Abstract
Mast cells (MCs) are key effector cells of IgE-FcεRI- or MrgprX2-mediated signaling event. Shuang-Huang-Lian (SHL), a herbal formula from Chinese Pharmacopoeia, has been clinically used in type I hypersensitivity. Our previous study demonstrated that SHL exerted a non-negligible effect on MC stabilization. Herein, we sought to elucidate the molecular mechanisms of the prominent anti-allergic ability of SHL. MrgprX2- and IgE-FcεRI-mediated MC activation in vitro and in vivo models were developed by using compound 48/80 (C48/80) and shrimp tropomyosin (ST), respectively. Our data showed that SHL markedly dampened C48/80- or ST-induced MC degranulation in vitro and in vivo. Mechanistic study indicated that cytosolic Ca2+ (Ca2+[c]) level decreased rapidly and sustainably after SHL treatment, and then returned to homeostasis when SHL was withdrawn. Moreover, SHL decreases Ca2+[c] levels mainly through enhancing the mitochondrial Ca2+ (Ca2+[m]) uptake. After genetically silencing or pharmacologic inhibiting mitochondrial calcium uniporter (MCU), the effect of SHL on the Ca2+[c] level and MC degranulation was significantly weakened. Simultaneously, the activation of SHL on Ca2+[m] uptake was completely lost. Collectively, by activating MCU, SHL decreases Ca2+[c] level to stabilize MCs, thus exerting a remarkable anti-allergic activity, which could have considerable influences on clinical practice and research.
Mast cells (MCs) originate from the haematopoietic progenitor cells that enter nearly all vascularized tissue, where they complete their maturation and, under some circumstances, can then migrate into epithelia1,2,3. As tissue-resident cells, MCs are strategically situated at host-environment interfaces, such as the skin, respiratory and gastrointestinal tracts, ready to respond to immunogenic stimuli4, indicating that they act as key contributors of innate and adaptive immune responses5,6. MCs are activated on IgE receptor (FcεRI) crosslinking, resulting in the release of a diverse array of preformed cytoplasmic granule-associated mediators (e.g., histamine and β-hexosaminidase, etc.), as well as newly synthesized proinflammatory lipid mediators, cytokines and chemokines7,8. In the FcεRI-independent pathways, MCs may be activated by numerous stimuli including basic secretagogues [e.g., substance P, compound 48/80 (C48/80) and mastoparan], peptidergic drugs (e.g., icatibant), THIQ motif drugs (e.g., atracurium) and fluoroquinolone family antibiotics (e.g., ciprofloxacin). Recently research revealed that they are all ligands of MrgprX2, an orthologue of the human G-protein coupled mas-related gene receptor9,10. But whichsoever, IgE-FcεRI- or MrgprX2-mediated MC signaling event, eventually results in the activation of protein kinase C (PKC) and the release of Ca2+ from the endoplasmic reticulum (ER), which in turn induces the stromal interaction molecule 1-mediated opening of the store-operated Ca2+ channel ORAI1 and then leads to the influx of extracellular Ca2+. The influx of Ca2+ is amplified by short transient potential Ca2+ channel 1. The increase in intracellular Ca2+ levels and the activation of PKC trigger MC degranulation10,11.
Thus, calcium mobilization is a critical event to the activation of MCs and intracellular Ca2+ pools are the determining factors of MC degranulation12. Mitochondrial Ca2+ (Ca2+[m]) uptake is considered to buffer local or bulk cytosolic Ca2+ (Ca2+[c]) rises13. But until recently, the uniporter’s veil began to be lifted. Now, it is known that the uniporter is a multi-subunit Ca2+ channel, with the Ca2+ pore formed by mitochondrial calcium uniporter (MCU) protein14,15 and accessory proteins, including MICU116, MICU217, MCUb18, MCUR119 and EMRE20. Although the precise roles of these accessory proteins is far from clear, they are required either for the channel activity or for regulating MCU under various conditions. MCU, an approximate 40-kDa protein, possesses two predicted transmembrane domains, which forms (through oligomerization) a gated ion channel21. Mutation of a single amino acid (serine 259) resulted in a uniporter that loses the ability to be deactivated by the classical inhibitor ruthenium red. Moreover, mutations in the acidic linker domain resulted in markedly diminished calcium uptake22. The fact that mitochondria buffer the Ca2+[c] rises by accumulating Ca2+ into their matrix raises the question whether the activating MCU may dampen MC degranulation for the treatment of allergy, anaphylaxis and asthma, etc.
Shuang-Huang-Lian (SHL), a formula containing Lonicerae Japonicae Flos, Scutellariae Radix and Fructus Forsythiae, is consistently prepared by stringent manufacturing procedure from Chinese Pharmacopoeia23. Clinically, SHL products, generally considered as the antimicrobial agent, are delivered through different routes (e.g., oral, injectable and pulmonary routes, etc.)23,24, and widely used to treat upper respiratory tract infection, pneumonia, tonsillitis, and other respiratory diseases caused by bacterium/viruses25. Our previous studies indicated that SHL protected lung tissue from infections via the potential anti-inflammatory and anti-oxidative activities25,26. In addition, SHL has also been applied in the type I hypersensitivity, including bronchial asthma27,28,29, vernal keratitis30, urticaria and eczema31, by using the aerosol inhalation or intravenous drip. Indeed, the excellent MC stabilization effect of SHL was observed. The present study focused on the underlying molecular mechanism of SHL. Our findings reveal that, for the first time, SHL potently stabilizes MCs through decreasing Ca2+[c] level by activating MCU independent of Ca2+[c] rise, which is different from the conventional MC stabilizers (e.g., cromolyn sodium and ketotifen).
Results
SHL exerts prominent effects on C48/80-induced MC activation in vitro and in vivo
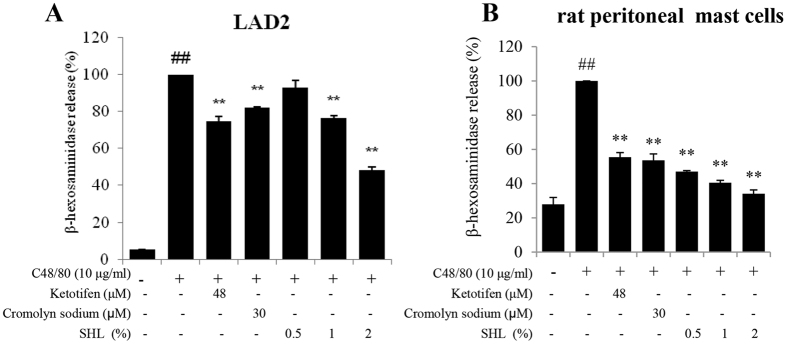
MC degranulation can be elicited by the synthetic C48/80, a direct and convenient reagent to study anaphylaxis32. We assessed the effect of SHL on C48/80-induced MC degranulation using β-hexosaminidase assay in vitro. As shown in Fig. 1, 10 μg/ml of C48/80 evoked a markedly β-hexosaminidase release in the LAD2 cells and rat peritoneal MCs (P < 0.01), while SHL potently inhibited the β-hexosaminidase release concentration-dependently (P < 0.01) without cytotoxicity (Fig. S1).
Figure 1. SHL suppressed C48/80-induced β-hexosaminidase releases in vitro.
The LAD2 cells (A) and rat peritoneal MCs (B) were stimulated by C48/80 with or without SHL. β-hexosaminidase release was determined 1.5 h after C48/80 challenge. Ketotifen and cromolyn sodium were used as a positive control. ##P < 0.01 vs. control; *P < 0.05 and **P < 0.01 vs. C48/80 alone.
Owing to the significant influence of SHL on the allergic mediator release in vitro, we next determined the effects of SHL on C48/80-induced anaphylactic shock in mice. As shown in Tables 1 and 2, intraperitoneal injection of C48/80 at 8 mg/kg caused a fatal anaphylactic shock with the mortality of 100%. In comparison, treatment of SHL either 30 min before or 5 min after C48/80 challenge under 2.5 ml/kg and 5 ml/kg dosages dramatically protected the mice against the anaphylactic shock and greatly reduced the mortality, showing the preventive and therapeutic effects of SHL on C48/80-induced anaphylactic shock in vivo.
