] [PubMed] [Google Scholar]
- 27.Holets VR, Hökfelt T, Rökaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24(3):893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 28.Melander T, et al. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6(12):3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu ZQ, Shi TJ, Hökfelt T. Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J Comp Neurol. 1998;392(2):227–251. doi: 10.1002/(sici)1096-9861(19980309)392:2<227::aid-cne6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Chan-Palay V, Jentsch B, Lang W, Höchli M, Asan E. Distribution of neuropeptide Y, C-terminal flanking peptide of NPY and galanin and coexistence with catecholamine in the locus coeruleus of normal human, Alzheimer’s nementia and Parkinson’s disease brains. Dementia. 1990;1:18–31. [Google Scholar]
- 31.Miller MA, Kolb PE, Leverenz JB, Peskind ER, Raskind MA. Preservation of noradrenergic neurons in the locus ceruleus that coexpress galanin mRNA in Alzheimer’s disease. J Neurochem. 1999;73(5):2028–2036. [PubMed] [Google Scholar]
- 32.Le Maître E, Barde SS, Palkovits M, Diaz-Heijtz R, Hökfelt TG. Distinct features of neurotransmitter systems in the human brain with focus on the galanin system in locus coeruleus and dorsal raphe. Proc Natl Acad Sci USA. 2013;110(6):E536–E545. doi: 10.1073/pnas.1221378110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu X, et al. A role for galanin in antidepressant actions with a focus on the dorsal raphe nucleus. Proc Natl Acad Sci USA. 2005;102(3):874–879. doi: 10.1073/pnas.0408891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuxe KAL, et al. Galanin/5-HT receptor interactions. A new integrative mechanism in the control of 5-HT neurotransmission in the central nervous system. In: Paoletti R, Vanhoutte PM, Brunello N, Maggi FM, editors. Serotonin. Springer; Dordrecht, The Netherlands: 1990. pp. 169–185. [Google Scholar]
- 35.Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux JF. Molecular cloning of a functional human galanin receptor. Proc Natl Acad Sci USA. 1994;91(21):9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang R, et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: Three decades of emerging diversity. Pharmacol Rev. 2015;67(1):118–175. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- 37.Skofitsch G, Sills MA, Jacobowitz DM. Autoradiographic distribution of 125I-galanin binding sites in the rat central nervous system. Peptides. 1986;7(6):1029–1042. doi: 10.1016/0196-9781(86)90133-6. [DOI] [PubMed] [Google Scholar]
- 38.Melander T, et al. Autoradiographic quantitation and anatomical mapping of 125I-galanin binding sites in the rat central nervous system. J Chem Neuroanat. 1988;1(4):213–233. [PubMed] [Google Scholar]
- 39.Köhler C, et al. Distribution of galanin-binding sites in the monkey and human telencephalon: Preliminary observations. Exp Brain Res. 1989;75(2):375–380. doi: 10.1007/BF00247944. [DOI] [PubMed] [Google Scholar]
- 40.Köhler C, Hallman H, Melander T, Hökfelt T, Norheim E. Autoradiographic mapping of galanin receptors in the monkey brain. J Chem Neuroanat. 1989;2(5):269–284. [PubMed] [Google Scholar]
- 41.Köhler C, Chan-Palay V. Galanin receptors in the post-mortem human brain. Regional distribution of 125I-galanin binding sites using the method of in vitro receptor autoradiography. Neurosci Lett. 1990;120(2):179–182. doi: 10.1016/0304-3940(90)90032-5. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: Distinct distribution from GALR1. J Comp Neurol. 1999;409(3):469–481. [PubMed] [Google Scholar]
- 43.Burazin TC, Larm JA, Ryan MC, Gundlach AL. Galanin-R1 and -R2 receptor mRNA expression during the development of rat brain suggests differential subtype involvement in synaptic transmission and plasticity. Eur J Neurosci. 2000;12(8):2901–2917. doi: 10.1046/j.1460-9568.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 44.Mennicken F, Hoffert C, Pelletier M, Ahmad S, O’Donnell D. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J Chem Neuroanat. 2002;24(4):257–268. doi: 10.1016/s0891-0618(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 45.Fuxe K, et al. On the existence and function of galanin receptor heteromers in the central nervous system. Front Endocrinol (Lausanne) 2012;3:127. doi: 10.3389/fendo.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, et al. Genomic organization and functional characterization of the mouse GalR1 galanin receptor. FEBS Lett. 1997;411(2-3):225–230. doi: 10.1016/s0014-5793(97)00695-9. [DOI] [PubMed] [Google Scholar]
- 47.Fuxe K, et al. Galanin modulates 5-hydroxytryptamine functions. Focus on galanin and galanin fragment/5-hydroxytryptamine1A receptor interactions in the brain. Ann N Y Acad Sci. 1998;863:274–290. doi: 10.1111/j.1749-6632.1998.tb10702.x. [DOI] [PubMed] [Google Scholar]
