Abstract
Background
Although public health programs have led to a substantial decrease in the prevalence of tobacco smoking, the adverse health effects of tobacco smoking is by no means a thing of the past. In the U.S, four out of 10 school aged children and 1 out of 3 adolescents are involuntarily exposed to second-hand tobacco smoke (SHS) with children of minority ethnic backgrounds and those living in low socioeconomic status households being disproportionately affected (68% and 43% respectively). Children are particularly vulnerable with little control over home and social environment and lack the understanding, agency, and ability to avoid SHS exposure on their own volition; they also have physiological or behavioral characteristics that render them especially susceptible to effects of SHS.
Side stream smoke (the smoke burned directly off the end of the cigarette), a major component of SHS, contains a higher concentration of some toxins than mainstream smoke (inhaled by the smoker directly), making SHS potentially more dangerous than direct smoking. Compelling animal and human evidence shows that SHS exposure during childhood is detrimental to arterial function and structure resulting in premature atherosclerosis and its cardiovascular consequences. Childhood SHS exposure is also related to impaired cardiac autonomic function and changes in heart rate variability. In addition, childhood SHS exposure is associated with clustering of cardiometabolic risk factors such as obesity, dyslipidemia, and insulin resistance. Individualized interventions to reduce childhood exposure to SHS are shown to be at least modestly effective, so are broader based policy initiatives such as community smoking bans and increased taxation.
Purpose
The purpose of this statement is to summarize the available evidence on the cardiovascular health consequences of childhood SHS exposure which will support ongoing efforts to reduce and eliminate SHS exposure in this vulnerable population. This statement reviews relevant data from epidemiologic studies; laboratory based experiments, and controlled behavioral trials, concerning SHS and cardiovascular disease risk in children. Information regarding the effects of SHS exposure on the cardiovascular system in animal and pediatric studies, including vascular disruption and platelet activation, oxidation and inflammation, endothelial dysfunction, increased vascular stiffness, changes in vascular structure, and autonomic dysfunction are examined.
Conclusion
The epidemiological, observational and experimental evidence accumulated to date, demonstrates the detrimental long-term cardiovascular consequences of SHS exposure in children.
Implications
Increased awareness of these adverse effects will facilitate the development of targeted individual, family-centered and community public health interventions to reduce and ideally eliminate SHS exposure in the vulnerable pediatric population. This evidence calls for a robust public health policy that embraces “zero tolerance” to childhood SHS exposure.
Keywords: Second hand tobacco smoke, children, vasculature, side-stream smoke, atherosclerosis
Introduction
Over the past 50 years, health care providers and public health professionals in the United States have increased awareness of the health risks associated with smoking tobacco. In 1964, approximately 40% of adults in the United States (US) were smokers, including one-third of women1. Although the percentage of US adults who smoke has decreased to an estimated 18.1%, over 45 million US adults still smoke cigarettes2, with approximately 500,000 dying each year from tobacco smoke-related illnesses, and millions of children are involuntarily exposed to second hand tobacco smoke (SHS) in the household or during transportation3. Recent reports from the Centers for Disease Control and Prevention (CDC) show disparities in SHS between children and adults of different socio-economic statuses (SES)3. Children, 3–11 years old, have the highest exposure to SHS, particularly children of minority ethnic backgrounds. Additionally, youth and adults of a lower SES have more SHS exposure than youth and adults of higher SES3. Direct tobacco smoking in the US has been estimated to cost $97 billion in medical costs annually,4 while SHS exposure is associated with $5.6 billion in lost productivity each year5. Cigarette smoke is a potent atherosclerosis promoting risk factor, atherosclerosis can begin as early as the first decade of life and is often mediated by risk factors such as obesity, dyslipidemia, hypertension, insulin resistance, and tobacco use 6, 7. Several studies have linked SHS exposure to accelerated atherosclerosis as well 8–13.
SHS is a mixture of gases and fine particles that emit from a burning tobacco product (such as a cigarette, cigar, or pipe), or from smoke that has been exhaled by an individual actively smoking tobacco14, 15. The scope of SHS exposure can span the life cycle, beginning in utero with exposure to maternal direct smoking or maternal SHS. SHS consists of many noxious chemicals including nicotine, carbon dioxide, carbon monoxide, carbonyls, hydrocarbons, polycyclic aromatic hydrocarbons, nitrosamines, and particulates collectively referred to as ‘tar’16. Exposure to SHS is associated with increased prevalence of respiratory infections, increased frequency and severity of asthma exacerbations, and a greater risk of sudden infant death syndrome (SIDS)14, 17. While the pulmonary consequences of SHS exposure are clinically apparent in childhood, the cardiovascular effects of SHS exposure are occult but substantial. Existing evidence suggests that SHS exposure in children and youth is detrimental to their cardiovascular health and that consequences attributable to SHS exposure may persist into adult life18. Furthermore, some studies speculate that, SHS exposure may be equally or even more harmful to cardiovascular health than direct cigarette use due to increasing concentrations of some chemicals than can promote inflammation and oxidation as SHS ages8, 19–21. Furthermore, there is substantial evidence that SHS has adverse cardiovascular consequences as early as the first decade of life including when the fetus is exposed to maternal smoking or maternal SHS in utero and in children who have no other atherosclerosis promoting risk factors22–24.
Children of smoking parents are significantly more likely to be exposed to SHS25, and are also more likely to smoke later in life. This may be associated with many interrelated factors, including SHS exposure itself 3, 26, 27, parental modeling28, 29, or physical sensitivities to SHS30. However, recent studies suggest that SHS in childhood is an independent factor in susceptibility to smoking initiation31; that home smoking bans may delay or prevent smoking initiation among children of smokers32; and, conversely, that SHS exposure may be a mediating factor making adolescent quitting less likely within the context of a smoking family25. Thus, childhood SHS exposure may also have indirect impacts on lifetime cardiovascular health by increasing the likelihood that these children will choose to smoke in adolescence and adulthood.
In the past 2 decades since the last publication of an American Heart Association Statement concerning the health of children exposed to SHS33, there has been substantial evolution of epidemiological and clinical research related to SHS. The current statement updates previous statements with recent data concerning SHS and cardiovascular disease (CVD) risk in children. Emphasis is placed on the adverse effects of SHS on cardiovascular health in children and includes discussions on mechanisms of vascular disruption and platelet activation, oxidation and inflammation as noted in animal studies, human endothelial dysfunction, increased vascular stiffness, changes in vascular structure and autonomic dysfunction. The effectiveness of currently available behavior modification techniques and smoking bans to reduce SHS exposure in children are also addressed. The purpose of this statement is to increase awareness amongst providers and policy makers regarding the substantial SHS exposure that continues to be prevalent in children and its lifelong, adverse cardiovascular consequence.
Epidemiology of SHS Exposure
Prevalence of SHS exposure in children
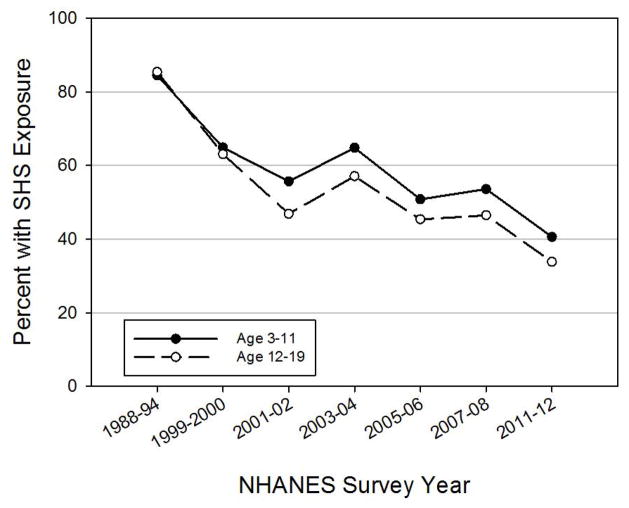
Approximately 24 million non-smoking children and adolescents in the US are currently exposed to SHS3. Nationally-representative data from 2011–2012 National Health and Nutrition Examination Survey (NHANES) shows that 40.6% of children aged 3–11 years and 33.8% of adolescents aged 12–19 years had detectable serum cotinine levels (>0.05 ng/mL), which is consistent with exposure to SHS3. Cotinine is a metabolite of nicotine found in biologic fluids and is a commonly used marker of tobacco smoke exposure34. These SHS exposure estimates represent a substantial reduction in prevalence since the NHANES III survey from 1988–199435 (Figure 1). The majority of this decline in SHS exposure in children appears to have occurred by the early 2000’s35–37, with a relative leveling off in the past decade36. However, a national sample of non-smoking middle- and high-school-aged adolescents who self-reported exposure to SHS revealed declines in SHS from 58.9% to 34.0% between 2000 and 2009 as well38. Despite these significant declines in children and adolescents exposed to SHS over the past 30 years, the prevalence remains substantial with approximately 1 in 3 children in the US still exposed to SHS.
Figure 1.
Trends in SHS exposure among non-smoking children and adolescents in US. Data compiled from NHANES periodic survey data3, 35, 48
Solid line: children aged 3–11 years; Dashed line: adolescents aged 12–19 years
These declining trends in SHS exposure among children in the US are not, however, consistent worldwide. A study of SHS exposure in rural areas of China identified that 68% of children were exposed to SHS at home, and that exposure prevalence was further amplified in households with low income or low educational status of the head of household 39. Prevalence of adult SHS exposure is approximately 35% in Shanghai, but was surprisingly higher in households with children under age 18 years40. A 43-country report “World Health Organization’s Global Youth Tobacco Survey of children aged 13–15 years” was released in 200241. Home SHS exposure in this age group exceeded 70% in six sites in India, was 69% in Indonesia, and was a median of 49% across all surveys 41. Updated nationwide representative data from India in 2009 showed lower prevalence at 22%, but remained high in Indonesia in 2014 (57%) 42, 43. Although public smoke-free bans were instituted in 2007 in Hong Kong and in 2011 in China, evidence from Hong Kong indicates that smoking bans have displaced smoking from public to private spaces, including the home, increasing home SHS exposure in children 44. Thus, childhood exposure to SHS both at home and in public places remains significant in many countries worldwide, particularly in southeast and east Asia and the Indian subcontinent. Results from a recent systematic review of SHS exposure and CVD reaffirm these findings with evidence indicating high prevalence of SHS exposure in both low- and middle-income countries 45. Taken together, available evidence supports the global need for public and provider education about childhood SHS.
Smoking inside the home
It has long been recognized that parental smoking is a major source of SHS exposure for non-smoking children and adolescents46. Children have less control over home and social environments leading to an increased likelihood of involuntary confined exposure to SHS. These developmental/behavioral barriers, in combination with physiological differences from adults discussed later47, reflect that non-smoking children have significantly higher objectively measurable exposure to SHS than non-smoking adults37.
The proportion of children aged 3–11 years living with someone who smokes inside the home declined from 38.2% in 1994–1998 to 23.8% in 1999–200435 to 18.2% in 2007–200848, with similar declines seen among non-smoking adolescents (35.4%, 19.5% and 17.1%, respectively). This is consistent with the secular decline in overall adult smoking prevalence, as well as an increase in voluntary smoke-free home rules49. These reductions in smoking inside the home contribute substantially to the overall decline in childhood SHS exposure. However, youth who remain in smoking home environments still face near-certain SHS exposure.
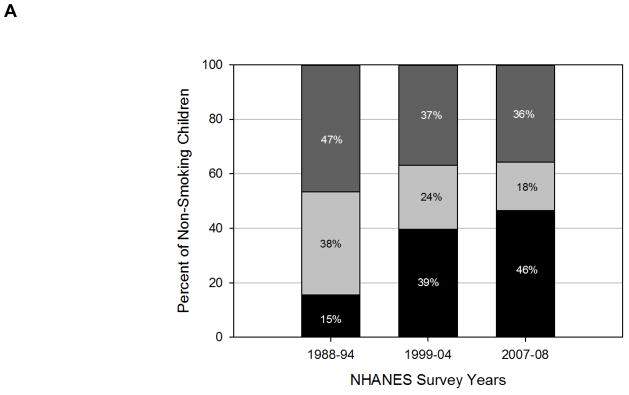
Epidemiological studies report that over 98% of children and adolescents living with someone who smokes at-home has detectable SHS exposure48, a proportion that has not appreciably declined since 198850. However, when the data of at-home SHS exposure35, 48, 50 with data on overall prevalence of SHS exposure among children35, 48 is combined, we estimate that only 1 in 3 SHS-exposed youth has exposure within the home environment (Figure 2A and 2B), suggesting that the majority of children and adolescents with detectable SHS exposure are exposed outside their home or in an automobile. This is consistent with a study of inner-city youth, where 95% reported SHS exposure outside the home, often in relatives’ or friends’ homes, or in cars51. Although both in-home and in-car smoking bans are associated with less SHS exposure in children52, 71% of smoking parents did not report having a smoke-free car policy, and only a third of parents who enforced a strict in-home smoking ban also strictly enforced a smoking ban in the car53. This is especially important, as SHS in a car can be significant, even with the windows open54, 55.
Figure 2.
Figure 2A and 2B. Prevalence and estimated source of SHS exposure in non-smoking A) children aged 3–11 years and B) adolescents aged 12–19 years by NHANES study years. Data for these figures were compiled using NHANES periodic survey data. Overall SHS exposure is presented as NHANES estimated prevalence of SHS exposure by age group 35, 48. To then estimate home-specific exposure, the proportion of non-smoking children or adolescents living with a smoker at home35, 48 was multiplied by the proportion of these children (98%) with detectable cotinine levels 48, 50; the remainder of SHS exposure was assumed to be not in the home.
Black indicates no SHS exposure (inside or outside of the home), light gray indicates SHS exposure in the home, and dark gray indicates SHS exposure outside of the home.
