Abstract
Individual genetic diversity is predicted to influence host–parasite interactions. Together with the genes directly associated with immune responses, variation in genes regulating vertebrate melanin-based pigmentation may play an important role in these interactions, mainly through the pleiotropic effects that affect colour-specific physiology, behaviour and immunity. Here, we test the hypothesis that the prevalence of avian malarial parasites differs between phenotypes in a raptor species in which the genetic basis of colour polymorphism and its pleiotropic effects over immune functions are known. We found that dark morphs had a higher prevalence of Plasmodium parasites than pale ones but detected no such association for Haemoproteus. This pattern may be associated with unequal exposure to vectors or, as suggested by our circumstantial evidence, to a differential ability to mount an immune response against blood parasites.
Keywords: colour polymorphism, haematozoa, host–parasite interactions, Plasmodium
1. Background
Understanding the role of individual genetic diversity in resistance to infectious diseases is crucial for forecasting evolutionary responses and long-term conservation of host populations [1,2]. In birds, genetic colour polymorphism—defined as a highly heritable variation in expressed plumage coloration that is independent of age and sex—is often associated with variation in life-history traits, including physiology, behaviour and immunity [3,4]. These associations may result from pleiotropic effects of genes regulating melanogenesis, such as the melanocortin-1-receptor (Mc1r). For example, pharmacological research has shown that melanocortin receptors and their ligands are key regulators of immune functions. Mc1r is constitutively expressed on monocytes/macrophages, but also on dendritic cells and lymphocytes with antigen-presenting and cytotoxic functions. The activation and binding of the peptide alpha-melanocyte-stimulating hormone (α-MSH) to its receptor MC1R in non-melanocytic immune cells modulates both the innate and the acquired immune responses, with overall anti-inflammatory and, apparently, immunosuppressive effects [5]. On the other hand, it has been proposed that the phagocytic function of melanocytes could confer higher protection from pathogens to more melanized individuals [6].
Parasites of the genera Plasmodium, Haemoproteus and Leucocytozoon are all pathogenic to some degree, yet Plasmodium is considered as the most virulent one [7]. Parasite lineages exhibit antigenic differences that will influence the effectiveness of the bird immune system. Consequently, virulence strongly depends on the interplay between specific lineages and the ability of the avian host to cope with the parasite infection [8]. In birds that survive infection, the initial acute phase, when severest fitness consequences generally occur, is followed by a rapid decline in parasitaemia to chronic levels with lower fitness consequences for the bearer [7,8]. Immune response to malarial infection is mainly cell-mediated through the lymphoid-macrophage system, while antibodies play an important supportive role [8]. Although the precise mechanism is unclear, a number of studies have proposed that the adaptive function of melanin-based colour polymorphism is associated with parasite resistance and could cause differences in vector-borne parasite loads between morphs (e.g. [9,10]).
Eleonora's falcon (Falco eleonorae) is a migratory raptor that breeds throughout the Mediterranean basin and winters in Madagascar. It occurs in two distinct melanin-based colour morphs owing to variation in the Mc1r gene [11]. Although the relationship between coloration and blood parasite infection in this species is unknown, both inflammatory and humoral immune responses are lower in dark than in pale nestlings [5,11]. Therefore, in the light of the link between Mc1r-genotypes and both arms of the immune system, we hypothesize that the two morphs will differ in parasite prevalence because dark morphs are less able to cope with parasite infections (genetic link hypothesis). Alternatively, parasite prevalence could differ due to morph-specific exposure to vectors, either if both morphs exploit different habitats with different vector abundances or if both morphs are differently appealing to vectors, thus creating unequal infection probabilities (exposure hypothesis).
2. Material and methods
Sampling was conducted in July–October in 2006–2014 on Alegranza islet (Canary Islands; 1050 ha, 289 m above sea level). Adult Eleonora's falcons were captured every year (mean = 23.22 individuals, range = 5–47), and their colour morph was determined visually [11]. All birds were weighed and their wing length measured. Blood samples were preserved in absolute ethanol and stored at −20°C until molecular analysis was performed. All birds were marked with numbered rings and released after manipulation.
(a). DNA extraction and blood parasite determinations
We analysed 209 blood samples from 183 individuals: 151 pale morphs (91 females and 60 males) and 32 dark morphs (22 females and 10 males). The remaining 26 samples belonged to 19 individuals recaptured in successive years. Genomic DNA was used to determine the prevalence of Plasmodium, Haemoproteus and Leucocytozoon parasites following [12] (see the electronic supplementary material).