Table 1. Preventive effect of SHL on C48/80-induced anaphylactic shock in mice (n = 12).
| Group | Dose | Survival rate (%) |
|---|---|---|
| Control | None (saline) | 0 |
| Ketotifen* | 47 μmol/kg | 41.7 |
| Cromolyn sodium* | 0.4 mmol/kg | 58.5 |
| SHL* | 1.25 ml/kg | 16.7 |
| SHL | 2.5 ml/kg | 75 |
| SHL | 5 ml/kg | 100 |
*SHL or the positive control (ketotifen or cromolyn sodium) was i.p. injected only once 30 min before the C48/80 administration.
Table 2. Therapeutic effect of SHL on C48/80-induced anaphylactic shock in mice (n = 12).
| Group | Dose | Survival rate (%) |
|---|---|---|
| Control | None (saline) | 0 |
| Ketotifen* | 47 μmol/kg | 25 |
| Cromolyn sodium* | 0.4 mmol/kg | 25 |
| SHL* | 1.25 ml/kg | 8.3 |
| SHL | 2.5 ml/kg | 58.3 |
| SHL | 5 ml/kg | 91.7 |
*SHL or the positive controls (ketotifen or cromolyn sodium) was i.p. injected only once 5 min after the C48/80 challenge.
SHL suppresses IgE-FcεRI-mediated MC activation in vitro and in vivo
Except for C48/80-induced anaphylactoid reaction, IgE-FcεRI-mediated allergic reactions are another kind of anaphylaxis33. Due to the surface expression of the high-affinity FcεRI receptor for IgE and the release of chemical mediators after crosslinking34, the sensitized RBL-2H3 cells were used to assess the effect of SHL on the shrimp tropomyosin (ST)-induced degranulation. Our data showed that pretreatment with SHL concentration-dependently dampened IgE-FcεRI-mediated β-hexosaminidase release (Fig. 2A).
Figure 2. SHL suppressed IgE-FcεRI-mediated MC activation in vitro and in vivo.
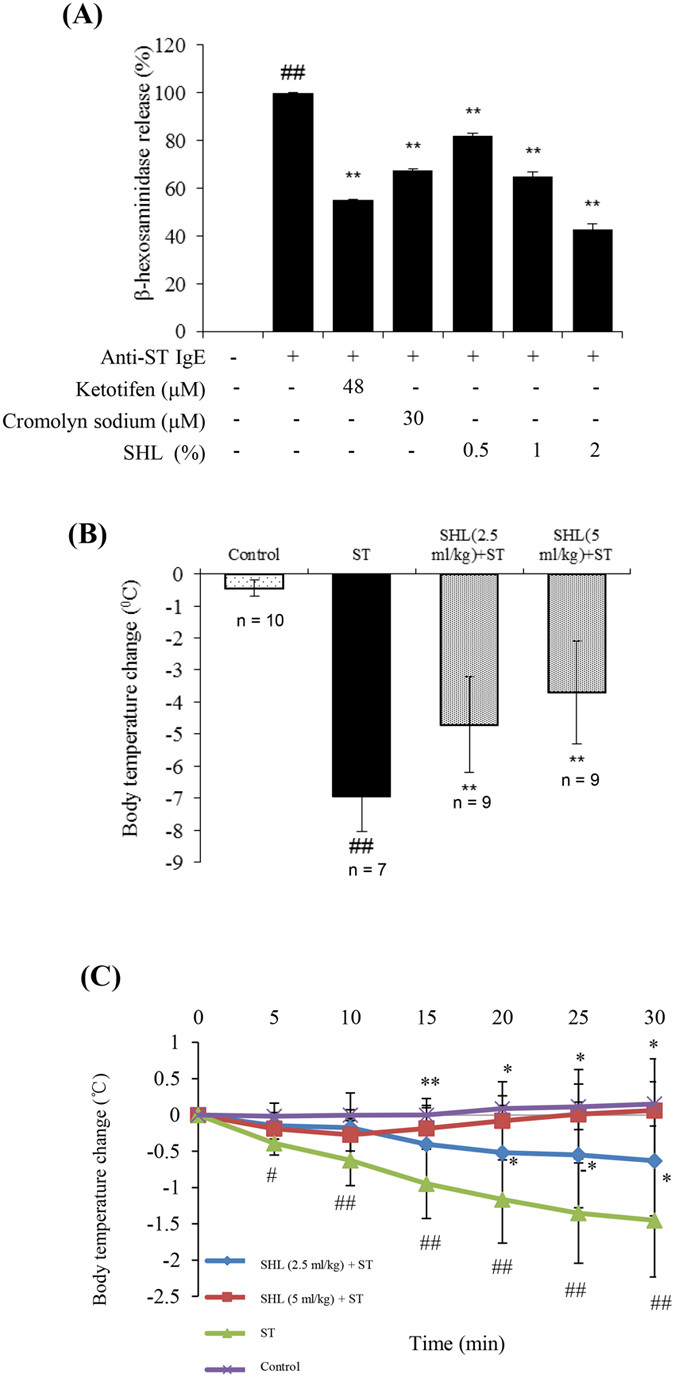
(A) SHL suppressed IgE-mediated β-hexosaminidase release in RBL-2H3 cells. The supernatants were assayed 1.5 h after ST challenge in the IgE-sensitized RBL-2H3 cells. Ketotifen and cromolyn sodium were used as a positive control. (B) Effects of SHL on the body temperature of ST-induced ASA mice. Rectal temperature was measured 30 min after ST challenge. (C) Effects of SHL on the body temperature of PSA mice (n = 6). #P < 0.05, ##P < 0.01 vs. control group; *P < 0.05, **P < 0.01 vs. ST group.
We next determined the effect of SHL on ST-induced active systemic anaphylaxis (ASA) in mice. As shown in Fig. 2B, robust hypothermia was observed after ST challenge (ΔT ≈ −7 °C) compared with the normal control group, while pretreatment with SHL significantly attenuated the body temperature decrease (P < 0.01). Next, the effect of SHL on passive systemic anaphylaxis (PSA) was shown in Fig. 2C and Fig. S2, after ST challenge, the body temperature of the sensitized mice gradually decreased about 1.5 °C within 30 min, while SHL markedly prevented the body temperature decrease.
SHL stabilizes MCs by decreasing Ca2+ [c] levels of resting cells
The above findings confirm that SHL dampens C48/80-MrgprX2 and IgE-FcεRI mediated MC degranulation9,35, both of which depends on the increase of Ca2+[c] concentration. Thus, we next investigated whether SHL could affect the Ca2+[c] level. As expected, ST challenge markedly elevated Ca2+[c] level in the sensitized RBL-2H3 cells, while pretreatment with SHL significantly reduced Ca2+[c] level in a concentration-dependent manner (Fig. 3A), without a direct chelation (data not shown). Of note, the Ca2+[c] levels before ST challenge (at 0 min) had been significantly reduced in response to pretreatment with SHL compared with the control (Fig. 3A), strongly suggesting that SHL decreased Ca2+[c] concentration before the IgE receptor cross-linking. To confirm this, we further measured the effects of SHL on the Ca2+[c] level in the resting RBL-2H3 cells. As shown in Fig. 3B, the Ca2+[c] level decreased rapidly and sustainably after SHL treatment, and then returned to homeostasis when SHL was withdrawn. Similar effect was observed in both human (LAD2) and mouse (P815) MCs (data not shown). In contrast, cromolyn sodium and ketotifen did not affect Ca2+[c] in the resting cells (data not shown) at their effective concentrations on MC degranulation (Figs 1 and 2A).