SHS exposure trends, demographics and socioeconomics
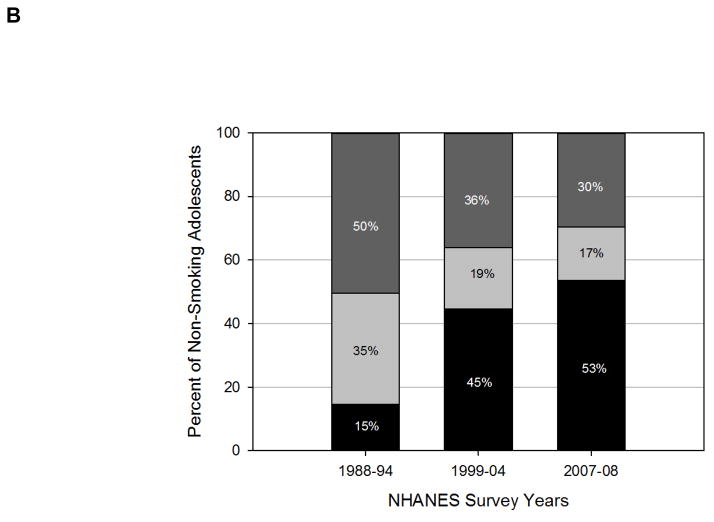
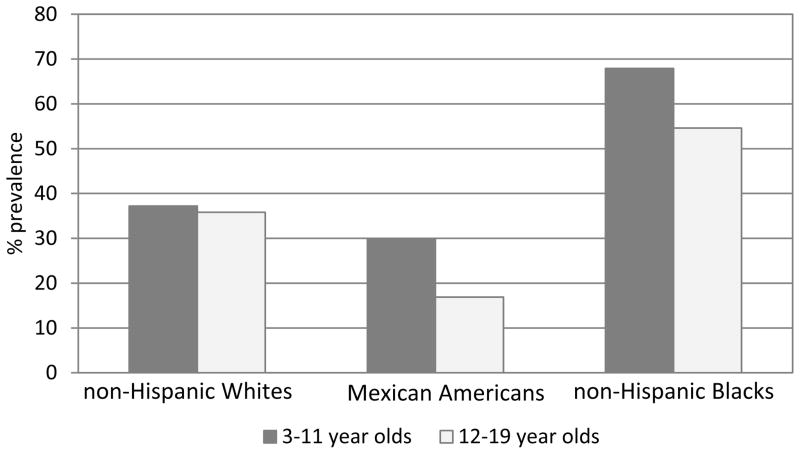
Although there has been reduction in overall SHS exposure, racial disparities persist3. There is some indication that SHS metabolism differs by race, for a given amount of exposure, cotinine levels may be higher in African-Americans56. NHANES data suggest that, 31.3 million non-Hispanic, white, non-smokers aged ≥3 years were SHS exposed in 2011–2012, including 7.2 million children aged 3–11 years. Additionally, 12.4 million, non-Hispanic, black non-smokers were SHS exposed including 3.4 million children and; 6.2 million Hispanic non-smokers aged ≥3 years were exposed to SHS, including 1.9 million children during the same time period. Specifically, the prevalence of SHS exposure declined comparably from 1999–2000 to 2011–2012 among non-Hispanic white children (−41.2%) and Hispanic children (−39.0%)3. The decline observed among non-Hispanic black children, however, was substantially less (−19.8%) (Figure 3). During 2011–2012, SHS exposure in 3–11 year old children was significantly higher among non-Hispanic blacks (67.9%) than non-Hispanic whites (37.2%) and Hispanics (29.9%) p< 0.05. Prevalence of SHS exposure in adolescents aged 12–19 years, was also significantly higher among non-Hispanic blacks (54.6%) than non-Hispanic whites (35.8%) and Hispanics (16.9%) p< 0.05 (Figure 4)3. So while cotinine metabolism differs by race, higher cotinine levels in African Americans may be due to increased exposure to SHS as well.
Figure 3.
Decline in SHS exposure prevalence from NHANES 1999–2000 to 2011–2012 by race
Figure 4.
NHANES 2011–2012 SHS exposure prevalence by age and race
In addition to differences in SHS exposure by race/ethnicity, there are significant differences by SES. NHANES data from 2011–2012 indicate that individuals living below the poverty level have greater exposure to SHS (43.2%) than those living above the poverty level (21.2%)3. By education, SHS exposure was highest among individuals with grade 11 or less education (27.6%) and lowest among those with a college diploma or graduate education (11.8%). Exposure to SHS was higher among individuals who rented their housing (36.8%) than individuals who owned housing (19.1%). Importantly, the prevalence of voluntary home smoking bans was also significantly lower for households with low income, single parent or lower educational attainment57, which may exacerbate the disparities in SHS exposure in these groups. Many individuals with low SES live in multi-unit housing, where SHS exposure can infiltrate smoke-free units and areas shared by others who smoke. Recent estimates indicate that 80 million individuals in the US live in multiunit housing and approximately 25% of these individuals are below the poverty level58. The living environment is recognized as a setting where substantial exposure to SHS occurs for children and youth59. There is substantial scientific evidence to support efforts designed to prohibit smoking in commonly shared areas in subsidized housing as summarized above 57, 58.
A recent systematic review generated from 41 studies across 21 countries adds to the NHANES findings indicating that parental smoking, low SES, and being less educated are frequently and consistently associated with SHS exposure in children and youth60. The significance of socio-demographic factors are also supported by data from Denmark61, the UK62, and Australia63. More recently, longitudinal studies have focused on the effects of in utero SHS exposure on developmental and health outcomes in childhood, adolescence, and young adulthood64–66 with these adversities also being disproportionately prevalent in minorities and lower SES groups. Taken together, these findings continue to underscore the importance of socio-demographic factors including household SES, educational levels of parents/guardians, and the home environment, in increasing the likelihood of childhood SHS exposure.
Influence of SHS exposure on childhood obesity, dyslipidemia and metabolic syndrome
Another area of emphasis has been the deleterious effect of in utero SHS exposure on weight status. The overall pattern appears to be one of lower weight at birth, larger postnatal weight gain, which is recognized to be a pattern that predicts future obesity. Supporting this theoretical pattern, data shows that in utero, exposure to tobacco smoke has obesogenic effects67. Results from the longitudinal Healthy Start Study, suggest that in utero exposure to SHS is associated with intrauterine growth retardation and rapid postnatal compensatory growth64. Specifically, at birth, those exposed to in utero SHS had reduced fat mass (p=0.007) and fat free mass (p=0.02). At 5 months of life, exposed and unexposed offspring were phenotypically similar in overall weight, length and body composition. However, after adjustment for birth weight, offspring exposed to in utero SHS had significantly greater fat free mass (p=0.04) and sum of skin folds (p=0.04) suggesting postnatal compensatory growth64.
Data from the National Institute of Child Health and Human Development Study, a longitudinal study that followed a cohort of children from birth, suggested a long-term combined effect of in utero SHS due to maternal smoking and exposure of the non smoking pregnant mother to SHS from a partner or other household member, on obesity among offspring at adolescence, independent of birth weight66. After adjustment for maternal and child factors, the odds of adolescent obesity increased both with in utero SHS exposure (OR=1.57; 95% CI=1.03–2.39) and exposure of the pregnant mother to SHS (OR=1.53; 95% CI= 1.04–2.27). Furthermore, the odds for obesity in adolescence increased two-fold among adolescents exposed to any in utero SHS (OR=2.10; 95% CI= 1.24–3.56) compared with those without any SHS exposure66.
The Southern California Children’s Health Study collected data on current SHS exposure and maternal smoking during pregnancy on 3,318 children who were around 10 years of age at study entry. Both in utero and current SHS exposure were associated with greater subsequent body mass index (BMI) over an 8-year period spanning adolescence through young adulthood68. In another study, maternal smoking during pregnancy was associated with a 60% greater chance of the child being overweight at age 4 years69. A Swedish cohort study of 5–15 year-old children has suggested that parental smoking is also associated with a 3–4% increase in BMI in children, compared with controls70. In a large study of German children, SHS exposure after birth was significantly associated with overweight status at age 6 years65. The mechanisms behind this association are not well understood. Taken together, results of cross-sectional and longitudinal studies suggest adverse effects of SHS exposure including in utero exposure on BMI in childhood and adolescence.
It appears that SHS exposure in early childhood, even in utero, can cause persistent lipoprotein changes later in life. Ayer et al have documented that in utero SHS exposure was associated with lower high-density lipoprotein cholesterol (HDL C) levels in 8 year-old children, even after adjusting for postnatal SHS exposure71. Neufeld et al72 documented a similar effect of SHS exposure and decreased HDL C level in SHS-exposed children, after adjusting for potential confounding atherosclerosis promoting risk factors. Abnormalities have been documented in low-density lipoprotein cholesterol (LDL C) as well. Yang et al, in their study of Tibetan adolescents, documented that Apolipoprotein-B values were significantly lower in SHS exposed teenagers compared with controls73. Furthermore, in an ex-vivo study performed in healthy non-smokers exposed to SHS for 5.5 hours, Valkonen and Kuusi noted that exposure of non-smoking subjects to SHS leads to accelerated lipid peroxidation, LDL C modification, and accumulation of LDL C in human macrophages74.
The effects of SHS exposure on blood pressure are less well studied but Simonetti et al22 have found that childhood exposure to SHS is associated with a significant (albeit modest) effect on blood pressure in 6 year-old children. Clustering of atherosclerosis promoting risk factors can result in metabolic syndrome and the association between SHS and metabolic syndrome has been examined in adolescents. Non SHS exposed children had a 5.6% incidence of metabolic syndrome whereas those exposed to SHS had a 19.6% incidence of metabolic syndrome, with SHS exposure being assessed by cotinine levels and this effect persisted after adjustment for confounders75.
Summary Table - Epidemiology of childhood SHS exposure
Up to 4 of 10 children have detectable cotinine
Parental smoking is a major source of SHS exposure
Nearly all children living with someone who smokes is SHS exposed
SHS exposure is highest in African Americans
SHS exposure is inversely linked to socio-economic status
In utero/post natal SHS exposure is associated with cardiovascular risk factors
Economic impact of SHS exposure
Tobacco smoke (from both smoking and SHS exposure) has a significant economic impact in direct health care costs and loss in productivity. Annual smoking-related economic costs in the US exceeded $289 billion, and exposure to SHS caused an estimated $5.6 billion yearly in lost productivity due to premature death over a 3-year period, between 2009 and 201214. Among children, living with at least one smoker, is associated with a modest impact on emergency department expenditures76 and inpatient use77. Some estimate that the additional cost associated with birth complications in pregnant women smoking, or being exposed to SHS, may be as high as $2 billion per year78. Lightwood et al. reported on health and economic benefits of smoking cessation during pregnancy, estimating savings of $572 million in direct pediatric medical expenses in 7 years79.
There is a negative economic impact of SHS exposure on the education system. Children exposed to SHS have higher rates of adverse behavioral and cognitive effects, including Attention Deficit Hyperactivity Disorder80. Max et al estimated that the costs to the education system from SHS may be 4 times higher than the annual healthcare cost attributable to Attention Hyperactivity Deficit Disorder80. With school absenteeism used as a surrogate measure of child health81–83, an article by Levy et al. showed, in a nationally representative sample of 6–11 year olds, that children living with two or more adults who smoke at home have 1.54 more days absent from school each year than children living with non smokers. Caregivers’ time tending to children absent from school is estimated to cost $227 million each year. The authors concluded that household SHS exposure is significantly associated caregivers’ loss of productivity and child school absenteeism84.
Tobacco Cessation Programs/Efforts
Resources spent towards education, counseling and health promotion are highly efficient, resulting in substantial health care cost savings and public health improvements. In 2015, an estimated $25 billion in revenue from tobacco settlements and taxes will be collected by all 50 states, with less than 2 percent of the revenue being spent on programs that help prevent children from smoking and help smokers quit85. These prevention programs are known to reduce smoking, save lives, and reduce tobacco-related healthcare costs that are estimated to run into hundreds of billions of dollars annually86.
Data from Washington State (2000–2009) indicated healthcare cost of tobacco prevention and cessation programs are not just a cost-effective healthcare intervention but actually have a savings multiplier effect, saving in excess of $5 for every dollar spent. Over the ten-year period, this program prevented almost 36,000 hospitalizations, a saving of $1.5 billion compared to $260 million spent on the program87. A 2013 study conducted in California, a leader in tobacco prevention, found that from 1989 to 2008, the state’s tobacco control programs reduced healthcare costs by $134 billion, compared to the $2.4 billion spent administering these programs88.
The economic impact of changing smoking locations is also substantial. For example, prohibiting smoking in all US subsidized and public housing is estimated to generate annual cost savings of approximately $500 million89. These figures likely underestimate true benefits as proper accounting of life course benefits cannot be modeled using currently available data. Thus, while the economic costs of smoking and SHS exposure are significant, well-designed and well-targeted programs can minimize these costs, at great benefit to society.
Summary Table - Childhood SHS exposure - Economic impacts
⇑ emergency room and inpatient use, and medical expenses
⇑ rates of cognitive disorders
⇑ rates of behavioral and cognitive adversities
⇑ school absenteeism
Cardiovascular dysfunction associated with SHS exposure in children
Chemical composition of second hand smoke and biochemical/mechanistic effects
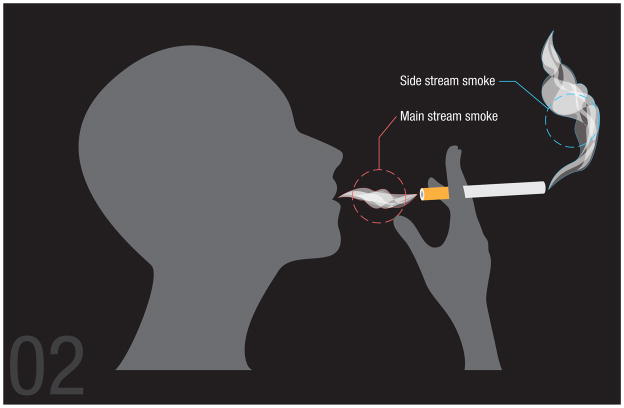
The multiple gases and chemicals of SHS make the causal attribution to any single component challenging16, 90, 91. Effects of tobacco smoke depend on whether the exposure is from direct smoking or SHS92, 93, the distance of those exposed from source, which is necessarily different between the direct smoking and SHS, length of time from when the constituents enter the environment and the individual is exposed (SHS aging)94, and whether it is mainstream versus side stream smoke95. Mainstream smoke is the inhaled component, some of which is then exhaled, while side stream smoke (containing 75% of the smoke generated by a cigarette96) is burned off directly from the cigarette and not immediately inhaled (Figure 5). Mitigation of CVD risk from SHS critically depends on the chemicals present in the side stream smoke. The precise composition of SHS depends on fluctuating conditions including pH, ambient temperature, atmospheric gas composition, and degree of combustion90, 95. For example, some constituents such as carbon monoxide and nicotine dissipate quickly, while others persist, and still others such as acrolein and certain organic chemicals concentrate and increase over time. In addition, the changes in these determinants over time affect the composition of SHS. This complex milieu synergistically results in the adverse consequences of SHS16, 90, 92. While interested stakeholders make much ado about filtered versus unfiltered distinctions or ‘low tar’ varieties, emitted chemical compositions of side stream smoke actually differ little among the various cigarette subtypes (i.e. low vs. high tar, filtered vs. unfiltered etc). Specific chemical constituents present in SHS number into the thousands; a few of the most prominent chemicals are presented in Table 2. Side stream smoke, containing approximately 3 times more toxins than main stream smoke, is equally as or potentially more dangerous than directly smoking depending on intensity of exposure 97, 98. Some studies suggest that the toxicity of some tobacco smoke constituents in side stream smoke actually increases over time due to ambient environmental reactions where certain compounds deposit onto surfaces resulting in continuing exposure and highly volatile gas constituents remain suspended in air95, 98–100. These compound specific features further obscure mechanisms of action since the degree of the exposure may vary based on the type of compound in question97, 101. While a panoply of noxious chemicals can result from SHS exposure, the precise degree of exposure varies from constituent to constituent and CVD relevant effects may vary accordingly. Regardless of mechanism, consensus has developed around quantifying the severity of smoke exposure by measuring nicotine and its metabolites in body fluids or hair, cotinine in body fluids, airborne nicotine and its metabolites, and particulate matter16, 34, 90, 102.
Figure 5.
Mainstream and side stream smoke
Table 2.