(b). Statistical analyses
The probability of different morphs being infected by blood parasites was assessed using generalized linear mixed models (GLMMs) with binomial error and logit link function in R v. 3.0.2 [13] using the dataset available in [14]. To prevent pseudoreplication, we used a random subsampling (1000 iterations) of the 19 resampled birds for each parasite genus (see the electronic supplementary material). The infection by Plasmodium and Haemoproteus, respectively, was defined as a binary variable (0/1) and used as the response variable. The morph type, sex (only for Plasmodium) and their interaction were included as fixed factors. We also included a body-condition index as a covariate, estimated for each sex separately as the standardized residuals of a linear regression of body mass on wing length. Year was included as a random term. We did not perform a third model for Leucocytozoon, because only one individual was found to be infected by this parasite. Parameter estimates and standard errors from the resulting GLMMs were averaged and the range of p-values and percentage of models where each term was statistically significant calculated.
3. Results
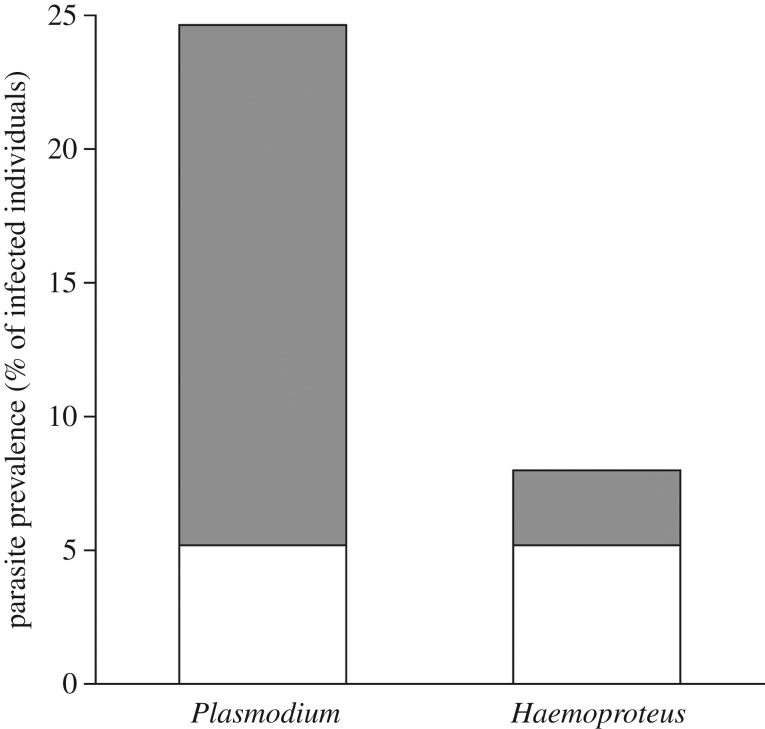
Overall, the prevalence of blood parasites was 12.9% (figure 1; electronic supplementary material, table S1 for details on parasite lineages, [14]). Of the 19 resampled individuals, 13 (10 pale females and three dark females) were never infected. However, one and two pale females became infected by Plasmodium and Haemoproteus, respectively, between the first and second sampling period. By contrast, three pale females, two infected by Haemoproteus and one by Plasmodium, were found to be uninfected 1 year later.
Figure 1.
Prevalence (number of infected/total × 100) of the two blood parasite genera infecting adult Eleonora's falcons of pale (white) and dark (grey) morph.
Dark falcons had a greater Plasmodium prevalence than pale ones (mean estimate = 2.50 ± 0.01 s.e., p-range = 0.001–0.01; figure 1). Sex was not significant (mean estimate = 0.75 ± 0.01 s.e., p-range = 0.59–0.99), whereas body condition (mean estimate = 0.011 ± 0.001 s.e., p-range = 0.02–0.59) and the interaction between sex and morph (mean estimate = −2.20 ± 0.012 s.e., p-range = 0.03–0.22) were significant only in 6.5% and 16.6% of the models, respectively. There was no significant relationship between the probability of infection by Haemoproteus and any explanatory variable (in all cases p-range = 0.30–0.99).
4. Discussion
We found that dark falcons had a higher prevalence of Plasmodium, the commonest parasite genus, than pale ones but found no significant relationship for Haemoproteus. Different factors such as differential exposure to vectors, the differing virulence of parasite genus/lineages and/or the host's capacity to fight infections may influence this result. In the support of the first possibility, Galeotti & Sacchi [9] found that rufous-morph tawny owls (Strix aluco) hosted higher total blood parasite burdens than grey morphs owing to both greater exposure to vectors and greater susceptibility to parasites. In feral pigeons (Columba livia), alternative morphs were distributed non-randomly across an urban gradient and had different parasite risks [15]. However, the different Eleonora's falcon morphs inhabit small islands sympatrically during the breeding season and local transmission of blood parasites at breeding grounds is absent, owing to the lack of suitable vectors [16]. Therefore, differences in the exposure to vectors must occur during migration and/or in their wintering quarters, where insect vectors abound [17] and the parasite transmission is likely to be higher.