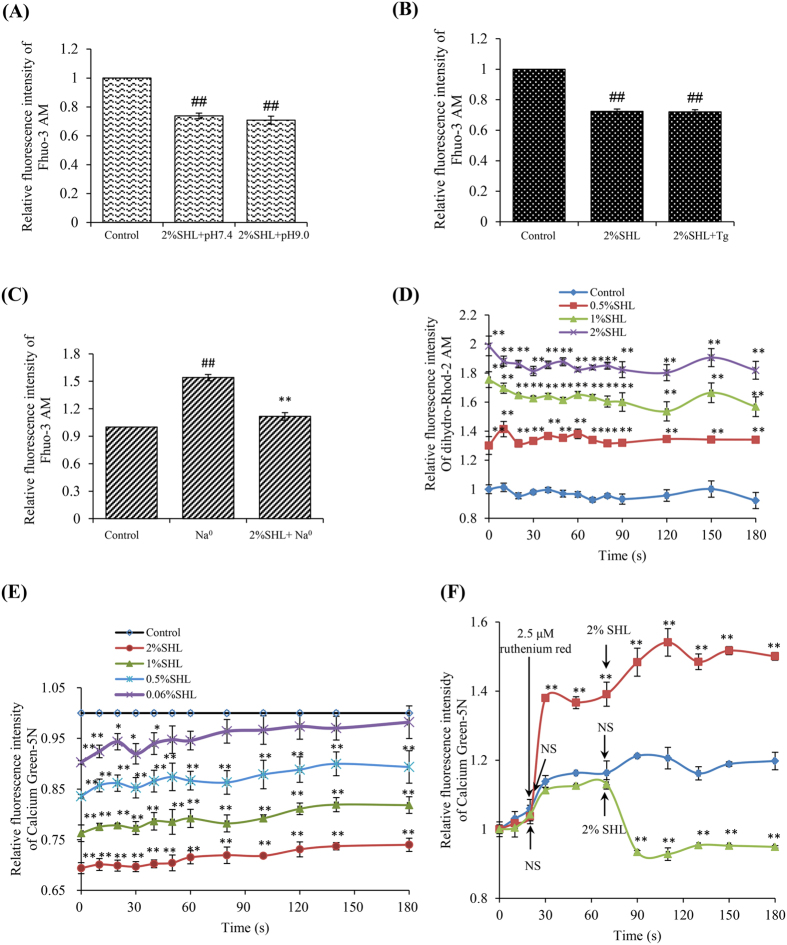
Figure 3. SHL reduced Ca2+[c] levels.
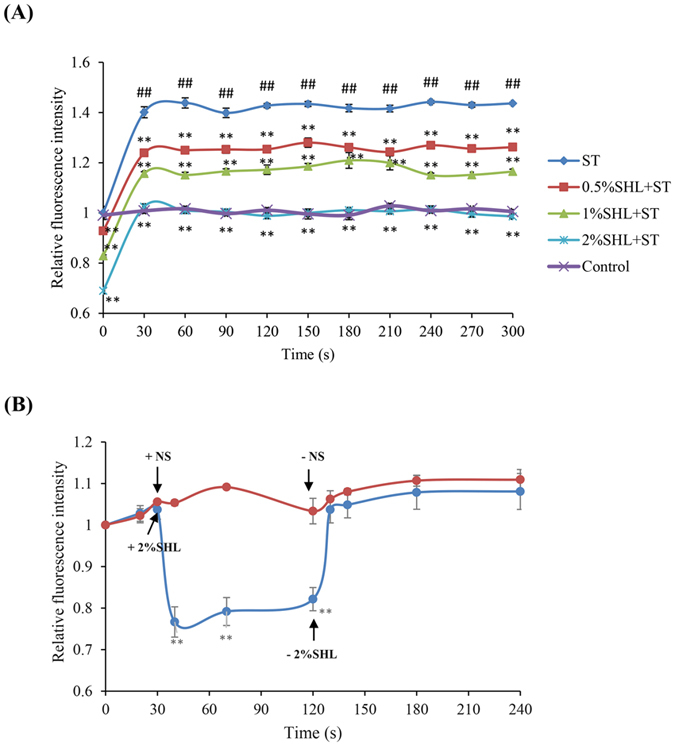
(A) Effect of SHL on IgE-mediated Ca2+[c] level in the sensitized RBL-2H3 cells. The sensitized cells were loaded with fluo-3 AM (4 μM) at 30 °C for 30 min. The stained cells were treated with or without SHL for 30 min and then exposed to ST (20 ng/ml). The fluorescent intensity (λex 485 nm and λem 538 nm) was recorded every 30 s. ##P < 0.01 vs. control; *P < 0.05, **P < 0.01 vs. ST alone. (B) SHL reduced Ca2+[c] levels in the resting RBL-2H3 cells. The cells were loaded with fluo-3 AM (4 μM) at 30 °C for 30 min. The stained cells were treated with or without SHL and the fluorescent intensity (λex 485 nm and λem 538 nm) was immediately recorded. **P < 0.01 vs. control.
SHL reduces Ca2+ [c] levels via enhancing the Ca2+ [m] uptake
The rapid and reversible effect of SHL on Ca2+[c] strongly suggested an underlying non-genomic mechanism. To our knowledge, two ways are recognized to reduce Ca2+ from the cytosol: extruding of Ca2+ through Na+-Ca2+ exchangers (NCX) and plasma membrane Ca2+-ATPase (PMCA), and (or) clearance of Ca2+ by resequestration into the ER and mitochondria36. Our findings showed that under either inhibition of PMCA activity by alkaline pH 9.037 or suppression of sarco/endoplasmic Ca2+-ATPase (SERCA) by thapsigargin38, the reduction of SHL on Ca2+[c] was not affected (Fig. 4A,B). Unexpectedly, SHL was still able to lower Ca2+[c] when the extracellular Na+ was withdrawn39 (Fig. 4C), seemingly suggesting that SHL inhibited rather than activated NCX. But anyhow, the reduction of Ca2+[c] by SHL in the resting cells is independent of PMCA, NCX and SERCA. Then, we found that SHL significantly enhanced the Ca2+[m] uptake in a concentration dependent manner (Fig. 4D). By using Calcium Green-5N, Ca2+[m] uptake was evaluated in the isolated mouse liver mitochondria, which is of advantage that Ca2+ uptake phenotypes can be directly attributed to mitochondria. In agreement with the results in Fig. 4C, SHL (≥0.06%) treatment led to a significant increase of Ca2+[m] uptake in response to extramitochondrial pulses of 50 μM of Ca2+ (Fig. 4E), which can be blocked by a MCU inhibitor ruthenium red15 (Fig. 4F). These results demonstrate that SHL decreases Ca2+[c] levels mainly through enhancing the Ca2+[m] uptake.
Figure 4. SHL decreased Ca2+[c] levels mainly through increasing the Ca2+[m] uptake.
(A) SHL did not affect the activity of PMCA. The effect of SHL on Ca2+[c] was determined by a shift to alkaline pH 9.0. ##P < 0.01 vs. control. (B) SHL did not affect the activity of SERCA. The effect of SHL on Ca2+[c] was determined in the present of thapsigargin (Tg, 5 μm), an inhibitor of the ER Ca2+-ATPase. ##P < 0.01 vs. control. (C) Effect of SHL on NCX. The effect of SHL on Ca2+[c] was measured in a Na+-free solution (Na0) containing 40 mM KCl. ##P < 0.01 vs. control; **P < 0.01 vs. Na0. (D) SHL concentration-dependently increased Ca2+[m] level in RBL-2H3 cells. The cells were loaded with 2 μM of dihydro-rhod-2/AM at 37 °C for 1 h and kept in primary culture for an additional 16 h. The fluorescence intensity of Rhod 2 was determined at λex 535 nm and λem 590 nm. (E) SHL increased Ca2+[m] uptake in the isolated mouse liver mitochondria. Ca2+[m] uptake in isolated mouse liver mitochondria was measured with Calcium Green-5N. (F) The increased effects of SHL on Ca2+[m] uptake can be blocked by ruthenium red. *P < 0.05 and **P < 0.01 vs. control.