Selected chemicals present in SHS and their strength in side stream versus mainstream smoke
| SHS* chemicals | Enrichment Ratio Side stream versus mainstream smoke |
|---|---|
| Carbon dioxide | 8–11 |
| Carbon monoxide | 2.5–4.7 |
| Nicotine | 2.6–3.3 |
| Carbonyls | |
| Acrolein | 8–15 |
| Formaldehyde | 0.1–50 |
| Hydrocarbons | |
| Toluene | 5.6–8.3 |
| Benzene | 5–10 |
| Pyridine | 6.5–20 |
| Ammonia | 40–170 |
| Nitrogen oxides | 4–10 |
| Hydrogen cyanide | 0.1–0.25 |
| Particulates: ‘Tar’ | |
| Larger PAH | 1.3–1.9 |
| Nitrosamines | 0.6–100 |
| Polonium | 1–4 |
| Nickel | 13–30 |
| Cadmium | 7.2 |
SHS – Secondhand tobacco smoke
Referenced from citation 213
Mechanisms of vascular disruption
Cigarette smoke both mainstream and side stream is understood by in vitro, in vivo, and epidemiological studies to have acute and sub acute effects leading to cardiovascular consequences. The specific mechanistic domains include acute effects on endothelial function, platelet function, vasoconstriction, autonomic function, heart rhythm, and inflammation16, 90. Sub acute effects can include inflammation via oxidative stress, dyslipidemia, thrombosis, insulin sensitivity, and endothelial dysfunction16, 90. While the sheer number of compounds precludes a full account of the mechanism of each compound here, a few key examples deserve mention. Nicotine alone is associated with hemodynamic alterations, dyslipidemia and insulin resistance103–105. Acrolein, a volatile organic chemical is highly reactive and causes oxidative stress, inflammation and is linked to hypertension, dyslipidemia, arrhythmia, and thrombosis while crotonaldehyde is an atherogenic compound that induces plaque instability, increases thrombosis, and may have direct negative inotropic effects106–108. Cadmium is documented to cause inflammation and facilitate atherosclerosis109, 110. Lead exposure may cause hypertension and predicts cardiovascular mortality111, 112. Various particulate matter is known to be arrythmogenic and precipitate CVD events113–115. Exposure to carbon monoxide and various metals is minimal in those exposed to SHS16. Nicotine exposures are also quite low, due in part to the fact that nicotine dissipates rapidly from SHS16. Conversely, acrolein and other organic chemicals do persist in SHS over time, are highly reactive, and are known to produce oxidative stress, inflammation, endothelial dysfunction, blood clotting16. Moreover, other substances can adhere to the smoking-elaborated particulate matter and enhance their toxicities100.
With respect to children specifically, investigators have demonstrated that SHS exposure markers are elevated in a graded fashion in concert with higher SHS exposure. Cotinine levels are detectable in fetal and cord blood serum and appear to be higher in younger children (compared to adults) either due to higher exposure from faster respiratory rates or inadequate cotinine metabolism47, 116–119. After adjustment for degree of exposure, children of African-American descent appear to have higher levels of SHS markers compared to children of Hispanic descent, underscoring race/ethnicity and metabolic differences50. SHS is thus an intricately interactive vector for producing cardiovascular consequences.
Summary Table - Effects of SHS exposure dependant on
Length of time exposed
Intensity of exposure
Aging of the SHS constituents in the environment
Race and age
Summary Table – Cardiovascular effects of key SHS components
| • Nicotine | Hemodynamic alterations |
| • Acrolein | Hypertension, arrhythmia |
| • Crotonaldehyde | Plaque instability, thrombosis |
| • Cadmium | Inflammation |
| • Lead | Hypertension |
| • Particulate matter | Arrhythmias |
Animal studies linking SHS exposure to mechanistics of atherosclerosis
Although the specific physiologic mechanisms of SHS exposure on cardiovascular health in humans are mostly unknown, animal studies have helped to shed light on potential mechanistic effects. Although experimental animals have substantial differences from humans in many respects (lipoprotein profiles, blood pressures, patterns of breathing such as nose versus mouth, and other biological dissimilarities), there is a remarkably consistent body of evidence from a variety of animal species concerning the potentially proatherogenic effects of SHS. Although most of the early work was done with SHS exposure in adult animals, often in high doses, recent studies have examined environmentally plausible levels of SHS and its effects on vascular biology and atherosclerosis lesion development. It should be noted, however, that most such studies do not provide a biochemical assessment of post-exposure levels. Although the respiratory properties of animals are different than humans, animal studies are especially important in studying the life course effects of SHS exposure due to their shorter lifespan. Thus, these studies have given sophisticated mechanistic insights into the possible pathogenesis of SHS exposure-related vascular disease.
1. Mouse studies
In 2001, Gairola et al found that side stream cigarette smoke accelerates atherogenesis in apo-E knockout mice. When these mice were maintained on a western diet and exposed to relatively high dose of SHS, there was a duration-dependent increase in atherosclerosis lesion development120. In 2004, Tani et al121 documented increased carotid-artery intima-media thickness (CIMT) in mice who were exposed to SHS compared to controls. This was associated with an excessive antibody response to oxidized LDL C, raising the possibility that SHS and high lipoprotein levels might have an additive proatherogenic effect. As human studies have linked SHS exposure to accelerated lipid peroxidation and LDL C modification, this additive effect could be relevant to children and young adults74. The mechanism of these effects has also been studied in mice. Zhang et al122 documented high levels of pro-inflammatory cytokines such as Interleukin-6 in adult mice exposed to SHS. Knight-Lozano et al123 studied the effect of SHS on normal or dyslipidemic mice and documented high levels of oxidative stress and increased mitochondrial DNA damage of aortic tissue in SHS exposed mice; this effect was maximal in mice with both dyslipidemia and SHS exposure.
Fetterman et al124 examined the effects of in utero and neonatal SHS exposure on both atherosclerosis lesion development in later life, as well oxidative stress and mitochondrial DNA damage in aortic tissue. Animals were exposed to 1mg/m3 of SHS (4–5 ppm carbon monoxide, and 200–300 μg/m3 nicotine), a level frequently found in smoky entertainment areas for adults, both in utero and early life exposure significantly increased adult atherosclerosis lesion development and resulted in significant alterations to the aortic tissue, mitochondria, and their genome. Oxidative stress levels were also markedly increased in the SHS exposed animals.
2. Rat studies
Mullick et al125 used quantitative fluorescent microscopy to study endothelial cell injury after SHS exposure. In rats, SHS exposure increased carotid artery LDL C accumulation more than 4 fold compared with filtered air exposure and was associated with marked ultra structural damage to the carotid artery endothelial cells. Biochemical studies implicated a potentially damaging role from highly reactive carbonyl components125.
Hutchison et al126 examined the effects of SHS on vascular reactivity in newborn rats. In utero or neonatal exposure to SHS resulted in enhanced constrictor sensitivity, reduced endothelium-dependent dilatation, and decreased sensitivity to nitroglycerin, suggesting that exposure to SHS might have detrimental effects on vascular endothelial and smooth muscle function in very young animals. Mechanistic studies in rats have also linked SHS exposure to increased LDL C accumulation in ex vivo perfused arteries127.
3. Rabbit studies
Zhu et al128 exposed cholesterol fed rabbits to low or high dose SHS compared with filtered air, finding a dose-dependent increase in percent aortic involvement with atherosclerosis-like lesions. An increase in bleeding time was also seen, suggesting an acquired platelet dysfunction in these animals. Hutchison et al129 later examined the effects of high cholesterol diet with or without SHS exposure in rabbits, finding both endothelial dysfunction and increased atherogenesis, with greater impairment of endothelial function in the combined high cholesterol and SHS-exposed animals, compared to those with high cholesterol only.
4. Cockerel studies
In 1993, Penn and Snyder130 exposed 6 week-old cockerels to side stream smoke or filtered air, finding markedly increased atherosclerotic plaque in the abdominal aorta. In a subsequent study, Penn et al then decreased the exposure of SHS to a level often observed in a smoky bars and again found enhanced atherosclerosis in the cockerels exposed to even this lower dose of SHS131.
Taken together, the data from these animal studies suggest that exposure to environmentally plausible levels of SHS in early life (including in utero) can conceivably increase atherosclerosis lesions later in life. Inferred mechanisms include increased oxidative stress, pro-inflammatory effects, mitochondrial damage, and impaired endothelial function.
Human Studies - Changes in vascular endothelial function
The vascular endothelium plays a central role in cardiovascular homeostasis, making or modifying a large number of chemicals that regulate arterial tone, thrombogenesis, cell proliferation, leukocyte adhesion, and platelet interaction, amongst others. A healthy endothelium maintains a normal dilator state and antithrombotic surface whereas endothelial dysfunction predisposes to vasoconstriction, thrombosis, cell proliferation, and leukocyte adhesion. These processes are thought to play a key role in early atherogenesis. Furthermore, in the presence of plaques, the loss of normal endothelium-dependent dilator function can predispose to vasoconstriction, plaque rupture and acute coronary events. Normal endothelial function and the consequences of endothelial dysfunction has been summarized in detail elsewhere132, 133. Endothelial dysfunction has been associated with a variety of common cardiovascular risk factors134 and predicts future cardiovascular events.
One method for assessing endothelial function in humans non-invasively is the measurement of arterial flow-mediated dilatation (FMD) by ultrasound. This vasodilator response to shear stress is due to the endothelial release of nitric oxide, a chemical entity, which is responsible for vasodilatation, inhibition of leukocyte adhesion, platelet aggregation, and smooth muscle cell proliferation. The measurement of FMD was first described in 1992 in children and young adults at high risk of atherosclerosis135 and has recently been shown to predict cardiovascular events in high risk patients136.
1. Acute arterial endothelial dysfunction with SHS exposure
Although the evidence on acute arterial endothelial dysfunction in children and adolescents is limited, adult studies suggest potential mechanistic changes. In 2001, Otsuka et al137 examined the acute effects of SHS on the coronary circulation, in healthy young adults. The authors measured coronary flow velocity reserve in response to Adenosine in young adult male non-smokers versus active smokers (mean age 27 years) before and after a 30-minute exposure to environmental tobacco smoke. They found that coronary flow velocity reserve was significantly impaired by exposure to SHS, in both the non-smokers and active smokers. This acute effect of SHS on coronary physiology has also been examined in women. Sumida et al138 examined the effects of SHS on endothelium-dependent coronary artery dilatation in 38 women aged 40–60 years, finding that acetylcholine caused coronary vasoconstriction in passive and active smokers whereas it led to a normal dilator response in non-smokers.
At a mechanistic level, the effects of cigarette smoke on endothelial function ex-vivo have been examined in a number of studies. Cigarette smoke increases adhesion molecule expression on human endothelial cells139, increases human monocyte adhesion to endothelial cells, an effect reversible by the nitric oxide precursor L-arginine140. Cigarette smokers have also been shown to have increased tissue factor expression in atherosclerotic plaques141.
Hausberg et al142 provided more insight when they studied the effect of acute short-term SHS exposure on muscle sympathetic nerve activity in 17 healthy young non-smokers (aged 28±6 years). One smoke inhalation session increased resting muscle sympathetic nerve activity by approximately 20% in the SHS exposed but not in the control group. This could underscore (in part) the association between SHS exposure in children and higher blood pressure22.
2. Effects of chronic SHS exposure on arterial endothelial function
In 1996, Celermajer et al studied 78 healthy teenagers and young adults (aged 15–30 years) comprising 26 active smokers, 26 who had never smoked but had been exposed to SHS for at least one hour daily for 3 or more years, and 26 controls who were not SHS exposed or actively smoking8. Arterial FMD showed profound impairment in the passive as well as active smoking teenagers. Arterial dilatation induced by nitroglycerin was similar in all groups, localizing the defect in vascular reactivity to the endothelium.
This finding was later confirmed by Kallio et al in younger subjects9. As part of a longitudinal study in Finland, children had annual serum cotinine concentrations measured between ages of 8 and 11 years to assess environmental tobacco smoke exposure, and at age 11 years, had FMD assessed. Exposure to SHS, documented by cotinine concentrations were associated with impaired endothelial function in a dose-dependent manner in pre-teenage children (Figure 6). This effect was also observed in a Chinese population of adolescents. Yang et al studied FMD in 16 year-old Tibetan school students and documented impaired endothelial function in those exposed to SHS73.
Figure 6.
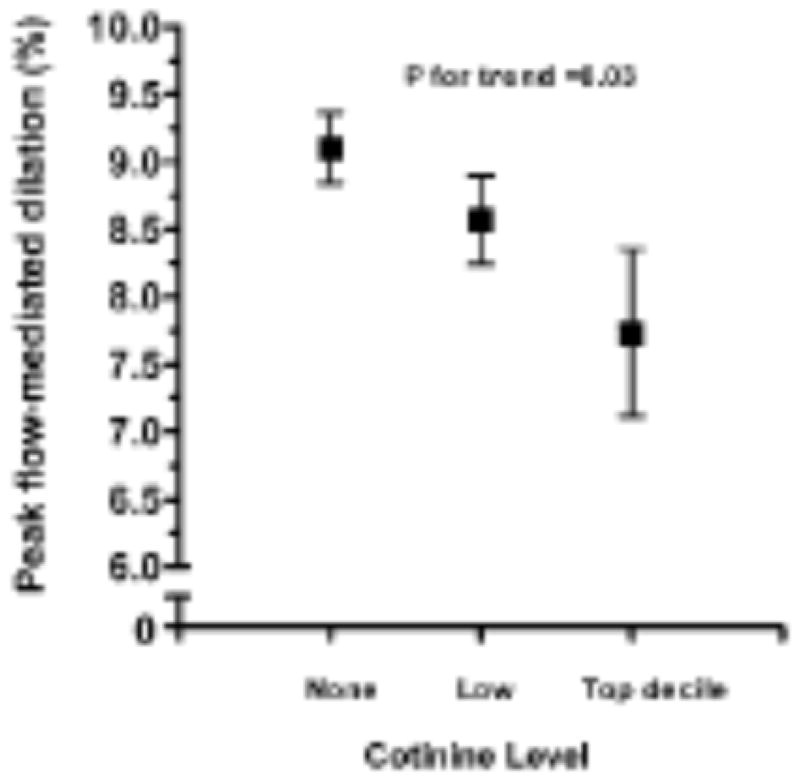
Peak FMD of brachial artery in 11-year-old children according to cotinine group. Values are mean ± SEM.
Kallio K, Jokinen E, Raitakari OT, Hamalainen M, Siltala M, Volanen I, Kaitosaari T, Viikari J, Ronnemaa T, Simell O. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–3212
Juonala et al examined the effects of parental smoking in childhood on endothelial function in adult life, 19–27 years following the period of SHS exposure in the home143. Two populations were studied, one from Finland and one from Australia, and both groups saw reduced FMD in participants whose parents had smoked compared to those whose parents had not smoked. These findings suggested that SHS exposure in childhood could cause impairment in endothelial function, observable many years later. However, the authors noted that arterial endothelial dysfunction related to passive smoking might be at least partially reversible in healthy young adults143. This reversibility was documented by Raitakari et al144 who examined endothelium-dependent dilatation in those formerly exposed to SHS compared with those currently exposed to SHS, aged 15–39 years of age. FMD was 2% in those currently exposed, 5% in those formerly exposed and 9% in controls, suggesting at least partial reversibility of SHS-related endothelial dysfunction, in those who were removed from SHS-containing environments.