The most prevalent Plasmodium LK6 is thought to be transmitted by Culex pipiens, while P_ACCTAC01 is transmitted by Coquillettidia aurites (see the electronic supplementary material, table S1), and these are common mosquitoes in Africa and Madagascar [17]. The lineage LK6 was recently isolated from passerines from Macaronesian archipelagoes, the Iberian Peninsula and Morocco (electronic supplementary material, table S1). Although the transmission areas remain unclear, it has been proposed that migratory birds such as Eleonora's falcon could spread blood parasites to resident birds on the main islands, where insect vectors are present [16]. It has been suggested that darker colours are more attractive to mosquitoes than light colours and so entirely dark plumages could increase host–vector contact rates. However, feeding preferences of these mosquitoes with regard to colour attractiveness are unknown. In addition, data on GPS-tagged falcons do not indicate the existence of morph-specific habitat exploitation during winter (L Gangoso, J Figuerola 2015, unpublished data).
This suggests that the difference in prevalence between morphs is unlikely to be due to morph-specific exposure to vectors but probably results from differential abilities to mount an immune response. Pale falcons could be more susceptible to Plasmodium infection than dark ones and their lower prevalence could in turn be the reflection of greater mortality. No study has addressed the effects of LK6 on host survival (electronic supplementary material, table S1). However, we cannot rule out a selective disappearance of pale morphs due to a higher mortality during the acute phase of infection. Dark Eleonora's falcons have poorer immune responses than pale ones from the nestling stage onwards [5,11]. It is thus likely that dark falcons have lower immune capacities in adulthood since this negative relationship is due to their Mc1r-derived genotype and not to the environment [5]. The fact that three infections found in pale females became undetectable in successive years partially supports the idea of greater immune competence in pale falcons for fighting infections. Nonetheless, the effects of infection can greatly depend on the parasite load. Previous studies addressing the relationship between plumage coloration and blood parasites have found differences in infection intensity rather than in prevalence, thereby suggesting that differences are due to resistance to parasites rather than exposure to vectors [6,10,18]. However, we estimated prevalence rather than infection intensity because birds were caught during a relatively long period (from arrival at breeding grounds to fledglings' emancipation) and across years. Infection intensity may vary greatly along and between breeding seasons [19], thus making between-individuals comparisons difficult to interpret. Further experimental approaches would be needed to clarify the relationship among colour polymorphism, blood parasite intensity and immune competence.
In conclusion, our results are in accordance with the genetic link hypothesis, yet we cannot completely rule out the exposure hypothesis and both mechanisms could contribute to the skewed prevalence of Plasmodium to the dark morph. To the best of our knowledge, this is the first study addressing the relationship between colour polymorphism and parasite prevalence in which both the gene responsible for colour polymorphism and its pleiotropic effects on immune functions are known, which thus enabled us to infer potential mechanisms underlying this covariation.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank J. J. Moreno and J. M. Grande for their help during the fieldwork, and L. Gómez and I. Martín for help during the laboratory work.
Ethics
All experimental procedures were approved by the CSIC ethics committee and Animal Health authorities as per Spanish law (no. 534/2014).
Data accessibility
The R code used in this article has been uploaded as part of the electronic supplementary material. Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.tk41q [14].
Authors' contributions
L.G., J.M.P. and J.F. designed the study; and R.G.L. and J.M.P. performed the molecular laboratory work. L.G. collected field data, analysed the data and wrote the paper with input from all other authors, who gave their final approval for publication. All authors agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
This study was partially funded by the Cabildo de Lanzarote, projects CGL2012-30759 and CGL2015-65055-P from the Spanish MINECO, and the Severo Ochoa programme for Centers of Excellence in R&D&I (SEV-2012-0262). R.G.L. was supported by an FPI grant (BES-2013-065274), J.M.P. by a Juan de la Cierva contract and L.G. by a contract under the Excellence Projects of the Junta de Andalucía (RNM-7800).