SHL enhances Ca2+ [m] uptake by activating MCU
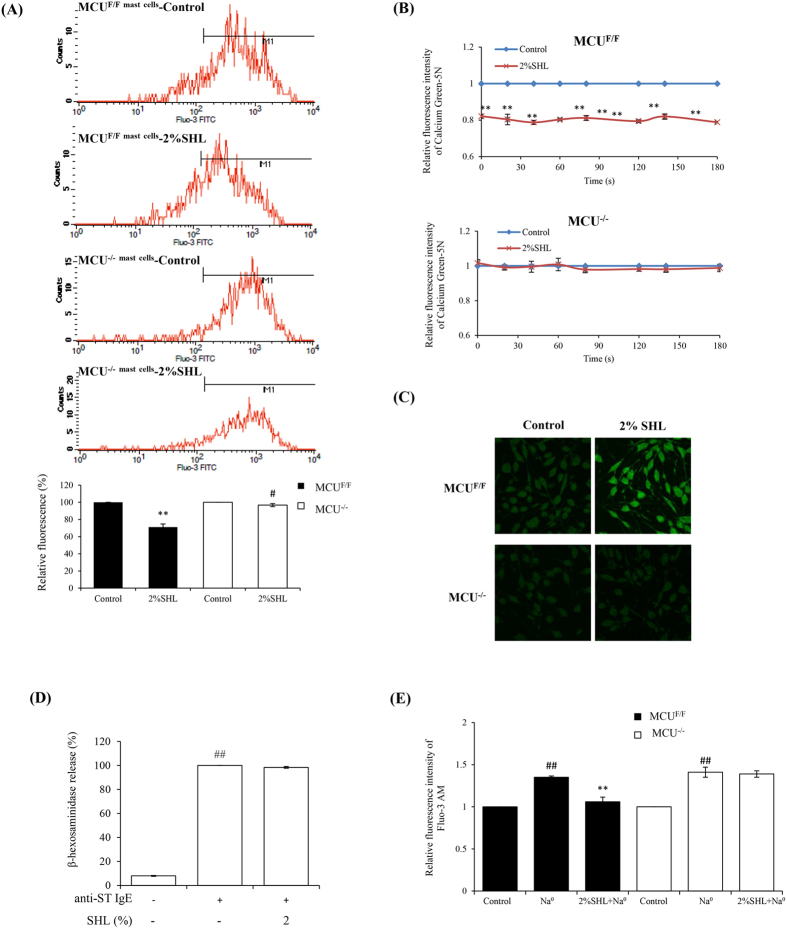
Calcium transport between the cytoplasm and the mitochondrial matrix involves the passage of Ca2+ across both the outer and inner mitochondrial membranes (OMM and IMM). The overall permeability of the OMM for Ca2+ is relatively high, while the IMM presents a tight barrier for Ca2+ 40. Early studies have revealed that MCU protein, which can form a Ca2+ channel in lipid bilayer in the IMM, forms the basis of the primary mechanism for Ca2+[m] transport14,15,41. Moreover, our above result (Fig. 4F) also showed that the effect of SHL on Ca2+[m] uptake can be completely blocked by ruthenium red, highly implicating that SHL enhanced Ca2+[m] uptake might through activating MCU. To verify whether MCU is indeed involved in the effect of SHL on Ca2+[m], we silenced MCU in mice using a Entranster TM in vivo transfection reagent. The resulting mice, termed MCU−/− mice, lack MCU protein in peritoneal MCs and liver mitochondria compared with the negative control mice (MCUF/F). The fluorescence intensity for the Ca2+[c] of FcεRI+ cells, namely MCs, was analyzed by a flow cytometer (FACSCalibur, BD, USA). It was found that SHL potently reduced Ca2+[c] levels of peritoneal MCs in MCUF/F mouse with a decreased percentage of 29%, while this effect was notably weakened in the MCU−/− cells with only a decreased percentage of 3.3% (Fig. 5A).
Figure 5. SHL increased Ca2+[m] uptake by activating MCU.
(A) The influence of SHL on Ca2+[c] was weakened upon silencing of MCU in mouse peritoneal MCs (n = 3). The cells in the mouse abdominal cavity were loaded with fluo-3/AM and stained with PerCP-eFluor 710 labeled anti-mouse FcεR1 antibody. The fluorescence intensity for the Ca2+[c] of MCs were analyzed by a flow cytometer. **P < 0.01 vs. Control of MCUF/F; #P < 0.05 vs. Control of MCU−/−. (B) The influence of SHL on Ca2+[m] was completely lost in the liver mitochondria from MCU−/− mice (n = 3). Ca2+ uptake in liver mitochondria following the addition of 50 μM CaCl2 was measured with Calcium Green-5N. **P < 0.01 vs. Control. (C) Representative images of SHL-mediated Ca2+[m] uptake in MCU−/− RBL-2H3 cells and its negative control cells MCUF/F. The Cells were infected with Ad.m-GCaMP2. 24 h later, the fluorescence signal in the mitochondria was captured by confocal microscopy using a 100× oil objective. (D) SHL did not suppress IgE-mediated β-hexosaminidase release in MCU−/− RBL-2H3 cells. (E) Effect of SHL on NCX in MCU−/− RBL-2H3 cells. ##P < 0.01 vs. control; **P < 0.01 vs. Na0.
In the isolated liver mitochondria from the MCUF/F and MCU−/− mice, the activation of SHL on the Ca2+[m] uptake was completely lost upon silencing of MCU (Fig. 5B). Moreover, in the MCU−/− RBL-2H3 cells, the effect of SHL on the Ca2+[m] uptake and MC degranulation also disappeared (Fig. 5C,D). It was in this MCU defective cells that we did not observe the effect of SHL in Fig. 4C (Fig. 5E), indicating that SHL did not affect NCX. Taken together, these findings reveal that SHL increases Ca2+[m] uptake through activating MCU to decrease Ca2+[c] level, thus dampens MC degranulation.
Quercetin, caffeic acid, ursolic acid, D-(-)-quinic acid and methyl salicylate lower Ca2+ [c] levels of resting cells
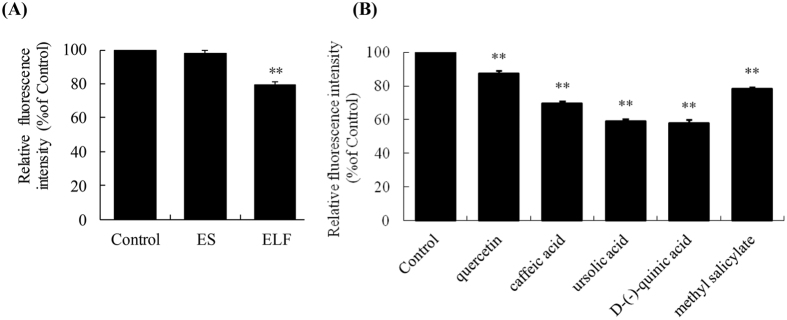
According to the Chinese Pharmacopoeia23, SHL is a mixture of the extract of Scutellariae Radix (ES) and the extract of Lonicerae Japonicae Flos and Fructus Forsythiae (ELF). Thus, to identify the active constituents in SHL, we first evaluated the effects of ES and ELF at the equivalent concentrations in 2% SHL on Ca2+[c] in the resting RBL-2H3 cells. As shown in Fig. 6A, ELF, rather than ES, significantly reduced Ca2+[c] levels compared with untreated control in a nontoxic manner (data not shown). Next, we tested the effects of 26 constituents from ELF (Table S1) on the Ca2+[c] levels. Figure 6B shows that Ca2+[c] levels were significantly decreased in the presence of quercetin, caffeic acid, ursolic acid, D-(-)-quinic acid and methyl salicylate at 10 μg/ml in a nontoxic manner (data not shown), and the fore 3 constituents could be detected by the HPLC-UV according to the Chinese Pharmacopoeia23 (Fig. S3), suggesting that they might be the major active constituents of SHL.