- 48.Fuxe K, et al. Galanin/5-HT interactions in the rat central nervous system. Relevance for depression. In: Hökfelt T, Bartfai T, Jacobowitz D, Ottoson D, editors. Galanin: A New Multifunctional Peptide in the Neuro-endocrine System. Macmillan Education UK; London: 1991. pp. 221–235. [Google Scholar]
- 49.Weiss JM, Bonsall RW, Demetrikopoulos MK, Emery MS, West CH. Galanin: A significant role in depression? Ann N Y Acad Sci. 1998;863:364–382. doi: 10.1111/j.1749-6632.1998.tb10707.x. [DOI] [PubMed] [Google Scholar]
- 50.Wrenn CC, Crawley JN. Pharmacological evidence supporting a role for galanin in cognition and affect. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(1):283–299. doi: 10.1016/s0278-5846(00)00156-1. [DOI] [PubMed] [Google Scholar]
- 51.Barrera G, et al. One for all or one for one: Does co-transmission unify the concept of a brain galanin “system” or clarify any consistent role in anxiety? Neuropeptides. 2005;39(3):289–292. doi: 10.1016/j.npep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Brunner SM, et al. GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc Natl Acad Sci USA. 2014;111(19):7138–7143. doi: 10.1073/pnas.1318066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmes A, Picciotto MR. Galanin: A novel therapeutic target for depression, anxiety disorders and drug addiction? CNS Neurol Disord Drug Targets. 2006;5(2):225–232. doi: 10.2174/187152706776359600. [DOI] [PubMed] [Google Scholar]
- 54.Kuteeva E, Hökfelt T, Wardi T, Ögren SO. Galanin, galanin receptor subtypes and depression-like behaviour. EXS. 2010;102:163–181. doi: 10.1007/978-3-0346-0228-0_12. [DOI] [PubMed] [Google Scholar]
- 55.Bing O, Möller C, Engel JA, Söderpalm B, Heilig M. Anxiolytic-like action of centrally administered galanin. Neurosci Lett. 1993;164(1-2):17–20. doi: 10.1016/0304-3940(93)90846-d. [DOI] [PubMed] [Google Scholar]
- 56.Juhasz G, et al. Brain galanin system genes interact with life stresses in depression-related phenotypes. Proc Natl Acad Sci USA. 2014;111(16):E1666–E1673. doi: 10.1073/pnas.1403649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5-HT1A receptor: Signaling, desensitization, and gene transcription. Neuropsychopharmacol. 1996;14(1):19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- 58.Artigas F, Bel N, Casanovas JM, Romero L. Adaptative changes of the serotonergic system after antidepressant treatments. Adv Exp Med Biol. 1996;398:51–59. doi: 10.1007/978-1-4613-0381-7_6. [DOI] [PubMed] [Google Scholar]
- 59.Stockmeier CA, et al. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18(18):7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drevets WC, et al. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27(5):499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 61.Gassen NC, et al. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci Signal. 2015;8(404):ra119. doi: 10.1126/scisignal.aac7695. [DOI] [PubMed] [Google Scholar]
- 62.Zimmermann N, et al. Antidepressants inhibit DNA methyltransferase 1 through reducing G9a levels. Biochem J. 2012;448(1):93–102. doi: 10.1042/BJ20120674. [DOI] [PubMed] [Google Scholar]
- 63.Bach-Mizrachi H, et al. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: Major depression and suicide. Neuropsychopharmacol. 2006;31(4):814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- 64.Underwood MD, et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46(4):473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 65.Jaspar M, et al. Influence of comt genotype on antero-posterior cortical functional connectivity underlying interference resolution. Cereb Cortex. 2016;26(2):498–509. doi: 10.1093/cercor/bhu188. [DOI] [PubMed] [Google Scholar]
- 66.Mathelier A, et al. JASPAR 2016: A major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2016;44(D1):D110–D115. doi: 10.1093/nar/gkv1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Land T, Langel U, Bartfai T. Hypothalamic degradation of galanin(1-29) and galanin(1-16): Identification and characterization of the peptidolytic products. Brain Res. 1991;558(2):245–250. doi: 10.1016/0006-8993(91)90775-q. [DOI] [PubMed] [Google Scholar]