In summary, there is compelling evidence that regular exposure to SHS in children and young adults is associated with arterial endothelial dysfunction, a likely predisposing factor to atherosclerosis and increased CVD risk in later life.
Mechanisms
A number of potential cellular mechanisms related to endothelial dysfunction have been documented in association with SHS. Studies have documented markers of oxidative stress in SHS and non-smoker, non SHS exposed groups, including increased levels of glutathione peroxidase and catalase in SHS exposed145. Inflammatory markers such as C-reactive protein and oxidized LDL C are also higher in those who are SHS exposed146. The presence of such reactive oxygen species and inflammatory markers are known to reduce the production and/or activity of endothelial nitric oxide synthase147. Following up on animal work implicating mitochondrial damage as a potential pathogenetic mechanism in SHS exposure, a recent review by Yang et al148 concerning fetal, childhood, and adult exposure to SHS and mitochondrial damage and dysfunction, concluded a potentially important role of mitochondrial abnormalities in mediating CVD susceptibility.
3. Changes in vascular tone/stiffness and structural vascular changes
Adequate arterial function includes the transmission of blood flow to downstream tissue capillary beds with minimal energy loss, and regulation of blood flow in those tissue beds with steady flow proportional to metabolic demand. These arterial actions are determined by the structure and function of large “conduit” and small “resistant” arteries. Assessment of structure includes, but is not limited, to measurement of CIMT, and arterial stiffness. SHS exposure appears to distort arterial structure. These distortions are of clinical relevance as recent reports indicate that peripheral artery disease is higher in those exposed to SHS in childhood adjusted for other adult predictors of peripheral artery disease149.
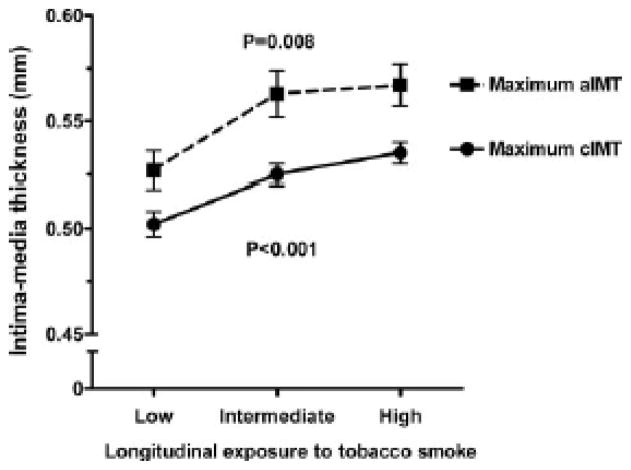
CIMT is assessed by ultrasound imaging of the carotid artery near the bifurcation into external and internal carotids. CIMT captures the effect of accumulated cardiovascular risk factors to the arterial wall. With every 1mm increase in CIMT measurement in adults, the hazard ratio for CVD increases by 2.46150. CIMT also measures localized plaque formation which may have independent prognostic information150. The Atherosclerosis Risk in Communities study demonstrated in adults a clear association between SHS exposure and thickening of CIMT151. Other studies suggest that SHS exposure is dangerous in younger adults and in those with low levels of other CVD risk factors as well152–154. In children with in utero SHS exposure, CIMT was thicker, while postnatal SHS exposure appeared to be less predictive of a higher CIMT10. Some studies suggest a positive relationship between higher postnatal SHS exposure (Figure 7) and thicker CIMT, while other investigators did not find this relationship71, 155, 156. It should be noted that although this evidence is not conclusive of cardiovascular disease as a result of SHS exposure, a relationship that will take decades to observe, this is compelling medium-term proxy evidence that supports the deleterious effects of SHS on the vasculature.
Figure 7.
Maximum cIMT (black circles) and maximum aIMT (black squares) in healthy 13-year-old adolescents according to longitudinal tobacco smoke exposure levels. Number of adolescents in exposure groups: cIMT: low (n=160), intermediate (n=171), high (n=163); aIMT: low (n=159), intermediate (n=167), high (n=161). Values are mean ± SEM
cIMT – carotid artery intima media thickness
aIMT – aortic intima media thickness
Kallio K, Jokinen E, Saarinen M, Hamalainen M, Volanen I, Kaitosaari T, Ronnemaa T, Viikari J, Raitakari OT, Simell O. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circulation. Cardiovascular quality and outcomes. 2010;3:196–203
Arterial stiffness is a measure of the material properties of the artery, and is associated with traditional CVD risk factors, predicts future CVD, and hypertension157, 158. Several methods can be used to measure arterial stiffness including carotid-femoral pulse wave velocity, the degree of pulse wave reflection called augmentation index, or arterial distensibility which is a change in diameter of the carotid artery from diastole to systole. In adults, chronic SHS exposure decreased carotid artery distensibility in persons already at risk of impaired distensibility including the obese, the elderly, and those with increased CIMT159. Moreover, experiments of acute SHS exposure in adults showed reduced aortic distensibility160. Children with in utero SHS exposure also appeared to have 15% less distensible arteries10. Adolescents from the Special Turku Coronary Risk Factor Intervention Project demonstrated associations between serum cotinine and decreased aortic elasticity (Figure 8)11. However, other population based studies do not show differences in childhood aortic stiffness on the basis of in utero smoking exposure161. So while SHS exposure appears to consistently induce acute and chronic alterations in endothelial function, the effects on arterial structure are not consistent and may depend on the nature and degree of exposure.
Figure 8.
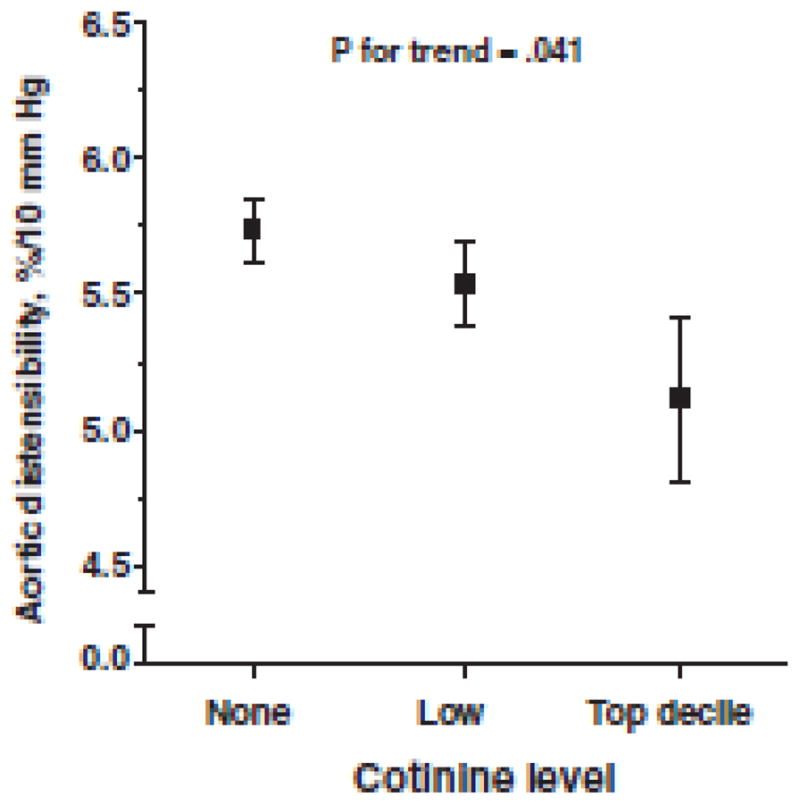
Aortic distensibility in children exposed to second hand tobacco smoke
Kallio K, Jokinen E, Hamalainen M, Saarinen M, Volanen I, Kaitosaari T, Viikari J, Ronnemaa T, Simell O, Raitakari OT. Decreased aortic elasticity in healthy 11-year-old children exposed to tobacco smoke. Pediatrics. 2009;123:e267–273
4. Arrhythmia and SHS exposure
The effects of SHS exposure on heart rate and rhythm in children are not well studied. Acute cardiovascular effects of SHS in the young include tachycardia137 and an increase in blood pressure22. An important potential pathway for tobacco exposure in children is in utero. Interestingly; the effects of in utero and postnatal SHS exposure on autonomic function seem to be gender specific. Schuetz et al162 examined the association between in utero SHS exposure and neonatal heart rate and heart rate variability assessed at 2–4 weeks of age. Neonates with in utero SHS exposure had higher heart rates and lower heart rate variation with breathing, than those in the non-exposed group. For unexplained reasons boys who were exposed to SHS either in utero or postnatally had higher heart rates and lower heart rate variation than SHS exposed girls at age 2–4 weeks162. A recent longitudinal study by Dixit and colleagues measured the effects of SHS exposure in childhood and in utero on arrhythmia development as an adult. The authors found that SHS exposure in utero and during childhood were associated with having atrial fibrillation later in life. The findings of this study indicated that SHS in early life may be an important, potentially modifiable risk factor for the development of late arrhythmia 163.
The cardiac effects of direct smoking and SHS exposure in adults are well documented and could suggest hypotheses for studying children exposed to SHS. Smoking is associated with release of the sympathetic neurotransmitter norepinephrine, and the adrenomedullary hormone epinephrine164. In addition, nicotine has a direct effect on sympathetic nerve endings, favoring catecholamine release. Thus, cigarette smoking has a powerful sympathetic excitatory effect influencing sympathetic drive to blood vessels, skin, and to the heart165.
Supporting these theoretical concerns, chronic adult smokers have a blunted heart rate response to exercise and decreased exercise tolerance compared to non-smokers, partially due to down-regulation of cardiac beta-adrenergic receptors166, 167. SHS exposure generates an autonomic system imbalance associated with increased cardiac vulnerability and may lead to arrhythmias such as atrial fibrillation, ventricular tachycardia, and ventricular fibrillation especially in those with other pre-existing CVD risks 163, 168–170. There is a higher incidence of arrhythmias in adults with coronary artery disease and cardiomyopathy who have been exposed to SHS and specific components of SHS (carboxyhemoglobin), including an increased frequency of premature ventricular contractions during supine bicycle exercise testing and ventricular arrhythmias168–170.
The pathophysiological mechanism of smoking-related arrhythmias is complex and influenced by several of the major components of tobacco smoke. It includes alteration in autonomic function and a pro-fibrotic effect of nicotine and carbon monoxide on myocardial tissue with consequent increased sensitivity to catecholamine171. As noted above, specific components of SHS such as carbon monoxide, may contribute to the generation of ventricular arrhythmias via endothelial dysfunction and coronary vasoconstriction171–173. Nicotine, carbon monoxide, and oxidative stress induce fibrosis at different cardiac sites, resulting in a structural remodeling that can predispose the patient to arrhythmias171.
Autonomic dysfunction and SHS exposure
In utero or postnatal SHS exposure increases the risk of Sudden Infant Death Syndrome (SIDS), particularly if postnatal exposure is in proximity to the infant1, 174–187. Compared with infants who were not exposed to SHS either in utero or postnatally, the risk of SIDS is greater in infants exposed in both in utero and postnatal periods (3 fold increase), and in those with only postnatal exposure (2 fold increase)176. Furthermore, the risk of SIDS increases with increasing dose of SHS exposure176. In the Wales Perinatal Survey, over half of the infants known to have died suddenly lived in households with SHS186. Behm et al studied the association between a higher prevalence of smoke-free homes and decreasing rates of SIDS, controlling for an important risk factor for SIDS, supine sleep position. On a population basis, for every 1% absolute increases in the prevalence of smoke-free homes, SIDS rates decreased 0.4% from 1995–2006188.
The mechanism behind SHS exposure and SIDS may include effects on the normal regulation of breathing. Experimental data from animal studies have shown that exposure to SHS can have adverse effects on brain cell development189–191. In addition, autonomic function is altered by in utero exposure to SHS. Newborns whose mother smoked during pregnancy have lower beat-to-beat, heart rate variability during quiet sleep192. Unexposed infants demonstrated increases in heart rate with head-up tilt and decreases in heart rate with head-down tilt, while infants exposed to SHS showed no such responses192. Maternal smoking induces changes in autonomic control and maturation in infants193. Cardiovascular stress reactivity is increased in newborn infants of active smokers194. The resting heart rate of a smoker’s infant is lower than non-exposed control, most likely secondary to excessive vagal tone23. Vagal potentiation is considered a predisposing factor in SIDS195–197. Any smoking during pregnancy or during the first year of the infant’s life increases the risk of SIDS186.
Summary Table - Childhood SHS exposure results in
Endothelial dysfunction
⇑ arterial stiffness
⇑ carotid artery intima-media thickness
Autonomic dysfunction
Late onset arrhythmia
Behavioral and community strategies to reduce/eliminate SHS exposure in children
Individual and population based approaches
Several controlled trials over the last 25 years have evaluated interventions aimed at reducing or eliminating SHS exposure in children. Intervention strategies fall into two broad groups, including those that (1) directly attempt to minimize SHS exposure to children, and (2) indirectly minimize children’s SHS exposure by assisting parents or caretakers to quit or reduce smoking. The majority of studies have targeted parents, rather than non-parental caregivers. Interventions that directly minimized child harm from SHS used a variety of strategies including, counseling (face to face or by telephone) or provision of materials to encourage parents not to allow smoking in the home or car, not smoke around their children, or remove the child from rooms in the home or other locations when smoking is taking place (hygienic smoking). Other strategies include providing air cleaners or biochemical feedback (as in cotinine measurements in children) of child SHS exposure.
Cessation/smoking reduction interventions typically have used behavioral counseling and/or pharmacotherapy such as nicotine replacement. A variety of outcomes have been assessed, including self-reported behavior change related to child SHS exposure (e.g., enforcement of home smoking ban, restricting smoking to designated smoking areas), self-reported smoking cessation or reduction (with or without biochemical verification), biochemical measures of children’s absorption of environmental tobacco smoke (typically cotinine assessed in urine, blood, saliva, or hair), and biochemical measures of ambient environmental tobacco smoke (usually nicotine or particulate matter) from rooms or other spaces where children are exposed.
Several literature reviews on this topic have been conducted. These include non-systematic narrative198–200; systematic narrative201, 202; and systematic quantitative (i.e., meta-analytic) reviews 203–206. The meta-analytic reviews include one originally published in the Cochrane Collaboration database in 2003203 and updated in 2008204 and 2014206. Arborelius et al. only included interventions that occurred in pediatric practices, but other reviews did not limit the setting of interventions198.
Two most recent reviews (Rosen et al.205 and Baxi et al.206), both published in 2014, included 57 and 30 trials, respectively. Rosen et al.’s systematic review205 included fewer studies than Baxi et al206, due to restricting eligibility to studies that delivered interventions to parents (not other family members, child care workers, and teachers), targeting children no older than 6 years of age (compared to 12 years of age in Baxi et al’s review), and focusing on interventions to help parents decrease child SHS exposure (Baxi et al. also included smoking cessation programs that measured child SHS exposure as an outcome but did not have child SHS reduction as an explicit goal).