Figure 6. Effects of the active fractions and constituents in SHL on Ca2+[c] levels in the resting RBL-2H3 cells.
(A) Effects of ES and ELF on Ca2+[c] levels in the resting RBL-2H3 cells. The final concentrations of ES and ELF are 192.4 μg/ml and 660 μg/ml, which are equivalent to the concentration in 2% SHL injection. (B) Effects of quercetin, caffeic acid, ursolic acid, D-(-)-quinic acid and methyl salicylate (10 μg/ml) in ELF on Ca2+[c] levels in RBL-2H3 cells. Ca2+[c] levels were expressed as the relative fluorescence intensity, and the values in control cells were arbitrarily normalized as 100%. **P < 0.01 vs. control.
Discussion
MCs are key effector cells that can act as potent initiators and amplifiers in allergy, immunity, and inflammation by secreting multiple mediators6,42. Our findings demonstrated that SHL markedly dampened C48/80- and IgE-mediated MC degranulation in vitro and in vivo (Figs 1 and 2 and Tables 1 and 2), showing an impressive influence on the MC activation. Further study indicated that SHL stabilized MC via a rapid, potent and reversible effect on Ca2+[c] level of resting cells (Fig. 3B), which is different from the conventional MC stabilizers (e.g., cromolyn sodium and ketotifen).
As a MC activator, C48/80 could induce a rapid release of allergic mediators and consequently lead to a systemic fatal anaphylaxis43,44. In accordance with previous studies45,46,47, intraperitoneal injection of C48/80 (8 mg/kg) induced a fatal anaphylactic shock with a mortality of 100% within 1 h. Unexpectedly but excitedly, by a single intraperitoneal treatment with 3.34 times adult oral dosage of SHL (5 ml/kg) or 600 times that of ketotifen (47 μmol/kg), the survival rate of SHL group were actually far more than that of ketotifen group (Tables 1 and 2).
At present, the commonly-used allergen in the IgE-FcεRI-mediated allergy research is ovalbumin (OVA) to mimic type I hypersensitivity48,49. However, our previous result showed that the sensibility of common mice response to OVA was not satisfactory, especially in the absence of an adjuvant (data not shown), which might be associated with the immune tolerance induced by a long-term consumption of eggs powder in rodents’ fodder50. As we known, seafood allergy is widely recognized as a universal health care issue51,52,53 and is one of the most common forms of food allergies54,55. Shrimp protein is a major allergen in the shellfish-induced allergy study56,57. Thus, we extracted a purified ST (Fig. S4) from the Metapenaeus ensis by isoelectric precipitation56. Satisfactorily, compared with OVA, ST dramatically elevated the total IgE level in the mouse sera, showing a more sensitive responsivity (data not shown). Therefore, ST instead of OVA was used in our study. In the IgE-FcεRI-mediated β-hexosaminidase release (in vitro) and PSA (in vivo), SHL exerted markedly anti-anaphylactic effects (Fig. 2A,C). In ASA mice, SHL also significantly attenuated the body temperature decrease (Fig. 2B). In particular, hypothermia in ASA mice was far more intense than that in PSA mice, and SHL exerted more effective protection on PSA than ASA (Fig. 2B), which may be attributed to the fact that ASA is mediated not only by IgE, but also by IgG58.
Theoretically, it is feasible for a drug to stabilize MCs through buffering the Ca2+[c] rises via accumulating Ca2+[c] into mitochondrial matrix. However, difficulties lie in the experimental practices. To our knowledge, MCU mediates Ca2+ uptake into the matrix to regulate metabolism, cytoplasmic Ca2+ signaling and cell death59. The uptake is electrogenic, driven by the large voltage present across the IMM (ΔΨ m) developed by proton pumping by the respiratory chain21,60. Balanced Ca2+[m] is critical for the regulation of mitochondrial functions such as fission, fusion and ATP production61. On one hand, Ca2+[m] rise is the stimulation of Ca2+-sensitive dehydrogenases of the Krebs cycle, tuning ATP synthesis to the increased needs of a cell; on the other hand, uncontrolled Ca2+[m] overload can lead to the opening of the mitochondrial permeability transition pore with disruption of mitochondrial membrane potential (MMP)62. Excess Ca2+ entry in mitochondria has been associated with apoptosis and necrosis in many pathological states63. Most recently, Vais and his colleagues found that mitochondria were protected from Ca2+ depletion and overload by a unique complex involving Ca2+ sensors on both sides of the IMM, coupled through EMRE59. Obviously, the dynamic regulation of Ca2+[m] is a highly sophisticated process. Thus, as a MC stabilizer through enhancing Ca2+[m] uptake, how to strike a better balance between the effectivity and toxicity is a serious challenge. Our findings indicate that it is through activating MCU that SHL, which has been using for the allergic diseases clinically, decreases Ca2+[c] level to stabilize MCs. Both the effectivity and safety (non-toxic) of SHL are compatible in vitro and in vivo, indicating that the Ca2+[m] increase induced by SHL through activating MCU is sustainable to a certain degree. Of course, the pharmacological reversibility of SHL is also an essential factor. Moreover, excess Ca2+ entry in mitochondria (Ca2+[m] overload) causes more reactive oxygen species (ROS) generation, a by-product of Krebs cycle, whose elevation is a key event that leads to further organelle depolarization and loss of MMP, thus resulting in a vicious cycle64,65. Perhaps not by coincidence, SHL possesses scavenging effect on the excess intracellular ROS thus protecting MMP25, which may also play an important role for striking the balance between effectivity and non-toxic.
It is generally recognized that MCU is a Ca2+-activated Ca2+ channel whose activation depends on the increase of Ca2+[c] concentration15. But, unlike the known MCU agonist histamine15, SHL can activate MCU independent of Ca2+[c] rise. Thus, we were able to observe that SHL rapidly reduced Ca2+[c] levels in the resting cells (Fig. 3B) and enhanced Ca2+[m] uptake in the isolated liver mitochondria (Fig. 4E), suggesting that the active constituents in SHL (e.g., quercetin, caffeic acid, ursolic acid, etc.) can rapidly enter into the cells to directly act on the mitochondria to active MCU. It is noteworthy that although five constituents reduced Ca2+[c] of resting cells (Fig. 6B), their effective concentrations (10 μg/ml) on Ca2+[c] was far higher than their equivalent concentrations in 2% SHL, indicating that there might be a series of active ingredients similar to these five constituents to collectively act on MCU to markedly stabilize MCs.
In summary, SHL is a potent inhibitor of MC activation through decreasing Ca2+[c] level by activating MCU. By virtue of the effect on resting Ca2+[c], the degree of MC activation was potently suppressed, which not only could limit allergic disease, but also might be beneficial to some non-allergic diseases involved MC activation, such as atherosclerosis66, obesity67,68, diabetes67,68, chronic obstructive pulmonary disease69, cancer70, postoperative ileus71 and fibromyalgia72, etc. However, our finding, together with the fact that SHL has already been used in the clinic for decades, may offer a suitable novel target for the clinical management of aberrant MC activation in diseases.
Methods
Materials
SHL injection and its 2 intermediate fractions (ES and ELF), which were prepared according to the Chinese Pharmacopoeia23 were from Duoduo Pharmaceutical Co., Ltd. (Jiamusi, Heilongjiang, China). C48/80, 4-Methylumbelliferyl N-acetyl-β-D-glucosaminide, and Pluonic F-127 were purchased from Sigma-Aldrich (St Louis, MO, USA). Fluo-3 AM Ester and rhod-2/AM were from Biotium (San Francisco, CA, USA). Ketotifen and cromolyn sodium were from TCI (Tokyo, Japan) and National Institutes for Food and Drug Control (Beijing, China), respectively. Calcium Green-5N and PerCP-eFluor 710 labeled anti-mouse FcεR1 antibody were obtained from Invitrogen (Carlsbad, CA, USA) and eBioscience (San Diego, CA, USA), respectively. The transfection reagents Entranster TM-in vivo and -H4000 were from Engreen Biosystem (Beijing, China). Balb/c mice (male, 18–20 g) and SD rats (male, 160–180 g) were from Vital River Experimental Animal Services (Beijing, China).
siRNA and plasmid
The MCU siRNA and their negative controls were synthesized by GenePharma Co., Ltd. (Shanghai, China). The plasmid pcDNA3.1-mito-GCaMP2 was a kind gift from Dr. Xianhua Wang (Institute of Molecular Medicine, Peking University, Beijing, China). In this plasmid, the GCaMP2 calcium indicator was ligated with a mitochondrial targeting sequence73.