- 68.Wray NR, et al. Genome-wide association study of major depressive disorder: New results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17(1):36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ripke S, et al. Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okbay A, et al. LifeLines Cohort Study Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet. 2016;48(6):624–633. doi: 10.1038/ng.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyde CL, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48(9):1031–1036. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kendler KS, Gardner CO. Depressive vulnerability, stressful life events and episode onset of major depression: A longitudinal model. Psychol Med. 2016;46(9):1865–1874. doi: 10.1017/S0033291716000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Misawa K, et al. Epigenetic inactivation of galanin receptor 1 in head and neck cancer. Clin Cancer Res. 2008;14(23):7604–7613. doi: 10.1158/1078-0432.CCR-07-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doufekas K, et al. GALR1 methylation in vaginal swabs is highly accurate in identifying women with endometrial cancer. Int J Gynecol Cancer. 2013;23(6):1050–1055. doi: 10.1097/IGC.0b013e3182959103. [DOI] [PubMed] [Google Scholar]
- 75.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacol. 2013;38(4):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bröhl D, et al. A transcriptional network coordinately determines transmitter and peptidergic fate in the dorsal spinal cord. Dev Biol. 2008;322(2):381–393. doi: 10.1016/j.ydbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez S, Binato R, Guida L, Mencalha AL, Abdelhay E. Conserved transcription factor binding sites suggest an activator basal promoter and a distal inhibitor in the galanin gene promoter in mouse ES cells. Gene. 2014;538(2):228–234. doi: 10.1016/j.gene.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 78.Kofler B, et al. Characterization of the 5′-flanking region of the human preprogalanin gene. DNA Cell Biol. 1995;14(4):321–329. doi: 10.1089/dna.1995.14.321. [DOI] [PubMed] [Google Scholar]
- 79.Rüegg J, et al. Epigenetic regulation of glucose transporter 4 by estrogen receptor β. Mol Endocrinol. 2011;25(12):2017–2028. doi: 10.1210/me.2011-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;Suppl 232:1–55. [PubMed] [Google Scholar]
- 81.Fu W, et al. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518(17):3464–3494. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- 82.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 83.Smith GS, et al. Distribution of messenger RNAs encoding enkephalin, substance P, somatostatin, galanin, vasoactive intestinal polypeptide, neuropeptide Y, and calcitonin gene-related peptide in the midbrain periaqueductal grey in the rat. J Comp Neurol. 1994;350(1):23–40. doi: 10.1002/cne.903500103. [DOI] [PubMed] [Google Scholar]
- 84.Kawa L, et al. Expression of galanin and its receptors are perturbed in a rodent model of mild, blast-induced traumatic brain injury. Exp Neurol. 2016;279:159–167. doi: 10.1016/j.expneurol.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 85.Wang P, et al. Depression-like behavior in rat: Involvement of galanin receptor subtype 1 in the ventral periaqueductal gray. Proc Natl Acad Sci USA. 2016;113(32):E4726–35. doi: 10.1073/pnas.1609198113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pizzagalli DA. Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacol. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith KE, et al. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273(36):23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- 89.Jones EG. Neurotransmitters in the cerebral cortex. J Neurosurg. 1986;65(2):135–153. doi: 10.3171/jns.1986.65.2.0135. [DOI] [PubMed] [Google Scholar]
- 90.Savasta M, Palacios JM, Mengod G. Regional localization of the mRNA coding for the neuropeptide cholecystokinin in the rat brain studied by in situ hybridization. Neurosci Lett. 1988;93(2-3):132–138. doi: 10.1016/0304-3940(88)90070-5. [DOI] [PubMed] [Google Scholar]
- 91.Morino P, et al. Cholecystokinin in cortico-striatal neurons in the rat: Immunohistochemical studies at the light and electron microscopical level. Eur J Neurosci. 1994;6(5):681–692. doi: 10.1111/j.1460-9568.1994.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 92.Hedlund PB, Yanaihara N, Fuxe K. Evidence for specific N-terminal galanin fragment binding sites in the rat brain. Eur J Pharmacol. 1992;224(2-3):203–205. doi: 10.1016/0014-2999(92)90806-f. [DOI] [PubMed] [Google Scholar]
- 93.Xu ZQ, Ma X, Soomets U, Langel U, Hökfelt T. Electrophysiological evidence for a hyperpolarizing, galanin (1-15)-selective receptor on hippocampal CA3 pyramidal neurons. Proc Natl Acad Sci USA. 1999;96(25):14583–14587. doi: 10.1073/pnas.96.25.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Millón C, et al. 2016. Galanin (1-15) enhances the antidepressant effects of the 5-HT1A receptor agonist 8-OH-DPAT: Involvement of the raphe-hippocampal 5-HT neuron system. Brain Struct Funct 221(9):4491–4504.