Rosen et al. combined and analyzed effects for three categories of outcomes: 1) parent-reported exposure to SHS of the child at follow-up and change from baseline to follow-up; 2) parent-reported number of cigarettes smoked around the child at follow-up; and 3) measured levels of cotinine or nicotine in urine, blood, saliva, or hair of the target child at follow-up205. Parent-reported SHS exposure to child at follow-up was collected by 17 studies and showed a small, statistically significant effect favoring intervention groups vs. controls (Relative risk [RR] = 1.12 [1.07–1.18]). The risk difference (RD) was 0.07 (0.05–0.09) indicating that 7% of intervention families benefited, on average. The effect for parent-reported exposure to SHS was positive but not statistically significant when analyzed as change from baseline to follow-up (RR 1.44 [0.90–2.29])205.
Parent-reported number of cigarettes to which children were exposed, decreased in both intervention and control groups, but to a greater extent in intervention groups (RD= −0.24 [−0.46 −0.03], p=0.03). Among thirteen studies that used biomarkers of child SHS as the outcome, eight showed positive effects, but statistical significance was reached in only one. The overall RD was −0.05 (−0.13 − 0.20, p=0.20) showing a non-significant trend toward a small benefit of intervention. No clear benefit was demonstrated in sub-group analyses that compared studies based on whether biochemical feedback was provided as an intervention strategy, intensity of the experimental intervention, intensity of the control intervention, and treatment fidelity205.
The authors concluded that interventions to reduce child SHS exposure showed “small benefits” when assessed by parent self-report, but this effect was not corroborated by objective, biochemical outcomes. It is unclear why an effect is observed for self-reported vs. objective outcomes, but may be related to greater statistical power for parent-reported outcomes (due to a greater number of studies), unreliability of parent reports due to lack of knowledge of their children’s exposure or intentional misreporting, or inadequate sensitivity of biomarkers to detect small changes in exposure levels205.
It is also noteworthy that many studies reported positive outcomes in control groups, which may be related to generally high levels of motivation in trial participants regardless of treatment allocation, effects of monitoring (Hawthorne Effect), or that parents tend to reduce their smoking around their children regardless of intervention (e.g., due to social pressure or secular trends that make childhood SHS exposure less acceptable). Regardless, the overall conclusion is that available interventions produce, at best, small effects, and there is no empirical basis to recommend specific intervention strategies, intensities, or delivery formats.
Baxi et al. reached similar conclusions. Among the 57 studies included in this review, 16 focused directly on minimizing child harm from SHS by changing parent/caregiver behaviors or attitudes, 20 focused exclusively on helping parents/caregivers to quit smoking or not relapse, and 21 used a combination of these two intervention approaches. Effects were not quantitatively combined due to heterogeneity of study design and characteristics. The resulting narrative review did not find evidence that any particular intervention strategies were more effective than others206. Only 14 of the 57 studies found a significant intervention treatment effect, and of these, 12 were judged to have either unknown or high risk of bias. These studies used a wide range of interventions including intensive counseling or motivational interviewing, telephone counseling, school-based strategies, picture books, and educational home visits. Similar to Rosen et al., this review found reductions in SHS exposure for children regardless of assignment to intervention or control groups205. Further, there was no evidence of difference in effectiveness according to age of the target child or whether targeted children were healthy or had respiratory or other illnesses. The authors concluded that effectiveness of interventions to reduce child SHS exposure has not been clearly demonstrated.
Expert Panel Recommendations
The Task Force on Community Preventive Services reviewed evidence for the effectiveness of community education to reduce exposure to SHS in the home. Community education approaches included enhancing motivation or providing skills to quit or reduce smoking or implementing home policies, such as bans, to reduce exposure to SHS. The task force concluded207 that there was insufficient evidence to make a recommendation about the effectiveness of community education to reduce SHS exposure in the home.
A more recent expert panel208 concluded that interventions in pediatric care settings to reduce children’s SHS exposure showed “mixed results.” However, due to the serious public health implications of SHS, it recommended that pediatric healthcare providers routinely intervene. The expert panel encouraged providers to identify parents and other caregivers who smoke and explicitly recommend that children not be exposed to tobacco smoke in the home, in automobiles, and in any other space where exposure can occur. Further, the expert panel concluded that parents and caregivers who smoke should be educated about the health consequences of tobacco use to the adult, the child, and (where appropriate) fetus, encouraged to quit, and referred for smoking cessation assistance. Adding routine biochemical screening to detect metabolites of SHS as part of health promotion may detect levels of exposure different than what is reported by parents. Such screening could be used by clinicians to identify high risk children for intervention.
Effect of smoking bans and taxes
Home smoking bans significantly reduce SHS exposure among children209, and may also lower the likelihood that children of smokers will take up smoking32. Unfortunately, approximately 16% of smoking households did not have any restrictions against indoor smoking by 2006–200757, with a similar proportion in 2010–201149 and children in these households were still likely be exposed to SHS.
The effect of smoking bans in public places and its effect on children’s exposure to SHS are less clear, with some suggesting that smoking bans could potentially increase SHS exposure in children, by displacing parental smoking from public places into their households. An observational study from England in 2007 by Jarvis et al using cotinine levels in children, found no evidence of increased household exposure following implementation of a legislative smoking ban. Adoption of smoke-free homes by smoking parents increased significantly after this ban, suggesting that it helped reinforce an emerging social norm favoring voluntary parental smoking restrictions210. Similar results have been seen in the US, where voluntary home-smoking bans in both smoking and non-smoking households were significantly more likely when community-wide 100% smoking bans were also in effect211.
In 2010, over 20 experts in economics, epidemiology, public policy, and tobacco-control concurred on the favorable effectiveness of increased tobacco excise taxes and cigarette prices in reducing overall tobacco consumption and improvement of public health, including prevention of initiation and uptake among young people212.
Summary Table - Interventional strategies to ⇓ childhood SHS
Tobacco cessation programs ⇓ health care costs
Home smoking bans and public smoking bans ⇓ childhood SHS exposure
⇑ taxes on tobacco products ⇓ tobacco consumption
Future directions
Children exposed to SHS experience both short and long-term adverse effects, including not only well-known respiratory complications but also cardiovascular consequences related to heart rate, blood pressure, autonomic effects and vascular dysfunction.
Questions remain about the mechanisms by which SHS exposure is related to adverse effects in childhood. For example, infants exposed to SHS are at higher risk for SIDS, but the mechanism by which SHS exposure increases risk of SIDS is not clear. Hypotheses include altered breathing patterns and diminished autonomic variability but more investigation is needed. Is the risk related to in utero or current SHS exposure, or are they both important? Similarly, epidemiological studies show increases in heart rate and blood pressure in children exposed to SHS. What is their clinical implication, if any, for an individual child, and for the population? How do the findings of increased heart rate and blood pressure integrate with the literature on chronic exposure to childhood stress, which may be an additional exposure for these children? Exposure to SHS is related to dyslipidemia and metabolic syndrome. Does SHS exposure cause these changes or are there confounding factors that mediate these relationships? Do epigenetic changes play a role? What is the impact of concomitant lifestyle factors, which may also increase the risk of CVD? Adults with SHS exposure are at risk for ventricular arrhythmias, possibly related to the release of catecholamines. Does this phenomenon also occur in childhood? In all these processes, are there particular components of SHS exposure that are to blame? Can we further tease out which components of SHS cause child-specific toxicities, particularly long-lasting ones, with a goal of minimizing or eliminating those in tobacco products as a plausible intervention?
Questions also remain with regard to the timing and durability of adverse effects of SHS exposure. What is reversible and what is persistent? Is there a tipping point after which the cardiovascular effects persist despite elimination of SHS exposure? Are there vulnerable periods where the effects are more pronounced or long lasting, perhaps infancy or adolescence? What about a threshold effect? How many years of SHS exposure are needed before the vascular changes are irreversible? More information is needed with regard to mechanisms, severity of effects, whether there are vulnerable periods for exposure, and if so, when they are, and whether all effects can be reversed. Despite these knowledge gaps about mechanisms and durability by which SHS exposure in children impacts the cardiovasculature, the sum of existing evidence suggests that reduction or elimination of SHS exposure in childhood will improve their cardiovascular health.
Therefore, new and better methods are needed to promote behavior change to reduce and ultimately eliminate SHS exposure using federal, state and local policy efforts, strategies within the healthcare system and interventions at the level of the individual. The concept of “hygienic smoking” which is defined as being as far away as possible from the smoker has been proposed, and some studies suggest a benefit, mitigating long term adverse vascular effects of childhood SHS exposure18. How exactly is “hygienic smoking” implemented? This will be important while we are perfecting prevention and cessation efforts directed at smoking parents and close contacts of children. Does banning smoking in public places increase exposure to SHS in children at home? Adolescents report that an important component of their SHS exposure occurs outside the home. What additional interventions can be brought to bear to address this high-risk population? Interventions to reduce SHS exposure in childhood are effective when parental report is the measurement outcome205, 206 but the effect is quite small and no significant benefit is seen when biochemical markers of SHS are used as the outcome (e.g. cotinine levels). How can we make a bigger impact? Future experimental research, such as SHS exposure reduction trials, should include specific cardiorespiratory outcomes or proxy outcomes in children.
Future directions for policy makers, health systems administrators, and providers should include systematic change methods to promote cessation such as electronic health records prompts, making cessation referral programs more easily available, training providers to be knowledgeable about best practices, working with “Head Start” to access parents outside of the medical system, providing real time feedback to parents about progress by way of changes in their child’s cotinine levels, focusing efforts to target high-risk minority and lower SES children living in multi unit housing, routine biochemical screening for tobacco metabolites in high risk children, and continued public health campaigns to spread information about the prevailing health and economic effects of SHS exposure, particularly as they relate to children.
Conclusion
A half a century following the first US Surgeon General warning about the harmful effects of cigarette smoking, we have seen dramatic reductions in incidence and prevalence of smoking as well as significant reductions in childhood SHS exposure. However, exposure to SHS in childhood remains high, and children remain especially vulnerable and are involuntarily exposed to SHS. Investments have been made in enforcing ordinances and bans that prohibit smoking in certain locations; increasing taxes on tobacco products and in education and behavior modification. While these have had a favorable impact in reducing smoking prevalence and improving awareness of the consequences of cigarette smoking, young and minority children remain disadvantaged. Based on the epidemiological, observational, and experimental evidence that is presented here, we conclude that the long-term cardiovascular consequences of SHS exposure in children are detrimental. Health care providers need to emphasize and promote heart healthy behaviors in caretakers of children at every encounter and encourage parents and caretakers to cease smoking for their own and their child’s wellbeing. According to recent US Surgeon General recommendations, interventions that include mass media campaigns, cigarette price increases including those that result from tax increases, school-based policies and programs, and statewide or community-wide changes in smoke free policies and norms are effective in reducing the initiation, prevalence, and intensity of smoking among youth and young adults and will in turn substantially decrease SHS exposure. Public health providers and communities need to promote interventions to lower and ultimately eliminate childhood SHS exposure. The evidence presented in this statement calls for a robust public health policy that embraces “zero tolerance” to childhood SHS exposure.
Table 1.