Cells
Rat basophilic leukemia cell line (RBL-2H3) and mouse mastocytoma cell line (P815) were purchased from the cell bank of Chinese Academy of Sciences (Shanghai, China). Human LAD2 cell line (from Michael D. Gershon, MD, Columbia University, USA) was presented as a gift from Prof. Renshan Sun (the Third Military Medical University, Chongqing, China). Peritoneal MCs were isolated from SD rats.
Isolation of ST
ST from the Metapenaeus ensis was extracted and purified by an isoelectric precipitation method as previously described56. Protein content of the purified fraction was assayed by Bradford method74, and the purity of the obtained ST was >98% (Fig. S4).
Production of mouse anti-ST monoclonal IgE
The preparation of antibody was similar to our previously described method75 except for the substitution of Freund’s adjuvant for Imject Alum.
β-hexosaminidase release assay
The β-hexosaminidase release assay was performed as previously described with some modifications76. For the measurements of IgE-induced β-hexosaminidase release, RBL-2H3 cells were seeded in the 48-well plates at 5.0 × 105 cells/well and sensitized with anti-ST monoclonal IgE (25 μg/ml) at 37 °C overnight. The cells were washed by Hank’s balanced salt solution (HBSS) supplemented with 0.1% (w/v) BSA and pre-incubated with SHL in 120 μl of HBSS at 37 °C for 30 min followed by adding 20 ng/ml of ST for further 1.5 h incubation. 30 μl of the supernatant was transferred to a 96-well black flat-bottom plate accompany with 50 μl of substrate solution (0.57 mg/ml 4-Methylumbelliferyl N-acetyl-β-D-glucosaminide in the buffer contained 133 mM sodium citrate and 133 mM NaCl, pH 4.3). The reaction proceeded at 37 °C for 1.5 h and was stopped by adding stop buffer (50 mM glycine and 5 mM EDTA-Na2, pH 10.5; 200 μl/well). Fluorescence was determined with a fluorescence microplate reader at λex 355 nm and λem 460 nm.
For the measurements of C48/80-induced β-hexosaminidase release, the LAD2 cells (6.0 × 104 cells/well) or the peritoneal MCs (1.0 × 106 cells/well) were seeded in the 96-well plates and pretreated with SHL at 37 °C for 30 min followed by adding C48/80 (10 μg/ml) for further 1.5 h incubation. β-hexosaminidase in the supernatant was determined.
C48/80-induced anaphylactic shock in mice
Balb/c mice were kept in standard laboratory conditions of temperature and humidity with a 12 h light/dark cycle. All experiments were carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Animals Ethics Committee of the Institute of Medicinal Plant Development of the Chinese Academy of Medical Sciences. The mice were given the intraperitoneal injection of C48/80 at 8 mg/kg77. For the preventive effect, SHL or the positive controls (ketotifen or cromolyn sodium) was i.p. injected only once 30 min before C48/80 administration (designated “single treatment”). For the therapeutic effect, SHL or the positive controls was i.p. injected only once 5 min after C48/80 challenge. Mortality was monitored for 1 h after induction of anaphylactic shock.
PSA
Mice were passively sensitized intravenously (i.v.) with 40 μg/mouse of anti-ST monoclonal IgE, while the control group were given the equal volume of physiologic saline. 24 h later, the mice were challenged (i.v.) with 20 μg/mouse of ST after pretreatment with SHL or physiologic saline (i.p.) for 20 min. The rectal temperature was measured by a thermal probe (ChengDu Instrument Factory, China) for 30 min using a polygraph (RM6240, Chengdu, China).
ASA
Mice received an i.p. injection of 100 μl of Imject Alum containing 60 μg/mouse ST and were immunized again 7 days later. 2 days after the second immunization, the mice were pretreated with SHL (2.5 ml/kg or 5 ml/kg) or physiologic saline (Control group and ST model group) for 30 min and then challenged by a rapid intravenous infusion (via the lateral tail vein) of 5 μg/mouse of ST. The mice in the control group were received the same solution without ST. To monitor changes in body temperature associated with anaphylaxis, rectal temperature was measured 30 min after ST challenge.
Measurement of Ca2+ [c] level
Measurement of the Ca2+[c] level was performed using the calcium-reactive fluorescence probe Fluo-3/AM as previously described with slight modifications78. Briefly, the cells were resuspended (2 × 106 cells/ml) and incubated for 30 min in the dark at 30 °C with Fluo-3/AM (4 μM) in the presence of 0.04% (w/v) Pluonic F-127 in HEPES buffer (10 mM HEPES, 135 mM NaCl, 1 mM Na2HPO4, 1 mM CaCl2, 5 mM KCl, 0.5 mM MgCl2, 5 mM glucose and 0.1%BSA, pH 7.4). 4 mM probenecid was added to avoid leakage of fluo-3. After removing the dye, the cells were treated with SHL or normal saline (the control group) and the fluorescent intensity was immediately determined at λex 485 nm and λem 538 nm using a spectrofluorimeter (Thermo Electron, Washington, USA).
To assay the Ca2+[c] levels in mouse peritoneal MCs, the cells in the mouse abdominal cavity were isolated and loaded with Fluo-3/AM (4 μM) at 30 °C for 30 min. MCs could be recognized and analyzed by a FACSCalibur flow cytometer after staining with PerCP-eFluor 710 labeled anti-mouse FcεR1 antibody at 25 °C for 15 min.
Measurement of Ca2+ [m] uptake
Measurement of Ca2+[m] level in RBL-2H3 cells was performed using the mitochondrially localizing Ca2+-reactive fluorescence probe, rhod-2/AM, as previously described79. To improve the discrimination between cytosolic and mitochondrially localized dye, 5 μM rhod-2/AM was reduced to the colorless, nonfluorescent dihydro-rhod-2/AM by sodium borohydride, according to the manufacturer’s protocol. RBL-2H3 cells were loaded with dihydro-rhod-2/AM (2 μM) at 37 °C for 1 h. The residual cytosolic fraction of the dye was eliminated when the cells were kept in primary culture for an additional 16 h after loading, whereas the mitochondrial dye fluorescence was maintained. The fluorescence intensity of rhod-2 was determined at λex 535 nm and λem 590 nm.
Mouse liver mitochondria were isolated and further purified80. Ca2+[m] uptake was measured using Calcium Green-5N according to previously described14.
The recombinant adenovirus (Ad.m-GCaMP2) based on the pcDNA3.1-mito-GCaMP2 was produced by Hanbio Biotechnology Co., Ltd. (Shanghai, China). Cells were infected with Ad.m-GCaMP2. 24 h later, the fluorescence signal in the mitochondria was captured by confocal microscopy (Fluoview FV1000, Olympus, Japan) using a 100× oil objective.
MCU siRNA transfected in vivo and in vitro
The mice were injected via tail vein with MCU siRNA (siRNA-MCU1#: sense 5′-GCG CCA GGA AUA UGU UUA UTT-3′ and antisense 5′-AUA AAC AUA UUC CUG GCG CTT-3′; siRNA-MCU2#: sense 5′-CCA AAG AGA GAC CUC CUA ATT-3′ and antisense 5′-UUA GGA GGU CUC UCU UUG GTT-3′) or the negative control siRNA (sense 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense 5′-ACG UGA CAC GUU CGG AGA ATT-3′) on days 1 and 4 (3 OD/mouse). Entranster TM-in vivo transfection reagent was used to deliver the siRNA according to the manufacturer′s recommendations. On day 5, the mice were sacrificed after anesthetization. Fresh liver and the peritoneal MCs were immediately isolated for the assay.