- 95.Peter CJ, Akbarian S. Balancing histone methylation activities in psychiatric disorders. Trends Mol Med. 2011;17(7):372–379. doi: 10.1016/j.molmed.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialogues Clin Neurosci. 2009;11(3):257–268. doi: 10.31887/DCNS.2009.11.3/wrenthal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lolak S, Suwannarat P, Lipsky RH. Epigenetics of depression. Prog Mol Biol Transl Sci. 2014;128:103–137. doi: 10.1016/B978-0-12-800977-2.00005-X. [DOI] [PubMed] [Google Scholar]
- 98.Korf J, Aghajanian GK, Roth RH. Increased turnover of norepinephrine in the rat cerebral cortex during stress: Role of the locus coeruleus. Neuropharmacology. 1973;12(10):933–938. doi: 10.1016/0028-3908(73)90024-5. [DOI] [PubMed] [Google Scholar]
- 99.Zigmond RE, Schon F, Iversen LL. Increased tyrosine hydroxylase activity in the locus coeruleus of rat brain stem after reserpine treatment and cold stress. Brain Res. 1974;70(3):547–552. doi: 10.1016/0006-8993(74)90267-4. [DOI] [PubMed] [Google Scholar]
- 100.Svensson TH. Stress, central neurotransmitters, and the mechanism of action of alpha 2-adrenoceptor agonists. J Cardiovasc Pharmacol. 1987;10(Suppl 12):S88–S92. [PubMed] [Google Scholar]
- 101.Holmes PV, Blanchard DC, Blanchard RJ, Brady LS, Crawley JN. Chronic social stress increases levels of preprogalanin mRNA in the rat locus coeruleus. Pharmacol Biochem Behav. 1995;50(4):655–660. doi: 10.1016/0091-3057(94)00334-3. [DOI] [PubMed] [Google Scholar]
- 102.Sweerts BW, Jarrott B, Lawrence AJ. Expression of preprogalanin mRNA following acute and chronic restraint stress in brains of normotensive and hypertensive rats. Brain Res Mol Brain Res. 1999;69(1):113–123. doi: 10.1016/s0169-328x(99)00095-9. [DOI] [PubMed] [Google Scholar]
- 103.Andersson PO, Bloom SR, Edwards AV, Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol. 1982;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lundberg JM, Anggård A, Fahrenkrug J, Hökfelt T, Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: Functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci USA. 1980;77(3):1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bean AJ, Roth RH. Extracellular dopamine and neurotensin in rat prefrontal cortex in vivo: Effects of median forebrain bundle stimulation frequency, stimulation pattern, and dopamine autoreceptors. J Neurosci. 1991;11(9):2694–2702. doi: 10.1523/JNEUROSCI.11-09-02694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Consolo S, et al. Impulse flow dependency of galanin release in vivo in the rat ventral hippocampus. Proc Natl Acad Sci USA. 1994;91(17):8047–8051. doi: 10.1073/pnas.91.17.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7(2):126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 108.Vila-Porcile E, et al. Dendritic synthesis and release of the neuropeptide galanin: Morphological evidence from studies on rat locus coeruleus neurons. J Comp Neurol. 2009;516(3):199–212. doi: 10.1002/cne.22105. [DOI] [PubMed] [Google Scholar]
- 109.Ma X, et al. Effects of galanin receptor agonists on locus coeruleus neurons. Brain Res. 2001;919(1):169–174. doi: 10.1016/s0006-8993(01)03033-5. [DOI] [PubMed] [Google Scholar]
- 110.Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37(19):6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- 111.Steiner RA, et al. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98(7):4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci. 2002;18(1-2):151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- 113.Hökfelt T. Neuropeptides in perspective: The last ten years. Neuron. 1991;7(6):867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 114.Plotsky PM, Owens MJ, Nemeroff CB. Neuropeptide alterations in mood disorders. In: Bloom FE, editor. Psychopharmacology: The Fourth Generation of Progress. 4th. Raven; New York: 1995. pp. 971–981. [Google Scholar]
- 115.Kramer MS, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281(5383):1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- 116.Ranga K, Krishnan R. Clinical experience with substance P receptor (NK1) antagonists in depression. J Clin Psychiatry. 2002;63(Suppl 11):25–29. [PubMed] [Google Scholar]
- 117.Frick A, et al. Increased neurokinin-1 receptor availability in the amygdala in social anxiety disorder: A positron emission tomography study with [11C]GR205171. Transl Psychiatry. 2015;5:e597. doi: 10.1038/tp.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ratti E, et al. Full central neurokinin-1 receptor blockade is required for efficacy in depression: Evidence from orvepitant clinical studies. J Psychopharmacol. 2013;27(5):424–434. doi: 10.1177/0269881113480990. [DOI] [PubMed] [Google Scholar]