Abbreviations
| Abbreviation | |
|---|---|
| Second-hand tobacco smoke | SHS |
| Centers for Disease Control and Prevention | CDC |
| Socio-economic status | SES |
| Sudden infant death syndrome | SIDS |
| Cardiovascular disease | CVD |
| National Health and Nutrition Examination Survey | NHANES |
| Body mass index | BMI |
| Carotid artery intima-media thickness | CIMT |
| Low-density lipoprotein cholesterol | LDL C |
| Flow mediated vasodilatation | FMD |
| High-density lipoprotein cholesterol | HDL C |
| Relative risk | RR |
| Confidence interval | CI |
| Risk difference | RD |
References
- 1.US Public Health Service. Smoking and health: A report of the surgeon general (dhew publication no.(phs) 79-50066) Washington, DC: US Department of Health, Education, and Welfare; 1979. [Google Scholar]
- 2.Centers for Disease Control Prevention. Vital signs: Current cigarette smoking among adults aged≥ 18 years--united states, 2005–2010. MMWR. Morbidity and mortality weekly report. 2011;60:1207. [PubMed] [Google Scholar]
- 3.Homa DM, Neff LJ, King BA, Caraballo RS, Bunnell RE, Babb SD, Garrett BE, Sosnoff CS, Wang L. Vital signs: Disparities in nonsmokers’ exposure to secondhand smoke - united states, 1999–2012. MMWR. Morbidity and mortality weekly report. 2015;64:103–108. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses--united states, 2000–2004. MMWR. Morbidity and mortality weekly report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 5.Max W, Sung HY, Shi Y. Deaths from secondhand smoke exposure in the united states: Economic implications. American journal of public health. 2012;102:2173–2180. doi: 10.2105/AJPH.2012.300805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.group PR. Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentration and smoking. A preliminary report from the pathobiological determinants of atherosclerosis in youth (payday) research group. Journal of the American Medical Assocaition. 1990;264:3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 7.Newman WP, Wattigney W, Berenson GS. Autopsy studies in united states children and adolescents: Relationship of risk factors to athersclerotic lesions. Annals of the New York Academy of Science. 1991;623:16–25. doi: 10.1111/j.1749-6632.1991.tb43715.x. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. New England Journal of Medicine. 1996;334:150–155. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 9.Kallio K, Jokinen E, Raitakari OT, Hamalainen M, Siltala M, Volanen I, Kaitosaari T, Viikari J, Ronnemaa T, Simell O. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–3212. doi: 10.1161/CIRCULATIONAHA.106.674804. [DOI] [PubMed] [Google Scholar]
- 10.Geerts CC, Bots ML, van der Ent CK, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in their 5-year-old children. Pediatrics. 2012;129:45–54. doi: 10.1542/peds.2011-0249. [DOI] [PubMed] [Google Scholar]
- 11.Kallio K, Jokinen E, Hamalainen M, Saarinen M, Volanen I, Kaitosaari T, Viikari J, Ronnemaa T, Simell O, Raitakari OT. Decreased aortic elasticity in healthy 11-year-old children exposed to tobacco smoke. Pediatrics. 2009;123:e267–273. doi: 10.1542/peds.2008-2659. [DOI] [PubMed] [Google Scholar]
- 12.Howard G, Burke GL, Szklo M. Active and passive smoking are associated with increased carotid wall thickness: The athersclerosis risk in communities study. Archives of Internal Medicine. 1994;154:1277–1282. [PubMed] [Google Scholar]
- 13.Guo X, Oldham MJ, Kleinman MT, Phalen RF, Kassab GS. Effect of cigarette smoing on nitric oxide, structural, and mechanical properties of mouse arteries. American Journal of Physiology. Heart and Circulatory Physiology. 2006;291:H2354–H2361. doi: 10.1152/ajpheart.00376.2006. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health Human Services. The health consequences of smoking—50 years of progress: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. p. 17. [Google Scholar]
- 15.National Toxicology Program. Ntp 12th report on carcinogens. Report on carcinogens: carcinogen profiles/US Dept. of Health and Human Services, Public Health Service, National Toxicology Program. 2011;12:iii. [PubMed] [Google Scholar]
- 16.Institute of Medicine (U.S.). Committee on Secondhand Smoke Exposure and Acute Coronary Events. Secondhand smoke exposure and cardiovascular effects : Making sense of the evidence. Washington, D.C: National Academies Press; 2010. [PubMed] [Google Scholar]
- 17.US Department of Health Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. p. 709. [Google Scholar]
- 18.West HW, Juonala M, Gall SL, Kahonen M, Laitinen T, Taittonen L, Viikari JS, Raitakari OT, Magnussen CG. Exposure to parental smoking in childhood is associated with increased risk of carotid atherosclerotic plaque in adulthood: The cardiovascular risk in young finns study. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.013485. [DOI] [PubMed] [Google Scholar]
- 19.Glantz SA, Parmley WW. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation. 1991;83:1–12. doi: 10.1161/01.cir.83.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Rubenstein D, Jesty J, Bluestein D. Differences between mainstream and sidestream cigarette smoke extracts and nicotine in the activation of platelets under static and flow conditions. Circulation. 2004;109:78–83. doi: 10.1161/01.CIR.0000108395.12766.25. [DOI] [PubMed] [Google Scholar]
- 21.Rubenstein DA, Morton BE, Yin W. The combined effects of sidestream smoke extracts and glycated serum albumin on endothelial cells and platelets. Cardiovascular diabetology. 2010;9:28. doi: 10.1186/1475-2840-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonetti GD, Schwertz R, Klett M, Hoffmann GF, Schaefer F, Wuhl E. Determinants of blood pressure in preschool children: The role of parental smoking. Circulation. 2011;123:292–298. doi: 10.1161/CIRCULATIONAHA.110.958769. [DOI] [PubMed] [Google Scholar]
- 23.Cohen G, Jeffery H, Lagercrantz H, Katz-Salamon M. Long-term reprogramming of cardiovascular function in infants of active smokers. Hypertension. 2010;55:722–728. doi: 10.1161/HYPERTENSIONAHA.109.142695. [DOI] [PubMed] [Google Scholar]
- 24.Geelhoed JJ, El Marroun H, Verburg BO, van Osch-Gevers L, Hofman A, Huizink AC, Moll HA, Verhulst FC, Helbing WA, Steegers EA, Jaddoe VW. Maternal smoking during pregnancy, fetal arterial resistance adaptations and cardiovascular function in childhood. BJOG : an international journal of obstetrics and gynaecology. 2011;118:755–762. doi: 10.1111/j.1471-0528.2011.02900.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang MP, Ho SY, Lo WS, Lam TH. Smoking family, secondhand smoke exposure at home, and quitting in adolescent smokers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2013;15:185–191. doi: 10.1093/ntr/nts109. [DOI] [PubMed] [Google Scholar]
- 26.Seo DC, Torabi MR, Weaver AE. Factors influencing openness to future smoking among nonsmoking adolescents. The Journal of school health. 2008;78:328–336. doi: 10.1111/j.1746-1561.2008.00310.x. quiz 356–328. [DOI] [PubMed] [Google Scholar]
- 27.Becklake MR, Ghezzo H, Ernst P. Childhood predictors of smoking in adolescence: A follow-up study of montreal schoolchildren. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2005;173:377–379. doi: 10.1503/cmaj.1041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racicot S, McGrath JJ, O’Loughlin J. An investigation of social and pharmacological exposure to secondhand tobacco smoke as possible predictors of perceived nicotine dependence, smoking susceptibility, and smoking expectancies among never-smoking youth. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2011;13:926–933. doi: 10.1093/ntr/ntr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang MP, Ho SY, Lo WS, Lam TH. Smoking family, secondhand smoke exposure at home, and nicotine addiction among adolescent smokers. Addict Behav. 2012;37:743–746. doi: 10.1016/j.addbeh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Lessov-Schlaggar CN, Wahlgren DR, Liles S, Jones JA, Ji M, Hughes SC, Swan GE, Hovell MF. Sensitivity to secondhand smoke exposure predicts smoking susceptibility in 8–13-year-old never smokers. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2011;48:234–240. doi: 10.1016/j.jadohealth.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntire RK, Nelson AA, Macy JT, Seo DC, Kolbe LJ. Secondhand smoke exposure and other correlates of susceptibility to smoking: A propensity score matching approach. Addict Behav. 2015;48:36–43. doi: 10.1016/j.addbeh.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Mathur C, Stigler MH, Erickson DJ, Perry CL, Forster JL. Transitions in smoking behavior during emerging adulthood: A longitudinal analysis of the effect of home smoking bans. Am J Public Health. 2014;104:715–720. doi: 10.2105/AJPH.2013.301642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Active and passive tobacco exposure: A serious pediatric health problem. A statement from the committee on atherosclerosis and hypertension in children, council on cardiovascular disease in the young, american heart association. Circulation. 1994;90:2581–2590. doi: 10.1161/01.cir.90.5.2581. [DOI] [PubMed] [Google Scholar]
- 34.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiologic Reviews. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Disparities in secondhand smoke exposure--united states, 1988–1994 and 1999–2004. MMWR. 2008;57:744–747. [PubMed] [Google Scholar]
- 36.Kit BK, Simon AE, Brody DJ, Akinbami LJ. Us prevalence and trends in tobacco smoke exposure among children and adolescents with asthma. Pediatrics. 2013;131:407–414. doi: 10.1542/peds.2012-2328. [DOI] [PubMed] [Google Scholar]
- 37.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the u.S. Population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agaku IT, Vardavas CI. Disparities and trends in indoor exposure to secondhand smoke among u.S. Adolescents: 2000–2009. PLoS One. 2013;8:e83058. doi: 10.1371/journal.pone.0083058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao T, Sung H-Y, Mao Z, Hu T-w, Max W. Secondhand smoke exposure at home in rural china. Cancer Causes & Control. 2012;23:109–115. doi: 10.1007/s10552-012-9900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng P, Berg CJ, Kegler MC, Fu W, Wang J, Zhou X, Liu D, Fu H. Smoke-free homes and home exposure to secondhand smoke in shanghai, china. International journal of environmental research and public health. 2014;11:12015–12028. doi: 10.3390/ijerph111112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Group GYTSC. Tobacco use among youth: A cross country comparison. Tobacco Control. 2002;11:252. doi: 10.1136/tc.11.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajalakshmi V, Kanimozhi C. A survey of 24,000 students aged 13–15 years in india: Global youth tobacco survey 2006 and 2009. Tobacco Use Insights. 2010;3:23. [Google Scholar]
- 43.World Health Organization, Regional Office for South-East Asia. Global youth tobacco survey (gyts): Indonesia report 2014. 2015. [Google Scholar]
- 44.Ho SY, Wang MP, Lo WS, Mak KK, Lai HK, Thomas GN, Lam TH. Comprehensive smoke-free legislation and displacement of smoking into the homes of young children in hong kong. Tobacco Control. 2010;19:129–133. doi: 10.1136/tc.2009.032003. [DOI] [PubMed] [Google Scholar]
- 45.Olasky SJ, Levy D, Moran A. Secondhand smoke and cvd in low-and middle-income countries: A case for action. Global heart. 2012;7:151–160. e155. doi: 10.1016/j.gheart.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. 2006. [PubMed] [Google Scholar]
- 47.Willers S, Skarping G, Dalene M, Skerfving S. Urinary cotinine in children and adults during and after semiexperimental exposure to environmental tobacco smoke. Archives of environmental health. 1995;50:130–138. doi: 10.1080/00039896.1995.9940890. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. Vital signs: Nonsmokers’ exposure to secondhand smoke--united states, 1999–2008. MMWR. 2010;59:1141–1146. [PubMed] [Google Scholar]
- 49.King BA, Patel R, Babb SD. Prevalence of smokefree home rules--united states, 1992–1993 and 2010–2011. MMWR. Morbidity and mortality weekly report. 2014;63:765–769. [PMC free article] [PubMed] [Google Scholar]
- 50.Mannino DM, Caraballo RS, Benowitz N, Repace J. Predictors of cotinine levels in us children: Data from the third national health and nutrition examination survey. Chest. 2001;120:718–724. doi: 10.1378/chest.120.3.718. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Stanton B, Hopper J, Khankari N. Sources, locations, and predictors of environmental tobacco smoke exposure among young children from inner-city families. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2011;25:365–372. doi: 10.1016/j.pedhc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Cartmell KB, Miner C, Carpenter MJ, Vitoc CS, Biggers S, Onicescu G, Hill EG, Nickerson BC, Alberg AJ. Secondhand smoke exposure in young people and parental rules against smoking at home and in the car. Public Health Rep. 2011;126:575–582. doi: 10.1177/003335491112600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nabi-Burza E, Regan S, Drehmer J, Ossip D, Rigotti N, Hipple B, Dempsey J, Hall N, Friebely J, Weiley V, Winickoff JP. Parents smoking in their cars with children present. Pediatrics. 2012;130:e1471–1478. doi: 10.1542/peds.2012-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones IA, St Helen G, Meyers MJ, Dempsey DA, Havel C, Jacob P, III, Northcross A, Hammond SK, Benowitz NL. Biomarkers of secondhand smoke exposure in automobiles. Tobacco Control. 2014;23:51–57. doi: 10.1136/tobaccocontrol-2012-050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raoof SA, Agaku IT, Vardavas CI. A systematic review of secondhand smoke exposure in a car: Attributable changes in atmospheric and biological markers. Chron Respir Dis. 2015 doi: 10.1177/1479972315575202. Epub ahead of print 2015 Mar 10. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. Jama. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Martinez-Donate AP, Kuo D, Jones NR, Palmersheim KA. Trends in home smoking bans in the u.S.A., 1995–2007: Prevalence, discrepancies and disparities. Tob Control. 2012;21:330–336. doi: 10.1136/tc.2011.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King BA, Babb SD, Tynan MA, Gerzoff RB. National and state estimates of secondhand smoke infiltration among u.S. Multiunit housing residents. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2013;15:1316–1321. doi: 10.1093/ntr/nts254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Office on SH. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. Atlanta (GA): Centers for Disease Control and Prevention (US); 2006. Publications and reports of the surgeon general. [PubMed] [Google Scholar]
- 60.Orton S, Jones LL, Cooper S, Lewis S, Coleman T. Predictors of children’s secondhand smoke exposure at home: A systematic review and narrative synthesis of the evidence. PLoS One. 2014;9:e112690. doi: 10.1371/journal.pone.0112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pisinger C, Hammer-Helmich L, Andreasen AH, Jorgensen T, Glumer C. Social disparities in children’s exposure to second hand smoke at home: A repeated cross-sectional survey. Environmental health : a global access science source. 2012;11:65. doi: 10.1186/1476-069X-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore GF, Currie D, Gilmore G, Holliday JC, Moore L. Socioeconomic inequalities in childhood exposure to secondhand smoke before and after smoke-free legislation in three uk countries. Journal of public health (Oxford, England) 2012;34:599–608. doi: 10.1093/pubmed/fds025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Longman JM, Passey ME. Children, smoking households and exposure to second-hand smoke in the home in rural australia: Analysis of a national cross-sectional survey. BMJ open. 2013:3. doi: 10.1136/bmjopen-2013-003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrod CS, Fingerlin TE, Chasan-Taber L, Reynolds RM, Glueck DH, Dabelea D. Exposure to prenatal smoking and early-life body composition: The healthy start study. Obesity (Silver Spring, Md) 2015;23:234–241. doi: 10.1002/oby.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raum E, Kupper-Nybelen J, Lamerz A, Hebebrand J, Herpertz-Dahlmann B, Brenner H. Tobacco smoke exposure before, during, and after pregnancy and risk of overweight at age 6. Obesity (Silver Spring, Md) 2011;19:2411–2417. doi: 10.1038/oby.2011.129. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Mamudu HM, Alamian A, Anderson JL, Brooks B. Independent and joint effects of prenatal maternal smoking and maternal exposure to second-hand smoke on the development of adolescent obesity: A longitudinal study. Journal of paediatrics and child health. 2014;50:908–915. doi: 10.1111/jpc.12667. [DOI] [PubMed] [Google Scholar]