The rat MCU siRNA (siRNA-MCU1#: sense 5′-GCC AGA GAC AGA CAA UAC UTT-3′ and antisense 5′-AGU AUU GUC UGU CUC UGG CTT-3′; siRNA-MCU2#: sense 5′-GGA GAA GGU ACG GAU UGA ATT-3′ and antisense 5′-UUC AAU CCG UAC CUU CUC CTT-3′) or the negative control (sense 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense 5′-ACG UGA CAC GUU CGG AGA ATT-3′) was transfected into RBL-2H3 cells using Entranster TM-H4000 as described in the manufacturer’s protocol.
Statistical analysis
Data represent the mean ± SD of at least three independent experiments. Statistical analysis was performed by one-way ANOVA. A student’s t test was used when only two groups were compared. The difference was considered to be statistically significant when P < 0.05.
Additional Information
How to cite this article: Gao, Y. et al. The Three-Herb Formula Shuang-Huang-Lian stabilizes mast cells through activation of mitochondrial calcium uniporter. Sci. Rep. 7, 38736; doi: 10.1038/srep38736 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos 81274163 and 81601385), Beijing Natural Science Foundation (No. 7142112), the CAMS Innovation Fund for Medical Sciences (2016-I2M-3-015) and the Open Research Fund of State Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources (Chengdu, P. R. China).
Footnotes
Author Contributions Y.G., Y.Q. and C.P. conceived the experiments and wrote the manuscript. Y.G., R.H. and Q.F. performed the main experiments. L.F. prepared all materials, and performed the Ca2+[c] analysis and confocal microscopy image. Y.H. statistically analyzed all data. R.C. cultured the cells. All authors reviewed the manuscript.
References
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu. Rev. Immunol. 7, 59–76 (1989). [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Baram D. & Mekori Y. A. Mast cells. Physiol. Rev. 77, 1033–1079 (1997). [DOI] [PubMed] [Google Scholar]
- Galli S. J., Zsebo K. M. & Geissler E. N. The kit ligand, stem cell factor. Adv. Immunol. 55, 1–96 (1994). [DOI] [PubMed] [Google Scholar]
- Tsai M., Grimbaldeston M. & Galli S. J. Mast cells and immunoregulation/immunomodulation. Adv. Exp. Med. Biol. 716, 186–211 (2011). [DOI] [PubMed] [Google Scholar]
- Galli S. J., Grimbaldeston M. & Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 8, 478–486 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. & Tsai M. IgE and mast cells in allergic disease. Nat. Med. 18, 693–704 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T. C., Bondy P. K., Tsakalos N. D. & Askenase P. W. Differential release of serotonin and histamine from mast cells. Nature 297, 229–231 (1982). [DOI] [PubMed] [Google Scholar]
- Grimbaldeston M. A. et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil B. D. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston M. A. Mast cell-MrgprB2: sensing secretagogues or a means to overreact? Immunol. Cell Biol. 93, 221–223 (2015). [DOI] [PubMed] [Google Scholar]
- Wernersson S. & Pejler G. Mast cell secretory granules: armed for battle. Nat. Rev. Immunol. 14, 478–494 (2014). [DOI] [PubMed] [Google Scholar]
- Beaven M. A. et al. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J. Biol. Chem. 259, 7129–7136 (1984). [PubMed] [Google Scholar]
- Pizzo P., Drago I., Filadi R. & Pozzan T. Mitochondrial Ca2+ homeostasis: mechanism, role, and tissue specificities. Pflugers Arch. 464, 3–17 (2012). [DOI] [PubMed] [Google Scholar]
- Baughman J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Stefani D., Raffaello A., Teardo E., Szabò I. & Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F. et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467, 291–296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plovanich M. et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One 8, e55785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A. et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 32, 2362–2376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K. et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 14, 1336–1343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y. et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago I., Pizzo P. & Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 30, 4119–4125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. et al. The ins and outs of mitochondrial calcium. Circ. Res. 116, 1810–1819 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- State Pharmacopoeia Committee. Chinese Pharmacopoeia, Vol. 1. 846–848 (Chemical Industry Press, 2010). [Google Scholar]
- Sun X. Z., Xie J. S. & Xie L. Y. The pharmacology and clinical application of Shuang-Huang-Lian. Hei Long Jiang Medicine Journal 19, 54–55 (2006). [Google Scholar]
- Gao Y. et al. Shuang-Huang-Lian exerts anti-inflammatory and anti-oxidative activities in lipopolysaccharide-stimulated murine alveolar macrophages. Phytomedicine 21, 461–469 (2014). [DOI] [PubMed] [Google Scholar]
- Fang L. et al. Shuang-huang-lian attenuates lipopolysaccharide-induced acute lung injury in mice involving anti-inflammatory and antioxidative activities. Evid. Based Complement Alternat. Med. 2015, 283939 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. W. Shuang-Huang-Lian injection by aerosol inhalation for the treatment of bronchial asthma. Modern Journal of Integrated Traditional Chinese and Western Medicine 17, 1448 (2008). [Google Scholar]
- Chen H. S. & Huang Q. M. Shuang-Huang-Lian powder-injection for the treatment of bronchial asthma (30 cases). Guangxi Journal of Traditional Chinese Medicine 21, 16 (1998). [Google Scholar]
- Liu X. S., Mei G. Y. & Ye X. Clinical observation of Shuang-Huang-Lian aerosol for the treatment of airway obstruction (106 cases). Chinese Journal of Integrated Traditional and Western Medicine Suppl, 169–170 (1994). [Google Scholar]
- Wang Y. Q. Study of the pharmacologic actions and clinical new applications of Shuang-Huang-Lian injection. For all Health 6, 107–108 (2012). [Google Scholar]
- Suqing Y. et al. Skin diseases therapy by Shuang-Huang-Lian injection (50 cases). Chinese medicine and Pharmacology 1, 28 (1998). [Google Scholar]
- Ennis M., Pearce F. L. & Weston P. M. Some studies on the release of histamine from mast cells stimulated with polylysine. Br. J. Pharmacol. 70, 329–334 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos V. & Gigi E. Anaphylactic and anaphylactoid reactions during the perioperative period. Hippokratia 15, 138–140 (2011). [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Mukai K., Tsujimura Y. & Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat. Rev. Immunol. 9, 9–13 (2009). [DOI] [PubMed] [Google Scholar]
- Gilfillan A. M. & Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6, 218–230 (2006). [DOI] [PubMed] [Google Scholar]
- Ma H. T. & Beaven M. A. Regulation of Ca2+ signaling with particular focus on mast cells. Crit. Rev. Immunol. 29, 155–186 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gover T. D., Moreira T. H., Kao J. P. & Weinreich D. Calcium homeostasis in trigeminal ganglion cell bodies. Cell Calcium 41, 389–396 (2007). [DOI] [PubMed] [Google Scholar]
- Peng Y. & Guo A. Novel stimulus-induced calcium efflux in Drosophila mushroom bodies. Eur. J. Neurosci. 25, 2034–2044 (2007). [DOI] [PubMed] [Google Scholar]
- Heise N. et al. Effect of dexamethasone on Na+/Ca2+ exchanger in dendritic cells. Am. J. Physiol. Cell Physiol. 300, C1306–1313 (2011). [DOI] [PubMed] [Google Scholar]
- Csordás G., Várnai P., Golenár T., Sheu S. S. & Hajnóczky G. Calcium transport across the inner mitochondrial membrane: molecular mechanisms and pharmacology. Mol. Cell Endocrinol. 353, 109–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hail D., Palty R. & Shoshan-Barmatz V. Measurement of mitochondrial Ca2+ transport mediated by three transport proteins: VDAC1, the Na+/Ca2+ exchanger, and the Ca2+ uniporter. Cold Spring Harb Protoc 2014, 161–166 (2014). [DOI] [PubMed] [Google Scholar]