- 119.Barr AM, et al. A novel, systemically active, selective galanin receptor type-3 ligand exhibits antidepressant-like activity in preclinical tests. Neurosci Lett. 2006;405(1-2):111–115. doi: 10.1016/j.neulet.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 120.Swanson CJ, et al. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci USA. 2005;102(48):17489–17494. doi: 10.1073/pnas.0508970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petschner P, et al. Chronic venlafaxine treatment fails to alter the levels of galanin system transcripts in normal rats. Neuropeptides. 2016;57:65–70. doi: 10.1016/j.npep.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 122.Asberg M, Thorén P, Träskman L, Bertilsson L, Ringberger V. “Serotonin depression”--a biochemical subgroup within the affective disorders? Science. 1976;191(4226):478–480. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- 123.Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508(6):906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: New evidence of anatomical and physiological specificity. Physiol Rev. 1983;63(3):844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 125.Zhu PC, Thureson-Klein A, Klein RL. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: A possible mechanism for neuropeptide release. Neuroscience. 1986;19(1):43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]
- 126.Dumais A, et al. Risk factors for suicide completion in major depression: A case-control study of impulsive and aggressive behaviors in men. Am J Psychiatry. 2005;162(11):2116–2124. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- 127.Paxinos G, Huang X-F. Atlas of the Human Brainstem. Academic; San Diego: 1995. [Google Scholar]
- 128.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27(2-3):126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 129.Tost J, Gut IG. DNA methylation analysis by pyrosequencing. Nat Protoc. 2007;2(9):2265–2275. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- 130.Unschuld PG, et al. Polymorphisms in the galanin gene are associated with symptom-severity in female patients suffering from panic disorder. J Affect Disord. 2008;105(1-3):177–184. doi: 10.1016/j.jad.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 131.Lesch KP, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 132.Canli T, Lesch KP. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 133.Misawa K, et al. Epigenetic inactivation of galanin and GALR1/2 is associated with early recurrence in head and neck cancer. Clin Exp Metastasis. 2016;33(2):187–195. doi: 10.1007/s10585-015-9768-4. [DOI] [PubMed] [Google Scholar]
- 134.Misawa Y, et al. Tumor suppressor activity and inactivation of galanin receptor type 2 by aberrant promoter methylation in head and neck cancer. Cancer. 2014;120(2):205–213. doi: 10.1002/cncr.28411. [DOI] [PubMed] [Google Scholar]
- 135.Kohno D, et al. Dnmt3a in Sim1 neurons is necessary for normal energy homeostasis. J Neurosci. 2014;34(46):15288–15296. doi: 10.1523/JNEUROSCI.1316-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ernst C, et al. The effects of pH on DNA methylation state: In vitro and post-mortem brain studies. J Neurosci Methods. 2008;174(1):123–125. doi: 10.1016/j.jneumeth.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 137.Nagy C, et al. Effects of postmortem interval on biomolecule integrity in the brain. J Neuropathol Exp Neurol. 2015;74(5):459–469. doi: 10.1097/NEN.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 138.Millon C, et al. A role for galanin N-terminal fragment (1-15) in anxiety- and depression-related behaviors in rats. Int J Neuropsychopharmacol. 2015;18(3):1–13. doi: 10.1093/ijnp/pyu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Z, Xu Y, Wu L, Zhang S. Evolution of galanin receptor genes: Insights from the deuterostome genomes. J Biomol Struct Dyn. 2010;28(1):97–106. doi: 10.1080/07391102.2010.10507346. [DOI] [PubMed] [Google Scholar]
- 140.Lang R, et al. Pharmacological and functional characterization of galanin-like peptide fragments as potent galanin receptor agonists. Neuropeptides. 2005;39(3):179–184. doi: 10.1016/j.npep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 141.Lu X, Lundström L, Bartfai T. Galanin (2-11) binds to GalR3 in transfected cell lines: Limitations for pharmacological definition of receptor subtypes. Neuropeptides. 2005b;39(3):165–167. doi: 10.1016/j.npep.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 142.Robinson J, et al. Development of a high-throughput screening-compatible cell-based functional assay to identify small molecule probes of the galanin 3 receptor (GalR3) Assay Drug Dev Technol. 2013;11(8):468–477. doi: 10.1089/adt.2013.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu X, Bartfai T. Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):417–420. doi: 10.1007/s00210-009-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Villar MJ, et al. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33(3):587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]