- 67.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: A national toxicology program workshop review. Environ Health Perspect. 2012;120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang CC, Lurmann F, Berhane K. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: The southern california children’s health study. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durmus B, Kruithof CJ, Gillman MH, Willemsen SP, Hofman A, Raat H, Eilers PH, Steegers EA, Jaddoe VW. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: The generation r study. The American journal of clinical nutrition. 2011;94:164–171. doi: 10.3945/ajcn.110.009225. [DOI] [PubMed] [Google Scholar]
- 70.Khanolkar AR, Byberg L, Koupil I. Parental influences on cardiovascular risk factors in swedish children aged 5–14 years. European journal of public health. 2012;22:840–847. doi: 10.1093/eurpub/ckr180. [DOI] [PubMed] [Google Scholar]
- 71.Ayer JG, Belousova E, Harmer JA, David C, Marks GB, Celermajer DS. Maternal cigarette smoking is associated with reduced high-density lipoprotein cholesterol in healthy 8-year-old children. European heart journal. 2011;32:2446–2453. doi: 10.1093/eurheartj/ehr174. [DOI] [PubMed] [Google Scholar]
- 72.Neufeld EJ, Mietus-Snyder M, Beiser AS, Baker AL, Newburger JW. Passive cigarette smoking and reduced hdl cholesterol levels in children with high-risk lipid profiles. Circulation. 1997;96:1403–1407. doi: 10.1161/01.cir.96.5.1403. [DOI] [PubMed] [Google Scholar]
- 73.Yang B, Li M, Chen B, Xu Y, Li TD. Deterioration of endothelial function and carotid intima-media thickness in tibetan male adolescents exposed to second-hand smoke. Journal of the renin-angiotensin-aldosterone system : JRAAS. 2012;13:413–419. doi: 10.1177/1470320312440901. [DOI] [PubMed] [Google Scholar]
- 74.Valkonen M, Kuusi T. Passive smoking induces atherogenic changes in low-density lipoprotein. Circulation. 1998;97:2012–2016. doi: 10.1161/01.cir.97.20.2012. [DOI] [PubMed] [Google Scholar]
- 75.Weitzman M, Cook S, Auinger P, Florin TA, Daniels S, Nguyen M, Winickoff JP. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005;112:862–869. doi: 10.1161/CIRCULATIONAHA.104.520650. [DOI] [PubMed] [Google Scholar]
- 76.Levy DE, Rigotti NA, Winickoff JP. Medicaid expenditures for children living with smokers. BMC health services research. 2011;11:125. doi: 10.1186/1472-6963-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill SC, Liang L. Smoking in the home and children’s health. Tobacco Control. 2008;17:32–37. doi: 10.1136/tc.2007.020990. [DOI] [PubMed] [Google Scholar]
- 78.Medical-care expenditures attributable to cigarette smoking during pregnancy -- united states, 1995. MMWR. Morbidity and mortality weekly report. 1997;46:1048–1050. [PubMed] [Google Scholar]
- 79.Lightwood JM, Phibbs CS, Glantz SA. Short-term health and economic benefits of smoking cessation: Low birth weight. Pediatrics. 1999;104:1312–1320. doi: 10.1542/peds.104.6.1312. [DOI] [PubMed] [Google Scholar]
- 80.Max W, Sung HY, Shi Y. Childhood secondhand smoke exposure and adhd-attributable costs to the health and education system. The Journal of school health. 2014;84:683–686. doi: 10.1111/josh.12191. [DOI] [PubMed] [Google Scholar]
- 81.Gutstadt LB, Gillette JW, Mrazek DA, Fukuhara JT, LaBrecque JF, Strunk RC. Determinants of school performance in children with chronic asthma. American Journal of Diseases of Children. 1989;143:471–475. doi: 10.1001/archpedi.1989.02150160101020. [DOI] [PubMed] [Google Scholar]
- 82.Ohlund LS, Ericsson KB. Elementary school achievement and absence due to illness. The Journal of genetic psychology. 1994;155:409–421. doi: 10.1080/00221325.1994.9914791. [DOI] [PubMed] [Google Scholar]
- 83.Richards W. Allergy, asthma, and school problems. Journal of School Health. 1986;56:151–152. doi: 10.1111/j.1746-1561.1986.tb05725.x. [DOI] [PubMed] [Google Scholar]
- 84.Levy DE, Winickoff JP, Rigotti NA. School absenteeism among children living with smokers. Pediatrics. 2011;128:650–656. doi: 10.1542/peds.2011-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: An update. Am J Prev Med. 2014;48:326–333. doi: 10.1016/j.amepre.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robert Wood Johnson Foundation and other partners. Broken promises to our children: The 1998 state tobacco settlement sixteen years later. 2014. [Google Scholar]
- 87.Dilley JA, Harris JR, Boysun MJ, Reid TR. Program, policy, and price interventions for tobacco control: Quantifying the return on investment of a state tobacco control program. Am J Public Health. 2012;102:e22–28. doi: 10.2105/AJPH.2011.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lightwood J, Glantz SA. The effect of the california tobacco control program on smoking prevalence, cigarette consumption, and healthcare costs: 1989–2008. PLoS One. 2013;8:e47145. doi: 10.1371/journal.pone.0047145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.King BA, Peck RM, Babb SD. National and state cost savings associated with prohibiting smoking in subsidized and public housing in the united states. Preventing chronic disease. 2014;11:E171. doi: 10.5888/pcd11.140222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.California Environmental Protection Agency. Part b: Health effects. 2005. Proposed identification of environmental tobacco smoke as a toxic air contaminant. [Google Scholar]
- 91.Hammond SK, Coghlin J, Gann PH, Paul M, Taghizadeh K, Skipper PL, Tannenbaum SR. Relationship between environmental tobacco smoke exposure and carcinogen-hemoglobin adduct levels in nonsmokers. Journal of the National Cancer Institute. 1993;85:474–478. doi: 10.1093/jnci/85.6.474. [DOI] [PubMed] [Google Scholar]
- 92.Guerin MR. Formation and physicochemical nature of sidestream smoke. IARC scientific publications. 1987:11–23. [PubMed] [Google Scholar]
- 93.Kritz H, Schmid P, Sinzinger H. Passive smoking and cardiovascular risk. Arch Intern Med. 1995;155:1942–1948. [PubMed] [Google Scholar]
- 94.Martins-Green M, Adhami N, Frankos M, Valdez M, Goodwin B, Lyubovitsky J, Dhall S, Garcia M, Egiebor I, Martinez B. Cigarette smoke toxins deposited on surfaces: Implications for human health. PloS one. 2014:9. doi: 10.1371/journal.pone.0086391. [DOI] [PMC free article] [PubMed]
- 95.Jenkins R, Guerin MR, Tomkins BA. The chemistry of environmental tobacco smoke: Composition and measurement. Boca Raton FL: Lewis Publishers; 2000. [Google Scholar]
- 96.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environmental Health Perspectives. 1999;107:349. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lodovici M, Akpan V, Evangelisti C, Dolara P. Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. Journal of applied toxicology : JAT. 2004;24:277–281. doi: 10.1002/jat.992. [DOI] [PubMed] [Google Scholar]
- 98.Schick S, Glantz S. Philip morris toxicological experiments with fresh sidestream smoke: More toxic than mainstream smoke. Tob Control. 2005;14:396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singer BC, Hodgson AT, Guevarra KS, Hawley EL, Nazaroff WW. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environmental Science Technology. 2002;36:846–853. doi: 10.1021/es011058w. [DOI] [PubMed] [Google Scholar]
- 100.Pritchard JN, Black A, McAughey JJ. The physical behavior of sidestream smoke under ambient conditions. Environ Technol Lett. 1988;9:545–552. [Google Scholar]
- 101.Benner C, Bayona JM, Caka FM, Tang H, Lewis L, Crawford J, Lamb L, Lee MI, Lewis EA, Hansen LD, Eatough DJ. Chemical composition of environmental tobacco smoke. 2. Particulate phase compounds. Environmental ScienceTechnology. 1989;23:688–699. [Google Scholar]
- 102.Council NR. Human biomonitoring for environmental chemicals. 2006. [Google Scholar]
- 103.Benowitz NL. Cigarette smoking and cardiovascular disease: Pathophysiology and implications for treatment. Progress in cardiovascular diseases. 2003;46:91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 104.Chelland Campbell S, Moffatt RJ, Stamford BA. Smoking and smoking cessation -- the relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis. 2008;201:225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 105.Therond P. Catabolism of lipoproteins and metabolic syndrome. Current opinion in clinical nutrition and metabolic care. 2009;12:366–371. doi: 10.1097/MCO.0b013e32832c5a12. [DOI] [PubMed] [Google Scholar]
- 106.Bhatnagar A. Cardiovascular pathophysiology of environmental pollutants. Am J Physiol Heart Circ Physiol. 2004;286:H479–485. doi: 10.1152/ajpheart.00817.2003. [DOI] [PubMed] [Google Scholar]
- 107.O’Toole TE, Zheng YT, Hellmann J, Conklin DJ, Barski O, Bhatnagar A. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicology and applied pharmacology. 2009;236:194–201. doi: 10.1016/j.taap.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–267. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 109.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 110.Subramanyam G, Bhaskar M, Govindappa S. The role of cadmium in induction of atherosclerosis in rabbits. Indian heart journal. 1992;44:177–180. [PubMed] [Google Scholar]
- 111.Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–2449. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 112.Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re-appraisal of the association between blood pressure and blood lead: A meta-analysis. Journal of human hypertension. 2002;16:123–131. doi: 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- 113.Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 114.Bhatnagar A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circulation research. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- 115.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 116.Jauniaux E, Gulbis B, Acharya G, Gerlo E. Fetal amino acid and enzyme levels with maternal smoking. Obstetrics and gynecology. 1999;93:680–683. doi: 10.1016/s0029-7844(98)00548-1. [DOI] [PubMed] [Google Scholar]
- 117.Nafstad P, Hagen JA, Oie L, Magnus P, Jaakkola JJ. Day care centers and respiratory health. Pediatrics. 1999;103:753–758. doi: 10.1542/peds.103.4.753. [DOI] [PubMed] [Google Scholar]
- 118.Irvine L, Crombie IK, Clark RA, Slane PW, Goodman KE, Feyerabend C, Cater JI. What determines levels of passive smoking in children with asthma? Thorax. 1997;52:766–769. doi: 10.1136/thx.52.9.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Preston AM, Ramos LJ, Calderon C, Sahai H. Exposure of puerto rican children to environmental tobacco smoke. Prev Med. 1997;26:1–7. doi: 10.1006/pmed.1996.9985. [DOI] [PubMed] [Google Scholar]
- 120.Gairola CG, Drawdy ML, Block AE, Daugherty A. Sidestream cigarette smoke accelerates atherogenesis in apolipoprotein e−/− mice. Atherosclerosis. 2001;156:49–55. doi: 10.1016/s0021-9150(00)00621-3. [DOI] [PubMed] [Google Scholar]
- 121.Tani S, Dimayuga PC, Anazawa T, Chyu K-Y, Li H, Shah PK, Cercek B. Aberrant antibody responses to oxidized ldl and increased intimal thickening in apoe−/− mice exposed to cigarette smoke. Atherosclerosis. 2004;175:7–14. doi: 10.1016/j.atherosclerosis.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 122.Zhang J, Jiang S, Watson RR. Antioxidant supplementation prevents oxidation and inflammatory responses induced by sidestream cigarette smoke in old mice. Environmental health perspectives. 2001;109:1007. doi: 10.1289/ehp.011091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation. 2002;105:849–854. doi: 10.1161/hc0702.103977. [DOI] [PubMed] [Google Scholar]
- 124.Fetterman JL, Pompilius M, Westbrook DG, Uyeminami D, Brown J, Pinkerton KE, Ballinger SW. Developmental exposure to second-hand smoke increases adult atherogenesis and alters mitochondrial DNA copy number and deletions in apoe−/− mice. PloS one. 2013;8:e66835. doi: 10.1371/journal.pone.0066835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mullick AE, McDonald JM, Melkonian G, Talbot P, Pinkerton KE, Rutledge JC. Reactive carbonyls from tobacco smoke increase arterial endothelial layer injury. American Journal of Physiology-Heart and Circulatory Physiology. 2002;283:H591–H597. doi: 10.1152/ajpheart.01046.2001. [DOI] [PubMed] [Google Scholar]
- 126.Hutchison SJ, Glantz SA, Zhu B-Q, Sun Y-P, Chou TM, Chatterjee K, Deedwania PC, Parmley WW, Sudhir K. In-utero and neonatal exposure to secondhand smoke causes vascular dysfunction in newborn rats. Journal of the American College of Cardiology. 1998;32:1463–1467. doi: 10.1016/s0735-1097(98)00217-4. [DOI] [PubMed] [Google Scholar]
- 127.Roberts K, Rezai A, Pinkerton K, Rutledge J. Effect of environmental tobacco smoke on ldl accumulation in the artery wall. Circulation. 1996;94:2248–2253. doi: 10.1161/01.cir.94.9.2248. [DOI] [PubMed] [Google Scholar]
- 128.Zhu B-Q, Sun Y-P, Sievers RE, Isenberg WM, Glantz SA, Parmley WW. Passive smoking increases experimental atherosclerosis in cholesterol-fed rabbits. Journal of the American College of Cardiology. 1993;21:225–232. doi: 10.1016/0735-1097(93)90741-i. [DOI] [PubMed] [Google Scholar]
- 129.Hutchison SJ, Sudhir K, Chou TM, Sievers RE, Zhu B-Q, Sun Y-P, Deedwania PC, Glantz SA, Parmley WW, Chatterjee K. Testosterone worsens endothelial dysfunction associated with hypercholesterolemia and environmental tobacco smoke exposure in male rabbit aorta 1. Journal of the American College of Cardiology. 1997;29:800–807. doi: 10.1016/s0735-1097(96)00570-0. [DOI] [PubMed] [Google Scholar]
- 130.Penn A, Snyder CA. Inhalation of sidestream cigarette smoke accelerates development of arteriosclerotic plaques. Circulation. 1993;88:1820–1825. doi: 10.1161/01.cir.88.4.1820. [DOI] [PubMed] [Google Scholar]
- 131.Penn A, Chen L-C, Snyder CA. Inhalation of steady-state sidestream smoke from one cigarette promotes arteriosclerotic plaque development. Circulation. 1994;90:1363–1367. doi: 10.1161/01.cir.90.3.1363. [DOI] [PubMed] [Google Scholar]
- 132.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 133.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S. The assessment of endothelial function from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? Journal of the American College of Cardiology. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 135.Celermajer DS, Sorensen K, Gooch V, Sullivan I, Lloyd J, Deanfield J, Spiegelhalter D. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. The Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 136.Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, Matsubara J, Kurokawa H, Fujisue K, Konishi M. Peripheral endothelial function and cardiovascular events in high-risk patients. Journal of the American Heart Association. 2013;2:e000426. doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Otsuka R, Watanabe H, Hirata K, Tokai K, Muro T, Yoshiyama M, Takeuchi K, Yoshikawa J. Acute effects of passive smoking on the coronary circulation in healthy young adults. Jama. 2001;286:436–441. doi: 10.1001/jama.286.4.436. [DOI] [PubMed] [Google Scholar]
- 138.Sumida H, Watanabe H, Kugiyama K, Ohgushi M, Matsumura T, Yasue H. Does passive smoking impair endothelium-dependent coronary artery dilation in women? 1. Journal of the American College of Cardiology. 1998;31:811–815. doi: 10.1016/s0735-1097(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 139.Shen Y, Rattan V, Sultana C, Kalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. American Journal of Physiology-Heart and Circulatory Physiology. 1996;39:H1624. doi: 10.1152/ajpheart.1996.270.5.H1624. [DOI] [PubMed] [Google Scholar]
- 140.Adams MR, Jessup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: Reversibility with oral l-arginine but not vitamin c. Journal of the American College of Cardiology. 1997;29:491–497. doi: 10.1016/s0735-1097(96)00537-2. [DOI] [PubMed] [Google Scholar]
- 141.Matetzky S, Tani S, Kangavari S, Dimayuga P, Yano J, Xu H, Chyu KY, Fishbein MC, Shah PK, Cercek B. Smoking increases tissue factor expression in atherosclerotic plaques: Implications for plaque thrombogenicity. Circulation. 2000;102:602–604. doi: 10.1161/01.cir.102.6.602. [DOI] [PubMed] [Google Scholar]
- 142.Hausberg M, Mark AL, Winniford MD, Brown RE, Somers VK. Sympathetic and vascular effects of short-term passive smoke exposure in healthy nonsmokers. Circulation. 1997;96:282–287. [PubMed] [Google Scholar]
- 143.Juonala M, Magnussen CG, Venn A, Gall S, Kähönen M, Laitinen T, Taittonen L, Lehtimäki T, Jokinen E, Sun C. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults the cardiovascular risk in young finns study and the childhood determinants of adult health study. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1024–1031. doi: 10.1161/ATVBAHA.111.243261. [DOI] [PubMed] [Google Scholar]