References
- 1.Carius HJ, Little TJ, Ebert D. 2001. Genetic variation in a host-parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136–1145. ( 10.1111/j.0014-3820.2001.tb00633.x) [DOI] [PubMed] [Google Scholar]
- 2.Thompson JN. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Ducrest AL, Keller L, Roulin A. 2008. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol. Evol. 23, 502–510. ( 10.1016/j.tree.2008.06.001) [DOI] [PubMed] [Google Scholar]
- 4.Roulin A, Ducrest AL. 2011. Association between melanism, physiology and behaviour: a role for the melanocortin system. Eur. J. Pharmacol. 660, 226–233. ( 10.1016/j.ejphar.2011.01.036) [DOI] [PubMed] [Google Scholar]
- 5.Gangoso L, Roulin A, Ducrest AL, Grande JM, Figuerola J. 2015. Morph-specific genetic and environmental variation in innate and acquired immune response in a color polymorphic raptor. Oecologia 178, 1113–1123. ( 10.1007/s00442-015-3306-6) [DOI] [PubMed] [Google Scholar]
- 6.Chakarov N, Boerner M, Krüger O. 2008. Fitness in common buzzards at the cross-point of opposite melanin–parasite interactions. Funct. Ecol. 22, 1062–1069. ( 10.1111/j.1365-2435.2008.01460.x) [DOI] [Google Scholar]
- 7.van Riper C III, Atkinson CT, Seed TM. 1994. Plasmodia of birds. In Parasitic protozoa, vol. 7 (ed Kreier JP.), pp. 73–140. New York, NY: Academic Press. [Google Scholar]
- 8.Bensch S, Waldenström J, Jonzén N, Westerdahl H, Hansson B, Sejberg D, Hasselquist D. 2007. Temporal dynamics and diversity of avian malaria parasites in a single host species. J. Anim. Ecol. 76, 112–122. ( 10.1111/j.1365-2656.2006.01176.x) [DOI] [PubMed] [Google Scholar]
- 9.Galeotti P, Sacchi R. 2003. Differential parasitaemia in the tawny owl (Strix aluco): effects of colour morph and habitat. J. Zool. 261, 91–99. ( 10.1017/S0952836903003960) [DOI] [Google Scholar]
- 10.Jacquin L, Lenouvel P, Haussy C, Ducatez S, Gasparini J. 2011. Melanin-based coloration is related to parasite intensity and cellular immune response in an urban free living bird: the feral pigeon Columba livia. J. Avian Biol. 42, 11–15. ( 10.1111/j.1600-048X.2010.05120.x) [DOI] [Google Scholar]
- 11.Gangoso L, Grande JM, Ducrest AL, Figuerola J, Bortolotti GR, Andrés JA, Roulin A. 2011. MC1R-dependent, melanin-based colour polymorphism is associated with cell-mediated response in the Eleonora's falcon. J. Evol. Biol. 24, 2055–2063. ( 10.1111/j.1420-9101.2011.02336.x) [DOI] [PubMed] [Google Scholar]
- 12.Hellgren O, Waldenström J, Bensch S. 2004. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 90, 797–802. ( 10.1645/GE-184R1) [DOI] [PubMed] [Google Scholar]
- 13.R Core Team. 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 14.Gangoso L, Gutiérrez-López R, Martínez-de la Puente J, Figuerola J. 2016. Data from: Genetic color polymorphism is associated with avian malarial infections. Dryad Digital Repository. ( 10.5061/dryad.tk41q) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacquin L, Récapet C, Prévot-Julliard AC, Leboucher G, Lenouvel P, Erin N, Corbel H, Frantz A, Gasparini J. 2013. A potential role for parasites in the maintenance of color polymorphism in urban birds. Oecologia 173, 1089–1099. ( 10.1007/s00442-013-2663-2) [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez-López R, et al. 2015. Low prevalence of blood parasites in a long-distance migratory raptor: the importance of host habitat. Parasit. Vectors 8, 189 ( 10.1186/s13071-015-0802-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tantely ML, Le Goff G, Boyer S, Fontenille D. 2016. An updated checklist of mosquito species (Diptera: Culicidae) from Madagascar. Parasite 23, 20 ( 10.1051/parasite/2016018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei B, Amar A, Koeslag A, Gous TA, Tate GJ. 2013. Differential haemoparasite intensity between black sparrowhawk (Accipiter melanoleucus) morphs suggests an adaptive function for polymorphism. PLoS ONE 8, e81607 ( 10.1371/journal.pone.0081607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merino S, Tomás G, Moreno J, Sanz JJ, Arriero E, Folgueira C. 2004. Changes in Haemoproteus sex ratios: fertility insurance or differential sex lifespan? Proc. R. Soc. Lond. B 271, 1605–1609. ( 10.1098/rspb.2004.2764) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R code used in this article has been uploaded as part of the electronic supplementary material. Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.tk41q [14].