- Weng Z., Patel A. B., Panagiotidou S. & Theoharides T. C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 135, 1044–1052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allansmith M. R., Baird R. S., Ross R. N., Barney N. P. & Bloch K. J. Ocular anaphylaxis induced in the rat by topical application of compound 48/80. Dose response and time course study. Acta Ophthalmol Suppl 192, 145–153 (1989). [DOI] [PubMed] [Google Scholar]
- Kim H. Y., Ko K. J., Nam S. Y., Jeong H. J. & Kim H. M. The Sound of a Buk (Korean Traditional Drum) Attenuates Anaphylactic Reactions by the Activation of Estrogen Receptor-β. Int. Arch. Allergy Immunol. 167, 242–249 (2015). [DOI] [PubMed] [Google Scholar]
- El-Agamy D. S. Anti-allergic effects of nilotinib on mast cell-mediated anaphylaxis like reactions. Eur. J. Pharmacol. 680, 115–121 (2012). [DOI] [PubMed] [Google Scholar]
- Kim S. H. et al. Suppression of mast cell-mediated allergic reaction by Amomum xanthiodes. Food Chem. Toxicol. 45, 2138–2144 (2007). [DOI] [PubMed] [Google Scholar]
- Shin T. Y., Kim S. H., Choi C. H., Shin H. Y. & Kim H. M. Isodon japonicus decreases immediate-type allergic reaction and tumor necrosis factor-alpha production. Int. Arch. Allergy Immunol. 135, 17–23 (2004). [DOI] [PubMed] [Google Scholar]
- Squaiella-Baptistão C. C., Teixeira D., Mussalem J. S., Ishimura M. E. & Longo-Maugéri I. M. Modulation of Th1/Th2 immune responses by killed Propionibacterium acnes and its soluble polysaccharide fraction in a type I hypersensitivity murine model: induction of different activation status of antigen-presenting cells. J. Immunol. Res. 2015, 132083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. M., Torrero M. N., Evans H. & Mitre E. Immunologic characterization of 3 murine regimens of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 135, 1341–1351 (2015). [DOI] [PubMed] [Google Scholar]
- Zheng S. S. et al. The immunoregulation of IgE antibody production in mice IV. Anti-ovalbumin IgE antibody response. Acta Academiae Medicinae Sinicae 9, 329–333 (1987). [PubMed] [Google Scholar]
- Leickly F. E. et al. Self-reported adherence, management behavior, and barriers to care after an emergency department visit by inner city children with asthma. Pediatrics 101, E8 (1998). [DOI] [PubMed] [Google Scholar]
- Low I. & Stables S. Anaphylactic deaths in Auckland, New Zealand: a review of coronial autopsies from 1985 to 2005. Pathology 38, 328–332 (2006). [DOI] [PubMed] [Google Scholar]
- Yamaguchi J. et al. A case of occupational contact urticaria and oral allergy syndrome due to seafood. Arerugi 56, 49–53 (2007). [PubMed] [Google Scholar]
- Rona R. J. et al. The prevalence of food allergy: a meta-analysis. J. Allergy Clin. Immunol. 120, 638–646 (2007). [DOI] [PubMed] [Google Scholar]
- Chiang W. C. et al. The changing face of food hypersensitivity in an Asian community. Clin. Exp. Allergy 37, 1055–1061 (2007). [DOI] [PubMed] [Google Scholar]
- Capobianco F. et al. Oral sensitization with shrimp tropomyosin induces in mice allergen-specific IgE, T cell response and systemic anaphylactic reactions. Int. Immunol. 20, 1077–1086 (2008). [DOI] [PubMed] [Google Scholar]
- Leung P. S. et al. Induction of shrimp tropomyosin-specific hypersensitivity in mice. Int. Arch. Allergy Immunol. 147, 305–314 (2008). [DOI] [PubMed] [Google Scholar]
- Tsujimura Y. et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity 28, 581–589 (2008). [DOI] [PubMed] [Google Scholar]
- Vais H. et al. EMRE is a Matrix Ca(2+) Sensor that Governs Gatekeeping of the Mitochondrial Ca(2+) Uniporter. Cell Rep. 14, 403–410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J. K. & Philipson B. The mitochondrial Ca(2+) uniporter complex. J. Mol. Cell Cardiol. 78, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom J. & Sheu S. S. Morphological dynamics of mitochondria-a special emphasis on cardiac muscle cells. J. Mol. Cell Cardiol. 46, 811–820 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally K. W., Peixoto P. M., Ryu S. Y. & Dejean L. M. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta 1813, 616–622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C. et al. Mitochondrial Ca(2+) and apoptosis. Cell Calcium 52, 36–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G., Xie W., Reiken S. R. & Marks A. R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 112, 11389–11394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. Y. et al. Interplay of reactive oxygen species, intracellular Ca2+ and mitochondrial homeostasis in the apoptosis of prostate cancer cells by deoxypodophyllotoxin. J. Cell Biochem. 114, 1124–1134 (2013). [DOI] [PubMed] [Google Scholar]
- Bot I. & Biessen E. A. Mast cells in atherosclerosis. Thromb. Haemost. 106, 820–826 (2011). [DOI] [PubMed] [Google Scholar]
- Wang J. & Shi G. P. Mast cell stabilization: novel medication for obesity and diabetes. Diabetes Metab. Res. Rev. 27, 919–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E., Folkerts G. & Redegeld F. Mast cells and COPD. Pulm. Pharmacol. Ther. 24, 367–372 (2011). [DOI] [PubMed] [Google Scholar]
- Ribatti D. Mast cells as therapeutic target in cancer. Eur. J. Pharmacol. 778, 152–157 (2016). [DOI] [PubMed] [Google Scholar]
- The F. O. et al. The role of mast cell stabilization in treatment of postoperative ileus: a pilot study. Am. J. Gastroenterol. 104, 2257–2266 (2009). [DOI] [PubMed] [Google Scholar]
- Ang D. C., Hilligoss J. & Stump T. Mast Cell Stabilizer (Ketotifen) in Fibromyalgia: Phase 1 Randomized Controlled Clinical Trial. Clin. J. Pain Nov 3, 10.1097/AJP.0000000000000169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. et al. Differential mitochondrial calcium responses in different cell types detected with a mitochondrial calcium fluorescent indicator, mito-GCaMP2. Acta Biochim. Biophys. Sin. (Shanghai) 43, 822–830 (2011). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Gao Y. et al. Preparation of highly specific anti-zearalenone antibodies by using the cationic protein conjugate and development of an indirect competitive enzyme-linked immunosorbent assay. Analyst 137, 229–236 (2012). [DOI] [PubMed] [Google Scholar]
- Han E. H., Park J. H., Kim J. Y., Chung Y. C. & Jeong H. G. Inhibitory mechanism of saponins derived from roots of Platycodon grandiflorum on anaphylactic reaction and IgE-mediated allergic response in mast cells. Food Chem. Toxicol. 47, 1069–1075 (2009). [DOI] [PubMed] [Google Scholar]
- Lee J. H. et al. Meliae cortex extract exhibits anti-allergic activity through the inhibition of Syk kinase in mast cells. Toxicol. Appl. Pharmacol. 220, 227–234 (2007). [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yoshimaru T., Inoue T., Nunomura S. & Ra C. The high-affinity immunoglobulin E receptor (FcepsilonRI) regulates mitochondrial calcium uptake and a dihydropyridine receptor-mediated calcium influx in mast cells: Role of the FcepsilonRIbeta chain immunoreceptor tyrosine-based activation motif. Biochem. Pharmacol. 75, 1492–1503 (2008). [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Robb-Gaspers L. D., Seitz M. B. & Thomas A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995). [DOI] [PubMed] [Google Scholar]
- Schmitt S., Eberhagen C., Weber S., Aichler M. & Zischka H. Isolation of Mitochondria from Cultured Cells and Liver Tissue Biopsies for Molecular and Biochemical Analyses. Methods Mol. Biol. 1295, 87–97 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.