- 144.Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS. Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Annals of internal medicine. 1999;130:578–581. doi: 10.7326/0003-4819-130-7-199904060-00017. [DOI] [PubMed] [Google Scholar]
- 145.Howard DJ, Ota RB, Briggs LA, Hampton M, Pritsos CA. Environmental tobacco smoke in the workplace induces oxidative stress in employees, including increased production of 8-hydroxy-2′-deoxyguanosine. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7:141–146. [PubMed] [Google Scholar]
- 146.Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Masoura C, Toutouzas P, Stefanadis C. Effect of exposure to secondhand smoke on markers of inflammation: The attica study. The American journal of medicine. 2004;116:145–150. doi: 10.1016/j.amjmed.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 147.Jaimes EA, Sweeney C, Raij L. Effects of the reactive oxygen species hydrogen peroxide and hypochlorite on endothelial nitric oxide production. Hypertension. 2001;38:877–883. [PubMed] [Google Scholar]
- 148.Yang Z, Harrison CM, Chuang GC, Ballinger SW. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutation research. 2007;621:61–74. doi: 10.1016/j.mrfmmm.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Priest JR, Nead KT, Wehner MR, Cooke JP, Leeper NJ. Self-reported history of childhood smoking is associated with an increased risk for peripheral arterial disease independent of lifetime smoking burden. PLoS One. 2014;9:e88972. doi: 10.1371/journal.pone.0088972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The atherosclerosis risk in communities (aric) study. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 152.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: The atherosclerosis risk in communities (aric) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 153.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The atherosclerosis risk in communities (aric) study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 154.Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Ronnemaa T, Raitakari OT. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: The cardiovascular risk in young finns study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 155.Kallio K, Jokinen E, Saarinen M, Hamalainen M, Volanen I, Kaitosaari T, Ronnemaa T, Viikari J, Raitakari OT, Simell O. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circulation. Cardiovascular quality and outcomes. 2010;3:196–203. doi: 10.1161/CIRCOUTCOMES.109.857771. [DOI] [PubMed] [Google Scholar]
- 156.Gall S, Huynh QL, Magnussen CG, Juonala M, Viikari JS, Kähönen M, Dwyer T, Raitakari OT, Venn A. Exposure to parental smoking in childhood or adolescence is associated with increased carotid intima-media thickness in young adults: Evidence from the cardiovascular risk in young finns study and the childhood determinants of adult health study. European heart journal. 2014;35:2484–2491. doi: 10.1093/eurheartj/ehu049. [DOI] [PubMed] [Google Scholar]
- 157.Kaess BM, Vasan RS, Mitchell GF. Aortic stiffness and incident hypertension--reply. JAMA. 2013;309:30. doi: 10.1001/jama.2012.68805. [DOI] [PubMed] [Google Scholar]
- 158.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mack WJ, Islam T, Lee Z, Selzer RH, Hodis HN. Environmental tobacco smoke and carotid arterial stiffness. Prev Med. 2003;37:148–154. doi: 10.1016/s0091-7435(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 160.Stefanadis C, Vlachopoulos C, Tsiamis E, Diamantopoulos L, Toutouzas K, Giatrakos N, Vaina S, Tsekoura D, Toutouzas P. Unfavorable effects of passive smoking on aortic function in men. Annals of internal medicine. 1998;128:426–434. doi: 10.7326/0003-4819-128-6-199803150-00002. [DOI] [PubMed] [Google Scholar]
- 161.Taal HR, de Jonge LL, van Osch-Gevers L, Steegers EA, Hofman A, Helbing WA, van der Heijden AJ, Jaddoe VW. Parental smoking during pregnancy and cardiovascular structures and function in childhood: The generation r study. Int J Epidemiol. 2013;42:1371–1380. doi: 10.1093/ije/dyt178. [DOI] [PubMed] [Google Scholar]
- 162.Schuetze P, Eiden RD. The association between maternal smoking and secondhand exposure and autonomic functioning at 2–4 weeks of age. Infant behavior & development. 2006;29:32–43. doi: 10.1016/j.infbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 163.Dixit S, Pletcher MJ, Vittinghoff E, Imburgia K, Maguire C, Whitman IR, Glantz SA, Olgin JE, Marcus GM. Secondhand smoke and atrial fibrillation: Data from the health eheart study. Heart rhythm : the official journal of the Heart Rhythm Society. 2015 doi: 10.1016/j.hrthm.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 165.Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation. 1998;98:528–534. doi: 10.1161/01.cir.98.6.528. [DOI] [PubMed] [Google Scholar]
- 166.Laustiola KE, Lassila R, Kaprio J, Koskenvuo M. Decreased beta-adrenergic receptor density and catecholamine response in male cigarette smokers. A study of monozygotic twin pairs discordant for smoking. Circulation. 1988;78:1234–1240. doi: 10.1161/01.cir.78.5.1234. [DOI] [PubMed] [Google Scholar]
- 167.Laustiola KE, Kotamaki M, Lassila R, Kallioniemi OP, Manninen V. Cigarette smoking alters sympathoadrenal regulation by decreasing the density of beta 2-adrenoceptors. A study of monitored smoking cessation. Journal of cardiovascular pharmacology. 1991;17:923–928. doi: 10.1097/00005344-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 168.Sheps DS, Herbst MC, Hinderliter AL, Adams KF, Ekelund LG, O’Neil JJ, Goldstein GM, Bromberg PA, Dalton JL, Ballenger MN, et al. Production of arrhythmias by elevated carboxyhemoglobin in patients with coronary artery disease. Annals of internal medicine. 1990;113:343–351. doi: 10.7326/0003-4819-113-5-343. [DOI] [PubMed] [Google Scholar]
- 169.Plank B, Kutyifa V, Moss AJ, Huang DT, Ruwald AC, McNitt S, Polonsky B, Zareba W, Goldenberg I, Aktas MK. Smoking is associated with an increased risk of first and recurrent ventricular tachyarrhythmias in ischemic and nonischemic patients with mild heart failure: A madit-crt substudy. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11:822–827. doi: 10.1016/j.hrthm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 170.Engstrom G, Hedblad B, Janzon L, Juul-Moller S. Ventricular arrhythmias during 24-h ambulatory ecg recording: Incidence, risk factors and prognosis in men with and without a history of cardiovascular disease. Journal of internal medicine. 1999;246:363–372. doi: 10.1046/j.1365-2796.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 171.D’Alessandro A, Boeckelmann I, Hammwhoner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: An overview. European journal of preventive cardiology. 2012;19:297–305. doi: 10.1177/1741826711411738. [DOI] [PubMed] [Google Scholar]
- 172.Kaul B, Calabro J, Hutcheon DE. Effects of carbon monoxide on the vulnerability of the ventricles to drug-induced arrhythmias. Journal of clinical pharmacology. 1974;14:25–31. doi: 10.1002/j.1552-4604.1974.tb02283.x. [DOI] [PubMed] [Google Scholar]
- 173.Aronow WS, Stemmer EA, Wood B, Zweig S, Tsao KP, Raggio L. Carbon monoxide and ventricular fibrillation threshold in dogs with acute myocardial injury. American heart journal. 1978;95:754–756. doi: 10.1016/0002-8703(78)90506-9. [DOI] [PubMed] [Google Scholar]
- 174.McMartin KI, Platt MS, Hackman R, Klein J, Smialek JE, Vigorito R, Koren G. Lung tissue concentrations of nicotine in sudden infant death syndrome (sids) The Journal of pediatrics. 2002;140:205–209. doi: 10.1067/mpd.2002.121937. [DOI] [PubMed] [Google Scholar]
- 175.Mitchell EA, Ford RP, Stewart AW, Taylor BJ, Becroft DM, Thompson JM, Scragg R, Hassall IB, Barry DM, Allen EM, et al. Smoking and the sudden infant death syndrome. Pediatrics. 1993;91:893–896. [PubMed] [Google Scholar]
- 176.Schoendorf KC, Kiely JL. Relationship of sudden infant death syndrome to maternal smoking during and after pregnancy. Pediatrics. 1992;90:905–908. [PubMed] [Google Scholar]
- 177.Pollack HA. Sudden infant death syndrome, maternal smoking during pregnancy, and the cost-effectiveness of smoking cessation intervention. Am J Public Health. 2001;91:432–436. doi: 10.2105/ajph.91.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anderson HR, Cook DG. Passive smoking and sudden infant death syndrome: Review of the epidemiological evidence. Thorax. 1997;52:1003–1009. doi: 10.1136/thx.52.11.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Comstock GW, Lundin FE., Jr Parental smoking and perinatal mortality. American journal of obstetrics and gynecology. 1967;98:708–718. doi: 10.1016/0002-9378(67)90185-8. [DOI] [PubMed] [Google Scholar]
- 180.Malloy MH, Kleinman JC, Land GH, Schramm WF. The association of maternal smoking with age and cause of infant death. Am J Epidemiol. 1988;128:46–55. doi: 10.1093/oxfordjournals.aje.a114957. [DOI] [PubMed] [Google Scholar]
- 181.Rush D, Kass EH. Maternal smoking: A reassessment of the association with perinatal mortality. Am J Epidemiol. 1972;96:183–196. doi: 10.1093/oxfordjournals.aje.a121447. [DOI] [PubMed] [Google Scholar]
- 182.Poswillo D. Report of the scientific committee on tobacco and health. London: The Stationery Office; 1998. [Google Scholar]
- 183.Office on SH. Women and smoking: A report of the surgeon general. Atlanta (GA): Centers for Disease Control and Prevention (US); 2001. Publications and reports of the surgeon general. [PubMed] [Google Scholar]
- 184.Schramm WF. Smoking during pregnancy: Missouri longitudinal study. Paediatric and perinatal epidemiology. 1997;11(Suppl 1):73–83. doi: 10.1046/j.1365-3016.11.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 185.Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period: Linked birth/infant death data set. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2004;53:1–29. [PubMed] [Google Scholar]
- 186.Davis P, Price L, Heatman B, Bailey L, John N, Rolfe K. Sudden unexpected death in infancy: A collaborative thematic review 2010–2012. Child Death Review Programme and All Wales Perinatal Survey. 2015 [Google Scholar]
- 187.US Department of Health Human Services. Us dept. Health and human services. Public Health Service, Office of the Assistant Secretary for Health, Office on Smoki; 1986. The health consequences of smoking for women. A report of the surgeon general. [Google Scholar]
- 188.Behm I, Kabir Z, Connolly GN, Alpert HR. Increasing prevalence of smoke-free homes and decreasing rates of sudden infant death syndrome in the united states: An ecological association study. Tob Control. 2012;21:6–11. doi: 10.1136/tc.2010.041376. [DOI] [PubMed] [Google Scholar]
- 189.Slotkin TA. Fetal nicotine or cocaine exposure: Which one is worse? The Journal of pharmacology and experimental therapeutics. 1998;285:931–945. [PubMed] [Google Scholar]
- 190.Slotkin TA, Pinkerton KE, Garofolo MC, Auman JT, McCook EC, Seidler FJ. Perinatal exposure to environmental tobacco smoke induces adenylyl cyclase and alters receptor-mediated cell signaling in brain and heart of neonatal rats. Brain research. 2001;898:73–81. doi: 10.1016/s0006-8993(01)02145-x. [DOI] [PubMed] [Google Scholar]
- 191.Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal environmental tobacco smoke exposure in rhesus monkeys: Critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ Health Perspect. 2006;114:34–39. doi: 10.1289/ehp.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fifer WP, Fingers ST, Youngman M, Gomez-Gribben E, Myers MM. Effects of alcohol and smoking during pregnancy on infant autonomic control. Developmental psychobiology. 2009;51:234–242. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Franco P, Chabanski S, Szliwowski H, Dramaix M, Kahn A. Influence of maternal smoking on autonomic nervous system in healthy infants. Pediatr Res. 2000;47:215–220. doi: 10.1203/00006450-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 194.Cohen G, Vella S, Jeffery H, Lagercrantz H, Katz-Salamon M. Cardiovascular stress hyperreactivity in babies of smokers and in babies born preterm. Circulation. 2008;118:1848–1853. doi: 10.1161/CIRCULATIONAHA.108.783902. [DOI] [PubMed] [Google Scholar]
- 195.Harper RM. Sudden infant death syndrome: A failure of compensatory cerebellar mechanisms? Pediatr Res. 2000;48:140–142. doi: 10.1203/00006450-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 196.Neff RA, Humphrey J, Mihalevich M, Mendelowitz D. Nicotine enhances presynaptic and postsynaptic glutamatergic neurotransmission to activate cardiac parasympathetic neurons. Circulation research. 1998;83:1241–1247. doi: 10.1161/01.res.83.12.1241. [DOI] [PubMed] [Google Scholar]
- 197.Huang Z-G, Wang X, Dergacheva O, Mendelowitz D. Prenatal nicotine exposure recruits an excitatory pathway to brainstem parasympathetic cardioinhibitory neurons during hypoxia/hypercapnia in the rat: Implications for sudden infant death syndrome. Pediatric research. 2005;58:562–567. doi: 10.1203/01.PDR.0000179380.41355.FC. [DOI] [PubMed] [Google Scholar]
- 198.Arborelius E, Hallberg AC, Hakansson A. How to prevent exposure to tobacco smoke among small children: A literature review. Acta paediatrica (Oslo, Norway : 1992). Supplement. 2000;89:65–70. doi: 10.1111/j.1651-2227.2000.tb03098.x. [DOI] [PubMed] [Google Scholar]
- 199.Klerman L. Protecting children: Reducing their environmental tobacco smoke exposure. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2004;6(Suppl 2):S239–253. doi: 10.1080/14622200410001669213. [DOI] [PubMed] [Google Scholar]
- 200.Wewers ME, Uno M. Clinical interventions and smoking ban methods to reduce infants’ and children’s exposure to environmental tobacco smoke. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN/NAACOG. 2002;31:592–598. doi: 10.1111/j.1552-6909.2002.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 201.Adair CE, Patten S. A review of interventions for reduction of residential environmental tobacco smoke exposures among children. Paediatrics & child health. 2001;6:70–79. doi: 10.1093/pch/6.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Emmons KM, Wong M, Hammond SK, Velicer WF, Fava JL, Monroe AD, Evans JL. Intervention and policy issues related to children’s exposure to environmental tobacco smoke. Prev Med. 2001;32:321–331. doi: 10.1006/pmed.2000.0822. [DOI] [PubMed] [Google Scholar]
- 203.Roseby R, Waters E, Polnay A, Campbell R, Webster P, Spencer N. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2003:Cd001746. doi: 10.1002/14651858.CD001746. [DOI] [PubMed] [Google Scholar]
- 204.Priest N, Roseby R, Waters E, Polnay A, Campbell R, Spencer N, Webster P, Ferguson-Thorne G. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2008:Cd001746. doi: 10.1002/14651858.CD001746.pub2. [DOI] [PubMed] [Google Scholar]
- 205.Rosen LJ, Myers V, Hovell M, Zucker D, Ben Noach M. Meta-analysis of parental protection of children from tobacco smoke exposure. Pediatrics. 2014;133:698–714. doi: 10.1542/peds.2013-0958. [DOI] [PubMed] [Google Scholar]
- 206.Baxi R, Sharma M, Roseby R, Polnay A, Priest N, Waters E, Spencer N, Webster P. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2014;3:Cd001746. doi: 10.1002/14651858.CD001746.pub3. [DOI] [PubMed] [Google Scholar]
- 207.Recommendations regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med. 2001;20:10–15. [PubMed] [Google Scholar]
- 208.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011;128(Suppl 5):S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reduced secondhand smoke exposure after implementation of a comprehensive statewide smoking ban--new york, june 26, 2003–june 30, 2004. MMWR. Morbidity and mortality weekly report. 2007;56:705–708. [PubMed] [Google Scholar]
- 210.Jarvis MJ, Sims M, Gilmore A, Mindell J. Impact of smoke-free legislation on children’s exposure to secondhand smoke: Cotinine data from the health survey for england. Tob Control. 2012;21:18–23. doi: 10.1136/tc.2010.041608. [DOI] [PubMed] [Google Scholar]
- 211.Cheng KW, Glantz SA, Lightwood JM. Association between smokefree laws and voluntary smokefree-home rules. Am J Prev Med. 2011;41:566–572. doi: 10.1016/j.amepre.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chaloupka FJ, Straif K, Leon ME. Effectiveness of tax and price policies in tobacco control. Tob Control. 2011;20:235–238. doi: 10.1136/tc.2010.039982. [DOI] [PubMed] [Google Scholar]
- 213.National Research Council Committee on Passive S. Environmental tobacco smoke: Measuring exposures and assessing health effects. Washington (DC): National Academies Press (US); [PubMed] [Google Scholar]